Highlights
-
•
Multifrequency UAT significantly reduced the thawing time of frozen samples.
-
•
Multifrequency UAT could convert more acoustic energy into thermal energy.
-
•
Multifrequency UAT treated samples maintained better quality attributes.
-
•
Multifrequency UAT improved the physicochemical properties and structural characteristics.
Keywords: Multifrequency, Ultrasound assisted thawing, TVB-N, Large yellow croaker
Abstract
The effects of mono-, dual- and tri-frequency ultrasound-assisted thawing (UAT) on the physicochemical quality, water-holding capacity, moisture migration and distribution and myofibrillary structure of frozen large yellow croaker (Larimichthys crocea) were detected. The results indicated that multifrequency UAT treatment significantly increased the thawing rate, maintained the stability of myofibrils and reduced the lipid oxidation. The multifrequency UAT samples had better water-holding capacity (higher water-holding capacity values, lower thawing loss and cooking loss) and physicochemical quality (higher hardness, springiness, resilience, chewiness and lower total volatile basic nitrogen (TVB-N) values, thiobarbituric acid reactive substances (TBARS) values), higher immobilized water content, and lower free water content. Therefore, the results provide a further understanding of the quality stability of frozen large yellow croaker treated by the multifrequency UAT.
1. Introduction
Large yellow croaker (Larimichthys crocea) is a kind of marine fish with important economic significance that has been cultured on a large scale on the southeast coast of China [1]. Fresh large yellow croaker is rich in nutrients such as proteins and lipids, but it is also associated with enzymes and many microorganisms. Due to protein degradation, lipid oxidation and decomposition, and microbial contamination, the shelf life of large yellow croaker during cold storage is very short [2]. Frozen preservation is extensively used for maintaining the quality of large yellow croaker, which can effectively hinder the activities of microorganisms and reduce the biological activities of enzymes, thus prolonging the shelf life of large yellow croaker. During the freezing process, the formation of ice crystals may damage the food microstructure and the changes in the structure of proteins after thawing [3], [4]. The thawing of frozen fish has always been a concern in the field of frozen food [5]. Therefore, it is necessary to select effective thawing methods to maintain the quality of frozen aquatic products.
In recent years, there have been many emerging thawing techniques used to improve the food quality after freezing, such as high pressure-assisted thawing, microwave thawing, ultrasound-assisted thawing (UAT), and high voltage electrostatic field-assisted thawing [6]. It is worth noting that the application of ultrasound in food storage and processing has been increasingly extensive, such as freezing, thawing, microbial inactivation and extraction [7]. UAT is an increasingly used method in food preservation. Sun et al. [8] investigated the effects of UAT of different powers on common carp (Cyprinus carpio) and showed that the most appropriate power (300 W) could accelerate the thawing process of frozen common carp and improve its muscle quality after thawing. The phenomenon of cavitation is the main physical mechanism of ultrasound working in liquid media. Cavitation is due to the asymmetric inclusion of bubbles formed by sound waves in a series of compression and decompression processes [9]. When this collapse occurs close to a solid surface, it creates microjets with defined pressure (100 MPa), temperature (5000 K), and velocity (400 km/s) characteristics, which increases the mass transfer rate [7]. In addition, ultrasound can also cause micro-stirring in the liquid, which can alter mass transfer. Moreover, the physical effect caused by ultrasound can convert sound energy into heat energy, improve the rate of heat transfer in the thawing process, facilitate the thawing process and significantly improve the efficiency of thawing [10]. The generation and intensity of ultrasonic cavitation are mainly affected by sound frequency and power, in which ultrasonic frequency plays a decisive role in the efficiency of ultrasonic chemical reactions [11]. However, high-power single-frequency ultrasound may damage muscle structure [12]. Therefore, the current research expored the application of multifrequency ultrasound. Ma et al. [13] found that the multifrequency ultrasonic treatment produced more cavitation nuclei, enhanced the cavitation effect, and could better retain the quality attributes.
In conclusion, the effect of multifrequency UAT on the quality and structure of large yellow croaker remains worth studying. Therefore, this research aimed to study the effects of UAT at different frequencies on the water migration, quality and myofibrillar protein properties of large yellow croaker after thawing under specific ultrasonic power.
2. Material and methods
2.1. Pretreatment of large yellow croaker samples
Fresh large yellow croakers (500 ± 20 g in weight, 30 ± 5 cm in length) were acquired from a local seafood market in Pudong New Area, Shanghai. The samples were packed using foam boxes with modest amounts of ice and were then taken back to the laboratory within 30 min.
The gills and viscera of the samples were discarded, and the samples were then rinsed with deionized water and packaged separately in polyethylene bags. Then, the packaged samples were rapidly frozen until the central temperature reached −18 °C using a screw freezing machine at −40 °C. The whole fish samples were frozen at −18 ± 1 °C for 7 days before the thawing experiment.
2.2. Different thawing treatments
The frozen samples were thawed in different ways, including air thawing (AT), flowing water thawing (FWT), single-frequency UAT at 20 kHz (SUAT), dual-frequency UAT at 20 and 28 kHz (DUAT), and tri-frequency UAT at 20, 28 and 40 kHz (TUAT). The specific thawing processes are as follows.
2.2.1. Air thawing (AT)
The frozen fish were placed flat on glass plates and thawed in a box with a constant temperature and humidity of 4 ± 1 °C until the central temperature of large yellow croakers reached approximately 4 ± 1 °C.
2.2.2. Flowing water thawing (FWT)
The frozen fish were thawed in a water thawing device where the water temperature was kept at 20 ± 1 °C until the central temperature of large yellow croakers reached approximately 4 ± 1 °C.
2.2.3. Ultrasound assisted thawing (UAT)
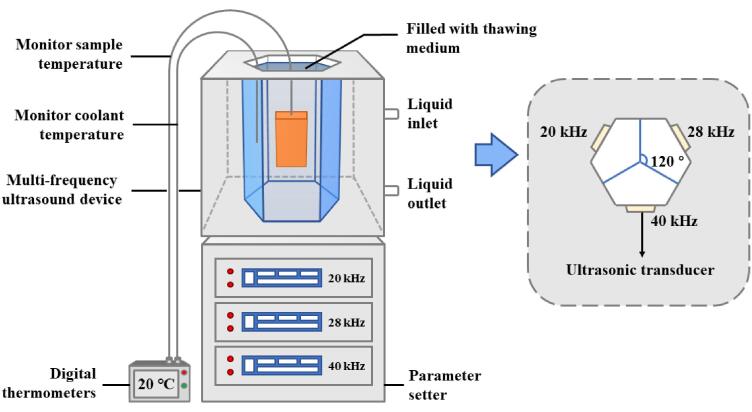
The frozen fish were thawed in a multifrequency ultrasonic assisted thawing (UAT) instrument (Fig. 1). The multifrequency UAT instrument was composed of a hexahedral ultrasonic processing system with a separate control panel and three ultrasound transducers. Water at 20 ± 1 °C was used as the propagation medium. The UAT parameters were set as follows: the ultrasonic power density was 0.9 W/L, single-frequency UAT at 20 kHz (SUAT), dual-frequency UAT at 20 and 28 kHz (DUAT), and tri-frequency UAT at 20, 28 and 40 kHz (TUAT). In addition, multifrequency UAT treatments were the simultaneous work of corresponding ultrasonic generators. The thawing process was completed when the central temperature of the samples had increased to 4 ± 1 °C.
Fig. 1.
Schematic diagram of multifrequency ultrasonic assisted thawing (UAT) system.
2.3. Thawing curve
The temperature of the samples was measured by a networked multipoint temperature collector (Fluke 2640A, Fluke Electronic Instrument Co., Ltd, USA), which records the temperature by a T-type thermocouple in real time. The thawing process from −18 °C to 4 °C was measured. The plotted thawing curves were recorded in time and temperature.
2.4. Thawing loss
Thawing loss was measured by the approach of Chevalier et al. [14]. The large yellow croaker samples were weighed before freezing (W0, g) and then thawed by different methods. The samples were wiped using filter paper, and the weight of the samples (W1, g) was accurately measured. The formula is as follows:
2.5. Cooking loss
Cooking loss was measured referring to the approach of Ma et al. [13]. The samples were cut into small pieces (approximately 5 g) after thawing and packed individually into polyethylene bags. The samples were put in the water at 80 °C and heated for 15 min. Then, the samples were wiped using filter paper. The sample was accurately weighed before and after cooking, and the weights were denoted as W0 and W1, respectively. The formula is as follows:
2.6. Water-holding capacity (WHC) determination
After thawing, the samples were sliced into small cubes (approximately 2 g) and placed in centrifuge tubes with filter paper that absorbed water during centrifugation. Samples were centrifuged at 5000×g for 10 min, and the temperature was maintained at 4 °C during the centrifugation process. The WHC value was represented by centrifugal loss. In addition, the calculation formula was as follows. The samples were accurately weighed before and after centrifugation, and the weights were denoted as W0 and W1, respectively.
2.7. pH value
Twenty millilitres of deionized water was homogenized with 2.0 g of minced flesh. The mixture obtained by homogenization was centrifuged at 3, 040 × g, and the temperature was maintained at 4 °C during the centrifugation process. After 15 min of centrifugation, a pH meter (PB-10, Sartorius, Germany) was used to measure the pH value of the samples.
2.8. Total volatile basic nitrogen (TVB-N)
TVB-N determination was performed with a Kjeldahl apparatus (Kjeltec8400, Foss, Hilleroed, Denmark). The microtitration method was used to detect the TVB-N value [15], and mg N/100 g was used to express the TVB-N value of large yellow croaker muscle.
2.9. Colour
A colorimeter (CR-400, Konica Minolta, Tokyo, Japan) was used to measure the colour of the thawed samples. Parameters such as L* (lightness), a* (the red-green degree) and b* (the yellow-blue degree) were detected. In addition, the total color difference (ΔE) was calculated using the following formula.
2.10. TPA
TPA was detected using the TA. XT Plus texture analyser (Stable Micro Systems, Ltd., Godalming, Surrey, UK) with a P/5 probe, and thawed samples of large yellow croaker were sliced into 2.0 × 1.5 × 1.5 cm sections. The sample deformation was 50%, and the detection speed was 1.0 m/s [16]. Each sample was tested for a minimum of six points.
2.11. Water distribution and migration
The thawed samples of large yellow croaker were cut into 2.0 × 1.5 × 1.5 cm pieces and then wrapped using polyethylene films. The measurements of transverse relaxation T2 were detected using an LF-NMR analyser (Niumag MesoMR23-060H. I, Suzhou, China). The frequency of proton resonance was 21 MHz, which corresponds to the pulse sequence of Carr-Purcell-MeiboomGill [17]. Each measurement included 16 scans and 3000 echoes. MRI were performed to obtain pseudocolour images of proton density weighted of large yellow croaker.
2.12. Determination of thiobarbituric acid reactive substances (TBARS)
TBARS was determined according to the approach of Li et al. [18]. Twenty millilitres of 20% TBA solution was homogenized evenly with 5.0 g of flesh and allowed to rest for 60 min at room temperature. The mixture obtained by homogenization was centrifuged at 11, 960×g, and the temperature was maintained at 4 °C during the centrifugation process. After 15 min of centrifugation, 5 mL of TBA solution (0.02 M) was mixed with 5 mL of supernatant. The mixture obtained after the above steps was put in water at 100 °C, heated for 40 min, and then cooled to room temperature. Finally, a spectrophotometer (Evolution 220, Thermo Fisher Scientific, MA, USA) was used to measure the absorbance at 532 nm. The value of TBARS was represented by the MDA content. It was calculated by the following formula and expressed as mg MDA/kg. The absorbance of the sample and the standard was denoted by A1 and A0, respectively, and the mass fraction was ω.
2.13. Free amino acids (FAAs)
FAAs were determined according to the approach of Zhou et al. [19]. Five grams of mashed large yellow croaker flesh supplemented with 15 mL of 15% TBA solution was homogenized and incubated for 60 min at 4 °C. The mixture was then centrifuged at 5,980×g, and the temperature was maintained at 4 °C during the centrifugation process. After 15 min of centrifugation, 5 mL of supernatant was diluted to 25 mL using deionized water. Finally, the mixture obtained after the above steps was filtered using a 0.22-µm filter and analysed using an ultrahigh-speed automatic amino acid analyser (Hitachi LA-8080, Tokyo, Japan).
2.14. Extraction of myofibrillar protein
Myofibril solution was obtained referring to the approach of Li et al. [20] with little modification. 20 mL of precooled Tris-buffer A (0.05 M sodium chloride) was homogenized with 2 g of flesh and then centrifuged at 11, 960 × g for 15 min, and the temperature was maintained at 4 °C during the centrifugation process. After discarding the supernatant, the above steps were repeated. The precipitate was then collected, and 20 mL of precooled Tris-buffer B (0.6 M sodium chloride) was added and stirred. After stirring for 60 min, the mixture was centrifuged at 11, 960 × g at 4 °C for 15 min. Then the supernatant was collected as myofibril solution.
2.15. Secondary structures of myofibrillar protein
The secondary structures of myofibrillar protein were determined by Fourier infrared spectrometer (Nicolet IS50, Thermo Scientific Inc., Waltham, MA, USA). The freeze-dried samples were ground using potassium bromide powder. The Gaussian curve fitting in Origin 2018 (OriginLab, Northampton, MA, USA) was used to deconvolute the spectra in the range from 1600 and 1700 cm−1, which represent the amide I region. The varied types of secondary structures were detected by dividing the peak area by the total area of the amide I area after deconvolution.
2.16. Tertiary structure of myofibrillar protein
A fluorescence spectrophotometer (F-7100, Hitachi, Tokyo, Japan) in the mode of emission scan was used to detect the tertiary structure of myofibrillar protein. The imaging parameters were set as follows: scan speed was 1200 nm/min, emission wavelength was 305–410 nm, excitation wavelength was 295 nm and slit width was 5 nm.
2.17. Statistical analysis
One-way analysis of variance (ANOVA) followed by Duncan's test using SPSS 23.0 was used for multiple comparisons, and the experimental results were reported as the mean ± standard deviation.
3. Results and discussion
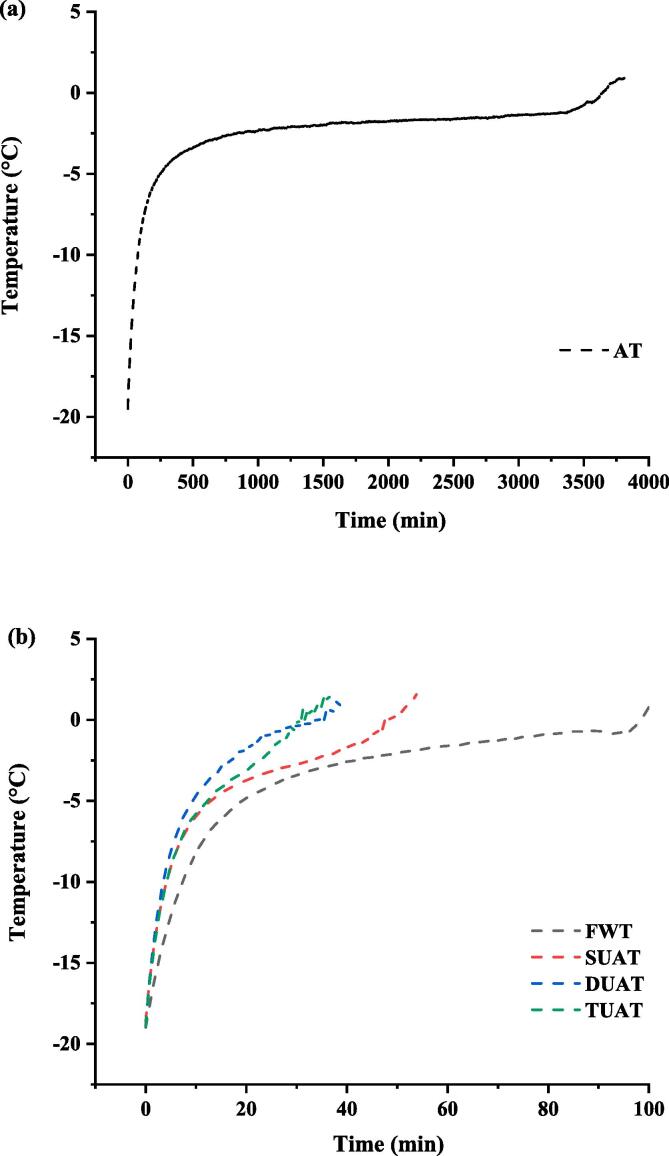
3.1. Thawing curve
The thawing curve is used to describe the change rate of temperature at the centre of thawed samples to express the thawing rate and the different stages of the thawing process. The thawing process can be divided into three parts: a heat absorption stage (−18 to −5 °C), a phase transformation stage (−5 to 0 °C) and an ice crystal melting stage (0–4 °C) [21]. The thawing time of UAT samples was an hour faster than that of FWT samples and only 1.25% of the time for AT samples (Fig. 2). The thawing rates of the three UAT samples were significantly faster than those of the AT and FWT samples, which was mainly due to the cavitation of ultrasound accelerating the melting of ice crystals [22]. In addition, ultrasound can enhance the transformation of heat during the thawing process. Gambuteanu et al. [23] deduced that increasing the water temperature and asymmetric bubble collapse could be effective for heat generation due to the physical effects of ultrasonic and high-speed jets. Among the UAT samples, the thawing times of the DUAT and TUAT samples were approximately 20 min shorter than that of the SUAT samples. The multifrequency UAT generated more bubbles to transfer more heat as they burst and reduced the time of thawing [12].
Fig. 2.
Thawing curves of frozen large yellow croaker with different thawing treatments. Fig. 1(a): Air thawing (AT). Fig. 1(b): Flowing water thawing (WT), the single frequency of UAT (SUAT), the dual-ultrasound frequencies of UAT (DUAT), the triple-ultrasound frequencies of UAT (TUAT). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
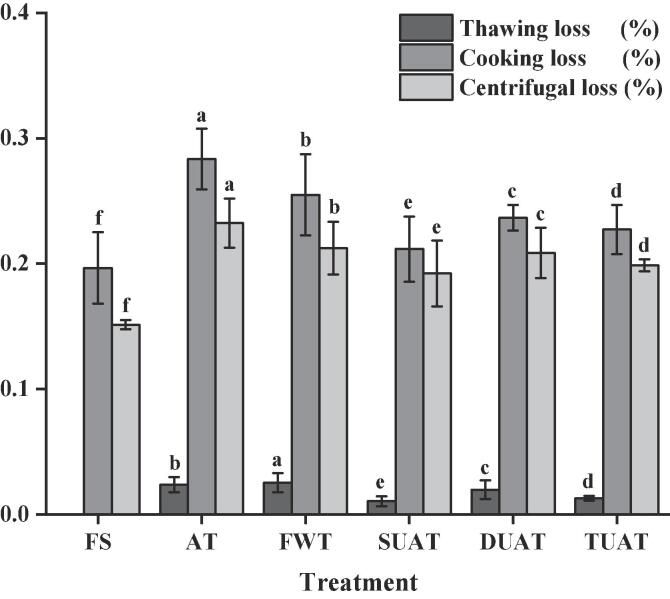
3.2. Thawing loss, cooking loss and WHC
The thawing loss, cooking loss and WHC of the large yellow croakers can reflect the water loss in the thawed fish tissue and the stability of the remaining water. UAT samples had lower thawing losses than FWT and AT samples (Fig. 3). The thawing loss of the DUAT samples was 0.91%, which was less than that of the SUAT and TUAT samples. During the thawing process, the damage to fish muscle and the degradation of protein are the major factors that cause the water loss of large yellow croakers after thawing. Ultrasound has been shown to improve myosin structural properties, convert the alpha-sheet structure to a looser beta-sheet structure and increase sulfhydryl group content, which is expected to reduce dense aggregation of muscle proteins and improve water retention [21]. However, ultrasound treatment can also cause some physical damage to the muscle tissue of large yellow croaker, resulting in a decrease in WHC. Thus, it was critical to select a suitable frequency and power when using UAT to thaw the frozen samples. Gambuteanu et al. [23] found that appropriate UAT power could reduce the thawing losses of pork longissimus dorsi muscle, which was similar to the findings of this study. The WHC of fresh samples was significantly higher than that of the frozen samples, which revealed that the freezing and thawing process caused some damage to the muscle structure of the samples (p < 0.05). In addition, the WHC of samples was strongly influenced by structural changes in myofibrillar proteins, especially myosin [24]. Ma et al. [13] pointed out that the ultrasound treatment had an obvious effect on the increase in WHC, possibly due to modification or mechanical destruction of the native structure of the protein, which led to more active groups becoming exposed, such as sulfhydryl and hydrophobic groups.
Fig. 3.
The changes of thawing loss, cooking loss and water holding capacity (centrifugal loss) of frozen large yellow croaker with different thawing treatments which include fresh sample (FS), air thawing (AT), flowing water thawing (FWT), the single frequency of UAT (SUAT), the dual-ultrasound frequencies of UAT (DUAT), the triple-ultrasound frequencies of UAT (TUAT). The letter from “a” to “f” are used to describe the significance of differences between the samples (p < 0.05).
There was a large loss of liquid and a small loss of soluble material in the sample, which was mainly due to the degeneration of myofibrillar protein causing damage to the muscle structure [25]. As shown in Fig. 3, the cooking losses of the AT and FWT samples were significantly higher than that of the UAT samples (p < 0.05). The cooking losses of SUAT, DUAT and TUAT were reduced by 7.19%, 4.69% and 5.62% compared to AT samples and 2.01%, 0.37% and 1.36% compared to FWT samples, meaning that UAT samples have less damage to the muscle structure and protein degradation. Li et al. [26] researched the effects of Peruvian squid thawed using different methods and also found that WHC had a similar change trend to the cooking loss.
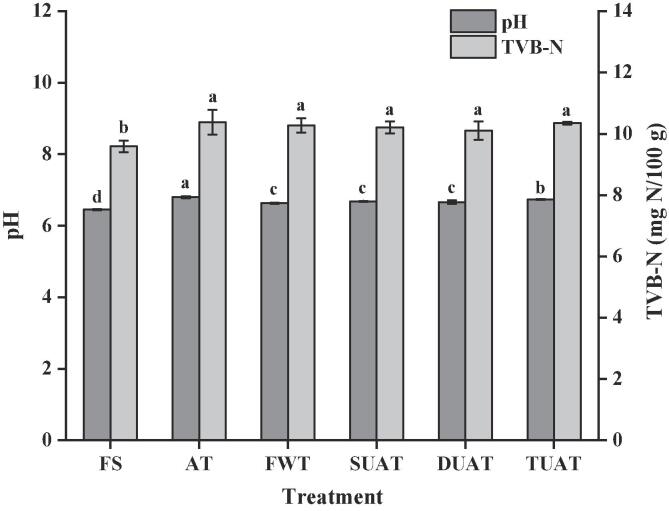
3.3. pH and TVB-N
TVB-N is often used to describe the degree of degradation of proteins and amines [27]. Enzyme-induced spoilage during the freezing process leads to the degradation of proteins and other nitrogen-containing compounds, resulting in the accumulation of organic amines, as shown by an increase in the value of TVB-N [28]. The TVB-N value of FS was 9.59 mg N/100 g and increased by 0.79, 0.69, 0.62, 0.51 and 0.76% for the AT, FWT, MUAT, DUAT and TUAT samples, respectively (Fig. 4). All samples had higher TVB-N values after freezing and thawing due to a mass of basic volatile compounds produced by the degradation of protein and nonprotein nitrogen compounds including ammonia, trimethylamine, methylamine, and dimethylamine [25]. The lower the TVB-N value is, the less tyrosine and methionine are destroyed in the protein, which means that the nutrients are better protected [29]. Therefore, these results indicated that the UAT samples showed less protein degradation and less nutritional loss than the AT and FWT samples.
Fig. 4.
The difference of pH and TVB-N of frozen large yellow croaker with different thawing treatments which include fresh sample (FS), air thawing (AT), flowing water thawing (FWT), the single frequency of UAT (SUAT), the dual-ultrasound frequencies of UAT (DUAT), the triple-ultrasound frequencies of UAT (TUAT). The letter from “a” to “d” are used to describe the significance of differences between the samples (p < 0.05).
Similarly, the pH value revealed the same trend as TVB-N, and no significant fluctuation was found during the freezing and thawing process. The pH value increased by 0.35, 0.18, 0.23, 0.21 and 0.28 for the AT, FWT, MUAT, DUAT and TUAT samples, respectively (Fig. 4). The increase in the pH of thawed fish might be due to conformational changes associated with denaturation of proteins by ultrasonic treatment and proteolysis of muscle fibres and the breakdown of chemical bonds involving imidazole, hydroxyl and sulfhydryl groups [23]. Cai et al. [29] detected the pH value of frozen largemouth bass (Micropterus salmoides) and came to the same conclusion; that is, there was no significant difference in pH value among different thawing treatments.
The generation of a considerable number of basic volatile compounds caused increases in not only the TVB-N value but also the pH value. Although the differences were not significant (P > 0.05), the multifrequency UAT samples had slightly lower pH and TVB-N values than the other samples, as the multifrequency ultrasound treatment might produce less damage to the integrity of muscle and the protein stability.
3.4. Colour
Colour is the first intuitive judgement of samples for consumers that affects people’s desire to buy [30]. The colour of the samples was analysed by detecting the parameters L* (lightness), a* (the red–green degree) and b* (the yellow–blue degree), and the total colour difference (ΔE) was also calculated (Table 1). Compared with FS samples, the L* of AT, FWT, SUAT, DUAT and TUAT samples all showed slight changes, but there was no significant difference between them. The change in L* might be due to the formation of ice crystals during freezing and water loss caused by the melting of ice crystals during thawing. The ice crystals were developed in the gaps between the muscle during the freezing process, and they melted as the samples were thawed. The light scattering caused by ice crystals might be the reason why the L* value changed [31]. In addition, during the thawing process, the melting rate of ice crystals affected the migration of water, and the distribution of water on the sample surface was also different, causing the change in L* [31]. Lan et al. [32] found that the L* value increased after thawing in their frozen pompano thawing research. In addition, the a* and b* values of UAT samples all increased after thawing. Lan et al. [32] also made a similar conclusion that the a* and b* values of samples undergoing UAT treatment were higher than those of fresh samples when studying the differences in the five thawing methods on frozen pompano. ΔE represents the magnitude of the total chromatic aberration, perceptible to the naked eye on a scale from 2 to 10 [33]. In this study, the ΔE values of the AT, FWT, SUAT, DUAT and TUAT samples were all greater than 5. The colour change of the samples after thawing can be detected by the naked eye, but there was no significant difference between the five thawing groups. Thus, it can be concluded that the multifrequency UAT causes no significant improvement in the colour of large yellow croaker.
Table 1.
The color results of frozen large yellow croaker under different thawing methods.
| Treatment | L* | a* | b* | △E |
|---|---|---|---|---|
| FS | 44.20 ± 3.92ab | −3.02 ± 0.35d | −2.24 ± 2.16ab | — |
| AT | 47.63 ± 1.33a | −0.44 ± 0.43c | −2.33 ± 1.12ab | 5.07 ± 1.98a |
| FWT | 43.09 ± 1.79b | 0.25 ± 0.10bc | −3.46 ± 0.03b | 4.64 ± 1.52a |
| SUAT | 46.40 ± 1.85ab | −0.25 ± 0.18c | −0.64 ± 0.86a | 4.46 ± 1.66a |
| DUAT | 43.09 ± 2.57b | 1.18 ± 0.91a | −1.16 ± 0.85ab | 5.02 ± 1.15a |
| TUAT | 42.45 ± 0.60b | 0.92 ± 0.19ab | −1.31 ± 1.24ab | 5.52 ± 1.43a |
The methods include fresh sample (FS), air thawing (AT), flowing water thawing (FWT), the single frequency of UAT (SUAT), the dual-ultrasound frequencies of UAT (DUAT), the triple-ultrasound frequencies of UAT (TUAT). The letter from “a” to “d” are used to describe the significance of differences between the samples (p < 0.05).
3.5. TPA
Texture is a critical index to evaluate the quality of aquatic products and can significantly affect the sensory characteristics of aquatic products [34]. All the TPA parameters were decreased after thawing (Table 2). Compared with the FS samples, the hardness levels of the AT, FWT, SUAT, DUAT and TUAT samples were reduced by 33.61%, 27.36%, 20.31%, 14.37% and 10.03%, respectively. It may be concluded that the denaturation of myofibrillar proteins such as aggregation or hydrolysis, possibly had a heavy effect on the fish texture during the thawing process [35]. There were significant differences in hardness among the different thawing samples, which might depend on the size and distribution of ice crystals produced during different thawing processes, as the crystals could cause heavy damage to the muscle tissue [36]. Hu et al. [37] also indicated that freezing and thawing processes could cause changes in texture when investigating the effect of liquid nitrogen freezing on long-term frozen hairtails. It is worth mentioning that multifrequency UAT samples had higher hardness values and were closer to FS samples than the other samples. It could be concluded that the multifrequency UAT samples had a better texture than the AT, FWT and SUAT samples, which was relatively close to fresh fish.
Table 2.
The TPA results of frozen large yellow croaker under different thawing methods.
| Treatment | Hardness | Springiness | Resilience | Chewiness |
|---|---|---|---|---|
| FS | 3091.02 ± 71.39a | 0.64 ± 0.02a | 0.20 ± 0.01a | 816.91 ± 95.30a |
| AT | 2052.22 ± 23.06f | 0.38 ± 0.01d | 0.08 ± 0.01e | 203.16 ± 64.29d |
| FWT | 2245.29 ± 46.44e | 0.44 ± 0.02c | 0.10 ± 0.01d | 285.12 ± 5.06 cd |
| SUAT | 2463.26 ± 52.75d | 0.45 ± 0.02c | 0.11 ± 0.01d | 312.17 ± 11.48c |
| DUAT | 2646.79 ± 16.33c | 0.49 ± 0.01b | 0.13 ± 0.01c | 364.87 ± 32.82bc |
| TUAT | 2780.97 ± 25.63b | 0.51 ± 0.02b | 0.15 ± 0.01b | 421.83 ± 5.12b |
The methods include fresh sample (FS), air thawing (AT), flowing water thawing (FWT), the single frequency of UAT (SUAT), the dual-ultrasound frequencies of UAT (DUAT), the triple-ultrasound frequencies of UAT (TUAT). The letter from “a” to “d” are used to describe the significance of differences between the samples (p < 0.05).
For springiness, the values of the AT, FWT, SUAT, DUAT and TUAT samples were 0.26, 0.20, 0.19, 0.15 and 0.13 less than that of the FS samples, respectively. Meanwhile, the resilience levels of the AT, FWT, SUAT, DUAT and TUAT samples were reduced by 0.12, 0.10, 0.09, 0.07 and 0.05, while the chewiness values were decreased by 75.13%, 65.10%, 61.79%, 55.34% and 48.36%, respectively. The reductions in springiness, resilience, and chewiness of the different thawed samples could be the result of water loss and protein aggregation caused by ice crystal growth and recrystallization during the freezing and thawing process [38]. Among the five different thawing treatments, the texture characteristics of the AT samples were most different from those of the FS samples. There was no significant difference in texture between the FWT and SUAT samples after thawing (p < 0.05), while the DUAT and TUAT treatments maintained the original elastic properties of the fillets. The above results indicated that multifrequency UAT treatment could lead to the preservation of the textural properties. This suggested that multifrequency UAT was better able to maintain the quality of fish after thawing.
3.6. Water distribution and migration
The water distribution and migration of samples were measured by LF-NMR transverse relaxation. The relaxation time reflects the state of hydrogen protons in fish samples, which is related to the degree of freedom and the binding force of hydrogen protons [39] and directly reflects the distribution of water molecules. Generally, there were three different types of water distributed in the fish muscles, including bound water, immobilized water, and free water, according to relaxation [24]. The T2 relaxation method was used to detect the proton relaxation behaviour, and pT21, pT22 and pT23 were used to represent bound water, immobilized water and free water, respectively [40]. After thawing, water migration was bound to occur because there was little disruption of the fibre structure with freezing and thawing treatment [29]. There was no obvious change in the proportions of bound water and free water of fresh/thawed samples, as indicated by pT21 and pT23 (Table 3). Protein molecules were combined with bound water and were not evidently affected by changes in temperature or mechanical stress. For the value of pT22, the values of DUAT and TUAT samples werer only reduced by 0.12 and 0.13 than that of FS samples, and the contents of immobilized water in DUAT and TUAT samples were statistically close to that of FS samples. However, the values of the AT, FWT and SUAT samples were less than those of the FS samples by 1.05, 0.77 and 0.66, respectively, and were significantly different from those of the FS samples (p < 0.05). The results illustrated that there was minimal damage to water mobility during multifrequency UAT processes. Among them, the moisture mobility of the AT samples was the highest, indicating that it caused a great damage to the fibre structure. This conclusion was consistent with the results of WHC.
Table 3.
Changes in water distribution in different thawing methods.
| Treatment | pT21/% | pT22/% | pT23/% |
|---|---|---|---|
| FS | 2.57 ± 0.06b | 95.24 ± 0.11a | 2.19 ± 0.06c |
| AT | 3.14 ± 0.12a | 94.19 ± 0.09c | 2.69 ± 0.04a |
| FWT | 2.98 ± 0.16a | 94.47 ± 0.21bc | 2.56 ± 0.05b |
| SUAT | 2.93 ± 0.01a | 94.58 ± 0.08b | 2.50 ± 0.07b |
| DUAT | 2.62 ± 0.07b | 95.12 ± 0.12a | 2.27 ± 0.06c |
| TUAT | 2.61 ± 0.08b | 95.11 ± 0.12a | 2.29 ± 0.04c |
The methods include fresh sample (FS), air thawing (AT), flowing water thawing (FWT), the single frequency of UAT (SUAT), the dual-ultrasound frequencies of UAT (DUAT), the triple-ultrasound frequencies of UAT (TUAT). The letter from “a” to “c” are used to describe the significance of differences between the samples (p < 0.05).
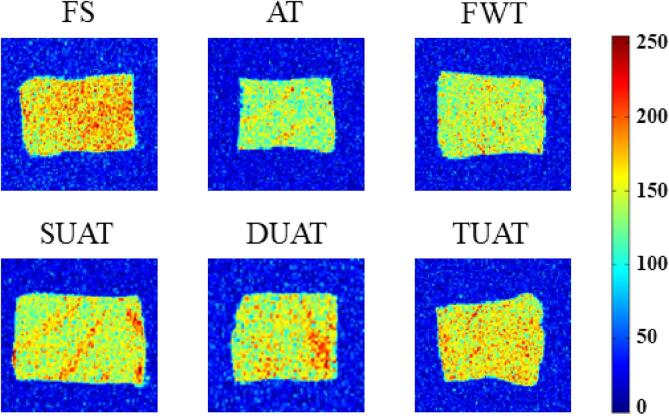
MRI was used as an intuitive and effective method to observe the internal water migration of the samples under different thawing treatments (Fig. 5). In the MRI results, the redness in the figure represents a high proton density region, and the blueness represents a low proton density region [41]. Because of the degradation of fish muscle during the freezing and thawing process, the MRI results in the thawed samples all became bluer and darker than that of the FS samples, indicating that the moisture contents in the samples were reduced. Obviously, the DUAT and TUAT samples were lighter than the other samples, which demonstrated that the figure showed a similar conclusion as the result of pT22.
Fig. 5.
The difference of magnetic resonance imaging (MRI) of frozen large yellow croaker with different thawing treatments which include fresh sample (FS), air thawing (AT), flowing water thawing (FWT), the single frequency of UAT (SUAT), the dual-ultrasound frequencies of UAT (DUAT), the triple-ultrasound frequencies of UAT (TUAT). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.7. TBARS
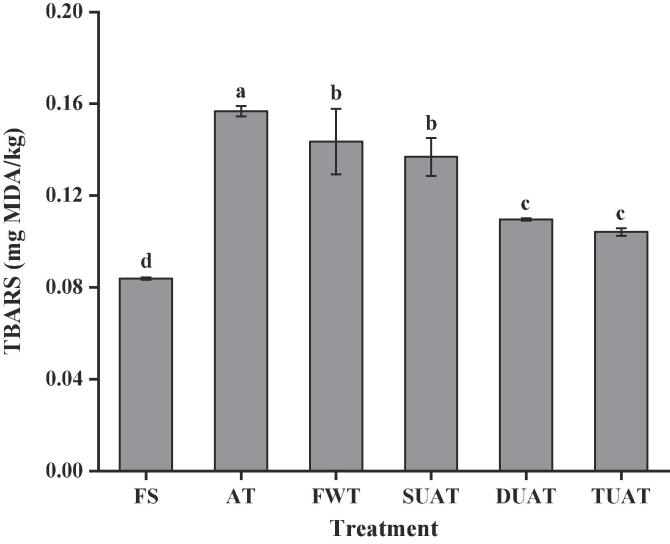
Lipid oxidation caused by the autoxidation of polyunsaturated fats is the main cause of frozen food spoilage [42]. Lipid oxidation leads to the formation of toxic compounds, which include the accumulation of volatile carbonyls, alcohols, and acids, as well as malondialdehyde and cholesterol oxidation products. Lipid oxidation not only produces undesirable odours and leads to rancidity, but may also produce potential precursors or catalysts for the formation of reactive oxygen species, which can lead to further food spoilage [43]. The content of TBARS in the FS samples was the lowest (Fig. 6). The TBARS values of the AT, FWT, SUAT, DUAT and TUAT samples had already increased by 0.07, 0.06, 0.05, 0.03 and 0.02 compared to those of the FS samples, meaning that all thawed samples produced different degrees of lipid oxidation after freezing and thawing, as well as an increased content of free fatty acids, which could also cause the acceleration of lipid oxidation [44]. As shown in Fig. 6, the samples under multifrequency UAT treatment had a significantly lower TBARS values than other samples (p < 0.05). Compared with the other groups, the samples thawed by multifrequency UAT experienced less muscle damage, which caused less lipid oxidation. In addition, the longer the thawing time, the more complete the lipid oxidation can become. Thus, the highest TBARS value was obtained in the AT sample. This was the same as the conclusion of Li et al. [45] that air thawing produced a sufficient oxidation reaction in the long thawing process when thawing bighead carp fillets. It could be concluded that the multifrequency UAT could reduce the damage to thawed fish and better improve the quality of fishes.
Fig. 6.
The difference of TBARS of frozen large yellow croaker with different thawing treatments which include fresh sample (FS), air thawing (AT), flowing water thawing (FWT), the single frequency of UAT (SUAT), the dual-ultrasound frequencies of UAT (DUAT), the triple-ultrasound frequencies of UAT (TUAT). The letter from “a” to “d” are used to describe the significance of differences between the samples (p < 0.05).
3.8. FAAs
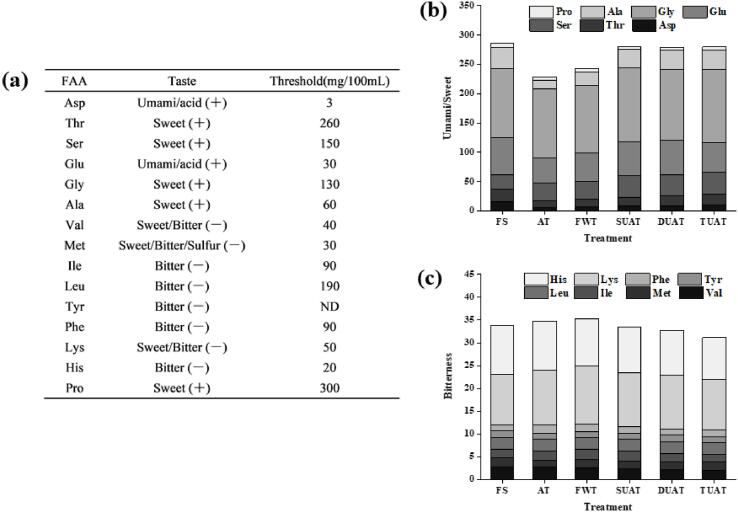
After freezing and thawing, the total content of FAAs had different degrees of reduction which might be because the protein structures were destroyed (Table 4). Whether an amino acid is exposed to oxidation depends largely on its position in the chain. Certain amino acids are easily converted to carbonyl derivatives and are more susceptible to reactive oxygen species [46]. FAAs can impart different flavours, such as flavour, sweetness and bitterness, and are thus important contributors to the development of flavour in aquatic products [47]. The taste of amino acids is closely related to the hydrophobicity of the R group of its side chain. When hydrophobic amino acids are small, such as glycine (Gly), alanine (Ala), serine (Ser), proline (Pro) and threonine (Thr), the imparted taste is mainly sweet; when hydrophobic amino acids are more abundant, such as leucine (Leu), isoleucine (Ile), glycine, phenylalanine (Phe), tyrosine (Tyr), valine (Val), methionine (Met), histidine (His), and lysine (Lys), the imparted taste is mainly bitter. When the R group of its side chain is acidic, such as aspartic acid (Asp) and glutamic acid (Glu), the imparted taste is mainly sour [48]. These two amino acids are also important preconditions for the formation of umami substances. As shown in Table 4, the total amount of FAAs has decreased after freezing and thawing which was probably related to the loss of water-soluble FAAs in the thawing process [49]. It was shown that the contents of Asp, Thr, Ser, Gly, Ala and Glu, which are related to the umami and sweet properties of the DUAT and TUAT samples, were significantly higher than those of the other samples (p < 0.05) (Fig. 7). Quick thawing could effectively reduce the thawing loss of fish [48], which was the main reason that the higher values of abovementioned FAAs were observed in the DUAT and TUAT samples. Thus, multifrequency UAT also played a role in preserving the flavour of the samples.
Table 4.
The FAAs results of frozen large yellow croaker under different thawing.
| Treatment | FAAs |
|||||||
|---|---|---|---|---|---|---|---|---|
| Asp | Thr | Ser | Glu | Gly | Ala | Val | Met | |
| FS | 16.21 ± 1.36a | 20.43 ± 0.80a | 25.59 ± 1.76c | 62.69 ± 2.61a | 117.07 ± 0.50bc | 37.25 ± 0.34a | 2.83 ± 0.53a | 1.89 ± 0.09a |
| AT | 5.52 ± 0.29d | 10.81 ± 0.22d | 31.41 ± 0.07b | 43.01 ± 0.65c | 117.87 ± 1.91bc | 14.34 ± 1.56d | 2.71 ± 0.10ab | 1.51 ± 0.56a |
| FWT | 6.99 ± 0.50 cd | 12.38 ± 0.33d | 30.22 ± 0.77b | 49.01 ± 2.53b | 115.80 ± 3.44c | 22.44 ± 4.14c | 2.50 ± 0.02abc | 1.91 ± 0.03a |
| SUAT | 8.01 ± 0.17c | 14.12 ± 1.38c | 37.33 ± 1.99a | 57.98 ± 1.55a | 127.24 ± 0.66a | 30.37 ± 0.89b | 2.29 ± 0.13abc | 1.75 ± 0.09a |
| DUAT | 8.49 ± 0.63bc | 17.14 ± 0.14b | 36.50 ± 0.44a | 58.67 ± 1.58a | 119.77 ± 0.19abc | 33.09 ± 0.88ab | 2.11 ± 0.05bc | 1.70 ± 0.17a |
| TUAT | 10.07 ± 0.28b | 17.83 ± 0.12b | 38.07 ± 0.35a | 50.25 ± 1.77b | 124.55 ± 6.17ab | 33.36 ± 1.76ab | 2.07 ± 0.20c | 1.70 ± 0.19a |
| Treatment | FAAs | |||||||
| Ile | Leu | Tyr | Phe | Lys | His | Pro | Total | |
| FS | 1.89 ± 0.15ab | 2.67 ± 0.11a | 1.43 ± 0.16a | 1.27 ± 0.06d | 11.12 ± 0.55b | 10.78 ± 0.04a | 6.23 ± 0.28a | 319.34 ± 4.26a |
| AT | 1.97 ± 0.23ab | 2.57 ± 0.07a | 1.39 ± 0.03a | 1.84 ± 0.04a | 12.03 ± 0.79ab | 10.70 ± 0.34ab | 6.06 ± 0.09ab | 263.74 ± 1.51c |
| FWT | 2.21 ± 0.24a | 2.60 ± 0.04a | 1.30 ± 0.28a | 1.73 ± 0.12ab | 12.74 ± 0.05a | 10.29 ± 0.29bc | 5.83 ± 0.06b | 277.95 ± 4.94b |
| SUAT | 2.14 ± 0.15ab | 2.60 ± 0.15a | 1.30 ± 0.16a | 1.57 ± 0.13bc | 11.80 ± 0.08dab | 9.92 ± 0.04c | 5.41 ± 0.03c | 313.83 ± 6.33a |
| DUAT | 1.93 ± 0.03ab | 2.62 ± 0.04a | 1.35 ± 0.15a | 1.36 ± 0.07 cd | 11.77 ± 0.06ab | 9.86 ± 0.01c | 5.27 ± 0.06c | 311.63 ± 2.30a |
| TUAT | 1.75 ± 0.14b | 2.59 ± 0.23a | 1.36 ± 0.23a | 1.34 ± 0.06d | 11.12 ± 0.37b | 9.20 ± 0.06d | 5.28 ± 0.04c | 310.55 ± 4.02a |
The methods include fresh sample (FS), air thawing (AT), flowing water thawing (FWT), the single frequency of UAT (SUAT), the dual-ultrasound frequencies of UAT (DUAT), the triple-ultrasound frequencies of UAT (TUAT). The letter from “a” to “c” are used to describe the significance of differences between the samples (p < 0.05).
Fig. 7.
The thresholds of different amino acids taste (a), and changes in the content of amino acids related to umami and sweet (b) and bitterness (c) of fresh fish group (FS), air thawing (AT), flowing water thawing (FWT), the single frequency of UAT (SUAT), the dual-ultrasound frequencies of UAT (DUAT), the triple-ultrasound frequencies of UAT (TUAT).
3.9. Secondary structure of protein
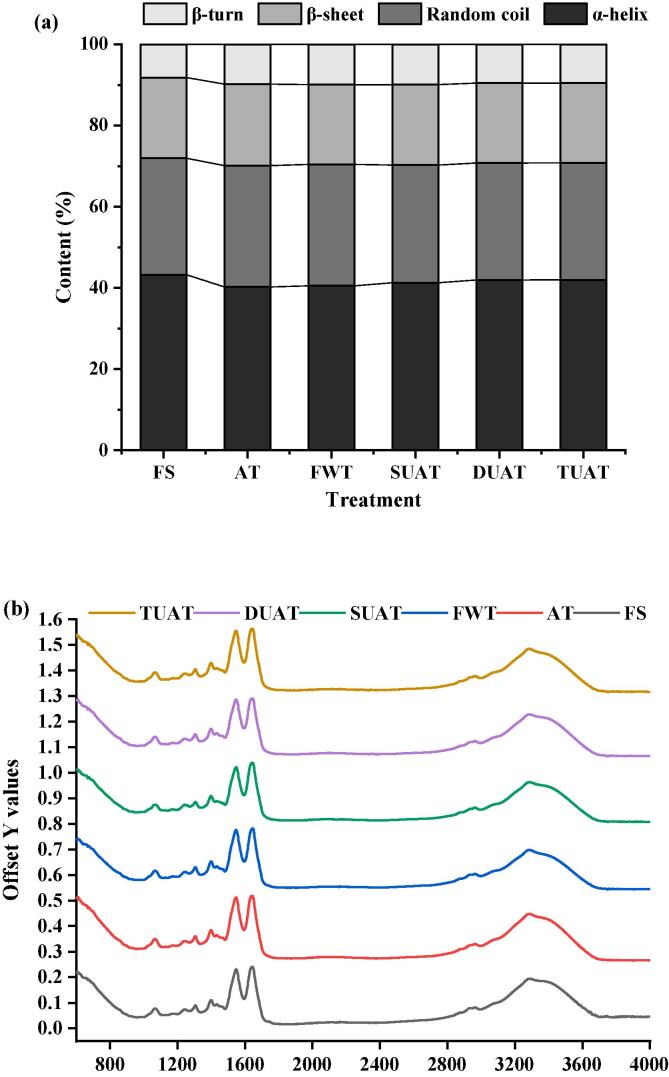
The secondary structure of MPs in different treatments mostly existed in the form of an α-helix. Compared with FS samples, the α-helix contents of MPs treated with AT, FWT, SUAT, DUAT, and TUAT decreased by 6.92%, 6.28%, 4.50%, 2.99% and 2.91%, while the contents of β-sheets increased by 19.68%, 20.88%, 20.81%, 16.39% and 16.59% (Fig. 8(a) and (b)). These changes occur because the spatial entanglement of protein molecules are altered under UAT, and part of the protein loses its structure to the point where it becomes soluble. When the protein residues are exposed to water molecules, new hydrogen bonds are formed, and the protein structures change from alpha-helical to beta-helical structures [50]. Cai et al. [51] similarly concluded that part of the α-helix structure of myofibrillar protein turned into β-sheets after thawing in a study of using multifrequency UAT treatment to thaw the small yellow croaker. Compared with the MPs obtained from the AT, FWT and SUAT samples, the MPs of the DUAT and TUAT samples had higher α-helix ratios, which were closest to that of the FS samples. It could be concluded that the multifrequency UAT could reduce the damage of thawing to MPs and better maintain the stability of fish protein.
Fig. 8.
The difference of secondary structure of protein of frozen large yellow croaker with different thawing treatments which include fresh sample (FS), air thawing (AT), flowing water thawing (FWT), the single frequency of UAT (SUAT), the dual-ultrasound frequencies of UAT (DUAT), the triple-ultrasound frequencies of UAT (TUAT). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
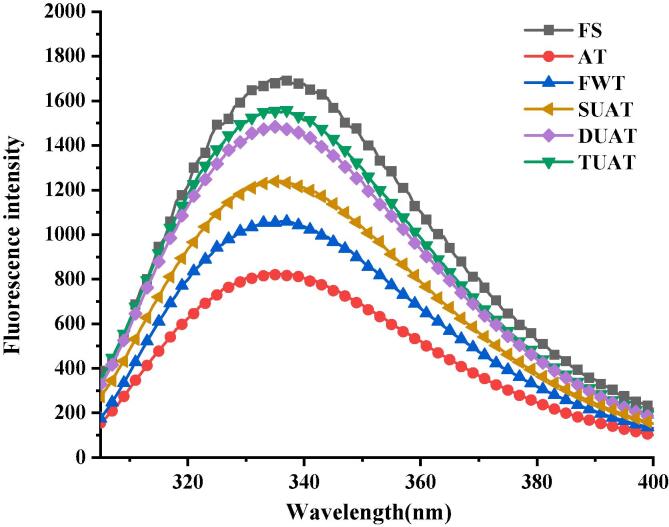
3.10. Tertiary structure of protein
The changes in the tertiary structure of proteins were reflected by the fluorescence spectra (Fig. 9). The fluorescence spectra of proteins usually reflects the contents of tryptophan, phenylalanine, and tyrosine residues in the protein [51]. The fluorescence intensities of the five different thawing samples had lower values than that of the fresh samples, which might be due to the aggregation and degradation of protein and the oxidation of tryptophan that occur after the freezing and thawing process [52]. Cai et al. [2] studied the effects of thawing red seabream (Pagrus major) fillets using ultrasound or microwave vacuum thawing methods and found that the fluorescence intensities of all frozen samples after different thawing treatments were lower than that of fresh samples. Among the five thawing methods, the fluorescence intensities of the DUAT and TUAT samples were relatively close to that of the fresh fish, which means that the UAT method seemed to cause slightly less damage to the protein.
Fig. 9.
The difference of protein tertiary structure of frozen large yellow croaker with different thawing treatments which include fresh sample (FS), air thawing (AT), flowing water thawing (FWT), the single frequency of UAT (SUAT), the dual-ultrasound frequencies of UAT (DUAT), the triple-ultrasound frequencies of UAT (TUAT). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Conclusions
This study evaluated the effects of different thawing treatments on the thawing rate and physical and chemical characteristics of large yellow croaker. The results showed that multifrequency UAT significantly reduced the thawing time of frozen samples. At the same time, the multifrequency UAT-treated samples retained better quality properties than other treated samples. The samples treated by UAT have better texture characteristics, lower TVB-N, and lower TBARS values. From the MRI images, it can be found that the fixed water content of DUAT and TUAT samples is statistically close to that of FS samples. Multifrequency UAT also plays a role in maintaining the flavour of the sample, preserving more sweet and delicious amino acids. It can also be seen from infrared detection and fluorescence intensity analysis that multifrequency UAT can reduce the damage of thawing to MPs and better maintain the stability of fish protein, similar to that of fresh samples. However, changing the ultrasonic frequency has no significant effect on colour. Thus, these results above indicated that multifrequency UAT is an effective method to accelerate the thawing process and improve the physical and chemical properties and structural characteristics of frozen large yellow croaker. It is worth noting that ultrasonic waves have the disadvantages of high-power consumption and instability in practical applications. In addition, the action mode of multifrequency ultrasound in the physical field and the influence of the relative position change of thawed samples on the experimental effect also need to be studied.
Funding
This research was funded by National Key R&D Program of China (2019YFD0901603).
CRediT authorship contribution statement
Chuhan Bian: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. Hao Cheng: Data curation, Formal analysis. Huijie Yu: Software, Writing – review & editing. Jun Mei: Conceptualization, Data curation, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. Jing Xie: Funding acquisition, Methodology, Project administration, Validation, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Jun Mei, Email: jmei@shou.edu.cn.
Jing Xie, Email: jxie@shou.edu.cn.
References
- 1.Zhang W., Zhu C., Chi H., Liu X., Gong H., Xie A., Zheng W., Chen J., Zhang N., Wu Y. Early immune response in large yellow croaker (Larimichthys crocea) after immunization with oral vaccine. Mol. Cell. Probes. 2021;56 doi: 10.1016/j.mcp.2021.101708. [DOI] [PubMed] [Google Scholar]
- 2.Cai L., Cao M., Cao A., Regenstein J., Li J., Guan R. Ultrasound or microwave vacuum thawing of red seabream (Pagrus major) fillets. Ultrason. Sonochem. 2018;47:122–132. doi: 10.1016/j.ultsonch.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Luan L., Wang L., Wu T., Chen S., Ding T., Hu Y. A study of ice crystal development in hairtail samples during different freezing processes by cryosectioning versus cryosubstitution method. Int. J. Refrig. 2018;87:39–46. [Google Scholar]
- 4.Li D., Zhu Z., Sun D.-W. Effects of freezing on cell structure of fresh cellular food materials: a review. Trends Food Sci. Technol. 2018;75:46–55. [Google Scholar]
- 5.Wang Y.-Y., Yan J.-K., Ding Y., Rashid M.T., Ma H. Effect of sweep frequency ultrasound and fixed frequency ultrasound thawing on gelling properties of myofibrillar protein from quick-frozen small yellow croaker and its possible mechanisms. LWT. 2021;150:111922. doi: 10.1016/j.lwt.2021.111922. [DOI] [Google Scholar]
- 6.Cai L., Zhang W., Cao A., Cao M. Effects of different thawing methods on the quality of largemouth bass (Micropterus salmonides) LWT. 2020;120:108908. doi: 10.1016/j.lwt.2019.108908. [DOI] [Google Scholar]
- 7.Bhargava N., Mor R.S., Kumar K., Sharanagat V.S. Advances in application of ultrasound in food processing: a review. Ultrason. Sonochem. 2021;70:105293. doi: 10.1016/j.ultsonch.2020.105293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Q., Kong B., Liu S., Zheng O., Zhang C. Ultrasound-assisted thawing accelerates the thawing of common carp (Cyprinus carpio) and improves its muscle quality. LWT. 2021;141:111080. doi: 10.1016/j.lwt.2021.111080. [DOI] [Google Scholar]
- 9.Antunes-Rohling A., Astráin-Redín L., Calanche-Morales J.B., Marquina P., Beltrán J.A., Raso J., Cebrián G., Álvarez I. Eco-innovative possibilities for improving the quality of thawed cod fillets using high-power ultrasound. Food Control. 2021;121:107606. doi: 10.1016/j.foodcont.2020.107606. [DOI] [Google Scholar]
- 10.Qiu L., Zhang M., Chitrakar B., Bhandari B. Application of power ultrasound in freezing and thawing processes: effect on process efficiency and product quality. Ultrason. Sonochem. 2020;68 doi: 10.1016/j.ultsonch.2020.105230. [DOI] [PubMed] [Google Scholar]
- 11.Xu B., Yuan J., Wang L., Lu F., Wei B., Azam R.S.M., Ren X., Zhou C., Ma H., Bhandari B. Effect of multi-frequency power ultrasound (MFPU) treatment on enzyme hydrolysis of casein. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2019.104930. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y.-Y., Yan J.-K., Rashid M.T., Ding Y., Chikari F., Huang S., Ma H. Dual-frequency sequential ultrasound thawing for improving the quality of quick-frozen small yellow croaker and its possible mechanisms. Innov. Food Sci. Emerg. 2021;68 [Google Scholar]
- 13.Ma X., Mei J., Xie J. Effects of multi-frequency ultrasound on the freezing rates, quality properties and structural characteristics of cultured large yellow croaker (Larimichthys crocea) Ultrason. Sonochem. 2021;76 doi: 10.1016/j.ultsonch.2021.105657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chevalier D., Le Bail A., Chourot J.M., Chantreau P. High pressure thawing of fish (whiting): Influence of the process parameters on drip losses. LWT. 1999;32:25–31. [Google Scholar]
- 15.Sadot M., Curet S., Chevallier S., Le-Bail A., Rouaud O., Havet M. Microwave assisted freezing part 2: Impact of microwave energy and duty cycle on ice crystal size distribution. Innov. Food Sci. Emerg. 2020;62 [Google Scholar]
- 16.Li P., Mei J., Xie J. Chitosan-sodium alginate bioactive coatings containing ε-polylysine combined with high CO2 modified atmosphere packaging inhibit myofibril oxidation and degradation of farmed pufferfish (Takifugu obscurus) during cold storage. LWT. 2021;140:110652. doi: 10.1016/j.lwt.2020.110652. [DOI] [Google Scholar]
- 17.Liu W., Mei J., Xie J. Effect of locust bean gum-sodium alginate coatings incorporated with daphnetin emulsions on the quality of Scophthalmus maximus at refrigerated condition. Int. J. Biol. Macromol. 2021;170:129–139. doi: 10.1016/j.ijbiomac.2020.12.089. [DOI] [PubMed] [Google Scholar]
- 18.Li P., Zhou Q., Chu Y., Lan W., Mei J., Xie J. Effects of chitosan and sodium alginate active coatings containing epsilon-polysine on qualities of cultured pufferfish (Takifugu obscurus) during cold storage. Int. J. Biol. Macromol. 2020;160:418–428. doi: 10.1016/j.ijbiomac.2020.05.092. [DOI] [PubMed] [Google Scholar]
- 19.Bao Y., Wang K., Yang H., Regenstein J.M., Ertbjerg P., Zhou P. Protein degradation of black carp (Mylopharyngodon piceus) muscle during cold storage. Food Chem. 2020;308 doi: 10.1016/j.foodchem.2019.125576. [DOI] [PubMed] [Google Scholar]
- 20.Li B., Wang X., Gao X., Mei J., Xie J. Xie, Effect of active coatings containing lippa citriodora kunth. essential oil on bacterial diversity and myofibrillar proteins degradation in refrigerated large yellow croaker. Polymers (Basel) 2021;13(11):1787. doi: 10.3390/polym13111787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Z., Ge X., Yang L., Ma G., Ma J., Yu Q.L., Han L. Ultrasound-assisted thawing of frozen white yak meat: effects on thawing rate, meat quality, nutrients, and microstructure. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singla M., Sit N. Application of ultrasound in combination with other technologies in food processing: a review. Ultrason. Sonochem. 2021;73:105506. doi: 10.1016/j.ultsonch.2021.105506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gambuteanu C., Alexe P. Comparison of thawing assisted by low-intensity ultrasound on technological properties of pork Longissimus dorsi muscle. J. Food Sci. Technol. 2015;52:2130–2138. doi: 10.1007/s13197-013-1204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan M., Ye J., Chu Y., Xie J. The effects of ice crystal on water properties and protein stability of large yellow croaker. Int. J. Refrig. 2021;130:242–252. [Google Scholar]
- 25.Sun Q., Sun F., Xia X., Xu H., Kong B. The comparison of ultrasound-assisted immersion freezing, air freezing and immersion freezing on the muscle quality and physicochemical properties of common carp (Cyprinus carpio) during freezing storage. Ultrason. Sonochem. 2019;51:281–291. doi: 10.1016/j.ultsonch.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Li X.X., Sun P., Jia J.Z., Cai L.Y., Li J.R., Lv Y.F. Effect of low frequency ultrasound thawing method on the quality characteristics of Peru squid Dosidicus gigas. Food Sci. Technol. Int. 2019;25:171–181. doi: 10.1177/1082013218809556. [DOI] [PubMed] [Google Scholar]
- 27.Bekhit A.-D., Holman B.W.B., Giteru S.G., Hopkins D.L. Total volatile basic nitrogen (TVB-N) and its role in meat spoilage: a review. Trends Food Sci. Technol. 2021;109:280–302. [Google Scholar]
- 28.Peng-cheng Z., Jing X. Effect of different thawing methods on the quality of mackerel Pneumatophorus japonicus. Food Sci. Biotechnol. 2021;30:1213–1223. doi: 10.1007/s10068-021-00966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai L., Wan J., Li X., Li J. Effects of different thawing methods on physicochemical properties and structure of largemouth bass Micropterus salmoides. J. Food Sci. 2020;85:582–591. doi: 10.1111/1750-3841.15029. [DOI] [PubMed] [Google Scholar]
- 30.Kono S., Kon M., Araki T., Sagara Y. Effects of relationships among freezing rate, ice crystal size and color on surface color of frozen salmon fillet. J. Food Eng. 2017;214:158–165. [Google Scholar]
- 31.Ottestad S., Enersen G., Wold J.P. Effect of freezing temperature on the color of frozen salmon. J. Food Sci. 2011;76(7):S423–S427. doi: 10.1111/j.1750-3841.2011.02313.x. [DOI] [PubMed] [Google Scholar]
- 32.Lan W., Zhao Y., Gong T., Mei J., Xie J. Xie, Effects of different thawing methods on the physicochemical changes, water migration and protein characteristic of frozen pompano (Trachinotus ovatus) J. Food Biochem. 2021;45(8) doi: 10.1111/jfbc.v45.810.1111/jfbc.13826. [DOI] [PubMed] [Google Scholar]
- 33.Inguglia E.S., Zhang Z., Burgess C., Kerry J.P., Tiwari B.K. Influence of extrinsic operational parameters on salt diffusion during ultrasound assisted meat curing. Ultrasonics. 2018;83:164–170. doi: 10.1016/j.ultras.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Liu D., Liang L., Xia W., Regenstein J.M., Zhou P. Biochemical and physical changes of grass carp (Ctenopharyngodon idella) fillets stored at -3 and 0 degrees C. Food Chem. 2013;140:105–114. doi: 10.1016/j.foodchem.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 35.Fattahi S., Zamindar N. Effect of immersion ohmic heating on thawing rate and properties of frozen tuna fish. Food Sci. Technol. Int. 2020;26:453–461. doi: 10.1177/1082013219895884. [DOI] [PubMed] [Google Scholar]
- 36.Hafezparast-Moadab N., Hamdami N., Dalvi-Isfahan M., Farahnaky A. Effects of radiofrequency-assisted freezing on microstructure and quality of rainbow trout (Oncorhynchus mykiss) fillet. Innov. Food Sci. Emerg. 2018;47:81–87. [Google Scholar]
- 37.Hu L., Ying Y., Zhang H., Liu J., Chen X., Shen N., Li Y., Hu Y. Advantages of liquid nitrogen freezing in long-term frozen preservation of hairtail (Trichiurus haumela): enzyme activity, protein structure, and tissue structure. J. Food Process Eng. 2021;44 [Google Scholar]
- 38.Zhang B., Cao H.J., Wei W.Y., Ying X.G. Influence of temperature fluctuations on growth and recrystallization of ice crystals in frozen peeled shrimp (Litopenaeus vannamei) pre-soaked with carrageenan oligosaccharide and xylooligosaccharide. Food Chem. 2020;306 doi: 10.1016/j.foodchem.2019.125641. [DOI] [PubMed] [Google Scholar]
- 39.Qi J., Zhang W.W., Xu Y., Xie X.F., Xiong G.Y., Xu X.L., Zhou G.H., Ye M. Enhanced flavor strength of broth prepared from chicken following short-term frozen storage. Food Chem. 2021;356 doi: 10.1016/j.foodchem.2021.129678. [DOI] [PubMed] [Google Scholar]
- 40.Li P., Peng Y., Mei J., Xie J. Effects of microencapsulated eugenol emulsions on microbiological, chemical and organoleptic qualities of farmed Japanese sea bass (Lateolabrax japonicus) during cold storage. LWT. 2020;118:108831. doi: 10.1016/j.lwt.2019.108831. [DOI] [Google Scholar]
- 41.Lan W., Liu J., Wang M., Xie J. Effects of apple polyphenols and chitosan-based coatings on quality and shelf life of large yellow croaker (Pseudosciaena crocea) as determined by low field nuclear magnetic resonance and fluorescence spectroscopy. J. Food Saf. 2021;41 [Google Scholar]
- 42.Cheng W., Sørensen K.M., Engelsen S.B., Sun D.-W., Pu H. Lipid oxidation degree of pork meat during frozen storage investigated by near-infrared hyperspectral imaging: Effect of ice crystal growth and distribution. J. Food Eng. 2019;263:311–319. [Google Scholar]
- 43.Faustman C., Sun Q., Mancini R., Suman S.P. Myoglobin and lipid oxidation interactions: mechanistic bases and control. Meat Sci. 2010;86:86–94. doi: 10.1016/j.meatsci.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 44.Cartagena L., Puértolas E., de Marañón I.M. Application of high pressure processing after freezing before frozen storage) or before thawing in frozen albacore tuna (Thunnus alalunga) Food Bioprocess Technol. 2020;13:1791–1800. [Google Scholar]
- 45.Li D., Zhao H., Muhammad A.I., Song L., Guo M., Liu D. The comparison of ultrasound-assisted thawing, air thawing and water immersion thawing on the quality of slow/fast freezing bighead carp (Aristichthys nobilis) fillets. Food Chem. 2020;320 doi: 10.1016/j.foodchem.2020.126614. [DOI] [PubMed] [Google Scholar]
- 46.Guyon C., Meynier A., de Lamballerie M. Protein and lipid oxidation in meat: A review with emphasis on high-pressure treatments. Trends Food Sci. Technol. 2016;50:131–143. [Google Scholar]
- 47.Chen D.-W., Zhang M. Non-volatile taste active compounds in the meat of Chinese mitten crab Eriocheir sinensis. Food Chem. 2007;104:1200–1205. [Google Scholar]
- 48.Yu D., Jing D., Yang F., Gao P., Jiang Q., Xu Y., Yu P., Xia W. The factors influencing the flavor characteristics of frozen obscure pufferfish (Takifugu Obscurus) during storage: ice crystals, endogenous proteolysis and oxidation. Int. J. Refrig. 2021;122:147–155. [Google Scholar]
- 49.Li N., Mei J., Shen Y., Xie J. Quality improvement of half-smooth tongue sole (Cynoglossus Semilaevis) fillets by chitosan coatings containing rosmarinic acid during storage. CyTA – J. Food. 2018;16:1018–1029. [Google Scholar]
- 50.Huang L., Ding X., Li Y., Ma H. The aggregation, structures and emulsifying properties of soybean protein isolate induced by ultrasound and acid. Food Chem. 2019;279:114–119. doi: 10.1016/j.foodchem.2018.11.147. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y.Y., Tayyab Rashid M., Yan J.K., Ma H. Effect of multi-frequency ultrasound thawing on the structure and rheological properties of myofibrillar proteins from small yellow croaker. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J., Zhang X., Chen Y., Zhao X., Anthony B., Xu X. Effects of different ultrasound frequencies on the structure, rheological and functional properties of myosin: Significance of quorum sensing. Ultrason. Sonochem. 2020;69:105268. doi: 10.1016/j.ultsonch.2020.105268. [DOI] [PubMed] [Google Scholar]