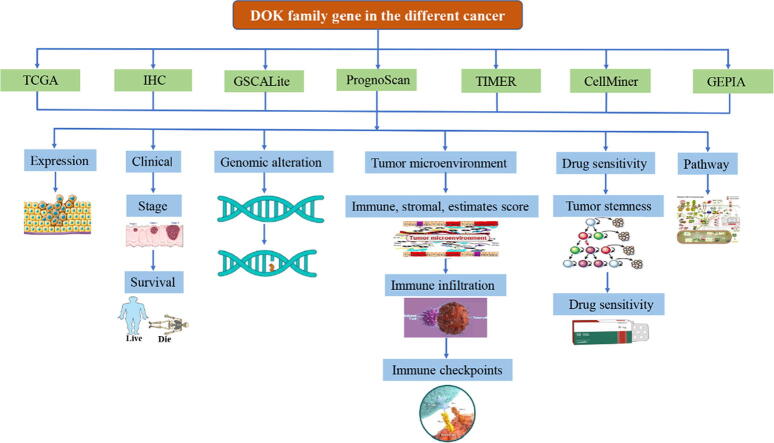
Graphical abstract
Keywords: DOK, Tumor microenvironment, Immune infiltration, Drug sensitivity
Highlights
-
•
The expression of DOK family genes is related to overall survival (OS), clinical stage, tumor mutation, methylation, CNV, and SNV.
-
•
DOK family genes are significantly associated with poor prognosis of UVM.
-
•
DOK1-DOK3 has obvious correlation with tumor immunity and tumor microenvironment.
-
•
DOK family gene is significantly related to tumor stemness and drug sensitivity.
-
•
The expression of DOK family genes is related to the activation of EMT and hormone ER pathways, and is related to the inhibition of DNA damage response, cell cycle, and hormone AR pathways.
-
•
DOK1 and DOK3, DOK2 and DOK3 have the significant correlation.
Abstract
Introduction
DOK is a new type of regulatory protein family that participates in the regulation of tumor cell growth. However, most of the studies are conducted in cell lines, and systematic studies have not been conducted in human tumors.
Objectives
We conducted a comprehensive analysis of DOK based on its expression profile and its relationship with patient survival, immune infiltration, tumor microenvironment, and drug sensitivity.
Methods
We used the TCGA database to analyze the correlation between DOK family gene expression and prognosis and clinical stage. The protein expression of DOK in tumor tissues was analyzed by immunohistochemistry. Use the cBioPortal database to analyze the alteration frequency in DOK family genes in human tumors. In addition, we used ESTIMATE algorithm and TIMER website to analyze the correlation between DOK family genes and tumor immunity. Finally, we further analyzed the relationship between DOK family genes and tumor stemness and the sensitivity of cancer cells to chemotherapy.
Results
We conducted a comprehensive analysis of DOK family genes based on its expression profile and its relationship with patient survival. We also confirmed this conclusion by immunohistochemistry. The expression of DOK family genes is related to OS, clinical stage, tumor mutation, methylation, CNV, and SNV. DOK family genes are significantly associated with poor prognosis of UVM. DOK1-DOK3 has obvious correlation with tumor immunity. DOK2 can increase the sensitivity of chemotherapy drugs, while DOK4 reduces the sensitivity of multiple chemotherapy drugs. In addition, the expression level of DOK family genes is significantly correlated with the activity of cancer marker-related pathways.
Conclusions
DOK plays a role of tumor suppressor gene or tumor-promoting gene in different tumors. However, DOK family genes play a role in promoting cancer in UVM. DOK family genes are significantly associated with drug sensitivity.
Introduction
DOK (downstream of tyrosine kinase/Docking protein) is a tyrosine residue phosphorylated protein, it belongs to a new type of regulatory protein family, and plays an important role in the receptor tyrosine kinase signaling pathway [1]. So far, it has been found that there are 7 members of the DOK family (DOK1- DOK7), which have similar structural characteristics, that is, they all have an N-terminal PH (pleckstrin homology) domain, a central PTB (phosphotyrosine binding) domain and a C-terminal SH2 (src homology2) target motif [2], [3], [4]. According to gene structure and expression pattern, DOK family can be divided into three categories: DOK1-DOK3, DOK4-DOK6 and DOK7. Among them, DOK1-DOK3 is preferentially expressed in hematopoietic and immune cells, and DOK1 is highly expressed in myeloid and lymphocytes, DOK2 is relatively highly expressed in T cells, and DOK3 is relatively highly expressed in B cells [5], [6]. DOK4-DOK6 are expressed in non-hematopoietic cells, DOK4 is expressed in heart, skeletal muscle and lung tissues, and DOK5 and DOK6 are highly expressed in neurocytes [5], [6]. Studies have found that DOK4, DOK5 and DOK6 are positive regulators of the MAPK pathway. DOK5 can promote the differentiation of PC12 cells by mediating RET, TrkB and other receptor signal transduction, thereby promoting neurite growth [7]. DOK5 can also positively regulate the PTK pathway and does not bind to p120 rasGAP [8]. DOK7 is preferentially expressed in skeletal muscle and heart, especially in the post-synaptic neuromuscular junction area, where it activates muscle-specific kinases to promote the accumulation of acetylcholine receptors on the postsynaptic membrane. Current studies have shown that the function of DOK7 is less related to other members of the family [5], [6].
Recent studies have shown that abnormal DOK gene expression is closely related to leukemia [9], lung cancer [10], gastric cancer [11], colorectal cancer [11], [12], and breast cancer [13], [14]. However, most of the studies are conducted in cell lines and/or animal models, and systematic studies have not been conducted in human tumors. In this study, TCGA cancer data were used to study the relationship between the expression of this family genes in 33 cancers and overall survival, and its expression was analyzed in combination with tumor microenvironment and pharmacology to determine its potential function and unique prognostic value.
Materials & methods
Human samples
Pathological sections were collected from Department of Pathology, Renmin Hospital of Wuhan University (Wuhan, Chian) from January 2019 to December 2020. Remove adjacent normal tissue from the area > 2 cm from the primary tumor.
Ethics statement
All experiments involving human were conducted according to the ethical policies and procedures approved by the ethics committee of the Renmin Hospital of Wuhan University, China (Approval no. WDRY2019-K092). All patients obtained written informed consent.
Evaluating the expression and prognosis of DOK gene variants in different cancer
Data of different types of cancers, including gene expression RNA-Seq (HTSeq-FPKM), clinical data, survival data, stemness scores based on mRNA (RNAss), and DNA-methylation (DNAss) were downloaded (March 2020) from xena browser(https://xenabrowser.net/datapages/). The cancer data of 33 primary tumors were described in supplementary Table 1. The datasets include adrenocortical carcinoma(ACC), bladder urothelial carcinoma(BLCA), invasive breast carcinoma(BRCA), cervical squamous cell carcinoma and endocervical adenocarcinoma(CESC), cholangiocarcinoma(CHOL), colon adenocarcinoma(COAD), lymphoid neoplasm diffuse large B-cell lymphoma(DLBC), esophageal carcinoma(ESCA), glioblastoma multiforme(GBM), head and neck squamous cell carcinoma(HNSC), kidney chromophobe(KICH), kidney renal clear cell carcinoma(KIRC), kidney renal papillary cell carcinoma(KIRP), acute myeloid leukemia(LAML), brain lower grade glioma(LGG), liver hepatocellular carcinoma(LIHC), lung adenocarcinoma(LUAD), lung squamous cell carcinoma(LUSC), mesothelioma(MESO), ovarian serous cystadenocarcinoma(OV), pancreatic adenocarcinoma(PAAD), pheochromocytoma and paraganglioma(PCPG), prostate adenocarcinoma(PRAD), rectum adenocarcinoma(READ), sarcoma(SARC), skin cutaneous melanoma(SKCM), stomach adenocarcinoma(STAD) , testicular germ cell tumors (TGCT), thyroid carcinoma(THCA), thymoma(THYM), uterine corpus endometrial carcinoma(UCEC), uterine carcinosarcoma(UCS), uveal melanoma(UVM). In total 14,319 samples were available for this study, and each dataset contains normal samples and tumor samples (Table 1). Among them, 15 cancer types had none or <5 associated normal tissue samples, so only the rest of the 18 cancer types were used to investigate whether there was altered gene expression in tumors compared to adjacent normal tissues with linear mixed effects models (Table 1). The Wilcoxon test was used to study the changes in gene expression in adjacent normal tissues for these 18 types of cancers.
Table 1.
Cancer types included in our study from TCGA database.
| TCGA ID | Primary disease types | Total | Tumor | Normal |
|---|---|---|---|---|
| ACC | Adrenocortical carcinoma | 79 | 79 | 0 |
| BLCA | Bladder urothelial carcinoma | 430 | 411 | 19 |
| BRCA | Breast invasive carcinoma | 1217 | 1097 | 120 |
| CESC | Cervical squamous cell carcinoma and endocervical adenocarcinoma | 309 | 306 | 3 |
| CHOL | Cholangiocarcinoma | 45 | 36 | 9 |
| COAD | Colon adenocarcinoma | 512 | 471 | 41 |
| DLBC | Lymphoid neoplasm diffuse large B-cell lymphoma | 48 | 48 | 0 |
| ESCA | Esophageal carcinoma | 173 | 162 | 11 |
| GBM | Glioblastoma multiforme | 173 | 168 | 5 |
| HNSC | Head and Neck squamous cell carcinoma | 546 | 502 | 44 |
| KICH | Kidney chromophobe | 89 | 65 | 24 |
| KIRC | Kidney renal clear cell carcinoma | 607 | 535 | 72 |
| KIRP | Kidney renal papillary cell carcinoma | 321 | 289 | 32 |
| LAML | Acute Myeloid Leukemia | 152 | 152 | 0 |
| LGG | Brain lower grade glioma | 529 | 529 | 0 |
| LIHC | Liver hepatocellular carcinoma | 424 | 374 | 50 |
| LUAD | Lung adenocarcinoma | 585 | 526 | 59 |
| LUSC | Lung squamous cell carcinoma | 550 | 501 | 49 |
| MESO | Mesothelioma | 86 | 86 | 0 |
| OV | Ovarian serous cystadenocarcinoma | 379 | 379 | 0 |
| PAAD | Pancreatic adenocarcinoma | 182 | 178 | 4 |
| PCPG | Pheochromocytoma and paraganglioma | 186 | 183 | 3 |
| PRAD | Prostate adenocarcinoma | 551 | 499 | 52 |
| READ | Rectum adenocarcinoma | 177 | 167 | 10 |
| SARC | Sarcoma | 265 | 263 | 2 |
| SKCM | Skin cutaneous melanoma | 472 | 471 | 1 |
| STAD | Stomach adenocarcinoma | 407 | 375 | 32 |
| TGCT | Testicular germ cell tumors | 156 | 156 | 0 |
| THCA | Thyroid carcinoma | 568 | 510 | 58 |
| THYM | Thymoma | 121 | 119 | 2 |
| UCEC | Uterine corpus endometrial carcinoma | 583 | 548 | 35 |
| UCS | Uterine carcinosarcoma | 56 | 56 | 0 |
| UVM | Uveal melanoma | 80 | 80 | 0 |
| Total | 11,058 | 10,321 | 737 |
Survival and clinical correlation analysis
Cox regression analysis was used to evaluate the correlation between DOK expression in 33 types of cancers and the overall survival (OS). After dividing the patients into high and low DOK groups, the Kaplan-Meier method was used to construct a survival curve for each cancer type. The PrognoScan database (www.prognoscan.org) combines several clinically annotated cancer microarray datasets from the GEO database and allows researchers to evaluate the expression of specific genes in cancer patients and their relationship with prognosis. We verified the relationship between DOK expression and prognosis in various types of cancer using the PrognoScan database, including OS, recurrence-free survival (RFS), and DSS, with P < 0.05 considered significant.
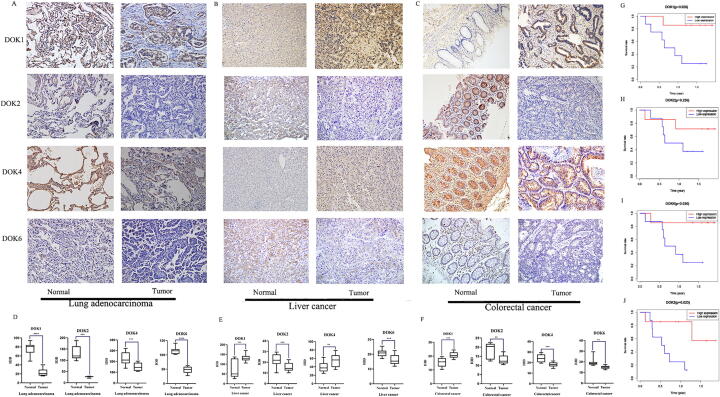
Immunohistochemistry
The incidence of lung adenocarcinoma, liver cancer, and colorectal cancer is relatively high, and DOK1, DOK2, DOK4, and DOK6 are differentially expressed in these three cancers. Therefore, Immunohistochemical method was used to detect the expression of DOK1, DOK2, DOK4, and DOK6 in lung adenocarcinoma, colorectal cancer, liver cancer patient tissues. The sections (4 μm) were deparaffinized and dehydrated in xylene and a series of gradient ethanol solutions, and then pretreated in 10 mM citrate buffer (pH 6.0) at 121 °C for 4 min. Hydrogen peroxide (0.3%) suspends the activity of endogenous peroxidase for 15 min at room temperature. Incubate the sections with DOK1 (1:200, cat. no. PA87543HU, Abebio, Wuhan, China), DOK2 (1:200, cat. no. PA84257HU Abebio, Wuhan, China), DOK4 (1:200, cat. no. PA85947HU, Abebio, Wuhan, China) and DOK6 (1:200, cat. no. PA85948HU, Abebio, Wuhan, China) primary antibodies at 4 °C overnight. Finally, the sections were counterstained with hematoxylin. Image Pro-Plus (version 6.0; Media Cybernetics, Inc. Rockville, Maryland, USA) was used to analyze IHC staining. Select the positive staining area as the target area (AOI). The brown reaction product represents the positive expression of DOK1, DOK2, DOK4, and DOK6. Randomly select the area in the microscope field of view in each area, and take photos with 200x magnification in each group. Analyze each image and use Image-Pro Plus 6.0 software to generate the mean of integral optical density (MOD) value. The average MOD value of all images taken in each group is used and finally the average expression index of each repeated sample is statistically analyzed.
Mutation and methylation analysis
We used the cBioPortal database (https://www.cbioportal.org) and the Cancer Cell Line Encyclopedia (CCLE) (https://portals.broadinstitute.org/ccle) to analyze the mutational changes in DOK family genes in a variety of cancers [15]. The cBioPortal data were obtained from the TCGA database and included data from 10,953 patients (10,967 samples). GSCALite (http://bioinfo.life.hust.edu.cn/web/GSCALite/) is a web-based analysis platform for genomic cancer analysis. We used GSCALite to analyze mRNA expression, Copy Number Variation (CNV), Single Nucleotide Variation (SNV), methylation, and pathway activity.
Tumor microenvironment and immune infiltration analysis
ESTIMATE is an algorithm that uses gene expression signatures to infer the fraction of stromal and immune cells in tumor samples. The estimate score from this program was used to describe tumor purity. More information can be acquired online at https://bioinformatics.mdanderson.org/public-software/estimate/.Spearman correlation was used to analyze the correlation between DOK expression and estimate score, immune score, stromal score, tumor purity. The tumor stem features extracted from the transcriptome and epigenetics of TCGA tumor samples were used to measure the stem cell-like features of tumor cells. The correlation between cancer stemness and DOK expression was investigated by Spearman correlation test. The TIMER database (https://cistrome.shinyapps.io/timer/) contains 10,897 samples across 32 cancer types from TCGA to allow the evaluation of the abundance of immune infiltration [16]. We analyzed DOK expression in different types of cancers and the correlation of DOK expression with the abundance of immune infiltrates, including B cells, CD4+ T cells, CD8+ T cells, neutrophils, macrophages, and dendritic cells. The relationship between the expression level of DOK and tumor purity was also determined.
Drug sensitivity analysis
The National Cancer Institute (NCI) established a screening platform for 60 kinds of whole-cell anticancer drugs (NCI-60 DTP Human Tumor Cell Line Drug Screen), which is a classic tool for in vitro drug screening. CellMiner provides the “NCI-60 Analysis Tool”, which can quickly retrieve the transcripts of 22,379 genes and 360 microRNAs, as well as activity reports of 20,503 compounds. Therefore, we can calculate the Pearson correlation coefficient (PCC) to analyze the relationship between the mRNA expression and the 50% growth inhibitory concentrations of the drugs. The correlation analyses used 262 FDA-approved drug reactions or drugs in clinical trials.
GEPIA dataset
GEPIA is a newly developed interactive web server that uses standard processing pipelines to analyze the RNA sequencing expression data of 9736 tumors and 8587 normal samples from the Cancer Genome Atlas and Genotype Tissue Expression (GTEx) project. We used GEPIA to analyze the correlation between DOK family genes.
Statistical analyses
We used t-test for normal distribution data. Non-normal distribution data sets were analyzed using Wilcoxon test. The Wilcoxon test was used to compare gene expression in normal tissues and in 18 types of cancers. All cancers had more than five adjacent normal samples and boxplots were used to describe gene expression data in each of the various cancers discussed in this study. Univariate Cox proportional hazards regression models were used to the association between gene expression and OS. Spearman or Pearson correlations were used to analyze the correlation between gene expression and Stemness score, stromal score, immune score, estimate score, immune checkpoints, and drug sensitivity.
Results
DOK expression in human tumors
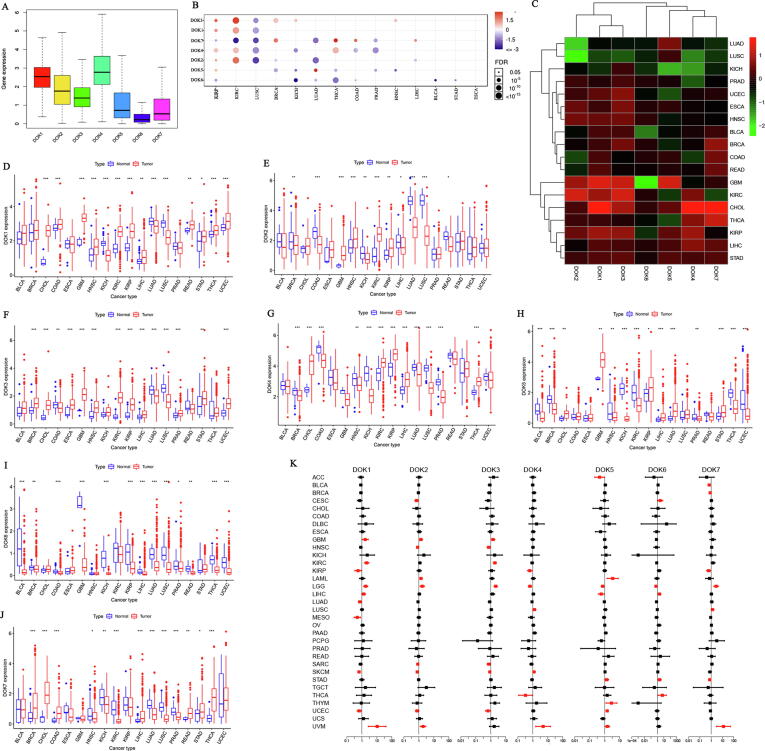
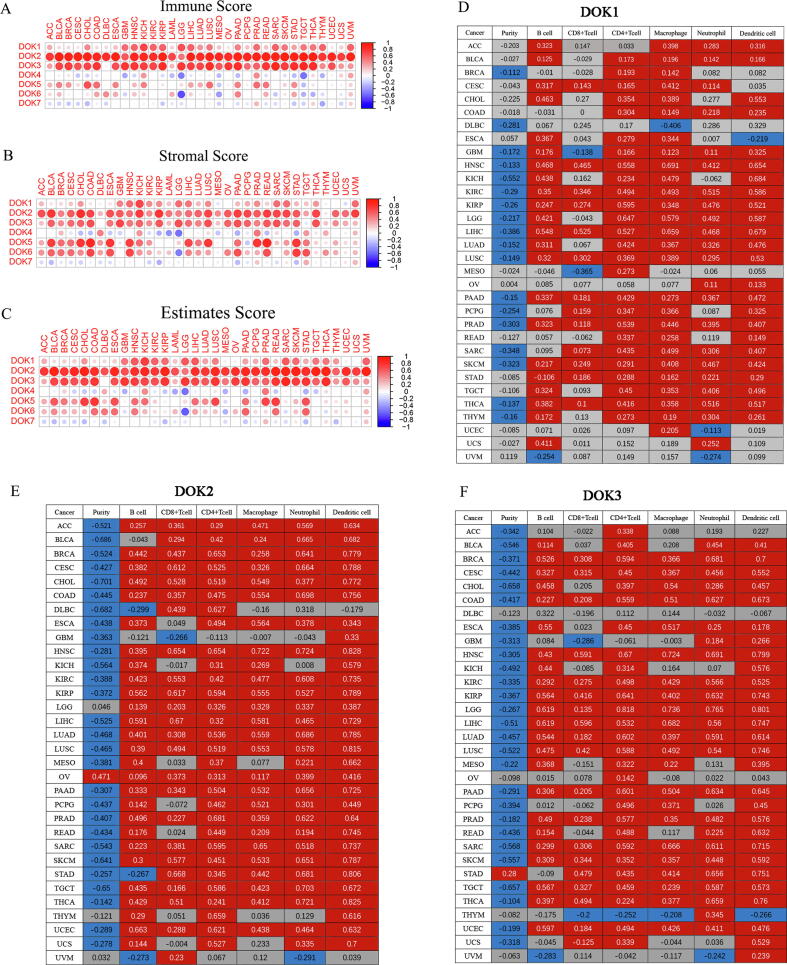
To understand the expression of the DOK in tumors, we analyzed 31 types of cancers in the TCGA database, and 18 of them have at least 5 pairs of normal control samples. The results showed that the expression levels of all genes in the DOK family members were significantly different in these 18 tumors (Fig. 1A-C). For example, the expression level of DOK4 exhibited the most apparent differences in various tumors, with the lowest expression level in BRCA, KICH, PRAD, LUSC, and the highest expression level in CHOL, KIRC, KIRP (Fig. 1G). Other members of the DOK family also showed different degrees of differences (Fig. 1D-J). Compared with other DOK family members (such as DOK1, DOK2, and DOK3), the average expression levels of DOK5, DOK6 and DOK7 in various cancers were lower. These analyses proved that there are apparent differences in the expression of DOK family members among different tumor types. However, the expression of each gene in the DOK family changes in different directions in various cancers (Fig. 1D-J). It was mainly up-regulated in GBM, KIRC, CHOL, THCA, KIRP, LIHC, and STAD, while down-regulated in LUSC and LUAD, but there were also some exceptions. In order to study the expression of DOK in different cancer, the expression of DOK1, DOK2, DOK4, and DOK6 in human liver cancer, lung adenocarcinoma and colorectal cancer was examined by immunohistochemical methods. We collected 15 samples of lung adenocarcinoma, 15 samples of liver cancer, and 10 samples of colorectal cancer. The expression of DOK1, DOK2, DOK4, and DOK6 was evaluated by IHC. The results showed that DOK1, DOK2, DOK4, and DOK6 were lowly expressed in lung adenocarcinoma (Fig. 2A). Consistently, the quantitative score of staining intensity showed that the staining of DOK1, DOK2, DOK4, and DOK6 in lung adenocarcinoma was significantly lower than the staining of DOK1, DOK2, DOK4, and DOK6 in adjacent normal tissues (Fig. 2D). These results indicate that DOK1, DOK2, DOK4, and DOK6 are lowly expressed in human lung adenocarcinoma tissues. The same method was used to analyze the expression of DOK1, DOK2, and DOK6 in liver cancer and colorectal cancer. The results show that DOK1 is highly expressed in liver cancer and colon cancer, while DOK2 and DOK6 are low in liver cancer and colorectal cancer (Fig. 2B, C, E, F). Subsequently, we analyzed the survival of these samples and found that DOK1, DOK2, and DOK6 were related to the OS of patients with lung adenocarcinoma, and patients with low expression had a poorer prognosis (Fig. 2G-I). DOK2 is related to the OS of liver cancer patients, and patients with low expression have a poorer prognosis (Fig. 2J).
Fig. 1.
The expression level of DOK family gene in cancer tissues and adjacent normal tissues. (A) The box plot shows the distribution of DOK family gene expression in all 33 cancer types. (B) The expression level of DOK family gene in cancer obtained from the GCSALite. (C) The heat map shows the difference in DOK expression between the primary tumor and adjacent normal tissues based on log2(fold change), for >5 adjacent normal samples of 18 cancer types. (D-J) Expression levels of DOK family gene in cancerous and adjacent normal tissues for all 18 cancer types. Boxplots represent the distribution of the DOK expression levels in primary tumor and normal tissues (if available) of different cancer types for each of the DOK. The band inside the box is the median expression values for the gene. (K) Association of DOK family gene expression with patient overall survival for different cancer types. The forest plots with the hazard ratios and 95% confidence intervals for overall survival for different cancer types to show survival advantage and disadvantage with increased gene expression of DOK family gene. Univariate Cox proportional hazard regression models were used for the association tests. ∗ P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 2.
Immunohistochemical expression of DOK1, DOK2, DOK4, and DOK6 in human lung cancer, liver cancer, and colorectal cancer. (A-C) The expression of DOK1, DOK2, DOK4, and DOK6 by IHC staining (Magnification× 200). (D–F) Compare the MOD of all groups DOK1, DOK2, and DOK6. (G) The expression of DOK1 is correlated with the prognosis of lung cancer. (H) the expression of DOK2 is correlated with the prognosis of lung cancer. (I) the expression of DOK6 is correlated with the prognosis of lung cancer. (J) the expression of DOK2 is correlated with the prognosis of liver cancer. ∗ P < 0.05; **P < 0.01; *** P < 0.001.
We analyzed the mRNA expression patterns of the DOK genes at different stages of these diseases and found that changes in the expression of various DOK genes are related to the clinical stage of various cancers (Supplementary 1). We found that DOK1 is not only linked to OS in LUAD and KIRC but can also indicate clinical stage in both of these diseases. DOK3 is a known prognostic marker for KIRC but is also linked to changes in the clinical stage of these cancers. DOK4 expression is linked to OS and cancer staging in UVM and KIRP. DOK5 is linked to OS and cancer staging in UVM. DOK6 is a prognostic marker for OS and cancer stage in BLCA and TGCT. DOK7 is a marker for OS and cancer stage in BLCA, KIRC, and UVM. In addition, DOK1, DOK2, and DOK3 are all markers for UCEC staging. DOK1, DOK2, and DOK6 are all linked to the clinical stage of LUAD. DOK1, DOK3, DOK4, and DOK6 expression are all markers for LGG staging. DOK4, DOK5, and DOK7 are all linked to the clinical stage in UVM. DOK3 and DOK5 transcription were also linked to SKCM stage and DOK1, DOK5, and DOK6 were determined to be markers of ESCA staging. The results of DOK1 and LUAD, READ staging, DOK4 and UVM staging, DOK6 and TGCT staging, are close to the significance threshold. Therefore, it is also worthy of our consideration.
The relationship between DOK family genes expression and patient survival rate
In order to determine the roles of genes in the DOK family genes in cancer progress and prognosis, the relationship between the expression of DOK family genes and the overall survival of patients in 33 cancers were analyzed. Using a univariate cox proportional hazard regression model, we claim that a P < 0.05 indicated a significant correlation, and it could be made consistent with the forest plot in Fig. 1K without multiple comparison adjustments (Supplementary 2). Our research found that the expression of DOK family genes was related to the overall survival rate of patients, but even the same gene showed different prognostic correlations with different tumors (Fig. 1K and Supplementary 3). Among them, DOK1 was related to poor prognosis of KIRC, LGG, UVM, while associated with higher survival rates in LUAD, MESO, SKCM, and UCEC. DOK2 was related to poor prognosis of GBM, LAML, LGG, UVM, while associated with higher survival rates in HNSC, LUAD, SARC, SKCM, and UCEC. DOK3 was associated with poor prognoses of GBM, KIRC, LGG, and THYM, while the prognoses were better in CESC, HNSC, PCPG, SARC, and UCEC. DOK4 was associated with poor prognosis of SKCM, UVM, and good prognosis of KIRP, LGG. DOK5 was associated with poor prognosis of LAML, THYM, STAD, UCEC, UVM and good prognosis of ACC, LGG, LIHC, PCPG. Although DOK6 predicted a good prognosis in LGG, it had a poor prognosis in other tumors. Except for DOK3, the other DOK family genes were all related to the poor prognosis of UVM. In addition, DOK4, DOK5 and DOK7 expression is linked to OS and cancer staging in UVM Therefore, DOK family genes are significantly associated with poor prognosis of UVM. We used the PrognoScan Database to analyze the relationship between DOK family gene expression and survival in different cancers. The results showed that DOK family genes are related to the prognosis of many tumors. and the results are summarized in Table 2 and Supplementary 4.
Table 2.
DOK1 expression was associated with the prognosis of different cancers in PrognoScan.
| Data set | Cancer Type | Endpoint | COX p-value | HR | 95% CI (low -high) |
|---|---|---|---|---|---|
| GSE12417-GPL570 | Blood cancer (AML) | OS | 0.005908 | 3.09 | 1.38–6.91 |
| GSE5122 | Blood cancer (AML) | OS | 0.049976 | 1.41 | 1.00–1.99 |
| MGH-glioma | Brain cancer (Glioma) | OS | 0.026259 | 1.87 | 1.08–3.24 |
| GSE4412-GPL96 | Brain cancer (Glioma) | OS | 0.004309 | 5.61 | 1.72–18.31 |
| GES12276 | Breast cancer | RFS | 0.000316 | 0.70 | 0.58–0.85 |
| GSE11121 | Breast cancer | DMFS | 0.000363 | 0.38 | 0.23–0.65 |
| GSE1378 | Breast cancer | RFS | 0.048402 | 0.71 | 0.51–1.00 |
| GSE1456-GPL96 | Breast cancer | DSS | 0.015867 | 0.57 | 0.37–0.90 |
| GSE1456-GPL96 | Breast cancer | RFS | 0.011870 | 0.30 | 0.12–0.77 |
| GSE7390 | Breast cancer | OS | 0.040569 | 0.73 | 0.54–0.99 |
| GES11595 | Esophagus cancer | OS | 0.003069 | 0.25 | 0.10–0.62 |
| Jacob-00182-HLM | Lung cancer (Adenocarcinoma) | OS | 0.006034 | 0.27 | 0.11–0.69 |
| Jacob-00182-MSK | Lung cancer (Adenocarcinoma) | OS | 0.028547 | 0.20 | 0.05–0.84 |
| GSE13213 | Lung cancer (Adenocarcinoma) | OS | 0.028070 | 0.69 | 0.49–0.96 |
| GSE3120 | Lung cancer (Adenocarcinoma) | RFS | 0.012020 | 5.07 | 1.43–18.02 |
| Jacob-00182-UM | Lung cancer (Adenocarcinoma) | OS | 0.032661 | 0.47 | 0.23–0.94 |
| GSE9891 | Ovarian cancer | OS | 0.036597 | 0.58 | 0.34–0.97 |
| GSE26712 | Ovarian cancer | DFS | 0.003239 | 0.59 | 0.41–0.84 |
| GSE26712 | Ovarian cancer | OS | 0.003871 | 0.57 | 0.39–0.83 |
| GSE19234 | Skin cancer (Melanoma) | OS | 0.041078 | 0.16 | 0.03–0.93 |
Annotation: OS, overall survival; RFS, relapse free survival; DSS, disease specific survival; DFS, Disease Free Survival; DMFS, Distant Metastasis Free Survival.
DOK family genes was related to genomic alteration
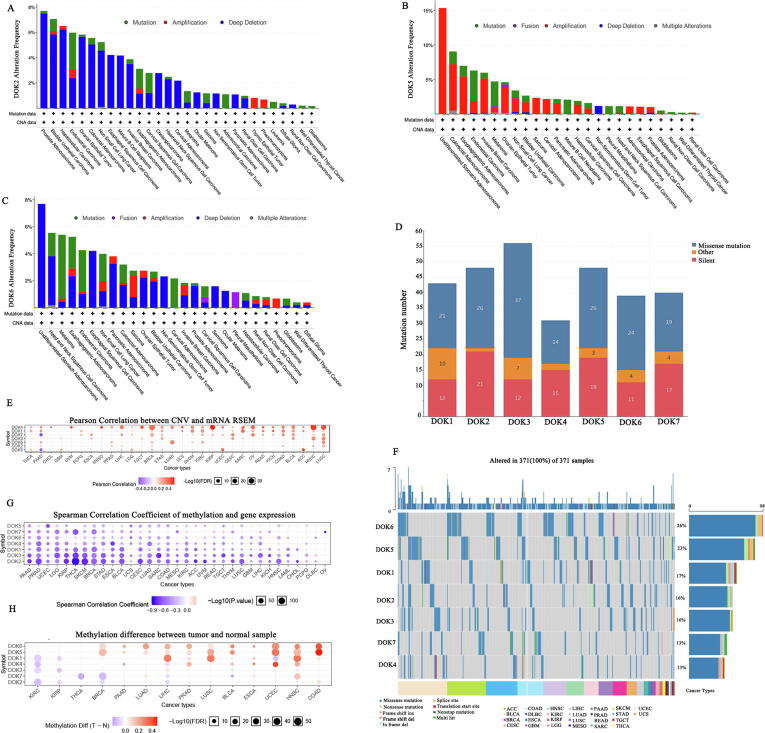
We evaluated the impact of the various DOK family genes alteration frequency using the cBio-Portal database. The alteration frequencies in DOK2, DOK5, DOK6, and DOK7 are relatively high, while the alteration frequency in DOK4 was shown to be the lowest. DOK2 has the highest alteration frequency in prostate cancer and bladder cancer, DOK3 has the highest alteration frequency in ccRCC, and DOK5 has the highest alteration frequency in colorectal cancer (Fig. 3A-C and Supplementary 5). Then we used CCLE to analyze mutation data in 967 cell lines of 23 cancers. These data revealed that DOK family genes have relatively high mutation frequencies in many types of cancers (Fig. 3D). Finally, the frequency of changes in CNV and SNV in DOK family genes was analyzed, and the results showed that CNV and SNV had a higher frequency of changes (Fig. 3E-F). Using methylation level and expression profile data, we further analyzed the effect of promoter methylation on DOK family gene changes in 33 types of cancer (Fig. 3G-H). Our results indicate that DOK family genes are methylated in a variety of tumors. Specifically, DOK5 and DOK6 are hypermethylated in most tumors. In addition, DOK family genes are mostly hypomethylated in KIRC and KIRP, but hypermethylated in HNSC and COAD. DOK family gene promoters are negatively correlated with their gene expression. These results reveal the highly heterogeneous inheritance and expression changes of DOK family genes in different types of cancer.
Fig. 3.
The alteration frequency of DOK family gene in different cancers, and the relationship between DOK family gene expression. (A-C) The alteration frequency of DOK family gene in different cancers obtained from the cBioPortal. (D) Mutation landscape of DOK family gene in cancer cell lines obtained from the CCLE. (E) The relationship between the Copy Number Variation (CNV)of DOK family genes and the expression level in different cancers. (F) SNV frequency of DOK family gene in different cancers. (G) The relationship between promoter methylation and expression levels of DOK family gene in different cancers. (H) The difference in promoter methylation of DOK family gene in different cancers.
DOK family genes was related to tumor microenvironment and immune response
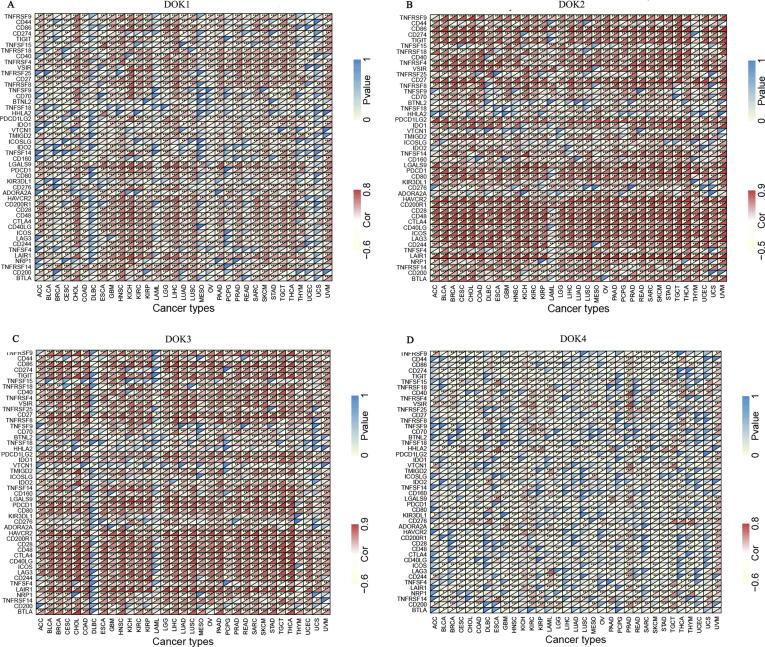
Studies have shown that the expression of DOK4 and DOK5 mRNA could be detected in human T cells, which implies that they are involved in immune regulation [8]. In addition, it has also been reported that DOK4 acts as a negative regulator of T cell activation [17]. To understand whether all DOK family members were related to tumor immunity, we analyzed the correlation between DOK and tumor immunity. DOK1-DOK3 has the most significant correlation with immune score in various cancers (Fig. 4A and Supplementary 6). Among them, DOK2 had the strongest correlation (r = 0.94, P < 0.001), followed by DOK3 (r = 0.86, P < 0.001) and DOK1 (r = 0.70, P < 0.001) (Supplementary 6). DOK4-DOK6 had both positive and negative correlations with immune score in various cancers. DOK7 was less correlated with immune score in different cancers. We further found that all members of the DOK family were correlated with immune scores in LGG, STAD, PAAD, THCA, and TGCT. In addition, we also analyzed the estimate score and stromal score in DOK and tumors, and the results were similar to that of immune scores (Fig. 4B-C and Supplementary 6). The correlation between DOK expression and immune infiltration levels in 32 cancers of TIMER was also analyzed (Fig. 4D-F and Supplementary 7). The results showed that DOK1-DOK7 were significantly correlated with infiltration of B cells, CD4+ T cells, CD8+ T cells, neutrophils, macrophages, and dendritic cells in different tumors, among which DOK1-DOK3 were the most significantly and positively correlated genes. DOK7 showed the least correlation with immune cell infiltration, and was only related to immune infiltration in THCA, THYM, and DLBC. In addition, DOK2 and DOK3 had a significant and negative correlation with tumor purity in most tumors, while DOK7 showed a small correlation with tumor purity. Finally, we analyze the relationship between DOK family genes expression and immune checkpoint gene expression (Fig. 5 and Supplementary 8). We found that there is a high correlation between DOK family genes and immune checkpoints (P < 0.05). Among them, the correlation between DOK1-DOK3 and immune checkpoint is the strongest and mostly positive correlation. The correlation between DOK6 and DOK7 and immune checkpoint is the weakest.
Fig. 4.
The correlation of DOK family gene expression with tumor microenvironment and immune infiltration. (A–C) The correlation matrix plots to shows the correlation between DOK family gene expression based on the ESTIMATE algorithm and the immune score, estimated score, and stromal score of 33 different cancer types. Spearman correlation is used for testing. The size of the dot represents the absolute value of the correlation coefficients. The larger the size, the higher the correlation (the higher the absolute correlation coefficient). This also applies to Fig. 6A–B. (D–F) The correlation between DOK1, DOK2, and DOK3 expression and immune infiltration levels in 32 cancers of TIMER. Red, positive correlation (P < 0.05); Blue, negative correlation (P < 0.05); Gray, not significant (P > 0.05).
Fig. 5.
Correlation between DOK family gene mRNA expression levels and acknowledged immune checkpoints’ mRNA expression in multiple tumors from TCGA database. (A) Correlation analysis of DOK1 expression level, (B) Correlation analysis of DOK2 expression level, (C) Correlation analysis of DOK3 expression level, (D) Correlation analysis of DOK4 expression level, with immune checkpoint gene levels in human cancer. The lower triangle in each tile indicates coefficients calculated by Pearson’s correlation test, and the upper triangle indicates log10 transformed P-value. *P<0.05; **P<0.01; *** P<0.001.
DOK family genes was related to tumor stemness and sensitivity of cancer cells to chemotherapy
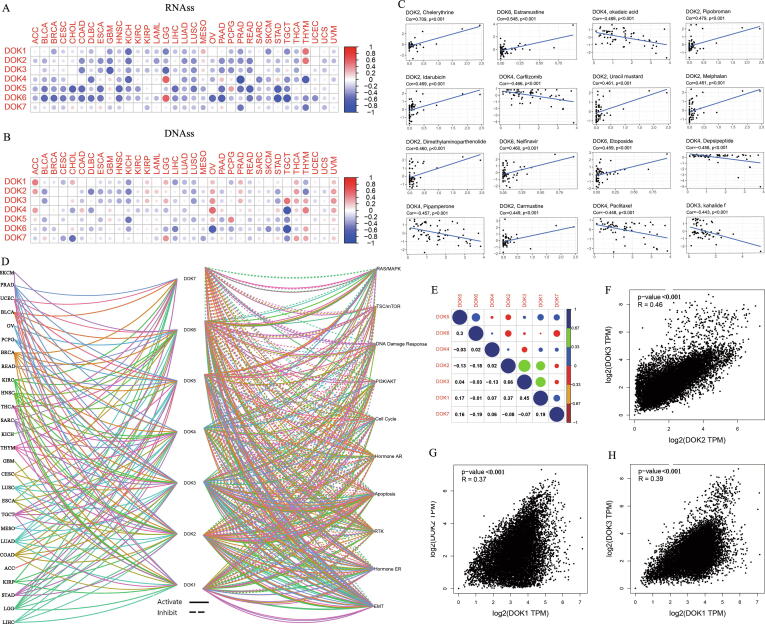
Tumors are a heterogeneous cell population. A small number of stem-like cells are similar to embryonic stem cells and have unlimited self-renewal and division capabilities, which facilitate the occurrence and development of tumors. In recent years, many studies have suggested that the existence of cancer stem cells is the leading cause of tumor recurrence and metastasis, and is closely related to chemotherapy resistance. Tumor stemness can be measured by RNA stemness score based on mRNA expression (RNAss) and DNA stemness based on DNA methylation pattern (DNAss). DOK family members showed various levels of association with RNAss and DNAss in different cancers (Fig. 6A-B and Supplementary 9). We found that DOK5 and DOK6 were negatively correlated with RNAss and DNAss (P < 0.05), and DOK6 has the most apparent correlation with RNAss (r = −0.73). DOK2, DOK3, and DOK4 were negatively correlated with RNAs, but were either positively or negatively correlated with DNAss in different tumors. DOK1 and DOK7 had relatively weak correlation with RNAs and DNAss (P < 0.05). Since DOK is related to stem cell-like characteristics, we next analyzed the expression of DOK in human cancer cell lines (NCI-60) as well as the sensitivity to chemotherapy drugs. Our study found that the increased expression of DOK was related to the increased sensitivity of a variety of chemotherapeutic drugs (r > 0.4 and P < 0.001), especially DOK2, DOK4, and DOK6 (Fig. 6C and Table 3). The increase in DOK2 expression was related to the increased sensitivity of cancer cells to Chelerythrine, Hydroxyurea, Fenretinide, Idarubicin, Uracil mustard, and Chlorambucil. In addition, increased expression of DOK6 also increased the sensitivity of cancer cells to a variety of chemotherapy drugs, including Ethinyl estradiol, Estramustine, Etoposide, Carmustine, and 7-Hydroxystaurosporine. However, the increase in DOK4 expression was associated with increased cell resistance to drugs, such as Carfilzomib, Depsipeptide, Pipamperone, Eribulin mesilate, Vinblastine, Actinomycin D.
Fig. 6.
Association of DOK family gene expression with tumor stemness, drug sensitivity, and signaling pathways. (A–B) Based on the Spearman correlation test, the correlation matrix between DOK family gene expression and cancer severity is RNAss (A) and DNAss (B). (C) Scatter plots to show the association between DOK family gene expression and drug sensitivity (Z-score from CellMiner interface) tested with Pearson Correlation using NCI-60 cell line data. (D) DOK family gene are associated with the activation and inhibition of 10 cancer pathways. The pie chart shows the correlation between DOK family gene and cancer pathways. Solid line indicates activation and dashed line indicates inhibition. (E) Correlation plot based on Spearman correlation test results, showing the correlation of gene expression among DOK family gene in all 33 cancer types. (F) Correlation analysis between DOK2 and DOK3 in GEPIA. (G) Correlation analysis between DOK1 and DOK2 in GEPIA. (H) Correlation analysis between DOK1 and DOK3 in GEPIA.
Table 3.
The relationship between DOK expression and drug sensitivity.
| Gene | Drug | correlation | p-value |
|---|---|---|---|
| DOK2 | Chelerythrine | 0.7085977 | 2.39E−10 |
| DOK6 | Estramustine | 0.5482401 | 5.77E−06 |
| DOK4 | okadaic acid | −0.488041 | 7.64E−05 |
| DOK2 | Pipobroman | 0.4788433 | 0.0001087 |
| DOK2 | Idarubicin | 0.4686688 | 0.0001589 |
| DOK4 | Carfilzomib | −0.466327 | 0.0001731 |
| DOK2 | Uracil mustard | 0.4610959 | 0.0002091 |
| DOK2 | Melphalan | 0.4607304 | 0.0002119 |
| DOK2 | Dimethylaminoparthenolide | 0.4603258 | 0.000215 |
| DOK6 | Nelfinavir | 0.4600371 | 0.0002172 |
| DOK6 | Etoposide | 0.4585132 | 0.0002293 |
| DOK4 | Depsipeptide | −0.458461 | 0.0002298 |
| DOK4 | Pipamperone | −0.457402 | 0.0002386 |
| DOK2 | Carmustine | 0.4488611 | 0.0003217 |
| DOK4 | Paclitaxel | −0.447562 | 0.0003365 |
| DOK3 | kahalide f | −0.44348 | 0.0003868 |
| DOK2 | Fludarabine | 0.4415362 | 0.0004132 |
| DOK2 | Thiotepa | 0.440709 | 0.0004249 |
| DOK6 | Carmustine | 0.4405261 | 0.0004275 |
| DOK2 | Carboplatin | 0.4359063 | 0.0004989 |
| DOK2 | Asparaginase | 0.4344543 | 0.0005235 |
| DOK2 | Arsenic trioxide | 0.4304928 | 0.0005962 |
| DOK2 | PX-316 | 0.4280999 | 0.0006445 |
| DOK4 | Eribulin mesilate | −0.426006 | 0.0006896 |
| DOK4 | Vinblastine | −0.425417 | 0.0007028 |
| DOK6 | 7-Hydroxystaurosporine | 0.4248457 | 0.0007158 |
| DOK4 | Actinomycin D | −0.420248 | 0.0008288 |
| DOK2 | Etoposide | 0.4176907 | 0.0008984 |
| DOK2 | Ifosfamide | 0.4162463 | 0.00094 |
| DOK2 | Triethylenemelamine | 0.4146959 | 0.0009865 |
| DOK1 | Gemcitabine | 0.4113606 | 0.0010938 |
| DOK6 | Megestrol acetate | 0.4074377 | 0.0012334 |
| DOK4 | Vinorelbine | −0.400945 | 0.0014998 |
| DOK4 | Dolastatin 10 | −0.394018 | 0.00184 |
| DOK6 | Salinomycin | 0.3939486 | 0.0018438 |
| DOK6 | Teniposide | 0.3923908 | 0.0019293 |
| DOK6 | AP-26113 | 0.3909877 | 0.0020094 |
| DOK2 | Parthenolide | 0.3900241 | 0.0020661 |
| DOK6 | Ethinyl estradiol | 0.3855035 | 0.0023517 |
| DOK2 | Lomustine | 0.3831947 | 0.0025107 |
| DOK6 | Arsenic trioxide | 0.3824409 | 0.0025647 |
| DOK6 | Epirubicin | 0.3809366 | 0.0026755 |
| DOK2 | Nitrogen mustard | 0.3794911 | 0.0027859 |
| DOK2 | Digoxin | 0.3773697 | 0.0029553 |
| DOK2 | Cytarabine | 0.3771083 | 0.0029768 |
| DOK7 | Pipamperone | −0.375646 | 0.0030997 |
| DOK6 | Isotretinoin | 0.3743589 | 0.0032115 |
| DOK2 | XK-469 | 0.3682948 | 0.0037879 |
| DOK7 | BN-2629 | −0.365256 | 0.0041097 |
| DOK2 | Cisplatin | 0.3616868 | 0.0045185 |
| DOK6 | Irofulven | −0.359741 | 0.0047561 |
| DOK7 | Midostaurin | −0.359726 | 0.004758 |
| DOK1 | Triapine | 0.3589469 | 0.0048562 |
| DOK7 | Epirubicin | −0.358925 | 0.004859 |
| DOK1 | Triethylenemelamine | 0.357197 | 0.0050833 |
| DOK4 | Tyrothricin | −0.356618 | 0.0051605 |
| DOK2 | BN-2629 | 0.356401 | 0.0051897 |
| DOK3 | ABT-199 | 0.35528 | 0.0053428 |
| DOK1 | 5-fluoro deoxy uridine 10mer | 0.3537224 | 0.0055622 |
| DOK3 | Carboplatin | 0.3534247 | 0.005605 |
| DOK4 | Carmustine | −0.352144 | 0.0057926 |
| DOK4 | Arsenic trioxide | −0.351821 | 0.0058407 |
| DOK2 | Dexrazoxane | 0.3498989 | 0.0061345 |
| DOK1 | Asparaginase | 0.3494209 | 0.0062096 |
| DOK6 | Ifosfamide | 0.3484718 | 0.006361 |
| DOK1 | LMP-400 | 0.3483937 | 0.0063736 |
| DOK1 | Thiotepa | 0.3474983 | 0.0065197 |
| DOK2 | Cladribine | 0.3462837 | 0.0067225 |
| DOK2 | Batracylin | 0.3459751 | 0.0067749 |
| DOK7 | Bortezomib | −0.344944 | 0.0069526 |
| DOK1 | Cladribine | 0.34487 | 0.0069655 |
| DOK4 | Lomustine | −0.342182 | 0.0074488 |
| DOK7 | Carmustine | −0.341285 | 0.0076165 |
| DOK4 | Mithramycin | −0.340635 | 0.0077401 |
| DOK2 | Calusterone | 0.3405351 | 0.0077592 |
| DOK4 | Ethinyl estradiol | −0.340444 | 0.0077768 |
| DOK1 | Uracil mustard | 0.3401498 | 0.0078335 |
| DOK2 | Teniposide | 0.3400573 | 0.0078514 |
Cancer-related pathways regulated by DOK family genes
In order to further understand the molecular mechanism of DOK family genes involved in cancer, we analyzed the correlation between the expression of DOK family genes and 10 cancer-related pathways (Fig. 6D). As a result, the expression of DOK family genes is related to the activation or inhibition of multiple oncogenic pathways. The expression of DOK family genes is related to the activation of EMT and hormone ER pathways, and is related to the inhibition of DNA damage response, cell cycle, and hormone AR pathways. In addition, genes cannot play a role alone. Interestingly, we found that there are highly correlated interaction patterns between DOK family genes. Among them, DOK1 and DOK3 (r = 0.45, P < 0.001), DOK2 and DOK3 (r = 0.66, P < 0.001) have the highest correlation among these seven genes, indicating that they may share some common features or functions (Fig. 6E). The results of the GEPIA database also confirmed that there is a strong correlation between the DOK family genes (Fig. 6F-H).
Discussion
DOK family genes disorders have been reported in many cancers [9], [18], [19], [20]. Although DOK family genes expression is closely associated related with pathological progression and poorer prognosis in some tumors, there has been no comprehensive analysis of DOK family genes in different cancer. This study is the first to explore the expression and prognostic value of DOK family genes in human tumors, as well as the mechanisms underlying tumor progression.
As the first family member discovered, DOK1 was named p62dok according to its molecular weight. Because DOK1 can bind to p120 rasGAP, thereby inhibiting the Ras-Raf-MEK-ERK signaling pathway [21], [22], DOK1 is considered to act as a tumor suppressor gene in most tumor [23], [24], [25]. Initially, DOK1 was only related to leukemia and Burkitt lymphoma. However, in recent years, studies have found that the low expression of DOK1 mRNA is also associated with non-hematological tumors. For example, knocking out DOK1 in epithelial ovarian cancer (EOC) can promote EOC cell migration, proliferation and cisplatin sensitivity [24]. In the study of mouse lung cancer models, it was found that knocking out DOK1, DOK2, and DOK3 alone can promote the occurrence of lung cancer. In addition, knocking out DOK1, DOK2, and DOK3 at the same time can significantly promote the formation of lung cancer, so DOK1-DOK3 can synergistically inhibit lung tumors. Our study found that DOK1 has a better prognosis in LUAD, MESO, SKCM, and UCEC, and may play a tumor suppressor function in these tumors. However, DOK1 does not play a tumor suppressor function in all tumors, and can play a tumor-promoting function in other tumors. For example, in glioma cells, DOK1 promotes the invasion of glioma cells through the p130Cas-Rap1 signaling pathway. Our study also found that DOK1 is associated with poor prognosis of KIRC, LGG, and UVM. In the study of tumor staging, it was found that DOK1 was highly expressed in low-grade LUAD and SKCM, but the opposite was true in KIRC. It is further confirmed that DOK1 plays a role of suppressing or promoting cancer in different tumors.
DOK2 and DOK3 are similar to DOK1 and act as tumor suppressor genes in most tumors, including lung cancer, gastric cancer, colorectal cancer, and acute myeloid leukemia [26], [11], [27]. Here, we found that DOK2 expression indicates an improved prognosis in HNSC, LUAD, SARC, SKCM, and UCEC, but a poor prognosis in GBM, LAML, and UVM. Similarly, DOK3 expression was linked to a better prognosis in CESC, HNSC, PCPG, SARC, and UCEC, but a worse prognosis in GBM, KIRC, LGG, and THYM. Therefore, DOK2 and DOK3 do not act exclusively as tumor suppressor genes and may promote the proliferation of certain tumors. In support of this hypothesis, we found that DOK2 expression could also be linked to tumor stage with both early stage COAD and TGCT exhibiting increased DOK2 expression and BLCA and KIRC demonstrating increased DOK2 expression in their later stages of development. DOK3 expression was higher in LUAD and TGCT in the earlier stages of diseases but higher in the later stages of both KIRP and STAD.
Since DOK family proteins have no obvious catalytic activity, their biological effects are likely attributed to the activity of their binding partners. Therefore, DOK1-DOK3 may interact with other proteins which affect tumor proliferation. Our study found that DOK1, DOK2, and DOK3 expression were all low in LUAD tumors and that this reduction was linked to poorer prognosis. The results of the correlation analysis showed that the expression of DOK1 and DOK2 are correlated and that DOK2 and DOK3 expression are also correlated. Niki [28] found that mice with a single deletion in DOK1 or DOK2 maintained normal hematopoiesis, but successive deletions in DOK1 and DOK2 led to abnormal hematopoiesis and activation of Ras/mitogen activated protein kinase in mice. In addition, mice with deleted DOK1 and DOK2 exhibited increased cellular proliferation and decreased apoptosis, thus facilitating the production of transplantable chronic myeloid leukemia-like bone marrow and external myeloproliferative diseases. Berger [26]confirmed that DOK1, DOK2, and DOK3 act as lung cancer suppressor genes and that DOK1, DOK2, and DOK3 single and (or) combined gene knockout promotes the occurrence of lung cancer in mice, while DOK1, DOK2, and DOK3 exerted a synergistic effect in suppressing lung cancer in these models. In addition, studies have found that the DOK1, DOK2, and DOK3 are closely associated with immunity, which we confirmed in our study when DOK1, DOK2, and DOK3 expression were all positively correlated with stromal scores and immune cell infiltration levels in a variety of cancers. However, the underlying mechanism allowing DOK1-DOK3 interactions to exert synergistic effects in tumor progression/suppression remains unknown. DOK4 -DOK6 have been less studied in tumors. Previous studies have shown that DOK4 and DOK6 are weakly expressed in breast cancer and play a tumor suppressor function [14], [13]. However, DOK5 and DOK6 are highly expressed in AML and are associated with poor prognosis [29]. Our study also found that UVM has a poor prognosis when DOK4-DOK6 are expressed and STAD has a poor prognosis when DOK4 and DOK6 are expressed. In contrast LGG has a better prognosis when DOK4 and DOK6 are expressed. Our research found that the expression of DOK5 and DOK6 are positively correlated with each other.
DOK7 is different from the other members of the DOK family in that it is preferentially expressed in muscle tissue and participates in the production and maintenance of neuromuscular junctions, making it essential for neuronal postsynaptic differentiation [30].Therefore, research on DOK7 has mainly focused on neuromuscular junction diseases, such as myasthenia gravis [31], [32]. However, recent studies have suggested that the DOK7 is closely related to the occurrence and development of lung and breast cancer [33], [34]. DOK7 expression decreases significantly in these tissues resulting in increased tumor cell proliferation and cloning ability, thereby accelerating pathological progression and worsening the prognosis. Our study showed that reduced expression of DOK7 was associated with poor prognosis in KIRC, UVM, and promoted a survival advantage in BLCA, BRCA, STAD, and THCA. The expression of DOK7 is also related to changes in the clinicopathological characteristics of several cancers. DOK7 is highly expressed in patients with stage III-IV CHOL, KIRC, THCA, and UVM, but reduced in stage I-II of these cancers. In contrast, BLCA and KIRP present with high levels of DOK7 in stage I–II which gradually reduces in stage III–IV. Based on this data, we can conclude that the DOK play an important role in suppressing or promoting cancer in different tumors. The reason for the differences in their activity may be explained by the heterogeneity among DOK family members, which results in differences in effector partners and signaling efficiencies. In addition, the expression level of a gene is related to the gene itself as well as to the upstream and downstream regulatory sequences (including promoters, enhancers, etc.) and introns of the gene. For example, it has been demonstrated that E2F1 and ATRA have a significant positive effect on the DOK1 promoter and promote DOK1 expression, while CREB1, SP1 and p53 can inhibit DOK1 expression [35].
In addition, the analysis of 118 breast cancer samples revealed that the expression levels of DOK1 mRNA were significantly reduced in breast cancer which was associated with changes in the clinicopathological characteristics of this samples, but in 6 of the 118 breast cancer samples, four of the coding sequences appeared to change [23]. These changes were located in the functional domain of the protein and only exist in tumor tissues. Therefore, these mutations may affect the function and/or cellular localization of the protein, which may promote the development of cancer. In addition, DOK1 mutations have also been found in a small number of colorectal cancer cases [36]. However, no obvious mutation in DOK2 were found in leukemia, colorectal cancers, and gastric cancer [11], [37]. Meanwhile, mutations in DOK7 have mainly been shown to be related to congenital myasthenic syndrome, which has not been studied in tumors [38]. Our study found that the expression of DOK family genes is not only related to tumor mutations, but also related to CNV, SNV, and promoter methylation. These changes may be one of the important mechanisms leading to the disturbance of DOK family genes expression.
Cancer stem cells are a small population of tumor cells which present with stem cell characteristics. They maintain self-renewal and multidirectional differentiation potential through asymmetric division, leading to continuous proliferation of tumor cells and promoting the production of tumor heterogeneity and diversity. These cells have strong tumorigenesis and invasive ability, and may be the root cause of tumor resistance, recurrence after treatment, and tumor metastasis [39], [40]. Previous studies have found that knocking out DOK1 in epithelial ovarian cancer (EOC) cells can increase their sensitivity to cisplatin [24]. Therefore, it is necessary to further evaluate the relationship between DOK family genes, cancer stem cells, and chemotherapeutic drug sensitivity. Our research found that the expression of DOK5 and DOK6 was the lowest in tumor tissues and that DOK5 and DOK6 expression was negatively correlated with RNAs and DNAss (P < 0.05), with the relationship between DOK6 and RNAss being the most obvious (r = −0.73). DOK5 is not linked to chemotherapy drug sensitivity but DOK6 is closely related to the sensitivity of multiple chemotherapy drugs, including ethinyl estradiol, estramustine, etoposide, carmustine, and 7-hydroxystaurosporine. DOK2, DOK3, and DOK4 are negatively correlated with RNAss, but positively or negatively correlated with DNAss in different tumors. Increased expression of DOK2 may increase tumor sensitivity to many chemotherapy drugs, including chelerythrine, hydroxyurea, fenretinide, idarubicin, uracil mustard, and chlorambucil. Previous studies have confirmed that in ovarian cancer, DOK2 deletion increases carboplatin resistance by reducing apoptosis [41], [19]. The increase in DOK4 expression reduces the sensitivity of cancer cells to multiple chemotherapy drugs. However, increased expression of DOK4 is related to resistance to several drugs, including carfilzomib, depsipeptide, pipamperone, ribulin mesilate, vinblastine, and actinomycin D. Therefore, DOK4 may play a role in drug resistance in some tumors and affect the survival of patients, but given that this is only a database analysis these hypotheses will need to be evaluated in in vivo and in vitro studies.
The tumor microenvironment is composed of tumor cells and infiltrating immune cells around the tumor, cancer-associated fibroblasts (CAFs), extracellular matrix, new blood vessels, and endothelial cells [42]. There are many factors in this microenvironment that facilitate the interaction between tumor tissues and the immune system, including immune cells which may identify and eliminate tumor cells inhibiting disease progression. Tumor cells express a variety of inhibitory molecules on their cell surface and secrete tumor-related cytokines to weaken the anti-tumor activity of these immune cells, thereby mediating the body's immune tolerance to tumors and enabling immune escape. CD4+ and CD8+ T cells are important members of TME and participate in specific anti-tumor immune responses. DCs are the most important antigen-presenting cells in the body, and can initiate immunity and determine the final development of the immune response channeling the environment towards immune activation or tolerance [43]. Neutrophils secrete MMP9 into TME, which contributes to angiogenesis, tumor progression and metastasis in mouse transplantation models. The degree of enrichment of mature dendritic cells and CD8+ T cells is closely related to the survival rate of tumor patients. The more DC and CD8+ T cells, the higher the survival rate. However, our analysis suggests that DOK1-DOK3 expression exhibits a significant correlation with the immune score, with DOK2 having the strongest correlation. DOK4-DOK6 were both positively and negatively correlated with immune score in different tumors, while DOK7 was shown to have a low degree of correlation with immune score in the microenvironment. In addition, we found that DOK1-DOK3 have a significant, positive correlation with B cell, CD4+ T cell, CD8+ T cell, neutrophil, macrophage, and dendritic cell (DCs) infiltration. Among them, the correlation was strongest between the DOK and DCs. DOK1-DOK3 may affect the development of tumors by regulating DCs and cD8+ T cells in some tumors [44]. Therefore, DOK1-DOK3 may play an important role in tumor immunity.
Studies have found that DOK2 and DOK5 expression is linked to changes in the tumor microenvironment [45], [46], but the correlation between other members of the DOK family gene and the tumor microenvironment have not been studied.
Tumor-associated macrophages (TAMs) occupy a large proportion of the immune cell population in the tumor microenvironment. The classical typology of TAMs can be divided into M1-type and M2-type macrophages. In most cases, the M1-type is thought to play a predominantly anti-tumor role, while the M2-type plays a role in promoting tumor progression [47]. Recent studies have found that DOK1 activates NF-κB conduction in macrophages and inhibits PD-L1 expression, thereby affecting the prognosis of gastric cancer patients [48]. Our research also found that there is a significant correlation between DOK1-DOK3 and DOK5 expression and macrophage activation. In addition, we also found that DOK1-DOK3 has a significant correlation with immune checkpoints. Our research further clarified that DOK1-DOK3 has broader tumor applicability, and confirmed that the expression of DOK1-DOK3 is closely related to the biological processes of immune cells and immune-related molecules in most cancers.
In summary, we found massive heterogeneity in DOK expression levels in different types of tumors. Among all the DOK family members, DOK4 showed the most abundant expression, and the highest degree of heterogeneity among the various tumors analyzed and was the most obvious marker for prognostic evaluations. Each gene in the DOK family members demonstrated a different expressional profile in each of the 18 cancers evaluated with most genes exhibiting some degree of upregulation in GBM, KIRC, CHOL, THCA, KIRP, LIHC and STAD, and downregulation in LUSC and LUAD, but there were some exceptions. We went on to analyzed the relationship between expression DOK family genes and the overall survival rate of patients in 30 cancer types. The relationship between the expression level of DOK family genes and the prognosis depended on the type of cancer. However, in general, DOK family genes were upregulated in GBM, KIRC, CHOL, THCA, KIRP, LIHC, and STAD, and linked to poorer prognosis, while they mainly downregulated in LUSC and LUAD, and linked to improved survival rate. We then used the ESTIMATE algorithm to evaluate the links between DOK family genes expression and stromal and immune cell infiltration. The correlation between DOK family genes expression and tumor stemness score and drug sensitivity score indicated that DOK4 reduces tumor the sensitivity for multiple chemotherapy drugs in tumors, while DOK2, DOK6, and DOK7 increased tumor sensitivity to multiple chemotherapy drugs. Although we conducted a comprehensive analysis of DOK family genes in human tumors, there are certain limitations. First, our data came from the TCGA database, which could not guarantee the quality of the samples included. Second, our current research is based on bioinformatic analysis, and many results have not been reported. Therefore, further clinical and laboratory studies are in urgently needed to verify our findings.
Conclusion
In summary, our research revealed the DOK family genes is not entirely a tumor suppressor gene, but also a tumor-promoting gene in some tumors. DOK family genes are significantly associated with poor prognosis of UVM. DOK1-DOK3 showed a significant correlation with tumor immunity. DOK2 and DOK6 were negatively correlated with tumor stemness and could increase the sensitivity of cancer cells to chemotherapy drugs, while DOK4 reduced the sensitivity of cancer cells to multiple chemotherapy drugs.
Compliance with Ethics Requirements
The clinical sample collection was approved by the local ethics committee of the Renmin Hospital of Wuhan University (WDRY2019-K092), and written informed consent was obtained from each patient.
Author contributions
GYJ, LM, WWX, and SQ came up with the design and conception. Material preparation, data collection, and analysis were performed by GYJ, QZD, XJH, ZYC, LM, HN, ZXZ, GWY, WWX, and SQ. Immunohistochemistry was performed by LM, SQ, and YJP . The first draft of the manuscript was written by GYJ, WWX, and SQ. GYJ and LM contributed equally to this study and shared co-first authors. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (No. 81800574; No. 81870442) and the Natural Science Foundation of Hubei Province (No. 2018CFB649). Funding sources for this study had no role in the study design; data collection, analyses, or interpretation; or writing of the manuscript.
Data availability
The following information was supplied regarding data availability: TCGA raw data is available at UCSC xena browser (https://xenabrowser.net/datapages/). NCI-60 cell line data is available at CellMiner (https://discover.nci.nih.gov/cellminer/home.do).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2021.06.008.
Contributor Information
Qiao Shi, Email: shiqiao614@whu.edu.cn.
Weixing Wang, Email: Wangwx@whu.edu.cn.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Janas J.A., Van Aelst L. Oncogenic tyrosine kinases target Dok-1 for ubiquitin-mediated proteasomal degradation to promote cell transformation. Mol Cell Biol. 2011;31(13):2552–2565. doi: 10.1128/MCB.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mashima R., Arimura S., Kajikawa S., Oda H., Nakae S., Yamanashi Y. Dok adaptors play anti-inflammatory roles in pulmonary homeostasis. Genes Cells. 2013;18(1):56–65. doi: 10.1111/gtc.12016. [DOI] [PubMed] [Google Scholar]
- 3.Carpino N., Wisniewski D., Strife A., Marshak D., Kobayashi R., Stillman B., et al. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell. 1997;88(2):197–204. doi: 10.1016/s0092-8674(00)81840-1. [DOI] [PubMed] [Google Scholar]
- 4.Yamanashi Y., Baltimore D. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein. Dok, Cell. 1997;88(2):205–211. doi: 10.1016/s0092-8674(00)81841-3. [DOI] [PubMed] [Google Scholar]
- 5.Mashima R., Hishida Y., Tezuka T., Yamanashi Y. The roles of Dok family adapters in immunoreceptor signaling. Immunol Rev. 2009;232(1):273–285. doi: 10.1111/j.1600-065X.2009.00844.x. [DOI] [PubMed] [Google Scholar]
- 6.Guittard G., Pontarotti P., Granjeaud S., Rodrigues M., Abi-Rached L., Nunès J.A. Evolutionary and expression analyses reveal a pattern of ancient duplications and functional specializations in the diversification of the Downstream of Kinase (DOK) genes. Dev Comp Immunol. 2018;84:193–198. doi: 10.1016/j.dci.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Grimm J., Sachs M., Britsch S., Di Cesare S., Schwarz-Romond T., Alitalo K., et al. Novel p62dok family members, dok-4 and dok-5, are substrates of the c-Ret receptor tyrosine kinase and mediate neuronal differentiation. J Cell Biol. 2001;154(2):345–354. doi: 10.1083/jcb.200102032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Favre C., Gérard A., Clauzier E., Pontarotti P., Olive D., Nunès J.A. DOK4 and DOK5: new Dok-related genes expressed in human T cells. Genes Immun. 2003;4(1):40–45. doi: 10.1038/sj.gene.6363891. [DOI] [PubMed] [Google Scholar]
- 9.He P.-F., Xu Z.-J., Zhou J.-D., Li X.-X., Zhang W., Wu D.-H., et al. Methylation-associated DOK1 and DOK2 down-regulation: potential biomarkers for predicting adverse prognosis in acute myeloid leukemia. J Cell Physiol. 2018;233(9):6604–6614. doi: 10.1002/jcp.26271. [DOI] [PubMed] [Google Scholar]
- 10.Chen M., Zhang J., Berger A.H., Diolombi M.S., Ng C., Bronson J., et al. Compound haploinsufficiency of Dok2 and Dusp4 promotes lung tumorigenesis. J Clin Invest. 2019;129(1):215–222. doi: 10.1172/JCI99699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An C.H., Kim M.S., Yoo N.J., Lee S.H. Mutational and expressional analysis of a haploinsufficient tumor suppressor gene DOK2 in gastric and colorectal cancers. APMIS. 2011;119(8):562–564. doi: 10.1111/j.1600-0463.2011.02749.x. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich T., Söhn M., Gutting T., Janssen K.-P., Behrens H.-M., Röcken C., et al. Burgermeister, Subcellular compartmentalization of docking protein-1 contributes to progression in colorectal cancer. EBioMedicine. 2016;8:159–172. doi: 10.1016/j.ebiom.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghanem T., Bracken J., Kasem A., Jiang W.G., Mokbel K. mRNA expression of DOK1-6 in human breast cancer. World J Clin Oncol. 2014;5(2):156–163. doi: 10.5306/wjco.v5.i2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidarizadi A., Salimi M., Mozdarani H. Study of DOK4 gene expression and promoter methylation in sporadic breast cancer. Neoplasma. 2020;67(4):916–921. doi: 10.4149/neo_2020_190811N747. [DOI] [PubMed] [Google Scholar]
- 15.Wu P., Heins Z.J., Muller J.T., Katsnelson L., de Bruijn I., Abeshouse A.A., et al. Integration and analysis of CPTAC proteomics data in the context of cancer genomics in the cBioPortal. Mol Cell Proteomics. 2019;18(9):1893–1898. doi: 10.1074/mcp.TIR119.001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li T., Fan J., Wang B., Traugh N., Chen Q., Liu J.S., et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 2017;77(21):e108–e110. doi: 10.1158/0008-5472.CAN-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gérard A., Ghiotto M., Fos C., Guittard G., Compagno D., Galy A., et al. Dok-4 is a novel negative regulator of T cell activation. J Immunol. 2009;182(12):7681–7689. doi: 10.4049/jimmunol.0802203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J., Peng X., Zhang K., Li C., Su B., Zhang Y., et al. Co-expression and significance of Dok2 and Ras p21 protein activator 1 in breast cancer. Oncol Lett. 2017;14(5):5386–5392. doi: 10.3892/ol.2017.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang F., Cardenas H., Huang H., Jiang G., Perkins S.M., Zhang C., et al. Genomic and epigenomic signatures in ovarian cancer associated with resensitization to platinum drugs. Cancer Res. 2018;78(3):631–644. doi: 10.1158/0008-5472.CAN-17-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshpande R.P., Chandra Sekhar Y.B.V.K., Panigrahi M., Babu P.P. Region-specific Dok2 overexpression associates with poor prognosis in human astrocytoma. Mol Neurobiol. 2018;55(1):402–408. doi: 10.1007/s12035-016-0324-2. [DOI] [PubMed] [Google Scholar]
- 21.Downer E.J., Johnston D.G., Lynch M.A. Differential role of Dok1 and Dok2 in TLR2-induced inflammatory signaling in glia. Mol Cell Neurosci. 2013;56:148–158. doi: 10.1016/j.mcn.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Oki S., Limnander A., Yao P.M., Niki M., Pandolfi P.P., Rothman P.B. Dok1 and SHIP act as negative regulators of v-Abl-induced pre-B cell transformation, proliferation and Ras/Erk activation. Cell Cycle. 2005;4(2):310–314. [PubMed] [Google Scholar]
- 23.Tuna E., Ersoy Y.E., Bulut P., Ozdemir F., Buyru N. Analysis of the DOK1 gene in breast cancer. Mol Biol Rep. 2020;47(3):1605–1612. doi: 10.1007/s11033-020-05247-3. [DOI] [PubMed] [Google Scholar]
- 24.Mercier P.L., Bachvarova M., Plante M., Gregoire J., Renaud M.C., Ghani K., et al. Characterization of DOK1, a candidate tumor suppressor gene, in epithelial ovarian cancer. Mol Oncol. 2011;5(5):438–453. doi: 10.1016/j.molonc.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saulnier A., Vaissière T., Yue J., Siouda M., Malfroy M., Accard R., et al. Inactivation of the putative suppressor gene DOK1 by promoter hypermethylation in primary human cancers. Int J Cancer. 2012;130(11):2484–2494. doi: 10.1002/ijc.26299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger A.H., Niki M., Morotti A., Taylor B.S., Socci N.D., Viale A., et al. Identification of DOK genes as lung tumor suppressors. Nat Genet. 2010;42(3):216–223. doi: 10.1038/ng.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carneiro F., Oliveira C., Leite M., Seruca R. Molecular targets and biological modifiers in gastric cancer. Semin Diagn Pathol. 2008;25(4):274–287. doi: 10.1053/j.semdp.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Niki M., Di Cristofano A., Zhao M., Honda H., Hirai H., Van Aelst L., et al. Role of Dok-1 and Dok-2 in leukemia suppression. J Exp Med. 2004;200(12):1689–1695. doi: 10.1084/jem.20041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L., Li R., Hu K., Dai Y., Pang Y., Jiao Y., et al. Prognostic role of DOK family adapters in acute myeloid leukemia. Cancer Gene Ther. 2019;26(9–10):305–312. doi: 10.1038/s41417-018-0052-z. [DOI] [PubMed] [Google Scholar]
- 30.Ueta R., Sugita S., Minegishi Y., Shimotoyodome A., Ota N., Ogiso N., et al. DOK7 Gene therapy enhances neuromuscular junction innervation and motor function in aged mice. iScience. 2020;23(8) doi: 10.1016/j.isci.2020.101385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bissay V., Maselli R.A. Phenotypic differences in 2 unrelated cases carrying identical DOK7 mutations. J Clin Neuromuscul Dis. 2019;21(1):30–34. doi: 10.1097/CND.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 32.Finsterer J. Congenital myasthenic syndromes. Orphanet J Rare Dis. 2019;14(1):57. doi: 10.1186/s13023-019-1025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen G., Yu H., Satherley L., Zabkiewicz C., Resaul J., Zhao H., et al. The downstream of tyrosine kinase 7 is reduced in lung cancer and is associated with poor survival of patients with lung cancer. Oncol Rep. 2017;37(5):2695–2701. doi: 10.3892/or.2017.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heyn H., Carmona F.J., Gomez A., Ferreira H.J., Bell J.T., Sayols S., et al. DNA methylation profiling in breast cancer discordant identical twins identifies DOK7 as novel epigenetic biomarker. Carcinogenesis. 2013;34(1):102–108. doi: 10.1093/carcin/bgs321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siouda M., Yue J., Shukla R., Guillermier S., Herceg Z., Creveaux M., et al. Transcriptional regulation of the human tumor suppressor DOK1 by E2F1. Mol Cell Biol. 2012;32(23):4877–4890. doi: 10.1128/MCB.01050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi E.J., Lee J.H., Kim M.S., Song S.Y., Yoo N.J., Lee S.H. Intratumoral heterogeneity of somatic mutations for NRIP1, DOK1, ULK1, ULK2, DLGAP3, PARD3 and PRKCI in colon cancers. Pathol Oncol Res. 2018;24(4):827–832. doi: 10.1007/s12253-017-0297-0. [DOI] [PubMed] [Google Scholar]
- 37.Kim M.S., Chung N.G., Yoo N.J., Lee S.H. Mutational analysis of DOK2 tumor suppressor gene in acute leukemias. Leuk Res. 2011;35(6):e87–e88. doi: 10.1016/j.leukres.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 38.Bevilacqua J.A., Lara M., Díaz J., Campero M., Vázquez J., Maselli R.A. Congenital Myasthenic syndrome due to DOK7 mutations in a family from Chile. Eur J Transl Myol. 2017;27(3):6832. doi: 10.4081/ejtm.2017.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X., Lewis M.T., Huang J., Gutierrez C., Osborne C.K., Wu M.F., et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100(9):672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 40.Creighton C.J., Li X., Landis M., Dixon J.M., Neumeister V.M., Sjolund A., et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A. 2009;106(33):13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lum E., Vigliotti M., Banerjee N., Cutter N., Wrzeszczynski K.O., Khan S., et al. Loss of DOK2 induces carboplatin resistance in ovarian cancer via suppression of apoptosis. Gynecol Oncol. 2013;130(2):369–376. doi: 10.1016/j.ygyno.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Klemm F., Joyce J.A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25(4):198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinman R.M., Nussenzweig M.C. Avoiding horror autotoxicus: the importance of dendritic cells in peripheral T cell tolerance. Proc Natl Acad Sci U S A. 2002;99(1):351–358. doi: 10.1073/pnas.231606698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips J.D., Knab L.M., Blatner N.R., Haghi L., DeCamp M.M., Meyerson S.L., et al. Preferential expansion of pro-inflammatory Tregs in human non-small cell lung cancer. Cancer Immunol Immunother. 2015;64(9):1185–1191. doi: 10.1007/s00262-015-1725-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pan L., Fang J., Chen M.Y., Zhai S.T., Zhang B., Jiang Z.Y., et al. Promising key genes associated with tumor microenvironments and prognosis of hepatocellular carcinoma. World J Gastroenterol. 2020;26(8):789–803. doi: 10.3748/wjg.v26.i8.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Z.Y., Zhao M., Chen W., Li K., Qin F., Xiang W.W., et al. Analysis of prognostic genes in the tumor microenvironment of lung adenocarcinoma. PeerJ. 2020;8:e9530. doi: 10.7717/peerj.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murri A.M., Hilmy M., Bell J., Wilson C., McNicol A.M., Lannigan A., et al. The relationship between the systemic inflammatory response, tumour proliferative activity, T-lymphocytic and macrophage infiltration, microvessel density and survival in patients with primary operable breast cancer. Br J Cancer. 2008;99(7):1013–1019. doi: 10.1038/sj.bjc.6604667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li T., Li B., Sara A., Ay C., Leung W.Y., Zhang Y., et al. Docking protein-1 promotes inflammatory macrophage signaling in gastric cancer. Oncoimmunology. 2019;8(11):e1649961. doi: 10.1080/2162402X.2019.1649961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability: TCGA raw data is available at UCSC xena browser (https://xenabrowser.net/datapages/). NCI-60 cell line data is available at CellMiner (https://discover.nci.nih.gov/cellminer/home.do).