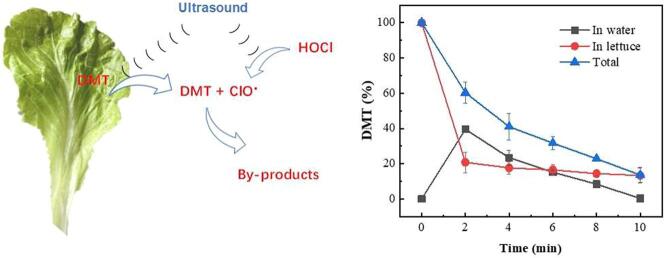
Graphical abstract
Keywords: Pesticide residue, Vegetables, FC/US process, Synergistic effect
Highlights
-
•
The selected pesticides were effectively removed from vegetables by FC/US process.
-
•
Synergistic effect was due to oxidation of pesticides in both vegetables and water.
-
•
High removal efficacy for five different vegetables verified universality of FC/US.
-
•
Slight reduction of chlorophyll content meant negligible damage to food quality.
-
•
FC/US is a promising AOP for pesticide removal from vegetables.
Abstract
Pesticide residue in vegetables has been considered as a serious food safety problem across the whole world. This study investigates a novel advanced oxidation process (AOP), namely the coupled free chlorine/ultrasound (FC/US) process for the removal of three typical pesticides from lettuce. The removal efficiencies of dimethoate (DMT), trichlorfon (TCF) and carbofuran (CBF) from lettuce reached 86.7%, 79.8% and 71.3%, respectively by the FC/US process. There existed a synergistic effect in the coupled FC/US process for pesticide removal and the synergistic factors reached 22.3%, 19.0% and 36.4% for DMT, TCF and CBF, respectively. Based on the analysis of mass balance of pesticides, the synergistic effect was probably attributed to the efficient oxidation of pesticides both in vegetables and in water by the generated free radicals and FC. The surface area and surface structure of vegetables strongly affected the removal of pesticides by FC/US. The removal efficiency of DMT increased from 80.9% to 88.1% as solution pH increased from 5.0 to 8.0, and then decreased to 84.1% when solution pH further increased to 9.0. When the ultrasonic frequency changed from 20 to 40 kHz, a remarkable improvement in pesticide removal by FC/US was observed. As the FC concentration increased from 0 to 15 mg L–l, the removal efficiencies of pesticides increased firstly, and then became stagnant when the FC concentration further increased to 25 mg L–l. The pesticide degradation pathways based on the identified intermediates were proposed. The total chlorophyll content was reduced by less than 5% after the FC/US process, indicating a negligible damage to the quality of vegetables. It suggests that the FC/US process is a promising AOP for pesticides removal from vegetables.
1. Introduction
Vegetables are one of the main sources of vitamins and minerals for human beings. A certain amount of fresh vegetables must be taken every day to maintain a balanced nutritional supply for the human body [1]. Because of the different eating habits, a proportion of vegetables is eaten raw or after minimally processing. However, pesticides are inevitably used during the growth of vegetables to increase production. According to the report by the Food and Agriculture Organization (FAO), the global agricultural pesticide use increased from 2.3 to 4.1 million tons from 1990 to 2018 [2]. The use of pesticides on such a large scale has led to the detection of various pesticide residues in vegetables around the world. For example, Qin et al. [3] made a survey on the contamination of vegetables with eleven pesticides in the Midwestern China and found out that the detection rates of pesticides in celery and cowpea were higher than 70 %. Diop et al. [4] investigated the impact of pesticide use practices on vegetable contamination in the Niayes zone of Dakar in Senegal, and reported that 65% of tomato samples, 71% of lettuce samples and 93% of cabbage samples contained at least one detectable pesticide residue. Therefore, the pesticide residue in vegetables has become a serious food safety problem in the whole world [5], [6], [7].
Ultrasound (US) is an acoustic wave with a frequency higher than 20 kHz. It has good directivity, strong reflectance and highly-concentrated energy. As a green technology, US process has good application in food cleaning industry [8], [9]. It can physically remove pesticides from the surface of vegetables through the cavitation effect. When the pressure of the sound waves propagated by the ultrasonic vibration in water reaches a certain level, the bubbles expand rapidly and then suddenly burst. At this moment, a shock wave is generated, causing great pressure. The tremendous pressure can break down the adhesive forces between pesticides and vegetables, causing the pesticides to disperse into water. Because US process does not need to add other cleaning agents and generally does not have a significant impact on the quality of vegetables, it has been considered as a clean and environment-friendly food processing technology [10], [11].
Although the removal efficiency of pesticide residues from vegetables and fruits by US is generally higher than that by the conventional water-washing (WW) process [12], [13], the removal efficiency varies significantly in different vegetables and fruits, leading to instability of the US process. For example, Zhou et al. [14] reported that the removal efficiencies of difenoconazole, azoxystrobin, thiamethoxam, abamectin and tebuconazole in grape were 82.0%, 72.1%, 85.9%, 100% and 88.4%, respectively by US process, while those in rape were only 49.2%, 59.8%, 14.7%, 55.7% and 27.1%, respectively under the same reaction conditions. It suggests that, due to the different surface properties of vegetables and fruits, the removal of pesticide residues by US alone is not always satisfactory [14]. In addition, the removal of pesticide residues by US is mainly achieved by transferring them into water, which will cause secondary pollution. Therefore, it is necessary to further improve the US process and one good way is to add free chlorine (FC) such as sodium hypochlorite (NaOCl), which is a common disinfectant for drinking water, to the US process to form the coupled free chlorine/ultrasound (FC/US) process [15]. As an advanced oxidation process (AOP), the FC/US process produces chlorine-contained free radicals via the sonolysis of FC (Eqs. (1), (2)) [16]. The generated free radicals can oxidize the pesticides not only in water but also in the surface of vegetables, which significantly improves the removal efficiency of pesticide residues. However, to the best of our knowledge, no studies on the removal of pesticide residues from vegetables by FC/US have been reported so far, and the reaction kinetics and mechanism still remain unknown. This study aims to fill this gap and investigate the removal of three pesticides, namely dimethoate (DMT), trichlorfon (TCF) and carbofuran (CBF) from lettuce by the coupled FC/US process. The reaction kinetics and mechanism for the removal of pesticide residues will be closely examined.
| (1) |
| (2) |
2. Materials and methods
2.1. Materials
Dimethoate (DMT, 99.2%), trichlorfon (TCF, 99.5%) and carbofuran (CBF,99.0%) were purchased from Dr. Ehrenstorfer, Germany. Sodium hypochlorite solution (Yongda Chemical Reagent Co., Ltd., Tianjin, China) was used as the source of FC. Methanol of high performance liquid chromatography (HPLC) grade (Aladdin, Shanghai, China) was used as the liquid phase for the detection of pesticides. Other chemicals of at least analytical grade were used without further purification. Fresh lettuce, spinach, celery, bean and tomato samples of the same size were purchased from the local market and stored in refrigerator at 4 °C before use.
2.2. Reaction conditions
All experiments were carried out in a conventional ultrasonic cleaner (SB25-12DTD, Scientz, China, 600 W) with two adjustable frequencies (20 and 40 KHz). The contaminated samples were prepared by soaking the vegetables into the dipping solution that contained DMT, TCF or CBF. Then the samples were naturally dried at room temperature of 22 ± 2 °C. The initial concentration of pesticides in vegetable samples was 3.5 ± 0.6 mg kg−1. In a typical run, 200 g contaminated vegetable sample was added to the bath of the ultrasonic cleaner that contained NaOCl solution with an FC concentration of 15 mg L–1. The frequency of the ultrasonic cleaner was kept at 40 KHz. The solution pH was 7.2 and was not controlled during the FC/US process. A sample was taken at a time interval of 2 min and was naturally dried in air at room temperature before the detection of pesticides.
2.3. Analytical methods
The extraction of pesticides was performed according to the standard method [17] with some modification. A sample (25.0 g) was accurately weighed and put into a homogenizer, and then 50.0 mL acetonitrile was added. After homogenizing for 2 min, the sample was filtered with 0.45 μm filter membrane and collected with a measuring cylinder. NaCl (5.0 g) was added to the cylinder. The sample was shaken violently for 1 min and kept at room temperature for 30 min to stratify the acetonitrile phase and water phase. The acetonitrile solution (2 mL) was filtered with 0.22 μm filter membrane for further analysis.
The concentration of pesticides was determined by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC/MS/MS, Xevo TQD, Waters, USA) [18], [19]. The injection volume was 10 μL. The mobile phase was a mixture of 0.2% formic acid in water (40%) and methanol (60%) at a flow rate of 0.3 mL min−1. The MS/MS detector was operated under the following conditions: positive electrospray ionization mode, cone gas flow rate 60 L h−1, capillary voltage 3.3 kV, cone voltage 16 V, desolvation temperature 500 °C, desolvation gas flow rate 500 L h−1. The quantitative precursor ion, product ion and collision energy for the three pesticides were listed in Table S1. The degradation intermediates were detected by a gas chromatography/mass spectrometry (GC/MS, ISQ QD, Thermo, USA). The injection volume was 3 μL with a split ratio of 10:1 and the injection temperature was 290 °C. The flow rate of carrier gas was set at 1 mL min−1. The programming for oven temperature was started at 60 °C and held for 4 min, and then ramped at 15 °C min−1 to 350 °C. The MS was operated in scan mode from 50 to 350 (m/z). Total organic carbon (TOC) was determined by a TOC analyzer (HTY-CT1000B, TAILIN Bioengineering Co., Ltd., Zhejiang Province, China). The concentration of FC was analyzed by a spectrophotometric method according to the Water Analysis Handbook by Hach [20].
The total chlorophyll, including chlorophyll a and chlorophyll b, was analyzed with a standard method published by the Ministry of Agriculture of China (NY/T 3082–2017) with some modification [13]. The crushed lettuce sample (0.50 g) was put in a conical flask and then 100 mL mixed solution of ethanol (50%) and acetone (50%) was added. The conical flask was sealed and placed in dark for 5 h. After filtration of the sample, the absorbance was measured at 663 nm and 645 nm by a spectrophotometer (UV-2600i, Shimadzu, Japan). The concentration of total chlorophyll was determined by Eq. (3):
| (3) |
Where ω is the concentration of total chlorophyll (mg kg−1); A1 and A2 are the absorbance of the sample at 663 nm and 645 nm, respectively; V (mL) is the volume of the sample and m (kg) is the weight of the sample.
3. Results and discussion
3.1. Pesticide removal by FC, US and FC/US
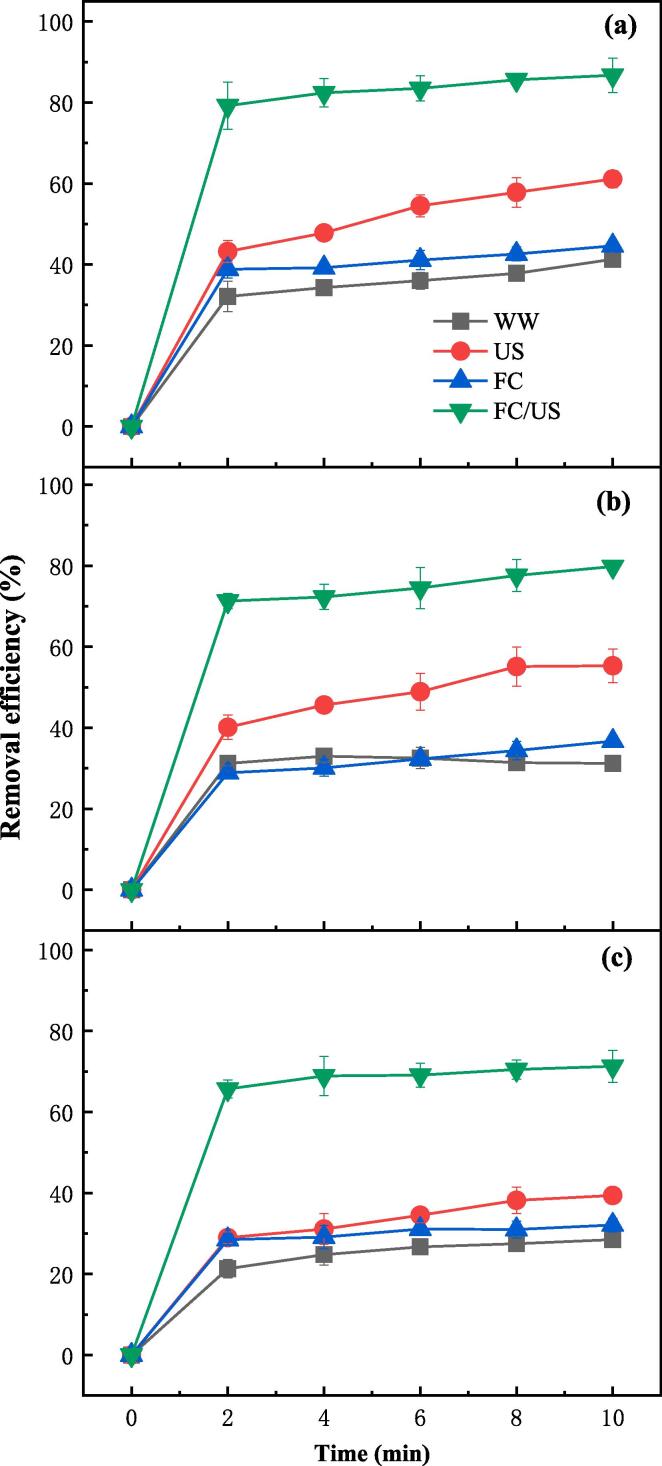
Washing with water is always recommended before eating vegetables since it is the easiest way to remove pesticide residues [21]. This was confirmed by the experimental results of removing pesticides from lettuce by the WW process. As Fig. 1 shows, the removal efficiencies of DMT, TCF and CBF from lettuce reached 41.3%, 31.2% and 28.5%, respectively, by the WW process. The variation of removal efficiencies among the pesticides was probably attributed to the different adhesive forces between pesticides and lettuce [22]. The different solubility of pesticides in water might be another important reason. Specifically, DMT had the highest removal efficiency in WW process for its high water solubility of 25 g L–1, while CBF had the lowest one for its low water solubility of 0.7 g L–1. Compared with the WW process, the removal efficiencies of DMT, TCF and CBF increased slightly to 44.6%, 36.7% and 32.1%, respectively by the FC process. This could be explained by the reason that FC oxidized not only the pesticides in lettuce but also the pesticides in water that were transferred from lettuce, which could reduce the mass transfer resistance and promote the removal of pesticides from lettuce. Comparatively, the removal efficiencies of DMT, TCF and CBF from lettuce increased significantly to 61.1%, 55.3% and 39.4%, respectively by the US process. This is because of the cavitation effect, which broke down the adhesive forces between pesticides and vegetables causing the pesticides to transfer into water. Surprisingly, when the contaminated lettuce was treated with the coupled FC/US process, the removal efficiencies of DMT, TCF and CBF increased dramatically to 86.7%, 79.8% and 71.3%, respectively. Compared with the WW process, the removal efficiencies of DMT, TCF and CBF by FC/US increased by 45.4%, 48.6% and 42.8%, respectively. The increments by the coupled FC/US process were actually higher than the sum of those by the separate FC and US processes. It suggests that there existed a synergistic effect in the FC/US process for pesticide removal. To quantitatively evaluate this synergistic effect, synergistic factor was defined as shown in Eq. (4). The pesticide removal efficiency by WW was used as blank control. After subtracting the blank control, the increments of pesticide removal efficiencies by FC, US and FC/US were obtained. Then the synergistic factor was determined by subtracting the sum of the increments of removal efficiencies by FC and US from that by FC/US. The results indicate that the synergistic factor reached 22.3%,19.0% and 36.4% for DMT, TCF and CBF, respectively, manifesting a significant synergistic improvement of the FC/US process for pesticide removal from lettuce. The synergistic effect can be explained by two reasons. On one hand, FC not only degraded the pesticides in water that were transferred from lettuce by US, which promoted the removal of pesticides via the reduction of mass transfer resistance, but FC could also penetrate into the surface of lettuce with the aid of US and degrade the pesticides therein [13]. On the other hand, the coupled FC/US process produced chlorine-contained radicals with strong oxidation capacity (Eqs. (1), (2)), which could effectively oxidize the pesticides in water as well as in lettuce.
| (4) |
Where RWW, RFC, RUS and RFC/US are the removal efficiencies of pesticides by WW, FC, US and FC/US processes.
Fig. 1.
Removal of DMT (a), TCF (b) and CBF (c) from lettuce by WW, FC, US and FC/US processes ([FC]0 = 15 mg L–1, pH = 7.2, ultrasonic frequency = 40 kHz).
3.2. Mass balance of DMT in FC, US and FC/US processes
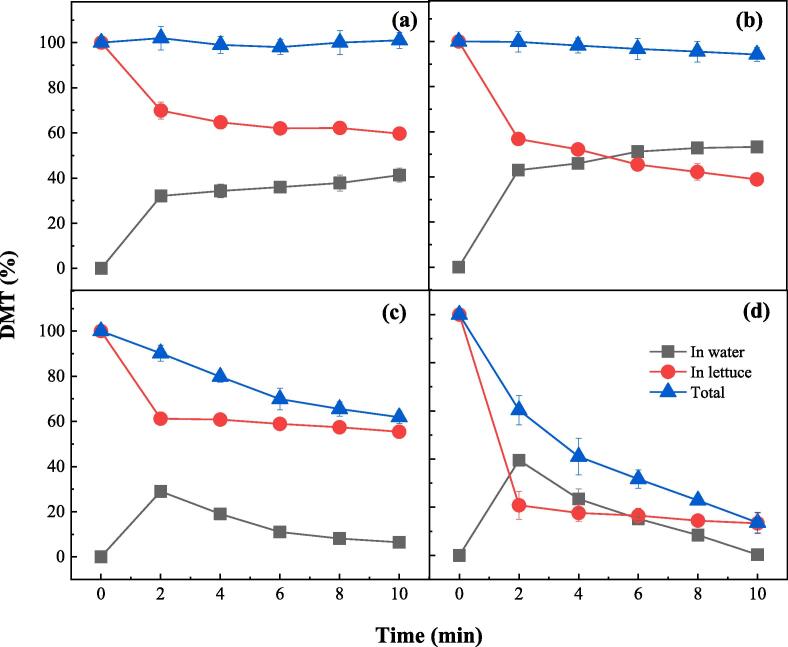
As discussed above, the synergistic effect observed in FC/US process might be caused by the efficient oxidation of pesticides both in lettuce and in water. Therefore, evaluation of the mass balance provides a good way to study the mechanism of the synergistic effect in FC/US process. As Fig. 2a shows, the total amount of DMT in lettuce and water remains constant of 100% during the WW process. Therefore, the DMT removed from lettuce in WW process was completely transferred into water. Similar behaviors for the mass balance of DMT were also observed in US process (Fig. 2b). The total amount of DMT decreased only slightly from 100% to 94.2%, while the amount of DMT in lettuce decreased dramatically from 100% to 38.9%. Therefore, the DMT removed from lettuce was also mainly transferred into water in the US process, and the only difference, compared with WW process, was that US accelerated the detaching of DMT from the lettuce via the cavitation effect. The slight decrease of total DMT was caused by two reasons. One reason is that the energy released in the hot spots was high enough to directly break the covalent bond of DMT molecules [23]; the other reason is that DMT was indirectly oxidized by the hydroxyl radicals (HO•) generated by sonolysis of water (Eq. (5)) [24], [25]. In FC process, however, the total amount of DMT decreased significantly from 100% to 61.9%. This is because DMT both in lettuce and in water could be oxidized by FC. It is further verified by the behavior of DMT in water, the amount of which firstly increased from 0% to 29.0% and then decreased to 6.5% after 10 min reaction in the FC process. The oxidation of DMT also accounted for the higher removal efficiency of DMT in FC process than that in WW process. In FC/US process, the total amount of DMT decreased dramatically from 100% to 13.6%, which means 86.4% of DMT in lettuce was actually oxidized. It is also verified by the behavior of DMT in water, the amount of which firstly reached as high as 39.5% and then decreased to 0% after 10 min. Therefore, the synergistic effect observed in FC/US process was mainly caused by the effective oxidation of DMT both in lettuce and in water instead of by physically transferring it into water. Not only was DMT degraded, but it was partially mineralized as well. Fig. S1 shows that 45.3% of TOC was removed by FC/US process, while only 11.6% of TOC was removed by FC and no TOC was removed by US or WW process. It shows another merit for the FC/US process that the DMT removed from lettuce was completely oxidized in water, which is environment-friendly, while in WW or US process, the DMT removed from lettuce was still in water, which needs further treatment or otherwise may cause secondary contamination.
| (5) |
Fig. 2.
Mass balance of DMT during the WW (a), US (b), FC (c) and FC/US (d) processes ([FC]0 = 15 mg L–1, pH = 7.2, ultrasonic frequency = 40 kHz).
3.3. Removal of pesticides from different vegetables by FC/US
To examine the universality of the FC/US process, four other vegetables, namely spinach, celery, bean and tomato were also chosen as the targets for pesticide removal. As Table 1 shows, the removal efficiencies and synergistic factors were different among the pesticides and vegetables. Generally, DMT had the highest removal efficiency from the selected vegetables by FC/US, while CBF had the lowest removal efficiency. It indicates that the property of different pesticides strongly affected their removal from the vegetables. For example, DMT had the highest removal efficiency for the reason that it had the highest value of water solubility to be detached from vegetables and had the fastest degradation rate in water (Fig. S2). However, the synergistic factors just turned the other way around, with those of CBF being the highest and those of DMT being the lowest. Although CBF had relatively low removal efficiencies in the FC/US process, it had even lower removal efficiencies in the separate FC and US processes, which would result in high synergistic factors according to Eq. (4). As for the vegetables, the removal efficiencies for all pesticides increased in the order of spinach < lettuce < celery < bean < tomato. This can be explained by the different surface properties of the vegetables. The specific surface area of the vegetables had a reverse order of spinach > lettuce > celery > bean > tomato. Pesticides could easily penetrate into the rough structure of vegetables with large surface area, while it was difficult for US to penetrate into these vegetables because US would lose energy while passing through any obstacles. Moreover, adhesive forces between the pesticides and vegetables also increased with large surface and small contact angle [26], [27]. Therefore, it was more difficult for pesticides to get detached from vegetables with larger surface area, resulting in the lower removal efficiency of pesticides from these vegetables. This is in good agreement with the study by Cengiz et al. [2] who reported that tomato had a higher pesticide removal percentage than that of lettuce that has a larger surface area.
Table 1.
Removal efficiencies and synergistic factors of DMT, TCF and CBF for the five different vegetables in the FC/US process.
| Vegetables | DMT |
TCF |
CBF |
|||
|---|---|---|---|---|---|---|
| Removal efficiency | Synergistic factor | Removal efficiency | Synergistic factor | Removal efficiency | Synergistic factor | |
| Spinach | 74.7% | 25.5% | 72.3% | 25.6% | 70.2% | 30.3% |
| Lettuce | 86.7% | 22.3% | 79.8% | 19.0% | 71.3% | 36.4% |
| Celery | 88.5% | 26.8% | 82.2% | 17.6% | 78.8% | 39.8% |
| Bean | 90.4% | 30.5% | 86.1% | 29.6% | 80.4% | 32.5% |
| Tomato | 93.2% | 24.3% | 86.4% | 24.0% | 81.0% | 33.5% |
The synergistic factors of the vegetables, however, had no obvious order as shown in Table 1, and it suggests that the synergistic factor mainly depended on the properties of pesticides, as discussed above, instead of the vegetables. Overall, the high removal efficiencies ranging from 70.2% to 93.2% for all pesticides and vegetables as well as the high synergistic factors of over 17% indicated that the FC/US process had good universality in pesticides and vegetables.
3.4. Effect of solution pH
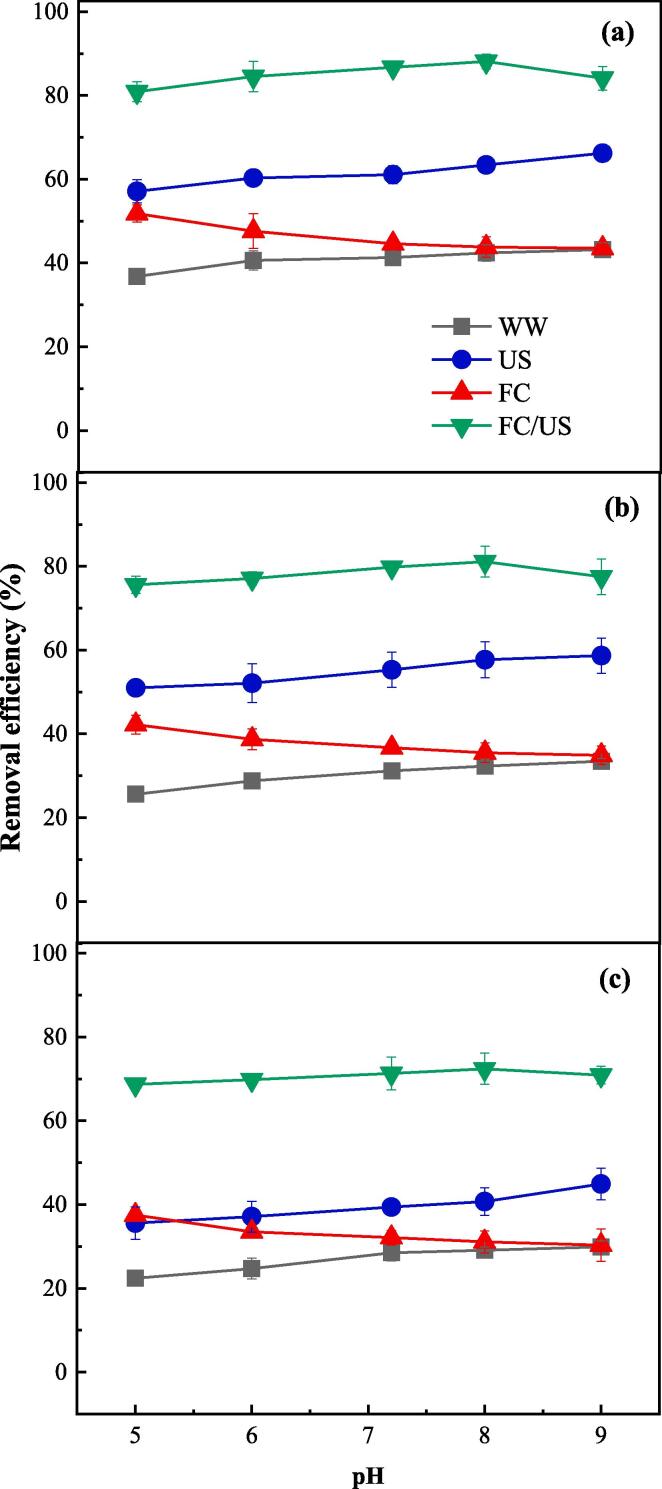
Solution pH is an important factor in the FC/US process, primarily affecting the redox potential of the reaction system. Fig. 3a shows that the removal efficiency of DMT from lettuce increased from 36.8% to 43.2% by WW after 10 min as the solution pH increased from 5.0 to 9.0. Since DMT has pKa1 value of −0.44 and pKa2 value of 16.6 (Fig. S3), it would remain unchanged in its neutral form during the variation of pH from 5.0 to 9.0. Therefore, the increasing of removal efficiency might be due to the reduction of adhesive forces between pesticides and vegetables in alkaline conditions. This could also account for the behaviors of DMT in the US process, the removal efficiency of which increased from 57.1% to 66.2% as pH increased from 5.0 to 9.0 (Fig. 3a). However, in the FC process, the removal efficiency decreased from 51.8% to 43.5% as pH increased from 5.0 to 9.0. Besides the physical removal of DMT like the cases in WW and US processes, chemical oxidation of DMT also played a role in the FC process. Since Eq. (6) has pKa value of 7.5, HClO dominated in the acidic conditions while ClO– dominated in the alkaline conditions. Because HClO has a much higher redox potential (1.48 V) than that of ClO– (0.81 V), the removal efficiency of DMT would consequently be higher in the acidic conditions where DMT could be oxidized more easily. In the FC/US process, however, the trend for removal efficiency of DMT as a function of pH behaved different. The removal efficiency of DMT by FC/US increased from 80.9% to 88.1% as pH increased from 5.0 to 8.0, and then decreased to 84.1% when pH further increased to 9.0. This is probably because of the two opposite influential factors, namely the rising trend of removal efficiency vs. pH for the US process and the declining trend of removal efficiency vs. pH for the FC process. The US process might overwhelm the FC process in the acidic conditions, while in alkaline conditions, the US process was overwhelmed by the FC process. Therefore, DMT removal by the coupled FC/US process was inhibited in either the acidic or the alkaline conditions. The net results came out that neutral condition with the solution pH around the pKa of Eq. (6) was optimal for DMT removal. Since the natural solution pH in this study was around 7.2, it could save much cost for adjusting the solution pH to get the optimal results. The trends for the removal of TCF and CBF from lettuce (Fig. 3b and c) were similar with that of DMT, which suggests that the solution pH was independent of the pesticides treated in the FC/US process.
Fig. 3.
Effect of solution pH on the removal of DMT (a), TCF(b) and CBF (c) from lettuce by WW, FC, US and FC/US processes ([FC]0 = 15 mg L–1, ultrasonic frequency = 40 kHz).
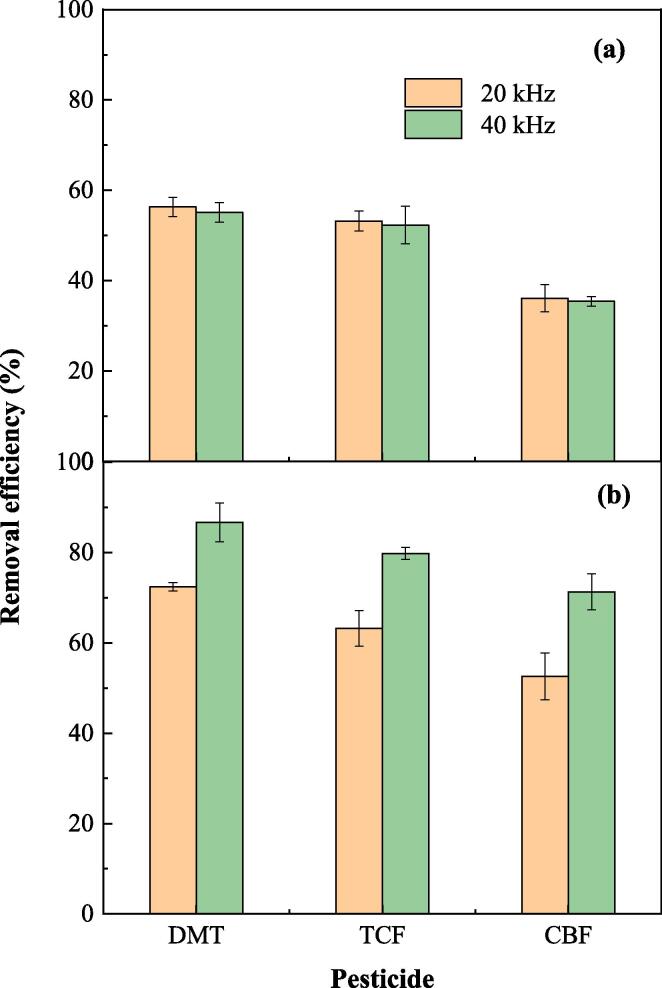
3.5. Effect of ultrasonic frequency
Commercial ultrasonic cleaner generally has two adjustable frequencies, and their influence on the pesticide removal by US and FC/US processes is shown in Fig. 4. When the ultrasonic frequency increased from 20 to 40 kHz, the removal efficiencies of DMT, TCF and CBF decreased slightly from 56.3%, 53.2% and 36.1% to 55.1%, 52.3% and 35.4%, respectively in the US process. This is in agreement with the study by Zhu et al. [28] who reported that the removal efficiencies of chlorothalonil, pyrazophos, and carbendazim from pakchoi decreased with the increasing of ultrasonic frequency in the US process. This is because the bubbles generated by low-frequency US had higher energy than those generated by high-frequency US, although low-frequency US generated less bubbles than did high-frequency US [1], [29]. However, increasing ultrasonic frequency in FC/US process promoted the removal of pesticides significantly. The removal efficiencies of DMT, TCF and CBF increased from 72.4%, 63.2% and 52.6% to 86.7%, 79.8% and 71.3%, respectively in FC/US process (Fig. 4b). A significant improvement (>13%) of pesticide removal efficiency was achieved by the FC/US process at 40 kHz compared with that of 20 kHz. The reason might be that increasing ultrasonic frequency promoted the chemical oxidation of pesticides in FC/US process via the acceleration of free radical production (Eqs. (1), (2)), as was reported that the rate of free radical production was positively related to the ultrasonic frequency [13], [30], [31].
Fig. 4.
Effect of ultrasonic frequency on the pesticide removal from lettuce by US (a) and FC/US (b) processes ([FC]0 = 15 mg L–1, pH = 7.2).
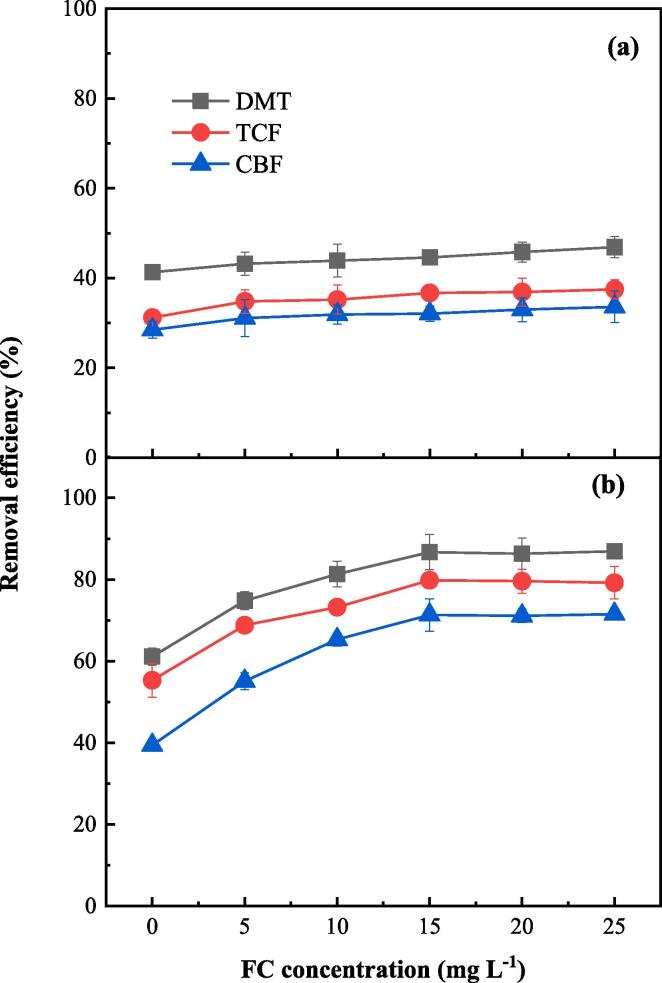
3.6. Effect of FC concentration
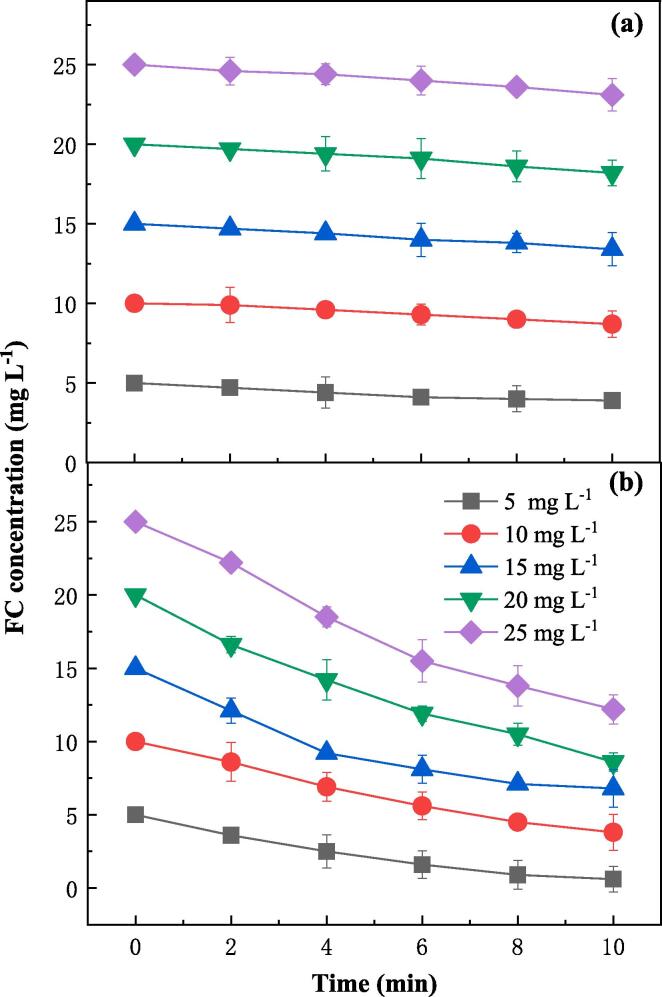
FC is a strong oxidant and is usually used as a disinfectant for drinking water. The influence of FC concentration on pesticide removal from lettuce by FC is shown in Fig. 5a. As FC concentration increased from 0 to 25 mg L–1, the removal efficiencies of DMT, TCF and CBF increased from 41.3%, 31.2% and 28.5% to 46.9%, 37.5% and 33.6%, respectively in the FC process. As chemical oxidation played a role in pesticide removal by FC process, there was a monotonous increase of removal efficiency with the increasing of FC concentration. It is worthwhile to note that less than 7% improvement of pesticide removal was achieved with an FC concentration of 25 mg L–l in the FC process compared with WW process. It suggests that the utilization rate of FC should be very low. To confirm this, the real-time concentration of FC was monitored during the FC process. Fig. 6a shows that the concentration of FC only decreased slightly after 10 min reaction, indicating the relatively low efficacy of the FC process. However, in the FC/US process, the improvement of pesticide removal was much more significant (Fig. 5b). The removal efficiencies of DMT, TCF and CBF from lettuce increased from 61.1%, 55.3% and 39.4% to 86.7%, 79.8% and 71.3%, respectively in the FC/US process as the initial concentration of FC increased from 0 to 15 mg L–l, and then the removal efficiencies became stagnant when the initial concentration of FC further increased to 25 mg L–l. A significant improvement of over 24% for all pesticides was achieved at an optimal FC concentration of 15 mg L–l. The high efficacy of the coupled FC/US process was probably attributed to the strong oxidation capacity of the chlorine-contained radicals generated by sonolysis of FC (Eqs. (1), (2)). In contrast with the slightly changing of FC concentration in FC process (Fig. 6a), the FC concentration decreased dramatically in FC/US process (Fig. 6b), confirming the high oxidative rates of pesticides. The stagnation of the removal efficiency with FC concentration higher than 15 mg L–l was due to the complete degradation of pesticides in water (Fig. 2d), and increasing the FC concentration would not further improve the removal efficiency of pesticides. It demonstrates that the FC/US process had a high utilization rate of FC and therefore a high efficacy for pesticide removal.
Fig. 5.
Effect of FC concentration on pesticide removal from lettuce by FC (a) and FC/US (b) processes (pH = 7.2, ultrasonic frequency = 40 kHz).
Fig. 6.
Changing of FC concentration during the pesticide removal from lettuce by FC (a) and FC/US (b) processes (pH = 7.2, ultrasonic frequency = 40 kHz).
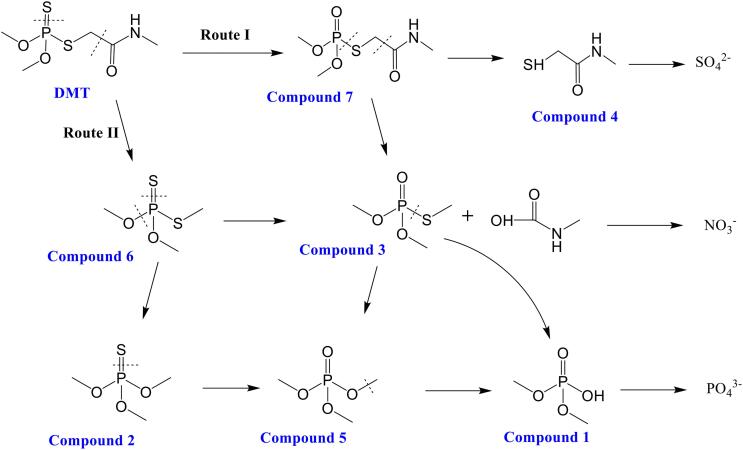
3.7. Pesticide degradation intermediates and pathways
Table 2 shows the intermediates identified by GC/MS during the degradation of DMT by FC/US. Possible pathways based on the identified intermediates were proposed as shown in Fig. 7. There were two main routes for the decomposition of DMT by FC/US. The first route was via the attack of S=P bond by ClO• to form Omethoate (Compound 7) [32], which was then oxidized to N-methyl mercaptoacetamide (Compound 4) that could be mineralized to SO42–. Omethoate could also be broken up to form O,O,S-trimethyl phosphorothioate (Compound 3) and methylcarbamic acid, which was likely an intermediate to form NO3– [33]. Compound 3 was an important intermediate and was also observed by Hu et al. [34] and Wu et al. [35] during the DMT degradation. Compound 3 could be directly oxidized to dimethyl hydrogen phosphate (Compound 1) or indirectly oxidized to Compound 1 via the formation of O,O,O-trimethyl phosphate (Compound 5). When the two other O-P bonds of Compound 1 were further hydroxylated, it was mineralized to form PO43–. The second route for the decomposition of DMT was via the formation of O,O,S-trimethyl dithiophosphate (Compound 6). The S=P bond of Compound 6 was then desulfurized to Compound 3 [32], or the S-P bond of Compound 6 was broken up to form O,O,O-trimethyl phosphorothioate (Compound 2), which could transform to Compound 5 and then again to Compound 1.
Table 2.
Intermediates identified by GC/MS during the DMT degradation.
| Compound No. | Compound name | Retention time (min) | Characteristic ions (m/z) |
|---|---|---|---|
| 1 | Dimethyl hydrogen phosphate | 7.93 | 126, 113, 85,58 |
| 2 | O,O,O-trimethyl phosphorothioate | 9.82 | 156,126,109,93 |
| 3 | O,O,S-trimethyl phosphorothioate | 10.6 | 156,126,110,47 |
| 4 | N-methyl mercaptoacetamide | 12.5 | 105,78,72,58, |
| 5 | O,O,O-trimethyl phosphate | 13.4 | 140,125, 79,65 |
| 6 | O,O,S-trimethyl dithiophosphate | 16.8 | 172,141, 109,93 |
| 7 | Omethoate | 19.1 | 213,156, 110,79 |
| 8 | Dimethoate | 20.7 | 229,143,125, 87 |
Fig. 7.
Possible pathways for the degradation of DMT by FC/US.
3.8. Change in total chlorophyll in FC/US process
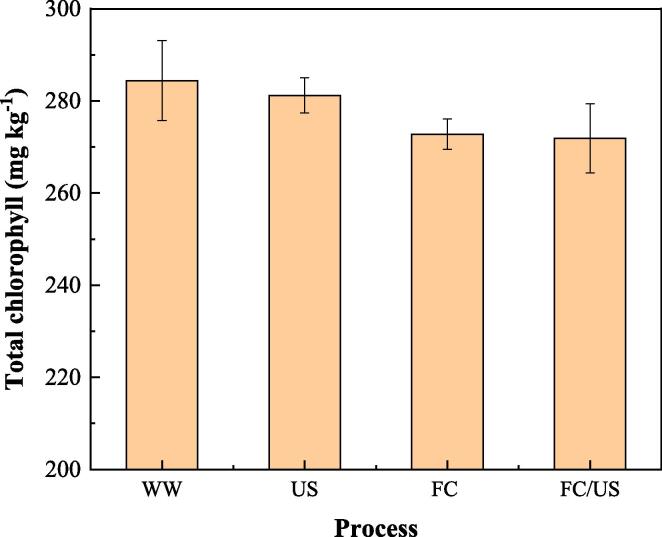
Chlorophyll is the primary pigment that comprises the greenness of lettuce. It is chemically unstable and sensitive to both light and heat. Therefore, the content of chlorophyll can be used as a good indicator for food quality of green vegetables [36]. Fig. 8 shows the changing of chlorophyll content in lettuce during the pesticide removal by WW, US, FC and FC/US processes. The content of chlorophyll in WW process was used as control. The total chlorophyll content decreased from 284.4 to 281.2, 272.8 and 270.4 mg kg−1 after the US, FC and FC/US processes, respectively. The negligible ecrease of chlorophyll content after the US process (1.1%) indicates that US would not damage the chemical structure of lettuce [13], while the slight decrease of chlorophyll content after the FC process (4.1%) was caused by the oxidation reaction between FC and chlorophyll. The decrease of chlorophyll content after the FC/US process (4.9%) was slightly higher than that of the FC process, and the extra loss of chlorophyll was probably due to the oxidation of chlorophyll by the generated radicals. Overall, the slight decrease of chlorophyll content of less than 5% after the FC/US process is acceptable considering its high efficacy for pesticide removal.
Fig. 8.
Changing of chlorophyll content in lettuce during the pesticide removal by WW, US, FC and FC/US processes ([FC]0 = 15 mg L–1, pH = 7.2, ultrasonic frequency = 40 kHz).
4. Conclusion
Removal of three pesticides from vegetables by the coupled FC/US process has been closely studied. Based on the results, the following conclusions are drawn:
-
•
The selected pesticides were effectively removed from vegetables by FC/US process and the synergistic factors reached 22.3%, 19.0% and 36.4% for DMT, TCF and CBF, respectively.
-
•
The synergistic effect observed in FC/US process was caused by the efficient oxidation of pesticides both in vegetables and in water by the generated free radicals and FC.
-
•
The high pesticide removal efficiencies for five different vegetables verified universality of the FC/US process.
-
•
Solution pH, ultrasonic frequency and FC concentration all had significant impacts on pesticide removal by FC/US. The slight reduction of chlorophyll content (<5%) after the FC/US process meant a negligible damage to the quality of vegetables.
-
•
FC/US process is a promising AOP for pesticides removal from vegetables.
CRediT authorship contribution statement
Laxiang Yang: Conceptualization, Methodology, Writing – original draft. Jieqiong Zhou: Data curation, Supervision. Yuxin Feng: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This paper was financially supported by the Education Department of Hunan Province (Grant No. 21B0687) and the Shaoyang University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105891.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Azam S.M.R., Ma H., Xu B., Devi S., Siddique M.A.B., Stanley S.L., Bhandari B., Zhu J. Efficacy of ultrasound treatment in the removal of pesticide residues from fresh vegetables: a review. Trends Food Sci. Technol. 2020;97:417–432. [Google Scholar]
- 2.Cengiz M.F., Basancelebi O., Baslar M., Certel M. A novel technique for the reduction of pesticide residues by a combination of low-intensity electrical current and ultrasound applications: A study on lettuce samples. Food Chem. 2021;354:129360. doi: 10.1016/j.foodchem.2021.129360. [DOI] [PubMed] [Google Scholar]
- 3.Qin G., Chen Y., He F., Yang B., Zou K., Shen N., Zuo B., Liu R., Zhang W., Li Y. Risk assessment of fungicide pesticide residues in vegetables and fruits in the mid-western region of China. J. Food Compos. Anal. 2021;95:103663. [Google Scholar]
- 4.Diop A., Diop Y.M., Thiaré D.D., Cazier F., Sarr S.O., Kasprowiak A., Landy D., Delattre F. Monitoring survey of the use patterns and pesticide residues on vegetables in the Niayes zone, Senegal. Chemosphere. 2016;144:1715–1721. doi: 10.1016/j.chemosphere.2015.10.058. [DOI] [PubMed] [Google Scholar]
- 5.Foong S.Y., Ma N.L., Lam S.S., Peng W., Low F., Lee B.H.K., Alstrup A.K.O., Sonne C. A recent global review of hazardous chlorpyrifos pesticide in fruit and vegetables: Prevalence, remediation and actions needed. J. Hazard. Mater. 2020;400:123006. doi: 10.1016/j.jhazmat.2020.123006. [DOI] [PubMed] [Google Scholar]
- 6.Zhou R., Zhou R., Yu F., Xi D., Wang P., Li J., Wang X., Zhang X., Bazaka K., Ostrikov K.(. Removal of organophosphorus pesticide residues from Lycium barbarum by gas phase surface discharge plasma. Chem. Eng. J. 2018;342:401–409. [Google Scholar]
- 7.Ikeura H., Kobayashi F., Tamaki M. Removal of residual pesticides in vegetables using ozone microbubbles. J. Hazard. Mater. 2011;186(1):956–959. doi: 10.1016/j.jhazmat.2010.11.094. [DOI] [PubMed] [Google Scholar]
- 8.Chemat F., Zill-e-Huma, Khan M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011;18(4):813–835. doi: 10.1016/j.ultsonch.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Raut-Jadhav S., Pinjari D.V., Saini D.R., Sonawane S.H., Pandit A.B. Intensification of degradation of methomyl (carbamate group pesticide) by using the combination of ultrasonic cavitation and process intensifying additives. Ultrason. Sonochem. 2016;31:135–142. doi: 10.1016/j.ultsonch.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 10.São José J.F.B.d., Andrade N.J.d., Ramos A.M., Vanetti M.C.D., Stringheta P.C., Chaves J.B.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control. 2014;45:36–50. [Google Scholar]
- 11.Lozowicka B., Jankowska M., Hrynko I., Kaczynski P. Removal of 16 pesticide residues from strawberries by washing with tap and ozone water, ultrasonic cleaning and boiling. Environ. Monit. Assess. 2016;188:51. doi: 10.1007/s10661-015-4850-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Zhang Z., Chen F., Zhang H., Hu X. Effect of sonication on eliminating of phorate in apple juice. Ultrason. Sonochem. 2012;19(1):43–48. doi: 10.1016/j.ultsonch.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Azam S.M.R., Ma H., Xu B., Devi S., Stanley S.L., Siddique M.A.B., Mujumdar A.S., Zhu J. Multi-frequency multi-mode ultrasound treatment for removing pesticides from lettuce (Lactuca sativa L.) and effects on product quality, LWT-Food. Sci. Technol. 2021;143:111147. [Google Scholar]
- 14.Zhou Q., Bian Y., Peng Q., Liu F., Wang W., Chen F. The effects and mechanism of using ultrasonic dishwasher to remove five pesticides from rape and grape. Food Chem. 2019;298:125007. doi: 10.1016/j.foodchem.2019.125007. [DOI] [PubMed] [Google Scholar]
- 15.Alenyorege E.A., Ma H., Ayim I., Aheto J.H., Hong C., Zhou C. Reduction of Listeria innocua in fresh-cut Chinese cabbage by a combined washing treatment of sweeping frequency ultrasound and sodium hypochlorite, LWT-Food. Sci. Technol. 2019;101:410–418. [Google Scholar]
- 16.Cotillas S., Clematis D., Cañizares P., Carpanese M.P., Rodrigo M.A., Panizza M. Degradation of dye Procion Red MX-5B by electrolytic and electro-irradiated technologies using diamond electrodes. Chemosphere. 2018;199:445–452. doi: 10.1016/j.chemosphere.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Agriculture of China, Pesticide multiresidue screen methods for determination of organophosphorus pesticides, organochlorine pesticides, pyrethroid pesticides and carbamate pesticides in vegetables and fruits, NY/T 761-2008.
- 18.Yang L., Feng Y., Gao Z. Degradation of organic micropollutant by vacuum ultraviolet process: a kinetics study. Korean J. Chem. Eng. 2021;38(8):1642–1647. [Google Scholar]
- 19.Yang L., Li M., Li W., Jiang Y., Qiang Z. Bench- and pilot-scale studies on the removal of pesticides from water by VUV/UV process. Chem. Eng. J. 2018;342:155–162. [Google Scholar]
- 20.Tian F., Liu W., Guo G., Qiang Z., Zhang C. Kinetics and mechanism of dimethoate chlorination during drinking water treatment. Chemosphere. 2014;103:181–187. doi: 10.1016/j.chemosphere.2013.11.061. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z., Huang J., Chen J., Li F. Effectiveness of dishwashing liquids in removing chlorothalonil and chlorpyrifos residues from cherry tomatoes. Chemosphere. 2013;92(8):1022–1028. doi: 10.1016/j.chemosphere.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Qi H., Huang Q., Hung Y.-C. Effectiveness of electrolyzed oxidizing water treatment in removing pesticide residues and its effect on produce quality. Food Chem. 2018;239:561–568. doi: 10.1016/j.foodchem.2017.06.144. [DOI] [PubMed] [Google Scholar]
- 23.Cengiz M.F., Başlar M., Basançelebi O., Kılıçlı M. Reduction of pesticide residues from tomatoes by low intensity electrical current and ultrasound applications. Food Chem. 2018;267:60–66. doi: 10.1016/j.foodchem.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 24.de Lima Leite R.H., Cognet P., Wilhelm A.-M., Delmas H. Anodic oxidation of 2,4-dihydroxybenzoic acid for wastewater treatment: study of ultrasound activation. Chem. Eng. Sci. 2002;57(5):767–778. [Google Scholar]
- 25.Sajjadi S., Khataee A., Bagheri N., Kobya M., Şenocak A., Demirbas E., Karaoğlu A.G. Degradation of diazinon pesticide using catalyzed persulfate with Fe3O4@MOF-2 nanocomposite under ultrasound irradiation. J. Ind. Eng. Chem. 2019;77:280–290. [Google Scholar]
- 26.Zhang Z.-Y., Liu X.-J., Hong X.-Y. Effects of home preparation on pesticide residues in cabbage. Food Control. 2007;18(12):1484–1487. [Google Scholar]
- 27.Saleh T.A., Gupta V.K. Synthesis and characterization of alumina nano-particles polyamide membrane with enhanced flux rejection performance. Sep. Purif. Technol. 2012;89:245–251. [Google Scholar]
- 28.Zhu Y., Zhang T., Xu D., Wang S., Yuan Y., He S., Cao Y. The removal of pesticide residues from pakchoi (Brassica rape L. ssp. chinensis) by ultrasonic treatment. Food Control. 2019;95:176–180. [Google Scholar]
- 29.Birmpa A., Sfika V., Vantarakis A. Ultraviolet light and ultrasound as non-thermal treatments for the inactivation of microorganisms in fresh ready-to-eat foods. Int. J. Food Microbiol. 2013;167(1):96–102. doi: 10.1016/j.ijfoodmicro.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Dalodière E., Virot M., Moisy P., Nikitenko S.I. Effect of ultrasonic frequency on H2O2 sonochemical formation rate in aqueous nitric acid solutions in the presence of oxygen. Ultrason. Sonochem. 2016;29:198–204. doi: 10.1016/j.ultsonch.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Pflieger R., Ndiaye A.A., Chave T., Nikitenko S.I. Influence of ultrasonic frequency on Swan band sonoluminescence and sonochemical activity in aqueous tert-butyl alcohol solutions. J. Phys. Chem. B. 2015;119(1):284–290. doi: 10.1021/jp509898p. [DOI] [PubMed] [Google Scholar]
- 32.Yao J.J., Hoffmann M.R., Gao N.Y., Zhang Z., Li L. Sonolytic degradation of dimethoate: kinetics, mechanisms and toxic intermediates controlling. Water Res. 2011;45:5886–5894. doi: 10.1016/j.watres.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 33.Echavia G.R.M., Matzusawa F., Negishi N. Photocatalytic degradation of organophosphate and phosphonoglycine pesticides using TiO2 immobilized on silica gel. Chemosphere. 2009;76(5):595–600. doi: 10.1016/j.chemosphere.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 34.Hu Y., Bai Y., Li X., Chen J. Application of dielectric barrier discharge plasma for degradation and pathways of dimethoate in aqueous solution. Sep. Purif. Technol. 2013;120:191–197. [Google Scholar]
- 35.Wu Z., Yang L., Tang Y., Qiang Z., Li M. Dimethoate degradation by VUV/UV process: Kinetics, mechanism and economic feasibility. Chemosphere. 2021;273:129724. doi: 10.1016/j.chemosphere.2021.129724. [DOI] [PubMed] [Google Scholar]
- 36.Alenyorege E.A., Ma H., Ayim I., Lu F., Zhou C. Efficacy of sweep ultrasound on natural microbiota reduction and quality preservation of Chinese cabbage during storage. Ultrason. Sonochem. 2019;59:104712. doi: 10.1016/j.ultsonch.2019.104712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.