Abstract
Sickle cell disease (SCD) is a blood disorder with few treatment options currently available. However, in recent years, there has been much progress toward developing new therapies and curative treatments to help patients with SCD. Stem cell transplant remains the only approved curative treatment for SCD, but new clinical trials are being initiated using gene therapy and gene editing. We surveyed patients with sickle cell disease (N=9) about attitudes toward stem cell transplant, gene therapy to add a new healthy gene, gene editing to up-regulate fetal hemoglobin, or gene editing to correct the point mutation. The participants read a fact sheet that included objective information on each curative treatment. When asked which curative treatment each participant would choose, all four options were selected at least once. The most highly selected treatment was gene correction gene editing (N=4). Participants generally agreed that the four treatment options are beneficial but were more mixed in their thoughts on whether the options are dangerous. Reasons for selecting a particular curative treatment were variable, but the most selected reasons were perception of a cure (N=4) or decreased severity (N=4), and not needing a donor (N=4). We are at the beginning stages of understanding how patients with SCD make decisions about curative treatments. Currently, patients may be interested in any of the four possibilities for curative treatments, with gene correction gene editing as the most popular choice. Reasons for choosing one treatment over another are mixed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12687-021-00562-z.
Keywords: Sickle cell disease, Decision-making, Gene editing, Gene therapy, Curative treatments, CRISPR
Introduction
Sickle cell disease (SCD) is a blood disorder that affects over 90,000 individuals in the USA (CDC 2015). This includes approximately 9,000 individuals in California (CDC 2015) and over 7,000 individuals in Georgia (RuSH - CDC 2012). The cause of hemoglobin S is a p.Glu6Val pathogenic variant in the β-globin gene, which causes hemoglobin to become sickle-shaped in the deoxygenated state; this leads to cell polymerization and aggregation (Inusa et al. 2019). At birth, about 70% of hemoglobin is fetal hemoglobin (HbF), which results in a milder, but not asymptomatic phenotype (Akinsheye et al. 2011).
For many years, the only treatment option for individuals with SCD was to treat the symptoms by prescribing pain reducers or by providing narcotics, antibiotics, fluids, hydroxyurea, and red blood cell transfusions (Lanzkron et al. 2008). The only current FDA-approved and widespread curative therapy for SCD is by hematopoietic stem cell transplantation (SCT) (Shenoy 2013). SCT involves replacing the bone marrow of an individual with SCD with bone marrow from a donor (Fitzhugh et al. 2014). SCT has successfully cured SCD for many patients and has been well studied for SCD and other diseases (Fitzhugh et al. 2014). SCT requires the use of chemotherapy to remove the transplant recipient’s cells, and those cells are then replaced with donor cells (Bolaños-Meade and Brodsky 2015). Graft-versus-host disease is a possible side effect of SCT (Bolaños-Meade and Brodsky 2015). More recently, the FDA approved clinical trials for gene therapy and gene editing for SCD (clinicaltrials.gov). There have been several successful gene therapy reports for SCD patients in recent years (Ribeil et al. 2017; Rubin 2019). Gene therapy as a cure for SCD works by inserting the correct β-globin sequence into an inactivated virus (Demirci et al. 2019). This virus is then introduced into a portion of the removed bone marrow of a patient with SCD (Demirci et al. 2019). The bone marrow is returned to the patient (Demirci et al. 2019). Researchers are also using CRISPR-Cas9 to either induce γ-globin production (Weber et al. 2020) or induce double stranded breaks and allow for repair of the HBB gene (Tasan et al. 2016). In both techniques, clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein 9 (Cas9) are used to induce a genetic change, which allows for a subsequent process that leads to SCD being cured (Antoniani et al. 2018).
Past studies have investigated how individuals with SCD make decisions about treatments (Hankins et al. 2007; Ross et al. 2016). Hankins et al. (2007) found that hydroxyurea was the most desirable treatment compared to blood transfusions and SCT due to the perception of safety and efficacy of hydroxyurea. Ross et al. (2016) found that individuals with higher disease severity and greater perception of poor prognosis were more likely to have an interest in undergoing SCT. Most recently, Persaud et al. (2018) found that individuals in the SCD community are interested in participating in clinical trials involving CRISPR, due to the possibility of reducing suffering, due to altruism, and because of a lack of other treatment options. At the same time, they had concerns about the dangers, the permanency, and the cost/access (Persaud et al. 2018).
There have not been any studies on how individuals with SCD will make treatment decisions in an era where gene therapy and gene editing will join SCT as a curative option. The purpose of this study is to ascertain how individuals with SCD might make decisions about treatments in order for healthcare providers to provide adequate counseling and education on the different options.
Methods
We conducted a descriptive quantitative research study using an online survey of SCD patients, recruiting from several support groups and community-based organizations in California and Georgia. California and Georgia were the two states chosen specifically due to the CDC’s publicly accessible data on SCD in these two states. Three groups were identified in each California and Georgia through online web searches and social media. There may be smaller groups and organizations that were not included in this initial recruitment stage. Support group coordinators were contacted via email with information on the study and requested that our survey be sent out to all participants on the support group contact list. Potential participants were emailed survey information through support group coordinators. Inclusion criteria included individuals with a diagnosis of SCD, those who are 18 years old or older, those with access to an electronic device to fill the survey out, and those who can read and type in English. Confirmation of SCD diagnosis was by self-report only, and not verified with medical records. The survey was available for completion from September 2019 through March 2020. The study coordinators requested that the survey be sent out twice to the support group members.
The participants were asked to read a consent document prior to completing the survey, and completion of the survey after this point was considered consent. Individuals who then met inclusion criteria were asked to complete a 27-question anonymous online survey administered by Qualtrics, an online survey software that is approved for HIPPA-related research. The survey measured demographic and social variables (Table 1). We used the CDC’s Health-Related Quality of Life (HRQOL) measure to assess disease severity; the number indicates how many days an individual perceives as being physically or mentally unhealthy (HRQOL - CDC 2018). The CDC commonly uses 14+ days per month to indicate a substantial level of impairment (HRQOL - CDC 2018). We measured the perception of the treatment options (efficacy, safety, fear, concerns, hopes, and dangers) using the Factors Influencing Preference Questionnaire (FIPQ; Hankins et al. 2007). The survey included a fact sheet, which included objective information on the four different treatment types including advantages and disadvantages of each. All potential participants (including those who did not complete the survey) had the opportunity to enter into a lottery for four $100 gift cards, which were used based in part on California requirements for reimbursement after research, and in part because we wished to offer potential participants a larger incentive, as compared to offering smaller honorarium for each respondent.
Table 1.
Demographics
| Participant | Age range | Race | Sex | State/location | Area classification | Religiosity | Religion practiced | Education level | Treatment chosen | Self-reported general health status | HRQOL unhealthy days |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40–50 | Black or African American | Male | CA | Urban | Moderately | Christian | Some college or university | Gene therapy to add a new healthy gene | Good | 15 |
| 2 | 30–40 | Black or African American | Male | CA | Urban | Very | Christian | Some college or university | Gene therapy to add a new healthy gene | Good | 30 |
| 3 | 30–40 | Black or African American | Female | CA | Urban | Very | Christian | College or university graduate | Fetal hemoglobin gene editing | Fair | 5 |
| 4 | 40–50 | Undisclosed | Female | CA | Urban | Slightly | Christian, Buddhism | College or university graduate | Gene correction gene editing | Good | 13 |
| 5 | 20–30 | Black or African American | Female | Canada | Urban | Moderately | Muslim | Some college or university | Gene correction gene editing | Fair | 7 |
| 6 | 20–30 | Black or African American | Male | CA | Suburban | Slightly | Agnostic | High school graduate or GED | Gene correction gene editing | Fair | 30 |
| 7 | 20–30 | Black or African American | Female | MN | Urban | Slightly | None | Graduate/professional degree | Gene correction gene editing | Good | 22 |
| 8 | 10–20 | Black or African American | Male | CA | Rural | Moderately | Muslim | High school graduate or GED | Stem cell transplant | Fair | 30 |
| 9 | 30–40 | Black or African American | Female | GA | Urban | Moderately | Spiritual | College or university graduate | Gene therapy to add a new healthy gene | Good | 28 |
Demographic information from each of the nine participants
Due to the nature of our survey data and the number of participants, we performed descriptive analysis of the data using version 26 of the Statistical Package for Social Sciences (SPSS) - 2019.
Results
Demographics
Of a total 23 people who began the survey, 17 met inclusion criteria and 9 people completed the entire survey; demographic details are described in Table 1. Eight people who met inclusion criteria did not complete the survey. These individuals did not provide information for why they did not submit answers for all questions. The mean age of participants was 31.89 (± 10.13) years. All but one participant (N=8) described themselves as Black and had at minimum graduated from high school or its equivalent. There was an even mix of females (N=5) and males (N=4). Most of our participants were from urban areas (N=7), with fewer being from suburban (N=1) or rural areas (N=1). The majority of participants were from California (N=6), with fewer being from other countries (Canada N=1), or other states (Georgia N=1 and Minnesota N=1). Most participants considered themselves religious, with about half of the participants (N=4) considering themselves Christian. Three of our participants (N=3) reported having one relative who also had SCD.
All participants stated that their general health was either good (N=4) or fair (N=5). Using the HRQOL, the mean number of unhealthy days (physical and mental) was 20.11 (± 10.25). All but one of our participants (N=8) have tried at least one treatment for sickle cell disease, typically blood transfusions (N=7) and/or hydroxyurea (N=4). None has undergone a bone marrow transplant themselves, though 4 report knowing someone who has, either for SCD or other reasons.
Treatment preferences
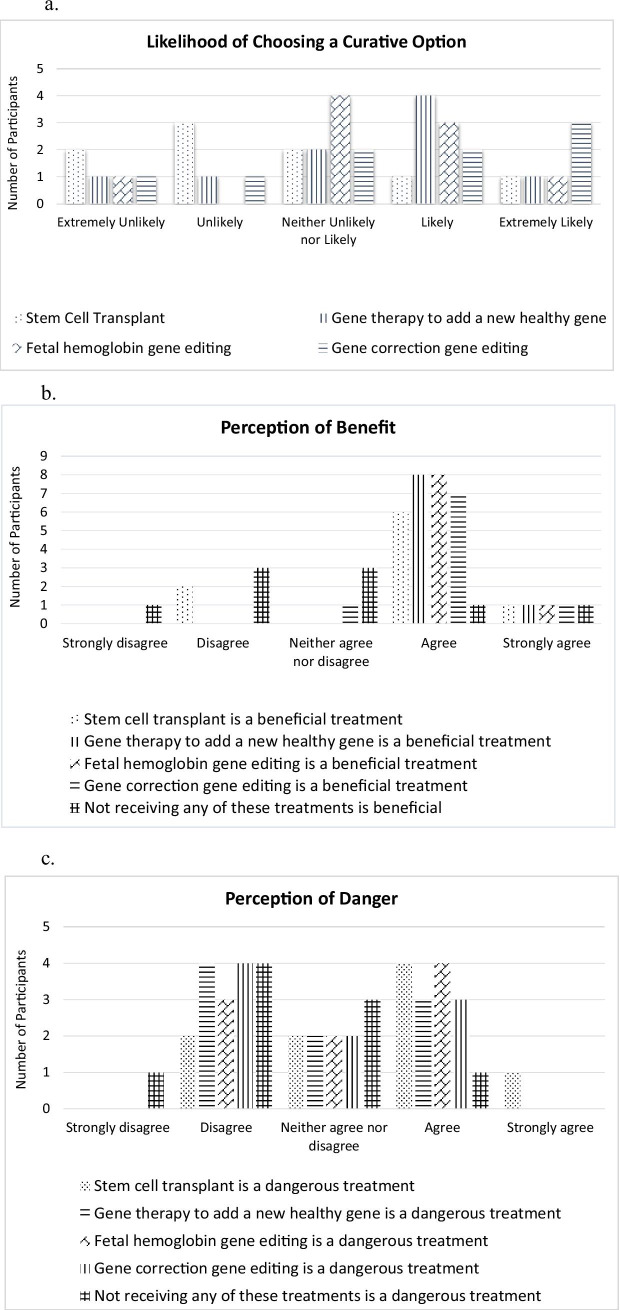
Summaries of each participant’s survey responses are in the supplementary materials (Appendix 1) and individual responses are described in Table 2. After reading a fact sheet (Appendix 2), participants were asked how likely they would be to choose a curative treatment for SCD if it were available at this time (Fig. 1). Participants were most likely to choose gene correction gene editing (N=4), followed by gene therapy to add a new healthy gene (N=3). One participant (N=1) chose fetal hemoglobin gene editing and one (N=1) chose stem cell transplant. Participants selected from a list of 38 reasons for their treatment choice; 22 reasons were chosen at least once. The most selected reasons for picking one of the curative options (bolded in Table 2) were because the treatment could cure them (N=4), the treatment could make their disease less severe (N=4), and because the treatment uses the participant’s own cells, and they would not need a donor (N=4). Other reasons given for picking an option were that the results of the treatment look very good (N=3), that a treatment will decrease the number of hospitalizations a participant has per year (N=3), that it sounds like it will be more likely to be successful (N=3), that a treatment will have fewer side effects (N=3), and that the participant can follow through with the treatment (get to visits, etc.) (N=3); see Table 2.
Table 2.
Treatments
| Participant | Familiar with | Likelihood of choosing any curative option | Most likely to choose | Beneficial treatments (agrees or strongly agrees) | Dangerous treatments (agrees or strongly agrees) | Reasoning |
|---|---|---|---|---|---|---|
| 1 |
SCT GT |
Likely | GT |
GT FH GC |
SCT | - This will have fewer side effects |
| 2 | All options | Unlikely | GT |
SCT (strongly) GT (strongly) FH (strongly) GC (strongly) |
SCT FH |
- The results of the treatment look very good - This treatment can decrease the number of hospitalizations I will have per year - This treatment can make my disease less severe - This is an easier treatment than the others - I think I can follow through on this treatment (get to the visits, etc.) - It uses my own cells and I would not need a donor - The goal is similar to the hydroxyurea treatment I have used/heard of - It sounds like it is more likely to be successful |
| 3 | None | Extremely unlikely | FH |
SCT GT FH GC No treatment |
SCT GT FH GC |
- This treatment can make my disease less severe |
| 4 |
SCT GT |
Likely | GC |
SCT GT FH GC |
None |
- This treatment can cure me - This will have fewer side effects - I think I can follow through on this treatment (get to the visits, etc.) - I am afraid I may die from sickle cell disease - It will decrease my risk of stroke - I won’t need as many transfusions - It uses my own cells and I would not need a donor - It would fix the root cause of the disease - It sounds like a more precise therapy - It sounds like it is more likely to be successful |
| 5 |
SCT GT |
Extremely likely | GC |
SCT GT FH GC No treatment (strongly) |
None |
- This treatment can cure me - I am worried that I am doing worse lately on my current treatment |
| 6 |
SCT GT GC |
Extremely likely | GC |
GT FH GC |
SCT (strongly) GT FH GC |
- The results of the treatment look very good - This treatment can cure me - I think it is safe - It sounds like what I would want |
| 7 |
SCT GT FH |
Neither likely nor unlikely | GC |
SCT GT FH GC |
SCT GT GC |
- This treatment can decrease the number of hospitalizations I will have per year - This treatment can decrease the days of missed work I will have - This treatment can make my disease less severe - It will have less of an impact on my family than the others - I am afraid I may die from sickle cell disease - It uses my own cells and I would not need a donor - I have heard about it being successful in the media for sickle cell disease |
| 8 |
SCT GT |
Extremely likely | SCT |
SCT GT FH GC |
None |
- This treatment can decrease the number of hospitalizations I will have per year - This treatment can cure me |
| 9 | SCT | Extremely likely | GT |
SCT GT FH |
FH No treatment |
- The results of the treatment look very good - This treatment can cure me - This treatment can make my disease less severe - I think it is safe - This will have fewer side effects - This is an easier treatment than the others - It will have less of an impact on my family than the others - I think I can follow through on this treatment (get to the visits, etc.) - I will be able to exercise more - It uses my own cells and I would not need a donor - It would fix the root cause of the disease - It sounds like it is more likely to be successful - It sounds like what I would want |
Includes which treatment each participants was familiar with prior to entering this study, the likelihood to choose each option, what their perceptions of benefit and danger were, and why they would choose the option they did
SCT stem cell transplant, GT gene therapy to add a new healthy gene, FH fetal hemoglobin gene editing, GC gene correction gene editing
Bold statements are the most highly selected reasons for choosing a particular treatment (N = 4)
Fig. 1.
a Displays the likelihood of participants to choose each individual treatment. b Displays the perceptions of benefit that the participants felt about each treatment. c Displays the perception of danger that the participants felt about each treatment
Discussion
With research into the decision-making process of patients with SCD lacking, our aim was to understand how individuals with SCD approach treatment decisions. This information is important to ascertain so that healthcare providers know what information to convey to patients once these treatments are all approved. This paper highlights where SCD patients have similarities and diversity in how they are thinking about current and future curative treatments for SCD.
Consistent with data from Hankins et al. (2007) that evaluated stem cell transplant, hydroxyurea, and red blood cell transfusions, participants in this study generally agreed that all four of the curative treatments are beneficial, although the participants in our study demonstrated mixed and nuanced views for the treatments they were asked to evaluate. The curative treatment that our participants chose the most was gene correction gene editing. The most common reasons that participants chose a particular treatment option were because the treatment is curative or could make the disease less severe, and there would be no need for a (stem cell) donor. Other studies have not evaluated the same measures, though Strong et al. (2017) showed that many SCD patients are hopeful about cures such as gene therapy and Persaud et al. (2018) found that patients with SCD are specifically hopeful about gene editing and the transition from hypothetical to approved treatments. While our study size is too small to draw generalizable conclusions, it was very interesting that several participants strongly agreed that all four potential curative treatments were beneficial, but reporting feeling unlikely to choose them (Participants 2 and 3), and that their reasons differed. Participant 2, for example, stated several reasons for choosing a curative option, including not needing a donor and the treatment they chose can make their disease less severe, and felt that stem cell transplant and fetal hemoglobin gene editing, but not the gene therapy and gene editing approaches, were dangerous. Participant 3 felt that all four curative treatments were dangerous, which may have influenced how likely they were to choose a cure.
There were several limitations of this study. Our small population size did not permit us to study correlations between different variables and choices made. Second, all of our participants were recruited from voluntary SCD patient support groups that may not represent the larger SCD patient population. Third, more of our participants were from California, possibly due to more individuals with SCD residing in California, compared to Georgia, and therefore may also not represent the larger SCD patient population. Despite these limitations, our results highlight the importance of conducting research on curative options and effectively educating patients with SCD on these curative treatments. Future studies diving further into the decision-making process of patients with SCD can shed further light on what is important to this patient population (and therefore where more outcomes data is needed) and how to better support their treatment decision-making. In particular, Riva and Pravettoni (2016) have looked at value-based decision-making in medicine. Further research could look at whether the values of patients with sickle cell disease correlate with the decisions that patients make.
Healthcare providers can continue to determine what is important to patients with sickle cell disease when making decisions around different treatment options. There are benefits and downsides to both the approved and experimental curative treatments, so further research into the decision-making process will help healthcare providers give useful and personalized information to each patient. This can be extended beyond sickle cell disease; as new technologies are proving to be successful, researchers can commence trials on other monogenic and polygenic diseases. We are in the beginning stages of understanding how patients make decisions about different treatment options and we anticipate these processes may change over time. In conclusion, our study showed that among our participants, gene correction gene editing was the most popular choice, participants generally agree that all four of these treatments are beneficial but had more mixed opinions on if they were dangerous, and there were a variety of factors that influenced one’s decision regarding which curative treatment they would be most likely to choose. Future research can further explore correlations between demographic and lifestyle factors with different treatment decisions.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge and thank all our participants who offered their time to participate in this study. This study was completed in partial fulfillment of the first author’s degree requirements.
Funding
The research was supported by the Stanford University School of Medicine Genetic Counseling Program. No outside funding was obtained.
Availability of data and material
Not applicable
Code availability
Not applicable
Declarations
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
The original version of this article was revised. Figure 1 is now corrected.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
12/23/2021
A Correction to this paper has been published: 10.1007/s12687-021-00569-6
References
- Akinsheye I, Alsultan A, Solovieff N, Ngo D, Baldwin C, Sebastiani P, Chui D, Steinberg M. Fetal hemoglobin in sickle cell anemia. Blood. 2011;118:19–27. doi: 10.1182/blood-2011-03-325258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniani C, Meneghini V, Lattanzi A, Felix T, Romano O, Magrin E, Weber L, Pavani G, Hoss S, Kurita R, Nakamura Y, Cradick T, Lundberg A, Porteus M, Amendola M, El Nemer W, Cavazzana M, Mavilio F, Miccio A. Induction of fetal hemoglobin synthesis by CRISPR/Cas9-mediated editing of the human β-globin locus. Blood. 2018;131(17):1960–1973. doi: 10.1182/blood-2017-10-811505. [DOI] [PubMed] [Google Scholar]
- Bolaños-Meade J, Brodsky R. Blood and marrow transplantation for sickle cell disease: overcoming barriers to success. Curr Opin Oncol. 2015;21(2):148–161. doi: 10.1097/CCO.0b013e328324ba04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention – Findings from RuSH (2012) Sickle Cell Disease in Georgia https://www.cdc.gov/ncbddd/sicklecell/documents/scd_in_ga_prov.pdf. Accessed Oct 2021
- Centers for Disease Control and Prevention (CDC) (2015) Longitudinal data collection for sickle cell disease in California: history, goals and challenges. https://www.cdc.gov/ncbddd/hemoglobinopathies/documents/donor-report-010416.pdf. Accessed Oct 2021
- Centers for Disease Control and Prevention (CDC) (2018) Health-Related Quality of Life (HRQOL) https://www.cdc.gov/hrqol/index.htm
- Clinicaltrials.gov. NIH U.S. National Library of Medicine. https://clinicaltrials.gov/ct2/results?cond=Sickle+Cell+Disease&term=gene+therapy&cntry=&state=&city=&dist. Accessed Oct 2021
- Demirci S, Uchida N, Tisdale J. Gene therapy for sickle cell disease: an update. Cytotherapy. 2019;20(7):899–910. doi: 10.1016/j.jcyt.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzhugh C, Abrahamn A, Tisdale J, Hsieh M. Hematopoietic stem cell transplantation for patients with sickle cell disease: progress and future directions. Hematol Oncol Clin North Am. 2014;28(6):1171–1185. doi: 10.1016/j.hoc.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins J, Hinds P, Day S, Carroll Y, Chin-Shang L, Garvie P, Wang W. Therapy preference and decision-making among patients with severe sickle cell anemia and their families. Pediatric Blood Cancer. 2007;48:705–710. doi: 10.1002/pbc.20903. [DOI] [PubMed] [Google Scholar]
- Inusa B, Hsu L, Kohli N, Patel A, Ominu-Evbota K, Anie K, Atoyebi W. Sickle cell disease – genetics, pathophysiology, clinical presentation and treatment. Int J Neonatal Screen. 2019;5(2):20. doi: 10.3390/ijns5020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzkron S, Strouse JJ, Wilson R, Beach MC, Haywood C, Park H, Witkop C, Bass E, Segal J. Systematic review: hydroxyurea for the treatment of adults with sickle cell disease. Ann Intern Med. 2008;148:939–955. doi: 10.7326/0003-4819-148-12-200806170-00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud A, Desine S, Blizinsky K, Bonham V. A CRISPR focus on attitudes and beliefs toward somatic genome editing from stakeholders within the sickle cell disease community. Genet Med. 2018;21:1726–1734. doi: 10.1038/s41436-018-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeil J, Hacein-Bey-Abina S, Payen E, et al. Gene therapy in a patient with sickle cell disease. N Engl J Med. 2017;376(9):848–855. doi: 10.1056/NEJMoa1609677. [DOI] [PubMed] [Google Scholar]
- Riva S, Pravettoni G. Value-based model: a new perspective in medical decision-making. Front Public Health. 2016;4:118. doi: 10.3389/fpubh.2016.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross D, Bakshi N, Khemani K, Sinha C, Loewenstein G, Krishnamurti L. What are the expectations of patients in decision making process for disease modifying therapies for sickle cell disease: do they care about shared decision making? Blood. 2016;128(22):59–68. doi: 10.1182/blood.V128.22.5968.5968. [DOI] [Google Scholar]
- Rubin R. Gene therapy for sickle cell disease shows promise. JAMA. 2019;321(4):334. doi: 10.1001/jama.2018.21119. [DOI] [PubMed] [Google Scholar]
- Serjeant GR. The natural history of sickle cell disease. Cold Spring Harb Perspect Med. 2013;3(10):a011783. doi: 10.1101/cshperspect.a011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S. Hematopoietic stem-cell transplantation for sickle cell disease: current evidence and opinions. Ther Adv Hematol. 2013;4(5):335–344. doi: 10.1177/2040620713483063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong H, Mitchell MJ, Goldstein-Leever A, Shook L, Malik P, Crosby LE. Patient perspectives on gene transfer therapy for sickle cell disease. Adv Ther. 2017;34(8):2007–2021. doi: 10.1007/s12325-017-0587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasan I, Jain S, Zhao H. Use of genome editing tools to treat sickle cell disease. Hum Genet. 2016;135(9):1011–1028. doi: 10.1007/s00439-016-1688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber L, Frati G, Felix T, Hardouin G, Casini A, Wollenschlaeger C, Meneghini V, Masson C, De Cian A, Chalumeau A, Mavilio F, Amendola M, Andre-Schmutz I, Cereseto A, El Nemer W, Concordet JP, Giovannangeli C, Cavazzana M, Miccio A. Editing a γ-globin repressor binding site restores fetal hemoglobin synthesis and corrects the sickle cell disease phenotype. Sci Adv. 2020;6(7):9392. doi: 10.1126/sciadv.aay9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable
Not applicable