Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive fibrotic interstitial pneumonia with unknown causes. The incidence rate increases year by year and the prognosis is poor without cure. Recently, phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB/AKT) signaling pathway can be considered as a master regulator for IPF. The contribution of the PI3K/AKT in fibrotic processes is increasingly prominent, with PI3K/AKT inhibitors currently under clinical evaluation in IPF. Therefore, PI3K/AKT represents a critical signaling node during fibrogenesis with potential implications for the development of novel anti-fibrotic strategies. This review epitomizes the progress that is being made in understanding the complex interpretation of the cause of IPF, and demonstrates that PI3K/AKT can directly participate to the greatest extent in the formation of IPF or cooperate with other pathways to promote the development of fibrosis. We further summarize promising PI3K/AKT inhibitors with IPF treatment benefits, including inhibitors in clinical trials and pre-clinical studies and natural products, and discuss how these inhibitors mitigate fibrotic progression to explore possible potential agents, which will help to develop effective treatment strategies for IPF in the near future.
KEY WORDS: Idiopathic pulmonary fibrosis, PI3K/AKT signaling, Pathogenesis, Coagulation cascade, Immune activation, Fibroblast accumulation, Therapeutic target, Drug therapy
Graphical abstract
PI3K/AKT signaling participates in the formation of idiopathic pulmonary fibrosis (IPF), and some promising PI3K/AKT inhibitors already show IPF treatment benefits, making targeting PI3K/AKT a novel anti-fibrotic strategy.
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is one of the chronic, progressive fibrotic interstitial lung diseases without an identifiable cause. It is identified as one of the most common and severe idiopathic interstitial pneumonia1, accompanied with difficult breathing, coughing, and worsening lung function. IPF is reported with quite a high prevalence of 58.7 per 100,000 person2,3, as well as a high mortality rate4. Respiratory failure contributes the most to the death of IPF patients, other causes include coronary heart disease, pulmonary embolism and lung cancer5. Currently, only pirfenidone and nintedanib are approved by the US Food and Drug Administration for IPF treatment. Both of them can interfere with fibroblast proliferation and migration, and reduce fibroblasts-embedded collagen gel contraction and excess extracellular matrix (ECM) secretion6,7. However, gastrointestinal and skin-related adverse events (AEs) are reported in pirfenidone treatment8, and diarrhea and increase of hepatic enzymes are common AEs for nintedanib therapy9, preventing the widespread use of these drugs. Therefore, it is urgent to seek for other appropriate therapeutic approaches for IPF.
Phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB/AKT) signaling pathway is one of the core signaling pathways in cells that regulates cell growth, proliferation, motility, metabolism and survival10. PI3K is a group of lipid kinases associated with the plasma membrane, and it can be divided into three categories: classes I, II, and III11. Class I PI3Ks are heterodimer formed by the p110 catalytic subunit and the P85 regulatory subunit, and exist in four isoforms including class IA (PI3Kα, PI3Kβ and PI3Kδ) and class IB (PI3Kγ). Among these four isoforms, which can be expressed in human lung fibroblasts12, PI3Kα is often up-regulated or mutated in lung-related diseases13, and PI3Kγ is usually found overexpressed in IPF lung homogenate and fibroblasts14, while class III PI3Ks are involved in the formation of autophagosome membranes which may affect pulmonary fibrosis15. AKT is a serine/threonine protein kinase with three subtypes: AKT1, AKT2 and AKT316, can be activated in response to upstream PI3K. Since AKT3 is predominantly expressed in brain tissue, research related to pulmonary fibrosis mainly focuses on AKT1 and AKT2 subtypes. AKT1-mediated mitophages contribute to alveolar macrophage apoptosis resistance, which is required for pulmonary fibrosis development17, and AKT2-deficient mice are protected against bleomycin (BLM)-induced pulmonary fibrosis and inflammation18, indicating that PI3K/AKT signaling plays important roles during IPF development.
Existing evidence suggested that overexpression of alpha-smooth muscle actin (α-SMA) in lung fibrosis was related to the activation of PI3K/AKT12, and the interaction between transforming growth factor-β (TGF-β) and PI3K/AKT promoted the formation of pulmonary fibrosis19. Besides, the activation of PI3K/AKT can participate in pulmonary fibrosis by regulating its downstreams such as mammalian target of rapamycin (mTOR), hypoxia inducible factor-1α (HIF-1α) and FOX family14,18. It is precisely because of the important role of PI3K/AKT in regulating receptor-mediated signal transduction, making targeting PI3K/AKT to be a new strategy for IPF treatment.
Here, we review the comprehensive pathogenesis of IPF, and systematically sort out the key role of PI3K/AKT signaling during the initial stage of epithelial cell damage, coagulation cascade, immune activation, and fibroblast accumulation. Based on the important role of PI3K/AKT signaling in IPF, we sum up the potential drugs targeting PI3K/AKT signaling which have been evaluated as therapeutic agents for IPF. Among them, GSK2126458, HEC68498 and rapamycin are currently under the clinical evaluation for the treatment of patients with IPF. Besides, substantial progresses are made in the treatment of IPF with other small molecules and natural compounds targeting PI3K/AKT signaling, broadening the treatment landscape of IPF and accelerating the advent of new promising drugs.
2. PI3K/AKT signaling in different development stage of IPF
The pathogenesis of IPF disease is largely unknown; however, singular strides have been made over the past few years. Numerous studies have suggested that some environmental and vocational exposures are related to the onset of IPF disease20, and corresponding speculation about the potential role of genetic mutations and interplay with assumed external factors has been made21,22. Currently, IPF is considered to be an abnormal wound healing response caused by damage to alveolar epithelial cells (AECs)23,24. Routine wound healing goes through four distinct phases: the clotting/coagulation phase, the inflammatory cell migration phase, the fibroblast migration/proliferation/activation phase, and tissue remodeling and decomposition25. Injured AECs activate multiple inflammatory responses, repair pathways and signaling pathways, including the PI3K–AKT pathway26, to release profibrotic mediators and disturb the balance between profibrotic and anti-fibrotic mediators27. This response is accompanied by abnormal epithelial–mesenchymal crosstalk20,28, fibroblast proliferation, and fibroblast to myofibroblast transformation29. Additionally, myofibroblasts secrete ECM, mainly collagen, which leads to chaotic lung remodeling30, and ultimately progressive pulmonary fibrosis and loss of function.
Pulmonary trauma repair is a complex, coordinated, and orderly process. If dysregulation or lung injury persists at any stage of the tissue repair process, fibrosis will develop, eventually leading to the development of multiple lung diseases (Fig. 1). Further investigation into the pathogenesis of IPF will provide a theoretical basis for the treatment of multiple fibrotic diseases26,29.
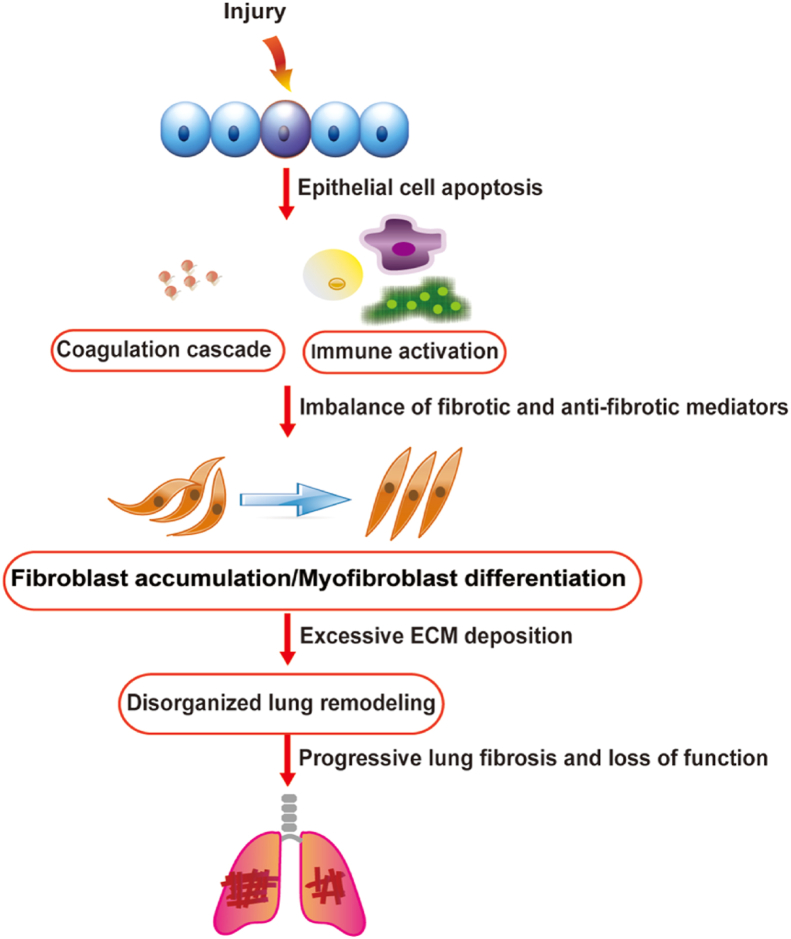
Figure 1.
The pathogenesis of IPF. Injured AECs can activate multiple inflammatory responses, coagulation cascade and repair programs, releasing quite a few profibrotic mediators and breaking the balance between profibrotic and anti-fibrotic mediators. Then fibroblasts will accumulate in large numbers and transdifferentiate into myofibroblasts. Myofibroblasts secrete excess extracellular matrix, causing chaotic lung remodeling, which eventually generates progressive pulmonary fibrosis and loses functions.
2.1. Disease initiation: Epithelial cell damage
Numerous studies have revealed the critical role of AECs in the pathogenesis of IPF, and several pieces of evidence support the opinion that injury to the alveolar epithelium is central to disease initiation. Initially, several genetic studies showed that defects of the alveolar epithelium are the basis of disease development31. The variant of the mucin 5B (MUC5B) gene has been reported to be one of the most relevant risk factors for familial and sporadic IPF. Moreover, the primary mucin-expressing cells in microscopic honeycomb cysts of IPF are AECs, suggesting that alveolar epithelium defects are the primary contributors to IPF32. Mutations in lung epithelial restricted genes (SFTPC, SFTPA2, and ABCA3) have also been implicated in familial forms of pulmonary fibrosis. Additionally, genome-wide association studies have also verified that variants in telomerase reverse transcriptase (TERT) and regulator of telomere elongation helicase 1 (RTEL1) notably shorten telomeres and increase the risk of IPF disease33. Defects in telomere maintenance have been linked to epithelial cell senescence and an impaired response to epithelial injury34. Further, established environmental exposures such as smoking35, inhaled particulates due to occupational factors (e.g., sawdust and metal dust), microbial (viral, bacterial, and fungal) infections36,37, and gastroesophageal reflux disease risk factors for pulmonary fibrosis may act as sources of recurrent damage to the alveolar epithelium. Together, these studies imply that AECs play prominent roles in driving early IPF disease pathogenesis.
It is common to observe abnormal epithelial cells, such as bronchial epithelial cells and proliferative type II AECs, lined with honeycomb fibrotic areas in IPF lung biopsies, and studies have shown that AECs damage is sufficient to cause pulmonary fibrosis38. Obvious AECs apoptosis in areas of positive remodeling and regions with high myofibroblast activity have also been found in IPF lung biopsies, suggesting that AECs apoptosis is associated with the onset of IPF39,40. Moreover, AECs produce key fibrogenic mediators, including connective tissue growth factor (CTGF), platelet-derived growth factor (PDGF), TGF-β41, 42, 43.
The interaction of predisposing risk factors, including genetic susceptibility and environmental exposure, plays a vital role in repeated micro-damage of epithelial cells followed by epithelial–mesenchymal transition (EMT), senescence, and apoptosis, which is considered to be the initial cause of IPF responses. The exact mechanisms of epithelial cell damage are complex and have not yet been elucidated. However, it is clear that EMT, senescence, and apoptosis of vulnerable alveolar epithelium are central to this process, among which, the PI3K/AKT signaling pathway is widely involved.
2.1.1. EMT
EMT is indispensable to the pathogenesis of IPF in that it allows epithelial cells to obtain a mesenchymal phenotype through disassembly of epithelial cell–cell contacts, resulting in the loss of cell polarity44. The role of EMT in fibrosis is pernicious, and activation of EMT in the lung has been advocated as one relevant mechanism leading to alveolar cell loss, myofibroblast accumulation, and lung fibrosis in both human and experimental studies45. Several studies have shown that the EMT was disrupted when PI3K/AKT is inhibited, and the use of AKT inhibitors can partially reverse EMT46. TGF-β is the most important EMT inducer in fibrosis and cancer. In renal fibrosis, EMT can be promoted by enhancing the expression of PI3K subunit p110δ induced by TGF-β and the phosphorylation of AKT. Activated AKT activates HIF-1α, which promotes the conversion of AECs to fibroblasts and mediates EMT to participate in pulmonary fibrosis47. Additionally, PI3K P85 was highly expressed in silica-induced lung fibrosis, HBE cells, and A549 cells. Indeed, siRNA mediated knockout of PI3K P85 in HBE and A549 cells reduced the severity of pulmonary fibrosis by weakening the process of EMT48. Recent research confirmed that the non-SMAD signaling pathway of PI3K/AKT plays a key role in BLM-induced EMT49. These findings demonstrated that PI3K/AKT promotes EMT and contributes to the pathogenesis of fibrosis.
2.1.2. Senescence
Interestingly, in the fibroblastic lesions and honeycomb areas of the IPF lung, increased aging markers were found mainly in epithelial cells50,51. Aging epithelial cells secrete several mediators in senescence-associated secretory phenotype (SASP), which directly affects the surrounding microenvironment, thereby triggering IPF; thus, epithelial cell senescence can be considered a pathological feature of IPF. Gene mutations related to telomere abrasion also promote the development of IPF by affecting the senescence of AECs52. Studies have found that in chronic obstructive pulmonary disease, oxidative stress dependent microRNA-34a can reduce the expression of anti-senescence-related sirtuin-1 and sirtuin-6 in epithelial cells through PI3Kα53. AKT signaling has also been reported to play a central role in controlling the proliferation and survival of fibroblasts54. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is a key negative regulator of the PI3K pathway that can dephosphorylate PIP3 to PIP2 and prevent the activation of downstream kinases such as AKT55. Research shows that AEC senescence induced by the loss of PTEN occurs in an AKT-dependent manner, which may help determine potential targets for anti-aging treatment of IPF56,57. Based on the above findings, inhibition of PI3K and AKT are postulated to be able to delay aging and reduce lung fibrosis.
2.1.3. Apoptosis
A growing body of evidence suggests that apoptosis of AECs plays an important role in the pathogenesis of lung diseases50. Many apoptosis incentives have been identified, and the most relevant to the pathobiology of IPF include autophagy, oxidative stress and endoplasmic reticulum (ER) stress58. Autophagy ameliorated BLM-induced pulmonary fibrosis by inhibiting the apoptosis of lung epithelial cells59. Insufficient autophagy was found in IPF, mainly in the AECs of the IPF lung, and further affects fibroblast differentiation60,61. Studies have indicated that the PI3K/AKT signaling pathway is up-regulated and autophagy regulator mTOR activation is increased to inhibit autophagy and exacerbate apoptosis of AECs to promote pulmonary fibrosis62. Oxidative stress is also important in the development of pulmonary fibrosis, and it has been proposed that the imbalance between the antioxidative and pro-oxidative state may promote apoptosis of epithelial cells and activation of fibrotic pathways63. Model studies of BLM-induced pulmonary fibrosis have also shown that the over-activated PI3K/AKT/HIF-1α pathway regulates abnormal cell proliferation and apoptosis through oxidative stress, thereby further affecting the normal repair of AECs, leading to the production of type III collagen and the formation of pulmonary fibrosis64. Studies have reported that markers (BIP, EDEM, and XBP-1) of ER stress and unfolded protein response activation were primarily elevated in the hyperplastic type II AECs overlying fibroblastic foci of patients with IPF65. The mechanisms by which ER stress regulates AEC apoptosis are not fully understood, and recent research has confirmed that ER stress and oxidative stress affect the apoptosis of type II AECs via activating PI3K/AKT pathway, leading to silicon dioxide nanoparticles-induced pulmonary fibrosis66. Taken together, these studies indicated that the increase in the apoptosis of AECs in IPF is closely related to the activation of PI3K/AKT.
2.2. Disease progression: Coagulation cascade
The coagulation cascade is responsible for fibrin formation at sites of damaged blood vessels, and functions to prevent blood loss67. In the early stages of wound healing, endothelial and epithelial damage can activate the coagulation cascade, resulting in the production of thrombin, followed by thrombin-mediated conversion of serum-derived fibrinogen to fibrin to form a pre-matrix68. Accumulating evidence suggests that the physiological function of the coagulation cascade is not limited to coagulation, and that this cascade also plays a key role in influencing inflammatory and tissue damage repair responses69. Therefore, uncontrolled coagulation contributes to the pathophysiology of various diseases, including acute and chronic lung injury70. Previous studies have shown that several zymogens of both the exogenous coagulation cascade and plasminergic systems, including factor X, thrombin, and plasminogen, are locally produced and activated in IPF fibrotic foci71, and that this cascade is closely related to fibrin deposition in the lungs of patients with IPF. Furthermore, increased pro-coagulant activity has been observed in the bronchoalveolar lavage fluids of patients with IPF72, and there is sufficient evidence to show that the balance of pro coagulation is increased in patients with IPF73, 74, 75. Under these coagulation promoting conditions, ECM degradation decreases, leading to fibrosis-promoting effects, and fibroblast differentiation into myofibroblasts is induced by protease-activated receptor25,76. It well recognized that activation of the coagulation cascade and PI3K/AKT may affect the pathogenesis of pulmonary fibrosis (Fig. 2).
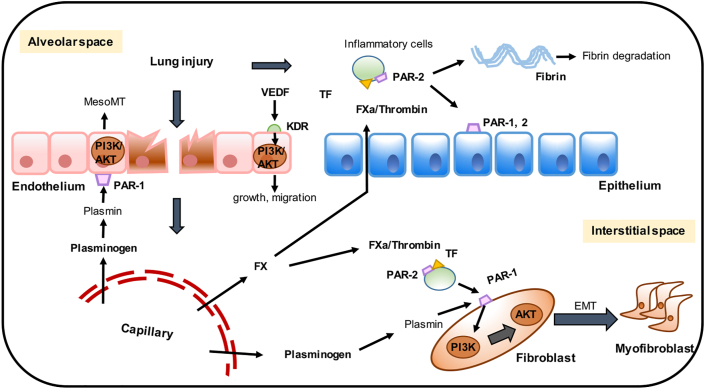
Figure 2.
The involvement of PI3K/AKT in coagulation cascade of IPF. Injured lung will cause the damage of the endothelium and epithelium, resulting in the activation of the coagulation cascade. The integrity of the alveolar-capillary barrier is disrupted, allowing factor X (FX), thrombin and plasminogen to enter the alveolar and interstitial spaces. Meanwhile, reactively increased VEGF combines with the receptor KDR in vascular endothelial cells to activate PI3K/AKT signaling pathway to advocate the growth and migration of endothelium. Endogenous tissue factor (TF) facilitates zymogen protease activation, causing fibrin deposition. Plasmin contributes to the activation of PI3K/AKT, which promotes the mesothelial–mesenchymal transition (MesoMT) and EMT via protease activated receptor 1 (PAR-1).
The alveolar blood vessels are affected by damage to the alveolar structure and removal of AECs in the basement membrane, which leads to increased vascular permeability. Extravasation of coagulation factors into the tissue leads to extravascular coagulation77. Subsequently, endothelial cells and endothelial progenitor cells will proliferate to form new blood vessels. Studies have shown that the quantity of endothelial progenitor cells in patients with IPF is significantly reduced, which to a large extent leads to the failure of reendothelialization, and may further lead to dysfunction of the alveolar-capillary barrier, profibrotic response, and increase in vascular endothelial growth factor (VEGF)78,79. VEGF combines with the receptor KDR on vascular endothelial cells to activate the PI3K/AKT signaling pathway, thereby promoting the growth and migration of vascular endothelial cells and the formation of new blood vessels80. Strong expression of VEGF can be detected in the lavage fluid and serum of patients with IPF, and pulmonary fibrosis can be reduced by inhibiting the expression of VEGF through the inhibition of PI3K/AKT80.
Additionally, endothelial cells may undergo mesenchymal transformation to promote the development of fibrosis81, and mesothelial–mesenchymal transition also contributes to BLM-induced pulmonary fibrosis. Previous studies have shown that tissue factor-dependent exogenous coagulation pathways are central to the pathogenesis of IPF, and both plasmin and thrombin are considered effective activators of the PI3K/AKT signaling pathway. Moreover, pleural mesothelial cells have been found to undergo thrombin-mediated PI3K/AKT activation through protease-activated receptor-1 activation to obtain mesothelial–mesenchymal transition, leading to increased expression of α-SMA and a characteristic fibrotic phenotype. Additionally, PI3Kβ is considered to be associated with thrombus formation, and PI3Kβ plays various roles in downstream G protein-coupled receptor-mediated thrombin and ADP signals, as well as in integrin and glycoprotein receptors; thus, it is speculated that β isoform is related to the coagulation cascade process of IPF82.
2.3. Disease maintenance: Immune activation
The immune response is divided into innate and adaptive immunity, both of which seem to be activated in IPF. Numerous studies have confirmed that inflammatory cells and lymphocytes and their related signals affect the pathophysiology of IPF83. Inflammation occurs in the early stage of wound healing and is characterized by continuous infiltration of inflammatory cells84. In IPF, macrophages and neutrophils are the most studied innate immune cells, while the role of lymphocytes in fibrosis is poorly understood and remains controversial85. This controversy mainly lies in the failure of IPF to improve in response to lymphocyte modulation therapies, but lymphocyte subsets and activation of lymphocytes are indeed found in the lungs and blood of IPF patients with abnormal prognosis83. Moreover, in patients with IPF, lymphocytes aggregate in lung tissue and autoantibodies are present in the serum, indicating that lymphocytes should still be regarded as a treatment target of IPF86.
2.3.1. Macrophage plasticity
Macrophages are the origin of tissue inhibitors of metalloproteinase, which can antagonize the degradation of the ECM mediated by metalloproteinase87. Macrophages have two phenotypes: M1 (classical activation) and M2 (alternative activation). According to the polarization, local microstructure, and fibrosis stage of alveolar macrophages, M1 and M2 play different roles in the process of fibrosis and exhibit obvious phenotypic plasticity88,89. At the early stages of inflammation, acute lung injury promotes an M1 phenotype, leading to the secretion of proinflammatory cytokines. The continuous inflammatory response serves as a trigger to initiate fibrotic responses in the lung90. However, M2 macrophages are important in wound healing processes and in terminating inflammatory responses in the lung91. The mechanism by which M2 macrophages improve IPF may be via the generation of TGF-β and PDGF, or by enhancing ECM degradation through matrix metalloproteinase (MMP) activity92,93. It is well known that interleukin (IL)-4 is the major inducer of M2 polarization via activation of one of its major downstream signals PI3K/AKT94. Studies have shown that IL-13 produced by M2 macrophages also plays a key role in the homeostasis control of normal lungs and the pathogenesis of pulmonary fibrosis. AKT1 regulates pulmonary fibrosis by inducing M2 macrophages to produce IL-13, suggesting that targeting AKT1 blocks the fibrotic process of IPF95. The O subclasses of the Forkhead box (FOXO) family, such as FOXO1, FOXO3, and FOXO4, are directly phosphorylated by AKT, causing them to be exported to the cytoplasm and degraded through the ubiquitin-proteasome pathway. AKT2 regulates pulmonary fibrosis by up-regulating the production of pro-fibrotic cytokines, TGF-β1, and IL-13, via the AKT2/FOXO3a signaling pathway18. It has also been reported that myeloid PTEN deficient mice induced by BLM exhibit sustained PI3K activation to enhance macrophage M2 polarization, which leads to increased morbidity96.
2.3.2. Formation of neutrophil extracellular traps
Neutrophils produce various proteases, especially serine proteases (neutrophil elastase [NE]) and MMPs, which degrade matrix components, but can also activate TGF-β and produce inhibitory factors through NE, thus promoting the accumulation of ECM97, 98, 99. NE can promote fibroblast proliferation and myofibroblast differentiation in vitro, while NE-deficient mice are protected from asbestos-induced pulmonary fibrosis100. Neutrophil extracellular traps (NETs) are released by neutrophils and consist of decolorized chromatin filaments and granular proteins101. The release of NETs may cause local tissue damage and inflammation, and plays a significant role in cystic fibrosis and acute virus-mediated lung injury102,103. If the damage is sustained, neutrophils and monocytes are recruited, and promote the production of reactive oxygen species (ROS) to intensify epithelial damage. PI3Kγ and PI3Kδ are mainly expressed in leukocytes, which leads to speculation that these are the primary isoforms in PI3K-mediated innate and adaptive immune response signals104. Neutrophils primed with tumor necrosis factor-α (TNFα) can be divided into two phases: PI3Kγ mainly mediates PIP3 accumulation at the leading edge of the cell in the chemokinetic phase, while the subsequent chemotactic phase depends on PI3Kδ105,106. PI3Kγ and PI3Kδ have also been shown to affect neutrophil degranulation and superoxide production107. Inhibition of PI3Kγ and PI3Kδ can restore the effective directionality of neutrophil movement to reduce the release of NETs and minimize potential diseases due to immunosuppression108.
2.3.3. Lymphocyte aggregates
Studies have confirmed that lymphocyte factors are related to IPF, but the role of lymphocytes is controversial. In IPF lungs, lymphocytes usually aggregate near the fibroblast foci. These aggregates are composed of CD3+ T lymphocytes and mature dendritic cells109,110. Th2 and Th17 cells promote pulmonary fibrosis by inducing elevated levels of IL-4, IL-13, and TGF-β1111, 112, 113, 114, while Th1, Th22, and γδ-T cells inhibit fibrosis by inducing IFN-γ and IL-12, IL-9, and CXCL10, respectively115, 116, 117, 118. Regulatory T cells and Th9 cells have been associated with anti-fibrosis effects116,117,119,120. An increase in CD20+ B cells has also been detected in the lungs of patients with IPF, and represent an important subset of aggregation. Additionally, many soluble factors that promote the growth and differentiation of B cells have been observed in the blood of patients with IPF, including B cell activating factor (also known as B lymphocyte stimulator), IL-6, and IL-13121. The inhibition of the PI3K/AKT pathway can inhibit the overproduction of pro-inflammatory cytokines, including TNFα, IL-1β, and IL-6, in bronchoalveolar lavage fluids and enhance the release of the anti-inflammatory cytokine IL-10. The specific loss of PTEN in myeloid cells is sufficient to reduce leukocyte recruitment, promote pathogenic inflammation, and drive TGF-β1 activation and subsequent expression of pro-fibrotic molecules96.
2.4. Disease evolution: Fibroblast accumulation/myofibroblast differentiation
Fibroblasts are tissue-derived mesenchymal cells whose central features are to secrete ECM proteins, providing an environment for regular repair events such as epithelial cell migration122. During the pathogenesis of IPF, activated fibroblasts secrete pro-fibrotic mediators to enhance the fibrotic environment, leading to excessive production of ECM and transdifferentiation to myofibroblasts123. Myofibroblasts have shrinkable attributes similar to smooth muscle cells and express α-SMA124. Compared to fibroblasts, myofibroblasts survive longer in damaged tissues and synthesize more ECM125. The strict connection between endothelial cytokines and interstitial cells is conducive to abnormal crosstalk and augments the role of the TGF-β1, PDGF, and WNT pathways, amplifies the fibrotic environment, and leads to a higher rate of transdifferentiation126. One unique pathological characteristic of IPF is the existence of fibroblastic foci, that is, an active synthetic cluster of fibroblasts in the vicinity of the air-tissue interface, which can be regarded as the site of new fibrosis127. Therefore, fibroblasts and myofibroblasts are deemed the potential therapeutic targets for IPF.
Lysophosphatidic acid (LPA) has been identified as a key fibroblast chemokine in experimental lung fibrosis, and LPA1 receptor KO mice are protected in this model128. Studies have found that PI3Kβ is a key downstream target of LPA in vivo. The activation of PI3Kβ downstream of LPA may help fibroblasts chemotax to sites of tissue damage in vivo129. Additionally, the increase in PI3Kγ expression in fibroblasts and basal cells is thought to be related to IPF14. In fibroblasts, activated AKT regulates the production of collagens I and III, and promotes human liver fibrosis and BLM-induced lung fibrosis in mice. In addition, AKT can maintain the low autophagy activity of fibroblasts by activating downstream mTOR signaling so that it can preserve the characteristics of high proliferation and anti-apoptosis130. Importantly, AKT inhibitors can effectively inhibit the expansion of fibroblasts and the formation of fibronectin matrix in lung tissue, reducing the levels of collagen I and collagen III and retaining lung compliance64. Because the fibroblasts in the fibrotic foci express low levels of PTEN, PI3K/AKT activity is enhanced in IPF fibroblasts131. Studies have found that PTEN inhibition and AKT/mTOR activation desensitize IPF fibroblasts from collagen matrix-induced cell death.
TGF-β is a member of a large family of polypeptides that modulate various cellular functions132. There are three subtypes of TGF-β (β1, β2, and β3), and TGF-β1 performs an essential function in the pathogenesis of IPF. It can promote the recruitment of fibroblasts, the differentiation and survival of myofibroblasts, and deposition of ECM, which is a powerful fibrotic promoting medium133, 134, 135, 136. Previous studies have shown that the PI3K/AKT signal pathway is involved in the TGF-β1 regulating pathway, and TGF-β1 could induce the activation of the PI3K/AKT pathway137. Furthermore, studies have confirmed that PI3K/AKT functions upstream of ER stress, affecting the proliferation of lung fibroblasts, leading to BLM-induced pulmonary fibrosis138. However, the relationship between ER stress and the PI3K/AKT pathway in regulating the proliferation of fibroblasts has not yet been elucidated. Moreover, pharmacological inhibition of ER stress reduces TGFβ1-induced myofibroblast differentiation, α-SMA expression, and collagen production in patients with IPF. ER stress-induced autophagy is partly attributable to the down-regulation of the AKT/mTOR pathway, and TGF-β1 inhibits autophagy of fibroblasts, at least in part, by activating mTORC1139, 140, 141, indicating that the activation of PI3K/AKT contribute to fibroblast accumulation and myofibroblast differentiation induced by TGF-β.
WNT protein is a secreted glycoprotein that can signal paracrine or autocrine through its frizzled receptors, low-density lipoprotein receptor-related protein 5 and 6, and disheveled to stabilize β-catenin and cause its nuclear translocation142. Activation of the WNT/β-catenin pathway is closely related to apoptosis resistance and proliferation76, and it is connected with EMT and fibrogenesis after initiation by TGF-β1, sonic Hedgehog, gremlin-1, and PTEN. It is worth noting that both TGF-β1 and the canonical WNT/β-catenin pathway can stimulate each other through PI3K/AKT signaling, and the WNT/β-catenin pathway is considered an upstream activator of the PI3K/AKT/mTOR pathway. More importantly, WNT/β-catenin signaling has been found to be activated in patients with IPF, in whom several WNT/β-catenin-dependent are up-regulated simultaneously143, 144, 145. Additionally, WNT target genes, such as stromelysin (MMP-7) and fibronectin, contribute to the transdifferentiation of fibroblasts in the development of pulmonary fibrosis143. Therefore, the WNT/β-catenin pathway and the PI3K/AKT pathway mediate each other to promote the pathogenesis of IPF.
BLM-induced pulmonary fibrosis depends on the production of ROS, and ROS can participate in lung injury through the PI3K/AKT signaling pathway. Studies have found that ROS can cause fibroblast proliferation and collagen production by activating the PI3K/AKT/HIF-1α pathway64. Additionally, AKT can act on fibroblasts after activation, causing the release of hydrogen peroxide and subsequent damage to adjacent type II AECs to participate in pulmonary fibrosis146. By inhibiting catalase and other products produced by ROS, the activation of the PI3K/AKT signaling pathway is inhibited to mediate anti-pulmonary fibrosis.
2.5. Disease formation: Tissue remodeling and decomposition
After the completion of the proliferative phase, wound repair enters its ultimate remodeling state, which can take several years. A major feature of this stage is the remodeling of the ECM into a structure resembling normal tissue147. The abnormal tissue remodeling is represented by considerable ECM accumulation. With the development of fibrosis, the biological characteristics of the ECM have changed, such as increased tissue elasticity (stiffness) and changes in matrix composition148. Increasing matrix stiffness increases the ability of myofibroblasts to differentiate, and, in turn, myofibroblasts can extend matrix stiffness, which may be accomplished by collagen synthesis and cross-linking to create a feedforward loop that drives the fibrosis process149. Studies on the ECM of IPF lungs have shown that type III collagen is mainly present in the alveolar septum and interstitial fibrosis area, while type I collagen is dominant in mature fibrosis area150,151. ECM turnover is tightly regulated by several protease families and their respective inhibitors152. MMPs include a family of proteases that target collagen and other matrix components for degradation. However, during fibrosis, collagen in the ECM is insoluble, and it is relatively resistant to deterioration by proteases153. Therefore, an understanding of this process is conducive to determining new targets for the treatment of IPF.
There is a substantial body of literature that support that PI3K/AKT can greatly regulate ECM154. Studies have found that when IPF fibroblasts interact with collagen-rich matrix, integrin receptors signal down-regulation of PTEN, which is followed by activation of the PI3K/AKT/mTORC1 phosphorylation cascade155. Additionally, focal adhesion kinase (FAK) plays a key role in regulating the integrin-mediated ECM signal156. The signaling between FAK and PI3K, which are also important in the focal adhesion pathway, could regulate cell survival, apoptosis, and cell cycle progression157,158.
CTGF (also known as CCN2) belongs to the connective tissue growth factor/cysteine-rich 61/nephroblastoma overexpressed (CCN) family. As an essential downstream mediator of TGF-β signaling, CTGF is also regarded as a component of the profibrotic matrix. CTGF is usually expressed at low levels in healthy individuals, but is strongly upregulated in mechanically stressed tissues159,160. In patients with IPF, high CTGF expression has been proven to promote ECM deposition, fibroblast activation, cell adhesion, and invasion, which are key in tissue remodeling and fibrosis161,162. Rapamycin can regulate the expression of CTGF in lung fibroblasts and epithelial cells through the PI3K signaling pathway, leading to excessive accumulation of ECM163. CCN5, another member of the CCN family, acts as a dominant-negative protein to suppress CCN2-mediated fibrogenesis. Emerging evidence has demonstrated that when CCN5 is overexpressed, it can down-regulate CCN2 to inhibit the PI3K/AKT signaling pathway and alleviate pulmonary fibrosis164.
3. Potential drugs targeting PI3K/AKT for IPF treatment
Based on this understanding of IPF, numerous therapeutic targets have been realized and the development of anti-IPF drugs has been improved. While several of these anti-IPF drugs have entered clinical trials, only nintedanib and pirfenidone are approved for IPF treatment, and the effects of these two drugs are limited to slowing down the disease process165. Three drugs target the PI3K signaling pathway, and many other experimental trials have also achieved promising results. A summary of these drugs is shown in Table 149,62,166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176.
Table 1.
Potential PI3K/AKT inhibitor for IPF treatment.
| Agent | Mechanism/target | Function description | Phase of development and status | Common adverse event | Ref. |
|---|---|---|---|---|---|
| GSK2126458 | PI3K/mTOR inhibitor | Reduce TGF-β-induced fibroblast proliferation and collagen I synthesis | Phase I completed (NCT01725139) | Diarrhoea, hyperglycaemia, nausea | 166 |
| HEC68498 | PI3K inhibitor | Anti-fibrosis and anti-inflammation | Phase I Active, not recruiting (NCT03502902) |
Not described | / |
| Rapamycin | mTOR inhibitor | Inhibit TGF-α and EGFR signaling | NA, completed (NCT01462006) | Hyperglycemia, hypophosphatemia, anemia | 167 |
| PX-866 | pan-PI3K inhibitor | Inhibit TGF-α | Pre-clinical | Rash, hyperglycemia, trans-aminase elevations | 168 |
| Derivatives of 4-methylquinaz-oline | PI3K inhibitor | Anti-fibrosis and anti-inflammation | Pre-clinical | Not described | 169 |
| LY294002 | AKT inhibitor | Inhibit fibroblasts expansion and fibronectin matrix formation | Pre-clinical | Not described | 49 |
| ASV | TGFβ1/PI3K/AKT pathway inhibition | Inhibit EMT | Pre-clinical | Raised total bilirubin and rash | 170,171 |
| Hyp | AKT/GSK3β pathway inhibition | Inhibit inflammation, oxidative stress and EMT | Pre-clinical | Not described | 172 |
| Ligustrazine | PI3K/AKT/mTOR pathway inhibition | Reduce ROS | Pre-clinical | Edema, hypertension, gastrointestinal bleeding | 62,173 |
| Quercetin | PI3K/AKT pathway inhibition | Anti-oxidation and anti-aging | Pre-clinical | Gastrointestinal effects, rash | 174, 175, 176 |
PI3K, phosphatidylinositol-3-kinase; mTOR, mammalian target of rapamycin; TGF-α, transforming growth factor-α; EGFR, epidermal growth factor receptor; TGF-β1, transforming growth factor-β1; ASV, astragaloside IV; EMT, epithelial–mesenchymal transition; Hyp, hyperin; GSK3β, glycogen synthase kinase 3β; ROS, reactive oxygen species.
NA, not available.
3.1. Clinical trials of PI3K/AKT inhibitors for IPF treatment
Based on the significance of PI3K/AKT in the pathogenesis of IPF, clinical trials began to reposition some PI3K/AKT inhibitors originally used for cancer treatment for the treatment of IPF. Although current drugs targeting PI3K to treat IPF are still in the early stages, their therapeutic effects are impressive. These clinical trials could provide important insights into the treatment of IPF and identify more PI3K/AKT inhibitors for lung fibrotic disorders.
3.1.1. Omipalisib (GSK2126458)
Omipalisib (GSK2126458), an effective small molecule inhibitor of the PI3K/mTOR pathway, was developed as an anti-tumor treatment and has been evaluated in phase I clinical trials in subjects with solid tumors and lymphomas177,178. The efficacy of omipalisib in IPF has also been evaluated179. In primary human lung fibroblasts derived from IPF lung tissue, omipalisib can reduce TGF-β-induced fibroblast proliferation and collagen I synthesis in vitro179. Additionally, omipalisib has an anti-fibrotic effect in IPF fibroblasts by inhibiting AKT phosphorylation. Moreover, some studies have shown that omipalisib changes the glycolysis in the IPF lung and fibroblasts isolated from fibrotic tissue, and can also reduce abnormal glucose signaling in IPF lung fibrosis areas180. A dose-finding, double-blind, placebo-controlled study (NCT01725139) to detect omipalisib in IPF subjects demonstrated the safety of the drug166. The results showed that orally dosed omipalisib exerts a measurable dose- and exposure-dependent inhibition of the PI3K/mTOR pathway in the systemic circulation and lungs of individuals with IPF. Reported treatment-related AEs mainly include diarrhea, hyperglycemia, and nausea, and no serious AEs were reported, as well as no AEs that led to early termination of treatment. However, there are some weaknesses in this study that limit its further research. The study only recruited a small number of subjects, and no formal evaluation of anti-fibrosis efficacy was performed due to the short duration of the study. Furthermore, it is designed to establish the pharmacological properties and biological relevance of the use of a PI3K inhibitor to alleviate IPF, and the evaluation of IPF therapeutic effects of this trial is inadequate. Further clinical trials are needed to determine more clinically relevant effects of GSK2126458 on attenuating IPF.
3.1.2. HEC68498
As a class I isoform inhibitor of PI3K and mTOR, HEC68498 has convincing and highly selective properties. It has a robust activity against fibrosis and inflammation, which can, at a lower effective dose, achieve a superior therapeutic effect. To study the application of HEC68498 IPF, a phase I, double-blind, placebo-controlled, single oral dose study is underway to assess safety, tolerability, and pharmacokinetics (NCT03502902). As of now, there has been no progress in related experiments and no relevant research results made public.
3.1.3. Rapamycin
Rapamycin, also known as sirolimus, is an mTOR inhibitor. Because of its anti-inflammatory and anti-immune effects, rapamycin is mainly used for immunosuppressive therapy. With the deepening of research on mTOR, the role of this important target in anti-fibrosis therapy has become increasingly clear. Rapamycin has antifibrotic properties in BLM-induced fibrosis in mice181. Moreover, previous studies have found that rapamycin can prevent and inhibit the progression of progressive pulmonary fibrosis caused by the expression of TGF-α and increased epidermal growth factor receptor (EGFR) signaling182,183. A double-blind, placebo-controlled trial is currently underway to evaluate the ability of rapamycin to act as a fibrosis inhibitor (NCT01462006). The results of the study have not been made public, but related studies have observed grade 3 or 4 AEs related to rapamycin, including hyperglycemia, hypophosphatemia, and anemia167. However, studies have also shown that rapamycin can effectively promote CCN2 expression in a PI3K-dependent manner to produce direct fibrotic activity163. Considering the controversial role of rapamycin for the treatment of IPF, there is no recent progress in the clinical treatment of IPF. It may due to the negative feedback of PI3K/AKT pathway by rapamycin as a single-target inhibitor of mTOR. Interestingly, the combination therapy of mTOR and PI3K/MAPK inhibitors showed superior anti-tumor activity184, suggesting that this combination therapy could be considered for treating IPF.
3.2. PI3K/AKT inhibitor in the treatment of IPF
Several PI3K/AKT inhibitors are being investigated in pre-clinical research and have shown positive progress. The advent of these inhibitors has profoundly expanded the treatment landscape of IPF, and novel PI3K/AKT inhibitor drugs are currently under evaluation. We believe that more PI3K pathway-targeted drugs will be available for the treatment of IPF in the future.
3.2.1. PX-866
PX-866, a pan-PI3K inhibitor, can down-regulate tumor phosphorylation of AKT and has anti-tumor activity in many human tumor xenograft models185. Recent studies indicate that PX-866 can prevent the progression of TGF-α-induced lung fibrosis in vivo168. Rash, hyperglycemia, and transaminase elevation are considered common AEs related to the treatment of PX-866186. At present, there has been no new progress in the use of PX-866 for the treatment of IPF.
3.2.2. Derivatives of 4-methylquinazoline
Rationally designed chemical derivatives of 4-methylquinazoline can be used as high-efficiency PI3K inhibitors for the potential treatment of IPF169. They have excellent resistance to proliferate mouse lung fibroblasts, and can significantly improve the lung function of BLM-induced pulmonary fibrosis mice by reducing the levels of α-SMA and hydroxyproline and exerting anti-fibrosis and anti-inflammation effects. These derivatives are expected to become popular drugs for IPF treatment, largely due to their limited adverse event reports.
3.2.3. LY294002
LY294002, a specific PI3K/AKT inhibitor, has been reported to significantly ease PI3K/AKT-mediated cellular processes by suppressing AKT phosphorylation. Numerous studies have confirmed that LY294002 can inhibit the expansion of fibroblasts and the formation of fibronectin matrix in lung tissue in the BLM-induced pulmonary fibrosis model, as well as reduce the content of collagens I and III49,187. These findings suggest that AKT inhibitors have anti-inflammatory and anti-fibrotic effects in pulmonary fibrosis, and no related AEs have yet been described.
3.3. Promising natural products targeting PI3K/AKT in IPF treatment
Recent studies have shown that active ingredients in natural compounds have anti-fibrotic effects188. These natural compounds may provide promising drug candidates for treating pulmonary fibrosis.
3.3.1. Astragaloside IV (ASV)
ASV is a natural saponin derived from astragalus, which has anti-fibrotic properties in BLM-induced pulmonary fibrosis189. The therapeutic effect of ASV is via the activation of FOXO3a by inhibiting the TGF-β1/PI3K/AKT pathway, thereby preventing EMT in BLM-induced pulmonary fibrosis170. Few studies have been conducted on the toxicity and AEs of AS-IV in vivo and in vitro, although preclinical trails indicate that ASV is safe and well tolerated, and that the AEs, such as raised total bilirubin and rash, were mild and resolved spontaneously171.
3.3.2. Hyperin (Hyp)
Hyp is extracted from rhododendron and has various biological effects, including anti-inflammatory, anti-oxidant, anti-fibrosis, and anti-cancer effects190,191. Hyp has been shown to reduce the development of pulmonary fibrosis in mice, potentially due to the inhibition of BLM-induced inflammation, oxidative stress, and EMT through the AKT/glycogen synthase kinase 3β (GSK3β) pathway172. Moreover, few treatment-related AEs have been reported following the use of Hyp.
3.3.3. Ligustrazine
Ligustrazine is extracted from the roots and stems of Ligusticum chuanxiong Hort. (Chuan Xiong), and has a protective effect by scavenging ROS, regulating the production of nitric oxide, and preventing the formation of peroxynitrite192. As ROS causes fibroblast proliferation and stimulates collagen synthesis, ROS play a pivotal role in IPF pathogenesis. Studies have found that ligustrazine can reduce pulmonary fibrosis by inhibiting PI3K/AKT/mTOR62. Common AEs reported following Ligustrazine use include edema, hypertension, and gastrointestinal bleeding173.
3.3.4. Quercetin
Quercetin is a member of the flavonoid family and can provide direct protection in the development of pulmonary fibrosis by resisting oxidative damage and inflammation193. Additionally, quercetin and its regulate the activities of PI3K and other kinases, and selectively reduce the viability of senescent endothelial cells. The combination of dasatinib and quercetin effectively reduce senescence and SASP markers in isolated AEC2 from BLM-treated mice174. Intriguingly, a recent study reported that quercetin might render senescent IPF fibroblasts susceptible to pro-apoptotic stimuli via up-regulation of caveolin-1 and inhibition of PI3K/AKT175. Common AEs associated with quercetin tend to be gastrointestinal (constipation, heartburn, bloating, diarrhea, nausea, and vomiting) and skin associated (rash, dryness, flushing)176.
4. Conclusions and future prospects
The PI3K/AKT pathway has gained growing recognition in the field of oncology due to its key roles in cell survival, growth, and proliferation. However, recent studies have found significant PI3K signaling activity in fibrotic lung lesions179. This review encapsulates the correlation between PI3K/AKT and IPF (Fig. 3) and explores potent PI3K/AKT inhibitors and novel anti-fibrotic agents in IPF. Although the pathogenesis of IPF remains largely unknown, the current findings are sufficient to deem the PI3K/AKT pathway a reasonable target for the treatment of IPF.
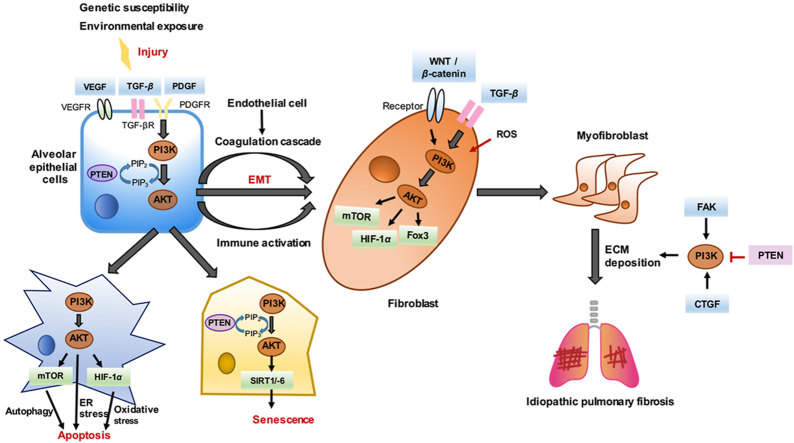
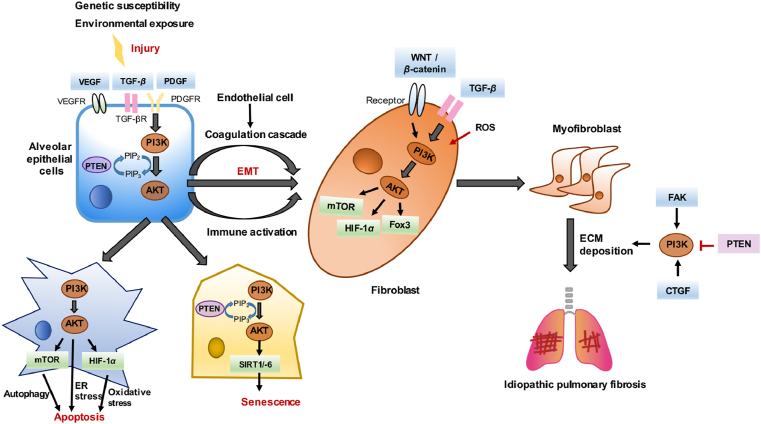
Figure 3.
PI3K/AKT in regulating IPF. The activation of PI3K/AKT can directly participate in the formation of IPF, and cooperate with other pathways including TGF, VEGF, PDGF, FAK, WNT, and mTOR, thus contributing to the pathogenesis of IPF. Specifically, activated PI3K/AKT is involved in aging, autophagy, EMT, immunity and other essential processes to promote IPF. Besides, endoplasmic reticulum stress (ER) and the release of reactive oxygen species (ROS) also contribute to the activation of PI3K/AKT to promote the deposition of excess extracellular matrix (ECM) in the lung fibrosis.
First, aside from regulating IPF alone, PI3K/AKT also has numerous crosstalk and interactions with signaling pathways, including TGF, VEGF, WNT, FAK, mTOR, Jun N-terminal kinase, CTGF, Hedgehog, and Notch pathway, thus participating in multiple links in the pathogenesis of IPF194. Second, there are few drugs that can directly target PI3K and AKT to treat pulmonary fibrosis, but these PI3K pathway-targeted drugs have entered the clinical research stage with encouraging results, and there is scope for improvement for treating IPF in the future. Some natural compounds also exhibit potent anti-fibrotic activity through the inhibition of PI3K/AKT, and are considered promising drug candidates for IPF treatment. It is worth noting that as the PI3K/AKT pathway contains a complex negative feedback system, compared to the suppression of specific isoforms in tumor treatment, inhibiting all four class I PI3K subtypes may produce better IPF treatment efficacy. This has higher requirements, that is, to reduce the systemic toxicity of pan-PI3K as much as possible while effectively treating IPF. Thus, it may be appropriate to design drugs that target pathological cells such as fibroblasts. Finally, the role of PI3K/AKT in IPF broadens the treatment horizons for IPF to establish novel targets to treat IPF, such as peroxisome proliferators-activated receptor γ and the eukaryotic translation initiation factor 4E-binding protein 1. Therefore, it is not difficult for us to conclude that the PI3K and AKT play a significant role in the pathogenesis of IPF. A clear understanding of the existing problems and finding new ways to cure IPF is still something that will require continued efforts for a long time to come.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 82003873), the Postdoctoral Science Foundation of China (No. 2020M681899) and the Zhejiang Provincial Natural Science Foundation of China (No. LR21H310001).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Jiajia Wang, Email: wangjiajia3301@zju.edu.cn.
Qinjie Weng, Email: wengqinjie@zju.edu.cn.
Author contributions
Qinjie Weng and Jiajia Wang designed the work. Jincheng Wang, Kaili Hu and Xuanyan Cai collected data and wrote the manuscript. Jincheng Wang and Kaili Hu designed and regenerated the conceptual pictures. Bo Yang and Qiaojun He gave some critical comments. Jincheng Wang, Kaili Hu, Jiajia Wang and Qinjie Weng in charge of checking and revision. All of the authors have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Meltzer E.B., Noble P.W. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis. 2008;3:8. doi: 10.1186/1750-1172-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esposito D.B., Lanes S., Donneyong M., Holick C.N., Lasky J.A., Lederer D., et al. Idiopathic pulmonary fibrosis in United States automated claims. Incidence, prevalence, and algorithm validation. Am J Respir Crit Care Med. 2015;192:1200–1207. doi: 10.1164/rccm.201504-0818OC. [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson J., Fogarty A., Hubbard R., McKeever T. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J. 2015;46:795–806. doi: 10.1183/09031936.00185114. [DOI] [PubMed] [Google Scholar]
- 4.Allen R.J., Guillen-Guio B., Oldham J.M., Ma S.F., Dressen A., Paynton M.L., et al. Genome-wide association study of susceptibility to idiopathic pulmonary fibrosis. Am J Resp Crit Care. 2020;201:564–574. doi: 10.1164/rccm.201905-1017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richeldi L., Collard H.R., Jones M.G. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 6.Jin J., Togo S., Kadoya K., Tulafu M., Namba Y., Iwai M., et al. Pirfenidone attenuates lung fibrotic fibroblast responses to transforming growth factor-β1. Respir Res. 2019;20:119. doi: 10.1186/s12931-019-1093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varone F., Sgalla G., Iovene B., Bruni T., Richeldi L. Nintedanib for the treatment of idiopathic pulmonary fibrosis. Expet Opin Pharmacother. 2018;19:167–175. doi: 10.1080/14656566.2018.1425681. [DOI] [PubMed] [Google Scholar]
- 8.Ruwanpura S.M., Thomas B.J., Bardin P.G. Pirfenidone: molecular mechanisms and potential clinical applications in lung disease. Am J Resp Cell Mol. 2020;62:413–422. doi: 10.1165/rcmb.2019-0328TR. [DOI] [PubMed] [Google Scholar]
- 9.Tepede A., Yogaratnam D. Nintedanib for idiopathic pulmonary fibrosis. J Pharm Pract. 2019;32:199–206. doi: 10.1177/0897190017735242. [DOI] [PubMed] [Google Scholar]
- 10.Yang J., Nie J., Ma X., Wei Y., Peng Y., Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18:26. doi: 10.1186/s12943-019-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engelman J.A., Luo J., Cantley L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 12.Conte E., Fruciano M., Fagone E., Gili E., Caraci F., Iemmolo M., et al. Inhibition of PI3K prevents the proliferation and differentiation of human lung fibroblasts into myofibroblasts: the role of class I P110 isoforms. PLoS One. 2011;6:e24663. doi: 10.1371/journal.pone.0024663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green S., Trejo C.L., McMahon M. PIK3CA(H1047R) accelerates and enhances KRAS(G12D)-driven lung tumorigenesis. Cancer Res. 2015;75:5378–5391. doi: 10.1158/0008-5472.CAN-15-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conte E., Gili E., Fruciano M., Korfei M., Fagone E., Iemmolo M., et al. PI3K p110gamma overexpression in idiopathic pulmonary fibrosis lung tissue and fibroblast cells: in vitro effects of its inhibition. Lab Invest. 2013;93:566–576. doi: 10.1038/labinvest.2013.6. [DOI] [PubMed] [Google Scholar]
- 15.Zhai C., Cheng J., Mujahid H., Wang H., Kong J., Yin Y., et al. Selective inhibition of PI3K/Akt/mTOR signaling pathway regulates autophagy of macrophage and vulnerability of atherosclerotic plaque. PLoS One. 2014;9:e90563. doi: 10.1371/journal.pone.0090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revathidevi S., Munirajan A.K. Akt in cancer: mediator and more. Semin Cancer Biol. 2019;59:80–91. doi: 10.1016/j.semcancer.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Larson Casey J.L., Deshane J.S., Ryan A.J., Thannickal V.J., Carter A.B. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity. 2016;44:582–596. doi: 10.1016/j.immuni.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie Y., Sun L., Wu Y., Yang Y., Wang J., He H., et al. AKT2 regulates pulmonary inflammation and fibrosis via modulating macrophage activation. J Immunol. 2017;198:4470–4480. doi: 10.4049/jimmunol.1601503. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y., Zhang Y., Chi P. Pirfenidone suppresses TGFbeta1induced human intestinal fibroblasts activities by regulating proliferation and apoptosis via the inhibition of the Smad and PI3K/AKT signaling pathway. Mol Med Rep. 2018;18:3907–3913. doi: 10.3892/mmr.2018.9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinoshita T., Goto T. Molecular mechanisms of pulmonary fibrogenesis and its progression to lung cancer: a review. Int J Mol Sci. 2019;20:1461. doi: 10.3390/ijms20061461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sgalla G., Iovene B., Calvello M., Ori M., Varone F., Richeldi L. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res. 2018;19:32. doi: 10.1186/s12931-018-0730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzouvelekis A., Gomatou G., Bouros E., Trigidou R., Tzilas V., Bouros D. Common pathogenic mechanisms between idiopathic pulmonary fibrosis and lung cancer. Chest. 2019;156:383–391. doi: 10.1016/j.chest.2019.04.114. [DOI] [PubMed] [Google Scholar]
- 23.Selman M., K T., Pardo A., American Thoracic Society; European Respiratory Society; American College of Chest Physicians Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 24.Bellaye P.S., K M. Why do patients get idiopathic pulmonary fibrosis? Current concepts in the pathogenesis of pulmonary fibrosis. BMC Med. 2015;13:176. doi: 10.1186/s12916-015-0412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betensley A., Sharif R., Karamichos D. A systematic review of the role of dysfunctional wound healing in the pathogenesis and treatment of idiopathic pulmonary fibrosis. J Clin Med. 2016;6:2. doi: 10.3390/jcm6010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maher T.M., Wells A.U., Laurent G.J. Idiopathic pulmonary fibrosis/multiple causes and multiple mechanisms?. Eur Respir J. 2007;30:835–839. doi: 10.1183/09031936.00069307. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y.M., Nepali K., Liou J.P. Idiopathic pulmonary fibrosis: current status, recent progress, and emerging targets. J Med Chem. 2017;60:527–553. doi: 10.1021/acs.jmedchem.6b00935. [DOI] [PubMed] [Google Scholar]
- 28.Hewlett J.C., Kropski J.A., Blackwell T.S. Idiopathic pulmonary fibrosis: epithelial–mesenchymal interactions and emerging therapeutic targets. Matrix Biol. 2018;71–72:112–127. doi: 10.1016/j.matbio.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolb M., Bonella F., Wollin L. Therapeutic targets in idiopathic pulmonary fibrosis. Respir Med. 2017;131:49–57. doi: 10.1016/j.rmed.2017.07.062. [DOI] [PubMed] [Google Scholar]
- 30.Selman M., Pardo A. Idiopathic pulmonary fibrosis: clinical behavior, pathogenic mechanisms and therapeutic approach. Barcelona Respir Network Rev. 2015;1:13–25. [Google Scholar]
- 31.Evans C.M., Fingerlin T.E., Schwarz M.I., Lynch D., Kurche J., Warg L., et al. Idiopathic pulmonary fibrosis: a genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol Rev. 2016;96:1567–1591. doi: 10.1152/physrev.00004.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selman M., Pardo A. The leading role of epithelial cells in the pathogenesis of idiopathic pulmonary fibrosis. Cell Signal. 2020;66:109482. doi: 10.1016/j.cellsig.2019.109482. [DOI] [PubMed] [Google Scholar]
- 33.Moore C., Blumhagent R.Z., Yang I.V., Walts A., Powers J., Walker T., et al. Resequencing study confirms that host defense and cell senescence gene variants contribute to the risk of idiopathic pulmonary fibrosis. Am J Resp Crit Care. 2019;200:199–208. doi: 10.1164/rccm.201810-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armanios M. Telomerase and idiopathic pulmonary fibrosis. Mutat Res. 2012;730:52–58. doi: 10.1016/j.mrfmmm.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spira A., Beane J., Shah V., Liu G., Schembri F., Yang X.M., et al. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci U S A. 2004;101:10143–10148. doi: 10.1073/pnas.0401422101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taskar V., Coultas D. Exposures and idiopathic lung disease. Semin Resp Crit Care. 2008;29:670–679. doi: 10.1055/s-0028-1101277. [DOI] [PubMed] [Google Scholar]
- 37.Chioma O.S., Drake W.P. Role of microbial agents in pulmonary fibrosis. Yale J Biol Med. 2017;90:219–227. [PMC free article] [PubMed] [Google Scholar]
- 38.Winters N.I., Burman A., Kropski J.A., Blackwell T.S. Epithelial injury and dysfunction in the pathogenesis of idiopathic pulmonary fibrosis. Am J Med Sci. 2019;357:374–378. doi: 10.1016/j.amjms.2019.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X.P., Shu R.J., Filippatos G., Uhal B.D. Apoptosis in lung injury and remodeling. J Appl Physiol. 2004;97:1535–1542. doi: 10.1152/japplphysiol.00519.2004. [DOI] [PubMed] [Google Scholar]
- 40.Uhal B.D., Joshi I., Hughes W.F., Ramos C., Pardo A., Selman M. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol. 1998;275:L1192–L1199. doi: 10.1152/ajplung.1998.275.6.L1192. [DOI] [PubMed] [Google Scholar]
- 41.Pan L.H., Yamauchi K., Uzuki M., Nakanishi T., Takigawa M., Inoue H., et al. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J. 2001;17:1220–1227. doi: 10.1183/09031936.01.00074101. [DOI] [PubMed] [Google Scholar]
- 42.Antoniades H.N., Bravo M.A., Avila R.E., Galanopoulos T., Neville-Golden J., Maxwell M., et al. Platelet-derived growth factor in idiopathic pulmonary fibrosis. J Clin Invest. 1990;86:1055–1064. doi: 10.1172/JCI114808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khalil N., OConnor R.N., Flanders K.C., Unruh H. TGF-beta(1), but not TGF-beta(2) or TGF-beta(3), is differentially present in epithelial cells of advanced pulmonary fibrosis: an immunohistochemical study. Am J Resp Cell Mol. 1996;14:131–138. doi: 10.1165/ajrcmb.14.2.8630262. [DOI] [PubMed] [Google Scholar]
- 44.Lamouille S., Xu J., Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill C., Jones M.G., Davies D.E., Wang Y. Epithelial–mesenchymal transition contributes to pulmonary fibrosis via aberrant epithelial/fibroblastic cross-talk. J Lung Health Dis. 2019;3:31–35. [PMC free article] [PubMed] [Google Scholar]
- 46.Lin G., Gai R., Chen Z., Wang Y., Liao S., Dong R., et al. The dual PI3K/mTOR inhibitor NVP-BEZ235 prevents epithelial–mesenchymal transition induced by hypoxia and TGF-beta1. Eur J Pharmacol. 2014;729:45–53. doi: 10.1016/j.ejphar.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Saito S., Zhuang Y., Shan B., Danchuk S., Luo F., Korfei M., et al. Tubastatin ameliorates pulmonary fibrosis by targeting the TGFbeta–PI3K–Akt pathway. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan W.W., Wu Q.Y., Yao W.X., Li Y., Liu Y., Yuan J.L., et al. MiR-503 modulates epithelial–mesenchymal transition in silica-induced pulmonary fibrosis by targeting PI3K p85 and is sponged by lncRNA MALAT1. Sci Rep. 2017;7:11313. doi: 10.1038/s41598-017-11904-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X.L., Xing R.G., Chen L., Liu C.R., Miao Z.G. PI3K/Akt signaling is involved in the pathogenesis of bleomycin-induced pulmonary fibrosis via regulation of epithelial–mesenchymal transition. Mol Med Rep. 2016;14:5699–5706. doi: 10.3892/mmr.2016.5960. [DOI] [PubMed] [Google Scholar]
- 50.Schafer M.J., White T.A., Iijima K., Haak A.J., Ligresti G., Atkinson E.J., et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minagawa S., Araya J., Numata T., Nojiri S., Hara H., Yumino Y., et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L391–L401. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu T., De Los Santos FGonzalez, Zhao Y., Wu Z., Rinke A.E., Kim K.K., et al. Telomerase reverse transcriptase ameliorates lung fibrosis by protecting alveolar epithelial cells against senescence. J Biol Chem. 2019;294:8861–8871. doi: 10.1074/jbc.RA118.006615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baker J.R., Vuppusetty C., Colley T., Papaioannou A.I., Fenwick P., Donnelly L., et al. Oxidative stress dependent microRNA-34a activation via PI3Kalpha reduces the expression of sirtuin-1 and sirtuin-6 in epithelial cells. Sci Rep. 2016;6:35871. doi: 10.1038/srep35871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pereira P.R., Oliveira-Junior M.C., Mackenzie B., Chiovatto J.E., Matos Y., Greiffo F.R., et al. Exercise reduces lung fibrosis involving serotonin/Akt signaling. Med Sci Sports Exerc. 2016;48:1276–1284. doi: 10.1249/MSS.0000000000000907. [DOI] [PubMed] [Google Scholar]
- 55.Papa A., Pandolfi P.P. The PTEN–PI3K axis in cancer. Biomolecules. 2019;9:153. doi: 10.3390/biom9040153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu T., Tian Y., Gao Y., Ma M., Li H., Liu X., et al. PTEN loss regulates alveolar epithelial cell senescence in pulmonary fibrosis depending on Akt activation. Aging. 2019;11:7492–7509. doi: 10.18632/aging.102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barnes P.J., Baker J., Donnelly L.E. Cellular senescence as a mechanism and target in chronic lung diseases. Am J Resp Crit Care. 2019;200:556–564. doi: 10.1164/rccm.201810-1975TR. [DOI] [PubMed] [Google Scholar]
- 58.Chambers R.C., Mercer P.F. Mechanisms of alveolar epithelial injury, repair, and fibrosis. Ann Am Thorac Soc. 2015;12 Suppl 1:S16–S20. doi: 10.1513/AnnalsATS.201410-448MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang K., Zhang T., Lei Y.L., Li X.F., Jiang J.W., Lan J., et al. Identification of ANXA2 (annexin A2) as a specific bleomycin target to induce pulmonary fibrosis by impeding TFEB-mediated autophagic flux. Autophagy. 2018;14:269–282. doi: 10.1080/15548627.2017.1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park S., Kim S., Kim M.J., Hong Y., Lee A.Y., Lee H., et al. GOLGA2 loss causes fibrosis with autophagy in the mouse lung and liver. Biochem Biophys Res Commun. 2018;495:594–600. doi: 10.1016/j.bbrc.2017.11.049. [DOI] [PubMed] [Google Scholar]
- 61.Gui X., Chen H., Cai H., Sun L., Gu L. Leptin promotes pulmonary fibrosis development by inhibiting autophagy via PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun. 2018;498:660–666. doi: 10.1016/j.bbrc.2018.03.039. [DOI] [PubMed] [Google Scholar]
- 62.Liu M.W., Su M.X., Tang D.Y., Hao L., Xun X.H., Huang Y.Q. Ligustrazin increases lung cell autophagy and ameliorates paraquat-induced pulmonary fibrosis by inhibiting PI3K/Akt/mTOR and hedgehog signalling via increasing miR-193a expression. BMC Pulm Med. 2019;19:35. doi: 10.1186/s12890-019-0799-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cameli P., Carleo A., Bergantini L., Landi C., Prasse A., Bargagli E. Oxidant/antioxidant disequilibrium in idiopathic pulmonary fibrosis pathogenesis. Inflammation. 2020;43:1–7. doi: 10.1007/s10753-019-01059-1. [DOI] [PubMed] [Google Scholar]
- 64.Lu Y., Azad N., Wang L., Iyer A.K., Castranova V., Jiang B.H., et al. Phosphatidylinositol-3-kinase/Akt regulates bleomycin-induced fibroblast proliferation and collagen production. Am J Respir Cell Mol Biol. 2010;42:432–441. doi: 10.1165/rcmb.2009-0002OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lawson W.E., Crossno P.F., Polosukhin V.V., Roldan J., Cheng D.S., Lane K.B., et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1119–L1126. doi: 10.1152/ajplung.00382.2007. [DOI] [PubMed] [Google Scholar]
- 66.Lee K.I., Su C.C., Fang K.M., Wu C.C., Wu C.T., Chen Y.W. Ultrafine silicon dioxide nanoparticles cause lung epithelial cells apoptosis via oxidative stress-activated PI3K/Akt-mediated mitochondria- and endoplasmic reticulum stress-dependent signaling pathways. Sci Rep. 2020;10:9928. doi: 10.1038/s41598-020-66644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin C., Borensztajn K., Spek C.A. Targeting coagulation factor receptors—protease-activated receptors in idiopathic pulmonary fibrosis. J Thromb Haemost. 2017;15:597–607. doi: 10.1111/jth.13623. [DOI] [PubMed] [Google Scholar]
- 68.Chambers R.C., Scotton C.J. Coagulation cascade proteinases in lung injury and fibrosis. Proc Am Thorac Soc. 2012;9:96–101. doi: 10.1513/pats.201201-006AW. [DOI] [PubMed] [Google Scholar]
- 69.Anthoni C., Russell J., Wood K.C., Stokes K.Y., Vowinkel T., Kirchhofer D., et al. Tissue factor: a mediator of inflammatory cell recruitment, tissue injury, and thrombus formation in experimental colitis. J Exp Med. 2007;204:1595–1601. doi: 10.1084/jem.20062354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sapru A., Wiemels J.L., Witte J.S., Ware L.B., Matthay M.A. Acute lung injury and the coagulation pathway: potential role of gene polymorphisms in the protein C and fibrinolytic pathways. Intensive Care Med. 2006;32:1293–1303. doi: 10.1007/s00134-006-0223-5. [DOI] [PubMed] [Google Scholar]
- 71.Jose R.J., Williams A.E., Chambers R.C. Proteinase-activated receptors in fibroproliferative lung disease. Thorax. 2014;69:190–192. doi: 10.1136/thoraxjnl-2013-204367. [DOI] [PubMed] [Google Scholar]
- 72.Wygrecka M., Kwapiszewska G., Jablonska E., von Gerlach S., Henneke I., Zakrzewicz D., et al. Role of protease-activated receptor-2 in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:1703–1714. doi: 10.1164/rccm.201009-1479OC. [DOI] [PubMed] [Google Scholar]
- 73.Kotani I., Sato A., Hayakawa H., Urano T., Takada Y., Takada A. Increased procoagulant and antifibrinolytic activities in the lungs with idiopathic pulmonary fibrosis. Thromb Res. 1995;77:493–504. doi: 10.1016/0049-3848(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 74.Günther A., Mosavi P., Ruppert C., Heinemann S., Temmesfeld B., Velcovsky H.G., et al. Enhanced tissue factor pathway activity and fibrin turnover in the alveolar compartment of patients with interstitial lung disease. Thromb Haemost. 2000;83:853–860. [PubMed] [Google Scholar]
- 75.Crooks M.G., Hart S.P. Coagulation and anticoagulation in idiopathic pulmonary fibrosis. Eur Respir Rev. 2015;24:392–399. doi: 10.1183/16000617.00008414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.King T.E., Pardo A., Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 77.Dvorak H.F. Tumors: wounds that do not heal—a historical perspective with a focus on the fundamental roles of increased vascular permeability and clotting. Semin Thromb Hemost. 2019;45:576–592. doi: 10.1055/s-0039-1687908. [DOI] [PubMed] [Google Scholar]
- 78.Ebina M. Pathognomonic remodeling of blood and lymphatic capillaries in idiopathic pulmonary fibrosis. Respir Investig. 2017;55:2–9. doi: 10.1016/j.resinv.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 79.Iyer A.K.V., Ramesh V., Castro C.A., Kaushik V., Kulkarni Y.M., Wright C.A., et al. Nitric oxide mediates bleomycin-induced angiogenesis and pulmonary fibrosis via regulation of VEGF. J Cell Biochem. 2015;116:2484–2493. doi: 10.1002/jcb.25192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laddha A.P., Kulkarni Y.A. VEGF and FGF-2: promising targets for the treatment of respiratory disorders. Respir Med. 2019;156:33–46. doi: 10.1016/j.rmed.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 81.Malli F., Koutsokera A., Paraskeva E., Zakynthinos E., Papagianni M., Makris D., et al. Endothelial progenitor cells in the pathogenesis of idiopathic pulmonary fibrosis: an evolving concept. PLoS One. 2013;8:e53658. doi: 10.1371/journal.pone.0053658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jackson S.P., Schoenwaelder S.M., Goncalves I., Nesbitt W.S., Yap C.L., Wright C.E., et al. PI 3-kinase p110beta: a new target for antithrombotic therapy. Nat Med. 2005;11:507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 83.Desai O., Winkler J., Minasyan M., Herzog E.L. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front Med. 2018;5:43. doi: 10.3389/fmed.2018.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Molyneaux P.L., Maher T.M. The role of infection in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir Rev. 2013;22:376–381. doi: 10.1183/09059180.00000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.LoRusso P.M. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 2016;34:3803–3815. doi: 10.1200/JCO.2014.59.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoyne G.F., Elliott H., Mutsaers S.E., Prele C.M. Idiopathic pulmonary fibrosis and a role for autoimmunity. Immunol Cell Biol. 2017;95:577–583. doi: 10.1038/icb.2017.22. [DOI] [PubMed] [Google Scholar]
- 87.Barron L., Wynn T.A. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am J Physiol Gastr Liver Physiol. 2011;300:G723–G728. doi: 10.1152/ajpgi.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun L., Louie M.C., Vannella K.M., Wilke C.A., LeVine A.M., Moore B.B., et al. New concepts of IL-10-induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am J Physiol Lung Cell Mol Physiol. 2011;300:L341–L353. doi: 10.1152/ajplung.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pechkovsky D.V., Prasse A., Kollert F., Engel K.M., Dentler J., Luttmann W., et al. Alternatively activated alveolar macrophages in pulmonary fibrosis-mediator production and intracellular signal transduction. Clin Immunol. 2010;137:89–101. doi: 10.1016/j.clim.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 90.Mills C.D., Ley K. M1 and M2 macrophages: the chicken and the egg of immunity. J Innate Immun. 2014;6:716–726. doi: 10.1159/000364945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hou J., Shi J., Chen L., Lv Z., Chen X., Cao H., et al. M2 macrophages promote myofibroblast differentiation of LR-MSCs and are associated with pulmonary fibrogenesis. Cell Commun Signal. 2018;16:89. doi: 10.1186/s12964-018-0300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van den Brule S., Heymans J., Havaux X., Renauld J.C., Lison D., Huaux F., et al. Profibrotic effect of IL-9 overexpression in a model of airway remodeling. Am J Respir Cell Mol Biol. 2007;37:202–209. doi: 10.1165/rcmb.2006-0397OC. [DOI] [PubMed] [Google Scholar]
- 93.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang S.C.C., Smith A.M., Everts B., Colonna M., Pearce E.L., Schilling J.D., et al. Metabolic reprogramming mediated by the mTORC2–IRF4 signaling axis is essential for macrophage alternative activation. Immunity. 2016;45:817–830. doi: 10.1016/j.immuni.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nie Y., Hu Y., Yu K., Zhang D., Shi Y., Li Y., et al. Akt1 regulates pulmonary fibrosis via modulating IL-13 expression in macrophages. Innate Immun. 2019;25:451–461. doi: 10.1177/1753425919861774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kral J.B., Kuttke M., Schrottmaier W.C., Birnecker B., Warszawska J., Wernig C., et al. Sustained PI3K activation exacerbates BLM-induced lung fibrosis via activation of pro-inflammatory and pro-fibrotic pathways. Sci Rep. 2016;6:23034. doi: 10.1038/srep23034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kruger P., Saffarzadeh M., Weber A.N.R., Rieber N., Radsak M., von Bernuth H., et al. Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takemasa A., Ishii Y., Fukuda T. A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. Eur Respir J. 2012;40:1475–1482. doi: 10.1183/09031936.00127011. [DOI] [PubMed] [Google Scholar]
- 99.Manoury B., Nenan S., Guenon I., Lagente V., Boichot E. Influence of early neutrophil depletion on MMPs/TIMP-1 balance in bleomycin-induced lung fibrosis. Int Immunopharm. 2007;7:900–911. doi: 10.1016/j.intimp.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 100.Gregory A.D., Kliment C.R., Metz H.E., Kim K.H., Kargl J., Agostini B.A., et al. Neutrophil elastase promotes myofibroblast differentiation in lung fibrosis. J Leukoc Biol. 2015;98:143–152. doi: 10.1189/jlb.3HI1014-493R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.O'Dwyer D.N., Ashley S.L., Moore B.B. Influences of innate immunity, autophagy, and fibroblast activation in the pathogenesis of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2016;311:L590–L601. doi: 10.1152/ajplung.00221.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Manzenreiter R., Kienberger F., Marcos V., Schilcher K., Krautgartner W.D., Obermayer A., et al. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J Cyst Fibros. 2012;11:84–92. doi: 10.1016/j.jcf.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 103.Narasaraju T., Yang E., Samy R.P., Ng H.H., Poh W.P., Liew A.A., et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Medina-Tato D.A., Ward S.G., Watson M.L. Phosphoinositide 3-kinase signalling in lung disease: leucocytes and beyond. Immunology. 2007;121:448–461. doi: 10.1111/j.1365-2567.2007.02663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yago T., Zhang N., Zhao L., Abrams C.S., McEver R.P. Selectins and chemokines use shared and distinct signals to activate β2 integrins in neutrophils. Blood Adv. 2018;2:731–744. doi: 10.1182/bloodadvances.2017015602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leisching G.R. PI3-kinase δγ catalytic isoforms regulate the Th-17 response in tuberculosis. Front Immunol. 2019;10:2583. doi: 10.3389/fimmu.2019.02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perry M.W.D., Abdulai R., Mogemark M., Petersen J., Thomas M.J., Valastro B., et al. Evolution of PI3Kgamma and delta inhibitors for inflammatory and autoimmune diseases. J Med Chem. 2019;62:4783–4814. doi: 10.1021/acs.jmedchem.8b01298. [DOI] [PubMed] [Google Scholar]
- 108.Sapey E., Greenwood H., Walton G., Mann E., Love A., Aaronson N., et al. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123:239–248. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Todd N.W., Scheraga R.G., Galvin J.R., Iacono A.T., Britt E.J., Luzina I.G., et al. Lymphocyte aggregates persist and accumulate in the lungs of patients with idiopathic pulmonary fibrosis. J Inflamm Res. 2013;6:63–70. doi: 10.2147/JIR.S40673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vuga L.J., Tedrow J.R., Pandit K.V., Tan J.N., Kass D.J., Xue J.M., et al. C-X-C motif chemokine 13 (CXCL13) is a prognostic biomarker of idiopathic pulmonary fibrosis. Am J Resp Crit Care. 2014;189:966–974. doi: 10.1164/rccm.201309-1592OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wynn T.A. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wynn T.A. Fibrotic disease and the TH1/TH2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Simonian P.L., Roark C.L., Wehrmann F., Lanham A.K., del Valle F.D., Born W.K., et al. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol. 2009;182:657–665. [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Y., Li C.Y., Weng D., Song L.Y., Tang W., Dai W.J., et al. Neutralization of interleukin-17A delays progression of silica-induced lung inflammation and fibrosis in C57BL/6 mice. Toxicol Appl Pharmacol. 2014;275:62–72. doi: 10.1016/j.taap.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 115.Arras M., Huaux F., Vink A., Delos M., Coutelier J.P., Many M.C., et al. Interleukin-9 reduces lung fibrosis and type 2 immune polarization induced by silica particles in a murine model. Am J Resp Cell Mol. 2001;24:368–375. doi: 10.1165/ajrcmb.24.4.4249. [DOI] [PubMed] [Google Scholar]
- 116.Arras M., Louahed J., Heilier J.F., Delos M., Brombacher F., Renauld J.C., et al. IL-9 protects against bleomycin-induced lung injury—involvement of prostaglandins. Am J Pathol. 2005;166:107–115. doi: 10.1016/S0002-9440(10)62236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Simonian P.L., Wehrmann F., Roark C.L., Born W.K., O'Brien R.L., Fontenot A.P. Gammadelta T cells protect against lung fibrosis via IL-22. J Exp Med. 2010;207:2239–2253. doi: 10.1084/jem.20100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pociask D.A., Chen K., Choi S.M., Oury T.D., Steele C., Kolls J.K. gamma delta T cells attenuate bleomycin-induced fibrosis through the production of CXCL10. Am J Pathol. 2011;178:1167–1176. doi: 10.1016/j.ajpath.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Galati D., De Martino M., Trotta A., Rea G., Bruzzese D., Cicchitto G., et al. Peripheral depletion of NK cells and imbalance of the Treg/Th17 axis in idiopathic pulmonary fibrosis patients. Cytokine. 2014;66:119–126. doi: 10.1016/j.cyto.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 120.Boveda-Ruiz D., D'Alessandro-Gabazza C.N., Toda M., Takagi T., Naito M., Matsushima Y., et al. Differential role of regulatory T cells in early and late stages of pulmonary fibrosis. Immunobiology. 2013;218:245–254. doi: 10.1016/j.imbio.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 121.Xue J., Kass D.J., Bon J., Vuga L., Tan J., Csizmadia E., et al. Plasma B lymphocyte stimulator and B cell differentiation in idiopathic pulmonary fibrosis patients. J Immunol. 2013;191:2089–2095. doi: 10.4049/jimmunol.1203476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zoz D.F., Lawson W.E., Blackwell T.S. Idiopathic pulmonary fibrosis: a disorder of epithelial cell dysfunction. Am J Med Sci. 2011;341:435–438. doi: 10.1097/MAJ.0b013e31821a9d8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Scotton C.J., Chambers R.C. Molecular targets in pulmonary fibrosis—the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 124.Abraham D.J., Eckes B., Rajkumar V., Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9:136–143. doi: 10.1007/s11926-007-0008-z. [DOI] [PubMed] [Google Scholar]
- 125.Desmouliere A., Chaponnier C., Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 126.Zolak J.S., de Andrade J.A. Idiopathic pulmonary fibrosis. Immunol Allergy Clin. 2012;32:473–485. doi: 10.1016/j.iac.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 127.Kuhn C., 3rd, Boldt J., King T.E., Jr., Crouch E., Vartio T., McDonald J.A. An immunohistochemical study of architectural remodeling and connective tissue synthesis in pulmonary fibrosis. Am Rev Respir Dis. 1989;140:1693–1703. doi: 10.1164/ajrccm/140.6.1693. [DOI] [PubMed] [Google Scholar]
- 128.Ninou I., Kaffe E., Muller S., Budd D.C., Stevenson C.S., Ullmer C., et al. Pharmacologic targeting of the ATX/LPA axis attenuates bleomycin-induced pulmonary fibrosis. Pulm Pharmacol Therapeut. 2018;52:32–40. doi: 10.1016/j.pupt.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 129.Fritsch R., de Krijger I., Fritsch K., George R., Reason B., Kumar M.S., et al. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell. 2013;153:1050–1063. doi: 10.1016/j.cell.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Spangle J.M., Roberts T.M., Zhao J.J. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim Biophys Acta Rev Cancer. 2017;1868:123–131. doi: 10.1016/j.bbcan.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xia H., Khalil W., Kahm J., Jessurun J., Kleidon J., Henke C.A. Pathologic caveolin-1 regulation of PTEN in idiopathic pulmonary fibrosis. Am J Pathol. 2010;176:2626–2637. doi: 10.2353/ajpath.2010.091117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aschner Y., Downey G.P. Transforming growth factor-beta: master regulator of the respiratory system in health and disease. Am J Respir Cell Mol Biol. 2016;54:647–655. doi: 10.1165/rcmb.2015-0391TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leask A., Abraham D.J. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 134.Border W.A., Noble N.A. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 135.Giri S.N., Hyde D.M., Hollinger M.A. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48:959–966. doi: 10.1136/thx.48.10.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sime P.J., Xing Z., Graham F.L., Csaky K.G., Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu L., Zhang Q., Mo W., Feng J., Li S., Li J., et al. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-beta1/Smads and PI3K/Akt pathways. Sci Rep. 2017;7:9289. doi: 10.1038/s41598-017-09673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hsu H.S., Liu C.C., Lin J.H., Hsu T.W., Hsu J.W., Su K., et al. Involvement of ER stress, PI3K/AKT activation, and lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Sci Rep. 2017;7:14272. doi: 10.1038/s41598-017-14612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lawrence J., Nho R. The role of the mammalian target of rapamycin (mTOR) in pulmonary fibrosis. Int J Mol Sci. 2018;19:778. doi: 10.3390/ijms19030778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baek H.A., Kim D.S., Park H.S., Jang K.Y., Kang M.J., Lee D.G., et al. Involvement of endoplasmic reticulum stress in myofibroblastic differentiation of lung fibroblasts. Am J Respir Cell Mol Biol. 2012;46:731–739. doi: 10.1165/rcmb.2011-0121OC. [DOI] [PubMed] [Google Scholar]
- 141.Qin L., Wang Z., Tao L., Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy. 2010;6:239–247. doi: 10.4161/auto.6.2.11062. [DOI] [PubMed] [Google Scholar]
- 142.Huang P., Yan R., Zhang X., Wang L., Ke X., Qu Y. Activating Wnt/beta-catenin signaling pathway for disease therapy: challenges and opportunities. Pharmacol Ther. 2019;196:79–90. doi: 10.1016/j.pharmthera.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 143.Zuo F.R., Kaminski N., Eugui E., Allard J., Yakhini Z., Ben-Dor A., et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci U S A. 2002;99:6292–6297. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pardo A., Gibson K., Cisneros J., Richards T.J., Yang Y., Becerril C., et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005;2 doi: 10.1371/journal.pmed.0020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Konigshoff M., Kramer M., Balsara N., Wilhelm J., Amarie O.V., Jahn A., et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Spassov S.G., Donus R., Ihle P.M., Engelstaedter H., Hoetzel A., Faller S. Hydrogen sulfide prevents formation of reactive oxygen species through PI3K/Akt signaling and limits ventilator-induced lung injury. Oxid Med Cell Longev. 2017;2017 doi: 10.1155/2017/3715037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Clarke D.L., Carruthers A.M., Mustelin T., Murray L.A. Matrix regulation of idiopathic pulmonary fibrosis: the role of enzymes. Fibrogene Tissue Repair. 2013;6:20. doi: 10.1186/1755-1536-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shimbori C., Gauldie J., Kolb M. Extracellular matrix microenvironment contributes actively to pulmonary fibrosis. Curr Opin Pulm Med. 2013;19:446–452. doi: 10.1097/MCP.0b013e328363f4de. [DOI] [PubMed] [Google Scholar]
- 149.Maharaj S., Shimbori C., Kolb M. Fibrocytes in pulmonary fibrosis: a brief synopsis. Eur Respir Rev. 2013;22:552–557. doi: 10.1183/09059180.00007713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Raghu G., Striker L.J., Hudson L.D., Striker G.E. Extracellular matrix in normal and fibrotic human lungs. Am Rev Respir Dis. 1985;131:281–289. doi: 10.1164/arrd.1985.131.2.281. [DOI] [PubMed] [Google Scholar]
- 151.Kaarteenaho-Wiik R., Lammi L., Lakari E., Kinnula V.L., Risteli J., Ryhanen L., et al. Localization of precursor proteins and mRNA of type I and III collagens in usual interstitial pneumonia and sarcoidosis. J Mol Histol. 2005;36:437–446. doi: 10.1007/s10735-006-9018-9. [DOI] [PubMed] [Google Scholar]
- 152.Lu P., Takai K., Weaver V.M., Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dancer R.C., Wood A.M., Thickett D.R. Metalloproteinases in idiopathic pulmonary fibrosis. Eur Respir J. 2011;38:1461–1467. doi: 10.1183/09031936.00024711. [DOI] [PubMed] [Google Scholar]
- 154.Xu W., Yang Z., Lu N. A new role for the PI3K/Akt signaling pathway in the epithelial-mesenchymal transition. Cell Adh Migr. 2015;9:317–324. doi: 10.1080/19336918.2015.1016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nho R.S., Polunovsky V. Translational control of the fibroblast–extracellular matrix association: an application to pulmonary fibrosis. Translation. 2013;1 doi: 10.4161/trla.23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tapial Martinez P., Lopez Navajas P., Lietha D. FAK structure and regulation by membrane interactions and force in focal adhesions. Biomolecules. 2020;10:179. doi: 10.3390/biom10020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ding Q., Gladson C.L., Wu H.J., Hayasaka H., Olman M.A. Focal adhesion kinase (FAK)-related non-kinase inhibits myofibroblast differentiation through differential MAPK activation in a FAK-dependent manner. J Biol Chem. 2008;283:26839–26849. doi: 10.1074/jbc.M803645200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Horowitz J.C., Rogers D.S., Sharma V., Vittal R., White E.S., Cui Z., et al. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19:761–771. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chaqour B., Whitbeck C., Han J.S., Macarak E., Horan P., Chichester P., et al. Cyr61 and CTGF are molecular markers of bladder wall remodeling after outlet obstruction. Am J Physiol Endocrinol Metab. 2002;283:E765–E774. doi: 10.1152/ajpendo.00131.2002. [DOI] [PubMed] [Google Scholar]
- 160.Chaqour B., Goppelt-Struebe M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins—implications in mechanical stress-associated pathologies. FEBS J. 2006;273:3639–3649. doi: 10.1111/j.1742-4658.2006.05360.x. [DOI] [PubMed] [Google Scholar]
- 161.Wang Q., Usinger W., Nichols B., Gray J., Xu L., Seeley T.W., et al. Cooperative interaction of CTGF and TGF-beta in animal models of fibrotic disease. Fibrogenesis Tissue Repair. 2011;4:4. doi: 10.1186/1755-1536-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lipson K.E., W C., Teng Y., Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogen Tissue Repair. 2012;5:S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu X., Dai H., Geng J., Wan X., Huang X., Li F., et al. Rapamycin increases CCN2 expression of lung fibroblasts via phosphoinositide 3-kinase. Lab Invest. 2015;95:846–859. doi: 10.1038/labinvest.2015.68. [DOI] [PubMed] [Google Scholar]
- 164.Zhang L., Li Y.N., Liang C.L., Yang W.L. CCN5 overexpression inhibits profibrotic phenotypes via the PI3K/Akt signaling pathway in lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis and in an in vivo model of lung fibrosis. Int J Mol Med. 2014;33:478–486. doi: 10.3892/ijmm.2013.1565. [DOI] [PubMed] [Google Scholar]
- 165.Galli J.A., Pandya A., Vega-Olivo M., Dass C., Zhao H., Criner G.J. Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: tolerability and adverse drug reactions. Respirology. 2017;22:1171–1178. doi: 10.1111/resp.13024. [DOI] [PubMed] [Google Scholar]
- 166.Lukey P.T., Harrison S.A., Yang S., Man Y., Holman B.F., Rashidnasab A., et al. A randomised, placebo-controlled study of omipalisib (PI3K/mTOR) in idiopathic pulmonary fibrosis. Eur Respir J. 2019;53:1801992. doi: 10.1183/13993003.01992-2018. [DOI] [PubMed] [Google Scholar]
- 167.Waqar S.N., Baggstrom M.Q., Morgensztern D., Williams K., Rigden C., Govindan R. A phase I trial of temsirolimus and pemetrexed in patients with advanced non-small cell lung cancer. Chemotherapy. 2016;61:144–147. doi: 10.1159/000442147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Le Cras T.D., Korfhagen T.R., Davidson C., Schmidt S., Fenchel M., Ikegami M., et al. Inhibition of PI3K by PX-866 prevents transforming growth factor-alpha-induced pulmonary fibrosis. Am J Pathol. 2010;176:679–686. doi: 10.2353/ajpath.2010.090123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lin S., Jin J., Liu Y., Tian H., Zhang Y., Fu R., et al. Discovery of 4-methylquinazoline based PI3K inhibitors for the potential treatment of idiopathic pulmonary fibrosis. J Med Chem. 2019;62:8873–8879. doi: 10.1021/acs.jmedchem.9b00969. [DOI] [PubMed] [Google Scholar]
- 170.Qian W., Cai X., Qian Q., Zhang W., Wang D. Astragaloside IV modulates TGF-beta1-dependent epithelial–mesenchymal transition in bleomycin-induced pulmonary fibrosis. J Cell Mol Med. 2018;22:4354–4365. doi: 10.1111/jcmm.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xu M., Yin J., Xie L., Zhang J., Zou C., Zou J., et al. Pharmacokinetics and tolerance of toal astragalosides after intravenous infusion of astragalosides injection in healthy Chinese volunteers. Phytomedicine. 2013;20:1105–1111. doi: 10.1016/j.phymed.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 172.Huang J., Tong X., Zhang L., Zhang Y., Wang L., Wang D., et al. Hyperoside attenuates bleomycin-induced pulmonary fibrosis development in mice. Front Pharmacol. 2020;11:550955. doi: 10.3389/fphar.2020.550955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wu X., Li W., Luo Z., Chen Y. Meta-analysis of clinical efficacy and safety of ligustrazine in the treatment of idiopathic pulmonary fibrosis. Evid Based Complement Alternat Med. 2020;2020:2416132. doi: 10.1155/2020/2416132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lehmann M., Korfei M., Mutze K., Klee S., Skronska-Wasek W., Alsafadi H.N., et al. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J. 2017;50:1602367. doi: 10.1183/13993003.02367-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hohmann M.S., Habiel D.M., Coelho A.L., Verri W.A., Jr., Hogaboam C.M. Quercetin enhances ligand-induced apoptosis in senescent idiopathic pulmonary fibrosis fibroblasts and reduces lung fibrosis in vivo. Am J Respir Cell Mol Biol. 2019;60:28–40. doi: 10.1165/rcmb.2017-0289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Andres S., Pevny S., Ziegenhagen R., Bakhiya N., Schafer B., Hirsch-Ernst K.I., et al. Safety aspects of the use of quercetin as a dietary supplement. Mol Nutr Food Res. 2018;62:1700447. doi: 10.1002/mnfr.201700447. [DOI] [PubMed] [Google Scholar]
- 177.Munster P., Aggarwal R., Hong D., Schellens J.H., van der Noll R., Specht J., et al. First-in-human phase I study of GSK2126458, an oral pan-class I phosphatidylinositol-3-kinase inhibitor, in patients with advanced solid tumor malignancies. Clin Cancer Res. 2016;22:1932–1939. doi: 10.1158/1078-0432.CCR-15-1665. [DOI] [PubMed] [Google Scholar]
- 178.Knight S.D., Adams N.D., Burgess J.L., Chaudhari A.M., Darcy M.G., Donatelli C.A., et al. Discovery of GSK2126458, a highly potent inhibitor of PI3K and the mammalian target of rapamycin. ACS Med Chem Lett. 2010;1:39–43. doi: 10.1021/ml900028r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mercer P.F., Woodcock H.V., Eley J.D., Plate M., Sulikowski M.G., Durrenberger P.F., et al. Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax. 2016;71:701–711. doi: 10.1136/thoraxjnl-2015-207429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kottmann R.M., Trawick E., Judge J.L., Wahl L.A., Epa A.P., Owens K.M., et al. Pharmacologic inhibition of lactate production prevents myofibroblast differentiation. Am J Physiol Lung Cell Mol Physiol. 2015;309:L1305–L1312. doi: 10.1152/ajplung.00058.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jin X., Dai H., Ding K., Xu X., Pang B., Wang C. Rapamycin attenuates bleomycin-induced pulmonary fibrosis in rats and the expression of metalloproteinase-9 and tissue inhibitors of metalloproteinase-1 in lung tissue. Chin Med J. 2014;127:1304–1309. [PubMed] [Google Scholar]
- 182.Korfhagen T.R., Le Cras T.D., Davidson C.R., Schmidt S.M., Ikegami M., Whitsett J.A., et al. Rapamycin prevents transforming growth factor-alpha-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2009;41:562–572. doi: 10.1165/rcmb.2008-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Molina-Molina M., Machahua-Huamani C., Vicens-Zygmunt V., Llatjos R., Escobar I., Sala-Llinas E., et al. Anti-fibrotic effects of pirfenidone and rapamycin in primary IPF fibroblasts and human alveolar epithelial cells. BMC Pulm Med. 2018;18:63. doi: 10.1186/s12890-018-0626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Roberts P.J., Usary J.E., Darr D.B., Dillon P.M., Pfefferle A.D., Whittle M.C., et al. Combined PI3K/mTOR and MEK inhibition provides broad antitumor activity in faithful murine cancer models. Clin Cancer Res. 2012;18:5290–5303. doi: 10.1158/1078-0432.CCR-12-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ihle N.T., Williams R., Chow S., Chew W., Berggren M.I., Paine-Murrieta G., et al. Molecular pharmacology and antitumor activity of PX-866, a novel inhibitor of phosphoinositide-3-kinase signaling. Mol Cancer Ther. 2004;3:763–772. [PubMed] [Google Scholar]
- 186.Levy B., Spira A., Becker D., Evans T., Schnadig I., Camidge D.R., et al. A randomized, phase 2 trial of docetaxel with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic non-small-cell lung cancer. J Thorac Oncol. 2014;9:1031–1035. doi: 10.1097/JTO.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 187.Hu X., Xu Q., Wan H., Hu Y., Xing S., Yang H., et al. PI3K–Akt–mTOR/PFKFB3 pathway mediated lung fibroblast aerobic glycolysis and collagen synthesis in lipopolysaccharide-induced pulmonary fibrosis. Lab Invest. 2020;100:801–811. doi: 10.1038/s41374-020-0404-9. [DOI] [PubMed] [Google Scholar]
- 188.Li L.C., Kan L.D. Traditional Chinese medicine for pulmonary fibrosis therapy: progress and future prospects. J Ethnopharmacol. 2017;198:45–63. doi: 10.1016/j.jep.2016.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li L., Hou X., Xu R., Liu C., Tu M. Research review on the pharmacological effects of astragaloside IV. Fundam Clin Pharmacol. 2017;31:17–36. doi: 10.1111/fcp.12232. [DOI] [PubMed] [Google Scholar]
- 190.Ye P., Yang X.L., Chen X., Shi C. Hyperoside attenuates OVA-induced allergic airway inflammation by activating Nrf2. Int Immunopharm. 2017;44:168–173. doi: 10.1016/j.intimp.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 191.Zou L., Chen S., Li L., Wu T. The protective effect of hyperoside on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2. Exp Toxicol Pathol. 2017;69:451–460. doi: 10.1016/j.etp.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 192.Li W., Tang Y., Chen Y., Duan J.A. Advances in the chemical analysis and biological activities of chuanxiong. Molecules. 2012;17:10614–10651. doi: 10.3390/molecules170910614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Boots A.W., Veith C., Albrecht C., Bartholome R., Drittij M.J., Claessen S.M.H., et al. The dietary antioxidant quercetin reduces hallmarks of bleomycin-induced lung fibrogenesis in mice. BMC Pulm Med. 2020;20:112. doi: 10.1186/s12890-020-1142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rosenbloom J., Mendoza F.A., Jimenez S.A. Strategies for anti-fibrotic therapies. Biochim Biophys Acta. 2013;1832:1088–1103. doi: 10.1016/j.bbadis.2012.12.007. [DOI] [PubMed] [Google Scholar]