Abstract
Early identification and treatment of active tuberculosis disease among high risk household contacts could limit new transmission and better clinical outcome, thus decreasing TB burden. Host iron homeostasis is an important yet underevaluated factor in pathophysiology of tuberculosis (TB). One such protein is hepcidin which internalizes ferroportin (membrane iron transporter), thus inhibiting iron export from macrophages which is utilised by bacteria leading to disease severity. Iron homeostasis markers were evaluated in 50 pulmonary tuberculosis patients (PTB) and their household contacts to assess their utility as biomarkers for TB development. Altered iron homeostasis with significantly lower haemoglobin levels despite optimum serum iron levels was observed in PTB compared to household contacts and healthy controls pointing towards anaemia of inflammation. Higher serum hepcidin with lower ferroportin expression and hence higher ferritin levels was seen in PTB compared to both household contacts and healthy controls due to IL-6 induced hepcidin production in TB. Transferrin levels were found to be significantly lower in PTB and household contacts as compared to healthy controls owing to higher ferritin levels in PTB group. Upon infection, regulation of iron absorption is disturbed via increased hepcidin levels leading to ferroportin internalization and thus inhibition of iron export from macrophages which may lead to favourable M.tb. survival and multiplication leading to tuberculosis. Some of these markers could be assessed for early identification and treatment of active tuberculosis among high risk household contacts limiting new transmission and better clinical outcome, thus decreasing TB burden.
Keywords: Tuberculosis, Iron homeostasis, Ferritin, Hepcidin, Ferroportin (FPN-1)
Introduction
Tuberculosis (TB) is a major global health problem caused by the bacteria, Mycobacterium tuberculosis (M.Tb.), that can evade the host immune system and can remain in the macrophages of the host in dormant stage until the immunity is suppressed. According to latest World Health Organization (WHO) global TB report (2019), an estimating 10 million new incident cases occurred globally in 2019, out of which 27% incident cases are from India [1].
Individuals exposed to a person with pulmonary TB within the same household (household contacts) are at high risk of developing active disease. However, there is still an enigma about how some individuals who are in close contact with TB patients may or may not develop TB. Variability in disease progression suggests that various host factors may play a role in the response to infection. One such factor is micronutrient status like iron which is an important contributor to immune function during infection [2]. During multiplication, M.tb. synthesizes iron-chelating compound called siderophore to acquire the iron available within the macrophages for its own growth, as iron is an obligate cofactor for its several enzymes [3].
The key regulator of iron homoeostasis in human body is hepcidin which acts on ferroportin (FPN-1), the only known mammalian iron exporter till date. Ferroportin is a membrane protein that is the major exporter of iron in mammalian cells, including macrophages that recycle iron, duodenal enterocytes that absorb iron, and hepatocytes that store iron [4]. Hepcidin binds to ferroportin, induces internalization and subsequent cellular degradation resulting in inhibition of iron export by the enterocytes and reticuloendothelial cells into the blood, thus maintaining iron homoeostasis [5, 6]. Elevated extracellular and stored iron or inflammatory stimuli enhance hepcidin expression. Hepcidin expression is inhibited by hypoxia and erythropoiesis [7, 8]. However, during inflammation/infection, hepcidin expression is induced by the cytokine IL-6 via the Janus kinase (JAK) & signal transducer and activator of transcription (STAT) 3 pathways [9]. Hepcidin expression is also stimulated by hemojuvelin (HJV) and bone morphogenic protein receptor (BMPR) complex, by SMAD signaling pathway [10].
Since there is paucity of literature worldwide and none in Indian population regarding these iron regulatory proteins in tuberculosis, this study was designed to explore the expression and localization of ferroportin and its correlation with hepcidin levels and deduce, if variable expression of FPN-1 along with hepcidin could alter the host response to M.tb. in health and disease. Also, there is no evidence towards using serum levels of hepcidin and iron indices as biomarkers in assessing progression of active tuberculosis among household contacts. Therefore, the present study was aimed at evaluating levels of ferroportin, its regulator hepcidin along with iron status and its associated molecules like transferrin and ferritin in active pulmonary tuberculosis patients and their household contacts compared to healthy controls.
Materials and Methods
Study Participants
The present cross-sectional study was carried out in the Department of Biochemistry at AIIMS. Active TB patients (Newly diagnosed laboratory confirmed cases of pulmonary tuberculosis) who had not received more than 2 weeks of ATT were recruited from DOTS Centre, All India Institute of Medical Sciences (AIIMS), New Delhi, India based on inclusion and exclusion criteria. A case of pulmonary TB was defined as a clinically diagnosed case of TB affecting the lungs, having symptoms of fever or cough and sputum smear that showed acid-fast bacilli or culture positive for M.Tb. or Gene Xpert Exclusion criteria included patients with extra-pulmonary and drug resistant tuberculosis, HIV, diabetes, hypertension, significant organ dysfunction of heart, liver and kidney, pregnant or lactating women. Additional exclusion criteria were known hypersensitivity to ATT, seizure disorder, abnormal hematologic function, inflammation like autoimmune disease, atopic dermatitis or any other uncontrolled concurrent illness. Patients with prior anti-microbial drug treatment of TB for longer than two weeks and any history of alcohol and drug abuse were also excluded.
Household contacts of TB patients aged between 18–60 years who had spent atleast 6 h per day for atleast 2 months prior with the TB patient before start of ATT with no clinical evidence of TB were recruited. The exclusion criteria were same as for the active TB patients. Mantoux test was done in household contacts. Mantoux negative individuals were directly recruited under household contacts group. Mantoux positive individuals were followed up by sputum test or chest X-ray. Individuals negative for these tests were recruited under household contacts. Individuals with positive test were further investigated for active disease. Individuals with altered clinical parameters like Hemogram, liver function test, kidney function test, blood glucose, etc. were excluded from the study. Normal healthy controls were recruited from the general population with no history of known contacts with TB patients and no clinical signs and symptoms of tuberculosis and no history of any other chronic disorder or inflammation. 50 PTB patients, 50 household contacts and 50 healthy controls were recruited from North Indian population for the study after obtaining written informed consent. The study was conducted adopting the ethical principles stated in the latest version of Helsinki Declaration as well as the applicable guidelines for good clinical practice (GCP). Ethical approval was obtained from Institutional Ethics Committee of All India Institute of Medical Sciences, New Delhi and V.M.M.C & Safdarjung Hospital, New Delhi (Ref NO: IEC/640.22.12.2016 and IEC/SH/VMMC/Project/Feb-2017/).
Sample Collection and Storage
6 mL of peripheral venous blood was collected from patients and controls. The blood specimen was given a personal identifier number that was used to link and maintain the biological information derived. 4 mL blood was for serum separation and was stored at −70 °C for further analysis. 5 mL blood was collected in EDTA vials for PBMC isolation.
Estimation of Serum Levels of Hepcidin, Iron, Transferrin and Ferritin
Serum levels of Hepcidin and transferrin was estimated using sandwich enzyme linked Immunosorbent assay (ELISA) using Human HEPCIDIN ELISA KIT (Cat No. 201-12-1020) and Human Transferrin (TRF) ELISA Kit (Cat No. MBS2600168). Serum Iron was measured using colorimetric assay-based kit (DIALAB). Iron bound to transferrin is released in an acidic medium as ferric iron and is then reduced to ferrous iron in the presence of ascorbic acid. Ferrous iron forms a blue complex with Ferene. The absorbance at 595 nm is directly proportional to the iron concentration. Serum levels of ferritin was measured using chemiluminiscencebased immunoassay (VITROS ECi, Johnson and Johnson Ortho Clinical Diagnostics).
Estimation of Hemoglobin
Hemoglobin levels were measured by automated machines designed to perform different tests on blood. Within the machine, the red blood cells are broken down to get the Hemoglobin into a solution. The free Hemoglobin is exposed to a chemical containing cyanide that binds tightly with the Hemoglobin molecule to form cyanomethemoglobin. By shining a light through the solution and measuring how much light is absorbed (specifically at a wavelength of 540 nm), the amount of Hemoglobinwas determined.
Estimation of Ferroportin Expression on Monocytes
PBMC was isolated from whole blood using ficoll hypaque followed by monocyte isolation using plastic adherence method. Ferroportin expression at protein levels was then estimated in monocytes using western blot. The Primary antibody used was anti human ab78060 and secondary antibody used was anti-rabbit ab6721.
Statistical analysis
All statistical analyses were performed on GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA). Non-parametric statistical analyses were performed throughout the study. Kruskal Wallis and Mann–Whitney U test was used for comparison of variables between three and two groups respectively. Values were expressed as median ± standard deviation. A p-value of less than 0.05 was considered significant.
Results
Study Population
The patient group consisted of 50 newly diagnosed active pulmonary tuberculosis (PTB) patients and 50 household contacts of active PTB patients based upon inclusion and exclusion criteria. The control group consisted of 50 healthy persons with no known history of tuberculosis and contact with active PTB patient. The mean age of PTB patients was 41.9 ± 8.72 years, household contacts was 38.2 ± 6.3 years and healthy controls was 31.2 ± 3.4 years. The socio demographic and clinical profile of study participants are shown in Table 1.
Table 1.
Socio demographic and clinical characteristics of study participants
| Demographic characteristics | PTB (n = 50) | Household contacts (n = 50) | Healthy controls (n = 50) |
|---|---|---|---|
| Age (Mean ± SD) | 41.9 ± 8.72 | 38.2 ± 6.3 | 31.2 ± 3.4 |
| Male (%) | 78 | 62 | 84 |
| Female (%) | 22 | 38 | 16 |
| Race | North Indian | North Indian | North Indian |
| Smoking (%) | 34 | 18 | 4 |
| Body mass index (Kg/m2) in mean ± SD | 18.7 ± 6.2 | 20.6 ± 5.7 | 20.7 ± 2.6 |
| *Gene Xpert positive, n (%) | 31 (62) | Not done | Not done |
| #AFB positive smear, n (%) | 19 (38) | Not done | Not done |
| 1 + sputum positivity (n) | 6 | NA | NA |
| 2 + sputum positivity (n) | 7 | ||
| 3 + sputum positivity (n) | 6 | ||
| Hb levels (g/dl) (Median ± SD) | 10.26 ± 1.88 | 12.77 ± 1.50 | 13.66 ± 1.12 |
*Gene Xpert positive: Positive for nucleic acid amplification (NAA) test for M.Tb. genome present in TB cases
#AFB positive smear: Positive for acid fast bacilli staining
Increased Serum Levels of Hepcidin in PTB Patients Compared to Household Contacts
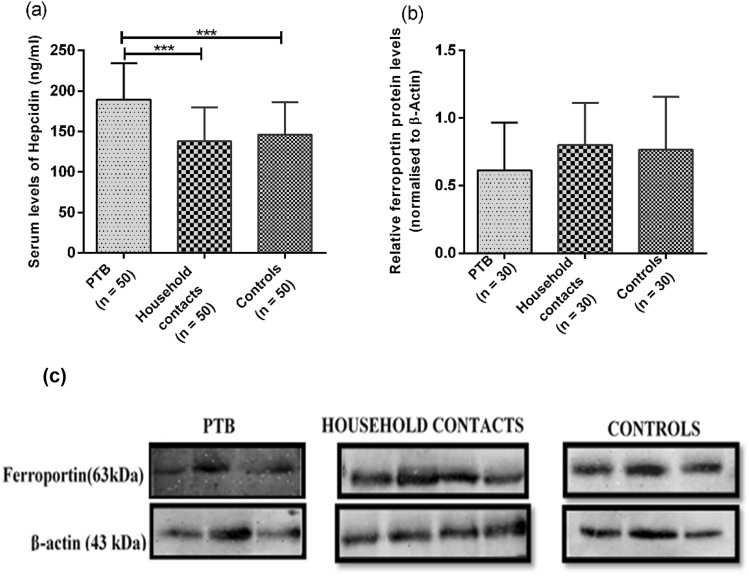
Serum hepcidin levels were measured using sandwich ELISA. Serum hepcidin levels varied among all the groups with significant increase in PTB patients (189.25 ± 45.18 ng/ml) as compared to household contacts (137.94 ± 41.61 ng/ml) and healthy controls (146.21 ± 40.15 ng/ml) with a p-value < 0.005 (Fig. 1a). However, the level of hepcidin was comparable in household contacts and control groups.
Fig. 1.
a Represents Serum levels of hepcidin in PTB, household contacts and control groups. Serum levels of hepcidin was measured by using sandwich ELISA. Data is represented as median ± standard deviation. b Showed the ferroportin protein expression using western blot in PTB, household contacts and control groups. c Shows representative blot image for ferroportin. Mann–Whitney U test was used for comparison of values between groups. One asterisk (*) indicates a p-value < 0.05; two asterisks (**) indicate a p-value < 0.01.PTB = active pulmonary TB; Household contacts = contacts living with the pulmonary TB patients for minimum of 2 months with no sign and symptoms of TB; Control = Healthy controls with no history of TB
Decreased Ferroportin Levels in PTB Patients Compared to Household Contacts
High levels of hepcidin leads to internalization of ferroportin and its degradation. Therefore, ferroportin levels were measured in all study groups using western blot. Lower expression of ferroportin was observed in PTB group as compared to household contacts and healthy controls, but the difference was not found to be statistically significant with a p-value > 0.05 (Fig. 1b and c). These findings could be due to a smaller sample size (n = 30).
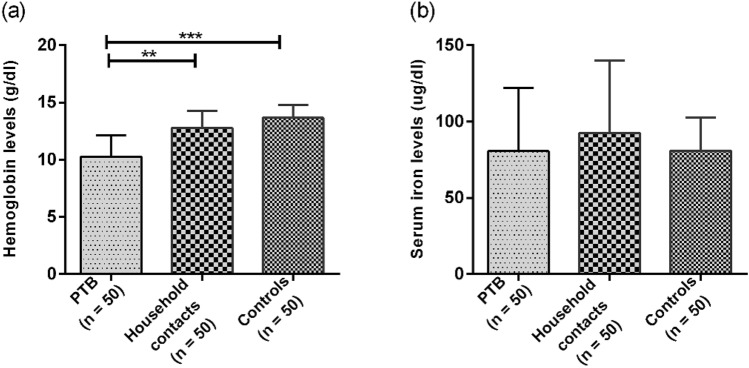
Decreased Hemoglobin and Iron Levels in PTB Patients Compared to Household Contacts
Significant difference was found between hemoglobin levels in all the three study groups with significantly lower levels in PTB patients (10.26 ± 1.88 g/dl) as compared to household contacts (12.77 ± 1.50 g/dl) and healthy control group (13.66 ± 1.12 g/dl) with p-value < 0.001 (Fig. 2a). Serum Iron levels also varied among all the groups with lowest levels seen in PTB patients (78.60 ± 42.55 µg/dl) as compared to household contacts (88.61 ± 49.89 µg/dl) and healthy controls (80.94 ± 21.89 µg/dl). However, the difference was not statistically significant with p-value > 0.05 (Fig. 2b).
Fig. 2.
a Represents levels of haemoglobin in PTB, household contacts and control groups. The levels of haemoglobin were measured using automated haematological flow cytometer in all groups. b Represents serum levels of iron in PTB, household contacts and control groups. The levels of Iron in serum was estimated using calorimetric assay. Mann–Whitney U test was used for comparison of values between groups. One asterisk (*) indicates a p-value < 0.05; two asterisks (**) indicate a p-value < 0.01, three asterisks (***) indicate a p-value < 0.001. PTB = Naïve active pulmonary TB; Household contacts = contacts living with the pulmonary TB patients for minimum of 2 months with no sign and symptoms of TB; Control = Healthy controls with no history of TB
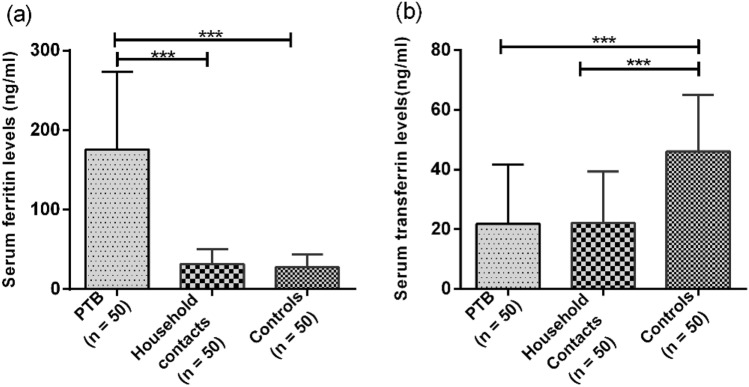
Increased Ferritin and Decreased Transferrin Levels in PTB Patients Compared to Household Contacts
Iron is stored in the form of ferritin in the body; therefore, ferritin levels were also measured. Serum Ferritin levels varied among all the groups with highest levels seen in PTB patient (175.74 ± 97.38 ng/ml) as compared to household contacts (31.74.35 ± 18.83 ng/ml) and healthy controls (27.35 ± 16.58 ng/ml). The difference was found to be statistically significant with p-value < 0.0001 (Fig. 3a). Since transferrin helps in transport of iron through the blood to various tissues, serum level of transferrin was also measured and it was found to be significantly lower in PTB group (21.77 ± 19.96 ng/ml) as compared to controls (46.06 ± 19.03 ng/ml) with a p-value < 0.001 (Fig. 3b). However, the level of transferrin was comparable in household contacts (22.11 ± 17.16 ng/ml) and patient groups (21.77 ± 19.96 ng/ml).
Fig. 3.
a Represents serum Levels of ferritin in PTB, household contacts and control groups. The serum levels of ferritin (the storage form of iron) was assessed by using chemiluminescence assay. b Represents serum transferrin levels in PTB, household contacts and control groups. Mann–Whitney U test was used for comparison of values between groups. One asterisk (*) indicates a p-value < 0.05; two asterisks (**) indicate a p-value < 0.01, three asterisks (***) indicate a p-value < 0.001 and four asterisks (****) indicate a p-value < 0.0001. PTB = Naïve active pulmonary TB; Household contacts = contacts living with the pulmonary TB patients for minimum of 2 months with no sign and symptoms of TB; Control = Healthy controls with no history of TB
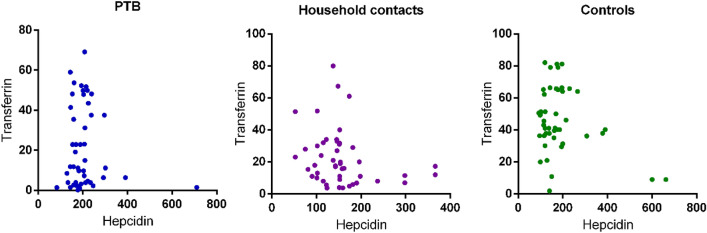
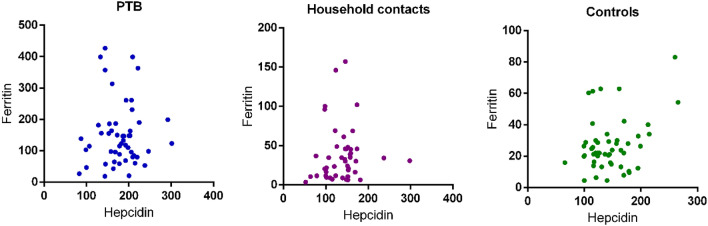
No Correlation of Transferrin and Ferritin with Hepcidin Levels
Correlation between serum levels of hepcidin and ferritin was also calculated to assess its potential as biomarker for active disease. However, no significant correlation was found for both ferritin and transferrin with hepcidin (Figs. 4 and 5).
Fig. 4.
Represents correlation of serum hepcidin levels with transferrin levels in PTB, household contacts and control groups. Spearman correlation test was performed for correlation analysis
Fig. 5.
Represents correlation of serum hepcidin levels with ferritin levels in PTB, household contacts and control groups. Spearman correlation test was performed for correlation analysis
Discussion
Upon infection, host and pathogen compete for the same iron pool, a crucial micronutrient for all living cells. Iron dysregulation in the host is strongly associated with poor outcomes in several infectious diseases, including tuberculosis. An inefficient iron scavenging by intracellular pathogen like M.tb. may severely affect its virulence. Numerous enzymes of the M.tb. require iron as a cofactor for its survival inside the host [11]. The ability of M.tb. to sequester and use the available iron inside the host is greatly altered by the host response to infection. One such mechanism is by regulating the level of iron present inside the host by a regulatory protein called hepcidin. Hepcidin, a 25 amino acid protein is produced in the liver to maintain iron homeostasis inside the body. Hepcidin inhibits iron transport by binding to the iron export channel, ferroportin which is located on the basolateral surface of gut enterocytes and the plasma membrane of macrophages. It regulates iron homeostasis during iron toxicity by mediating the internalization and degradation of the iron export protein ferroportin 1, thereby, inhibiting iron absorption from the small intestine and release of iron from macrophages [12]. During infection, M.tb. present inside the macrophage escapes Fe toxicity by storing iron in the form of siderophore and utilizes it for its own growth and multiplication [13]. Therefore, in the present study, Fe regulatory proteins namely hepcidin along with ferroportin in tuberculosis patients and their household contacts (who are at high risk for developing into active disease) were explored. Increased hepcidin levels were observed in PTB patients as compared to household contacts and healthy controls which might lead to reduction of free serum iron via inhibition of iron transport through ferroportin.
An increased hepcidin level in the present study is attributed to its synthesis, plausibly due to release of IL-6 by hepatocytes owing to infection. This is in accordance with a previous study where the macrophages from mouse produced increased levels of hepcidin upon M.Tb infection [14]. In, another study, it was shown that macrophages from humans with type I hemochromatosis have lower iron accumulation and lower bacterial burden when infected with M.tb. as compared to cells from healthy donors; conversely, M.Tb acquire iron more efficiently from macrophages of healthy subjects as compared to cells from patients with hemochromatosis [15]. These data suggest that hepcidin-mediated increase in intracellular iron may be harmful to the host during mycobacterial infections, but this hypothesis has not been addressed directly in vivo. Our study has shown similar results in vivo in tuberculosis patients. Since ferroportin is the only mammalian iron transporter, which is regulated by hepcidin, expression of ferroportin was studied. It was found to be lower in PTB patients compared to household contacts and healthy controls, but the difference was not found to be statistically significant. This may be attributed to small sample size. Present findings are supported with another study where it was shown that ferroportin overexpression limits early intracellular M. tuberculosis growth [16]. A lower hepcidin and ferroportin in household contacts may suggest protection from active disease in these individuals despite contact with patients. Also, a comparable level of hepcidin in household contacts and healthy controls indicates no active infection in these individuals.
Since hepcidin ferroportin axis plays role in iron homeostasis, the status of iron and its associated molecules were further analysed. Low serum Fe and plasma haemoglobin levels suggested anemia of inflammation in PTB group due to active infection. Ferritin, which is the stored form of iron, is another important molecule in iron homeostasis and has been shown to be associated with infectious diseases, including tuberculosis. In the present study, increased ferritin levels in PTB patients compared to household contacts and healthy controls were seen. This is in accordance with previous study in which it was concluded that the ferritin levels were higher in the presence of M.Tb. infection [17]. In another study, patients with Diabetes mellitus had increased levels of ferritin as compared to healthy controls [18].This is attributed to the fact that free iron is sequestered within macrophages and enterocytes as ferritin in the presence of infection which is one of the reasons for considering ferritin as positive acute phase reactant. As suspected, transferrin levels were found to be lower in PTB patients compared to other groups.
To conclude, in PTB, regulation of Fe absorption is disturbed in terms of increase in hepcidin levels which lead to internalization of ferroportin as shown in the present study with higher level of hepcidin and lower level of ferroportin in PTB group. Ferritin levels were found to be higher in active TB group. With the findings in the present study, it could be proposed that hepcidin along with ferritin could be used as biomarker for activation of disease in household contacts. However, the same needs to be further studied in cohort of household contacts in a prospective study design.
Acknowledgements
We thank the DOTS center, AIIMS and Safdarjung Hospital and all the study subjects for participation in the study. We thank All India Institute of Medical Sciences for providing intramural research grant for the study (A-409).
Author Contributions
AS, SP, SF, KKS and AS conceptualized and designed the study. SP and SF drafted the manuscript. SP and KKS carried out recruitment of patients under guidance of AS and NKG. SP, SF, KKS carried out sample collection, standardisation and execution of experimental work along with data acquisition and interpretation of data under guidance of AS, AS, NKG and SD. AS and AS critically reviewed and contributed to the final version of manuscript. AS gave the final approval of manuscript submission and supervised the project.
Funding
This work was supported by an intramural research Grant (A-409) from All India Institute of Medical Sciences, New Delhi.
Availability of Data and Materials
All the data used for making the conclusions are in the manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare no potential conflict of interests.
Consent to Participate
50 PTB patients, 50 household contacts and 50 healthy controls were recruited from North Indian population for the study. Informed consent was obtained from all individuals participants included in the study.
Consent for Publication
The authors affirm that human research participants provided informed consent for publication of data.
Ethical Approval
The study was conducted adopting the ethical principles stated in the latest version of Helsinki Declaration as well as the applicable guidelines for good clinical practice (GCP). Ethical approval was obtained from Institutional Ethics Committee of All India Institute of Medical Sciences, New Delhi and V.M.M.C & Safdarjung Hospital, New Delhi (Ref NO: IEC/640.22.12.2016 and IEC/SH/VMMC/Project/Feb-2017/).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sudhasini Panda and Shah Faisal have contributed equally to this work.
References
- 1.WHO|Global tuberculosis report 2019 [Internet]. WHO. World Health Organization; [cited 22 Sep 2020]. http://www.who.int/tb/publications/global_report/en/
- 2.Boelaert JR, Vandecasteele SJ, Appelberg R, Gordeuk VR. The effect of the host’s iron status on tuberculosis. J Infect Dis. 2007;195(12):1745–1753. doi: 10.1086/518040. [DOI] [PubMed] [Google Scholar]
- 3.De Voss JJ, Rutter K, Schroeder BG, Su H, Zhu Y, Barry CE. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc Natl Acad Sci USA. 2000;97(3):1252–1257. doi: 10.1073/pnas.97.3.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward DM, Kaplan J. Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta. 2012;1823(9):1426–1433. doi: 10.1016/j.bbamcr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 6.De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, et al. The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell. 2007;18(7):2569–2578. doi: 10.1091/mbc.e07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–1044. doi: 10.1172/JCI0215686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276. doi: 10.1172/JCI200420945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38(5):531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 11.Pandey M, Talwar S, Bose S, Pandey AK. Iron homeostasis in Mycobacterium tuberculosis is essential for persistence. Sci Rep. 2018;8(1):17359. doi: 10.1038/s41598-018-35012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122(2–3):78–86. doi: 10.1159/000243791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones CM, Niederweis M. Mycobacterium tuberculosis can utilize heme as an iron source. J Bacteriol. 2011;193(7):1767–1770. doi: 10.1128/JB.01312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sow FB, Nandakumar S, Velu V, Kellar KL, Schlesinger LS, Amara RR, et al. Mycobacterium tuberculosis components stimulate production of the antimicrobial peptide hepcidin. Tuberculosis (Edinb) 2011;91(4):314–321. doi: 10.1016/j.tube.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Olakanmi O, Schlesinger LS, Britigan BE. Hereditary hemochromatosis results in decreased iron acquisition and growth by Mycobacterium tuberculosis within human macrophages. J Leukoc Biol. 2007;81(1):195–204. doi: 10.1189/jlb.0606405. [DOI] [PubMed] [Google Scholar]
- 16.Johnson EE, Sandgren A, Cherayil BJ, Murray M, Wessling-Resnick M. Role of ferroportin in macrophage-mediated immunity. Infect Immun. 2010;78(12):5099–5106. doi: 10.1128/IAI.00498-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Visser A, van de Vyver A. Severe hyperferritinemia in Mycobacteria tuberculosis infection. Clin Infect Dis. 2011;52(2):273–274. doi: 10.1093/cid/ciq126. [DOI] [PubMed] [Google Scholar]
- 18.Momeni A, Behradmanesh MS, Kheiri S, Abasi F. Serum ferritin has correlation with HbA1c in type 2 diabetic patients. Adv Biomed Res. 2015;4:74. doi: 10.4103/2277-9175.153900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data used for making the conclusions are in the manuscript.