Abstract
Lead (Pb) and cadmium (Cd) are toxic heavy metals commonly found in aqueous environments. Biochar as a green adsorbent generated from biomass feedstock may be used for effective removal of these heavy metals. This study investigated the adsorption kinetics and isotherms of Pb2+ and Cd2+ in aqueous solutions at different pH by biochar prepared from banana stem and leaf (BSL-BC) at 400 °C. Characterizations using scanning electron microscope, X-ray diffraction, and Fourier-transform infrared spectroscopy showed that the synthesized BSL-BC had rough surface, porous structure, and oxygen-containing functional groups. The adsorption of Pb2+ and Cd2+ onto BSL-BC reached equilibrium in 8 h and 200 min, respectively, with faster adsorption attained at higher pH and the optimum pH occurred at 5 (Pb2+) and 8 (Cd2+). All adsorption kinetic data followed the pseudo-second-order rate model. The adsorption isotherm data of Pb2+ and Cd2+ could be well-described by the Langmuir and Freundlich models, respectively, whereas neither the Temkin or Dubinin–Radushkevich models provided satisfactory fitting results. The maximum adsorption capacities for Pb2+ and Cd2+ were 302.20 and 32.03 mg/g, respectively. The calculated mechanism contributions showed that complexation with oxygen-containing functional groups, ion exchange, mineral precipitation, and Pb2+/Cd2+-π coordination accounted for 0.1%, 8.4%, 88.8%, and 2.6% to Pb2+ adsorption, and 0.4%, 6.3%, 83.0%, and 10.4% to Cd2+ adsorption, respectively. Therefore, mineral precipitation was likely the major mechanism responsible for adsorption of both Pb2+ and Cd2+ by BSL-BC. The results suggest that the synthesized BSL-BC has great potential for adsorption of Pb2+ and Cd2+ from aqueous solutions.
Subject terms: Environmental sciences, Materials science, Chemical physics
Introduction
Heavy metals are widely present in aqueous environments due to water discharged from anthropogenic activities such as applications of fertilizer and pesticide, smelting and mining, and manufacture of electrical appliances, resulting in serious groundwater and surface water pollution1. Cadmium (Cd) and lead (Pb) are the most common and toxic heavy metals found in aqueous environments, posing acute and chronic effects on ecosystems and human health as they are persistent, migratable, bio-accumulative, and carcinogenic2. Therefore, it is important to treat water contaminated with heavy metals originated from domestic, agricultural, and industrial sources prior to its discharge into surface water or groundwater environments.
Heavy metals can be removed from wastewater and contaminated aqueous environments by various techniques, including reverse osmosis, ion-exchange, chemical precipitation, coagulation, electrochemical treatment, and physical adsorption3–5. Among them, adsorption is a promising approach for removing heavy metals from aqueous systems, with the benefits of cost-effectiveness, wide applicability, and ease of operation6. Various types of conventional (e.g., activated carbon, amorphous silica, clay minerals, diatomite, biochar, zeolites, and polymers) and novel nanosized (e.g., carbon nanotubes, graphene oxide, and reduced graphene oxide) adsorbents have been developed for metal treatment4,7. Compared with biochar, activated carbon and nanomaterials are relatively more expensive, while other conventional adsorbents such as natural zeolite/clay minerals generally have low adsorption efficiency; meanwhile, nanomaterials are difficult to be retrieved after adsorption of heavy metals4. Development of green adsorbents such as biochar from waste recycling that possess local availability, low cost, and high adsorption efficiency would be an environmental-friendly approach for heavy metal remediation.
Recent studies have highlighted biochar as an effective and green absorbent for removing heavy metals from water, due to its unique physicochemical properties including porous structure with abundant functional groups (e.g., carboxyl, carbonyl, and phenolic groups), large specific surface area (SSA), and readily available feedstock resources as raw material for adsorbent synthesis8–10. Biochar can be generated from incomplete combustion of organic matter under oxygen-limited conditions and at relatively low temperatures (i.e., 350–700 °C). Theoretically, biomass feedstock can include any organic waste materials, such as industrial wastes and by-products, forest and crop residues, algae, domestic solid wastes, sewage sludge, and manures11. Among different types of feedstocks, the derivation of biochar from crop residues (e.g., rice, corn, wheat straw, and woodchips) is gaining increasing attention as an approach for recycling agricultural wastes and achieving sustainable development12.
Banana is widely available and ranks the fourth most grown food crop worldwide after rice, wheat, and corn13. China is one of the countries with the longest history of banana cultivation. In 2017, the global banana production reached a record of 114 million tons, among which China accounted for ~ 10% (~ 11 million tons per year) and ranked the second largest banana producer13. However, banana fruit only weights ~ 12% of the whole plant, generating huge amounts (~ 220 t/hectare) of waste residues (i.e., stems, leaves, and rachis) during production14. Ortiz-Ulloa et al.15 reported that the average ratio of waste residue (i.e., above-ground biomass) to product (i.e., fruit) was 3.79, and that the biomass of stems and leaves contributed 78% and 17% to the above ground biomass, respectively. After harvesting banana fruits, the stems and leaves are usually abandoned in the field, taking months for natural degradation16, as the banana stem is primarily lignocellulosic in nature and mainly composed of 35–40% cellulose, 25–35% hemicellulose, and 8–13% lignin17. As banana stem and leaf (BSL) contain high lignin and low cellulose contents, they could be favorably considered as a raw material for producing biochar, which should ideally have a high production yield, large SSA, porous structure, and high fixed carbon content18,19. Such application can recycle the banana waste residues effectively for preparing adsorbents for treatment of heavy metals in contaminated water.
Therefore, this study prepared biochar from BSL (BSL-BC) via oxygen-limited pyrolysis under optimized conditions at 400 °C, and investigated the adsorption of Cd2+ and Pb2+ by the synthesized adsorbent in aqueous solutions. The objectives of this study were to: (1) characterize the physicochemical properties of BSL-BC and BSL; (2) examine and compare the adsorption rates and capacities of Cd2+ and Pb2+ onto BSL-BC in adsorption kinetic and isotherm experiments; (3) elucidate the adsorption mechanisms and quantify their relative contributions; and (4) compare the metal adsorption capacities of BSL-BC with other plant-based biochar with previous studies. The results would promote agricultural waste recycling and novel adsorbent development, as well as provide useful insights on adsorption mechanisms of heavy metals onto biochar.
Materials and methods
Preparation of adsorbent
The banana (Musa acuminata) stem and leaf sample was obtained from Dongguan city in Guangdong province of China (23° 3 N, 113° 5 E) with the consent of the crop owner, and was identified by Dr. Ping Li (South China Agricultural University) as banana (Musa acuminata). After washing thrice with double-distilled water, BSL was chopped to about 2-cm in length and dried at 80 °C for 24 h, and then milled to pass through a 0.154-mm sieve. Since the recovery rate of biochar after pyrolysis was ~ 50%, 50 g powdered BSL biomass was packed into a 304 stainless steel vessel, filled with nitrogen gas, and tightly capped, yielding ~ 25 g biochar in one pyrolysis cycle. The pyrolysis process of BSL for producing BSL-BC was conducted in a muffle furnace under the optimum pyrolysis condition of heating BSL at a rate of 10 °C/min to 400 °C, which was maintained for 3 h. The optimum pyrolysis conditions on temperature, residence time, and heating rate were determined from the experiments as described in S1 of the Supplementary Material, taking into considerations of both energy consumption and adsorption performance.
Characterization of BSL and BSL-BC before adsorption
The chemical contents of BSL and BSL-BC were thoroughly characterized. The contents of total C, H, N, and P were measured with an elemental analyzer (Thermo Scientific FLASH 2000). The ash content was determined by the difference between the mass of 1 g BSL-BC heated at 750 °C for 6 h and the mass of the remaining material20. The total oxygen content (%) was determined by subtracting 100% by the contents (%) of ash, C, H, and N. The pH and electrical conductivity (EC) of BSL-BC were measured with a pH meter (Mettler Toledo 320-S) and a conductivity meter (DDB303A), respectively, by mixing BSL-BC with double-distilled water in a ratio 1:20 (w:v)20. The zeta potential of BSL-BC as a function of solution pH in double-distilled water was determined with a Zetasizer Nano ZS90 (Malvern, UK).
To determine the total contents of Pb and Cd on BSL-BC before adsorption, 0.5 g BSL-BC was digested with solution containing 7 mL HNO3, 3 mL HCL, and 3 mL HF. The mixture was sequentially heated in a microwave digester (ETHOS UP, Milestones Helping Chemists, Italy) at 130 °C for 5 min, 170 °C for 5 min, and 190 °C for 35 min. The Pb and Cd contents in the digested liquid were measured with a flame atomic absorption spectrophotometer (AAS, Z-2300, Hitachi, Japan).
The morphology and size of BSL and BSL-BC were characterized by scanning electron microscopy (SEM, Zeiss Sigma 300), with their element analysis conducted on the SEM equipped with energy dispersive X-ray spectrometry (SEM–EDS, Bruker Electric Cooling X-ray Spectrometer XFlash6). The SSA, total pore volume, and pore size distribution of BSL-BC were assessed by Brunauer–Emmett–Teller (BET) analysis using a NOVA 1200 surface area pore analyzer (Mike ASAP2020).
Fourier-transform infrared spectroscopy (FTIR) analysis was performed to determine the major organic functional groups on the surface of BSL-BC. The FTIR spectra between 400 and 4000 cm−1 for BSL-BC prepared in pellets of fused KBr were measured with Bruker Vector 22 spectrometer (PE FT-IR Frontier). The valance of specific elements was analyzed by energy dispersive X-ray spectroscopy (XPS, Thermo Fisher Scientific K-Alpha), and all binding energies were calibrated using C 1 s peak (284.8 eV). The crystallite phase composition was analyzed with powder X-ray diffraction (XRD), which was performed by an X’Pert PRO diffractor (BRUKER D8 Advance) with the tube parameters set at 40 kV of voltage and 40 mA of current.
Adsorption experiments
The Cd2+ and Pb2+ adsorption experiments were performed using a batch equilibration technique for triplicate samples at room temperature. Stock solutions of Cd2+ and Pb2+ at 1000 mg/L were prepared with CdCl2·2.5H2O and Pb(NO3)2 in double-distilled water, respectively. The equilibrium adsorption amounts (Qe, mg/g) of Cd2+ or Pb2+ onto BSL-BC and the adsorption efficiencies (%) were calculated using Eqs. (1) and (2), respectively:
| 1 |
| 2 |
where C0 and Ce are the initial and equilibrium aqueous concentrations of Cd2+ or Pb2+ (mg/L), respectively, V is the solution volume (mL), and M is the mass of adsorbent (g).
Adsorption kinetics
To investigate the adsorption rate, 40 mg BSL-BC was mixed with 50 mL solution containing 200 mg/L Pb2+ at an initial pH of 5.0 ± 0.1 (unadjusted) or 50 mg/L Cd2+ at an initial pH of 5.5 ± 0.1 (unadjusted) in 150 mL conical flasks. The mixture was agitated at 180 rpm on a reciprocating shaker at 25 °C. Replicate flasks containing Pb2+ or Cd2+ were sampled at regular time intervals (5–1440 min), and filtered with 0.30–0.50 μm Double Ring quantitative filter paper. The filtrate was acidified with 1% (v/v) HNO3 (Guaranteed reagent, GR) and the concentration of Pb2+ or Cd2+ was determined by AAS (Z-2300, Hitachi, Japan).
The pseudo-first-order (PFO) (Eq. 3) and pseudo-second-order (PSO) (Eq. 4) kinetic models are two most frequently used models for fitting the adsorption rate data of metal ions21. We also investigated the rate-limiting step of adsorption by fitting the adsorption rate data with an intra-particle diffusion model (Eq. 5)22.
| 3 |
| 4 |
| 5 |
where Qt (mg g−1) is the amount adsorbed at time t (min), K1 (min−1) and K2 (g mg−1 min−1) are the PFO and PSO rate constants, respectively, Kd (mg g−1 h−1/2) is the rate constant of the intra-particle diffusion model, and I (mg g−1) is a constant corresponded to the boundary layer thickness. The values of Kd and I were obtained from the slope and intercept of the second linear regime of the intra-particle diffusion model, respectively.
Adsorption isotherms
To initiate the adsorption isotherm experiments, 50 mL solution with different initial concentrations of Pb2+ (10, 50, 100, 200, 300, 400, 500, 600, and 700 mg/L) or Cd2+ (10, 25, 50, 75, 100, 125, 150, and 200 mg/L) was added into a series of conical flasks, followed by addition of 40 mg BSL-BC. Adsorption experiments were conducted at initial pH values of 5.0 for Pb2+ and 5.5 for Cd2+. The mixture was shaken at 180 rpm and 25 °C for 8 h, after which it was filtered with 0.30–0.50 μm Double Ring quantitative filter paper, and the concentrations of Pb2+ and Cd2+ in the filtrate were analyzed by AAS (Z-2300, Hitachi, Japan).
The adsorption isotherm data were fitted with the Langmuir (Eq. 6), Freundlich (Eq. 7), Temkin (Eq. 8), and Dubinin–Radushkev (D–R) (Eq. 9) isotherm models:
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
| 11 |
where Qm (mg g−1) is the maximum adsorption amount, Kl (L mg−1) is the Langmuir model constant, Kf (L g−1) is the Freundlich model constant, n is a constant related to the adsorption strength, R is the gas constant (8.314 (J mol−1 K−1), T is absolute temperature (K), b is the Temkin constant related to the adsorption heat (J mol−1), A is the Temkin isotherm constant (L g−1), K is the D–R isotherm parameter used for estimating the mean free energy (E = ) to distinguish the type of adsorption process23, and ε is the D–R isotherm parameter.
Effects of pH
The pH effect on Pb2+ adsorption was studied by mixing 50 mg BSL-BC and 50 mL solutions containing 200 mg/L Pb2+ at different initial pH (2.0–6.0) in 100 mL centrifuge tubes, as Pb2+ may precipitate as hydroxides at pH ≥ 724. To investigate the pH effect on Cd2+ adsorption, 50 mg BSL-BC was mixed with 50 mL solutions containing 50 mg/L Cd2+ at different initial pH (2.0–8.0). The solution pH was adjusted with 0.1 M HCl or NaOH before adding BSL-BC. After shaken at 180 rpm and 25 °C for 8 h, the mixture was filtered with 0.30–0.50 μm Double Ring quantitative filter paper, and the concentrations of Pb2+ and Cd2+ in the filtrate were analyzed by AAS (Z-2300, Hitachi, Japan).
Characterization of BSL-BC after adsorption
To investigate the adsorption mechanisms of Pb2+or Cd2+ onto BSL-BC, 50 mg BSL-BC was added into 50 mL solutions containing 200 mg/L Pb2+ (pH 5.0) or 50 mg/L Cd2+ (pH 5.6) in 150 mL conical flasks. The mixture was shaken at 180 rpm and 25 °C for 8 h. After filtration, the BSL-BC samples loaded with Pb2+ or Cd2+ were recovered and dried at 40 °C. The changes in morphology and functional groups of BSL-BC after adsorption were characterized by SEM–EDS, FTIR, and XRD.
Determination of mechanism contributions to adsorption
The contribution of different mechanisms to Pb2+/Cd2+ adsorption onto BSL-BC was calculated based on the modified method proposed by Wang et al.20 and Cui et al.25. Firstly, BSL-BC was demineralized by soaking for 30 min in 1 M HCl, rinsed with double-distilled water until stable solution pH, air-dried, and weighed. The demineralization rate, Y (%), was calculated according to the mass before and after demineralization. Fifty milligrams of the original BSL-BC or the demineralized BSL-BC were weighted into a 150 mL triangular flask containing 50 mL of 50 mg/L Cd2+ or 200 mg/L Pb2+. The mixture was shaken at 180 rpm and 25 °C for 8 h, and then filtered through Double Ring quantitative filter paper. The filtrate was collected for analysis of concentrations for Pb2+, Cd2+, K+, Na+, Ca2+, and Mg2+ by AAS (Z-2300, Hitachi, Japan). Double-distilled water was used as a control group.
The adsorption capacities attributed to complexation with oxygen-containing functional group (Qco), metal ion exchange (Qcme), mineral precipitation (Qcmp), and Pb2+/Cd2+-π coordination (Qcπ) were determined as follows:
| 12 |
| 13 |
| 14 |
| 15 |
| 16 |
where Qcm is the amount of Pb2+/Cd2+ adsorption attributed to interaction with minerals (mg/g), Qt1 and Qa are the total adsorption capacities before and after demineralization (mg/g), respectively, and QK, QNa, QCa, and QMg are the amounts of cations (K+, Na+, Ca2+, and Mg2+, respectively) released from biochar (mg/g) into Pb2+/Cd2+ solution after subtracting those leached into double-distilled water. Since the amount of H+ released could be determined by the decrease of pH, the unadjusted adsorbed amount of Pb2+ or Cd2+ via complexation with oxygen-containing functional group (Qco1, mg/g) was calculated accordingly, which was multiplied by Y to offset the concentration effect.
Results and discussion
Characteristics of BSL and BSL-BC
Both BSL and BSL-BC were characterized to examine the basic properties of the raw material and to reveal the change in properties after preparation into biochar. The major characteristics of the raw material (BSL) and synthesized adsorbent (BSL-BC) are presented in Table 1, with their photos presented in Fig. S1a,b, respectively. Compared with BSL, BSL-BC had higher contents of C, O, and N but lower H content. The molar ratios of H/C and O/C on BSL-BC were about 0.058 and 0.34, respectively, indicating that BSL-BC had high aromaticity and hydrophobicity26. The cation contents for K+, Na+, Ca2+, and Mg2+ on BSL-BC were 5.91, 1.26, 0.37, and 5.71 mg/g, respectively. The digestion analysis shows that before adsorption, BSL-BC had trace amounts of Cd (0.0054 mg/g) and Pb (0.10 mg/g).
Table 1.
Physicochemical properties of BSL and BSL-BC before adsorption.
| Sample | pH | EC a | SSA b | Pore volume | Pore diameter | Ash content | Elements (%) | Heavy metals (mg/kg) | Other cations (mg/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (μS/cm) | (m2/g) | (cm3/g) | (nm) | (%) | C | H | N | O | P | Cd | Pb | K | Ca | Na | Mg | ||
| BSL | – | – | 0.7860 | 0.002000 | 9.309 | – | 42.34 | 5.520 | 1.080 | – | 0.0004200 | – | – | – | – | – | – |
| BSL-BC | 9.980 | 5.520 | 15.73 | 0.06800 | 17.04 | 17.27 | 58.19 | 3.380 | 1.380 | 19.78 | 0.001000 | 0.005400 | 0.1000 | 5.910 | 1.260 | 0.3700 | 5.710 |
aEC Electrical conductivity.
bSSA Specific surface area.
The double-distilled water containing only BSL-BC had solution pH of 10.2. Fig. S2 shows that BSL-BC remained negatively charged under most pH conditions in double-distilled water, yielding a point of zero charge (PZC) at pH 1.2. As shown in the FTIR spectrum (Fig. S3), the surface of BSL-BC mainly contained C=C (1320 cm−1) and –CH (780 cm−1) as well as oxygen-containing functional groups including –OH (3430 cm−1) and C=O (1615 cm−1), which would contribute to the adsorption of positively charged heavy metals such as Pb2+ and Cd2+. The hydroxyl and carboxylic groups should be responsible for the deprotonation of BSL-BC in water that resulted in its negatively charged surface.
The SEM image (Fig. S4a) shows that the surface of BSL was covered with cracks and it had irregular lamellar structures stacked in layers. After pyrolysis, BSL-BC displayed many wrinkles and irregular pore-like structures uniformly distributed on the surface (Fig. S4b). The inner walls of these pores in BSL-BC were relatively smooth, which may provide surface area for adsorption. Table 1 shows that BSL-BC had larger SSA, greater total pore volume, and smaller average pore diameter (15.73 m2/g, 0.06800 cm3/g, and 17.04 nm, respectively) than BSL (0.7860 m2/g, 0.002000 cm3/g, and 9.309 nm, respectively), indicating that BSL-BC could provide more available sites for adsorption and storage of metal ions1. The SSA of BSL-BC (15.73 m2/g) was similar to the SSA values (1–50 m2/g) reported in the literature for biochar made from eucalyptus sawdust27, activated sludge, cow biosolids28, and banana peel29; however, it was significantly smaller than the SSA values (50–500 m2/g) for biochar made from other plant residues such as charcoal, sugarcane bagasse, rape straw, wheat straw, Miscanthus straw, and soft wood26,30.
Adsorption kinetics
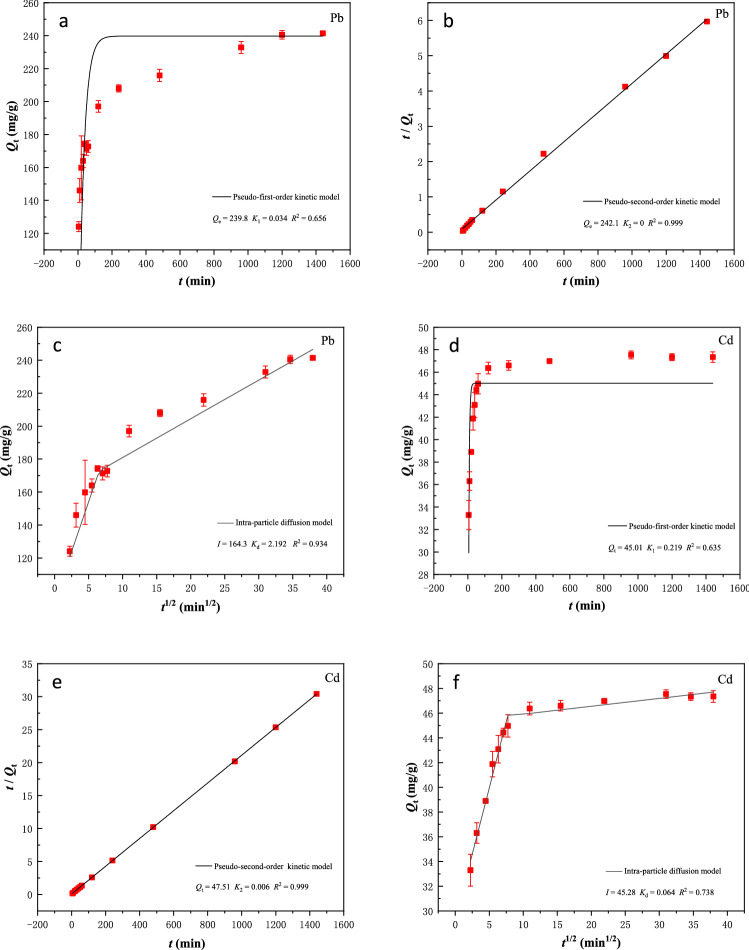
The adsorption kinetics of 200 mg/L Pb2+ at pH 5.0 and 50 mg/L Cd2+ at pH 5.5 by 0.8 g/L BSL-BC within the first 24 h are presented in Fig. 1. As shown in Fig. 1a, high adsorption rate of Pb2+ onto BSL-BC was observed in the first 5 min and then gradually decreased after 200 min of contact. The adsorption amounts of Pb2+ still slowly increased until ~ 960 min, when adsorption equilibrium was attained. However, BSL-BC adsorbed Cd2+ rapidly in 200 min and approached equilibrium (Fig. 1d). The adsorption of Pb2+ appeared to be faster than Cd2+ by BSL-BC, as 63.4% of 200 mg/L Pb2+ was rapidly removed within the first 5 min, during which only 6.3% of 50 mg/L Cd2+ was removed from solution. At this stage, the adsorbed amounts of Pb2+ and Cd2+ onto BSL-BC were 152.8 and 3.140 mg/g, respectively, indicating that the adsorption capacity of BSL-BC for Pb2+ was much stronger than that for Cd2+.
Figure 1.
Adsorption kinetic data of Pb2+ onto BSL-BC fitted by the (a) PFO and (b) PSO kinetic models and (c) intra-particle diffusion model. Adsorption kinetic data of Cd2+ onto BSL-BC fitted by the (d) PFO and (e) PSO kinetic models and (f) intra-particle diffusion model. The solutions containing 0.8 g/L BSL-BC and initial concentrations of 200 mg/L Pb2+ (pH 5.0) or 50 mg/L Cd2+ (pH 5.5) were mixed at 180 rpm and 25 °C.
The adsorption kinetic data of both Pb2+ (Fig. 1b) and Cd2+ (Fig. 1e) onto BSL-BC could be well-described by the PSO model, showing high regression coefficients of R2 = 0.999 for both Pb2+ and Cd2+ (Table 2). Meanwhile, the theoretical Qe values fitted by PSO model were 239.8 and 45.01 mg/g for Pb2+ and Cd2+, respectively, which were consistent with their experimental results of 242.1 and 47.51 mg/g (Table 2). In comparison, the PFO model provided less ideal fitting for both metal ions (Fig. 1a–d), with R2 of 0.656 and 0.635 for Pb2+ and Cd2+, respectively. The better fitting results of PSO model than the PFO model indicated that chemical adsorption was mainly responsible for the removal of Pb2+ and Cd2+ by BSL-BC31.
Table 2.
Fitting parameters of adsorption kinetic models for the adsorption of Pb2+ and Cd2+ onto BSL-BC.
| Kinetic model | Fitting parameter | Pb2+ | Cd2+ |
|---|---|---|---|
| Pseudo-first-order | Qe (mg g−1) | 239.8 | 45.01 |
| K1 | 0.034 | 0.219 | |
| R2 | 0.656 | 0.635 | |
| Pseudo-second-order | Qe (mg g−1) | 242.1 | 47.51 |
| K2 | 0 | 0.006 | |
| Intra-particle diffusion | R2 | 0.999 | 0.999 |
| Kd (mg g−1 h−1/2) | 2.192 | 0.064 | |
| I (mg g−1) | 164.3 | 45.28 | |
| R2 | 0.934 | 0.738 |
The values of Kd and I were obtained from the slope and intercept of the second linear regime of the intra-particle diffusion model, respectively. The solutions containing 0.8 g/L BSL-BC and initial concentrations of 200 mg/L Pb2+ (pH 5.0) or 50 mg/L Cd2+ (pH 5.5) were mixed at 180 rpm and 25 °C.
The intra-particle diffusion model was further fitted to the kinetic data for investigating the rate-limiting step of adsorption. The results show that the adsorption of both Pb2+ and Cd2+ on BSL-BC could be divided into two linear regimes, with the first regime showing a steeper slope than the second one. This suggests that the adsorption proceeded through two steps: the first linear regime describes a fast bulk diffusion step due to boundary effects, whereas the second regime describes a slow equilibrium attainment due to intra-particle diffusion processes2. The fitted correlation coefficients of R2 for adsorption of Pb2+ (0.934) was larger than Cd2+ (0.738), indicating that the intra-particle diffusion model was more suitable for describing the adsorption process of Pb2+ onto BSL-BC than Cd2+ (Table 2). The larger Kd value of Pb2+ (2.192 mg g−1 h−1/2) than that Cd2+ (0.064 mg g−1 h−1/2) suggested a faster diffusion of Pb2+ into the porous structure of BSL-BC. Meanwhile, the greater I value of Pb2+ (164.3 mg/g) than Cd2+ (45.28 mg/g) indicated that the adsorption of Pb2+ by BSL-BC experienced a stronger boundary layer effect (i.e., molecular diffusion in solution) than Cd2+ 32.
Adsorption isotherms
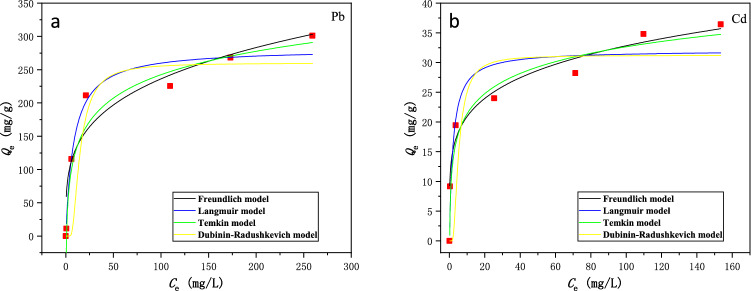
The adsorption isotherm experiments were conducted to investigate the equilibrium adsorption behaviors of Pb2+ and Cd2+ onto BSL-BC. Figure 2 shows that upon adsorption equilibrium, the solid phase adsorbed amount (Qe) of both metals increased drastically with their aqueous concentration (Ce) at low Ce range. Such an increase became less significant at higher Ce likely due to adsorbent saturation. As the initial Pb2+ aqueous concentration (C0) increased from 10 to 500 mg/L, Qe increased from 11.30 to 301.02 mg/g, with removal efficiencies decreasing from 94.1 to 46.4% (Fig. 2a). For Cd2+, Qe increased from 9.17 to 28.23 mg/g and the removal efficiencies decreased from 97.5 to 28.4% as C0 increased from 10 to 200 mg/L (Fig. 2b). The results showed that the Qe values for Pb2+ were an order of magnitude higher than those for Cd2+ under the tested conditions, indicating the stronger adsorption of BSL-BC for Pb2+ than Cd2+. Since Pb could be classified as a hard Lewis acid compared with Cd, the hydroxyl and carboxyl groups (hard Lewis bases) on the adsorbent likely had higher affinity for Pb; in addition, the smaller hydration radius and lower pKH (negative logarithm of the hydrolysis constant) of Pb compared with Cd may also contribute to the stronger adsorption of Pb, as previously shown for another adsorbent (MgBC400)33.
Figure 2.
Adsorption isotherms of (a) Pb2+ and (b) Cd2+ on BSL-BC fitted with 4 isotherm models. The solutions containing 1 g/L BSL-BC and initial concentrations of 10–700 mg/L Pb2+ (pH 5.0) or 10–200 mg/L Cd2+ (pH 5.5) were mixed for 8 h at 180 rpm and 25 °C.
The Langmuir, Freundlich, Temkin, and D–R isotherm models widely adopted for evaluation of adsorption behaviors21,34 were used to fit the isotherm data (Fig. 2). The fitting parameters for each isotherm model are compiled in Table 3. All 4 isotherm models could well-describe the adsorption of Pb2+ by BSL-BC, with the Langmuir model showing the best fit (R2 = 0.961) followed by Temkin (R2 = 0.957), D–R (R2 = 0.713), and Freundlich (R2 = 0.900) models (Fig. 2a and Table 2). However, the Freundlich (R2 = 0.968) and Temkin (R2 = 0.936) models provided better fitting results to the adsorption of Cd2+ onto BSL-BC as compared with the Langmuir (R2 = 0.791) and D–R (R2 = 0.437) models (Fig. 2b and Table 3). As shown in Fig. 2, the adsorption isotherms of both Pb2+ and Cd2+ onto BSL-BC displayed similar “L” type shapes.
Table 3.
Fitting parameters of adsorption isotherm models for the adsorption of Pb2+ and Cd2+ onto BSL-BC.
| Isotherm model | Fitting parameter | Pb2+ | Cd2+ |
|---|---|---|---|
| Langmuir | Qm (mg g−1) | 302.2 | 32.03 |
| K1 (L mg−1) | 0.062 | 0.667 | |
| R2 | 0.961 | 0.791 | |
| Freundlich | Kf (L g−1) | 62.35 | 12.96 |
| 1/n | 0.272 | 0.192 | |
| R2 | 0.900 | 0.968 | |
| Temkin | A (L g−1) | 1.260 | 15.91 |
| b (J mol−1) | 21.52 | 247.6 | |
| R2 | 0.747 | 0.673 | |
| Dubinin–Radushkevich | Qm (mg g−1) | 217.9 | 28.04 |
| K2 (mol2 J−2) | 5.040 × 10–7 | 6.888 × 10–8 | |
| E (kJ mol−1) | 1.000 | 2.694 | |
| R2 | 0.713 | 0.437 |
The solutions containing 1 g/L BSL-BC and initial concentrations of 10–700 mg/L Pb2+ (pH 5.0) or 10–200 mg/L Cd2+ (pH 5.5) were mixed for 8 h at 180 rpm and 25 °C.
Since the Langmuir model provided the best fit on Pb2+ adsorption by BSL-BC, the above result indicates that monolayer adsorption of Pb2+ occurred at homogeneous sites with equal energy on BSL-BC35–37. On the other hand, according to the assumptions of Freundlich model, multilayer adsorption of Cd2+ should have taken place on heterogeneous surface with different binding energies on BSL-BC35,38–41. The values of 1/n obtained from the Freundlich model were 0.272 and 0.192 for Pb2+ and Cd2+, respectively (Table 3). This parameter represents the relative distribution of energy sites and relates to the favorable level of the adsorption system. For instance, the adsorption is generally pseudo-irreversible when 1/n < 0.01, strongly favorable between 0.01 and 0.1, favorable between 0.1 and 0.5, pseudo-reversible between 0.5 and 1, and unfavorable when above 142. Therefore, the results imply that both the adsorption of Pb2+ and Cd2+ by BSL-BC was favorable.
The Temkin model assumes a uniform distribution of binding energies at the adsorbent surface, and that the adsorption heat decreases linearly with B in Eq. (8) rather than logarithmically owing to sorbate-sorbent interactions21. The fitting results in Table 3 demonstrate that the Temkin model did not provide satisfactory fitting to the adsorption isotherm data. Low correlation coefficients (R2) were obtained for Pb2+ (0.747) and Cd2+ (0.673). Meanwhile, the Temkin isotherm constants (b) (Eq. 10) of 21.52 and 247.6 J/mol for Pb2+ and Cd2+, respectively, describing the adsorption heat were also quite low43.
The D–R isotherm model generally applies to heterogeneous adsorbent surfaces44, and can be used to estimate the free energy, apparent porosity, and biosorption characteristics45. The biosorption mean free energy (E) calculated from the D–R isotherm model provides insights on the biosorption mechanism. The biosorption is a chemical process via ion exchange if E is 8–16 kJ/mol, and is a physical process if E < 8 kJ/mol46. The fitted E values for Pb2+ and Cd2+ were 1.000 and 2.694 kJ/mol, respectively (Table 3), indicating that the adsorption a physical process. This contradicts with the earlier PSO adsorption kinetic model fitting results that the adsorption was a chemical process. Considering the low R2 of the D–R model fitting, the adsorption should be mainly a chemical process. The higher value of biosorption coefficient (K2) for Pb2+ (5.040 × 10–7 mol2 J−2) than Cd2+ (6.888 × 10–8 mol2 J−2) indicates that the free energy for adsorption of Pb2+ was larger than Cd2+ on BSL-BC.
The maximum monolayer adsorption capacities (Qm) obtained from the Langmuir model were 302.2 and 32.03 mg/g Cd2+ and Pb2+, respectively, which were slightly higher than those obtained by the D–R model (217.9 and 28.04 mg/g Cd2+ and Pb2+, respectively) (Table 3). The adsorption capacities of BSL-BC for Cd2+ and Pb2+ derived from the Langmuir model were compared with other biochar reported in the literature, with the experimental conditions given (Table S4). The results show that BSL-BC is an effective adsorbent for two heavy metals. Compared with other biochar, BSL-BC exhibited superior maximum adsorption capacity especially for Pb2+ (302.2 mg/g), which was higher than the camellia seed husk biochar (109.7 mg/g)47, peanut shell biochar (52.80 mg/g)20, and wheat straw biochar (100.00 mg/g)36. Meanwhile, the maximum adsorption capacity of Cd2+ by BSL-BC (32.03 mg/g) was also higher than the rice husk biochar (9.670 mg/g)31 and the wheat straw biochar (19.72 mg/g)36, yet lower than the dairy manure biochar (51.40 mg/g)48. Consistent with our above-mentioned results, other biochar materials in the literature also had stronger adsorption capacity for Pb2+ than Cd2+ (Table S4).
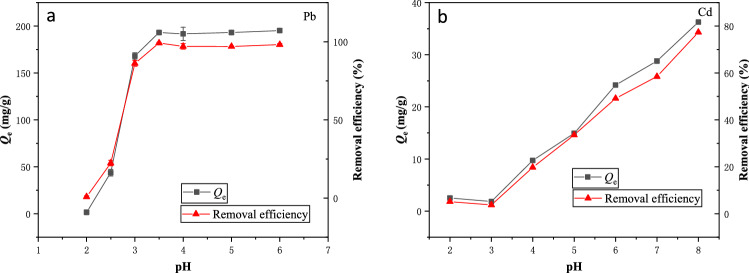
Effects of solution pH on adsorption
Figure 3 shows the variation of heavy metal adsorption onto BSL-BC with different initial solution pH. For both Pb2+ and Cd2+, their adsorption capacities and removal efficiencies by BSL-BC all greatly increased at higher pH. The most significant increase in Pb2+ adsorption onto BSL-BC occurred as pH increased from 2.0 to 3.5, after which the adsorption became steady with the maximum adsorption amount reaching 195.3 mg/g and removal efficiency approaching 100% at pH 6 (Fig. 3a). In comparison, low removal efficiency (< 5.2%) of Cd2+ by BSL-BC was observed at pH 2.0–3.0. Further increase in solution pH from 3.0 to 8.0 resulted in gradually enhancement of Cd2+ adsorption by BSL-BC, yielding the maximum adsorption amount and removal efficiency of 36.3 mg/g and 77.4% at pH 8.0, respectively (Fig. 3b).
Figure 3.
Effects of initial solution pH on the equilibrium adsorption amount (Qe) and removal efficiency of (a) Pb2+ and (b) Cd2+ by BSL-BC. The solutions containing 1 g/L BSL-BC and initial concentrations of 10–700 mg/L Pb2+ (pH 5.0) or 10–200 mg/L Cd2+ (pH 5.5) were mixed for 8 h at 180 rpm and 25 °C.
The above results indicate that the initial solution pH strongly influenced both the adsorption of Pb2+ and Cd2+ by BSL-BC. The pH effects were mainly ascribed to their influence on the distribution of Pb2+ and Cd2+ in solution as well as the deprotonation state of surface functional groups on BSL-BC49. Pb2+ is the major species at solution pH < 7.5, with some fractions of lead present as Pb(OH)+ and PbHCO33+ at pH > 5.047. Similarly, Cd2+ also predominates in solution at pH 2.0–8.0 as Cd has low hydrolysis tendency at pH < 8.039. Therefore, under acidic conditions (e.g., pH 2.0–3.0), abundant amounts of H+ in solution can effectively compete with Pb2+ and Cd2+ cations for active adsorption sites on the surface of BSL-BC50.
Meanwhile, Fig. S2 shows that with a PZC of 1.2, BSL-BC had low negative surface charge density under pH < 3.0. Its zeta potential changed from − 30 to − 40 mV as the solution pH increased from 3.0 to 4.0 and remained stable near − 40 mV at higher pH, which was due to deprotonation of functional groups such as –OH and –COOH as indicated by the FTIR spectra (Fig. S3). Consequently, in acidic solutions, weak electrostatic attraction existed between the adsorbent (BSL-BC) surface and the adsorbate (Pb2+ and Cd2+)51, with H+ also competing for adsorption sites, leading to the low removal efficiency. The results also indicate that electrostatic attraction and complexation with functional groups might be involved in the Pb2+ and Cd2+ adsorption process by BSL-BC.
Adsorption mechanisms
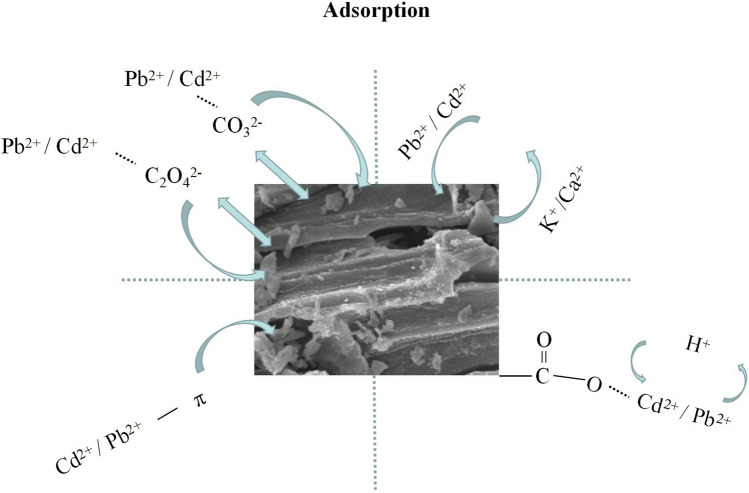
The above adsorption kinetic and isotherm results (Figs. 1, 2, 3) have confirmed the effective adsorption of Pb2+ and Cd2+ onto BSL-BC. It is generally recognized that biochar adsorbs Pb2+ and Cd2+ via complexation with oxygen-containing functional groups (e.g., carboxyl and hydroxyl), cation exchange (e.g., with K+, Na+, Ca2+, and Mg2+), precipitation with minerals (e.g., CO32−, PO43−, and OH−), and coordination with π electrons38,52–54. The potential mechanisms responsible for the adsorption of Pb2+ and Cd2+ onto surface of BSL-BC were further explored at the microscale level based on FTIR, XPS, and XRD analyses, with the contribution of each mechanism quantified.
Functional group complexation
The FTIR spectra of BSL-BC before and after adsorption of Pb2+ or Cd2+ are presented in Fig. S3. The results show that the C–O absorption peak (1318 cm−1) was shifted to the right and the –OH peak (3430 cm−1) on BSL-BC was reduced significantly after contact with Pb2+. Similarly, these two peaks on Cd2+-loaded BSL-BC also obviously weakened. Since these oxygen-containing functional groups were consumed during the adsorption of Pb2+ and Cd2+, they likely participated in the complexation with the metal ions31.
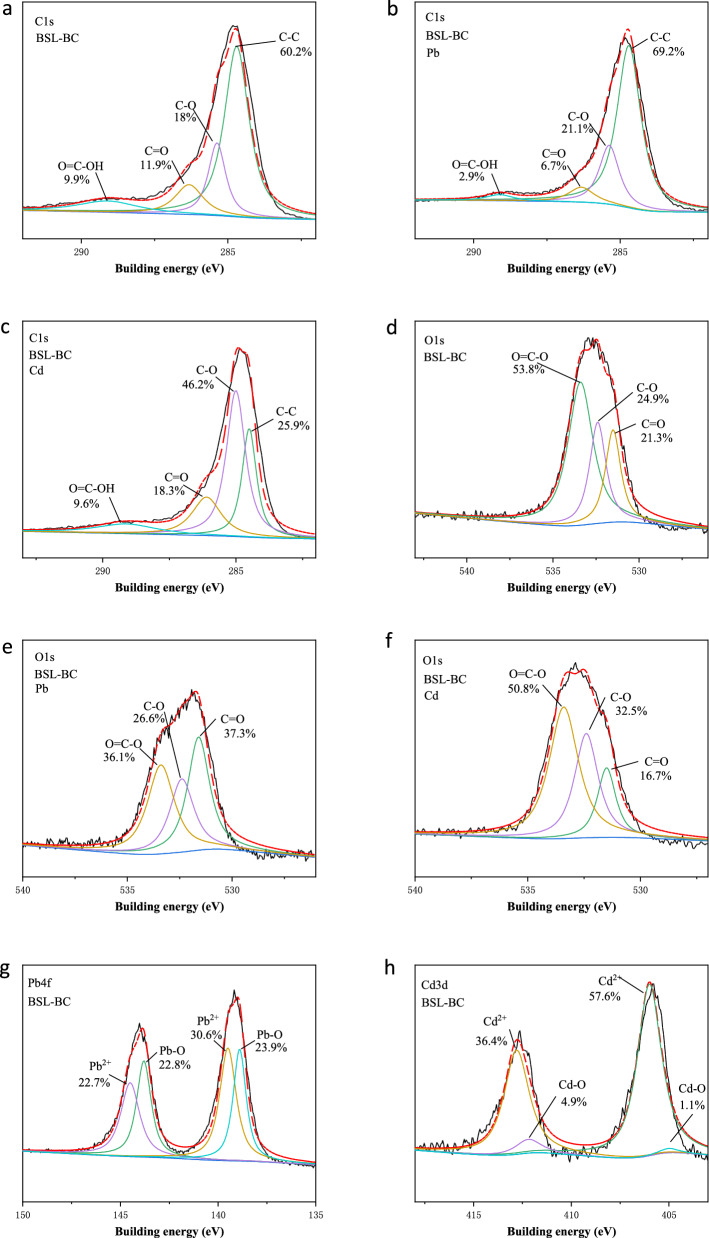
The XPS results presented in Fig. 4 also confirmed with the FTIR results that complexation with oxygen-containing functional groups was responsible for the adsorption of both metals. For Pb2+, comparing the high-resolution C1s spectra of BSL-BC before (Fig. 4a) and after (Fig. 4b) adsorption shows that the peak area of –COOH decreased from 9.9 to 2.9% while the peak area of C–O increased slightly from 18 to 21.1%. This corresponds to the high-resolution O1s spectra before (Fig. 4d) and after adsorption of Pb2+ (Fig. 4e), which also suggest that the peak area of − COOH decreased from 53.8 to 36.1% while the peak area of C–O increased slightly from 24.9 to 26.6%. These results indicate that the adsorbed Pb2+ formed bidentate complexes (–O–Pb–O–) rather than monodentate complexes (–O–Pb–OH)55. Furthermore, the Pb4f. spectra after Pb2+ adsorption clearly identified the presence of Pb2+ (53.3%) and Pb–O (46.7%) on the surface of BSL-BC (Fig. 4g), since XPS analysis mainly probes the sample surface elements. The chemical formations may be due to the precipitation of Pb oxalate and Pb hydroxide during the adsorption of Pb2+.
Figure 4.
XPS spectra of elemental scan of C1s on BSL-BC (a) before and after adsorption of (b) Pb2+ or (c) Cd2+; XPS spectra of elemental scan of O1s on BSL-BC (d) before and after adsorption of (e) Pb2+ or (f) Cd2+; (g) XPS spectra of elemental scan of Pb4f. on BSL-BC after Pb2+ adsorption; (h) XPS spectra of elemental scan of Pb4f. on BSL-BC after Cd2+ adsorption.
For Cd2+, comparing the high-resolution C1s spectra of BSL-BC before (Fig. 4a) and after (Fig. 4c) adsorption shows that the peak area of C–O increased from 18.2 to 46.2%. Consistently, the C–O peak area in the O1s spectra also increased from 24.9 to 32.5% after Cd2+ adsorption (Fig. 4d,f). In addition, the Cd3d spectra after adsorption prove the presence of Cd2+ (94%) or Cd–O (6%) on the BSL-BC surface (Fig. 4h), which could be attributed to the precipitation of Cd carbonates (pebbles) and/or Cd hydroxides. The results indicate the involvement of C–O in the complexation process during the adsorption of Cd2+ and the formation of dentate complexes (–O–Cd–O–). Surface complexation of Pb2+ and Cd2+ with oxygen-containing functional groups (e.g., –OH and –COOH) has been suggested as a crucial mechanism for the adsorption of metal ions by biochar38. The above characterization results fully demonstrate that Cd2+ interacts with the oxygen-containing functional groups on the surface of BSL-BC during adsorption to form the CdCO3 complex.
Ion exchange
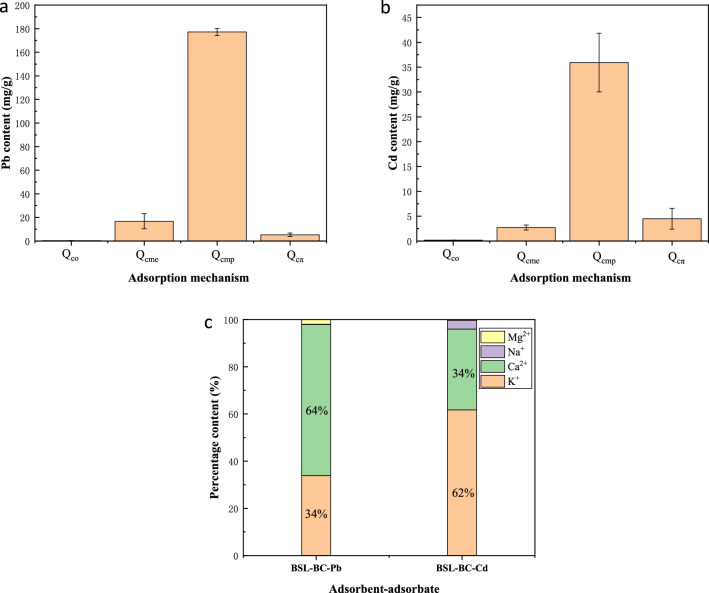
Abundant metal cations (e.g., K+, Na+, Ca2+, and Mg2+) typically retain on the biochar surface through electrostatic attraction and complexation with carboxyl and hydroxyl groups. These cations can exchange with Pb2+ and Cd2+ in solution and promote heavy metal adsorption25. As shown in Fig. 5c, significant amounts of K+ and Ca2+ were released from BSL-BC into solution after adsorption of Pb2+ and Cd2+. For the adsorption of Pb2+, 98% of K+ and Ca2+ were released into solution from BSL-BC, and 96% for adsorption of Cd2+; however, only a trace amount of Na+ was released and almost all Mg2+ was retained on BSL-BC. The release of Na+ and Mg2+ into solution accounted for 2% for adsorbed Pb2+ and 4% for adsorbed Cd2+ by BSL-BC.
Figure 5.
Mechanism contributions of complexation with oxygen-containing functional groups (Qco), metal ion exchange (Qcme), mineral precipitation (Qcmp), and Pb2+/Cd2+-π coordination (Qcπ) to the adsorption of (a) Pb2+ and (b) Cd2+ onto BSL-BC. (c) Percentage release of elements (Mg2+, Na+, Ca2+, and K+) from BSL-BC into solution after adsorption of Pb2+ or Cd2+.
Mineral precipitation
It was reported that anions (e.g., C2O42−, CO32−, PO43−, and OH−) released from biochar may react with metal cations in solution to form mineral precipitates20,51. In this study, the XPS spectra identified the formation of Pb–O (Fig. 4g) and Cd–O (Fig. 4h) on BSL-BC surface after adsorption of Pb2+ and Cd2+, respectively. Consistently, the SEM–EDS analysis (Fig. S5) show scattered white granular crystals on the BSL-BC surface after adsorption process, with the spectra of elemental compositions confirming the presence of Pb2+ or Cd2+, P, C, and O on these crystals. These above characterization results indicate that precipitation of Pb(OH)2 and Cd(OH)2 may occur during adsorption.
According to the XRD analysis, CaCO3 and CaC2O4(H2O) crystals were present on the surface of BSL-BC before adsorption (Fig. S6a). After adsorption, PbC2O4 crystal was formed on the Pb-loaded BSL-BC (Fig. S6b), whereas CdCO3 and CdC2O4 crystals were identified on the Cd-loaded BSL-BC (Fig. S6c). These results are consistent with those aforementioned in analyzing the functional group complexation mechanisms. Although some previous studies20,25,38 reported the formation of Pb3(PO4)2 or Cd3(PO4)2 after biochar adsorption of Pb2+ or Cd2+, respectively, these precipitates were not present in the XRD pattern in this study, probably because they were below the detect limit of XRD.
Other potential mechanisms
Except for the above mechanisms, Pb2+/Cd2+-π coordination and electrostatic attraction also potentially contributed to the adsorption process. According to the FTIR analysis in Fig. S3, the peaks of –CH (700–900 cm−1), C=C (1318 cm−1), and C=O (1615 cm−1) on BSL-BC were reduced after adsorption of Pb2+ and Cd2+. These results indicate that Pb2+ and Cd2+ may interact with the π electrons during the adsorption process. Furthermore, determination of the PZC of BSL-BC suggested that its surface was neutral at pH 1.2 (Fig. S2). This indicates that at solution pH above 1.2, the surface of BSL-BC was negatively charged, which was favorable for adsorbing the Pb2+ and Cd2+ cations. Therefore, electrostatic attraction may occur that also contributed to the adsorption process.
Contribution of each adsorption mechanism
The contribution of each adsorption mechanisms was calculated with Eqs. (12–16) and the results are presented in Fig. 5a,b. The schematic diagram of each adsorption mechanism is shown in Fig. 6. It is noted that electrostatic attraction was neglected during calculation of the mechanism contribution. The results show that mineral precipitation (Qcmp), metal ion exchange (Qcme), complexation with oxygen-containing functional group (Qco), and π-electron coordination (Qcπ) accounted for 88.8%, 8.4%, 0.1%, and 2.6%, respectively, for Pb2+ adsorption onto BSL-BC; and were 83.0%, 6.3%, 0.4%, and 10.4%, respectively, for Cd2+ adsorption. Therefore, mineral precipitation was the major adsorption mechanism that accounted for above 80% in both Pb2+ and Cd2+ adsorption.
Figure 6.
Mechanism of Pb2+ / Cd2+ adsorption onto BSL-BC.
The result is supported by the crystal formation of PbC2O4, CdCO3, and CdC2O4 on BSL-BC after adsorption as identified in the XRD spectra (Fig. S6). It is also consistent with literature studies. For instance, Wang et al.20 found that the dominant mechanism for Pb2+ adsorption by peanut shell biochar was mineral precipitation; similarly, Gao et al.56 also reported that the adsorption of Cd2+ by rice biochar was dominated by mineral precipitation, with relatively small contributions from complexation of Cd2+ with functional groups and coordination with π electrons on biochar.
Conclusions
In this study, BSL-BC was successfully prepared from BSL as a recycling product from agricultural waste, with major physicochemical properties characterized. The synthesized BSL-BC exhibited strong adsorption for Pb2+ and Cd2+ in water. The adsorption rate data of Pb2+ and Cd2+ onto BSL-BC both followed the PSO kinetic model, indicating the process was chemisorption. The adsorption isotherm data of Pb2+ and Cd2+ could be well-described by the Langmuir and Freundlich models, respectively, whereas neither the Temkin or Dubinin–Radushkevich models provided satisfactory fitting results. The results indicate that monolayer and homogeneous adsorption occurred for Pb2+ onto BSL-BC, while the adsorption of BSL-BC for Cd2+ was multilayer and heterogeneous. The adsorption for Pb2+ and Cd2+ reached equilibrium after 8 h and 200 min, respectively, yielding maximum adsorption capacities of 302.2 and 32.03 mg/g. The optimum adsorption pH values were 5 and 8 for Pb2+ and Cd2+, respectively. Various characterization techniques show that the adsorption occurred via functional group complexation, cation exchange, mineral precipitation, π-electron coordination, and electrostatic attraction. Mineral precipitation was the major mechanism for the adsorption of Pb2+ and Cd2+ by BSL-BC, accounting for above 80% of mechanism contribution. This study provides a theoretical basis for recycling BSL waste to produce BSL-BC, which could be effectively applied in removal of Pb2+ and Cd2+ from contaminated water. Future study should examine the adsorption of BSL-BC for Pb2+ and Cd2+ under co-existing state or presented at lower metal concentrations in actual situation.
Supplementary Information
Acknowledgements
This work was financially supported by National Key Research and Development Program of China [Grant Number 2017YFD0801000]; Program for Guangdong Introducing Innovative and Enterpreneurial Teams [Grant Number 2019ZT08N291]; Guangdong Basic and Applied Basic Research Foundation [Grant Number 2021A1515011503]; Science and Technology Planning Project of Guangzhou [Grant Number 202002020072]; National Natural Science Foundation of China (NSFC) [Grant Number 41807451]; Guangzhou Young Talents Lifting Program [Grant Number X20200301025]; and National Natural Science Foundation of China (NSFC) [Grant Number 42177014].
Author contributions
X.L.: Data Curation, Formal Analysis, Writing—Original Draft. G.L.: Methodology, Data Curation, Formal Analysis. X.Z.: Methodology, Data Curation. C.C.: Writing—Review & Editing, Funding Acquisition. Z.K.: Data Curation. X.L.: Conceptualization, Methodology, Funding Acquisition, Writing—Review & Editing.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chengyu Chen, Email: cychen@scau.edu.cn.
Xinxian Long, Email: longxx@scau.edu.cn.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-05652-7.
References
- 1.Shakoor MB, et al. A review of biochar-based sorbents for separation of heavy metals from water. Int. J. Phytoremediation. 2020;22:111–126. doi: 10.1080/15226514.2019.1647405. [DOI] [PubMed] [Google Scholar]
- 2.Chen C, Geng X, Huang W. Adsorption of 4-chlorophenol and aniline by nanosized activated carbons. Chem. Eng. J. 2017;327:941–952. doi: 10.1016/j.cej.2017.06.183. [DOI] [Google Scholar]
- 3.Gunatilake SK. Methods of removing heavy metals from industrial wastewater. J. Multidiscip. Eng. Sci. Stud. 2015;1:12–18. [Google Scholar]
- 4.Burakov AE, et al. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018;148:702–712. doi: 10.1016/j.ecoenv.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Fu F, Wang Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manage. 2011;92:407–418. doi: 10.1016/j.jenvman.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Hasanpour M, Hatami M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid. Interface. Sci. 2020;284:102247. doi: 10.1016/j.cis.2020.102247. [DOI] [PubMed] [Google Scholar]
- 7.Selvaraj M, Hai A, Banat F, Haija MA. Application and prospects of carbon nanostructured materials in water treatment: A review. J. Water Process. Eng. 2020 doi: 10.1016/j.jwpe.2019.100996. [DOI] [Google Scholar]
- 8.Ahmed MB, Zhou JL, Ngo HH, Guo W, Chen M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016;214:836–851. doi: 10.1016/j.biortech.2016.05.057. [DOI] [PubMed] [Google Scholar]
- 9.Gupta S, Sireesha S, Sreedhar I, Patel CM, Anitha KL. Latest trends in heavy metal removal from wastewater by biochar based sorbents. J. Water Process. Eng. 2020 doi: 10.1016/j.jwpe.2020.101561. [DOI] [Google Scholar]
- 10.Xiang W, et al. Biochar technology in wastewater treatment: A critical review. Chemosphere. 2020;252:126539. doi: 10.1016/j.chemosphere.2020.126539. [DOI] [PubMed] [Google Scholar]
- 11.Xiong X, et al. Value-added chemicals from food supply chain wastes: State-of-the-art review and future prospects. Chem. Eng. J. 2019 doi: 10.1016/j.cej.2019.121983. [DOI] [Google Scholar]
- 12.Domingues RR, et al. Properties of biochar derived from wood and high-nutrient biomasses with the aim of agronomic and environmental benefits. PLoS ONE. 2017;12:e0176884. doi: 10.1371/journal.pone.0176884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FAO, Banana facts and figures, http://www.fao.org/economic/est/est-commodities/bananas/bananafacts/en/#.YTh1wWgzaUm (2017).
- 14.Suhag M, Kumar A, Singh J. Saccharification and fermentation of pretreated banana leaf waste for ethanol production. SN Appl. Sci. 2020 doi: 10.1007/s42452-020-03215-x. [DOI] [Google Scholar]
- 15.Ortiz-Ulloa JA, Abril-Gonzalez MF, Pelaez-Samaniego MR, Zalamea-Piedra TS. Biomass yield and carbon abatement potential of banana crops (Musa spp.) in Ecuador. Environ. Sci. Pollut. Res. Int. 2021;28:18741–18753. doi: 10.1007/s11356-020-09755-4. [DOI] [PubMed] [Google Scholar]
- 16.Chanakya HN, Sreesha M. Anaerobic retting of banana and arecanut wastes in a plug flow digester for recovery of fiber, biogas and compost. Energy Sustain. Dev. 2012;16:231–235. doi: 10.1016/j.esd.2012.01.003. [DOI] [Google Scholar]
- 17.Cordeiro N, Belgacem MN, Torres IC, Moura JCVP. Chemical composition and pulping of banana pseudo-stems. Ind. Crops. Prod. 2004;19:147–154. doi: 10.1016/j.indcrop.2003.09.001. [DOI] [Google Scholar]
- 18.Kumar A, Bhattacharya T. Biochar: A sustainable solution. Environ. Dev. Sustain. 2020;23:6642–6680. doi: 10.1007/s10668-020-00970-0. [DOI] [Google Scholar]
- 19.Li Y, Xing B, Ding Y, Han X, Wang S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2020;312:123614. doi: 10.1016/j.biortech.2020.123614. [DOI] [PubMed] [Google Scholar]
- 20.Wang Z, et al. Investigating the mechanisms of biochar's removal of lead from solution. Bioresour. Technol. 2015;177:308–317. doi: 10.1016/j.biortech.2014.11.077. [DOI] [PubMed] [Google Scholar]
- 21.Beni AA, Esmaeili A. Biosorption, an efficient method for removing heavy metals from industrial effluents: A Review. Environ. Technol. Inno. 2020 doi: 10.1016/j.eti.2019.100503. [DOI] [Google Scholar]
- 22.Duan Z, et al. Magnetic Fe3O4/activated carbon for combined adsorption and Fenton oxidation of 4-chlorophenol. Carbon. 2020;167:351–363. doi: 10.1016/j.carbon.2020.05.106. [DOI] [Google Scholar]
- 23.Hu Q, Zhang Z. Application of Dubinin–Radushkevich isotherm model at the solid/solution interface: A theoretical analysis. J. Mol. Liq. 2019;277:646–648. doi: 10.1016/j.molliq.2019.01.005. [DOI] [Google Scholar]
- 24.Ahmad Z, et al. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 2018;180:437–449. doi: 10.1016/j.jclepro.2018.01.133. [DOI] [Google Scholar]
- 25.Cui X, et al. Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci. Total. Environ. 2016;562:517–525. doi: 10.1016/j.scitotenv.2016.03.248. [DOI] [PubMed] [Google Scholar]
- 26.Chun Y, Sheng G, Chiou CT, Xing B. Compositions and sorptive properties of crop residue-derived chars. Environ. Sci. Technol. 2004;38:4649–4655. doi: 10.1021/es035034w. [DOI] [PubMed] [Google Scholar]
- 27.Martins AF, Cardoso Ade L, Stahl JA, Diniz J. Low temperature conversion of rice husks, eucalyptus sawdust and peach stones for the production of carbon-like adsorbent. Bioresour. Technol. 2007;98:1095–1100. doi: 10.1016/j.biortech.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 28.Shinogi Y, Kanri Y. Pyrolysis of plant, animal and human waste: Physical and chemical characterization of the pyrolytic products. Bioresour. Technol. 2003;90:241–247. doi: 10.1016/s0960-8524(03)00147-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhou N, et al. Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization. Bioresour. Technol. 2017;232:204–210. doi: 10.1016/j.biortech.2017.01.074. [DOI] [PubMed] [Google Scholar]
- 30.Soria RI, Rolfe SA, Betancourth MP, Thornton SF. The relationship between properties of plant-based biochars and sorption of Cd(II), Pb(II) and Zn(II) in soil model systems. Heliyon. 2020;6:e05388. doi: 10.1016/j.heliyon.2020.e05388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun C, et al. Enhanced adsorption for Pb(II) and Cd(II) of magnetic rice husk biochar by KMnO4 modification. Environ. Sci. Pollut. Res. Int. 2019;26:8902–8913. doi: 10.1007/s11356-019-04321-z. [DOI] [PubMed] [Google Scholar]
- 32.Smith YR, Bhattacharyya D, Willhard T, Misra M. Adsorption of aqueous rare earth elements using carbon black derived from recycled tires. Chem. Eng. J. 2016;296:102–111. doi: 10.1016/j.cej.2016.03.082. [DOI] [Google Scholar]
- 33.Ni BJ, et al. Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere. 2019;219:351–357. doi: 10.1016/j.chemosphere.2018.12.053. [DOI] [PubMed] [Google Scholar]
- 34.Lim JY, Mubarak NM, Khalid M, Abdullah EC, Arshid N. Novel fabrication of functionalized graphene oxide via magnetite and 1-butyl-3-methylimidazolium tetrafluoroborate. Nano-Struct. Nano-Obj. 2018;16:403–411. doi: 10.1016/j.nanoso.2018.10.005. [DOI] [Google Scholar]
- 35.Abdelfattah I, Ismail AA, Sayed FA, Almedolab A, Aboelghait KM. Biosorption of heavy metals ions in real industrial wastewater using peanut husk as efficient and cost effective adsorbent. Environ. Nanotechnol. Monit. Manage. 2016;6:176–183. doi: 10.1016/j.enmm.2016.10.007. [DOI] [Google Scholar]
- 36.Trakal L, Bingol D, Pohorely M, Hruska M, Komarek M. Geochemical and spectroscopic investigations of Cd and Pb sorption mechanisms on contrasting biochars: Engineering implications. Bioresour. Technol. 2014;171:442–451. doi: 10.1016/j.biortech.2014.08.108. [DOI] [PubMed] [Google Scholar]
- 37.Zhan W, et al. Green synthesis of amino-functionalized carbon nanotube-graphene hybrid aerogels for high performance heavy metal ions removal. Appl. Surf. Sci. 2019;467–468:1122–1133. doi: 10.1016/j.apsusc.2018.10.248. [DOI] [Google Scholar]
- 38.Deng Y, Huang S, Laird DA, Wang X, Meng Z. Adsorption behaviour and mechanisms of cadmium and nickel on rice straw biochars in single- and binary-metal systems. Chemosphere. 2019;218:308–318. doi: 10.1016/j.chemosphere.2018.11.081. [DOI] [PubMed] [Google Scholar]
- 39.Li H, et al. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere. 2017;178:466–478. doi: 10.1016/j.chemosphere.2017.03.072. [DOI] [PubMed] [Google Scholar]
- 40.Rajamohan N, Rajasimman M. Biosorption of Selenium using activated plant based sorbent: Effect of variables, isotherm and kinetic modeling. Biocatal. Agric. Biotechnol. 2015;4:795–800. doi: 10.1016/j.bcab.2015.10.013. [DOI] [Google Scholar]
- 41.Tounsadi H, Khalidi A, Abdennouri M, Barka N. Biosorption potential of Diplotaxis harra and Glebionis coronaria L. biomasses for the removal of Cd(II) and Co(II) from aqueous solutions. J. Environ. Chem. Eng. 2015;3:822–830. doi: 10.1016/j.jece.2015.03.022. [DOI] [Google Scholar]
- 42.Ojedokun AT, Bello OS. Sequestering heavy metals from wastewater using cow dung. Water Resour. Ind. 2016;13:7–13. doi: 10.1016/j.wri.2016.02.002. [DOI] [Google Scholar]
- 43.Nadeem R, Manzoor Q, Iqbal M, Nisar J. Biosorption of Pb(II) onto immobilized and native Mangifera indica waste biomass. J. Ind. Eng. Chem. 2016;35:185–194. doi: 10.1016/j.jiec.2015.12.030. [DOI] [Google Scholar]
- 44.Malik R, Dahiya S, Lata S. An experimental and quantum chemical study of removal of utmostly quantified heavy metals in wastewater using coconut husk: A novel approach to mechanism. Int. J. Biol. Macromol. 2017;98:139–149. doi: 10.1016/j.ijbiomac.2017.01.100. [DOI] [PubMed] [Google Scholar]
- 45.Srivastava S, Agrawal SB, Mondal MK. Biosorption isotherms and kinetics on removal of Cr(VI) using native and chemically modified Lagerstroemia speciosa bark. Ecol. Eng. 2015;85:56–66. doi: 10.1016/j.ecoleng.2015.10.011. [DOI] [Google Scholar]
- 46.Naiya TK, Bhattacharya AK, Mandal S, Das SK. The sorption of lead(II) ions on rice husk ash. J. Hazard. Mater. 2009;163:1254–1264. doi: 10.1016/j.jhazmat.2008.07.119. [DOI] [PubMed] [Google Scholar]
- 47.Wu J, Wang T, Zhang Y, Pan WP. The distribution of Pb(II)/Cd(II) adsorption mechanisms on biochars from aqueous solution: Considering the increased oxygen functional groups by HCl treatment. Bioresour. Technol. 2019;291:121859. doi: 10.1016/j.biortech.2019.121859. [DOI] [PubMed] [Google Scholar]
- 48.Xu X, et al. Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar. Environ. Sci. Pollut. Res. Int. 2013;20:358–368. doi: 10.1007/s11356-012-0873-5. [DOI] [PubMed] [Google Scholar]
- 49.Lian W, et al. Utilization of biochar produced from invasive plant species to efficiently adsorb Cd (II) and Pb (II) Bioresour. Technol. 2020;317:124011. doi: 10.1016/j.biortech.2020.124011. [DOI] [PubMed] [Google Scholar]
- 50.Amiri-Yazani T, Zare-Dorabei R, Rabbani M, Mollahosseini A. Highly efficient ultrasonic-assisted pre-concentration and simultaneous determination of trace amounts of Pb (II) and Cd (II) ions using modified magnetic natural clinoptilolite zeolite: Response surface methodology. Microchem. J. 2019;146:498–508. doi: 10.1016/j.microc.2019.01.050. [DOI] [Google Scholar]
- 51.Teng D, et al. Efficient removal of Cd(II) from aqueous solution by pinecone biochar: Sorption performance and governing mechanisms. Environ. Pollut. 2020;265:115001. doi: 10.1016/j.envpol.2020.115001. [DOI] [PubMed] [Google Scholar]
- 52.Ahmad M, et al. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere. 2014;99:19–33. doi: 10.1016/j.chemosphere.2013.10.071. [DOI] [PubMed] [Google Scholar]
- 53.Lu H, et al. Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res. 2012;46:854–862. doi: 10.1016/j.watres.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 54.Sohi, S. P., Krull, E., Lopez-Capel, E. & Bol, R. Chapter 2-a review of biochar and its use and 325 function in soil. Adv. Agron. 47–82 (2010).
- 55.Wu J, Wang T, Wang J, Zhang Y, Pan WP. A novel modified method for the efficient removal of Pb and Cd from wastewater by biochar: Enhanced the ion exchange and precipitation capacity. Sci. Total. Environ. 2021;754:142150. doi: 10.1016/j.scitotenv.2020.142150. [DOI] [PubMed] [Google Scholar]
- 56.Gao LY, et al. Relative distribution of Cd(2+) adsorption mechanisms on biochars derived from rice straw and sewage sludge. Bioresour. Technol. 2019;272:114–122. doi: 10.1016/j.biortech.2018.09.138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.