Abstract
Larval zebrafish have been established as an excellent model for examining vertebrate biology, with many researchers using the system for neuroscience. Controlling a fast escape response of the fish, the Mauthner cells and their associated network are an attractive model, given their experimental accessibility and fast development, driving ethologically relevant behavior in the first five days of development. Here, we describe methods for immunostaining electrical and chemical synapse proteins at 3-7 days post fertilization (dpf) in zebrafish using tricholoracetic acid fixation. The methods presented are ideally suited to easily visualize neural circuits and synapses within the fish.
Keywords: Immunostaining, Zebrafish, Larvae, TCA, Antibody
Background
Immunostaining tissue is a useful method to analyze protein distribution in situ in organisms. Using the transparent larval zebrafish, this process presents a valuable window into the construction of vertebrate tissues, here with a focus on neural circuit development. We used a protocol that is optimal for visualizing the electrical and chemical synapse proteins within the Mauthner cell neural network ( Lasseigne et al., 2021 ). Here, we present methods for fixing and immunostaining this structure using tricholoracetic acid (TCA). TCA fixes zebrafish larvae in a much stiffer way than paraformaldehyde (PFA), allowing for an easy microdissection. During this process, the dorsal and ventral halves are bisected, leaving the entire central nervous system intact, and giving access to imaging the brain from the ventral side, thereby avoiding pigmentation located on the dorsal surface of the animal. Additionally, TCA and PFA result in different antibody access to proteins of interest, and staining that fails using one can work well with another. Overall, this method is an excellent alternative to PFA fixation and is a helpful tool for zebrafish immunohistochemistry.
Materials and Reagents
22 × 22 mm (7/8 × 7/8") Micro Cover Glasses, Square, No.1 (VWR, catalog number: 48366-067)
Superfrost Microscope Slides, 25 × 75 × 1 (Fisher, catalog number: 12-550-143)
-
ProLong Gold Antifade reagent (Fisher, catalog number: P10144)
Note: For all below reagents, see Recipes for appropriate dilutions/mixes to create working solutions.
Tricaine methanesulfonate (MS-222 sometimes called MESAB, Tricaine-S, Syndel)
TCA (trichloroacetic acid, Sigma, catalog number: T6399-500G)
Triton X-100 (VWR, catalog number: 0694-1L)
Western Block (Sigma, catalog number: 11921673001)
Glycerol (VWR, catalog number: AAAA16205-0F)
Phosphate-buffered saline (PBS)
Equipment
Dumont Medical Tweezers, Style 5, High Precision, 0.01 mm × 0.05 mm (EMS, catalog number: 72877-D)
Benchtop tube rocker
Procedure
-
DAY ONE – Fix and block
Note: The days noted are for the standard procedure. Step lengths can be modified (see below) and will alter the days in which a particular step is performed.
Anesthetize your fish (using tricaine methanesulfonate, fish will be anesthetized in less than a minute), then sort into 1.5 mL microcentrifuge tubes (less than 100 fish per tube).
Make a 2% TCA solution fresh from frozen aliquots.
Using a Pasteur pipette, remove any excess fish water from the microcentrifuge tubes.
Add 1 mL of 2% TCA to your larvae and rock for 3-4 h at room temperature (RT). Ensure the liquid and fish are moving inside the microcentrifuge tube.
Wash in 1 mL of PBS for 5 × 5 min.
Block using 1 mL of Western Block overnight at 4°C on a benchtop tube rocker.
-
DAY TWO – Primary antibody stain
-
(Option A) – Dissect, then stain
Note: An alternative dissection method is at the end of the protocol, see F.
Place embryos in a Sylgard plate with 1× PBS.
Prepare new microcentrifuge tubes with Western Block. Dissected larvae will be placed into these tubes.
Use pins to hold larvae during dissection.
Remove eye and skin with tungsten wire and forceps.
Use wire to cut off cerebellum, optic tectum, and telencephon. This decreases the thickness of the brain by approximately half when mounting.
Place the dissected brain into microcentrifuge with Western Block.
Block for 3 h at RT.
Remove Western Block.
Sonicate and vortex primary antibodies (at least twice).
Add primary antibody mix (primary antibodies in Western Block). Rock overnight at 4°C. Ensure liquid and fish are moving inside the microcentrifuge tube.
-
(Option B) – Whole larvae stain
Remove Western Block.
Sonicate/mix primary antibodies (at least twice).
Add primary antibody mix (500 μL to 1 mL of primary antibodies in Western Block). Rock overnight at 4°C. Ensure liquid and fish are moving inside the microcentrifuge tube (Alternatively, you can rock overnight at RT).
Note: The above protocols give the best results. Alternatively, steps can be truncated. Rather than blocking overnight, the block can be added for one hour, and then the fish transferred to the primary antibody to rock overnight. Instead of incubating with primary antibody overnight at 4°C, this can be done at RT for 5 h up to overnight.
-
-
DAY THREE – Wash and add the fluorescent secondary antibody
Remove primary antibody. [Save the antibodies as they can be re-used many times. Staining becomes better with several uses (three to five times), as the antibodies are pre-absorbed. Over many uses, the staining will degrade (ten times). Store at 4°C. We advise adding sodium azide, as it is key to preserving the antibody long-term.]
Wash with PBSTx at least 5 × 15 min, but preferably all afternoon, with five changes of 1 mL of PBSTx.
Remove PBSTx. (Alternatively, you can block/rock for 1 h at RT, then proceed.)
Sonicate/mix secondary antibodies (at least twice).
Add secondary antibody mix (500 μL to 1 mL of secondary antibodies in Western Block).
-
Rock overnight at 4 °C in the dark. Ensure liquid and fish are moving inside the microcentrifuge tube.
Note: The secondary antibody can be added for 3 h up to overnight. Note that all steps that shorten antibody incubations tend to decrease image quality, while those that increase the length or temperature tend to decrease the life of the antibodies.
-
DAY FOUR – Wash
Remove secondary antibody. (Save as noted above for primary antibodies.)
Wash with 1 mL of PBSTx at least 4 × 15 min.
Final change into new PBSTx, wash overnight at 4°C in the dark. (Alternatively, you can skip this final wash and move to the mounting stage.)
-
DAY FIVE – Mount for imaging
-
(Option A) – Mount using bridge
Remove PBSTx and replace with 1 mL of fresh PBSTx.
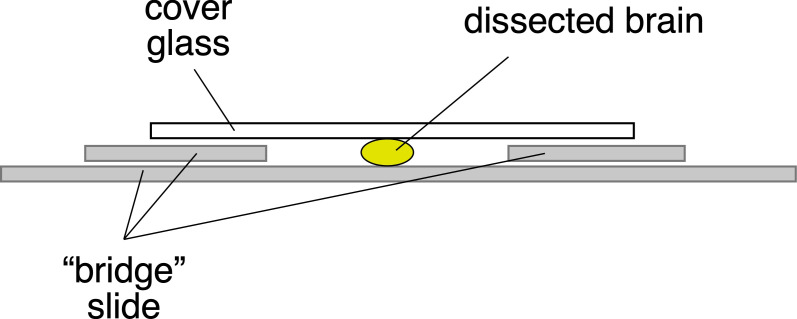
Create ‘bridge’ slide for mounting (Figure 1). Add one coverslip glued on either side of a slide using crazy glue. This becomes a bridge for your sample, so that you do not crush it.
Move brains into Prolong Gold antifade and mount the brain ventral side up. Place a coverslip on top of the bridge. Ensure there is enough Prolong Gold antifade to cover the sample and watch out for air bubbles.
Seal edges with nail polish.
Image.
-
(Option B) – Mount using vacuum grease pillars on coverslip
Dehydrate through 1 mL of 25%, 50%, and 75% glycerol in PBS (do not use PBSTx, as it creates bubbles with glycerol). (After the addition of the next higher concentration of glycerol solution, wait for the embryos to ‘fall’ to the bottom of the tube before moving to the next dilution.)
Dissect animals if necessary. See alternative dissection protocol below (F).
-
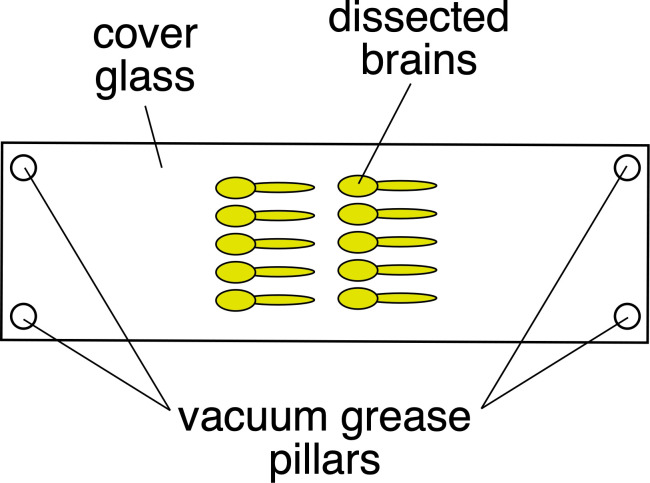
Mount the brains, using the minimal amount of vacuum grease to form pillars on the coverslip so that the tissue is not distorted (Figure 2).
Mount brains with the ventral side to the coverslip. You can mount multiple brains if you are careful.
Remove excess glycerol if present (use Kimwipe).
Use vacuum grease to make pillars on each corner of the coverslip.
Carefully place a slide onto the prepared coverslip. Gently push down on the slide, until the vacuum grease compresses and the brains barely touch the slide. The brains should be very carefully sandwiched between coverslip and slide, just touching each one.
Turn slide/coverslip over.
Touch a small drop of Prolong Gold antifade to the edge of coverslip – it will ‘wick’ into the coverslip to surround the brains. Before applying, gently squeeze the tube and check to ensure you are not adding bubbles. Allow 3-4 h or overnight to set.
Seal edges with nail polish.
Image.
-
-
Additional zebrafish brain/spine dissection method after TCA fixation and immunostaining
Insert forceps just above the notochord, near the posterior end of the fish laying on its side. Separate forceps, to split the fish into two halves.
Using these separated dorsal and ventral (D/V) larval halves, grab the end of each half with forceps, and pull the fish in two – the break should follow just dorsal to the notochord. The best imaging occurs when the notochord goes with the ventral half.
Pull gently, but persistently – sometimes forceps must be repositioned. The otolith is often challenging for the D/V separation – ideally, the otolith will separate with the ventral half. If not, dissect it off. Ideally, the brain will separate from the eyes anteriorly. If not, dissect them off.
The ventral half can be discarded (jaws, yolk, etc.).
The dorsal half contains the brain/spine, with a clear view from the ventral side into the brain. Mount this dorsal half with its ventral portion facing the cover glass imaging surface.
Figure 1. Mounting the brain on a bridge slide.
Figure 2. Mounting brains using grease pillars.
Recipes
-
Trichloroacetic Acid (TCA)
Make 20% TCA and freeze in useful aliquots for future use.
For a 2% working solution, dilute 1:10 in 1× PBS.
-
Tricaine methanesulfonate (MS-222 sometimes called MESAB)
4 g/L of Tricaine-S to make a stock solution, 170 mg/L to make a working solution, ~50 mg/L for sedation.
-
PBSTx
1× PBS, 0.5% Triton-X
-
Western Block with Sodium Azide
Sodium Azide prevents microorganism growth.
Add 1 mL of 20% Sodium Azide to a 100 mL bottle of Western Blocking Reagent for the 10× Western Block, and freeze down in 1 mL aliquots at -20°C. For the working solution, make up 1× with PBS-Tx.
Primary and secondary antibodies are diluted in Western Block.
-
Glycerol dilutions
(25%, 50%, and 75%) in PBS
Acknowledgments
This protocol was used in the companion paper Lasseigne et al. (2021) Elife (DOI: 10.7554/eLife.66898).
Eunice Kennedy Shriver National Institute of Child Health and Human Development (F32HD102182) to E Anne Martin.
National Institute on Deafness and Other Communication Disorders (R01DC011099) to Alberto Pereda.
National Institute of Neurological Disorders and Stroke (R21NS085772) to Alberto Pereda.
National Institute of Mental Health (RF1MH120016) to Alberto Pereda and Adam C Miller.
National Institute of Neurological Disorders and Stroke (R01NS105758) to Adam C Miller.
Competing interests
We declare no competing interests.
Ethics
Studies using this protocol were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All of the animals were handled according to approved institutional animal care and use committee (IACUC) protocols (#AUP-18-35) of the University of Oregon.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
Q&A
Post your question about this protocol in Q&A and get help from the authors of the protocol and some of its users.
References
- 1. Lasseigne A. M., Echeverry F. A., Ijaz S., Michel J. C., Martin E. A., Marsh A. J., Trujillo E., Marsden K. C., Pereda A. E. and Miller A. C.(2021). Electrical synaptic transmission requires a postsynaptic scaffolding protein. Elife 10: e66898. [DOI] [PMC free article] [PubMed] [Google Scholar]