SUMMARY
Using tyrosine hydroxylase immunohistochemistry we examined the structure of the pontine, or rostral rhombencephalic, catecholaminergic cells groups, which may be collectively termed the locus coeruleus complex (LC), in the bottlenose dolphin. The present study is the first to describe the LC in a cetacean species and, at 1.3 kg, represents the largest non-human brain to date in which the LC has been investigated. We identified four catecholaminergic cell groups in the dorsal pontine tegementum and peri-aqueductal gray matter: A6 dorsal (locus coeruleus), A6 ventral (locus coeruleus alpha), A7 (subcoeruleus), and A5 (fifth arcuate nucleus). No patterns of cellular distribution, nuclear subdivision, or cellular morphology indicate specialization of the LC, which might have been anticipated because of the large absolute brain size and unihemispheric sleep phenomenology of cetaceans.
Keywords: cetacean, evolution, norepinephrine, rapid eye movement, sleep, slow wave sleep, thermoregulation
INTRODUCTION
The locus coeruleus complex (LC) has been identified in all species of vertebrates studied (Smeets and González 2000) and is composed of one or more groups of catecholaminergic neurons located in the rostral rhombencephalon. In mammals, the LC can be composed of up to four or more subdivisions, termed A4, A5 (fifth arcuate nucleus), A6 (locus coeruleus) and A7 (subcoeruleus) in standardized (and anatomical) nomenclature (Hokfelt et al. 1984a, b). The axons of LC neurons innervate the entire central nervous system (CNS), and the LC is the major producer of norepinephrine (Amaral and Sinnamon 1977). The present study examines the LC of the bottlenose dolphin (Tursiops truncatus) using tyrosine hydroxylase immunohistochemistry.
The brains of cetaceans have evolved to a very large size (Marino 1998; Pilleri and Gihr 1970; Ridgway and Brownson 1984), the reasons for which are not yet clear. One canonical evolutionary precept is that increased size equals increased complexity (Bonner 1988; Kaas 1995). Thus, one might anticipate the LC of cetaceans to show a complex arrangement of subdivisions.
The discharge rate of LC neurons is closely aligned with the sleep–wake cycle (Siegel 2000), although the functional role of the LC in sleep control sleep remains uncertain. It is established that several species of cetaceans, most likely all, sleep in an apparently obligatory unihemispheric fashion (Mukhametov 1987; Mukhametov and Lyamin 1994; Mukhametov et al. 1977; Lyamin et al. 2000, 2002). Unihemispheric slow wave sleep (USWS) occurs nocturnally, and the contrasting slow wave/desynchronized electroencephalogram (EEG) activity alternates with a periodicity of approximately 1 h (Mukhametov 1987). Furthermore, rapid eye movement (REM) sleep is either absent in cetaceans or occupies an extremely small proportion of the day – an absolute maximum of 15 min each day (Mukhametov 1988, 1995; Lyamin et al. 1998, 2000). In other eutherian mammals, LC neurons discharge at a high constant rate during wake, slowing during slow wave sleep (SWS), and minimally during REM sleep (and cataplexy) at which time a complete loss of muscle tone is experienced (Wu et al. 1999). For this and other reasons the LC has been implicated in the maintenance of muscle tone (Pompeiano 2001). This functional attribute is an important aspect in the present study on the dolphin, as they swim continuously during sleep, never losing muscle tone (Mukhametov and Lyamin 1994; Mukhametov et al. 1977).
Hypotheses regarding the evolution of unihemispheric sleep in marine mammals suggest postural maintenance in the water column and the requirement of coming to the surface to breathe as the selection pressures (Mukhametov 1987, 1988, 1995). However, these hypotheses are difficult to test, and are inconsistent with the available data, as the true seals (the Phocids) do not exhibit USWS (Lyamin 1993; Lyamin et al. 1993; Milsom et al. 1996; Mukhametov et al. 1984; Ridgway et al. 1975), and the manatee spends just 25% of total sleep time in USWS (Mukhametov et al. 1992). No studies have addressed the neural mechanisms underlying USWS phenomenology. Thus, in order to begin to understand the mechanisms and purpose of USWS in marine mammals we examined the morphology of the LC in the bottlenose dolphin.
MATERIALS AND METHODS
One specimen of adult bottlenose dolphin (T. truncatus) was used in the present study. This animal (NOR) was a 151-kg adult female, 245 cm in length that was collected from the Northern Gulf of Mexico as a young adult and maintained by the US Navy Marine Mammal Program headquartered in San Diego, California, for 13 years. The animal died of natural causes (age approximately 23 years) because of the complications of a second pregnancy and parturition, despite a successful pregnancy 3 years earlier. The brain was removed 5 h postmortem, weighed (1305 g; smaller than the overall species average of about 1500 g) and partially sectioned along the interhemispheric cleft to expose the ventricles. The brain was immerse fixed in 10 l of Streck Tissue Fixative (Streck Laboratories Inc., Omaha, NB, USA). The whole brain, in the fixative, was placed on a floating pier for 2 months so that wave action produced a rocking motion to improve fixation of the large brain. Five years following fixation the brainstem (midbrain, pons and medulla oblongata) was dissected away from the remainder of the brain and sectioned as described below.
Serial 50-µm sections of the brainstem (from the level of the enlarged posterior commissure through to the cervical spinal cord) were made in a coronal plane. Three consecutive sections from every 10 were stained for Nissl substance with cresyl violet, fibers (Gallyas 1979), or immunocytochemically stained for tyrosine hydroxylase (TH). For TH staining, the sections were rinsed three times in 0.1 m Trizma buffered saline (TBS) followed by a 48-h incubation at 4°C with a 1/500 dilution of primary rabbit antiserum to TH (Eugene Tech International Inc., Ridgefield Park, NJ, USA). The dilutions were prepared with a solution of 1% normal goat serum (NGS) and 0.25% Triton X-100 in 0.1 m Tris-saline. This was followed by a 2.5-h incubation with biotinylated goat antirabbit immunoglobulin G (IgG) (Vector Laboratories, Burlingame, CA, USA) diluted 1/200 with 1% NGS in Tris-saline. The tissue was then incubated for 2 h with the avidin–biotin complex diluted 1/100 with 1% NGS in Tris-saline (Vector). Between each incubation, the sections were rinsed three times with 1% NGS in Tris-saline. The sections were then treated for 6 min with a 0.05% solution of 3,3′diamino-benzidine and 0.01% hydrogen peroxide, rinsed in phosphate buffer, mounted to gel-coated slides, cleared in xylene and coverslipped with Depex mounting medium. The stained sections were examined under a low-power dissecting microscope, cell bodies marked using a camera lucida, and then matched to architectural boundaries determined from the adjacent Nissl and fiber-stained sections. High power photomicrographs were taken of approximately 100 cells in each architectonic region and the somatal area determined using the freely available program NIH Image. Only cells with a visible nucleus were included in the analysis.
RESULTS
Tyrosine hydroxylase immunohistochemistry revealed the neurons of the LC complex as well as those of catecholaminergic neuronal groups in adjacent regions of the bottlenose dolphin brain. The present report is limited to those neuronal clusters found in the pontine region, which arguably belong to the LC complex. In the dolphin, we found four catecholaminergic cell groups that form the LC. These include the A6 dorsal subdivision (the locus coeruleus proper), the A6 ventral subdivision (sometimes referred to as locus coeruleus alpha), the A7 subdivision (the subcoeruleus) and the A5 subdivision (the fifth arcuate nucleus). These groups were found to extend from the posteriormost level of the inferior colliculus to the anteriormost level of the inferior olive.
A6 dorsal subdivision (A6d, locus coeruleus)
This group of putatively noradrenergic neurons were located entirely within the caudal peri-aqueductal gray matter (Fig. 1). The most anterior of these neurons are found at the posteriormost level of the inferior colliculus. At this level a few scattered neurons are found. However, 2 mm posterior to this level the number of neurons rises substantially, and a tightly packed cluster is found within the gray matter, between 2.5 and 5 mm from the midline. These neurons form a continuous column within the gray matter, extending posteriorly to the level of the decussation of the brachium conjunctivum and trigeminal motor nucleus. At this level the number of neurons drops dramatically over an antero-posterior distance of 1 mm. No TH immunoreactive neurons were found within the gray matter posterior to this level, and this is the basis on which we report a lack of an A4 subdivision (Fig. 1).
Figure 1.
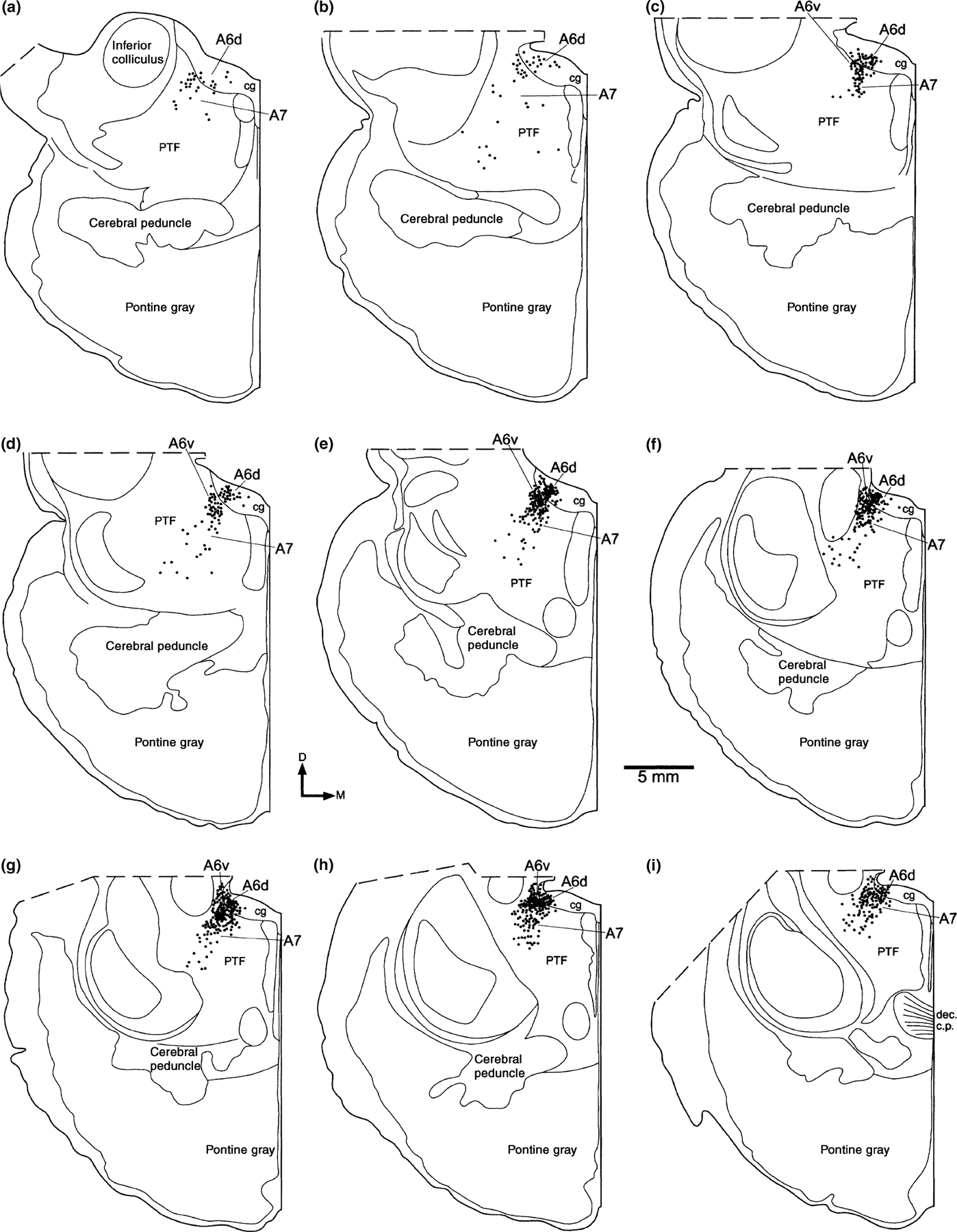
Diagrams of the cellular distribution of the tyrosine hydroxylase immunoreactive cells which make up the locus coeruleus complex of the bottlenose dolphin. Sections are approximately 1 mm apart and are shown from rostral (a) to caudal (i). Each dot represents a single immunoreactive cell. cg, central, or peri-aqueductal gray matter; D, dorsal; dec c.p., decussation of the cerebral peduncle or brachium conjunctivum; PTF, pontine tegemental field.
Four-sided polygons and irregular triangles were the most common shapes observed for soma in this subdivision (Fig. 2a). Three to four primary dendrites were seen to emerge from various points around the neuron body, and ramified within a single neuron body length to form an extremely dense local plexus. No specific dendritic orientations were observed, except for those at the ventron lateral portion of the nucleus, where dendrites gathered as fascicles to bridge the gap across an antero-posterior running fiber bundle (located external, but adjacent, to the peri-aqueductal gray matter) separating this subdivision from the A6 ventral subdivision. The soma of this subdivision are the smallest of the four subdivisions of the dolphin LC with an average somatal area of 469.5 µm2 (SD 127.6 µm2).
Figure 2.
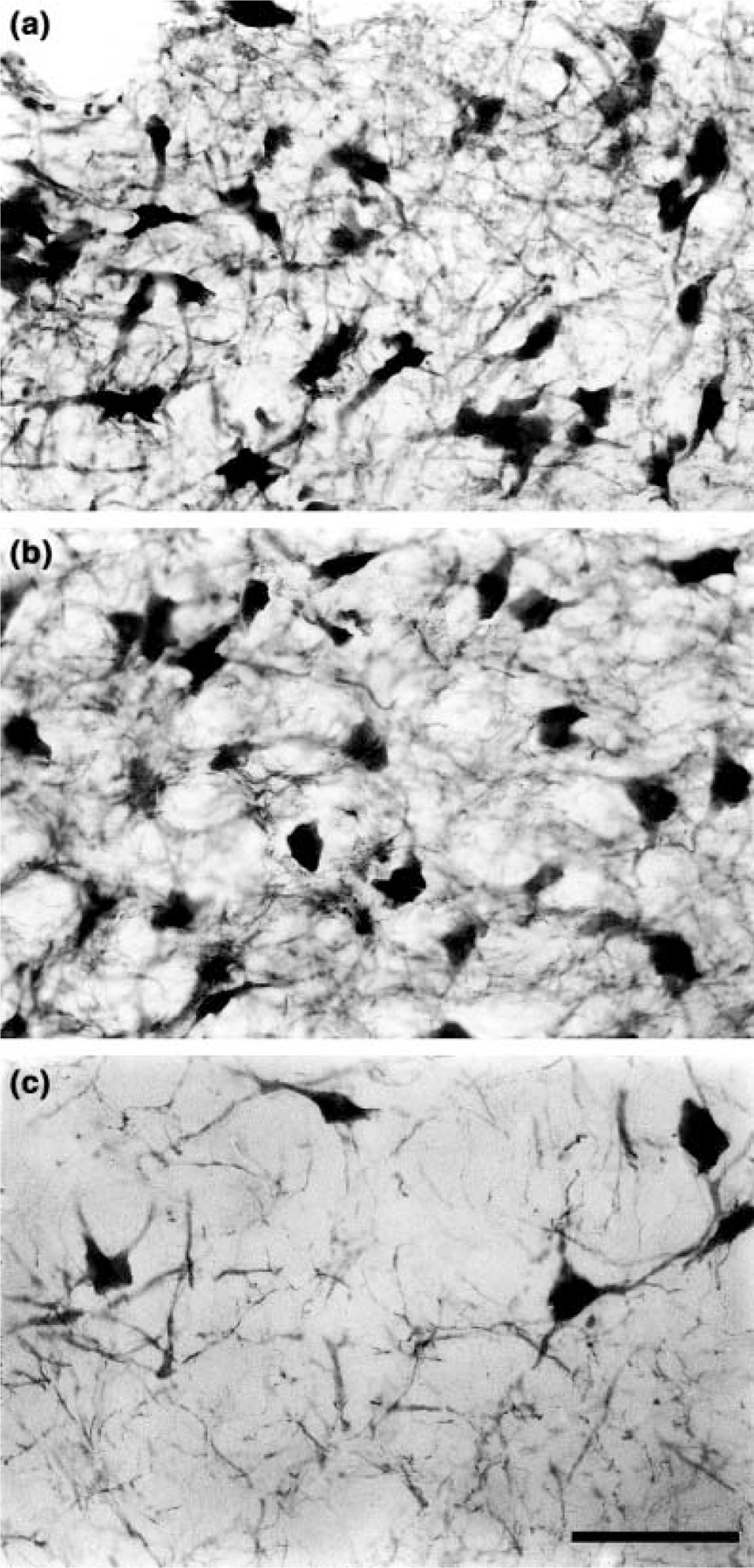
High power photomicrographs of cells in three of the subdivisions described in the present study. (a) Tyrosine hydroxylase immunoreactive cells in the A6 dorsal subdivision. Note the high cellular density and extensive local plexus formed by the dendrites of these cells. (b) Cells of the A6 ventral subdivision. Note the lower cellular density and the similarly reduced density of the local dendritic plexus. (c) Cells of the A7 subdivision. Here the cellular density is lowest, however, this varies across this subdivision, with the periphery exhibiting the lowest cellular density. The density of the dendritic plexus is also significantly lower. Scale bar = 50 µm, applies to all plates.
A6 ventral subdivision (A6v, locus coeruleus alpha)
The neurons of A6v appear as a tightly packed cluster located adjacent to those neurons of A6d, except that they lie outside of the peri-aqueductal gray matter, ventral and slightly lateral to the A6d subdivision (Fig. 1). The A6v subdivision is a tightly packed cluster of neurons that begins at a level 4 mm posterior to the first neurons of A6d, and forms an anteroposterior column of 4 mm, ending 1 mm anterior to the level of decussation of the brachium conjunctivum. This division lies 3–5.5 mm from the midline, and is located 1.5–4 mm below the ependymal surface of the peri-aqueductal gray matter (Fig. 1).
Polygonal shaped soma characterized the neurons of the A6 ventral division, with four to five sides making up the polygon (Fig. 2b). Three to four primary dendrites emerged from the corners of the polygon, all of similar size. These primary dendrites were seen to ramify within a single neuronal body length of the soma and form a dense local plexus. No specific dendritic orientation could be seen. However, several dendrites on the dorsal lateral surface were observed to project as fascicles across a larger bundle of fibers that separated this division from the A6d subdivision. The soma of these neurons were slightly larger than those of the A6 dorsal subdivision, with an average somatal area of 630.1 µm2 (SD 114.4 µm2).
A7 subdivision (locus subcoeruleus)
The A7 subdivision is the most expansive of those of the LC complex of the dolphin, as in other mammals. It extends from the posteriormost level of the inferior colliculus to the level of the decussation of the brachium conjunctivum (Fig. 1). The neurons of A7 are distributed throughout the pontine tegmental field, and exhibit a higher density dorsally within this region. These neurons form a loosely arranged column located between 3 and 7.5 mm from the midline and 2–7.5 mm from the ependymal surface of the aqueduct. They are limited in their lateral extent by the edge of the pontine tegmental field; however, no clear architectonic boundary corresponds to the observed medial limit. The neurons of A7 are located entirely outside of the peri-aqueductal gray matter, and encapsulate the tightly packed A6v subdivision on all surfaces except the dorsolateral surface (Fig. 1).
The soma of the neurons of the A7 subdivision were polygonal in shape, mostly with four sides, but occasionally with five (Fig. 2c). Three to four primary dendrites were seen to emerge, one from each corner of the polygon, one or two of which were quite large and emerged from the same side of the neuron. The dendrites were observed to ramify within one or two neuronal body lengths from the soma and form a local plexus. There did not appear to be any consistent orientation of the dendrites in relation to the location of the neuron body. The soma of these neurons are the largest of the four subdivisions with an average somatal area of 749.6 µm2 (SD 163.5 µm2).
A5 subdivision (fifth arcuate nucleus)
The TH immunoreactive neurons that make up the A5 subdivision are found within the ventromedial portion of the pontine tegmentum, from just medial to the anterior border of the inferior olive, to a level adjacent to the posterior limit of the trigeminal motor nucleus. The neurons are not numerous, and form a loose latticework of interconnected dendritic branches, through which large bundles of fibers pass. The soma are small and triangular in shape with three primary dendrites, and have an average somatal area of 634.4 µm2 (SD 127.1 µm2).
DISCUSSION
The present study provides the first anatomical description of the LC of the cetacean. We introduced two issues that the present study addresses. (1) As a general principle, the evolution of large brain size has been associated with neo-Darwinian gradualistic increases in neural complexity (Kaas 1995; Kaas and Catania 2002) – does this apply to the mammalian LC? (2) Cetaceans exhibit an apparently obligatory USWS phenomenology – are there features of the anatomical organization of the LC that might help explain the function of USWS in marine mammals? The observations made in this study allow us to introduce the hypothesis that the neural activity of the LC, as the result of cetacean USWS phenomenology, may play a role in thermogenesis and general thermal adaptation to a wide range of water temperatures.
Locus coeruleus complex organization and increased brain size
A recent review of the LC of eutherian mammals is provided by Kitahama et al. (1994). Eutherian mammals generally have four subdivisions of the LC, which include the A4–7 subdivisions. However, this is not the complete picture. In rodents, all four subdivisions are present. However, the A6 group can be divided into a dorsal and ventral portion. In carnivores, in addition to the four basic subdivisions, several extra groups have been identified, which include the locus coeruleus alpha (or ventral division of locus coeruleus), medial and lateral parabrachial nuclei, and the Kölliker-Fuse nucleus. In contrast to carnivores, the primate brain shows a more simple nuclear differentiation of the LC, with dorsal and ventral divisions of A6, A4, A5 and A7 subdivisions. In ungulates, a dorsal division of the A6 group was found, as well as the A5 and A7 subdivisions; however, the A4 group was absent.
In the present study of the bottlenose dolphin LC complex, we identified the dorsal and ventral divisions of the A6 group and the A5 and A7 groups. An A4 group could not be identified. The most striking feature of the comparative data is that species belonging to different orders exhibit different subdivisions of LC. However, species within the same order exhibit the same subdivisions. Thus, despite differences, for example in the number of neurons in the A6 dorsal division of the cat and dog (Kitahama et al. 1994), the number of LC subdivisions is identical. The cat brain (~30 g) weighs approximately one-third of the dog brain (~90 g), and we can hypothesize that the subdivisions described for the dog and cat are likely to be the same for all carnivores, irrespective of brain weight (a testable hypothesis). The macaque monkey brain, at around 90 g, is significantly smaller than the human brain (~1300 g), yet Kitahama et al. (1994) state that the monkey has ‘a distributional pattern similar to, but more compact than that of humans’.
Thus, simply increasing brain size does not appear to increase the complexity of the nuclear subdivisions of LC. In the dolphin, with a brain weight of around 1300 g we identified four subdivisions, less than that identified in the 1 g brain of the mouse (Kitahama et al. 1994). These comparative results do not lend support to the ‘increased size equals increased complexity’ paradigm commonly found in biology (Bonner 1988), and more specifically in neurobiology (Kaas 1995; Kaas and Catania 2002). What emerges from this comparison is that it is likely that changes in the complexity of LC, in terms of the number of subdivisions, occurs during the evolution of a new order, and these subdivisions, established at the emergence of a new order, are retained in all subsequently evolved species of that order, despite gradualistic changes such as differences in total neuron numbers in each subdivision.
Cetacean unihemispheric slow wave sleep and the locus coeruleus complex
One of the striking adaptations to an aquatic existence exhibited by cetaceans is the faculty to sleep with one hemisphere (unihemispheric EEG synchronization) while the opposite hemisphere is apparently awake (unihemispheric EEG desynchronization) (Mukhametov et al. 1977). In addition, dolphins have a very small amount of REM sleep, which has proved difficult to identify physiologically (Mukhametov 1988, 1995). The activity of LC neurons during the mammalian sleep–wake cycle has been determined in single unit recording studies in a variety of eutherian species (e.g. Aston-Jones and Bloom 1981), but not cetaceans. During unambiguous waking behavior, the LC neurons fire continuously (2–4 Hz). During SWS the firing rate of the LC neurons slows (1–2 Hz), and during REM sleep, the LC neurons cease to discharge (0.01–0.1 Hz) (Aston-Jones and Bloom 1981); this lack of activity is one of the defining features of REM sleep (Siegel 2000).
Our anatomical observations indicate no specialization in the organization of the cetacean LC complex that might be directly related to unihemispheric sleep phenomenology. At this point, one might be tempted to say that the present observations add nothing new to our understating of cetacean sleep phenomenology. However, as we will attempt to show, this would be a premature conclusion. The LC complex is one of the first anatomically recognizable nuclei of the mammalian brain to develop (Clancy et al. 2001). Interestingly, the peak of LC cell generation is coincident with what has been termed the ‘phylotypic stage’ in vertebrate development (Sander 1983). It has been shown that at this stage of development, disturbances in the temporal and spatial pattern of genetic expression leads to a greater probability of lethal mutations of the phenotype than at other developmental stages (Galis and Metz 2001). Thus, the conserved anatomical appearance of the adult cetacean LC suggests that the physiology and function of the LC is similarly unchanged.
This allows us to hypothesize that the discharge pattern of LC neurons in the dolphin is aligned to the sleep–wake cycle in a manner not dissimilar to other eutherian mammals. If this is the case, as each cerebral hemisphere of the dolphin is awake for 20 h per day, the LC neurons must be discharging at a high constant rate during this period. During USWS, the discharge rate must slow down, but potentially only for one hemisphere. Only during REM sleep do LC neurons cease to discharge, and this occupies a very small percentage of the total sleep time of the dolphin. Thus, we can propose that the LC neurons must almost never cease to discharge during the life of the dolphin.
The proposed adaptive purposes of unihemispheric sleep in cetaceans are: (1) to allow the animals to come to the surface to breathe, and (2) to maintain posture in a continuously moving medium (Mukhametov 1988, 1995). While these suggestions have an immediacy value to an air-breathing, sleep-requiring, aquatic mammal, the teleological nature of these suggestions do not explain the adaptive evolutionary rational of cetacean USWS. As pointed out earlier, not all marine mammals exhibit USWS (i.e. Phocids), and some exhibit only a limited amount of USWS (i.e. Sirenians). Thus, these proposed adaptive functions do not provide a consistent evolutionary rationale for the emergence of USWS phenomenology in marine mammals.
One of the major obstacles facing a homeotherm in adapting to an aquatic existence is that of thermoregulation. In water, conductive heat loss is 90.8 times the rate it is in air at the same ambient temperature (Downhower and Blumer 1988). It has been shown that the descending projections of the LC play a facilitatory role in the regulation of muscle tone, i.e. discharges of LC neurons maintain muscle tone (e.g. Kiyashchenko et al. 2001; Pompeiano 2001; and the references therein). As we have hypothesized above, the cetacean LC neurons may be discharging more or less continuously. This leads to the conclusion that heat is produced constantly by cetacean musculature in part, because of the maintenance of basal muscle tone by the discharge of LC neurons (30% of heat production in terrestrial mammals is generated through basal muscle tone, and up to 70% of heat production can be generated through the activity of skeletal muscles, see Gisolfi and Mora 2000). Thus, we propose, that in cetaceans the normal activity pattern of LC neurons, which is theoretically aligned with USWS phenomenology, may have been co-opted for continuous heat production by maintaining basal muscle tone. This maintenance of basal muscle tone also allows for continuous swimming during sleep (permitting the cetaceans to surface for breathing, maintain posture in the water column, and maintain pod coherence), and for maintained heat production by the active skeletal muscle. Thus, the need to maintain body temperature might have been the selection pressure leading to the evolution of USWS phenomenology in cetaceans, and possibly in the other aquatic mammalian species that exhibit this form of sleep.
ACKNOWLEDGEMENTS
This work was supported by the Medical Research Service of the Veterans Administration, NIH grants NS42947 and HL41370, and NSF grant 0234687. We thank Drs William Van Bonn and Raymond Tarpley for help in dissection and brain removal, and Cheryl Short for attending the fixing brain. The authors would also like to thank Heidi Fahringer and Brenda Chestnut for expert technical assistance in the preparation of the histological sections used in this study.
Abbreviations:
- A4
division of the locus coeruleus complex
- A5
fifth arcuate nucleus
- A6
locus coeruleus
- A6d
dorsal subdivision of the locus coeruleus
- A6v
ventral subdivision of the locus coeruleus
- A7
subcoeruleus
- LC
locus coeruleus complex
- NGS
normal goat serum
- REM
rapid eye movement
- SWS
slow wave sleep
- TBS
Trisma buffered saline
- TH
tyrosine hydroxylase
- USWS
unihemispheric slow wave sleep
REFERENCES
- Amaral DG and Sinnamon HM The locus coeruleus. neurobiology of a central noradrenergic nucleus. Prog. Neurobiol, 1977, 9: 147–196. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G and Bloom FE Activity of norepinephrinecontaining locus coeruleus cells neurons in behaving rats anticipates fluctuations in the sleep–waking cycle. J. Neurosci, 1981, 1: 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JT The Evolution of Complexity by Means of Natural Selection Princeton University Press, Princeton, NJ, 1988. [Google Scholar]
- Clancy B, Darlington RB and Finlay BL Translating developmental time across mammalian species. Neuroscience, 2001, 105: 7–17. [DOI] [PubMed] [Google Scholar]
- Downhower JF and Blumer LS Calculating just how small a whale can be. Nature, 1988, 335: 675.3173490 [Google Scholar]
- Galis F and Metz JAJ Testing the vulnerability of the phylotypic stage: on modularity and evolutionary conservatism. J. Exp. Zool. (Mol. Dev. Evol.), 2001, 291: 195–204. [DOI] [PubMed] [Google Scholar]
- Gallyas F Silver staining of myelin by means of physical development. Neurolog. Res, 1979, 1: 203–209. [DOI] [PubMed] [Google Scholar]
- Gisolfi CV and Mora F The Hot Brain. Survival, Temperature, and the Human Body The MIT Press, London, 2000. [Google Scholar]
- Hokfelt T, Johansson O and Goldstein M Central catecholamine neurons as revealed by immunohistochemistry with special reference to adrenaline neurons. In: Bjorklund A and Hokfelt T. (Eds) Handbook of Chemical Neuroanatomy, Vol. 2 Classical Neurotransmitters in the CNS, Part 1. Elsevier, Amsterdam, 1984a: 157–276. [Google Scholar]
- Hokfelt T, Martensson R, Bjorklund A, Kleinau S and Goldstein M Distributional maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Bjorklund A and Hokfelt T. (Eds) Handbook of Chemical Neuroanatomy, Vol. 2 Classical Neurotransmitters in the CNS, Part 1. Elsevier, Amsterdam, 1984b: 277–379. [Google Scholar]
- Kaas JH and Catania KC How do features of sensory representations develop? Bioessays, 2002, 24: 334–343. [DOI] [PubMed] [Google Scholar]
- Kaas JH The evolution of isocortex. Brain Behav. Evol, 1995, 46: 187–196. [DOI] [PubMed] [Google Scholar]
- Kitahama K, Nagatsu I and Pearson J Catecholamine systems in mammalian midbrain and hindbrain: theme and variations. In: Smeets WJAJ and Reiner A. (Eds) Phylogeny and Development of Catecholamine Systems in the CNS of Vertebrates Cambridge University Press, Cambridge, 1994: 183–205. [Google Scholar]
- Kiyashchenko LI, Mileykovskiy BY, Lai YY and Siegel JM Increased and decreased muscle tone with orexin (hypocretin) microinjections in the locus coeruleus and pontine inhibitory area. J. Neurophysiol, 2001, 85: 2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamin OI Sleep in the harp seal (Pagophilus groenlandica). Comparisons of sleep on land and in water. J. Sleep Res, 1993, 2: 170–174. [DOI] [PubMed] [Google Scholar]
- Lyamin OI, Oleksenko AI and Polyakova IG Sleep in the harp seal (Pagophilus groenlandica). Peculiarities of sleep in pups during the first month of their lives. J. Sleep Res, 1993, 2: 163–169. [DOI] [PubMed] [Google Scholar]
- Lyamin OI, Shpak OV, Nazarenko EA and Mukhametov LM Behavioral signs of paradoxical sleep in the beluga whale. J. Sleep Res, 1998, 7: 166. [Google Scholar]
- Lyamin OI, Manger PR, Mukhametov LM, Siegel JM and Shpak OV Rest and activity states in a gray whale. J. Sleep Res, 2000, 9: 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyamin OI, Mukhametov LM, Siegel JM, Nazarenko EA, Polyakova IG and Shpak OV Unihemispheric slow wave sleep and the state of the eyes in a white whale. Behav. Brain Res, 2002, 129: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino L A comparison of encephalization between odontocete cetaceans and anthropoid primates. Brain Behav. Evol, 1998, 51: 230–238. [DOI] [PubMed] [Google Scholar]
- Milsom W, Castellini M, Harris M, Castellini J, Jones D, Berger R, Bahrma S, Rea L and Costa D Effects of hypoxia and hypercapnia on patterns of sleep-associated apnea in elephant seal pups. Am. J. Physiol, 1996, 271: R1017–R1024. [DOI] [PubMed] [Google Scholar]
- Mukhametov LM Unihemispheric slow-wave sleep in the Amazonian dolphin, Inia geoffrensis. Neurosci. Lett, 1987, 79: 128–132. [DOI] [PubMed] [Google Scholar]
- Mukhametov LM The absence of paradoxical sleep in dolphins. In: Koella WP, Obal F, Schulz H and Visser P. (Eds) Sleep 1986, Gustav Fischer Verlag, New York, 1988: 154–156. [Google Scholar]
- Mukhametov LM Paradoxical sleep peculiarities in aquatic mammals. Sleep Res, 1995, 24A: 202. [Google Scholar]
- Mukhametov LM and Lyamin OI Rest and active states in bottlenose dolphins (Tursiops truncatus). J. Sleep Res, 1994, 3: 174. [Google Scholar]
- Mukhametov LM, Supin AY and Polyakova IG Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins. Brain Res, 1977, 134: 581–584. [DOI] [PubMed] [Google Scholar]
- Mukhametov LM, Supin AY and Polyakova IG Sleep in Caspian seals (Phoca caspica). J. High. Nerve Activity, 1984, 34: 259–264 (In Russian). [PubMed] [Google Scholar]
- Mukhametov LM, Lyamin OI, Chetyrbok IS, Vassilyev AA and Diaz R Sleep in an Amazonian manatee, Trichechus inunguis. Experientia, 1992, 48: 417–419. [DOI] [PubMed] [Google Scholar]
- Pilleri G and Gihr M The central nervous system of the Mysticete and Odontocete whales. Invest. Ceteacea, 1970, 2: 89–135. [Google Scholar]
- Pompeiano O Role of locus coeruleus in the static and dynamic control of posture. Arch. Ital. Biol, 2001, 139: 109–124. [PubMed] [Google Scholar]
- Ridgway SH and Brownson RH Relative brain sizes and cortical surface areas in odontocetes. Acta. Zool. Fennica, 1984, 172: 149–152. [Google Scholar]
- Ridgway SH, Harrison RJ and Joyce PL Sleep and cardiac rhythm in the gray seal. Science, 1975, 187: 553–555. [DOI] [PubMed] [Google Scholar]
- Sander K The evolution of patterning mechanisms: gleanings from insect embryogenesis and spermatogenesis. In: Goodwin BC and Holder N and Wylie CC. (Eds) Development and Evolution Cambridge University Press, Cambridge, 1983: 137–159. [Google Scholar]
- Siegel JM Brainstem Mechanisms Generating REM Sleep. In: Kryger MK, Roth T and Dement WC. (Eds) Principals and Practice of Sleep Medicine, 2nd edn. Saunders, New York, 2000: 112–133. [Google Scholar]
- Smeets WJAJ and González A Catecholamine systems in the brain of vertebrates: new perspectives through a comparative approach. Brain Res. Rev, 2000, 33: 308–379. [DOI] [PubMed] [Google Scholar]
- Wu MF, Gulyani SA, Yai E, Mignot E, Phan B and Siegel JM Locus coeruleus neurons: cessation of activity during cataplexy. Neuroscience, 1999, 91: 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]


