Abstract
Rutin was nano-encapsulated in date [En-Ru(D)] and mushroom [En-Ru(M)] β-glucan matrix to protect it from the harsh gastrointestinal environment and to enhance its bioavailability and biological activity upon digestion. The encapsulation was carried using green technology i.e., ultra-sonication. The En-Ru(D) and En-Ru (M) showed the hydrodynamic diameter of 314.04 and 482.21 nm with polydispersity index of 0.21 and 0.33. The in vitro release behaviour followed the Higuchi model. The antimicrobial activity of En-Ru(D) and En-Ru(M) were evaluated against gram negative E. coli (ATCC 25922) and gram positive (Staphylococcus aureus) bacteria. Furthermore, En-Ru(D) and En-Ru(M) exhibited increased bioavailability of rutin in intestinal fluid with retention of anti-obesity and antioxidant activities after digestion (p < 0.05). Therefore, β-glucan matrix can efficiently encapsulate flavonoids and regulate the release of functional bioactive ingredients in the simulated human digestive conditions.
Keywords: Flavonoids, Release kinetics, Anti-obesity, Antioxidant
1. Introduction
Flavonoids are a group of secondary plant metabolites, considered as a vital constituent having health-promoting properties. These flavonoids can be used in food fortification, preservation, and designing of healthy food products. Among the various flavonoids existing in plants, rutin (quercetin-3-rhamnosyl glucoside) is of distinct attention to food scientists due of its immense nutraceutical potential such as antioxidant, anti-inflammatory, antimicrobial, anticancerous and antiobesity effect [1]. However, there are many constrains associated with bioavailability of rutin because of its low stability and solubility in both hydrophilic and lipohillic solution [2]. Also, rutin is unpleasant due to its astringent taste and undesirable sensorial attributes which further limits its use in food systems [3], [4]. To overcome such obstacles, rutin has been entrapped within different carriers like starch, egg albumin, olive oil, and liposomes [5], [6], [7]. Among different food grade delivery systems, β-glucan has gained focus as a wall material because of its honeycomb structure which can easily hold bioactive compounds resistance to heat, acid and enzymes, broad spectrum of biological activities and is a prebiotic [8], [9]. The stability and harmless character of β-glucan makes it a GRAS substance certified by the FDA (US Food and Drug Administration) [8]. Further, the limitations of native β-glucan like low solubility, high viscosity, less bioavailability etc can be efficiently overcome by reducing its size as previously reported by Ashraf et al. [8]: Ashraf et al. [9]. The structural variation of mushroom and date β-glucan results in difference in their properties. The β-1,6-linkages have been found in mushroom β-glucan however, both β-1,6 and β-1,4-linkages have been found in date β-glucan [10]. This could lead to difference in the ability of β-glucan to encapsulate the bioactive compounds [10].
Among physical techniques, ultrasonication is a promising technique for encapsulation of polyphenols as it is simple, rapid and produces a high yield without any need of purification [11]. Ultrasonication involves the use of high intensity sound waves of frequency > 16 kHz to produce periodical waves when propagated through the medium. The agitation produces bubbles which later on collapse, generating high temperature and pressure and breaking the macropolymer chain [11]. It also facilitates the entry of encapsulant and water within the internal channels of wall material by removal of air.
Hence, the purpose of this study is to explore the use of β-glucan from mushroom and dates as a carrier for rutin using a more environment-friendly ultrasound-based method. The nano encapsulated β-glucan particles loaded with rutin were studied for its release behaviour and retention of its biological activity in simulated gastric and intestinal juices.
1.1. Materials
Dates (Phoenix dactylifera L.) and mushrooms (Agaricus bisporus,) were purchased from the local market of Srinagar, India. The chemicals and enzymes such as α-amylase (Aspergillus oryzea 30 U/mg), pancreatin (porcine pancreas 8 USP), pepsin (porcine gastric mucosa 250 U/mg) and bile salts were bought from Sigma-Aldrich.
1.1.1. Extraction of β-glucan
Alkaline method was used for the extraction of β-glucan from dates [12]. Briefly, sample was dried, milled and mixed with deionized water, pH was adjusted to 7 using sodium hydroxide and stirred vigorously for 1 h at 90 °C. The extract was filtered through muslin cloth and then centrifuged. The pH of supernatant was adjusted to 4.5 followed by re-centrifugation. The 99% ethanol was used for precipitation of supernatant followed by enzymatic treatment. The solution was again suspended in ethanol.
β-Glucan from the mushrooms was extracted following the method of Chaiyasut et al. [13] with some modifications. Fresh fruiting body of mushroom was washed in deionised water, sliced and dried out in a hot air oven at 36 °C for 11 h. Ground dried mushrooms were dissolved in NaOH (1 M) and heated at 80 °C for 6 h. This was followed by centrifugation (6000×g) at 4 °C for 25 min and pellet was washed with deionised water thrice and re-centrifugated to separate compounds such as heteropolysaccharides, phenols and glycogen. The recovered fraction was dissolved in acetic acid (1 M) and reheated at 80 °C for 2 h with constant stirring. After heating, the suspension was re-centrifuged. Ethanol (99%) was used to precipitate the supernatant followed by enzymatic treatment. The mixture was again suspended in ethanol. The recovered β-glucan was dried and stored for further analysis. β-Glucan Assay Kit (Mixed Linkage), megazyme, Ireland, Pro Lab Marketing PVT. LTD) was used to examine the purity of β-Glucan which was in the range of 94–97%.
1.2. Nano-encapsulation of rutin in β-glucan matrix
Mushroom and dates β-glucan (D-Glu& M−Glu) was dissolved the in 2% NaOH solution and heated up to 50 °C until a paste-like solution was formed. The mixture was allowed to cool. Rutin (4 mg/mL) was dissolved in ethanol and added drop wise to the β-glucan solution with constant stirring on magnetic stirrer at 30 °C. This was followed by ultrasonication for 15 min at 40 KHz for 5 s “onset” and 3 s “offset” at 28 °C. Further, the whole mixture was freeze-dried to get a fine powder. Samples were labelled as En-Ru(D) and En-Ru(M) for rutin nanocapsules from dates and mushroom β-glucan.
1.3. Particle size and polydispersity index
The particle size and polydispersity index was determined using Dynamic Light Scattering (Anton Paar, Litesizer). Sample (1.0 mg/mL) was dissolved in distilled water and sonicated at 20 KHz for 30 min to completely dissolve the polymer.
1.4. Structural elucidation by ATR-FTIR
Spectra of En-Ru (D) and En-Ru (M) was obtained using FTIR spectrometer system (Cary 630 FTIR, Agilent Technologies, USA), coupled to an ATR accessory. Analysis was carried out at room temperature, spectra was acquired in 4000–500 cm−1 range at resolution of 4 cm−1, using Resolution Pro software version 2.5.5 (Agilent Technologies, USA).
1.5. Encapsulation efficiency
The encapsulation efficiency was determined following the method described by Ashraf et al. [8]. Encapsulated sample (50 mg) was washed using distilled water (5 mL) followed by centrifugation at 4000 rpm for 5 min. The supernatant was discarded and the pellet was collected and re-dissolved in distilled water followed by sonication at 40 kH for 30 min. This was followed by centrifugation and filtration of the supernatant using Watman filter no 1. The absorbance of the filtrate was spectrophotometrically measured at 283 nm to estimate rutin content.
The encapsulation efficiency (E.E) was estimated by using the below equation
E.E (%) = Total amount of rutin entrapped in the β-glucan matrix /Total amount of rutin loaded in β-glucan × 100
1.6. Swelling behaviour
Freeze dried samples 0.1 g (W1) were dissolved in 10 mL of phosphate buffer saline at different pH values (3, 4, and 6.5) and incubated for 2 h at 37 °C. After incubation the samples were centrifuged at 1000 × g for 10 min and supernatant was discarded and the pellet was weighed (W2). Swelling Behaviour is the gain in weight and was calculated by this formula
1.7. Release behaviour of rutin from β-glucan
Rutin release profile from β-glucan matrix was determined using dialysis membrane following the method of Costa et al. [14] with some modifications. Dialysis bags (molecular weight cut-off 14KD) were cut to a quantified length and rinsed (outer and inner surface) with sodium chloride (0.9%). After rinsing, 200 mg of each sample was added into a dialysis membrane and its ends were sealed properly followed by placing it into 200 mL of PBS (phosphate buffer solution) on magnetic stirring. After regular time intervals, 1 mL of sample was taken from PBS and rutin concentration was measured as a function of time by calculating the absorbance (Elisa Biotech Synergy HT) at 283 nm, which represents the maximum absorbance peak of rutin.
1.7.1. Release Kinetics
The release of rutin was modelled using the Higuchi equation [15]. According to the Higuchi equation, the quantity of bioactive released from the polymer matrix is proportional to the square root of time when based purely on Fickian diffusion.
where Q is the amount of drug released in time t per unit area, k is a constant
1.8. Antimicrobial assay
1.8.1. Agar disc diffusion method
Antibacterial ability of En-Ru(D) and En-Ru(M) was determined using method described by Ashraf et al. [9] with slight modifications. Briefly, En-Ru(D) and En-Ru(M) were tested against gram negative E.coli (ATCC 25922) and gram positive (Staphylococcus aureus). A suspension of 0.1 mL of 106 CFU/mL of each microorganism was spread on the solid medium of specific agar. Sterile disc of diameter 10 mm was placed on the agar plate inoculated with microbial strains. The sample extracts were placed on these sterile discs. Plates were then kept at room temperature for 1 h to facilitate diffusion of En-Ru(D) and En-Ru(M) extract followed by incubation at 28 °C for 24 h. After incubation, diameter of clear zone around sample was measured with a calliper and expressed in millimetres (disc diameter included) as its antibacterial activity. Erythromycin discs of the same diameter as that of films were used as positive control.
1.9. Retention of the biological activity
The encapsulated rutin was exposed to simulated gastric juice (SGJ) and simulated intestinal juice (SIJ) environment and its biological activity such as anti-oxidant and anti-obesity by calculating percent inhibition activity of cholesterol esterase & pancreatic lipase. Sample (50 mg) was dissolved in 5 mL of SGJ and incubated at 37°C for 1 h. After incubation, the sample was exposed to SIJ (5 mL) and re-incubated again for 3 h. This was followed by centrifugation for 10 min and recovered supernatant was used for biological activity.
1.9.1. Antiobesity assay
1.9.1.1. Pancreatic lipase inhibition assay
The Pancreatic lipase inhibition assay was done using the method followed by Ahmad et al. [16] with some modifications. Briefly, a sample mixture (50 µL) including 25 µL of p- nitrophenol butyrate (pNPB), used as a substrate was incubated for 10 min. After incubation, 25 µL of pancreatic lipase solution (50 mg/mL) was added to the mixture to start the enzymatic reaction and was again incubated for 30 min at 37 °C. The release of p-Vitrophenol was analysed at 405 nm. % inhibition was calculated using the following equation 1.
where,
B = absorbance of the control (enzyme and without test sample); C = absorbance of control blank i.e, without enzyme and test sample; D = absorbance of reaction (with enzyme and test sample); E = absorbance of reaction blank (with test sample but without enzyme).
1.9.1.2. Cholesterol esterase (CE) inhibition assay
The cholesterol lipase inhibition was done using the method followed by Ahmad et al. [16] with some modifications. A mixture containing 25 µL of p-Nitrophenol butyrate (pNPB), 50 µL cholesterol esterase enzyme solution (10 µg/mL) prepared in sodium phosphate buffer (0.2 M: pH 7.2) and sample (50 µL) was incubated for 30 min at 37 °C. The released p-Nitrophenol was monitored at 405 nm and percentage of enzyme inhibition was calculated using the Equation 1.
1.10. Antioxidant assay
1.10.1. Inhibition of lipid peroxidation
The Inhibition of lipid peroxidation was done according to the method followed by Gani et al. [20]. Briefly, reaction mixture consisting of linoleic acid (1 mL), ascorbic acid (0.2 mL), H2O2 (0.2 mL), ferric nitrite (0.2 mL) and sample solution was incubated for 1 h at 37℃. After incubation TCA (1.0 mL) and TBA (1.0 mL) was used to terminate the reaction and re-incubated for 20 min. The whole mixture was then centrifuged and malonaldehyde content formed was evaluated by calculating the absorbance of the supernatant at 535 nm.
The Percentage inhibition was calculated by using the Eq. 2:
where (p) is the absorbance of the control and (a)is the absorbance of the sample
1.10.1.1. Ferric reducing antioxidant power activity assay (FRAP)
Briefly FRAP reagent consisting of 0.5 mL of ferrous chloride (20 mM), 1 mL of a 10 mM TPTZ and 1 mL of sodium acetate buffer (300 mM) at pH 3.6. To 1 mL of sample solution, 3 mL of FRAP reagent was added. The mixture was incubated in dark for 15 min at room temperature followed by addition of 2.5 mL of trichloro-acetic acid solution (10% W/V) to reaction mixture. After incubating the reaction mixture for 4 min at room temperature, the absorbance of each reaction mixture was measured at 593 nm against distilled water using UV–vis spectrophotometer.
1.10.1.2. Ferrous chelating activity
The ferrous ion chelating ability of En-Ru(D) and En-Ru(M) was determined using the ferrozine method. The reaction mixture consists of ferrous chloride (0.5 mL), ferrozine (0.25 mL) and sample solution (1 mL), to initiate the reaction. The volume of the mixture was adjusted to 2 mL with deionised water and the whole mixture was incubated for 10 min at room temperature and absorbance was measured at 562 nm The percentage inhibition of ferrozine–Fe2+ complex formation was calculated using the equation 2.
1.11. Statistical analysis
All the experiments were carried in triplicate and results were expressed as mean ± SD. Data was analysed using analysis of variance (ANOVA) and Duncan’s multiple range test at 95% level of significance.
2. Results and discussion
2.1. Particle size and polydispersity index (PDI)
The size of the particle in suspension is the most important characteristic feature to know the bioavailability and release behaviour. A suitable size of particle is associated with the stability against gravity because of Brownian motion. The particle size of En-Ru (D) and En-Ru (M) are presented in Table 1. The hydrodynamic particle size was found to be 314.04 and 482.21 nm with a PDI of 0.21 and 0.33 for En-Ru (D) and En-Ru (M), respectively. The nanosize of capsule may enhance bioavailability and prevent aggregation of particles [18]. Our results are somehow in accordance with the study of Ahmad et al. [17] which reported the average size of chitosan loaded rutin nanoparticles in the range of 98–366.85 nm.
Table 1.
Particle size, Polydispersity Index and encapsulation efficiency of En-Ru(D) and En-Ru(M).
| En-Ru(D) | En-Ru(M) | |
|---|---|---|
| Particle Size (nm) | 314.04 | 482.21 |
| Polydispersity index (PDI) | 0.21 | 0.33 |
| Encapsulation Efficiency (%) | 89 | 91 |
Where En-Ru(D) = rutin encapsulated dates β-glucan.
En-Ru(M) = rutin encapsulated mushroom β-glucan.
The polydispersity index (PDI) measures the average homogeneity of particles in dispersion. Higher PDI value shows a larger size distribution of particles and vice versa. PDI value of<0.4 indicates narrow size distribution of particles [18]. The values of PDI of En-Ru(D) and En-Ru(M) were found to be<0.4 which corresponds to a narrow size distribution.
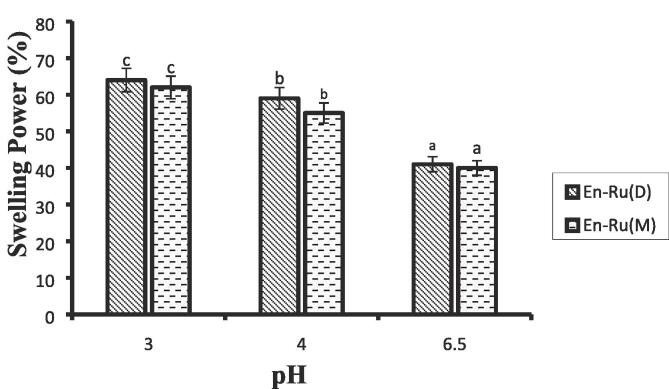
2.2. Swelling behaviour
Swelling behaviour (%) is a significant factor that tells us about the capability of an encapsulation medium to hold the bioactive compound during its transit through the gastrointestinal tract. It involves the rehydration of encapsulated particles in phosphate buffer at two altered pH 3 and 6 to simulate the environment of the stomach and intestine as shown in Fig. 1. It can be hypothesized that if encapsulated capsule swells over a time deprived of dissolution, higher will be a swelling index which indicates that the wall material is stable and hence, higher will be the interruption in the release of rutin. Further, if swelling power is lower, higher will be the dissolution of the capsule and the release of rutin will be instant [19]. En- Ru(M) and En-Ru(D) showed a higher degree of swelling under the simulated condition of the stomach at pH 3 and 4. Furthermore, under simulated conditions of intestines at pH 6.5 En- Ru(M) and En-Ru(D) capsules started its dissolution followed by the release of rutin. The mushroom and date β-glucan (Glu-M &Glu-D) units comprising of β-(1,6)- and β-(1,4)- linkage have the capability for interchain association and permits to have poor solubility [20]. These results show that the release of rutin from the β-glucan matrix of dates and mushroom is pH-dependent and started its release in the intestines.
Fig. 1.
Swelling index of En-Ru(D) and En-Ru(M) at different pH. Where En-Ru(D) = rutin encapsulated dates β-glucan; En-Ru(M) = rutin encapsulated mushroom β-glucan.
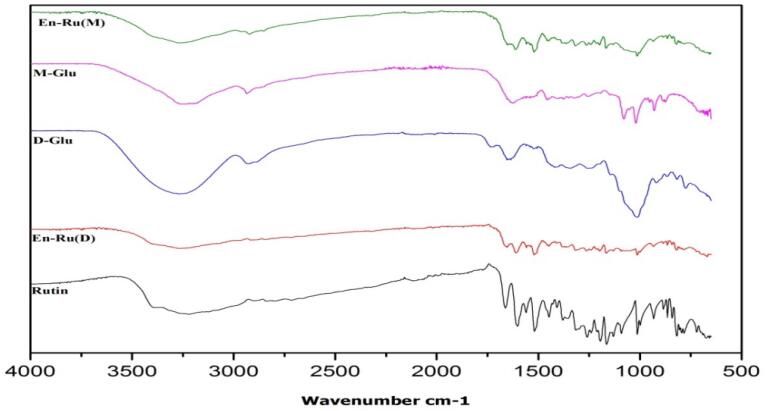
2.3. Structural elucidation using FTIR (Fourier transform infrared spectroscopy)
The ATR- FTIR provides the knowledge of the molecular arrangement, interaction and characterization of nanocapsules. The FTIR results presented the probable interaction between β-glucan from dates (D-Glu) and mushroom (M−Glu) with flavonol rutin as shown in Fig. 2. The Infrared (IR) band of rutin shows specific characteristic peaks in the range of 500–1500 cm−1 with the representative peak of OH stretching at 3423.97 cm−1 and other functional groups such as carboxyl, and carbonyl groups were also shown. However, IR spectra of the En-Ru(D) and En-Ru(M) showed significant changes in comparison to pure rutin. The rutin loaded capsules displayed a swing in the - OH stretching at 3393 cm−1 for En-Ru (D) and 3308 cm−1for En-Ru(M). This new band was absent in rutin or D-Glu and M−Glu. The formation of a new peak in En-Ru(D) and En-Ru(M) might be because of the encapsulation of rutin inside the β-glucan matrix. Further a slight vibration in the range of 1300–1500 cm−1 was observed in the En-Ru(D) and En-Ru(M). A similar peak shift was reported by Patil & Jobanputra for rutin encapsulated in chitosan nanoparticles [23]. Moreover, in D-Glu and M−Glu stretching vibrations of C—O at 1025 and 1205 cm−1 were observed However, these bands showed lower intensity in En-Ru(D) and En-Ru(M).These positional changes in the peaks might be due to incorporation of the rutin within a carrier material [24]. Our results are in accordance with the previous study reported by Mei et al [8] which showed a similar result for encapsulation of rutin in lipid carrier. Remanan et al. [21] and Natarajanet al. [22] reported the similar behaviour of encapsulated rutin in carrier material.
Fig. 2.
The ATR-FTIR Spectra of En-Ru(D) and En-Ru(M).. Where En-Ru(D) = rutin encapsulated dates β-glucan; En-Ru(M) = rutin encapsulated mushroom β-glucan.
2.4. Encapsulation efficiency
Encapsulation efficiency is the mass percentage of rutin entrapped in the β-glucan matrix. The encapsulation efficiency of β-glucan from dates and mushroom was 89% [En-Ru(D)] and 91% [En-Ru(M)] showing significant (p < 0.05) difference in the rutin holding ability. The factors which affect the encapsulation efficiency are size, source of wall material, and process of encapsulation [25]. The rutin loading capacity of the date β-glucan (D-Glu) was higher than the mushroom β-glucan (M−Glu). This may be attributed to a structural difference of the β-glucan from various sources and extent of interaction between rutin and β-glucan. Farrag et al. [7] reported the difference in encapsulation efficiency of folic acid loaded on starch nanoparticles. These results displayed the capability of β-glucan to hold the rutin in its core due to the honeycomb structure [24]. However, Cahyonol et al. [26] reported the encapsulation efficiency of rutin in chitosan matrix in the range of 60 to 90%.
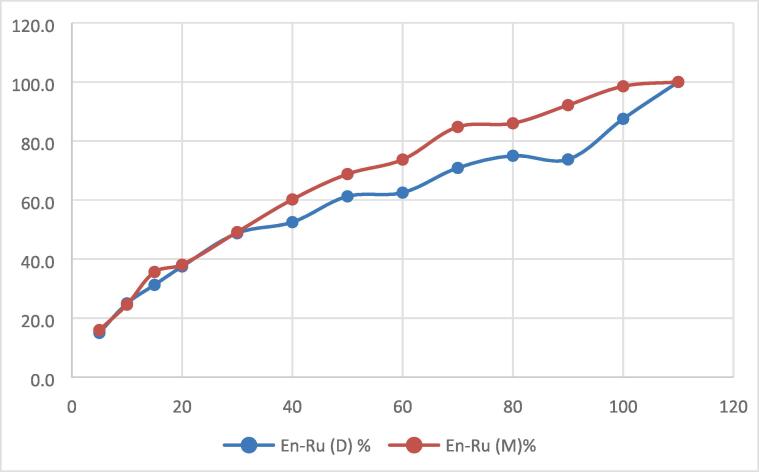
2.5. Release behavior of rutin from beta-glucan matrix
To maintain an actual concentration of the bioactive compound inside the blood, plasma or to the targeted site, the control release designs are made. Different mathematical models are applied for the calculation of release kinetics of the bioactive compound under simulated gastrointestinal conditions. The En-Ru (D) and En-Ru (M) were placed in simulated gastrointestinal conditions for 180 min, and the cumulative release rate of rutin was interpreted using different mathematical models like Hixson- Crowell, Higuchi, and Korsmeyer-Peppas in order to evaluate the kinetics and the mechanism of release. The model that best fits the release data is selected based on correlation coefficient value. The Higuchi fitted the best for the release rate of rutin from the β-glucan matrix of dates and mushroom under simulated digestion conditions as shown in Fig. 3. Higuchi's model involves the release of active ingredients from a solid matrix by diffusion process [15]. It is applicable to water soluble and sparingly water soluble drugs incorporated into solid or semisolid matrix [27]. The data indicate that the release of rutin was best represented by the Higuchi model thereby following the Fick’s release behaviour. According to the Higuchi model, there is a linear relationship between the cumulative release rate of rutin and the square root of the simulated digestion time. As, it is clearly visible in Fig. 3 that En-Ru(D) nano-capsules showed different rutin release profiles than En-Ru(M). The difference in the release profile of En-Ru (D) and En-Ru (M) may be because of the structural difference of β-glucan obtained from various sources. The initial burst release of rutin from the β-glucan matrix may be because of the rutin adhered to the surface followed with a constant prolonged release of rutin signifying hydration and swelling of β-glucan matrix [28]. Similar results were reported by Ahmad et al. [17] for chitosan encapsulated rutin.
Fig. 3.
Fitting of release kinetics equation (Q = kt1/2) to rutin released from β-glucan from dates and mushroom in simulated gastrointestinal conditions. Where En-Ru(D) = rutin encapsulated dates β-glucan; En-Ru(M) = rutin encapsulated mushroom β-glucan.
Where K is the release constant
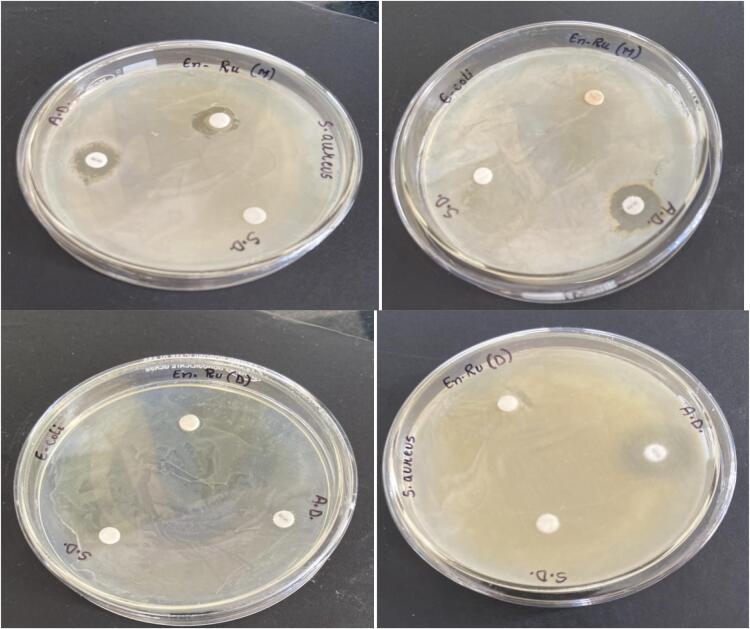
2.6. Antimicrobial activity
En-Ru(D) and En-Ru(M) showed antimicrobial property against the screened strains, namely gram-positive bacteria- Staphylococcus aureus and gram-negative bacteria- Escherichia coli. These strains exhibited comparable inhibition zones with positive control erythromycin. En-Ru(D) and En-Ru(M) showed 4 mm & 5 mm for Escherichia coli and 8 mm & 9 mm for Staphylococcus aureus at the concentrations of 20 μg/mL as shown in Fig. 4. A decreased but detectable activity was noticed for Escherichia coli in comparison to staphylococcus aureus. The antimicrobial activity of flavonoids is due to interaction of flavonoids with bacterial cell wall and disintegration of lipopolysaccharide layer hence impairing the permeability of cell membrane [29]. In addition the β-glucan has been also reported to exhibit antimicrobial activity [30].
Fig. 4.
Antimicrobial activity of En-Ru(D) and En-Ru(M). Where En-Ru(D) = rutin encapsulated dates β-glucan; En-Ru (M) = rutin encapsulated mushroom β-glucan.
2.7. Retention of biological activity
The biological activity of nanocapsules of En-Ru (D) and En-Ru (M) was evaluated in terms of antioxidant and antiobesity activities before and after absorption under simulated gastrointestinal conditions. Biological activity of rutin have been assessed subsequently before and after their separation from plants but it is attention-grabbing to know about their meticulous biological potential after being consumed into the human body [16]. Bioactivity of encapsulated rutin using β-glucan as wall material is expected to retain after its transit through simulated gastrointestinal conditions. The biological activity of En-Ru (D) and En-Ru (M) after digestion was evaluated.
2.7.1. Antioxidant activity
The antioxidant potential of En-Ru (D) and En-Ru (M) were examined before and after in vitro digestion using lipid peroxidation, ferrous chelating, and FRAP assays (Table 2). The antioxidant potential of En-Ru (D) and En-Ru (M) before in vitro digestion was 58.86 and 56.39% for Frap, 68.66 and 71.7% for Ferrous chelating assay and 71.78 and 73.17 for lipid peroxidation respectively. However, the antioxidant potential of the En-Ru (D) and En-Ru (M) after in vitro digestion were found to be 66.12 and 62.31% for lipid peroxidation assay, 58.12 and 47.3% for FRAP, and 49.12 and 50.1% for Ferrous chelating assay respectively. Results revealed good antioxidant activity was retained by nanocapsules of rutin encapsulated in dates and mushroom β-glucan. An increase in the antioxidant potential of En-Ru (D) and En-Ru (M) under simulated gastro-intestinal condition after digestion may be due to the conversion of rutin to quercetin (a flavonoid aglycone) during digestion [19] and partial breakdown of β-glucan due to enzymes leads to exposure of more functional groups [24].
Table 2.
Nutraceutical property of En-Ru(D) and En-Ru(M) before and after digestion.
| En-Ru(D) (Undigested) | En-Ru(M) (Undigested) | En-Ru(D) (Digested) | En-Ru(M) (Digested) | |
|---|---|---|---|---|
| Antioxidant assay | ||||
| Lipid peroxidation (%) | 71.78 ± 0.1c | 73.17 ± 0.3c | 66.12 ± 2.1b | 62.31 ± 2 a |
| Ferrous chelating (%) | 68.66 ± 4c | 71.7 ± 1.1d | 58.12 ± 0.1b | 47.1 ± 3.4a |
| FRAP (%) | 58.86 ± 2.8b | 56.39 ± 0.9b | 49.1 ± 02a | 50.3 ± 2.4a |
| Antiobesity activity | ||||
| Pancreatic lipase (%) | 74.9 ± 2.2d | 71.12 ± 3.3c | 62.3 ± 0.1a | 68.5 ± 0.2b |
| Cholesterol esterase (%) | 76.1 ± 2.1d | 70.4 ± 1.1c | 63.1 ± 1.9a | 67.1 ± 1.1b |
Results are expressed means (n = 3) ± S.D. Values followed by same letter in a row do not differ significantly (p < 0.05).
Where En-Ru(D) = rutin encapsulated dates β-glucan.
En-Ru(M) = rutin encapsulated mushroom β-glucan.
2.7.2. Antiobesity activity of En-Ru (D) and En-Ru (M)
The antiobesity activity of En-Ru (D) and En-Ru (M) was examined before and after in vitro digestion by evaluating the percent inhibition of pancreatic lipase and cholesterol esterase enzymes. Before digestion percent inhibition activity of En-Ru (D) and En-Ru (M) was found to be 74.9% and 71.12% for pancreatic lipase and 76.1 and 70.4% for cholesterol esterase. After digestion, it was found to be 62.3 and 68.5% for pancreatic lipase and 63.1 and 67.1% for cholesterol lipase under simulated gastro-intestinal conditions (Table 2). The release of bioactive compounds was not significantly affected by pH along the digestion process. As encapsulates are aimed for potential incorporation into food products, so it is important that release behaviour should be controlled by wall material during gastrointestinal digestion, as they protect the bioactivity of encapsulated bioactive compound from degradation. These results are as per the literature studies, which shows the release of bioactive compounds from encapsulates exposed to simulated gastrointestinal conditions. Mudasir et al. [16] reported the maximum quantity of resveratrol released under simulated digestion conditions with high anti-obesity and anti-diabetic activity. The results indicate that β-glucan nanoparticles are desirable carriers for delivery of bioactive compounds which decrease the release in gastric fluid and enhance the same in the intestinal fluid [18].
3. Conclusion
The use of β-glucan from dates and mushroom as a carrier for rutin molecules has multiple benefits of target delivery, prevention from acidic environment of stomach, wide range of biological activities and prebiotic properties. The encapsulation efficiency of β-glucan from dates and mushroom were 89% and 91%, revealing the considerable interaction of hydroxyl groups of rutin with β-glucan. The FT-IR analyses of the rutin-loaded β-glucan nanoparticles confirmed entrapment of rutin within its matrix. Rutin encapsulated in β-glucan matrix exhibited enhanced anti-obesity, antioxidant and antimicrobial activity. The results obtained from the present invitro study suggested that the encapsulation of rutin in β-glucan can be a better way for targeted release of rutin within the gastrointestinal tract. However future research is needed to explore the use of rutin capsules in different food systems for its enhanced health benefits.
CRediT authorship contribution statement
Asima Shah: Supervision, Resources, Conceptualization. Zanoor ul Ashraf: Investigation, Writing – original draft. Asir Gani: Writing – review & editing. F.A. Masoodi: Supervision. Adil Gani: Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Dr. Asima Shah is highly grateful to the Department of Science & Technology, GOI (INSPIRE fellowship scheme Grant number: DST/INSPIRE/04/2017/000969) for financial support.
References
- 1.Yang J., Guo J., Yuan J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008;41:1060–1066. [Google Scholar]
- 2.Ren F.N., Ma Z., Li X., Hu X. Preparation of rutin-loaded microparticles by debranched lentil starch-based wall materials: Structure, morphology and in vitro release behaviour. Int. J. Biol. Macromol. 2021;173:293–306. doi: 10.1016/j.ijbiomac.2021.01.122. [DOI] [PubMed] [Google Scholar]
- 3.Luksic L., Bonafaccia G., Timoracka M., Vollmannova A., Trcek J., Nyambe T.K., Melini V., Acquistucci R., Germ R., Kreft I. Rutin and quercetin transformation during preparation of buckwheat sourdough bread. J. Cereal Sci. 2016;69:71–76. [Google Scholar]
- 4.Zhu F. Interactions between starch and phenolic compound. Trends Food Sci. Technol. 2015;43:129–143. [Google Scholar]
- 5.Zhu F. Encapsulation and delivery of food ingredients using starch based systems. Food Chem. 2017;229:542–552. doi: 10.1016/j.foodchem.2017.02.101. [DOI] [PubMed] [Google Scholar]
- 6.Mel M.M.R.D., Gunathilake K.D.P.P., Fernand C.A.N. Formulation of microencapsulated rutin and evaluation of bioactivity and stability upon in vitro digestive and dialysis conditions. Int. J. Biol. Macromol. 2020;159:316–323. doi: 10.1016/j.ijbiomac.2020.05.085. [DOI] [PubMed] [Google Scholar]
- 7.Jhan F., Gani A., Noor N., Ashraf Z.U., Gani A., Shah A. Characterisation and utilisation of nano-reduced starch from underutilised cereals for delivery of folic acid through human GI tract. Sci. Rep. 2021;11:1–15. doi: 10.1038/s41598-021-81623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashraf Z.U., Shah A., Gani F.A.M., Noor N. Effect of nano-reduction on properties of β-glucan and its use as encapsulating agent for release of α-tocopherol. Bioact. Carbohydr. Diet. Fibre. 2020;100230 [Google Scholar]
- 9.Ashraf Z.U., Shah A., Gani A., Gani A., Masoodi F.A., Noor N. N, Nanoreduction as a technology to exploit β-Glucan from cereal and fungal sources for enhancing its nutraceutical potential. Carbohydr. Polym. 2021;258 doi: 10.1016/j.carbpol.2021.117664. [DOI] [PubMed] [Google Scholar]
- 10.D. Mudgil, The Interaction Between Insoluble and Soluble Fiber, Dietary Fiber for the Prevention of Cardiovascular Disease (2017) 35-59.
- 11.Noor N., Gani A., Jhan F., Jenno J.L.H., Dar M.A. Resistant starch type 2 from lotus stem: ultrasonic effect on physical and nutraceutical properties. Ultrason. Sonochem. 2021;76 doi: 10.1016/j.ultsonch.2021.105655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noor N., Shah A., Gani A., Gani A., Masoodi F.A. Microencapsulation of caffeine loaded in polysaccharide based delivery systems. Food Hydrocoll. 2017;171:992–996. [Google Scholar]
- 13.Chaiyasut C., Pengkumsri N., Sundaram B., Sivamaruthi S., Sirilun S., Kesika P., Saelee M., Chaiyasut K., Peeraja S. Extraction of β-glucan of Hericiumerinaceus, Avenasativa L., and Saccharomyces cerevisiae and in vivo evaluation of their immunomodulatory effects. J. Food Sci. Technol. 2018;38:1678–2457. [Google Scholar]
- 14.Costa S.B., Duarte C., Bourbon A.I., Pinheiro A.C., Serra A.T., Martins M.M., Vicente M.I.N.J.A., Delgadillo I., Duarte C., Costa M.L.B. Effect of the matrix system in the delivery and in vitro bioactivity of microencapsulated Oregano essential oil. J. Food Eng. 2012:190–199. [Google Scholar]
- 15.Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad M., Gani A. Ultrasonicated resveratrol loaded starch nanocapsules: characterization, bioactivity and release behaviour under in-vitro digestion. Carbohydr. Polym. 2021;251 doi: 10.1016/j.carbpol.2020.117111. [DOI] [PubMed] [Google Scholar]
- 17.Ahmada N., Ahmadb R., Naqvic A.A., Alamd M.A., Ashafaqe M., Samimf M., Iqbalg Z., Ahmad F.J. Rutin-encapsulated chitosan nanoparticles targeted to the brain in the treatment of Cerebral Ischemia. Int. J. Biol. Macromol. 2016;91:640–655. doi: 10.1016/j.ijbiomac.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Gani A., Ashraf Z.U., Shah A., Noor N., Gani A. Encapsulation of vitamin D3 into β-glucan matrix using the supercritical carbon dioxide. ACS Food Sci. Technol. 2021 doi: 10.1021/acsfoodscitech.1c00233. [DOI] [Google Scholar]
- 19.Asfour M.H., Mohsen A.M. Formulation and evaluation of pH-sensitive rutinnanospheres against colon carcinoma using HCT-116 cell line. J. Adv. Res. 2018;9:17–26. doi: 10.1016/j.jare.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheow W.S., Kiew T.Y., Hadinoto K. K, Controlled release of Lactobacillus rhamnosus biofilm probiotics from alginate-locust bean gum microcapsules. Carbohydr. Polym. 2014;103:587–595. doi: 10.1016/j.carbpol.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 21.Gani A., Benjakul S., Ashraf Z.U. Nutraceutical profiling of surimi gel containing β-glucan stabilized virgin coconut oil with and without antioxidants after simulated gastro-intestinal digestion. J. Food Sci. Technol. 2018;57:3132–3141. doi: 10.1007/s13197-020-04347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remanan M.K., Zhu F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chem. 2020;353 doi: 10.1016/j.foodchem.2020.128534. [DOI] [PubMed] [Google Scholar]
- 23.Natarajan V., Krithica N., Madhan B., Sehgal P.K. Formulation and evaluation of quercetinpolycaprolactone microspheres for the treatment of rheumatoid arthritis. J. Pharm. Sci. 2011;100:195–205. doi: 10.1002/jps.22266. PMID: 20607810. [DOI] [PubMed] [Google Scholar]
- 24.Patil A.G., Jobanputra A.H. Rutin-chitosan nanoparticles: fabrication, characterization and application in dental disorders. Polym Plast. Technol. Eng. 2015;54:202–208. [Google Scholar]
- 25.Shah A., Gani A., Masoodi F.A., Wani S.M., Ashwar B.A. Structural, rheological and nutraceutical potential of β-glucan from barley and oat. Bioact. Carbohydr. Diet. Fibre. 2017;10:10–16. [Google Scholar]
- 26.Farraga Y., Ide W., Montero B., Ricoa M., Rodríguez-Llamazares S., Barral L., Bouzaa R. Preparation of starch nanoparticles loaded with quercetin using nanoprecipitation technique. Int. J. Biol. Macromol. 2018;114:426–433. doi: 10.1016/j.ijbiomac.2018.03.134. [DOI] [PubMed] [Google Scholar]
- 27.Remanan M.K., Zhu F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chem. 2019;20:32396–32397. doi: 10.1016/j.foodchem.2020.128534. [DOI] [PubMed] [Google Scholar]
- 28.Cahyono B., Pratiwi S.B., Hadiyanto, Suzery M. Encapsulation rutin with chitosan-NATPP Using coaservation method. Reaktor. 2017;17(2017):215–220. [Google Scholar]
- 29.C.B. Subal, Modelling of Drug release: The Higuchi equation and its application. Pharmabiz com (2006).
- 30.Alam Z.I., Khan G., Mustafa M., Kumar F., Islam A., Bhatnagar F.J. Ahmad, development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: a pharmacoscintigraphic study. Int. J. Nanomed. 2012;7:5705–5718. doi: 10.2147/IJN.S35329. [DOI] [PMC free article] [PubMed] [Google Scholar]