Highlights
-
•
Ultrasound-assisted chlorine washing disinfects winter jujube with great efficiency.
-
•
Ultrasonication-assisted chlorine washing does not affect quality of winter jujube.
-
•
The combination treatment reduces the cross-contamination incidence.
Abbreviations: FC, free chlorine; PAL, phenylalanine ammonia-lyase; PPO, polyphenol oxidase; TA, titratable acid; AMC, aerobic mesophilic count; APC, aerobic psychrotrophic count; M&Y, molds and yeasts
Keywords: Decontamination, Fresh produce, Ultrasonication, Chlorine treatment
Abstract
Wash water is circulated for use in the minimal processing industry, and inefficient disinfection methods can lead to pathogen cross-contamination. Moreover, few disinfection strategies are available for ready-to-eat fruits that do not need to be cut. In this study, the use of chlorine and ultrasound, two low-cost disinfection methods, were evaluated to disinfect winter jujube, a delicious, nutritious, and widely sold fruit in China. Ultrasound treatment (28 kHz) alone could not decrease the cross-contamination incidence of Escherichia coli O157:H7, non-O157 E. coli, and Salmonella Typhimurium, and free chlorine treatment at 10 ppm decreased the incidence from 55.00% to 5.00% for E. coli O157:H7, 65.00% to 6.67% for non-157 E. coli, and 70.00% to 6.67% for S. Typhimurium. The cross-contamination incidence was completely reduced (pathogens were not detected in sample) when the treatments were combined. The counts of aerobic mesophiles, aerobic psychrophiles, molds, yeasts, and three pathogens in the group subjected to combination treatment (28 kHz ultrasound + 10 ppm free chlorine) were significantly lower than those in the control, chlorine-treated, and ultrasound-treated groups during storage (0–7 d at 4 °C). Analysis of weight loss, sensory quality (crispness, color, and flavor), instrument color (a*/b*), soluble matter contents (total soluble solids, reducing sugar, total soluble sugar, and titratable acid), and nutritional properties (ascorbic acid and polyphenolic contents) indicated that treatment with ultrasound, chlorine, and their combination did not lead to additional quality loss compared with properties of the control. Additionally, the activities of phenylalanine ammonia-lyase and polyphenol oxidase were not significantly increased in the treatment group, consistent with the quality analysis results. These findings provide insights into disinfection of uncut ready-to-eat fruits using a minimum dose of disinfectant for cross-contamination prevention under ultrasonication. The use of ultrasound alone to decontaminate fresh produce is accompanied by a high risk of pathogen contamination, and the use of sanitizers to decrease cross-contamination incidence is recommended.
1. Introduction
Consumption of fresh vegetable and fruit produce provides vitamins, minerals, and fiber to the body. The US Food and Drug Administration recommends consuming 2–4 different vegetables and 3–5 different fruits every day. Ready-to-eat fresh produce have convenient characteristics; however, because they have not been heat-treated, they also have many safety hazards, the most important being food-borne pathogen contamination. In Europe and the United States, Salmonella is the most common contaminant present in fresh produce and causes food-borne diseases, followed by Escherichia coli [1]. According to the US Centers for Disease Control and Prevention report, in July 2021, salad vegetables contaminated with Salmonella were recalled, the contamination caused illness in 11 people, and two individuals were hospitalized in three states [2]; From August 10 to October 31, 2020, due to the consumption of leafy greens infected with E. coli O157: H7, 40 food safety incidents occurred in 19 states in the USA [3]. Therefore, disinfection is an effective strategy required for ensuring the safety of fresh produce.
Various novel disinfection technologies for ready-to-eat agricultural products have been studied, such as the application of cold plasma, pulsed light, microbial-microbial interactions, and bacteriophages; however, owing to their high costs, they have not yet been applied on a large scale [4], [5], [6]. Ready-to-eat fresh produce need to be washed to remove the dirt and kill the attached bacteria before packaging. Owing to cost constraints, chlorine disinfectants are used extensively in the industry [7]. It is generally believed that the effect of chlorine disinfection is positively correlated with the concentration of free chlorine (FC). However, a review, which summarized the characteristics of chlorine disinfection for fresh produce, concluded that high and low concentrations of chlorine have similar disinfection effects at the industrial scale [8]; in addition, no chemical disinfection method can kill pathogens to an undetectable levels, mainly owing to the formation of biofilms and the presence of pathogens in difficult-to-clean sites such as stomata on the surface of produce [8], [9], [10]. Therefore, recent studies have focused on using a minimum amount of disinfectant to prevent cross-contamination during fresh produce washing, because if fresh produce are infected with pathogens before washing, the pathogens may enter the circulating wash water and infect additional produce that enter the washing tank. Thus, the disinfection of wash water is very important, and the use of chlorine-based disinfectants has not been challenged in this regard [10], [11]. Additionally, a reduction in sanitizer dosage is required to adhere to the cost requirements of minimal processing industries [12], [13]. Therefore, the use of low-concentration chlorine to disinfect fresh produce is a current hotspot in the field of minimal processing.
Most disinfection studies have focused on cut produce, which exhibit characteristics of cut-based wounds, short shelf life, and rapid consumption of oxidizing disinfectants caused by outflow solids. In addition, certain common fresh-cut fruits, such as mango, strawberry, and papaya, cannot be disinfected in aqueous solution, and can only be disinfected by gaseous disinfectants or radiation, such as ozone and ultraviolet-C radiation. Moreover, few fruits are not suitable for cutting; however, these fruits have a large demand. For example, currently in China, fine-packaged fruits are purchased by consumers at railway stations, airports, scenic spots, supermarkets, and during transportation (airplanes and trains). However, to the best of our knowledge, there are no ready-to-eat fruit products that can be directly consumed, and additional washing is needed. Therefore, it is necessary to establish a low-cost washing method for uncut, ready-to-eat fruits. Among these uncut fruits, winter jujube is a popular fruit that has the highest frequency on the shelf (Fig. 1) since it is sweet, crisp, juicy, profitable, and has a low decay rate. Ultrasound (US) treatment has been widely used in the disinfection of fresh produce, and most studies combining US with other disinfection strategies have shown improved disinfection efficacy. Recently, Takundwa et al [14] combined US with nisin and oregano to disinfect E. coli and Listeria monocytogenes on lettuce and observed improved disinfection effects as compared to those with US alone. Li et al [15] used 55 ℃ hot water to improve the disinfection efficacy of US against Rhizopus stolonifer in sweet potato. Similarly, combination of 55 ℃ hot water with US showed a better effect on the control of E. coli O157:H7 on sprouting Brassicaceae seeds [16]. Moreover, slightly acidic electrolyzed water was found to improve the disinfection efficacy of US, killing naturally present microbes on cherry tomato and strawberries, without negatively affecting the quality [17]. However, little is known about the effects of the combination of disinfection treatments (US + low-concentration chlorine) on cross-contamination prevention, disinfection efficacy, and quality during washing. In this study, winter jujube was selected as the model for these evaluations.
Fig. 1.
Packaged winter jujube in supermarket.
2. Materials and methods
2.1. Sample preparation
Winter jujube (Zizyphus jujuba Mill. cv. Dongzao) was purchased from a local market on the day of the experiment, and the sample with a red index (as calculated according to [18]) of 43.5 ± 3.8% and size of 20 ± 1 g were selected for further experiments.
2.2. Pathogen inoculation
E. coli O157:H7 (NCTC12900), a non-toxic strain that was previously used in fresh produce inoculation experiments [19], [20], [21], was selected in this experiment. Non-O157 E. coli (ATCC25922) and Salmonella Typhimurium (ATCC14028), two quality control strains recommended by the FDA for food safety testing [22], [23], were selected as well. The inoculation experiment was performed according to our previous study [6], with minor modifications. Pure cultures of E. coli O157:H7, non-O157 E. coli, and Salmonella Typhimurium stored in 50% glycerol were cultured on modified sorbitol MacConkey agar (Hopebio, Qingdao, China), eosin methylene blue agar (Hopebio), and xylose lysine deoxycholate agar (Hopebio), respectively. After incubation for 24 h at 37 °C, one bacterial colony was cultured in nutrient broth (Hopebio) overnight at 37 °C, and the cell density of the suspension was adjusted to 109 colony forming units (CFU)/mL. The adjusted suspension (6.5 mL) was added to a stomacher bag containing sterilized 0.85% NaCl (200 mL) and 10 jujubes and massaged for 20 min. After air drying in a biological safety cabinet, infected samples were placed at 4 °C for 24 h. The cell counts of the pathogen on the sample were 105–106 CFU/g.
2.3. Disinfection
2.3.1. Wash water preparation
Since the washing process causes mechanical damage and leads to the leakage of soluble solids into the wash water, many studies recommend using fresh produce homogenates to prepare the wash water instead of clean water to simulate real conditions [12], [24], [25]. Winter jujube was cut into two parts, the core was removed, and it was immediately placed into the analytical mill (A11 basic; IKA, Germany) for 20 s processing. The resulting jujube homogenate was filtered under a vacuum and then stored at − 20 °C until use. Before the experiment, sterilized tap water was mixed with the homogenate and the chemical oxygen demand (COD) was adjusted to 415 ± 11 mg/L. The concentration of FC and pH in the washing water was adjusted to 5 and 10 ppm, and pH 5.5 using sodium hypochlorite (Sinopharm, Beijing, China) and phosphoric acid (Sinopharm), respectively [12]. The COD and FC concentrations were determined using a COD and N,N-diethyl-p-phenylenediamine test kit (Lohand, Hangzhou, China), respectively.
2.3.2. Disinfection time screening
A US frequency ranging from 25 to 40 kHz is generally used for produce surface disinfection [26], [27], [28], and before the experiment, we screened the disinfection effect with a 7 min treatment time and frequencies of 20, 28, and 40 kHz with a power ranging from 200 to 600 W. We found that the disinfection effect at 40 kHz was significantly lower than that at 20 and 28 kHz. For 20 and 28 kHz, the disinfection efficacy was not further improved when the power exceeded 400 W, and similar disinfection effects between 20 and 28 kHz with 400 W were observed. Thus, disinfection time screening was carried out using a US parameter of 28 kHz and 400 W. Two cages (18 cm × 15 cm × 5 cm; Fig. 2) containing infected samples (20 samples/cage) were immersed in an ultrasonic washer (JM-30D-28; Skymen, Shenzhen, China) containing 8 L of prepared wash water (4 ± 1 °C), and a submersible pump (3,500 L/h; Chuangning, China) was used to simulate the water flow. After processing for 1, 4, and 7 min, six samples (three samples/cage) were randomly selected and homogenized for 2 min in a stomacher bag containing 240 mL of 0.85% NaCl. A serially diluted suspension (0.1 mL) was surface plated on the agar as described in Section 2.2.
Fig. 2.
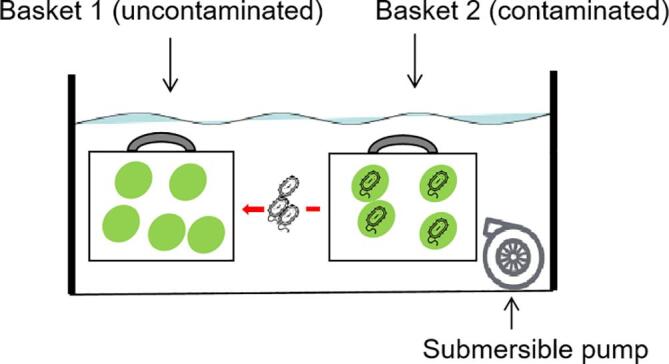
Schematic diagram of ultrasonic washing.
2.3.3. Cross-contamination incidence analysis
The winter jujube sample was immersed in 75% ethanol for 5 min, rinsed with sterile water thrice, and then placed in cage 1 (Fig. 2) as an uncontaminated group. The same amount of inoculated sample was placed into cage 2 (a clamp was used to tightly fix it together with cage 1) to reach a contamination incidence of 50% in the ultrasonic washer. The FC concentrations tested were 5 and 10 ppm, and the other treatment conditions were as described in Section 2.3.2. After processing for 4 min, each sample in cage 1 was analyzed using the agar method, as described in Section 2.2. Regardless of the cell count number, pathogen growth on the agar was recorded as infected, and no pathogen growth was recorded as uninfected. The cross-contamination incidence was calculated based on the total number of samples in cage 1.
2.3.4. Disinfection efficacy for winter jujube
After processing for 4 min in washing water with an FC concentration of 10 ppm, the sample was dewatered using an alcohol-sterilized salad spinner and sealed in a polyethylene terephthalate box using polyvinyl chloride cling film [29]. The samples were stored at 4 °C and analyzed on days 0, 3, and 7. Pathogens were analyzed as described in Section 2.3.2. For naturally present microbes, 0.1 mL suspension was surface plated on Rose Bengal agar (Hopebio) and incubated at 30 °C for 3 d to quantify molds and yeasts (M&Y); 1 mL bacterial suspension was pour-plated onto plate count agar (Hopebio) and incubated at 37 °C for 2 d to obtain the aerobic mesophilic counts (AMCs) and at 7 °C for 10 d to obtain the aerobic psychrophilic counts (APCs).
2.4. Quality analysis
2.4.1. Liquid nitrogen grinding
Eight samples were randomly selected from each package. After cutting it into two parts and removing the core, each sample was further cut into eight parts and one-eighth of each sample was combined for liquid nitrogen grinding. The ground powder was immediately transferred into a pre-cooled tube for subsequent analysis (Sections 2.4.2–2.4.5). Peels from additional eight samples were ground under liquid nitrogen, and the ground powder was immediately transferred into a pre-cooled tube for analysis, as described in Section 2.4.6.
2.4.2. Polyphenolic content analysis
The ground powder was mixed with 80% methanol at a ratio of 1:15. After extraction for 2 h, centrifugation was performed at 12,000 × g for 10 min. The supernatant (50 μL) was mixed with 250 μL of Folin-Ciocalteu reagent (Sinopharm) and 3 mL distilled water. The reaction was allowed to stand for 6 min, and then 750 μL of 20% sodium carbonate solution was added, and the mixture was incubated for 90 min in the dark. The absorbance was recorded at 765 nm, and the results were expressed as gallic acid equivalents (GAE, mg/kg) expressed on a fresh weight basis.
2.4.3. Ascorbic acid content analysis
The 2,6-dichlorophenolindophenol titration method was used for analysis of ascorbic acid contents, referring to GB5009.86–2016.
2.4.4. Total soluble solid (TSS) and titratable acid (TA) analysis
The ground powder was mixed with distilled water at a ratio of 1:5 and centrifuged at 12,000 × g for 10 min. The supernatant was analyzed using a refractometer (Suwei, Guangzhou, China) to determine the TSS content. TA analysis was performed according to GB/T 12293–1990.
2.4.5. Total soluble and reducing sugar analysis
The ground powder was mixed with distilled water at a ratio of 1:5 and centrifuged at 12,000 × g for 10 min. The supernatant was analyzed according to GB/T 15038–2006.
2.4.6. Polyphenol oxidase (PPO) and phenylalanine ammonia-lyase (PAL) analysis
The ground powder was mixed with 0.1 mol/L potassium phosphate buffer (pH 7.0; containing 2 mmol/L EDTA, 1% polyvinylpolypyrrolidone, and 1 mmol/L phenylmethylsulfonyl fluoride) at a ratio of 1:2 and centrifuged at 12,000 × g for 10 min at 4 °C. The protein content in the supernatant was analyzed using a total protein quantitative assay kit (Jiancheng, Nanjing, China).
For PAL analysis, 0.1 mL supernatant was mixed with 1.2 mL of 100 mmol/L Tris–HCl buffer (containing 1 mmol/L 2-mercaptoethanol; pH 8.5), 0.2 mL 15 mmol/L L-phenylalanine, and reacted for 30 min at 45 °C. The reaction was stopped using 6 mol/L HCl, and the absorbance was measured at 278 nm. The results were defined as the amount required to transform 1 mmol of L-phenylalanine to t-cinnamic acid per min and expressed as U/mg protein. For polyphenol oxidase (PPO) analysis, 0.1 mL supernatant was mixed with 0.9 mL 10 mmol/L phosphate buffer (pH 7.0) and 0.5 mL 60 mmol/L catechol. After 2 min, the absorbance was measured at 420 nm to determine the initial formation rate of quinone. PPO activity (U) is expressed as an increase in absorbance by 0.001 per min per mg protein.
2.4.7. Weight loss analysis
Weight loss was analyzed using following formula:
2.4.8. Sensory analysis
A three-point scale method was used for sensory evaluation at the end of storage (day 7), where 0 indicates extremely poor without any desirable characteristics, 5 indicates acceptability threshold, and 10 indicates excellent without any defects. Ten trained panelists from Shijiashike Co. Ltd. (Liaoyang, China) evaluated the sensory crispness, flavor, and color. The samples were placed on a white plate marked at the bottom and reordered. Only one person was allowed to enter the room (no windows, with white wall, and equipped with a 40 W white fluorescent lamp) during the evaluation and were not allowed to communicate with each other after the evaluation. For flavor analysis, the panelists gargled thrice after evaluation, and the next evaluation was performed after 30 s.
2.4.9. Instrument color analysis
The values of a* and b* were analyzed using a colorimeter (CR400; Konica Minolta, Osaka, Japan). The illuminant was D65, and the color space used was the CIELab system. Before use, the colorimeter was calibrated using a white standard plate (Y = 82.80, X = 0.3194, Y = 0.3264). Ten samples were randomly selected from each package and analyzed four times per sample for a total of 40 readings per replicate. The results are expressed as a*/ b* [30].
2.5. Statistical analysis
Each experiment was independently replicated thrice. Differences between the mean values of groups were evaluated via one-way analysis of variance using SPSS v.20 (SPSS, Chicago, IL, USA), and differences in mean values were analyzed via post hoc Duncan's multiple range tests. P values < 0.05 were considered significant.
3. Results and discussion
3.1. Wash time screening
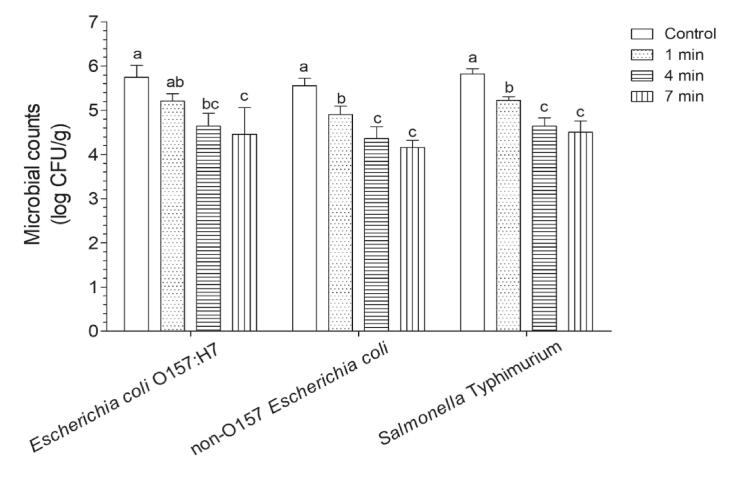
Low-frequency ultrasound is considered suitable for surface decontamination. A recent study compared the disinfection efficacy between 20 kHz + generally recognized as safe (GRAS) antibacterial substances and 1 MHz + GRAS antibacterial substances against E. coli and L. monocytogenes, and found that the 20 kHz treatment is more effective than the 1 MHz treatment, mainly because low-frequency ultrasound can cause cell membrane damage, whereas high-frequency ultrasound mainly induces intracellular oxidative stress [31]. The purpose of the present study was to perform surface disinfection, and the US time screening experiment showed that the counts of E. coli O157:H7, non-O157 E. coli, and S. Typhimurium were 5.21, 4.90, and 5.22 log CFU/g after washing for 1 min, respectively, and no significant difference was observed in counts between the E. coli O157:H7 and the control groups (Fig. 3). After washing for 4 min, the counts of these three pathogens were all significantly lower than that of the control; however, similar counts of these pathogens were observed after washing for 7 min. In the context of industrialization, disinfection is one step of the entire processing line; therefore, it is recommended that the washing time does not exceed 5 min [32]. Therefore, 4 min was used in further experiments.
Fig. 3.
Effects of different ultrasonic-assisted wash times on Escherichia coli O157:H7, non-O157 E. coli, and Salmonella Typhimurium in winter jujube. Bars show mean ± standard deviation values, and different lowercase letters in the same group indicate significant differences (P < 0.05).
3.2. Cross-contamination prevention
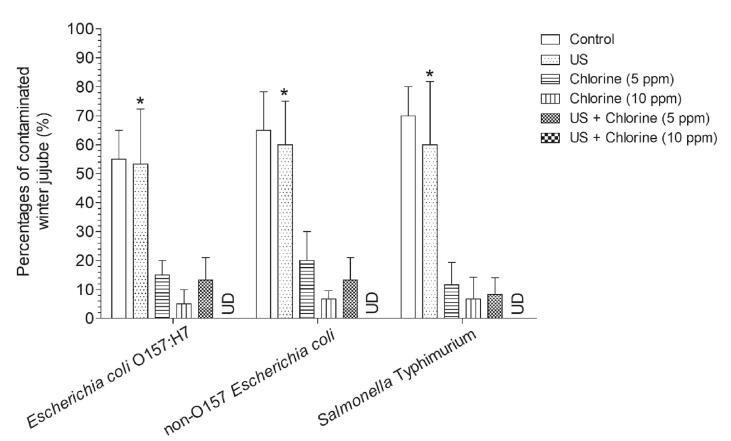
Once the pathogen enters the wash water and is not killed immediately, the entire batch of produce may get infected; thus, the incidence of cross-contamination should be as low as possible. We found that the cross-contamination incidence was not significantly reduced after US treatment alone (Fig. 4). When the sample was washed with 5 ppm FC alone, the cross-contamination incidence of E. coli O157:H7, non-O157 E. coli, and S. Typhimurium decreased from 55.00% to 15.00%, 65.00% to 20.00%, and 70.00% to 11.67%, respectively, and further decreased to 5.00%, 6.67%, and 6.67% as FC concentration increased to 10 ppm. In a previous study, the minimum FC concentration required for cross-contamination prevention has been reported to be associated with the type of produce. For example, Luo et al. [24] found that maintaining at least 10 mg/L FC at an industrial scale can strongly reduce bacterial survival in lettuce wash water. Additionally, Gómez-López et al. [11] found that 7 mg/L FC is an effective concentration for preventing E. coli O157:H7 cross-contamination when washing spinach. In this study, we found that 10 ppm FC was insufficient to completely prevent cross-contamination of winter jujube; however, the pathogen was not detected when 10 ppm FC was combined with US treatment (Fig. 4). Previously, 20 kHz US treatment has been shown to be ineffective in inactivating Salmonella growth [31]; however, a synergistic inactivation effect is observed using the combination of carvacrol, limonene, geraniol, and citral. Another study reported a synergistic inactivation effect when using ultraviolet-A radiation and curcumin [33]. Taken together, these results indicate that treatment for 4 min using US + 10 ppm FC is an effective method to prevent cross-contamination.
Fig. 4.
Cross-contamination incidence of Escherichia coli O157:H7, non-O157 E. coli, and Salmonella Typhimurium during winter jujube washing. Bars show mean ± standard deviation values, and asterisks above the column indicate non-significant differences (P>0.05) as compared with control. US, ultrasonication; UD, undetected.
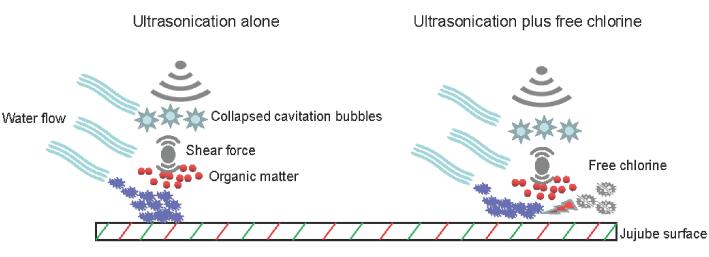
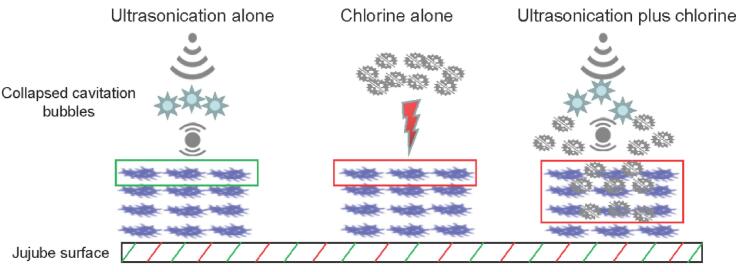
The transduction of US in water is associated with the organic matter content [34]. US can induce the generation of cavitation bubbles, and the collapsing bubbles lead to the generation of shear force to weaken the adhesion of pathogens to the jujube surface (Fig. 5). However, during fresh produce washing, the leakage of soluble solids dissolved in water from produce can weaken the transduction of the shear force. In a study by Huang et al [35], homogenate was used to prepare wash water and simulate the water flow, and they found that the contamination incidence of Salmonella in the US-treated group was similar to that in the control. In this study, the jujube homogenate in the washing water might have weakened the transduction of the shear force (Fig. 5). Moreover, water flow was needed during produce washing, and we speculate that this might disperse the cavitation bubbles and the shear force and consequently incompletely prevent pathogen adhesion to the jujube surface (Fig. 5). In contrast, in a study by Huang et al [36], the water flow was not simulated, and they found that US treatment alone could significantly lower the cross-contamination incidence. Based on the inclusion of FC in washing water, the pathogen was inactivated via the combined effects of FC and US before the adhesion between the pathogen and the jujube surface occurred, thus completely preventing the occurrence of cross-contamination.
Fig. 5.
Schematic diagram of proposed pathogen cross-contamination process under conditions of ultrasonication alone and ultrasonication plus free chlorine.
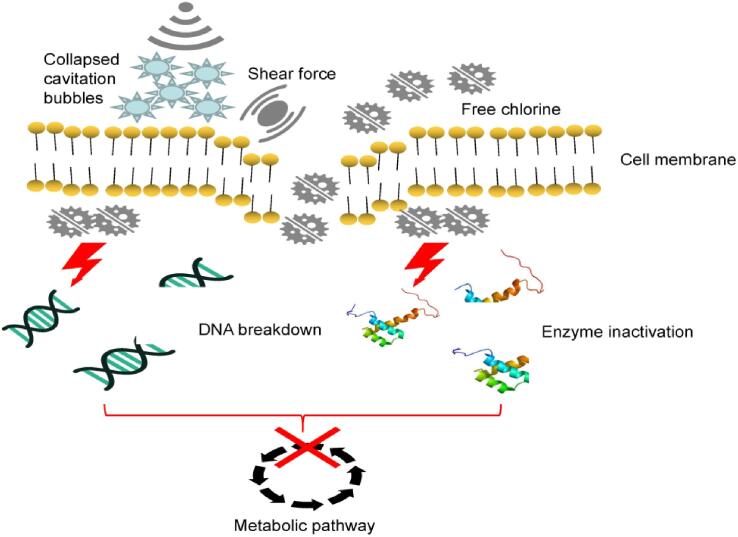
The disinfection mechanism of US mainly includes sonoporation and sonochemistry. When low-frequency (20–100 kHz) ultrasound is used, physical effects including shear force and shock waves were induced to cause membrane perforation; meanwhile, a high frequency can induce the generation of hydrogen peroxide and ROS to inactivate pathogens, termed sonochemistry [37]. The antibacterial mechanism of FC is oxidization of the cell membrane [38]. When 5 and 10 ppm FC were used, limited cell membrane damage was achieved, and thus, cross-contamination was not completely prevented. However, when low frequency US was combined with 5 ppm FC, cell membrane damage was accelerated, and when the FC concentration was increased to 10 ppm, the cell membrane was completely damaged and FC was able to enter the cell to damage the intracellular component resulting in DNA breakdown and enzyme inactivation (Fig. 6). Similarly, Guo et al [39] found that US + FC can damage E. coli membranes and induce changes in the intracellular organization and protein conformation. These speculations might explain why 10 ppm FC + US could completely prevent the occurrence of cross-contamination, which was superior to the effects with 5 ppm FC, 10 ppm FC, and 5 ppm FC + US.
Fig. 6.
Schematic diagram of proposed antibacterial mechanism of ultrasonication plus free chlorine.
3.3. Effects of different treatments on the quality of winter jujube
3.3.1. Effects on weight loss, nutritional properties, and soluble matter
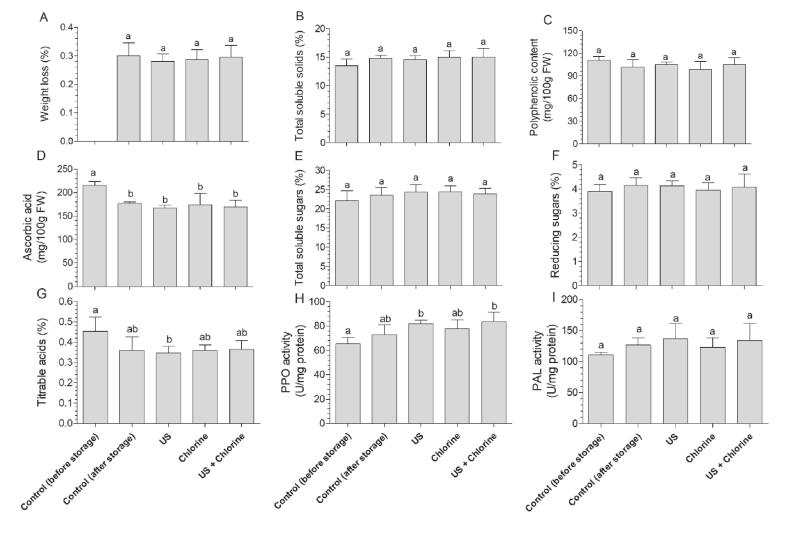
The shear force and pressure may cause mechanical damage, which can be applied in the medical field. A previous study improved the anticancer efficacy of titanium dioxide based on mechanical damage caused by high-intensity focused ultrasound [40]. Moreover, hypotonia has been shown to increase the sensitivity of cancer cells to ultrasonic-induced mechanical damage [41]. However, ultrasonic-induced mechanical damage may deteriorate the quality of produce, leading to browning, and weight and nutrition loss. Such deterioration cannot be observed immediately after treatment; thus, quality analysis was performed at the end of storage (day 7), which is consistent with findings of previous studies [4], [29], [42]. The weight loss was not significantly different between the treatment and control groups (Fig. 7A). TSS mainly include soluble sugars; the TSS content reflects the ripening extent of winter jujube [30]. Generally, TSS increases as ripening progresses and decreases with senescence [43]. We found that US and US + FC treatment did not negatively affect TSS content (Fig. 7B), which was consistent with the results of total soluble and reducing sugars (Fig. 7E, F). In addition, the TA content in fruits decreases with ripening, and in this study, TA content was not negatively affected by different treatments at the end of storage (Fig. 7G). Similarly, the two main nutrition indicators, ascorbic acid and polyphenols, were not negatively affected (Fig. 7C,D). However, studies have shown that mechanical damage caused by food processing, such as fresh cut, can induce intracellular peroxidation, and ascorbic acid as a stress response substance reacts with reactive oxygen species, leading to a decrease in concentration during subsequent storage [44].
Fig. 7.
Effects of different treatments on the quality of winter jujube. (A) Weight loss, (B) total soluble solids, (C) polyphenolic content, (D) ascorbic acid, and (I) PAL activity of winter jujube. Bars show mean ± standard deviation values, and different lowercase letters indicate significant differences (P < 0.05). PAL, phenylalanine ammonia-lyase; PPO, polyphenol oxidase; US, ultrasonication.
3.3.2. Effects on PAL and PPO
In undamaged fruit tissues, PPO and phenols are not in contact with each other; PPO catalyzes the conversion of polyphenols to quinones when mechanical damage occurs, leading to poor color and odor [45]. As a plant stress response enzyme, PAL is involved in phenol synthesis, and when fruit and vegetable tissues are damaged, such as in cut and ultraviolet radiation-treated fruits, PAL activity increases to increase phenol synthesis to mediate self-repair [46], [47]. US treatment primarily affects the surface of fruits and vegetables, especially leafy vegetables, and thin leaves are more sensitive to ultrasound than thick leaves. For example, a study showed that the quality of iceberg lettuce treated with sonication does not significantly differ from that of the control, whereas in Romaine lettuce, treatment without US treatment has a significantly higher overall quality score than that obtained using US treatment [48]. In this study, PAL and PPO activities in the peel were analyzed, and did not significantly differ between the treatment and control groups (Fig. 7H,I), which is consistent with the analysis of polyphenolic content (Fig. 7C).
3.3.3. Effects on sensory quality and instrument color
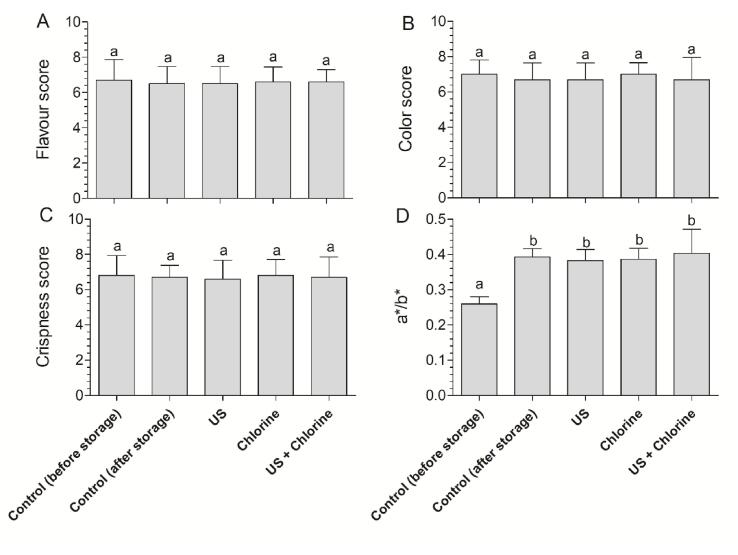
The sensory crispness, color, and flavor were not significantly affected by the different treatments at the end of the storage period (Fig. 8A–C), which was consistent with the findings of a previous study [48]. Color change is a key indicator for evaluating the extent of ripening in winter jujube. Instrument color analysis showed that the a*/b* value in the control group was significantly higher than that on day 0 (Fig. 8D), indicating post-ripening for 7 d, which was consistent with previous findings [18], [49]. When comparing the treatment groups with the control group at day 7, the results indicated that US did not negatively affect the instrument color, consistent with sensory analysis. Mechanical damage has been reported to accelerate the ripening process by stimulating ethylene production [50]. When analyzing a*/b* in combination with TSS and TA contents, our results suggest that US does not cause significant damage to accelerate the ripening process of winter jujube.
Fig. 8.
Effects of different treatments on the sensory quality of winter jujube. (A) Flavour, (B) color, (C) crispness, and (D) instrument color of winter jujube. Bars show mean ± standard deviation values, and different lowercase letters indicate significant differences (P < 0.05). US, ultrasonication.
Previous studies have shown that coating and fumigation are effective methods to control weight loss, decay, and nutrition loss in winter jujube [18], [30]; however, US treatment is only effective during washing, without leaving any residue on jujube to affect its quality during storage and was considered a low-cost and effective decontamination method for fresh produce. In this study, we confirmed that US treatment did not deteriorate the quality during storage, by analyzing nutrients, soluble matter, instrument color, sensory quality, and stress response enzymes. Oxidizing sanitizers can cause the loss of fresh produce quality. For example, 10 ppm aqueous ozone can lead to ascorbic acid loss in fresh-cut rocket leaves during storage [51], whereas for FC, in general, fresh produce quality will not decline with an FC concentration that does not exceed 200 ppm [52]. In this study, a low concentration of FC (10 ppm) was used, and we found that US + 10 ppm FC did not cause additional quality loss as compared to that with the control, which was in consistent with the results of US treatment. However, for other fragile fruits, US treatment leads to significant quality loss. A previous study used low-frequency US (25 kHz) to process fresh-cut mangoes and found that the color, firmness, soluble solids, and sugar content are negatively affected [53].
3.4. Decontamination efficacy of different treatments for winter jujube
3.4.1. Effects on food-borne pathogens
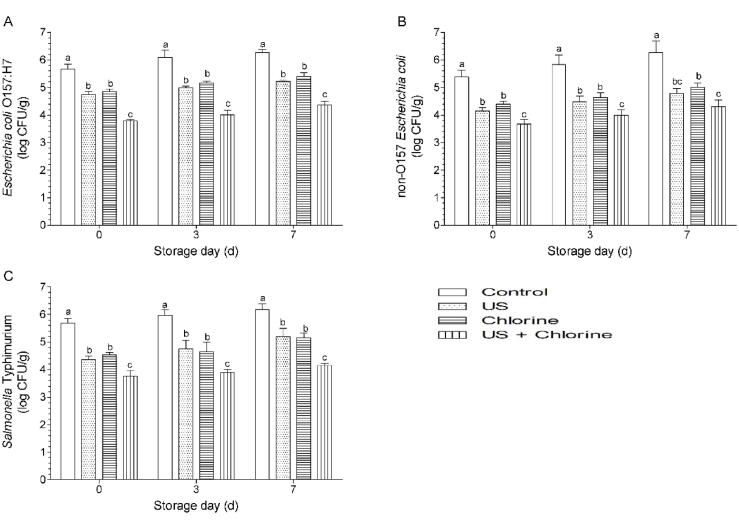
No significant difference was observed between US and FC treatment for disinfecting samples containing E. coli O157: H7, non-O157 E. coli, and Salmonella (Fig. 9) at day 0. However, when comparing the results regarding cross-contamination prevention and disinfection efficacy (Fig. 4 vs. Fig. 9), cross-contamination incidence did not decrease after US treatment, whereas 10 ppm chlorine treatment significantly decreased the incidence. Therefore, the decontamination of fresh produce using US treatment alone has a minor contribution to prevent cross-contamination. Therefore, it is important to use sanitizers during ultrasonic washing to prevent cross-contamination.
Fig. 9.
Effects of different treatments on food-borne pathogen of winter jujube. (A) Escherichia coli O157:H7, (B) non-O157 E. coli, and (C) Salmonella Typhimurium of winter jujube. Bars show mean ± standard deviation values, and different lowercase letters indicate significant differences (P < 0.05). US, ultrasonication.
When US was used in combination with FC disinfection, the counts of E. coli O157: H7, non-O157 E. coli, and Salmonella were 3.78, 3.67, and 3.75 log CFU/g, respectively, which were significantly lower than those obtained using US and FC treatments separately (Fig. 9); however, a synergistic effect was not observed. Many studies have shown that hurdle technology cannot provide synergistic effects on the disinfection of produce; however, the technology enables additional microbial reduction when compared with single disinfection methods [54], [55], [56].
When fresh produce is contaminated by a pathogen, a layer-by-layer state of the pathogen is formed on the produce surface [34]. The use of US and FC alone will detach and inactivate the pathogen from the upper layer, respectively (Fig. 10); however, when US and FC are combined, shear force can weaken the adhesion between each pathogen layer, making it easier for FC to inactivate the pathogen in deeper layers (Fig. 10) [34]. This can explain why US + FC led to the highest disinfection efficacy against the pathogen on the surface of winter jujube.
Fig. 10.
Schematic diagram of proposed antibacterial mechanism of ultrasonication, free chlorine, and ultrasonication plus free chlorine against the pathogens present on the winter jujube surface.
During storage, the treatment with US, FC, and their combination still led to significantly lower pathogen counts than that in the control group, and the count in the combination group was significantly lower than that in the US and FC-treated groups. In few cases, insufficient disinfection may stimulate pathogen growth on produce. A previous study found that L. monocytogenes shows growth on lettuce after washing with 0.5% propionic acid (PA), whereas 1% PA significantly reduces the cell counts of this bacterium; the authors suggested that this result may be explained by the fact that L. monocytogenes is more resistant to 0.5% PA than native microflora and is more competitive, whereas 1% PA can create an acidic environment that exceeds the resistance limit of the bacterium [57].
3.4.2. Effects on naturally present microbes
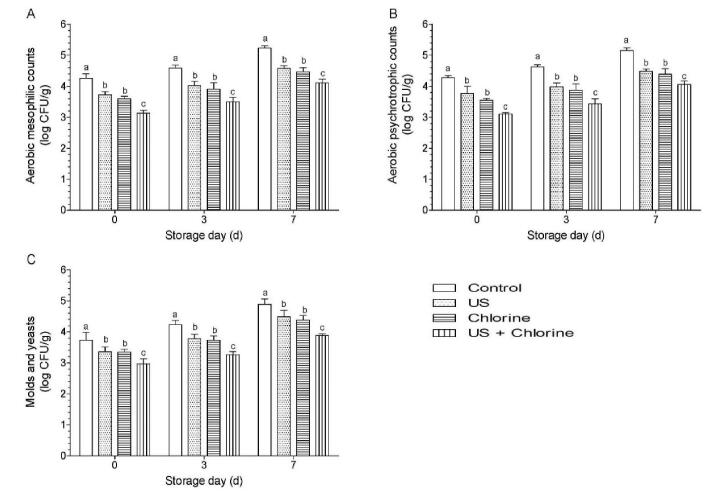
A combination of different decontamination methods considerably inhibits the growth of microbes naturally present on fresh produce. A study found that 0.6% citric acid and 2% H2O2 can reduce AMC and M&Y by<1 log when used alone; however, they reduce the cell counts by 2.26 and 1.28 log, respectively, when used in combination [58]. The combined use of chlorine, ozone, and electrolyzed water with organic acids exhibits better disinfection effects for lowering the AMC than that obtained using individual treatments [29], [59]. It was observed that the AMC, APC, and M&Y in the treatment group were significantly lower than those in the control group, and the counts in the combination group were significantly lower than those in the US and FC groups (Fig. 11).
Fig. 11.
Effects of different treatments on naturally present microbes of winter jujube. (A) aerobic mesophilic counts, (B) aerobic psychrotrophic counts, and (C) molds and yeasts of winter jujube. Bars show mean ± standard deviation values, and different lowercase letters indicate significant differences (P < 0.05). US, ultrasonication.
When comparing microbial reduction between AMC and M&Y, the treatment with US, FC, and their combination reduced the AMC by 0.53, 0.66, and 1.22 log CFU/g, respectively, and reduced M&Y by 0.38, 0.39, and 0.77 log CFU/g, respectively. The relatively weaker effect against fungi may be due to the relatively greater resistance of the fungal membrane to the treatment, which is the main target of the oxidizing agent, chlorine [4]. Similarly, the use of oxidizing sanitizers showed lower disinfection effects against fungi than that obtained using other sanitizers. Aqueous ozone reduces the M&Y by 0.78, 0.99, and 0.5-log for strawberries, lettuce, and durum wheat, respectively, which are significantly lower than those obtained using organic acids [60], [61]. A study compared the disinfection efficacies of chlorine, aqueous ozone, and lactic acid (LA), and found that LA is more effective than ozone and chlorine in reducing M&Y during storage [51]. During storage, the combination group still had the lowest AMC, APC, and AMC, which were significantly lower than those of the other groups, which is consistent with the results of the pathogen experiment (Fig. 11).
4. Conclusion
In this study, we proposed a decontamination method combining low-concentration chlorine with US treatment and determined its efficiency in preventing cross-contamination. US treatment (28 kHz) combined with 10 ppm FC could effectively control cross-contamination during winter jujube decontamination; however, US treatment alone could not decrease the cross-contamination incidence. When analyzing the disinfection efficacy against winter jujube, we considered the microbial reduction in winter jujube to be mainly attributed to physical effects (shear force and pressure), unlike membrane damage caused by chlorine. Therefore, it is necessary to use sanitizers to control cross-contamination when washing fresh produce under ultrasonic conditions. The disinfection efficacy of US + FC can lead to the lowest cell counts of E. coli O157: H7, non-O157 E. coli, and Salmonella during storage (0–7 d). The cell counts of the combination group were significantly lower than those of the control, US, and FC groups. Quality analysis including apparent indicators (nutrition, soluble matter, weight loss), sensory quality, and enzymatic activity suggest that US does not lead to quality loss in winter jujube. Currently, studying the relationship between disinfection and ecological changes has been proposed as an emerging strategy to elucidate the antimicrobial mode of action against naturally present microbes on fresh produce, and metatranscriptomic analysis can be performed to evaluate the disinfection mechanism of US + FC in the future.
CRediT authorship contribution statement
Jiayi Wang: Conceptualization, Supervision, Funding acquisition, Writing – original draft, Writing – review & editing. Kun Huang: Investigation, Methodology. Zhaoxia Wu: Data curation. Yougui Yu: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the Scientific Research Foundation of Hunan Provincial Education Department (No. 20B527), Special Funding for Innovative Province Construction of Hunan (No. 2021NK4258), and The Science and Technology Innovation Program of Hunan Province (No. 2020RC1011).
References
- 1.Callejón R.M., Rodríguez-Naranjo M.I., Ubeda C., Hornedo-Ortega R., Garcia-Parrilla M.C., Troncoso A.M. Reported foodborne outbreaks due to fresh produce in the United States and European Union: trends and causes. Foodborne Pathog. Dis. 2015;12(1):32–38. doi: 10.1089/fpd.2014.1821. [DOI] [PubMed] [Google Scholar]
- 2.Centers fo Diseases Control and Prevention of United States, Salmonella outbreak linked to BrightFarms packaged salad greens. https://www.cdc.gov/salmonella/typhimurium-07-21/index.html, 2021 (accessed 2 November 2021).
- 3.Centers fo Diseases Control and Prevention of United States, Outbreak of E. coli infections linked to leafy greens. https://www.cdc.gov/ecoli/2020/o157h7-10-20b/index.html, 2020 (accessed 2 November 2021).
- 4.Wang J., Zhang Y., Yu Y., Wu Z., Wang H. Combination of ozone and ultrasonic-assisted aerosolization sanitizer as a sanitizing process to disinfect fresh-cut lettuce. Ultrason. Sonochem. 2021;76 doi: 10.1016/j.ultsonch.2021.105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma L., Zhang M., Bhandari B., Gao Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017;64:23–38. [Google Scholar]
- 6.Wang J., Yu Y., Dong Y. Combination of polyhexamethylene guanidine hydrochloride and potassium peroxymonosulfate to disinfect ready-to-eat lettuce. RSC Adv. 2020;10:40316–40320. doi: 10.1039/d0ra08356a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gombas D., Luo Y., Brennan J., Shergill G., Petran R., Walsh R., Hau H., Khurana K., Zomorodi B., Rosen J., Varley R., Deng K. Guidelines to validate control of cross-contamination during washing of fresh-cut leafy vegetables. J. Food Prot. 2017;80:312–330. doi: 10.4315/0362-028X.JFP-16-258. [DOI] [PubMed] [Google Scholar]
- 8.Gil M.I., Selma M.V., López-Gálvez F., Allende A. Fresh-cut product sanitation and wash water disinfection: problems and solutions. Int. J. Food Microbiol. 2009;134(1-2):37–45. doi: 10.1016/j.ijfoodmicro.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Ölmez H., Kretzschmar U. Potential alternative disinfection methods for organic fresh-cut industry for minimizing water consumption and environmental impact. LWT - Food Sci. Technol. 2009;42:686–693. [Google Scholar]
- 10.Castro-Ibáñez I., Gil M.I., Allende A. Ready-to-eat vegetables: Current problems and potential solutions to reduce microbial risk in the production chain. LWT - Food Sci. Technol. 2017;85:284–292. [Google Scholar]
- 11.Gómez-López V., Lannoo A.S., Gil M.I., Allende A.J.F.C. Minimum free chlorine residual level required for the inactivation of Escherichia coli O157:H7 and trihalomethane generation during dynamic washing of fresh-cut spinach. Postharvest Biol. Technol. 2014;42:132–138. [Google Scholar]
- 12.Garrido Y., Marín A., Tudela J.A., Allende A., Gil M.I.J.P.B. Chlorate uptake during washing is influenced by product type and cut piece size, as well as washing time and wash water content. Postharvest Biol. Technol. 2019;151:45–52. [Google Scholar]
- 13.Gómez-López V., Marín A., Medina-Martínez M., Gil M.I., Allende A.J.P.B. Generation of trihalomethanes with chlorine-based sanitizers and impact on microbial, nutritional and sensory quality of baby spinach. Postharvest Biol. Technol. 2013;85:210–217. [Google Scholar]
- 14.Takundwa B.A., Bhagwat P., Pillai S., Ijabadeniyi O.A. Antimicrobial efficacy of nisin, oregano and ultrasound against Escherichia coli O157:H7 and Listeria monocytogenes on lettuce. LWT - Food Sci. Technol. 2021;139:110522. [Google Scholar]
- 15.Li L., Zhang M., Sun H.-N., Mu T.-H. Contribution of ultrasound and conventional hot water to the inactivation of Rhizopus stolonifer in sweet potato. LWT - Food Sci. Technol. 2021;148 [Google Scholar]
- 16.Dong M., Park H.K., Wang Y., Feng H. Control Escherichia coli O157:H7 growth on sprouting brassicacae seeds with high acoustic power density (APD) ultrasound plus mild heat and calcium-oxide antimicrobial spray. Food Control. 2022;132 [Google Scholar]
- 17.Ding T., Ge Z., Shi J., Xu Y.-T., Jones C.L., Liu D.-H. Impact of slightly acidic electrolyzed water (SAEW) and ultrasound on microbial loads and quality of fresh fruits. LWT - Food Sci. Technol. 2015;60:1195–1199. [Google Scholar]
- 18.Zhu S., Sun L., Zhou J. Effects of nitric oxide fumigation on phenolic metabolism of postharvest Chinese winter jujube (Zizyphus jujuba Mill. cv. Dongzao) in relation to fruit quality. LWT - Food Sci. Technol. 2009;42:1009–1014. [Google Scholar]
- 19.Marilyn C., Erickson J.-Y., Liao S.A., Payton W.P., Cook H.C.D. Survival of Salmonella enterica and Escherichia coli O157:H7 sprayed onto the foliage of field-grown cabbage plants. J. Food Prot. 2019;82:479–485. doi: 10.4315/0362-028X.JFP-18-326. [DOI] [PubMed] [Google Scholar]
- 20.Williams T.R., Moyne A.-L., Harris L.J., Marco M.L., Ibekwe A.M. Season, irrigation, leaf age, and Escherichia coli inoculation influence the bacterial diversity in the lettuce phyllosphere. PLoS One. 2013;8(7):e68642. doi: 10.1371/journal.pone.0068642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moyne A.-L., Blessington T., Williams T.R., Koike S.T., Cahn M.D., Marco M.L., Harris L.J. Conditions at the time of inoculation influence survival of attenuated Escherichia coli O157:H7 on field-inoculated lettuce. Food Microbiol. 2020;85:103274. doi: 10.1016/j.fm.2019.103274. [DOI] [PubMed] [Google Scholar]
- 22.American Type Culture Collection, Salmonella enterica subsp. enterica (ex Kauffmann and Edwards) Le Minor and Popoff serovar Typhimurium. https://www.atcc.org/products/14028, 2021 (accessed 16 December 2021).
- 23.American Type Culture Collection, Escherichia coli (Migula) Castellani and Chalmers. https://www.atcc.org/products/25922-mini-pack, 2021 (accessed 16 December 2021).
- 24.Luo Y., Zhou B., Van Haute S., Nou X., Zhang B., Teng Z., Turner E.R., Wang Q., Millner P.D. Association between bacterial survival and free chlorine concentration during commercial fresh-cut produce wash operation. Food Microbiol. 2018;70:120–128. doi: 10.1016/j.fm.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Van Haute S., Tryland I., Escudero C., Vanneste M., Sampers I. Chlorine dioxide as water disinfectant during fresh-cut iceberg lettuce washing: Disinfectant demand, disinfection efficiency, and chlorite formation. LWT - Food Sci. Technol. 2017;75:301–304. [Google Scholar]
- 26.São José J.F.B.D., Andrade N.J.D., Ramos A.M., Vanetti M.C.D., Stringheta P.C., Chaves J.B.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control. 2014;45:36–50. [Google Scholar]
- 27.Fan K., Wu J., Chen L. Ultrasound and its combined application in the improvement of microbial and physicochemical quality of fruits and vegetables: A review. Ultrason. Sonochem. 2021;80:105838. doi: 10.1016/j.ultsonch.2021.105838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen F., Zhang M., Yang C.-hui. Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason Sonochem. 2020;63:104953. doi: 10.1016/j.ultsonch.2019.104953. [DOI] [PubMed] [Google Scholar]
- 29.Wang J., Wang S., Sun Y., Li C., Li Y., Zhang Q., Wu Z. Reduction of Escherichia coli O157:H7 and naturally present microbes on fresh-cut lettuce using lactic acid and aqueous ozone. RSC Adv. 2019;9:22636–22643. doi: 10.1039/c9ra03544c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H., Li F., Wang L., Sheng J., Xin Z., Zhao L., Xiao H., Zheng Y., Hu Q. Effect of nano-packing on preservation quality of Chinese jujube (Ziziphus jujuba Mill. var. inermis (Bunge) Rehd) Food Chem. 2009;114:547–552. [Google Scholar]
- 31.Zhang H., Wang S., Goon K., Gilbert A., Nguyen Huu C., Walsh M., Nitin N., Wrenn S., Tikekar R.V. Inactivation of foodborne pathogens based on synergistic effects of ultrasound and natural compounds during fresh produce washing. Ultrason Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.104983. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Yu Y., Dong Y. Disinfection of ready-to-eat lettuce using polyhexamethylene guanidine hydrochloride. Microorgansism. 2020;8:272. doi: 10.3390/microorganisms8020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Oliveira E.F., Tosati J.V., Tikekar R.V., Monteiro A.R., Nitin N. Antimicrobial activity of curcumin in combination with light against Escherichia coli O157:H7 and Listeria innocua: Applications for fresh produce sanitation. Postharvest Biol. Technol. 2018;137:86–94. [Google Scholar]
- 34.Yu H., Liu Y., Li L., Guo Y., Xie Y., Cheng Y., Yao W. Ultrasound-involved emerging strategies for controlling foodborne microbial biofilms. Trends Food Sci. Technol. 2020;96:91–101. [Google Scholar]
- 35.Huang R., Chen H. Evaluation of inactivating Salmonella on iceberg lettuce shreds with washing process in combination with pulsed light, ultrasound and chlorine. Int J Food Microbiol. 2018;285:144–151. doi: 10.1016/j.ijfoodmicro.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Huang K., Wrenn S., Tikekar R., Nitin N. Efficacy of decontamination and a reduced risk of cross-contamination during ultrasound-assisted washing of fresh produce. J. Food Eng. 2018;224:95–104. [Google Scholar]
- 37.Dai J., Bai M., Li C., Cui H., Lin L. Advances in the mechanism of different antibacterial strategies based on ultrasound technique for controlling bacterial contamination in food industry. Trends Food Sci. Technol. 2020;105:211–222. [Google Scholar]
- 38.Bridges D.F., Lacombe A., Wu V.C.H. Integrity of the Escherichia coli O157:H7 cell wall and membranes after chlorine dioxide treatment. Front. Microbiol. 2020;11:888. doi: 10.3389/fmicb.2020.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo L., Sun Y., Zhu Y., Wang B., Xu L., Huang M., Li Y., Sun J. The antibacterial mechanism of ultrasound in combination with sodium hypochlorite in the control of Escherichia coli. Food Res. Int. 2020;129 doi: 10.1016/j.foodres.2019.108887. [DOI] [PubMed] [Google Scholar]
- 40.Tachibana K., Endo H., Feril L.B., Nejad S.M., Takahashi H., Narihira K., Kikuta T. Enhanced mechanical damage to in vitro cancer cells by high-intensity-focused ultrasound in the presence of microbubbles and titanium dioxide. J. Med Ultrason. 2015;42:449–455. doi: 10.1007/s10396-015-0626-4. [DOI] [PubMed] [Google Scholar]
- 41.Feril L.B., Tachibana K., Kondo T., Ogawa R., Zhao Q.-L., Yamaguchi K., Ogawa K., Endo H., Irie Y., Harada Y. Hypotonia-induced cell swelling enhances ultrasound-induced mechanical damage to cancer cells. J. Med Ultrason. 2010;37:3–8. doi: 10.1007/s10396-009-0241-3. [DOI] [PubMed] [Google Scholar]
- 42.Wang J., Lei Y., Yu Y., Yin L., Zhang Y. Use of acetic acid to partially replace lactic acid for decontamination against Escherichia coli O157:H7 in fresh produce and mechanism of action. Foods. 2021;10:2406. doi: 10.3390/foods10102406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X., Long Q., Gao F., Han C., Jin P., Zheng Y. Effect of cutting styles on quality and antioxidant activity in fresh-cut pitaya fruit. Postharvest Biol. Technol. 2017;124:1–7. [Google Scholar]
- 44.Wang Y., Zhou F., Zuo J., Zheng Q., Gao L., Wang Q., Jiang A. Pre-storage treatment of mechanically-injured green pepper (Capsicum annuum L.) fruit with putrescine reduces adverse physiological responses. Postharvest Biol. Technol. 2018;145:239–246. [Google Scholar]
- 45.Chen C., Liu C., Jiang A., Guan Q., Sun X., Liu S., Hao K., Hu W. The effects of cold plasma-activated water treatment on the microbial growth and antioxidant properties of fresh-cut pears. Food. Bioprocess Technol. 2019;12:1842–1851. [Google Scholar]
- 46.Moreno C., Andrade-Cuvi M.J., Zaro M.J., Darre M., Vicente A.R., Concellón A. Short UV-C treatment prevents browning and extends the shelf-life of fresh-cut Carambola. J. Food Qual. 2017;2017:2548791. [Google Scholar]
- 47.Liu C., Chen C., Jiang A., Sun X., Guan Q., Hu W. Effects of plasma-activated water on microbial growth and storage quality of fresh-cut apple. Innov. Food Sci. Emerg. 2020;59 [Google Scholar]
- 48.Salgado S.P., Pearlstein A.J., Luo Y., Feng H. Quality of Iceberg (Lactuca sativa L.) and Romaine (L. sativa L. var. longifolial) lettuce treated by combinations of sanitizer, surfactant, and ultrasound. LWT - Food Sci. Technol. 2014;56:261–268. [Google Scholar]
- 49.Kou X., He Y., Li Y., Chen X., Feng Y., Z Xue, Effect of abscisic acid (ABA) and chitosan/nano-silica/sodium alginate composite film on the color development and quality of postharvest Chinese winter jujube (Zizyphus jujuba Mill. cv. Dongzao) Food Chem. 2019;270:385–394. doi: 10.1016/j.foodchem.2018.06.151. [DOI] [PubMed] [Google Scholar]
- 50.Spricigo P.C., Freitas T.P., Purgatto E., Ferreira M.D., Correa D.S., Bai J., Brecht J.K. Visually imperceptible mechanical damage of harvested tomatoes changes ethylene production, color, enzyme activity, and volatile compounds profile. Postharvest Biol. Technol. 2021;176 [Google Scholar]
- 51.Martínez-Sánchez A., Allende A., Bennett R.N., Ferreres F., Gil María.I. Microbial, nutritional and sensory quality of rocket leaves as affected by different sanitizers. Postharvest Biol. Technol. 2006;42(1):86–97. [Google Scholar]
- 52.De Corato U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020;60(6):940–975. doi: 10.1080/10408398.2018.1553025. [DOI] [PubMed] [Google Scholar]
- 53.Santos J.G.., Fernandes F.A.N., de Siqueira Oliveira L., de Miranda M.R.A. Influence of ultrasound on fresh-cut mango quality through evaluation of enzymatic and oxidative metabolism. Food. Bioprocess Technol. 2015;8(7):1532–1542. [Google Scholar]
- 54.Khan I., Tango C.N., Miskeen S., Lee B.H., Oh D.-H. Hurdle technology: A novel approach for enhanced food quality and safety–A review. Food Control. 2017;73:1426–1444. [Google Scholar]
- 55.Ngnitcho P.-F., Khan I., Tango C.N., Hussain M.S., Oh D.H. Inactivation of bacterial pathogens on lettuce, sprouts, and spinach using hurdle technology. Innov. Food Sci. Emerg. 2017;43:68–76. [Google Scholar]
- 56.Luo K., Kim S.Y., Wang J., Oh D.-H. A combined hurdle approach of slightly acidic electrolyzed water simultaneous with ultrasound to inactivate Bacillus cereus on potato. LWT - Food Sci. Technol. 2016;73:615–621. [Google Scholar]
- 57.Samara A., Koutsoumanis K.P. Effect of treating lettuce surfaces with acidulants on the behaviour of Listeria monocytogenes during storage at 5 and 20 ℃ and subsequent exposure to simulated gastric fluid. Int. J. Food Microbiol. 2009;129:1–7. doi: 10.1016/j.ijfoodmicro.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 58.Zhang J., Yang H. Effects of potential organic compatible sanitisers on organic and conventional fresh-cut lettuce (Lactuca sativa Var. Crispa L) Food Control. 2017;72:20–26. [Google Scholar]
- 59.Huang Y.-R., Hung Y.-C., Hsu S.-Y., Huang Y.-W., Hwang D.-F. Application of electrolyzed water in the food industry. Food Control. 2008;19:329–345. [Google Scholar]
- 60.B. Dhillon D. Wiesenborn C. Wolf-Hall F. Manthey Development and evaluation of an ozonated water system for antimicrobial treatment of durum wheat 74 7 2009 E396 E403. [DOI] [PubMed]
- 61.Wei K., Zhou H., Zhou T., Gong J. Comparison of aqueous ozone and chlorine as sanitizers in the food processing industry: Impact on fresh agricultural produce quality. Ozone-Sci. Eng. 2007;29:113–120. [Google Scholar]