Graphical abstract

Keywords: Dysprosium oxide, SiO2, Nanostructure, Ultrasonic irradiation, Photocatalytic performance
Highlights
-
•
Introducing a facile sonochemical approach for the efficient preparation of new photocatalytic nanocomposites (Dy2O3-SiO2).
-
•
Remarkable effect of SiO2 on improving photocatalytic performance of Dy2O3.
-
•
Excellent photocatalytic performance of sonochemically fabricated Dy2O3-SiO2 nanocomposite in removal of organic pollutants under sunlight, for the first time.
-
•
Enhanced optical absorption and having a high specific surface area were responsible for enhanced photocatalytic performance.
-
•
Significant effect of sonication on the synthesis of high-performance binary Dy2O3-SiO2 nanophotocatalys.
Abstract
The present study is on the fabrication of new photocatalytic nanocomposites (Dy2O3-SiO2) employing a basic agent, tetraethylenepentamine (Tetrene), through a simple, efficient and, quick sonochemical approach. The features of the fabricated photocatalytic nanocomposite were examined employing a variety of microscopic and spectroscopic methods such as XRD, EDS, TEM, FTIR, DRS, and FESEM. The outcomes of morphological studies demonstrated that by proper tuning of sonication time and ultrasonic power (10 min and 400 W), a porous nanocomposite composed of sphere-shaped nanoparticles with a particle size in the range of 20 to 60 nm could be fabricated. The energy gap for the binary Dy2O3-SiO2 nanophotocatalyst was determined to be 3.41 eV, making these nanocomposite favorable for removing contaminants. The photocatalytic performance of the optimal nanocomposite sample was tested for photodecomposition of several contaminants including erythrosine, thymol blue, eriochrome black T, Acid Red 14, methyl orange, malachite green, and Rhodamine B. The binary Dy2O3-SiO2 nanophotocatalyst exhibited superior efficiency toward the decomposition of the studied contaminants. It was able to degrade the erythrosine pollutant more effectively (92.9%). Optimization studies for the photocatalytic decomposition of each contaminant demonstrated that the best performance could be achieved at a specific amount of contaminant and nanocatalyst. Trapping experiments illustrated that hydroxyl radicals were more effectively involved in the decomposition of contaminant molecules by Dy2O3-SiO2 nanophotocatalyst.
1. Introduction
Rapid population growth, along with the emergence of various industries, has led to the discharge of effluents containing toxic organic contaminants such as various dyes into the environment [1], [2]. Release of effluents containing toxic organic compounds such as erythrosine [3], thymol blue [4], eriochrome black T [5], Acid Red 14 [6], methyl orang [7], malachite green [8], and Rhodamine B [9] can have adverse impacts on the environment and human life, as these organic compounds have poor biodegradability [10], [11], [12]. Among the various solutions reported so far, photocatalysis can be beneficial through the direct usage of solar energy to decompose and remove toxic contaminants, including dyes [13], [14], [15]. The solar photocatalytic process does not require any additional chemical agents or the usage of any other energy to treat the contaminated water [16], [17], [18]. Thus, with the increasing demand for the decomposition and elimination of toxic and unsafe contaminants, the design and development of high-performance nanostructured photocatalysts have become one of the most popular research topics [19], [20], [21]. So far, a wide range of nanostructures and nanocomposites have been explored to treat water contaminated with organic compounds [22], [23], [24]. Nevertheless, there is still a strong need to design and produce new high-efficiency photocatalytic compounds through reproducible and large-scale approaches.
Dysprosium oxide with the formula Dy2O3 is a compound based on rare earth elements that has special features that are derived from their 4f electrons [25]. It is a basic substance, with exceptional thermal stability, and high insolubility properties [26], [27]. This oxide compound crystallizes in three different phases: cubic, hexagonal, and monoclinic [28]. What is more, dysprosium oxide and compounds based on this oxide have unique features, making their wide use in luminescent compounds [29], [30], catalysis [31], MRI contrast agents [32], fuel cells [33], and ceramics [34] possible. Since Dy2O3 has a wide energy gap (4.8 eV) [35], efforts have been made to improve its photocatalytic ability by doping it with other compounds, such as zinc oxide, which tunes its energy gap [36]. Up to present, a variety of methods have been reported for the fabrication of dysprosium oxide structures, including precipitation [25], thermal decomposition [37], [38], combustion [30], sol–gel process [26], and hydrothermal [39]. The methods employed so far to fabricate nanostructured Dy2O3 have drawbacks such as high time and energy consumption, the need for costly precursors, and multiple and sometimes complex steps [26], [37], [38]. The use of ultrasound waves to fabricate a variety of nanostructures is an area of interest to many scientists because it has significant benefits [40]. Ultrasonic radiation accelerates the reactions because high temperatures as well as high pressures are fabricated in the reaction medium, which causes the reactions to take place in a short time [41]. In addition, low energy consumption, good ability to tune the dimensions, architecture, and morphology of different compounds, and simplicity are other unique features of the use of sonochemistry, which has made it very popular for the fabrication of nanostructured compounds [42].
In order to eliminate the shortcomings including secondary contamination owing to high dispersion of nanostructured catalysts in the aqueous medium or the impossibility of effective binding of contaminant molecules to larger photocatalyst clusters, the combination of photocatalysts with adsorbents has been considered as a desirable approach [43]. In the nanocomposite formed, the adsorbent component will efficiently adsorb the contaminant molecules and the nanophotocatalyst component with active sites will cause the decomposition of the contaminant molecules. So far, silicon dioxide has been employed as a support to improve the performance of titanium dioxide catalyst [44].
Very few reports are available for the fabrication of Dy2O3-SiO2 nanocomposites, which have drawbacks such as high energy consumption as well as a long process [45]. The present study on the fabrication of new photocatalytic nanocomposites (Dy2O3-SiO2) employing a basic agent, tetraethylenepentamine (Tetrene), through a simple, efficient, and quick sonochemical approach. The combination of silicon dioxide with a porous structure with Dy2O3 can both form a nanocomposite photocatalyst with a narrower energy gap and enhance the rate of adsorption of pollutant molecules. To the best of the author's knowledge, no previous experimental research has focused on the efficient production of Dy2O3-SiO2 nanocomposites using ultrasonic waves and Tetrene and the study of its photocatalytic efficiency in the decomposition of several toxic contaminants. The effect of altering the sonication time and ultrasonic power on the architecture, dimensions, and morphology of photocatalytic nanocomposite was explored to select the optimal nanostructure. Furthermore, in order to achieve the best photocatalytic efficiency in the decomposition of each contaminant, the effects of the amount of contaminant as well as the dose of the composite nanostructure were investigated.
2. Experimental
2.1. Materials and characterization
All reagents employed in this experimental study, including dysprosium (III) nitrate, tetraethylenepentamine (Tetrene), and Si(OC₂H₅)₄ (TEOS), were of analytical purity and were all purchased from Merck. A diffractometer of Philips Company was employed to record the X-ray diffraction (XRD) outcome of the prepared nanocomposite photocatalyst. Morphological studies and chemical composition analysis of the produced nanocomposite samples were performed by a field emission scanning electron microscopy (MIRA3 FEG-SEM) coupled with energy-dispersive X-ray spectroscopy (EDS). A UV–visible spectrophotometer (Shimadzu, UV-2550, Japan) was employed to examine the optical absorption feature of the fabricated Dy2O3-SiO2 nanocomposite. The morphology and structure of the fabricated nanocatalyst were examined in more detail with a transmission electron microscopy (TEM, FEI F20). Fourier Transform Infrared Spectroscopy (FTIR) investigation was performed with a Magna-IR, spectrometer 550 Nicolet.
2.2. Preparation of binary Dy2O3-SiO2 nanophotocatalyst
A simple, efficient, and quick sonochemical approach was employed to produce binary Dy2O3-SiO2 nanophotocatalyst. First, 1 mmol of TEOS was dissolved in ethanol, and then it was added dropwise to a solution containing 1 mmol of dysprosium nitrate dissolved in ethanol. Then the pH of the resulting mixture was tuned to about 10 with the help of a new alkaline agent (Tetrene), and it was irradiated with the ultrasonic probe at 400 W for 10 min. The resulting precipitate was then dried after washing several times with ethanol. Binary Dy2O3-SiO2 nanophotocatalyst (sample 5) was fabricated by calcination of the residual mass at 1000 °C within 8 h (see Scheme 1). The effect of change in the sonication time and ultrasonic power on the architecture, dimensions, and morphology of binary Dy2O3-SiO2 nanophotocatalyst was explored (see Table 1).
Scheme 1.
Schematic diagram illustrating the production route of binary Dy2O3-SiO2 nanocomposite (sample 5).
Table 1.
Reaction conditions for sonochemical synthesis of all samples.
| Sample no | Dy:Si ratio | Basic agent | Sonication time (min) | Sonication power (W) | Figure of FESEM images |
|---|---|---|---|---|---|
| 1 | 1:1 | Tetrene | 10 | 160 | 1a and b |
| 2 | 1:1 | Tetrene | 15 | 160 | 1c and d |
| 3 | 1:1 | Tetrene | 20 | 160 | 1e and f |
| 4 | 1:1 | Tetrene | 10 | 280 | 2a and b |
| 5 | 1:1 | Tetrene | 10 | 400 | 2c and d |
2.3. Photocatalytic performance of binary Dy2O3-SiO2 nanocatalyst
The photocatalytic performance of the binary Dy2O3-SiO2 nanophotocatalyst was tested for photodecomposition of several contaminants, including erythrosine, thymol blue, eriochrome black T, Acid Red 14, methyl orange, malachite green, and Rhodamine B. In each test, a certain amount of the binary Dy2O3-SiO2 nanophotocatalyst was dispersed in 50 ml of a solution comprising a specified quantity of the target contaminant. Then the resulting suspension was stirred in the darkness for half an hour to establish adsorption–desorption equilibrium [18]. The light source (400 W mercury lamp) was then switched on, and the suspension irradiated [46]. The efficiency of the sonochemically prepared Dy2O3-SiO2 nanophotocatalyst was evaluated and reported with ٪efficiency = (At /A0) × 100 [18]. A0 and At signify the absorbance of each contaminant before and after light irradiation [18].
3. Results and discussion
3.1. FESEM studies
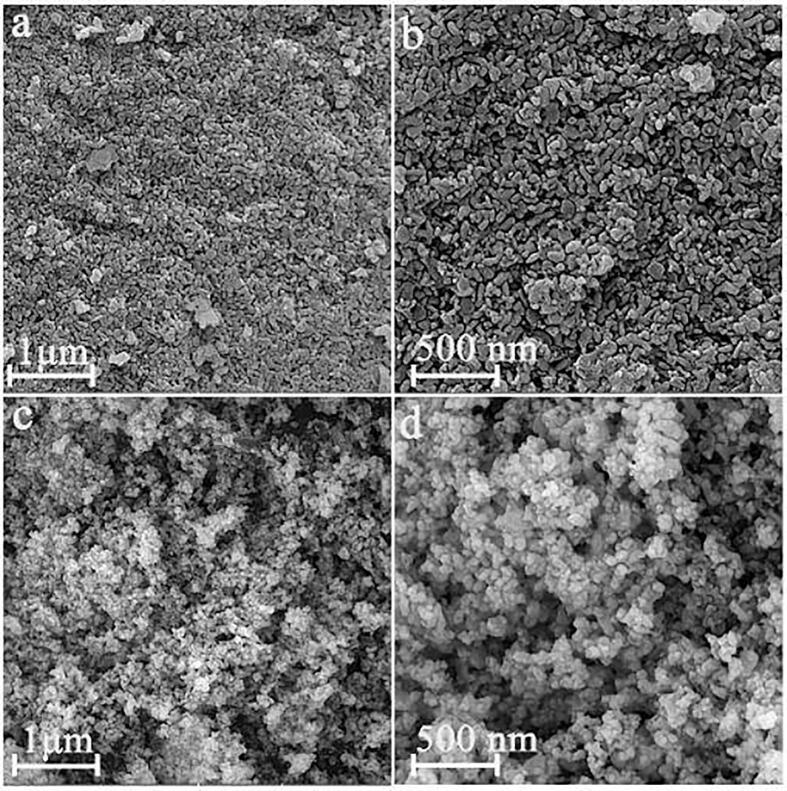
In this experimental work, a new alkaline agent, tetraethylenepentamine (Tetrene) was utilized to prepare the binary Dy2O3-SiO2 nanocomposite via a quick sonochemical approach. First, instrumental variables, including tuning sonication time as well as ultrasound power, were tuned to determine the proper conditions for creating a nanostructured product with favorable features in terms of uniformity and particle size (see Fig. 1, Fig. 2). FESEM outcomes for composite samples 1, 2, and 3 fabricated by sonication for 10, 15, and 20 min are displayed in Fig. 1. By sonication for 10 min, sphere-shaped nanoparticles were fabricated that are relatively uniform (Fig. 1a and b). With sonication longer than 10 min, the tendency of the prepared nanoparticles to aggregate enhanced, and irregular micro/nanostructures were formed (Fig. 1c–f). It seems that nanoparticles that have been fabricated in a shorter time owing to their high surface energy can act as initial nuclei and have grown through the Ostwald process, resulting in irregular micro/nanostructures [47], [48]. Enhancing the sonication time to 20 min also did not have a positive effect on improving the uniformity of the nanostructures and diminishing the particle size. It led to the creation of irregularly shaped nanostructures. It seems that with the prolongation of sonication, the effect of the Ostwald process has been greater than the mechanical effects of ultrasound waves. As a result, it has enhanced the particle size [48], [49]. Therefore, it can be concluded that sonication for 10 min can be proper for the preparation of sphere-shaped Dy2O3-SiO2 nanoparticles.
Fig. 1.
FESEM images of composite samples 1, 2, and 3 fabricated by sonication for 10 (a and b), 15 (c and d), and 20 (e and f) minutes.
Fig. 2.
FESEM images of composite samples 4 and 5 made with 280 (a and b) and 400 (c and d) W ultrasonic power.
FESEM outcomes for composite samples 4 and 5 made with 280 and 400 W ultrasonic power are exhibited in Fig. 2. By enhancing the ultrasonic power from 160 W (Fig. 1a and b) to 280 (Fig. 2a and b) and then 400 W (Fig. 2c and d), the accumulation and aggregation of nanoparticles diminishes, the uniformity improves, and the particle size reduces. It can be seen that sphere-shaped composite nanoparticles with excellent uniformity and also tinier particle size are fabricated by tuning the ultrasound power to 400 W. Hence, enhancing the ultrasonic power remarkably affects the uniformity of the composite nanostructure as well as its architecture and particle size. Applying ultrasonic power above 160 W seems to accelerate the collapse of the cavitation bubbles, bringing in a stronger shock wave that hinders nanoparticles from accumulating more effectively [50]. So, the outcomes of morphological studies demonstrate that by proper tuning of sonication time and ultrasonic power (10 min and 400 W), a porous nanocomposite composed of sphere-shaped nanoparticles with a narrow size distribution can be fabricated. This composite nanostructure was selected for further investigation as well as photocatalytic decomposition of various contaminants.
3.2. Formation mechanism of sphere-shaped Dy2O3-SiO2 nanoparticles
Effective help to control the architecture of various nanostructures has been reported to be one of the beneficial applications of ultrasound waves. Desirable and distinctive nanoscale structures with great uniformity can be fabricated by the cavitation fabricated by the application of sonication [50]. According to the hot-spot theory, the conversion of huge structures into tiny particles can be easily facilitated and accelerated by creating very high temperatures as well as releasing enormous amounts of energy that occur during the collapse of bubbles [50]. So, as the above outcomes showed, tuning the ultrasonic radiation conditions can be advantageous in controlling the composite nanostructure architecture. Due to the adsorption of ultrasonic waves by water molecules, hydroxyl radical species are fabricated that can play an efficient role in the hydrolysis of dysprosium and silicon precursors and thus have a substantial contribution to the sonochemical fabrication of composite nanophotocatalyst (Dy2O3-SiO2) [51]. The possible mechanism for sonochemical creation of sphere-shaped Dy2O3-SiO2 nanoparticles can be summarized as follows [42], [52]:
| H2O + ultrasound waves → H. + OH. |
| OH. + H2N(CH2CH2NH)3CH2CH2NH2 + 2H2O → H3N+(CH2CH2NH)3CH2CH2N+H3 + 2OH– + by products |
| 3OH– + Dy(NO3)3 → 3NO3–+ Dy(OH)3 |
| Dy(OH)3 Dy2O3 |
| TEOS + H2O + OH– → Si-(OH)4 + by-product |
| 2Si-(OH)4 → SiO2 + by-product |
| Dy2O3 + SiO2 → Dy2O3/SiO2[42], [52] |
3.3. TEM, EDS, XRD, and FTIR studies
TEM was utilized for the sake of minutely probing the architecture and microstructure of the selected composite nanostructure (Fig. 3). The sonochemically fabricated oxide sample consists of approximately sphere-shaped particles with a particle size in the range of 20 to 60 nm. The elemental composition of the samples fabricated via sonochemistry in three different ultrasonic powers, samples 1, 4, and 5 was determined by EDS. In Fig. 4, only the signals of the elements oxygen, silicon, and dysprosium can be clearly seen, corroborating their presence and the purity of the nanostructures.
Fig. 3.
TEM images of binary Dy2O3-SiO2 nanocomposite (sample 5).
Fig. 4.

EDS patterns of samples 1 (a), 4 (b), and 5 (c).
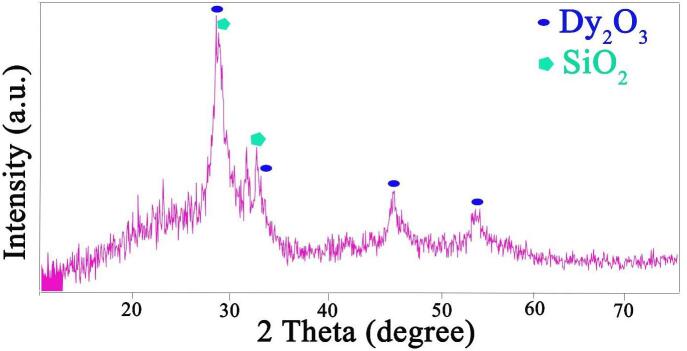
As an efficient and exact tool, XRD was employed to evaluate the crystal phase of the selected sample (Fig. 5). The characteristic diffraction lines of cubic Dy2O3 (JCPDS no. 01-074-1985) and tetragonal SiO2 (JCPDS no. 00-047-1300) can be found in Fig. 5, which manifests the fabrication of the binary Dy2O3-SiO2 nanocomposite with poor crystallization. The crystal size of the selected Dy2O3-SiO2 nanostructure was estimated to be about 17.4 nm employing the Scherrer formula [50]. No signal indicative of the impurity was observed in XRD profile, signifying the binary nanocomposite is pure.
Fig. 5.
XRD pattern of binary Dy2O3-SiO2 nanocomposite (sample 5).
The selected sample was also examined by FTIR to further corroborate the fabrication of composite nanophotocatalyst (Dy2O3-SiO2). Signals around 990, 916, and 650 cm−1 correspond to Si-O-Si bonds that manifest the creation of the SiO2 phase (see Fig. 6) [53], [54]. Characteristic signals of Dy2O3 are observed near 545 and 499 cm−1 [31]. The absorption signals at 3603, 3424, and 1632 cm−1 demonstrate the physisorbed water molecules [41]. Thus, FTIR findings are in line with XRD and EDS outcomes.
Fig. 6.
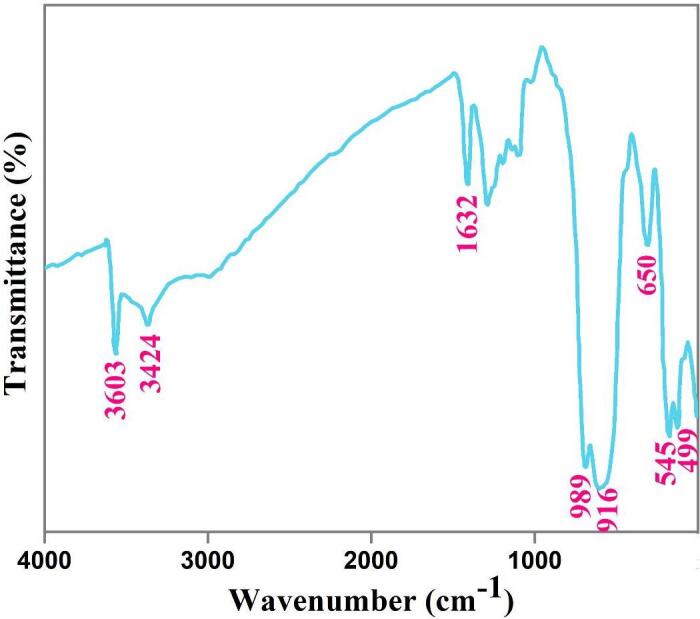
FT-IR spectrm of binary Dy2O3-SiO2 nanocomposite (sample 5).
3.4. Optical and textural properties of sphere-shaped Dy2O3-SiO2 nanoparticles
The optical absorption features of the selected composite nanostructure were explored by DRS (see Fig. 7). The binary Dy2O3-SiO2 nanocomposite exhibits an absorption edge at 367 nm. The energy gap of about 3.41 eV for the selected binary nanocomposite was determined employing DRS outcomes and Tauc’s formula [18]. The sonochemically fabricated binary Dy2O3-SiO2 nanocomposite has a narrower energy gap than the pure dysprosium oxide previously reported (4.8 eV) [35]. It can be concluded that the addition of silicon dioxide into Dy2O3 effectively tuned its energy gap, and the prepared binary Dy2O3-SiO2 nanocomposite could possibly manifest improved photocatalytic performance in removing contaminants.
Fig. 7.
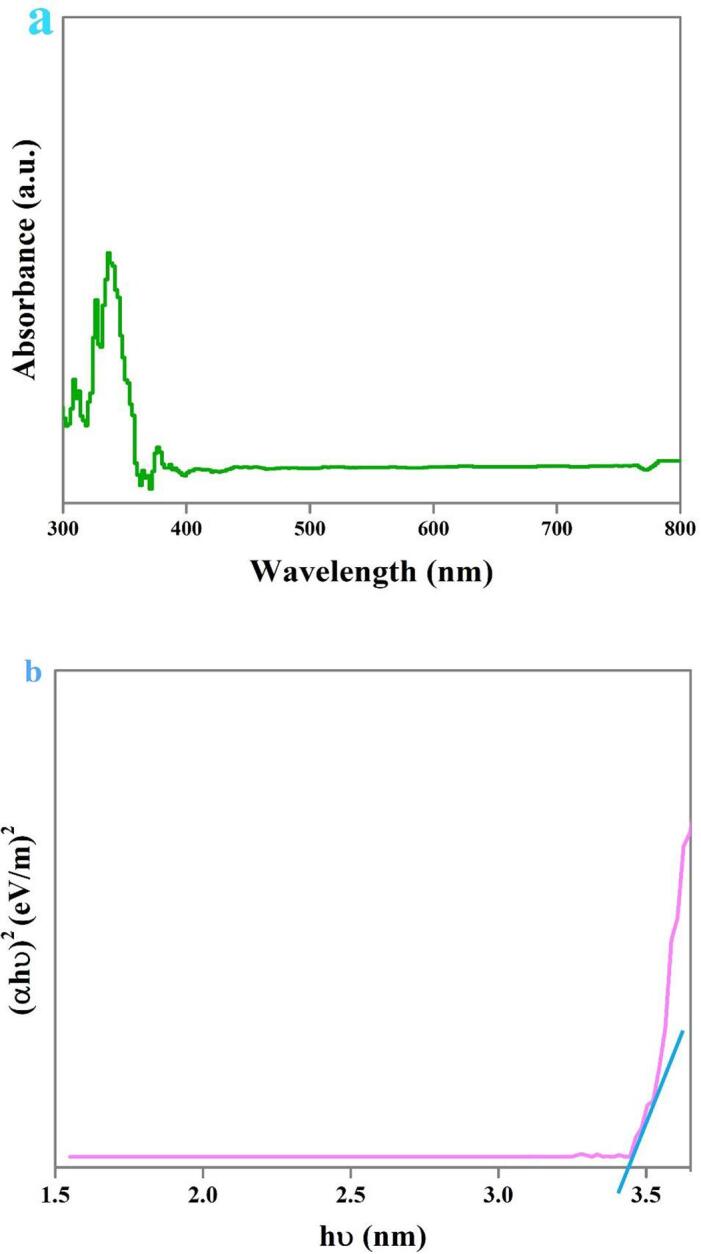
DRS spectrm (a), plot to determine the band gap (b) of binary Dy2O3-SiO2 nanocomposite (sample 5).
The textural features of the sonochemically fabricated binary Dy2O3-SiO2 nanocomposite were also examined, and the outcomes are presented in Fig. 8 and Table 2. It can be seen that the prepared binary nanocomposite sample is mesoporous in nature. The compounds with the great specific surface area can more efficiently adsorb contaminant molecules and also provide a large number of active sites for the decomposition of contaminant, resulting in excellent photocatalytic efficiency [55]. With a good specific surface area, it can be expected that binary nanocomposites can denote outstanding photocatalytic performance.
Fig. 8.

N2 adsorption/desorption isotherm (a) and pore size distribution curve (b) of binary Dy2O3-SiO2 nanocomposite (sample 5).
Table 2.
The textural properties of the binary Dy2O3-SiO2 nanocomposite (sample 5).
| Sample no | BET area (m2g−1) | Pore volume (cm3 g−1) | Pore diameter (nm) |
|---|---|---|---|
| 5 | 19.847 | 0.1285 | 25.904 |
3.5. Evaluation of the photocatalytic activity of binary Dy2O3-SiO2 nanocomposite
Due to the proper energy gap of the selected composite nanostructure, its photocatalytic performance was tested under ultraviolet light for photodecomposition of water contaminant. Several dyes including erythrosine, thymol blue, eriochrome black T, Acid Red 14, methyl orange, malachite green, and Rhodamine B were selected as target contaminants to test the performance of the binary Dy2O3-SiO2 nanocatalyst. In order to achieve the greatest efficiency in photocatalytic decomposition of each contaminant, the impact of variables including contaminant concentration and quantity of Dy2O3-SiO2 nanophotocatalyst was explored (see Fig. 9, Fig. 10). Solutions with different concentrations of 5, 10, and 15 ppm were employed as target contaminants. Various quantities of binary Dy2O3-SiO2 nanophotocatalyst, 0.015 and 0.03 g, were applied for photocatalytic testing. Decomposition of any contaminants was negligible without UV light or without the usage of binary Dy2O3-SiO2 nanocatalyst. Thus, the presence of both factors was necessary to decompose each of the pollutants. It can be seen that the studied variables, the concentration of the contaminant solution and the quantity of Dy2O3-SiO2 nanophotocatalyst, both significantly affect the percentage of decomposition. The effect of these variables for the percentage of decomposition of each contaminant is different from other contaminants. By increasing the quantity of binary Dy2O3-SiO2 nanocomposite from 0.015 g to 0.03, in the case of contaminants with a concentration of 5 ppm, the percentage of decomposition of all 4 contaminants was enhanced (Fig. 9, Fig. 10a). It can be observed in the case of contaminant with a concentration of 5 ppm, employing 0.03 g of binary Dy2O3-SiO2 nanocatalyst, 78.75, 71.43, 28.16, and 68.08% of thymol blue, methyl orange, Rhodamine B, and malachite green were decomposed after 120 min of UV exposure (Fig. 10a). In contrast, the percentage of decomposition of Acid Red 14, erythrosine, and eriochrome black T contaminant diminished to 51.04, 86.11, and 75.45 %. For contaminants with a concentration of 10 ppm, by altering the amount of binary Dy2O3-SiO2 nanocatalyst from 0.015 g to 0.03 g, the decomposition percentage of thymol blue, methyl orange, Rhodamine B, and malachite green enhanced to 81.70, 31.81, 20.48, and 61.98 (Fig. 9, Fig. 10b). In comparison, the percentage of decomposition of Acid Red 14, erythrosine, and eriochrome black T contaminant diminished to 25.64, 78.59, and 44.16%. In the case of contaminants with a concentration of 15 ppm, utilizing 0.015 g of binary Dy2O3-SiO2 nanocomposite, 73.97, 32.65, 92.05, 66.19, 29.42, 19.05, and 60% of thymol blue, Acid Red 14, erythrosine, eriochrome black T, methyl orange, Rhodamine B, and malachite green were removed after 120 min of UV exposure. Applying 0.03 g of the composite nanostructure, a lower percentage of decomposition was observed for all contaminants (Fig. 9, Fig. 10c). Based on the above outcomes, it can be concluded that the concentration of 5 ppm of contaminant, as well as the quantity of 0.015 g of Dy2O3-SiO2 nanocatalyst, is the most appropriate conditions to have the highest percentage of degradation of Acid Red 14, erythrosine, and eriochrome black T contaminants (Fig. 9a). In contrast, the highest percentage of decomposition of methyl orange, Rhodamine B, and malachite green occurred under optimal conditions, including a concentration of 5 ppm of contaminant and 0.03 g of Dy2O3-SiO2 nanocatalyst (Fig. 10a). In the case of thymol blue, conditions including 10 ppm of contaminant and 0.03 g of Dy2O3-SiO2 nanocomposite were more proper to achieve the highest percentage of decomposition (Fig. 10b). Under optimized conditions, the binary Dy2O3-SiO2 nanophotocatalyst exhibited superior efficiency toward the decomposition of the studied contaminants, and the highest percentage of erythrosine contaminant (about 92.99) was decomposed. The addition of silicon dioxide into Dy2O3 results in the formation of a binary Dy2O3-SiO2 nanocomposite with a good specific surface area that can be very beneficial for efficient absorption of light as well as enhancing the adsorption of target contaminant molecules. It also tunes its energy gap, which results in more separation of the charge carrier [56], [57]. For the above reasons, it seems that sonochemically prepared Dy2O3-SiO2 nanocatalyst manifest superior efficiency in the decomposition of pollutants. Fig. 11a–c exhibit UV–vis absorption spectra of erythrosine, eriochrome black T, and thymol blue with respect to time over the binary Dy2O3-SiO2 nanocomposite, which illustrates that the absorption intensity diminishes as illumination time enhances and; the decomposition of all three contaminant occurs continuously.
Fig. 9.

Photocatalytic degradation of various pollutants with different concentrations of 5 (a), 10 (b), and 15 (c) ppm in the presence of 0.015 g binary Dy2O3-SiO2 nanocomposite (sample 5), under UV light irradiation.
Fig. 10.

Photocatalytic degradation of various pollutants with different concentrations of 5 (a), 10 (b), and 15 (c) ppm in the presence of 0.03 g of binary Dy2O3-SiO2 nanocomposite (sample 5), under UV light irradiation.
Fig. 11.
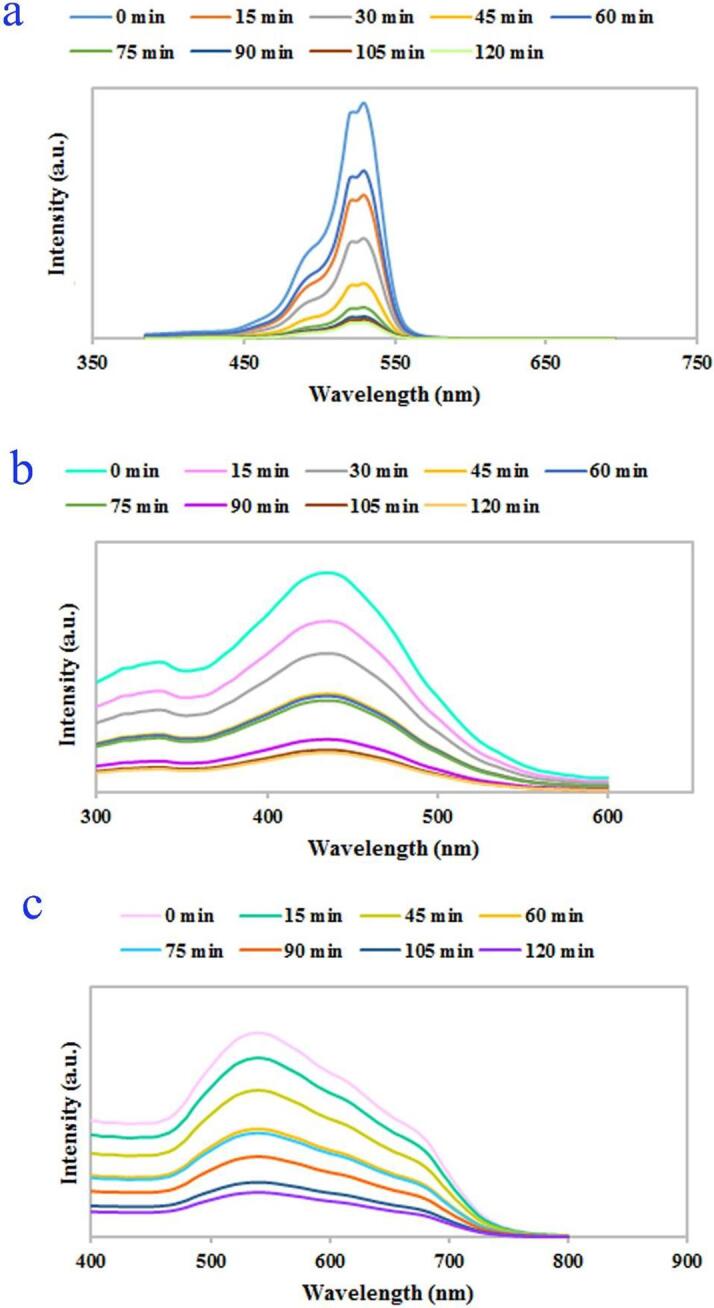
UV–vis absorption spectra depicting decomposition of (a) erythrosine (0.015 g of catalyst, concentration of 5 ppm of dye), (b) thymol blue (0.03 g of catalyst, concentration of 10 ppm of dye), and (c) eriochrome black T (0.015 g of catalyst, concentration of 5 ppm of dye) under UV light irradiation.
Since among the studied contaminants, even in optimal conditions, the lowest percentage of decomposition was observed for Rhodamine B contaminant, in order to achieve a greater percentage of degradation, higher quantities of Dy2O3-SiO2 nanophotocatalyst were employed to degrade the solution with a concentration of 5 ppm (see Fig. 12a). With altering the quantity of Dy2O3-SiO2 nanocomposite from 0.03 g to 0.05 g, an increment in Rhodamine B degradation from 28.16 to 65.07% was observed, and the percentage of Rhodamine B degradation almost doubled. A possible reason for the enhancement in Rhodamine B degradation with increasing nanocatalyst dose could be the increment in the number of active sites as well as the improvement in the adsorption of Rhodamine B molecules on the surface of Dy2O3-SiO2 nanocomposite [58], [59]. However, with a further enhancement in the quantity of Dy2O3-SiO2 nanocomposite to 0.07 g, a decrement in the degradation efficiency of the Rhodamine B was observed, and about 52.82% of the contaminant was degraded. It seems that with the addition in the amount of Dy2O3-SiO2 nanocomposite, owing to the accumulation and precipitation of nanocomposite particles, the light scattering inside the suspension is enhanced, and as a result, the degradation efficiency is diminished [60]. Also, the agglomeration of nanocomposite particles can reduce the number of active photocatalytic sites and be a possible reason for declining the percentage of Rhodamine B decomposition [60]. Thus, the appropriate quantity of Dy2O3-SiO2 nanocomposite for efficient decomposition of Rhodamine B is confirmed, 0.05 g, is confirmed.
Fig. 12.

Effects of the quantity of photocatalytic nanostructure (binary Dy2O3-SiO2 nanocomposite) as well as the duration of ultraviolet light on the photocatalytic degradation of Rhodamine B.
Also, to explore the effect of light irradiation time on the enhancement of degradation efficiency, a test was performed in a condition including 0.05 g of Dy2O3-SiO2 nanocomposite and 5 ppm of Rhodamine B for 165 min (see Fig. 12b). It was observed that by prolonging the UV exposure time, the percentage of Rhodamine B degradation could enhance from 54.77 to 61.53%.
The effects of various scavengers upon the photodecomposition of Rhodamine B by binary Dy2O3-SiO2 nanocomposite are illustrated in Fig. 13. Benzoic acid, EDTA, and p-benzoquinone were utilized to quench OH., h+, and O2–, correspondingly [18]. Without the presence of a scavenger, about 54.77% of Rhodamine B molecules were decomposed by the binary Dy2O3-SiO2 nanophotocatalyst. The addition of different scavengers, to varying degrees, prevented the photocatalytic decomposition of Rhodamine B molecules. P-benzoquinone had the least inhibitory effect on the decomposition of Rhodamine B molecules, because in its presence, 49.76% of Rhodamine B could be decomposed. The presence of benzoic acid and EDTA diminished the photocatalytic efficiency to 13.92% and 38.19%, correspondingly, signifying that OH. radicals are the most active degradative species of Rhodamine B molecules. Of course, holes are also involved in the photocatalytic decomposition of Rhodamine B to a lesser degree than hydroxyl radicals [18]. The feasible mechanism involved in the photodecomposition of Rhodamine B molecules is as below [18] (see Scheme 2):
Fig. 13.
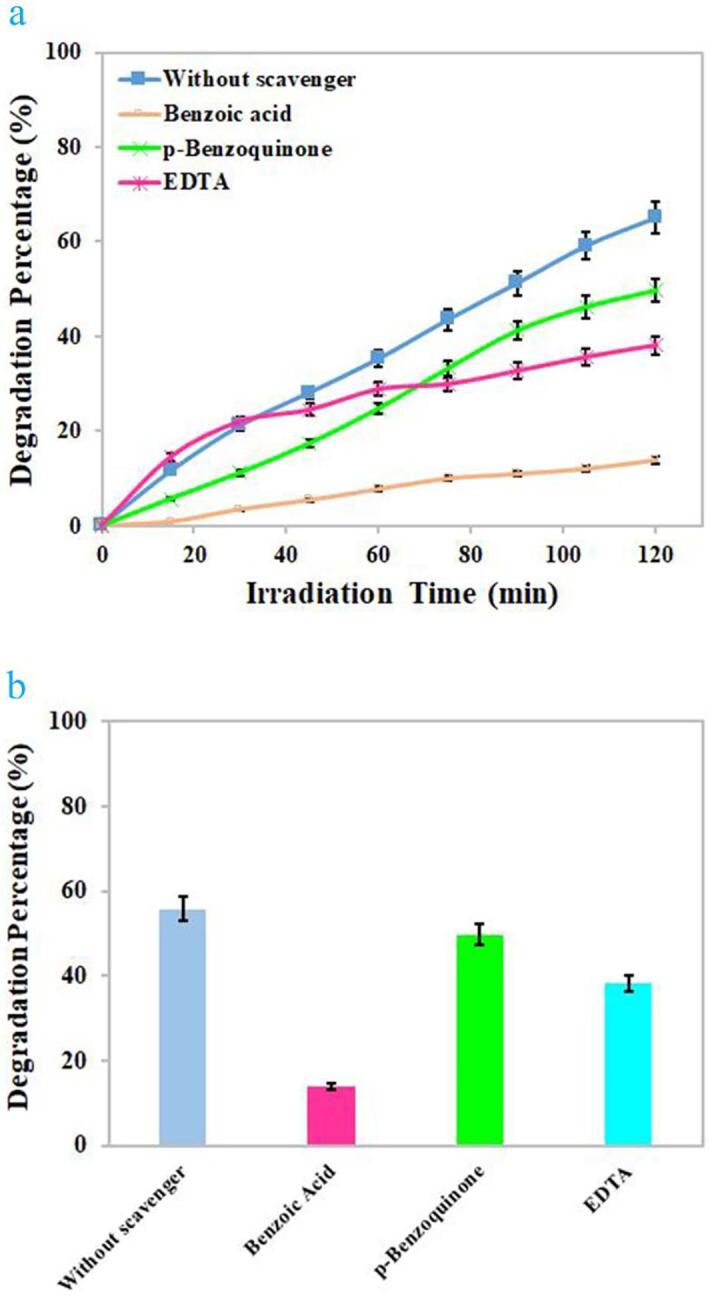
Radical trapping experiment of active species in the photocatalytic degradation of Rhodamine B over binary Dy2O3-SiO2 nanocomposite (sample 5).
Scheme 2.
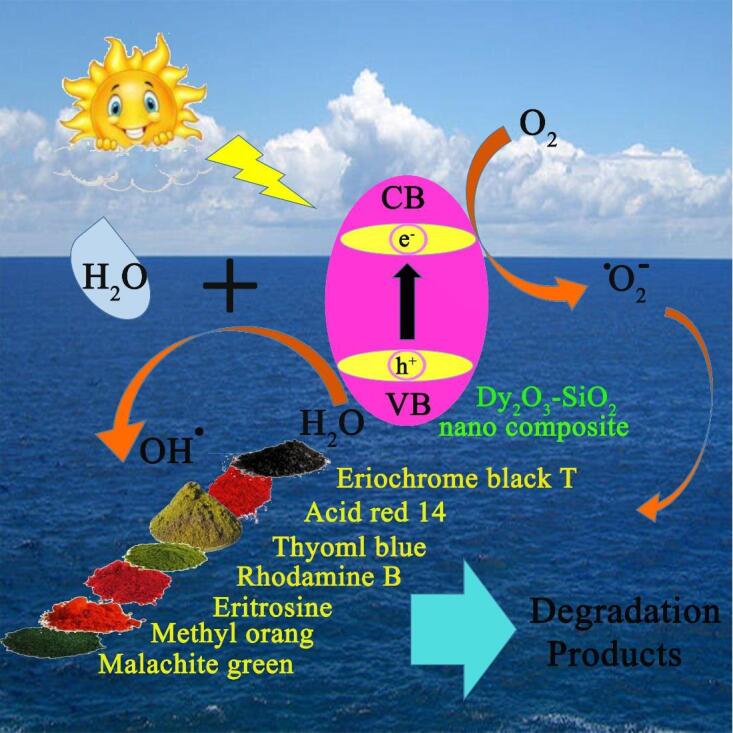
Schematic diagram of the mechanism for the photocatalytic decomposition of various pollutants over binary Dy2O3-SiO2 nanocomposite (sample 5).
Dy2O3-SiO2 nanophotocatalyst + hν → Dy2O3-SiO2 nanophotocatalyst* + e− + h+
| h+ + H2O → OH. + H+ |
| 2 h+ + 2H2O → H2O2 + 2H+ |
| H2O2 → 2OH. |
| e− + O2 → O2–. |
| O2–. + 2OH. + H+→ H2O2 + O2 |
| H2O2 → 2OH. |
| OH. + Rhodamine B molecules → Degradation products [18] |
The reusability of the binary Dy2O3-SiO2 nanocomposite (sample 5) was tested for ten cycles in the photodecomposition of Rhodamine B under UV illumination. It was noted that the photodecomposition efficiency diminishes, but was still about 49.5% after the repeated experiments (see Fig. 14).
Fig. 14.
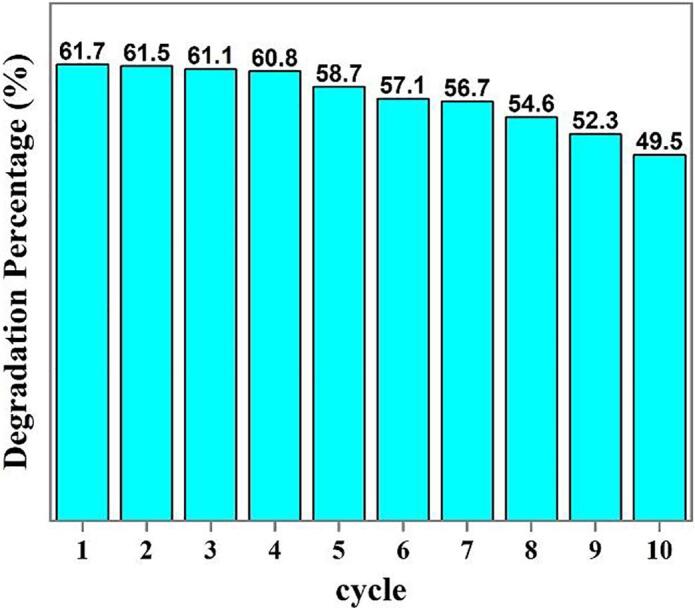
Reusability studies in the degradation of Rhodamine B over binary Dy2O3-SiO2 nanocomposite (sample 5) under UV illumination.
Table 3 exhibits the photocatalytic efficiency of different compounds for the decomposition of various contaminants under ultraviolet illumination. In this investigation, a new photocatalytic nanocomposite (Dy2O3-SiO2) with enhanced catalytic efficiency toward toxic contaminants was efficiently fabricated employing a basic agent, tetraethylenepentamine (Tetrene), through a simple and quick sonochemical approach. As observed in Table 3, the sonochemically fabricated Dy2O3-SiO2 nanocomposite can compete with other compounds as photocatalysts. We can nominate the porous Dy2O3-SiO2 nanocomposite as a new kind of high-performance nanocatalyst in the field of water remediation and environmental cleaning.
Table 3.
Comparison of photocatalytic efficiency for decomposition of various pollutants between Dy2O3-SiO2 nanocomposite (sample 5) with other compounds under ultraviolet illumination.
| Material | Pollutant | Photocatalytic efficiency (%) & duration of decomposition (min) | Reference |
|---|---|---|---|
| La2Sn2O7 nanoparticles | Erythrosine | 84 & 120 | [61] |
| TiO2-P25 | Methyl orange | 90.5 & 180 | [62] |
| TiO2 particles | Acid Red 14 | 88 & 150 | [63] |
| SnO2 nanoparticles | Eriochrome black T | 77 & 270 | [64] |
| CdS nanostructures | Malachite green | 42 & 60 | [65] |
| ZnO-CdO nanocomposite | Thymol blue | 46 & 120 | [66] |
| Dy2O3-SiO2 nanocomposite | Thymol blue | 81.7 & 120 | This work |
| Dy2O3-SiO2 nanocomposite | Malachite green | 71.08 & 120 | This work |
| Dy2O3-SiO2 nanocomposite | Eriochrome black T | 77.69 & 120 | This work |
| Dy2O3-SiO2 nanocomposite | Acid Red 14 | 62.2 & 120 | This work |
| Dy2O3-SiO2 nanocomposite | Methyl orange | 71.43 & 120 | This work |
| Dy2O3-SiO2 nanocomposite | Erythrosine | 92.99 & 120 | This work |
4. Conclusions
In summary, a new photocatalytic nanocomposite (Dy2O3-SiO2) with enhanced catalytic efficiency toward toxic contaminants was efficiently fabricated employing a basic agent, tetraethylenepentamine (Tetrene), through a simple and quick sonochemical approach. The features of the fabricated photocatalytic nanocomposite were examined employing a variety of microscopic and spectroscopic methods. According to the outcomes of morphological studies demonstrated, it was found that by properly tuning the sonication time and ultrasound power (10 min and 400 W), a porous nanocomposite composed of sphere-shaped nanoparticles with a narrow size distribution can be made. The optimal nanocomposite sample was tested as a nanostructured catalyst for the photodecomposition of several contaminants. The binary Dy2O3-SiO2 nanophotocatalyst demonstrated superior efficiency toward the decomposition of the studied contaminants, and the highest percentage of erythrosine contaminant (about 92.99) was decomposed. Optimization studies for the photocatalytic decomposition of each contaminant illustrated that the best performance could be achieved at a specific amount of contaminant and nanocatalyst. Trapping experiments illustrated that hydroxyl radicals were more effectively involved in the decomposition of contaminant molecules by Dy2O3-SiO2 nanophotocatalyst. The outcomes of this experimental work demonstrate that the addition of silicon dioxide into Dy2O3 and the sonochemically fabrication of porous Dy2O3-SiO2 nanocomposite brought a new kind of high-performance nanocatalyst in the field of water remediation and environmental cleaning.
CRediT authorship contribution statement
Kamran Mahdavi: Investigation, Methodology, Formal analysis, Software. Sahar Zinatloo-Ajabshir: Writing – original draft, Visualization, Writing – review & editing. Qahtan A. Yousif: Methodology, Data curation, Writing – review & editing. Masoud Salavati-Niasari: Writing – original draft, Writing – review & editing, Conceptualization, Supervision, Project administration, Visualization, Investigation, Methodology, Data curation, Validation, Resources.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are grateful to University of Bonab, the council of Iran National Science Foundation (97017837) and University of Kashan for supporting this work by Grant No (159271/KM1).
Contributor Information
Sahar Zinatloo-Ajabshir, Email: s.zinatloo@ubonab.ac.ir.
Masoud Salavati-Niasari, Email: salavati@kashanu.ac.ir.
References
- 1.Olagunju M.O., Zahran E.M., Reed J.M., Zeynaloo E., Shukla D., Cohn J.L., Surnar B., Dhar S., Bachas L.G., Knecht M.R. Halide Effects in BiVO4/BiOX Heterostructures Decorated with Pd Nanoparticles for Photocatalytic Degradation of Rhodamine B as a Model Organic Pollutant. ACS Appl. Nano Mater. 2021;4(3):3262–3272. [Google Scholar]
- 2.Soltaninejad V., Ahghari M.R., Taheri-Ledari R., Maleki A. Bifunctional PVA/ZnO/AgI/Chlorophyll Nanocomposite Film: Enhanced Photocatalytic Activity for Degradation of Pollutants and Antimicrobial Property under Visible-Light Irradiation. Langmuir. 2021;37(15):4700–4713. doi: 10.1021/acs.langmuir.1c00501. [DOI] [PubMed] [Google Scholar]
- 3.Khan M., Khan A., Khan H., Ali N., Sartaj S., Malik S., Ali N., Khan H., Shah S., Bilal M. Development and characterization of regenerable chitosan-coated nickel selenide nano-photocatalytic system for decontamination of toxic azo dyes. Int. J. Biol. Macromol. 2021;182:866–878. doi: 10.1016/j.ijbiomac.2021.03.192. [DOI] [PubMed] [Google Scholar]
- 4.Sajadi S.M., Kolo K., Pirouei M., Mahmud S.A., Ali J.A., Hamad S.M. Natural iron ore as a novel substrate for the biosynthesis of bioactive-stable ZnO@CuO@iron ore NCs: a magnetically recyclable and reusable superior nanocatalyst for the degradation of organic dyes, reduction of Cr(vi) and adsorption of crude oil aromatic compounds, including PAHs. RSC Adv. 2018;8:35557–35570. doi: 10.1039/c8ra06028b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honarmand M., Golmohammadi M., Naeimi A. Green synthesis of SnO2-bentonite nanocomposites for the efficient photodegradation of methylene blue and eriochrome black-T. Mater. Chem. Phys. 2020;241:122416. doi: 10.1016/j.matchemphys.2019.122416. [DOI] [Google Scholar]
- 6.Sadegh F., Politakos N., González De San Román E., Sanz O., Perez-Miqueo I., Moya S.E., Tomovska R. A green synthesis of nanocatalysts based on reduced graphene oxide/magnetic nanoparticles for the degradation of Acid Red 1. RSC Adv. 2020;10:38805–38817. doi: 10.1039/d0ra06311h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safavi A., Momeni S. Highly efficient degradation of azo dyes by palladium/hydroxyapatite/Fe3O4 nanocatalyst. J. Hazard. Mater. 2012;201–202:125–131. doi: 10.1016/j.jhazmat.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 8.Prado A.G.S., Costa L.L. Photocatalytic decouloration of malachite green dye by application of TiO2 nanotubes. J. Hazard. Mater. 2009;169:297–301. doi: 10.1016/j.jhazmat.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 9.Su S., Guo W., Leng Y., Yi C., Ma Z. Heterogeneous activation of Oxone by CoxFe3−xO4 nanocatalysts for degradation of rhodamine B. J. Hazard. Mater. 2013;244–245:736–742. doi: 10.1016/j.jhazmat.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Ji R., Liu J., Zhang T., Peng Y., Li Y., Chen D., Xu Q., Lu J. Construction of a ternary Z-scheme In2S3@Au@P3HT photocatalyst for the degradation of phenolic pollutants under visible light. Sep. Purif. Technol. 2021;272:118787. [Google Scholar]
- 11.Zheng Q., Lee H.-J., Lee J., Choi W., Park N.-B., Lee C. Electrochromic titania nanotube arrays for the enhanced photocatalytic degradation of phenol and pharmaceutical compounds. Chem. Eng. J. 2014;249:285–292. [Google Scholar]
- 12.Alshabib Muntathir, Onaizi Sagheer A. A review on phenolic wastewater remediation using homogeneous and heterogeneous enzymatic processes: Current status and potential challenges. Sep. Purif. Technol. 2019;219:186–207. [Google Scholar]
- 13.Zhang G., Chen D., Li N., Xu Q., Li H., He J., Lu J. Fabrication of Bi2MoO6/ZnO hierarchical heterostructures with enhanced visible-light photocatalytic activity. Appl. Catal. B. 2019;250:313–324. [Google Scholar]
- 14.Zinatloo-Ajabshir S., Mousavi-Kamazani M. Recent advances in nanostructured Sn−Ln mixed-metal oxides as sunlight-activated nanophotocatalyst for high-efficient removal of environmental pollutants. Ceram. Int. 2021;47:23702–23724. [Google Scholar]
- 15.Piao C., Chen L., Liu Z., Tang J., Liu Y., Lin Y., Fang D., Wang J. Construction of solar light-driven dual Z-scheme Bi2MoO6/Bi2WO6\AgI\Ag photocatalyst for enhanced simultaneous degradation and conversion of nitrogenous organic pollutants. Sep. Purif. Technol. 2021;274:119140. [Google Scholar]
- 16.Mahadadalkar Manjiri A., Gosavi Suresh W., Kale Bharat B. Interstitial charge transfer pathways in a TiO2/CdIn2S4 heterojunction photocatalyst for direct conversion of sunlight into fuel. J. Mater. Chem. A. 2018;6(33):16064–16073. [Google Scholar]
- 17.Chen Ping, Blaney Lee, Cagnetta Giovanni, Huang Jun, Wang Bin, Wang Yujue, Deng Shubo, Yu Gang. Degradation of Ofloxacin by Perylene Diimide Supramolecular Nanofiber Sunlight-Driven Photocatalysis. Environ. Sci. Technol. 2019;53(3):1564–1575. doi: 10.1021/acs.est.8b05827. [DOI] [PubMed] [Google Scholar]
- 18.Salavati-Niasari Masoud, Mir Noshin, Davar Fatemeh. Synthesis and characterization of NiO nanoclusters via thermal decomposition. Polyhedron. 2009;28(6):1111–1114. [Google Scholar]
- 19.Miao Fang, Wang Qianqian, Zhang Lai-Chang, Shen Baolong. Magnetically separable Z-scheme FeSiB metallic glass/g-C3N4 heterojunction photocatalyst with high degradation efficiency at universal pH conditions. Appl. Surf. Sci. 2021;540:148401. doi: 10.1016/j.apsusc.2020.148401. [DOI] [Google Scholar]
- 20.Liang Shun-Xing, Zhang Wenchang, Zhang Lina, Wang Weimin, Zhang Lai-Chang. Remediation of industrial contaminated water with arsenic and nitrate by mass-produced Fe-based metallic glass: Toward potential industrial applications. Sustainable Mater. Technol. 2019;22:e00126. doi: 10.1016/j.susmat.2019.e00126. [DOI] [Google Scholar]
- 21.Salavati-Niasari Masoud, Davar Fatemeh, Loghman-Estarki Mohammad Reza. Long chain polymer assisted synthesis of flower-like cadmium sulfide nanorods via hydrothermal process. J. Alloy. Compd. 2009;481(1-2):776–780. [Google Scholar]
- 22.Schneider Jenny, Matsuoka Masaya, Takeuchi Masato, Zhang Jinlong, Horiuchi Yu, Anpo Masakazu, Bahnemann Detlef W. Understanding TiO2 photocatalysis: mechanisms and materials. Chem. Rev. 2014;114(19):9919–9986. doi: 10.1021/cr5001892. [DOI] [PubMed] [Google Scholar]
- 23.Lee Kian Mun, Lai Chin Wei, Ngai Koh Sing, Juan Joon Ching. Recent developments of zinc oxide based photocatalyst in water treatment technology: a review. Water Res. 2016;88:428–448. doi: 10.1016/j.watres.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 24.Gogoi Debika, Makkar Priyanka, Ghosh Narendra Nath. Solar light-irradiated photocatalytic degradation of model dyes and industrial dyes by a magnetic CoFe2O4–gC3N4 S-scheme heterojunction photocatalyst. ACS Omega. 2021;6(7):4831–4841. doi: 10.1021/acsomega.0c05809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abu-Zied Bahaa M., Asiri Abdullah M. Synthesis of Dy2O3 nanoparticles via hydroxide precipitation: effect of calcination temperature. J. Rare Earths. 2014;32(3):259–264. [Google Scholar]
- 26.Sreethawong T., Chavadej S., Ngamsinlapasathian S., Yoshikawa S. A simple route utilizing surfactant-assisted templating sol–gel process for synthesis of mesoporous Dy2O3 nanocrystal. J. Colloid Interface Sci. 2006;300:219–224. doi: 10.1016/j.jcis.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 27.Pan T.-M., Chang W.-T., Chiu F.-C. Structural properties and electrical characteristics of high-k Dy2O3 gate dielectrics. Appl. Surf. Sci. 2011;257:3964–3968. [Google Scholar]
- 28.Tang M., Valdez J.A., Lu P., Gosnell G.E., Wetteland C.J., Sickafus K.E. A cubic-to-monoclinic structural transformation in the sesquioxide Dy2O3 induced by ion irradiation. J. Nucl. Mater. 2004;328:71–76. [Google Scholar]
- 29.Chandrasekhar M., Sunitha D.V., Dhananjaya N., Nagabhushana H., Sharma S.C., Nagabhushana B.M., Shivakumara C., Chakradhar R.P.S. Structural and phase dependent thermo and photoluminescent properties of Dy(OH)3 and Dy2O3 nanorods. Mater. Res. Bull. 2012;47:2085–2094. [Google Scholar]
- 30.Chandrasekhar M., Sunitha D.V., Dhananjaya N., Nagabhushana H., Sharma S.C., Nagabhushana B.M., Shivakumara C., Chakradhar R.P.S. Thermoluminescence response in gamma and UV irradiated Dy2O3 nanophosphor. J. Lumin. 2012;132:1798–1806. [Google Scholar]
- 31.Krishna Chandar N., Jayavel R. Wet chemical synthesis and characterization of pure and cerium doped Dy2O3 nanoparticles. J. Phys. Chem. Solids. 2012;73(9):1164–1169. [Google Scholar]
- 32.Kattel Krishna, Park Ja Young, Xu Wenlong, Kim Han Gyeol, Lee Eun Jung, Bony Badrul Alam, Heo Woo Choul, Jin Seonguk, Baeck Jong Su, Chang Yongmin, Kim Tae Jeong, Bae Ji Eun, Chae Kwon Seok, Lee Gang Ho. Paramagnetic dysprosium oxide nanoparticles and dysprosium hydroxide nanorods as T2 MRI contrast agents. Biomaterials. 2012;33(11):3254–3261. doi: 10.1016/j.biomaterials.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 33.He Beibei, Zhao Ling, Wang Wendong, Chen Fanglin, Xia Changrong. Electro-catalytic activity of Dy2O3 as a solid oxide fuel cell anode material. Electrochem. Commun. 2011;13(2):194–196. [Google Scholar]
- 34.Aung Y.L., Ikesue A., Yasuhara R., Iwamoto Y. Magneto-optical Dy2O3 ceramics with optical grade. Opt. Lett. 2020;45(16):4615. doi: 10.1364/OL.396397. [DOI] [PubMed] [Google Scholar]
- 35.Strehlow W.H., Cook E.L. Compilation of Energy Band Gaps in Elemental and Binary Compound Semiconductors and Insulators. J. Phys. Chem. Ref. Data. 1973;2(1):163–200. [Google Scholar]
- 36.Josephine G.A.S., Sivasamy A. Nanocrystalline ZnO Doped on Lanthanide Oxide Dy2O3: A Novel and UV Light Active Photocatalyst for Environmental Remediation. Environ. Sci. Technol. Lett. 2014;1:172–178. [Google Scholar]
- 37.Hussein G.A.M., Kroenke W.J., Goda B., Miyaji K. Formation of dysprosium oxide from the thermal decomposition of hydrated dysprosium acetate and oxalate Thermoanalytical and microscopic studies. J. Anal. Appl. Pyrol. 1997;39:35–51. [Google Scholar]
- 38.Hussein G.A.M., Korban H., Goda B., Miyaji K. Physicochemical characterization of the formation course of dysprosium oxide from hydrated dysprosium nitrate; thermoanalytical and microscopic studies. Colloids Surf., A. 1997;125(1):63–71. [Google Scholar]
- 39.Song X.C., Zheng Y.F., Wang Y. Selected-control synthesis of dysprosium hydroxide and oxide nanorods by adjusting hydrothermal temperature. Mater. Res. Bull. 2008;43:1106–1111. [Google Scholar]
- 40.Abkar E., Hassanpour M., Amiri O., Ghanbari M., Salavati-Niasari M. Photocatalytic and antibacterial activities of Tl–Hg–I nanocomposites: sonochemical synthesis and characterization. RSC Adv. 2021;11:22238–22249. doi: 10.1039/d1ra03666a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salavati-Niasari M. Nanoscale microreactor-encapsulation 14-membered nickel (II) hexamethyl tetraaza: synthesis, characterization and catalytic activity. J. Mol. Catal. A: Chem. 2005;229(1–2):159–164. [Google Scholar]
- 42.Zinatloo-Ajabshir S., Morassaei M.S., Salavati-Niasari M. Eco-friendly synthesis of Nd2Sn2O7–based nanostructure materials using grape juice as green fuel as photocatalyst for the degradation of erythrosine. Compos. B Eng. 2019;167:643–653. [Google Scholar]
- 43.Wu Y., Du X., Kou Y., Wang Y., Teng F. Mesoporous SiO2 nanostructure: Light-induced adsorption enhancement and its application in photocatalytic degradation of organic dye. Ceram. Int. 2019;45:24594–24600. [Google Scholar]
- 44.Wang W., Chen H., Fang J., Lai M. Large-scale preparation of rice-husk-derived mesoporous SiO2@TiO2 as efficient and promising photocatalysts for organic contaminants degradation. Appl. Surf. Sci. 2019;467–468:1187–1194. [Google Scholar]
- 45.Kendir E., Tekgül A., Küçük İ., Yaltkaya Ş. Structural, optical and magnetic properties of α-Fe2O3-SiO2 and Dy2O3-SiO2 composites produced by a Facile method. J. Electron. Mater. 2020;49:798–806. [Google Scholar]
- 46.Salehi Z., Zinatloo-Ajabshir S., Salavati-Niasari M. Dysprosium cerate nanostructures: facile synthesis, characterization, optical and photocatalytic properties. J. Rare Earths. 2017;35:805–812. [Google Scholar]
- 47.Nanda K., Maisels A., Kruis F., Fissan H., Stappert S. Higher surface energy of free nanoparticles. Phys. Rev. Lett. 2003;91:106102. doi: 10.1103/PhysRevLett.91.106102. [DOI] [PubMed] [Google Scholar]
- 48.Namvar F., Abass S.K., Soofivand F., Salavati-Niasari M., Moayedi H. Sonochemical synthesis of Pr6MoO12 nanostructures as an effective photocatalyst for waste-water treatment. Ultrason. Sonochem. 2019;58:104687. doi: 10.1016/j.ultsonch.2019.104687. [DOI] [PubMed] [Google Scholar]
- 49.Zinatloo-Ajabshir Sahar, Morassaei Maryam Sadat, Amiri Omid, Salavati-Niasari Masoud. Green synthesis of dysprosium stannate nanoparticles using Ficus carica extract as photocatalyst for the degradation of organic pollutants under visible irradiation. Ceram. Int. 2020;46(5):6095–6107. [Google Scholar]
- 50.Zinatloo-Ajabshir S., Baladi M., Salavati-Niasari M. Enhanced visible-light-driven photocatalytic performance for degradation of organic contaminants using PbWO4 nanostructure fabricated by a new, simple and green sonochemical approach. Ultrason. Sonochem. 2021;72:105420. doi: 10.1016/j.ultsonch.2020.105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad Krishnamurthy, Pinjari D.V., Pandit A.B., Mhaske S.T. Synthesis of zirconium dioxide by ultrasound assisted precipitation: effect of calcination temperature. Ultrason. Sonochem. 2011;18(5):1128–1137. doi: 10.1016/j.ultsonch.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Masjedi-Arani M., Salavati-Niasari M. Cd2SiO4/graphene nanocomposite: ultrasonic assisted synthesis, characterization and electrochemical hydrogen storage application. Ultrason. Sonochem. 2018;43:136–145. doi: 10.1016/j.ultsonch.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Innocenzi P., Falcaro P., Grosso D., Babonneau F. Order− disorder transitions and evolution of silica structure in self-assembled mesostructured silica films studied through FTIR spectroscopy. J. Phys. Chem. B. 2003;107:4711–4717. [Google Scholar]
- 54.Sun S., Li C., Sun Z., Wang J., Wang X., Ding H. In-situ design of efficient hydroxylated SiO2/g-C3N4 composite photocatalyst: Synergistic effect of compounding and surface hydroxylation. Chem. Eng. J. 2021;416:129107. [Google Scholar]
- 55.Ma M., Yang Y., Chen Y., Ma Y., Lyu P., Cui A., Huang W., Zhang Z., Li Y., Si F. Photocatalytic degradation of MB dye by the magnetically separable 3D flower-like Fe3O4/SiO2/MnO2/BiOBr-Bi photocatalyst. J. Alloy. Compd. 2021;861:158256. [Google Scholar]
- 56.Hu Shaozheng, Li Fayun, Fan Zhiping. Preparation of SiO2-coated TiO2 composite materials with enhanced photocatalytic activity under UV light. Bull. Korean Chem. Soc. 2012;33(6):1895–1899. [Google Scholar]
- 57.Arai Y., Tanaka K., Khlaifat A.L. Photocatalysis of SiO2-loaded TiO2. J. Mol. Catal. A: Chem. 2006;243(1):85–88. [Google Scholar]
- 58.Choina J., Kosslick H., Fischer Ch., Flechsig G.-U., Frunza L., Schulz A. Photocatalytic decomposition of pharmaceutical ibuprofen pollutions in water over titania catalyst. Appl. Catal. B. 2013;129:589–598. [Google Scholar]
- 59.Parida K.M., Parija S. Photocatalytic degradation of phenol under solar radiation using microwave irradiated zinc oxide. Sol. Energy. 2006;80(8):1048–1054. [Google Scholar]
- 60.Shubha J. Pranesh, Adil Syed F., Khan Mujeeb, Hatshan Mohammad R., Khan Aslam. Facile Fabrication of a ZnO/Eu2O3/NiO-Based Ternary Heterostructure Nanophotocatalyst and Its Application for the Degradation of Methylene Blue. ACS Omega. 2021;6(5):3866–3874. doi: 10.1021/acsomega.0c05670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ansari Fatemeh, Sobhani Azam, Salavati-Niasari Masoud. Green synthesis of magnetic chitosan nanocomposites by a new sol–gel auto-combustion method. J. Magn. Magn. Mater. 2016;410:27–33. [Google Scholar]
- 62.Guettaï N., Ait Amar H. Photocatalytic oxidation of methyl orange in presence of titanium dioxide in aqueous suspension. Part I: Parametric study. Desalination. 2005;185(1-3):427–437. [Google Scholar]
- 63.Daneshvar N., Salari D., Khataee A.R. Photocatalytic degradation of azo dye acid red 14 in water: investigation of the effect of operational parameters. J. Photochem. Photobiol., A. 2003;157(1):111–116. [Google Scholar]
- 64.Najjar M., Hosseini H.A., Masoudi A., Sabouri Z., Mostafapour A., Khatami M., Darroudi M. Green chemical approach for the synthesis of SnO2 nanoparticles and its application in photocatalytic degradation of Eriochrome Black T dye. Optik. 2021;242:167152. [Google Scholar]
- 65.Munyai S., Tetana Z.N., Mathipa M.M., Ntsendwana B., Hintsho-Mbita N.C. Green synthesis of Cadmium Sulphide nanoparticles for the photodegradation of Malachite green dye. Sulfisoxazole and removal of bacteria. Optik. 2021;247:167851. [Google Scholar]
- 66.Kumar S., Kaushik R.D., Purohit L.P. ZnO-CdO nanocomposites incorporated with graphene oxide nanosheets for efficient photocatalytic degradation of bisphenol A, thymol blue and ciprofloxacin. J. Hazard. Mater. 2022;424:127332. doi: 10.1016/j.jhazmat.2021.127332. [DOI] [PubMed] [Google Scholar]