Abstract
The immune system is involved in the initiation and progression of cancer. Research on cancer and immunity has contributed to the development of several clinically successful immunotherapies. These immunotherapies often act on a single step of the cancer–immunity cycle. In recent years, the discovery of new nanomaterials has dramatically expanded the functions and potential applications of nanomaterials. In addition to acting as drug-delivery platforms, some nanomaterials can induce the immunogenic cell death (ICD) of cancer cells or regulate the profile and strength of the immune response as immunomodulators. Based on their versatility, nanomaterials may serve as an integrated platform for multiple drugs or therapeutic strategies, simultaneously targeting several steps of the cancer–immunity cycle to enhance the outcome of anticancer immune response. To illustrate the critical roles of nanomaterials in cancer immunotherapies based on cancer–immunity cycle, this review will comprehensively describe the crosstalk between the immune system and cancer, and the current applications of nanomaterials, including drug carriers, ICD inducers, and immunomodulators. Moreover, this review will provide a detailed discussion of the knowledge regarding developing combinational cancer immunotherapies based on the cancer–immunity cycle, hoping to maximize the efficacy of these treatments assisted by nanomaterials.
KEY WORDS: Cancer immunotherapy, Nanomaterials, Cancer‒immunity cycle, Drug delivery, ICD inducers, Immunomodulators, Photothermal therapy, Photodynamic therapy, Radio sensitizer
Graphical abstract
Nanomaterials-assisted immunotherapy restores the cancer–immunity cycle by boosting one or several steps of cancer–immunity cycle or ease of immunosuppression in tumor macroenvironment, in which nanomaterials function as drug delivery platforms, ICD nano-inducers or immunomodulators.
1. Introduction
Cancer, one of the most fatal diseases, threatens the lives of about 20 million people worldwide currently1. Traditionally, surgery, chemotherapy, and radiotherapy have been the main theranostics for patients with cancer. However, systemic toxicity, cancer recurrence and metastasis affect patients' prognosis2. As our understanding of the interaction between oncology and immunology has increased, it has become feasible to utilize patients’ immune systems to defend against cancer. Cancer immunotherapies that can induce immunological memory have demonstrated a lasting inhibitory effect on cancer growth, recurrence, and metastasis3. Cancer immunotherapies, such as immune checkpoint blockade (ICB)4, 5, 6, 7 and chimeric antigen receptor T (CAR-T)8, 9, 10 cell therapy, have improved overall survival in a subset of patients, especially in those with hematological cancers. However, these treatments induce limited responses in solid tumors11 and are associated with systemic inflammation12. After the clinical success of ICB and CAR-T therapy, numerous immunotherapeutic agents and combinatorial strategies have been developed. Immunotherapy is redefining cancer theranostics and is not limited to the treatment of in situ or existing cancers. However, incomplete immunological knowledge as well as technical limitations still restricts the development of more efficient cancer immunotherapies. Novel immunological targets, drug delivery methods, and synergistic therapies are likely to lead to new breakthroughs in cancer immunotherapy.
Recently, discoveries in cancer immunology have broadened the horizon of cancer immunotherapy. Neoantigens, derived from mutations arising during the rapid proliferation of cancer cells, significantly increase the immunogenicity of tumor antigens13. Neoantigen vaccines have been shown to activate cytotoxic T (CD8+ T) cells14. In addition, a high cancer mutation burden is an important prognostic indicator of cancer immunotherapy15,16. During ICB therapy, the amount of tumor-infiltrating CD8+ T cells is directly linked to the therapeutic effect17. “Hot tumors”, with higher numbers of infiltrating CD8+ T cells against tumor antigens, present a greater response to ICB therapy18. In addition to activating an immune response against cancer cells, regulation of the tumor immunosuppressive microenvironment is also necessary. Various cytokines and immune cells are involved in the development and maintenance of tumor immunosuppressive microenvironments. These include interleukin (IL)-10, transforming growth factor (TGF)-β, immune checkpoints overexpressed on the surface of cancer cells, regulatory T (Treg) cells, and M2-type tumor-associated macrophages (TAMs)19. Recently, cancer–immunity cycle that describes the interaction of cancer tissues and immune system has been come up, and this concept is constantly being updated and improved20, 21, 22. Basically, cancer–immune cycle describes the process how tumor antigens that are released from damaged cancer cells are captured by APC cells and primed to CD8+ T cells, and how CD8+ T cells infiltrate into cancer tissues and kill cancer cells. For cancer immunotherapy, every step of the cancer–immunity cycle should be well considered. Moreover, optimizing the temporal and spatial activation of the immune response is the basis for achieving a safe and long-lasting anticancer effect20.
Cancer immunotherapy is generally administered systemically to ensure that it reaches all potential tumors. However, this can be accompanied by severe immune-related adverse events, such as colitis, diarrhea, and endocrinopathy23,24. Therefore, targeting and specifically activating cancer-related immune cells are critical. Due to the concerted efforts of clinicians, biologists, chemists, and material scientists, nanomaterials now play important and diverse roles in cancer immunotherapy25, 26, 27, 28. Nanomaterials can be enriched in cancer tissues compared to free small molecular drugs, which is termed an enhanced permeability and retention (EPR) effect29. The EPR effect was originally believed to result from the hyperpermeable tumor vasculature and impaired lymphatic drainage30. Recent reports have suggested that most nanomaterials enter tumor tissues via active trans-endothelial pathways31,32. A more detailed study on the mechanism of EPR will enable nanomaterials to be optimized for more efficient enrichment within cancer tissues. As an ideal platform, nanomaterials have the capacity to integrate multiple drugs for combination or synergistic treatment strategies33,34, meanwhile a part of them possessing their own functionality, including photothermal35, photodynamic36 and magnetic response capabilities37. In addition, some nanomaterials can stimulate the immune system, partially by inducing antigen uptake and presentation by APCs38. These properties of nanomaterials make it possible to simultaneously activate several steps in the cancer–immunity cycle with spatial and temporal accuracy, which helps in controlling immune-related adverse events and effectively amplifies the anticancer immune response by synergistically activating different stages of the immune process. Current applications of nanomaterials in cancer immunotherapy include use as drug carriers (delivery of apoptosis inducer, immunostimulants, photothermal or photodynamic molecules, ICB antibodies), functional materials (induction of photothermal or photodynamic processes), and immunomodulators. This review summarized the immune mechanisms and knowledge about the cancer–immunity cycle, meanwhile discussing in detail the application of nanomaterials to promote cancer immunotherapies based on cancer–immunity cycle. Finally, we hope to identify a breakthrough to further promote the combination and application of nanomaterials in cancer immunotherapy.
2. Game between cancer and immunity
Cancer immunotherapy is a complicated interdisciplinary field, involving the interaction and crosstalk between tumors and the immune system at various stages of cancer development. It was initially believed that there was no clear association between immune processes and cancer development. In the past few decades, an increasing amount of evidence has been published to support the involvement of immune processes in cancer39,40. Additionally, cancer has been shown to influence immune processes and lead to immune escape or immune suppression41. Based on these discoveries, numerous studies have focused on activating patients’ immune systems or adopting powerful immune cells to monitor, inhibit, and reverse cancer growth42. However, the effects of cancer immunotherapy against a single component of the immune process can be compromised by blocking other parts of the immune process induced by cancer. Therefore, there is an urgent need to elucidate a detailed understanding of immune responses associated with the development and treatment of cancer.
2.1. Cancer‒immunity cycle
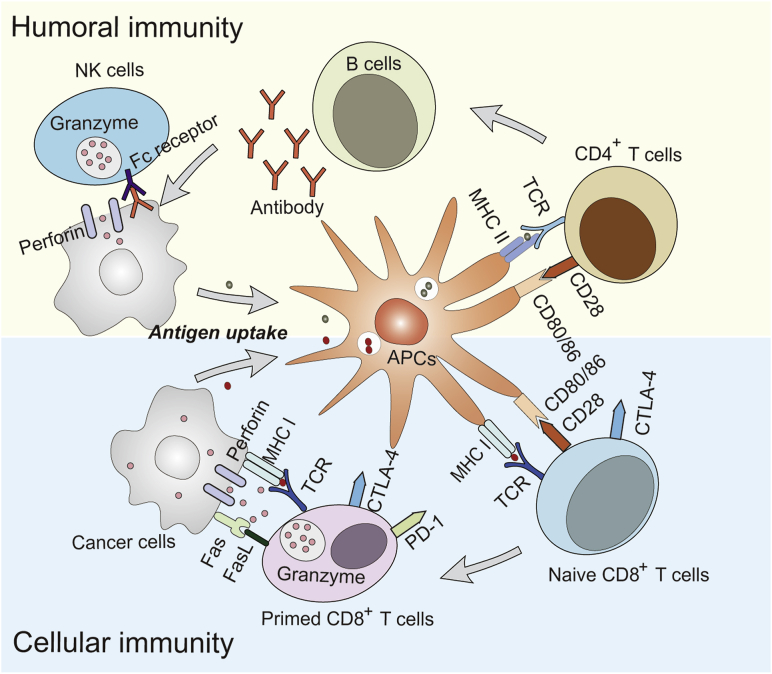
Cancer‒immunity cycle was first summarized by Chen et al.20 in 2013. Basically, it describes the cellular immunity process against cancer tissues. It includes several steps. Step 1, tumor antigens are released from damaged cancer cells and captured by dendritic cells (DCs) for processing; Step 2, DCs present tumor antigens to MHCI and MHCII molecules on T cells; Step 3, the priming and activation the effector T cell response; Step 4, effector T cells circulate to tumors; Step 5, effector T cells infiltrate into tumor tissues; Step 6, effector T cells recognize cancer cells by TCR and MHC I complex; Step 7, effector T cells kill cancer cells. The final step of killing cancer cells contribute to the release of tumor antigens to initiate a new round of cancer–immunity cycle. Therefore, the cancer–immunity cycle has the capacity to self-sustain upon initiation. The original cancer–immunity cycle emphasizes the critical function of cellular immunity in cancer therapy. However, lots of evidence proves that humoral immunity and innate immunity play important roles in inhibiting cancer development43. As described in Fig. 1, the tumor antigens from cancer cells are captured by APCs. As exogenous antigens, tumor antigens that are endocytosed into the endosome–lysosome system usually bind MHC II molecules that are rich in the endosome, which further induce the priming and activation CD4+ T cells. This pathway is classical humoral immunity, which kill cancer cells by antibody–antigen co-precipitation or antibody-dependent cell-mediated cytotoxicity (ADCC) mediated by NK cells. However, specific DCs, like CD8α+ DCs44, or special circumstances, like endosome leakage of tumor antigens45, induce the cross-presentation of tumor antigens. In the situation of cross-presentation, tumor antigens that exist in the cytoplasm are transported by the transporter of antigenic peptides (TAP) to the endoplasmic reticulum (ER), and are associated with newly assembled MHC I molecules. The presentation of MHC I/antigen complex eventually leads to the activation of CD8+ T cells.
Figure 1.
Adaptive immunity in cancer therapy. Humoral immunity: APCs take up and present antigens by MHC II molecules to activate CD 4+ T cells; CD4+ T cells present antigens to B cells, resulting in the secretion of antigen-specific antibodies; antibodies associate with antigens and co-precipitate for digestion by macrophages or induce ADCC effect mediated by NK cells. Cellular immunity: cancer cells are engulfed by APCs; APCs cross-present antigens to naïve CD8+ T cells by MHC I molecules, which is accompanied by CTLA-4 expression on primed CD8+ T cells; primed CD8+ T cells recognize cancer cells via an MHC I/antigen complex and kill cells via the perforin, granzyme and Fas/FasL pathway; however, the association of CTLA-4 or PD-1 with their ligands can induce the dysfunction of primed CD8+ T cells.
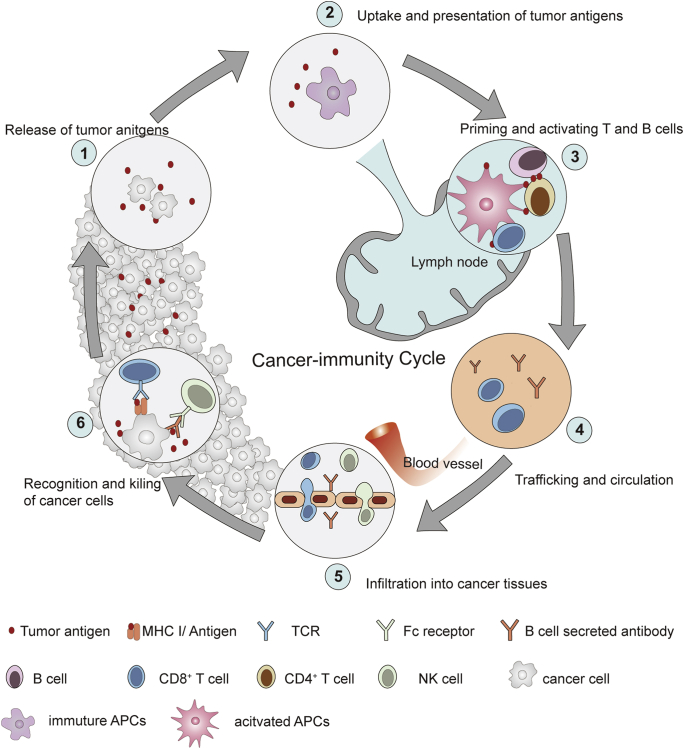
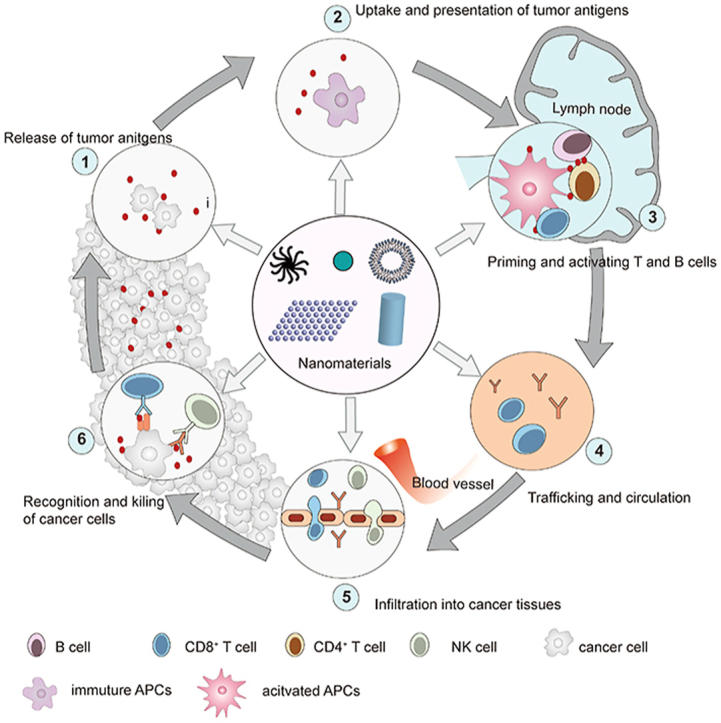
Therefore, we reorganized and amplified the content of cancer–immunity cycle in this review (Fig. 2). We described the cancer–immunity cycle as following steps. (1) Release of tumor antigens from damaged or dying cancer cells; (2) uptake and presentation of tumor antigens by APCs; (3) priming and activation of CD4+ and CD8+ T cells to trigger anticancer humoral and cellular immunity; (4) trafficking of NK cells, tumor antigen-specific antibodies, and CD8+ T cells; (5) infiltration and enrichment of NK cells, tumor antigen-specific antibodies, and CD8+ T cells into cancer tissues; (6) recognition and eradication of cancer cells via the cytotoxicity of CD8+ T cells and ADCC effect mediated by NK cells.
Figure 2.
Cancer-immunity cycle. (1) Release of tumor antigens from damaged or dying cancer cells; (2) uptake and presentation of tumor antigens by APCs; (3) priming and activation of CD4+ and CD8+ T cells to trigger anticancer humoral and cellular immunity; (4) trafficking of NK cells, tumor antigen-specific antibodies, and CD8+ T cells; (5) infiltration and enrichment of NK cells, tumor antigen-specific antibodies, and CD8+ T cells into cancer tissues; (6) recognition and eradication of cancer cells via the cytotoxicity of CD8+ T cells and antibody-dependent cell-mediated cytotoxicity (ADCC) mediated by NK cells. The design of Fig. 2 was inspired by Fig. 1 of Ref. 20 with the copyright permission. Copyright © 2013 Elsevier Inc.
2.2. Immune escape and immunosuppression in cancer tissues
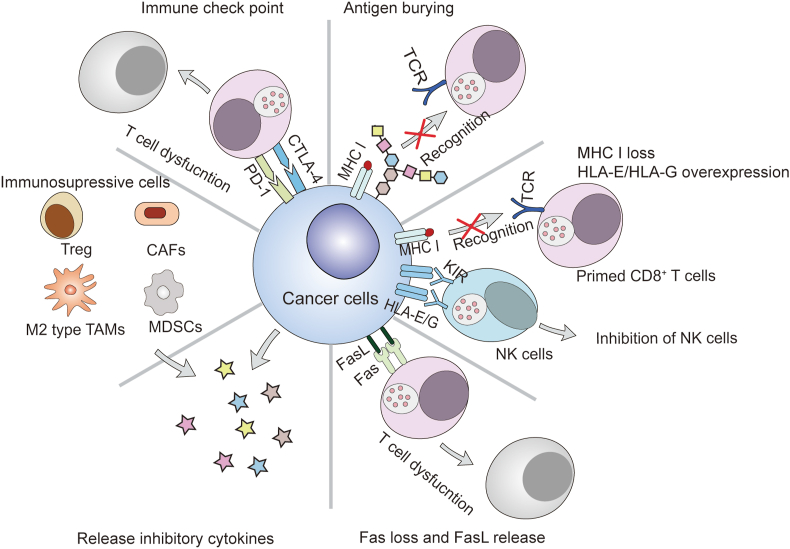
The relationship between cancer and immunity is extremely complicated. Recent research has suggested that chronic inflammation contributes to the initiation and growth of cancer. Gene mutations or metabolite variations occur in cancer cells during tumorigenesis. CD8+ T cells recognize and destroy cancer cells by monitoring the abnormal antigens presented by MHC I molecules on cancer cells, which ensures a low frequency of cancers considering that mutations occur in approximately 107–109 human cells every day. Commonly, the killing process of CD8+ T cells on cancer cells could start up the cancer–immunity cycle and efficiently inhibit the occurrence of cancer. However, CD8+ T cells sometimes are blind to a subset of mutated cells, which is termed the immune escape of cancer cells46. Moreover, the tumor immunosuppressive macroenvironment also impede the operation of cancer–immunity cycle. The main reasons of immune escape and immunosuppression are summarized as follows (Fig. 3).
-
(1)
Immune selection allows tumors with relatively weak immunogenicity to escape immune surveillance and selectively proliferate47. Cancers induced by oncogenic viruses and chemical carcinogens are highly immunogenic and easily cleared by the immune system, while spontaneous cancers of animals bear weak immunogenicity and tend to be retained48.
-
(2)
Antigen blockade or burying on the surface of cancer cells affects the recognition and attack by immune cells49. Some cancer cells overexpress mucopolysaccharides50, such as sialic acid51 or glycoproteins52, preventing CD8+ T cells from recognizing antigens presented by MHC I. Clearing sialic acid was found to enhance the anticancer immune response53,54.
-
(3)
Reduced expression of MHC I molecules on the surface of cancer cells can limit primed CD8+ T cell recognition55,56. However, MHC I molecules also inhibit NK cells by binding killer-cell inhibitory receptors (KIR) on NK cells. A lack of MHC I molecules activates NK cells to mediate the lysis or apoptosis of cancer cells57. Therefore, cancer cells express non-classical MHC I molecules (HLA-E and HLA-G) to associate with KIR and inhibit the activity of NK cells58.
-
(4)
Disordered Fas expression on the surface of cancer cells limits the ability CD8+ T cells to induce cancer cell apoptosis via the Fas/FasL pathway59. Moreover, some cancers overexpress and secrete FasL to bind Fas molecules on T cells and induce the death of T cells60.
-
(5)
Cancer cells secrete inhibitory factors, such as IL-10 and TGF-β to suppress the host immune response61,62. These inhibitory molecules accumulate in cancer tissues, forming a strong immunosuppressive microenvironment, which inactivates and kills infiltrating immune cells63. In addition, in cancer tissues, stromal cells secrete indoleamine-2,3-dioxygenase (IDO) to inhibit the proliferation of T cells64. IDO is the rate-limiting enzyme for tryptophan metabolism and exhausts tryptophan in the microenvironment to inhibit effector T cell proliferation65. Common cancer-related cytokines are listed in Table 1 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77.
-
(6)
Suppressive immune cells exist in tumor tissues, including cancer-associated fibroblasts (CAFs), regulatory T (Treg) cells, myeloid derived suppressor cells (MDSCs), and M2 type tumor-associated macrophages (TAMs). Cancer, which can be considered a non-healing wound, can induce an injury-like response, including the continued activation of fibroblasts. During cancer progression, cancer cells secrete vascular endothelial growth factor (VEGF), and recruit fibroblasts, endothelial cells, and inflammatory cells. Fibroblasts and inflammatory cells are the main resources of host-derived VEGF, which forms an autocrine circuit in cancer tissues78. However, the reduced fibroblast and inflammatory cell activity observed after wound healing does not occur in cancer.
-
(7)
Immune checkpoints
Figure 3.
Immunosuppression in cancer tissues.
Table 1.
Cancer-related cytokines.
| Cytokine | Main source | Function | Ref. |
|---|---|---|---|
| IL-6 | Macrophages Endothelial cells | Participates in acute inflammation and promote tumorigenesis | 66, 67, 68 |
| IL-10 | Th2 cells Monocytes | Inhibits activated monocytes to produce cytokines Inhibits the production of Th1 cytokines |
69 |
| TGF-β | Monocytes T cells | Regulates the differentiation of Treg and Th17 cells Inhibits the proliferation and differentiation of most immune cells and the production of cytokines Promotes the healing of damaged tissue |
61,63 |
| TNF-α | Monocytes Macrophages DCs | Pathogenic mediator of several autoimmune diseases; chronic exposure to TNF-α promotes tumor growth by mediating activation-induced cell death of effector T cells | 70 |
| IL-2 | Th1 cells | Activates T cells, NK cells, and macrophages | 71 |
| IL-12 | DCs Macrophages | Activates NK cells and induces Th1 differentiation | 72,73 |
| IL-15 | Activated myeloid cells | Activates T cells and NK cells | 74 |
| IFN-γ | Activated T cells NK cells | Activates macrophages and MHC expression Induces the differentiation of Th1 cells and inhibits Th2 cells |
75 |
| GM-CSF | Macrophages T cells | Induces the proliferation and differentiation of DCs Activates macrophages |
76,77 |
T cell activation requires the binding of antigen-bound MHC I molecules to TCRs, and is regulated by costimulatory or inhibitory signals, or an immunity checkpoint (Table 2). Immune checkpoint pairs are important strategies to achieve self-tolerance and prevent the immune system from damaging the surrounding normal tissue during anti-pathogen immunity. Immune checkpoints transmit “self” and “do not eat me” signals to T cells. Cancer cells escape immune surveillance by upregulating immune checkpoint signals. ICB strategies provide powerful therapies to facilitate anticancer immunity.
Table 2.
Immune regulatory pairs.
| Regulator | Ligand | Source of ligand | Function |
|---|---|---|---|
| CTLA-4 | CD80 CD86 | APCs cancer cells | Limit T cell activity |
| PD-1 | PD-L1 PD-L2 | T and B cells cancer cells | Induce T cell exhaustion |
| BTLA | HVEM | T cells and APCs cancer cells | Inhibit T cell proliferation |
| TIM3 | GAL9 | Tregs cancer cells | Inhibit T cell proliferation |
| TIGHT | PVR PVRL2 | DCs | Inhibit T cell activation |
| LAG3 | MHC complexes | APC cells | Induce T cell exhaustion |
| CD40L | CD40 | APCs | Induce CTL priming |
| OX40 | OX40L | APCs | Promote T cell division and survival |
| CD27 | CD70 | DCs | Induce T cell priming |
| CD28 | CD80 CD86 | APCs | Induce T cell priming of |
| ICOS | B7RP1 | B cells macrophages | T cell co-stimulation |
CTLA-4 is expressed dominantly in primed CD8+ T cells and shares the B7 ligand with CD28. The association of B7 with CD28, accompanied by antigen presentation, activates naïve T cells. Conversely, the binding of B7 to CTLA prevents T cell activation. The up-regulation of CTLA-4 on primed CD8+ T cells prevents the overaction of cellular immunity79. Although CTLA-4 is mainly expressed on primed CD8+ T cells, it has also been found on Th and Treg cells80. The engagement of CTLA-4 on Th cells reduces Th activity, while the expression of CTLA-4 on Treg cells enhances their immunosuppressive effect81. The principal function of PD-1 is to limit T cell activity in peripheral tissues in anti-pathogen inflammatory response. Nevertheless, it has an immunosuppressive function during cancer progression. PD-1 is expressed in many tumor-infiltrating lymphocytes (TILs), including CD8+ T, Treg, B, and NK cells82. The common ligands of PD-1 are PD-L1 and PD-L2, which are commonly overexpressed on cancer cells83. The association between PD-1 and its ligands can induce the dysfunction of CD8+ T and NK cells. However, PD-1 enhances the proliferation of Treg cells in the presence of ligands84. In solid cancers, PD-L1 is the major ligand of PD-1. However, the level of PD-L1 expression is heterogeneous in different cancers, which may be important when considering the feasibility of therapeutic strategies against PD-1 and PD-L1. The anticancer effects of CTLA-4 or PD-1 blockade arise from the synergistic effect of CD8+ T and NK cell activation and Treg cell inhibition.
2.3. Cancer immunotherapy restoring cancer–immunity cycle
The role of the immune system during cancer initiation, development, and metastasis has received increasing attention. The identification of cancer–immunity cycle involved in cancer has supported the use of immunotherapies in patients. As immunotherapies have become more targeted, the clinical outcomes of patients receiving anticancer immunotherapies are gradually improving, meanwhile the safety profile is improving. Commonly, the cancer immunotherapies that directly reinitiate the cancer–immunity cycle or relief the immunosuppressive effect on cancer–immunity cycle, show great potentials to eradicate cancer. Because the cancer–immunity cycle has the capacity to self-sustain, any immunotherapies that promote any steps of cancer–immunity cycles may achieve self-amplified anticancer effect. Currently used cancer immunotherapies restoring cancer–immunity cycle include non-specific immunotherapy, monoclonal antibody therapy, adoptive cell therapy (ACT), and anti-tumor vaccine therapy.
2.3.1. Non-specific immunotherapy (cytokines and immunostimulants)
Non-specific immunotherapy, mainly including cytokines and immunostimulants, usually systematic activate immune response against cancer cells. Non-specific immunotherapy usually has diverse mechanisms of promoting cancer–immunity cycle, including inducing the tumor antigen uptake of APCs, promoting the activation of CD8+ T cells and easing the tumor immunosuppressive environment by stimulatory cytokines.
Coley85 first administered bacterial extracts (Coley's toxins) as adjuvants in patients with cancer at the end of the nineteenth century. Coley's toxins altered cytokine levels and leaded to tumor clearance in some patients. Subsequently, numerous cytokines have been demonstrated to possess anticancer effects, including IL-2, IFN-γ, and GM-CSF86. IL-2 and IFN-γ have demonstrated promising anticancer potential. However, the clinical application of IL-2 and IFN-γ are hindered by severe toxicity following systemic administration87. Fusing cytokines with targeting proteins was shown to increase the accumulation in tumors and improve the subsequent outcomes, while reducing systematic toxicity88. Nevertheless, fusion strategies have distinct effects on different cytokines. The combination of transgene technology and cytokines provides a novel treatment strategy. Cancer cells modified with cytokine genes have been evaluated based on the protective effect against subsequent challenges with wild-type cancer cells89,90. Except for stimulatory cytokines, immunostimulants, such as agonists of TLRs or STING protein, are widely utilized to activate the function of APCs91, 92, 93.
2.3.2. Monoclonal antibodies (mAbs)
In cancer immunotherapy, monoclonal antibodies (mAbs) constitute a substantial proportion of US Food and Drug Administration (FDA)-approved drugs. mAbs associate with targets at high affinity, which ensures the accuracy and efficiency of these agents. Moreover, mAbs have the capacity to mediate ADCC of NK cells, which further supports the anticancer effects of mAbs. The mAbs targeting on cancer–immunity cycles are mainly divided into two categories: immune checkpoint inhibitors for relieving the immunosuppression on cancer–immunity cycle and antibody–drug conjugates (ADCs) for inducing the death and antigen release of cancer cells.
The existence of immune checkpoints on cancer cells significantly limits the effectiveness of cancer immunotherapy. To restore the function of primed CD8+ T cells, antibodies that inhibit the association of CTLA-4 or PD-1 with respective ligands have been widely studied. Clinical data promote the commercialization of mAbs against CTLA-4 (ipilimumab), PD-1 (nivolumab, pembrolizumab), and PD-L1 (atezolizumab)94. Compared with CTLA-4 and PD-1 mAbs, PD-L1 mAbs have demonstrated lower toxicity.
Recently, ADCs have attracted attention, and three types of ADCs have been commercialized since 2019. Traditional chemical drugs have no selectivity and require a relatively high dose to achieve curative effects. High-affinity antibodies can be accurately associated with their targets. ADCs, composed of an antibody “warhead”, a cleavable linker, and a cytotoxic drug, combine the advantages of antibody and chemical drugs. Currently approved ADCs target biomarkers that are overexpressed on cancer cells, such as HER-2, CD30, CD33, and CD2295. The development of ADC technologies has expanded the indications for these therapies from leukemia to solid malignancies. Enhertu, a newer ADC, has been used in patients with HER-2 positive breast, stomach, and non-small cell lung cancers, with an objective response rate (ORR) of approximately 60%96. The FDA-approved ADCs are summarized in Table 3.
Table 3.
FDA-approved ADCs for cancer therapy.
| Product | ADC | Approval date | Target | FDA-approved indication |
|---|---|---|---|---|
| Adcertris | Brentuximab vedotin | 2011 | CD-30 | Hodgkin's lymphoma |
| Kadcyla | Trastuzumab emtansine | 2013 | HER-2 | HER2-positive advanced breast cancer |
| Besponsa | Inotuzumab ozogamicin | 2017 | CD22 | Relapsed or refractory CD22-positive acute lymphoblastic leukemia |
| Mylotarg | Gemtuzumab ozogamicin | 2017 | CD33 | CD33-positive newly diagnosed acute myeloid leukemia |
| Lumoxiti | Moxetumomab pasudotox-tdfk | 2018 | CD22 | Adult patients with relapsed or refractory hairy cell leukemia |
| Policy | Polatuzumab vedotin-piiq | 2019 | CD-79 b | Adult patients with relapsed or refractory diffuse large B-cell lymphoma |
| Padcev | Enfortumab vedotin-ejfv | 2019 | Nectin-4 | Patients with locally advanced or metastatic urothelial cancer |
| Enhertu | Fam-trastuzumab Deruxtecan-nxki | 2019 | HER-2 | Adult patients with HER2-positive unresectable or metastatic breast cancer |
2.3.3. ACT
ACT involves the activation and expansion of autologous immune cells in vitro, which are then reintroduced into the patient to enhance the anticancer capacity of the immune system. ACT directly carries out the identification and killing of cancer cells and reinitiates the cancer–immunity cycle by supplying a large amount of tumor antigens.
The effector cells of ACT are mainly lymphokine-activated killer (LAK) cells97, cytokine-induced killer (CIK) cells98, tumor-infiltrating lymphocytes (TIL), DC, NK, TCR-T, and CAR-T99. In non-specific ACT therapy, the effector immune cells, including LAK, CIK, DC, and NK, do not recognize specific tumor antigens and have no MHC I restriction. Although non-specific ACT therapy has demonstrated excellent anticancer activity against cancer cells lacking MHC I molecules, the potential toxicity to normal tissue cannot be ignored. The effector immune cells in specific ACT therapy, including TIL and TCR-T, can recognize tumor antigens. The recognition and subsequent lethal effect in cancer cells are dependent on MHC I molecules. Due to the MHC I restriction, TIL- and TCR-T-based specific ACT therapies are ineffective against cancer cells lacking MHC I. Nevertheless, CAR-T utilizes an antibody-antigen recognition model to replace the association of TCR-CD3 with MHC I/antigen complex, which avoids the MHC I restriction. However, CAR-T can only target cancer cells with surface antigens, and not those with internal antigens. CAR-T therapies approved by the FDA, such as Kymriah and Yescarta, mainly target lymphomas. The application of CAR-T to solid tumors remains challenging. Identifying better cancer biomarkers and the rational design of CAR are the main determinants for the success of CAR-T therapy. Moreover, similar technologies have been utilized in CAR-NK cells, which have been utilized in many preclinical studies100.
Patients bearing with cancer often have a weak immune system, with limitation of number and activity of self-lymphocytes. Therefore, the development of lymphocytes from other sources is considered a breakthrough. Recently, induced pluripotent stem cell (iPSC) technology has expanded the sources and doses of primary lymphocytes. Perhaps due to epigenetic memory, compared to somatic cells, iPSCs from cord blood or peripheral blood lymphocytes increase the efficiency of CD4+ CD8+ lymphocyte production101. CAR-T or CAR-NK therapies based on iPSCs have demonstrated significant curative effects in patients with B-cell malignancies and ovarian cancer102. Furthermore, universal CAR-T overcomes the limitations associated with cell source, providing widely applicable ACT without HLA-matching. Thus, MHC I, MHC II, and TCR molecules are eliminated to avoid transplant rejection or graft–versus–host reactions through mature gene editing. Furthermore, HLA-E or HLA-G is introduced to avoid immune attack by patients’ NK cells103. Clinical trials investigating iPSC-derived ACT or universal CAR-T therapies are currently ongoing.
2.3.4. Anticancer vaccines
Tumor initiation and progression are commonly accompanied by genetic mutations, which generate unique antigens that differ from those on normal autologous cells. The release of tumor antigens is supposed to start up the cancer–immunity cycle. However, the inability of APCs in tumor macroenvironment or low immunogenicity of tumor antigens impedes the capture and presentation of tumor antigens. Cancer vaccines, which are composed of tumor antigens and adjuvants, aim to overcome the tumor immunosuppressive environment, enhance the immunogenicity of tumor antigens, activate autologous cellular and humoral immunity, and thereby control or eliminate cancers104. Traditionally, cancer vaccines have included whole cell (cancer cell vaccines and DC vaccines), peptide, and nucleic acid vaccines.
With recent developments in gene sequencing and bioinformatics, personalized tumor neoantigens can be rapidly identified105. Relative to tumor-associated antigens, neoantigens are derived from cancer cell mutations and are therefore, completely new antigens. These neoplastic neoantigens are usually polypeptide fragments that possess a certain binding capacity with HLA; however, their immunogenicity is uncertain, and should be determined in vivo experiments. Peptide vaccines and mRNA vaccines based on neoantigens can induce anticancer humoral and cellular immunity14,106.
An in-depth study of cancer cell death found that some cancer cells can cause an immune response after death, which is termed immunogenic cell death (ICD). When cancer cells die normally, their antigens and immunostimulatory components are degraded via the apoptosis pathway. During ICD, cancer cells expose their antigens and release damage-associated molecular patterns (DAMPs), including ATP, high-mobility group protein 1 (HMGB1), and calreticulin107. These DMAPs induce the uptake and presentation of tumor antigens by APC cells, thereby producing anticancer effects108. In situ cancer vaccines based on the ICD process have revealed new anticancer mechanisms of traditional chemotherapeutic drugs, such as doxorubicin (DOX)109. Furthermore, several external cancer therapies, such as photothermal therapy, photodynamic therapy, and radiotherapy, have been shown to induce ICD in cancer cells. However, the effect of the in situ vaccine strategy is not guaranteed, and it depends directly on the immunogenicity of the tumor antigens. Cancers with greater mutation burden present a greater response to in-situ vaccines. Moreover, cancer vaccines that efficiently activate cellular immunity have good anticancer outcomes. The cross-presentation of APCs for exogenous tumor antigens is critical for activating cellular immunity. The cancer vaccines that could target CD8α+ DCs in lymph nodes or release tumor antigens from endosome–lysosome system to cytoplasm, provoke cross-presentation of APCs and better anticancer cellular immunity.
3. Nanomaterials provoking cancer‒immunity cycle
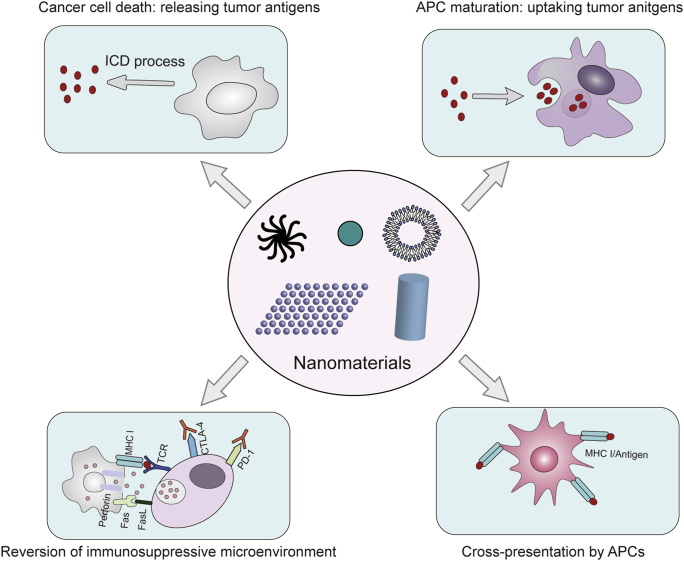
Nanomaterials have versatile advantages, such as controllable size, high biocompatibility, and excellent load capacity. As the mechanisms underlying cancer immunology are gradually elucidated, nanomaterials are expected to have potential to optimize many aspects of cancer immunotherapy based on cancer–immunity cycle. The targeting capacity of nanomaterials can ensure that the different steps of the cancer–immunity cycle are activated with temporal and spatial precision, which minimizes side effects while ensuring an anti-tumor immune response. The identification of EPR effects makes nanomaterials (20–200 nm) a suitable partner for cancer-targeted drug delivery110. Applying nanomaterials as carriers may enrich immune regulatory compounds in cancer tissues to enhance the immune response and reduce systemic toxicity. In addition, nanomaterials with a size of about 25 nm are more likely to target lymph nodes, resulting in the potent immune activation induced by cancer vaccines111,112. In addition to participating in cancer immunotherapy as drug carriers, an increasing number of nanomaterials are indicated to mediate external anticancer treatments, including photothermal and photodynamic therapies, which induce ICD in cancer cells to form an in-situ anticancer vaccine and reinitiate cancer–immunity cycle. In addition, partial nanomaterials have demonstrated unique adjuvant effects, which can stimulate the body to produce a stronger immune response and relieve the suppression of cancer–immunity cycle. The following will introduce in detail how nanomaterials as drug carriers, ICD inducers and immune adjuvants enhance cancer immunotherapy based on cancer‒immunity. The versatile functions of nanomaterials raise up the possibility of developing combinatorial cancer immunotherapy for simultaneously targeting several steps of cancer–immunity cycle to achieve more potent anti-cancer outcome (Fig. 4).
Figure 4.
Nanomaterials target different stages of the cancer–immunity cycle individually or simultaneously. Currently used nanomaterials mainly induce the ICD of cancer cells, promoting the antigen uptake and maturation of APCs, enhancing the cross-presentation of APCs, and regulating the immunosuppressive microenvironment of cancer tissues.
3.1. Drug delivery platform
In cancer immunotherapy, nanomaterials are used widely to target and enrich immune stimulatory compounds or cancer vaccines in cancer tissues or immune tissues (such as lymph nodes). The enriched drugs enhance immune system activation because of the increased concentration, and also limit damage to normal tissue caused by a systemic immune response. Nanomaterials that target cancer tissues and lymph nodes can be divided into passive and active targeting agents, according to the mechanism used. Passive targeting relies on the uptake of specific-sized nanomaterials by cancer tissues or lymph nodes, while active targeting predominantly relies on the overexpression of receptor molecules. For example, αv-integrins or folate receptors are overexpressed in some cancer tissues, and the αv-integrin ligand (iRGD) or folic acid can be modified on the surface of nanomaterials to achieve active targeting. Mannose receptors are overexpressed on the surface of APCs in lymph nodes. Therefore, mannose can be modified on nanomaterials to actively target APCs. Cancer-targeted nanomaterials are usually loaded with immune activators that can overcome the immune suppressive microenvironment, including cytokines, immune stimulants, and ICB antibodies. Nanomaterials that target lymph nodes are usually loaded with tumor antigens to promote the processing and cross-presentation of antigens by DCs, and to activate cancer-specific CD8+ T cells in the lymph nodes. The identification and development of ICD inducers have led to the use of nanomaterials to transport these agents to cancer tissues. In addition, the rational combination of different immunomodulators in nanomaterials will further improve the efficacy of cancer immunotherapy.
3.1.1. Cytokines and immunostimulants
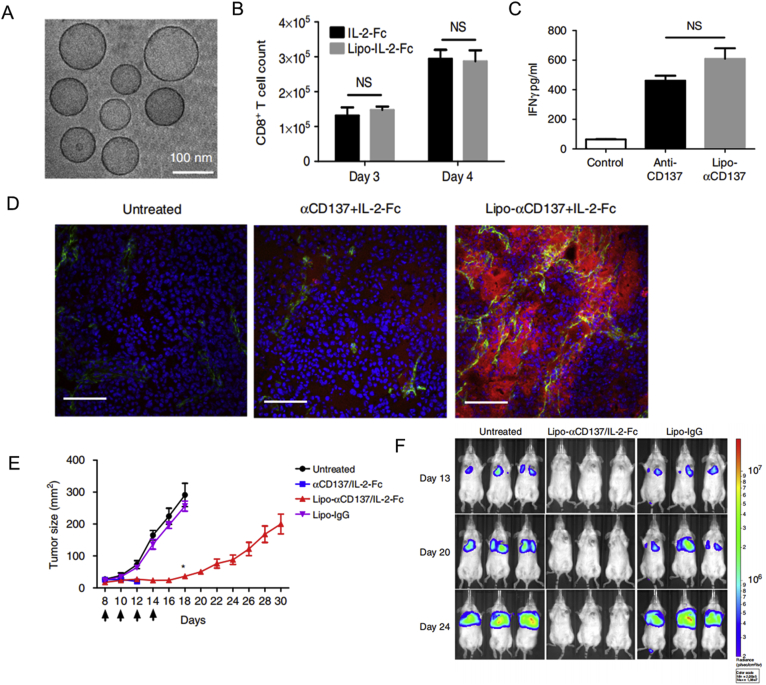
Systemic administration of free cytokines and immunostimulants leads to an uncontrollable immune storm. Cancer-targeted nanomaterials can restrict immune activation occurring inside cancer tissues. Following the assembly of low molecular-weight polyethyleneimine (600 Da), linkage with β-cyclodextrin and the IL-2 gene, and association with folate, polymeric nanoparticles about 100 nm in diameter, were shown to induce the activation of CD4+ T cells, CD 8+ T cells and NK cells, resulting in the regression of B16–F1 melanoma grafts113. A combination of the IL-2 gene, IL-12 gene, endosomally cleavable lipid, and endosomally cleavable RGD peptide generated nanoparticles approximately 100 nm in size, which led to increased leukocyte infiltration and necrotic cancerous areas114. In addition, loading IL-2-fused Fc proteins and an agonistic CD137 antibody on liposomes could retain the potent anticancer effects of IL-2 and an agonistic CD137 antibody, while significantly reducing systemic toxicity caused by circulating leukocytes115 (Fig. 5).
Figure 5.
Liposomes anchoring IL-2-fused Fc and an agonistic CD137 antibody resulted in anticancer immunity without systemic toxicity. (A) Cryo-TEM image of a IL-2-Fc-liposome (anti-CD137 liposomes were similar). (B) CD8+ T cell counts were determined following the treatment of polyclonal T cells from C57Bl/6 mice with soluble or liposomal IL-2-Fc (10 ng/mL of protein). (C) secreted IFN-γ was analyzed and then activated T cells were incubated with soluble anti-CD137 or Lipo-αCD137 (final αCD137 concentration: 10 μg/mL). (D) frozen sections of tumor after injections of Alexa-568-labeled αCD137 and IL-2-Fc and Lipo-αCD137 + Lipo-IL2-Fc. (E) tumor sizes in C57Bl/6 mice following treatment with αCD137 + IL-2-Fc, Lipo-αCD137 + Lipo-IL-2-Fc, or Lipo-IgG. (F) Bioluminescence images of C57BL/6 mice carrying luciferase-expressing B16F10 tumors, after treatment with Lipo-αCD137 + Lipo-IL-2-Fc or Lipo-IgG. Reprinted with the permission from Ref. 115. Copyright © 2019 Nature Publishing Group.
Except for cytokines, nanomaterials combined with immunostimulants ensure the local activation of tumor-infiltrating leukocytes. Intravenous administration of cyclic-di GMP-loaded cationic lipids was shown to efficiently trigger the production of interferon (IFN) and the activation of NK cells, which inhibited lung metastasis in a B16-F10 xenograft mouse model116. In addition, immunomodulator-loaded nanomaterials have the potential to target cancer-related leukocytes. For example, nanoparticles loaded with a TLR7/8 agonist specifically targeted DCs in cancer tissues and draining lymph nodes through passive targeting due to the size effects117. PLGA-PEG polymeric nanomaterials associated with an anti-PD-1 antibody (aPD1) or a CD8 antibody were shown to specifically bind to PD-1 positive CD8+ T cells. These PLGA-PEG polymeric nanomaterials were shown to deliver TGF-β inhibitors, reversing the suppressive effect of TGF-β on CD 8+ T cells118. Similar nanoparticles decorated with aPD1 were used to deliver a NF-κB inhibitor to PD-1 positive TILs to reduce the production of IL-10 and TGF-β, thereby easing the immunosuppressive conditions119.
3.1.2. mAbs
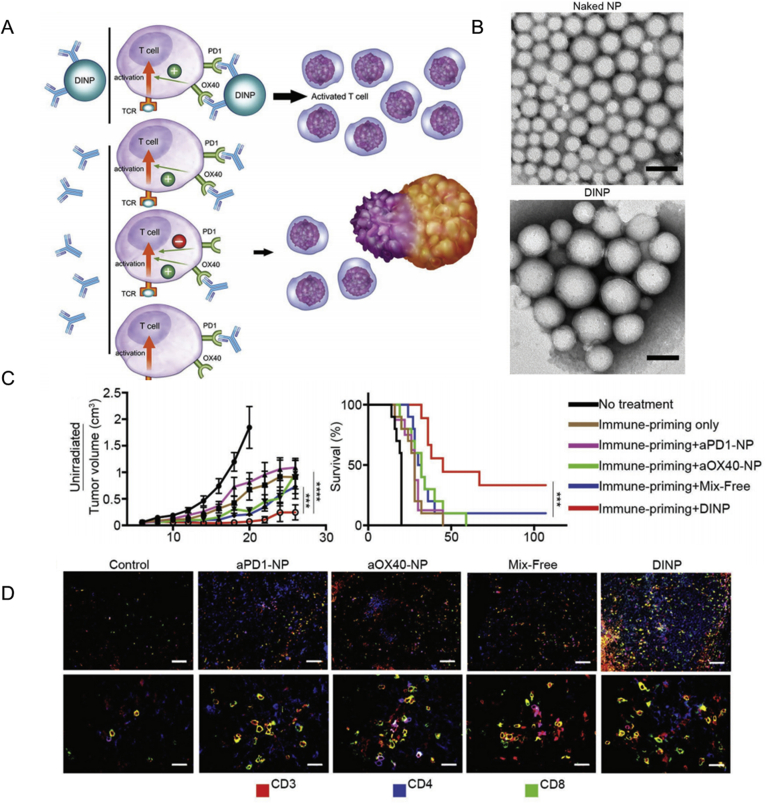
ICB, the most successful mAb therapy used in cancer immunotherapy, acts on both TIL and circulatory leukocytes, which induce anticancer effects along with autoimmune inflammation. Nanomaterials can transport ICB antibodies to cancer tissues and ameliorate toxicity. Self-degradable microneedles encapsulating aPD1 were shown to release aPD1 to melanoma tissues and significantly inhibit cancer progress120. In addition to controlled release, nanomaterials also endow ICB antibodies with multivalent effects, which strengthens the interaction between antibodies and their targets. Construction of a multivalent anti-PD-L1 antibody (aPD-L1) by conjugating aPD-L1 with hyperbranched poly-(amidoamine) dendrimers, enhanced the binding avidity with PD-L1 six-fold compared with free aPD-L1121. Moreover, fusion of recombinant scFv of aPD1 with immune-tolerant elastin-like polypeptide (iTEP) resulted in the self-assembly of aPD1 nanoparticles, which blocked the PD-1 immune checkpoint in vitro and in vivo122. Furthermore, nanoparticles co-loaded with different mAbs, such as aPD1 and anti-OX40 antibody (aOX40) demonstrated synergistic and improved anticancer outcomes compared to the two free antibodies123 (Fig. 6).
Figure 6.
A dual immunotherapy nanoparticle targeting PD-1 and OX40 improved anticancer immunity. (A) Schematic of DINP-facilitated enhancement of combination immunotherapy. (B) images of nanoparticles before and after antibody conjugation (scale bar: 100 nm). (C) tumor size and survival curves of C57BL/6 mice with B16F10 tumors following treatment with different drugs. (D) immunofluorescent images of tumors after treatment of different drugs. Reprinted with the permission from Ref. 123. Copyright © 2018 WILEY-VCH Publishing Group.
3.1.3. Cancer vaccines
Tumor antigens and adjuvants are required for cancer vaccines. Auxiliary ingredients, such as immunostimulants, could further improve the immunogenicity of cancer vaccines. Nanomaterials are multifunctional platforms that provide versatile advantages for the construction of cancer vaccines, as follows124,125: (1) the capacity to simultaneously deliver different vaccine components to the same APCs to boost an specific immune response; (2) enrich cancer vaccines to APCs in lymph nodes or cancer tissues by passive or active targeting; (3) mediate size and multivalence effects of cancer vaccines to trigger a potent immune response; (4) the controlled and sustainable release of tumor antigens ensuring long-term activation of the immune system; (5) cytosolic delivery of tumor antigens to promote cross-presentation of APCs to efficiently prime naïve CD8+ T cells.
Tumor-associated antigens (TAAs) and model antigens have been widely utilized for the primary identification and development of cancer vaccines, since identification of tumor-specific antigens is relatively difficult. Based on TAAs and model antigens (such as ovalbumin, OVA), numerous nanoparticles have been used to develop cancer nanovaccines. For example, biodegradable PLGA126, lipid–calcium–phosphate (LCP) nanoparticles127, glutathione-depletion mesoporous organosilica nanoparticles128 and protein nanoparticles129 have successfully loaded TAAs and model antigens, and shown to mediate tumor antigen-specific immunity. Following the discovery that DCs play important roles in the uptake and handling of cancer vaccines, an increasing number of cancer nanovaccines have been designed to target DCs130. For example, golden nanoparticles approximately 14 nm in size were used to load red fluorescent protein (RFP) as a model antigen and CPG-ODN as adjuvants. The formulation resulted in the enrichment of nanoparticles in draining lymph nodes, a high titer of anti-RFP antibody, and the regression of RFP-expressing B16-F10 tumors131. In addition, DC-targeting molecules, such as mannose or CD40 antibodies, are commonly modified on nanoparticles to deliver cancer nanovaccines to DCs. For example, PLGA-nanoparticles containing Pam3CSK4, Poly (I:C), and OVA, were associated with anti-CD40 antibody, which resulted in efficient vaccine delivery to DCs and the potent activation of CD8+ T cells132.
Under normal circumstances, DCs present antigens to CD4+ T cells to induce humoral immunity after engulfing exogenous antigens. However, cellular immunity facilitated by the cross-presentation of DCs with the aid of MHC I molecules represents a more efficient anticancer immune response. Nanomaterials that have the capacity to deliver tumor antigens into the cytoplasm greatly improve the probability and efficiency of cross-presentation. Positively charged nanomaterials, such as polyethyleneimine (PEI) and chitosan/calcium phosphate nanosheets, have been shown to trigger the endosomal escape of cargos via a proton sponge effect45,133. Nanomaterials loaded with endosomal-disrupting agents, such as pore-forming peptides, can mediate the escape of co-delivered cargos. For example, Kong and Liu et al.134 constructed nanovaccines by loading PLGA with OVA and hydroxychloroquine (HCQ). HCQ induced membrane permeabilization of the endosome and facilitated the release of OVA. Compared to PLGA/OVA nanoparticles, nanovaccines enhanced the expression of MHC-I and the costimulatory molecule CD86 of BMDCs, increased the frequency of IFN-γ+ CD8+ T cells, IFN-γ+ CD4+ T cells, and central memory T cells, and promoted the significant regression of tumors. In 2019, Xu et al.135 constructed another nanovaccine facilitating cross-presentation by loading OVA and CpG-ODN on a polyamidoamine dendrimer modified with guanidinobenzoic acid (DGBA). This nanovaccine induced potent antigen-specific cellular immunity and prevented the re-challenge of B16-OVA melanoma. Moreover, this nanovaccine demonstrated robust anticancer efficacy against B16-OVA melanomas when combined with the ICB strategy of aPD-1.
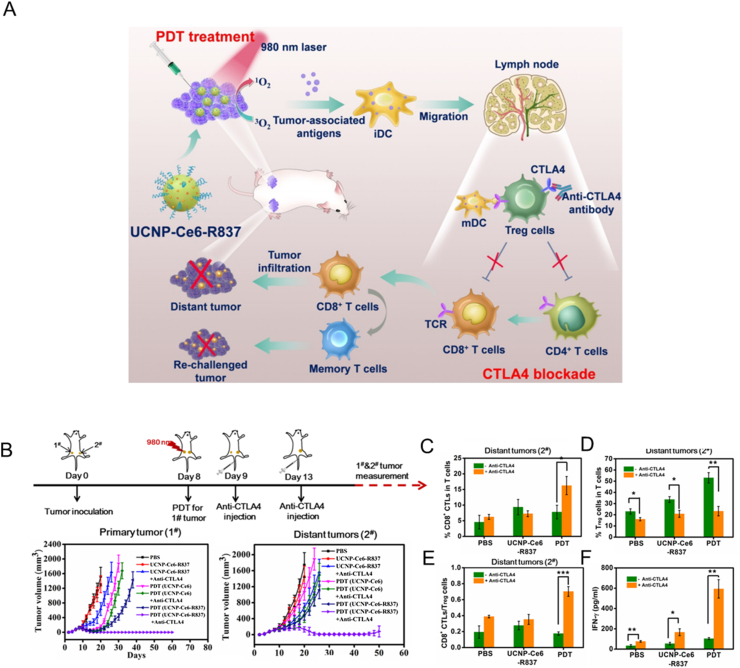
Following the maturation of identification technology for neoantigens, cancer neoantigens are now being utilized to formulate cancer nanovaccines. Nanodiscs coated with neoantigens and CPG-ODN were demonstrated to enriched in lymphoid organs, and induced up to 47-fold more neoantigen-specific CTLs than soluble vaccines136. In addition, the use of T7 bacteriophages as nanocarriers for the expression of neoantigens could obtain nanovaccines containing diverse neoantigens. These nanovaccines elicited high titers of anti-neoantigen antibodies and B cell responses137. In addition, the construction of cancer nanovaccines by the self-assembly of neoantigens and immunostimulants ensured efficient codelivery and high loading capacity. Conjugation of neoantigen peptides with an imidazoquinoline-based TLR-7/8 agonist could self-assemble into nanoparticles about 20 nm in size. Administration of this nanoparticles elicited functional CD8+ T cells against approximately 50% of neoantigens and led to enhanced tumor clearance138.
Neoantigens are specific for different tumor types, making them ideal antigen sources for cancer vaccines. Nevertheless, common neoantigen cancer vaccines are difficult to include all tumor antigens owing to the heterogenicity and frequent mutation of cancer cells. Therefore, cancer cell lysis and membrane structures have attracted attention for being rich in antigens. Polydopamine nanoparticles (PDA NPs) conjugated with cancer cell lysates with a high loading capacity were demonstrated to efficiently inhibit cancer progression139. In addition, cancer biofilms may be a suitable antigen donor for developing cancer vaccines. In 2014, Fang et al.140 utilized PLGA as the core material, encapsulated within cancer cell membranes derived from murine melanoma cells, and monophosphoryl lipid A (MPLA) as an adjuvant. This PLGA-membrane system retained most of the membrane-bound protein antigens and potently activated DCs with the aid of MPLA. Notably, the PLGA–membrane system without MPLA rarely induced DC activation, which might result from the low immunogenicity of the membrane alone.
In addition, the cell membrane from murine melanoma after PEGylation and association with CPG-ODN as an adjuvant, was found to induce a 3.7-fold greater antigen-specific cytotoxic CD8+ T cell response, compared to cancer cell lysates. Moreover, the PEGylated cancer vaccine combined with aPD-1 potently inhibited melanoma development141. Cancer vaccines derived from the cell membrane elicited a more efficient anticancer effect when combined with DC targeting. For example, PLGA loaded with the TLR7 agonist R837 significantly triggered DCs activation, IL-12 release, and TNF-α production after coating with cancer cytomembrane and mannose142. Fusion cell membranes from cancer cells and DCs have provided new insights into the construction of biofilm-based cancer vaccines. The fused cell membrane presented both tumor antigens and costimulatory molecules of DCs, producing potent DC-mimicking nanoparticles to directly present tumor antigens to T cells, and protect against re-challenge by cancer cells143. However, the fusion efficiency and stability of fused cells should be optimized.
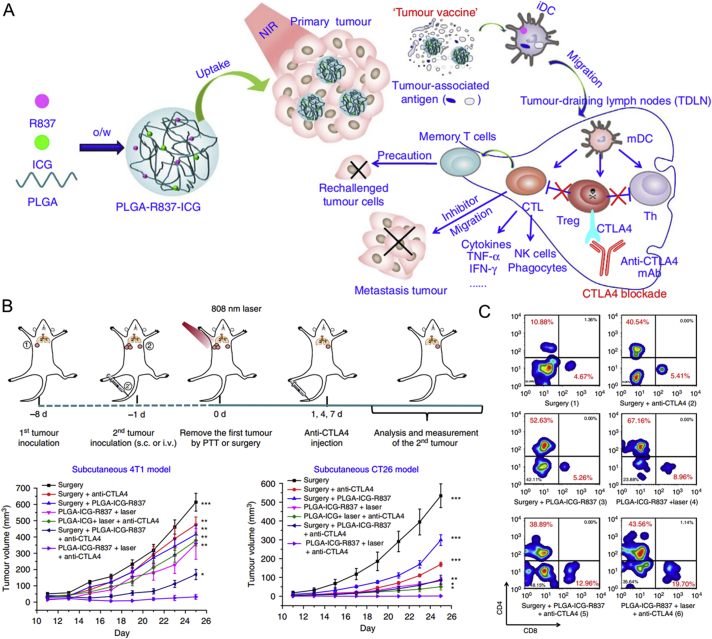
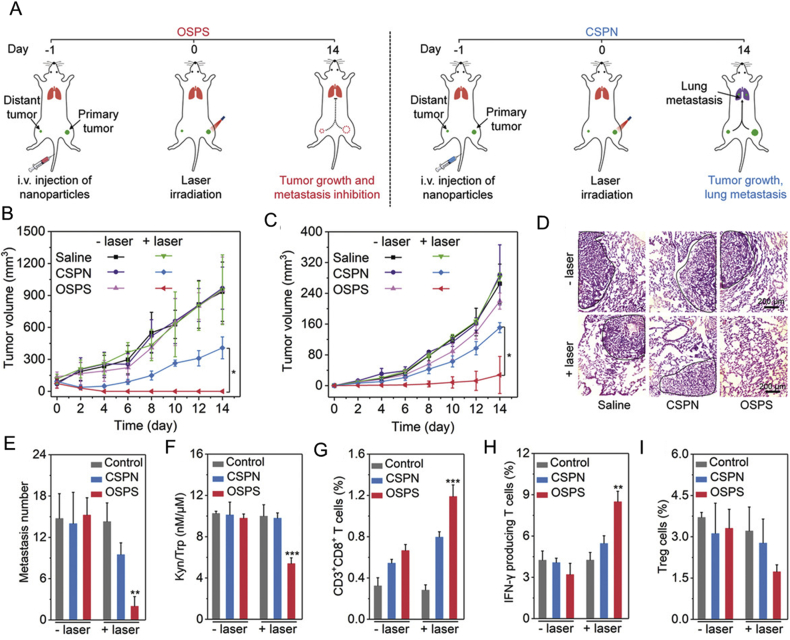
Theoretically, ICD inducers cause the release of tumor antigens and DAMPs, which function as in situ vaccines to activate APCs107. However, the immunogenicity of in situ vaccines is usually not strong, and depends on the mutation burden of cancer cells. Nanomaterials can target cancer tissues to deliver ICD inducers and co-load versatile immunostimulants to enhance the immunogenicity of in situ cancer vaccines. In addition to traditional chemotherapy drugs that induce ICD in cancer cells, such as DOX, oxaliplatin, and mitoxantrone, other types of molecules have also been shown to induce ICD, including photothermal and photodynamic agents144. Indocyanine green (ICG) is an FDA-approved dye used to assess liver function and blood flow, and is widely utilized as a photothermal agent in cancer immunotherapy. For example, PLGA loaded with ICG and R837 was found to mediate local tumor regression after irradiation with near-infrared light, and further inhibited tumor recurrence via the activation of cellular immunity against cancer cells145 (Fig. 7). A similar strategy was used to carry ICG and R837 on magnetic nanoparticles. With the aid of a magnetic field, the nanoparticles could be efficiently enriched in cancer tissues for MRI imaging. Moreover, the magnetic nanoparticles potently inhibited the progression of localized cancer and metastasis146.
Figure 7.
Photothermal therapy with immune-adjuvant nanoparticles induced anticancer immunity. (A) Schematic of immune-adjuvant nanoparticle constructed by PLGA loaded with ICG and R837 and its effect on immune system. (B) tumor volume of 4T1 and CT26 distant tumors following the indicated treatment of the primary tumor. (C) CD4+ and CD8+ T cells counts of distant tumors following the indicated treatment of the primary tumor. Reprinted with the permission from Ref. 145. Copyright © 2016 Nature Publishing Group.
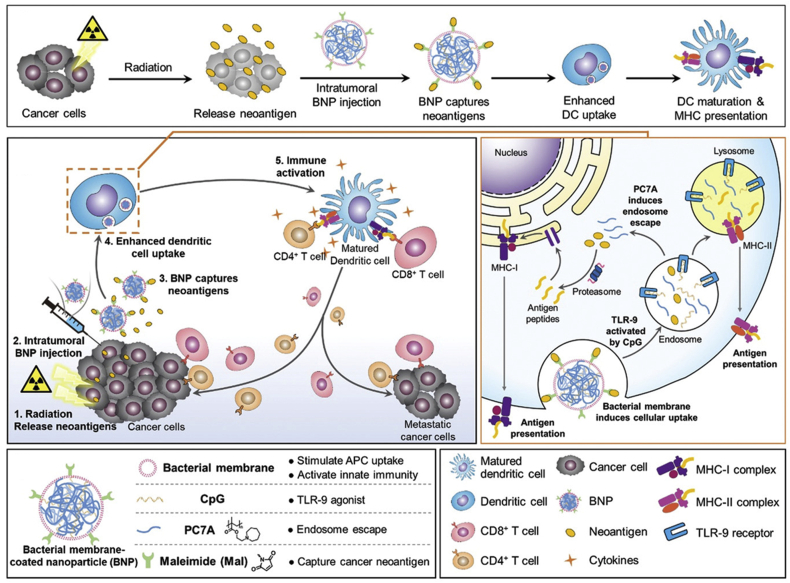
Photofrin and seven other photosensitizers have been approved by the FDA for use in photodynamic therapy. New-generation photosensitizers with longer excitation wavelengths and improved light stability are currently under development. Chlorin e6 (Ce6), which efficiently produces singlet oxygen, has attracted significant attention in basic research. As photosensitizers, O2 and light are necessary for the effective production of singlet oxygen and other reactive oxygen species (ROS). Nanomaterials are commonly used to co-deliver photosensitizers and O2 to cancer tissues to overcome the hypoxic environment. For example, hemoglobin (Hb) covalently bound to Ce6 and loaded with sorafenib (SRF, ferroptosis promotor) was shown to recruit immune cells to secrete IFN-γ and mediate potent anticancer effects147. Recently, nanomaterials were applied as antigen capture carriers in vivo for the construction of in situ cancer nanovaccines148. After the release of tumor antigens induced by radiotherapy, different surface-modified PLGA nanoparticles were injected intratumorally and their ability to capture tumor antigens and activate DC and CD8+ T cells was assessed. Screening demonstrated that maleimide polyethylene glycol PLGA (Mal AC-NPs) could elicit potent cellular immunity and a subsequent anticancer outcome149.
In addition, nanomaterials are designed to recruit and associate cancer cells with immune cells such as APCs. For example, Yuan et al.150 constructed a bi-specific nanobioconjugate engager equipped with an antibody against HER2 on cancer cells and CRT proteins to recruit APCs. This nanoparticle induced HER-2-mediated phagocytosis and resulted in a durable anticancer response against HER2-positive cancer cells.
3.2. Functional material as an ICD inducer
The types and functions of nanomaterials are diverse. They can be used as drug carriers (such as liposomes, mesoporous silicon, and polymers), and also have various other functions, such as photothermal effects, photo dynamic effects, chemical kinetic effects, magnetothermal effects, and radiation enhancement effects. A part of nanomaterials with these functions have been shown to induce ICD in cancer cells, thereby releasing tumor antigens and DMAPs151. The combination of these nanomaterials with traditional immunotherapy, such as immunostimulants and immune checkpoint therapy, has the potential to efficiently promote several steps of cancer–immunity cycle and eventually achieve better anticancer effect. The following part of this review will introduce photothermal, photodynamic, radiotherapeutic, chemodynamic, and other functional nanomaterials, and discuss their applications in cancer immunotherapy based on cancer–immunity cycle.
3.2.1. Photothermal nanomaterial
Photothermal agents (PTAs) transform light energy into thermal energy. PTA nanomaterials are usually divided into metal-based inorganic agents, carbon-based inorganic agents, phosphorene-based agents, polymeric agents, and other new PTAs152, 153, 154. Metal-based inorganic PTAs include conventional noble metal materials (including Au, Ag, Pd, and Pt) and semiconductor materials (containing CuS, MoS2, and WS2). Metal-based inorganic PTAs are easily synthesized with adjustable sizes and shapes, but have disadvantages such as slow metabolism rate and unclear long-term toxicity profiles. Carbon-based inorganic PTAs are mainly composed of graphene, carbon nanotubes, and fullerene. While carbon-based inorganic PTAs have high photothermal conversion efficiency and stability, they have potential to induce pneumonia and are difficult to produce on a large-scale. Phosphorene-based PTAs, newly developed nanomaterials, contain two-dimensional black phosphorene and black phosphorous quantum dots. Phosphorene-based PTAs have high photothermal conversion efficiency and excellent biodegradation properties. Nevertheless, issues with stability, large-scale production, and storage capacity remain to be resolved. Moreover, the acute toxicity and immune effects associated with phosphorene-based PTAs are still unclear. Polymeric PTAs, including polypyrrole (PPy) and polydopamine (PDA), are easily synthesized with adjustable molecular weights. Regarding other novel PTAs, several two-dimensional materials have been generated with high photothermal conversion efficiency, such as C3N4 and MXenes with the general formula Mn+1Xn. In Mn+1Xn, M indicates a transition metal (Ti, V, Ta, Nb, Mo, and Zr) and X represents C or N.
The PTAs suitable for PTT in cancer immunotherapy should satisfy the following requirements: (1) relatively high photothermal conversion efficiency to avoid laser damage to normal tissue; (2) excellent biocompatibility and biodegradation to avoid systemic toxicity; and (3) light absorption in the NIR region, which is optimal in the second NIR (NIR-II) window (1000–1350 nm). To date, photothermal therapies function as two models: high-temperature PTT and low-temperature PTT. For high temperature PTT, cancer tissues are ablated at temperatures exceeding 50 °C155. High temperature and heat transduction may damage adjacent normal tissue156. Normally, mammalian cells respond to heat shock by overexpressing heat shock proteins (HSPs), such as HSP70 and HSP90. Therefore, research has focused on sensitizing cancer cells for low-temperature PTT by inhibiting the expression and activity of HSPs157.
Recent studies have confirmed that PTT can induce ICD in cancer cells144. The higher temperature induced by irradiation resulted in more efficient cell death. However, ICD biomarkers did not increase with raising temperature. ICD markers, such as ATP release, HMGB1 release, and calreticulin expression, emerged more frequently at 63.3–66.4 °C than at higher (83.0–83.5 °C) and lower (50.7–52.7 °C) temperatures. Moreover, subsequent vaccination with different PTT-treated neuroblastomas confirmed in vitro findings. Challenging immunized mice with neuroblastoma cells within an optimal temperature window resulted in improved long-term survival compared to higher or lower temperature groups158.
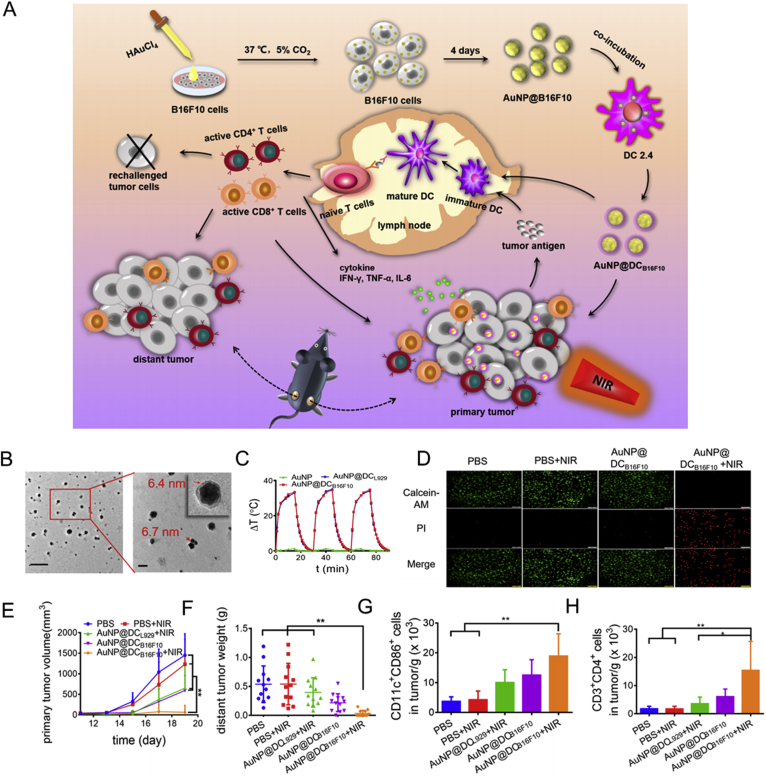
However, it is difficult to elicit a potent anticancer immune response only depending on antigen immunogenicity resulting from PTT-induced ICD. Therefore, PTA nanomaterials are frequently combined with other traditional immune strategies to improve the outcome of cancer immunotherapy. Utilization of carbon nanotubes as PTAs, combined with systemic administration of an anti CTLA-4 antibody effectively inhibited distant cancer and cancer metastasis under irradiation159. Hollow CuS nanoparticles coated with chitosan and CpG-ODN were found to activate NK cells and DCs in cancer tissues and draining lymph nodes, leading to the inhibition of localized and distant cancer160. Recently, mammalian cells have been utilized for the in situ generation of PTAs, Au nanoparticles (AuNPs). Treatment of B16F10 cells with HAuCl4 induced the intracellular generation of AuNPs. After exocytosis, AuNPs were encapsulated in B16F10 membranes containing diverse tumor antigens. Then, the AuNP@B16F10 cells were incubated with DC2.4 cells to further decorate the DC membrane and form AuNP@DCB16F10. Administration of AuNP@DCB16F10 to B16F10-bearing mice significantly inhibited cancer development and activated DCs and CD8+ T cells after irradiation161 (Fig. 8).
Figure 8.
Gold nanoparticles in situ generated in B16F10 and DCs for the combination of PPT and immunotherapy. (A) Schematic of construction and immunological functions of AUNP@DCB16F10. (B) TEM images of AUNP@DCB16F10. (C) temperature change (ΔT) of AuNP, AuNP@DCL929, and AuNP@DCB16F10. (D) images presenting live/dead cells after treatment with AuNP@DCB16F10 or/and laser. (E) primary tumor volume following the indicated treatment. (F) distant tumor weight following the indicated treatment. (G) DC maturation following the indicated treatment. (H) CD4+ T cell count after the indicated treatment. Reprinted with the permission from Ref. 161. Copyright © 2019 ACS Publishing Group.
Although nanomaterial-mediated PTT has been used widely in basic research for its non-invasive feature, there are still no successful clinical applications. The obstacles of nanomaterial-mediated PTT in clinical transformation can be summarized into six categories. (1) The limitation derived from the material features of most nanomaterials, including poor blood stability and dispersion, limited long circulation capacity, liver enrichment and inflammation, and unclear pharmacokinetic process. (2) The limitation of nanomaterial-based PTAs, including low photothermal efficiency and poor photo stability, especially for gold-based PTAs. (3) The obstacles derived from the irradiation process of light, including light toxicity and superficial tissue penetration. (4) What temperature is beneficial to the ICD process? Is there general principle for all nanomaterials-based PTAs (5) The limitation of knowledge about ICD process induced by nanomaterials-assisted PTT, for example, whether all the nanomaterials-assisted PTT cause ICD of cancer cells and how to evaluate and predict the capacity of nanomaterial-based PTAs to induce ICD process. (6) The intensity of ICD process induced by nanomaterials-assisted PTT is not enough to potently reinitiate the cancer–immunity cycle, and the combination of nanomaterials-assisted PTT with other cancer immunotherapy is necessary, which increases the complexity of the treatment system.
3.2.2. Photodynamic nanomaterial
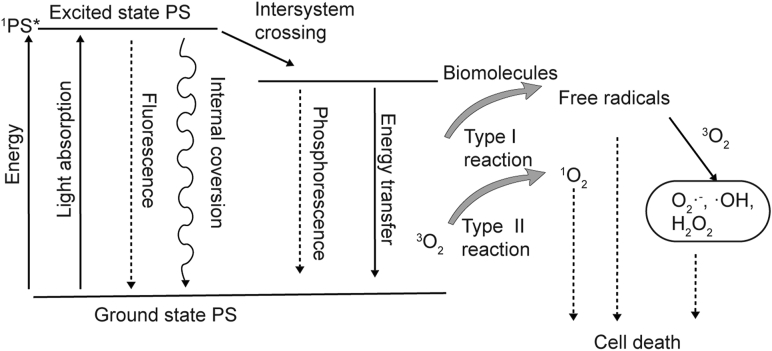
In PDT, photosensitizers (PSs) can absorb photons and transform them from a ground state to an excited state. Under an excited state, the PS is usually unstable and easily transfers high-energy electronics to other substrates. In type I reactions, PS in an excited state, reacts with the cell membrane or other biomolecules to form radicals, which further react with O2 to generate oxygenated products. In type II reactions, PS in an excited state directly reacts with O2 to form singlet oxygen, which is a highly active ROS162 (Fig. 9). Therefore, the PDT output is closely related to O2 concentration. Under a hypoxic tumor environment, it is difficult to demonstrate high efficiency with PDT. Although PDT emerged in the 1970s and was successfully used to treat superficial cancers, PDT-mediated immune activation was confirmed in the late 20th century, and therapies are still under development163, 164, 165. In more recent studies, PDT has been shown to be an effective method of inducing ICD in cancer cells166,167. Notably, it appears that ROS are required for ICD, because the immunogenicity of the process is largely inhibited in the presence of antioxidants. The PS of PDT includes organic dyes and nanomaterials. Organic dyes have several intrinsic drawbacks, including hydrophobicity, low penetration depth, and low specificity for cancer cells. Utilizing nanomaterials as carriers for organic PSs can overcome their several shortcomings, and related content have been introduced in Section 3.1.3. Herein, we will introduce nanomaterials that have an intrinsic capability to elicit photodynamic processes and their application in cancer immunotherapy.
Figure 9.
ROS generation in photodynamic therapies.
Common PDT nanomaterials include noble metallic nanomaterials, carbon-based nanomaterials, black phosphorene, and nanoscale metal–organic frameworks (MOFs)168. Briefly, noble metallic nanomaterials are represented by gold and silver nanoparticles. Gold nanorods, for example, were reported to produce singlet oxygen under NIR light at 915 nm, which destroyed B16F0 melanoma tumors in a mouse model. In addition, these gold nanorods induced an increase in temperature around cancer tissues following irradiation with NIR light at 780 nm169. The switch in excitation light could transform the therapeutic model between PTT and PDT. Carbon-based PS nanomaterials contain carbon nanotubes, fullerenes, and graphene quantum dots. Native carbon-based PS produces limited singlet oxygen under NIR irradiation. However, doping and surface modification can endow carbon-based PS with excellent quantum conversion efficiency under NIR irradiation170, 171, 172. Black phosphorene with a tunable band gap, excellent biocompatibility, and biodegradation was first applied as PS in 2015. Black phosphorene demonstrated an approximately 0.91 quantum yield of singlet oxygen upon 660 nm irradiation, and caused significant cell death and tumor suppression173. MOFs assembled with organic PSs as ligand, and metal ions (Hf, Fe, Zn, and Zr ions) as metal centers, were shown to function as a PS nanomaterial174,175. For example, a new porphyrin derivative, 5,15-di (p-benzoato) porphyrin (H2DBP) was reacted with HfCl4 through a solvothermal reaction to generate a DBP-UiO MOF structure. The DBP-UiO-O MOF showed enhanced PDT efficacy and eliminated cancer in approximately half of mice following a single administration, and once exposure of 640 nm irradiation176. Subsequent research developed chlorin-based MOF by replacing H2DBP with 5,15-di (p-benzoato)-chlorin (H2DBC) to obtain DBC-UiO, which had red shift excitation and an 11-fold greater extinction coefficient compared to DBP-UiO177. Compared to PTAs, the types and application potentials of current PS nanomaterials are limited. Most PS nanomaterials are excited under visible light or the NIR-I region, which limits the depth of tissue penetration. Two-photon excitation PDT nanomaterials provide a solution of NIR-II irradiation178. Conventional one-photon excitation of PSs absorbs a single photon to trigger PSs. However, two-photon excitation PSs are capable of absorbing two low-energy photons simultaneously to achieve the band-gap energy of PSs by the sum of the two photon energies. Two-photon excitation allows for deeper tissue penetration and reduced photobleaching of PSs. For example, CdSe QDs were used as two-photon excitation nanomaterials that could be excited under an 1100 nm laser and emit photons with a wavelength of 635 nm. The silicon phthalocyanine 4 (Pc 4) conjugated on CdSe QDs was able to absorb 635 nm photons and functioned as a PS via a fluorescence resonance energy transfer (FRET) process between QD and Pc 4179. Although current PS nanomaterials can potently inhibit localized cancers, the prevention of distant and metastatic cancers are dependent on the combination with other cancer immunotherapies. For example, Fe-TBP MOF was constructed from [Fe3O(OAc)6(H2O)3] OAc and the 5,10,15,20-tetra (p-benzoato) porphyrin (TBP) ligand. Fe3+ can interact with H2O2, which is abundant in cancer tissues, to generate O2 and ease hypoxia in cancer tissues, resulting in improved PDT efficiency. Fe-TBP combined with aPD-1 inhibited both localized primary cancers and distant cancers via abscopal effects180. Recently, poly (γ-glutamic acid) @glucose oxidase @carbon dot nanoparticles were constructed and combined with aPD-1. This nanomaterial generated O2 from H2O2 under Mn2+ catalysis and mediated carbon dot-based PDT, which further induced an anticancer immune response against treated and untreated distant tumors181.
Compared to conventional organic PSs, functional nanomaterial-mediated PDT is relatively rare. Development of NIR-II-elicited PDT nanomaterials with excellent biocompatibility and biodegradation may provide new opportunities for PDT. Except for the general limitations of nanomaterials, nanomaterials-assisted PDT owns its unique obstacles. First, it has photo toxicity and poor tissue penetration depth, which are common problems in photo irradiation therapy. Secondly, better intracellular uptake of PDT nanomaterials is necessary, for the ROS produced in PDT is active and only effective in the nanometer range. Thirdly, it should achieve a balance between PTT and PDT process induced by nanomaterials, for many nanomaterials have the ability to convert photons into heat and high-energy free radicals at the same time. Fourthly, O2 is necessary in PDT and hypoxia in cancer tissues impedes the efficiency of PDT. However, delivery of O2 with nanomaterial-based PS complicates the drug system and is not sustainable.
3.2.3. Radiotherapy nanomaterial
Radiotherapy is a mature treatment for cancer. Some nanomaterials are believed to enhance the effect of radiotherapy. Combined with its cancer-targeting ability, nanomaterial-based radiotherapy can reduce the damage to surrounding normal tissue182. Radiotherapy-enhanced nanomaterials are usually composed of high-Z elements183. The most prevailing radiosensitizers are gold-based nanoparticles184. Other studied radiosensitizers include lanthanide-based NPs185,186, Bi2Se3 nanoparticles187 and Hf-based MOF nanomaterials188,189. These radiosensitizing nanoparticles have been shown to improve the efficacy of radiotherapy by enhancing the photoelectric and Compton effects, which further increase the emission of secondary electrons and the production of ROS. During radiotherapy, DNA radicals need to react with O2 to induce DNA double-strand breaks. Therefore, the hypoxic environment of cancer tissues weakens the anticancer effects of radiotherapy. To circumvent the hypoxic microenvironment, radiosensitizers are usually accompanied by O2 delivery molecules, such as MnO2 nanoparticles or perfluorocarbons. For example, core–shell Au@MnO2-PEG was constructed to combine radiosensitizer high-Z atoms and O2 generators. The Au core, a well-known radiosensitizer, can enhance the production of DNA radicals. MnO2 has the capacity to decompose H2O2 to O2 to overcome hypoxia-mediated resistance to radiotherapy190. In addition, perfluorocarbon-coated hollow Bi2Se3 nanoparticles were shown to enhance the efficacy of radiotherapy via three mechanisms: perfluorocarbon, as an O2 carrier, reduced the hypoxic condition in solid tumors; Bi2Se3 nanoparticles, as radiosensitizers with high Z atom Bi, efficiently enhanced the photoelectric effect of RT; Bi2Se3 absorbed NIR light and produced a photothermal effect to increase the intertumoral blood flow, thus enhancing the concentration of O2 in tumor tissues191.
The abscopal effect, first proposed in 1953, hints at the evolution of the immune system under radiotherapy192. Subsequent studies found that radiotherapy could upregulate the expression of MHC I molecules and TAAs, thereby inducing the antigen presentation of DCs and the activation and trafficking of CD8+ T cells193. Further studies demonstrated that radiotherapy enhanced the immune response via ICD194. Combining radiotherapy with other immunotherapeutic strategies strengthens the immune response and induces synergistic anticancer efficacy. For example, Hf-based nMOFs with radiosensitizing effects induced potent CRT exposure and activation of immune effector cells (including DC, CD4+ T, CD8+ T, and NK cells), which further inhibited the growth of primary and distant tumors. Moreover, combining Hf-based nMOFs with aPD-L1 clearly improved the immune response and almost eradicated primary and abscopal tumors188.
Radiotherapy achieves deeper tissue penetration than photo irradiation. The immune elicitation mediated by radiotherapy is relatively well documented compared with photo irradiation. However, the tumor's hypoxic microenvironment is an obstacle for radiotherapy to produce enough ROS. Moreover, radiotherapy resistance mechanisms in cancer cells, like up-regulating DNA repair enzyme, reduce the anticancer outcome of radiotherapy. Besides, it is necessary to carefully evaluate the anti-cancer effect brought by the enhanced immune response and the cost of nanomaterial. Moreover, the general limitations of nanomaterials cannot be ignored in radiotherapy.
3.2.4. Other functional nanomaterials
In addition to improving the efficiency of PTT, PDT, and radiotherapy, nanomaterials also present other functionalities, including chemodynamic effect, inducing ferroptosis and magnetic hyperthermia (MHT) effects. The chemodynamic effect is mainly derived from the Fenton reaction, which primarily describes the reaction of Fe2+ with H2O2 to produce Fe3+ and hydroxyl radicals (·OH) with high oxidizing ability. High concentrations of ·OH are lethal to cancer cells. Except for ferrous ions, other cations, such as Cu2+, Mn2+, V2+, and Cr4+, are capable of catalyzing Fenton-like reactions195. Compared to stable Fe3O4 nanoparticles that only utilize surficial Fe2+ to catalyze the Fenton reaction, amorphous Fe nanoparticles (AFeNPs), which would be more efficiently ionized under an acidic tumor microenvironment and release active Fe2+, enhanced the inhibition of cancer development196. Although H2O2 is present at much higher levels in cancer cells compared with in normal cells, endogenous H2O2 seems not enough to mediate a lethal chemodynamic effect on cancer cells in vitro. For many CDT therapies, the supply of additional H2O2 is necessary to induce cancer cell death and tumor regression197. Therefore, CDT is usually combined with other cancer therapies. For example, our group36 developed a Z-scheme heterojunction with a FeS2 core and Fe2O3 shell. The novel 2D thermally oxidized pyrite nanosheets (TOPY NSs) were able to kill cancer cells through glutathione consumption, the Fenton reaction, heterojunction-mediated PDT, and PTT. Moreover, TOPY NSs almost eradicated HepG2 xenograft tumors under irradiation at 650 and 808 nm. As CDT generates ROS as PDT does, it may induce ICD in cancer cells as well. However, the interaction between CDT and the immune system requires further study.
Ferroptosis is a new type of programmed cell death. Under the action of divalent iron or ester oxygenase, it catalyzes the high expression of unsaturated fatty acids on the cell membrane to cause lipid peroxidation, thereby inducing cell death. The ROS production in ferroptosis and non-apoptotic nature of ferroptosis imply its ability to modulate the immune system. Ferroptocide, a newly identified natural product, was proved to induce ferroptosis via covalent conjugation on thioredoxin, a critical component of the antioxidant system. The ferroptocide induced 40% tumor retardation in 4T1 bearing BALB/c mice but had rare inhibition in 4T1 bearing nude mice, which suggested the participation of T and B cells in ferroptocide meditated in vivo tumor inhibition198. The nanomaterials inducing chemodynamic effects could start up the ferroptosis process, because Fenton reaction can initiate liposome peroxidation199. However, several nanomaterials that not contain divalent iron show the potential to induce ferroptosis. PEGylated untrasmall silica nanoparticles (about 6 nm) were demonstrated to induce ferroptosis in nutrient-deprived cancer cells. The cell death induced by silica nanoparticles was inhibited by treatment of scavengers of lipid ROS (liproxstatin-1) or glutathione repletion via the addition of glutathione or N-acetylcysteine (NAC). Moreover, intravenous injection of the silica particles (12 nmol per dose) significantly inhibited the growth of 786-O and HT-1080 xenograft tumors in nude mice. And intraperitoneal doses of liproxstatin-1 significantly reduced the particle induced tumor inhibition200. Recently, arginine-rich manganese silicate nanobubbles (AMSNs) have been proved to induce ferroptosis by highly efficiently depletion of glutathione (GSH) and thereby inducing the inactivation of glutathione-dependent peroxidases 4 (GPX4). Manganese in AMSNs mediated the depletion of GSH, and ariginine modification provide tumor targeting ability. The AMSNs induced tumor inhibition by ferroptosis mechanism in vitro and in vivo201. Recently, a hybrid core‒shell vesicles (HCSVs) was constructed by utilizing ascorbic acid (AA) as core and PLGA as shell decorated with iron oxide nanocubes (IONCs). HCSVs induced the exposure of calreticulin via Fenton reaction and ferroptosis-like cell death after magnetic field treatment. Moreover, intratumoral injection of HCSVs boosted significant proliferation of splenocytes, DC activation in inguinal LNs, and T cells activation in tumors and LN202.
MHT mainly depends on superparamagnetic materials, which can achieve magnetic targeting and transform electromagnetic to thermal energy under an alternating magnetic field. Compared to PDT and PTT, MHT has a deeper penetration capacity and is associated with lower toxicity to surrounding tissue203. Fe3O4 nanoparticles are the most widely applied superparamagnetic nanomaterials, which can heat tumors above 43 °C and trigger the activation and proliferation of CD4+ and CD8+ T cells. The inhibition of distal and secondary tumors by Fe3O4 nanoparticles suggests the involvement of the immune system204. Utilizing Fe nanoparticles (FeNPs) as MHT agents, local administration of PLGA-R837 and systemic administration of aCTLA-4 checkpoint blockade were found to efficiently prevent cancer metastasis205.
In conclusion, functional nanomaterials that induce photothermal, photodynamic, radiosensitizing, chemodynamic, ferroptotic and magnetic hyperthermia effect, show great potentials to induce the ICD process of cancer cells in which dying cancer cells could release tumor antigens and present immunostimulatory signals to activate the APCs. The potential ICD nano-inducers are summarized in Table 4. For many functional nanomaterials, whether they induce ICD of cancer cells and whether ICD strength is sufficient to restart cancer–immunity cycle remains to be studied. In addition, a small number of reports on functional nanomaterials inducing ICD are difficult to explain that all functional nanomaterials of the same type can induce ICD. However, lack of standardized characterization and research on the ICD process mediated by nanomaterials impede the rational optimization of ICD nano-inducers.
Table 4.
Potential ICD nano-inducers.
| Nanomaterial | Component | Function | ICD biomarker | Ref. |
|---|---|---|---|---|
| FAL-ICG-HAuNS | ER-targeting pardaxin (FAL) peptides-modified, ICG-conjugated hollow gold nanospheres | PTT PDT |
ROS generation and CRT exposure Increased CD8+ T cells, reduced CD4+ T cells and Tregs in tumor, increased TNF-α and IFN-γ in blood |
144 |
| Prussian blue nano-particles | Coordination compound between Fe2+, Fe3+ and CN | PTT | ATP release, HMGB1 release and CRT exposure, vaccination mediated prevention of tumor challenge | 158 |
| Single-walled nanotubes (SWNTs) | PEG-grafted amphiphilic polymer-decorated SWNTs | PTT | DC maturation and the expression of pro-inflammatory cytokines, no direct ICD biomarkers are evaluated, primary and metastatic tumor inhibition through combination of CTLA-4 mAbs | 159 |
| Fe-TBP MOF | Solvothermal synthesis from [Fe3O(OAc)6(H2O)3] OAc (OAc = acetate) and H4TBP |
PDT | Exposure of calreticulin, proliferation of tumor-antigen Specific cytotoxic T cells and CD4+ T cells, inhibition of distal tumors, better anticancer outcome combined with α-PD-L1 |
180 |
| PGA@glucose oxidase@carbon dot nanoparticles | Poly (γ-glutamic acid)@glucose oxidase@carbon dot nanoparticles | PDT PTT |
No direct ICD biomarkers are evaluated, inhibition of distal tumors, better anticancer outcome combined with α-PD-L1 | 181 |
| Hf-based nMOF | Hf6-DBA with a formula of Hf6(μ3-O)4 (μ3-OH)4 (DBA)6 and Hf12-DBA with a formula of Hf12(μ3-O)8 (μ3-OH)8 (μ2-OH)6 (DBA)9 | RT | Exposure of calreticulin, release of HMGB1, proliferation of CD8+ and CD4+ T cells, inhibition of distal tumors, prevention of tumor challenge | 188 |
| A hybrid core‒shell vesicle (HCSVs) | Ascorbic acid (AA) in the core and poly (lactic-co-glycolic acid) shell incorporating iron oxide nanocubes (IONCs) | CDT ferroptosis | CRT exposure, GPX4 downregulation, the maturation of DCs, proliferation of CD8+ T cells in DLN, inhibition of primary tumor | 202 |
| Ferrihydrite nanoparticles | PEGylation of ferrihydrite nanoparticles | Ferroptosis | Glutathione peroxidase 4 (GPX4) inhibition, increasing of ROS level, TAM polarization from M2 to M1, inhibition of tumor metastasis | 199 |
| Iron oxide nanoparticles | Iron oxide nanoparticles | MHT | Activation of DCs and CD8+ T cells in LN, production of cytokines and chemokines, inhibition of distal tumor, prevention of tumor challenge | 204 |
| FeNPs | Pure iron nanoparticles functionalized with polyethylene glycol (PEG)/dopamine (DA) -cografted polymer | MHT | Increase of CD8+ T cells in secondary tumor, slight inhibition of secondary tumor, better anticancer effect after combination with R837 and α-CTLA-4 | 205 |
3.3. Immunomodulatory adjuvants
Adjuvants are essential components of modern vaccines, which strengthen and/or shape the immune response against pathogens or malignancies. In the field of cancer vaccines, it is critical to trigger potent cellular immunity against tumor antigens. Nanomaterials with various sizes, shapes, and surface modifications may function as adjuvants via the following mechanisms206: delivery and consistent release of antigens, targeting APCs in a passive or active way, the cytosolic delivery of antigens to enhance the cross-presentation of APCs, and modulate the immune response. The use of nanomaterials as delivery platforms to transport tumor antigens to immune organs and the cytoplasm of DCs is described in Section 3.1.3. Herein, we will discuss the immunomodulatory effects of nanomaterials and their application in cancer immunotherapy.
The immunomodulatory effects of nanomaterials mainly include inflammasome activation, complement system activation, and the recruitment of immune cells207. Alum adjuvants, which are widely used clinically, have been shown to induce NLPR3 inflammasome activation. Upon exposure to danger, such as pathogens, DAMPs or PAMPs, NLPR3, and other related proteins will self-interact to form high-molecular-weight complexes that induce the autocleavage of caspase-1. This further regulates the secretion of IL-β and IL-18208. In addition to alum, numerous nanomaterials have been shown to induce NLRP3 inflammasome activation, including carbon black nanoparticles209, SiO2210,211 and TiO2 nanoparticles212. Inflammasomes are activated in response to the danger signal provided by nanoparticles, such as lysosomal destabilization and ROS production. Complement proteins exist in the serum and tissue fluid in humans and vertebrates. Complements can be activated by antigen–antibody complexes or microorganisms, leading to the lysis or phagocytosis of pathogenic microorganisms. Recent studies have demonstrated that opsonin, a type of complement, could adsorb onto nanoparticles to mediate the recognition and endocytosis of particles by phagocytic cells. After ingestion by phagocytes, nanomaterials may induce phagocytes to synthesize and secrete proinflammatory cytokines and chemokines to recruit immune cells, such as macrophages, DCs, and T cells. For example, in 2010, Yang et al.213 reported an [Gd@C82(OH)22]n nanoparticles, which induced cytokine production (including IL-12p70), co-stimulatory molecule expression, and MHC molecule expression in DCs. Furthermore, mice immunized with OVA and [Gd@C82(OH)22]n presented a robust Th1 immune response. In 2017, Luo et al.214 constructed novel polymeric PC7A nanoparticles, which were ultra-pH sensitive and had diameters of 20–50 nm for lymph node targeting. Using PC7A as a carrier without other immunostimulatory agents to deliver tumor antigens to lymph nodes potently inhibited the growth of melanoma and colon cancer. These PC7A nanoparticles were shown to regulate the immune response, including the promotion of DC maturation through the STING pathway.
The immunoregulatory function of nanomaterials is usually ignored in application of nanomaterials as drug delivery. Recently, consensus that lots of nanomaterials affect immune system has been reached. The influence on immune response of nanomaterials is diverse. Although we mainly introduced the immunostimulatory effect of nanomaterials, other nanomaterials that provoked inflammation or immunosuppression were reported. Large amount of immune evaluation of nanomaterials should be accumulated. The relationship between structure of nanomaterials and immunoregulatory function remains to be uncovered.
3.4. Combinatorial cancer immunotherapy enabled by nanomaterials
Based on the cancer–immunity cycle, successful cancer immunotherapies should focus on the following aspects.
-
(1)
Making tumor antigens available for DCs. Apoptosis of cancer cells removes most antigens, and in situ ICD or exogenous cancer vaccines can retain or provide tumor antigens.
-
(2)
Enhancing the immunogenicity of tumor antigens. Usually, tumor antigens have relatively low immunogenicity, making it difficult to trigger an antigen-specific immune response. Therefore, immunostimulatory agents such as CpG-ODN, R837, CDG, and Pam3CSK4 are necessary to enhance the immunogenicity of tumor antigens and increase the antigen-uptake of DCs.
-
(3)
Co-delivery of tumor antigens and immunostimulatory agents to DCs. The simultaneous uptake of both tumor antigens and immunostimulatory agents by APCs will enhance the strength of antigen-specific immune responses.
-
(4)
Enhancing the cross-presentation of tumor antigens by DCs. Targeting lymph nodes and the cytoplasmic delivery of tumor antigens in DCs are essential for enhancing cross-presentation efficiency, which is directly related to the strength of the cellular immune response.
-
(5)
Overcoming the immunosuppressive microenvironment of cancer tissues. After activating and recruiting antigen-specific CD8+ T cells, the immunosuppressive microenvironment tends to trigger the dysfunction or exhaustion of CD8+ T cells. Therefore, ICB inhibitors, IDO inhibitors, or other agents that can overcome the immunosuppressive microenvironment, have potential in cancer immunotherapy.
-
(6)
Combination therapies may lead to additional or synergistic anticancer effects. However, the toxicity of combination therapies should be carefully evaluated and controlled.
Combination immunotherapies that act simultaneously on the different components detailed above, or on different steps of the cancer–immunity cycle, have an increased possibility of triggering robust antigen-specific cellular immunity. Nanomaterials, which act as carriers, ICD inducers, and immunomodulatory agents, have the potential to integrate different anticancer functions into a single platform and to achieve relatively efficient anticancer outcome.
Initially, nanomaterials were used to co-deliver tumor antigens and immunostimulatory agents for the development of potent anticancer vaccines in combination immunotherapies. For example, Xu et al.127 in 2013 constructed lipid-calcium-phosphate (LCP) nanoparticles modified with mannose to target DCs, which were co-loaded with tyrosinase-related protein 2 (Trp 2) peptide as melanoma antigens and CPG ODN as an adjuvant.
More recently, nanomaterials equipped with inducers of ICD and immunostimulatory agents have been used in combination with ICB therapies. For example, in 2017, Chen et al.145 prepared a nanosystem composed of PLGA loaded with ICG and R837. ICG generated a strong photothermal effect, which mediated the release of tumor antigens. R837, a robust TLR-7 agonist, potently activated DCs. Utilization of PLGA to co-deliver ICG and R837 was shown to induce the strongest effect on DC maturation and TNF-α production under 808 nm irradiation. Following combination treatment with aCTLA-4, PLGA-ICG-R837 with laser irradiation almost eradicated 4T1 and CT26 distant xenograft tumors, and efficiently inhibited the metastasis and recurrence of tumors. The inhibition of cancer growth, metastasis, and recurrence was enhanced by the activation and proliferation of CD4+ and CD8+ T cells, and a reduction of Treg cells. Thereafter, Xu et al.215 constructed a multifunctional nanosystem combining upconversion nanoparticles (UCNPs), Ce6, R837, and aCTLA-4. The UCNPs were modified with amphiphilic polymers to load Ce6 and R837. UCNPs were able to absorb light at 980 nm and emit light at 550 nm to activate Ce6 and produce ROS. UCNP-Ce6-R837 was shown to potently induce DC maturation and pro-inflammatory cytokine secretion under 908 nm irradiation. Furthermore, UCNP-Ce6-R837 combined with systemic administration of aCTLA-4 robustly inhibited the growth of primary, distant, and metastatic tumors (Fig. 10).
Figure 10.
NIR triggered PDT combinatorial therapy with immune checkpoint blockade. (A) Schematic showing the anticancer function of UCNP-Ce6-R837. (B) tumor volume of primary and distant CT26 tumors following the indicated treatment; the level of CD8+ CTL cells (C), Treg cells (D) and the CD8+ CTL/Treg ratio (E) in distant tumors, and IFN-γ cytokine levels in sera (F) from mice following the indicated treatment. Reprinted with the permission from Ref. 215. Copyright © 2017 ACS Publishing Group.
Furthermore, Chen et al.216 in 2019 combined enhanced radiotherapy, immunostimulatory agents, and aCTLA-4. PLGA was used to encapsulate R837 and catalase, which could convert tumor-rich H2O2 to O2, thereby ameliorating the hypoxic environment of cancer tissues. PLGA-R837@cat nanoparticles under X-ray irradiation induced ICD and reverted the immunosuppressive environment, which further contributed to the therapeutic efficacy against cancer growth, metastasis, and recurrence. Recently, Patel et al.217 constructed BNP nanoparticles by using PC7A nanoparticles as carriers to load CpG and bacterial membranes, meanwhile capturing tumor antigens released during radiation via a maleimide modification on bacterial membranes. This combination enhanced the antigen uptake and cross-presentation of DCs and robustly activated effector T cells to induce clear tumor regression (Fig. 11). Qin et al.148 constructed cyclodextrin (CD)-based gel system, DOX/ICG/CpG-P-ss-M/CD, which was load with DOX, ICG and CpG-P-ss-M. CpG-P-ss-M was indicated polyamidoamine (PAMAM) dendrimer decorated with reductive 4-aminophenyl-α-D-mannopyranoside (MAN)–polyethylene glycol (PEG) chain and CpG. The DOX/ICG/CpG-P-ss-M/CD released tumor antigens by DOX treatment and PTT, meanwhile capturing the tumor antigens by CpG-P-ss-M nanoparticle to form in situ vaccine for inducing antigen uptake of DCs. Furthermore, DOX/ICG/CpG-P-ss-M/CD system induced the activation of DCs in spleen and the proliferation of CD8+ T cells, and eventually inhibited the growth of primary and distant tumors.
Figure 11.
In situ vaccine elicited by combined RT + BNP. Reprinted with the permission from Ref. 217. Copyright © 2019 John Wiley and Sons Group.
In addition, nanomaterials that induce ICD are combined with immunostimulants. For example, Li et al.218 developed an organic semiconducting pro-nano-stimulant (OSPS) with a semiconducting polymer nanomaterial as the core and an immunostimulant (NLG919) as the coating layer (Fig. 12). This OSPS showed excellent photothermal and photodynamic efficiency, which ensured the potent activation of CD8+ T cells and inhibition of primary and distant tumors (Fig. 13).
Figure 12.
The scheme of OSPS mediated combinatory cancer therapy. Reprinted with the permission from Ref. 218. Copyright © 2019 John Wiley and Sons Group.
Figure 13.
Anticancer immune response induced by OSPS. (A) OSPS-mediated tumor inhibition and lung metastasis. (B) Growth curves of primary tumors in 4T1 tumor-bearing mice. (C) Growth curves of distant tumors in 4T1 tumor-bearing mice. (D) H&E staining of lung metastasis in 4T1 tumor-bearing mice. (E) Number of metastatic nodules in 4T1 tumor-bearing mice (F) The Kyn/Trp ratio in primary tumors in 4T1 tumor-bearing mice. (G) Population of CD3+CD8+ T cells in distant tumors. (H) IFN-γ producing T-cells in distant tumors. (I) Treg cells in distant tumors. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, n = 5. CSPN, nanoparticles without NLG919. Reprinted with the permission from Ref. 218. Copyright © 2019 John Wiley and Sons Group.
4. Conclusions
The interaction of cancer with the immune system is complicated. Tumor formation usually requires long-term immune screening and an immune-tolerant microenvironment. Therefore, an individual's immune system may not be sufficient by itself to eliminate cancer cells. The use of external agents that reinitiate the cancer–immunity cycle at one or multiple steps is the basis of cancer immunotherapy. Restoring the cancer–immunity cycle includes the following key steps: making tumor antigens available for APCs, inducing the maturation of APCs, promoting the cross-presentation of APCs, and ameliorating the immunosuppressive microenvironment. Targeting multiple key points simultaneously is an effective means to overcome compensation mechanisms and promote potent anticancer immunity.
Nanomaterials are used widely in the fields of diagnostics and drug delivery because of their controllable size, shape, and surface properties. Development and in-depth research on nanomaterials show that nanomaterials can not only be used for drug delivery, but also have versatile properties, such as their photothermal effect, photodynamic effect, ability to enhance radiotherapy, magnetic hyperthermia effect, and immunomodulatory effect. These properties allow nanomaterials to be used as a comprehensive platform to integrate diverse drugs or strategies focusing on different steps of cancer–immunity cycle, which promotes additional, or even synergistic anticancer outcomes. As excellent drug delivery platform, nanomaterials have the capacity to load and sustainably release of multiple immunomodulators simultaneously to cancer tissues or lymph nodes to potently activate the different processes of cancer–immunity cycle, like supply of tumor antigens, activation of APCs and inhibition of immune checkpoint or immunosuppressive regulatory immune cell. As functional nanomaterials, nanomaterials are able to trigger the ICD process of cancer cells, thereby inducing the uptake of immunogenic tumor antigens by APCs and the activation of APCs. As for immunomodulators, nanomaterials could function as adjuvant and directly induce the activaiton of inflammasome and production of stimulatory cytokines for activation of immune system and ease of immunosuppression. The three faces of nanomaterials make them excellent candidate for manually regulating cancer–immunity cycle. Moreover, nanomaterials can possess multiple roles at the same time, for example, functional nanomaterials are able to deliver small molecular immunomodulators.
5. Challenges and future perspectives
Nanomaterials have unique advantages in promoting cancer immunotherapy. In recent years, many basic scientific studies have been performed in this field. However, nanomaterial-assisted immunotherapy is still rarely used clinically. The main limitations include unclear mechanisms underlying the toxicity of nanomaterials, pharmacokinetics, and interactions with the immune system. Although the cytotoxicity and acute toxicity of nanomaterials have been systematically studied, the chronic toxicity of nanomaterials is often not addressed. Some nanomaterials easily accumulate in tissues, such as the liver and lungs, causing chronic inflammation. The pharmacokinetics of nanomaterials also need to be studied. For example, for how long are they retained in the body? Will they cause damage to metabolic organs? In addition, the relationship between nanomaterials and immunity remains unclear. Some nanomaterials promote inflammation, and some suppress immune responses. Therefore, each nanomaterial should be systematically evaluated to determine its toxicity, pharmacokinetics, and mechanisms of immune regulation before application. In addition, the stability of nanomaterials is also an important aspect that restricts their clinical applications. Many nanomaterials, especially inorganic nanomaterials, have poor stability in saline or serum and aggregate easily, which is unfavorable for clinical applications. Besides, the protein corona of nanomaterials has significant effects on the drug releasing capacity, stability, tissue distribution and pharmacokinetics. The different species of nanomaterials and diverse surface modifications on nanomaterials lead to different components of protein corona.
Except for these general limitations of nanomaterials, ICD nano-inducers possess their own problems. The most important issue of ICD nano-inducers is lack of standardized characterization and research on the ICD process mediated by nanomaterials. Currently, lots of nanomaterials-based PTAs, PSs and radiosensitizers are combined with immunotherapies, like ICBs, IDO inhibitors or immunostimulatory molecules. Although, these combinations achieve enhanced proliferation of CD8+ T cells and significant inhibition of primary, distant and metastatic tumors, the immune related mechanisms are roughly studied. It's hard to differentiate the main cause of the activation of anticancer immune response. Is it derived from the ICD process induced by functional nanomaterials or because of the introduction of ICBs or immunostimulatory molecules? We are cautious about whether all nanomaterials-based PTAs or PSs can induce ICD. Detailed experimental evaluation needs to be performed to explain the structure–activity relationship of nanomaterials and ICD inducing capacity. Besides, currently designed nanomaterials targeting on several steps of cancer–immunity cycle often need complex composition and several steps of modifications or drug loading, which seriously hinders the clinical application of these systems. The multifunctional nanomaterials that could simultaneously possess better cancer targeting ability, ICD inducing capacity and immune regulatory potential should be identified and designed.
Increased knowledge of cancer immunology has resulted in the availability of several immunotherapeutic anticancer strategies, such as CAR-T and ICB therapy. However, our understanding of the immune environment in cancer tissues, and the relationship between the immune system and cancer remain incomplete. The low efficiency of CAR-T in solid tumors and the low response rate of ICB therapy remain to be solved. The identification and classification of cancer markers is likely to promote the use of cancer immunotherapy, for example, detecting the level of PD-L1 expression in cancer cells to determine the suitability of PD-L1 antibody treatments. Even though PD-L1 or PD-1 positivity is necessary for a good prognosis in response to ICB therapy, it is not a sufficient condition to guarantee its antitumor outcome. Cancer is commonly divided into “cold” or “hot” types. Hot tumors are rich in TILs, which are important biomarkers for ICB therapy. Activating these TILs may efficiently eliminate cancer cells. However, some studies have found that most of the TILs act on viruses, but not cancer cells219. These limitations of cancer immunotherapies highlight the need for further research, and emphasize that cancer immunotherapy is not a panacea. Some malignancies have achieved the ability to ignore immune killing during the long-term battle with the immune system. For example, the immune system is unable to identify and kill cancer cells that lack MHC I and meanwhile overexpress NK inhibitory biomarkers (such as HLA-E). Therefore, rational combinations of cancer immunotherapy with other traditional cancer theranostics, and the artificial transformation of effector immune cells may provide an efficient method of treating cancer.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (22007106, 31800842, 31922042 and 81771966), Technology & Innovation Commission of Shenzhen Municipality (JCYJ20180507181654186 and JCYJ20170818162637217, China), the Fundamental Research Funds for the Central Universities (2020-RC320-002 and 2019PT320028), and the China Postdoctoral Science Foundation (2019TQ0396, China).
Author contributions
Under the supervision of Prof. Lin Mei and Prof. Dunwan Zhu, Qianqian Li summarized related literatures and wrote this review. Zhaoqing Shi, Zengzeng Wei and Fan Zhang helped investigate the literatures and checked the spelling and formatting errors of this review. All authors read and contributed to the manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Dunwan Zhu, Email: zhudunwan@bme.pumc.edu.cn.
Lin Mei, Email: meilin@bme.pumc.edu.cn.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. Ca - Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Liang C., Xu L., Song G., Liu Z. Emerging nanomedicine approaches fighting tumor metastasis: animal models, metastasis-targeted drug delivery, phototherapy, and immunotherapy. Chem Soc Rev. 2016;45:6250–6269. doi: 10.1039/c6cs00458j. [DOI] [PubMed] [Google Scholar]
- 3.Mellman I., Coukos G., Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P., Allison J.P. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 6.Hodi F.S., O'Day S.J., McDermott D.F., Weber R.W., Sosman J.A., Haanen J.B., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.June C.H., O'Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 9.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newick K., O'Brien S., Moon E., Albelda S.M. CAR T cell therapy for solid tumors. Annu Rev Med. 2017;68:139–152. doi: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 12.Martins F., Sofiya L., Sykiotis G.P., Lamine F., Maillard M., Fraga M., et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16:563–580. doi: 10.1038/s41571-019-0218-0. [DOI] [PubMed] [Google Scholar]
- 13.Schumacher T.N., Schreiber R.D. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 14.Ott P.A., Hu Z., Keskin D.B., Shukla S.A., Sun J., Bozym D.J., et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–221. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan T.A., Yarchoan M., Jaffee E., Swanton C., Quezada S.A., Stenzinger A., et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30:44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simoni Y., Becht E., Fehlings M., Loh C.Y., Koo S.L., Teng K.W.W., et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557:575–579. doi: 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 18.Pigeon J.C., Jegede O., Mahoney K.M., Moreira R.B., Novak J., Conen H. Impact of immune checkpoint protein expression in tumor cells and tumor infiltrating CD8+ T cells on clinical benefit from PD-1 blockade in metastatic clear cell renal cell carcinoma (mccRCC) J Clin Oncol. 2017;35:477. [Google Scholar]
- 19.Whiteside T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen D.S., Mellman I. Oncology meets immunology: the cancer‒immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Manieri N.A., Chiang E.Y., Grogan J.L. TIGIT: a key inhibitor of the cancer immunity cycle. Trends Immunol. 2017;38:20–28. doi: 10.1016/j.it.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Ellmark P., Mangsbo S.M., Furebring C., Totterman T.H., Norlen P. Kick-starting the cancer‒immunity cycle by targeting CD40. OncoImmunology. 2015;4:1011484. doi: 10.1080/2162402X.2015.1011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brahmer J.R., Lacchetti C., Schneider B.J., Atkins M.B., Brassil K.J., Caterino J.M., et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y.H., Zang X.Y., Wang J.C., Huang S.S., Xu J., Zhang P. Diagnosis and management of immune related adverse events (irAEs) in cancer immunotherapy. Biomed Pharmacother. 2019;120:109437. doi: 10.1016/j.biopha.2019.109437. [DOI] [PubMed] [Google Scholar]
- 25.Irvine D.J., Dane E.L. Enhancing cancer immunotherapy with nanomedicine. Nat Rev Immunol. 2020;20:321–334. doi: 10.1038/s41577-019-0269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X., Wang X., Ito A. Tailoring inorganic nanoadjuvants towards next-generation vaccines. Chem Soc Rev. 2018;47:4954–4980. doi: 10.1039/c8cs00028j. [DOI] [PubMed] [Google Scholar]
- 27.Park W., Heo Y.J., Han D.K. New opportunities for nanoparticles in cancer immunotherapy. Biomater Res. 2018;22:24. doi: 10.1186/s40824-018-0133-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song W., Musetti S.N., Huang L. Nanomaterials for cancer immunotherapy. Biomaterials. 2017;148:16–30. doi: 10.1016/j.biomaterials.2017.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang J., Nakamura H., Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery factors involved and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress challenges and opportunities. Nat Rev Cancer. 2017;17:20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sindhwani S., Syed A.M., Ngai J., Kingston B.R., Maiorino L., Rothschild J., et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19:566–575. doi: 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 32.Price L.S.L., Stern S.T., Deal A.M., Kabanov A.V., Zamboni W.C. A reanalysis of nanoparticle tumor delivery using classical pharmacokinetic metrics. Sci Adv. 2020;6 doi: 10.1126/sciadv.aay9249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng W., Nie J., Gao N., Liu G., Tao W., Xiao X., et al. A multifunctional nanoplatform against multidrug resistant cancer: merging the best of targeted chemo/gene/photothermal therapy. Adv Funct Mater. 2017;27:1704135. [Google Scholar]
- 34.Shi Z.Q., Li Q.Q., Mei L. pH-Sensitive nanoscale materials as robust drug delivery systems for cancer therapy. Chin Chem Lett. 2020;31:1345–1356. [Google Scholar]
- 35.Ye X., Liang X., Chen Q., Miao Q., Chen X., Zhang X., et al. Surgical tumor-derived personalized photothermal vaccine formulation for cancer immunotherapy. ACS Nano. 2019;13:2956–2968. doi: 10.1021/acsnano.8b07371. [DOI] [PubMed] [Google Scholar]
- 36.Pan C., Ou M., Cheng Q., Zhou Y., Yu Y., Li Z., et al. Z-Scheme heterojunction functionalized pyrite nanosheets for modulating tumor microenvironment and strengthening photo/chemodynamic therapeutic effects. Adv Funct Mater. 2019;30:1906466. [Google Scholar]
- 37.Zhang F., Li F., Lu G.H., Nie W., Zhang L., Lv Y., et al. Engineering magnetosomes for ferroptosis/immunomodulation synergism in cancer. ACS Nano. 2019;13:5662–5673. doi: 10.1021/acsnano.9b00892. [DOI] [PubMed] [Google Scholar]
- 38.Fang R.H., Kroll A.V., Zhang L. Nanoparticle-based manipulation of antigen-presenting cells for cancer immunotherapy. Small. 2015;11:5483–5496. doi: 10.1002/smll.201501284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantovani A., Romero P., Palucka A.K., Marincola F.M. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371:771–783. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 40.Grivennikov S.I., Greten F.R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunn G.P., Bruce A.T., Ikeda H., Old L.J., Schreiber R.D. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 42.Zitvogel L., Apetoh L., Ghiringhelli F., Andre F., Tesniere A., Kroemer G. The anticancer immune response: indispensable for therapeutic success?. J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demaria O., Cornen S., Daeron M., Morel Y., Medzhitov R., Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574:45–56. doi: 10.1038/s41586-019-1593-5. [DOI] [PubMed] [Google Scholar]
- 44.Cerovic V., Houston S., Westlund J., Utriainen L., Davison E., Scott C., et al. Lymph-borne CD8 alpha+ dendritic cells are uniquely able to cross-prime CD8+ T cells with antigen acquired from intestinal epithelial cells. Mucosal Immunol. 2014;8:38–48. doi: 10.1038/mi.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J.A., Li Z.R., Huang H., Yang Y.Z., Ding Q.A., Mai J.H., et al. Improved antigen cross-presentation by polyethyleneimine-based nanoparticles. Int J Nanomed. 2011;6:77–84. doi: 10.2147/IJN.S15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beatty G.L., Gladney W.L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin Cancer Res. 2015;21:687–692. doi: 10.1158/1078-0432.CCR-14-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gavin D., Allen B., Hiroaki I., Lloyd O., Robert S. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 48.DuPage M., Mazumdar C., Schmidt L.M., Cheung A.F., Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482:405–409. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fukuda M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996;56:2237–2244. [PubMed] [Google Scholar]
- 50.Paszek M.J., DuFort C.C., Rossier O., Bainer R., Mouw J.K., Godula K., et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 2014;511:319–325. doi: 10.1038/nature13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumoto A., Cabral H., Sato N., Kataoka K., Miyahara Y. Assessment of tumor metastasis by the direct determination of cell-membrane sialic acid expression. Angew Chem Int Ed. 2010;49:5494–5497. doi: 10.1002/anie.201001220. [DOI] [PubMed] [Google Scholar]
- 52.Kufe D.W. Mucins in cancer: function, prognosis and therapy. Nat Rev Canc. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J.Y., Tang Y.A., Huang S.M., Juan H.F., Wu L.W., Sun Y.C., et al. A novel sialyltransferase inhibitor suppresses FAK/paxillin signaling and cancer angiogenesis and metastasis pathways. Cancer Res. 2011;71:473–483. doi: 10.1158/0008-5472.CAN-10-1303. [DOI] [PubMed] [Google Scholar]
- 54.Xiao H., Woods E.C., Vukojicic P., Bertozzi C.R. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc Natl Acad Sci U S A. 2016;113:10304–10309. doi: 10.1073/pnas.1608069113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paulson K.G., Tegeder A., Willmes C., Iyer J.G., Afanasiev O.K., Schrama D., et al. Downregulation of MHC-I expression is prevalent but reversible in merkel cell carcinoma. Canc Immunol Res. 2014;2:1071–1079. doi: 10.1158/2326-6066.CIR-14-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angell T.E., Lechner M.G., Jang J.K., LoPresti J.S., Epstein A.L. MHC class I loss is a frequent mechanism of immune escape in papillary thyroid cancer that is reversed by interferon and selumetinib treatment in vitro. Clin Cancer Res. 2014;20:6034–6044. doi: 10.1158/1078-0432.CCR-14-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 58.Kochan G., Escors D., Breckpot K., Guerrero-Setas D. Role of non-classical MHC class I molecules in cancer immunosuppression. OncoImmunology. 2013;2 doi: 10.4161/onci.26491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kornmann M., Ishiwata T., Kleeff J., Beger H.G., Korc M. Fas and Fas-ligand expression in human pancreatic cancer. Ann Surg. 2000;231:368–379. doi: 10.1097/00000658-200003000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohta T., Elnemr A., Kitagawa H., Kayahara M., Takamura H., Fujimura T., et al. Fas ligand expression in human pancreatic cancer. Oncol Rep. 2004;12:749–754. [PubMed] [Google Scholar]
- 61.Mariathasan S., Turley S.J., Nickles D., Castiglioni A., Yuen K., Wang Y., et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544–548. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamichhane P., Karyampudi L., Shreeder B., Krempski J., Bahr D., Daum J., et al. IL10 release upon PD-1 blockade sustains immunosuppression in ovarian cancer. Cancer Res. 2017;77:6667–6678. doi: 10.1158/0008-5472.CAN-17-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tauriello D.V.F., Palomo-Ponce S., Stork D., Berenguer-Llergo A., Badia-Ramentol J., Iglesias M., et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 64.Munn D.H., Mellor A.L. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uyttenhove C., Pilotte L., Theate I., Stroobant V., Colau D., Parmentier N., et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 66.Hodge D.R., Hurt E.M., Farrar W.L. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 67.Kumari N., Dwarakanath B.S., Das A., Bhatt A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016;37:11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 68.Tsukamoto H., Fujieda K., Miyashita A., Fukushima S., Ikeda T., Kubo Y., et al. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78:5011–5022. doi: 10.1158/0008-5472.CAN-18-0118. [DOI] [PubMed] [Google Scholar]
- 69.Llanes-Fernandez L., Alvarez-Goyanes R.I., Arango-Prado M.D., Alcocer-Gonzalez J.M., Mojarrieta J.C., Perez X.E., et al. Relationship between IL-10 and tumor markers in breast cancer patients. Breast. 2006;15:482–489. doi: 10.1016/j.breast.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 70.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–416. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 71.Rosenberg S.A. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–5458. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zitvogel L., Tahara H., Robbins P.D., Storkus W.J., Clarke M.R., Nalesnik M.A., et al. Cancer immunotherapy of established tumors with IL-12. Effective delivery by genetically engineered fibroblasts. J Immunol. 1995;155:1393–1403. [PubMed] [Google Scholar]
- 73.Tahara H., Lotze M.T. Antitumor effects of interleukin-12 (IL-12): applications for the immunotherapy and gene therapy of cancer. Gene Ther. 1995;2:96–106. [PubMed] [Google Scholar]
- 74.Steel J.C., Waldmann T.A., Morris J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beatty G.L., Paterson Y. Regulation of tumor growth by IFN-gamma in cancer immunotherapy. Immunol Res. 2001;24:201–210. doi: 10.1385/IR:24:2:201. [DOI] [PubMed] [Google Scholar]
- 76.Dai S., Wei D., Wu Z., Zhou X., Wei X., Huang H., et al. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev. 2002;188:147–154. doi: 10.1034/j.1600-065x.2002.18813.x. [DOI] [PubMed] [Google Scholar]
- 78.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 79.Alegre M.L., Frauwirth K.A., Thompson C.B. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 80.Chan D.V., Gibson H.M., Aufiero B.M., Wilson A.J., Hafner M.S., Mi Q.S., et al. Differential CTLA-4 expression in human CD4+versus CD8+ T cells is associated with increased NFAT1 and inhibition of CD4+ proliferation. Gene Immun. 2014;15:25–32. doi: 10.1038/gene.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sojka D.K., Hughson A., Fowell D.J. CTLA-4 is required by CD4+CD25+ Treg to control CD4+ T-cell lymphopenia-induced proliferation. Eur J Immunol. 2009;39:1544–1551. doi: 10.1002/eji.200838603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muenst S., Soysal S.D., Gao F., Obermann E.C., Oertli D., Gillanders W.E. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139:667–676. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Konishi J., Yamazaki K., Azuma M., Kinoshita I., Dosaka-Akita H., Nishimura M. B7-h1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 84.Leung C.S., Yang K.Y., Li X.S., Chan V.W., Ku M.C., Waldmann H., et al. Single-cell transcriptomics reveal that PD-1 mediates immune tolerance by regulating proliferation of regulatory T cells. Genome Med. 2018;10:71. doi: 10.1186/s13073-018-0581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coley W.B. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. Clin Orthop Relat Res 1991. 1893:3–11. [PubMed] [Google Scholar]
- 86.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 87.Parton M., Gore M., Eisen T. Role of cytokine therapy in 2006 and beyond for metastatic renal cell cancer. J Clin Oncol. 2006;24:5584–5592. doi: 10.1200/JCO.2006.08.1638. [DOI] [PubMed] [Google Scholar]
- 88.Halin C., Rondini S., Nilsson F., Berndt A., Kosmehl H., Zardi L., et al. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat Biotechnol. 2002;20:264–269. doi: 10.1038/nbt0302-264. [DOI] [PubMed] [Google Scholar]
- 89.Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cavallo F., Signorelli P., Giovarelli M., Musiani P., Modesti A., Brunda M.J., et al. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J Natl Cancer Inst. 1997;89:1049–1058. doi: 10.1093/jnci/89.14.1049. [DOI] [PubMed] [Google Scholar]
- 91.Vacchelli E., Eggermont A., Sautes-Fridman C., Galon J., Zitvogel L., Kroemer G., et al. Trial watch Toll-like receptor agonists for cancer therapy. OncoImmunology. 2013;2 doi: 10.4161/onci.25238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu J.J., Li W.H., Chen P.G., Zhang B.D., Hu H.G., Li Q.Q., et al. Targeting STING with cyclic di-GMP greatly augmented immune responses of glycopeptide cancer vaccines. Chem Commun. 2018;54:9655–9658. doi: 10.1039/c8cc04860f. [DOI] [PubMed] [Google Scholar]
- 93.Ramanjulu J.M., Pesiridis G.S., Yang J.S., Concha N., Singhaus R., Zhang S.Y., et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564:439–443. doi: 10.1038/s41586-018-0705-y. [DOI] [PubMed] [Google Scholar]
- 94.Hargadon K.M., Johnson C.E., Williams C.J. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharm. 2018;62:29–39. doi: 10.1016/j.intimp.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 95.Abdollahpour-Alitappeh M., Lotfinia M., Gharibi T., Mardaneh J., Farhadihosseinabadi B., Larki P., et al. Antibody-drug conjugates (ADCs) for cancer therapy: strategies, challenges and successes. J Cell Physiol. 2019;234:5628–5642. doi: 10.1002/jcp.27419. [DOI] [PubMed] [Google Scholar]
- 96.Two drugs for advanced HER2-positive breast cancer (Enhertu and Tukysa) Med Lett Drugs Ther. 2020;62:182–184. [PubMed] [Google Scholar]
- 97.Margolin K.A., Rayner A.A., Hawkins M.J., Atkins M.B., Dutcher J.P., Fisher R.I., et al. Interleukin-2 and lymphokine-activated killer cell therapy of solid tumors: analysis of toxicity and management guidelines. J Clin Oncol. 1989;7:486–498. doi: 10.1200/JCO.1989.7.4.486. [DOI] [PubMed] [Google Scholar]
- 98.Schmidt-Wolf I.G., Negrin R.S., Kiem H.P., Blume K.G., Weissman I.L. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J Exp Med. 1991;174:139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rosenberg S.A., Restifo N.P., Yang J.C., Morgan R.A., Dudley M.E. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rezvani K., Rouce R., Liu E.L., Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther. 2017;25:1769–1781. doi: 10.1016/j.ymthe.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Themeli M., Kloss C.C., Ciriello G., Fedorov V.D., Perna F., Gonen M., et al. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31:928–933. doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y., Hermanson D.L., Moriarity B.S., Kaufman D.S. Human iPSC-derived natural killer cells engineered with chimeric antigen receptors enhance anti-tumor activity. Cell Stem Cell. 2018;23:181–192. doi: 10.1016/j.stem.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cho J.H., Collins J.J., Wong W.W. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell. 2018;173:1426–1438. doi: 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rosenberg S.A., Yang J.C., Restifo N.P. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hu Z., Ott P.A., Wu C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18:168–182. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sahin U., Derhovanessian E., Miller M., Kloke B.P., Simon P., Lower M., et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–226. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 107.Krysko D.V., Garg A.D., Kaczmarek A., Krysko O., Agostinis P., Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–875. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 108.Tesniere A., Panaretakis T., Kepp O., Apetoh L., Ghiringhelli F., Zitvogel L., et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15:3–12. doi: 10.1038/sj.cdd.4402269. [DOI] [PubMed] [Google Scholar]
- 109.Obeid M., Tesniere A., Ghiringhelli F., Fimia G.M., Apetoh L., Perfettini J.L., et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 110.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzym Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 111.Jiang H., Wang Q., Sun X. Lymph node targeting strategies to improve vaccination efficacy. J Control Release. 2017;267:47–56. doi: 10.1016/j.jconrel.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 112.Reddy S.T., van der Vlies A.J., Simeoni E., Angeli V., Randolph G.J., O'Neill C.P., et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 113.Yao H., Ng S.S., Huo L.F., Chow B.K.C., Shen Z., Yang M., et al. Effective melanoma immunotherapy with interleukin-2 delivered by a novel polymeric nanoparticle. Mol Cancer Therapeut. 2011;10:1082–1092. doi: 10.1158/1535-7163.MCT-10-0717. [DOI] [PubMed] [Google Scholar]
- 114.Tagalakis A.D., Grosse S.M., Meng Q.H., Mustapa M.F.M., Kwok A., Salehi S.E., et al. Integrin-targeted nanocomplexes for tumour specific delivery and therapy by systemic administration. Biomaterials. 2011;32:1370–1376. doi: 10.1016/j.biomaterials.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Y., Li N., Suh H., Irvine D.J. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6. doi: 10.1038/s41467-017-02251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakamura T., Miyabe H., Hyodo M., Sato Y., Hayakawa Y., Harashima H. Liposomes loaded with a STING pathway ligand, cyclic di-GMP, enhance cancer immunotherapy against metastatic melanoma. J Control Release. 2015;216:149–157. doi: 10.1016/j.jconrel.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 117.Nuhn L., De Koker S., Van Lint S., Zhong Z.F., Catani J.P., Combes F., et al. Nanoparticle-conjugate TLR7/8 agonist localized immunotherapy provokes safe antitumoral responses. Adv Mater. 2018;30:1803397. doi: 10.1002/adma.201803397. [DOI] [PubMed] [Google Scholar]
- 118.Schmid D., Park C.G., Hartl C.A., Subedi N., Cartwright A.N., Puerto R.B., et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat Commun. 2017;8:1747. doi: 10.1038/s41467-017-01830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xiao Z.C., Su Z.W., Han S.S., Huang J.S., Lin L.T., Shoai X.T. Dual pH-sensitive nanodrug blocks PD-1 immune checkpoint and uses T cells to deliver NF-kappa B inhibitor for antitumor immunotherapy. Sci Adv. 2020;6 doi: 10.1126/sciadv.aay7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang C., Ye Y.Q., Hochu G.M., Sadeghifar H., Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16:2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- 121.Bu J.Y., Nair A., Iida M., Jeong W.J., Poellmann M.J., Mudd K., et al. An avidity-based PD-L1 antagonist using nanoparticle-antibody conjugates for enhanced immunotherapy. Nano Lett. 2020;20:4901–4909. doi: 10.1021/acs.nanolett.0c00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao P., Atanackovic D., Dong S.Y., Yagita H.D., He X., Chen M.N. An anti-programmed death-1 antibody (alpha PD-1) fusion protein that self-assembles into a multivalent and functional alpha PD-1 nanoparticle. Mol Pharm. 2017;14:1494–1500. doi: 10.1021/acs.molpharmaceut.6b01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mi Y., Smith C.C., Yang F.F., Qi Y.F., Roche K.C., Serody J.S., et al. A dual immunotherapy nanoparticle improves T-cell activation and cancer immunotherapy. Adv Mater. 2018;30 doi: 10.1002/adma.201706098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fan Y.C., Moon J.J. Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy. Vaccines-Basel. 2015;3:662–685. doi: 10.3390/vaccines3030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kapadia C.H., Tian S.M., Perry J.L., Sailer D., Luft J.C., DeSimone J.M. Extending antigen release from particulate vaccines results in enhanced antitumor immune response. J Control Release. 2018;269:393–404. doi: 10.1016/j.jconrel.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 126.Zhang Z.P., Tongchusak S., Mizukami Y., Kang Y.J., Ioji T., Touma M., et al. Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials. 2011;32:3666–3678. doi: 10.1016/j.biomaterials.2011.01.067. [DOI] [PubMed] [Google Scholar]
- 127.Xu Z.H., Ramishetti S., Tseng Y.C., Guo S.T., Wang Y.H., Huang L. Multifunctional nanoparticles co-delivering Trp 2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. J Control Release. 2013;172:259–265. doi: 10.1016/j.jconrel.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 128.Lu Y., Yang Y.N., Gu Z.Y., Zhang J., Song H., Xiang G.Y., et al. Glutathione-depletion mesoporous organosilica nanoparticles as a self-adjuvant and co-delivery platform for enhanced cancer immunotherapy. Biomaterials. 2018;175:82–92. doi: 10.1016/j.biomaterials.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 129.Neek M., Tucker J.A., Kim T.I., Molino N.M., Nelson E.L., Wang S.W. Co-delivery of human cancer-testis antigens with adjuvant in protein nanoparticles induces higher cell-mediated immune responses. Biomaterials. 2018;156:194–203. doi: 10.1016/j.biomaterials.2017.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Joshi M.D., Unger W.J., Storm G., van Kooyk Y., Mastrobattista E. Targeting tumor antigens to dendritic cells using particulate carriers. J Control Release. 2012;161:25–37. doi: 10.1016/j.jconrel.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 131.Lee I.H., Kwon H.K., An S., Kim D., Kim S., Yu M.K., et al. Imageable antigen-presenting gold nanoparticle vaccines for effective cancer immunotherapy in vivo. Angew Chem Int Ed. 2012;51:8800–8805. doi: 10.1002/anie.201203193. [DOI] [PubMed] [Google Scholar]
- 132.Rosalia R.A., Cruz L.J., van Duikeren S., Tromp A.T., Silva A.L., Jiskoot W., et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials. 2015;40:88–97. doi: 10.1016/j.biomaterials.2014.10.053. [DOI] [PubMed] [Google Scholar]
- 133.Pei M.Y., Liang J.Y., Zhang C., Wang X.L., Zhang C.N.A., Ma G.L., et al. Chitosan/calcium phosphates nanosheet as a vaccine carrier for effective cross-presentation of exogenous antigens. Carbohydr Polym. 2019;224:115172. doi: 10.1016/j.carbpol.2019.115172. [DOI] [PubMed] [Google Scholar]
- 134.Liu J.L., Liu X.X., Han Y.F., Zhang J., Liu D., Ma G.L., et al. Nanovaccine incorporated with hydroxychloroquine enhances antigen cross-presentation and promotes antitumor immune responses. Acs Appl Mater Inter. 2018;10:30983–30993. doi: 10.1021/acsami.8b09348. [DOI] [PubMed] [Google Scholar]
- 135.Xu J., Wang H., Xu L.G., Chao Y., Wang C.Y., Han X., et al. Nanovaccine based on a protein-delivering dendrimer for effective antigen cross-presentation and cancer immunotherapy. Biomaterials. 2019;207:1–9. doi: 10.1016/j.biomaterials.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 136.Kuai R., Ochyl L.J., Bahjat K.S., Schwendeman A., Moon J.J. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16:489–496. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shukla G.S., Sun Y.J., Pero S.C., Sholler G.S., Krag D.N. Immunization with tumor neoantigens displayed on T7 phage nanoparticles elicits plasma antibody and vaccine-draining lymph node B cell responses. J Immunol Methods. 2018;460:51–62. doi: 10.1016/j.jim.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 138.Lynn G.M., Sedlik C., Baharom F., Zhu Y.L., Ramirez-Valdez R.A., Coble V.L., et al. Peptide-TLR-7/8a conjugate vaccines chemically programmed for nanoparticle self-assembly enhance CD8 T-cell immunity to tumor antigens. Nat Biotechnol. 2020;38:320–332. doi: 10.1038/s41587-019-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang X.L., Wang N., Yang Y., Wang X.X., Liang J.Y., Tian X.X., et al. Polydopamine nanoparticles carrying tumor cell lysate as a potential vaccine for colorectal cancer immunotherapy. Biomater Sci. 2019;7:3062–3075. doi: 10.1039/c9bm00010k. [DOI] [PubMed] [Google Scholar]
- 140.Fang R.H., Hu C.M.J., Luk B.T., Gao W.W., Copp J.A., Tai Y.Y., et al. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ochyl L.J., Bazzill J.D., Park C., Xu Y., Kuai R., Moon J.J. PEGylated tumor cell membrane vesicles as a new vaccine platform for cancer immunotherapy. Biomaterials. 2018;182:157–166. doi: 10.1016/j.biomaterials.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang R., Xu J., Xu L.G., Sun X.Q., Chen Q., Zhao Y.H., et al. Cancer cell membrane-coated adjuvant nanoparticles with mannose modification for effective anticancer vaccination. ACS Nano. 2018;12:5121–5129. doi: 10.1021/acsnano.7b09041. [DOI] [PubMed] [Google Scholar]
- 143.Liu W.L., Zou M.Z., Liu T., Zeng J.Y., Li X., Yu W.Y., et al. Cytomembrane nanovaccines show therapeutic effects by mimicking tumor cells and antigen presenting cells. Nat Commun. 2019;10:3199. doi: 10.1038/s41467-019-11157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li W., Yang J., Luo L., Jiang M., Qin B., Yin H., et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat Commun. 2019;10:3349. doi: 10.1038/s41467-019-11269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen Q., Xu L.G., Liang C., Wang C., Peng R., Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193. doi: 10.1038/ncomms13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang F., Lu G., Wen X., Li F., Ji X., Li Q., et al. Magnetic nanoparticles coated with polyphenols for spatio-temporally controlled cancer photothermal/immunotherapy. J Control Release. 2020;326:131–139. doi: 10.1016/j.jconrel.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 147.Xu T., Ma Y.Y., Yuan Q.L., Hu H.X., Hu X.K., Qian Z.Y., et al. Enhanced ferroptosis by oxygen-boosted phototherapy based on a 2-in-1 nanoplatform of ferrous hemoglobin for tumor synergistic therapy. ACS Nano. 2020;14:3414–3425. doi: 10.1021/acsnano.9b09426. [DOI] [PubMed] [Google Scholar]
- 148.Qin L., Cao J., Shao K., Tong F., Yang Z.H., Lei T., et al. A tumor-to-lymph procedure navigated versatile gel system for combinatorial therapy against tumor recurrence and metastasis. Sci Adv. 2020;6 doi: 10.1126/sciadv.abb3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Min Y.Z., Roche K.C., Tian S.M., Eblan M.J., McKinnon K.P., Caster J.M., et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12:877–882. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yuan H.F., Jiang W., von Roemeling C.A., Qie Y.Q., Liu X.J., Chen Y.X., et al. Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat Nanotechnol. 2017;12:763–769. doi: 10.1038/nnano.2017.69. [DOI] [PubMed] [Google Scholar]
- 151.Duan X.P., Chan C., Lin W.B. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew Chem Int Ed. 2019;58:670–680. doi: 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang Z.Z., Sun Z.R., Ren Y., Chen X., Zhang W., Zhu X.H., et al. Advances in nanomaterials for use in photothermal and photodynamic therapeutics. Mol Med Rep. 2019;20:5–15. doi: 10.3892/mmr.2019.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Doughty A.C.V., Hoover A.R., Layton E., Murray C.K., Howard E.W., Chen W.R. Nanomaterial applications in photothermal therapy for cancer. Materials. 2019;12 doi: 10.3390/ma12050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hu J.J., Cheng Y.J., Zhang X.Z. Recent advances in nanomaterials for enhanced photothermal therapy of tumors. Nanoscale. 2018;10:22657–22672. doi: 10.1039/c8nr07627h. [DOI] [PubMed] [Google Scholar]
- 155.Zhang Y.J., Zhan X.L., Xiong J., Peng S.S., Huang W., Joshi R., et al. Temperature-dependent cell death patterns induced by functionalized gold nanoparticle photothermal therapy in melanoma cells. Sci Rep. 2018;8:8720. doi: 10.1038/s41598-018-26978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhu X.J., Feng W., Chang J., Tan Y.W., Li J.C., Chen M., et al. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat Commun. 2016;7:10437. doi: 10.1038/ncomms10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Deng X.Y., Guan W., Qing X.C., Yang W.B., Que Y.M., Tan L., et al. Ultrafast low-temperature photothermal therapy activates autophagy and recovers immunity for efficient antitumor treatment. Acs Appl Mater Inter. 2020;12:4265–4275. doi: 10.1021/acsami.9b19148. [DOI] [PubMed] [Google Scholar]
- 158.Sweeney E.E., Cano-Mejia J., Fernandes R. Photothermal therapy generates a thermal window of immunogenic cell death in neuroblastoma. Small. 2018;14:1800678. doi: 10.1002/smll.201800678. [DOI] [PubMed] [Google Scholar]
- 159.Wang C., Xu L.G., Liang C., Xiang J., Peng R., Liu Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv Mater. 2014;26:8154–8162. doi: 10.1002/adma.201402996. [DOI] [PubMed] [Google Scholar]
- 160.Guo L.R., Yan D.D., Yang D.F., Li Y.J., Wang X.D., Zalewski O., et al. Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles. ACS Nano. 2014;8:5670–5681. doi: 10.1021/nn5002112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang D., Wu T.T., Qin X.Y., Qiao Q., Shang L.H., Song Q.L., et al. Intracellularly generated immunological gold nanoparticles for combinatorial photothermal therapy and immunotherapy against tumor. Nano Lett. 2019;19:6635–6646. doi: 10.1021/acs.nanolett.9b02903. [DOI] [PubMed] [Google Scholar]
- 162.Chen J.M., Fan T.J., Xie Z.J., Zeng Q.Q., Xue P., Zheng T.T., et al. Advances in nanomaterials for photodynamic therapy applications: status and challenges. Biomaterials. 2020;237:119827. doi: 10.1016/j.biomaterials.2020.119827. [DOI] [PubMed] [Google Scholar]
- 163.Korbelik M. Induction of tumor immunity by photodynamic therapy. J Clin Laser Med Surg. 1996;14:329–334. doi: 10.1089/clm.1996.14.329. [DOI] [PubMed] [Google Scholar]
- 164.Castano A.P., Mroz P., Hamblin M.R. Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Thong P.S.P., Ong K.W., Goh N.S.G., Kho K.W., Manivasager V., Bhuvaneswari R., et al. Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. Lancet Oncol. 2007;8:950–952. doi: 10.1016/S1470-2045(07)70318-2. [DOI] [PubMed] [Google Scholar]
- 166.Garg A.D., Agostinis P. ER stress autophagy and immunogenic cell death in photodynamic therapy-induced anti-cancer immune responses. Photochem Photobiol Sci. 2014;13:474–487. doi: 10.1039/c3pp50333j. [DOI] [PubMed] [Google Scholar]
- 167.Zheng Y.H., Yin G.F., Le V., Zhang A.L., Chen S.Y., Liang X., et al. Photodynamic-therapy activates immune response by disrupting immunity homeostasis of tumor cells which generates vaccine for cancer therapy. Int J Biol Sci. 2016;12:120–132. doi: 10.7150/ijbs.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hong E.J., Choi D.G., Shim M.S. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm Sin B. 2016;6:297–307. doi: 10.1016/j.apsb.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vankayala R., Huang Y.K., Kalluru P., Chiang C.S., Hwang K.C. First demonstration of gold nanorods-mediated photodynamic therapeutic destruction of tumors via near infra-red light activation. Small. 2014;10:1612–1622. doi: 10.1002/smll.201302719. [DOI] [PubMed] [Google Scholar]
- 170.Murakami T., Nakatsuji H., Inada M., Matoba Y., Umeyama T., Tsujimoto M., et al. Photodynamic and photothermal effects of semiconducting and metallic-enriched single-walled carbon nanotubes. J Am Chem Soc. 2012;134:17862–17865. doi: 10.1021/ja3079972. [DOI] [PubMed] [Google Scholar]
- 171.Mroz P., Xia Y.M., Asanuma D., Konopko A., Zhiyentayev T., Huang Y.Y., et al. Intraperitoneal photodynamic therapy mediated by a fullerene in a mouse model of abdominal dissemination of colon adenocarcinoma. Nanomed Nanotechnol. 2011;7:965–974. doi: 10.1016/j.nano.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wang L., Shi J.J., Liu R.Y., Liu Y., Zhang J., Yu X.Y., et al. Photodynamic effect of functionalized single-walled carbon nanotubes: a potential sensitizer for photodynamic therapy. Nanoscale. 2014;6:4642–4651. doi: 10.1039/c3nr06835h. [DOI] [PubMed] [Google Scholar]
- 173.Wang H., Yang X.Z., Shao W., Chen S.C., Xie J.F., Zhang X.D., et al. Ultrathin black phosphorus nanosheets for efficient singlet oxygen generation. J Am Chem Soc. 2015;137:11376–11382. doi: 10.1021/jacs.5b06025. [DOI] [PubMed] [Google Scholar]
- 174.He M.E., Chen Y.A., Tao C., Tian Q.Q., An L., Lin J.M., et al. Mn-porphyrin-based metal-organic framework with high longitudinal relaxivity for magnetic resonance imaging guidance and oxygen self-supplementing photodynamic therapy. ACS Appl Mater Interfaces. 2019;11:41946–41956. doi: 10.1021/acsami.9b15083. [DOI] [PubMed] [Google Scholar]
- 175.Park J., Jiang Q., Feng D.W., Mao L.Q., Zhou H.C. Size-controlled synthesis of porphyrinic metal-organic framework and functionalization for targeted photodynamic therapy. J Am Chem Soc. 2016;138:3518–3525. doi: 10.1021/jacs.6b00007. [DOI] [PubMed] [Google Scholar]
- 176.Lu K.D., He C.B., Lin W.B. Nanoscale metal-organic framework for highly effective photodynamic therapy of resistant head and neck cancer. J Am Chem Soc. 2014;136:16712–16715. doi: 10.1021/ja508679h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lu K.D., He C.B., Lin W.B. A chlorin-based nanoscale metal-organic framework for photodynamic therapy of colon cancers. J Am Chem Soc. 2015;137:7600–7603. doi: 10.1021/jacs.5b04069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Secret E., Maynadier M., Gallud A., Chaix A., Bouffard E., Gary-Bobo M., et al. Two-photon excitation of porphyrin-functionalized porous silicon nanoparticles for photodynamic therapy. Adv Mater. 2014;26:7643–7648. doi: 10.1002/adma.201403415. [DOI] [PubMed] [Google Scholar]
- 179.Dayal S., Burda C. Semiconductor quantum dots as two-photon sensitizers. J Am Chem Soc. 2008;130:2890–2891. doi: 10.1021/ja0781285. [DOI] [PubMed] [Google Scholar]
- 180.Lan G.X., Ni K.Y., Xu Z.W., Veroneau S.S., Song Y., Lin W.B. Nanoscale metal-organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J Am Chem Soc. 2018;140:5670–5673. doi: 10.1021/jacs.8b01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang M., Wang W.T., Wu F., Zheng T., Ashley J., Mohammadniaei M., et al. Biodegradable poly(gamma-glutamic acid)@glucose oxidase@carbon dot nanoparticles for simultaneous multimodal imaging and synergetic cancer therapy. Biomaterials. 2020;252:120106. doi: 10.1016/j.biomaterials.2020.120106. [DOI] [PubMed] [Google Scholar]
- 182.Song G., Cheng L., Chao Y., Yang K., Liu Z. Emerging nanotechnology and advanced materials for cancer radiation therapy. Adv Mater. 2017;29:1700996. doi: 10.1002/adma.201700996. [DOI] [PubMed] [Google Scholar]
- 183.Coulter J.A., Hyland W.B., Nicol J., Currell F.J. Radiosensitising nanoparticles as novel cancer therapeuticspipe dream or realistic prospect?. Clin Oncol. 2013;25:593–603. doi: 10.1016/j.clon.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 184.Her S., Jaffray D.A., Allen C. Gold nanoparticles for applications in cancer radiotherapy: mechanisms and recent advancements. Adv Drug Deliv Rev. 2017;109:84–101. doi: 10.1016/j.addr.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 185.Sancey L., Lux F., Kotb S., Roux S., Dufort S., Bianchi A., et al. The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. Br J Radiol. 2014;87:20140134. doi: 10.1259/bjr.20140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen Y.Y., Li N., Wang J.B., Zhang X., Pan W., Yu L.H., et al. Enhancement of mitochondrial ROS accumulation and radiotherapeutic efficacy using a Gd-doped titania nanosensitizer. Theranostics. 2019;9:167–178. doi: 10.7150/thno.28033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ma M., Huang Y., Chen H.R., Jia X.Q., Wang S.G., Wang Z.Z., et al. Bi2S3-embedded mesoporous silica nanoparticles for efficient drug delivery and interstitial radiotherapy sensitization. Biomaterials. 2015;37:447–455. doi: 10.1016/j.biomaterials.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 188.Ni K.Y., Lan G.X., Chan C., Quigley B., Lu K.D., Aung T., et al. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat Commun. 2018;9:2351. doi: 10.1038/s41467-018-04703-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Retif P., Pinel S., Toussaint M., Frochot C., Chouikrat R., Bastogne T., et al. Nanoparticles for radiation therapy enhancement: the key parameters. Theranostics. 2015;5:1030–1045. doi: 10.7150/thno.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yi X., Chen L., Zhong X.Y., Gao R.L., Qian Y.T., Wu F., et al. Core-shell Au@MnO2 nanoparticles for enhanced radiotherapy via improving the tumor oxygenation. Nano Res. 2016;9:3267–3278. [Google Scholar]
- 191.Song G., Liang C., Yi X., Zhao Q., Cheng L., Yang K., et al. Perfluorocarbon-loaded hollow Bi2Se3 nanoparticles for timely supply of oxygen under near-infrared light to enhance the radiotherapy of cancer. Adv Mater. 2016;28:2716–2723. doi: 10.1002/adma.201504617. [DOI] [PubMed] [Google Scholar]
- 192.Jeong H., Bok S., Hong B.J., Choi H.S., Ahn G.O. Radiation-induced immune responses: mechanisms and therapeutic perspectives. Blood Res. 2016;51:157–163. doi: 10.5045/br.2016.51.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Reits E.A., Hodge J.W., Herberts C.A., Groothuis T.A., Chakraborty M., Wansley E.K., et al. Radiation modulates the peptide repertoire enhances MHC class I expression and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Formenti S.C., Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718–726. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ranji-Burachaloo H., Gurr P.A., Dunstan D.E., Qiao G.G. Cancer treatment through nanoparticle-facilitated Fenton reaction. ACS Nano. 2018;12:11819–11837. doi: 10.1021/acsnano.8b07635. [DOI] [PubMed] [Google Scholar]
- 196.Zhang C., Bu W.B., Ni D.L., Zhang S.J., Li Q., Yao Z.W., et al. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction. Angew Chem Int Ed. 2016;55:2101–2106. doi: 10.1002/anie.201510031. [DOI] [PubMed] [Google Scholar]
- 197.Ying W., Zhang Y., Gao W., Cai X., Wang G., Wu X., et al. Hollow magnetic nanocatalysts drive starvation-chemodynamic-hyperthermia synergistic therapy for tumor. ACS Nano. 2020;14:9662–9674. doi: 10.1021/acsnano.0c00910. [DOI] [PubMed] [Google Scholar]
- 198.Llabani E., Hicklin R.W., Lee H.Y., Motika S.E., Crawford L.A., Weerapana E., et al. Diverse compounds from pleuromutilin lead to a thioredoxin inhibitor and inducer of ferroptosis. Nat Chem. 2019;11:521–532. doi: 10.1038/s41557-019-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang Y., Tian Q., Wu S., Li Y., Yang K., Yan Y., et al. Blue light-triggered Fe2+-release from monodispersed ferrihydrite nanoparticles for cancer iron therapy. Biomaterials. 2021;271:120739. doi: 10.1016/j.biomaterials.2021.120739. [DOI] [PubMed] [Google Scholar]
- 200.Kim S.E., Zhang L., Ma K., Riegman M., Chen F., Ingold I., et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol. 2016;11:977–985. doi: 10.1038/nnano.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang S., Li F., Qiao R., Hu X., Liao H., Chen L., et al. Arginine-rich manganese silicate nanobubbles as a ferroptosis-Inducing agent for tumor-targeted theranostics. ACS Nano. 2018;12:12380–12392. doi: 10.1021/acsnano.8b06399. [DOI] [PubMed] [Google Scholar]
- 202.Yu B., Choi B., Li W., Kim D.H. Magnetic field boosted ferroptosis-like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat Commun. 2020;11:3637. doi: 10.1038/s41467-020-17380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kumar C.S.S.R., Mohammad F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv Drug Deliv Rev. 2011;63:789–808. doi: 10.1016/j.addr.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Toraya-Brown S., Sheen M.R., Zhang P.S., Chen L., Baird J.R., Demidenko E., et al. Local hyperthermia treatment of tumors induces CD8+ T cell-mediated resistance against distal and secondary tumors. Nanomed Nanotechnol. 2014;10:1273–1285. doi: 10.1016/j.nano.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chao Y., Chen G.B., Liang C., Xu J., Dong Z.L., Han X., et al. Iron nanoparticles for low-power local magnetic hyperthermia in combination with immune checkpoint blockade for systemic antitumor therapy. Nano Lett. 2019;19:4287–4296. doi: 10.1021/acs.nanolett.9b00579. [DOI] [PubMed] [Google Scholar]
- 206.Oyewumi M.O., Kumar A., Cui Z.R. Nano-microparticles as immune adjuvants: correlating particle sizes and the resultant immune responses. Expert Rev Vaccines. 2010;9:1095–1107. doi: 10.1586/erv.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhu M.T., Wang R.F., Nie G.J. Applications of nanomaterials as vaccine adjuvants. Hum Vaccines Immunother. 2014;10:2761–2774. doi: 10.4161/hv.29589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li H.F., Willingham S.B., Ting J.P.Y., Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Reisetter A.C., Stebounova L.V., Baltrusaitis J., Powers L., Gupta A., Grassian V.H., et al. Induction of inflammasome-dependent pyroptosis by carbon black nanoparticles. J Biol Chem. 2011;286:21844–21852. doi: 10.1074/jbc.M111.238519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dostert C., Petrilli V., Van Bruggen R., Steele C., Mossman B.T., Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang X.P., Li X., Yoshiyuki K., Watanabe Y., Sogo Y., Ohno T., et al. Comprehensive mechanism analysis of mesoporous-silica-nanoparticle-induced cancer immunotherapy. Adv Healthc Mater. 2016;5:1169–1176. doi: 10.1002/adhm.201501013. [DOI] [PubMed] [Google Scholar]
- 212.Morishige T., Yoshioka Y., Tanabe A., Yao X., Tsunoda S., Tsutsumi Y., et al. Titanium dioxide induces different levels of IL-1 beta production dependent on its particle characteristics through caspase-1 activation mediated by reactive oxygen species and cathepsin B. Biochem Biophys Res Commun. 2010;392:160–165. doi: 10.1016/j.bbrc.2009.12.178. [DOI] [PubMed] [Google Scholar]
- 213.Yang D., Zhao Y.L., Guo H., Li Y.N., Tewary P., Xing G.M., et al. [Gd@C82(OH)22]n nanoparticles induce dendritic cell maturation and activate Th1 immune responses. ACS Nano. 2010;4:1178–1186. doi: 10.1021/nn901478z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Luo M., Wang H., Wang Z.H., Cai H.C., Lu Z.G., Li Y., et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12:648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xu J., Xu L.G., Wang C.Y., Yang R., Zhuang Q., Han X., et al. Near-infrared-triggered photodynamic therapy with multitasking upconversion nanoparticles in combination with checkpoint blockade for immunotherapy of colorectal cancer. ACS Nano. 2017;11:4463–4474. doi: 10.1021/acsnano.7b00715. [DOI] [PubMed] [Google Scholar]
- 216.Chen Q., Chen J.W., Yang Z.J., Xu J., Xu L.G., Liang C., et al. Nanoparticle-enhanced radiotherapy to trigger robust cancer immunotherapy. Adv Mater. 2019;31:1802228. doi: 10.1002/adma.201802228. [DOI] [PubMed] [Google Scholar]
- 217.Patel R.B., Ye M.Z., Carlson P.M., Jaquish A., Zangl L., Ma B., et al. Development of an in situ cancer vaccine via combinational radiation and bacterial-membrane-coated nanoparticles. Adv Mater. 2019;31:1902626. doi: 10.1002/adma.201902626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Li J.C., Cui D., Huang J.G., He S.S., Yang Z.B., Zhang Y., et al. Organic semiconducting pro-nanostimulants for near-infrared photoactivatable cancer immunotherapy. Angew Chem Int Ed. 2019;58:12680–12687. doi: 10.1002/anie.201906288. [DOI] [PubMed] [Google Scholar]
- 219.Scheper W., Kelderman S., Fanchi L.F., Linnemann C., Bendle G., de Rooij M.A.J., et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat Med. 2019;25:89–94. doi: 10.1038/s41591-018-0266-5. [DOI] [PubMed] [Google Scholar]