Abstract
Aim of the Study:
To identify plasma biomarkers associated with cardiac arrest in a cohort of children with acute respiratory distress syndrome (ARDS), and to assess the association of these biomarkers with mortality in children with cardiac arrest and ARDS (ARDS+CA).
Methods:
This was a secondary analysis of a single-center prospective cohort study of children with ARDS from 2014-2019 with 17 biomarkers measured. Clinical characteristics and biomarkers were compared between subjects with ARDS+CA and ARDS with univariate analysis. In a sub-cohort of ARDS+CA subjects, the association between biomarker levels and mortality was tested using univariate and bivariate logistic regression.
Results:
Biomarkers were measured in 333 subjects: 301 with ARDS (median age 5.3 years, 55.5% male) and 32 ARDS+CA (median age 8 years, 53.1% male). More arrests (69%) occurred out-of-hospital with a median CPR duration of 11 (IQR 5.5, 25) minutes. ARDS severity, PRISM III score, vasoactive-ionotropic score and extrapulmonary organ failures were worse in the ARDS+CA versus ARDS group. Eight biomarkers were elevated in the ARDS+CA versus ARDS cohort: sRAGE, nucleosomes, SP-D, CCL22, IL-6, HSP70, IL-8, and MIP-1b. sRAGE, SP-D, and CCL22 remained elevated when the cohorts were matched for illness severity. When controlling for severity of ARDS and cardiac arrest characteristics, sRAGE, IL-6 and granzyme B were associated with mortality in the ARDS+CA group.
Conclusion:
sRAGE, IL-6 and granzyme B were associated with cardiac arrest mortality when controlling for illness severity. sRAGE was consistently higher in the ARDS+CA cohort compared to ARDS and retained independent association with mortality.
Keywords: cardiac arrest, child, biomarkers
INTRODUCTION
Cardiac arrest occurs in more than 20,000 children per year in the United States and is associated with high mortality and morbidity [1]. The post-cardiac arrest syndrome (PCAS) is characterized by brain injury, myocardial dysfunction, and a systemic ischemic-reperfusion response [2]. The pathophysiology of pediatric post-arrest brain injury [3-5] and myocardial dysfunction [6, 7] has been studied; however there are little data characterizing the pediatric post-arrest systemic ischemic perfusion response.
The post-arrest systemic ischemic-reperfusion response is a sepsis-like syndrome that clinically manifests as vasoplegia, hypotension, coagulopathy, adrenal insufficiency, hyperglycemia, and fever – all of which can contribute to multisystem organ dysfunction [2, 8]. This process is a combination of reperfusion-induced free radical-mediated injury, endothelial activation, and systemic inflammation post-arrest. In adults, elevated levels of IL-6, TNF-α and other cytokines characterize this sepsis-like state of inflammation [9-11]. While brain-specific biomarkers have been evaluated following pediatric cardiac arrest to prognosticate outcomes [12-14], limited pediatric studies to date have focused on the association of biomarkers of systemic inflammation with clinical outcomes; to date elevated levels IL-17 have been associated with unfavorable 6-month neurologic outcome in cardiac arrest survivors [15].
Given the reported associations between innate immune markers and poor outcomes in adult cardiac arrest [10, 16, 17], we theorized a similar association in pediatric cardiac arrest. Therefore, we leveraged an established plasma biobank with multiple potentially informative markers of systemic inflammation measured in children with acute respiratory distress syndrome (ARDS) [18, 19] to investigate their utility in the sub-cohort of subjects with ARDS who also suffered a cardiac arrest (ARDS+CA). We aimed to: (1) identify plasma biomarkers that are associated with cardiac arrest in a cohort of subjects with ARDS and, (2) to identify plasma biomarkers that are associated with mortality in children with ARDS+CA. We specifically hypothesized that subjects ARDS+CA would have biomarkers reflecting innate immunity associated with mortality.
METHODS
This study was approved by the Children’s Hospital of Philadelphia (CHOP) Institutional Review Board (IRB). Subjects for this study were previously enrolled in a biomarker study in pediatric ARDS at CHOP from 2014-2019 [20].
Population:
Subjects were eligible for the parent ARDS study if they were greater than 1 month and less than 18 years of age, required invasive ventilation, had arterial access, and had ARDS defined as two consecutive PaO2/FiO2 measurements less than or equal to 300 at least one hour apart on positive end-expiratory pressure greater than or equal to 5cm H2O, and bilateral parenchymal infiltrates on chest radiograph. We identified the subset of subjects who had a cardiac arrest (ARDS+CA), defined as receiving CPR at least one minute either prior to or during admission to the PICU. Cardiac arrest could have occurred prior to hospital admission and the diagnosis of ARDS or after ARDS diagnosis [21].
Clinical Variables, Definitions & Outcomes:
Demographics, severity of illness defined by the Pediatric Risk of Mortality (PRISM) III [22] were abstracted from the primary ARDS study [20]. Cardiac arrest characteristics were collected for our institution’s post-cardiac arrest database [7, 23]. Abstracters for both databases used their standardized data collection and were blinded to the outcome of mortality.
Metrics of oxygenation utilized were PaO2/FiO2 (PF ratio) and oxygenation index (OI): [mean airway pressure (mPaw)×FiO2× 100]/PaO2 at pediatric ARDS onset and at 24 hours. The maximum vasopressor-inotrope score (VIS) score at 72 hours was calculated using: dopamine dose (μg kg min)×1 + dobutamine (μg kg min)×1 + epinephrine (μg kg min)×100 + norepinephrine (μg kg min)×100 + phenylephrine (μg kg min)×100 + milrinone (μg kg min)×10 + vasopressin (U/kg/min)×10,000 [24]. The number of extrapulmonary organ failures at time of ARDS diagnosis were identified by accepted definitions in children [25].
Biomarker Analyses:
Blood samples were collected within 24 hours of ARDS diagnosis. Samples were collected in citrated tubes, centrifuged within 30 minutes of collection (2000 G, 20 minutes, 20°C) and stored at −80°C until analysis. Analysis of biomarkers was performed using a combination of single- and multiplex enzyme-linked immunosorbent assays (ELISAs). Granzyme B, HSP70, IL-1A, IL-8, CCL3/MIP-1A, MIP-1B, and MM8 were measured using an established Luminex platform [26]. CCL22, CCL7, IL-6, sTNFR1, and TNF-alpha were measured using a custom multiplex on the Ella (Biotechne) platform. The remaining analytes (Ang2, sRAGE, nucleosomes, SPD1, and OFM4) were measured using commercially available singleplex ELISAs (R&D Systems and Sigma-Aldrich). Overall variability was minimal between plates (<10%), with samples above and below lower limits of detection set to equal the highest or lowest value for that plate.
Statistical Analyses:
Analysis was performed with Stata/SE 16 (College Station, TX) and Prism 9 (GraphPad Software, San Diego, CA, USA). Descriptive statistics included numbers with percentages for categorical variables and medians with interquartile ranges (IQR) for continuous variables. Demographic and clinical characteristics were compared between groups using Wilcoxon rank-sum and Fisher’s exact tests.
We initially compared ARDS subjects without CA to ARDS+CA. To address the impact of illness severity between the ARDS and ARDS+CA cohorts, we performed a separate analysis comparing the cohorts matched 1:1 to nearest neighbor without replacement, based on PRISM III score (+/− 2), immunosuppression status at time of arrest (yes/no), and age.
As sample size was small in the ARDS+CA cohort, univariate and stepwise bivariate logistic regressions with log-transformed biomarker values were performed to assess the associations between biomarkers and PICU mortality after adjusting for potential confounders: worst OI in 24 hours, PRISM III score, VIS, number of extrapulmonary organ failures, ARDS category (pneumonia, sepsis, aspiration or other), witnessed arrest, CPR duration, initial rhythm. These factors were selected because they are markers for ARDS and post-arrest circulatory failure severity.
RESULTS
Our total cohort included 333 subjects with the diagnosis of ARDS (Table 1). Thirty-two (9.6%) of the ARDS subjects experienced a cardiac arrest (ARDS+CA), 19/32 (59%) of whom died prior to PICU discharge.
Table 1:
Demographics of Subjects with ARDS with and without Cardiac Arrest (n=333)
| ARDS+CA (n=32) | ARDS (n=301) | p-value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 8 (3.4, 13.375) | 5.3 (1.75, 13.05) | 0.105 |
| Weight (kg) | 11 (8, 16.75) | 20 (11, 43.5) | 0.263 |
| Male gender | 17 (53.1%) | 167 (55.5%) | 0.790 |
| Race & Ethnicity | 0.583 | ||
| White | 14 (43.8%) | 133 (44.2%) | |
| Black | 10 (41.3%) | 83 (27.6%) | |
| Hispanic | 3 (9.4%) | 42 (14.0%) | |
| Asian | 0 (0%) | 13 (4.3%) | |
| Other | 5 (15.6%) | 30 (10.0%) | |
| Co-Morbidities | |||
| None | 20 (62.5%) | 90 (30.0%) | <0.001 |
| Prematurity | 3 (9.4%) | 34 (11.3%) | 1.000 |
| Cerebral palsy | 7 (21.9%) | 51 (16.9%) | 0.467 |
| Epilepsy | 2 (6.3%) | 44 (14.6%) | 0.283 |
| Genetic syndrome | 2 (6.3%) | 65 (21.6%) | 0.038 |
| Hematologic disease | 1 (3.1%) | 19 (6.3%) | 0.706 |
| Solid cancer | 0 (0%) | 15 (5.0%) | 0.378 |
| Liquid cancer | 2 (6.3%) | 25 (8.3%) | 0.402 |
| Stem cell transplant | 1 (3.1%) | 36 (12.0%) | 0.150 |
| Chronic lung disease | 0 (0%) | 7 (2.3%) | 1.000 |
| Organ transplant | 0 (0%) | 7 (2.3%) | 1.000 |
| Rheumatologic disease | 0 (0%) | 2 (0.7%) | 1.000 |
| Immunologic disease | 0 (0%) | 2 (0.7%) | 1.000 |
| Immunosuppressed | 2 (6.25%) | 82 (27.2%) | 0.009 |
| ARDS Characteristics | |||
| ARDS Category | |||
| Viral pneumonia | 3 (9.4%) | 107 (35.5%) | <0.001 |
| Aspiration pneumonia | 15 (46.9%) | 35 (11.6%) | |
| Bacterial pneumonia | 0 (0%) | 49 (16.3%) | |
| Fungal pneumonia | 0 (0%) | 3 (1.0%) | |
| Sepsis | 2 (6.3%) | 81 (27.0%) | |
| Trauma | 2 (6.3%) | 11 (3.7%) | |
| Medication-related | 0 (0%) | 7 (2.3%) | |
| TRALI | 1 (3.1%) | 1 (0.3%) | |
| Drowning | 5 (15.6%) | 3 (1.0%) | |
| Smoke inhalation | 2 (6.3%) | 2 (0.7%) | |
| Pancreatitis | 0 (0%) | 2 (0.7%) | |
| Cardiac arrest | 2 (6.3%) | 0 (0%) | |
| PRISM III @ 12H | 26 (21.25, 33) | 10 (5, 15) | <0.001 |
| Initial PaO2/FiO2 | 92.5 (61.25, 154) | 158 (105.5, 228) | <0.001 |
| Initial OI | 24.5 (11.65, 43.95) | 10.6 (7.2, 17.25) | <0.001 |
| Worst mPaw in 24 hours (cmH2O) | 26.5 (21, 30.75) | 19 (16, 23) | <0.001 |
| iNO within 72 hours | 14 (43.8%) | 195 (64.8%) | 0.019 |
| Vasoactive Use at 72 hours | |||
| # of vasopressors | 1 (1,2) | 1 (0,2) | 0.014 |
| VIS | 21 (12.25, 67.5) | 8 (0, 15) | <0.001 |
| Ventilator days | 6.5 (3.25, 14.5) | 9 (6, 15) | 0.059 |
| Outcomes | |||
| ECMO | 0.599 | ||
| VV | 2 (6.25%) | 12 (4.0%) | |
| VA | 1 (3.13% | 4 (1.3%) | |
| PICU length of stay (days) | 9 (3.25, 21.75) | 14 (8, 24) | 0.012 |
| PICU death | 19 (59.4%) | 50 (16.6%) | <0.001 |
Results presented as median (interquartile range) or n (%). P-value calculated by either Fisher’s exact test or Wilcoxon rank-sum, as appropriate.
Abbreviations include: ARDS = acute respiratory distress syndrome, TRALI = transfusion-associated lung injury, PRISM = Pediatric Risk of Mortality, OI = oxygenation index, PIP = peak inspiratory pressure, PEEP = positive end-expiratory pressure, mPaw = mean airway pressure, iNO = inhaled nitric oxide, VIS = vasoactive-inotrope score, VV ECMO = veno-venous extracorporeal membrane oxygenation, VA ECMO = veno-arterial extracorporeal membrane oxygenation, PICU = pediatric intensive care unit
Comparison between ARDS and ARDS+CA cohorts
When comparing the unmatched ARDS and ARDS+CA groups, there was no difference in gender, race, ethnicity, or weight at time of ARDS diagnosis (Table 1). ARDS+CA subjects were more likely to have aspiration and drowning as cause of lung injury, as compared to infectious pneumonia or sepsis in ARDS alone. Markers of severity of illness in the ARDS+CA group were higher, including PRISM III and VIS scores. Markers of ARDS severity were also higher in the ARDS+CA group, including PaO2/FiO2 and OI at time of ARDS diagnosis, and the worst PaO2/FiO2, OI, and highest mPaw in the first 24 hours. Subjects with cardiac arrest were less likely to be immunosuppressed or have comorbidities. Mortality was higher in the ARDS+CA group (p <0.001).
Eight biomarkers were found to be elevated in ARDS+CA subjects compared to ARDS subjects: sRAGE, nucleosomes, SP-D, CCL22, IL-6, HSP70, IL-8, and MIP-1b (Table 2).
Table 2:
Biomarker Profiles of Subjects with ARDS with and without Cardiac Arrest
| ARDS + Cardiac Arrest (n=32) | ARDS (n=301) | p | |
|---|---|---|---|
| Ang2 (pg/mL) | 7065.25 (4988.35, 14381.52) | 5356.55 (2670.85, 13302.15) | 0.079 |
| sRAGE (pg/mL) | 9075.36 (3622.42, 15784.49) | 2113.11 (1023.80, 4687.77) | <0.001 |
| Nucleosomes | 0.24 (0.16, 0.47) | 0.13 (0.05, 0.33) | 0.017 |
| SP-D (ng/mL) | 16.62 (10.33, 39.57) | 11.97 (6.28, 21.83) | 0.017 |
| CCL22 (pg/mL) | 925.98 (673.58, 1463.15) | 485.56 (286.07, 713.27) | <0.001 |
| CCL7 (pg/mL) | 11.85 (5.06, 29.02) | 10.21 (2.94, 39.75) | 0.519 |
| IL-6 (pg/mL) | 982.78 (109.96, 5397.36) | 159.8 (36.09, 773.65) | <0.001 |
| TNFR1 (pg/mL) | 2045.11 (1389.95, 4052.27) | 2536.89 (1417.09, 4848.62) | 0.593 |
| TNF-alpha (pg/mL) | 17.60 (9.15, 41.54) | 15.87 (9.53, 32.10) | 0.741 |
| OFM4 (ng/mL) | 2.97 (1.54, 8.41) | 2.19 (0.59, 6.82) | 0.234 |
| Granzyme B (pg/mL) | 10.05 (3.50, 32.45) | 5.62 (2.69, 18.09) | 0.112 |
| HSP70 (pg/mL) | 321590.35 (161870.43, 1165861.65) | 149476.9 (76048.0, 321383.84) | <0.001 |
| IL-1a (pg/mL) | 0.33 (0.12, 2.27) | 0.33 (0.12, 1.42) | 0.213 |
| IL-8 (pg/mL) | 270.35 (105.98, 19179.34) | 102.21 (38.55, 504.60) | 0.003 |
| CCL3/MIP-1a (pg/mL) | 31.07 (14.9, 89.49) | 22.3 (14.9, 43.42) | 0.343 |
| MIP-1b (pg/mL) | 64.16 (33.56, 122.37) | 40.51 (25.81, 76.81) | 0.021 |
| MMP8 (pg/mL) | 10168.35 (3210.48, 17540.64) | 7729.6 (2874.48, 21125.18) | 0.764 |
The matched cohort included 50 subjects (n=25 in each ARDS+CA and ARDS cohort; Supplemental Table A). sRAGE, SP-D, and CCL22 remained higher in cardiac arrest when comparing the ARDS+CA and ARDS cohorts matched on illness severity (Supplemental Table B).
Mortality in the ARDS+CA Subgroup
Most cardiac arrests were out-of-hospital (OHCA; 22/32, 69%) and unwitnessed (Table 3). The median duration of CPR was 11 (IQR 5.5, 25) minutes. For those with an initial documented rhythm (n=20), the most common initial rhythms were bradycardia (9/20, 28%) and pulseless electrical activity (7/20, 22%). Only 2/32 (6%) subjects received defibrillation.
Table 3:
Demographics of Subjects with ARDS+CA based on PICU Mortality
| Survivors (n=13) | Non-Survivors (n=19) | p-value | |
|---|---|---|---|
| Patient Characteristics | |||
| Age (years) | 8 (5.2, 13) | 8 (3.2, 13.4) | 0.970 |
| Weight (kg) | 30 (20, 40) | 25 (13, 40) | 0.687 |
| Male gender | 8 (61.5%) | 9 | 0.491 |
| Race & Ethnicity | 0.365 | ||
| White | 7 (53.8%) | 7 (36.8%) | |
| Black | 5 (39.6%) | 5 (26.3%) | |
| Hispanic | 0 (0%) | 3 (16.8%) | |
| Asian | 0 (0%) | 0 (0%) | |
| Other | 1 (7.7%) | 4 (21.1%) | |
| Co-Morbidities | |||
| None | 6 (46.2%) | 14 (73.7%) | 0.114 |
| Prematurity | 2 (15.4%) | 1 (5.3%) | 0.356 |
| Cerebral palsy | 3 (23.1%) | 4 (21.0%) | 1.000 |
| Epilepsy | 2 (15.4%) | 0 (0%) | 0.157 |
| Genetic syndrome | 1 (7.7%) | 1 (5.3%) | 1.000 |
| Hematologic disease | 1 (7.7%) | 0 (0%) | 0.406 |
| Solid cancer | 0 (0%) | 0 (0%) | -- |
| Liquid cancer | 1 (7.7%) | 1 (5.3%) | 1.000 |
| Stem cell transplant | 0 (0%) | 1 (5.3%) | 1.000 |
| Chronic lung disease | 0 (0%) | 0 (0%) | -- |
| Organ transplant | 0 (0%) | 0 (0%) | -- |
| Rheumatologic disease | 0 (0%) | 0 (0%) | -- |
| Immunologic disease | 0 (0%) | 0 (0%) | -- |
| Immunosuppressed | 1 (7.7%) | 1 (5.3%) | 1.000 |
| ARDS Characteristics | |||
| ARDS Category | 0.782 | ||
| Viral pneumonia | 2 (15.4%) | 1 (5.3%) | |
| Bacterial pneumonia | 0 (0%) | 0 (0%) | |
| Fungal pneumonia | 0 (0%) | 0 (0%) | |
| Aspiration pneumonia | 7 (53.8%) | 8 (42.1%) | |
| Sepsis | 0 (0%) | 2 (10.5%) | |
| Trauma | 1 (7.7%) | 1 (5.3%) | |
| Medication-related | 0 (0%) | 0 (0%) | |
| TRALI | 1 (7.7%) | 0 (0%) | |
| Drowning | 1 (7.7%) | 5 (26.3%) | |
| Smoke inhalation | 0 (%) | 2 (10.5%) | |
| Pancreatitis | 0 (0%) | 0 (0%) | |
| Cardiac arrest | 1 (7.7%) | 1 (5.3%) | |
| Initial PaO2/FiO2 | 122 (66, 168) | 88 (58, 137) | 0.347 |
| Initial OI | 22.6 (12.4, 39.3) | 25.3 (11.4, 50.8) | 0.545 |
| Worst mPaw (cmH2O) | 27 (21, 28) | 26 (21, 33) | 0.602 |
| iNO within 72 hours | 8 (%) | 15 (%) | 0.725 |
| Ventilator days | 16 (7, 18) | 4 (3, 7) | 0.0001 |
| Other Organ Involvement | |||
| Extrapulmonary organ failure | 3 (2, 3) | 4 (3, 5) | <0.001 |
| New organ failure | |||
| Neurologic | 9 (69.2%) | 18 (94.7%) | 0.132 |
| Cardiovascular | 8 (61.5%) | 16 (84.2%) | 0.219 |
| Renal | 4 (30.8%) | 11 (57.9%) | 0.166 |
| Hepatic | 8 (61.5%) | 16 (84.2%) | 0.219 |
| Hematologic | 4 (30.8%) | 8 (42.1%) | 0.713 |
| PRISM 3 @ 12H | 23 (21, 26) | 31 (23, 36) | 0.060 |
| Vasoactive Use at 72 hours | |||
| # of vasopressors | 1 (1, 1) | 2 (1, 2) | 0.060 |
| VIS | 12 (5, 20) | 50 (20, 100) | 0.0009 |
| Cardiac Arrest Details | |||
| Arrest Location | 0.049 | ||
| OHCA | 6 (46.2%) | 16 (84.2%) | |
| IHCA | 7 (53.8%) | 3 (15.8%) | |
| Witnessed (n=30) | 8 (61,5%) | 6 (31.6%) | 0.269 |
| CPR duration (minutes) (n=25) | 8 (4.5, 12.5) | 17 (10, 30) | 0.019 |
| Initial Rhythm | 0.080 | ||
| Bradycardia | 7 (53.8%) | 2 (10.5%) | |
| PEA | 2 (15.4%) | 5 (26.3%) | |
| Asystole | 1 (7.7%) | 3 (15.8%) | |
| Unknown/Undocumented | 3 (23.0%) | 9 (47.7%) | |
| Doses of Epinephrine | 1.5 (0, 3.5) | 3 (3, 6) | 0.039 |
| Defibrillation | 0 (0 %) | 2 (10.5%) | 0.488 |
| Outcomes | |||
| ECMO | 0.058 | ||
| VV | 1 (%) | 0 (0%) | |
| VA | 2 (%) | 0 (0%) | |
| PICU length of stay (days) | 24 (11, 64) | 4 (3, 7) | <0.001 |
Results presented as median (interquartile range) or n (%). P-value calculated by either Fisher’s exact test or Wilcoxon rank-sum, as appropriate.
Abbreviations include: ARDS = acute respiratory distress syndrome, TRALI = transfusion-associated lung injury, PRISM = Pediatric Risk of Mortality, OI = oxygenation index, PIP = peak inspiratory pressure, PEEP = positive end-expiratory pressure, mPaw = mean airway pressure, iNO = inhaled nitric oxide, VIS = vasoactive-inotrope score, VV ECMO = veno-venous extracorporeal membrane oxygenation, VA ECMO = veno-arterial extracorporeal membrane oxygenation, PICU = pediatric intensive care unit
When comparing clinical characteristics of the ARDS+CA subjects by PICU mortality, there were no differences in gender, race, or co-morbidities (Table 3). Non-survivors had more extrapulmonary organ failures, higher PRISM III scores and required more vasoactive medications. ARDS+CA non-survivors were more likely to have an OHCA, longer duration of CPR, and received more doses of epinephrine. Eight log-transformed biomarkers were associated with mortality in the ARDS+CA cohort on univariate regression: sRAGE, CCL7, IL-6, TNF-alpha, granzyme B, HSP70, IL-8 and MIP-1b (Table 4). On bivariate analyses, sRAGE, IL-6 and granzyme B retained association with mortality after adjustment for VIS and extrapulmonary organ failure, ARDS category and characteristics of the cardiac arrest (Table 5, Figure 1).
Table 4:
Univariate Logistic Regression for PICU Mortality by Log-Transformed Biomarker in Cardiac Arrest (n=32)
| Unadjusted OR (95% CI) | p-value | |
|---|---|---|
| Log [Ang2] (pg/mL) | 2.062 (0.686, 6.189) | 0.197 |
| Log [sRAGE] (pg/mL) | 3.478 (1.345, 8.987) | 0.010 |
| Log [Nucleosomes] | 2.111 (0.986, 4.522) | 0.054 |
| Log [SP-D] (ng/mL) | 1.739 (0.757, 3.991) | 0.192 |
| Log [CCL22] (pg/mL) | 2.793 (0.929, 8.396) | 0.067 |
| Log [CCL7] (pg/mL) | 2.624 (1.145, 6.010) | 0.023 |
| Log [IL-6] (pg/mL) | 1.749 (1.128, 2.710) | 0.012 |
| Log [TNFR1] (pg/mL) | 2.110 (0.784, 5.675) | 0.139 |
| Log [TNF-alpha] (pg/mL) | 3.275 (1.127, 9.516) | 0.029 |
| Log [OFM4] (ng/mL) | 1.568 (0.881, 2.790) | 0.126 |
| Log [Granzyme B] (pg/mL) | 3.738 (1.460, 9.571) | 0.006 |
| Log [HSP70] (pg/mL) | 2.467 (1.081, 5.628) | 0.032 |
| Log [IL-1a] (pg/mL) | 1.151 (0.729, 1.817) | 0.547 |
| Log [IL-8] (pg/mL) | 1.992 (1.074, 3.697) | 0.029 |
| Log [CCL3/MIP-1a] (pg/mL) | 1.922 (0.901, 4.100) | 0.091 |
| Log [MIP-1b] (pg/mL) | 3.596 (1.171, 11.041) | 0.025 |
| Log [MMP8] (pg/mL) | 1.254 (0.709, 2.218) | 0.436 |
Table 5:
Univariate and Bivariate Logistic Regression for PICU Mortality by Log-Transformed Biomarker in Cardiac Arrest (n=32)
| OR (95% CI) | p-value | |
|---|---|---|
| Log [sRAGE] (unadjusted) | 3.478 (1.346, 8.987) | 0.010 |
| + Worst OI | 3.689 (1.278, 10.651) | 0.016 |
| + PRISM III | 2.995 (1.138, 7.877) | 0.026 |
| + VIS | 2.930 (1.099, 7.808) | 0.032 |
| + Organ failure | 3.593 (1.077, 11.981) | 0.037 |
| + ARDS category | 5.599 (1.560, 20.087) | 0.008 |
| + Witnessed arrest | 3.623 (1.288, 10.190) | 0.015 |
| + Initial rhythm | 3.767 (1.161, 12.221) | 0.027 |
| + CPR duration | 2.304 (0.774, 6.855) | 0.134 |
| Log [CCL7] (unadjusted) | 2.624 (1.145, 6.010) | 0.023 |
| + Worst OI | 2.637 (1.065, 6.535) | 0.036 |
| + PRISM III | 2.239 (0.901, 5.566) | 0.083 |
| + VIS | 2.213 (0.912, 5.373) | 0.008 |
| + Organ failure | 1.766 (0.662, 4.714) | 0.256 |
| + ARDS category | 2.601 (1.049, 6.447) | 0.039 |
| + Witnessed arrest | 3.073 (1.188, 7.946) | 0.021 |
| + Initial rhythm | 2.919 (1.126, 7.568) | 0.020 |
| + CPR duration | 2.201 (0.930, 5.206) | 0.072 |
| Log [IL-6] (unadjusted) | 1.749 (1.128, 2.710) | 0.012 |
| + Worst OI | 1.761 (1.107, 2.799) | 0.017 |
| + PRISM III | 1.654 (1.049, 2.607) | 0.030 |
| + VIS | 1.649 (1.016, 2.677) | 0.043 |
| + Organ failure | 1.744 (1.054, 2.886) | 0.030 |
| + ARDS category | 1.668 (1.088, 2.556) | 0.019 |
| + Witnessed arrest | 1.696 (1.089, 2.642) | 0.019 |
| + Initial rhythm | 1.651 (1.052, 2.593) | 0.031 |
| + CPR duration | 1.610 (0.997, 2.599) | 0.051 |
| Log [TNF-alpha] (unadjusted) | 3.275 (1.127, 9.516) | 0.029 |
| + Worst OI | 3.102 (1.040, 9.252) | 0.042 |
| + PRISM III | 2.596 (0.806, 8.358) | 0.110 |
| + VIS | 2.469 (0.767, 7.948) | 0.130 |
| + Organ failure | 2.375 (0.726, 7.770) | 0.153 |
| + ARDS category | 3.133 (1.024, 9.583) | 0.045 |
| + Witnessed arrest | 4.324 (1.167, 16.010) | 0.028 |
| + Initial rhythm | 4.621 (1.110, 19.219) | 0.030 |
| + CPR duration | 3.902 (1.004, 15.162) | 0.049 |
| Log [Granzyme B] (unadjusted) | 3.738 (1.460, 9.571) | 0.006 |
| + Worst OI | 3.721 (1.417, 9.773) | 0.008 |
| + PRISM III | 3.355 (1.245, 9.040) | 0.017 |
| + VIS | 3.361 (1.168, 9.669) | 0.025 |
| + Organ failure | 2.810 (1.057, 7.472) | 0.038 |
| + ARDS category | 3.687 (1.389, 9.787) | 0.009 |
| + Witnessed arrest | 3.774 (1.413, 10.076) | 0.008 |
| + Initial rhythm | 3.738 (1.401, 9.969) | 0.011 |
| + CPR duration | 2.881 (1.111, 7.468) | 0.029 |
| Log [HSP70] (unadjusted) | 2.467 (1.081, 5.628) | 0.032 |
| + Worst OI | 2.430 (1.026, 5.754) | 0.044 |
| + PRISM III | 2.044 (0.871, 4.799) | 0.100 |
| + VIS | 2.035 (0.873, 4.742) | 0.100 |
| + Organ failure | 1.714 (0.709, 4.143) | 0.231 |
| + ARDS category | 2.615 (1.040, 6.579) | 0.041 |
| + Witnessed arrest | 2.179 (0.983, 4.831) | 0.055 |
| + Initial rhythm | 1.927 (0.908, 40.087) | 0.087 |
| + CPR duration | 1.609 (0.700, 3.702) | 0.263 |
| Log [IL-8] (unadjusted) | 1.993 (1.074, 3.697) | 0.029 |
| + Worst OI | 2.086 (1.044, 4.167) | 0.037 |
| + PRISM III | 1.809 (0.951, 3.437) | 0.071 |
| + VIS | 1.830 (0.911, 3.678) | 0.090 |
| + Organ failure | 1.672 (0.887, 3.152) | 0.112 |
| + ARDS category | 2.058 (1.085, 3.904) | 0.027 |
| + Witnessed arrest | 2.179 (0.983, 4.831) | 0.043 |
| + Initial rhythm | 1990 (0.906, 4.374) | 0.069 |
| + CPR duration | 1.609 (0.700, 3.702) | 0.013 |
| Log [MIP-1b] (unadjusted) | 3.595 (1.170, 11.041) | 0.025 |
| + Worst OI | 3.463 (1.102, 10.881) | 0.033 |
| + PRISM III | 2.837 (0.848, 9.495) | 0.091 |
| + VIS | 3.432 (0.976, 12.063) | 0.055 |
| + Organ failure | 2.182 (0.636, 7.485) | 0.215 |
| + ARDS category | 4.337 (1.195, 15.733) | 0.026 |
| + Witnessed arrest | 3.603 (1.047, 12.404) | 0.042 |
| + Initial rhythm | 5.226 (0.894, 30.556) | 0.066 |
| + CPR duration | 5.268 (0.985, 28.202) | 0.052 |
Abbreviations include: OI = oxygenation index, PRISM = Pediatric Risk of Mortality, VIS = vasoactive-inotrope score, ARDS = acute respiratory distress syndrome, CPR = cardiopulmonary resuscitation.
Figure 1:
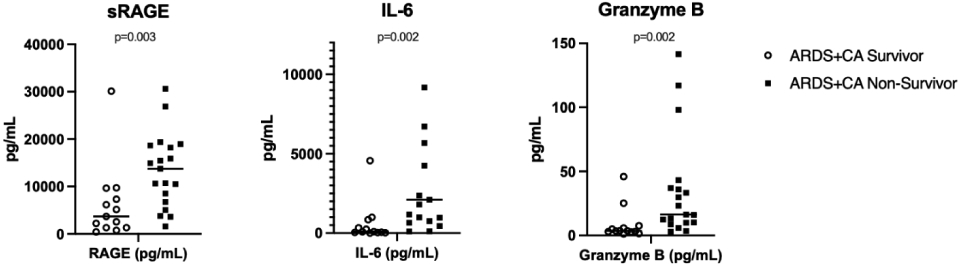
Scatter plots with median line for the three biomarkers associated with PICU mortality in ARDS+CA cohort after bivariate analysis.
P-values presented from Wilcoxon rank-sum comparison. Extreme values truncated for ease of visualization, but all outliers were included for analysis.
DISCUSSION
In this study, we identified eight biomarkers (sRAGE, nucleosomes, SP-D, CCL22, IL-6, HSP70, IL-8, and MIP-1b) that were elevated in the ARDS+CA cohort, with sRAGE, SP-D and CCL22 significantly higher in the ARDS+CA cohort than ARDS cohort after a matched analysis accounting for severity of illness. In the sub-cohort of ARDS+CA subjects, sRAGE, IL-6, and granzyme B were associated with mortality after controlling for measures of ARDS and PCAS severity.
Identification, classification, and treatment of PCAS is a growing area of focus in post-cardiac arrest care in pediatrics. The recent 2020 scientific statement from the American Heart Association emphasizes the management of the four critical components of this state: “post–cardiac arrest brain injury, post-cardiac arrest myocardial dysfunction, systemic ischemia/reperfusion response, and persistent precipitating pathophysiology” [2]. Specifically, the ischemia and reperfusion response after cardiac arrest may trigger a systemic inflammatory state, similar to sepsis [9] and other critical illness syndromes, as interleukins and cytokines have been elevated in adult patients after cardiac arrest [27]. The use of biomarkers to assess pediatric PCAS is novel and, as in other multisystem inflammatory conditions, studying biomarkers may allow for improved characterization of inflammatory states and propose therapies to address molecular pathophysiology [28].
In this work, we identified three markers of inflammation associated with mortality in ARDS+CA in multiple analyses that controlled for disease severity: sRAGE, IL-6 and granzyme B. Soluble RAGE (receptor for advanced glycation end products, sRAGE) is a receptor for fats and proteins that have undergone glycosylation and oxidation due to inflammatory stress and is present in multiple tissues [29]. Upregulation of sRAGE can be seen in other inflammatory states, including diabetes, renal insufficiency, Alzheimer’s disease and in certain cancers [30]. sRAGE has been shown to be associated with sepsis and endothelial damage associated with disseminated intravascular coagulopathy [31], as well as sepsis-related ARDS in adults [32]. sRAGE was not found to be as strongly associated with mortality in pediatric ARDS related to sepsis [33], but has not been examined in the cardiac arrest population. In adult patients with chronic heart failure, sRAGE has been shown to be associated with mortality, is sensitive, and independent of other heart failure scores based on clinical and standard laboratory data [34]. Elevated sRAGE levels in our post-cardiac arrest cohort may be a signal of the body’s response to the inflammatory stress after ischemia and reperfusion, and thus serve as a mediator in the pathway affecting organ dysfunction, eventually leading to mortality, as has been suspected in multiple other diseases [35].
IL-6 has been better studied the adult cardiac arrest population. IL-6 has both pro- and anti-inflammatory properties and plays important roles in the immune system, regulating fever and promoting B-cell development in bone marrow. In addition to elevated levels in pediatric sepsis [36, 37], cytokines, including IL-6, are elevated in the systemic ischemic-reperfusion response after cardiac arrest [38], and in post-arrest brain injury. In adults after cardiac arrest, serum levels of IL-6 have been found to be associated with markers of severity of disease [39], mortality [40], and associated with neurologic outcomes [41, 42]. Non-specific corticosteroid and specific IL-6 targeting monoclonal antibodies have been used in other inflammatory disease processes [43, 44] including sepsis [45, 46], but neither specifically have not proven beneficial in improving post-cardiac arrest outcomes [47-49]. More work examining the prognostic strength of IL-6 is needed, in assessing both survival and survival with preserved neurologic function, after pediatric cardiac arrest.
Lastly, granzyme B was also associated with post-cardiac arrest mortality in our study. Granzyme B is one of the cytotoxic granules within cytotoxic T cells and natural kill cells, important in programming and induction for cell death [50]. In a murine sepsis model, granzymes were associated with the proinflammatory cascade associated with multisystem organ dysfunction after bacterial infection; mice that were null for granzyme had less organ dysfunction but could still appropriately clear bacterial infection [51]. In post-cardiac arrest rat models, granzyme B is associated with neuronal apoptosis of the hippocampus [52]. This suggests that granzyme B may contribute to organ dysfunction and poor outcomes, including mortality, after cardiac arrest via systemic cell death signaling. The specific relationship with the hippocampus or other brain structures also raise question if granzyme B could be specific for neurologic injuries and outcomes in cardiac arrest.
These three biomarkers are a unique addition to the existing, though limited, data about the post-cardiac arrest inflammatory state in pediatrics. The only other pediatric study examining the inflammatory biomarkers of PCAS found ciliary neurotrophic factor (CNTF) and IL-17 to be associated with poor 6-month neurologic outcomes after pediatric cardiac arrest [15]. The lack of overlap in biomarkers of significance between the studies may be due to outcomes of interest: our study examined factors associated with PICU mortality and in an ARDS population, while they compared outcomes in a group of survivors after cardiac arrest.
As this study is exploratory, there were some limitations. There was a small sample size, as cardiac arrest is relatively rare in pediatric ARDS [21, 53]. Because of the small sample, a fully adjusted multivariate analysis was not able to be performed given that a limited number of variables that could be included for logistic regression with only 19 non-survivors in the ARDS+CA cohort, For this study, we were restricted to subjects with ARDS and cardiac arrest diagnosis, and these markers may not be generalizable to all patients after cardiac arrest. Recognizing that the time after cardiac arrest could affect biomarker levels [16], we were limited in the ability to time biomarker collection after cardiac arrest because many arrests were out-of-hospital and therefore exact time was not available. Given these limitations, a prospective study of biomarkers in all subjects with cardiac arrest would allow for a more complete assessment of PCAS. With this work, however, we were able to utilize a densely-phenotyped cohort with a known inflammatory process and find markers useful after cardiac arrest and associated with mortality, which may assist with the design of future studies in a general pediatric cardiac arrest biobank.
Further identification and classification of important plasma biomarkers of the post-cardiac arrest inflammatory state is needed. This could allow for improved prognostication, design of specific therapies to target inflammatory pathways, and to develop methods to identify subjects at high risk for poor outcomes to enrich patient selection for future clinical trials design.
CONCLUSIONS
This pilot study identified novel biomarkers of systemic inflammation in the PCAS of pediatric subjects with cardiac arrest and ARDS, and specifically three that were associated with mortality: sRAGE, IL-6 and granzyme B.
Supplementary Material
FUNDING
We appreciate the support from the following sources: NIH NHLBI K23HL153759 (ASH), NIH NHLBI K23-HL136688 (NY), and NIH NHLBI R01148054 (NY).
Footnotes
CONFLICTS OF INTEREST: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Holmberg MJ, et al. , Annual Incidence of Adult and Pediatric In-Hospital Cardiac Arrest in the United States. Circ Cardiovasc Qual Outcomes, 2019. 12(7): p. e005580. [PMC free article] [PubMed] [Google Scholar]
- 2.Topjian AA, et al. , Pediatric post-cardiac arrest care: a scientific statement from the American Heart Association. Circulation, 2019. 140(6): p. e194–e233. [DOI] [PubMed] [Google Scholar]
- 3.Fink EL, et al. , Regional brain injury on conventional and diffusion weighted MRI is associated with outcome after pediatric cardiac arrest. Neurocritical care, 2013. 19(1): p. 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topjian AA, et al. , Early Electroencephalographic Background Features Predict Outcomes in Children Resuscitated From Cardiac Arrest. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies, 2016. 17(6): p. 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topjian AA, et al. , Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med, 2013. 41(1): p. 215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Checchia PA, et al. , Myocardial injury in children following resuscitation after cardiac arrest. Resuscitation, 2003. 57(2): p. 131–7. [DOI] [PubMed] [Google Scholar]
- 7.Conlon TW, et al. , Association of left ventricular systolic function and vasopressor support with survival following pediatric out-of-hospital cardiac arrest. Pediatr Crit Care Med, 2015. 16(2): p. 146–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neumar RW, et al. , Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation, 2008. 118(23): p. 2452–83. [DOI] [PubMed] [Google Scholar]
- 9.Adrie C, et al. , Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care, 2004. 10(3): p. 208–12. [DOI] [PubMed] [Google Scholar]
- 10.Bro-Jeppesen J, et al. , Level of systemic inflammation and endothelial injury is associated with cardiovascular dysfunction and vasopressor support in post-cardiac arrest patients. Resuscitation, 2017. 121: p. 179–186. [DOI] [PubMed] [Google Scholar]
- 11.Mentzelopoulos SD and Zakynthinos SG, Post-cardiac arrest syndrome: pathological processes, biomarkers and vasopressor support, and potential therapeutic targets. Resuscitation, 2017. 121: p. A12–a14. [DOI] [PubMed] [Google Scholar]
- 12.Fink EL, et al. , Serum biomarkers of brain injury to classify outcome after pediatric cardiac arrest*. Crit Care Med, 2014. 42(3): p. 664–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fink EL, et al. , Exploratory study of serum ubiquitin carboxyl-terminal esterase L1 and glial fibrillary acidic protein for outcome prognostication after pediatric cardiac arrest. Resuscitation, 2016. 101: p.65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirschen MP, et al. , Circulating Neurofilament Light Chain Is Associated With Survival After Pediatric Cardiac Arrest. Pediatr Crit Care Med, 2020. 21(7): p. 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kernan KF, et al. , An Exploratory Assessment of Serum Biomarkers of Post-Cardiac Arrest Syndrome in Children. Resuscitation, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C-H, et al. , Predicting the outcomes for out-of-hospital cardiac arrest patients using multiple biomarkers and suspension microarray assays. Scientific Reports, 2016. 6(1): p. 27187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel JK, et al. , Association of post-resuscitation inflammatory response with favorable neurologic outcomes in adults with in-hospital cardiac arrest. Resuscitation, 2021. 159: p. 54–59. [DOI] [PubMed] [Google Scholar]
- 18.Yehya N, Thomas NJ, and Margulies SS, Circulating nucleosomes are associated with mortality in pediatric acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol, 2016. 310(11): p. L1177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yehya N, et al. , Circulating markers of endothelial and alveolar epithelial dysfunction are associated with mortality in pediatric acute respiratory distress syndrome. Intensive Care Med, 2016. 42(7): p. 1137–45. [DOI] [PubMed] [Google Scholar]
- 20.Yehya N, et al. , Plasma Nucleosomes Are Associated With Mortality in Pediatric Acute Respiratory Distress Syndrome. Crit Care Med, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirschen MP, et al. , Circulating Neurofilament Light Chain Is Associated With Survival After Pediatric Cardiac Arrest. Pediatr Crit Care Med, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollack MM, Patel KM, and Ruttimann UE, PRISM III: an updated Pediatric Risk of Mortality score. Critical care medicine, 1996. 24(5): p. 743–752. [DOI] [PubMed] [Google Scholar]
- 23.Starling RM, et al. , Early Head CT Findings Are Associated With Outcomes After Pediatric Out-of-Hospital Cardiac Arrest. Pediatr Crit Care Med, 2015. 16(6): p. 542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaies MG, et al. , Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med, 2010. 11(2): p. 234–8. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein B, Giroir B, and Randolph A, International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med, 2005. 6(1): p. 2–8. [DOI] [PubMed] [Google Scholar]
- 26.Yehya N and Wong HR, Adaptation of a Biomarker-Based Sepsis Mortality Risk Stratification Tool for Pediatric Acute Respiratory Distress Syndrome. Crit Care Med, 2018. 46(1): p. e9–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adrie C, et al. , Successful cardiopulmonary resuscitation after cardiac arrest as a "sepsis-like" syndrome. Circulation, 2002. 106(5): p. 562–8. [DOI] [PubMed] [Google Scholar]
- 28.Carcillo JA, et al. , Pathophysiology of Pediatric Multiple Organ Dysfunction Syndrome. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies, 2017. 18(3_suppl Suppl 1): p. S32–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramasamy R, Yan SF, and Schmidt AM, Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Annals of the New York Academy of Sciences, 2011. 1243: p. 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt AM, et al. , The biology of the receptor for advanced glycation end products and its ligands. Biochimica et Biophysica Acta (BbA) - Molecular Cell Research, 2000. 1498(2): p. 99–111. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto H, et al. , The clinical significance of circulating soluble RAGE in patients with severe sepsis. J Trauma Acute Care Surg, 2015. 78(6): p. 1086–93; discussion 1093-4. [DOI] [PubMed] [Google Scholar]
- 32.Jones TK, et al. , Plasma sRAGE acts as a genetically regulated causal intermediate in sepsis-associated acute respiratory distress syndrome. American journal of respiratory and critical care medicine, 2020. 201(1): p. 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitney JE, et al. , Endothelial Biomarkers Are Associated With Indirect Lung Injury in Sepsis-Associated Pediatric Acute Respiratory Distress Syndrome. Crit Care Explor, 2020. 2(12): p. e0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raposeiras-Roubín S, et al. , Relation of soluble receptor for advanced glycation end products to predict mortality in patients with chronic heart failure independently of Seattle Heart Failure Score. Am J Cardiol, 2011. 107(6): p. 938–44. [DOI] [PubMed] [Google Scholar]
- 35.Erusalimsky JD, The use of the soluble receptor for advanced glycation-end products (sRAGE) as a potential biomarker of disease risk and adverse outcomes. Redox Biol, 2021. 42: p. 101958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng PC, et al. , Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clinical chemistry, 2006. 52(6): p. 1181–1189. [DOI] [PubMed] [Google Scholar]
- 37.Fioretto JR, et al. , Interleukin-6 and procalcitonin in children with sepsis and septic shock. Cytokine, 2008. 43(2): p. 160–164. [DOI] [PubMed] [Google Scholar]
- 38.Jou C, et al. , The role of inflammatory cytokines in cardiac arrest. Journal of intensive care medicine, 2020. 35(3): p. 219–224. [DOI] [PubMed] [Google Scholar]
- 39.Samborska-Sablik A, Sablik Z, and Gaszynski W, The role of the immuno-inflammatory response in patients after cardiac arrest. Arch Med Sci, 2011. 7(4): p. 619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peberdy MA, et al. , Inflammatory markers following resuscitation from out-of-hospital cardiac arrest—a prospective multicenter observational study. Resuscitation, 2016. 103: p. 117–124. [DOI] [PubMed] [Google Scholar]
- 41.Oda Y, et al. , The cutoff values of intrathecal interleukin 8 and 6 for predicting the neurological outcome in cardiac arrest victims. Resuscitation, 2009. 80(2): p. 189–193. [DOI] [PubMed] [Google Scholar]
- 42.Chong JY, et al. , Interleukin-6 as a Potential Predictor of Neurologic Outcomes in Cardiac Arrest Survivors Who Underwent Target Temperature Management. The Journal of Emergency Medicine, 2020. 59(6): p. 828–835. [DOI] [PubMed] [Google Scholar]
- 43.Nakahara H and Nishimoto N, Anti-interleukin-6 receptor antibody therapy in rheumatic diseases. Endocr Metab Immune Disord Drug Targets, 2006. 6(4): p. 373–81. [DOI] [PubMed] [Google Scholar]
- 44.Cortegiani A, et al. , Rationale and evidence on the use of tocilizumab in COVID-19: a systematic review. Pulmonology, 2021. 27(1): p. 52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minneci PC, et al. , Meta-analysis: the effect of steroids on survival and shock during sepsis depends on the dose. Annals of internal medicine, 2004. 141(1): p. 47–56. [DOI] [PubMed] [Google Scholar]
- 46.Casserly B, et al. , Low-dose steroids in adult septic shock: results of the Surviving Sepsis Campaign. Intensive care medicine, 2012. 38(12): p. 1946–1954. [DOI] [PubMed] [Google Scholar]
- 47.Grafton ST and Longstreth W, Steroids after cardiac arrest: a retrospective study with concurrent, nonrandomized controls. Neurology, 1988. 38(8): p. 1315–1315. [DOI] [PubMed] [Google Scholar]
- 48.Mentzelopoulos SD, et al. , Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. Jama, 2013. 310(3): p. 270–279. [DOI] [PubMed] [Google Scholar]
- 49.Meyer MAS, et al. , Treatment Effects of Interleukin-6 Receptor Antibodies for Modulating the Systemic Inflammatory Response After Out-of-Hospital Cardiac Arrest (The IMICA Trial) A Double-Blinded, Placebo-Controlled, Single-Center, Randomized, Clinical Trial. Circulation, 2021. 143(19): p. 1841–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chowdhury D and Lieberman J, Death by a thousand cuts: granzyme pathways of programmed cell death. Annual review of immunology, 2008. 26: p. 389–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arias MA, et al. , Elucidating sources and roles of granzymes A and B during bacterial infection and sepsis. Cell Rep, 2014. 8(2): p. 420–9. [DOI] [PubMed] [Google Scholar]
- 52.Ji N-N, et al. , CTL-Derived Granzyme B Participates in Hippocampal Neuronal Apoptosis Induced by Cardiac Arrest and Resuscitation in Rats. Frontiers in neurology, 2019. 10: p. 1306–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dowell JC, et al. , Epidemiology of Cause of Death in Pediatric Acute Respiratory Distress Syndrome. Critical care medicine, 2018. 46(11): p. 1811–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


