Abstract
Introduction
In the event-driven GRIPHON randomised-controlled trial, the oral prostacyclin receptor agonist selexipag significantly reduced the risk of disease progression (composite primary endpoint of morbidity/mortality), compared with placebo, in patients with pulmonary arterial hypertension (PAH). The ongoing open-label extension study (GRIPHON OL) collects further data on long-term safety, tolerability, and survival of PAH patients treated with selexipag.
Methods
Patients randomised to selexipag or placebo in GRIPHON could enter GRIPHON OL either after experiencing a morbidity event during double-blind treatment or at the end of the study. Patients were followed for adverse events (AE) and survival from selexipag initiation up to 3 days and 30 days after end of treatment, respectively. Data are presented up to a cut-off date of 1 September 2019.
Results
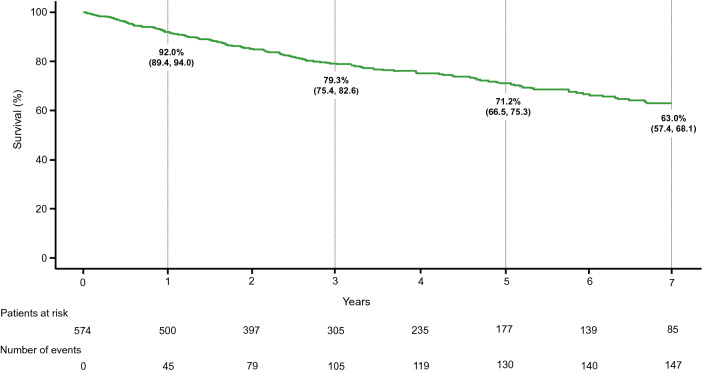
Overall, 953 patients in GRIPHON and GRIPHON OL were treated with selexipag. At the time of selexipag initiation, 81.2% of patients were receiving background PAH therapy. Median (min, max) exposure to selexipag was 31.7 months (0, 106), corresponding to a total of 3054.4 patient-years. The most frequently reported AEs were related to known prostacyclin-related effects or underlying disease. There were 305 (32.0%) patients who experienced an AE leading to treatment discontinuation. Survival during GRIPHON and GRIPHON OL was assessed for the 574 patients randomised to selexipag in GRIPHON. Kaplan–Meier survival estimates (95%CI) at 1, 3, 5 and 7 years were 92.0% (89.4, 94.0), 79.3% (75.4, 82.6), 71.2% (66.5, 75.3) and 63.0% (57.4, 68.1), respectively.
Conclusions
These results provide the longest follow-up period published to date for a PAH therapy. The safety profile of selexipag over this extended treatment period was consistent with that observed in GRIPHON. A large proportion of the population was receiving background therapy at selexipag initiation, providing further insight into the long-term safety of selexipag as part of a combination therapy regimen.
Trial Registration
ClinicalTrials.gov Identifiers: NCT01106014 and NCT01112306
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-021-01898-1.
Keywords: Selexipag, Pulmonary arterial hypertension, PAH, GRIPHON, Open-label extension, Safety, Tolerability, Survival, Combination therapy, Long-term outcomes
Key Summary Points
| Why carry out this study? |
| The GRIPHON open-label extension study provides data on long-term safety, tolerability and survival for patients with pulmonary arterial hypertension (PAH) treated with the oral prostacyclin receptor agonist, selexipag. |
| What did the study ask? |
| This ongoing open-label extension study collected long-term data on adverse events (AEs) and vital status of PAH patients treated with selexipag. |
| What were the study outcomes/conclusions? |
| Over the 7-year follow-up period, the median (min, max) exposure to selexipag in the study population (n = 953) was 31.7 months (0, 106), corresponding to a total of 3054.4 patient-years. The most frequently reported AEs were related to known prostacyclin-related effects and/or underlying disease. Kaplan–Meier survival estimates at 1, 3, 5 and 7 years in the population of patients randomised to selexipag in GRIPHON (n = 574) were 92.0%, 79.3%, 71.2% and 63.0%, respectively. |
| What has been learned from the study? |
| These results provide the longest follow-up period published to date for PAH therapies. The long-term safety and tolerability profile of selexipag observed in this study was in line with previously published data over shorter time periods. As the majority of the population was receiving combination PAH therapy with an endothelin receptor antagonist and/or a phosphodiesterase type 5 inhibitor, these analyses also provide further insights into the long-term safety of selexipag as part of a combination therapy regimen. |
Introduction
Pulmonary arterial hypertension (PAH) is a rare, progressive disease and, while outcomes remain poor, the availability of targeted therapies has led to improved prognosis [1, 2]. In this context, it is important to understand the long-term safety and tolerability of PAH therapies, as well as their impact on survival. Selexipag is an oral selective prostacyclin receptor (IP receptor) agonist approved for the treatment of PAH [World Health Organization (WHO) Group I] to delay disease progression and to reduce the risk of hospitalisation for PAH [3]. In the event-driven randomised-controlled trial (RCT) GRIPHON, selexipag significantly delayed the progression of PAH [4]. The risk of a primary endpoint event of morbidity/mortality was reduced by 40% with selexipag versus placebo [hazard ratio 0.60; 99% confidence interval (CI) 0.46–0.78; p < 0.001] [4]. GRIPHON is, to date, the largest and longest RCT in PAH, with 1156 patients enrolled and a median follow-up time of 98.1 weeks. Its ongoing open-label extension study collects further data on long-term safety, tolerability and survival in patients treated with selexipag. The following report describes an analysis of long-term outcomes for patients treated with selexipag in GRIPHON and/or its open-label extension study.
Methods
The data sharing policy of the Sponsor is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to study data can be submitted through the Yale Open Data Access Project site at https://yoda.yale.edu.
Study Design
GRIPHON (NCT01106014) was a global, multicentre, double-blind, randomised, placebo-controlled event-driven phase 3 study, which assessed the safety and efficacy of selexipag in patients with PAH [4]. Briefly, selexipag/placebo were titrated over 12 weeks to an individualised dose of 200–1600 µg twice daily (b.i.d), based on tolerability. Patients received double-blind treatment until they experienced a morbidity/mortality (primary endpoint) event, discontinued prematurely, or until the end of the study (reached when 331 primary endpoint events had occurred). Primary endpoint events included disease progression or worsening of PAH that resulted in hospitalisation, initiation of parenteral prostanoid therapy or long-term oxygen therapy, need for lung transplantation or balloon atrial septostomy, or death from any cause. Disease progression was defined as a decrease from baseline of at least 15% in the 6-min walk distance (6MWD) accompanied by a worsening in WHO functional class (FC) (for the patients with WHO FC II or III at baseline) or the need for additional treatment of PAH (for the patients with WHO FC III or IV at baseline). All events were adjudicated by a blinded independent critical-event committee [4].
GRIPHON OL (NCT01112306) is an open-label, multicentre study to assess the long-term safety and tolerability of selexipag in patients with PAH (Supplementary Fig. 1). Patients enrolled in GRIPHON could enter the GRIPHON OL study either after experiencing a morbidity event during double-blind treatment or at the end of the study if they were still receiving study treatment.
The dose at which selexipag was started in GRIPHON OL depended on the reason to enter the OL and/or the treatment allocation in GRIPHON. For patients who entered GRIPHON OL following a primary endpoint event in GRIPHON, the study treatment allocation and dose in GRIPHON remained blinded to preserve the integrity of the double-blind study, hence in these patients selexipag was started at the lowest dose (200 μg b.i.d), with up-titration to an individualised dose as described above. In contrast, for patients who entered GRIPHON OL at the end of the GRIPHON double-blind study, the study treatment allocation and dose in GRIPHON were known. Hence, patients randomised to selexipag in GRIPHON entered GRIPHON OL at the same dose received at the end of the study, and patients randomised to placebo started selexipag at the lowest dose (200 μg b.i.d), with up-titration of selexipag to an individualised dose, as described above [4]. In GRIPHON OL, patients received selexipag until either selexipag became commercially available for PAH in the patient’s country or the patient/investigator decided to discontinue selexipag. At the end of selexipag treatment, an end-of-study visit was performed, followed by a post-treatment safety follow-up period of 30 days.
Ethics
GRIPHON and GRIPHON OL were conducted in accordance with the Declaration of Helsinki. The protocols were approved by the institutional review board/independent ethics committee at each site (Supplementary Table 1). GRIPHON OL was monitored until 30 June 2016 by the same independent data and safety monitoring committee as in GRIPHON [4]. Written informed consent was obtained from all patients at entry into GRIPHON and GRIPHON OL.
Patient Population
Full inclusion/exclusion criteria for GRIPHON have been described [4]. Briefly, enrolled patients were adults with a diagnosis of one of the following types of PAH, confirmed by right heart catheterisation: idiopathic PAH, heritable PAH, or PAH associated with either connective tissue disease, repaired congenital systemic-to-pulmonary shunts, human immunodeficiency virus infection, drug use or toxin exposure. Further inclusion criteria included a 6MWD of 50–450 m at screening. Background therapy with an endothelin receptor antagonist (ERA) and/or a phosphodiesterase type 5 inhibitor (PDE5i) was permitted at a stable dose at the start of GRIPHON [4]. In GRIPHON OL, concomitant PAH therapies permitted during the study included ERAs, PDE5is and/or riociguat; prostacyclin and/or its analogues were permitted, if deemed medically indicated by the investigator, to stabilise a patient with worsening of PAH or to switch a patient to intravenous or subcutaneous treatment.
Assessments
Patients were followed for adverse events (AEs) from selexipag initiation up to 3 days after end of treatment and for serious AEs and vital status from selexipag initiation up to 30 days after end of treatment. Collection of vital status after this time was not mandated; however, if death was reported to the study sponsor, it was included in the survival analysis.
Statistical Analyses
All analyses were performed using data from both GRIPHON and GRIPHON OL up to a cut-off date of 1 September 2019 and were descriptive in nature. For the analyses of safety and tolerability, all patients treated with at least one dose of selexipag in GRIPHON or GRIPHON OL were included, defined as the safety/tolerability set. For the post hoc analyses of long-term survival, all patients randomised to selexipag in GRIPHON were included, defined as the survival analysis set. The Kaplan–Meier (KM) method was used to estimate time from selexipag initiation to death. This analysis included deaths that occurred more than 30 days after end of treatment, if reported to the study sponsor. Analyses were performed on all patients, as well as on subsets of patients grouped according to REVEAL 2.0 risk category (based on REVEAL 2.0 risk score; low: ≤ 6, intermediate: 7–8, high: ≥ 9; Supplementary Methods) [5] or WHO FC at baseline (WHO FC II or WHO FC III).
Results
Patient Characteristics
Overall, 953 patients in the GRIPHON and GRIPHON OL studies were treated with selexipag and were included in the safety/tolerability set (Fig. 1). Of these, 330 patients received selexipag in both GRIPHON and GRIPHON OL, 244 received selexipag in GRIPHON only (i.e. randomised to selexipag in GRIPHON and did not enter GRIPHON OL), and 379 received selexipag in GRIPHON OL only (i.e. randomised to placebo and entered GRIPHON OL). As of 1 September 2019, 216 (22.7%) patients continued to receive treatment in GRIPHON OL (Table 1).
Fig. 1.
Patient disposition. Data cut-off 1 September 2019. PAH pulmonary arterial hypertension, OL open-label. aFour patients did not receive placebo as assigned. bCompleted study treatment in GRIPHON or GRIPHON OL: Patients who performed the end of study assessment. Green shading indicates patients who received selexipag
Table 1.
Treatment disposition at the cut-off date (1 Sep 2019)
| Selexipag treated patients, (n = 953) | |
|---|---|
| Ongoing in study, n (%) | 216 (22.7) |
| Completed study treatment, n (%) | 163 (17.1) |
| Discontinued study treatment, n (%) | 574 (60.2) |
| Reason for discontinuationa, n (%) | |
| Adverse event | 251 (26.3) |
| Death | 152 (15.9) |
| Withdrawal by patient | 107 (11.2) |
| Progression of PAH | 24 (2.5) |
| Physician decision | 18 (1.9) |
| Lost to follow-up | 10 (1.0) |
| Other | 12 (1.3) |
Data presented for the safety/tolerability set
PAH pulmonary arterial hypertension
aA patient may have discontinued selexipag for multiple reasons, but only the primary reason for discontinuation is reported here
At selexipag initiation, patients in the safety/tolerability set (n = 953) had a mean (SD) age of 48.0 (15.3) years; the majority of patients were female (80.8%) and in WHO FC II (43.5%) or III (50.3%). 81.2% of patients were treated with an ERA and/or PDE5i: 33.3% with both an ERA and a PDE5i, 14.7% with an ERA and 33.3% with a PDE5i (Table 2).
Table 2.
Demographics and clinical characteristics at time of selexipag initiation
| Characteristic | Selexipag-treated patients, (n = 953) |
|---|---|
| Female, n (%) | 770 (80.8) |
| Age, years, mean ± SD | 48.0 ± 15.3 |
| Time from diagnosis of PAHa, years, mean ± SD | 2.4 ± 3.7 |
| PAH classification, n (%) | |
| Idiopathic PAH | 532 (55.8) |
| Heritable PAH | 22 (2.3) |
| Associated with connective tissue disease | 273 (28.6) |
| Associated with congenital heart disease | 96 (10.1) |
| Associated with HIV | 8 (0.8) |
| Drug or toxin induced | 22 (2.3) |
| 6MWD, m, mean ± SD | 346.4 ± 99.2 |
| WHO FC, n (%) | |
| I | 12 (1.3) |
| II | 415 (43.5) |
| III | 479 (50.3) |
| IV | 47 (4.9) |
| Background PAH therapy, n (%) | |
| ERA and PDE5i combination therapy | 317 (33.3) |
| ERA monotherapy | 140 (14.7) |
| PDE5i monotherapy | 317 (33.3) |
| None | 179 (18.8) |
Data presented for the safety/tolerability set
6MWD 6-min walk distance, ERA endothelin receptor antagonist, HIV human immunodeficiency virus, m metres, PAH pulmonary arterial hypertension, PDE5i phosphodiesterase 5 inhibitor, SD standard deviation, WHO FC World Health Organization functional class
aConfirmed by right heart catheterisation
Safety and Tolerability
In the safety/tolerability set, the median (min, max) exposure to selexipag was 31.7 (0.0, 106.0) months, corresponding to a total exposure of 3054.4 patient-years. At the time of data cut-off, 99 (10.4%) patients had been receiving selexipag for at least 7 years and 286 (30.0%) patients had been receiving selexipag for at least 5 years. During the observation period, 99.6% of patients experienced at least one treatment-emergent AE and 60.1% experienced at least one serious AE. The most frequently reported AEs were headache (67.9%), diarrhoea (44.6%), nausea (32.8%) and PAH worsening (32.6%) (Table 3); these were either known prostacyclin-related effects and/or associated with progression of underlying disease. After adjusting for exposure, the incidences per year per 100-treated patients for these AEs were 66.3, 23.0, 14.3 and 11.9, respectively (Table 3).
Table 3.
Safety and exposure
| Selexipag treated patients, (n = 953) | |
|---|---|
| Selexipag exposure, months, median (range) | 31.7 (0.0–106.0) |
| Adverse events, n (%) | |
| Patients with ≥ 1 adverse event | 949 (99.6) |
| Patients with ≥ 1 serious adverse event | 573 (60.1) |
| Patients with ≥ 1 adverse event leading to selexipag discontinuationa | 305 (32.0) |
| Most frequentb adverse events | n (%) | Incidence rate per year per 100 treated patients |
|---|---|---|
| Headache | 647 (67.9) | 66.3 |
| Diarrhoea | 425 (44.6) | 23.0 |
| Nausea | 313 (32.8) | 14.3 |
| PAH worsening | 311 (32.6) | 11.9 |
| Pain in jaw | 268 (28.1) | 12.0 |
| Pain in extremity | 175 (18.4) | 6.7 |
| Vomiting | 174 (18.3) | 6.8 |
| Dyspnoea | 172 (18.0) | 6.4 |
| Oedema peripheral | 159 (16.7) | 5.8 |
| Myalgia | 157 (16.5) | 6.1 |
| Dizziness | 156 (16.4) | 5.9 |
| Nasopharyngitis | 149 (15.6) | 5.6 |
| Right ventricular failure | 147 (15.4) | 5.1 |
| Upper respiratory tract infection | 136 (14.3) | 5.1 |
| Cough | 130 (13.6) | 4.8 |
| Arthralgia | 119 (12.5) | 4.4 |
| Flushing | 117 (12.3) | 4.4 |
| Anaemia | 108 (11.3) | 3.9 |
| Bronchitis | 100 (10.5) | 3.6 |
Data presented for the safety/tolerability set
PAH pulmonary arterial hypertension
aAll adverse events leading to discontinuation of selexipag are reported here and not only those considered the primary reason for discontinuation as presented in Table 1
bOccurring in ≥ 10% of patients
Of the 953 patients in the safety/tolerability set, there were 305 (32.0%) patients who experienced an AE leading to treatment discontinuation, with an incidence rate of 10 AEs leading to discontinuation per year per 100 patients. The most common AEs leading to discontinuation were PAH worsening (12.9%), right ventricular failure (2.9%), headache (3.4%), diarrhoea (2.0%), nausea (1.4%), dyspnoea (1.5%) and pain in extremity (1.0%). There were 65 (6.8%) patients who discontinued due to a prostacyclin-associated AE. Overall, during the study, 212 (22.2%) patients had died by end of treatment + 30 days. The most common (> 1%) reasons for death were PAH worsening (6.4%), right ventricular failure (4.4%), sudden death (1.7%) and cardiac arrest (1.2%).
Survival
Analyses of time to death were performed in all patients randomised to selexipag in GRIPHON [survival analysis set (n = 574)]. Patients in the survival analysis set had a mean age (SD) of 48.2 (15.2) years; the majority of patients were female (79.6%) and in WHO FC II (47.7%) or III (51.0%). A total of 80.5% of patients were treated with a stable dose of an ERA and/or a PDE5i: 31.2% with both an ERA and a PDE5i, 16.4% with an ERA, and 32.9% with a PDE5i [4]. The median (min, max) exposure to selexipag in the survival analysis set was 35.8 (0.0, 106.0) months, corresponding to a total exposure of 1983 patient-years. During this time in GRIPHON and GRIPHON OL, 163 (28.4%) patients initiated a new class of PAH therapy. The most common PAH therapies newly initiated were an ERA (n = 40; 7.0%), a PDE5i (n = 42; 7.3%) and prostacyclin or its analogue (n = 55; 9.6%). However, most (86.3%) of the selexipag exposure in the survival analysis set (n = 574) was accumulated without or prior to the addition of any new PAH therapies.
KM estimates (95%CI) for the survival analysis set at 1, 2, 3, 5 and 7 years were 92.0% (89.4, 94.0), 85.3% (82.0, 88.0), 79.3% (75.4, 82.6), 71.2% (66.5, 75.3) and 63.0% (57.4, 68.1) respectively (Fig. 2). Additional survival analyses were performed on patients grouped according to REVEAL 2.0 risk category (Supplementary Fig. 2) or WHO FC at baseline (Supplementary Fig. 3). Survival estimates [KM (95%CI)] in the REVEAL 2.0 low- (n = 284), intermediate- (n = 145) and high-risk (n = 145) categories, respectively, were: 97.1% (94.3, 98.5), 96.5% (91.7, 98.5), and 77.7% (69.9, 83.7) at 1 year, 89.1% (84.5, 92.4), 82.9% (75.0, 88.6), and 55.7% (46.3, 64.1) at 3 years, and 83.7% (77.9, 88.1), 78.5% (69.1, 85.3), and 35.2% (24.7, 45.9) at 5 years. Seven-year survival estimates [KM (95%CI)] were calculated for the low- and intermediate-risk categories only and were 76.7% (69.2, 82.5) and 59.8% (46.3, 70.9), respectively (Supplementary Fig. 2). Patients in WHO FC II (n = 273) had an estimated survival [KM (95%CI)] of 96.6% (93.5, 98.2), 91.2% (86.9, 94.1), 84.7% (79.4, 88.7), 80.2% (74.1, 85.0), and 70.0% (62.0, 76.6), at 1, 2, 3, 5, and 7 years respectively. For patients in WHO FC III (n = 294), estimated survival [KM (95%CI)] was 88.0% (83.7, 91.2), 79.8% (74.5, 84.1), 74.5% (68.6, 79.4), 61.8% (54.3, 68.4) and 56.0% (47.6, 63.6) at 1, 2, 3, 5 and 7 years respectively (Supplementary Fig. 3).
Fig. 2.
Survival in selexipag treated patients. Analyses performed in the survival analysis set. Kaplan–Meier curve for time from selexipag initiation to death up to data cut-off (1 September 2019). Kaplan–Meier estimates (95% CI) are shown at 1, 3, 5 and 7 years
Discussion
These analyses of GRIPHON and its open-label extension study (GRIPHON OL) provide long-term survival, tolerability and safety data in patients with PAH treated with selexipag. The 7-year observation period in this selexipag study is the longest follow-up period published to date for PAH patients treated with any PAH medication. These data are of clinical importance in the setting of a rare disease, where long-term survival data in a well-characterised population are limited.
The combination of the long follow-up period and the large patient population makes this the most extensive study of safety and tolerability for a PAH therapy published to date, as previous long-term OL studies in PAH typically report safety in the range of 2–3 years [6–9]. Tolerability for selexipag over the extended treatment period was in line with that observed in GRIPHON and in real-world clinical settings [4, 10]. The median exposure time to selexipag in this analysis was over 2.5 years (31.7 months), with 30% of patients having received selexipag for at least 5 years, providing evidence for long-term tolerability of selexipag in patients with PAH. Overall, 305 (32%) patients experienced at least one AE leading to treatment discontinuation, with 6.8% discontinuing due to a prostacyclin related AE. However, of the patients that discontinued due to an AE, the largest proportion (12.9%; n = 123) did so due to PAH worsening, reflecting the progressive nature of the disease over the extended observation period. Furthermore, taking into consideration the study’s uniquely long exposure period, the incidence rate of AEs leading to discontinuation was 10 AEs per year per 100 patients.
Previous long-term OL studies in PAH typically report survival estimates in the range of 2–3 years [6–9]. Our post hoc analyses provide up to 7 years contemporary survival data, making this a unique long-term analysis of outcomes from trial data in PAH. As over 80% of patients in GRIPHON/GRIPHON OL were receiving selexipag as combination therapy, including a third on triple oral combination therapy, these results provide contemporary data on the long-term survival of patients treated with combination therapy. These data also suggest that patients in GRIPHON/GRIPHON OL were managed in a way that broadly reflects the treatment paradigm of combination therapy for patients with PAH, recommended by the European Society of Cardiology/European Respiratory Society guidelines and the World Symposium on Pulmonary Hypertension proceedings [11–13]. Patients in the low- or intermediate-REVEAL 2.0 risk categories at baseline had better long-term survival than those in the high-risk category. Similarly, patients in WHO FC II at baseline had better long-term survival compared to those in WHO FC III, in line with previous observations [1, 14]. These data further highlight the importance of proactively treating less severe patients with multiple therapies for delaying the progression of PAH. With 5-year estimated survival rates of approximately 80%, these results also suggest that patients treated with selexipag in WHO FC II or at low/intermediate-risk have good long-term survival.
One limitation to these analyses, inherent to OL extension studies, is the uncontrolled nature of the results. Furthermore, the number of patients included in the survival analyses was lower than the total number exposed to selexipag. These analyses were performed in patients originally randomised to selexipag in the GRIPHON trial to ensure robust and non-biased assessment of survival in selexipag treated patients. Patients originally randomised to placebo who received selexipag in GRIPHON OL were not included as they initiated selexipag at different timepoints and with varying disease characteristics, which would lead to significant challenges for interpretation of the results. In addition, although the duration of follow-up is extensive, there is a lack of follow-up data after discontinuation of selexipag, including for patients who switched from receiving selexipag in GRIPHON OL to commercial selexipag.
Conclusions
This analysis provides novel insights into long-term outcomes of PAH patients treated with selexipag. Furthermore, as these analyses include a population of patients where the majority were receiving combination therapy, they further our understanding of the long-term benefit–risk implications of combination therapy including selexipag.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Funding
Funding for the study and for the journal’s rapid service and open access fees was provided by Actelion Pharmaceuticals, Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson.
Medical Writing and/or Editorial Assistance
Medical writing support was provided by Jatta Huotari and Laura Corbett of eluSCIdate ltd (Meggen, Switzerland) and was funded by Actelion Pharmaceuticals, Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to the writing of the manuscript. Nazzareno Galiè, Sean Gaine, Richard Channick, J. Gerry Coghlan, Marius M. Hoeper, Irene M. Lang, Vallerie V. McLaughlin, Lewis J. Rubin, Olivier Sitbon, Victor F. Tapson and Kelly M. Chin contributed to collection of the data as investigators and to interpretation of the data. Shu-Fang Hsu Schmitz contributed to statistical analyses and interpretation of the data. Cheryl Lassen contributed to collection and interpretation of the data.
List of Investigators
Investigators for GRIPHON were as previously described [4]. Investigators for GRIPHON OL were: Argentina – Hospital Privado Centro Medico de Cordoba, Cordoba: O Salomone; Instituto de Cardiologia de Corrientes, Corrientes: E Perna; Servicio De Diálisis Y Nefrología, Buenos Aires: GR Bortman; Hospital Britanico, Buenos Aires: FJ Chertcoff; Sanatorio Otamendi y Miroli S.A., Buenos Aires: R Gene; CIPREC, Buenos Aires: JO Caneva. Australia – Pulmonary Arterial Hypertension Clinic, Hobart: D Kilpatrick; Royal Adelaide Hospital, Adelaide: P Steele; Flinders Medical Centre, Bedford Park: R Minson; St Vincent’s Hospital, Darlinghurst: E Kotlyar; John Hunter Hospital, New Lambton Heights: G Reeves; Advanced Lung Disease Unit, Murdoch: M Lavender; Queensland Lung Transplant Service, Chermside: F Kermeen; St. Vincent’s Hospital (Melbourne), Fitzroy: W Stevens; Royal Melbourne Hospital, Parkville: D Smallwood; Concord Hospital, Concord: L Kritharides. Austria – Medical University of Vienna, Vienna: I Lang; Medical University of Graz, Graz: H Olschewski. Belarus – Minsk Regional Clinical Hospital Of The Red Banner Of Labor, Minsk: I Adzerikho; 9th Healthcare Institution Minsk Clinical Hospital, Minsk: N Soroka; Republican Scientific and Practical Center “Cardiology,” Minsk: O Polonetsky. Belgium – Erasme Hospital, Brussels: J-L Vachiéry; UZ Leuven, Leuven: M Delcroix. Canada – Institut Universitaire De Cardiologie Et De Pneumologie De Québec, Québec, QC: S Provencher; Health Sciences Center, Winnipeg, MB: Z Bshouty; University Health Network – Toronto General Hospital, Toronto, ON: J Granton; Royal University Hospital, Saskatoon, SK: K Laframboise; University of Ottawa Heart Institute, Ottawa, ON: L Mielniczuk; Vancouver General Hospital, Vancouver, BC: J Swiston; University of Alberta Hospital, Edmonton, AB: D Lien; Jewish General Hospital, Montreal, QC: K Boutet. Chile – Hospital Clínico De La Pontificia Universidad Católica De Chile, Santiago: F Baraona; Hospital San Juan De Dios, Santiago: P Sepúlveda Varela; Asesorias e Investigaciones Medicas TASOL Ltda., Santiago: M Zagolín; Instituto Nacional Del Torax, Santiago: M Zagolín. China Peking Union Medical College Hospital, Beijing: X Zeng; Shanghai Pulmonary Hospital, Shanghai: J Liu; Beijing Fuwai Hospital, Beijing; J He; Guangdong Provincial People's Hospital, Guangzhou, Guangdong: H Yao; Renji Hospital, Shanghai: J Shen; Shanghai Renji Hospital, Shanghai: C Bao; Beijing Shijitan Hospital, Beijing: Y Wang. Colombia – Fundacion Abood Shaio, Bogota: J Rubén Dueñas; Fundacion Cardio Infantil IC, Bogota: C Aguirre. Czech Republic – General Faculty Hospital, Prague: M Aschermann. Denmark – Aarhus University Hospital, Aarhus: JE Nielsen-Kudsk; Rigshospitalet Kardiologisk Klinisk, Copenhagen: J Carlsen. France – Bicêtre Hospital, Le Kremlin-Bicêtre: X Jaïs; Hospital Larrey CHU de Toulouse, Toulouse: L Têtu; Hospital Claude Huriez CHRU de Lille, Lille: E Hachulla; Hospital Louis Pradel, Bron: V Cottin. Germany – University of Greifswald, Greifswald: R Ewert; Universitätsklinikum Carl Gustav Carus Medizinische Klinik und Poliklinik 1-Pneumologie, Dresden: M Halank; DRK Kliniken Westend, Berlin: C Opitz; Klinik Löwenstein gGmbH, Löwenstein: G Staehler; Universitätsklinikum Regensburg, Regensburg: T Lange; Medizinische Hochschule Hannover Zentrum Innere Medizin Klinik für Pneumologie, Hannover: M Hoeper; Universitätsklinikum Giessen, Giessen: H-A Ghofrani; Thoraxklinik am Universitätsklinikum Heidelberg, Heidelberg: E Grünig; Praxis Für Pneumologie Und Allergologie, Leipzig: J Winkler; Herzzentrum Uniklinik Köln Klinik III für Innere Medizin, Cologne: S Rosenkranz. Greece – Onassis Cardiac Surgery Center, Athens: V Voudris; University Hospital of Alexandroupolis, Alexandroupoli: S Konstantinidis; Ahepa University General Hospital Of Thessaloniki, Thessaloniki: I Styliadis. Hungary – Semmelweis Egyetem,Pulmonológiai Klinika, Budapest: K Karlócai;Pecsi Tudomanyegyetem Klinikai Kozpont, Pécs: A Komócsi; Kenézy Gyula Kórház Rendelőintézet, Debrecen: I Czuriga; Szegedi Tudományegyetem Áok Szent-Györgyi Alb Klin Központ, Szeged: T Forster; Gottsegen Gyorgy National Cardiological Institute, Budapest: A Temesvári. India – Apollo Hospitals Chennai, Chennai: A Oomman; Care Hospitals Nampally, Hyderabad: BKS Sastry; Sanjivani Hospital Cardiology, Ahmedabad: K Sharma; Frontier Lifeline Hospitals, Chennai: A Kannaiyan. Ireland – Mater Misericordiae Hospital, Dublin: S Gaine. Israel – Hadassah Medical Center, Jerusalem: N Berkman; The Chaim Sheba Medical Center, Tel-Hashomer: M Segel; Tel Aviv Sourasky Medical Center, Tel Aviv: Y Schwarz; Kaplan Medical Center, Pulmonary Institute, Rehovot: G Fink; Rabin Medical Centre, Petach Tikvah: M Kramer; Carmel Medical Center, Haifa: Y Adir. Italy – Second University of Naples, Naples: G Valentini; Sant’Orsola-Malpighi Polyclinic, Bologna: N Galiè; Ospedali Dei Colli (Monaldi, Cotugno, Cto) - Ospedale Monaldi, Naples: M D’Alto; Mediterranean Institute for Transplantation and Advanced Specialized Therapies, Palermo: P Vitulo. Malaysia – Institut Jantung Negara, Kuala Lumpur: CK Teoh. Mexico – Instituto Nacional De Cardiologia, Mexico City: T Pulido; Instituto Nacional De Ciencias Medicas y Nutricion Salvador Zubirán, Mexico City: JL Hernández-Oropeza. The Netherlands – Hospital Maastricht University Medical Center, Maastricht: V Van Empel; VUMC Amsterdam, Amsterdam: A Boonstra; Erasmus University Medical Center, Rotterdam: L Van Den Toorn. Peru – Centro De Investigación Enfermedades Respiratorios Y Medicina Intensiva, Lima: M Camere; Hospital Nacional Edgardo Rebagliati Martins, Lima: JG Matheus. Poland – University Clinical Centre, Gdańsk: G Raczak; Krakow Specialist John Paul II Hospital, Kraków: P Podolec; European Health Centre, Otwock: A Torbicki; Wojewódzki Specjalistyczny Szpital im. dr. Wł. Biegańskiego, Łódź: J Kasprzak. Republic of Korea (South) – Severance Hospital, YonSei University Health System, Seoul: H-J Chang; Gachon University Gil Medical Center, Incheon: W-J Chung; The Catholic University of Korea Yeouido St. Mary's Hospital, Seoul: HO Jung; Seoul National University Hospital, Seoul: H-K Kim; Samsung Medical Center, Seoul: S-A Chang; The Catholic University of Korea Seoul St. Mary’s Hospital, Seoul: HO Jung. Romania – National Institute of Pneumology “Marius Nasta”, Bucharest: MA Bogdan; Iasi Pneumophysiology Clinical Hospital, Iasi: T Mihaescu. Russian Federation – Federal State Budgetary Institution, St Petersburg: O Moiseeva; Federal State Budgetary Scientific Instit, Moscow: A Volkov; National Medical Research Center of Cardiology of MoH of Russian Federation, Moscow: I Chazova; Federal State Budgetary Institution, Moscow: S Avdeev; Regional State Autonomous Healthcare Institution of Tomsk Regional Clinical Hospital, Tomsk: L Lenskaya; Sverdlovsk Regional Clinical Hospital #1, Ekaterinburg: M Arkhipov; Federal State Budget Scientific Institution, Kemerovo: O Barbarash; Federal State Budget Scientific Institution, Tomsk: A Evtushenko; Municipal Health Care Institution City Hospital #5, Barnaul: TI Martynenko; State Autonomic Health Care Institution Of Novosibirsk Region: E Zonova. Serbia – Clinic for Pulmonology, University Clinical Centre of Serbia, Belgrade: B Milenkovic; Clinical Hospital Center Zemun, Belgrade: B Putniković. Singapore – Changi General Hospital, Singapore: R Jagadesan; National Heart Centre, Singapore: ST Lim. Slovakia – Eastern Slovak Institute of Cardiovascular Diseases, Košice: M Studenčan; National Institute of Cardiovascular Diseases, Bratislava: E Goncalvesová. Spain – Hospital Clinic of Barcelona, Barcelona: JA Barbera; Vall d’Hebron University Hospital, Barcelona: A Roman; University Hospital 12 Octubre, Madrid: P Escribano. Sweden – Sahlgrenska University Hospital, Göteborg: B Rundqvist; Linköping University Hospital, Garnisonsvägen: L Hübbert; Norrlands Universitetssjukhus, Umeå: S Söderberg; Uppsala University Hospital, Uppsala: G Wikström. Switzerland – University Hospital Basel, Basel: M Tamm; University Hospital Geneva, Geneva: F Lador; Lausanne University Hospital, Lausanne: L Nicod; University Hospital of Bern, Bern: T Geiser. Taiwan – National Taiwan University Hospital, Taipei: H-H Hsu; Kaohsiung Veterans General Hospital, Kaohsiung: C-C Cheng. Thailand – Srinagarind Hospital Khon Kaen University, Khon Kaen: A Mahakkanukrauh. Turkey – Dokuz Eylul University Hospital, Izmir: B Akdeniz; Çukurova University Balcali Hospital, Balcali: M Demir; Istanbul University Cardiology Institute, Istanbul: S Kucukoglu; Marmara University School of Medicine, Istanbul: B Mutlu. Ukraine – Lviv Regional Clinical Hospital, Lviv: L Solovey; Dnipropetrovsk Medical Academy, Dnepropetrovsk: Vasilieva L; National Institute of Phthisiology and Pulmonology Yanovskiy AMS, Kyiv: V Gavrysyuk; Kharkiv City Clinical Hospital 13, Kharkiv: V Blazhko. UK – National Waiting Times Centre Board Golden Jubilee National Hospital, Glasgow: A Peacock; Royal Free Hospital, London: G Coghlan. USA – Massachusetts General Hospital, Boston, MA: R Channick; University of Michigan, Ann Arbor, MI: V Mclaughlin; UT Southwestern Medical Center, Dallas, TX: K Chin; Arizona Pulmonary Specialists Ltd, Phoenix, AZ: J Feldman; Christiana Care Health Services Inc., Newark, DE: M Saltzberg; Mercy Hospital, Iowa City, IA: R Oren; University of Arizona, Tuscon, AZ: F Rischard; Doylestown Cardiology Associates-VIAA, Doylestown, PA: R Sangrigoli; UC Davis Health System, Sacramento, CA: R Allen; Cedars-Sinai Medical Center, Los Angeles, CA: G Chaux; University of Texas Houston Health Center, Houston, TX: B Patel; Columbia University Medical Center, New York, NY: E Rosenzweig; University of Wisconsin Hospital, Madison, WI: J Runo; Henry Ford Hospital, Detroit, MI: R Awdish; Johns Hopkins University Medical Center, Baltimore, MD: P Hassoun; Mayo Clinic, Rochester, MN: R Frantz; LSU Health Sciences Center, New Orleans, LA: B Deboisblanc; Vanderbilt University Medical Center, Nashville, TN: I Robbins; Lindner Clinical Trial Center, Cincinnati, OH: PJ Engel; University of California San Diego Medical Center, La Jolla, CA: D Poch; University of Kansas Medical Center, Kansas, KS: T Williamson; Penn Presbyterian Medical Center, Philadelphia, PA: JS Fritz; University of Cincinnati, Cincinnati, OH: J Elwing; Sentara Cardiovascular Research Institute, Norfolk, VA: P Mahoney; Kentuckiana Pulmonary Associates, Louisville, KY: J McConnell; Ohio State University Medical Center, Columbus, OH: N Sood; Bend Memorial Clinic, Bend, OR: R Sussmane-Stubbs; Boston University School of Medicine, Boston, MA: HW Farber; Stony Brook University Medical Center, Islandia, NY: P Strachan; Washington University School of Medicine, St Louis, MO: M Chakinala; Pulmonary Associates Of Richmond, Richmond, VA: J Hey; Wake Forest University School of Medicine, Winston-Salem, NC: E Bleecker; St. Luke'S Medical Group Cardio-Pulmonary Associates, Chesterfield, MO: N Ettinger; Georgia Regents University, Augusta, GA: J Gossage; Newark Beth Israel Medical Center, Newark, NJ: C Migliore; William Beaumont Hospital Troy PH Center, Troy, MI: S Allen; York Hospital, York, PA: D Zubkus; Houston Methodist Hospital, Houston, TX: Z Safdar; Indiana University North Hospital, Carmel, IN: W Harvey; Duke University Medical Center, Durham, NC: T Fortin; St Luke’s Medical Center, Milwaukee, WI: R Tumuluri; Tufts Medical Center, Boston, MA: I Preston; Liu Center for the Study and Treatment of Pulmonary Hypertension, Torrance, CA: R Oudiz; Oregon Pulmonary Associates, Portland, OR: J Butler; Emory University School of Medicine, Atlanta, GA: M Fisher; GLVA Healthcare Center, Los Angeles, CA: S Shapiro; Montefiore Medical Center – JD Weiler Hospital, Bronx, NY: J Tauras; University of South Alabama, Mobile, AL: K Fagan; Thomas Jefferson University Hospital, Philadelphia, PA: M Scharf; University Hospitals – Case Medical Center, Cleveland, OH: R Schilz; Piedmont Healthcare, Austell, GA: C Miller; University of Iowa Hospitals and Clinics, Iowa City, IA: L Cadaret; Medical College of Wisconsin, Froedtert Hospital, Milwaukee, WI: K Presberg; Houston Methodist Hospital Lung Center, Houston TX: A Frost.
Prior Presentations
These data have been partially previously presented at the ESC Congress 2018 (25–29 August 2018, Munich, Germany), the BTS Winter Meeting 2018 (5–7 December 2018, London, UK) and SEPAR 2019 (13–16 June, Santiago de Compostela, Spain).
Disclosures
Nazzareno Galiè is a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received grant support, personal fees and non-financial support from Janssen Pharmaceutical Companies of Johnson & Johnson; and has received grant support and personal fees from Bayer Healthcare, Pfizer and GlaxoSmithKline. Sean Gaine has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson; has received advisory board fees from Janssen Pharmaceutical Companies of Johnson & Johnson, and Daiichi-Sankyo; and has served on a data and safety monitoring board for United Therapeutics. Richard Channick has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has served on an advisory board for Janssen Pharmaceutical Companies of Johnson & Johnson and Bayer; has received consultancy fees from Bayer and Arena Pharmaceuticals; and has received research grants from Janssen Pharmaceutical Companies of Johnson & Johnson and United Therapeutics. J. Gerry Coghlan has received grant support, speakers’ fees and travel support from Janssen Pharmaceutical Companies of Johnson & Johnson and has served as a member of working groups for Janssen Pharmaceutical Companies of Johnson & Johnson, Acceleron and Bayer. Marius M. Hoeper has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received speaker and consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer, GlaxoSmithKline, Merck Sharp & Dohme, and Pfizer; and has received research grants from Janssen Pharmaceutical Companies of Johnson & Johnson. Irene M. Lang has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Merck Sharp & Dohme, and AOP Orphan Pharmaceuticals; and has received research grants from Janssen Pharmaceutical Companies of Johnson & Johnson and AOP Orphan Pharmaceuticals. Vallerie V. McLaughlin has served as a as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson. She receives consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Acceleron, Bayer, Caremark LLC, CiVi Biopharma, United Therapeutics, Altavant, Gossamer Bio, and Liquidia and research/grant support from Janssen Pharmaceutical Companies of Johnson & Johnson, Acceleron, United Therapeutics, Reata Pharmaceuticals, SoniVie and NIH. Cheryl Lassen is an employee of Actelion Pharmaceuticals Ltd. Lewis J. Rubin has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; and has received consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Arena Pharmaceuticals, GENO Pharmaceuticals, Gilead, Karos Pharmaceuticals, Pfizer, and SoniVie Ltd. Shu-Fang Hsu Schmitz is an employee of Actelion Pharmaceuticals Ltd. Olivier Sitbon has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has served as an advisory board member for and received research grants from Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer, GlaxoSmithKline, and Merck Sharp & Dohme; has received consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Arena, Bayer, GlaxoSmithKline and Merck Sharp & Dohme; has received speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer, GlaxoSmithKline, and Merck Sharp & Dohme; has served on a scientific advisory board for Arena Pharmaceuticals and Gossamer Bio; and has received writing assistance from Janssen Pharmaceutical Companies of Johnson & Johnson and GlaxoSmithKline. Victor F. Tapson has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer, and United Therapeutics; has received consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Arena Pharmaceuticals, Bayer, Daiichi-Sankyo, EKOS/BTG, Gilead Sciences, Janssen, Reata, and United Therapeutics; has received research grants from Arena Pharmaceuticals, Arena, Bayer, EKOS/BTG, and Riata; has received speaker fees from Bayer, Gilead Sciences, and Janssen. Kelly M. Chin has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received research grants from Janssen Pharmaceutical Companies of Johnson & Johnson, NIH, Ironwood Pharmaceuticals, National Institutes of Health and SoniVie Ltd; has served on an advisory board for Bayer Healthcare (through UCSD) and Flowonix; has served as an adjudication committee member for Arena Pharmaceuticals; is Circulation Associate Editor for the American Heart Association; and has received consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson.
Compliance with Ethics Guidelines
GRIPHON and GRIPHON OL were conducted in accordance with the Declaration of Helsinki. The protocols were approved by the institutional review board/independent ethics committee at each site (Supplementary Table 1). GRIPHON OL was monitored until 30 June 2016 by the same independent data and safety monitoring committee as in GRIPHON [4]. Written informed consent was obtained from all patients at entry into GRIPHON and GRIPHON OL.
Data Availability
The datasets generated during and/or analysed during the current study are available; requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site http://yoda.yale.edu.
References
- 1.Farber HW, Miller DP, Poms AD, Badesch DB, Frost AE, Muros-Le Rouzic E, et al. Five-year outcomes of patients enrolled in the REVEAL Registry. Chest. 2015;148:1043–1054. doi: 10.1378/chest.15-0300. [DOI] [PubMed] [Google Scholar]
- 2.Benza RL, Miller DP, Barst RJ, Badesch DB, Frost AE, McGoon MD. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142:448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 3.Uptravi USPI. Uptravi® (selexipag) prescribing information. 2021.
- 4.Sitbon O, Channick R, Chin KM, Frey A, Gaine S, Galie N, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med. 2015;373:2522–2533. doi: 10.1056/NEJMoa1503184. [DOI] [PubMed] [Google Scholar]
- 5.Benza RL, Gomberg-Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest. 2019;156:323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Benza RL, Seeger W, McLaughlin VV, Channick RN, Voswinckel R, Tapson VF, et al. Long-term effects of inhaled treprostinil in patients with pulmonary arterial hypertension: the Treprostinil Sodium Inhalation Used in the Management of Pulmonary Arterial Hypertension (TRIUMPH) study open-label extension. J Heart Lung Transplant. 2011;30:1327–1333. doi: 10.1016/j.healun.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Ghofrani HA, Grimminger F, Grunig E, Huang Y, Jansa P, Jing ZC, et al. Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016;4:361–371. doi: 10.1016/s2213-2600(16)30019-4. [DOI] [PubMed] [Google Scholar]
- 8.Simonneau G, Rubin LJ, Galiè N, Barst RJ, Fleming TR, Frost A, et al. Long-term sildenafil added to intravenous epoprostenol in patients with pulmonary arterial hypertension. J Heart Lung Transplant. 2014;33:689–697. doi: 10.1016/j.healun.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Rubin LJ, Badesch DB, Fleming TR, Galie N, Simonneau G, Ghofrani HA, et al. Long-term treatment with sildenafil citrate in pulmonary arterial hypertension: the SUPER-2 study. Chest. 2011;140:1274–1283. doi: 10.1378/chest.10-0969. [DOI] [PubMed] [Google Scholar]
- 10.Kim NH, Hemnes AR, Chakinala MM, Highland KB, Chin KM, McLaughlin V, et al. Patient and disease characteristics of the first 500 patients with pulmonary arterial hypertension treated with selexipag in real-world settings from SPHERE. J Heart Lung Transplant. 2021 doi: 10.1016/j.healun.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 12.Galiè N, Humbert M, Vachiéry JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 13.Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53:1801889. doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heresi GA, Rao Y. Follow-up functional class and 6-minute walk distance identify long-term survival in pulmonary arterial hypertension. Lung. 2020;198:933–938. doi: 10.1007/s00408-020-00402-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available; requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site http://yoda.yale.edu.