Abstract
This study aims to assess the effects of central and general adiposity on development of cardiovascular diseases (CVDs) mediated by cardiometabolic risk factors and to analyze their degree of dependency for mediating their effects. To this end, data from the the Tehran Lipid and Glucose Study cohort with 6280 participants were included in this study. The hazard ratios were calculated using a 2-stage regression model in the context of a survival model. Systolic blood pressure (BP), total serum cholesterol, and fasting plasma glucose were designated as mediators. Assessing the interactions revealed that BP was the most important mediator for general ( (HRNIE: 1.11, 95% CI 1.17–1.24) and central obesity (CO) (HRNIE: 1.11, 95% CI 1.07–1.15) with 60% and 36% proportion of the effects mediated in the total population, respectively. The proportion of mediated risk for all three metabolic risk factors was 46% (95% CI 31–75%) for overweight, 66% (45–100%) for general obesity and 52% (39–87%) for central obesity. BP was the most important mediator for overweight and central obesity in men, comprising 29% and 36% of the risk, respectively. The proportion of the risk mediated through all three metabolic risk factors in women was 23% (95% CI 13–50%) for overweight, 36% (21–64%) for general obesity and 52% (39–87%) for central obesity. Based on the results of this study, cardiometabolic mediators have conciliated more than 60% of the adverse effects of high BMI on CVDs in men. Controlling the metabolic risk factors in women does not efficiently contribute to decreasing CVDs as effectively.
Subject terms: Computational biology and bioinformatics, Cardiology, Diseases, Risk factors
Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide1. Mortality and morbidity from CVDs are expected to rise in low and middle income countries as well as high income countries over the next few decades2. The combination of socio-economic and lifestyle changes has contributed to the development of CVDs over the past decades3. Likewise, economic growth, industrialization, increased sedentary lifestyle and nutritional transition has lead to increased prevalence of being overweight and obese. Allied to that, their incidence has doubled and even quadrupled over the last 30 years4. It has been shown that overweight and obese individuals have an elevated risk of developing CVDs, particularly those with central obesity5. This global increase in prevalence of being overweight and obese and the elevated risk of CVDs has raised concerns in many countries6.
The association of obesity with dyslipidemia, hypertension, diabetes, insulin resistance and systemic inflammation which also contribute to risk of developing CVDs themselves, has also been substantiated5. However, the mechanisms linking body mass index (BMI) to CVDs have not been clearly understood. The question remains as what proportion of the risks associated with high BMI directly affects cardiovascular disease and how much of it is conciliated by its associated metabolic mediators? In order to clarify this question, we need to first understand how much the effects of obesity could be mediated per se or through other metabolic factors e.g. blood pressure, glucose, cholesterol together or separately. The combined proportion of mediated effect of BP, cholesterol, and diabetes on the association between BMI and incidence of CVDs has been examined in some of the previous studies7; However, the effects of individual mediators or possible combination of these risk factors are neglected in these studies8,9.
In this study, we have conducted a mediation analysis to examine the degree of effect of overweight and obesity i.e. general and abdominal adiposity on developing CVDs mediated through blood pressure, cholesterol, and blood glucose as single mediators or in varying combinations; along with the assessment of correlation between BMI itself and the mediators. Furthermore, we assessed whether sex-specific analyses could alter overall findings.
Methods
Study population
Current research was performed using data derived from The Tehran Lipid and Glucose Study (TLGS) which was a population-based longitudinal cohort study to determine the local epidemiology of non-communicable diseases in Tehran, Iran. In the TLGS, patients were recruited in two phases i.e. the first (1999–2001) and the second (2002–2005) with an approximately 3-year interval10.
The eligibility criteria for the current study were as follows: (1) age ≥ 30 years; (2) individuals with at least 1 year follow up; (3) subjects who had data regarding anthropometric and metabolic measurements including BP, waist circumference (WC), fasting plasma glucose (FPG) and 2-h post-challenge plasma glucose (2 h-PCPG), systolic blood pressure (SBP), diastolic blood pressure (DBP) and total cholesterol (TC) at baseline; (4) and data necessary for CVD event assessment during follow-up.
Of the 9560 eligible participants aged ≥ 30 years, those with BMI < 18.5 (n = 113), CVD at baseline (n = 602), history of cancer (n = 57), and hospitalization at baseline (n = 109), and pregnant women (n = 43) and participants lost to follow-up or with missing data regarding metabolic mediators and other covariates (n = 1584) were excluded from the study. Final analyses were performed in 6280 individuals (5357 individuals from exam 1 and 923 new participants from exam 2), who were followed till March 20, 2014. Sequential imputation using chained equations were used to manage missing data in the main variables such as exposures, mediators, and covariates used in the models11.
This study obtained ethical approval from the ethics committee at Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences (IRB approval No.: 240/25) and was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent.
Clinical and laboratory measurements
Data related to demographics, smoking habits, physical activity, medical history and medication use were obtained through questionnaires administered by trained physicians12. The SBP, DBP and WC were measured at the baseline and every three years intervals which has been described elsewhere10. Participants’ fasting venous blood samples were taken after overnight fasting (12–14 h) between 07:00 and 09:00 AM10. Serum TC was measured enzymatically via cholesterol oxidase. Measurement protocol for other biochemical variables including FPG and 2hPG, high density lipoprotein cholesterol (HDL-C), and triglyceride (TG) has been described elsewhere13.
Definition of covariates
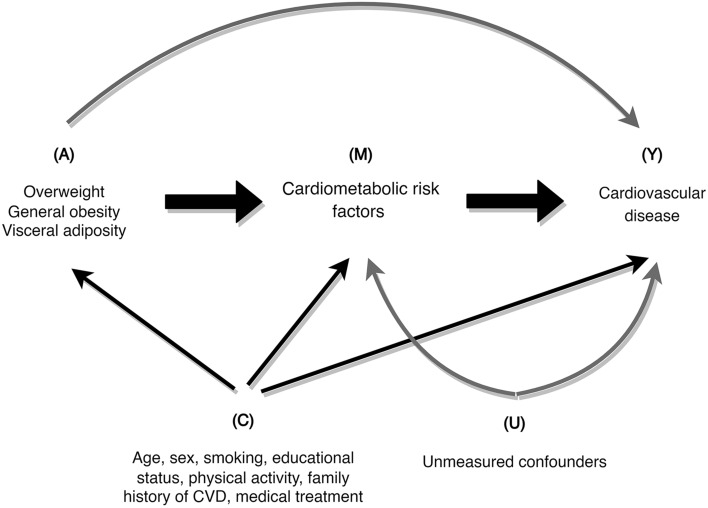
Subjects were classified as current smokers if smoked either daily or occasionally, and non-smoker who had never smoked or were ex-smokers. Education levels were categorized as follows: illiterate and those with primary school education, those with and without diploma certificate, and those with a university degree. Central/Visceral adiposity was defined as having WC of ≥ 90 cm for both men and women based on the Iranian National Committee of Obesity reports14. Obesity was determined based on BMI and categorized as follows: normal weight (BMI ≤ 25.0 kg/m2), overweight (25.0 < BMI < 30.0 kg/m2) and general obesity (BMI ≥ 30.0 kg/m2). Individuals who had metabolic equivalent task (MET) of less than 600 or exercising less than 3 days a week were considered as insufficiently physically active. Individuals with hypertension were defined as participants with SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, or those receiving antihypertensive medication. Hyperlipidemia was described as total cholesterol ≥ 200 mmol/L. Study variables definition has been detailed elsewhere12. In the current study, CVD events were described as a composite count of cases with definite myocardial infarction (MI), probable MI, unstable angina, angiographic-proven coronary heart disease (CHD), CHD death, defnite or possible stroke, transient ischemic attack or cerebrovascular death15. The interview guide used in the current investigation was developed for the purpose of this study which has been detailed elsewhere16. Demographics, family history of CVDs, and physical activity were regarded as potential factors counfounding the association of central obesity and BMI with CVDs, obesity (both central and general) and mediators, and mediators and CVDs (Fig. 1)17–23. Cardiometabolic markers including SBP, TC, and FPG were regarded as mediators24.
Figure 1.
The relationship between exposures (A), mediators (M), outcome (Y), and measured confounders (C) as well unmeasured confounders (U).
Statistical analysis
The differences between baseline continuous and categorical variables in men and women were compared using the t test and chi-square test, respectively. In order to specify the relationship between variables and their appropriate scale to be included in the final model, a fractional polynomial model was utilized25.
The inverse probability-of-censoring weighting (IPCW) method was employed to adjust for the selection bias resulted from censoring during follow up i.e. loss to follow-up or competing risk26–28. We used pooled logistic regression model to assess the inverse probability of loss to follow-up censoring weights in which the censoring variable was regarded as the outcome and other covariates as predictors29. The effect of unmeasured mediator-outcomes, confounding direct and indirect effects30 as well as the setting of mild and strong confounders were also assessed. Detailed description of the sensitivity analysis is published elsewhere18.
A two-stage regression method proposed by VanderWeele was used to estimate the direct and indirect effects31. We performed a sensitivity analysis to determine the impact of violations to the no-unmeasured-confounding assumption. The model will provide valid estimates of direct and indirect effects if the occurrence of the outcome is relatively rare, assuming that there are no unmeasured confounding and model misspecifications30,32.
First, we fitted three linear regression models, one for each mediator (M), conditional on BMI categories (A) and confounders (C):
| 1 |
We then fitted a Cox proportional hazards regression model for CVD risk on BMI categories (A), mediators (M), a BMI–mediator interaction term, and confounders (C) using age as the time scale:
| 2 |
where in the equation, λ0(t) was the baseline hazard at age t for a normal weight participant when all the mediators and confounders were set to 0 and θ3 is the vector of coefficients for the interaction between overweight/obesity and its mediators. The natural direct and indirect effects were estimated using the coefficients of the above regressions. The detailed explanation was provided by Lu et al24.
Following convention, the direct and indirect effects at the mean level of confounders in the TLGS cohort was estimated and “proportion of the risk mediated” for each mediator was calculated based on the natural direct and indirect effects, using the formula (HRTE–HRNDE)/(HRTE–1), where HRTE is the total effect hazard ratio and is calculated as HRTE = HRNDE × HRNIE33. In the final step, a bootstrap with 1000 samples was used for the total, direct, and indirect effects. Statistical analyses were performed using Stata 13.0 MP (Stata corp, College Station, TX, USA) and R 3.04.
Results
Out of 6280 included subjects, 710 developed CVDs during follow up. The average age of study population was 46.2 ± 11.7 years. The final analysis was performed on 2859 males (45.53%) and 3421 (54.47%) females. No significant differences were found between the men and women in WC, SBP & DBP (p > 0.05) while women were more likely to be overweight and have lower education levels as well as higher serum TC compared to men in this study (Table 1). The estimations from the initial parametric regression model showed that being overweight was associated with 61% of the overall increased risk of CVDs. According to our analyses, the indirect hazard ratio for high cholesterol levels was 1.09 (1.06–1.12) with a mediated risk proportion of 22% which was the most important intermediate risk factor in the relation between the overweight and CVDs. Our findings indicated general obesity increased the risk of incident CVDs by 68%. Considering the relation between general obesity and CVDs, the intermediate variables of blood pressure, TC, and FPG were responsible for 17, 14, and 5% of increased risk, respectively; and accounted for the most indirect effects in our analyses. In obese individuals, high blood pressure, TC, and FPG was indirectly accompanied by 33% elevated risk for incident CVDs [HRNIE = 1.33 (1.26–1.42)], comprising 66% of effect of obesity on developing CVDs. Moreover, central obesity increased the risk of CVDs by 59%. The results demonstrated that 52% of total effects of central obesity on developing CVDs were exerted through blood pressure, TC, and FPG mediators (Table 2).
Table 1.
Baseline characteristics of participants according to gender status, Tehran Lipid and Glucose Study (1999–2015).
| Variable | Men (n = 2859) | Women (n = 3421) | Total population (n = 6280) | P value* |
|---|---|---|---|---|
| Age (years) | 47.1 ± 12.5 | 45.5 ± 10.9 | 46.2 ± 11.7 | < 0.001 |
| Body mass index (kg/m2) | 26.3 ± 3.7 | 28.6 ± 4.6 | 27.5 ± 4.4 | < 0.001 |
| 30 > BMI > 25 (Overweight) | 1324 (46%) | 1444 (42%) | 2768 (44%) | |
| BMI > 30 (Obese) | 455 (16%) | 1208 (35%) | 1663 (26%) | |
| Waist circumference(cm) | 90.9 ± 10.3 | 90.4 ± 11.9 | 90.6 ± 11.2 | 0.095 |
| WC ≥ 90 (Visceral adiposity) | 1583 (55%) | 1793 (52%) | 3376 (54%) | 0.019 |
| Systolic blood pressure (mmHg) | 120.9 ± 18.4 | 120.1 ± 19.3 | 120.5 ± 18.9 | 0.08 |
| Diastolic blood pressure (mmHg) | 78.5 ± 11.2 | 78.8 ± 10.6 | 78.6 ± 10.9 | 0.43 |
| Hypertension (yes) | 634 (22%) | 844 (25%) | 1478 (24%) | 0.02 |
| Fasting plasma glucose (mg/dl) | 5.29 ± 1.17 | 5.21 ± 1.1 | 5.25 ± 1.13 | 0.009 |
| Diabetes mellitus (yes) | 196 (7%) | 256 (7%) | 452 (7%) | 0.33 |
| Triglycerides (mmol/L) | 2.19 ± 1.56 | 1.91 ± 1.18 | 2.04 ± 1.37 | < 0.001 |
| Total cholesterol (mmol/L) | 5.38 ± 1.1 | 5.63 ± 1.2 | 5.51 ± 1.16 | < 0.001 |
| Total cholesterol ≥ 200 mmol/L (Hyperlipidemia) | 46 (1%) | 115 (3%) | 161 (3%) | < 0.001 |
| HDL-cholesterol (mmol/L) | 0.98 ± 0.24 | 1.15 ± 0.28 | 1.07 ± 0.27 | < 0.001 |
| Family history of CVDS (%) | 417 (14.59) | 606 (17.71) | 1023(16.29) | 0.001 |
| Low physical activity (%) | 2061 (72.09) | 2402 (70.21) | 4463(71.07) | 0.10 |
| Education (%) | ||||
| Illiterate/primary school | 824 (28.82) | 1563 (45.6) | 2387(38.01) | < 0.001 |
| Below diploma/diploma | 1497 (52.36) | 1595 (46.62) | 3092(49.24) | |
| Higher than diploma | 538 (18.82) | 263 (7.69) | 801(12.75) | |
| Smoking (%) | ||||
| Never | 1528 (53.45) | 3196 (93.42) | 4724(75.22) | < 0.001 |
| Past | 453 (15.84) | 68 (1.99) | 521(8.3) | |
| Current | 878 (30.71) | 157 (4.59) | 1035(16.48) | |
*Differences in continuous and categorical variables between males and females were assessed using the independent t-test and Chi-square test, respectively.
Table 2.
Total, direct, and indirect effects of overweight and adiposity on cardiovascular diseases (CVDs) using a parametric method without considering exposure-mediator interaction.
| Exposures | Mediators | Total effecta,b | Natural direct effect | Natural indirect effect | Proportion mediatedc (95% CI) |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Overweight | Blood pressure (mmHg) | 1.61 (1.34–1.95) | 1.42 (1.18–1.73) | 1.08 (1.06–1.11) | 21 (14–37) |
| Cholesterol (mmol/L) | 1.5 (1.23–1.81) | 1.09 (1.06–1.12) | 22 (15–34) | ||
| Glucose (mmol/L) | 1.53 (1.27–1.86) | 1.02 (1.03–1.04) | 8 (5–15) | ||
| Blood pressure, cholesterol, and glucose | 1.29 (1.07–1.58) | 1.19 (1.15–1.24) | 46 (31–75) | ||
| General obesity | Blood pressure (mmHg) | 1.68 (1.35–2.08) | 1.37 (1.08–1.70) | 1.17 (1.11–1.21) | 38 (26–70) |
| Cholesterol (mmol/L) | 1.5 (1.21–1.85) | 1.14 (1.10–1.18) | 29 (20–45) | ||
| Glucose (mmol/L) | 1.58 (1.26–1.96) | 1.05 (1.03–1.07) | 12 (7–20) | ||
| Blood pressure, cholesterol, and glucose | 1.20 (0.96–1.52) | 1.33 (1.26–1.42) | 66 (45–100) | ||
| Visceral adiposity | Blood pressure (mmHg) | 1.59 (1.33–1.85) | 1.40 (1.18–1.63) | 1.10 (1.07–1.13) | 27 (18–43) |
| Cholesterol (mmol/L) | 1.44 (1.19–1.67) | 1.09 (1.06–1.12) | 23 (16–38) | ||
| Glucose (mmol/L) | 1.50 (1.25–1.74) | 1.04 (1.02–1.06) | 11 (8–19) | ||
| Blood pressure, cholesterol, and glucose | 1.25 (1.03–1.46) | 1.22 (1.17–1.27) | 52 (39–87) |
MI body mass index; CI confidence interval; HR hazard ratio; WC waist circumference.
aCompared with normal-weight participants for general adiposity and WC < 90 cm as a reference for central adiposity.
bAll models were adjusted for age, gender, smoking, physical activity level, educational status, and family history of CVDs.
cThe direct, indirect, and total effects were estimated for each bootstrap resampling.
Median duration of follow up was 13.9 years in the included participants. The proportion of censored cases during the follow-up period was 8.36%. The result of IPCW demonstrated that the amount of participants censored due to loss to follow-up for increased BMI, adiposity, and central adiposity was less than 5% (Supplementary Table S1). Furthermore, multiple imputation of missing data didn't show any significant differences (less than 5%) with observed estimates (see Supplementary Table S2).
Tables 3 and 4 demonstrate the results of the gender-based parametric model. The general estimated effects of the overwight, general and visceral obesity were associated with 11, 28 and 17% decrease in risk of developing CVDs in men compared to the whole population. In addition, our finding showed high blood pressure, TC and FPG with an intermediate contribution percentage of 29, 24 and 11%, respectively, were the most important cardio-metabolic intermediate variables in the relation between the overweight and CVDs in men. We also observed that 60% of the association of being overweight with incident CVDs was mediated by the concurrent presence of hypertension, high TC, and FPG. It is worth noting that the same effect for the whole studied population was calculated to be around 46% (Tables 3, 4).
Table 3.
Total, direct, and indirect effects of overweight and adiposity on cardiovascular diseases (CVDs) using a parametric method not considering exposure-mediator interaction in men.
| Exposures | Mediators | Total effecta,b | Natural direct effect | Natural indirect effect | Proportion mediatedc (95% CI) |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Overweight | Blood pressure (mmHg) | 1.50 (1.22–1.86) | 1.32 (1.07–1.64) | 1.10 (1.06–1.14) | 29 (16–62) |
| Cholesterol (mmol/L) | 1.40 (1.13–1.75) | 1.09 (1.05–1.13) | 24 (14–48) | ||
| Glucose (mmol/L) | 1.42 (1.15–1.77) | 1.04 (1.02–1.05) | 11 (6–26) | ||
| Blood pressure, cholesterol, and glucose | 1.17 (0.93–1.47) | 1.23 (1.17–1.29) | 60 (30–100) | ||
| General obesity | Blood pressure (mmHg) | 1.40 (1.06–1.85) | 1.12 (0.82–1.52) | 1.11 (1.17–1.24) | 60 (1–100) |
| Cholesterol (mmol/L) | 1.27 (0.95–1.67) | 1.14 (1.08–1.22) | 40 (22–99) | ||
| Glucose (mmol/L) | 1.28 (0.97–1.70) | 1.06 (1.03–1.10) | 22 (10–99) | ||
| Blood pressure, cholesterol, and glucose | 0.95 (0.70–1.28) | 1.39 (1.29–1.51) | 98 (10–100) | ||
| Visceral adiposity | Blood pressure (mmHg) | 1.42 (1.16–1.73) | 1.24 (1.02–1.56) | 1.11(1.07–1.15) | 36 (19–83) |
| Cholesterol (mmol/L) | 1.30 (1.05–1.60) | 1.09 (1.06–1.13) | 29 (15–67) | ||
| Glucose (mmol/L) | 1.34 (1.11–1.66) | 1.04 (1.02–1.06) | 14 (7–30) | ||
| Blood pressure, cholesterol, and glucose | 1.11 (0.90–1.41) | 1.25 (1.18–1.32) | 71 (41–100) |
MI body mass index; CI confidence interval; HR hazard ratio; WC waist circumference.
aCompared with normal-weight participants for general adiposity and WC < 90 cm as a reference for central adiposity.
bAll models were adjusted for age, gender, smoking, physical activity level, educational status, and family history of CVDs.
cThe direct, indirect, and total effects were estimated for each bootstrap resampling.
Table 4.
Total, direct, and indirect effects of overweight and adiposity on cardiovascular diseases (CVDs) using a parametric method not considering exposure-mediator interaction in women.
| Exposures | Mediators | Total effecta,b | Natural direct effect | Natural indirect effect | Proportion mediated (95% CI) |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Overweight | Blood pressure (mmHg) | 1.91 (1.32–2.93) | 1.76 (1.21–2.75) | 1.05 (1.02–1.08) | 10 (4–22) |
| Cholesterol (mmol/L) | 1.80 (1.26–2.81) | 1.06 (1.03–1.10) | 12 (1–25) | ||
| Glucose (mmol/L) | 1.83 (1.25–2.79) | 1.02 (1.01–1.03) | 5 (2–11) | ||
| Blood pressure, cholesterol, and glucose | 1.64 (1.13–2.56) | 1.11 (1.07–1.17) | 23 (13–50) | ||
| General obesity | Blood pressure (mmHg) | 2.13 (1.44–3.20) | 1.82 (1.23–2.79) | 1.13 (1.07–1.19) | 22 (11–41) |
| Cholesterol (mmol/L) | 1.96 (1.35–3.03) | 1.08 (1.04–1.13) | 14 (7–26) | ||
| Glucose (mmol/L) | 2.04 (1.39–3.04) | 1.03 (1.01–1.03) | 6 (3–14) | ||
| Blood pressure, cholesterol, and glucose | 1.70 (1.15–2.64) | 1.21 (1.14–1.31) | 36 (21–64) | ||
| Visceral adiposity | Blood pressure (mmHg) | 1.72 (1.39–2.39) | 1.59 (1.16–2.20) | 1.08 (1.04–1.12) | 18 (10–40) |
| Cholesterol (mmol/L) | 1.62 (1.19–2.27) | 1.05 (1.02–1.08) | 13 (5–25) | ||
| Glucose (mmol/L) | 1.64 (1.19–2.24) | 1.03 (1.01–1.06) | 9 (4–22) | ||
| Blood pressure, cholesterol, and glucose | 1.47 (1.07–2.06) | 1.14 (1.09–1.21) | 31 (18–69) |
MI body mass index; CI confidence interval; HR hazard ratio; WC waist circumference.
aCompared with normal-weight participants for general adiposity and WC < 90 cm as a reference for central adiposity.
bAll models were adjusted for age, gender, smoking, physical activity level, educational status, and family history of CVDs.
cThe direct, indirect, and total effects were estimated for each bootstrap resampling.
The results of the association between obesity and CVDs revealed that the estimated total effects were lower in men compared to women. The presence of cardiometabolic risk factors in men was associated with less harmful effects on the incidence of CVDs compared to women. We found that the effects of obesity on incident CVDs in men having the three analyzed cardiometabolic risk factors were not mediated via direct natural effects but mostly through causal route (intermediate share 98%).
Furthermore, our analyses indicate the most crucial intermediate variables in men with abdominal obesity were the same as those in overweight and obese individuals and was 71%. We observed that controlling intermediate risk factors in men with general obesity came with better results in controlling and preventing CVDs rather than those with abdominal obesity. The results of the parametric model showed that overweight, general, and abdominal obesity were associated with higher risks of developing CVDs in women compared to men and exerted their effects mostly independent of the cardiometabolic risk factors. The increased risk was 40% higher for overweight, 60% for general obesity and 30% for abdominal obesity compared to men. The direct natural effects had a more significant proportion of the whole effects and the mediators played a weaker role in conducting the harmful effects on developing CVDs in women. For example, in the obese individuals, the intermediate contribution of high BP, TC, and FPG in incident CVDs, if co-occurred, was 36% among women compared to 98% in men.
Table 5 illustrates the parametric model analyses indicating the interaction between the exposures, namely, overweight, general and visceral obesity and the intermediate variables were not statistically significant. The reason behind the minute changes of the whole effects in this table compared to the results observed in Table 1 (Table 2) was the incorporation of possible interactions in models. There were no interactions between any mediator and the exposure in the models. The sensitivity analysis for unmeasured mediator-outcome confounding suggested the variations were less than 5% in the two scenarios (Supplementary Table S3).
Table 5.
Total, direct, and indirect effects of overweight and adiposity on cardiovascular diseases (CVDs) using a parametric method considering exposure-mediator interaction.
| Exposures | Mediators | Total effecta,b | Natural direct effect | Natural indirect effect | Multiplicative interaction (95% CI) |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Overweight | Blood pressure (mmHg) | 1.50 (1.29–1.90) | 1.46 (1.17–1.74) | 1.08 (1.05–1.12) | 1 (0.99–1.07) |
| Cholesterol (mmol/L) | 1.64 (1.35–1.94) | 1.51 (1.24–1.82) | 1.08 (1.04- 1.12) | 0.93 (0.83–1.04) | |
| Glucose (mmol/L) | 1.57 (1.30–1.87) | 1.53 (1.27–1.82) | 1.03 (1.01–1.04) | 0.95 (0.87–1.02) | |
| Blood pressure, cholesterol, and glucose | 1.61 (1.30–1.94) | 1.38 (1.10–1.67) | 1.17 (1.12–1.22) | 1 (0.99–1.007) | |
| 0.90 (0.80–1.10) | |||||
| 0.92 (0.85–1.01) | |||||
| General obesity | Blood pressure (mmHg) | 1.69 (1.36–2.07) | 1.50 (1.20–1.91) | 1.12 (1.05–1.19) | 1 (0.99–1.00) |
| Cholesterol (mmol/L) | 1.67 (1.36–2.05) | 1.42 (1.15–1.78) | 1.17 (1.11–1.24) | 0.93 (0.83–1.04) | |
| Glucose (mmol/L) | 1.60 (1.31–1.94) | 1.48 (1.21–1.79) | 1.07 (1.04–1.11) | 0.95 (0.87–1.02) | |
| Blood pressure, cholesterol, and glucose | 1.66 (1.33–2.07) | 1.20 (0.94–1.54) | 1.37 (1.26–1.50) | 1.08 (0.99–1.07) | |
| 0.90 (0.80–1.01) | |||||
| 1.02 (0.85–1.04) | |||||
| Visceral adiposity | Blood pressure (mmHg) | 1.59 (1.34–1.79) | 1.41 (1.18–1.65) | 1.07 (1.07–1.13) | 1.01 (0.98–1.04) |
| Cholesterol (mmol/L) | 1.57 (1.31–1.82) | 1.42 (1.17–1.67) | 1.09 (1.06–1.15) | 1.02 (0.97–1.08) | |
| Glucose (mmol/L) | 1.60 (1.29–1.84) | 1.51 (1.26–1.76) | 1.05 (1.04–1.07) | 1.00 (0.99–1.01) | |
| Blood pressure, cholesterol, and glucose | 1.63 (1.35–1.83) | 1.22 (1.03–1.42) | 1.23 (1.16–1.23) | 0.98 (0.99–1.002) | |
| 0.99 (0.96–1.03) | |||||
| 1.00 (0.99–1.01) |
MI body mass index; CI confidence interval; HR hazard ratio; WC waist circumference.
aCompared with normal-weight participants for general adiposity and WC < 90 cm as a reference for central adiposity.
bAll models were adjusted for age, gender, smoking, physical activity level, educational status, and family history of CVDs.
cThe direct, indirect, and total effects were estimated for each bootstrap resampling.
A separate analysis was done while taking into account the effect of medications for diabetes mellitus, hypertension, and dyslipidemia (hypercholesterolemia) to provide proportion of mediated effects adjusted for medications for each mediator (Supplementary Table S4).
Discussion
The results of this study demonstrated that a proportion of increased risk of CVDs in overweight individuals and subjects with increased general or visceral adiposity is exerted independent of the analyzed intermediate metabolic risk factors, which was greater in women compared to men. Cardiometabolic risk factors, including hypertension, high FPG, and TC levels contributed to 46, 66, and 52% of the increased risk of incidence of CVDs in overweight and obese individuals, respectively. The most important variable intermediating the relation between overweight and CVDs was high serum TC concentration (22%), while hypertension was identified as the most important factor which mediated the effects of general obesity on CVD incidence in our data (38%). Hypertension was also the most important mediator variable between visceral obesity and development of CVDs. In men, a total of 60% of the incresaed risk of CVDs of overweight subjects, 98% of those with general obesity, and 71% of those visceral obesity were mediated via hypertension and high serum TC and FPG. However, in women, the effects of obesity on incidence of CVDs were mostly exerted directly and independent of the risk factors. An interesting observation in this study was the effect of therapeutic medications in proportion of mediated risk for each risk factor. The secondary analysis (Supplementary Table S4) in which the results were adjusted for the use of medications for hypertension, diabetes mellitus, and hypercholestrolemia, led to different proportion of mediated risk for mediators.
While BMI is considered as an excellent index of general obesity, using WC to assess central obesity is regarded as a better index to show visceral fat deposition and consequently worsening metabolic profile34. Several studies have been conducted on the effects of central obesity on cardiovascular events35,36. In a study by Bogers et al. showed overweight can increase the effects of high cholesterol and hypertension on CVDs up to 45%37. It was also reported that the overweight and obesity increased the mortality risk caused by coronary heart diseases (CHDs) in both patients previously diagnosed with CHDs and those without a history of CHDs; although this effect is faster in the latter group38. In a study by Jousilahti et al. showed that each 1 kg increase in weight was accompanied by 1–1.5% elevated risk of mortality caused by CHDs39. In another study performed in 221,934 people in 17 countries indicated BMI, WC, and waist to hip ratio, either alone or in combination with other mediators, did not increase the risk of CVDs after further adjustment for baseline SBP, history of diabetes, total and high-density lipoprotein (HDL) cholesterol levels40.
In the study conducted by Kazempour‐Ardebili on Tehran residents aged ≥ 65 years revealed that visceral rather than general obesity contributes to development of CVDs and CHDs which was partially mediated via cardiometabolic risk factors, specifically hypertension41. However, the small sample size and lack of use of standardized models of causal mediation analyses could have contributed to the varying results, considering that both studies were performed in the the same population.
The incidence and prevalence of general and visceral obesity has risen in the Iranian adults in the recent years42 and the current interventions and policies have failed to control this health problem.; Therefore, there is a growing interest to recognize the causal patterns of obesity and its its metabolic mediators43. Investigaring the causal patterns in which how and from what route the potential risk factors exert their protective or harmful effects on the outcome is called mediation analysis44. The primary purpose of these methods is delineate the interventions’ effects with removing components that do not have any impact on the outcome45.
In a study by Lu et al. revealed that hypertension mediated 22% and 36% of adverse effects of overweight and abdominal obesity on CHDs, respectively; while for obesity, an elevated blood sugar levels, accounted for 65% of its effects on the incident outcome as the most important mediator. Furthermore, it was shown that overweight, obesity, and a waist circumference > 90 cm contributed their adverse effects on CHDs through the three cardiometabolic risk factors including hypertension (54%), elevated blood sugar (81%), and cholesterol (62%)24.
Lu et al. in a pooled analyses of 97 prospective cohort studies reported that each 5 kg/m2 higher BMI was accompanied with 27% elevated risk for CHDs which was mediated through high blood pressure, cholesterol, and glucose. In addition, it is indicated that obesity and overweight increase the risk for CHDs independent of these selected metabolic risk factors by 54%17.
Several studies have been performed to investigate the the impacts of visceral obesity on incident CVDs2,3. For example in a study by Bakhtiyari et al. the relationship between obesity and CVDs was determined by employing nonparametric methods. It was revealed that the essential mediators which linked the relationship between overweight, general, and visceral obesity with CVDs, were hypertension (PM = 22), high cholesterol (PM = 65) and blood glucose concentations (PM = 36). They also showed that 81% of the effects caused by obesity on incident CVDs were conducted via three mediators including hypertension, high cholesterol, and blood glucose levels. The nonparametric methods used in the study by Bakhtiyari et al. may contribute to the different results of their analyses18.
The findings of current study suggested that the general and central obesity indices were accompanied by the increased risk of CVDs, independent of the previously mentioned cardiometabolic risk factors. While our findings contradict the results of the previous studies41,46, It could be postulated that failure to adjust for potential confounding variables and different age groups in prior studies may have contributed to the observed dissimilarities47. Another factor that may have influenced the results of sties is the age pattern of the study population. It is of note to say that BMI is not a good index for adiposity in the elderly, as skeletal muscle mass reduce and abdominal obesity increases with aging48.
Different mechanisms link general and abdominal obesity to CVD via cardiometabolic risk factors. When excessive fat accumulates, even in the absence of systematic hypertension and underlying cardiac disease, remarkable changes in the structure and function of the heart occurs. To overcome the metabolic needs, circulating blood and plasma volume, as well as cardiac output increases. The increase in blood volume leads to an increase in the venous return to the left ventricle, which will lead to cardiac chambers diastolic compliance reduction and an increase in the left ventricle filling time and left ventricle enlargement. As long as left ventricular hypertrophy is synced with the left ventricular hypertrophy(LVH), the systolic activity of the heart is preserved. When LVH cannot keep up with the progressive increase in heart size, the increased cardiac wall pressure may lead to systolic dysfunction. An increase in systematic and pulmonary blood pressure (left ventricle failure and chronic hypoxia) and CHDs can all occur due to the impact of obesity on the structure and function of the heart. Also, the risk of sudden cardiac death increases with progression of obesity 49. Another mechanism is the release of bioactive mediators from the adipose tissue that, by acting on blood lipids, blood pressure, inflammation, and coagulation, will eventually lead to blood vessel dysfunction and atherosclerosis50.
After sensitivity analysis and considering (U) energy-adjusted glycemic load as an unmeasured confounder variable in this study, the results did not show a tangible change, and this is a support to the validity of the results observed in the current analyses. No difference was indicated in the sensitivity analysis conducted Lu et al.17.
The strength of the methods used in the current study are freedom in input exposure and outcome types, intermediate variable count, applicability in most basic regression equations including survival models, and the ability to assess any interaction between exposure and the mediators.
This study however had initially met several limitations, some of which were addressed throughout the text. The wide intervals for the proportion of mediated risks estimated indices calculated in this study may be attributable to high variation and instability of the indices themselves51. Furthermore, the small sample size24 and the changes in the levels of the measured risk factors at baseline and during follow up may have confound our findings. Moreover, information bias due to incomplete data recording and assumption of “no correlation” between the potential risk factors for CVDs should also be considered as limiting factors in the current study. For example, studies have reported that an increase in patients’ blood glucose levels could lead to an increase in their blood pressure which may subsequently affect kidneys function and circulating cholesterol levels52. In order to achieve a valid estimation of the direct and indirect natural effects in the current study, it was assumed that there is no confounding variable between the following relations; (A): BMI (or WC) and CVDs, (B): Mediator and CVDs, (C): BMI (or WC) and mediator, and that (D): No confounder is present in the relation between the mediators and CVDs that is being affected by the exposures. Individuals with a BMI < 18.5 and history of hospital admission were excluded to avoid any confounding effects between BMI (or WC) and CVDs. The effects of unmeasured mediator–CVD confounding was determined by calculating a bias factor which revealed no significant changes in the estimations were. To assess the assumption “C,” the main reasons relating to BMI and mediators such as physical activity, smoking, education, and history of CVDs were included in the analyses. Finally, assumption “D” was not verified if, for instance, physical activity affects both hypertension (diabetes) and CVDs wherase it was affected by obesity. To address this, in one instance, the physical activity was treated as as a new variable, whereas in another instance, its direct effects were estimated using marginal structural and structural nested models which do not divide the effects into direct and indirect ones.
Conclusions
In conclusion, the results of this study demonstrated that the negative effects of obesity in development of CVDs were mostly via hyprtension, high blood glucose, and cholesterol levels in men. Thus management of these three cardiometabolic factors should be considered as alternative, effective interventions compared to normally inefficient modalities targeting obesity in reduction of CVDs in men53–55. However, the greater role of direct effects of obesity in women should not be neglected. In other words, Obesity is considered as a crucial risk factor for development of CVDs. Current strategies for controlling weight including behavioral, medication and surgical interventions have been subject of criticism due to their insufficient questionable efficacy56. On the other hand, hypertention and dyslipidemia could be effectively managed via antihypertensive or lipid-lowering agents. Therefore, understanding the casual pathway of obesity and its cardiometabolic risk factors could provide effective and practical solutions to decrease the obesity related comorbidities57–59.
Supplementary Information
Acknowledgements
The authors would like to thank Dr. Noora Enayati for their valuable contributions.
Author contributions
M.B., F.A., E.K. and M.A.M. interpreted the data and drafted the manuscript. F.A., F.H., K.K., S.A., P.M. and N.T.G. designed the study, interpreted the data, reviewed the article critically, and revised it for important intellectual content. A.G., F.A., M.A.M., E.K. and M.B. analyzed and interpreted the data, reviewed the article critically, and revised it for important intellectual content. all authors have read and approved the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Farzad Hadaegh, Email: farzad.hadaegh@gmail.com.
Mohammad Ali Mansournia, Email: mansournia_ma@yahoo.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-05536-w.
References
- 1.Guilbert, J. J. The world health report 2002—Reducing risks, promoting healthy life. Education for health (Abingdon, England)16, 230. 10.1080/1357628031000116808 (2003) [DOI] [PubMed]
- 2.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 3.Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr. Probl. Cardiol. 2010;35:72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hruby A, Hu FB. The epidemiology of obesity: A big picture. Pharmacoeconomics. 2015;33:673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akil L, Ahmad HA. Relationships between obesity and cardiovascular diseases in four southern states and Colorado. J. Health Care Poor Underserv. 2011;22:61–72. doi: 10.1353/hpu.2011.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wahl S, et al. Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature. 2017;541:81–86. doi: 10.1038/nature20784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogers RP, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: A meta-analysis of 21 cohort studies including more than 300 000 persons. Arch. Intern. Med. 2007;167:1720–1728. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- 8.Etminan M, Brophy JM, Collins G, Nazemipour M, Mansournia MA. To adjust or not to adjust: The role of different covariates in cardiovascular observational studies. Am. Heart J. 2021;237:62–67. doi: 10.1016/j.ahj.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Mansournia MA, et al. A CHecklist for statistical Assessment of Medical Papers (the CHAMP statement): Explanation and elaboration. Br. J. Sports Med. 2021;55:1009. doi: 10.1136/bjsports-2020-103652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azizi F, et al. Prevention of non-communicable disease in a population in nutrition transition: Tehran Lipid and Glucose Study phase II. Trials. 2009;10:1. doi: 10.1186/1745-6215-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Resche-Rigon M, White IR. Multiple imputation by chained equations for systematically and sporadically missing multilevel data. Stat. Methods Med. Res. 2018;27:1634–1649. doi: 10.1177/0962280216666564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azizi F, et al. Prevention of non-communicable disease in a population in nutrition transition: Tehran Lipid and Glucose Study phase II. Trials. 2009;10:5. doi: 10.1186/1745-6215-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadaegh F, Harati H, Ghanbarian A, Azizi F. Association of total cholesterol versus other serum lipid parameters with the short-term prediction of cardiovascular outcomes: Tehran Lipid and Glucose Study. Eur. J. Cardiovasc. Prev. Rehabil. 2006;13:571–577. doi: 10.1097/01.hjr.0000216552.81882.ca. [DOI] [PubMed] [Google Scholar]
- 14.Azizi F, et al. Appropriate waist circumference cut-off points among Iranian adults: The first report of the Iranian National Committee of Obesity. Arch. Iran. Med. 2010;13:243. [PubMed] [Google Scholar]
- 15.Khajavi A, Khalili D, Azizi F, Hadaegh F. Impact of temperature and air pollution on cardiovascular disease and death in Iran: A 15-year follow-up of Tehran Lipid and Glucose Study. Sci. Total Environ. 2019;661:243–250. doi: 10.1016/j.scitotenv.2019.01.182. [DOI] [PubMed] [Google Scholar]
- 16.Azizi F, et al. Tehran Lipid and Glucose Study (TLGS): Rationale and design. Iran. J. Endocrinol. Metabol. 2000;2:77–86. [Google Scholar]
- 17.Lu, Y. et al. (Elsevier, 2014).
- 18.Bakhtiyari M, et al. Direct and indirect effects of central and general adiposity on cardiovascular diseases: The Tehran Lipid and Glucose Study. Eur. J. Prev. Cardiol. 2018;25:1170–1181. doi: 10.1177/2047487318780030. [DOI] [PubMed] [Google Scholar]
- 19.Etminan M, Collins GS, Mansournia MA. Using causal diagrams to improve the design and interpretation of medical research. Chest. 2020;158:S21–s28. doi: 10.1016/j.chest.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Mansournia, M. A., Hernán, M. A. & Greenland, S. Matched designs and causal diagrams. Int J Epidemiol.42(3), 860–869. 10.1093/ije/dyt083 (2013). [DOI] [PMC free article] [PubMed]
- 21.Mansournia, M. A., Higgins, J. P. T., Sterne, J. A. C. & Hernán, M. A. Biases in Randomized Trials. Epidemiology28(1), 54–59. 10.1097/EDE.0000000000000564 (2017). [DOI] [PMC free article] [PubMed]
- 22.Mansournia, M. A., Nazemipour, M. & Etminan, M. Causal diagrams for immortal time bias. Int J Epidemiol.50(5), 1405–1409. 10.1093/ije/dyab157 (2021). [DOI] [PubMed]
- 23.Etminan, M., Nazemipour, M., Candidate, M. S. & Mansournia, M. A. Potential Biases in Studies of Acid-Suppressing Drugs and COVID-19 Infection. Gastroenterology160(5), 1443–1446. 10.1053/j.gastro.2020.11.053 (2021). [DOI] [PMC free article] [PubMed]
- 24.Lu Y, Hajifathalian K, Rimm EB, Ezzati M, Danaei G. Mediators of the effect of body mass index on coronary heart disease: Decomposing direct and indirect effects. Epidemiology. 2015;26:153–162. doi: 10.1097/EDE.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 25.Royston, P. & Sauerbrei, W. Multivariable model-building: A pragmatic approach to regression anaylsis based on fractional polynomials for modelling continuous variables. Vol. 777 (Wiley, 2008).
- 26.Mansournia, M. A. & Altman D. G. Inverse probability weighting. BMJ.10.1136/bmj.i189 (2016). [DOI] [PubMed]
- 27.Mansournia, M. A., Etminan, M., Danaei, G., Kaufman, J. S. & Collins, G. Handling time varying confounding in observational research. BMJ.10.1136/bmj.j4587 (2017). [DOI] [PubMed]
- 28.Mohammad, A. et al. Effect of Physical Activity on Functional Performance and Knee Pain in Patients With Osteoarthritis. Epidemiology23(4), 631–640. 10.1097/EDE.0b013e31824cc1c3 (2012). [DOI] [PubMed]
- 29.Breskin, A., Cole, S. R. & Westreich, D. Exploring the subtleties of inverse probability weighting and marginal structural models. Epidemiology (Cambridge, MA)29, 352–355 (2018). [DOI] [PMC free article] [PubMed]
- 30.VanderWeele TJ. Unmeasured confounding and hazard scales: Sensitivity analysis for total, direct, and indirect effects. Eur. J. Epidemiol. 2013;28:113–117. doi: 10.1007/s10654-013-9770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VanderWeele, T. J. Causal mediation analysis with survival data. Epidemiology (Cambridge, Mass.)22, 582 (2011). [DOI] [PMC free article] [PubMed]
- 32.Lange, T. & Hansen, J. V. Direct and indirect effects in a survival context. Epidemiology, 575–581 (2011). [DOI] [PubMed]
- 33.Hafeman DM. “Proportion explained”: A causal interpretation for standard measures of indirect effect? Am. J. Epidemiol. 2009;170:1443–1448. doi: 10.1093/aje/kwp283. [DOI] [PubMed] [Google Scholar]
- 34.Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004;89:2595–2600. doi: 10.1210/jc.2004-0372. [DOI] [PubMed] [Google Scholar]
- 35.Goh, L. G., Dhaliwal, S. S., Welborn, T. A., Lee, A. H. & Della, P. R. Anthropometric measurements of general and central obesity and the prediction of cardiovascular disease risk in women: a cross-sectional study. BMJ open4, e004138 (2014). [DOI] [PMC free article] [PubMed]
- 36.Coutinho T, et al. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: Role of “normal weight central obesity”. J. Am. Coll. Cardiol. 2013;61:553–560. doi: 10.1016/j.jacc.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 37.Bogers RP, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: A meta-analysis of 21 cohort studies including more than 300,000 persons. Arch. Intern. Med. 2007;167:1720–1728. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- 38.Batty GD, et al. Obesity and overweight in relation to disease-specific mortality in men with and without existing coronary heart disease in London: the original Whitehall study. Heart. 2006;92:886–892. doi: 10.1136/hrt.2005.072637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jousilahti P, Tuomilehto J, Vartiainen E, Pekkanen J, Puska P. Body weight, cardiovascular risk factors, and coronary mortality: 15-year follow-up of middle-aged men and women in eastern Finland. Circulation. 1996;93:1372–1379. doi: 10.1161/01.CIR.93.7.1372. [DOI] [PubMed] [Google Scholar]
- 40.Collaboration ERF. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. The Lancet. 2011;377:1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kazempour-Ardebili S, et al. Metabolic mediators of the impact of general and central adiposity measures on cardiovascular disease and mortality risks in older adults: Tehran Lipid and Glucose Study. Geriatr. Gerontol. Int. 2017;17:2017–2024. doi: 10.1111/ggi.13015. [DOI] [PubMed] [Google Scholar]
- 42.Jahangiri-Noudeh, Y. et al. Trends in cardiovascular disease risk factors in people with and without diabetes mellitus: A Middle Eastern cohort study. PloS one9, e112639 (2014). [DOI] [PMC free article] [PubMed]
- 43.Finucane MM, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. The Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu. Rev. Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards JR, Lambert LS. Methods for integrating moderation and mediation: A general analytical framework using moderated path analysis. Psychol. Methods. 2007;12:1. doi: 10.1037/1082-989X.12.1.1. [DOI] [PubMed] [Google Scholar]
- 46.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 47.Willett WC, Hu FB, Thun M. Overweight, obesity, and all-cause mortality. JAMA. 2013;309:1681–1682. doi: 10.1001/jama.2013.3075. [DOI] [PubMed] [Google Scholar]
- 48.Wannamethee SG, Atkins JL. Muscle loss and obesity: The health implications of sarcopenia and sarcopenic obesity. Proc. Nutr. Soc. 2015;74:405–412. doi: 10.1017/S002966511500169X. [DOI] [PubMed] [Google Scholar]
- 49.Poirier P, et al. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 50.Van Gaal LF, Mertens IL, Christophe E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 51.MacKinnon, D. Introduction to Statistical Mediation Analysis. (Routledge, 2008).
- 52.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 53.Association, A. D. 8. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes-2018. Diabetes care41, S73 (2018). [DOI] [PubMed]
- 54.Nayor M, Vasan RS. Recent update to the US cholesterol treatment guidelines: A comparison with international guidelines. Circulation. 2016;133:1795–1806. doi: 10.1161/CIRCULATIONAHA.116.021407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nerenberg KA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can. J. Cardiol. 2018;34:506–525. doi: 10.1016/j.cjca.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 56.Franz MJ, et al. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 2007;107:1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 57.Anand SS, Yusuf S. Stemming the global tsunami of cardiovascular disease. The Lancet. 2011;377:529–532. doi: 10.1016/S0140-6736(10)62346-X. [DOI] [PubMed] [Google Scholar]
- 58.Avenell A, et al. What interventions should we add to weight reducing diets in adults with obesity? A systematic review of randomized controlled trials of adding drug therapy, exercise, behaviour therapy or combinations of these interventions. J. Hum. Nutr. Diet. 2004;17:293–316. doi: 10.1111/j.1365-277X.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 59.Sjöström L, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N. Engl. J. Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.