Abstract
Dopamine transporter takes released dopamine back into presynaptic terminals and has been implicated in several aging disorders including depression. The present study was designed to demonstrate dopamine gene polymorphism, its circulatory levels, biochemical and oxidative stress parameters in geriatric population with and without depression. Thirty geriatric patients with depression and thirty age and sex matched normal controls were genotyped for Dopamine Active Transporter (DAT TaqA1 and DAT VNTR) gene polymorphisms using the Polymerase Chain Reaction-Restriction Fragment Length Polymorphism method. The frequency of genotypes and alleles were compared in study groups. Biochemical markers, oxidative stress parameters, and dopamine levels were also measured using standard protocols and compared between patients and controls. The frequency distribution of DAT TaqA1 and DAT VNTR genotypes and alleles in patients were not statistically significant as compared to controls. At DAT TaqA1 gene polymorphism we found that the levels of dopamine were significantly high in genotypes A1A2 as compared to A2A2 (p ≤ 0.01). The present study demonstrated elevated levels of Catalase, Lipid Peroxide, and Glutathione Reductase, whereas decreased levels of Superoxide Dismutase, Dehydroepiandrosterone, Glutathione Peroxidase and Melatonin, in depressive patients as compared to controls. Our results clearly suggested that elevated mean levels of Catalase, Lipid Peroxides and Glutathione Reductase and decreased levels of Dehydroepiandrosterone, Superoxide Dismutase, Glutathione Peroxidase and Melatonin in depressed individuals may be a consequence of depression. Moreover, DAT TaqA1 allele A1 has a protective effect with high dopamine levels and DAT VNTR genotype 10R/10R has the highest protective effect followed by 9R/10R and 10R/11R.
Keywords: Depression, Dopamine, Oxidative stress, Neurotransmitter, DAT TaqA1, DAT VNTR
Introduction
With the increase in elderly population, depression is becoming one of the most common health problems. According to recent reports nearly 8–16% of ageing population (> 65) living in the society are suffering from depressive symptoms [1], and is accountable for high morbidity and early mortality [2]. According to World Health Organization (WHO), depression is one of the main causes of suicide and will become the leading cause of global disease burden soon. According to WHO reports the burden of depression is 50% higher for females as compared to males and Indians are reported to be among the world’s most depressed. The overall prevalence of depression is 9%, of major depressive episode is 36%, and the average age of onset of depression in India is 31.9 years [3]. Ageing population with depression is an indicator of poor clinical course and there is an increased possibility of relapse [3–5] with minimal chance of remission [6]. Genetic component accounts for about 40% in progression, heredity and clinical course of the disease [7]. Many recent studies have shown the role of disturbed gene expression or single nucleotide polymorphism of neurotransmitter genes in the progression of disease [8]. Neurotransmitters have important role in brain disorders, thus are targets for pharmacological interventions. Dysfunction in dopaminergic neurotransmission has also been suggested in the pathophysiology of depression. Several studies consequently reported reduced plasma levels of dopamine metabolite Homovanillic Acid (HVA) in depressed patients [9]. In contrast, suicide victims without a history of depression have normal levels of HVA, and dopamine as reported by some autopsy studies [10].
Genes which are involved in metabolic and signaling pathways of dopamine are reported to have significant role in the Emotional Instability [11], Depression [12], and Attention Deficit Hyperactivity Disorder (ADHD) [13]. Moreover, oxidative stress is reported to be associated with neurodegenerative diseases and psychiatric disorders in addition to various cell signaling pathways [14]. Oxidative stress has also been reported to have potential to cause abnormality in biomolecules like lipids, proteins, and Deoxyribo Nucleic Acid (DNA) [15].
In view of the pressing need to prevent depression in geriatric population, and to elucidate the genetic basis for depression and its complications current case control study was designed to investigate Dopamine transporter gene polymorphisms (Variable Number Tandem Repeats (VNTR) and Taq polymorphism), their circulatory levels, oxidative stress parameters, biochemical markers of ageing (HbA1C, DHEAS, Cortisol, Melatonin) and depression in Geriatric population of North India.
Materials and Methods
Thirty (30) geriatric patients with depression according to Diagnostic and Statistical Manual of Mental Disorder-Fourth Edition criteria, with ≥ 10 points on Geriatric Depression Scale (GDS) and ≥ 60 years of age who were free from any type of psychotic drugs for last 4 weeks were enrolled from the outdoor patients OPD of Department of Psychiatry, Era’s Lucknow Medical College and Hospital, Era University, Lucknow. Critically ill patients or known cases of other neurodegeneratory or psychiatry disorders like Alzheimer’s disease, Parkinson’s disease, Schizophrenia, Epilepsy, or any type of substance abuse, or age < 60 years or individuals on psychotic drugs in last 4 months were excluded from the present study. Thirty (30) age and sex matched controls with no previous psychiatric history, without any substance dependence and ≥ 60 years of age were also enrolled and included geriatric volunteers from surrounding areas of Era University, Lucknow.
Medical, Demographic, Family, Nutritional and Substance Dependence information of each subject was collected and filled on predetermined structured Performa. The study was started after obtaining ethical clearance from institutional ethical committee of Era’s Lucknow Medical College and Hospital, Era University, Lucknow. Moreover, written informed consent was taken from all the subjects.
Laboratory Measurements
About 6 ml blood sample was collected from all the enrolled subjects. Serum was separated from 2 ml of blood collected in plain vial by centrifugation at 2000 rpm, lysate was prepared from 2 ml of blood sample taken in Ethylene Diamine Tetra Acetic Acid (EDTA) vial, Isolation of DNA was done from 1 ml of blood collected in EDTA vial and remaining 1 ml of blood in EDTA vial was used for the estimation of HbA1C. Thereafter Serum, Plasma, Lysate and DNA were stored at − 20 °C until analyzed for biochemical markers, oxidative stress parameters, dopamine levels and genetic polymorphism study.
The percent of hemoglobin A1c (HbA1c) in human whole blood was determine by using ion—exchange high performance liquid chromatography (HPLC) Bio-Rad D-10. Estimation of DHEAS, Melatonin, and Cortisol was done by ELISA kit method as per the standard protocol (Abcam Cambridge, MA, USA).
The estimation of Dopamine was done using HPLC. The mobile phase used was buffer acetate (pH 4.66) along with methanol with a flow rate of 0.8 ml/min [16]. 1 mg/ml of each sample was launched into chromatography column. All estimations were done at 4 °C and 40 °C and Dopamine, HVA and DOPAC were estimated at different wavelengths of 220, 260 and 280 respectively. Flow rate was kept at 1.0 ml/min and injection volume at 5 μl. The results were presented as mg/dl.
The estimation of catalase (CAT) was done using the method as described by Aebi et al. [17] with H2O2 taken as substrate. The catalase activity was presented as nmole H2O2 catabolized per min per mg of protein. Likewise Superoxide Dismutase activity was measured by its capacity to reduce nitro blue tetrazolium in the presence of nitro blue tetrazolium as per the method of Mc Chord and Fridovich [18]. The reading was taken at 560 nm on spectrophotometer and presented as U/mg protein. The estimation of glutathione peroxidase (GSH-Px) was done according to the method of Pagila and Valentine [19]. The reactants used were GSH, NADPH and H2O2 and presented as nmoles of NADPH oxidized per min per mg of protein. Glutathione Reductase was estimated as per the procedure described by Hazelton and Lang [20]. Lipid Peroxide was also estimated by the procedure described by Okhawa et al. [21]. Lipid Peroxide was measured using Thiobarbiuteric acid (TBA) which acts on lipid peroxides, fatty acid, and lipid hydroperoxides to give coloured product which is then measured through spectrophotometer at 532 nm and are then presented as MDA/mg protein.
DAT VNTR and Taq Polymorphism
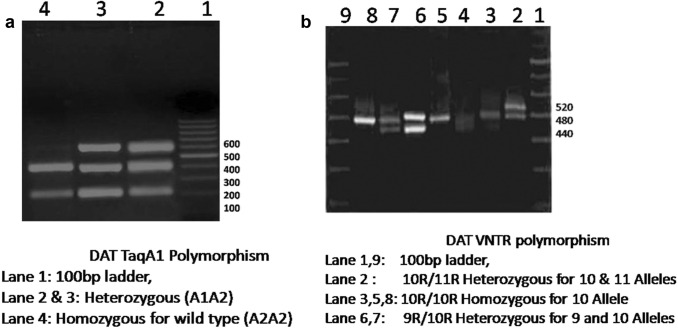
The DNA of all subjects was isolated using the DNA extraction kit from Qiagen, USA. For Taq polymorphism primers used were F 5′CCCTTCCTGAGTGTCATCA3′ and R 5′CGGCTGGCCAAGTTGTCTA 3′. The PCR amplification was done for 35 cycles (98 °C for 10 s, 58 °C for 60 s, 72 °C for 60 s) and final extension of 5 min at 72 °C. After amplification the PCR product was digested with restriction Enzyme TaqI and detected using 2% Agarose gel electrophoresis. A1A1 genotype resulted in band size of 623 bp whereas A2A2 genotype resulted in two bands of 423 + 200 bp (Fig. 1a). For DAT VNTR polymorphism the primers used were F 5′TGTGGTGTAGGGAACGGCCTGAG3′ and R 5′CTTCCTGGAGGTCACGGCTCAAGG3′. The PCR amplification was done with Initial denaturation at 94 °C for 2 min, than 40 cycle of denaturation at 94 °C for 15 secs, annealing at 62 °C for 15 secs and 72 °C for 45 s and final extension of 5 min at 72 °C. The PCR products were then checked directly using 3% Agarose gel electrophoresis. The band size of 440 bp (9R allele), 480 bp (10R allele), and 520 bp (11R allele) were included as per the add health data [22] (Fig. 1b).
Fig. 1.
Gel photograph of DAT TaqA1 Polymorphism and DAT VNTR polymorphism
Statistical Analysis
INSTAT 3.0 [23] Software (San Diego, CA) was used for statistical analysis. χ2 test was performed for univariate analysis to establish the interrelation between polymorphism, dopamine levels and depression. Genotype count was used to estimate the frequencies of Alleles and Genotypes. All analysis were two tailed and p value less than 0.05 was considered statistically significant. Variables which were quantitative were expressed as mean ± SD and Welch’s corrected unpaired t test was used to investigate the difference in blood parameters between patient and controls groups.
Results
Biochemical Profile
Table 1 demonstrates different biochemical parameters in study groups. We found statistically significant difference between patient and control group in all biochemical parameters except HbA1C, Cortisol and Dopamine levels. We found significantly increased mean levels of Catalase, Glutathione Reductase and Lipid Peroxide whereas decreased level of DHEA, Melatonin, SOD, and Glutathione Peroxidase (Gpx) in patients as compared to controls. Furthermore, we also found decreased level of dopamine and increased levels of HbA1C and cortisol in patients but not to a statistically significant level.
Table 1.
Biochemical characteristics of patients and controls
| S. no. | Variables | Patients N = 30 |
Controls N = 30 |
p value |
|---|---|---|---|---|
| 1 | Dopamine (mg/dl) | 0.101 ± 0.028 | 0.102 ± 0.038 | 0.908 |
| 2 | HbA1C (%) | 5.73 ± 1.67 | 4.47 ± 0.65 | 0.412 |
| 3 | DHEA (ng/ml) | 2.57 ± 1.38 | 3.74 ± 2.65 | 0.049* |
| 4 | Cortisol (ng/ml) | 28.61 ± 12.59 | 27.85 ± 12.97 | 0.818 |
| 5 | Melatonin (ng/ml) | 16.40 ± 8.15 | 27.89 ± 8.0 | < 0.001* |
| 6 | SOD (unit/mg) | 10.06 ± 4.62 | 30.50 ± 7.50 | < 0.001* |
| 7 | CAT (unit/mg) | 19.15 ± 8.60 | 6.28 ± 6.12 | < 0.001* |
| 9 | GR (units/min/mg) | 23.06 ± 6.99 | 16.48 ± 8.10 | 0.001* |
| 10 | GPx (units/min/mg) | 7.93 ± 6.22 | 19.78 ± 6.68 | < 0.001* |
| 11 | LPO (nmole/mda/mg) | 31.76 ± 18.66 | 17.41 ± 7.28 | 0.0004* |
All data are shown as mean ± SD
*p < 0.05 is considered statistically significant
Frequency Distribution of DAT Taq A1 Genotypes and Alleles
Table 2 demonstrates the frequency distribution of DAT Taq A1 alleles and genotypes among patient and healthy control group. We found that frequency distribution of TaqA1 genotypes and alleles did not differ significantly (TaqA1 (A2A2 vs. A1A2): p = 0.795; OR = 1.14), and (TaqA1 (A1 vs. A2): p = 0.838, OR = 1.10) between patient and control group. DAT TaqA1 genotype frequencies were not in hardy–Weinberg equilibrium and this may be because of small sample size (p = 0.046).
Table 2.
Frequency distribution of genotypes and alleles of DAT TaqA1 polymorphism in Patients and controls
| Genotypes | Patients N = 30 |
Controls N = 30 |
OR (95% CI) | χ2 | p value |
|---|---|---|---|---|---|
| A2A2 | 14 (46.67%) | 13 (43.34%) | 1.14 (0.413–3.167) | 0.067 | 0.795 |
| A1A2 | 16 (53.33%) | 17 (56.66%) | |||
| A1A1 | 0 (0%) | 0 (0%) | |||
| Alleles | |||||
| A2 | 44 (73.33%) | 43 (71.66%) | 1.10 (0.466–2.594) | 0.041 | 0.838 |
| A1 | 16(26.67%) | 17 (28.34%) | |||
*p < 0.05 is considered statistically significant
Frequency Distribution of DAT VNTR Genotypes and Alleles
Table 3 demonstrates the frequency of DAT VNTR genotypes and alleles in patient and control group. We found that frequency of DAT VNTR genotypes and alleles did not differ significantly (VNTR (10R/10R vs. 9R/10R vs. 10R/11R): p = 0.722; χ2 = 0.650) and (VNTR (9R vs. 10R vs. 11R): p = 0.778, χ2 = 0.500) between patient and control group. DAT VNTR genotype frequencies are consistent with Hardy–Weinberg equilibrium (p = 0.46).
Table 3.
Frequency distribution of genotypes and alleles of DAT VNTR polymorphism in Patients and controls
| Genotypes | Patients (N=30) | Controls (N = 30) | χ2 | p value |
|---|---|---|---|---|
| 10 R/10R | 9 (30%) | 11 (36.66%) | 0.650 | 0.722 |
| 10R/11R | 13 (43.34%) | 10 (33.34%) | ||
| 9R/10R | 8 (26.66%) | 9 (30 %) | ||
| Alleles | ||||
| 9R | 8 (13.34%) | 9 (15%) | 0.500 | 0.778 |
| 10R | 39 (65%) | 41 (68.34%) | ||
| 11R | 13 (21.66%) | 10 (16.66%) | ||
*p < 0.05 is considered statistically significant
Dopamine Levels in Different DAT A1 and DAT VNTR Genotypes
At DAT TaqA1 gene polymorphism we found that the levels of dopamine are significantly high in genotypes A1A2 as compared to A2A2 (p ≤ 0.01) thereby showing that allele A1 is having a protective effect with high dopamine levels. On comparing dopamine levels in DAT VNTR polymorphism we found that individuals with genotype 10R/10R have significantly higher dopamine levels when compared with 10R/11R (p ≤ 0.01) or 9R/10R (0.02). Furthermore, on comparing 9R/10R with 10R/11R we found significantly higher levels in 9R/10R as compared to 10R/11R (p ≤ 0.01). The highest level of dopamine was observed in 10R/10R followed by 9R/10R and than by 10R/11R (Table 4).
Table 4.
Dopamine levels in different DAT A1 and DAT VNTR genotypes
| Variable | Genotypes | p value | 95%CI | |
|---|---|---|---|---|
| Dopamine (mg/dL) | DAT TaqA1 A1A2 | DAT TaqA1 A2A2 | ||
| 0.120 ± 0.034 | 0.080 ± 0.013 | < 0.001 | − 0.053 to − 0.026 | |
| DAT VNTR 10R/10R (N = 20) | DAT VNTR 10R/11R (N = 23) | |||
| 0.135 ± 0.037 | 0.078 ± 0.015 | < 0.001 | − 0.074 to − 0.037 | |
| DAT VNTR 10R/10R (N = 20) | DAT VNTR 9R/10R (N = 17) | |||
| 0.135 ± 0.037 | 0.095 ± 0.004 | < 0.002 | − 0.057 to − 0.021 | |
| DAT VNTR 10R/11R (N = 23) | DAT VNTR 9R/10R (N = 17) | |||
| 0.0788 ± 0.015 | 0.095 ± 0.004 | < 0.001 | − 0.010 to − 0.023 | |
*Values entered as mean ± SD
*p < 0.05 is considered statistically significant
Discussion
In conditions with pain, anxiety, acute tissue damage or fright, numerous metabolic as well as endocrine changes take place, of which increase in the circulatory levels of cortisol is one of the most significant physiological effect [24]. We in our study found an increased level of cortisol in patient group as compared to control group; however, it was not statistically significant. Our results are in concordance with an earlier study by Theodor Moica et al. [25]. Serum cortisol levels have been used as an index of stress in a wide range of studies [26] and have been taken as a biochemical marker of stress [27]. A study by Cowen et al. [28] showed that around 50% of patients having Major Depressive Disorder (MDD) comprise hypersecretion of cortisol. There is an increased neurotoxic effect of cortisol in hippocampus and prefrontal cortex, where hyper-cortisolemia causes cell death by declining the quantity of dendritic spines and synapses, by dropping the number of glial cells and by causing dendritic atrophy [29].
Observational studies suggest that physiological levels of melatonin, a pineal gland hormone are reduced in demented or depressed patients [30]. Our findings are in concordance with other studies demonstrating decreased physiological melatonin levels in subjects with mental illness or major depressive disorder [31]. However, previous study conducted by Obayashi et al. [32] demonstrated that elevated melatonin levels within its reference range were linked with lesser incidence of Cognitive impairment and dejected mood. The plausible mechanisms underlying the relationship between circulatory levels of melatonin and cognitive function include melatonin’s antioxidative action, antiamyloidogenic properties, antidepressive and atheroprotective effects. Melatonin acts as a potent antioxidant by free radical scavenging at extra and intracellular levels and may have a protective effect against oxidative injury to the brain and contribute to neuroprotection against amyloid neurotoxicity [33].
Steroid hormone Dehydroepiandrosterone (DHEA) in contrast to cortisol, tends to turn down with aging [34]. It has been reported to be associated with anxiety [35] and depression [36]. In the present study significant negative association was observed between DHEA levels and stressful life events. Moreover, DHEA is also linked to stress response process because it has inducible effects on catecholamine synthesis and secretion and at the same time has protective role in inflammation and act as glucocorticoid antagonist at molecular as well as physiological levels [37]. Therefore, DHEA plays an essential part in regulating physiological regulatory systems concerned with stress response specifically the hypothalamic pituitary adrenal axis (HPA) and some studies demonstrated negative correlation between DHEA levels and chronic stress [38]. Subjects with increased psychological stress exhibited elevated levels of DHEA and Cortisol. Our results are in concordance with studies on other populations.
In our present study, we found low levels of dopamine in patients as compared to controls but not to statistically significant level. Studies using electrophysiological, brain imaging, and pharmacological procedure in both human and animal models of depression have provided evidence for the presence of Dopamine (DA) dysfunctions as demonstrated by Yadid and Friedman [39]. Reduction in DA release into the synapse notably reduce homovanillic acid, which is a DA metabolite and also decreases striatal dopaminergic action as reported in depressed patients when compared with controls [40].
Our results revealed that there is a significant elevated level of plasma LPO in patients when compared with healthy controls. Similar results were obtained by Sarandol et al. [41]. The increased malondialdehyde level signifies membrane injury resulting from lipid peroxidation and elevated oxidative stress in depressive patients. The brain has comparatively weak anti-oxidative defenses mechanism against such type of free radical attack [42]. Damaged antioxidant defense systems alongwith elevated monoamine catabolism in depression possibly may result in rise of ROS generation [43]. This may defend the increasing in serum MDA levels in our present study.
We observed that the patients with depression have lower activity of antioxidant enzyme SOD as compared with healthy controls. SOD acts as reactive oxygen species scavenger, thus eliminates the reactive oxygen species and maintains the oxidative balance [44]. Earlier, Rybka et al. [45] also reported reduced levels of SOD in depression patients as compared to healthy controls but it was not statistically significant. Constantly reduced level of SOD may signify some principal pathophysiological conditions in the variety of bipolar disorders or inability of coping with oxidative stress in bipolar disorders [46].
Catalase activity was found significantly increased in depression patients as compared with healthy controls in our study which is in concordance with study of Tsai and Huang [47] and it may be an offsetting mechanism for the higher oxidative stress in MDD patients in a critical phase. Moreover, significantly decreased activity of glutathione peroxidase and increased activity of glutathione reductase were also found in our study and that may due to depression induced ROS generation which in turn may have perturbed antioxidant enzymes levels.
In our study, we did not find any association at DRD2 gene Taq1A gene polymorphism with depression. A study conducted by He et al. [48] in Chinese Han population also did not report any association between DRD2 gene Taq1A. Moreover, the findings of our study are in concordance with the study by He et al. [48]. Furthermore, at DAT1 VNTR polymorphism also we did not find any significant results in the frequency distribution of genotypes and alleles in patients with control groups. These findings are in concordance with the finding of Frisch et al. [49] conducted in unipolar MDD patients of Ashkenazi and non-Ashkenazi origin.
In conclusion, our results clearly suggested that elevated mean levels of Glutathione Reductase, Lipid Peroxides and Catalase, and lower mean levels of DHEA, Melatonin, SOD, and Gpx in depressed individuals may be a consequence of depression. As far as DAT taqA1 genetic polymorphism is concerned it may be pointed out that as our sample size was quite small therefore we may not have been able to observe any mutant genotype, and no significant results were obtained when genotype and allele frequency distribution was compared between the patients and controls. At DAT VNTR level also we did not get any significant results on comparing the genotype and allele frequency distribution among patients and controls. However, we may add that DAT TaqA1 allele A1 has a protective effect with high dopamine levels and DAT VNTR genotype 10R/10R has the highest protective effect followed by 9R/10R. The limitation of the present study was small sample size.
Acknowledgements
Support from Indian Council of Medical Research Short Term Studentship Program 2018 (Reference ID: 2018-01998).
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed Consent
Signed informed consent was obtained from all enrolled subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58:249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- 2.Schulz R, Drayer RA, Rollman BL. Depression as a risk factor for non-suicide mortality in the elderly. Biol Psychiatry. 2002;52:205–225. doi: 10.1016/s0006-3223(02)01423-3. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell AJ, Subramaniam H. Prognosis of depression in old age compared to middle age: a systematic review of comparative studies. Am J Psychiatry. 2005;162:1588–1601. doi: 10.1176/appi.ajp.162.9.1588. [DOI] [PubMed] [Google Scholar]
- 4.Licht-Strunk E, Van Der Windt DA, Van Marwijk HW, De Haan M, Beekman AT. The prognosis of depression in older patients in general practice and the community. A systematic review. Fam Pract. 2007;24:168–180. doi: 10.1093/fampra/cml071. [DOI] [PubMed] [Google Scholar]
- 5.Tedeschini E, Levkovitz Y, Iovieno N, Ameral VE, Nelson JC, Papakostas GI. Efficacy of antidepressants for late-life depression: a meta-analysis and meta-regression of placebo-controlled randomized trials. J Clin Psychiatry. 2011;72:1660–1668. doi: 10.4088/JCP.10r06531. [DOI] [PubMed] [Google Scholar]
- 6.Beekman AT, Geerlings SW, Deeg DJ, Smit JH, Schoevers RS, De Beurs E, et al. The natural history of late-life depression: a 6-year prospective study in the community. Arch Gen Psychiatry. 2002;59:605–611. doi: 10.1001/archpsyc.59.7.605. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and metaanalysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 8.Bufalino C, Hepgul N, Aguglia E, Pariante CM. The role of immune genes in the association between depression and inflammation: a review of recent clinical studies. Brain Behav Immun. 2003;31:31–47. doi: 10.1016/j.bbi.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Mitani H, Shirayama Y, Yamada T, Kawahara R. Plasma levels of homovanillic acid, 5- hydroxyindoleacetic acid and cortisol, and serotonin turnover in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:531–534. doi: 10.1016/j.pnpbp.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 10.Beskow J, Gottfries CG, Roos BE, Winblad B. Determination of monoamine and monoamine metabolites in the human brain: post mortem studies in a group of suicides and in a control group. Acta Psychiatr Scand. 1976;53:7–20. doi: 10.1111/j.1600-0447.1976.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 11.Beiderbeck DI, Reber SO, Havasi A, Bredewold R, Veenema AH, Neumann ID. High and abnormal forms of aggression in rats with extremes in trait anxiety—involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology. 2012;37:1969–1980. doi: 10.1016/j.psyneuen.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression; a postmortem study. Biol Psychiatry. 2002;552:740–748. doi: 10.1016/s0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- 13.Levy F. The dopamine theory of attention deficit hyperactivity disorder (ADHD) Aust N Z J Psychiatry. 1991;25:277–283. doi: 10.3109/00048679109077746. [DOI] [PubMed] [Google Scholar]
- 14.Durackova Z. Free radicals and antioxidants for non-experts. In: Laher I, editor. Systems biology of free radicals and antioxidants. Berlin: Springer; 2014. pp. 3–38. [Google Scholar]
- 15.Galecki P. Oxidative stress in depression. In: Laher I, editor. Systems biology of free radicals and antioxidants. Berlin: Springer; 2014. pp. 2369–2395. [Google Scholar]
- 16.Baranowska I, Zydron M. Liquid chromatography in the analysis of neurotransmitters and alkaloids. J Chromatogr Sci. 2002;40(4):224–228. doi: 10.1093/chromsci/40.4.224. [DOI] [PubMed] [Google Scholar]
- 17.Aebi H. Catalase in vitro methods. Enzymologia. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 18.McCord JM, Fridovich I. SOD enzyme function for erythrocuprein. J Biol Chem. 1969;224:6049–6055. [PubMed] [Google Scholar]
- 19.Pagila DE, Valentine WN. Studies on the quantitation and qualitation characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 20.Hazelton GA, Lang CA. GSH content of tissue in ageing mouse. Biochem J. 1985;188:25–30. doi: 10.1042/bj1880025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohkawa H, Oshiba N, Yagi K. Assay of lipid peroxides in animal tissue by thiobarbutyric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 22.Hoper CJ, Timberlake D, Haberstick B, Lessem JM, Ehringer MA, Smolen A, et al. Genetic influences on quantity of alcohol consumed by adolescents and young adults. Drug Alcohol Depend. 2005;78:187–193. doi: 10.1016/j.drugalcdep.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Wass John. Software: statistics, fast and easy. Science. 1998;282:5394–5652. [Google Scholar]
- 24.Koray M, Dulger O, Ak G, Horasanli S, Ucok A, Tanyeri H, et al. The evaluation of anxiety and salivary cortisol in patients with oral lichenplanus. Oral Dis. 2003;9:298–301. doi: 10.1034/j.1601-0825.2003.00960.x. [DOI] [PubMed] [Google Scholar]
- 25.Moica T, Grecu IG, Moica S, Grecu MG, Buicu GE. Cortisol and hippocampal volume as predictors of active suicidal behavior in major depressive disorder: case report. Balkan Med J. 2016;33(6):706–708. doi: 10.5152/balkanmedj.2016.150842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheriff MJ, Krebs CJ, Boonstra R. Assessing stress in animal populations: do fecal and plasma glucocorticoids tell the same story. Gen Comp Endocrinol. 2010;166(3):614–619. doi: 10.1016/j.ygcen.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Jameel MK, Joshi AR, Dawane J, Padwal M, Joshi AR, Pandit VA, et al. Effect of various physical stress models on serum cortisol level in wistar rats. J Clin Diagn Res. 2014;8(3):181–183. doi: 10.7860/JCDR/2014/7210.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cowen PJ. Cortisol, serotonin and depression: all stressed out. Br J Psychiatry. 2002;180:99–100. doi: 10.1192/bjp.180.2.99. [DOI] [PubMed] [Google Scholar]
- 29.Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Wu YH, Feenstra MGP, Zhou JN, Liu RY, Torano JS, Van Kan HJM, et al. Molecular changes underlying reduced pineal melatonin levels in Alzheimer disease: alterations in preclinical and clinical stages. J Clin Endocrinol Metab. 2003;88(12):5898–5906. doi: 10.1210/jc.2003-030833. [DOI] [PubMed] [Google Scholar]
- 31.Ionov M, Burchell V, Klajnert B, Bryszewska M, Abramov AY. Mechanism of neuroprotection of melatonin against-amyloid neurotoxicity. Neuroscience. 2011;180:229–237. doi: 10.1016/j.neuroscience.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 32.Obayashi K, Saeki K, Iwamoto J, Tone N, Tanaka K, Kataoka H, et al. Physiological levels of melatonin relate to cognitive function and depressive symptoms: the HEIJO-KYO cohort. J Clin Endocrinol Metab. 2015;100(8):3090–3096. doi: 10.1210/jc.2015-1859. [DOI] [PubMed] [Google Scholar]
- 33.Hennebert O, Chalbot S, Alran S, Morfin R. Dehydroepiandrosterone 7alpha-hydroxylation in human tissues: possible interference with type 1 11beta-hydroxysteroid dehydrogenase-mediated processes. J Steroid Biochem Mol Biol. 2007;104(3–5):326–333. doi: 10.1016/j.jsbmb.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Front Neuroendocrinol. 2009;30(1):65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markopoulou K, Papadopoulos A, Juruena M, Poon L, Pariante C, Cleare A. The salivary ratio of cortisol/DHEA in treatment resistant unipolar depression. Psychoneuroendocrinology. 2009;34(1):19–26. doi: 10.1016/j.psyneuen.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Reddy PL, Khanna S, Subhash MN, Channabasavanna SM, Rao BS. CSF amine metabolites in depression. Biol Psychiatry. 1992;31:112–118. doi: 10.1016/0006-3223(92)90198-9. [DOI] [PubMed] [Google Scholar]
- 37.Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2006;114(3):187–193. doi: 10.1111/j.1600-0447.2006.00801.x. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton LD, Meston CM. The role of salivary cortisol and DHEA-S in response to sexual, humorous, and anxiety-inducing stimuli. Hormones Behav. 2011;59(5):765–771. doi: 10.1016/j.yhbeh.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res. 2008;172:265–286. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- 40.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C] raclopride. J Neurosci. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sarandol A, Sarandol E, Eker S, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative system. Hum Psychopharmacol. 2007;22:67–73. doi: 10.1002/hup.829. [DOI] [PubMed] [Google Scholar]
- 42.Stocks J, Gutteridge JMC, Sharp RJ, Dormandy TL. Assay using brain homogenate for measuring the antioxidant activity of biological fluids. Clin Sci Mol Med. 1974;47:215–222. doi: 10.1042/cs0470215. [DOI] [PubMed] [Google Scholar]
- 43.Pandey GN, Sharma RJ, Janicak PG, Davis JM. Monoamine oxidase and cortisol response in depression and schizophrenia. Psychiatr Res. 1992;44:1–8. doi: 10.1016/0165-1781(92)90064-a. [DOI] [PubMed] [Google Scholar]
- 44.Stefanescu C, Ciobica A. The relevance of oxidative stress status in first episode and recurrent depression. J Affect Disord. 2012;143(1–3):34–38. doi: 10.1016/j.jad.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 45.Rybka J, Kornatowska KK, Lezanska PB, Majsterek I, Carvalho LA, Cattaneo A, et al. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic Biol Med. 2013;63:187–194. doi: 10.1016/j.freeradbiomed.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 46.Selek S, Savas HA, Gergerlioglu HS, Bulbul F, Uz E, Yumru M. The course of nitric oxide and superoxide dismutase during treatment of bipolar depressive episode. J Affect Disord. 2008;107(1–3):89–94. doi: 10.1016/j.jad.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Tsai MC, Huang TL. Increased activities of both superoxide dismutase and catalase were indicators of acute depressive episodes in patients with major depressive disorder. Psychiatry Res. 2015;235:38–42. doi: 10.1016/j.psychres.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 48.He M, Yan H, Duan ZX, Qu W, Gong HY, Fan ZL, et al. Genetic distribution and association analysis of DRD2 gene polymorphisms with major depressive disorder in the Chinese Han population. Int J Clin Exp Pathol. 2013;6(6):1142–1149. [PMC free article] [PubMed] [Google Scholar]
- 49.Frisch A, Michaelovsky E, Rockah R, Amir I, Hermesh H, Laor N, et al. Association between obsessive-compulsive disorder and polymorphisms of genes encoding components of the serotonergic and dopaminergic pathways. Eur Neuropsychopharmacol. 2000;10(3):205–209. doi: 10.1016/s0924-977x(00)00071-7. [DOI] [PubMed] [Google Scholar]