Abstract
Introduction
Levothyroxine monotherapy (Synthroid® or multiple generic levothyroxine [GL] formulations) is standard treatment for hypothyroidism. Our objective was to compare effectiveness (as measured by achievement of thyroid-stimulating hormone [TSH] levels) and economic outcomes of Synthroid vs. any one of multiple GLs in patients with hypothyroidism.
Methods
Data for this retrospective cohort study were obtained from the HealthCore Integrated Research Database®. All study patients had ≥ 2 claims between 1 January 2006 and 31 December 2017 with ICD-9/10-CM diagnosis codes for hypothyroidism; were persistent users of Synthroid vs. any GL; and had ≥ 1 TSH laboratory result during 12-month follow-up. Patients were divided into one of two cohorts based on index medication and were 1:1 matched using propensity scores. The primary outcome was the proportion of patients with last TSH laboratory result during follow-up within the reference range (0.3–4.12 mIU/L). Secondary outcomes included all-cause and hypothyroidism-related healthcare resource utilization (HCRU) and costs.
Results
After propensity score matching, the Synthroid and GL cohorts each contained 18,382 patients. At follow-up, significantly more patients receiving Synthroid were in the TSH reference range vs. GL (78.5% vs. 77.2%, respectively, p = 0.002). HCRU and costs were broadly similar between the cohorts in terms of all-cause inpatient hospitalizations, emergency department visits, outpatient services, and pharmacy fills. Irrespective of index medication, patients with TSH within the reference range had significantly lower hypothyroidism-related medical and total costs compared to those outside the range.
Conclusions
This real-world data study showed Synthroid was associated with better TSH target achievement vs. GL in a US managed care population. Achieving TSH goals may provide substantial economic value by reducing hypothyroidism-related HCRU and costs.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01969-3.
Keywords: Hypothyroidism, Levothyroxine, Comparative effectiveness, TSH
Key Summary Points
| Why carry out this study? |
| Hypothyroidism (overt and subclinical) is common in the US and associated with adverse clinical outcomes. |
| Levothyroxine is considered standard of care, with branded (Synthroid®) and multiple generic versions (GL) available. |
| Prior preliminary research indicated that TSH outcomes may differ between patients with hypothyroidism treated with Synthroid or GL. |
| What was learned from the study? |
| After 12 months from initiation, significantly more patients receiving Synthroid were in the TSH reference range (0.3–4.12 mIU/L) vs. GL (78.5% vs. 77.2%, p = 0.002). |
| Patients with TSH within the reference range had significantly lower hypothyroidism-related medical and total costs compared to those outside the range. |
| Use of Synthroid may offer improved health and economic outcomes relative to generic formulations. |
Introduction
Hypothyroidism is a common disease that currently affects 4.6% of the US population [1] and will affect more than 12% of Americans at some point during their lifetimes [2]. The condition most commonly affects women and individuals > 60 years and people with certain autoimmune conditions (e.g., Hashimoto’s thyroiditis, lupus, rheumatoid arthritis), and those with a family history of thyroid disease are more likely to develop hypothyroidism [3]. Effective treatment largely depends on an accurate diagnosis [4], and the most reliable screening test is measurement of thyroid-stimulating hormone (TSH) levels [5, 6]. TSH levels are also an indicator of the effectiveness of hypothyroid treatment, and achievement of a TSH level within the normal reference range is a goal of thyroid replacement therapy [4, 6, 7]. According to the American Thyroid Association Task Force on Thyroid Hormone Replacement, the recommended treatment goal for TSH is in the range of 0.4 mIU/L to 4.0 mIU/L; the limits may vary with age, pregnancy, and race [1, 4–6].
Levothyroxine (LT4) monotherapy is considered the standard of care for the treatment of hypothyroidism [6], with Synthroid® (levothyroxine sodium tablets, AbbVie) and any one of multiple generic levothyroxine (GL) formulations most often prescribed in the US. Levothyroxine has a narrow therapeutic window, and slight variations in dose or differences in bioavailability between products can impact clinical effectiveness [8–10]. In the US, the current regulatory process for assessing the bioequivalence of levothyroxine products has raised concerns that products with clinically significant differences in bioavailability could still be labeled as therapeutically equivalent; therefore, professional organizations recommend consistent use of the same (branded or generic) formulation [11]. Previous real-world research using administrative claims data demonstrated differences in outcomes between patients with hypothyroidism treated with Synthroid or GL [9, 10]. Compared with patients who received GL, significantly fewer patients in the Synthroid treatment group had TSH levels outside the recommended range (0.3 to 4.12 mIU/L) and a smaller proportion was undertreated [9, 10].
To determine whether achievement of TSH goals was more consistent (i.e., more likely to be within the reference range) with Synthroid than GL, we analyzed administrative claims for patients with hypothyroidism over a 12-month follow-up period. We also examined the economic outcomes for patients who achieved TSH goals compared with patients who did not achieve TSH goals.
Methods
Data for this retrospective cohort study were obtained from claims contained in the HealthCore Integrated Research Database (HIRD®). The HIRD is a health insurance database that contains claims integrated across data sources and service types (i.e., professional claims, facility claims, outpatient pharmacy claims, outpatient laboratory results, and enrollment information) as well as across years. Data are derived from a large national commercial payer with membership in all 50 US states.
Researchers only accessed data in the format of a limited data set for which a data use agreement was in place with the covered entities in compliance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. An Institutional Review Board did not review the study since only this limited data set was accessed.
Patient Identification
To be included in the study, patients were required to have at least two distinct claims between 1 January 2006 and 31 December 2017 with ICD-9/10-CM diagnosis codes for hypothyroidism (see Supplementary Material Table 1). Patients were also required to have two or more fills for either Synthroid or GL (same or multiple). The date of the first fill that occurred on or after the first hypothyroid diagnosis was used as the index date. Patients were divided into one of two cohorts based on their index medication (Synthroid or GL).
All patients were ≥ 18 years old on the index date, and all had at least 6 months of pre-index (baseline) and 12 months of post-index (follow-up) health plan enrollment. Patients were required to have at least 1 TSH laboratory result during the 12-month follow-up period and be persistent in their index therapy through follow-up. Persistence was defined as continuous use of the index therapy without either a switch from brand to generic levothyroxine or vice versa (switching between generic levothyroxines in the GL arm was permitted) or a gap in levothyroxine treatment greater than the days’ supply on the last claim. Patients were excluded from the study if they had a diagnosis of thyroid cancer or pregnancy at any time during the study period; had fills for both Synthroid and GL on the index date; used liothyronine (LT3) and/or desiccated thyroid within the 12-month follow-up period; or had a baseline claim for a levothyroxine other than Synthroid or any GL (i.e., patients with Synthroid or GL fills prior to their first hypothyroid diagnosis were not excluded).
Outcome Measures
The primary outcome was the proportion of patients with last TSH laboratory result during follow-up between 0.3 mIU/L and 4.12 mIU/L (reference range) [1, 4, 12]. This outcome was assessed in patients who persistently followed index therapy and were matched 1:1 (Synthroid vs. GL) using propensity scores.
Secondary outcomes included all-cause and hypothyroidism-related healthcare resource utilization (HCRU) and costs. All-cause HCRU and costs included all medical and pharmacy claims, and costs were the sum of plan-paid and patient-paid amounts. Hypothyroidism-related HCRU and costs were based on medical claims with a hypothyroid diagnosis code and pharmacy claims for hypothyroid medications (see Supplementary Table 1 for a list of codes). HCRU and costs were stratified by place of service (inpatient hospitalization, stand-alone emergency department [ED] visits, outpatient visits and services, and pharmacy dispensing). Pharmacy costs, total medical costs (the sum of inpatient, ED, and outpatient costs), and total costs (the sum of medical and pharmacy costs) were assessed. Costs are reported per patient and were adjusted to 2017 USD price levels using the medical care index provided by the Bureau of Labor Statistics. Secondary outcomes were measured in a subset of matched patients from the primary objective with complete pharmacy cost capture.
All-cause and hypothyroidism-related HCRU and costs were also separately compared between patients who were persistent and who achieved target TSH levels during follow-up vs. those who did not achieve target TSH levels (achievers vs. non-achievers). No matching was performed for this comparison.
Statistical Analysis
All variables were summarized using descriptive statistics. Means and standard deviations (SD) were reported for continuous variables (for select variables, medians were also reported) and absolute and relative frequencies for categorical variables.
Due to the observational nature of the study and lack of randomization of patients into the two study cohorts (Synthroid vs. GL), it was necessary to account for potential treatment selection bias. This was achieved through 1:1 nearest neighbor matching on propensity scores, with the propensity score defined as the probability of initiating Synthroid (vs. GL) given the baseline patient characteristics and estimated using logistic regression (refer to Supplemental Material Table 2 for a list of included variables) [13]. Matching was finalized before the outcome analyses were conducted.
For this matched cohort comparison of Synthroid vs. GL, differences at baseline were evaluated using standardized differences, with an absolute value < 0.1 indicating balance in variables across cohorts [13]. Differences at follow-up were evaluated using hypothesis testing. Categorical variables were compared using χ2 tests; TSH laboratory results, pharmacy fills, and costs were compared using t-tests. The odds of having TSH laboratory tests outside of the specified range were calculated using logistic regression with cohort (Synthroid vs. GL) as the only independent variable.
For the comparison of TSH achievers and non-achievers, where no matching was performed, differences at baseline were also assessed using standardized differences, while differences at follow-up were assessed via hypothesis testing using χ2 tests and t-tests. Costs at follow-up were also compared using a GLM regression with gamma distribution and log link function and several baseline variables as covariates (refer to Supplemental Material Table 3 for a list of included variables).
An alpha level of 0.05 was used to identify statistical significance. No adjustments for multiple comparisons were made in this study. The statistical analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute Inc., Cary, NC, USA, 2014). Sensitivity analyses of the primary outcome were performed using a narrower TSH range (0.4 mIU/L to 4.0 mIU/L [6]) and also among a subset of patients with at least two TSH results at follow-up.
Results
Patient Selection
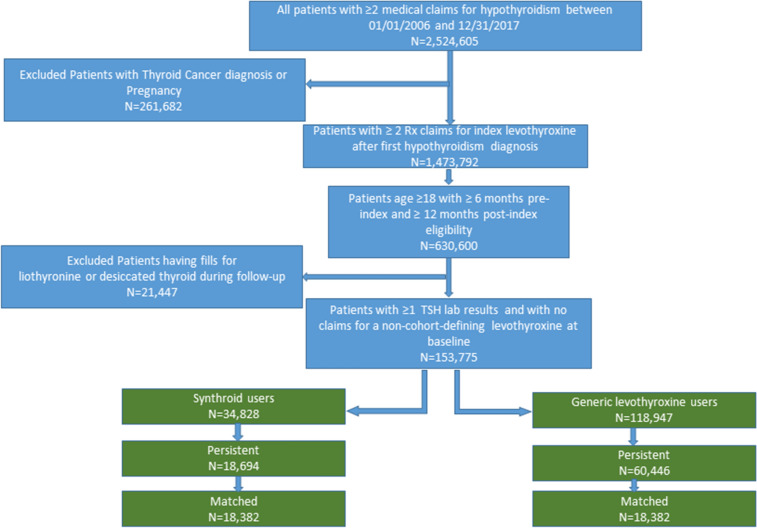
A total of 153,775 patients (34,828 in the Synthroid cohort and 118,947 in the GL cohort) were initially identified for potential study inclusion (Fig. 1). Of these, roughly half persistently followed index therapy (79,140 total; 18,694 Synthroid; 60,446 GL). After propensity score matching, the Synthroid and GL cohorts each contained 18,382 patients. This population was used for analysis of the primary outcome.
Fig. 1.
Patient selection flow chart: full study population
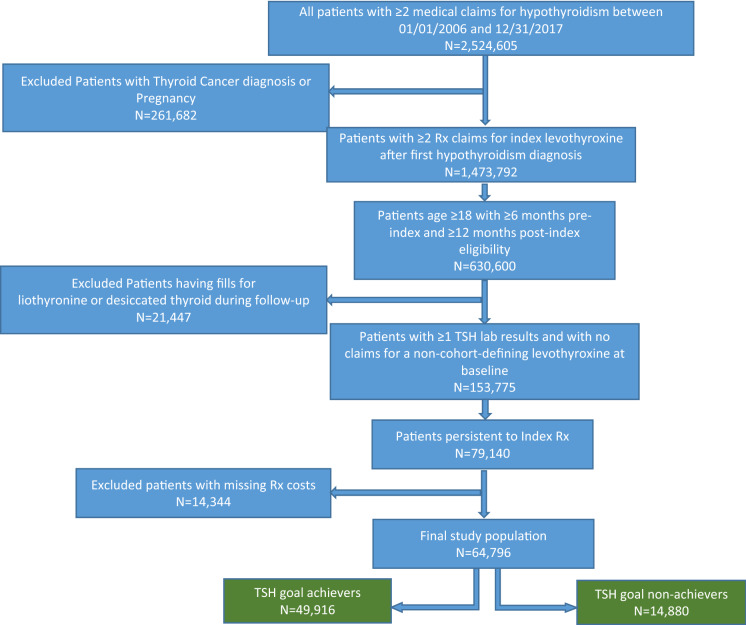
Of the 79,140 patients who were persistent to their index therapy, 64,796 had complete pharmacy claims information and comprised the population used in the analysis of HCRU and costs (Fig. 2). These patients were stratified into those who achieved TSH goals (achievers, n = 49,916) and those who did not reach TSH goals (non-achievers, n = 14,880) for further analysis of HCRU and costs.
Fig. 2.
Patient selection flow chart: economic analysis subset
Patient Characteristics
After matching, the Synthroid and GL cohorts were balanced on baseline characteristics (Tables 1, 2). The average age of study participants was 53 years, and 82% were female. Preferred provider organization (PPO) health plans were the most common in both cohorts, and approximately 8% had Medicare Advantage plans.
Table 1.
Baseline patient demographics and clinical characteristics in the matched cohorts
| Demographic and clinical characteristics | Synthroid persistent users (n = 18,382) |
Generic levothyroxine persistent users (n = 18,382) |
Standardized differencea |
|---|---|---|---|
| n (%)/mean (SD) | n (%)/mean (SD) | ||
| Age at index (years), mean (SD) | 53.4 (12.13) | 53.4 (11.77) | 0.000 |
| Age categories, n (%) | |||
| 18–39 | 2100 (11.4) | 2046 (11.1) | 0.009 |
| 40–64 | 13,806 (75.1) | 14,152 (77.0) | –0.044 |
| 65–74 | 1629 (8.9) | 1407 (7.7) | 0.044 |
| 75 + | 847 (4.6) | 777 (4.2) | 0.019 |
| Female, n (%) | 15,083 (82.1) | 15,085 (82.1) | 0.000 |
| Health plan type, n (%) | |||
| PPO | 10,919 (59.4) | 10,270 (55.9) | 0.071 |
| HMO | 5769 (31.4) | 6154 (33.5) | –0.045 |
| CDHP | 1694 (9.2) | 1957 (10.6) | –0.048 |
| Medicare Advantage plan, n (%) | 1442 (7.8) | 1390 (7.6) | 0.011 |
| Geographic region of patientb, n (%) | |||
| Northeast | 5176 (28.2) | 5028 (27.4) | 0.018 |
| Midwest | 2313 (12.6) | 2291 (12.5) | 0.004 |
| South | 6835 (37.2) | 6937 (37.7) | –0.011 |
| West | 4056 (22.1) | 4125 (22.4) | –0.009 |
| Prescribing/treating physician specialtyc, n (%) | |||
| PCP | 10,104 (55.0) | 10,388 (56.5) | –0.031 |
| Endocrinologist | 4961 (27.0) | 4604 (25.0) | 0.044 |
| Others/unknown | 3317 (18.0) | 3390 (18.4) | –0.010 |
| Comorbidities | |||
| QCI, mean (SD) | 0.5 (1.10) | 0.5 (1.06) | 0.005 |
| Other comorbidities of interest, n (%) | |||
| Alopecia | 347 (1.9) | 335 (1.8) | 0.005 |
| Anemia | 1590 (8.6) | 1580 (8.6) | 0.002 |
| Celiac disease | 59 (0.3) | 41 (0.2) | 0.019 |
| Constipation | 363 (2.0) | 401 (2.2) | –0.014 |
| Goiter | 2497 (13.6) | 2353 (12.8) | 0.023 |
| Hyperlipidemia | 7292 (39.7) | 7373 (40.1) | –0.009 |
| Hypertension | 5763 (31.4) | 5761 (31.3) | 0.000 |
| Inflammatory bowel disease | 99 (0.5) | 116 (0.6) | –0.012 |
| Obesity | 1051 (5.7) | 1195 (6.5) | –0.033 |
| Psoriasis | 147 (0.8) | 140 (0.8) | 0.004 |
| Rheumatoid arthritis | 273 (1.5) | 288 (1.6) | –0.007 |
| Urticaria | 145 (0.8) | 147 (0.8) | –0.001 |
| Laboratory testing | |||
| Patients with TSH resultd, n (%) | 11,909 (64.8) | 11,795 (64.2) | 0.013 |
| TSH result, mean (SD) [median], mIU/L | 5.0 (11.82) [2.6] | 5.6 (13.01) [2.9] | –0.048 |
| TSH results between 0.3 and 4.12 mIU/L, n (%) | 6919 (37.6) | 6440 (35.0) | 0.054 |
CDHP consumer-driven health plan, HMO health maintenance organization, PCP primary care physician, PPO preferred provider organization, SD standard deviation, TSH thyroid-stimulating hormone, mIU/L milli-international units per liter
aStandardized difference = difference in means or proportions divided by the pooled standard deviation. An absolute value of the standardized difference < 0.1 was used to indicate balance in a variable across the cohorts
bFor the remainder, geographic region information was not available
cOn index prescription claim
dMost recent serum TSH result in the 6-month pre-index period
Table 2.
Baseline HCRU and costs in the matched cohorts (subset with complete pharmacy cost capture)
| HCRU and costs | Synthroid persistent users (n = 14,140) |
Generic levothyroxine persistent users (n = 15,891) |
Standardized differencea |
|---|---|---|---|
| n (%)/mean (SD) | n (%)/mean (SD) | ||
| All-cause HCRU | |||
| Inpatient hospitalizations, n (%) | 826 (5.8) | 918 (5.8) | 0.003 |
| ED visits, n (%) | 1088 (7.7) | 1181 (7.4) | 0.010 |
| Outpatient services, n (%) | 13,942 (98.6) | 15,661 (98.6) | 0.004 |
| Pharmacy fills, n (%) | 13,354 (94.4) | 15,187 (95.6) | –0.052 |
| Fills per patient, mean (SD) | 9.2 (7.45) | 9.3 (7.58) | –0.020 |
| Hypothyroidism-related HCRUb | |||
| Inpatient hospitalizations, n (%) | 456 (3.2) | 503 (3.2) | 0.003 |
| ED visits, n (%) | 299 (2.1) | 240 (1.5) | 0.045 |
| Outpatient services, n (%) | 11,511 (81.4) | 13,072 (82.3) | –0.022 |
| Pharmacy fills, n (%) | 9325 (65.9) | 10,779 (67.8) | –0.040 |
| Fills per patient, mean (SD) | 2.3 (2.27) | 2.2 (2.18) | 0.032 |
| All-cause healthcare costs, per patient in 2017 USD | |||
| Inpatient hospitalizations, mean (SD) | 1288 (9910) | 1695 (16,956) | –0.029 |
| ED visits, mean (SD) | 162 (863) | 165 (984) | –0.003 |
| Outpatient services, mean (SD) [median] | 2861 (6395) [1099] | 2668 (5754) [1024] | 0.032 |
| Pharmacy fills, mean (SD) [median] | 1478 (3618) [613] | 1448 (3332) [585] | 0.008 |
| Total medical costsc, mean (SD) [median] | 4311 (12,585) [1,215] | 4528 (18,492) [1,121] | –0.014 |
| Total costsd, mean (SD) [median] | 5788 (13,534) [2346] | 5976 (19,143) [2211] | –0.011 |
| Hypothyroidism-related costsb, per patient in 2017 USD | |||
| Inpatient hospitalizations, mean (SD) | 631 (5061) | 786 (8722) | –0.022 |
| ED visits, mean (SD) | 49 (539) | 34 (501) | 0.029 |
| Outpatient services, mean (SD) [median] | 291 (1352) [102] | 277 (1166) [100] | 0.011 |
| Pharmacy fills, mean (SD) [median] | 77 (76) [67] | 35 (41) [25] | 0.697 |
| Total medical costsc, mean (SD) [median] | 971 (5271) [114] | 1097 (8820) [109] | –0.017 |
| Total costsd, mean (SD) [median] | 1049 (5272) [211] | 1132 (8819) [148] | –0.011 |
ED emergency department, HCRU healthcare resource utilization, SD standard deviation, USD US Dollars
aStandardized difference = difference in means or proportions divided by the pooled standard deviation. An absolute value of the standardized difference < 0.1 was used to indicate balance in a variable across the cohorts
bHypothyroidism-related healthcare utilization and cost were based on medical claims with an ICD-9-CM diagnosis code of (244.0x, 244.1x, 244.8x, or 244.9x) or ICD-10-CM diagnosis code of (E01.8%, E03.8%, E89.0%, or E03.9%) and pharmacy claims for hypothyroidism medications (including levothyroxine, liothyronine, desiccated thyroid, liotrix, and thyroglobulin)
cTotal medical costs included inpatient, ED, and outpatient costs
dTotal costs were the sum of medical and pharmacy costs
The overall comorbidity burden was low (mean Quan-Charlson index score < 1) [14]. Of those who had comorbidities, the most commonly reported were hyperlipidemia (39.7% Synthroid, 40.1% GL), hypertension (31.4% Synthroid, 31.3% GL), and goiter (13.6% Synthroid, 12.8% GL). Among the subset of patients with available TSH at baseline (~ 64–65%), i.e., before their first levothyroxine claim following hypothyroidism diagnosis, mean/median levels were 5.0/2.6 and 5.6/2.9 mIU/L, with 37.6% and 35.0% being within the reference range, for Synthroid vs. GL users, respectively.
At baseline, hospitalizations and ED visits were rare (6–8% all-cause), while most patients in both cohorts had outpatient visits and pharmacy fills (94–99% all-cause), with mean number of all-cause pharmacy fills per patient of 9.2–9.3. Total all-cause costs were approximately $5800–6000 per patient in 2017 USD (Table 2).
TSH at Follow-Up
At 12-month follow-up after starting LT4, the proportion of patients achieving TSH levels within the goal range was significantly higher in the Synthroid cohort compared with the GL cohort (78.5% vs. 77.2%, respectively, p = 0.002; Table 3). The mean TSH level was 2.4 mIU/L in the Synthroid cohort and 2.6 mIU/L in the GL cohort (p < 0.001). The odds of a patient having a TSH level outside the reference range were 0.92 (95% CI 0.88–0.97; Fig. 3) for Synthroid vs. GL.
Table 3.
TSH levels at 12-month follow-up in the matched cohorts
| Synthroid persistent users (n = 18,382) |
Generic levothyroxine persistent users (n = 18,382) |
p valuea | |
|---|---|---|---|
| n (%)/mean (SD) | n (%)/mean (SD) | ||
| Patients with ≥ 1 TSH resultb, n (%) | 18,382 (100.0) | 18,382 (100.0) | – |
| Mean (SD) [median] TSH, mIU/L | 2.4 (3.77) [1.8] | 2.6 (3.94) [2.0] | < 0.001 |
| TSH laboratory results between 0.3 and 4.12 mIU/L, n (%) | 14,436 (78.5) | 14,184 (77.2) | 0.002 |
| TSH laboratory results between 0.4 to 4.0 mIU/L, n (%) | 13,828 (75.2) | 13,578 (73.9) | 0.003 |
| Patients with ≥ 2 TSH resultsb, n (%) | 3910 (100.0) | 3910 (100.0) | – |
| Mean (SD) [median] TSH, mIU/L | 2.5 (3.49) [1.9] | 2.7 (4.00) [2.0] | 0.002 |
| TSH laboratory results between 0.3 and 4.12 mIU/L, n (%) | 3055 (78.1) | 3016 (77.1) | 0.290 |
| TSH laboratory results between 0.4 to 4.0 mIU/L, n (%) | 2910 (74.4) | 2875 (73.5) | 0.367 |
SD standard deviation, TSH thyroid-stimulating hormone, mIU/L milli-international units per liter
ap values were based on t-tests for continuous variables and χ2 tests for categorical variables
bMost recent serum TSH laboratory result in the 12-month post-index period
Fig. 3.
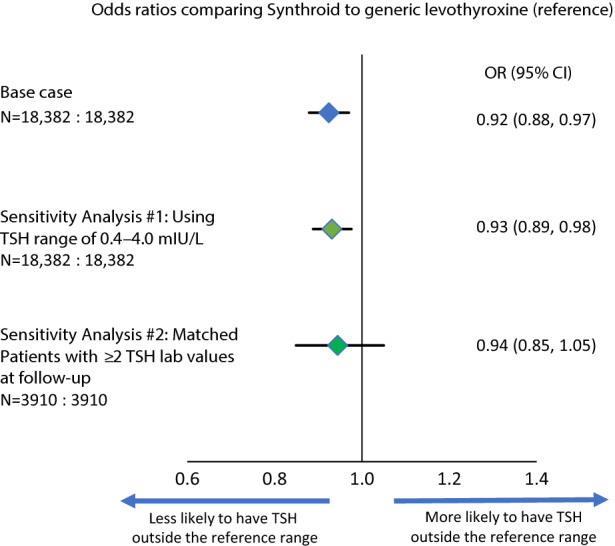
Odds of having TSH results outside the reference range with Synthroid compared with generic levothyroxine. N sample sizes of Synthroid: generic levothyroxine
These results remained consistent when a narrower TSH range (0.4–4.0 mIU/L, as suggested in the 2014 ATA guidelines [6]) was applied: 75.2% of patients in the Synthroid cohort and 73.9% in the GL cohort had TSH levels within the narrower range (p = 0.003), and the odds of having a TSH level outside the range were 0.93 (95% CI 0.89–0.98) for Synthroid vs. GL. In a smaller subset of patients with at least two TSH results at follow-up, the results were also directionally consistent, but the difference was not statistically significant (78.1% vs. 77.1%, p = 0.290; OR 0.94, 95% CI 0.85–1.05; see Table 3 and Fig. 3).
Utilization and Costs at Follow-Up
At follow-up, HCRU and costs were broadly similar between the two cohorts in terms of inpatient hospitalizations, ED visits, outpatient services, and pharmacy fills (Table 4). In most cases, statistically significant differences were associated with small effect sizes, except for all-cause inpatient hospitalizations and associated costs (lower in the Synthroid cohort by ~ 20% or $500) and hypothyroidism-related pharmacy costs (higher in the Synthroid cohort by ~ 40% or $192, driven by the underlying medication costs of the branded and generic formulations).
Table 4.
HCRU and costs at 12 months follow-up in the matched cohorts (subset with complete pharmacy cost capture)
| HCRU and costs | Synthroid persistent users (n = 14,140) |
Generic levothyroxine persistent users (n = 15,891) |
p valuea |
|---|---|---|---|
| n (%)/mean (SD) | n (%)/mean (SD) | ||
| All-cause HCRU | |||
| Inpatient hospitalizations, n (%) | 965 (6.8) | 1208 (7.6) | 0.009 |
| ED visits, n (%) | 1692 (12.0) | 1985 (12.5) | 0.166 |
| Outpatient services, n (%) | 14,136 (100.0) | 15,886 (100.0) | 0.874 |
| Pharmacy fills, n (%) | 14,140 (100.0) | 15,891 (100.0) | – |
| Fills per patient, mean (SD) | 23.9 (14.33) | 24.0 (14.81) | 0.411 |
| Hypothyroidism-related HCRUb | |||
| Inpatient hospitalizations, n (%) | 670 (4.7) | 779 (4.9) | 0.508 |
| ED visits, n (%) | 464 (3.3) | 531 (3.3) | 0.772 |
| Outpatient services, n (%) | 12,572 (88.9) | 14,167 (89.2) | 0.506 |
| Pharmacy fills, n (%) | 14,140 (100.0) | 15,891 (100.0) | - |
| Fills per patient, mean (SD) | 10.0 (3.48) | 9.4 (3.59) | < 0.001 |
| All-cause healthcare costs, per patient in 2017 USD | |||
| Inpatient hospitalizations, mean (SD) | 1585 (9519) | 2033 (13,485) | 0.007 |
| ED visits, mean (SD) | 259 (1134) | 309 (1310) | 0.096 |
| Outpatient services, mean (SD) [median] | 5490 (13,768) [2460] | 5339 (13,948) [2330] | 0.002 |
| Pharmacy costs, mean (SD) [median] | 3433 (7213) [1665] | 3387 (7006) [1535] | < 0.001 |
| Total medical costsc, mean (SD) [median] | 7334 (18,635) [2708] | 7681 (21,342) [2597] | 0.050 |
| Total costsd, mean (SD) [median] | 10,767 (21,291) [5260] | 11,067 (23,702) [5121] | < 0.001 |
| Hypothyroidism-related healthcare costsb, per patient in 2017 USD | |||
| Inpatient hospitalizations, mean (SD) | 1049 (7066) | 1183 (8799) | 0.476 |
| ED visits, mean (SD) | 76 (612) | 84 (670) | 0.747 |
| Outpatient services, mean (SD) [median] | 604 (1989) [227] | 612 (2313) [223] | 0.133 |
| Pharmacy costs, mean (SD) [median] | 328 (99) [313] | 136 (79) [131] | < 0.001 |
| Total medical costsc, mean (SD) [median] | 1729 (7554) [251] | 1879 (9280) [244] | 0.278 |
| Total costsd, mean (SD) [median] | 2057 (7557) [589] | 2015 (9282) [381] | < 0.001 |
ED emergency department, HCRU healthcare resource utilization, SD standard deviation, USD US Dollars
ap values were based on t-tests for continuous variables and χ2 tests for categorical variables
bHypothyroidism-related healthcare utilization and cost were based on medical claims with an ICD-9-CM diagnosis code of [244.0x; 244.1x, 244.8x, or 244.9x] or ICD-10-CM diagnosis code of [E0.8%, E03.8%, E89.0%, E03.9%] and pharmacy claims for hypothyroidism medications (including levothyroxine, liothyronine, desiccated thyroid, liotrix, thyroglobulin)
cTotal medical costs included inpatient, ED, and outpatient costs
dTotal costs were the sum of medical and pharmacy costs
TSH Achievers vs. Non-Achievers
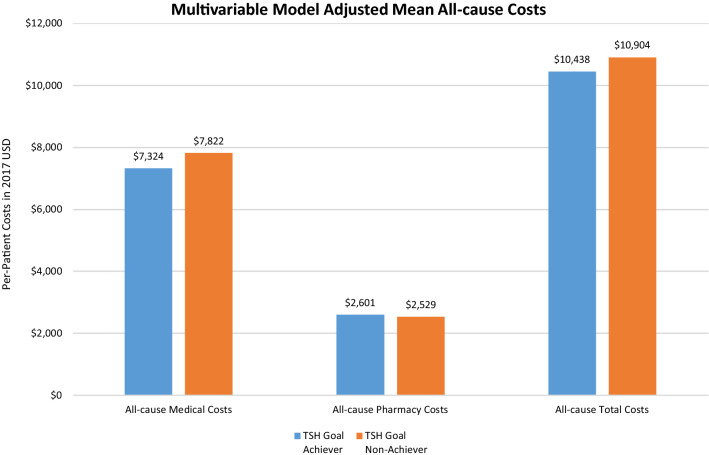
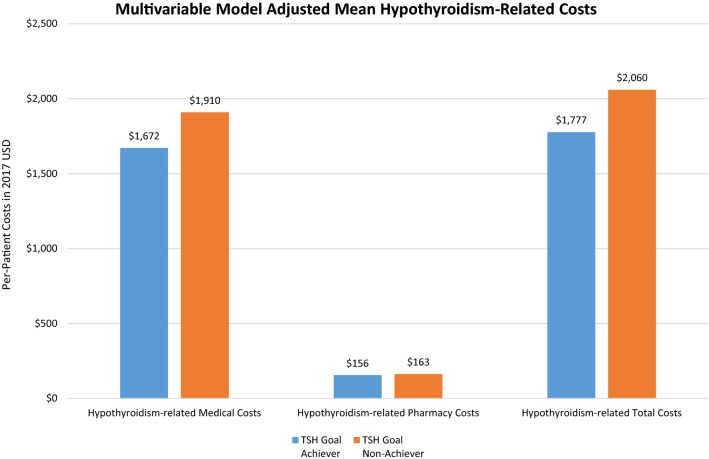
Patients achieving TSH goals (i.e., normal reference range 0.3 mIU/L to 4.12 mIU/L) were of similar age (~ 54 years) but more likely to be female (78% vs. 71%) compared to those not achieving TSH goals (Supplemental Material Table 4), and their mean baseline TSH was lower (5.3 vs. 8.8 mIU/L). At follow-up, TSH goal-achievers had significantly lower all-cause medical costs than those who did not reach TSH goals (adjusted mean $7324 vs. $7822, respectively, p ≤ 0.001; Fig. 4; for unadjusted results, see Supplemental Material Table 5). The same pattern was also observed for total costs (adjusted mean $10,438 TSH achievers vs. $10,904 TSH non-achievers, p ≤ 0.001). All-cause pharmacy costs, however, were significantly higher for TSH achievers than for non-achievers (adjusted mean $2601 vs. $2529, p ≤ 0.001). Similarly, hypothyroidism-related medical costs (adjusted mean $1672 TSH achievers vs. $1910 non-achievers, p ≤ 0.001) and hypothyroidism-related total costs (adjusted mean $1777 TSH achievers vs. $2060 TSH non-achievers, p ≤ 0.001; Fig. 5) were significantly lower among patients who achieved TSH goals than among those who did not. Hypothyroidism-related pharmacy costs were also lower for TSH achievers than for non-achievers (adjusted mean $156 vs. $163, p ≤ 0.001).
Fig. 4.
Comparison of multivariable model adjusted mean all-cause costs in the 1-year follow-up period. p ≤ 0.001 for all comparisons. Adjusted mean costs were calculated using a generalized linear model regression (gamma distribution with log link), which controlled for index levothyroxine type (Synthroid vs. generic levothyroxine), age, gender, insurance type, region, baseline Quan-Charlson Comorbidity Index, year of index date, baseline treating physician specialty (endocrinologist, PCP, others), baseline hypothyroidism-related comorbidities, and other baseline HCRU and costs (flag for hypothyroidism-related inpatient hospitalizations and pharmacy dispensing; all-cause outpatient, pharmacy, and total costs; and hypothyroidism-related inpatient, ER, outpatient, and total costs)
Fig. 5.
Comparison of multivariable model adjusted mean hypothyroidism-related costs in the 1-year follow-up period. Note: p ≤ 0.001 for all comparisons. Adjusted mean costs were calculated using a generalized linear model regression (gamma distribution with log link), which controlled for index levothyroxine type (Synthroid vs. generic levothyroxine), age, gender, insurance type, region, baseline Quan-Charlson Comorbidity Index, year of index date, baseline treating physician specialty (endocrinologist, PCP, others), baseline hypothyroidism-related comorbidities, and other baseline HCRU and costs (flag for hypothyroidism-related inpatient hospitalizations and pharmacy dispensing; all-cause outpatient, pharmacy and total costs; and hypothyroidism-related inpatient, ER, outpatient, and total costs)
Discussion
This study reported on the real-world comparative effectiveness of persistent Synthroid compared with persistent GL treatment in a managed care setting using a large longitudinal sample. At 12-month follow-up, a significantly higher proportion of patients in the Synthroid cohort were within both the broad (78.5% vs. 77.2%) and narrower (75.2% vs. 73.9%) TSH reference ranges compared with the GL cohort. Given that levothyroxine is one of the most commonly prescribed drugs in the US, with use rates between 5 and 7% of adults [15] (an estimated 15 m users in 2021), the absolute difference of ~ 1.3 percentage points may translate into tens of thousands of patients with suboptimal outcomes.
These results are consistent with previous claims-based studies using different databases, which reported improvements in target TSH levels achieved with Synthroid vs. GL. A previous study using the Clinformatics® Data Mart database compared TSH levels at 1-year follow-up among patients receiving Synthroid (n = 14,017) or GL (n = 28,034). The findings demonstrated a smaller proportion of patients in the Synthroid cohort failed to achieve TSH goals compared with the GL cohort (20.9% vs. 22.6%, respectively, p < 0.0001) [10]. A second study using the claims database of a large commercial health insurer (Humana) reported similar proportions of inadequately treated patients at 1-year follow-up (19.4% Synthroid vs. 21.4% GL, p < 0.0461); the study population was smaller than in the previous study (n = 1595 Synthroid; n = 3190 GL), and the patients were older (mean age 54 years vs. 74 years) [9]. In the current study, 21.5% of patients receiving Synthroid failed to achieve TSH target levels compared with 22.8% receiving GL (p = 0.002). The potential differences across generics in their true clinical effectiveness [11] together with the possibility of a patient using different generic formulations [16] may contribute to the differences in TSH target achievement observed in our study.
Another recent study [16] examined short-term (3 months) goal achievement for a cohort consisting of multiple branded vs. multiple generic levothyroxines using claims data from OptumLabs® and found no significant differences between the cohorts. There are several methodological differences between this study and the current one that may explain these findings (e.g., time windows for goal assessment; width of the reference range; composition of cohorts).
In a subpopulation with at least 2 TSH values recorded over 12 months follow-up, we found directionally consistent results that were not statistically significant. In this analysis, the sample size was considerably reduced (containing only 21% of the base case cohort), which may contribute to statistical uncertainty. Also, the patients in this subcohort may have multiple TSH values due to titration efforts, which our study did not further explore.
With few exceptions, all-cause and hypothyroidism-related HCRU was comparable between the Synthroid and GL cohorts, most notably in terms of hospitalizations, outpatient services, and ED visits. Whereas no significant difference between the two groups in all-cause pharmacy fills was observed, the Synthroid cohort had significantly more hypothyroidism-related prescription fills per patient than the GL cohort. The rate of hypothyroidism-related prescription fills in the Synthroid cohort may reflect improved medication adherence or consistency compared with the GL cohort [17]. A previous economic analysis reported that although patients with hypothyroidism who switched from Synthroid to GL had lower medication costs than patients who remained on Synthroid therapy, hypothyroidism-related medical and overall total costs were higher [18]. The researchers suggested the elevated costs in the GL group may be attributed to complications resulting from inadequate treatment or to the need for increased monitoring [18].
The effect of consistent treatment on HCRU and costs was further demonstrated in our comparison of patients who achieved TSH goals versus those who did not. Patients who achieved TSH goals were significantly less likely to have all-cause or hypothyroidism-related inpatient hospitalizations as well as hypothyroidism-related outpatient services than patients who did not achieve TSH goals. Additionally, TSH goal achievers incurred significantly lower average medical and total all-cause and hypothyroidism-related costs than non-achievers. To our knowledge, this is the first real-world study looking into outcomes for goal achievers vs. non-achievers.
Limitations
In this observational study, TSH laboratory values were only available for a subset of the population in the claims database; therefore, a number of patients with hypothyroidism were excluded because of the lack of TSH values during the follow-up period. This may affect the generalizability of the results to patients without observable TSH values. Approximately a third of patients with TSH available at baseline were within the reference range when initiating levothyroxine treatment, which is similar to recent results from a different commercially insured US population [17]; details on the etiology of their hypothyroidism or the clinical treatment plan rationale were not available. The study focused on TSH levels and did not differentiate between overt hypothyroidism and subclinical hypothyroidism (where TSH levels are abnormal while T4 levels are normal). Propensity score matching was used to address confounding of the treatment-outcome relationship by ensuring balance in the baseline characteristics. However, as with other observational studies, this study was limited by the potential for unmeasured confounders (for example, patient socioeconomic status, disease severity/longevity, or physician treatment preferences). All patients in the study were enrolled in commercial health plans. These results may not be generalizable to patients with other types of health insurance, those who are uninsured, or those living outside the US. Our study examined GL utilization as a single cohort and did not differentiate between different generics, whose effectiveness may not be identical. We also did not assess titration patterns; however, it is reasonable to assume these patterns to be similar across the cohorts given the limited follow-up time frame and the patients’ initially similar demographic and clinical profiles generated through the propensity score matching process. Lastly, limitations typically associated with claims studies, such as undetected errors in coding, may have had an effect on the results. Additionally, the presence of a diagnosis code on a claim does not guarantee positive presence of a condition, and pharmacy claims only show a prescription was filled; they do not indicate whether the medication was consumed or taken as directed.
Conclusions
This real-world study explored and refined comparative effectiveness research of Synthroid and GL. Consistent with previous findings using other real-world data, persistent Synthroid use was associated with better TSH target achievement vs. persistent GL use in a US managed care population in the majority of the analyses. These results provide evidence that achieving TSH outcomes within the reference range for persistent levothyroxine users may provide substantial economic value by reducing hypothyroidism-related HCRU and costs. This analysis confirms that the use of Synthroid may offer improved health and economic outcomes relative to generic formulations in the treatment and management of patients with hypothyroidism.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Study funding was provided by AbbVie, Inc., to HealthCore, Inc. The study sponsor is also funding the journal’s Rapid Service and Open Access Fees.
Medical Writing/Editorial Assistance
The authors thank Cheryl Jones (employee of HealthCore, Inc., at the time the manuscript was created) for writing and editorial support on this manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
MG, BN, LW contributed to the design of the study; collected, analyzed, and interpreted the data; and drafted the manuscript. AB, RE, YC, JH contributed to the design of the study; interpreted the data; and provided critical revisions of the manuscript.
Prior Presentation
Partial findings from this study were previously presented at the 88th Annual Meeting of the American Thyroid Association, October 3–7, 2018, Washington, DC.
Disclosures
Michael Grabner and Bal Nepal are employees of HealthCore, Inc., which received funding from AbbVie, Inc., for the conduct of the study. Michael Grabner is a shareholder of Anthem, Inc. Liya Wang was an employee of HealthCore at the time the study was conducted and is currently an employee of Merck and Co., Inc. Amit Bodhani and Ramon Espaillat are employees of AbbVie, Inc. Yaozhu J. Chen was an employee of AbbVie, Inc. at the time the study was conducted, and is currently an employee of Takeda Pharmaceuticals USA, Inc. James V. Hennessey is affiliated with Beth Israel Deaconess Medical Center, Boston, MA, and was under contract with AbbVie, Inc., to provide consulting services to the study.
Compliance with Ethics Guidelines
Researchers only accessed data in the format of a limited data set for which a data use agreement was in place with the covered entities in compliance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. An Institutional Review Board did not review the study since only this limited data set was accessed.
Data Availability
The datasets generated and/or analyzed in this study are not publicly available due to their proprietary nature and the associated restrictions that apply to their availability to external sources. Datasets may be made available through the corresponding author upon reasonable request and with permission of HealthCore.
References
- 1.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 2.American Thyroid Association. General Information. https://www.thyroid.org/media-main/press-room/. Accessed 11 Nov 2021.
- 3.National Institute of Diabetes and Digestive and Kidney Diseases. Hypothyroidism (underactive thyroid). https://www.niddk.nih.gov/health-information/endocrine-diseases/hypothyroidism. Accessed October 29, 2019, 2019.
- 4.Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18(8):989–1028. doi: 10.4158/EP12280.GL. [DOI] [PubMed] [Google Scholar]
- 5.Faix JD, Thienpont LM. Thyroid-stimulating hormone: why efforts to harmonize testing are critical to patient care. Clinical Laboratory News. 2013. https://www.aacc.org/publications/cln/articles/2013/may/tsh-harmonization. Published May 1. Accessed October 10, 2019.
- 6.Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24(12):1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thayakaran R, Adderley NJ, Sainsbury C, et al. Thyroid replacement therapy, thyroid stimulating hormone concentrations, and long term health outcomes in patients with hypothyroidism: longitudinal study. BMJ. 2019;366:l4892. doi: 10.1136/bmj.l4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Girolamo G, Keller GA, de los Santos AR, Schere D, Gonzalez CD. Bioequivalence of two levothyroxine tablet formulations without and with mathematical adjustment for basal thyroxine levels in healthy Argentinian volunteers: a single-dose, randomized, open-label, crossover study. Clin Ther. 2008;30(11):2015–2023. doi: 10.1016/j.clinthera.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Hepp Z, Castelli-Haley J, Wang S, Chen YJ, Espaillat R. Comparative effectiveness of Synthroid(R) vs generic levothyroxine on TSH lab outcomes: a confirmatory analysis of US Medicare claims data. In: American Association of Clinical Endocrinologists 27th Annual Scientific and Clinical Congress; May 16–20, 2018; Boston, MA.
- 10.Hepp Z, Wang S, Espaillat R. Comparative effectiveness of Synthroid(R) vs generic levothyroxine on TSH lab outcomes: a retrospective claims database analysis. In: American Association of Clinical Endocrinologists 26th Annual Scientific and Clinical Congress; May 3–7, 2017; Austin, TX.
- 11.Benvenga S, Carlé A. Levothyroxine formulations: pharmacological and clinical implications of generic substitution. Adv Ther. 2019;36(Suppl 2):59–71. doi: 10.1007/s12325-019-01079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pecina J, Bernard M, Furst J, Rohrer J. Hypothyroidism management: is an annual check of TSH level always necessary? J Fam Pract. 2012;61(10):E1–E5. [PubMed] [Google Scholar]
- 13.Austin PC. An introduction to propensity score methods for reducing the effects of confoundng in observational studies. Multivar Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 15.Ross JS, Rohde S, Sangaralingham L, et al. Generic and brand-name thyroid hormone drug use among commercially insured and medicare beneficiaries, 2007 through 2016. J Clin Endocrinol Metab. 2019;104(6):2305–2314. doi: 10.1210/jc.2018-02197. [DOI] [PubMed] [Google Scholar]
- 16.Brito JP, Ross JS, Sangaralingham L, et al. Comparative effectiveness of generic vs brand-name levothyroxine in achieving normal thyrotropin levels. JAMA Netw Open. 2020;3(9):e2017645. doi: 10.1001/jamanetworkopen.2020.17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hepp Z, Wyne K, Manthena SR, Wang S, Gossain V. Adherence to thyroid hormone replacement rherapy: a retrospective, claims database analysis. Curr Med Res Opin. 2018;34(9):1673–1678. doi: 10.1080/03007995.2018.1486293. [DOI] [PubMed] [Google Scholar]
- 18.Khandelwal N, Johns B, Hepp Z, Castelli-Haley J. The economic impact of switching from Synthroid for the treatment of hypothyroidism. J Med Econ. 2018;21(5):518–524. doi: 10.1080/13696998.2018.1443110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed in this study are not publicly available due to their proprietary nature and the associated restrictions that apply to their availability to external sources. Datasets may be made available through the corresponding author upon reasonable request and with permission of HealthCore.