Summary
Background
Tuberculosis is a major contributor to the global burden of disease, causing more than a million deaths annually. Given an emphasis on equity in access to diagnosis and treatment of tuberculosis in global health targets, evaluations of differences in tuberculosis burden by sex are crucial. We aimed to assess the levels and trends of the global burden of tuberculosis, with an emphasis on investigating differences in sex by HIV status for 204 countries and territories from 1990 to 2019.
Methods
We used a Bayesian hierarchical Cause of Death Ensemble model (CODEm) platform to analyse 21 505 site-years of vital registration data, 705 site-years of verbal autopsy data, 825 site-years of sample-based vital registration data, and 680 site-years of mortality surveillance data to estimate mortality due to tuberculosis among HIV-negative individuals. We used a population attributable fraction approach to estimate mortality related to HIV and tuberculosis coinfection. A compartmental meta-regression tool (DisMod-MR 2.1) was then used to synthesise all available data sources, including prevalence surveys, annual case notifications, population-based tuberculin surveys, and tuberculosis cause-specific mortality, to produce estimates of incidence, prevalence, and mortality that were internally consistent. We further estimated the fraction of tuberculosis mortality that is attributable to independent effects of risk factors, including smoking, alcohol use, and diabetes, for HIV-negative individuals. For individuals with HIV and tuberculosis coinfection, we assessed mortality attributable to HIV risk factors including unsafe sex, intimate partner violence (only estimated among females), and injection drug use. We present 95% uncertainty intervals for all estimates.
Findings
Globally, in 2019, among HIV-negative individuals, there were 1·18 million (95% uncertainty interval 1·08–1·29) deaths due to tuberculosis and 8·50 million (7·45–9·73) incident cases of tuberculosis. Among HIV-positive individuals, there were 217 000 (153 000–279 000) deaths due to tuberculosis and 1·15 million (1·01–1·32) incident cases in 2019. More deaths and incident cases occurred in males than in females among HIV-negative individuals globally in 2019, with 342 000 (234 000–425 000) more deaths and 1·01 million (0·82–1·23) more incident cases in males than in females. Among HIV-positive individuals, 6250 (1820–11 400) more deaths and 81 100 (63 300–100 000) more incident cases occurred among females than among males in 2019. Age-standardised mortality rates among HIV-negative males were more than two times greater in 105 countries and age-standardised incidence rates were more than 1·5 times greater in 74 countries than among HIV-negative females in 2019. The fraction of global tuberculosis deaths among HIV-negative individuals attributable to alcohol use, smoking, and diabetes was 4·27 (3·69–5·02), 6·17 (5·48–7·02), and 1·17 (1·07–1·28) times higher, respectively, among males than among females in 2019. Among individuals with HIV and tuberculosis coinfection, the fraction of mortality attributable to injection drug use was 2·23 (2·03–2·44) times greater among males than females, whereas the fraction due to unsafe sex was 1·06 (1·05–1·08) times greater among females than males.
Interpretation
As countries refine national tuberculosis programmes and strategies to end the tuberculosis epidemic, the excess burden experienced by males is important. Interventions are needed to actively communicate, especially to men, the importance of early diagnosis and treatment. These interventions should occur in parallel with efforts to minimise excess HIV burden among women in the highest HIV burden countries that are contributing to excess HIV and tuberculosis coinfection burden for females. Placing a focus on tuberculosis burden among HIV-negative males and HIV and tuberculosis coinfection among females might help to diminish the overall burden of tuberculosis. This strategy will be crucial in reaching both equity and burden targets outlined by global health milestones.
Funding
Bill & Melinda Gates Foundation.
Introduction
Tuberculosis is an important contributor to morbidity and mortality globally.1 Despite being preventable and treatable, tuberculosis is a leading cause of death from a single infectious agent.2, 3 The Sustainable Development Goals (SDGs) and the WHO End TB Strategy have defined targets for ending the tuberculosis epidemic that aim to reduce tuberculosis mortality by 95% and incidence by 90% by 2035.4, 5 An understanding of the trends in tuberculosis burden is crucial to evaluate progress towards ending the epidemic and informing policy and programmes.
Research in context.
Evidence before this study
Globally, tuberculosis is a leading cause of death from a single infectious agent, contributing more than a million deaths annually. The global sex-specific burden of tuberculosis has been estimated by various groups, including the WHO Global Tuberculosis Programme and the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD). However, differences in the levels and trends of the global burden of tuberculosis by HIV status, along with the contribution of modifiable risk factors by sex, have not been extensively characterised. We searched PubMed with the terms “tuberculosis” AND (“burden” OR “estimates”) AND (“sex” OR “gender”) AND (“differences” OR “discrepancies” OR “disparities”), with no language restrictions, for publications up to Dec 17, 2020. Our search identified ten studies that reported population-based tuberculosis burden estimates and, of these studies, two closely examined sex differences. One of these studies quantified sex differences in tuberculosis prevalence surveys from 2001 to 2014 for selected countries. Neither of these studies systematically examined sex differences in tuberculosis mortality and incidence, along with the contribution of modifiable risk factors, over time.
Added value of this study
This iteration of GBD provides tuberculosis mortality and morbidity estimates for nine new small or island countries and territories (Cook Islands, Monaco, San Marino, Nauru, Niue, Palau, Saint Kitts and Nevis, Tokelau, and Tuvalu). It also includes many new data sources and a multitude of advances in tuberculosis modelling methods. Moreover, we report for the first time how sex differences vary by sociodemographic development and the burden of HIV and tuberculosis coinfection attributable to potentially modifiable HIV risk factors (unsafe sex, intimate partner violence, and injection drug use). We also report a detailed assessment of sex differences across different measures of tuberculosis burden disaggregated by HIV status to enhance understanding of excess tuberculosis burden by sex globally. Finally, we report for the first time age-standardised, risk-deleted mortality rates along with the male-to-female ratio of risk-deleted mortality rates. Our data confirm findings from previous studies that the burden of tuberculosis is greater in males than in females. We found that males experienced substantially greater tuberculosis burden globally, with many countries estimated to have 50% higher incidence and 100% higher mortality rates for males than for females among HIV-negative individuals. Across all sociodemographic development levels, tuberculosis mortality rates were consistently greater among HIV-negative males than HIV-negative females. Among individuals with HIV and tuberculosis coinfection, mortality was higher among females than males, with unsafe sex and intimate partner violence being large contributors to the differences in the countries with the highest HIV burden.
Implications of all the available evidence
Smoking, alcohol consumption, and timely access to tuberculosis services represent challenges and opportunities for reducing the excess burden of tuberculosis among men. Developing strategies to prevent tuberculosis among males, while also reducing the burden of HIV and tuberculosis coinfection, especially among females, will be crucial in achieving equity targets outlined by the WHO End TB Strategy.
Despite an emphasis on equity in access to tuberculosis diagnosis and treatment by the WHO End TB Strategy, differences in tuberculosis burden by sex have received little attention. The available evidence indicates that men are less likely to seek or have access to tuberculosis care than women.6, 7, 8 A systematic review and meta-analysis of tuberculosis prevalence surveys in 28 countries found that males have 2·21 times higher prevalence of bacteriologically confirmed tuberculosis than females.6 For global case notifications, studies have estimated that males have a 50–70% higher incidence rate than females in selected low-income and middle-income countries.6, 9 Although previous Global Burden of Diseases, Injuries, and Risk Factors Study (GBD)2, 10 and WHO3 tuberculosis modelling efforts have presented sex-specific tuberculosis burden estimates, there has yet to be a systematic comparable quantification of these sex differences by HIV status, along with the contribution of modifiable risk factors. Few global sex differences are observed in age-adjusted prevalence of latent tuberculosis infection;11 this finding is a possible indicator that men have heightened exposure to risk factors for the development of active tuberculosis that might partially explain the sex differences. One study found that cigarette consumption explains 33% of the variation in the sex ratio of tuberculosis notifications.12 Although indicative, other important risk factors for tuberculosis, such as diabetes, alcohol consumption, and HIV infection, have yet to be considered in analyses of sex differences at the global level.
For GBD 2019, we assess the levels and trends of the global burden of tuberculosis, with an emphasis on investigating differences by sex. We focus on reporting tuberculosis mortality and incidence by HIV status and sex from 1990 to 2019, for 204 countries and territories. We also analyse how sex differences vary by the Socio-demographic Index (SDI),13 a composite indicator based on income, education, and fertility. Finally, we estimate the percentage of tuberculosis deaths, among HIV-negative individuals, attributable to the independent effects of risk factors including smoking, alcohol consumption, and diabetes. We also examine the proportion of deaths among people with HIV and tuberculosis coinfection that are attributable to HIV risk factors, including unsafe sex, intimate partner violence, and injection drug use.
Methods
Overview
Detailed methods for GBD and on tuberculosis estimation in GBD have been previously published.1, 2, 10 Here, we provide a brief description of the methods and estimation strategy for tuberculosis and risk factors. Detailed descriptions for each step of the estimation process with flow charts are provided in the appendix (pp 5–6). In compliance with the Guidelines for Accurate and Transparent Health Estimates Reporting, input data sources and code for each step of the estimation process are available on the Global Health Data Exchange. This manuscript was produced as part of the GBD Collaborator Network and in accordance with the GBD protocol.
Tuberculosis mortality
The GBD Cause of Death database collates all available data from vital registration systems, surveillance systems, and verbal autopsies. For modelling mortality due to tuberculosis among HIV-negative individuals, we included 21 505 site-years of vital registration data, 705 site-years of verbal autopsy data, 825 site-years of sample-based vital registration data, and 680 site-years of mortality surveillance data. We processed raw data to reconcile discrepancies in coding schemes, redistribute garbage codes (eg, ill-defined codes and intermediate causes) to underlying causes of death, disaggregate data by age and sex, and adjust for misclassified HIV deaths.1
We modelled tuberculosis mortality among HIV-negative individuals using the Cause of Death Ensemble model (CODEm) framework with separate models by sex.1, 14 CODEm produces a wide range of submodels with different functional forms for the outcome variable (mortality rate or cause fraction), with varying combinations of predictive covariates (eg, alcohol consumption, diabetes, smoking prevalence, and Healthcare Access and Quality [HAQ] Index15). A full list of covariates used in the CODEm framework can be found in the appendix (p 13). The ensemble of models was then selected on the basis of CODEm performance on out-of-sample predictive validity tests. Tuberculosis mortality among HIV-positive individuals was estimated using a population attributable fraction approach consistent with previous GBD cycles.2, 11
Tuberculosis morbidity
Consistent with previous GBD iterations,2, 11 we used a Bayesian meta-regression modelling tool, DisMod-MR 2.11 to simultaneously model tuberculosis incidence, prevalence, and cause-specific mortality. Details on case definitions and how these data are processed are in the appendix (pp 3–4, 20–22).
We made several improvements to our modelling approach in GBD 2019. First, we standardised our approach to adjust prevalence data when the case definition was smear-positive tuberculosis rather than bacteriologically positive tuberculosis, using all available prevalence surveys that provided comparisons. We used the same approach to compute an adjustment factor to recalibrate studies that used symptoms only as a screening method compared with studies using both symptoms and chest x-ray during screening (appendix pp 21–22). Second, we used a novel Bayesian meta-regression method16 using age-sex-specific mortality-to-incidence ratios (logit transformed) from locations with high-quality data ratings on cause of death data as input. In our meta-regression analysis, we used the HAQ Index15 as the primary covariate, with GBD super-region fixed effects. After model calibration, we predicted age-sex-specific mortality-to-incidence ratios as a function of the HAQ Index for all locations and years similar to the previous GBD iteration.11 Third, we used tuberculosis duration and excess mortality rate data as priors in our morbidity modelling strategy to help create coherent estimates in modelling (appendix pp 22–23).
We used age-sex-specific case notifications as input data for locations with high-quality data ratings on causes of death data,1 predicted mortality-to-incidence ratio-based incidence for locations with low-quality data ratings for causes of death data, prevalence survey data where available, and estimates of excess mortality rate, remission, and cause-specific mortality as input to DisMod-MR 2.117 to generate estimates that are consistent with each other. Sex-specific results were generated in DisMod-MR 2.1 by using sex-specific inputs and incorporating a covariate for sex. As in previous GBD iterations, we applied the proportion of cases of HIV and tuberculosis coinfection among all cases of tuberculosis to incidence and prevalence to distinguish between HIV and tuberculosis coinfection and tuberculosis without HIV.4, 11
Risk factors
Methods for risk factor attribution to tuberculosis disaggregated by HIV status have previously been published in detail.10, 18 Briefly, we included risk–outcome pairs with convincing or probable evidence to compute the proportion of disease burden attributable to risk factors by estimating the relative risk of the outcome as a function of exposure values, estimating the global prevalence of exposures, and determining the theoretical minimum risk exposure level (TMREL) using all available global data sources (appendix pp 138–170). Next, estimates of attributable mortality were computed by multiplying the number of tuberculosis deaths by the population attributable fraction (PAF) for the risk–outcome pair for a given age, sex, location, and year. The PAF is the fraction of tuberculosis deaths that would be reduced if the exposure to the risk factor was at the TMREL. In addition to PAFs, we computed risk-deleted mortality rates, defined as the death rate that would had been observed if the risk factors of interest were set to their TMREL.18 For tuberculosis among HIV-negative individuals, we examined the fractions of deaths attributable to alcohol consumption, smoking, and diabetes. For HIV and tuberculosis coinfection, we focused on the fractions of deaths attributable to HIV risk factors including unsafe sex, intimate partner violence (only estimated among females), and injection drug use.
SDI and data presentation
The SDI is a composite indicator, ranging from 0 to 1, of a country's total fertility rate for individuals younger than 25 years, average years of schooling, and lag-distributed income per capita.1 The index was categorised by quintiles using country-level estimates of the SDI in 2019. To investigate differences by sex, we present male-to-female (sex) ratios as the quotient of tuberculosis burden in males divided by tuberculosis burden in females. These ratios were computed for tuberculosis mortality rates, incidence rates, and PAFs. We use the GBD world population age standard to derive age-standardised rates for mortality and incidence. We present 95% uncertainty intervals (UIs) for every tuberculosis estimate based on the 2·5th and 97·5th percentiles of the posterior distributions that are carried over from each step in analyses.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Globally, in 2019, among HIV-negative individuals, we estimated that there were 8·50 million (95% UI 7·45–9·73) tuberculosis incident cases and 1·18 million (1·08–1·29) deaths due to tuberculosis. In 1990, these figures were 8·76 million (7·59–10·06) tuberculosis incident cases and 1·78 million (1·63–1·92) deaths. Among HIV-positive individuals, we estimated that there were 1·15 million (1·01–1·32) tuberculosis incident cases and 217 000 (153 000–279 000) deaths due to tuberculosis in 2019 (appendix pp 62–77). The corresponding figures in 1990 for HIV-positive individuals were 507 000 (449 000–571 000) cases and 119 000 (89 700–159 000) deaths. Overall, HIV and tuberculosis coinfection constituted 12·0% (11·3–12·4) of the global 9·65 million (8·48–11·03) incident cases of tuberculosis and 15·5% (9·6–21·3) of the global 1·40 million (1·28–1·53) deaths due to tuberculosis in 2019.
Globally, in 2019, more incident cases and deaths occurred in males than in females among HIV-negative individuals, with 1·01 million (95% UI 0·82–1·23) more incident cases and 342 000 (234 000–425 000) more deaths among males than females (table). Among HIV-positive individuals, however, 81 100 (63 300–100 000) more incident cases and 6250 (1820–11 400) more deaths occurred among females than among males (appendix p 62). Globally, 56·0% (54·4–57·5) of incident cases and 64·5% (59·8–67·8) of deaths occurred among males in 2019.
Table.
Tuberculosis incident cases and deaths and age-standardised rates of tuberculosis incidence and mortality per 100 000 population among HIV-negative males and females by GBD regions and SDI quintiles, 2019
|
Incidence |
Mortality |
|||||||
|---|---|---|---|---|---|---|---|---|
| Female cases | Rate (per 100 000 females) | Male cases | Rate (per 100 000 males) | Female deaths | Rate (per 100 000 females) | Male deaths | Rate (per 100 000 males) | |
| Global | 3 740 000 (3 270 000–4 310 000) | 94·9 (83·0–109) | 4 750 000 (4 160 000–5 440 000) | 120 (105–136) | 419 000 (366 000–502 000) | 10·1 (8·81–12·1) | 761 000 (688 000–836 000) | 19·7 (17·9–21·6) |
| Low SDI | 938 000 (815 000–1 080 000) | 196 (173–223) | 964 000 (847 000–1 110 000) | 228 (201–259) | 135 000 (115 000–168 000) | 43·4 (37·2–54·7) | 234 000 (204 000–269 000) | 77·5 (68·7–87·6) |
| Low-middle SDI | 1 460 000 (1 260 000–1 700 000) | 170 (148–196) | 1 660 000 (1 420 000–1 920 000) | 202 (175–233) | 176 000 (144 000–220 000) | 23·8 (19·5–29·8) | 312 000 (271 000–353 000) | 45·2 (39·2–51·1) |
| Middle SDI | 1 020 000 (897 000–1 160 000) | 83·3 (73·2–94·5) | 1 340 000 (1 180 000–1 520 000) | 107 (95·3–121) | 86 700 (76 700–103 000) | 7·06 (6·25–8·42) | 168 000 (153 000–186 000) | 14·7 (13·5–16·2) |
| High-middle SDI | 265 000 (230 000–304 000) | 33·8 (29·4–39·1) | 487 000 (423 000–561 000) | 58·5 (51·1–67·1) | 15 000 (13 400–17 700) | 1·53 (1·37–1·79) | 38 300 (34 600–42 500) | 4·33 (3·92–4·80) |
| High SDI | 49 600 (43 100–56 900) | 8·33 (7·20–9·62) | 74 500 (65 100–85 600) | 11·9 (10·4–13·8) | 5450 (4380–6250) | 0·470 (0·395–0·551) | 8450 (7620–9180) | 1·02 (0·918–1·11) |
| Central Europe, eastern Europe, and central Asia | 78 200 (66 300–92 800) | 34·4 (29·1–41·0) | 159 000 (133 000–191 000) | 69·7 (58·3–83·3) | 5210 (4610–5920) | 1·95 (1·72–2·22) | 15 700 (14 100–17 500) | 6·40 (5·74–7·12) |
| Central Asia | 21 500 (18 500–24 900) | 44·9 (38·8–52·1) | 30 600 (26 200–35 800) | 67·7 (58·4–78·2) | 1910 (1630–2250) | 4·09 (3·49–4·81) | 4140 (3690–4700) | 9·69 (8·67–10·9) |
| Central Europe | 6710 (5790–7790) | 9·67 (8·30–11·3) | 13 400 (11 300–15 900) | 18·8 (16·1–22·1) | 519 (451–591) | 0·495 (0·430–0·570) | 1620 (1390–1850) | 1·96 (1·70–2·24) |
| Eastern Europe | 50 000 (41 400–60 500) | 43·1 (35·8–52·2) | 115 000 (94 400–141 000) | 99·2 (81·9–120) | 2780 (2290–3290) | 1·90 (1·56–2·25) | 9960 (8480–11 500) | 7·80 (6·65–9·01) |
| High income | 39 600 (34 400–45 500) | 6·10 (5·26–7·05) | 58 000 (50 300–67 100) | 8·95 (7·75–10·4) | 5430 (4350–6140) | 0·391 (0·329–0·434) | 7750 (6990–8280) | 0·825 (0·748–0·882) |
| Australasia | 933 (801–1100) | 6·19 (5·30–7·34) | 929 (808–1070) | 6·04 (5·20–7·04) | 47·7 (36·3–59·0) | 0·166 (0·128–0·202) | 64·8 (51·2–82·8) | 0·280 (0·221–0·356) |
| High-income Asia Pacific | 18 800 (16 100–21 900) | 13·0 (11·1–15·1) | 28 500 (24 300–33 600) | 19·9 (17·1–23·3) | 2830 (2130–3290) | 0·797 (0·635–0·908) | 4260 (3710–4660) | 2·02 (1·79–2·22) |
| High-income North America | 3930 (3360–4610) | 1·88 (1·60–2·22) | 5990 (5200–6960) | 2·85 (2·46–3·32) | 440 (393–480) | 0·129 (0·117–0·139) | 689 (639–725) | 0·254 (0·235–0·267) |
| Southern Latin America | 3660 (3180–4260) | 10·4 (8·91–12·2) | 4660 (4010–5460) | 13·7 (11·8–16·0) | 469 (411–534) | 1·05 (0·930–1·19) | 770 (726–817) | 2·15 (2·03–2·29) |
| Western Europe | 12 300 (10 600–14 300) | 5·78 (4·88–6·83) | 17 900 (15 400–20 900) | 8·30 (6·98–9·86) | 1640 (1350–1870) | 0·266 (0·227–0·299) | 1970 (1800–2130) | 0·483 (0·445–0·519) |
| Latin America and Caribbean | 67 700 (58 200–78 400) | 21·9 (18·9–25·3) | 102 000 (87 100–119 000) | 34·7 (29·9–40·3) | 6280 (5360–7660) | 2·01 (1·72–2·45) | 11 800 (10 500–13 300) | 4·25 (3·79–4·78) |
| Andean Latin America | 17 900 (15 300–20 900) | 55·9 (47·7–65·2) | 24 000 (20 500–28 100) | 76·3 (65·5–88·7) | 1610 (1270–2070) | 5·36 (4·24–6·87) | 2620 (2050–3280) | 9·26 (7·26–11·5) |
| Caribbean | 8410 (7200–9710) | 35·3 (30·1–40·9) | 7160 (6190–8280) | 30·1 (26·0–34·8) | 1010 (755–1510) | 4·04 (3·02–6·07) | 1300 (1050–1570) | 5·49 (4·42–6·59) |
| Central Latin America | 19 400 (16 800–22 300) | 14·8 (12·8–16·9) | 26 000 (22 400–30 100) | 21·6 (18·7–24·9) | 2040 (1650–2620) | 1·60 (1·29–2·05) | 3730 (3150–4410) | 3·34 (2·82–3·94) |
| Tropical Latin America | 21 900 (18 500–25 900) | 18·0 (15·2–21·2) | 44 800 (37 800–53 200) | 38·4 (32·5–45·3) | 1620 (1440–1810) | 1·26 (1·12–1·41) | 4110 (3880–4350) | 3·64 (3·43–3·86) |
| North Africa and Middle East | 79 600 (68 000–94 200) | 28·1 (24·1–32·9) | 73 900 (63 300–86 300) | 24·6 (21·4–28·4) | 7470 (5800–10 800) | 3·37 (2·66–5·09) | 8030 (6510–9890) | 3·50 (2·90–4·23) |
| South Asia | 1 750 000 (1 500 000–2 040 000) | 201 (174–232) | 2 070 000 (1 770 000–2 410 000) | 240 (206–278) | 188 000 (152 000–239 000) | 25·6 (20·7–32·8) | 334 000 (280 000–393 000) | 46·5 (39·2–54·5) |
| Southeast Asia, east Asia, and Oceania | 707 000 (628 000–791 000) | 62·3 (54·9–69·8) | 1 180 000 (1 060 000–1 320 000) | 99·5 (88·8–111) | 67 600 (59 600–77 700) | 5·30 (4·69–6·09) | 133 000 (120 000–148 000) | 11·8 (10·7–13·0) |
| East Asia | 260 000 (230 000–294 000) | 31·7 (27·9–35·5) | 503 000 (449 000–567 000) | 57·4 (51·4–64·1) | 11 400 (9270–14 300) | 1·15 (0·945–1·43) | 29 300 (24 000–35 200) | 3·40 (2·84–4·01) |
| Oceania | 6480 (5630–7440) | 107 (95–121) | 5840 (5180–6490) | 102 (91·9–111) | 679 (373–1030) | 15·7 (8·75–23·6) | 1190 (813–1620) | 27·4 (18·9–37·0) |
| Southeast Asia | 440 000 (390 000–495 000) | 130 (115–145) | 671 000 (593 000–753 000) | 207 (185–230) | 55 500 (48 200–64 400) | 17·9 (15·6–20·7) | 103 000 (90 000–117 000) | 38·2 (33·9–42·9) |
| Sub-Saharan Africa | 1 020 000 (875 000–1 190 000) | 209 (182–239) | 1 110 000 (983 000–1 270 000) | 276 (245–312) | 139 000 (115 000–171 000) | 47·5 (40·0–60·2) | 251 000 (217 000–290 000) | 94·3 (82·7–107) |
| Central sub-Saharan Africa | 169 000 (145 000–195 000) | 289 (253–328) | 155 000 (136 000–175 000) | 325 (291–362) | 27 800 (19 600–40 900) | 75·3 (51·6–112) | 41 400 (31 600–53 400) | 136 (105–172) |
| Eastern sub-Saharan Africa | 409 000 (347 000–481 000) | 231 (202–266) | 502 000 (437 000–579 000) | 334 (295–380) | 58 900 (47 800–76 500) | 59·4 (48·8–77·9) | 113 000 (92 200–133 000) | 115 (94·6–135) |
| Southern sub-Saharan Africa | 160 000 (130 000–193 000) | 372 (307–447) | 130 000 (113 000–148 000) | 321 (285–360) | 10 300 (8860–12 100) | 29·2 (25·5–33·9) | 24 900 (22 100–27 800) | 88·2 (79·8–97·3) |
| Western sub-Saharan Africa | 286 000 (247 000–330 000) | 152 (134–174) | 328 000 (288 000–379 000) | 210 (184–240) | 41 600 (29 700–56 600) | 36·3 (25·0–53·4) | 72 000 (59 000–86 100) | 67·6 (56·1–80·2) |
95% uncertainty intervals are shown in parentheses. GBD=Global Burden of Diseases, Injuries, and Risk Factors Study. SDI=Socio-demographic Index.
In HIV-negative people, the global age-standardised tuberculosis incidence rate per 100 000 population in 2019 was 120 (95% UI 105–136) among males and 94·9 (83·0–109) among females, and the global age-standardised tuberculosis mortality rate per 100 000 population was 19·7 (17·9–21·6) among males and 10·1 (8·81–12·1) among females (table). At the regional level, among HIV-negative individuals, age-standardised incidence rates were nearly two times higher in eastern Europe, tropical Latin America, and central Europe, and age-standardised mortality rates were more than three times higher in eastern Europe, central Europe, and southern sub-Saharan Africa, among males than among females.
Among HIV-positive individuals, the global age-standardised tuberculosis incidence rate per 100 000 population in 2019 was 13·2 (95% UI 11·6–15·0) among males and 15·5 (13·5–17·7) among females, and the global age-standardised tuberculosis mortality rate per 100 000 population was 2·61 (1·83–3·35) among males and 2·81 (1·98–3·60) among females (appendix pp 61–75). The regions where males with HIV had the largest relative excess burden compared with females with HIV were in high-income Asia Pacific, Australasia, and central Latin America for both incidence and mortality, but females had higher age-standardised rates for both measures in almost every GBD region in Africa.
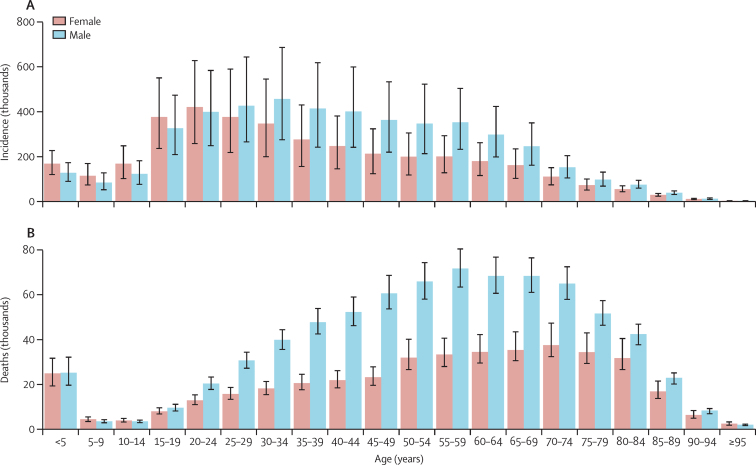
The majority of incident cases (78·0% [95% UI 73·5–81·8]) and deaths (58·3% [56·8–59·8]) in 2019 were in individuals aged 15–64 years (figure 1). For tuberculosis incidence among HIV-negative individuals, females had slightly greater numbers of incident cases for age groups younger than 25 years, but more incident cases occurred in males throughout most adult age groups. The differences between sexes became smaller in the older age groups. For tuberculosis mortality in HIV-negative individuals, trends were similar to those observed for incident infections, but compared with females, males had more deaths starting at age 15–19 years (figure 1). Among HIV-positive people, females generally had more cases and deaths than males in the reproductive age groups, in which most cases and deaths occurred (appendix p 46).
Figure 1.
Global age-sex distribution of tuberculosis incident cases (A) and deaths (B) in HIV-negative individuals, 2019
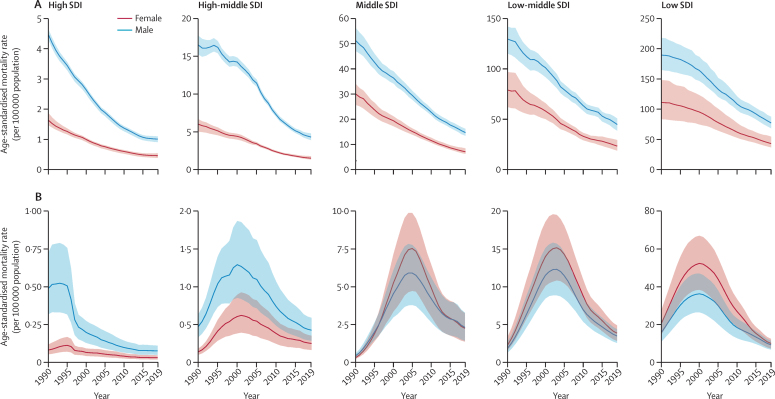
Figure 2 shows trends in age-standardised tuberculosis mortality rate per 100 000 population among HIV-negative individuals and HIV-positive individuals by SDI quintile and sex. Across SDI quintiles, sharp declines in age-standardised tuberculosis mortality from 1990 to 2019 occurred in both males and females among HIV-negative individuals. Males consistently had higher mortality than females across the time period. Although the relative gap between males and females without HIV declined for the high SDI quintile, the gap remained the same or slightly increased in the remaining SDI quintiles (appendix p 41). For people with HIV and tuberculosis coinfection, there were sharp declines in age-standardised mortality after each SDI quintile's peak in tuberculosis mortality. In the high SDI and high-middle SDI quintiles, males with HIV and tuberculosis coinfection consistently had higher mortality than females. In the low and middle SDI quintiles, however, females with HIV and tuberculosis coinfection had higher mortality than males throughout most of the time series. The relative gap between females and males diminished substantially in the low and middle SDI quintiles after peaks in HIV and tuberculosis coinfection mortality (appendix p 41).
Figure 2.
Temporal trends of age-standardised tuberculosis mortality rate per 100 000 population among HIV-negative individuals (A) and HIV-positive individuals (B) by SDI quintile and sex, 1990–2019
SDI=Socio-demographic Index.
Among HIV-negative individuals, age-standardised tuberculosis incidence rates were more than 300 per 100 000 population in 21 countries for males and in ten countries for females (appendix pp 42, 48–61). Age-standardised tuberculosis mortality rates were more than 100 per 100 000 population in 19 countries for males and in three for females (appendix pp 43, 48–61). Among HIV-positive individuals, the estimated age-standardised incidence rate was more than 300 per 100 000 population in eight countries for females and four for males (appendix pp 44, 62–77). There were similar sex differences in mortality in people with HIV and tuberculosis coinfection: nine countries had an estimated age-standardised mortality rate of more than 50 per 100 000 population for females compared with eight countries for males (appendix pp 45, 62–77).
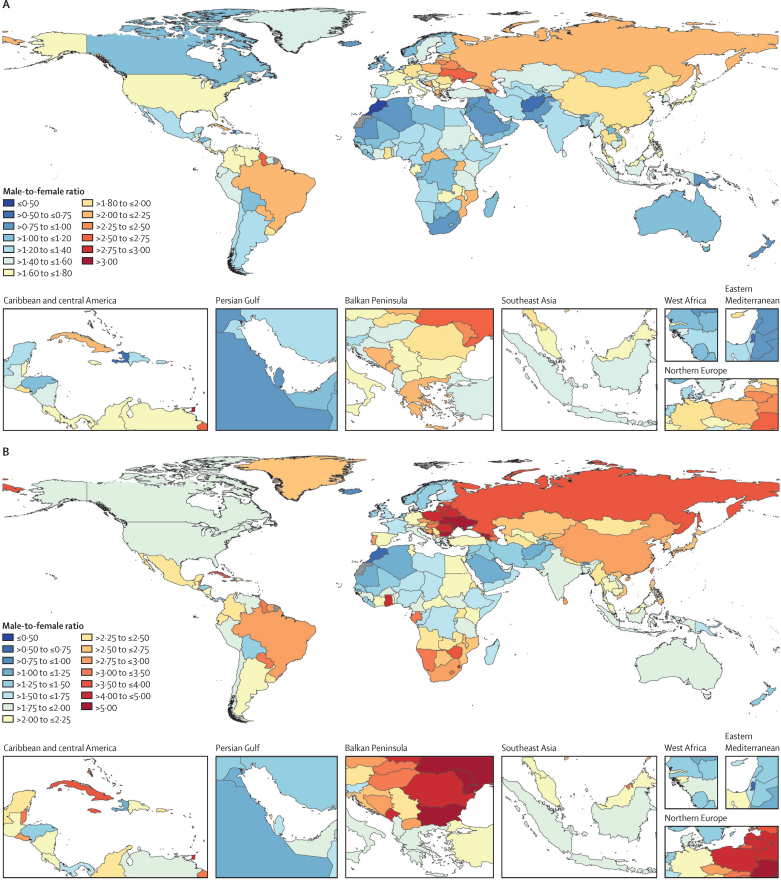
Figure 3 maps the ratio between males and females for age-standardised incidence rates and mortality rates for tuberculosis in HIV-negative individuals in 2019. 74 countries had age-standardised incidence rates that were at least 1·5 times greater and 21 countries had age-standardised incidence rates that were at least two times greater among males than females. The countries with the largest ratios between males and females for age-standardised incidence rates were Armenia, Trinidad and Tobago, Guyana, Ukraine, and Belarus. For age-standardised mortality rates, 105 countries had rates more than two times greater and 17 countries had rates more than four times greater among males than females. Armenia, Georgia, Ukraine, Saint Vincent and the Grenadines, and Moldova had the greatest ratios in age-standardised mortality rates between males and females in 2019. The ratios between males and females for age-standardised incidence and mortality were similar between countries with high and low estimated tuberculosis burdens among HIV-negative individuals. For example, the respective ratios for incidence and mortality were 1·81 (95% UI 1·76–1·88) and 3·04 (2·12–3·98) for China, 1·77 (1·70–1·84) and 2·67 (1·84–3·72) for the Philippines, and 1·24 (1·20–1·29) and 1·91 (1·34–2·62) for India, whereas the respective ratios in countries with lower tuberculosis burden were 1·62 (1·54–1·72) and 2·00 (1·80–2·17) for the USA, 1·61 (1·53–1·69) and 2·55 (2·30–2·96) for Japan, and 1·26 (1·16–1·37) and 1·55 (1·33–2·02) for Finland.
Figure 3.
Male-to-female ratio of age-standardised incidence (A) and mortality (B) rates among HIV-negative individuals by geography, 2019
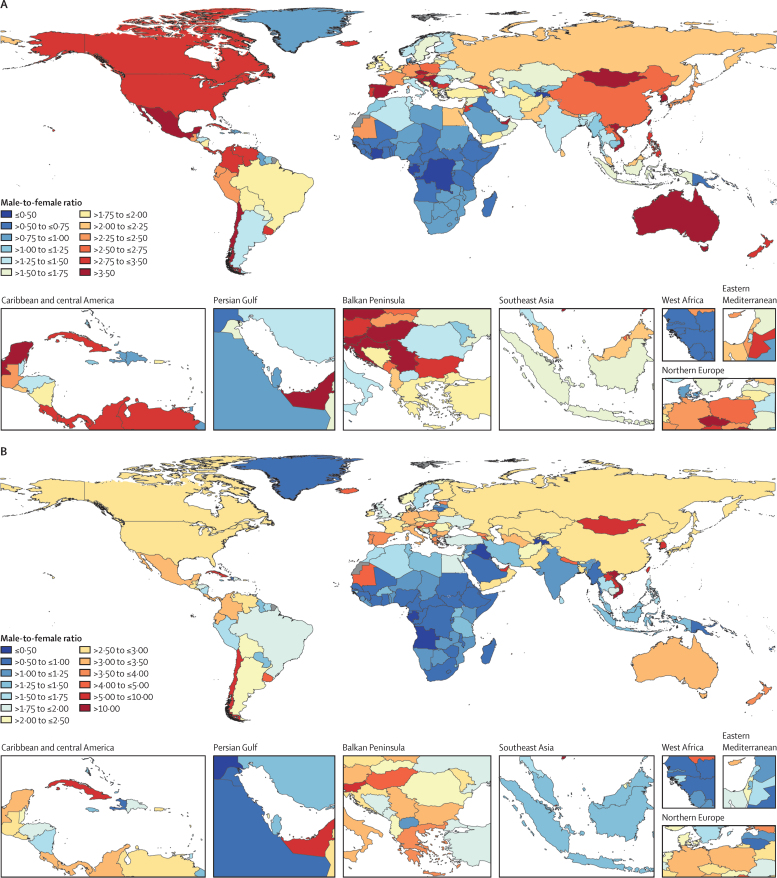
Among HIV-positive individuals, 66 countries and 24 countries had age-standardised incidence rates that were more than two times and three times greater, respectively, among males than females (figure 4A). For age-standardised mortality rates, 87 countries and 16 countries had rates that were more than two times and four times greater, respectively, among males than females (figure 4B). Countries where overall estimated HIV and tuberculosis coinfection burden was low had the largest male-to-female ratios. Conversely, the countries with the highest estimated HIV and tuberculosis coinfection burden (eg, Lesotho, Eswatini, Botswana, Zimbabwe, and South Africa) had male-to-female ratios between 0·90 and 1·24 for age-standardised mortality, but these same countries had ratios below 1 for age-standardised incidence (ratios between 0·76 and 0·94). Overall, 35 and 56 countries had male-to-female ratios below 0·75 (female-to-male ratio 1·33) for age-standardised incidence and mortality among HIV-positive individuals, respectively.
Figure 4.
Male-to-female ratio of age-standardised incidence (A) and mortality (B) rates among HIV-positive individuals by geography, 2019
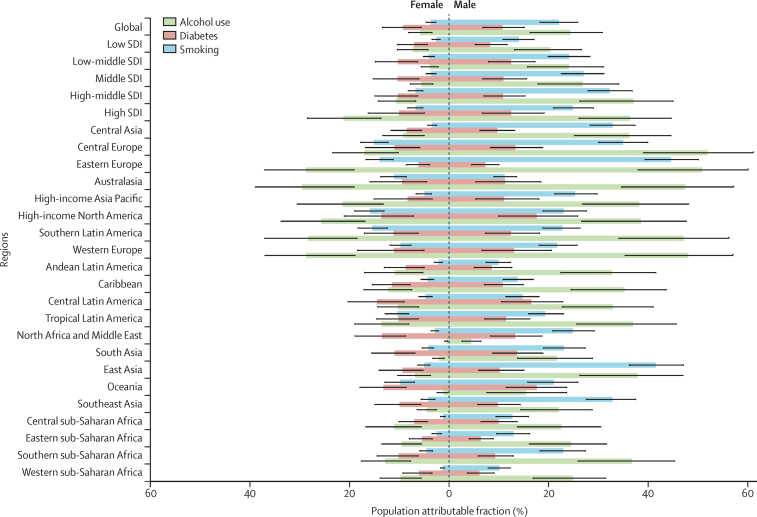
Globally, in 2019, among HIV-negative individuals, the PAF of tuberculosis deaths due to alcohol was 17·76% (95% UI 11·60–22·83), due to smoking was 15·52% (12·67–18·33), and due to diabetes was 10·20% (6·37–14·47). The global all-age PAFs for tuberculosis deaths due to alcohol, smoking, and diabetes were 4·27 (3·69–5·02), 6·17 (5·48–7·02), and 1·17 (1·07–1·28) times higher, respectively, among males than females in 2019 (figure 5). The regions with the largest sex ratios in the PAF of tuberculosis deaths due to alcohol were in south Asia (11·20 [8·38–15·08]), north Africa and the Middle East (8·53 [6·66–11·53]), and east Asia (5·77 [4·32–7·81]); those due to smoking were in central sub-Saharan Africa (10·59 [8·61–13·08]), central Asia (9·80 [8·18–12·15]), and north Africa and the Middle East (8·86 [7·57–10·99]); and those due to diabetes were in central sub-Saharan Africa (1·44 [1·28–1·62]), high-income Asia Pacific (1·36 [1·14–1·66]), and Oceania (1·34 [1·20–1·50]). If the combined effects of alcohol, smoking, and diabetes were removed, HIV-negative males would still have a modestly higher age-standardised mortality rate than HIV-negative females at the global level (sex ratio 1·28 [1·05–1·49]; appendix pp 104–18).
Figure 5.
All-age population attributable fractions of tuberculosis deaths due to alcohol use, diabetes, and smoking among HIV-negative men and women by GBD region, 2019
GBD=Global Burden of Diseases, Injuries, and Risk Factors Study.
Among both male and female HIV-positive individuals in 2019, the global PAF of tuberculosis deaths due to unsafe sex was 82·47% (95% UI 80·42–84·25), due to drug use was 5·59% (4·44–7·00), and due to intimate partner violence was 8·04% (4·22–12·42). The global all-age PAF for tuberculosis mortality in people with HIV due to injection drug use was 2·23 (2·03–2·44) times greater among males than females, and the PAF due to unsafe sex was 1·06 (1·05–1·08) times greater among females than males (appendix p 47). The regions with the largest sex ratios in the PAF due to injection drug use between male and females with HIV were in east Asia (5·30 [4·00–6·85]), southeast Asia (5·05 [4·23–5·70]), and central Europe (3·02 [2·82–3·27]). Southern sub-Saharan Africa (19·71% [12·03–29·94]), central sub-Saharan Africa (18·95% [11·06–29·55]), and Oceania (17·41% [11·49–25·16]) are estimated to have the highest PAFs for intimate partner violence among females with HIV (appendix p 47). After removal of the combined effects of unsafe sex, injection drug use, and intimate partner violence, males with HIV would have a 1·21 (1·15–1·28) times higher global age-standardised tuberculosis mortality rate than females with HIV (appendix pp 119–33).
Discussion
GBD 2019 provides a comprehensive assessment of the global tuberculosis burden, with an emphasis on characterising sex differences across different metrics of tuberculosis burden. We found that age-standardised incidence rates among HIV-negative individuals were more than 1·5 times greater in 74 countries, and age-standardised mortality rates were more than two times greater in 105 countries, in males than in females in 2019. Among HIV-positive individuals, age-standardised incidence and mortality rates were 1·33 times larger in 35 and 56 countries, respectively, for females than males.
These results align with the findings from previous studies that males have a greater burden of tuberculosis than females due to men exhibiting lower levels of health-care use, presenting more advanced stages of the disease when care is sought, and having poorer adherence to anti-tuberculosis treatment.8, 19, 20 Previous work has highlighted that men may delay seeking health care for tuberculosis-suggestive symptoms due to fear or to avoid diagnosis of a serious condition such as tuberculosis or HIV.20, 21 These fears and anxieties intersect with masculinity expectations, wherein men face societal pressure to neglect symptoms to remain physically and financially strong and capable to satisfy leadership roles.22, 23, 24 The combination of late initiation of tuberculosis treatment and poor treatment adherence might be contributing to age-standardised mortality rates being nearly two times greater among males than among females in the GBD.
Risk factors are also important to the observed sex differences in tuberculosis mortality. Previous work has found that alcohol use25 and smoking12 are substantial contributors to sex differences in tuberculosis burden. The global fraction of tuberculosis deaths attributable to alcohol consumption and smoking was more than four times and six times higher, respectively, among males than among females. Despite the global diabetes prevalence being very similar for males and females,11 we found that the fraction of tuberculosis deaths attributable to diabetes was modestly higher for males. Some evidence suggests that the higher fraction among males might be due to men having poorer tuberculosis treatment outcomes than women in the presence of diabetes comorbidities.26, 27 Together, the findings underscore that risk factors, particularly alcohol and smoking, along with late treatment initiation, are probably major contributors to the sex differences in tuberculosis mortality among HIV-negative people. Indeed, our results show that the excess tuberculosis mortality among males would decrease from 1·97 (95% UI 1·60–2·26) to 1·28 (1·05–1·49) if the effects of risk factors for disease progression were removed. The heightened exposure to risk factors that contribute to disease progression among men might also be a factor for why males have 50% higher age-standardised incidence rates than females in more than 70 countries.
Although our study found that tuberculosis burden and risk factors are higher among males than females, the burden among females is still unnecessarily large; we estimated more than 500 000 deaths among HIV-negative and HIV-positive females in 2019. Evidence suggests that men indeed delay seeking health care for tuberculosis, contributing to poor case detection among men, but that women generally encounter more barriers to receiving appropriate tuberculosis care.19 Across various settings, women, compared with men, face a substantially longer time to tuberculosis diagnosis28, 29, 30, 31, 32 due to lower priority or attention paid when women present non-specific tuberculosis symptoms,19 evidence of tuberculosis diagnostic tools being less sensitive for women,33 and gender norms requiring women to negotiate with husbands to seek tuberculosis care.34
Moreover, we found that the global burden of tuberculosis was higher among females than among males in HIV-positive individuals. In the countries with the highest HIV and tuberculosis coinfection burden (eg, countries in southern sub-Saharan Africa), our results show that females generally had a greater HIV and tuberculosis coinfection burden than males, with unsafe sex and intimate partner violence being significant contributors. The larger incidence rates among females in these countries might be due to HIV disproportionately affecting women in countries with high HIV burden.35, 36, 37 The larger burden of HIV among women, combined with HIV being the strongest risk factor for progression from latent to active tuberculosis,38 compounds the observed differences in these countries.
These findings underscore the need for HIV prevention and treatment to diminish HIV and tuberculosis coinfection burden. Interventions are needed that address multiple causes of women's vulnerability to HIV infection, including poverty, exposure to intimate partner violence, cultural factors that disempower women, such as encouraging marriage to older men, and laws deterring reproductive health services.39, 40, 41
The End TB Strategy sought to reduce tuberculosis incidence to below 85 new cases per 100 000 population by 2020. However, our findings suggest that substantial additional progress needs to be made, with 40 countries having age-standardised incidence rates greater than 170 per 100 000 population, more than double the target of 85 per 100 000, in 2019. Males are particularly at risk for not reaching this target, as the global age-standardised tuberculosis incidence rate for males without HIV was 120 per 100 000 population, compared with 95 per 100 000 population for HIV-negative females in 2019. The excess tuberculosis burden among men found in this study strengthens the view that men should be targeted in screening services and routine diagnostics6, 8 to achieve equity targets outlined by the SDGs and End TB Strategy. Interventions are needed to engage men in tuberculosis care, with social protections (eg, prevention of loss of employment while receiving care and cash transfers) for families, while actively communicating the importance of early diagnosis to men.24 Interventions should also place efforts on improving communication and preventive strategies to support reducing behavioural risk factors such as alcohol use and smoking.
Although the results reported in GBD 2019 reflect tuberculosis burden before the emergence of the COVID-19 pandemic, the worldwide pandemic has important consequences for the global burden of tuberculosis that need to be investigated by tuberculosis programmes. Modelling studies have suggested that tuberculosis deaths could increase up to 20% in the next 5 years.42, 43 Physical-distancing and mask-wearing policies might help to reduce tuberculosis transmission, but this beneficial effect might be offset by increased opportunities for household transmission.44 Prolonged exposure to household contacts is an important risk factor for tuberculosis transmission, and the duration of infectiousness might be further extended if there are disruptions to tuberculosis treatment due to health system overload as a result of COVID-19. Tuberculosis programmes, once they have evaluated the impact of COVID-19 on tuberculosis for any severe setbacks, might need to consider the recalibration of global targets.
Overall, despite different estimation methods, the global burden of tuberculosis estimated by GBD is similar to that produced by WHO.3 A concern raised previously was that past iterations of GBD and WHO estimates produced discrepancies in estimated tuberculosis mortality.45 For example, in earlier iterations, GBD 20162 estimated 1·45 million tuberculosis deaths, whereas WHO 201746 estimated 1·67 million tuberculosis deaths in 2016. However, the latest estimates show that the differences are decreasing at the global level. Although GBD 2019 estimated slightly lower global incident cases at 9·65 million (95% UI 8·48–11·03) than WHO's estimate of 10·0 million (9·0–11·1), both GBD 2019 and WHO estimated approximately 1·40 million deaths in 2019 (1·40 million deaths by GBD 2019, and 1·39 million deaths by WHO). However, there are some differences in sex-specific estimates: GBD 2019 estimated that 56% of global incident tuberculosis cases and 65% of global tuberculosis deaths were among males, whereas, for the same year, WHO estimated that 63% of global incident tuberculosis cases and 61% of global tuberculosis deaths were among males. The higher percentage for tuberculosis mortality than for tuberculosis incidence in males in GBD 2019 might be expected due to several factors, such as men presenting advanced stages of tuberculosis when care is sought and heightened exposure to risk factors.19, 47, 48, 49 Previous work has shown that smoking and alcohol consumption further contribute to poor tuberculosis treatment outcomes and compliance among men.47, 48, 49
The tuberculosis burden results should be interpreted in the context of the following limitations. First, there are gaps in data availability across countries, age groups, and time periods. In locations without reliable vital registration data, mortality estimates are driven by verbal autopsy studies, which have been shown to have modest sensitivity in identifying tuberculosis deaths.50, 51, 52 We believe that this bias has been minimised through excluding verbal autopsy studies from countries with high HIV burden. Second, because the GBD 2019 tuberculosis results are based on available data and modelling, lags in reporting of vital registration data indicate that tuberculosis mortality estimates for most recent years rely on modelling processes. Estimates for locations and years where data are sparse are reflected by wide UIs. Third, we have not yet been able to quantify the burden of tuberculosis attributable to indoor air pollution due to a lack of objectively measured longitudinal data.53 Similarly, the burden attributable to malnutrition has not yet been quantified because there is insufficient evidence of a causal relationship and limited data on the risk of tuberculosis across different levels of malnutrition.54 Fourth, there are challenges in accurately estimating sex differences in tuberculosis burden due to the way age-sex-specific estimates are reported in tuberculosis prevalence surveys. Prevalence surveys often report data separately by age (for both sexes combined) and by sex (for all ages combined). To extract age-sex-specific data from these surveys, we used the age distribution for both sexes in the study to split the all-age sex-specific data. Data extraction would be more accurate if tuberculosis prevalence surveys reported data by age and sex to improve modelling inputs. Finally, there remain major challenges in our statistical triangulation approach, in which there are difficulties in computing consistent estimates between tuberculosis death rates and prevalence data from national surveys. This is particularly problematic for locations in sub-Saharan Africa where there are few prevalence surveys or reliable cause of death data. Closer analyses have shown that even when prevalence and cause of death data are available, there are inconsistencies in the sources. In Bangladesh, for example, we found that a national prevalence survey55 estimated tuberculosis prevalence to be approximately 300 per 100 000 population, but verbal autopsy sources56, 57 reported mortality rates of less than 10 per 100 000 population. Together, the two sources suggest that Bangladesh has one of the lowest case fatality rates in the world for tuberculosis. In GBD 2019, we resolved inconsistencies in data sources by excluding less reliable sources that contradicted others.
GBD 2019 has made several methodological improvements compared with earlier GBD iterations.2, 10 First, we used a novel Bayesian meta-regression method to incorporate uncertainty from the input data in our mortality-to-incidence ratio approach. Second, we addressed the potential for misclassification of tuberculosis deaths as pneumonia deaths for children younger than 15 years. We made this adjustment using data from a systematic review58, 59, 60, 61, 62, 63, 64, 65 to redistribute pneumonia deaths in cause of death data to tuberculosis. Third, we standardised our adjustment to prevalence surveys that used smear-positive tuberculosis as the case definition rather than bacteriologically confirmed tuberculosis, while incorporating a novel adjustment to recalibrate studies that used symptoms only as the screening method compared with using both symptoms and chest x-ray. Finally, our statistical triangulation approach was improved by incorporating data on remission and excess mortality, while also resolving discrepancies in morbidity and mortality data.
As countries refine national tuberculosis programmes and strategies to end the tuberculosis epidemic, the excess burden experienced by males should be more widely realised and monitored. The greater incidence and mortality rates among men found in GBD 2019 indicate that men are not fully accessing tuberculosis services and are remaining infectious in the community for substantial periods of time. Targeting the burden of disease in males will diminish the overall burden of tuberculosis and will be crucial in reaching both equity and burden targets outlined by the SDGs and the End TB Strategy. Furthermore, tuberculosis programmes should examine key risk factors that are contributing to disproportionate tuberculosis burden in males, such as alcohol and smoking, and liaise with risk factor control initiatives aiming to reduce alcohol use and smoking. Reaching equity goals will also require identifying and addressing inequalities in health facilities where women often encounter barriers to accessing tuberculosis diagnostic services. These efforts should occur in parallel with addressing risk factors for women's vulnerability to HIV infection to minimise excess HIV and tuberculosis coinfection burden among females in the countries with highest HIV burden.
Data sharing
To download the data used in these analyses, please visit the Global Health Data Exchange GBD 2019 website.
Declaration of interests
JMR reports grants or contracts from the US National Institutes of Health (NIH) and consulting fees from the US Agency for International Development, all outside the submitted work. KEL reports support for the present manuscript from the Bill & Melinda Gates Foundation. CATA reports grants or contracts and consulting fees from Johnson & Johnson (Philippines), all outside the submitted work. IF reports payment or honoraria for lectures, presentations, speaker's bureaus, manuscript writing, or educational events from Avicenna Medical and Clinical Research Institute, all outside the submitted work. AmG reports support for the present manuscript, grants, and contracts from Sistema Nacional de Investigadores de Panamá, outside the submitted work. KeK reports other support from UGC Centre of Advanced Study, CAS II, Department of Anthropology, Panjab University, Chandigarh, India, outside the submitted work. MJP reports grants or contacts from Merck Sharp & Dohme, GlaxoSmithKline, Pfizer, Boehringer Ingelheim, Novavax, Bayer, Bristol Myers Squibb, AstraZeneca, Sanofi, IQVIA, BioMerieux, WHO, EU, Seqirus, FIND, Antilope, DIKTI, LPDP, and Budi; consulting fees from Merck Sharp & Dohme, GlaxoSmithKline, Pfizer, Boehringer Ingelheim, Novavax, Quintiles, Bristol Myers Squibb, AstraZeneca, Sanofi, Novartis, Pharmerit, IQVIA, and Seqirus; participation on a data safety monitoring board or advisory board for Asc Academics as an adviser; and stock or stock options in Ingress Health, Health-Ecore, and Pharmacoeconomics Advice Goningen, all outside the submitted work. AmR reports payment or honoraria for lectures, presentations, speaker's bureaus, manuscript writing, or educational events from Avicenna Medical and Clinical Research Institute, outside the submitted work. OR reports grants or contracts from the Research Foundation of Rigshospitalet and A P Møller Foundation, all outside the submitted work. JAS reports grant support, paid to their institution, for the present manuscript from the Bill & Melinda Gates Foundation.
Acknowledgments
Acknowledgments
Funding for this study was obtained from the Bill & Melinda Gates Foundation. SaiA acknowledges the support of the Community Medicine Department King Edward Medical University (Lahore, Pakistan) in facilitating this research and provision of qualified human resources to work on this research. FC and EF acknowledge support with funding from Fundação para a Ciência e Tecnologia/Ministério da Ciência, Tecnologia e Ensino Superior (FCT/MCTES; UID/MULTI/04378/2019 and UID/QUI/50006/2019) through national funds. MG acknowledges being a project lead of the NITI Aayog, Government of India-funded project Impact of the National Health Mission on Governance, Health System, and Human Resources for Health, and being a project lead of a Department of Science and Technology-funded project on the development and implementation of Heat Action Plan in Indian Cities under the National Mission on Strategic Knowledge for Climate Change. VKG acknowledges funding support from the Australian National Health and Medical Research Council. YJK was supported by the Research Management Centre at Xiamen University Malaysia (XMUMRF/2020/C6/ITCM/0004). SLKL acknowledges institutional support by the Manipal Academy of Higher Education (India). KeK is supported by the UGC Centre of Advanced Study (Phase II), awarded to the Department of Anthropology, Panjab University, Chandigarh, India. IL acknowledges being member of the Sistema Nacional de Investigación, supported by the Secretaría Nacional de Ciencia, Tecnología e Innovación, Panama. MarM is supported by the National Institute for Health Research (NIHR) Biomedical Research Center at Guy's and St Thomas' National Health Service Foundation Trust and King's College London (UK). OOO acknowledges support from the Fogarty International Center of the US National Institutes of Health (NIH; award number K43TW010704). AMS acknowledges support from the Egyptian Fulbright Mission Program. JPS acknowledges support from the Applied Molecular Biosciences Unit (UCIBIO; grant number UIDB/04378/2020), supported through Portuguese national funds via FCT/MCTES. AlZ acknowledges support from the European and Developing Countries Clinical Trials Partnership (EDCTP2) Programme, Horizon 2020, the EU's Framework Programme for Research and Innovation (grants PANDORA-ID-NET, TESA-2, and CANTAM-2); is in receipt of an NIHR senior investigator award; and is a Mahathir Science Award Laureate. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations
Contributors
JRL, AN, MHB, SIH, CJLM, and HHK managed the estimation or publications process. JRL, JM, AV, and HHK wrote the first draft of the manuscript. JRL, DanaB, and DJ had primary responsibility for applying analytical methods to produce estimates. JRL, JM, AV, ERM, KEL, and RF had primary responsibility for seeking, cataloguing, extracting, or cleaning data, and designing or coding figures and tables. Members of the core Institute for Health Metrics and Evaluation (IHME) research team for this topic area had full access to the underlying data used to generate estimates presented in this paper. All other authors had access to, and reviewed, estimates as part of the Global Burden of Disease Study and research evaluation process, which includes additional stages of internal IHME and external formal collaborator review. Contributions for the remaining authors can be found in the appendix (p 172).
GBD 2019 Tuberculosis Collaborators
Jorge R Ledesma, Jianing Ma, Avina Vongpradith, Emilie R Maddison, Amanda Novotney, Molly H Biehl, Kate E LeGrand, Jennifer M Ross, Deepa Jahagirdar, Dana Bryazka, Rachel Feldman, Hassan Abolhassani, Akine Eshete Abosetugn, Eman Abu-Gharbieh, Oladimeji M Adebayo, Qorinah Estiningtyas Sakilah Adnani, Saira Afzal, Bright Opoku Ahinkorah, Sajjad Ahmad Ahmad, Sepideh Ahmadi, Tarik Ahmed Rashid, Yusra Ahmed Salih, Addis Aklilu, Chisom Joyqueenet Akunna, Hanadi Al Hamad, Fares Alahdab, Yosef Alemayehu, Kefyalew Addis Alene, Beriwan Abdulqadir Ali, Liaqat Ali, Vahid Alipour, Hesam Alizade, Rajaa M Al-Raddadi, Nelson Alvis-Guzman, Saeed Amini, Arianna Maever L Amit, Jason A Anderson, Sofia Androudi, Carl Abelardo T Antonio, Catherine M Antony, Razique Anwer, Jalal Arabloo, Asrat Arja, Mulusew A Asemahagn, Sachin R Atre, Gulrez Shah Azhar, Darshan B B, Zaheer-Ud-Din Babar, Atif Amin Baig, Maciej Banach, Hiba Jawdat Barqawi, Fabio Barra, Amadou Barrow, Sanjay Basu, Uzma Iqbal Belgaumi, Akshaya Srikanth Bhagavathula, Nikha Bhardwaj, Pankaj Bhardwaj, Natalia V Bhattacharjee, Krittika Bhattacharyya, Ali Bijani, Boris Bikbov, Archith Boloor, Nikolay Ivanovich Briko, Danilo Buonsenso, Sharath Burugina Nagaraja, Zahid A Butt, Austin Carter, Felix Carvalho, Jaykaran Charan, Souranshu Chatterjee, Soosanna Kumary Chattu, Vijay Kumar Chattu, Devasahayam J Christopher, Dinh-Toi Chu, Mareli M Claassens, Omid Dadras, Amare Belachew Dagnew, Xiaochen Dai, Lalit Dandona, Rakhi Dandona, Parnaz Daneshpajouhnejad, Aso Mohammad Darwesh, Deepak Dhamnetiya, Mostafa Dianatinasab, Daniel Diaz, Linh Phuong Doan, Sahar Eftekharzadeh, Muhammed Elhadi, Amir Emami, Shymaa Enany, Emerito Jose A Faraon, Farshad Farzadfar, Eduarda Fernandes, Lorenzo Ferro Desideri, Irina Filip, Florian Fischer, Masoud Foroutan, Tahvi D Frank, Alberto L Garcia-Basteiro, Christian Garcia-Calavaro, Tushar Garg, Biniyam Sahiledengle Geberemariyam, Keyghobad Ghadiri, Ahmad Ghashghaee, Mahaveer Golechha, Amador Goodridge, Bhawna Gupta, Sapna Gupta, Veer Bala Gupta, Vivek Kumar Gupta, Mohammad Rifat Haider, Samer Hamidi, Asif Hanif, Shafiul Haque, Harapan Harapan, Arief Hargono, Ahmed I Hasaballah, Abdiwahab Hashi, Shoaib Hassan, Hadi Hassankhani, Khezar Hayat, Kamal Hezam, Ramesh Holla, Mehdi Hosseinzadeh, Mihaela Hostiuc, Mowafa Househ, Rabia Hussain, Segun Emmanuel Ibitoye, Irena M Ilic, Milena D Ilic, Seyed Sina Naghibi Irvani, Nahlah Elkudssiah Ismail, Ramaiah Itumalla, Jalil Jaafari, Kathryn H Jacobsen, Vardhmaan Jain, Fatemeh Javanmardi, Sathish Kumar Jayapal, Shubha Jayaram, Ravi Prakash Jha, Jost B Jonas, Nitin Joseph, Farahnaz Joukar, Zubair Kabir, Ashwin Kamath, Tanuj Kanchan, Himal Kandel, Patrick DMC Katoto, Gbenga A Kayode, Parkes J Kendrick, Amene Abebe Kerbo, Himanshu Khajuria, Rovshan Khalilov, Khaled Khatab, Abdullah T Khoja, Jagdish Khubchandani, Min Seo Kim, Yun Jin Kim, Adnan Kisa, Sezer Kisa, Soewarta Kosen, Parvaiz A Koul, Sindhura Lakshmi Koulmane Laxminarayana, Ai Koyanagi, Kewal Krishan, Burcu Kucuk Bicer, Avinash Kumar, G Anil Kumar, Narinder Kumar, Nithin Kumar, Alexander Kwarteng, Hassan Mehmood Lak, Dharmesh Kumar Lal, Iván Landires, Savita Lasrado, Shaun Wen Huey Lee, Wei-Chen Lee, Christine Lin, Xuefeng Liu, Platon D Lopukhov, Rafael Lozano, Daiane Borges Machado, Shilpashree Madhava Kunjathur, Deepak Madi, Preetam Bhalchandra Mahajan, Azeem Majeed, Ahmad Azam Malik, Francisco Rogerlândio Martins-Melo, Saurabh Mehta, Ziad A Memish, Walter Mendoza, Ritesh G Menezes, Hayimro Edemealem Merie, Amanual Getnet Mersha, Mohamed Kamal Mesregah, Tomislav Mestrovic, Nour Mheidly Mheidly, Sanjeev Misra, Prasanna Mithra, Masoud Moghadaszadeh, Mokhtar Mohammadi, Abdollah Mohammadian-Hafshejani, Shafiu Mohammed, Mariam Molokhia, Mohammad Ali Moni, Ahmed Al Montasir, Catrin E Moore, Ahamarshan Jayaraman Nagarajan, Sanjeev Nair, Suma Nair, Atta Abbas Naqvi, Sreenivas Narasimha Swamy, Biswa Prakash Nayak, Javad Nazari, Sandhya Neupane Kandel, Trang Huyen Nguyen, Molly R Nixon, Chukwudi A Nnaji, Mpiko Ntsekhe, Virginia Nuñez-Samudio, Bogdan Oancea, Oluwakemi Ololade Odukoya, Andrew T Olagunju, Eyal Oren, Mahesh P A, Ramakrishnan Parthasarathi, Fatemeh Pashazadeh Kan, Sanjay M Pattanshetty, Rajan Paudel, Pintu Paul, Shrikant Pawar, Veincent Christian Filipino Pepito, Norberto Perico, Majid Pirestani, Roman V Polibin, Maarten J Postma, Akram Pourshams, Akila Prashant, Dimas Ria Angga Pribadi, Amir Radfar, Alireza Rafiei, Fakher Rahim, Vafa Rahimi-Movaghar, Mahfuzar Rahman, Mosiur Rahman, Amir Masoud Rahmani, Priyanga Ranasinghe, Chythra R Rao, David Laith Rawaf, Salman Rawaf, Marissa B Reitsma, Giuseppe Remuzzi, Andre M N Renzaho, Melese Abate Reta, Nima Rezaei, Omid Rezahosseini, Mohammad sadegh Rezai, Aziz Rezapour, Gholamreza Roshandel, Denis O Roshchin, Siamak Sabour, KM Saif-Ur-Rahman, Nasir Salam, Hossein Samadi Kafil, Mehrnoosh Samaei, Abdallah M Samy, Satish Saroshe, Benn Sartorius, Brijesh Sathian, Susan M Sawyer, Subramanian Senthilkumaran, Allen Seylani, Omid Shafaat, Masood Ali Shaikh, Kiomars Sharafi, Ranjitha S Shetty, Mika Shigematsu, Jae Il Shin, João Pedro Silva, Jitendra Kumar Singh, Smriti Sinha, Valentin Yurievich Skryabin, Anna Aleksandrovna Skryabina, Emma Elizabeth Spurlock, Chandrashekhar T Sreeramareddy, Paschalis Steiropoulos, Mu'awiyyah Babale Sufiyan, Takahiro Tabuchi, Eyayou Girma Tadesse, Zemenu Tamir, Elvis Enowbeyang Tarkang, Yohannes Tekalegn, Fisaha Haile Tesfay, Belay Tessema, Rekha Thapar, Imad I Tleyjeh, Ruoyan Tobe-Gai, Bach Xuan Tran, Berhan Tsegaye, Gebiyaw Wudie Tsegaye, Anayat Ullah, Chukwuma David Umeokonkwo, Sahel Valadan Tahbaz, Bay Vo, Giang Thu Vu, Yasir Waheed, Magdalene K Walters, Joanna L Whisnant, Mesfin Agachew Woldekidan, Befikadu Legesse Wubishet, Seyed Hossein Yahyazadeh Jabbari, Taklo Simeneh Yazie Yazie, Yigizie Yeshaw, Siyan Yi, Vahit Yiğit, Naohiro Yonemoto, Chuanhua Yu, Ismaeel Yunusa, Mikhail Sergeevich Zastrozhin, Anasthasia Zastrozhina, Zhi-Jiang Zhang, Alimuddin Zumla, Ali H Mokdad, Joshua A Salomon, Robert C Reiner Jr, Stephen S Lim, Mohsen Naghavi, Theo Vos, Simon I Hay, Christopher J L Murray*, and Hmwe Hmwe Kyu*.
*Joint last authors.
Affiliations
Institute for Health Metrics and Evaluation (J R Ledesma MPH, J Ma MS, A Vongpradith BA, E R Maddison BS, A Novotney MPH, K E LeGrand MPH, J M Ross MD, D Jahagirdar PhD, D Bryazka BA, R Feldman BS, J A Anderson BS, C M Antony MA, G S Azhar PhD, N V Bhattacharjee PhD, A Carter MPH, X Dai PhD, Prof L Dandona MD, Prof R Dandona PhD, P J Kendrick BS, C Lin BS, Prof R Lozano MD, M R Nixon PhD, M B Reitsma BS, E E Spurlock BA, M K Walters BS, J L Whisnant MPH, Prof A H Mokdad PhD, R C Reiner Jr PhD, Prof S S Lim PhD, Prof M Naghavi MD, Prof T Vos PhD, Prof S I Hay FMedSci, Prof C J L Murray DPhil, H H Kyu PhD), Department of Global Health (J M Ross MD), Department of Medicine (J M Ross MD), Department of Health Metrics Sciences, School of Medicine (Prof R Dandona PhD, Prof R Lozano MD, B Sartorius PhD, Prof A H Mokdad PhD, R C Reiner Jr PhD, Prof S S Lim PhD, Prof M Naghavi MD, Prof T Vos PhD, Prof S I Hay FMedSci, Prof C J L Murray DPhil, H H Kyu PhD), University of Washington, Seattle, WA, USA (Prof E Oren PhD); Division of Data, Analytics, and Delivery for Impact (M H Biehl MPH), World Health Organization (WHO), Genève, Switzerland; Department of Laboratory Medicine (H Abolhassani PhD), Karolinska University Hospital, Huddinge, Sweden; Research Center for Immunodeficiencies (H Abolhassani PhD, Prof N Rezaei PhD), Non-communicable Diseases Research Center (Prof F Farzadfar DSc), Digestive Diseases Research Institute (Prof A Pourshams MD), Metabolomics and Genomics Research Center (F Rahim PhD), Sina Trauma and Surgery Research Center (Prof V Rahimi-Movaghar MD), Tehran University of Medical Sciences, Tehran, Iran; Department of Public Health (A E Abosetugn MPH), Debre Berhan University, Debre Brehan, Ethiopia; Department of Clinical Sciences (E Abu-Gharbieh PhD), Clinical Sciences Department (H J Barqawi MPhil), University of Sharjah, Sharjah, United Arab Emirates; College of Medicine (O M Adebayo MD), University College Hospital, Ibadan, Ibadan, Nigeria; Department of Midwifery (Q E S Adnani PhD), Karya Husada Institute of Health Sciences, Kediri, Indonesia; Department of Midwifery (Q E S Adnani PhD), Auckland University of Technology, Auckland, New Zealand; Department of Community Medicine (Prof S Afzal PhD), King Edward Memorial Hospital, Lahore, Pakistan; Department of Public Health (Prof S Afzal PhD), Public Health Institute, Lahore, Pakistan; The Australian Centre for Public and Population Health Research (ACPPHR) (B O Ahinkorah MPH), University of Technology Sydney, Sydney, NSW, Australia; Foundation University Medical College (S A Ahmad PhD, Prof Y Waheed PhD), Foundation University Islamabad, Islamabad, Pakistan; School of Advanced Technologies in Medicine (S Ahmadi PhD), Department of Epidemiology (S Sabour PhD), Shahid Beheshti University of Medical Sciences, Tehran, Iran; Department of Computer Science and Engineering (T Ahmed Rashid PhD), University of Kurdistan Hewler, Erbil, Iraq; Database Technology Department (Y Ahmed Salih PhD), College of Informatics (Y Ahmed Salih PhD), Sulaimani Polytechnic University, Sulaymaniyah, Iraq; Department of Medical Laboratory Sciences (A Aklilu MSc), Department of Midwifery (Y Alemayehu MSc), Department of Biomedical Sciences (E G Tadesse MSc), Arba Minch University, Arba Minch, Ethiopia; Department of Public Health (C J Akunna DMD), The Intercountry Centre for Oral Health (ICOH) for Africa, Jos, Nigeria; Department of Public Health (C J Akunna DMD), Federal Ministry of Health, Garki, Nigeria; Geriatric and Long Term Care Department (H Al Hamad MD, B Sathian PhD), Rumailah Hospital (H Al Hamad MD), Hamad Medical Corporation, Doha, Qatar; Mayo Evidence-based Practice Center (F Alahdab MSc), Mayo Clinic Foundation for Medical Education and Research, Rochester, MN, USA; Faculty of Health Sciences (K A Alene MPH), Curtin University, Perth, WA, Australia; Wesfarmers Centre of Vaccines and Infectious Diseases (K A Alene MPH), Telethon Kids Institute, Perth, WA, Australia; Erbil Technical Health College (B A Ali PhD), Erbil Polytechnic University, Erbil, Iraq; School of Pharmacy (B A Ali PhD), Tishk International University, Erbil, Iraq; Department of Biological Sciences (L Ali PhD), Multidisciplinary Department (A Ullah MS), National University of Medical Sciences (NUMS), Rawalpindi, Pakistan; Health Management and Economics Research Center (V Alipour PhD, J Arabloo PhD, A Ghashghaee BSc, M Hosseinzadeh PhD, A Rezapour PhD), Department of Health Economics (V Alipour PhD), Student Research Committee (A Ghashghaee BSc), Iran University of Medical Sciences, Tehran, Iran (F Pashazadeh Kan BSN); Infectious and Tropical Disease Research Center (H Alizade PhD), Hormozgan University of Medical Sciences, Bandar Abbas, Iran; Department of Community Medicine (R M Al-Raddadi PhD), Rabigh Faculty of Medicine (A A Malik PhD), King Abdulaziz University, Jeddah, Saudi Arabia; Research Group in Hospital Management and Health Policies (Prof N Alvis-Guzman PhD), Universidad de la Costa (University of the Coast), Barranquilla, Colombia; Research Group in Health Economics (Prof N Alvis-Guzman PhD), University of Cartagena, Cartagena, Colombia; Department of Health Services Management (S Amini PhD), Department of Pediatrics (J Nazari MD), Arak University of Medical Sciences, Arak, Iran; School of Medicine and Public Health (A L Amit BS), Ateneo De Manila University, Manila, Philippines; College of Medicine (A L Amit BS), Department of Health Policy and Administration (C T Antonio MD, E A Faraon MD), University of the Philippines Manila, Manila, Philippines; Department of Medicine (S Androudi PhD), University of Thessaly, Volos, Greece; Department of Applied Social Sciences (C T Antonio MD), Hong Kong Polytechnic University, Hong Kong, China; Department of Pathology (R Anwer PhD), Department of Public Health (A T Khoja MD), Imam Mohammad Ibn Saud Islamic University, Riyadh, Saudi Arabia; National Data Management Center for Health (NDMC) (A Arja MPH, M A Woldekidan MPH), Ethiopian Public Health Institute, Addis Ababa, Ethiopia; School of Public Health (M A Asemahagn PhD), Department of Nursing (A B Dagnew MSc), College of Medicine and Health Sciences (G W Tsegaye MPH), Bahir Dar University, Bahir Dar, Ethiopia; Dr DY Patil Medical College, Hospital and Research Centre (S R Atre PhD), Dr DY Patil Vidyapeeth, Pune, Pune, India; Center for Clinical Global Health Education (S R Atre PhD), Department of Pathology (P Daneshpajouhnejad MD), Department of Health Policy and Management (A T Khoja MD), Department of Radiology and Radiological Science (O Shafaat MD), Johns Hopkins University, Baltimore, MD, USA; Badan Pusat Statistik (BPS) (Central Bureau of Statistics) (G S Azhar PhD), The RAND Corporation, Santa Monica, CA, USA; Kasturba Medical College, Mangalore (D B B MD, R Holla MD, A Kamath MD), Kasturba Medical College (Prof S Nair MD), Department of Health Policy (S M Pattanshetty MD), Department of Community Medicine (C R Rao MD, R S Shetty MD), Manipal Academy of Higher Education, Manipal, India (A Kamath MD); Department of Pharmacy (Prof Z Babar PhD), University of Huddersfield, Huddersfield, UK; Unit of Biochemistry (A A Baig PhD), Universiti Sultan Zainal Abidin (Sultan Zainal Abidin University), Kuala Terengganu, Malaysia; Department of Hypertension (Prof M Banach PhD), Medical University of Lodz, Lodz, Poland; Polish Mothers' Memorial Hospital Research Institute, Lodz, Poland (Prof M Banach PhD); Academic Unit of Obstetrics and Gynecology (F Barra MD), University Eye Clinic (L Ferro Desideri MD), University of Genoa, Genoa, Italy; Department of Public & Environmental Health (A Barrow MPH), University of The Gambia, Brikama, The Gambia; Epidemiology and Disease Control Unit (A Barrow MPH), Ministry of Health, Kotu, The Gambia; Center for Primary Care (S Basu PhD), TH Chan School of Public Health (I Yunusa PhD), Harvard University, Boston, MA, USA; School of Public Health (S Basu PhD), Department of Primary Care and Public Health (Prof A Majeed MD, Prof S Rawaf MD), WHO Collaborating Centre for Public Health Education and Training (D L Rawaf MD), Imperial College London, London, UK; Department of Oral Pathology and Microbiology (U I Belgaumi MD), Krishna Institute of Medical Sciences “Deemed To Be University”, Karad, India; Department of Social and Clinical Pharmacy (A S Bhagavathula PharmD), Charles University, Hradec Kralova, Czech Republic; Institute of Public Health (A S Bhagavathula PharmD), United Arab Emirates University, Al Ain, United Arab Emirates; Department of Anatomy (Prof N Bhardwaj MD), Government Medical College Pali, Pali, India; Department of Community Medicine and Family Medicine (P Bhardwaj MD), School of Public Health (P Bhardwaj MD), Department of Pharmacology (J Charan MD), Department of Forensic Medicine and Toxicology (T Kanchan MD), Department of Surgical Oncology (Prof S Misra MCh), All India Institute of Medical Sciences, Jodhpur, India; Department of Statistical and Computational Genomics (K Bhattacharyya MSc), National Institute of Biomedical Genomics, Kalyani, India; Department of Statistics (K Bhattacharyya MSc), University of Calcutta, Kolkata, India; Social Determinants of Health Research Center (A Bijani PhD), Babol University of Medical Sciences, Babol, Iran; Mario Negri Institute for Pharmacological Research, Ranica, Italy (B Bikbov MD); Department of Internal Medicine (A Boloor MD, D Madi MD), Department of Community Medicine (N Joseph MD, N Kumar MD, P Mithra MD, R Thapar MD), Department of Anaesthesiology (S Sinha DNB), Manipal Academy of Higher Education, Mangalore, India; Department of Epidemiology and Evidence-Based Medicine (Prof N I Briko DSc, P D Lopukhov PhD, R V Polibin PhD), IM Sechenov First Moscow State Medical University, Moscow, Russia; Department of Woman and Child Health and Public Health (D Buonsenso MD), Fondazione Policlinico Universitario A. Gemelli IRCCS (Agostino Gemelli University Polyclinic IRCCS), Roma, Italy; Global Health Research Institute (D Buonsenso MD), Università Cattolica del Sacro Cuore (Catholic University of Sacred Heart), Roma, Italy; Department of Community Medicine (Prof S Burugina Nagaraja MD), Employee State Insurance Post Graduate Institute of Medical Sciences and Research, Bangalore, India; School of Public Health and Health Systems (Z A Butt PhD), University of Waterloo, Waterloo, ON, Canada; Al Shifa School of Public Health (Z A Butt PhD), Al Shifa Trust Eye Hospital, Rawalpindi, Pakistan; Research Unit on Applied Molecular Biosciences (UCIBIO) (Prof F Carvalho PhD, J P Silva PhD), Associated Laboratory for Green Chemistry (LAQV) (Prof E Fernandes PhD), University of Porto, Porto, Portugal; Department of Microbiology & Infection Control (S Chatterjee MD), Medanta Medicity, Gurugram, India; Department of Public Health (S Chattu PhD), Texila American University, Georgetown, Guyana; Department of Medicine (V Chattu MD), University of Toronto, Toronto, ON, Canada; Global Institute of Public Health (GIPH), Thiruvananthapuram, India (V Chattu MD); Department of Pulmonary Medicine (Prof D J Christopher MD), Christian Medical College and Hospital (CMC), Vellore, India; Center for Biomedicine and Community Health (D Chu PhD), VNU-International School, Hanoi, Vietnam; Department of Biochemistry and Microbiology (M M Claassens PhD), University of Namibia, Windhoek, Namibia; Department of Paediatrics and Child Health (M M Claassens PhD), Stellenbosch University, Tygerberg, South Africa; School of Public Health (O Dadras DrPH), Walailak University, Nakhon Si Thammarat, Thailand; Graduate School of Medicine (O Dadras DrPH), Kyoto University, Kyoto, Japan; Public Health Foundation of India, Gurugram, India (Prof L Dandona MD, Prof R Dandona PhD, G Kumar PhD, D K Lal MD); Indian Council of Medical Research, New Delhi, India (Prof L Dandona MD); Department of Pathology (P Daneshpajouhnejad MD), Department of Radiology and Interventional Neuroradiology (O Shafaat MD), Isfahan University of Medical Sciences, Isfahan, Iran; Department of Information Technology (A M Darwesh PhD), University of Human Development, Sulaymaniyah, Iraq; Department of Community Medicine (D Dhamnetiya MD), Dr. Baba Sahib Ambedkar Medical College and Hospital, Delhi, India; Department of Epidemiology and Biostatistics (M Dianatinasab MSc), Shahroud University of Medical Sciences, Shahroud, Iran; Department of Epidemiology (M Dianatinasab MSc), Bacteriology and Virology Department (A Emami PhD), Burn and Wound Healing Research Center (F Javanmardi MSc), Shiraz University of Medical Sciences, Shiraz, Iran; Center of Complexity Sciences (Prof D Diaz PhD), National Autonomous University of Mexico, Mexico City, Mexico; Faculty of Veterinary Medicine and Zootechnics (Prof D Diaz PhD), Autonomous University of Sinaloa, Culiacán Rosales, Mexico; Institute for Global Health Innovations (L P Doan MSc, T H Nguyen MSc), Faculty of Medicine (L P Doan MSc, T H Nguyen MSc), Duy Tan University, Da Nang, Vietnam; Division of Urology (S Eftekharzadeh MD), Children's Hospital of Philadelphia, Philadelphia, PA, USA; Faculty of Medicine (M Elhadi MD), University of Tripoli, Tripoli, Libya; Department of Microbiology and Immunology (S Enany PhD), Suez Canal University, Ismailia, Egypt; Psychiatry Department (I Filip MD), Kaiser Permanente, Fontana, CA, USA; School of Health Sciences (I Filip MD), AT Still University, Mesa, AZ, USA; Institute of Gerontological Health Services and Nursing Research (F Fischer PhD), Ravensburg-Weingarten University of Applied Sciences, Weingarten, Germany; Department of Medical Parasitology (M Foroutan PhD), Abadan Faculty of Medical Sciences, Abadan, Iran; Vagelos College of Physicians and Surgeons (T D Frank MPH), Columbia University Medical Center, New York, NY, USA; Department of Tuberculosis (A L Garcia-Basteiro PhD), Manhiça Health Research Center (CISM), Manhiça, Mozambique; Viral and Bacterial Infections Research Program (A L Garcia-Basteiro PhD), Barcelona Institute for Global Health, Barcelona, Spain; Programa Centro de Salud Pública,(C Garcia-Calavaro DrPH), Universidad de Santiago de Chile (School of Public Health, University of Santiago Chile), Santiago, Chile; Department of Radiology (T Garg MBBS), King Edward Memorial Hospital, Mumbai, India; Department of Public Health (B S Geberemariyam MPH, Y Tekalegn MPH), Madda Walabu University, Bale Robe, Ethiopia; Infectious Disease Research Center (Prof K Ghadiri MD), Pediatric Department (Prof K Ghadiri MD), Research Center for Environmental Determinants of Health (K Sharafi PhD), Kermanshah University of Medical Sciences, Kermanshah, Iran; Indian Institute of Public Health, Gandhinagar, India (M Golechha PhD); Tuberculosis Biomarker Research Unit (A Goodridge PhD), Instituto de Investigaciones Científicas y Servicios de Alta Tecnología (Institute for Scientific Research and High Technology Services), City of Knowledge, Panama; Department of Public Health (B Gupta PhD), Torrens University, Melbourne, VIC, Australia; Toxicology Department (S Gupta MSc), Shriram Institute for Industrial Research, Delhi, Delhi, India; School of Medicine (V Gupta PhD), Deakin University, Geelong, VIC, Australia; Department of Clinical Medicine (Prof V K Gupta PhD), Macquarie University, Sydney, NSW, Australia; Department of Social and Public Health (M Haider PhD), Ohio University, Athens, OH, USA; School of Health and Environmental Studies (Prof S Hamidi DrPH), Hamdan Bin Mohammed Smart University, Dubai, United Arab Emirates; University Institute of Public Health (A Hanif PhD, A A Malik PhD), The University of Lahore, Lahore, Pakistan; Research & Scientific Studies Unit (S Haque PhD), Jazan University, Jazan, Saudi Arabia; Medical Research Unit (H Harapan PhD), Universitas Syiah Kuala (Syiah Kuala University), Banda Aceh, Indonesia; Department of Epidemiology (A Hargono Dr), Universitas Airlangga (Airlangga University), Surabaya, Indonesia; Department of Zoology and Entomology (A I Hasaballah PhD), Al Azhar University, Cairo, Egypt; Department of Public Health (A Hashi PhD), Jigjiga University, Jijiga, Ethiopia; Center for International Health (CIH) (S Hassan MPhil), Bergen Center for Ethics and Priority Setting (BCEPS) (S Hassan MPhil), University of Bergen, Bergen, Norway; School of Nursing and Midwifery (H Hassankhani PhD), Biotechnology Research Center (M Moghadaszadeh PhD), Molecular Medicine Research Center (M Moghadaszadeh PhD), Drug Applied Research Center (H Samadi Kafil PhD), Tabriz University of Medical Sciences, Tabriz, Iran; Independent Consultant, Tabriz, Iran (H Hassankhani PhD, S N Irvani MD); Institute of Pharmaceutical Sciences (K Hayat MS), University of Veterinary and Animal Sciences, Lahore, Pakistan; Department of Pharmacy Administration and Clinical Pharmacy (K Hayat MS), Xian Jiaotong University, Xian, China; Department of Applied Microbiology (K Hezam PhD), Taiz University, Taiz, Yemen; Department of Microbiology (K Hezam PhD), Nankai University, Tianjin, China; Internal Medicine Department (M Hostiuc PhD), Carol Davila University of Medicine and Pharmacy, Bucharest, Romania; College of Science and Engineering (Prof M Househ PhD), Hamad Bin Khalifa University, Doha, Qatar; School of Pharmaceutical Sciences (R Hussain PhD), University of Science Malaysia, Penang, Malaysia; Department of Health Promotion and Education (S E Ibitoye MPH), University of Ibadan, Ibadan, Nigeria; Faculty of Medicine (I M Ilic PhD), University of Belgrade, Belgrade, Serbia; Department of Epidemiology (Prof M D Ilic PhD), University of Kragujevac, Kragujevac, Serbia; Department of Clinical Pharmacy (Prof N Ismail PhD), MAHSA University, Bandar Saujana Putra, Malaysia; Department of Health Management (R Itumalla PhD), University of Hail, Hail, Saudi Arabia; Department of Environmental Health Engineering (J Jaafari PhD), Gastrointestinal and Liver Diseases Research Center (F Joukar PhD), Caspian Digestive Disease Research Center (F Joukar PhD), Guilan University of Medical Sciences, Rasht, Iran; Department of Global and Community Health (K H Jacobsen PhD), George Mason University, Fairfax, VA, USA; Department of Internal Medicine (V Jain MD, H Lak MD), Lerner Research Institute (X Liu PhD), Cleveland Clinic, Cleveland, OH, USA; Centre of Studies and Research (S Jayapal PhD), Ministry of Health, Muscat, Oman; Department of Biochemistry (Prof S Jayaram MD), Government Medical College, Mysuru, India; Department of Community Medicine (R P Jha MSc), Dr. Baba Saheb Ambedkar Medical College & Hospital, Delhi, India; Department of Community Medicine (R P Jha MSc), Banaras Hindu University, Varanasi, India; Department of Ophthalmology (Prof J B Jonas MD), Heidelberg University, Heidelberg, Germany; Beijing Institute of Ophthalmology (Prof J B Jonas MD), Beijing Tongren Hospital, Beijing, China; School of Public Health (Z Kabir PhD), University College Cork, Cork, Ireland; Save Sight Institute (H Kandel PhD), University of Sydney, Sydney, NSW, Australia; Sydney Eye Hospital (H Kandel PhD), South Eastern Sydney Local Health District, Sydney, NSW, Australia; Centre for Tropical Diseases and Global Health (P D Katoto PhD), Catholic University of Bukavu, Bukavu, Democratic Republic of the Congo; Department of Global Health (P D Katoto PhD), Stellenbosch University, Cape Town, South Africa; International Research Center of Excellence (G A Kayode PhD), Institute of Human Virology Nigeria, Abuja, Nigeria; Julius Centre for Health Sciences and Primary Care (G A Kayode PhD), Utrecht University, Utrecht, Netherlands; School of Public Health (A A Kerbo PhD), Wolaita Sodo University, Wolaita Sodo, Ethiopia; Amity Institute of Forensic Sciences (H Khajuria PhD, B P Nayak PhD), Amity University, Noida, India; Department of Biophysics and Biochemistry (Prof R Khalilov PhD), Baku State University, Baku, Azerbaijan; Russian Institute for Advanced Study (Prof R Khalilov PhD), Moscow State Pedagogical University, Moscow, Russia; Faculty of Health and Wellbeing (K Khatab PhD), Sheffield Hallam University, Sheffield, UK; College of Arts and Sciences (K Khatab PhD), Ohio University, Zanesville, OH, USA; Department of Public Health (Prof J Khubchandani PhD), New Mexico State University, Las Cruces, NM, USA; Department of Genomics and Digital Health (M Kim MD), Samsung Advanced Institute for Health Sciences & Technology (SAIHST), Seoul, South Korea; Public Health Center (M Kim MD), Ministry of Health and Welfare, Wando, South Korea; School of Traditional Chinese Medicine (Y Kim PhD), Xiamen University Malaysia, Sepang, Malaysia; School of Health Sciences (Prof A Kisa PhD), Kristiania University College, Oslo, Norway; Department of Global Community Health and Behavioral Sciences (Prof A Kisa PhD), Tulane University, New Orleans, LA, USA; Department of Nursing and Health Promotion (S Kisa PhD), Oslo Metropolitan University, Oslo, Norway; Independent Consultant, Jakarta, Indonesia (S Kosen MD); Department of Internal and Pulmonary Medicine (Prof P A Koul MD), Sheri Kashmir Institute of Medical Sciences, Srinagar, India; Kasturba Medical College, Udupi, India (S Koulmane Laxminarayana MD); Network Centre for Biomedical Research in Mental Health (A Koyanagi MD), San Juan de Dios Sanitary Park, Sant Boi de Llobregat, Spain; Catalan Institution for Research and Advanced Studies (ICREA), Barcelona, Spain (A Koyanagi MD); Department of Anthropology (K Krishan PhD), Panjab University, Chandigarh, India; Faculty of Medicine (B Kucuk Bicer PhD), Gazi University, Ankara, Turkey; Department of Community Medicine (A Kumar MD), Manipal Academy of Higher Education, Jamshedpur, India; Department of Orthopaedics (Prof N Kumar MS), Medanta Hospital, Lucknow, India; Department of Biochemistry and Biotechnology (A Kwarteng PhD), Kwame Nkrumah University of Science and Technology, Kumasi, Ghana; Unit of Genetics and Public Health (Prof I Landires MD), Unit of Microbiology and Public Health (V Nuñez-Samudio PhD), Institute of Medical Sciences, Las Tablas, Panama; Department of Public Health (V Nuñez-Samudio PhD), Ministry of Health, Herrera, Panama (Prof I Landires MD); Department of Otorhinolaryngology (S Lasrado MS), Father Muller Medical College, Mangalore, India; School of Pharmacy (S W H Lee PhD), Monash University, Bandar Sunway, Malaysia; School of Pharmacy (S W H Lee PhD), Taylor's University Lakeside Campus, Subang Jaya, Malaysia; The Office of Health Policy & Legislative Affairs (W Lee PhD), University of Texas, Galveston, TX, USA; Department of Quantitative Health Science (X Liu PhD), Case Western Reserve University, Cleveland, OH, USA; Center for Integration of Data and Health Knowledge (D B Machado PhD), Oswald Cruz Foundation (FIOCRUZ), Salvador, Brazil; Centre for Global Mental Health (CGMH) (D B Machado PhD), London School of Hygiene & Tropical Medicine, London, England; Department of Biochemistry (S Madhava Kunjathur MD), BGS Global Institute of Medical Sciences, Bengaluru, India; Department of Community Medicine (P B Mahajan MD), Jawaharlal Institute of Postgraduate Medical Education and Research, Karaikal, India; Campus Caucaia (F R Martins-Melo PhD), Federal Institute of Education, Science and Technology of Ceará, Caucaia, Brazil; Division of Nutritional Sciences (S Mehta DSc), Cornell University, Ithaca, NY, USA; College of Medicine (Prof Z A Memish MD), Alfaisal University, Riyadh, Saudi Arabia; Research & Innovation Center (Prof Z A Memish MD), Ministry of Health, Riyadh, Saudi Arabia; Peru Country Office (W Mendoza MD), United Nations Population Fund (UNFPA), Lima, Peru; Forensic Medicine Division (Prof R G Menezes MD), Department of Pharmacy Practice (A Naqvi PhD), Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; Department of Statistics (H E Merie MSc), Debre Markos University, Debre Markos, Ethiopia; School of Medicine and Public Health (A G Mersha MD), Research Centre for Generational Health and Ageing (B Wubishet MPH), University of Newcastle, Newcastle, NSW, Australia; School of Medicine (A G Mersha MD), Department of Medical Microbiology (B Tessema PhD), Department of Epidemiology and Biostatistics (Y Yeshaw MPH), University of Gondar, Gondar, Ethiopia; Department of Orthopaedic Surgery (M K Mesregah MSc), University of Southern California, Los Angeles, CA, USA; Department of Orthopaedic Surgery (M K Mesregah MSc), Menoufia University, Shebin El-Kom, Egypt; Clinical Microbiology and Parasitology Unit (T Mestrovic PhD), Dr Zora Profozic Polyclinic, Zagreb, Croatia; University Centre Varazdin (T Mestrovic PhD), University North, Varazdin, Croatia; Department of Communication and Journalism (N M Mheidly BA), Autonomous University of Barcelona, Barcelona, Spain; Department of Information Technology (M Mohammadi PhD), Lebanese French University, Erbil, Iraq; Department of Epidemiology and Biostatistics (A Mohammadian-Hafshejani PhD), Shahrekord University of Medical Sciences, Shahrekord, Iran; Health Systems and Policy Research Unit (S Mohammed PhD), Department of Community Medicine (M B Sufiyan MD), Ahmadu Bello University, Zaria, Nigeria; Department of Health Care Management (S Mohammed PhD), Technical University of Berlin, Berlin, Germany; Faculty of Life Sciences and Medicine (M Molokhia PhD), King's College London, London, UK; Department of Computer Science and Engineering (M A Moni PhD), Pabna University of Science and Technology, Pabna, Bangladesh; Department of Medicine (A A Montasir FMD), TMSS Medical College, Bogura, Bangladesh; Department of Medicine (A A Montasir FMD), Sofia Ismail Memorial Medical Centre, Bogura, Bangladesh; Big Data Institute (C E Moore PhD), Centre for Tropical Medicine and Global Health (B Sartorius PhD), Nuffield Department of Medicine (B Sartorius PhD), University of Oxford, Oxford, UK; Research and Analytics Department (A J Nagarajan MTech), Initiative for Financing Health and Human Development, Chennai, India; Department of Research and Analytics (A J Nagarajan MTech), Bioinsilico Technologies, Chennai, India; Department of Pulmonary Medicine (S Nair MD), Government Medical College Trivandrum, Trivandrum, India; Health Action by People, Trivandrum, India (S Nair MD); Discipline of Social & Administrative Pharmacy (A Naqvi PhD), University of Science, Malaysia, Penang, Malaysia; Mysore Medical College and Research Institute (Prof S Narasimha Swamy MD), Government Medical College, Mysore, India; Bupa Clemton Park (S Neupane Kandel BSN), Bupa, Sydney, NSW, Australia; South African Medical Research Council, Cape Town, South Africa (C A Nnaji MPH); School of Public Health and Family Medicine (C A Nnaji MPH), Division of Cardiology (Prof M Ntsekhe PhD), University of Cape Town, Cape Town, South Africa; The Cardiac Clinic (Prof M Ntsekhe PhD), Groote Schuur Hospital, Cape Town, South Africa; Administrative and Economic Sciences Department (Prof B Oancea PhD), University of Bucharest, Bucharest, Romania; Department of Community Health and Primary Care (O O Odukoya MSc), University of Lagos, Idi Araba, Nigeria; Department of Family and Preventive Medicine (O O Odukoya MSc), University of Utah, Salt Lake City, UT, USA; Department of Psychiatry and Behavioural Neurosciences (A T Olagunju MD), McMaster University, Hamilton, ON, Canada; Department of Psychiatry (A T Olagunju MD), University of Lagos, Lagos, Nigeria; Graduate School of Public Health (Prof E Oren PhD), San Diego State University, San Diego, CA, USA; Department of Respiratory Medicine (Prof M P A DNB), Jagadguru Sri Shivarathreeswara Academy of Health Education and Research, Mysore, India; CSIR-Indian Institute of Toxicology Research (R Parthasarathi PhD), Council of Scientific & Industrial Research, Lucknow, India; Central Department of Public Health (R Paudel MPH), Tribhuvan University, Kathmandu, Nepal; Centre for the Study of Regional Development (P Paul MPhil), Jawahar Lal Nehru University, New Delhi, India; Department of Genetics (S Pawar PhD), Yale University, New Haven, CT, USA; Center for Research and Innovation (V F Pepito MSc), Ateneo De Manila University, Pasig City, Philippines; Mario Negri Institute for Pharmacological Research, Bergamo, Italy (N Perico MD, Prof G Remuzzi MD); Department of Parasitology and Entomology (M Pirestani PhD), Tarbiat Modares University, Tehran, Iran; University Medical Center Groningen (Prof M J Postma PhD), School of Economics and Business (Prof M J Postma PhD), University of Groningen, Groningen, Netherlands; Department of Biochemistry (Prof A Prashant PhD), Jagadguru Sri Shivarathreeswara University, Mysuru, India; Health Sciences Department (D R A Pribadi MSc), Muhammadiyah University of Surakarta, Sukoharjo, Indonesia; College of Medicine (A Radfar MD), University of Central Florida, Orlando, FL, USA; Department of Immunology (Prof A Rafiei PhD), Molecular and Cell Biology Research Center (Prof A Rafiei PhD), Pediatric Infectious Diseases Research Center (Prof M Rezai MD), Mazandaran University of Medical Sciences, Sari, Iran; Thalassemia and Hemoglobinopathy Research Center (F Rahim PhD), Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran; Pure Earth, Dhaka, Bangladesh (M Rahman PhD); Department of Population Science and Human Resource Development (M Rahman DrPH), University of Rajshahi, Rajshahi, Bangladesh; Future Technology Research Center (A Rahmani PhD), National Yunlin University of Science and Technology, Yunlin, Taiwan; Department of Pharmacology (P Ranasinghe PhD), University of Colombo, Colombo, Sri Lanka; NIHR-Biomedical Research Centre (NIHR-BRC) (Prof A Zumla PhD), University College London Hospitals, London, UK (D L Rawaf MD); Academic Public Health England (Prof S Rawaf MD), Public Health England, London, UK; School of Medicine (Prof A M N Renzaho PhD), Translational Health Research Institute (Prof A M N Renzaho PhD), Western Sydney University, Campbelltown, NSW, Australia; Department of Medical Laboratory Science (M A Reta MSc), Woldia University, Woldia, Ethiopia; Department of Medical Microbiology (M A Reta MSc), University of Pretoria, Pretoria, South Africa; Network of Immunity in Infection, Malignancy and Autoimmunity (NIIMA) (Prof N Rezaei PhD), Universal Scientific Education and Research Network (USERN), Tehran, Iran; Department of Infectious Diseases (O Rezahosseini MD), University of Copenhagen, Copenhagen, Denmark; Golestan Research Center of Gastroenterology and Hepatology (GRCGH) (G Roshandel PhD), Golestan University of Medical Sciences, Gorgan, Iran; Department of Lifestyle Research and Public Health (D O Roshchin PhD), NA Semashko National Research Institute of Public Health, Moscow, Russia; Health Systems and Population Studies Division (K Saif-Ur-Rahman MPH), International Centre for Diarrhoeal Disease Research, Bangladesh, Dhaka, Bangladesh; Department of Public Health and Health Systems (K Saif-Ur-Rahman MPH), Nagoya University, Nagoya, Japan; Department of Microbiology (N Salam PhD), Central University of Punjab, Bathinda, India; Emergency Department (M Samaei MD), Brown University, Providence, RI, USA; Department of Entomology (A M Samy PhD), Ain Shams University, Cairo, Egypt; Department of Community Medicine (S Saroshe MD), Mahatma Gandhi Memorial Medical College, Indore, India; Faculty of Health & Social Sciences (B Sathian PhD), Bournemouth University, Bournemouth, UK; Department of Paediatrics (Prof S M Sawyer MD), University of Melbourne, Parkville, VIC, Australia; Centre for Adolescent Health (Prof S M Sawyer MD), Murdoch Childrens Research Institute, Parkville, VIC, Australia; Emergency Department (S Senthilkumaran MD), Manian Medical Centre, Erode, India; National Heart, Lung, and Blood Institute (A Seylani BS), National Institute of Health, Rockville, MD, USA; Independent Consultant, Karachi, Pakistan (M A Shaikh MD); National Institute of Infectious Diseases, Tokyo, Japan (M Shigematsu PhD); College of Medicine (Prof J Shin MD), Yonsei University, Seoul, South Korea; Department of Community Medicine & Public Health (J Singh PhD), Tribhuvan University, Janakpur, Nepal; Department No.16 (V Y Skryabin MD), Laboratory of Genetics and Genomics (Prof M S Zastrozhin PhD), Moscow Research and Practical Centre on Addictions, Moscow, Russia; Therapeutic Department (A A Skryabina MD), Balashiha Central Hospital, Balashikha, Russia; Division of Community Medicine (C T Sreeramareddy MD), International Medical University, Kuala Lumpur, Malaysia; Department of Medicine (P Steiropoulos MD), Democritus University of Thrace, Alexandroupolis, Greece; Cancer Control Center (T Tabuchi MD), Osaka International Cancer Institute, Osaka, Japan; Department of Medical Laboratory Science (Z Tamir MSc), Addis Ababa University, Addis Ababa, Ethiopia; Department of Population and Behavioral Science (E E Tarkang PhD), University of Health and Allied Sciences, Ho, Ghana; HIV Health Promotion Division (E E Tarkang PhD), HIV/AIDS Prevention Research Network, Cameroon, Kumba, Cameroon; School of Public Health (F H Tesfay PhD), School of Pharmacy (B Wubishet MPH), Mekelle University, Mekelle, Ethiopia; Southgate Institute for Health and Society (F H Tesfay PhD), Flinders University, Adelaide, SA, Australia; Department of Medical Specialties: Infectious Diseases Section (Prof I I Tleyjeh MD), King Fahad Medical City, Riyadh, Saudi Arabia; Division of Infectious Diseases and Department of Epidemiology (Prof I I Tleyjeh MD), Mayo Clinic, Rochester, MN, USA; Department of Social Security Empirical Research (Prof R Tobe-Gai PhD), National Institute of Population and Social Security Research, Tokyo, Japan; Department of Health Economics (B X Tran PhD), Hanoi Medical University, Hanoi, Vietnam; School of Midwifery (B Tsegaye MSc), Hawassa University, Hawassa, Ethiopia; Department of Community Medicine (C D Umeokonkwo MPH), Alex Ekwueme Federal University Teaching Hospital Abakaliki, Abakaliki, Nigeria; Clinical Cancer Research Center (S Valadan Tahbaz PhD, S Yahyazadeh Jabbari MD), Milad General Hospital, Tehran, Iran; Department of Microbiology (S Valadan Tahbaz PhD), Islamic Azad University, Tehran, Iran; Faculty of Information Technology (B Vo PhD), Ho Chi Minh City University of Technology (HUTECH), Ho Chi Minh City, Vietnam; Center of Excellence in Behavioral Medicine (G T Vu BA), Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam; Department of Pharmacy (T S Y Yazie MSc), Debre Tabor University, Debre Tabor, Ethiopia; Saw Swee Hock School of Public Health (S Yi PhD), National University of Singapore, Singapore, Singapore; KHANA Center for Population Health Research (S Yi PhD), Khana, Phnom Penh, Cambodia; Department of Health Management (V Yiğit PhD), Süleyman Demirel Üniversitesi (Süleyman Demirel University), Isparta, Turkey; Department of Neuropsychopharmacology (N Yonemoto MPH), National Center of Neurology and Psychiatry, Kodaira, Japan; Department of Public Health (N Yonemoto MPH), Juntendo University, Tokyo, Japan; Department of Epidemiology and Biostatistics (Prof C Yu PhD), School of Medicine (Z Zhang PhD), Wuhan University, Wuhan, China; Department of Clinical Pharmacy and Outcomes Sciences (I Yunusa PhD), University of South Carolina, Columbia, SC, USA; Addictology Department (Prof M S Zastrozhin PhD), Pediatrics Department (A Zastrozhina PhD), Russian Medical Academy of Continuous Professional Education, Moscow, Russia; Department of Infection (Prof A Zumla PhD), University College London, London, UK; Center for Health Policy & Center for Primary Care and Outcomes Research (Prof J A Salomon PhD), Stanford University, Stanford, CA, USA.
Contributor Information
GBD 2019 Tuberculosis Collaborators:
Jorge R Ledesma, Jianing Ma, Avina Vongpradith, Emilie R Maddison, Amanda Novotney, Molly H Biehl, Kate E LeGrand, Jennifer M Ross, Deepa Jahagirdar, Dana Bryazka, Rachel Feldman, Hassan Abolhassani, Akine Eshete Abosetugn, Eman Abu-Gharbieh, Oladimeji M Adebayo, Qorinah Estiningtyas Sakilah Adnani, Saira Afzal, Bright Opoku Ahinkorah, Sajjad Ahmad Ahmad, Sepideh Ahmadi, Tarik Ahmed Rashid, Yusra Ahmed Salih, Addis Aklilu, Chisom Joyqueenet Akunna, Hanadi Al Hamad, Fares Alahdab, Yosef Alemayehu, Kefyalew Addis Alene, Beriwan Abdulqadir Ali, Liaqat Ali, Vahid Alipour, Hesam Alizade, Rajaa M Al-Raddadi, Nelson Alvis-Guzman, Saeed Amini, Arianna Maever L Amit, Jason A Anderson, Sofia Androudi, Carl Abelardo T Antonio, Catherine M Antony, Razique Anwer, Jalal Arabloo, Asrat Arja, Mulusew A Asemahagn, Sachin R Atre, Gulrez Shah Azhar, Darshan B B, Zaheer-Ud-Din Babar, Atif Amin Baig, Maciej Banach, Hiba Jawdat Barqawi, Fabio Barra, Amadou Barrow, Sanjay Basu, Uzma Iqbal Belgaumi, Akshaya Srikanth Bhagavathula, Nikha Bhardwaj, Pankaj Bhardwaj, Natalia V Bhattacharjee, Krittika Bhattacharyya, Ali Bijani, Boris Bikbov, Archith Boloor, Nikolay Ivanovich Briko, Danilo Buonsenso, Sharath Burugina Nagaraja, Zahid A Butt, Austin Carter, Felix Carvalho, Jaykaran Charan, Souranshu Chatterjee, Soosanna Kumary Chattu, Vijay Kumar Chattu, Devasahayam J Christopher, Dinh-Toi Chu, Mareli M Claassens, Omid Dadras, Amare Belachew Dagnew, Xiaochen Dai, Lalit Dandona, Rakhi Dandona, Parnaz Daneshpajouhnejad, Aso Mohammad Darwesh, Deepak Dhamnetiya, Mostafa Dianatinasab, Daniel Diaz, Linh Phuong Doan, Sahar Eftekharzadeh, Muhammed Elhadi, Amir Emami, Shymaa Enany, Emerito Jose A Faraon, Farshad Farzadfar, Eduarda Fernandes, Lorenzo Ferro Desideri, Irina Filip, Florian Fischer, Masoud Foroutan, Tahvi D Frank, Alberto L Garcia-Basteiro, Christian Garcia-Calavaro, Tushar Garg, Biniyam Sahiledengle Geberemariyam, Keyghobad Ghadiri, Ahmad Ghashghaee, Mahaveer Golechha, Amador Goodridge, Bhawna Gupta, Sapna Gupta, Veer Bala Gupta, Vivek Kumar Gupta, Mohammad Rifat Haider, Samer Hamidi, Asif Hanif, Shafiul Haque, Harapan Harapan, Arief Hargono, Ahmed I Hasaballah, Abdiwahab Hashi, Shoaib Hassan, Hadi Hassankhani, Khezar Hayat, Kamal Hezam, Ramesh Holla, Mehdi Hosseinzadeh, Mihaela Hostiuc, Mowafa Househ, Rabia Hussain, Segun Emmanuel Ibitoye, Irena M Ilic, Milena D Ilic, Seyed Sina Naghibi Irvani, Nahlah Elkudssiah Ismail, Ramaiah Itumalla, Jalil Jaafari, Kathryn H Jacobsen, Vardhmaan Jain, Fatemeh Javanmardi, Sathish Kumar Jayapal, Shubha Jayaram, Ravi Prakash Jha, Jost B Jonas, Nitin Joseph, Farahnaz Joukar, Zubair Kabir, Ashwin Kamath, Tanuj Kanchan, Himal Kandel, Patrick DMC Katoto, Gbenga A Kayode, Parkes J Kendrick, Amene Abebe Kerbo, Himanshu Khajuria, Rovshan Khalilov, Khaled Khatab, Abdullah T Khoja, Jagdish Khubchandani, Min Seo Kim, Yun Jin Kim, Adnan Kisa, Sezer Kisa, Soewarta Kosen, Parvaiz A Koul, Sindhura Lakshmi Koulmane Laxminarayana, Ai Koyanagi, Kewal Krishan, Burcu Kucuk Bicer, Avinash Kumar, G Anil Kumar, Narinder Kumar, Nithin Kumar, Alexander Kwarteng, Hassan Mehmood Lak, Dharmesh Kumar Lal, Iván Landires, Savita Lasrado, Shaun Wen Huey Lee, Wei-Chen Lee, Christine Lin, Xuefeng Liu, Platon D Lopukhov, Rafael Lozano, Daiane Borges Machado, Shilpashree Madhava Kunjathur, Deepak Madi, Preetam Bhalchandra Mahajan, Azeem Majeed, Ahmad Azam Malik, Francisco Rogerlândio Martins-Melo, Saurabh Mehta, Ziad A Memish, Walter Mendoza, Ritesh G Menezes, Hayimro Edemealem Merie, Amanual Getnet Mersha, Mohamed Kamal Mesregah, Tomislav Mestrovic, Nour Mheidly Mheidly, Sanjeev Misra, Prasanna Mithra, Masoud Moghadaszadeh, Mokhtar Mohammadi, Abdollah Mohammadian-Hafshejani, Shafiu Mohammed, Mariam Molokhia, Mohammad Ali Moni, Ahmed Al Montasir, Catrin E Moore, Ahamarshan Jayaraman Nagarajan, Sanjeev Nair, Suma Nair, Atta Abbas Naqvi, Sreenivas Narasimha Swamy, Biswa Prakash Nayak, Javad Nazari, Sandhya Neupane Kandel, Trang Huyen Nguyen, Molly R Nixon, Chukwudi A Nnaji, Mpiko Ntsekhe, Virginia Nuñez-Samudio, Bogdan Oancea, Oluwakemi Ololade Odukoya, Andrew T Olagunju, Eyal Oren, Mahesh P A, Ramakrishnan Parthasarathi, Fatemeh Pashazadeh Kan, Sanjay M Pattanshetty, Rajan Paudel, Pintu Paul, Shrikant Pawar, Veincent Christian Filipino Pepito, Norberto Perico, Majid Pirestani, Roman V Polibin, Maarten J Postma, Akram Pourshams, Akila Prashant, Dimas Ria Angga Pribadi, Amir Radfar, Alireza Rafiei, Fakher Rahim, Vafa Rahimi-Movaghar, Mahfuzar Rahman, Mosiur Rahman, Amir Masoud Rahmani, Priyanga Ranasinghe, Chythra R Rao, David Laith Rawaf, Salman Rawaf, Marissa B Reitsma, Giuseppe Remuzzi, Andre M N Renzaho, Melese Abate Reta, Nima Rezaei, Omid Rezahosseini, Mohammad sadegh Rezai, Aziz Rezapour, Gholamreza Roshandel, Denis O Roshchin, Siamak Sabour, KM Saif-Ur-Rahman, Nasir Salam, Hossein Samadi Kafil, Mehrnoosh Samaei, Abdallah M Samy, Satish Saroshe, Benn Sartorius, Brijesh Sathian, Susan M Sawyer, Subramanian Senthilkumaran, Allen Seylani, Omid Shafaat, Masood Ali Shaikh, Kiomars Sharafi, Ranjitha S Shetty, Mika Shigematsu, Jae Il Shin, João Pedro Silva, Jitendra Kumar Singh, Smriti Sinha, Valentin Yurievich Skryabin, Anna Aleksandrovna Skryabina, Emma Elizabeth Spurlock, Chandrashekhar T Sreeramareddy, Paschalis Steiropoulos, Mu'awiyyah Babale Sufiyan, Takahiro Tabuchi, Eyayou Girma Tadesse, Zemenu Tamir, Elvis Enowbeyang Tarkang, Yohannes Tekalegn, Fisaha Haile Tesfay, Belay Tessema, Rekha Thapar, Imad I Tleyjeh, Ruoyan Tobe-Gai, Bach Xuan Tran, Berhan Tsegaye, Gebiyaw Wudie Tsegaye, Anayat Ullah, Chukwuma David Umeokonkwo, Sahel Valadan Tahbaz, Bay Vo, Giang Thu Vu, Yasir Waheed, Magdalene K Walters, Joanna L Whisnant, Mesfin Agachew Woldekidan, Befikadu Legesse Wubishet, Seyed Hossein Yahyazadeh Jabbari, Taklo Simeneh Yazie Yazie, Yigizie Yeshaw, Siyan Yi, Vahit Yiğit, Naohiro Yonemoto, Chuanhua Yu, Ismaeel Yunusa, Mikhail Sergeevich Zastrozhin, Anasthasia Zastrozhina, Zhi-Jiang Zhang, Alimuddin Zumla, Ali H Mokdad, Joshua A Salomon, Robert C Reiner Jr, Stephen S Lim, Mohsen Naghavi, Theo Vos, Simon I Hay, Christopher J L Murray, and Hmwe Hmwe Kyu
Supplementary Material
References
- 1.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kyu HH, Maddison ER, Henry NJ, et al. Global, regional, and national burden of tuberculosis, 1990–2016: results from the Global Burden of Diseases, Injuries, and Risk Factors 2016 Study. Lancet Infect Dis. 2018;18:1329–1349. doi: 10.1016/S1473-3099(18)30625-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . World Health Organization; Geneva: 2020. Global tuberculosis report 2020. [Google Scholar]
- 4.Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med. 2018;6:299–314. doi: 10.1016/S2213-2600(18)30057-2. [DOI] [PubMed] [Google Scholar]
- 5.Castro KG, Colvin CE. Updated global tuberculosis targets: a welcome ambition in need of attention to quality of care. Int J Tuberc Lung Dis. 2018;22:709. doi: 10.5588/ijtld.18.0344. [DOI] [PubMed] [Google Scholar]
- 6.Horton KC, MacPherson P, Houben RMGJ, White RG, Corbett EL. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borgdorff MW, Nagelkerke NJ, Dye C, Nunn P. Gender and tuberculosis: a comparison of prevalence surveys with notification data to explore sex differences in case detection. Int J Tuberc Lung Dis. 2000;4:123–132. [PubMed] [Google Scholar]
- 8.Horton KC, Sumner T, Houben RMGJ, Corbett EL, White RG. A Bayesian approach to understanding sex differences in tuberculosis disease burden. Am J Epidemiol. 2018;187:2431–2438. doi: 10.1093/aje/kwy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hertz D, Schneider B. Sex differences in tuberculosis. Semin Immunopathol. 2019;41:225–237. doi: 10.1007/s00281-018-0725-6. [DOI] [PubMed] [Google Scholar]
- 10.Kyu HH, Maddison ER, Henry NJ, et al. The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect Dis. 2018;18:261–284. doi: 10.1016/S1473-3099(17)30703-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watkins RE, Plant AJ. Does smoking explain sex differences in the global tuberculosis epidemic? Epidemiol Infect. 2006;134:333–339. doi: 10.1017/S0950268805005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyu HH, Abate D, Abate KH, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foreman KJ, Lozano R, Lopez AD, Murray CJ. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. 2012;10:1. doi: 10.1186/1478-7954-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fullman N, Yearwood J, Abay SM, et al. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet. 2018;391:2236–2271. doi: 10.1016/S0140-6736(18)30994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng P, Aravkin AY, Barber R, Sorensen RJD, Murray CJL. Trimmed constrained mixed effects models: formulations and algorithms. arXiv. 2019 http://arxiv.org/abs/1909.10700 published online Sept 24. (preprint). [Google Scholar]
- 17.Flaxman AD, Vos T, Murray CJ. University of Washington Press; Seattle, WA: 2015. An integrative metaregression framework for descriptive epidemiology. [Google Scholar]
- 18.Murray CJL, Aravkin AY, Zheng P, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van den Hof S, Najlis C, Bloss E, Straetemans M. A systematic review on the role of gender in tuberculosis control. 2010. https://www.kncvtbc.org/uploaded/2015/09/Role_of_Gender_in_TB_Control.pdf
- 20.Kumwenda M, Desmond N, Hart G, et al. Treatment-seeking for tuberculosis-suggestive symptoms: a reflection on the role of human agency in the context of universal health coverage in Malawi. PLoS One. 2016;11 doi: 10.1371/journal.pone.0154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mavhu W, Dauya E, Bandason T, et al. Chronic cough and its association with TB-HIV co-infection: factors affecting help-seeking behaviour in Harare, Zimbabwe. Trop Med Int Heal. 2010;15:574–579. doi: 10.1111/j.1365-3156.2010.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chikovore J, Hart G, Kumwenda M, Chipungu GA, Corbett L. ‘For a mere cough, men must just chew Conjex, gain strength, and continue working’: the provider construction and tuberculosis care-seeking implications in Blantyre, Malawi. Glob Health Action. 2015;8 doi: 10.3402/gha.v8.26292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chikovore J, Hart G, Kumwenda M, Chipungu GA, Desmond N, Corbett L. Control, struggle, and emergent masculinities: a qualitative study of men's care-seeking determinants for chronic cough and tuberculosis symptoms in Blantyre, Malawi. BMC Public Health. 2014;14 doi: 10.1186/1471-2458-14-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chikovore J, Pai M, Horton KC, et al. Missing men with tuberculosis: the need to address structural influences and implement targeted and multidimensional interventions. BMJ Glob Heal. 2020;5 doi: 10.1136/bmjgh-2019-002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marçôa R, Ribeiro AI, Zão I, Duarte R. Tuberculosis and gender—factors influencing the risk of tuberculosis among men and women by age group. Pulmonology. 2018;24:199–202. doi: 10.1016/j.pulmoe.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Sulaiman SAS, Khan AH, Ahmad N, Iqubal MS, Muttalif AR, Hassali MA. Impact of diabetes mellitus on treatment outcomes of tuberculosis patients in tertiary care setup. Am J Med Sci. 2013;345:321–325. doi: 10.1097/MAJ.0b013e318288f8f3. [DOI] [PubMed] [Google Scholar]
- 27.Golub JE, Mok Y, Hong S, Jung KJ, Jee SH, Samet JM. Diabetes mellitus and tuberculosis in Korean adults: impact on tuberculosis incidence, recurrence and mortality. Int J Tuberc Lung Dis. 2019;23:507–513. doi: 10.5588/ijtld.18.0103. [DOI] [PubMed] [Google Scholar]
- 28.Miller CR, Davis JL, Katamba A, et al. Sex disparities in tuberculosis suspect evaluation: a cross-sectional analysis in rural Uganda. Int J Tuberc Lung Dis. 2013;17:480–485. doi: 10.5588/ijtld.12.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thongraung W, Chongsuvivatwong V, Pungrassamee P. Multilevel factors affecting tuberculosis diagnosis and initial treatment. J Eval Clin Pract. 2008;14:378–384. doi: 10.1111/j.1365-2753.2007.00871.x. [DOI] [PubMed] [Google Scholar]
- 30.Karim F, Islam MA, Chowdhury AM, Johansson E, Diwan VK. Gender differences in delays in diagnosis and treatment of tuberculosis. Health Policy Plan. 2007;22:329–334. doi: 10.1093/heapol/czm026. [DOI] [PubMed] [Google Scholar]
- 31.Cheng G, Tolhurst R, Li RZ, Meng QY, Tang S. Factors affecting delays in tuberculosis diagnosis in rural China: a case study in four counties in Shandong province. Trans R Soc Trop Med Hyg. 2005;99:355–362. doi: 10.1016/j.trstmh.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 32.Datiko DG, Jerene D, Suarez P. Patient and health system delay among TB patients in Ethiopia: nationwide mixed method cross-sectional study. BMC Public Health. 2020;20 doi: 10.1186/s12889-020-08967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kivihya-Ndugga LEA, van Cleeff MRA, Ng'ang'a LW, Meme H, Odhiambo JA, Klatser PR. Sex-specific performance of routine TB diagnostic tests. Int J Tuberc Lung Dis. 2005;9:294–300. [PubMed] [Google Scholar]
- 34.Krishnan L, Akande T, Shankar AV, et al. Gender-related barriers and delays in accessing tuberculosis diagnostic and treatment services: a systematic review of qualitative studies. Tuberc Res Treat. 2014;2014 doi: 10.1155/2014/215059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegdahl HK, Fylkesnes KM, Sandøy IF. Sex differences in HIV prevalence persist over time: evidence from 18 countries in sub-Saharan Africa. PLoS One. 2016;11 doi: 10.1371/journal.pone.0148502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magadi MA. Understanding the gender disparity in HIV infection across countries in sub-Saharan Africa: evidence from the Demographic and Health Surveys. Sociol Health Illn. 2011;33:522–539. doi: 10.1111/j.1467-9566.2010.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sia D, Onadja Y, Hajizadeh M, Heymann SJ, Brewer TF, Nandi A. What explains gender inequalities in HIV/AIDS prevalence in sub-Saharan Africa? Evidence from the Demographic and Health Surveys. BMC Public Health. 2016;16 doi: 10.1186/s12889-016-3783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aaron L, Saadoun D, Calatroni I, et al. Tuberculosis in HIV-infected patients: a comprehensive review. Clin Microbiol Infect. 2004;10:388–398. doi: 10.1111/j.1469-0691.2004.00758.x. [DOI] [PubMed] [Google Scholar]
- 39.Ramjee G, Daniels B. Women and HIV in sub-Saharan Africa. AIDS Res Ther. 2013;10:30. doi: 10.1186/1742-6405-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdool Karim Q, Sibeko S, Baxter C. Preventing HIV infection in women: a global health imperative. Clin Infect Dis. 2010;50(suppl 3):S122–S129. doi: 10.1086/651483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birdthistle I, Schaffnit SB, Kwaro D, et al. Evaluating the impact of the DREAMS partnership to reduce HIV incidence among adolescent girls and young women in four settings: a study protocol. BMC Public Health. 2018;18:912. doi: 10.1186/s12889-018-5789-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8:e1132–e1141. doi: 10.1016/S2214-109X(20)30288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cilloni L, Fu H, Vesga JF, et al. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alene KA, Wangdi K, Clements ACA. Impact of the COVID-19 pandemic on tuberculosis control: an overview. Trop Med Infect Dis. 2020;5:123. doi: 10.3390/tropicalmed5030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.García-Basteiro AL, Brew J, Williams B, Borgdorff M, Cobelens F. What is the true tuberculosis mortality burden? Differences in estimates by the World Health Organization and the Global Burden of Disease study. Int J Epidemiol. 2018;47:1549–1560. doi: 10.1093/ije/dyy144. [DOI] [PubMed] [Google Scholar]
- 46.WHO . World Health Organization; Geneva: 2017. Global tuberculosis report 2017. [Google Scholar]
- 47.Muture BN, Keraka MN, Kimuu PK, Kabiru EW, Ombeka VO, Oguya F. Factors associated with default from treatment among tuberculosis patients in Nairobi province, Kenya: a case control study. BMC Public Health. 2011;11:696. doi: 10.1186/1471-2458-11-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas BE, Thiruvengadam KSR, Kadam D, et al. Smoking, alcohol use disorder and tuberculosis treatment outcomes: a dual co-morbidity burden that cannot be ignored. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rehm J, Samokhvalov AV, Neuman MG, et al. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health. 2009;9:450. doi: 10.1186/1471-2458-9-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lozano R, Lopez AD, Atkinson C, Naghavi M, Flaxman AD, Murray CJ. Performance of physician-certified verbal autopsies: multisite validation study using clinical diagnostic gold standards. Popul Health Metr. 2011;9:32. doi: 10.1186/1478-7954-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.James SL, Flaxman AD, Murray CJ. Performance of the Tariff Method: validation of a simple additive algorithm for analysis of verbal autopsies. Popul Health Metr. 2011;9:31. doi: 10.1186/1478-7954-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray CJ, Lozano R, Flaxman AD, et al. Using verbal autopsy to measure causes of death: the comparative performance of existing methods. BMC Med. 2014;12:5. doi: 10.1186/1741-7015-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sumpter C, Chandramohan D. Systematic review and meta-analysis of the associations between indoor air pollution and tuberculosis. Trop Med Int Health. 2013;18:101–108. doi: 10.1111/tmi.12013. [DOI] [PubMed] [Google Scholar]
- 54.Narasimhan P, Wood J, Macintyre CR, Mathai D. Risk factors for tuberculosis. Pulm Med. 2013;2013 doi: 10.1155/2013/828939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bangladesh Bureau of Statistics, Centers for Disease Control and Prevention, Directorate General of Health Services, Ministry of Health and Family Welfare (Bangladesh), Global Fund to Fight Aids Tuberculosis and Malaria, et al. Bangladesh National Tuberculosis Prevalence Survey 2015–2016. Dhaka, Bangladesh; 2016.
- 56.Alam N, Chowdhury HR, Ahmed A, Rahman M, Streatfield PK. Distribution of cause of death in rural Bangladesh during 2003–2010: evidence from two rural areas within Matlab Health and Demographic Surveillance site. Glob Health Action. 2014;7 doi: 10.3402/gha.v7.25510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarker M, Homayra F, Rawal LB, et al. Urban-rural and sex differentials in tuberculosis mortality in Bangladesh: results from a population-based survey. Trop Med Int Health. 2019;24:109–115. doi: 10.1111/tmi.13171. [DOI] [PubMed] [Google Scholar]
- 58.Adegbola RA, Falade AG, Sam BE, et al. The etiology of pneumonia in malnourished and well-nourished Gambian children. Pediatr Infect Dis J. 1994;13:975–982. doi: 10.1097/00006454-199411000-00008. [DOI] [PubMed] [Google Scholar]
- 59.Chisti MJ, Graham SM, Duke T, et al. A prospective study of the prevalence of tuberculosis and bacteraemia in Bangladeshi children with severe malnutrition and pneumonia including an evaluation of Xpert MTB/RIF assay. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madhi SA, Petersen K, Madhi A, Khoosal M, Klugman KP. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin Infect Dis. 2000;31:170–176. doi: 10.1086/313925. [DOI] [PubMed] [Google Scholar]
- 61.McNally LM, Jeena PM, Gajee K, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. Lancet. 2007;369:1440–1451. doi: 10.1016/S0140-6736(07)60670-9. [DOI] [PubMed] [Google Scholar]
- 62.Moore DP, Klugman KP, Madhi SA. Role of Streptococcus pneumoniae in hospitalization for acute community-acquired pneumonia associated with culture-confirmed Mycobacterium tuberculosis in children: a pneumococcal conjugate vaccine probe study. Pediatr Infect Dis J. 2010;29:1099–1104. doi: 10.1097/inf.0b013e3181eaefff. [DOI] [PubMed] [Google Scholar]
- 63.Nantongo JM, Wobudeya E, Mupere E, et al. High incidence of pulmonary tuberculosis in children admitted with severe pneumonia in Uganda. BMC Pediatr. 2013;13:16. doi: 10.1186/1471-2431-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zar HJ, Hanslo D, Tannenbaum E, et al. Aetiology and outcome of pneumonia in human immunodeficiency virus-infected children hospitalized in South Africa. Acta Paediatr. 2001;90:119–125. [PubMed] [Google Scholar]
- 65.Moore DP, Higdon MM, Hammitt LL, et al. The incremental value of repeated induced sputum and gastric aspirate samples for the diagnosis of pulmonary tuberculosis in young children with acute community-acquired pneumonia. Clin Infect Dis. 2017;64(suppl 3):S309–S316. doi: 10.1093/cid/cix099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To download the data used in these analyses, please visit the Global Health Data Exchange GBD 2019 website.