Abstract
Introduction
Outcomes remain poor in patients with diffuse large B cell lymphoma (DLBCL) who overexpress BCL-2 protein. We present population pharmacokinetics (PopPK) and exposure–response (ER) analyses for venetoclax (a selective BCL-2 inhibitor) administered with rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in patients with relapsed/refractory (R/R) and previously untreated (1L) non-Hodgkin lymphoma (NHL) from the phase 1b/2 CAVALLI study, to confirm dose selection for future studies.
Methods
Analyses included 216 patients with R/R or 1L NHL treated for eight 21-day cycles with 400–800 mg venetoclax (cycle 1: days 4–10; cycles 2–8: days 1–10) in combination with R for eight cycles and CHOP for 6–8 cycles. A legacy PopPK model for venetoclax was used to describe the observed data and provide post hoc PK parameters. Venetoclax steady-state exposure (AUCss) was used to predict clinical efficacy, safety, or tolerability. To isolate the effect of venetoclax, ER analyses referenced data from the R-CHOP arm of a historical control study, GOYA, in 1L DLBCL.
Results
There was no significant association between venetoclax AUCss and progression-free survival or complete response either for all-comers or the BCL-2-immunohistochemistry-positive subpopulation. No statistically significant trends were observed with venetoclax AUCss and the key grade ≥ 3 adverse events and serious adverse events. Similar dose intensities were observed for venetoclax and R-CHOP components across venetoclax exposures, suggesting venetoclax did not impact delivery of the R-CHOP backbone.
Conclusions
The PopPK and ER analyses, in addition to the positive benefit–risk observed in the clinical data, support the selection of 800 mg venetoclax given with R-CHOP for future studies in BCL-2-immunohistochemistry-positive patients with 1L DLBCL.
Trial Registration
ClinicalTrials.gov Identifier NCT02055820.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01919-z.
Keywords: Diffuse Large B cell Lymphoma, Lymphoma, Pharmacokinetics, R-CHOP, Venetoclax
Key Summary Points
| The pharmacokinetics (PK) of venetoclax in the CAVALLI study were adequately described using a previously developed legacy population pharmacokinetics (PopPK) model, with study-specific (CAVALLI) effects on apparent clearance (CL/F) and apparent central volume of distribution (V2/F) added to the PopPK model to account for the possible differences between the population of CAVALLI study patients relative to the population of patients from the prior analysis. |
| Encouraging outcomes for efficacy were achieved without compromising delivery of rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) therapy; increased venetoclax exposures did not translate into improved efficacy for investigator-assessed progression free survival (INV-PFS) and positron emission tomography-computed tomography assessed complete response (PET-CR) rates in all-comers or BCL-2-IHC-positive subpopulations. |
| Venetoclax plus R-CHOP demonstrated increased but manageable toxicity versus R-CHOP alone, with adverse events expected on the basis of the mechanism of action of venetoclax and consistent with the toxicity profile of R-CHOP. Data did not suggest that higher doses of venetoclax would cause additional toxicity compared with lower doses of venetoclax within the CAVALLI study. |
Introduction
The B cell lymphoma 2 protein (BCL-2) family proteins comprise the sentinel network that regulates the mitochondrial or intrinsic apoptotic response [1]. A recognized hallmark of cancer development, overexpression of anti-apoptotic BCL-2 family proteins mediates resistance to chemotherapy in experimental models of lymphoma, and is associated with poorer prognosis in patients with diffuse large B cell lymphoma (DLBCL) treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) [4]. Venetoclax, a selective inhibitor of BCL-2 [5], is approved globally for monotherapy or combination therapy in patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), as well as treatment-naïve unfit patients with acute myeloid leukemia [6, 7]. Venetoclax has also demonstrated significant clinical activity across a broad range of non-Hodgkin lymphoma (NHL) subtypes [8].
CAVALLI is a phase 1b/2 multicenter, open-label study investigating the safety and efficacy of venetoclax plus an anti-CD20 antibody (rituximab [R] or obinutuzumab [GA101; G]) in combination with standard CHOP chemotherapy in patients with NHL. The phase 1b portion of the study included two parallel treatment arms, with planned venetoclax doses ranging from 200 to 800 mg orally at two different dosing schedules: once daily (21 days per cycle) or a noncontinuous dosing schedule of 10 doses in a 21-day cycle (10/21-day dosing), plus standard cycles of R-CHOP or G-CHOP in relapsed/refractory (R/R) or previously untreated patients with NHL [9]. A greater than anticipated incidence of hematologic toxicity was evidenced by dose-limiting toxicities (DLTs) at the initial starting dose of 200 mg administered daily in a 21-day cycle for eight cycles. The phase 1b part of CAVALLI established the recommended phase 2 dose of venetoclax as 800 mg in a 10/21-day dosing schedule, i.e., days 4–10 of cycle 1 and days 1–10 of cycles 2–8, in combination with R for eight cycles and CHOP [9] for 6–8 cycles in patients with first-line (1L) DLBCL. The incidence of hematologic toxicity observed in patients following this schedule was lower than in patients following the once-daily dosing schedule; as such, the noncontinuous dosing schedule was preferred, given that it allowed administration of higher doses of venetoclax. The G-CHOP arm was not expanded to phase 2 in the light of results from the phase 3 GOYA study in 1418 patients with previously untreated DLBCL, which showed no improvement in progression-free survival (PFS) with G-CHOP over R-CHOP in 1L therapy [9, 10].
After 30.8 months’ median follow-up, venetoclax plus R-CHOP continued to be associated with improved investigator-assessed (INV)-PFS in the all-comer 1L DLBCL population (adjusted Cox regression hazard ratio of 0.61; 95% confidence interval [CI] of 0.43–0.87), compared with matched controls from the GOYA study. Promising efficacy (PFS) was evident in the poor prognostic BCL-2-immunohistochemistry (IHC)-positive subpopulation (adjusted Cox proportional hazards [CPH] ratio of 0.55; 95% CI of 0.34–0.89 in CAVALLI versus the R-CHOP arm of GOYA) [11].
As a complementary report to the initial efficacy, safety, and biomarker analyses of CAVALLI phase 2 [11], the present study investigated population pharmacokinetics (PopPK) and exposure–response (ER) characteristics of venetoclax from CAVALLI to confirm the dose selection of venetoclax in combination with R-CHOP chemoimmunotherapy for future studies.
Methods
Compliance with Ethics Guidelines
All procedures involving human participants in the CAVALLI and GOYA studies were in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments. The CAVALLI study was approved by the institutional review boards/ethics committees at all participating institutions including Comite de Protection des Personnes, WIRB, and CEIC Hospital Universitario (Table S1 in the supplementary material). This article does not contain any studies with animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study. This article does not contain any identifying information about participants and therefore consent was not sought from participants for publication.
Studies
Fifty-six patients with R/R or 1L NHL (arm A with R-CHOP, N = 24; arm B with G-CHOP, N = 32) were enrolled in the phase 1b portion and 211 patients with 1L DLBCL were enrolled in the phase 2 portion of the CAVALLI study, previously described by Zelenetz et al. [9]. The data cutoff date for the PopPK and ER analyses in this manuscript for CAVALLI was April 12, 2019.
To isolate the venetoclax effect in the CAVALLI study, the R-CHOP arm of the phase 3 GOYA trial (patients with International Prognostic Index [IPI] 2–5) was used as a historical control for comparative analyses of efficacy, safety, and tolerability. In GOYA, 1418 patients with 1L DLBCL were randomized to receive eight 21-day cycles of G or R plus 6–8 cycles of standard CHOP [10]. Overall, patient characteristics are similar for CAVALLI and GOYA studies. For the efficacy analyses, double-robust methods were applied to the adjustment of the baseline prognostic covariates in the CAVALLI and GOYA studies [12]. The data cutoff date for GOYA was April 29, 2016.
Analytical Methods
Plasma concentrations of venetoclax were determined using validated liquid chromatography methods with tandem mass spectrometry. The lower limit of quantification (LLOQ) for venetoclax was 2.08 ng/mL or 2.18 ng/mL [13]. BCL-2 protein expression was assessed by IHC (BCL-2 cutoff, 50% medium/high expression) and BCL-2 translocations by fluorescence in situ hybridization (FISH) [12]. Intensive PK sampling was performed for venetoclax on cycle 1 day 4 and cycle 2 day 1 at pre- and up to 8 h post-dose and at pre-dose on cycle 1 day 8 and cycle 2 day 10.
PopPK Modeling and Evaluation
A previously developed PopPK legacy model for venetoclax in 505 subjects with R/R CLL/SLL, R/R NHL, and healthy volunteers [13] enrolled in eight phase 1/2 studies was used. Parameter values were fixed at prior estimates to describe the observed PK and provide post hoc predicted PK parameters for venetoclax in the CAVALLI study. The appropriateness of the legacy model for predicting exposures in CAVALLI was evaluated using diagnostic plots, visual predictive checks (VPCs), and normalized prediction distribution errors plots [14–16].
Determination of Venetoclax Exposure
Individual venetoclax values for area under the curve (AUC) at steady state (AUCss nominal) were estimated using empirical Bayesian post hoc estimates of the relevant PK parameters, the protocol-assigned dose, and covariate values on day 1 as follows:
| 1 |
where Dnom is the nominal dose (protocol assigned dose), CL/F is apparent clearance, and F1 is relative bioavailability. Given the dependence of bioavailability on dose that was defined in the legacy PopPK model, Dnom was used to compute the F1 parameter for the exposure measure. Values of covariates at baseline were used to compute CL/F. Patients without evaluable PK data and those not included in the PopPK analysis were excluded.
ER Analyses
To isolate the venetoclax effect, the ER analyses referenced data from patients in the R-CHOP arm of the GOYA trial [10]. Three types of ER analyses were conducted using CAVALLI study data: (1) exposure–efficacy and (2) exposure–dose intensity analyses (using phase 2 patients [1L DLBCL, n = 199] receiving 800 mg venetoclax on the 10/21-day dosing regimen), and (3) exposure–safety analysis (using phase 1b/2 patients receiving 400, 600, or 800 mg venetoclax on the 10/21-day dosing regimen). Further, venetoclax AUCss nominal (Eq. 1) was used as the exposure metric for predicting clinical response.
Exposure–efficacy relationships were conducted using (1) Kaplan–Meier (KM) analysis stratified by venetoclax exposure AUCss nominal, (2) CPH modeling, and (3) logistic regression analysis. Logistic regression and CPH models were used to assess the impact of exposure on the key efficacy endpoints of positron emission tomography-computed tomography-assessed complete response (PET-CR) and investigator-assessed PFS (INV-PFS), respectively, in both all-comers and BCL-2-positive patients. PET-CR was assessed at the end of treatment (EOT) visit, 6–8 weeks from day 1 of the last cycle received. If a patient has not experienced clinical response or death at the data cutoff date, PFS was censored at the day of the last tumor assessment.
Relationships between venetoclax exposure and PFS were first characterized using base Cox models that characterize the marginal effect of venetoclax exposure on PFS without considering covariates. A significance level of α = 0.05 was used to evaluate the exposure coefficient. The hazard function in the CPH model was expressed as:
| 2 |
where λ0(t) is the baseline hazard function and is a vector of predictor variables (continuous exposure [AUCss nominal] included in the base model). The parameter vector β is estimated by maximum partial-likelihood.
Logistic regression models were implemented to assess correlations between venetoclax exposure (AUCss nominal); the probability of PET-CR and treatment-emergent safety endpoints, i.e., grade ≥ 3 adverse events (AEs) like neutropenia, thrombocytopenia, infections, and febrile neutropenia; and serious AEs (SAEs), in the intent-to-treat population. These analyses include the treatment-emergent grade ≥ 3 AEs reported through 30 days after the last dose of venetoclax or CHOP, or 90 days after the last dose of R, whichever is later; SAEs reported until the cutoff date of July 13, 2018 were included. A significance level of α = 0.05 was used to evaluate the exposure coefficient. A covariate analysis was also carried out, in which a forward addition (with α = 0.01) stepwise procedure was implemented.
Covariates tested in the ER analyses for efficacy and safety included demographics (sex, age, body mass index [BMI]), and baseline disease characteristics: Eastern Cooperative Oncology Group performance status (ECOG PS; 0–1 vs 2 +), IPI (high [4, 5] vs non-high [2, 3]), bulky disease (> 7.5 mm: yes vs no), stage (IV vs I–III), lactate dehydrogenase (low/normal vs high), and cell-of-origin (COO), based on likely associations with the clinical efficacy and/or safety endpoints. Missing continuous covariates were imputed by study using the median value of the covariate, and the missing categorical covariates by the most frequent population value. There were no covariates with missing data exceeding 15% of study data. The covariate analysis was conducted using the forward addition procedure. A significance level of α = 0.01 (objective function change of 6.63 points for one parameter) was used. Significance levels of covariate effects (associated p values) were presented.
Relative dose intensity (RDI) for the venetoclax or R-CHOP components was calculated from the first day of venetoclax or R-CHOP treatment until the date of the planned EOT as follows:
Thus, dose holds and reductions with penalty for early treatment discontinuations due to AEs were accounted for by imputing the actual doses as zero until EOT. The effect of venetoclax exposure (AUCss nominal) on dose intensity of individual R-CHOP and venetoclax components was also evaluated.
Software
PopPK analysis was carried out using nonlinear mixed effects modeling with NONMEM software, Version 7.3.0 (ICON Development Solutions) [17]. The first-order conditional estimation method with interaction (FOCEI) was used for all NONMEM model runs. All ER analyses were performed using R software, version 3.3.3, for Windows (R project, http://www.r-project.org/). The function glm() with logit link was used for the logistic regression analysis, and coxph() of the survival package was used for CPH modeling.
Results
The baseline demographics and covariates are shown in Table 1.
Table 1.
Continuous and categorical covariates at baseline
| Continuous covariates | Measure | Result |
|---|---|---|
| Patients | Number | 232 |
| Age, years | Mean (SD) | 61.2 (12.6) |
| Median (range) | 64 (18–85) | |
| Body weight, kg | Mean (SD) | 77.8 (16.8) |
| Median (range) | 77.0 (46.0–182) |
| Categorical covariates | Level | Number (%) |
|---|---|---|
| Sex | Male | 129 (55.6) |
| Female | 103 (44.4) | |
| Study population | DLBCL | 218 (94.0) |
| FL | 10 (4.3) | |
| Transformed lymphoma | 2 (0.9) | |
| Other | 2 (0.9) | |
| Race | White | 165 (71.1) |
| Asian | 5 (2.2) | |
| Black/African American | 4 (1.7) | |
| Native Hawaiian or Pacific Islander | 3 (1.3) | |
| Unknown | 55 (23.7) | |
| Dose | 200 mg | 7 (3.0) |
| 400 mg | 3 (1.3) | |
| 600 mg | 8 (3.4) | |
| 800 mg | 214 (92.2) | |
| ECOG PS | 0 | 103 (44.4) |
| 1 | 94 (40.5) | |
| 2 | 35 (15.1) | |
| IPI | 0–1 (Low) | 26 (11.2) |
| 2 (Low intermediate) | 86 (37.1) | |
| 3 (High intermediate) | 69 (29.7) | |
| 4–5 (High) | 51 (22.0) | |
| Stage | I | 5 (2.2) |
| II | 30 (12.9) | |
| III | 44 (19.0) | |
| IV | 153 (65.9) | |
| COO | ABC | 51 (27.1) |
| GCB | 113 (60.1) | |
| Unclassified | 24 (12.8) | |
| BCL-2 IHC | Positive | 120 (60.3) |
| Negative | 79 (39.7) | |
| MYC IHC | Positive | 146 (72.6) |
| Negative | 55 (27.4) | |
| DP | DP | 91 (45.7) |
| Non-DP | 108 (54.3) | |
| DH | DH | 7 (4.5) |
| Non-DH | 150 (95.5) | |
| C3AHIB | Not administered | 5 (2.2) |
| Weak but no moderate or strong | 152 (66.7) | |
| Moderate but no strong | 65 (28.5) | |
| Strong | 6 (2.6) | |
| OATP3HIB | Not administered | 225 (98.7) |
| Administered | 3 (1.3) | |
| Region | North America | 80 (34.5) |
| Western Europe | 100 (43.1) | |
| Eastern Europe | 37 (15.9) | |
| Other | 15 (6.5) |
Missing continuous covariates were imputed by the median value of the covariate. Missing categorical covariates were imputed by the most frequent value in the population. There were no covariates with missing data fraction exceeding 15% of study data
BCL-2 B cell lymphoma 2 protein, C3AHIB CYP3A inhibitor flag, COO cell-of-origin, DH doubt hit, DLBCL diffuse large B cell lymphoma, DP double positive, ECOG PS Eastern Cooperative Oncology Group performance status, FL follicular lymphoma, IHC immunohistochemistry, OATP3HIB OATP1B3 inhibitor flag, SD standard deviation
PopPK Analysis
Among the 232 patients who received venetoclax in combination with R-CHOP in CAVALLI, 223 (97.8%) had at least one evaluable PK sample, i.e., had at least one quantifiable PK sample, and were included in the analysis (1150 samples in total); all the 32 patients enrolled on the venetoclax + G-CHOP arm in phase 1b had at least one evaluable PK sample (179 samples in total). Exclusion of PK samples obtained more than 10 days after the last dose, those with concentrations below the LLOQ, and/or those with missing time left 1100 quantifiable samples from 260 patients for use in the PopPK analysis. This resulted in a proportion of missing samples of 19%.
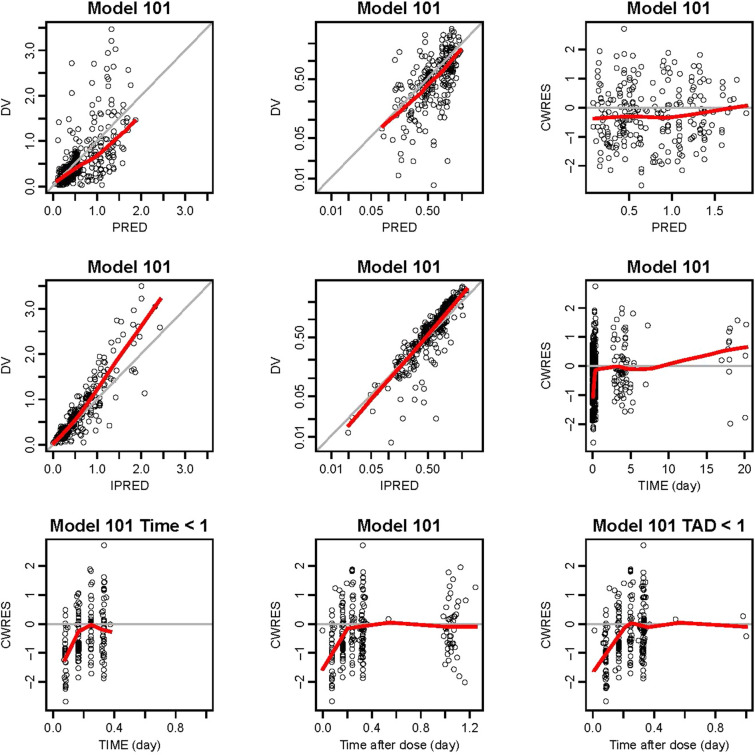
The legacy model was applied to the CAVALLI study data. All model parameters were fixed, including the inter-individual and residual variability. The basic diagnostic plots are shown in Fig. 1. The legacy model generally fitted the new data from the CAVALLI study. However, there were some biases (described in the supplementary material); therefore, the base model was improved by adding a multiplicative “study effect” covariate to each of CL/F and apparent central volume of distribution (V2/F) to account for possible differences in PK between CAVALLI and the historical studies used to generate the legacy model.
Fig. 1.
Basic diagnostic plots for model 101 (Legacy Model Applied to the Data of CAVALLI). The gray solid y = x or y = 0 lines are included for reference. The bold red lines are the lowess (local regression smoother) trend lines. Bottom plot on the right is a truncated version of the bottom middle plot. DV observed concentrations, PRED population predictions of the model, IPRED individual predictions of the model, CWRES conditional weighted residuals, TIME time after the first dose, TAD time after the most recent dose
The study effect covariate was shown to be significant on both parameters. The effect on CL/F was estimated at 1.63 (95% confidence interval [CI] 1.49–1.76) and that on V2/F at 2.33 (95% CI 2.17–2.54). Another model using a similar study effect on bioavailability (instead of separate effects on CL/F and V2/F) was not as good. The parameter estimates and the variability for venetoclax PK are shown in Table 2.
Table 2.
Parameter estimates for the final current model
| Parameter | Final model | |||
|---|---|---|---|---|
| Legacy model estimate | RSE (%) | Variability | Shrinkage | |
| CL/F (L/day) | 447 | 3.51 | – | – |
| θCL/F, strong CYP3A inhibitor | 0.18 | 6.03 | – | – |
| θCL/F, moderate CYP3A inhibitor | 0.84 | 4.24 | – | – |
| θCL/F,OATP1B3 inhibitor | 0.85 | 2.54 | – | – |
| θCL/F, GO27878 | 1.63 | 4.25 | – | – |
| V2/F (L) | 118 | 13.7 | – | – |
| θV2/F, CLL/SLL/NHL | 1.71 | 12.5 | – | – |
| θV2/F,SEX=female | 0.68 | 6.68 | – | – |
| θV2/F,GO27878 | 2.33 | 4.67 | – | – |
| Q/F (L/day) | 97.2 | 5.54 | – | – |
| V3/F (L) | 119 | 4.16 | – | – |
| ka(1/day) | 3.72 | 4.09 | – | – |
| θF1,FOOD=fasting | 0.34 | 0.97 | – | – |
| θF1,FOOD=moderate fat | 1.31 | 8.24 | – | – |
| θF1,FOOD=high fat | 1.43 | 1.3 | – | – |
| θF1,FOOD=fed | 1.23 | 4.29 | – | – |
| F1,DOSE nonlinearity (400 mg as reference) | − 0.18 | 2.38 | – | – |
| ω2CL/F | 0.15 | 9.35 | CV = 39.1% | 19.0% |
| ω2V2/F | 0.21 | 6.34 | CV = 45.3% | 27.7% |
| ω2F1 | 0.10 | 13.37 | CV = 31.2% | 28.3% |
| σ2prop | 0.22 | 1.67 | – | – |
| σ2add | 3.07 × 10–7 | 39.7 | – | – |
| t1/2 (day) | 1.05 | – | – | – |
95% CI 95% confidence interval, C3AHIB CYP3A inhibitor indicator variable, CL clearance, CL/F apparent clearance, CV coefficient of variation, F1 relative bioavailability, ka first-order absorption rate constant, OATP3HIB OATP1B3 indicator variable, Q/F apparent inter-compartmental clearance, RSE residual standard error, V2/F apparent central volume of distribution, V3/F apparent peripheral volume of distribution, ω2 inter-individual variances, σ2 residual variances
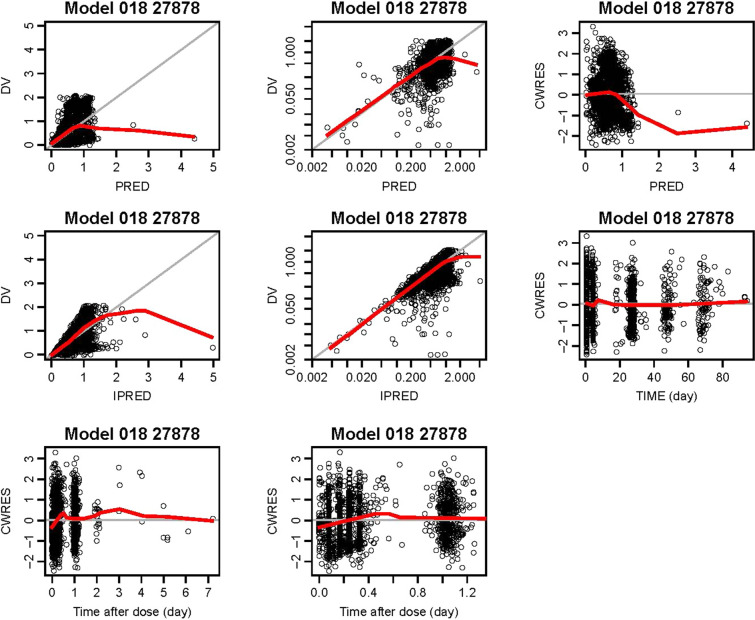
In the final model evaluation, goodness-of-fit of the data was confirmed (Fig. 2; described further in the supplementary material, Figs. S1 and S2), with no apparent model deficiencies.
Fig. 2.
Final model (018) evaluation: goodness-of-fit plots for final model. Top row: observed versus population predicted venetoclax concentrations (linear and log scales); CWRES versus population predicted venetoclax concentrations. Second row: observed versus individual predicted venetoclax concentrations (linear and log scales); CWRES versus time. Bottom row: CWRES versus time after dose. The gray solid lines (showing plots of y = x or y = 0, as appropriate) are included for reference. The bold red lines are the lowess (local regression smoother) trend lines. CWRES conditional weighted residuals, DV observed concentrations, IPRED individual predictions of the model, PRED population predictions of the model, TIME time after the first dose
Exposure–Efficacy
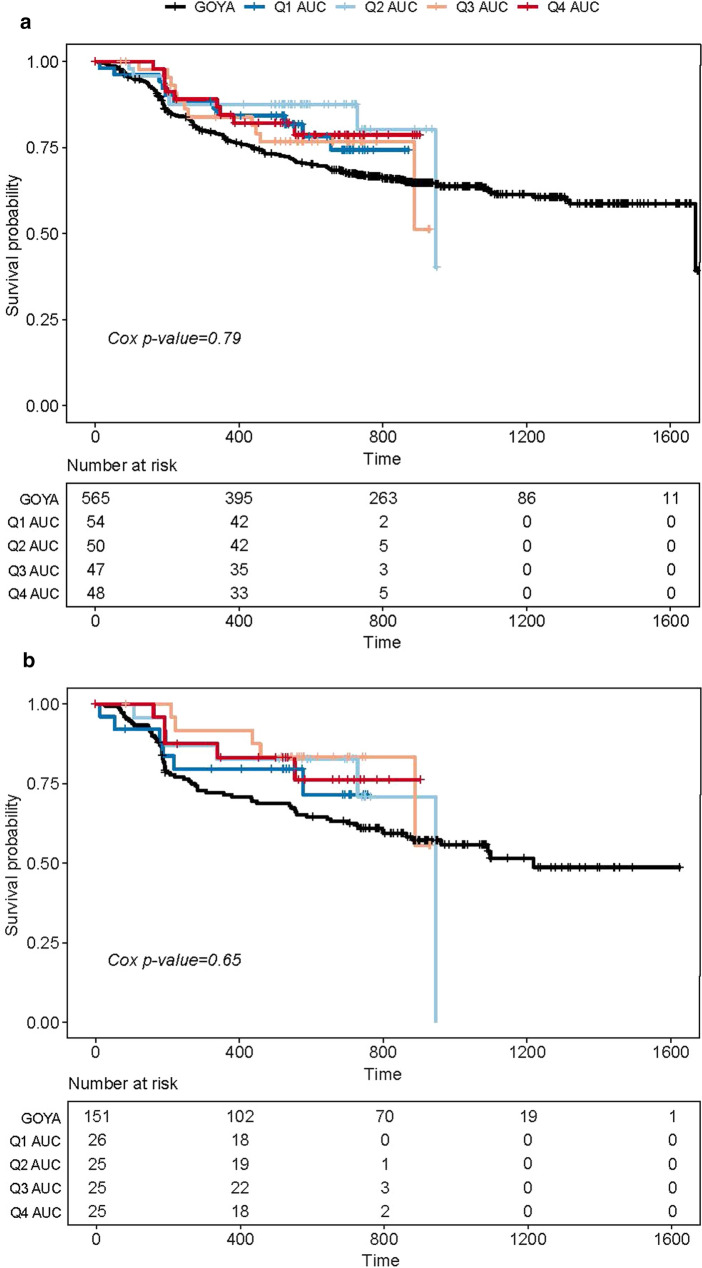
The 800 mg venetoclax dose was evaluated for impact on efficacy in the phase 2 part of CAVALLI. To support the previously published clinical findings [11], exposure–efficacy analysis was conducted in both BCL-2 positive (N = 101) and all-comer (regardless of biomarker status, N = 199) patients with 1L DLBCL enrolled in CAVALLI phase 2, who received venetoclax at 800 mg (10-day dosing) plus R-CHOP. KM plots stratified by quartiles of exposure indicated no apparent exposure–INV-PFS relationships (Fig. 3a, b). Furthermore, CPH models using exposure (AUCss nominal) as a continuous variable were tested for both the BCL-2-IHC-positive subpopulation and all-comers population; the exposure parameters of the base CPH model were not a significant predictor of INV-PFS (P > 0.05). Moreover, none of the tested covariates showed significant impact on INV-PFS (Table 3).
Fig. 3.
a Kaplan–Meier plot of PFS by investigator assessment according to venetoclax exposure (AUCss nominal) quartiles for all-comers. b PFS by investigator assessment according to venetoclax exposure quartiles for BCL-2-positive patients. AUC area under the curve of plasma concentration, BCL-2 B cell lymphoma 2, PFS progression-free survival
Table 3.
Base Cox proportional hazard models for investigator-assessed PFS in all-comers and BCL-2-positive population
| Model | Parameter | β | SE | RSE (%) | p value |
|---|---|---|---|---|---|
| All-comers | |||||
| Base PFS-AUCss nominal model | AUCss nominal | − 0.00181 (μg × day/mL) | 0.37 | 20,359.24 | 0.79 |
| BCL-2-positive population | |||||
| Base PFS-AUCss nominal model | AUCss nominal | − 0.2108 (μg × day/mL) | 0.46 | 21,761 | 0.65 |
AUCss venetoclax individual predicted steady-state area under the curve following the cohort-assigned dose, BCL-2 B cell lymphoma 2 protein, HR hazard ratio computed as exp(β), HR95CI 95% confidence intervals on hazard ratio, PFS progression-free survival, RSE relative standard error of β estimate (%), SE standard error of β estimate
Similar efficacy (PET-CR rate assessed at EOT) was reported for the all-comers population (69% CAVALLI vs 63% GOYA) and the BCL-2-positive subpopulation (BCL-2 by IHC: 65% CAVALLI vs 60% GOYA). The exposure–efficacy analyses using logistic regression were conducted in both BCL-2-IHC-positive patients (BCL-2 IHC: n = 101 and BCL-2 FISH: n = 40) and the all-comers (N = 199) population. No clear association was observed between venetoclax AUCss nominal and PET-CR rates in both the all-comers and BCL-2-IHC-positive subpopulations. Although this analysis did not show a clear correlation between venetoclax AUCss nominal and PET-CR rate, a wide CI was observed around the model prediction in the BCL-2-IHC-positive subpopulations (Fig. 4a–c). Given that all patients were treated at the 800 mg venetoclax dose and also the wide CI, it was not possible to confidently predict efficacy at other doses. None of the tested covariates significantly affected CR rates.
Fig. 4.
CR rate by PET at the EOT visit as a function of venetoclax nominal exposure (AUCss nominal) in a all-comers. *Black square represents the CR rates in GOYA, which was plotted, but not included in the ER analysis fit. Black unfilled circles = observed data in the CAVALLI study; blue solid line = logistical regression fitted curve; gray shaded area = 90% confidence interval (CI) of the blue solid line; probability of CR = 1: patients with CR; probability of CR = 0: patients with partial response/stable/progressive disease/no EOT assessments; black circles: observed probability of an event for each quartile of mean exposure plotted at the mean value within each exposure quartile. Dashed vertical lines show bounds of exposure groups. CIs were defined using 1000 bootstrap samples, and the logistic regression was fitted to each of these samples. The 90% CI for the logistic regression was defined as the 5th and 95th percentiles of the model predictions of the bootstrap data sets. AUCss unit: μg/mL × day. b BCL-2+ by FISH. *Red square represents the CR rates for the BCL-2 high (IHC scores 2+/3+) patients and blue square represents the CR rates for BCL-2 low (IHC scores 0/1+) patients in GOYA which was plotted, but not included in the ER analysis fit. Blue and red unfilled circles = observed data for the BCL-2 high and low subjects in the CAVALLI study; blue and red solid line = logistical regression fitted curve for the BCL-2 high and low subjects; green and pink shaded area = 90% CI of the blue and red regression line for the BCL-2 high and low subjects; probability of CR = 1: patients with CR; probability of CR = 0: patients with partial response/stable/progressive disease/no EOT assessments; blue and red circles: observed probability of an event for each quartile of mean exposure plotted at the mean value within each exposure quartile in the BCL-2 high and low subjects. CIs were defined using 1000 bootstrap samples, and the logistic regression was fitted to each of these samples. The 90% CI for the logistic regression was defined as the 5th and 95th percentiles of the model predictions of the bootstrap data sets. AUCss nominal unit: μg/mL × day. c BCL-2+ by IHC subpopulations. AUCss area under the curve of plasma concentration versus time at steady state, CI confidence interval, CR complete response, ER exposure–response, FISH fluorescence in situ hybridization, IHC immunohistochemistry, PET positron emission tomography, EOT end of treatment
Exposure–Safety
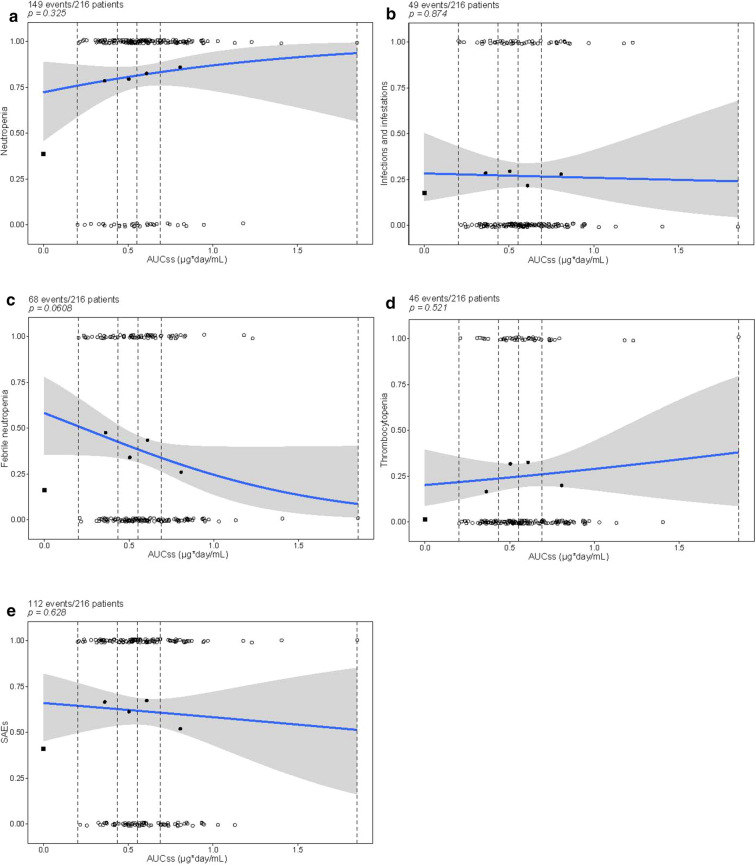
Exposure–safety analyses were conducted across all the 10-day venetoclax dosing regimens for the R-CHOP combination in both phase 1b and phase 2 portions of the CAVALLI study, and included both patients with 1L and R/R NHL (N = 3 at 400 mg, N = 8 at 600 mg, N = 205 at 800 mg), using logistic regression analyses. Grade 3–4 AEs occurred in 86.1% of patients in the phase 2 portion of CAVALLI (179/208) versus 66.1% in GOYA (373/564), mainly driven by a higher rate of cytopenia, febrile neutropenia, and infections. No statistically significant exposure–safety trends were associated with clinical manifestations of toxicity (i.e., grade ≥ 3 febrile neutropenia, grade ≥ 3 infections, and/or SAEs), or grade ≥ 3 neutropenia or grade ≥ 3 thrombocytopenia (Fig. 5).
Fig. 5.
Incidence of grade ≥ 3 a neutropenia, b infections and infestations, c febrile neutropenia, d thrombocytopenia, and e SAEs as a function of venetoclax nominal exposure (AUCss nominal). *Black square represents the observed fraction of patients with events in GOYA, which was plotted, but not included in the ER analysis fit. The blue solid line and gray shaded area = logistic regression model prediction and 90% CI of predictions. The unfilled circles = exposure of individual patients with events (p = 1) and without events (p = 0); black circles = observed probability of an event for each quartile of mean exposure plotted at the mean value within each exposure quartile in the CAVALLI study. Dashed vertical lines show bounds of exposure groups. CIs were defined using 1000 bootstrap samples, and the logistic regression was fitted to each of these samples. The 90% CI for the logistic regression was defined as the 5th and 95th percentiles of the model predictions of the bootstrap data sets. AUCss nominal unit: μg/mL × day. Venetoclax doses tested were 400 mg (N = 3), 600 mg (N = 8), 800 mg (N = 205) given for 10 days of 21-day cycles. AUCss area under the curve of plasma concentration versus time at steady state, CI confidence interval, ER exposure–response, SAE serious adverse event
Of the tested covariates, high (4, 5) IPI scores were significantly correlated with increased incidence of grade ≥ 3 febrile neutropenia (p < 0.001); increased age was significantly associated with increased incidence of grade ≥ 3 infections (p < 0.01) and grade ≥ 3 thrombocytopenia (p < 0.0001); and ECOG PS scores ≥ 2 were significantly correlated with increased incidence of grade ≥ 3 thrombocytopenia (p < 0.0001; Table 4). Despite the significant relationships observed for the patient-level covariates, no substantial impact on the exposure–safety relationship for venetoclax was evident upon including these covariates into the final logistic regression models. The observed AEs when venetoclax was given in combination with R-CHOP were manageable and consistent with the known safety profile and mechanism of action of venetoclax.
Table 4.
Logistic regression analysis for adverse events
| Parameter | Estimate | SE | RSE (%) | 95% CI | p value |
|---|---|---|---|---|---|
| Grade ≥ 3 neutropenia | |||||
| Intercept | − 0.20 | 0.44 | 223.9 | − 1.07; 0.67 | 0.66 |
| Slope of AUCss nominal (μg × day/mL) | 1.76 | 0.76 | 42.9 | 0.28; 3.25* | 0.325 |
| Grade ≥ 3 infection | |||||
| Intercept | − 3.65 | 1.06 | 29.07 | − 5.73; − 1.57 | 0.00058 |
| Slope of AUCss nominal (μg × day/mL) | − 0.20 | 0.72 | 353.88 | − 1.62; 1.21* | 0.874 |
| Age | 0.04 | 0.02 | 36.89 | 0.01; 0.07 | 0.0067 |
| Grade ≥ 3 febrile neutropenia | |||||
| Intercept | − 0.81 | 0.42 | 51.53 | − 1.63; 0.01 | 0.052 |
| Slope of AUCss nominal (μg × day/mL) | − 0.46 | 0.66 | 144.05 | − 1.75; 0.84* | 0.0608 |
| IPI (high) [4, 5]a | 1.20 | 0.33 | 27.36 | 0.55; 1.84 | 0.00026 |
| Grade ≥ 3 thrombocytopenia | |||||
| Intercept | − 8.70 | 1.59 | 18.33 | − 11.82; − 5.57 | 0.000000049 |
| Slope of AUCss nominal (μg × day/mL) | 0.59 | 0.75 | 127.03 | − 0.88; 2.05* | 0.521 |
| Age | 0.11 | 0.02 | 20.66 | 0.06; 0.15 | 0.0000013 |
| ECOG (2+)b | 1.65 | 0.45 | 27.62 | 0.75; 2.54 | 0.00029 |
| Serious adverse events | |||||
| Intercept | − 3.07 | 0.87 | 28.36 | − 4.77; − 1.36 | 0.00042 |
| Slope of AUCss nominal (μg × day/mL) | − 0.51 | 0.63 | 123.64 | − 1.75; 0.73* | 0.628 |
| Age | 0.05 | 0.01 | 25.93 | 0.02; 0.07 | 0.00011 |
95% CI 95% confidence interval on the parameter estimate, AUCss venetoclax individual predicted area under the curve at steady state based on the cohort-assigned dose, RSE relative standard error of the parameter estimate (%), SE standard error
*95% CI of the exposure parameter spans zero, indicating statistical nonsignificance
aIPI (high) [4, 5] represents patients with IPI scores of 4 and 5 and in the covariate analysis were compared with those with the IPI scores of 0, 1, and 2
bECOG (2+) represents patients with ECOG PS score ≥ 2 and in the covariate analysis were compared with those with ECOG PS score < 2
*95% CI of the exposure parameter spans zero, indicating statistical nonsignficance
Exposure–Dose Intensity
The 800 mg 10-day dose regimen of venetoclax was evaluated for potential impact on the R-CHOP components for the population of patients with 1L DLBCL. The percentage of patients with greater than 90% RDI for the R-CHOP components in the CAVALLI study was generally similar to the R-CHOP arm in the GOYA study across exposure cohorts [9, 10]. Importantly, the exposure–dose intensity analyses did not show any relationship between venetoclax AUCss nominal and RDIs for the individual components of venetoclax and R-CHOP in the CAVALLI study (Fig. 6).
Fig. 6.
RDIs for R-CHOP and venetoclax in CAVALLI as a function of venetoclax nominal exposure (AUCss nominal). Values in the lower right of each panel represent the correlation coefficient. Circles correspond to individual dose intensity values. AUCss defined by Eq. (1) was used as a measure of exposure. Red lines are the lowess trend lines. Plus sign represents an individual patient’s relative dose intensity values. AUCss area under the curve of plasma concentration versus time at steady state, CHOP cyclophosphamide, doxorubicin, vincristine, and prednisone, R rituximab, RDI relative dose intensity
Discussion
In the CAVALLI study, evidence of the positive benefit–risk profile for venetoclax administered at a dose of 800 mg in a 10/21-day cycle was demonstrated, particularly for BCL-2-IHC-positive patients with 1L DLBCL, when compared with historical data from the R-CHOP arm of the GOYA study [10].
The PK of venetoclax in CAVALLI were adequately described using a previously developed legacy PopPK model. However, upon evaluation of the legacy model (when applied to the CAVALLI data) using diagnostic plots, lower exposures for the patients enrolled in CAVALLI were noticeable compared with the patients with R/R NHL previously evaluated in the legacy model at similar dose levels. It is known that increased fat intake increases venetoclax exposure [18] and therefore it could be speculated that the lower exposures in the CAVALLI study may be attributed to the lower fat content in the meal-type, i.e., 23% and 3.5% of fat calories in the meal as recommended in the CAVALLI protocol, compared with the 30% of fat calories following the completion of the standard low-fat breakfast for the patients with R/R NHL included in the legacy PopPK model. Moreover, we were not able to consider these differences in fat content of 23% and 3.5% in the PopPK model as both meals were classified as low-fat meal type in the CAVALLI study. Subsequently, a study (CAVALLI) effect of 1.63 on CL/F and 2.33 on V2/F (values higher than those reported for the historical studies) was added in the updated PopPK model to account for the possible differences between the population of CAVALLI study patients relative to the population of patients from the prior analysis.
Furthermore, in contrast to the legacy model, the present PopPK model did not include the co-administration of R as a covariate on CL/F of venetoclax. In the legacy model, concomitant R administration was estimated to increase the venetoclax CL/F by 1.21-fold based on a small sample size of 50 subjects receiving venetoclax + R [13]. However, subsequent analysis in a much larger patient population (the phase 3 MURANO study, where 181 patients with R/R CLL were randomized to receive venetoclax plus R) showed a minimal 7% increase in the CL/F for venetoclax when co-administered with R, supporting removal of the R/G covariate from the model [19]. The legacy model and phase 3 MURANO study suggest that neither R, which leads to a rapid depletion of CD20+ B cells, nor indication type (whether CLL, SLL, or NHL; all of which are characterized by varying levels of circulating B cell lymphocytes) has any apparent impact on venetoclax PK [13, 19]. Taken together, this indicates minimal impact of B cell subtypes on venetoclax PK (although this was not specifically tested as a covariate in the PopPK model).
Previous studies have evaluated the venetoclax ER relationships in subjects with CLL, NHL, or multiple myeloma [20–24]. ER analyses were performed to confirm the dose selection of venetoclax in combination with R-CHOP chemotherapy for future studies. PopPK-predicted AUCss nominal was used as an exposure metric for predicting clinical response, i.e., efficacy, safety, or tolerability of venetoclax. We used AUCss nominal as the exposure metric for the ER analysis rather than average plasma concentration to the time of the event (Caverage) to avoid bias due to a correlation between lower exposures from dose reductions and response, both of which are more likely the longer a patient is on study. Moreover, AUCss nominal isolated the impact of assigned target venetoclax dose and associated steady-state exposure on safety and efficacy, and was not subject to confounding by complex interactions between time and treatment-related or disease-related changes to venetoclax or R-CHOP dosing. To isolate the treatment effect of venetoclax, the ER analyses referenced clinical data from the R-CHOP arm of GOYA.
Promising efficacy for PFS and improved efficacy for PET-CR rate assessed at EOT in CAVALLI compared with matched GOYA controls were consistent with the venetoclax mechanism of action [12]. However, the graphical and CPH analysis of the patients treated at the 800 mg dose in the phase 2 portion of the CAVALLI study showed no statistically significant relationship between venetoclax exposure and PFS in both all-comers or BCL-2-IHC-positive subpopulations, which suggests no evidence of additional efficacy with increasing venetoclax exposure in this trial. This is further confirmed by the logistic regression analyses using PET-CR rates, where increased venetoclax exposure did not translate into improved efficacy in both the all-comers or BCL-2-IHC-positive subpopulations at the 800 mg (10/21-day cycle) dose.
Although no statistically significant exposure-PFS or exposure-PET-CR trends were observed in these analyses, the results are not considered sufficiently strong enough to confidently predict efficacy at alternative venetoclax doses, as the majority of patients in the exposure-efficacy analyses were studied at a single venetoclax dose level of 800 mg and a wide CI was observed around exposure-efficacy predictions for the BCL-2-IHC-positive subpopulations.
Furthermore, compared with CLL, where the labeled venetoclax dose is 400 mg, a venetoclax dose resulting in a higher steady-state exposure in NHL may be required because of a potential decreased sensitivity to venetoclax of lymphocytes located in the lymph node compared with the blood as reflected in the lower half maximum effective concentration (EC50) values of venetoclax for circulating lymphocyte counts (0.00863 μg/mL) compared with tumor size (0.146 μg/mL) [21]. This increased sensitivity of circulating CLL cells than CLL cells within a tumor mass may be attributed to the reduced blood flow and subsequent delivery of systemic venetoclax within a tumor, and due to survival signals cells receive when in direct contact with other cells within the tumor microenvironment. It is therefore not surprising that DLBCL sensitivity is lower compared with CLL, as the bulk of the disease is non-circulating (in lymph nodes and other tissues in the body).
As long as delivery of the potentially curative standard of care R-CHOP therapy is not compromised, the addition of venetoclax is considered to pose a low risk for negatively impacting outcome in patients with 1L DLBCL. In the current study, patients with higher venetoclax exposures showed similar dose intensities for the individual components of venetoclax and R-CHOP combination therapy compared with patients with lower venetoclax exposures, suggesting that patients with higher initial venetoclax exposure did not have higher probability to maintain or reduce their venetoclax or R-CHOP backbone dose. This finding supports the proposal that addition of venetoclax to R-CHOP did not compromise delivery of R-CHOP therapy.
Logistic regression analysis of exposure-safety data from the patients in the CAVALLI study showed no statistically significant associations between venetoclax exposure and the probability of clinical manifestations of toxicity (i.e., grade ≥ 3 febrile neutropenia, grade ≥ 3 infections, and/or SAEs), or grade ≥ 3 neutropenia or grade ≥ 3 thrombocytopenia. The prognostic impact of the tested covariates has been previously reported. [25–28]
Although a higher incidence of AEs was generally seen for patients with 1L DLBCL in the CAVALLI study compared with the GOYA historical control (R-CHOP), the reported AEs were manageable and predictable on the basis of the mechanism of action of venetoclax, or consistent with the known safety profile of R-CHOP [29, 30]. The interpretation of these events could be confounded by the additional mandatory mid-cycle laboratory tests and clinical evaluations required for the CAVALLI study but not required in the GOYA trial. Taken together, there was limited evidence to suggest that administering 800 mg of venetoclax would cause additional toxicity compared with the lower venetoclax doses administered to patients in the CAVALLI study; however, it is possible that higher doses could lead to increased safety events.
Conclusions
The early promising efficacy data from the CAVALLI study of venetoclax treatment in combination with R-CHOP, together with the acceptable early safety and tolerability in patients treated with this combination, support the selection of venetoclax at 800 mg in cycle 1 on days 4–10 and cycles 2–8 on days 1–10 in combination with R-CHOP for 21-day cycles in the BCL-2-IHC-positive 1L DLBCL population in future studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Venetoclax is being developed in collaboration between Genentech, Inc. and AbbVie Inc. The authors thank the patients who participated in the CAVALLI and GOYA studies and their families, as well as the study co-investigators, research nurses, and coordinators at each of the clinical sites. The authors also thank Sandhya Girish for her input into data analyses and manuscript development.
Funding
Genentech, Inc. and AbbVie Inc. provided financial support for the CAVALLI study and participated in the design, study conduct, analysis and interpretation of data. The GOYA study was supported by research funding from F. Hoffmann-La Roche Ltd, with scientific support from the Fondazione Italiana Linfomi. The analyses presented here were supported by Genentech, Inc. The journal’s Rapid Service and Open Access fees were funded by F. Hoffmann-La Roche Ltd.
Medical Writing and Editorial Assistance
Medical writing and editorial support were provided by Anshin Biosolutions and funded by Genentech, Inc. Under the direction of the authors, further editorial assistance was provided by Rachel Dobb PhD of Ashfield MedComms, an Ashfield Health company, and funded by F. Hoffmann-La Roche Ltd.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
Disclosures
Divya Samineni, Weize Huang, Hao Ding, Rong Zhang, Chunze Li, Sandhya Girish, and Dale Miles are employees of Genentech, Inc., and may hold stock or stock options with the Roche Group. Richa Rajwanshi was previously an employee of Genentech Inc and may have held stock or stock options with the Roche Group; new affiliation is Amgen, Thousand Oaks, CA, USA. Kathryn Humphrey and Alexandra Bazeos are employees of Roche Products Ltd and may hold stock or stock options with the Roche Group. Arijit Sinha was previously an employee of Roche Products Ltd and may have held stock or stock options with the Roche group; new affiliation is AstraZeneca, Melbourn Science Park, Melbourn, Herts, UK. Ahmed Hamed Salem is an employee of AbbVie Inc. and may hold stock or stock options with AbbVie. Leonid Gibiansky is an employee of QuantPharm LLC.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments. This article does not contain any studies with animals performed by any of the authors.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
This article does not contain any identifying information about participants and therefore consent was not sought from participants for publication.
Data Availability
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, visit https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
References
- 1.Hata AN, Yeo A, Faber AC, et al. Failure to induce apoptosis via BCL-2 family proteins underlies lack of efficacy of combined MEK and PI3K inhibitors for KRAS-mutant lung cancers. Cancer Res. 2014;74(11):3146–3156. doi: 10.1158/0008-5472.CAN-13-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell KJ, Tait SWG. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 2018 doi: 10.1098/rsob.180002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang HW, Cheng NL, Chen ZW, Wang JF, Li SH, Bai W. Clinical impact of t(14;18) in diffuse large B-cell lymphoma. Chin J Cancer Res. 2011;23(2):160–164. doi: 10.1007/s11670-011-0160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson MA, Tsui A, Wall M, Huang DC, Roberts AW. Current challenges and novel treatment strategies in double hit lymphomas. Ther Adv Hematol. 2016;7(1):52–64. doi: 10.1177/2040620715608091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 6.Deeks ED. Venetoclax: first global approval. Drugs. 2016;76(9):979–987. doi: 10.1007/s40265-016-0596-x10.1007/s40265-016-0596-x. [DOI] [PubMed] [Google Scholar]
- 7.Venclexta (venetoclax) [package insert]. North Chicago, IL: AbbVie Inc; 2020. https://www.rxabbvie.com/pdf/venclexta.pdf
- 8.Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-hodgkin lymphoma. J Clin Oncol. 2017;35(8):826–833. doi: 10.1200/JCO.2016.70.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zelenetz AD, Salles G, Mason KD, et al. Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: results from the CAVALLI phase 1b trial. Blood. 2019 doi: 10.1182/blood-2018-11-880526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vitolo U, Trneny M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017;35(31):3529–3537. doi: 10.1200/JCO.2017.73.3402. [DOI] [PubMed] [Google Scholar]
- 11.Franck Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600–609. doi: 10.1182/blood.2020006578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zelenetz AD, Feugier P, Flinn, I et al. Improved outcomes with venetoclax plus rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone (R-CHOP) in previously untreated patients with BCL-2-positive diffuse large B-cell lymphoma (DLBCL): updated desults from the phase II CAVALLI Study. (Abstract). 61st ASH Annual meeting; December 7−10. Orlando, Florida. 2019
- 13.Jones AK, Freise KJ, Agarwal SK, Humerickhouse RA, Wong SL, Salem AH. Clinical predictors of venetoclax pharmacokinetics in chronic lymphocytic leukemia and non-hodgkin’s lymphoma patients: a pooled population pharmacokinetic analysis. AAPS J. 2016;18(5):1192–1202. doi: 10.1208/s12248-016-9927-9. [DOI] [PubMed] [Google Scholar]
- 14.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13(2):143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brendel K, Comets E, Laffont C, Laveille C, Mentre F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res. 2006;23(9):2036–2049. doi: 10.1007/s11095-006-9067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yano Y, Beal SL, Sheiner LB. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn. 2001;28(2):171–192. doi: 10.1023/A:1011555016423. [DOI] [PubMed] [Google Scholar]
- 17.Beal S, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM User’s Guides (1989–2011). Ellicott City, MD, USA: Icon Development Solutions; 2011
- 18.Salem AH, Agarwal SK, Dunbar M, et al. Effect of low- and high-fat meals on the pharmacokinetics of venetoclax, a selective first-in-class BCL-2 inhibitor. J Clin Pharmacol. 2016;56(11):1355–1361. doi: 10.1002/jcph.741. [DOI] [PubMed] [Google Scholar]
- 19.Deng R, Gibiansky L, Lu T, et al. Bayesian population model of the pharmacokinetics of venetoclax in combination with rituximab in patients with relapsed/refractory chronic lymphocytic leukemia: results from the phase III MURANO study. Clin Pharmacokinet. 2019 doi: 10.1007/s40262-019-00788-8. [DOI] [PubMed] [Google Scholar]
- 20.Agarwal S, Gopalakrishnan S, Mensing S, et al. Optimizing venetoclax dose in combination with low intensive therapies in elderly patients with newly diagnosed acute myeloid leukemia: an exposure-response analysis. Hematol Oncol. 2019 doi: 10.1002/hon.2646. [DOI] [PubMed] [Google Scholar]
- 21.Freise KJ, Dunbar M, Jones AK, et al. Venetoclax does not prolong the QT interval in patients with hematological malignancies: an exposure-response analysis. Cancer Chemother Pharmacol. 2016;78(4):847–853. doi: 10.1007/s00280-016-3144-1. [DOI] [PubMed] [Google Scholar]
- 22.Freise KJ, Jones AK, Menon RM, et al. Relationship between venetoclax exposure, rituximab coadministration, and progression-free survival in patients with relapsed or refractory chronic lymphocytic leukemia: demonstration of synergy. Hematol Oncol. 2017;35(4):679–684. doi: 10.1002/hon.2373. [DOI] [PubMed] [Google Scholar]
- 23.Freise KJ, Jones AK, Verdugo ME, Menon RM, Maciag PC, Salem AH. Moving beyond maximum tolerated dose for targeted oncology drugs: use of clinical utility index to optimize venetoclax dosage in multiple myeloma patients. Clin Pharmacol Ther. 2017;102(6):970–976. doi: 10.1002/cpt.712. [DOI] [PubMed] [Google Scholar]
- 24.Parikh A, Gopalakrishnan S, Freise KJ, et al. Exposure-response evaluations of venetoclax efficacy and safety in patients with non-Hodgkin lymphoma. Leuk Lymphoma. 2018;59(4):871–879. doi: 10.1080/10428194.2017.1361024. [DOI] [PubMed] [Google Scholar]
- 25.Choi YW, Jeong SH, Ahn MS, et al. Patterns of neutropenia and risk factors for febrile neutropenia of diffuse large B-cell lymphoma patients treated with rituximab-CHOP. J Korean Med Sci. 2014;29(11):1493–1500. doi: 10.3346/jkms.2014.29.11.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyman DM, Eaton AA, Gounder MM, et al. Predictors of early treatment discontinuation in patients enrolled on phase I oncology trials. Oncotarget. 2015;6(22):19316–19327. doi: 10.18632/oncotarget.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matzdorff A, Meyer O, Ostermann H, et al. Immune thrombocytopenia—current diagnostics and therapy: recommendations of a joint working group of DGHO, OGHO, SGH, GPOH, and DGTI. Oncol Res Treat. 2018;41(Suppl 5):1–30. doi: 10.1159/000492187. [DOI] [PubMed] [Google Scholar]
- 28.Pfreundschuh M. How I treat elderly patients with diffuse large B-cell lymphoma. Blood. 2010;116(24):5103–5110. doi: 10.1182/blood-2010-07-259333. [DOI] [PubMed] [Google Scholar]
- 29.Davies A, Berge C, Boehnke A, et al. Subcutaneous rituximab for the treatment of B-cell hematologic malignancies: a review of the scientific rationale and clinical development. Adv Ther. 2017;34(10):2210–2231. doi: 10.1007/s12325-017-0610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salles G, Barrett M, Foà R, et al. Rituximab in B-cell hematologic malignancies: a review of 20 years of clinical experience. Adv Ther. 2017;34(10):2232–2273. doi: 10.1007/s12325-017-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche’s criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, visit https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.