Graphical abstract
Keywords: Polymeric nanocarriers, Gene delivery, Microneedles, Drug delivery, Squamous cell carcinoma, Basal cell carcinoma
Abbreviations: SC, Skin cancer; MSC, Melanoma skin cancer; NMSC, Non melanoma skin cancer; NPs, Nano Particles; SCC, Squamous cell Carcinoma; BCC, Basal cell carcinoma; cAMP, Cyclic adenosine monophosphate; MCIR, Melanocortin-1 receptor; CREB, response element-binding protein; HH, Hedgehog; UV, Ultra Violet; PATCH1, Patch; SMO, Smoothen; DDS, Drug delivery system; 5-ALA, 5-aminolevulinic acid; 5-FU, 5-fluorouracil; AIDS, Acquired immune deficiency syndrome; BCCs, Basal cell carcinomas; dPG, Dendritic polyglycerol; DIM-D, Di indolyl methane derivative; Gd, Gadolinium; hTERT, Human telomerase reverse transcriptase; HPMC, Hydroxypropyl methylcellulose; IPM, Isopropyl myristate; MNPs, Magnetic nanoparticle; MRI, Magnetic Resonance Imaging; Mn, Manganese; MNs, Microneedles; OTR, Organ transplant recipients; GNR-PEG-MN, PEGylated gold nanorod microneedle; PDT, Photodynamic therapy; PLA-HPG, Poly (d-l-lactic acid)-hyperbranched polyglycerol; PLGA, Poly (lactide-co-glycolide) copolymers; PLA, Poly lactic acid; PLL, Poly (L-lysine); PCL, Poly (ε-caprolactone); PAN, Polyacrylonitrile; PAMAM, Poly-amidoamines; PEG, Polyethylene glycol; QDs, Quantum dots; SPIO, Superparamagnetic iron oxide
Highlights
-
•
Skin cancer is a fatal public health concern rising continuously all over the world.
-
•
Several environmental and genetic risk factors are associated with cutaneous carcinogenesis.
-
•
Use of nanocarriers for targeted delivery of anticancer agents is the most advanced approach.
-
•
Polymeric structures are suitable for tumor selective delivery of drugs, genes and imaging agents.
-
•
Polymeric micro/nanostructures have successfully used for combination anticancer therapies.
Abstract
Background
Skin cancer has been the leading type of cancer worldwide. Melanoma and non-melanoma skin cancers are now the most common types of skin cancer that have been reached to epidemic proportion. Based on the rapid prevalence of skin cancers, and lack of efficient drug delivery systems, it is essential to surge the possible ways to prevent or cure the disease.
Aim of review
Although surgical modalities and therapies have been made great progress in recent years, however, there is still an urgent need to alleviate its increased burden. Hence, understanding the precise pathophysiological signaling mechanisms and all other factors of such skin insults will be beneficial for the development of more efficient therapies.
Key scientific concepts of review
In this review, we explained new understandings about onset and development of skin cancer and described its management via polymeric micro/nano carriers-based therapies, highlighting the current key bottlenecks and future prospective in this field. In therapeutic drug/gene delivery approaches, polymeric carriers-based system is the most promising strategy. This review discusses that how polymers have successfully been exploited for development of micro/nanosized systems for efficient delivery of anticancer genes and drugs overcoming all the barriers and limitations associated with available conventional therapies. In addition to drug/gene delivery, intelligent polymeric nanocarriers platforms have also been established for combination anticancer therapies including photodynamic and photothermal, and for theranostic applications. This portfolio of latest approaches could promote the blooming growth of research and their clinical availability.
Introduction
Present time, skin cancer is a global public health challenge and its burden is continuously rising that may lead to profound effects on both the global economy and manpower. Skin primarily comprises of two major layers, the epidermis and dermis. The epidermis is the outermost layer of skin that consists of melanocytes, keratinocytes, merkel cells and langerhans cells [1]. Any abnormality occurring in this layer will lead to various kinds of skin insults and cancer is one of them. The incidence, morbidity and mortality rates of skin cancer continue to increase in various geographical regions of the world; in United States, 5.4 million new cases of skin cancer are being reported every year [2]. In general, skin cancer is broadly divided into two major types: melanoma (cancers arising from melanocytes dysfunction) and non-melanoma skin cancers (from the epidermal derived cells) [3].
Melanoma occurs due to abnormal proliferation of human melanocytes; pigment containing cells, comprised of 90%, 5% and 1% in skin, eyes, and intestine, respectively [4], [5], [6]. As compared to other skin insults, melanoma accounts only for 1% of all skin malignant tumors. Despite recent advances in therapeutic approaches, still melanoma is the most aggressive skin cancer, showing only 15–20% of five-year survival rate [7], [8]. Non-melanoma skin cancer (NMSC) caused by genetic and environmental factors represents approximately 95% of the skin cancers [9], [10], [11]. Generally, non-melanoma skin cancer encompasses many other cancerous types but these types are mainly divided into two main subtypes; cutaneous squamous cell carcinoma (SCC), and basal cell carcinoma (BCC) make up 99% of all NMSCs [10], [12]. Several studies have suggested that the incidence rate of NMSC has been increased 3–8% around the world annually since 1960 and it is 18–20 time higher than that of melanoma [13], [14]. Men are more at risk of NMSC than women and the risk of progression of NMSC depends on genotypic, phenotypic and environmental factors [15]. Based on the raising prevalence of skin cancers, and challenges in efficient drug delivery systems, it is indispensable to surge the possible ways to prevent or cure the disease.
Current literature on the disease especially raising burden and challenges for efficient drug delivery against the disease urges to highlight and reconsider the issue to develop better understanding about both the prevention and remedy. In this review, we presented the current understanding about biology of skin cancer and leading risk factors with the main focus on barriers to treatment and ability of polymer-based micro and nanostructures to cope the disease. Owing to their promising potential among the variety of strategies being explored by researchers, these structures are considered the best possible drug delivery approach. Herein, we have summarized that how polymer-based micro and nanostructures have successfully been exploited for efficient delivery of anticancer drugs and genes to the skin cancers for possible management.
Risk factors associated with skin cancer
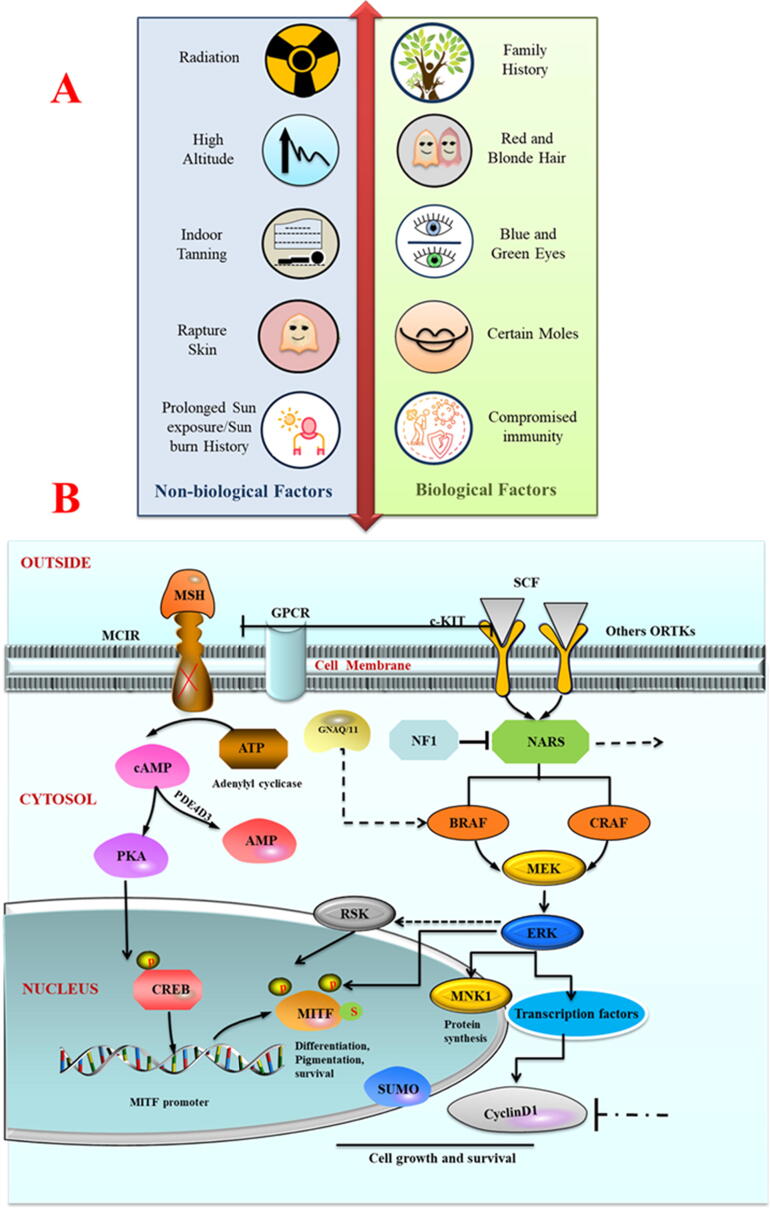
There are multiple factors involved in cancer genesis. However, two major risk factors related to the pathogenesis of many cutaneous cancers are biological (non-modifiable) and non-biological (modifiable) [16], and are represented in Fig. 1A.
Fig. 1.
(A) Biological (non-modifiable) and non-biological (modifiable) risk factors associated with the pathophysiology of many cutaneous carcinogenesis. (B) Figurative description of membrane receptor to protein transcription pathways in melanomas. Expression of MITF; master transcriptional regulator in melanocyte development gets dysregulated by mutational functional loss in three different sites (a- MCIR receptor (red cross, b- BRAF, NRAS or NF1(black lines) and c- GNAQ/11(block dot lines) that mediate the mechanisms to turn them in melanomas.
Biological factors (Non-modifiable)
The human skin is the largest and outermost organ of the body, strategically providing an interface between external and internal worlds. It provides a dynamic, mechanical, physical, and defensive barrier against external insults: infectious microorganisms, ultraviolet (UV) radiations, toxic chemicals and mechanical stresses [17]. It also coordinates sensory perception and mediates thermo‐regulatory and immune responses [18]. Biological factors which contribute in the onset of skin cancers are mainly involved in altering the protein synthesis which negatively impacts the skin cells proliferation; ultimately, results in various skin diseases including the melanoma and NMSC [19].
As compared to the general population, immunosuppressed patients are at enhanced risk of developing cutaneous malignancies. Management of such patients requires an integrated multidisciplinary approach with dermatologic surgery, radiation oncology and medical oncology [20].
There are associations between skin cancers and those viral infectious diseases like acquired immune deficiency syndrome (AIDS). It has been observed that the risk of progression of non-melanoma skin cancer increases 3 to 5 times in AIDS patients [21]. Moreover, it has been documented that the incidence of BCC is 11.4 times more common in HIV-infected hemophiliac individuals than in the general population. The SSC in HIV patients show a high risk of metastasis and recurrence together with a 50% mortality in the age of 6–84 months [22]. Molecular studies unravel the complicity and describe that approximately 90% of NMSC in immuno-compromised patients and up to 50% in immuno-competent patients detected as contain DNA origin from cutaneous or β-HPV types [23]. Additionally, it is considered that these viruses might be indirectly incorporated in the pathogenesis of NMSC [24]. In a largest reported series of ocular surface biopsies with xeroderma pigmentosa, it has been found that patients are predisposed to an increase in sunburn, freckling, and childhood skin malignancies [25].
Numerous signaling pathways associated with the regulation of gene expressions are frequently dysregualted in diverse cancers including the melanoma and non‐melanoma skin cancers. One on such dysregulation is the mutation in the PTCH1 gene (under an autosomal dominant condition) that leads to uncontrollable proliferation of skin cells and results in the development of multiple BCCs [26]. Similarly, in men, mutation in the CDKN2A gene is the most commonly identified cause, whereas in women mutations in the MDM2 gene are predisposed to establish an earlier age melanoma [27].
Human telomerase RNA and human telomerase reverse transcriptase (hTERT) might be involved in the pathogenesis of BCC, SCC and melanomas. It has been found that as compared to normal cells, most tumor cells have substantial telomerase activity and demonstrate no net loss in telomere length during proliferation and progression [28]. These characteristics of the telomerase make a link and indicate its involvement in NMSC. However, understanding of the complicated telomerase activities in human cancer still remains, in fact, ambiguous [29].
Non-biological factors (Modifiable)
Environmental stressors such as exposure to air pollutants, noise and artificial lights at night are contributing to rising cancer rates [30]. The skin is the prime protective barrier that protects humans against environmental stressors: chemical, biological and physical. Moreover, these stressors negatively affect the skin and enhance the risk of cutaneous diseases, especially skin cancer. As a result of exposure to these external environmental stressors, molecular pathways start to be involved in skin aging and other related abnormalities [31].
Although there are several factors associated with skin cancer but incessantly UV radiations from sunlight is the predominant etiologic agent in the development and progression of skin cancers worldwide [32] there is a cascade of molecular mechanisms involved in UV‐induced skin cancers include: activation of the p53 pathways, increased DNA damage, inflammatory responses, genetic mutations, oxidative stress, immunosuppression and apoptotic pathway induction, which remarkably modify cell physiology to arbitrate cell cycle arrest [33]. In UVR exposure, ultraviolet A (UVA) produced reactive oxygen species (ROS), which interact with lipids and proteins molecules and subsequently producing intermediates capable of combining with DNA to make adducts and result in breakage of DNA [9]. UVB is the most carcinogenic UVR reaching the earth’s surface and induce structural damage of DNA and RNA. It initiates covalent bond formation between neighboring pyrimidines that subsequently generates genotoxic photoproducts like pyrimidine-pyrimidine adducts and cyclo-pyrimidine dimers that later on cause inflammatory responses and tumor genesis [34]. While lastly, ultraviolet C (UVC) damage is repairable for DNA repair enzymes and in rare instances, is responsible for skin cancer [34]. Geographic variations and UVR (lifetime sunlight exposure) incidence are key aspects in skin cells proliferation and progression where they contribute in skin carcinogenesis by antigen-presenting cell dysfunction and inducing immunosuppressive cytokine production [35], [36].
In term of diet, several epidemiological studies have consistently provided the evidence of the relationship between diet and skin cancer. It has been observed that the reduction of tumor latency and augmentation of tumor multiplicity, diets rich in omega-6 fat endorse tumorigenesis [37]. However, low fat diet could significantly decrease occurrence of non-melanoma skin cancers [38]. Findings revealed that, polyunsaturated fats supply arachidonic acids as substrates for the formation of prostanoids and it can make structural or physiological modifications in immune responses to UV radiation, thus promoting the growth of skin cancer [39].
Exposure to environmental trace elements (arsenic, selenium and zinc) confirms the risk of keratinocyte carcinoma and melanoma in humans [40]. Conclusively, as for the correlation between diet and skin cancer, there are still insufficient evidences and the existing studies contain limitations. Further well-designed studies are required to unveil the role of diet in the establishments and advancements of skin cancers.
In contrast to general population, skin cancer is the most recurrent malignancy among organ transplant recipients (OTR) [41]. Most keratinocyte carcinomas cause low mortality but relevant morbidity is more prominent. In transplant recipients, NMSC accounts for 90% of all skin cancers. In western countries about 40–50% and in Australia about 70–80% of causcasian transplant recipients have established at least one NMSC [42]. White skinned transplant recipients exhibit a 65–250 fold high susceptibility to SCC and a 10–16-fold risk of BCC [43], [44].
Biology of melanoma skin cancer
Aforementioned, melanocytes are known for synthesizing color pigments called melanin in their specific organelle called melanosomes which are then transferred to neighboring keratinocytes via dendritic processes [45], [46]. During the developmental stages, melanocytes originate from neural crest, thus, they migrated to various localizations in the body but mainly, they are present on skin where any abnormality in their functions leads to malignant transformation [47], [48].
Melanocytes are considered as the endogenous protective shield of skin against harmful radiations. Aberrations in melanocytes functions are associated with their amplification and growth. Several details are involved that give pledge to these skin insults such as self-sufficiency of growth factors, evasion of cellular apoptosis, insensitivity to growth inhibitors, sustained angiogenesis, limitless replicative potential, metastasis and tissue invasion [47], [49], [50]. These factors drive the events of activating oncogenes or suppress the tumor suppressor genes by means of molecular mechanisms such as dotted mutation, deletions and translocation or epigenetic mechanisms such as microRNA expression and promoter methylation [47].
In molecular picture, G protein-coupled melanocortin-1 receptors (MCIRs) in the membrane of melanocytes are key components of melanocytes physiology and are well characterized in the leading risk of melanoma: UV-induced tanning pathways [51], [52]. MCIR regulates the melanocytes proliferation in response to the external signal of melanocytes-stimulating hormone (a-MSH). Any UV exposure damage to melanocytes initiate a subsequent cascade of molecular events; P53 stabilization and transcriptional activation of pro-opiomelanocortin that further process into several signaling molecules and turn on the melanocytes-stimulating hormone (a-MSH) production [53], [54]. Production of a-MSH triggers another cascade of melanocyte proliferation via MCIR. MCIR activation increases the cyclic adenosine monophosphate (cAMP) and subsequent cAMP response element-binding protein (CREB) regulates the expression of transcriptional activation factor microphthalmia (MITF) via CREB regulated transcript activator (CRTC) [55], [56].
MCIR polymorphism is second major factor that dramatically increases the melanoma risk. Patients with MCIR variants, display less shielding from UV by pheomelanin and carry the improved UV signature mutation burden [57], [58].
Evidence proposed that pheomelanin synthesis mediates the reactive oxygen damage and promotes melanoma development in a UV independent fashion in the red hair/fair skin background. This association between pheomelanin and melanomagenesis is an aberrant pigment pathway that needs attention for restoring functional melanogenesis that is eumelanogenesis [59], [60]. Clinical findings of a recent study reveals that any foreign body entry and trauma stimulate the excessive corticosteroid secretion, coagulopathy in blood, accelerate reactive oxygen species and suppress the immune system of host that may mediate the onset of melanoma metastases in specific area [61]. Melanoma cells show similarity of build in developmental program with neural crest cells and exhibit the same gene expression patterns. Findings of a novel study demonstrated that loss of neural crest regulator- highly conserved transcriptional factor PRDM1 accelerates the development of melanoma and confirmed its role of tumor suppressor in p53 muted models [62]. Other study validating in those in vitro and in vivo experiments, oncogene- induced (BRAFV600E) senescence presents a genuine protective physiological process and senescent proliferation of melanocytes also leads to a benign in the form of nevi or moles [63]. Similarly, mutations of BRAFV600E and NRAS are proved as the primary pathogenic event in acral naevi. Mutation in these genes is reported in the acquired and congenital nevi respectively and both contribute in the onset of melanoma formation [64], [65], [66], [67], [68], [69]. Fig. 1B gives figurative description of membrane receptor to protein transcription pathways in melanomas.
Current standard therapies for melanoma face the challenge of tumor heterogeneity; display variable responses. Investigations give emphasis on interpreting the communications between different phenotypes (e.g. melanocytic and mesenchymal) of melanoma at different stages of tumor progression and it could act as therapeutic target along with other receptor and ligand targets in involved signaling pathways [70].
Biology of NMSC
NMSC develops in epidermal keratinocytes cells. Chronic sun and physical carcinogenic –ultraviolet radiations (UVR) exposure to skin has the potential to directly drive the malignant transformation of progenitor cells [71], [72]. Adsorption of UVR by the keratinocytes leads to immune-suppression and p53 mutations that further mediate other carcinogenesis processes [73], [74], [75]. Traditionally, NMSC is divided into two major types; BCC and SCC.
Basal cell carcinoma
Basal cell carcinoma also called Jacob’s ulcer, rodent ulcer, basal cell epithelioma or basalioma cancer arising from basal membrane of epidermis [76]. BCC is very low –grade malignancy in all skin cancers, needs lineage specific immunohistochemical analysis for correct diagnosis and suggested to be completely excised at early stages [77]. It develops in the 80% of patients, mostly in the head/neck regions. BCC rarely metastasizes but frequently shows local invasion and tissue destruction, thus resulting in high morbidity [78]. It has been found that genetic susceptibility in development of BCC increase the skin ageing and shared connections in molecular pathological features [79]. Molecular understandings about BCC indicate that Hedgehog (HH) pathway is the key regulator of these cells and mutational errors in its transmembrane receptor pathed1 can inhibit the signal headway and result in carcinoma development [80], [81], [82], [83].
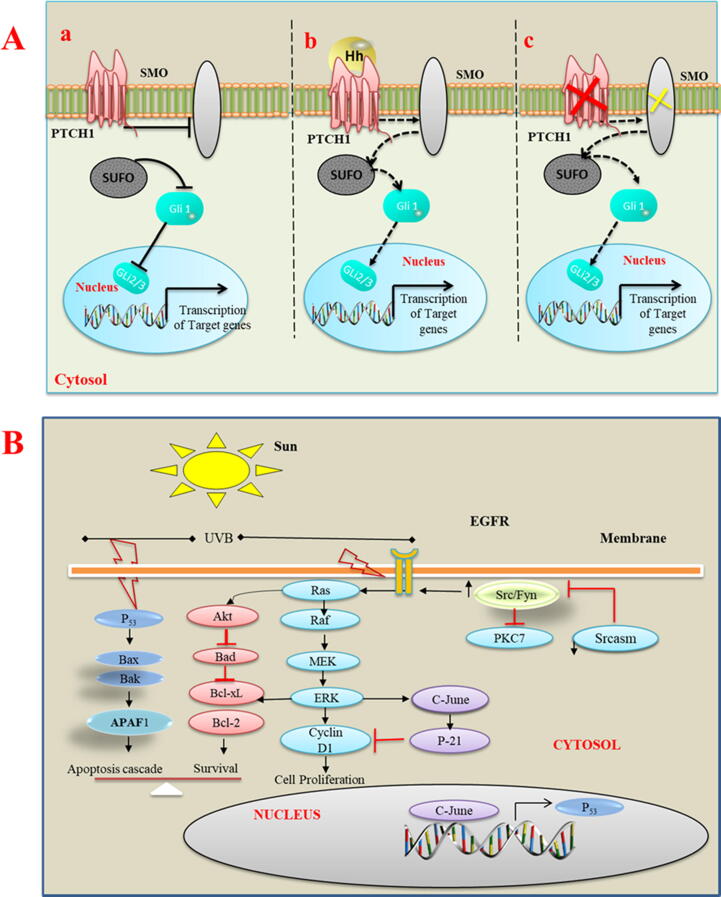
Mutational analysis studies have confirmed that functional mutation in any component of HH pathway; sonic hedgehog (HH) ligand, smoothened (SMO), pathed1 (PTCH1) receptor, glioma-associated (GLI) oncogenes, Cyclin-D1, Myc and Bcl-2 protein will mediate the BCC development [84], [85]. Furthermore, it has been recognized that, aberrant HH pathway trigger a complex signature that actuates PTCH1, SMO, and cytoplasmic-released mechanism of GLI that further drives the proliferation and angiogenesis in the development of BCC [80], [86], [87], [88]. On other hand, up regulation of the HH signaling initiates pathogenic events involved in the 90% of BCC and it got further confirmed in infundibulocystic variant of BCC [89], [90]. Heddgehog signalling inhibitors are being used as target cancer therapeutics and research community is continuously trying to explore their role in treatments modality for BCC e.g. periocular BCC patients [91]. Addition to the intrinsic factors (genetic mutation), there are evidences about the enhanced risks of early onset of BCC and other cancers in the consumers of unauthorized, illegal cosmetic brands and cosmetic tattoos with high loads of lead, mercury, copper, and others hazardous compounds [91], [92]. Fig. 2A explains hedgehog signalling mechanisms in onset of BCC.
Fig. 2.
(A) Hedgehog signalling mechanisms in onset of basal cell carcinoma. a) In the absence of SHH ligand, HH signalling is inactive and receptor patched 1 inhibit SMO action that allow SUFO to hold GLI1 in cytoplasm and prevents its signalling for the transcription GLI target proteins. b) Binding of SHH ligand activates the SMO that activates the SMO and allows SUFO to release the GLi1 for its action in nucleus. c) Any functional mutation in pathed1 (red cross) or activating mutation in (green cross) initiate HH pathway in the absence of ligand that leads to tumor formation. (B) Key signalling pathways involved in squamous cell carcinogenesis followed by UVB exposure-mutations. Block arrows are indication of activation of protein while red T-bars shows inhibitory relationship.
Squamous cell carcinoma
Human squamous cell carcinoma originates from epidermal keratinocytes. Cumulative UVR, chronic inflammatory dermatologic conditions, burn scars, human papillovirsus (HPV), and human immunodeficiency virus (HIV) infections are the risk factors for the onset of SCC [93]. SCC is one of the highly mutated human cancers and more aggressive and speedily metastasizes to regional lymph nodes as compared to BCC [94]. 95% of SCC cases carry the mutations of tumor suppressor gene TP53 that are induced mostly by UVR and other environmental risk factors [95], [96], [97].
Recently, molecular analysis revealed that, key downstream transcriptional cofactor involved in cell growth regulation; phosphorylated Yes associated protein (YAP1) and splicing factor derived circular RNA circUHRF1 are potent drivers in the development and progression of oral SCC and the finders says that these cofactors could act as therapeutic target in treating NMSC [98], [99]. In other mutations that contributes in the onset of SCC, mutations in CDKN2, NOTCH and in oncogenes; RAS are crucial and need to explore further. [50]. Investigations show that other epigenetic factors like expression of certain microRNAs in the skin cells also have significant role in mediating those pathogenic events that induced skin insult in the form of SCC [100]. Findings of other studies emphasize to elucidate the relationship between tumour heterogeneity and therapeutic response and it needs to adopt the tumor microenvironment approaches for their applications in the carcinogenesis of SCC [101], [102]. Fig. 2B illustrates the key signaling pathways involved in squamous cell carcinogenesis followed by UVB exposure- mutations.
Barriers to the use of nanomedicine in skin cancer treatment
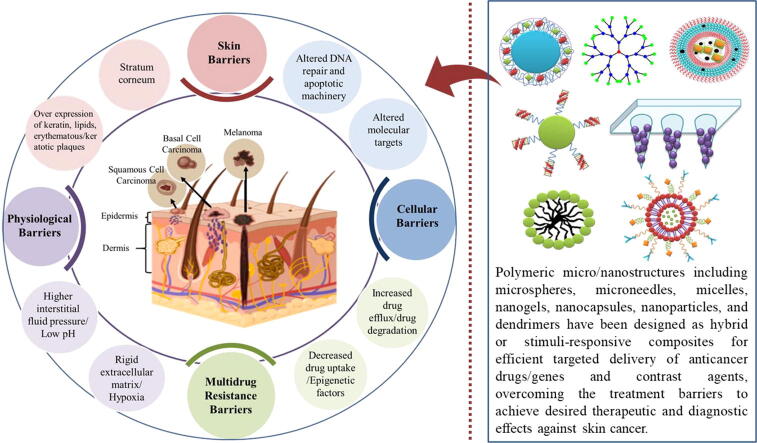
Skin barriers
To cope with rising skin cancer burden, technologies are being replaced with their better and advanced forms. Use of nanomedicine in the form nanoparticles (NPs) loaded with anticancer agents for accurate delivery at target tissues of the body is the most common one. These administrative approaches are used for different skin malignancies; pre-malignant lesions, like AK; skin cancer at superficial level, non-persistent BCC and SCC. In addition, these routes are also applied to cure the melanoma at primary stages [103]. Anatomy of skin reveals that, the uppermost layer of skin; stratum corneum (SC) is main barrier that resists the entry of anticancer agents to target sites thus; interrupts the response of topical treatments [104]. In addition to the said phenomenon, corneocytes surround by lipids, namely fatty acids, triglycerides, cholesterols and ceramides arrange into a complex network that further control the movement of macro and micro molecules across the skin. Usually, AK lesions form excessive keratin, resulting in the thicker SC layer and consequently a stronger barrier for NPs entry. Thus, NPs employed against AK require proper designing to reach the deep epidermal layers, where the langerhans cells are present, to show immune response [105]. A layer below the SC is an epidermal layer called as viable epidermis and it reaches down to the thin basal layer dermal-epidermal junction [106]. This deep layer of skin is vascularized and encloses keratinocytes, melanocytes, cells of langerhans and, merkel [107]. Different skin cancer studies observed higher levels of keratin, lipids and the appearance of keratotic papules, and erythematous plaques in cancer cells as compared to normal tissues, which provide an extra remarkable barrier to the passive transport of NPs to the target site [108], [109], [110].
Physiological barriers
In systemic applications of nanomedicine for the treatments of skin cancer, liver and kidney provide another barrier of drug delivery to target site by removing the NPs from circulatory system of blood [109]. It has also been noted that, normally, structural arrangement of blood vessels hinders the NP entry and tightened retention level in tumor site [111]. In tumor tissues, interstitial spaces filled with proteins, collagen, glycosaminoglycan and elastic fibers uplift interstitial fluid pressure making extracellular matrix rigid thus provide another biological barrier for the efficient transport of anticancer drugs at tumor sites [109], [112].
Cellular barriers
Tumor cell-NPs interaction studies show that set of NPs characteristics affect their interactions with tumor cells. Main physiological interactions of NPs and tumor cells include adsorption/attachment, cellular uptake leading to endosomal transport, metabolism, and degradation [111]. Cell membrane is an overall uptake barrier for all types of biological cell transports and acts as selective permeable membrane due to its negative charge nature, thus also provide a biological barrier for NPs loaded with anticancer drug. Such barriers can easily be overcome by different mechanisms like phagocytosis, endocytosis, micropinocytosis, clathrin-dependent endocytosis, caveolin-dependent endocytosis and clathrin-independent endocytosis [113], [114], [115]. Beside their defined target site actions, designing of NPs is aimed to surpass the challenges of intracellular transport such as pH changes, redox condition and lysosome encounter. For these challenges, biocompatible and the thermo responsive cargo systems are developed [116], [117].
Multidrug resistance (MDR) barriers
Tumor cells developed MDR when the cells get confrontation of various drugs used in therapy. MDR lessened the therapeutic ability of a drug that play role in disease progression. So, MDR proved to be an impediment for an effective therapy in various cancers for instance, skin cancer, especially melanoma [118], [119], [120]. There are several intrinsic factors that may lead to MDR; degradation of drug, changes in prodrugs, changes in receptors and target of drugs, and also reduced in drug-receptor interactions. In addition, mechanisms like changes in membrane, alteration of metabolic process, cell division changes, changes in repair of DNA damage, and modifications in efflux pumps can contribute to MDR [118], [119]. By reason of these developed intrinsic factors in a patient with acquired resistance showed less treatment efficacy with the passage of time.
In clinical manifestation, these medical complexities are driven mostly by genomic instability, environmental and lifestyle base factors. To deal with these diverse mechanisms of MDR for attaining maximum efficacy in cancer therapy, advanced technologies have been emerged that are capable of characterizing cancer MDR and help in appropriate diagnosis and treatments; atomic force microscopy, next generation sequencing, single live- cell tracking for identification of drug resistance, microfluid technology and microfluid based 3D cell culture [121].
To explore more about the mechanisms of MDR developments and to bring in control, researchers are engaged to try multi-ends approaches and achieved considerable success. For example, very recent, a novel research demonstrated that, some selected Nigerian medicinal plants are endowed with significant in vitro cytotoxicity and mediate the pathways to inhibit the MDR mechanisms in skin cancers [122]. Similarly, it is also clearly postulated that known biological active natural products: Silybin and Nobiletin are potent MDR reversal agents in cancer [123], [124].
Polymer-based structures: Management of skin cancer
In spite of a remarkable progress in cancer genomics, biology, and proteomics during the last several decades, cancer treatment is still not satisfactory and overall survival rate of many of cancer patients stays low [125]. To the date, due to the difficulties in clinical trials, there is no FDA registered topical treatments of melanoma and other related cancerous lesions. However, there are several ways to treat the skin cancer. In current skin cancer therapies, clinically, chemotherapy is still one of the most frequently practiced approaches. Primarily, chemotherapeutic agents are toxic compounds and usually administrated systemically. Notwithstanding, these chemotherapeutic agents often present some limitations, including poor solubility and bioavailability, unsuitable pharmacokinetics, and non-selective biodistribution, which in turn can complicate their clinical use resulting in unwanted side effects [126], [127]. In other available therapies conventional photothermal therapy (PTT) is considered as the earliest approach to treat the cancers. These days, it has proven efficacy for certain types of NMSC but still there is need of new strategies for improving the efficacy and minimize the factor of pain to patient [128]. Application of ionizing radiation and laser exposure to the cancer parts of the body to manage the skin cancer is also a common practice of time, however, their clinical efficacy is variable and not accepted as standard of care. [129], [130]. Skin cancers are also being treated with anti-PD-1 immune checkpoint inhibitors to support the immunity responses against viral gene expression or other mutations. Despite great progress of immunotherapy approaches, most of the patients are not cured by these treatments [131]. Surgery is most viable option and considered as the traditional mainstay of treatment. It gives high cure rates with clear identification of tumor margins. However, decision to perform surgery can be affected by various considerations [132]. To treat the skin cancers, there are numerous anticancer agents including 5-fluorouracil (Pyrimidine synthesis inhibitor), Imiquimod, (Inhibitor of herpes simplex virus replication), Ingenol mebutate (Protein kinase C activator), sinecatechins (Camellia sinensis leaves extract, known as green tea), epigallocatechin-3-gallate (Flavonoid of plants) and betulinic acid (Acidic molecules consist of betulin) that have proved their amazing results to cure the different cancers [133], [134], [135], [136], [137], [138]. All these ways of skin cancer treatment are under continuous development to manage the challenges both at patient level and target site level.
To cope the above-mentioned drawbacks, challenges to skin treatments and other limitations, presently, nanoscale drug delivery systems have shown their excellence in diagnosis, drug delivery, and therapy, specifically, in cancer management. In contrast to conventional drug delivery approaches, micro/nanostructures have shown great potential in enhancement of drug bioavailability, prolonged circulation time, controlled drug release, and tumor targeting. In addition to these primary functions, the newly emerging materials and technology can offer additional opportunities or functions to micro/nanocarriers for cancer diagnosis and treatment [139], [140].
Drug delivery through skin is an attractive alternative route to conventional drug delivery systems such as oral and parenteral. It provides many advantages over other routes of administration because; it is non-invasive drug delivery system which maintains drug level within the therapeutic window for prolonged periods of time, avoids degradation of the drug in a gastrointestinal tract, eliminates first pass effect and offers easy application, improves patient compliance and acceptability of drug therapy [141]. For topical treatment of skin cancers, micro/nanoparticle-based systems have been widely tested due to their potential to enhance the penetration of bioactive molecules into tumor cells. Micro/nanostructures enhance drug retention in the tumor and skin; thus, resulting in improved patient compliance, minimal toxicity and reduced dosage [142].
Several nanocarriers have been established, of which the most commonly employed are: (1) inorganic nanocarriers, including quantum dots (QDs), carbon nanotubes, silica NPs, gold NPs, and magnetic NPs, and (2) organic nanocarriers like liposomes, polymeric micelles, and dendrimers. Polymeric structures have been considered as one of the most studied drug delivery carriers as they offer several biological and physicochemical advantages over other types of nanostructures, including simple structures, easy synthesis, drug solubilization, enhanced biocompatibility, improved pharmacokinetics and biodistribution. Additionally, these have potential for further engineering and can be successfully used to exploit cancer microenvironment to design stimuli responsive drug delivery systems including pH responsive, light responsive, redox-responsive, temperature-responsive, ultrasound responsive, enzyme responsive, magnetic field responsive, and multi responsive systems for tumor selective delivery of drug and genes to prevent the emergence of multi drug resistant cancers [143], [144], [145]. Table 1 Table 1 provides the comprehensive view of latest studies on polymer-based micro/nanostructures in different skin cancers. Hence, in this review, due to promising potential for enhanced delivery, we are primarily focusing on polymer-based carriers for the delivery of anticancer cargoes across the skin to the target tissues among the other available approaches. Following the general classification, a brief overview of how polymeric micro and nano carriers; microspheres, microemulsions, microneedles, micelles, nanogels, nanocapsules, nanoparticles, and dendrimers have been exploited for efficient delivery of drugs and genes is discussed here.
Table 1.
Role of polymer-based micro/nanostructures in management of different skin cancers.
| Polymer-based micro/nanostructures | Anti-cancer agent | Size of material | Polymer used/Important characteristics | Enhancement of drug delivery action | Cell line/Model | Effects against skin cancer | Ref |
|---|---|---|---|---|---|---|---|
| Self-assembled pH-sensitive, folic acid-cholesterol sodium alginate NPs (FCA NPs) | Metformin (MET) and Doxorubicin (DOX) | < 180 nm | Sodium alginate, a linear and anionic polysaccharide consisting of two 1,4-linked hexuronic acid residues, forms the hydrophilic shell of FCA NPs. This material has been widely used for cancer drug delivery therapies because of its biocompatibility, low cytotoxicity, and ability to self-assemble into NPs under mild condition. | Tumor targeting was achieved by grafting folic acid onto cholesterol-sodium alginate to deliver functional drugs into folate receptor-overexpressing melanoma cancer cells. | A375 and SK-MEL-28 cells and in vivo unilateral melanoma tumor model. | The developed nano system has co-delivered a combination of MET/DOX into melanoma tumors to trigger pyroptosis, apoptosis, and necroptosis of the melanoma cells, thus blocking melanoma progression, and proved a promising vector for effective drug delivery into melanoma. | [146] |
| Polymeric nanoparticles based non-aqueous dispersions | Cisplatin | < 150 nm | PNVP (poly(vinylpyrrolidone) is an FDA approved, hydrophilic polymer and has a good efficiency to control therelease rate of poorly water-soluble drugs. PCL (poly(∊-caprolactone)), also approved by the FDA, and have a high hydrophobicity, biocompatibility, biodegradability, non-toxicity, and it is permeable to low-molecular-weight drugs. |
Controlled drug delivery through polymeric nanoparticles to achieve improved efficiency, and reduced toxicity. Moreover, a biocompatible non-aqueous emulsion polymerization approach was used to develop polymeric nanoparticles. | A-375 skin cancer cell line | The drug release rate from the hydrophilic cross-linked PNVP-based NPs is higher than that from the hydrophobic PCL-based NPs. Moreover, results showed that NPs have a good compatibility with the blood. Furthermore, Both types of NPs had no cytotoxic effect but, at a concentration of 500 µg/mL, presented an apoptotic effect similar to that of the free drug. | [147] |
| α-terpineol-loaded PMMA nanoparticles | α-terpineol | 50–150 nm | Poly (methyl methacrylate) (PMMA) is a synthetic polymer having widespread applications in biological systems, since it is biocompatible and non-toxic. Its most important characteristic that can make it a promising carrier of drugs since its circulation time in the bloodstream is increased. PMMA is approved by FDA for medical use. | The miniemulsion technique is suitable for the synthesis of polymers in the form of NPs or nanocapsules. This system allows the encapsulation of liquids or solids (hydrophilic/hydrophobic) during the formation of the polymer structure, and can be adjusted for different forms of polymerization, such as: anionic, cationic, ring-opening, radical, condensation and others. The high hydrophobicity of α-terpineol limits its direct application, since it has been encapsulated into polymeric nanoparticles for enhanced delivery. | Melanoma cell lines from mice (B16-F10) and human (SK-MEL-28) | The toxicological profile of PMMA containing 400 mg of α-terpineol in Artemia salina, erythrocytes and normal animal cells like macrophages and fibroblasts (MRC-5), suggested the high pharmacological security of the drug. Moreover, its cytotoxic effects were demonstrated against melanoma cell lines suggesting the potential of these NPs for melanoma therapy. | [148] |
| Chitosan nanoparticles containing S-nitrosomercaptosuccinic acid (S-nitroso-MSA-CS) | S-nitrosomercaptosuccinic acid | --------- | Chitosan is a biocompatible, nontoxic, and biodegradable polymer with pharmaceutical applications, and has been widely used for nanoparticle preparation. | Considering that NO releasing polymeric nanomaterials are emerging as a promising strategy in cancer chemotherapy, biocompatible chitosan NPs were developed and used to encapsulate low molecular weight mercaptosuccinic acid (MSA), a thiol containing small molecule. Free thiol groups on mercaptosuccinic chitosan NPs (MSA-CS) were nitrosated to form S-nitroso-MSA-containing chitosan NPs (S-nitrosoMSA-CS). | Melanoma B16-F10 Cells | Cytotoxic effects were selective to tumor cells in comparison to normal melanocytes and dependent on the entire nanoparticle composition; only CS, free MSA, or free S-nitroso-MSA did not exhibit significant cytotoxicity. Additionally, S-nitroso-MSA-CS induced an apoptotic cell death profile, dependent on caspase activation, and associated with a cellular and mitochondrial oxidative stress. | [149] |
| Eudragit nanoparticles | Imiquimod | 249.3 ± 12.6 nm | Polymethacrylate copolymer has been widely used in drug delivery systems based on its mucoadhesive properties, proteolytic enzyme inhibition properties, tight junction opening, and drug absorption enhancement. | Imiquimod was encapsulated in polymeric NPs to improve cutaneous permeation and reduce imiquimod adverse effects following the topical use and evaluate antiangiogenic effect and chemopreventive activity of this system compared to the market formulation. | Multistage DMBA and croton oil model of skin carcinogenesis in mice. | The designed stable nanocarriers were capable of improving imiquimod skin permeation and their chemopreventive activity as well as antiangiogenic effect represented a promising alternative for the management of malignant skin lesions. | [150] |
| Hybrid nanocomplexes (AgNP@CMC-DOX) Silver NPs embedded in the carboxymethylcellulose (CMC) polymer cross-linked networks conjugated with doxorubicin (DOX) |
Doxorubicin | 10 nm | Carboxymethyl cellulose (CMC) is a polysaccharide whose hydrogens on the hydroxyl groups are partly substituted by carboxymethyl groups. This polysaccharide is soluble in water and therefore has been widely used in manufacturing of biopolymer-based hydrogels for biomedical applications. Herein, CMC was used simultaneously as reducing agent and polymer ligand for producing colloidal silver nanoparticles complexed with doxorubicin in aqueous dispersions. |
An innovative platform was designed and developed based on nanoparticle − polysaccharide − drug nanostructures for producing anticancer and antibacterial hybrid hydrogels. Importantly, these hybrids were produced by means of a fully green chemistry strategy aiming at nanomedicine applications against skin cancer. | A375 and HEK 293 T cells | Hydrogels demonstrated tuned kinetics of intracellular releaseof DOX in vitro for killing melanoma cancer cells evidencing a synergistic effect with AgNPs incorporated in the matrices. Moreover, these hybrid nanocomposites proved antimicrobial activity against Gram-positive and Gram-negative bacteria. |
[151] |
| Surface functionalized hydroxypropyl cellulose-sliver nanoparticles (HPC-SNPs) | miR-148b | 58.03 ± 15.5 nm | Hydroxypropyl cellulose is a derivative of cellulose having a combination of hydrophobic and hydrophilic groups and shows both water solubility and organic solubility. | Light- inducible nucleic acid gene regulation system, in which particles penetrate via irradiation and precisely deliver the drug for tumor ablation. Technique has lowest side effects to healthy tissues while treatment, improve immunity and cellular uptake. | Epidermal skin cells (Pam 212 cell)/ Transgenic mice with HRasG12V-driven skin tumors | Increased apoptosis in Ras-expressing keratinocytes in epidermal squamous cell carcinoma. A sustained and rapid reduction in tumor (92.8%), and potent immunomodulation both local and systemic was achieved. | [152] |
| Lipid NPs loaded dissolving microneedles array | PD-1-cisplatin | 55.5 nm, PVP based MNs of 800 µm | Polyvinylpyrrolidone (PVP) is a water-soluble polymer made up of monomer N-vinylpyrrolidone. Based on its water solubility, it is widely being used in fabrication of dissolving microneedles. Here in, microneedle tips dissolved within 5 min to show rapid direct delivery. | Direct deep layer delivery using microneedle patch loaded with tumor targeted NPs. | FaDu and CAL skin cancer cell lines | Robust immune response in targeted skin cancer. | [153] |
| Gold nanocage-microneedle | Doxorubicin | AuNC of 59.2 nm Hyaluronic acid (HA) based MNs. | Hyaluronic acid (HA) a polysaccharide is being used in MNs fabrication due to its excellent biocompatibility, biodegradability and solubility. Despite the promising features, weak mechanical properties of the polysaccharides limited their applications. Increasing polymer concentration in the preparing procedure makes the manufacturing process more difficult because of the significantly enhanced viscosity of the polymer solution. However, crosslinking endows MNs with enhanced strength along with decreased solubility as well. | Nanocage-microneedles can efficiently penetrate inside the skin. System released loaded drug precisely and get dissolved after delivery of cargo | C 57 mice/Mouse melanoma cell line BI 6 F10ice | With DOX and laser exposure of NIR, nanocage-MN system has showed significant synergistic chemo-photothermal effects for inhibition of superficial skin tumor cells with lower side effects | [154] |
| Microneedle patch | Genes | 1000 µm | Polycaprolactone (PCL), an FDA approved bioresorbable polymer, is suitable as a needle material for photothermally triggered drug release because of its high biocompatibility and relatively low melting point. The encapsulated LaB6@SiO2 nanostructures acted as a local heat source and increasedthe temperature of the PCL microneedles after NIR irradiation. When the temperature (50 C) wass close to the melting point of the PCL, the MNs undergo rapid thermal transitions from a solid to a liquid state, thus increasing the mobility of the polymer chains and enabling the release of the molecules. | Microneedles coated with polyelectrolyte multilayers can efficiently release the genes after insertion into the skin. pH-responsive polyelectrolyte multilayers (PEM) were coated on the surface of PCL MNs by layer-by-layer assembly to realize rapid gene release. Dimethylmaleic anhydride-modified polylysine (PLL-DMA), a charge reversible polymer, was introduced to PEM. The PEM composed of two parts: the transition layers of (PLL-DMA/polyethyleneimine) and the gene-loaded layers of (p53 expression plasmid/polyethyleneimine). | Mice model/ Human oral epidermoid cancer cell line | Microneedles proved to be an excellent carrier for DNA delivery and its quick dispatch upon insertion in deep dermal area. As compared to control group, MN genes delivery showed greater inhibition of skin tumor cells up to to 90.1%. | [155] |
| Polyvinylpyrrolidone-co-vinyl acetate (PVPVA) based MNs | Imiquimod, | 1000 µm | PVPVA is a biocompatible polymer that is widely used in the pharmaceutical industry as a dry binder in tableting, as a film-forming agent in tablet coating, as well as a film-forming agent in topical drug delivery systems. Besides that, being a derivative of PVP, PVPVA is a chemically and biologically inert polymer which obviates the issues of polymer drug compatibility along with biological toxicity. | Less invasive co-localization of polymeric MNs with Imiquimod is viable approach to enhance the dermal delivery of Imiquimod for the treatment of nodular basal cell carcinoma (BCC). | Porcine skin via Fran diffusion cell method | MNs showed similar intradermal permeation of imiquimod as from Aldara™ cream, in spite of having six-fold lower drug loading than the clinical dose of Aldara™. Moreover, skin cross sections showed intradermal co-localization of the PVPVA polymer, with imiquimod within the MN channels, illustrating it a viable approach for efficient delivery of imiquimod for nodular BCC. | [156] |
| PLGA NPs | Peptide P20 (CSSRTMHHC) and combined peptide C. Combined peptide C comprised of a tumor-homing peptide “C” (CVNHPAFAC), conjugated to (HTMYYHHYQHHL) an antiangiogenic peptide with a GYG. |
800–100 nm | Poly(lactide-co-glycolide) acid (PLGA), is a biodegradable copolymer approved by FDA for use in humans. The PLGA NPs have been commonly used asnanocarriers, due to their ability to encapsulate and deliver drugs. Additionally, the controlled release profile of PLGA in response to biological signals enables prolonged treatment with low doses of the drug. | Effectiveness of peptides is usually hampered by their fast degradation in the biological system. PLGA NPs conjugated to peptide C on the NPs surface and loaded with peptide P20 were applied as a dual‐peptide carrier for application in cancer therapy to achieve synergistic effects of two peptides. | B 16-F10 melanoma cell line | The inhibitory effect of P20‐PLGANPs was almost same to the effect of non‐encapsulated P20 in fivefold higher dose. The inhibitory effects were even higher with P20PLGA NPs functionalized with combined peptide C, showing 28% reduction in lung nodules in a syngeneic model of metastatic melanoma in comparison to untreated animals. | [157] |
| Dual targeted polymeric micellar NPs | Dasatinib | 100–200 nm | Three polymers were used to build the micellar nanoparticles: the matrix metalloproteinase MMP2-sensitive polymer (PEG5k-pp-PE), FR-targeted polymer (FA-PEG2k-PE), and micelle building block (PEG2k-PE). | Targeted micellar approach ensures the stability and efficient release of cargo at target site. It significantly prolongs the systemic circulation of drug and decrease the non-uniform bio distribution of drug in healthy tissues. Matrix metalloproteinase 2 (MMP2), a major enzyme responsible for cancer initiation, growth and metastasis, is up-regulated in many cancer tissues. MMP2 has been used as a biomarker for cancer diagnosis and as a stimulus for tumor-targeted delivery of imaging agents and drugs. | mice model /Murine B 16-F10 melanoma cell | MMP2-mediated PEG5k de-shielding and FA exposure significantly improved cellular uptake and anti-cancer effects of the micellar NPs in FR and MMP2 expressing cells, including multidrug resistant (MDR) cancer cells. Additionally, MMP/FR micelles showed remarkable MMP2-dependent tissue penetration, uptake and cytotoxicity in 3D MDR tumor spheroids. Moreover, the MMP2 and FR dual targeting approach resulted in prolonged systemic circulation, decreased non-specific biodistribution, and increased tumor accumulation of the NPs in a melanoma xenograft mouse model. | [158] |
| Low molecular weight heparin (LMWH)-coated and dendrimer-based core–shell nanoplatform | CPG and doxorubicin | 35 ± 4.2 nm | Polyamidoamine (PAMAM) dendrimers are hyperbranched polymers with unparalleled molecular uniformity, narrow molecular weight distribution, defined size and shape characteristics and a multifunctional terminal surface. Herein, nanoplatform with G4 PAMAM was serving as the main support to conjugate DOX involved in immune activation and anti-metastatic activities. | Chemoimmunotherapy to treat highly aggressive melanoma. It is newly developed multifunctional approach in which immunoadjuvant cytosine-phosphate-guanine oligonucleotides (CpG ODNs) are used to boost the doxorubicin (DOX)-elicited immune responses, which synergistically suppressed tumor. Additionally, anti-metastatic LMWH was also used, to achieve multiple anti-metastatic activity against tumor metastasis. | Xenograft mice model (B16-F10). | Multifunctional nanoplatform of dendrimer-based core shell showed anti-metastatic results. This anti-metastatic activity significantly inhibited melanoma tumor growth in B16-F10 tumor bearing mice model (C57 mice). This nanoplatform could broadly applied for the co-delivery of other chemotherapeutics drugs to treat highly aggressive tumors. | [159] |
Polymer-based micro/nanostructures for drug delivery to skin cancers
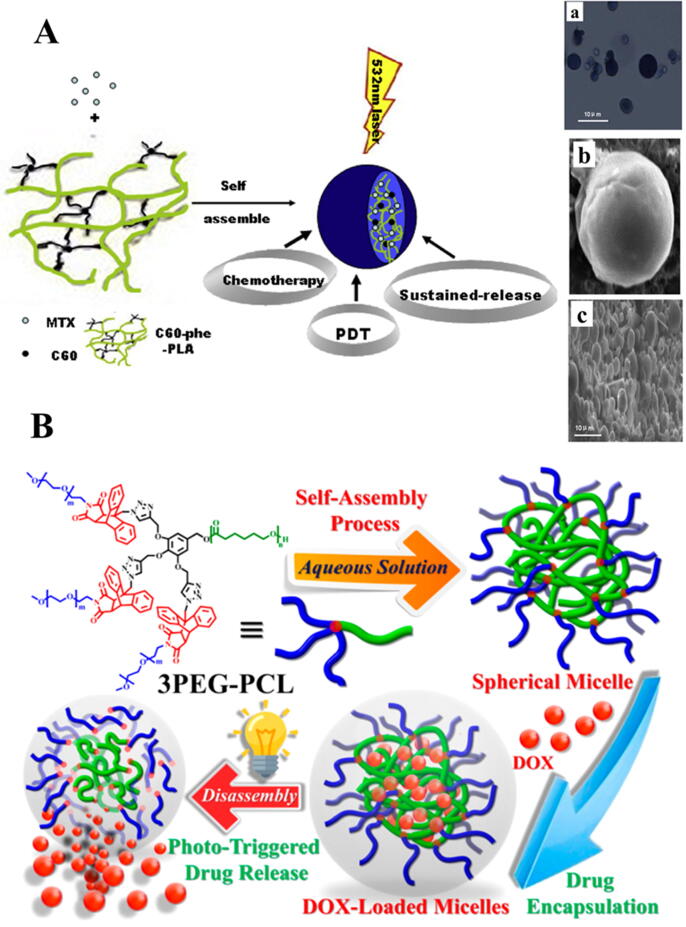
Polymeric chains have a hydrophilic shell and a hydrophobic core. The hydrophobic core is employed to encapsulate poorly soluble compounds and the hydrophilic shell provides the stability to core in its aqueous environment [160]. Fullerene (C60), a nano scale carbon material, can show photochemical properties under visible light or UV irradiation. It also possesses low systemic toxicity profile. A biodegradable and biocompatible polymer, Poly (lactic acid) (PLA), has been employed to fabricate a multifunctional implant of fullerene C60 and an anticancer drug Mitoxantrone (MTX). Fullerene (C60) L-phenylalanine derivative functionalized with PLA (C60-phe-PLA) self-assembled to formulate microspheres of a hydrophilic antitumor agent MTX and a hydrophobic block (C60) via dispersion–solvent diffusion method. Visual illustration of the self-assembly property of the microspheres is provided in Fig. 3A. The microspheres demonstrated sustained in vitro release pattern approximately for 15 days. Moreover, high anticancer effects without toxic effects to normal organs, thanks to the remarkably improved tumor retention time of MTX, less biodistribution to other organs and strong photodynamic activity of PLA-phe-C60 were achieved. Hence, the results demonstrated that these microspheres might be a potential approach for combined delivery of chemotherapy and photodynamic therapy (PDT) [161].
Fig. 3.
Visual representation of self-assembled polymeric micro/nanostructures-based delivery systems. (A) Schematic illustration of fullerene-based multi-functional sustained-release microspheres and their bio-functions. Characterization of microspheres based on C60-PHE-PLA (a): Photomicrographs of MTX loaded microspheres, (b) and (c) SEM images of MTX loaded microspheres at different magnifications. Figure is reproduced from the reference [161] copyright © 2015 Elsevier. (B) Structural and graphical presentations of controlled drug loading and release by light-sensitive 3PEG − PCL. Figure is reproduced from the reference [173] copyright © 2019 American Chemical Society.
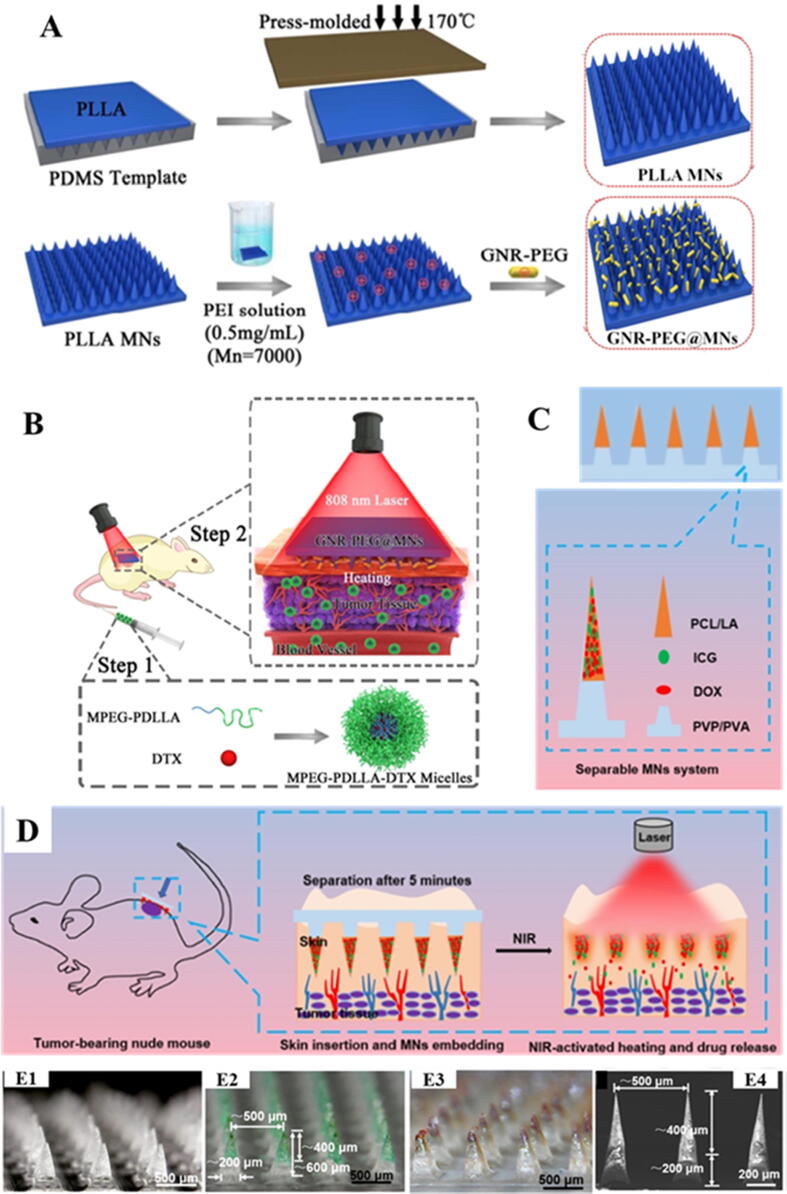
The microneedles (MNs) are one of the most advanced polymeric microstructures which are extensively being investigated in recent years. Physical drug delivery enhancement improves skin cancer treatment efficacy. MNs are proved quite useful in managing actinic keratoses and NMSC [162]. The MNs technique has often been used to promote noninvasive intradermal/transdermal delivery of drugs. They have potential to favor the delivery of drug molecules across the skin. MNs enhance systemic drug absorption and improve bioavailability by acting as a microinjection device [163]. MNs are categorized into five categories: coated, solid, hollow, dissolvable and hydrogel-forming MNs usually made up of polymers. Recently, MNs are frequently being explored to deliver the anticancer drugs to skin tumors [164]. Considering the potential of combination of photothermal therapy (PTT) and chemotherapy, Hao et al., have reported a novel smart delivery system to overcome the limitations associated with high doses of photothermal and chemotherapeutic agents. A near-infrared (NIR) responsive PEGylated gold nanorod (GNR-PEG) were fabricated and then coated on poly (l-lactide) microneedle (PLLA MNs) system (GNR-PEG@MNs). On the other hand, docetaxel-loaded MPEG-PDLLA (MPEG-PDLLA-DTX) polymeric micelles were fabricated. The developed MNs system was used to improve the anticancer effectiveness of intravenously administered MPEG-PDLLA-DTX polymeric micelles to treat an A431 tumor. Schematic illustration of the smart delivery system developed here is provided in Fig. 4. Both in vitro and in vivo studies showed an excellent heating efficiency of GNR-PEG@MNs containing only 31.83 ± 1.22 μg of GNR-PEG/patch. Moreover, GNR-PEG@MN of 480 μm height showed good skin insertion properties, and in vivo heat transferring ability, demonstrating that tumor site could attain 50 °C within 5 min. In contrast to PTT and chemotherapy alone, the combination of GNR-PEG@MNs and low dose MPEG-PDLLA-DTX micelles has eradicated an A431 tumor completely, showing the significant synergetic effects. Thus, GNR-PEG@MNs may prove a promising polymeric carrier for enhancement of antitumor activity of DTX loaded polymeric micelles for the treatment of superficial tumors and exhibits potential for clinical translation for epidermoid cancer treatment [165]. Following this, in another study, Hao et al, have developed an improved version of their previously established synergistic system. Herein, the authors have fabricated NIR responsive 5-fluorouracil (5-Fu; an anticancer) and indocyanine green (ICG; photothermal agent) encapsulated monomethoxy-poly (ethylene glycol) polycaprolactone (MPEG-PCL) NPs (5-Fu-ICG-MPEG-PCL). Subsequently, 5-Fu-ICG-MPEG-PCL NPs loaded hyaluronic acid (HA) dissolving MN arrays (HA MNs) were developed to treat melanomas and human epidermoid cancers. HA, an FDA approved pharmaceutical ingredient, used to fabricate MNs, possesses good skin insertion properties. The results indicated the potential of HA MNs to efficiently deliver 5-FuICG-MPEG-PCL NPs to the skin, and NIR controlled release of drug from the NPs. Hence, the developed system has shown the potential for achieving a single-dose therapy of skin cancer [166].
Fig. 4.
Utilization of different polymeric MNs based systems to achieve synergistic effects of chemo and photothermal therapy. (A) Schematic presentation of the preparation of PLLA MNs and GNR-PEG@MNs, (B) Working protocol of the novel synergetic system to treat A431 tumors by the combination of NIR responsive GNR-PEG@MNs and MPEG-PDLLA-DTX micelles. (Step 1: Injected the DTX loaded micelles; Step 2: After the injection, pressed the GNR-PEG@MNs at the tumor sites and under 2 W/cm2 irradiation by 808 nm laser within 5 min). Images are reproduced from the reference [165] copyright © 2017 American Chemical Society. (C) Illustration of the composition of the separable MNs system. (D) Schematic presentation of the working of ICG/DOX loaded separable MNs system for synergistic chemo-photothermal therapy against superficial skin tumors. Digital microscopic images of separable MNs without ICG and DOX (E1 and E4), with ICG (E2) and with ICG and DOX (E3). Images are reproduced from the reference [167] copyright © 2020 American Chemical Society.
In another recent study, a combination chemo-photothermal synergistic system has been developed for superficial skin tumors (SST). For the purpose, two-stage separable MNs were fabricated. Polycaprolactone and lauric acid (PCL/LA) as phase change materials were employed to develop the arrowheads of the two-stage separable MNs. Arrowheads were loaded with doxorubicin and ICG. The arrowheads were capped on the dissolvable bases comprised of polyvinyl pyrrolidone and poly (vinyl alcohol) (PVP/PVA). Upon insertion into skin, the PVP/PVA support bases were quickly dissolved resulting in the separation of arrowheads in the skin layers. Upon NIR irradiation, the embedded arrowheads were ablated owing to the photothermal conversion of the ICG, which in turn liberated the DOX from the MNs for penetration into the tumor site. In vivo studies in melanoma mouse model showed synergistic effects of two-stage separable MNs based chemo and phototherapy in treating skin cancers [167]. Visual representation of the developed system is provided in Fig. 4.
For cancer treatment, Novel 3D printed polymeric MN arrays are also being utilized for augmented cisplatin delivery to A-431 epidermoid skin tumors. The use of 3D printed MNs confirmed the capability of the system for dermal delivery of anticancer agents via tumor inhibition effect [168]. Topical 5-fluorouracil (5-FU) is an approved treatment for superficial BCC. By pretreatment with polymeric MNs having 50 µm base width and 50 µm height, 4.5-fold increased permeation flux of 5-FU through the full thickness skin was achieved. In an in vivo mouse model containing B16-F10 mouse melanoma cells, the anticancer efficacy of a market product of 5-FU (5% topical cream) was remarkably improved upon the application of cream on a MNs pretreated skin, in contrast to the skin not treated with the MNs. 5-FU has not been approved for melanoma therapy, but the clinical effectiveness of topical 5-FU against BCC can be enhanced by combining it with MNs technology [169].
In the past several decades, amphiphilic block copolymers with both hydrophilic and hydrophobic segments, and an ability to spontaneously self-assemble into spherical micelles, have been emerged as promising nanocarriers to improve the efficacy of chemotherapy. Their ability to control the self-assembly in an aqueous environment provides adaptability and versatility to modify the fabrication of different micellar nanostructures, including nanocapsules, core − shell nanospheres, vesicular structures and hollow spheres [170], [171]. Polymeric micelles, typically, ranged from 10 to 80 nm in size penetrate skin through follicular pathway via accumulation in hair follicles. Functionalization of these micelles with ligands (antibodies, carbohydrates, and aptamers) or by using block copolymers, their efficacy can be improved [172].
A recent study has reported the synthesis of rapidly self-assembled spherical micelles. A light-responsive, ultrasensitive block copolymer, consisting of multiarmed poly (ethylene glycol)-b-poly(caprolactone) (PEG-b-PCL) polymer was used as a water-soluble segment and maleimide-anthracene linkers were employed as a photosensitive element, to fabricate the desired self-assembled spherical micellar NPs. The developed smart micelles showed unique characteristics, including extremely low critical micelle concentration, modifiable drug-loading capacity, desirable structural stability, and ultrasensitive light-responsive drug delivery. Additionally, cellular studies illustrated that upon UV irradiation for 10 s, the drug-loaded micelles completely and rapidly released the drug within the cells in result of degradation of the maleimide − anthracene cycloadduct linkers, subsequently, the released drug exhibited strong cytotoxic activity in oral SCC as the micelles had effectively delivered the drug into the cellular nucleus. Given the simplicity of their design, and the quality performance, these new light-sensitive polymeric micelles could be a promising approach for the establishment of a multifunctional nanocarrier system [173]. Schematic illustration of the designed intelligent system is provided in Fig. 3.
In another study, Wan et al., have reported the synthesis of reduction-responsive polymeric micelles for the targeted delivery of drug to cancerous cells. For the purpose, D-α-Tocopheryl polyethylene glycol succinate-folate (TPGS) was used as a copolymer. TPGS has been used in drug delivery based on its amphiphilic property. However, TPGS having PEG1000 does not keep a long blood circulation owing to short chains of PEG1000. Therefore, authors have fabricated TPGS3350 with PEG3350 for stabilization of the micelles, enhanced blood circulation time and prevention of their non-specific cellular uptake. Following the accumulation of the micelles in tumor region by EPR effect, tumor cells may ingest these via endocytosis mediated by folate-receptors. Based on the higher content of glutathione (the reducing agent) in cancer cells, breakage of disulphide link favors the rapid release of docetaxel to show its anticancer effect. The developed micelles exhibited different functions including reduction responsively, active targeting, extended blood circulation, and rapid intracellular drug release, thus, enhanced the drug delivery efficiency [174]. Among different available therapeutic options for the treatment of skin melanomas, localized application of imiquimod (IMQ), provides a non-invasive, acceptable option. IMQ acts as an immune response modulator. IMQ is available in the market as Aldara® (5% cream) which is primarily indicated to treat the BCC. The block copolymer ‘methoxy-poly (ethylene glycol)-)-hexyl-substituted lactide (mPEG-hexPLA)’ self-assembled micelles have been used to encapsulate poorly aqueous soluble IMQ in order to enhance its cutaneous bioavailability. 0.05% of IMQ micelles (27 nm size) were loaded into carboxymethyl cellulose (CMC) based gel. The formulation showed > 17-fold selective cutaneous retention in contrast to transdermal permeation. The developed 0.05% gel showed remarkable delivery efficiency into human skin in comparison to Aldara® cream. Hence, allowing therapeutically relevant IMQ concentrations to be delivered at target site in spite of a 100-fold dose reduction [175]. Thus, these aforementioned studies reporting the designing of self-assembled polymeric micelles exhibited the potential of amphiphilic block copolymers for establishment of smart delivery systems for controlled drug targeting.
Nanogels are aqueous soluble cross-linked polymeric networks having dimensions in nanometer range which can be developed to incorporate various kinds of compounds to design potential carrier systems for biological molecules and drugs. Hydrophilic and thermo responsive three-dimensional cross linked nanogels with dendritic polyglycerol (dPG) have been used to enhance the skin penetration of anticancer drugs [176], [177]. In a recent study, in response to the tumor’s acidic environment, pH-responsive double walled PLGA-chitosan nanogels were developed to load 5-FU. 5-FU was encapsulated into PLGA core. To achieve interaction with anionic cancer cell membrane, these were further coated with chitosan. Additionally, eucalyptus oil as a penetration enhancer was coated on the biodegradable polymeric nanogels. The results showed the bio and cytocompatibility of drug loaded double walled nanogel in human keratinocyte (HaCaT) cell lines, and its high skin penetration and cellular uptake, suggesting that this novel polymeric nanogel could be a promising approach for treatment of skin cancers [178].
Polymeric nanocapsules have been exhibited to have potential to reduce side effects associated with some drugs. However, these are formed as liquid formulations, hence, presenting difficult skin application owing to their low viscosity. To address this problem these are loaded into polymeric hydrogels composed of bio adhesive polymers including Carbopol®, chitosan and hydroxypropyl methylcellulose to make their topical application possible. Recently, Gazzi et al., have used pectin (a natural polymer), to formulate a semisolid formulation. Firstly, they have developed IMQ-loaded polymeric nanocapsules then incorporated into pectin-based hydrogel. The investigations showed that IMQ-nanocapsules loaded hydrogel displayed better cell viability, skin penetration and adhesiveness, release profile, and cytotoxic effects in contrast to the solution of the drug, proving the promising potential of nanoencapsulation for drug delivery efficiency [179].
Chitosan is a cationic biodegradable polymer. The positive charge favors strong interaction with the negatively charged skin surface; hence, alter the barrier function and deliver cargoes [172]. Nanoemulsions of 5FU were prepared using Capyrol (propylene glycol monocaprylate) as oil, polyethylene glycol (PEG) 400 as a co-surfactant, and transcutol as a surfactant. A remarkable increase in permeation was achieved with nanoemulsions in contrast to the control conventional gel. The results suggested that the developed formulation could safely be used to enhance skin permeability of 5FU following the topical application [180].
For site-specific areas of SCC, the effectiveness of conventional treatments, including surgery, is not satisfactory. PDT through topical application of prodrug 5-aminolevulinic acid (ALA) is could be a simple and effective alternative for skin carcinomas. In spite of promising potential of ALA PDT for therapy of superficial SCC, it is not being preferred for invasive SCC, primarily owing to its limited skin bioavailability. ALA-loaded PLGA-NPs were fabricated to address this aforementioned problem. The results indicated that the designed NPs improved the protoporphyrin IX production in cutaneous SCC, suggesting that ALA PLGA NPs induced topical PDT found to be more effective in treating SCC as compared to free [181]. A recent study has reported fabrication of lipid coated chitosan NPs to load ferrous chlorophyllin (Fe-CHL), a photosensitizer in order to improve the Fe-CHL delivery for effective PDT of SCC. The NPs showed their promising PDT effects in human SCC monolayers. The skin retention and cytotoxicity results indicated the potential of nanocarriers for treatment of SCC employing PDT [182].
Decoration of NPs with complementary ligands facilitates the cancerous cell specific targeting. Once the NPs bind with the receptors, they rapidly undergo receptor-mediated endocytosis or phagocytosis by cells, resulting in cellular internalization of the loaded drug. Dendrimers are mono dispersed and multivalent NPs possessing a central core that provides a symmetrical arrangement of repeating units. Highly branched structures of dendrimers provide a great number of surface functional groups. Their structure favors the inclusion of both hydrophilic and lipophilic drugs and imaging agents [183]. The cationic dendrimers have the ability to modify skin permeation via interaction with its lipids. Dendrimers are also used for efficient delivery of anticancer, antiviral, antimicrobial and antihypertensive drugs [184].
Dendrimers incorporating 5-FU, have also been investigated for topical application. Amine-terminated dendrimers may act as polymeric skin enhancers for hydrophilic drugs like 5-FU. Pre-treatment of skin with dendrimers with isopropyl myristate (IPM) enhanced the tissue internalization of 5-FU; hence, increasing its permeability coefficient by reducing drug solubility in IPM [185]. Hu et al., have developed dual pH and redox responsive system by introducing redox-sensitive disulfide linkages between poly (ethylene glycol) (PEG) and poly (amidoamine) dendrimers (PAMAM) to achieve both long circulating time and efficient intracellular drug release. Doxorubicin (DOX) was loaded into the hydrophobic core of the conjugates to get PAMAM-SS-PEG/DOX complexes. The results of release studies displayed the acid-triggered release of DOX from the established complexes. Moreover, cellular uptake mechanism of the complexes was determined to be caveolae and clathrin-mediated endocytosis. Lastly, in vivo studies in B16 tumor-bearing mice indicated that these could remarkably enhance anticancer effectiveness offering a good safety profile [186].
Polymeric micro/nano-structures for gene delivery to skin cancers
The conventional therapeutic options for skin melanomas include chemotherapy, surgical removal, radiation therapy, immunotherapy, and biotherapy [187]. Notwithstanding, their therapeutic effectiveness is limited owing to their high toxicity, associated drug resistance, and poor selectivity [188]. Gene therapy is a powerful approach for the treatment of skin cancers as it targets the source of the disease rather symptomatic relief, offering high specificity and low toxicity [189]. However, there are some problems associated with cutaneous gene delivery, including skin barriers and the small number of cells getting transfected. Recent advancements have presented the potential of non-viral delivery systems for cutaneous gene delivery, demonstrating their superiority over viral vectors, - ‘the current gold standard’ – in terms of manufacturing and safety concerns [190]. Some of these advanced non-viral, polymeric micro and nanostructures-based gene delivery systems have been described here.
In most of the malignant tumors, signal transducer and activator of transcription 3 (STAT3) plays a pivotal role in tumor proliferation, survival, angiogenesis, metastasis, and immune evasion, and is reported to be hyperactive in skin melanomas [191]. Gene therapy employing short interfering RNA (siRNA) targeting STAT3 is a promising therapeutic option for skin cancers. A recent study has reported the fabrication of novel polymeric delivery system for topical delivery of STAT3 siRNA. Polyethylenimine (PEI) has been used as a carrier to encapsulate STAT 3 siRNA to increase its cellular uptake, and STAT3-siRNA-PEI complex has further loaded in to the dissolving microneedles (MNs) composed of biocompatible polymers: dextran, hyaluronic acid (HA), and polyvinylpyrrolidone (PVP), with intention to increase its intradermal penetration. The results of the study have demonstrated that MNs can efficiently penetrate in the skin and rapidly dissolve there. In vitro B16F10 cell experiments showed the enhanced cellular uptake and transfection of siRNA, increased gene silencing and tumor inhibition. Moreover, in vivo studies in mouse melanoma model demonstrated that topical delivery of STAT3 siRNA PEI complex via dissolving MNs can efficiently suppress the melanoma through silencing STAT3 gene, proving its potential for treatment of skin melanoma with targeted inhibition efficiency and minimum adverse effects [192]. In many studies, p53 tumor suppressor gene (p53 DNA) has also been widely explored for cancer treatment. However, its low therapeutic efficacy and lack of sustained delivery, demands for synergistic therapies to treat cancer efficiently [193].
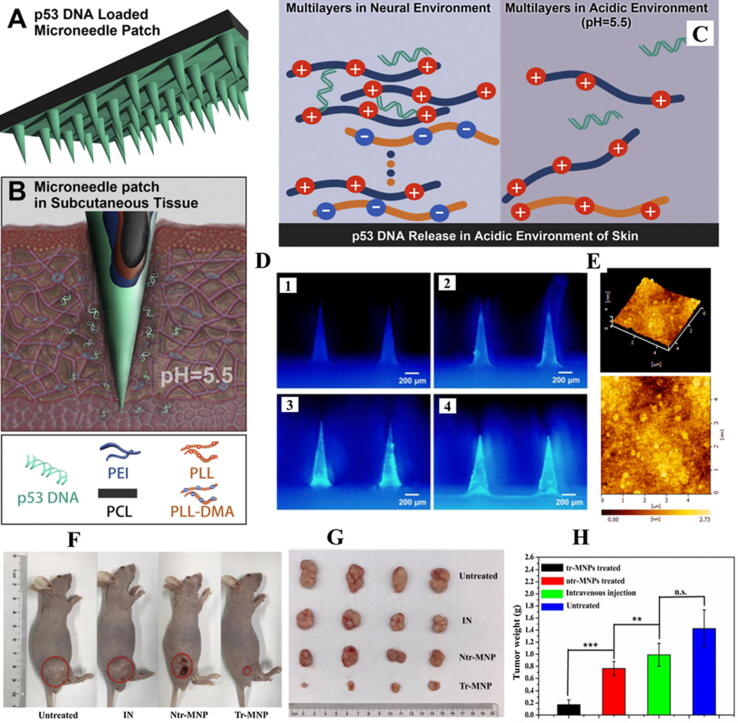
Photothermal therapy (PTT) has been reported to have great potential to treat cancer. Near-infrared (NIR) light has been extensively employed in PTT due to its capability to penetrate tissues above the tumor site and relatively lesser side effects. PTT with NIR light exhibits low systemic toxicity and inhibits tumor growth through spatiotemporally controlled photothermal effects [194], [195]. A recent study has reported the design of polymeric MNs patch to simultaneously deliver IR820 (a derivative of NIR dye, as a photothermal agent) and p53 DNA to a tumor site. p53 DNA and IR820 were co-loaded into the tips of HA based MNs to reduce waste. The results of the study demonstrated the efficient penetration of MNs in the stratum corneum, and rapid dissolution to deliver IR820 and p53 DNA to the tumor site. At the site of application of MNs patches temperature of the tumor site increased by 14.7℃ owing to the photothermal efficacy of IR820, upon NIR light irradiation. The excellent synergistic in vivo antitumor effects of p53 DNA/IR820 based MN patch have proved this novel delivery system to be a promising approach for the treatment of subcutaneous tumors [196]. Another recent study has explored the potential of polymeric microneedles combined with acidic cutaneous environment for direct and rapid delivery of p53 DNA to the skin for enhanced patient compliance. For the purpose, polycaprolactone (PCL) MNs were designed by layer-by-layer assembly and coated with pH-responsive polyelectrolyte multilayers (PEM). A charge reversible polymer, dimethylmaleic anhydride-modified polylysine (PLL-DMA) was introduced to PEM. The PEM was made up of two parts: the transition layers of (PLL-DMA/polyethyleneimine) 12 and the gene loaded layers of (p53 expression plasmid/polyethyleneimine) 16. The modified MNs patch with the PEM (coded as tr-MNP) could load 31 μg model DNA and improve gene release, in contrast to the control (MNP without transition layers, coded as ntr-MNP). Tr-MNP showed 33% release of model DNA in simulative cutaneous environment (pH = 5.5) in comparison to only 4% by ntr-MNP, based on the reason that PLL-DMA could achieve charge reversal in cutaneous acidic environment, resulting in the collapse of transition layers and subsequently the release of the gene. In vivo studies have displayed the enhanced tumor inhibitory effects of tr-MNP treated mice (90.1%) as compared to the ntr-MNP treated mice (46.4%) and intravenously administered mice (30.5%). Hence, the pH-responsive, DNA loaded MN patch could potentially treat the subcutaneous tumors (155). Visual representation of the polymeric microneedles-based microenvironment responsive delivery platform designed in the study is provided in Fig. 5.
Fig. 5.
Visual representation of the polymeric microneedles-based microenvironment responsive delivery platform for rapid release of the gene. (A, B and C) Schematic illustration of the microneedle patch modified with pH-responsive transition layers and gene (p53 DNA)-loaded layers, via layer-by-layer assembly. (D) The fluorescence images of tr-MNP modified with 4, 8, 12, 16 bilayers of modal DNA (D1, D2, D3 and D4 respectively). (E) The topography of silicon pieces modified with transition layers and gene-loaded layers. (F) Image of representative mice of four groups showing the very rare change in the weight, depicting the safety of the designed delivery system. (G) Image of isolated tumor after 21 days treatment of four groups. (H) Weight of isolated tumor after 21 days treatment of four groups (n = 4, * p <0.05), demonstrating that the p53 DNA loaded tr-MNP showed a great tumor suppression in comparison to the intravenous (IV) administration, because MNs can enhance drug utilization by avoiding gene loss in systemic circulation. Thus, both ntr-MNP and tr-MNP treated mice showed better tumor suppression in contrast to IV administration. Figure is reproduced from the reference [155] copyright © 2019 Elsevier.
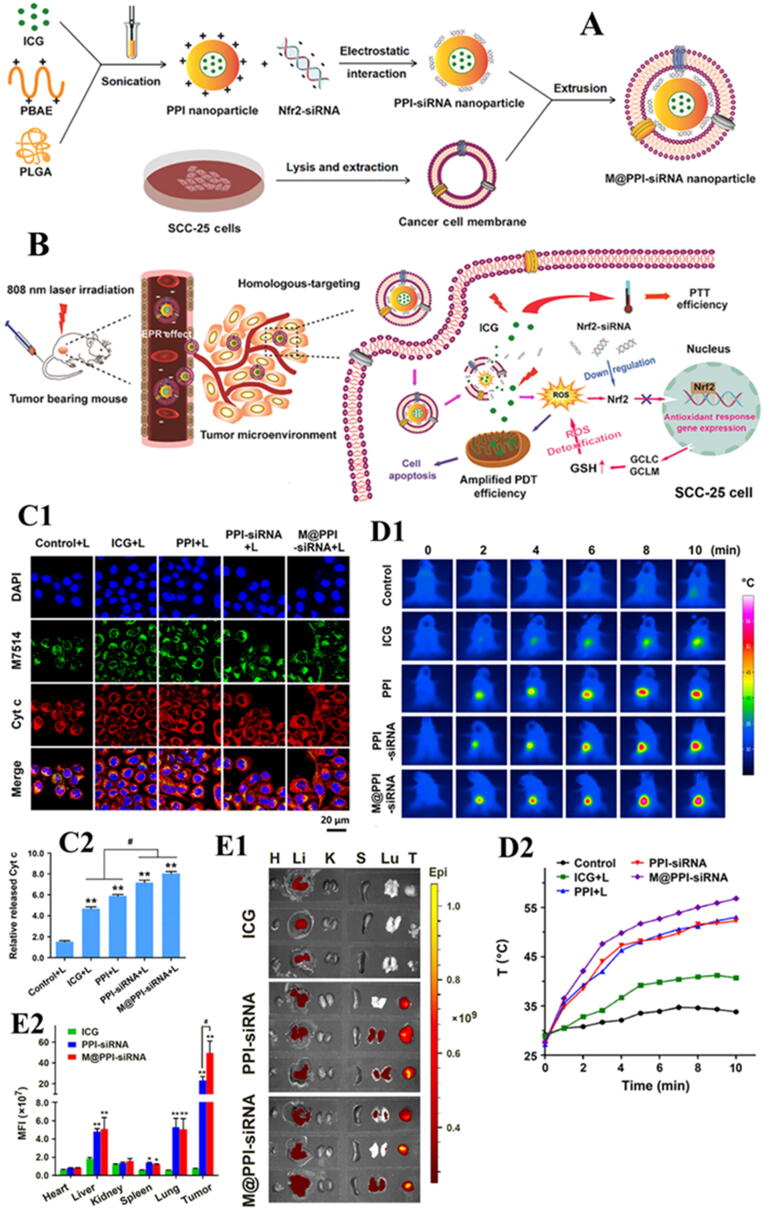
Recently, Shurui Shi et al. reported a novel biomimetic NPs delivery platform for synergistic effects of PTT and photodynamic therapy (PDT). PTT and PDT have been selected based on their advantages including non-invasive property, site selectivity and very less chances of drug resistance for the treatment of oral tongue squamous cell carcinoma (SCC). Nevertheless, the anticancer activity of PDT to some extent is compromised by activation of intracellular antioxidant responses. Considering the role of Nrf2, a redox regulated transcription factor, in the tumor resistance against PDT, Nrf2-siRNA has been chosen to design a biomimetic NPs system. Poly (β-amino ester) (PBAE)/poly lactic-co-glycolic acid (PLGA) blended NPs co-loaded with photosensitizer indocyanine green (ICG) and Nrf2-siRNA were fabricated and then coated with cancer cell membrane (CCM), specifically derived from homologous oral tongue SCC, hence, finally named as M@PPI-siRNA. Schematic illustration of the preparation of M@PPI-siRNA NPs is provided in Fig. 6. Both in vitro and in vivo, M@PPI-siRNA NPs showed strong SCC-targeting ability owing to tumor-homing effect of homologous CCM. A significant down-regulation of the expressions of Nrf2 and its regulated genes responsible for ROS detoxification, including glutamatecysteine ligase catalytic subunit (GCLC) and modifier subunit (GCLM) has been achieved upon laser irradiation (at 808 nm) of M@PPI-siRNA NPs. Hence, the designed NPs enhanced the anticancer effects of PDT indirectly, by preserving the intracellular ROS accumulation. M@PPI-siRNA NPs with combined effects of PTT and Nrf2-siRNA amplified PDT showed a remarkable suppression of tumor growth and angiogenesis in oral tongue SCC tumor-bearing mice. In short, this study provided a promising polymer-based SCC-targeted delivery platform for both ICG and gene, and also confirmed the efficiency of Nrf2-siRNA to be a potential synergist for PDT amplification (197].
Fig. 6.
Visual representation of the novel biomimetic NPs based delivery system. (A) Schematic illustration of the steps involved in the preparation of biomimetic NPs utilizing various polymers and cancer cell membrane derived from the oral tongue SCC. (B) Scheme presenting the mechanisms through which the designed delivery system worked to show the combined effects of gene (Nrf2-siRNA) delivery and amplification of PDT through the delivered gene. (C1) Confocal images of the cells after 48-hour treatments with free ICG, PPI, PPI-siRNA, and M@PPI-siRNA NPs combined with laser irradiation (+L). Mitochondria stained with MitoTracker green (M7514) and Cyt c stained with anti-Cyt c antibody emitted green and red fluorescence. (C2) Quantitative analysis of released Cyt c from mitochondria in the SCC-25 cells following the treatment with NPs and laser irradiation. Red fluorescence in the merged confocal images indicated Cyt c released from mitochondria into cytoplasm. Relative released Cyt c is defined as the fluorescence intensity ratio of release Cyt c in the treated cells to that in the control cells without laser irradiation. Scale bars present 20 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.) (D) PTT and PDT efficacies of M@PPI-siRNA NPs in SCC-25 tumor-bearing mice: (D1) IR thermal images of the mice and (D2) temperature changes inside the tumors during 10 min-laser irradiations at 8 h after IV administration of normal saline (the control), free ICG, PPI, PPI-siRNA, and M@PPI-siRNA NPs. Upon laser irradiation, M@PPI-siRNA NPs showed an improved heating efficiency as compared to other treatments, increasing the temperature up to 56.5 °C, that is sufficient for tumor ablation. (E) Tissue distributions and tumor accumulation of M@PPI-siRNA NPs in SCC-25 tumor bearing mice: (E1) Fluorescence images and (E2) mean fluorescence intensities (MFIs) of tumors and main organs collected from the mice at 24 h after various treatments. ** P < 0.01 compared to the control; # P < 0.05 for the comparison between two treatment groups. Figure is reproduced from the reference [197] copyright © 2020 Elsevier.
In a nutshell, polymers of both synthetic and natural origin have been successfully employed to fabricate the micro and nanostructures for efficient gene delivery to the skin cancers. Additionally, polymer’s chemistry is being exploited to design the smart delivery systems having potential to combine multiple therapies in a single micro/nano-platform, for full utilization of the gene/drug and the ultimate enhanced patient compliance.
Theranostic applications of polymer-based micro/nanostructures
Micro and nanostructures are considered most favorable types of theranostic agents based on their tunable optoelectronic properties. High doses of the drugs and biomolecules can be encapsulated in hollow nanostructures due to their high surface to volume ratio and porosity. These intrinsic properties make them eligible in various bioimaging applications as contrast agents and photon-triggered therapies (photothermal and photodynamic therapies). For diagnostic purpose, polymeric particles can be incorporated with paramagnetic metals, such as gadolinium (Gd) or manganese (Mn), as contrast agents for imaging. Nanoshells are one of the most efficient candidates to be used in multiple-purpose platforms that enable synergistic multimodal performance [198]. NPs with superparamagnetic iron oxide (SPIO) can be used to detect cancer. As MRI contrast agents, MNPs have been extensively examined to enhance the detection, diagnosis and therapeutic management of tumors.
Hou et al., have successfully developed theranostic NPs by chemically connecting IR820 (a promising NIR dye for cancer diagnosis and therapy) onto the surface of chitosan-coated magnetic iron oxide. The developed IR820-CS-Fe3O4 NPs showed an excellent MRI capability and cytotoxicity against melanoma upon irradiation with NIR laser (808 nm), along with reasonable stability for up to 8 days. Hence, a novel chitosan based theranostic platform for potential detection and therapy of melanoma has been established [199]. Upponi et al., have fabricated a nanosized combination platform for cancer diagnosis and treatment using polymeric polyethylene glycol phosphatidylethanolamine-based micelles incorporated with paclitaxel (poorly aqueous soluble anticancer) and hydrophobic superparamagnetic iron oxide NPs (SPION), as a contrast agent. The co-loading of SPION and paclitaxel in polymeric micelles had not affected functional activities of both agents, showing both apoptotic activity and MRI contrast properties in breast and melanoma tumor mice models. The developed theranostic system can play an important role in the combined diagnosis and treatment leading to a more effective and personalized treatment [200].
Recently, Shen et al., have designed a complex PLGA nanocomposite for co-loading of QDs, Fe3O4 nanocrystals, and doxorubicin (DOX). PLGA nanocomposites were fabricated via double emulsion solvent evaporation method, then coupled to the amine group of polyethyleneimine pre-modified with PEG-folic acid (PEI-PEG-FA [PPF]) segments, following the adsorption of vascular endothelial growth factor (VEGF)-targeted small hairpin RNA (shRNA). VEGF is essential for tumor metastasis. These drug-loaded luminescent PLGA-based nanocomposites have been designed for tumor-selective targeting, gene/drug delivery, and imaging. The results indicated the potential of nanocomposites for co-delivery of DOX and VEGF shRNA into tumor cells and effective suppression of VEGF expression, showing significant enhanced antitumor effects. Additionally, both fluorescence and magnetic resonance imaging potential of the hybrid nanocomposites suggested that it can be employed as an efficient nanoprobe. Hence, these multifunctional polymeric nanocomposites have exhibited a promising potential to be an effective theranostic platform for codelivery of genes/drugs and dual-modality imaging for cancer therapy [201].
Conclusion and future prospective
Among the various approaches used to deliver the anticancer agents to cure skin cancer, use of nanomedicine has successfully dealt with limitations associated with conventional therapies. Dermal drug delivery system is an attractive, non-invasive approach to prevent and treat skin cancers. Nanomedicine treatment is favorable for patients for whom surgery is not suitable or those who cannot survive highly intensive non-specific therapies. Notwithstanding, barrier properties of the hyperkeratotic SCC lesion along with its intra-tumoral obstructions limit the performance of nanomedicine by reducing the level of drug molecule within tumor core. Moreover, different individual responses in the form of systemic toxicity to the exposure of applied doses also harmful for the development of these therapies. Polymer-based nanostructures of anticancer drugs can cross the SC and deliver the cargo to the target site without posing any serious side effect to skin cells. Polymer’s chemistry has enabled the synthesis of a wide variety of polymeric micro/nanostructures to combat with limitations associated with conventional therapies and to overcome the drug delivery barriers. Polymeric nanocarriers have played their role in improving skin targeting, drug’s ability to reach and penetrate into tumor cells. Furthermore, nanocarriers offered improved drug stability and reduced skin irritation by avoiding direct contact of cargoes with the skin’s surface. Most of the patients could have developed drug resistance against some anticancer agents, when these are used alone, like BRAF (oncogene v-Raf murine sarcoma viral oncogene homolog B1) inhibitors. However, their targeted delivery has remarkably improved their therapeutic response (5% to 80%), indicating the importance of selective delivery. Hence, for selective delivery of cargos to the tumor, polymeric micro/nanostructures have been designed to exploit specific cancer microenvironment like, temperature, specific enzymes, redox reaction, and pH to establish targeted drug delivery systems. The patients suffering from metastasized melanoma show average survival time of 6 to10 months only, with < 20% achieving 5-years survival rate. Thus, effective therapy of melanoma is required. To achieve this, enhancements in both the diagnosis and treatment are badly needed. Its timely diagnosis can lead to surgical removal prior to metastasis. For the purpose, polymeric structures have also exploited for delivery of contrast agents, for efficient imaging and detection of the disease pathology. Although, NPs face challenges, yet to improve these drawbacks continuous research could deliver exciting and promising results in coming days. The combination approaches including combination of passive and active targeting (tumor microenvironment responsive and targeted delivery), combination of chemotherapy with PTT and PDT, and combination of diagnostics and therapeutics (theranostics), and combination of drug and gene delivery could be a potential strategy to overcome the limitations/barriers faced by nanoparticles. In biomedical sciences, polymer-based nanostructures are highly versatile in their functioning and mechanisms of actions that lead to provide tremendous opportunities to magnificently translate the novel therapies at clinical levels.
Promising role of polymeric-nanomedicine in revolutionizing the cancer therapeutics–sciences of today, especially in skin cancer and fronting challenges demand highly interdisciplinary approaches in choosing the right cargo and the right carrier to develop and translate treatments more effectively. It also urges to explore further about the cargo-carrier combination, formulation strategies and their physical enhancement strategies for improved delivery through lesions and surrounding skin. Hence, the knowledge gained, would make the application of these approaches possible not only in therapeutic with better penetration/retention and controlled release in the skin but also for diagnosis and prophylaxis. Furthermore, recognition of the responsible gene mutations and comprehension of impairment in the primary signaling pathways could be helpful in designing the suitable therapeutic regimes. Successful therapy will also depend on the discovery of specific, more potent pharmacological agents, and importantly, novel intelligent smart delivery systems to achieve synergistic effects. We are expecting that all aimed approaches will be there in near future where next generation of skin cancer diagnostic and drug delivery system based on polymeric nanostructures will incorporate several features into a single system of personalized medicines.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
CRediT authorship contribution statement
Nazeer Hussain Khan: Conceptualization, Data curation, Writing - original draft. Maria Mir: Conceptualization, Visualization, Writing - original draft, Writing - review & editing. Lei Qian: Data curation, Writing - original draft. Mahnoor Baloch: Writing - original draft. Muhammad Farhan Ali Khan: Writing - original draft. Asim-ur- Rehman: Writing - review & editing, Supervision. Ebenezeri Erasto Ngowi: Data curation, Writing - original draft. Dong-Dong Wu: Supervision, Writing - review & editing. Xin-Ying Ji: Supervision, Funding acquisition, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to thank the journals/publishers that granted us permission to reuse their data. NHK pays thanks to his Piayree Lala for his presence and support in the long challenging journey of PhD.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Nos. 81802718, 81670088), the Foundation of Science & Technology Department of Henan Province, China (Nos. 202102310480, 192102310151), and the Training Program for Young Backbone Teachers of Institutions of Higher Learning in Henan Province, China (No. 2020GGJS038).
Ethics approval
Not applicable.
Biographies

Nazeer Hussain Khan is a PhD student at Henan International Joint Key Laboratory for Nuclear Protein Regulation, School of Life Sciences Henan University China. Before arriving at Henan, Hussain earned his MS in Animal and Human physiology from a Quaid I Azam University Islamabad Pakistan: a prestigious institute of the country. He is actively engaged in exploring the different mechanisms of cancer onset and regulation of nuclear proteins especially PCNP with their potential involvement in cancer cells proliferation, migration and invasion. Up to now, along with laboratory work, he published many scientific articles under the guidance of supervisor.

Maria Mir has earned her PhD in Pharmaceutics from the Department of Pharmacy, Quaid-i-Azam University Islamabad, Pakistan. Her research is focussing on the development of stimuli responsive polymeric nanoparticles and microneedles for site-specific delivery of drug cargoes. She also worked as a visiting research associate in the Microneedle Drug Delivery Group, School of Pharmacy, Queens University Belfast, United Kingdom, where she had extensive experience in the development of various types of polymeric microneedles and had published her work in Q1 journals.

Lei Qian is MS student of Henan International Joint Laboratory for Nuclear Protein Regulation at Henan University China. The research direction is the regulation effect of PCNP on the growth of human hepatocellular carcinoma cells. At present, it has been found that PCNP has an inhibitory effect on human hepatocellular carcinoma cells after overexpression, and the relevant mechanism is being explored to study the effect mechanism of PCNP on the poor prognosis of carcinoma.

Mahnoor Baloch is a postgraduate, young researcher at school of Natural Sciences, National University of Science and Technology Islamabad, Pakistan. She is passionate about caring for the environment and pursuing her career as environmental chemist and activist on current environmental dilemmas related to environmental hazards and radiation as a threat to human health issues including cancer. Cureently, she is exploring about different environmental pollutants and assessing their consequence to human health.

Muhammad Farhan Ali Khan graduated from College of Pharmacy, University of The Punjab, Pakistan. Then, he worked as Research Associate in Quaid-i-Azam University Islamabad. He has expertise in Solid lipid and Polymeric nanoformulations. He has, also, worked in Transdermal drug delivery. Currently, he is designing a project on Microneedles for transdermal delivery of peptides to treat metabolic diseases.

sim-ur-Rehman is an associate professor in the Department of Pharmacy at Quaid-i-Azam University Islamabad, Pakistan since 2014. Dr. Asim’s key interests include nanomedicines, pharmaceutical technology and drug delivery. His research focuses on the development of polymeric micro and nanostructures including microneedles, nanoparticles and nanogels/emulsions for enhancement of drug delivery through skin. He directly supervises numerous PhD, MPhil and graduate students. Currently, he has more than 80 publications including articles in peer-reviewed journals, invited articles, book chapters, conference papers and abstracts related to nanotechnology based polymeric drug delivery systems.

Ebenezeri Erasto Ngowi, a young promising researcher from Tanzania, graduated from the University of Dar es Salaam (UDSM), and served as a tutorial assistant in the field of animal physiology at the University of Dar es Salaam (DUCE). He is currently, pursuing Masters of Science in Biochemistry and Molecular Biology in Henan University, China. So far, he has 4 publications in zone 2 journals published between October, 2020 and March, 2021. His research focus is on the therapeutic potential of hydrogen sulfide and PEST-containing nuclear protein (PCNP) in cancer treatment.

Dong-Dong Wu obtained his PhD from Department of Physiology and Pathophysiology, Shanghai Medical College, Fudan University, Shanghai, China. Currently he serves as Associate Professor of Physiology and Vice President of School of Stomatology at Henan University and Deputy Director of Henan International Joint Laboratory for Nuclear Protein Regulation (at Henan University). He has been elected to membership within a number of scientific committees across China, including Chinese Society of Immunology, Chinese Anti-Cancer Association, and Chinese Society for Microbiology. Dr Dong-Dong Wu has authored more than 50 original research papers and review articles, focusing role of hydrogen sulfide and nucleoprotein in metabolic diseases and cancer.

Xin-Ying Ji, MD, PhD, is a professor in Henan University College of Medicine, focusing on the functions of novel nuclear protein PCNP (PEST Proteolytic Signal Containing Nuclear Protein). He is vice dean for international affairs. As the founder of Henan International Joint Laboratory for Nuclear Protein, Dr. Ji has made fundamental progress in PCNP associated with tumor.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Dong-Dong Wu, Email: ddwubiomed2010@163.com.
Xin-Ying Ji, Email: 10190096@vip.henu.edu.cn.
References
- 1.Losquadro W.D. Anatomy of the skin and the pathogenesis of nonmelanoma skin cancer. Facial Plastic Surgery Clinics. 2017;25(3):283–289. doi: 10.1016/j.fsc.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Urban K., Mehrmal S., Uppal P., Giesey R.L., Delost G.R. The global burden of skin cancer: A longitudinal analysis from the Global Burden of Disease Study, 1990–2017. JAAD International. 2021;2:98–108. doi: 10.1016/j.jdin.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, et al. Dermatologist-level classification of skin cancer with deep neural networks. nature. 2017;542(7639):115-8. [DOI] [PMC free article] [PubMed]
- 4.Gray-Schopfer V., Wellbrock C., Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445(7130):851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 5.Davids L, Kleemann B. The menace of melanoma: a photodynamic approach to adjunctive cancer therapy. Melanoma-From Early Detection to Treatment: IntechOpen; 2013.
- 6.Pópulo H., Soares P., Lopes J.M. Insights into melanoma: targeting the mTOR pathway for therapeutics. Expert opinion on therapeutic targets. 2012;16(7):689–705. doi: 10.1517/14728222.2012.691472. [DOI] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7-30. [DOI] [PubMed]
- 8.Society AC. Cancer Facts & Figures. 2017.
- 9.Didona D., Paolino G., Bottoni U., Cantisani C. Non melanoma skin cancer pathogenesis overview. Biomedicines. 2018;6(1):6. doi: 10.3390/biomedicines6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barton V., Armeson K., Hampras S., Ferris L.K., Visvanathan K., Rollison D., et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309(4):243–251. doi: 10.1007/s00403-017-1724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craythorne E., Al-Niami F. Skin cancer. Medicine. 2017;45(7):431–434. [Google Scholar]
- 12.Green A.C., Olsen C. Cutaneous squamous cell carcinoma: an epidemiological review. Br J Dermatol. 2017;177(2):373–381. doi: 10.1111/bjd.15324. [DOI] [PubMed] [Google Scholar]
- 13.Apalla Z., Lallas A., Sotiriou E., Lazaridou E., Ioannides D. Epidemiological trends in skin cancer. Dermatology practical & conceptual. 2017;7(2):1. doi: 10.5826/dpc.0702a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eide M.J., Krajenta R., Johnson D., Long J.J., Jacobsen G., Asgari M.M., et al. Identification of patients with nonmelanoma skin cancer using health maintenance organization claims data. Am J Epidemiol. 2010;171(1):123–128. doi: 10.1093/aje/kwp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green A. Changing patterns in incidence of non-melanoma skin cancer. Epithelial Cell Biol. 1992;1(1):47–51. [PubMed] [Google Scholar]
- 16.Carr S., Smith C., Wernberg J. Epidemiology and risk factors of melanoma. Surgical Clinics. 2020;100(1):1–12. doi: 10.1016/j.suc.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Coleman WB, Tsongalis GJ. The molecular basis of human cancer: Springer Science & Business Media; 2001.
- 18.Dawes S.M., Tsai S., Gittleman H., Barnholtz-Sloan J.S., Bordeaux J.S. Racial disparities in melanoma survival. J Am Acad Dermatol. 2016;75(5):983–991. doi: 10.1016/j.jaad.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Wu S., Cho E., Li W.-Q., Weinstock M.A., Han J., Qureshi A.A. History of severe sunburn and risk of skin cancer among women and men in 2 prospective cohort studies. Am J Epidemiol. 2016;183(9):824–833. doi: 10.1093/aje/kwv282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins L., Quinn A., Stasko T. Skin cancer and immunosuppression. Dermatol Clin. 2019;37(1):83–94. doi: 10.1016/j.det.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Yeung H., Balakrishnan V., Luk K.M.H., Chen S.C. Risk of skin cancers in older persons living with HIV: a systematic review. The Journal of the Association of Nurses in AIDS Care: JANAC. 2019;30(1):80. doi: 10.1097/JNC.0000000000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang A.Y., Doiron P., Maurer T. Cutaneous malignancies in HIV. Current Opinion in HIV and AIDS. 2017;12(1):57–62. doi: 10.1097/COH.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 23.Rollison D.E., Viarisio D., Amorrortu R.P., Gheit T., Tommasino M. An emerging issue in oncogenic virology: the role of beta human papillomavirus types in the development of cutaneous squamous cell carcinoma. J Virol. 2019;93(7) doi: 10.1128/JVI.01003-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols A.J., Gonzalez A., Clark E.S., Khan W.N., Rosen A.C., Guzman W., et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA dermatology. 2018;154(8):927–930. doi: 10.1001/jamadermatol.2018.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vekinis J., Morley A.M.S. Ocular surface biopsies of patients with xeroderma pigmentosum in the United Kingdom: a retrospective observational case series. Br J Ophthalmol. 2020 doi: 10.1136/bjophthalmol-2020-316125. [DOI] [PubMed] [Google Scholar]
- 26.Lova Navarro M., Vera Casaño Á., Benito López C., Fernández Ballesteros M.D., Godoy Díaz D.J., Crespo Erchiga A., et al. Transient Neonatal Zinc Deficiency Due to a New Autosomal Dominant Mutation in Gene SLC 30A2 (ZnT-2) Pediatr Dermatol. 2014;31(2):251–252. doi: 10.1111/pde.12257. [DOI] [PubMed] [Google Scholar]
- 27.Leachman S.A., Lucero O.M., Sampson J.E., Cassidy P., Bruno W., Queirolo P., et al. Identification, genetic testing, and management of hereditary melanoma. Cancer Metastasis Rev. 2017;36(1):77–90. doi: 10.1007/s10555-017-9661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shay J.W. Role of telomeres and telomerase in aging and cancer. Cancer discovery. 2016;6(6):584–593. doi: 10.1158/2159-8290.CD-16-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boscolo-Rizzo P., Da Mosto M.C., Rampazzo E., Giunco S., Del Mistro A., Menegaldo A., et al. Telomeres and telomerase in head and neck squamous cell carcinoma: from pathogenesis to clinical implications. Cancer Metastasis Rev. 2016;35(3):457–474. doi: 10.1007/s10555-016-9633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flies E.J., Mavoa S., Zosky G.R., Mantzioris E., Williams C., Eri R., et al. Urban-associated diseases: Candidate diseases, environmental risk factors, and a path forward. Environ Int. 2019;133 doi: 10.1016/j.envint.2019.105187. [DOI] [PubMed] [Google Scholar]
- 31.Parrado C., Mercado S., Perez-Davo A., Gilaberte Y., Gonzalez S., Juarranz A. Environmental stressors on skin aging. Mechanistic insights Frontiers in pharmacology. 2019;10:759. doi: 10.3389/fphar.2019.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt J., Haufe E., Trautmann F., Schulze H.J., Elsner P., Drexler H., et al. Is ultraviolet exposure acquired at work the most important risk factor for cutaneous squamous cell carcinoma? Results of the population-based case–control study FB-181. Br J Dermatol. 2018;178(2):462–472. doi: 10.1111/bjd.15906. [DOI] [PubMed] [Google Scholar]
- 33.Downs NJ, Axelsen T, Schouten P, Igoe D, P, Parisi A, V, Vanos J. Biologically effective solar ultraviolet exposures and the potential skin cancer risk for individual gold medalists of the 2020 Tokyo Summer Olympic Games. Temperature. 2020;7(1):89-108. [DOI] [PMC free article] [PubMed]
- 34.Ouhtit A., Konrad Muller H., Gorny A., Ananthaswamy H.N. UVB-induced experimental carcinogenesis: dysregulation of apoptosis and p53 signalling pathway. Redox Rep. 2000;5(2–3):128–129. doi: 10.1179/135100000101535447. [DOI] [PubMed] [Google Scholar]
- 35.Moan J., Grigalavicius M., Baturaite Z., Dahlback A., Juzeniene A. The relationship between UV exposure and incidence of skin cancer. Photodermatol Photoimmunol Photomed. 2015;31(1):26–35. doi: 10.1111/phpp.12139. [DOI] [PubMed] [Google Scholar]
- 36.Hart P.H., Norval M. Ultraviolet radiation-induced immunosuppression and its relevance for skin carcinogenesis. Photochem Photobiol Sci. 2018;17(12):1872–1884. doi: 10.1039/c7pp00312a. [DOI] [PubMed] [Google Scholar]
- 37.Ruan L., Cheng S.-P., Zhu Q.-X. Dietary fat intake and the risk of skin cancer: a systematic review and meta-analysis of observational studies. Nutr Cancer. 2020;72(3):398–408. doi: 10.1080/01635581.2019.1637910. [DOI] [PubMed] [Google Scholar]
- 38.Black H.S., Rhodes L.E. Potential benefits of omega-3 fatty acids in non-melanoma skin cancer. Journal of clinical medicine. 2016;5(2):23. doi: 10.3390/jcm5020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park M.K., Li W.-Q., Qureshi A.A., Cho E. Fat intake and risk of skin cancer in US adults. Cancer Epidemiology and Prevention Biomarkers. 2018;27(7):776–782. doi: 10.1158/1055-9965.EPI-17-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matthews N.H., Koh M., Li W.-Q., Li T., Willett W.C., Stampfer M.J., et al. A prospective study of toenail trace element levels and risk of skin Cancer. Cancer Epidemiology and Prevention Biomarkers. 2019;28(9):1534–1543. doi: 10.1158/1055-9965.EPI-19-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stenz N.A., Stampf S., Arnold A.W., Cozzio A., Dickenmann M., Gaide O., et al. Skin Cancer Development in Solid Organ Transplant Recipients in Switzerland (Swiss Transplant Cohort Study) Dermatology. 2020;1–11 doi: 10.1159/000510685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rizvi S.M.H., Aagnes B., Holdaas H., Gude E., Boberg K.M., Bjørtuft Ø., et al. Long-term change in the risk of skin cancer after organ transplantation: a population-based nationwide cohort study. JAMA dermatology. 2017;153(12):1270–1277. doi: 10.1001/jamadermatol.2017.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howard M.D., Su J.C., Chong A.H. Skin cancer following solid organ transplantation: a review of risk factors and models of care. Am J Clin Dermatol. 2018;19(4):585–597. doi: 10.1007/s40257-018-0355-8. [DOI] [PubMed] [Google Scholar]
- 44.Cajanding R. Immunosuppression following organ transplantation. Part 2: complications and their management. British Journal of Nursing. 2018;27(18):1059–1065. doi: 10.12968/bjon.2018.27.18.1059. [DOI] [PubMed] [Google Scholar]
- 45.Wu X., Hammer J.A. Melanosome transfer: it is best to give and receive. Curr Opin Cell Biol. 2014;29:1–7. doi: 10.1016/j.ceb.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu X., Bowers B., Rao K., Wei Q., Hammer J.A., III Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function in vivo. The Journal of cell biology. 1998;143(7):1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vuković P., Lugović-Mihić L., Ćesić D., Novak-Bilić G., Šitum M., Spoljar S. Melanoma development: current knowledge on melanoma pathogenesis. Acta Dermatovenerologica Croatica. 2019;27(3):163-. [PubMed] [Google Scholar]
- 48.Coricovac D., Dehelean C., Moaca E.-A., Pinzaru I., Bratu T., Navolan D., et al. Cutaneous melanoma—a long road from experimental models to clinical outcome: a review. Int J Mol Sci. 2018;19(6):1566. doi: 10.3390/ijms19061566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kosmidis C., Baka S., Sapalidis K., Mixalopoulos N., Atmatzidis S., Koulouris H., et al. Melanoma from molecular pathways to clinical treatment: an up to date review. J Biomed. 2017;2:94–100. [Google Scholar]
- 50.Leong S.P., Mihm M.C., Murphy G.F., Hoon D.S., Kashani-Sabet M., Agarwala S.S., et al. Progression of cutaneous melanoma: implications for treatment. Clin Exp Metastasis. 2012;29(7):775–796. doi: 10.1007/s10585-012-9521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frändberg P.A., Doufexis M., Kapas S., Chhajlani V. Amino acid residues in third intracellular loop of melanocortin 1 receptor are involved in G-protein coupling. IUBMB Life. 1998;46(5):913–922. doi: 10.1080/15216549800204462. [DOI] [PubMed] [Google Scholar]
- 52.Robbins L.S., Nadeau J.H., Johnson K.R., Kelly M.A., Roselli-Rehfuss L., Baack E., et al. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72(6):827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- 53.Cui R., Widlund H.R., Feige E., Lin J.Y., Wilensky D.L., Igras V.E., et al. Central role of p53 in the suntan response and pathologic hyperpigmentation. Cell. 2007;128(5):853–864. doi: 10.1016/j.cell.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 54.Millington G. Proopiomelanocortin (POMC): the cutaneous roles of its melanocortin products and receptors. Clinical and Experimental Dermatology: Clinical dermatology. 2006;31(3):407–412. doi: 10.1111/j.1365-2230.2006.02128.x. [DOI] [PubMed] [Google Scholar]
- 55.Mujahid N., Liang Y., Murakami R., Choi H.G., Dobry A.S., Wang J., et al. A UV-independent topical small-molecule approach for melanin production in human skin. Cell reports. 2017;19(11):2177–2184. doi: 10.1016/j.celrep.2017.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horike N., Kumagai A., Shimono Y., Onishi T., Itoh Y., Sasaki T., et al. Downregulation of SIK2 expression promotes the melanogenic program in mice. Pigment cell & melanoma research. 2010;23(6):809–819. doi: 10.1111/j.1755-148X.2010.00760.x. [DOI] [PubMed] [Google Scholar]
- 57.Robles-Espinoza C.D., Roberts N.D., Chen S., Leacy F.P., Alexandrov L.B., Pornputtapong N., et al. Germline MC1R status influences somatic mutation burden in melanoma. Nat Commun. 2016;7:12064. doi: 10.1038/ncomms12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valverde P., Healy E., Sikkink S., Haldane F., Thody A.J., Carothers A., et al. The Asp84Glu variant of the melanocortin 1 receptor (MC1R) is associated with melanoma. Hum Mol Genet. 1996;5(10):1663–1666. doi: 10.1093/hmg/5.10.1663. [DOI] [PubMed] [Google Scholar]
- 59.Mitra D., Luo X., Morgan A., Wang J., Hoang M.P., Lo J., et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491(7424):449–453. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Napolitano A., Panzella L., Monfrecola G., d'Ischia M. Pheomelanin-induced oxidative stress: bright and dark chemistry bridging red hair phenotype and melanoma. Pigment cell & melanoma research. 2014;27(5):721–733. doi: 10.1111/pcmr.12262. [DOI] [PubMed] [Google Scholar]
- 61.De Giorgi V., Maida P., Salvati L., Scarfì F., Trane L., Gori A., et al. Trauma and foreign bodies may favour the onset of melanoma metastases. Clin Exp Dermatol. 2020 doi: 10.1111/ced.14202. [DOI] [PubMed] [Google Scholar]
- 62.Iwanaga R., Truong B.T., Hsu J.Y., Lambert K.A., Vyas R., Orlicky D., et al. Loss of prdm1a accelerates melanoma onset and progression. Mol Carcinog. 2020;59(9):1052–1063. doi: 10.1002/mc.23236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Michaloglou C., Vredeveld L.C., Soengas M.S., Denoyelle C., Kuilman T., Van Der Horst C.M., et al. BRAF E600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436(7051):720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 64.Jansen P., Cosgarea I., Murali R., Möller I., Sucker A., Franklin C., et al. Frequent occurrence of NRAS and BRAF mutations in human Acral Naevi. Cancers. 2019;11(4):546. doi: 10.3390/cancers11040546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pollock P.M., Harper U.L., Hansen K.S., Yudt L.M., Stark M., Robbins C.M., et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33(1):19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- 66.Krengel S., Scope A., Dusza S.W., Vonthein R., Marghoob A.A. New recommendations for the categorization of cutaneous features of congenital melanocytic nevi. J Am Acad Dermatol. 2013;68(3):441–451. doi: 10.1016/j.jaad.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 67.da Silva V.M., Martinez-Barrios E., Tell-Martí G., Dabad M., Carrera C., Aguilera P., et al. Genetic abnormalities in large to giant congenital nevi: beyond NRAS mutations. J, Invest Dermatol. 2019;139(4):900–908. doi: 10.1016/j.jid.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 68.Polubothu S., McGuire N., Al-Olabi L., Baird W., Bulstrode N., Chalker J., et al. Does the gene matter? Genotype–phenotype and genotype–outcome associations in congenital melanocytic naevi. Br J Dermatol. 2020;182(2):434–443. doi: 10.1111/bjd.18106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kinsler V, O'hare P, Bulstrode N, Calonje J, Chong W, Hargrave D, et al. Melanoma in congenital melanocytic naevi. British Journal of Dermatology. 2017;176(5):1131-43. [DOI] [PMC free article] [PubMed]
- 70.Rowling E.J., Miskolczi Z., Nagaraju R., Wilcock D.J., Wang P., Telfer B., et al. Cooperative behaviour and phenotype plasticity evolve during melanoma progression. Pigment cell & melanoma research. 2020;33(5):695. doi: 10.1111/pcmr.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chuang T.-Y., Popescu N.A., Su W.-P.D., Chute C.G. Squamous cell carcinoma: a population-based incidence study in Rochester. Minn Archives of dermatology. 1990;126(2):185–188. doi: 10.1001/archderm.126.2.185. [DOI] [PubMed] [Google Scholar]
- 72.Nikolouzakis T.K., Falzone L., Lasithiotakis K., Krüger-Krasagakis S., Kalogeraki A., Sifaki M., et al. Current and Future Trends in Molecular Biomarkers for Diagnostic, Prognostic, and Predictive Purposes in Non-Melanoma Skin Cancer. Journal of Clinical Medicine. 2020;9(9):2868. doi: 10.3390/jcm9092868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang N.-B., Feng R., Gao Z., Gao W. Skin cancer incidence is highly associated with ultraviolet-B radiation history. Int J Hyg Environ Health. 2010;213(5):359–368. doi: 10.1016/j.ijheh.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Coelho SG, Choi W, Brenner M, Miyamura Y, Yamaguchi Y, Wolber R, et al., editors. Short-and long-term effects of UV radiation on the pigmentation of human skin. Journal of Investigative Dermatology Symposium Proceedings; 2009: Elsevier. [DOI] [PMC free article] [PubMed]
- 75.Benjamin CL, Melnikova VO, Ananthaswamy HN. P53 protein and pathogenesis of melanoma and nonmelanoma skin cancer. Sunlight, Vitamin D and Skin Cancer: Springer; 2008. p. 265-82. [DOI] [PubMed]
- 76.Sari Z.A.L., Yahya Y.F., Toruan T.L. The applicability of Sonic hedgehog in mixed type basal cell carcinoma. Journal of General-Procedural Dermatology and Venereology. Indonesia. 2020:86–90. [Google Scholar]
- 77.Bisceglia M., Panniello G., Nirchio V., Sanguedolce F., Centola M., Ben-Dor D.J. Metastatic Cutaneous Basal Cell Carcinoma: Report of 2 Cases Preceding the Hedgehog Pathway Antagonists Era. Advances In Anatomic Pathology. 2020;27(2):98–111. doi: 10.1097/PAP.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 78.Cameron M.C., Lee E., Hibler B.P., Barker C.A., Mori S., Cordova M., et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80(2):303–317. doi: 10.1016/j.jaad.2018.03.060. [DOI] [PubMed] [Google Scholar]
- 79.van der Poort E.K., Gunn D.A., Beekman M., Griffiths C.E., Slagboom P.E., van Heemst D., et al. Basal cell carcinoma genetic susceptibility increases the rate of skin ageing: a Mendelian randomization study. J Eur Acad Dermatol Venereol. 2020;34(1):97–100. doi: 10.1111/jdv.15880. [DOI] [PubMed] [Google Scholar]
- 80.Scales S.J., de Sauvage F.J. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30(6):303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Wong S.Y., Reiter J.F. The primary cilium: at the crossroads of mammalian hedgehog signaling. Curr Top Dev Biol. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.di Magliano M.P., Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3(12):903–911. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 83.Otsuka A., Levesque M.P., Dummer R., Kabashima K. Hedgehog signaling in basal cell carcinoma. J Dermatol Sci. 2015;78(2):95–100. doi: 10.1016/j.jdermsci.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 84.Raleigh D.R., Choksi P.K., Krup A.L., Mayer W., Santos N., Reiter J.F. Hedgehog signaling drives medulloblastoma growth via CDK6. J Clin Investig. 2018;128(1):120–124. doi: 10.1172/JCI92710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Monkkonen T., Lewis M.T. New paradigms for the Hedgehog signaling network in mammary gland development and breast Cancer. Biochimica et Biophysica Acta (BBA)-Reviews on. Cancer. 2017;1868(1):315–332. doi: 10.1016/j.bbcan.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li C., Chi S., Xie J. Hedgehog signaling in skin cancers. Cell Signal. 2011;23(8):1235–1243. doi: 10.1016/j.cellsig.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bonilla X., Parmentier L., King B., Bezrukov F., Kaya G., Zoete V., et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016;48(4):398–406. doi: 10.1038/ng.3525. [DOI] [PubMed] [Google Scholar]
- 88.Gupta S., Takebe N., LoRusso P. Targeting the Hedgehog pathway in cancer. Therapeutic advances in medical oncology. 2010;2(4):237–250. doi: 10.1177/1758834010366430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pellegrini C., Maturo M.G., Di Nardo L., Ciciarelli V., Gutiérrez García-Rodrigo C., Fargnoli M.C. Understanding the molecular genetics of basal cell carcinoma. Int J Mol Sci. 2017;18(11):2485. doi: 10.3390/ijms18112485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Russell-Goldman E., MacConaill L., Hanna J. Hedgehog Pathway Alterations Downstream of Patched-1 Are Common in Infundibulocystic Basal Cell Carcinoma. The. Am J Dermatopathol. 2020 doi: 10.1097/DAD.0000000000001746. [DOI] [PubMed] [Google Scholar]
- 91.Ashraf D.C., Vagefi M.R. Hedgehog Pathway Inhibitors for Periocular Basal Cell Carcinoma. Int Ophthalmol Clin. 2020;60(2):13–30. doi: 10.1097/IIO.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 92.Khan N.H., Ullah F., Khan T.A., Zafar U., Khan M.F.A., Mustaqeem M., et al. Personal-Care Cosmetic Practices in Pakistan: Current Perspectives and Management. Clinical, Cosmetic and Investigational Dermatology. 2021;14:9. doi: 10.2147/CCID.S270667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fahradyan A, Howell AC, Wolfswinkel EM, Tsuha M, Sheth P, Wong AK, editors. Updates on the management of non-melanoma skin cancer (NMSC). Healthcare; 2017: Multidisciplinary Digital Publishing Institute. [DOI] [PMC free article] [PubMed]
- 94.Kang S.Y., Toland A.E. High risk cutaneous squamous cell carcinoma of the head and neck. World journal of otorhinolaryngology-head and neck surgery. 2016;2(2):136–140. doi: 10.1016/j.wjorl.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brash D.E., Rudolph J.A., Simon J.A., Lin A., McKenna G.J., Baden H.P., et al. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci. 1991;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ziegler A., Jonason A.S., Leffellt D.J., Simon J.A., Sharma H.W., Kimmelman J., et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372(6508):773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 97.Nelson M.A., Einspahr J.G., Alberts D.S., Balfour C.A., Wymer J.A., Welch K.L., et al. Analysis of the p53 gene in human precancerous actinic keratosis lesions and squamous cell cancers. Cancer Lett. 1994;85(1):23–29. doi: 10.1016/0304-3835(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 98.Omori H, Nishio M, Masuda M, Miyachi Y, Ueda F, Nakano T, et al. YAP1 is a potent driver of the onset and progression of oral squamous cell carcinoma. Science Advances. 2020;6(12):eaay3324. [DOI] [PMC free article] [PubMed]
- 99.Zhao W., Cui Y., Liu L., Qi X., Liu J., Ma S., et al. Splicing factor derived circular RNA circUHRF1 accelerates oral squamous cell carcinoma tumorigenesis via feedback loop. Cell Death Differ. 2020;27(3):919–933. doi: 10.1038/s41418-019-0423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.García-Sancha N., Corchado-Cobos R., Pérez-Losada J., Cañueto J. MicroRNA dysregulation in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20(9):2181. doi: 10.3390/ijms20092181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 102.Junttila M.R., de Sauvage F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501(7467):346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 103.Shimizu I., Cruz A., Chang K.H., Dufresne R.G. Treatment of squamous cell carcinoma in situ: a review. Dermatol Surg. 2011;37(10):1394–1411. doi: 10.1111/j.1524-4725.2011.02088.x. [DOI] [PubMed] [Google Scholar]
- 104.Matsui T., Amagai M. Dissecting the formation, structure and barrier function of the stratum corneum. Int Immunol. 2015;27(6):269–280. doi: 10.1093/intimm/dxv013. [DOI] [PubMed] [Google Scholar]
- 105.Shende P., Vaidya J., Gaud R. Pharmacotherapeutic approaches for transportation of anticancer agents via skin. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S423–S433. doi: 10.1080/21691401.2018.1498349. [DOI] [PubMed] [Google Scholar]
- 106.McGrath J, Eady R, Pope F. Anatomy and Organization of Human Skin. Rook’s Textb Dermatology. Blackwell Publishing company; 2004.
- 107.Depieri LV, Garcia Praça FS, Campos PM, Lopes Badra Bentley MV. Advances in the bioanalytical study of drug delivery across the skin. Therapeutic Delivery. 2015;6(5):571-94. [DOI] [PubMed]
- 108.Lima A.M.F., Daniel C.R., Navarro R.S., Bodanese B., Pasqualucci C.A., Pacheco M.T.T., et al. Discrimination of non-melanoma skin cancer and keratosis from normal skin tissue in vivo and ex vivo by Raman spectroscopy. Vib Spectrosc. 2019;100:131–141. [Google Scholar]
- 109.Santos I.P., van Doorn R., Caspers P.J., Schut T.C.B., Barroso E.M., Nijsten T.E., et al. Improving clinical diagnosis of early-stage cutaneous melanoma based on Raman spectroscopy. Br J Cancer. 2018;119(11):1339–1346. doi: 10.1038/s41416-018-0257-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Barua S., Mitragotri S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: a review of current status and future prospects. Nano Today. 2014;9(2):223–243. doi: 10.1016/j.nantod.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bertrand N., Wu J., Xu X., Kamaly N., Farokhzad O.C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jain R.K., Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Donahue N.D., Acar H., Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv Drug Deliv Rev. 2019;143:68–96. doi: 10.1016/j.addr.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 114.Kaksonen M., Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19(5):313. doi: 10.1038/nrm.2017.132. [DOI] [PubMed] [Google Scholar]
- 115.Wang Z., Tiruppathi C., Minshall R.D., Malik A.B. Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano. 2009;3(12):4110–4116. doi: 10.1021/nn9012274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu W., Luo L., Wang Y., Wu Q., Dai H.-B., Li J.-S., et al. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics. 2018;8(11):3038. doi: 10.7150/thno.23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gerecke C., Edlich A., Giulbudagian M., Schumacher F., Zhang N., Said A., et al. Biocompatibility and characterization of polyglycerol-based thermoresponsive nanogels designed as novel drug-delivery systems and their intracellular localization in keratinocytes. Nanotoxicology. 2017;11(2):267–277. doi: 10.1080/17435390.2017.1292371. [DOI] [PubMed] [Google Scholar]
- 118.Nikolaou M., Pavlopoulou A., Georgakilas A.G., Kyrodimos E. The challenge of drug resistance in cancer treatment: a current overview. Clin Exp Metastasis. 2018;35(4):309–318. doi: 10.1007/s10585-018-9903-0. [DOI] [PubMed] [Google Scholar]
- 119.Kartal-Yandim M., Adan-Gokbulut A., Baran Y. Molecular mechanisms of drug resistance and its reversal in cancer. Crit Rev Biotechnol. 2016;36(4):716–726. doi: 10.3109/07388551.2015.1015957. [DOI] [PubMed] [Google Scholar]
- 120.Kalal B.S., Upadhya D., Pai V.R. Chemotherapy resistance mechanisms in advanced skin cancer. Oncology reviews. 2017;11(1) doi: 10.4081/oncol.2017.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Andrei L., Kasas S., Garrido I.O., Stanković T., Korsnes M.S., Vaclavikova R., et al. Advanced technological tools to study multidrug resistance in cancer. Drug Resist Updates. 2020;48 doi: 10.1016/j.drup.2019.100658. [DOI] [PubMed] [Google Scholar]
- 122.AlQathama A., Ezuruike U.F., Mazzari A.L., Yonbawi A., Chieli E., Prieto J.M. Effects of Selected Nigerian Medicinal Plants on the Viability, Mobility, and Multidrug-Resistant Mechanisms in Liver, Colon, and Skin Cancer Cell Lines. Front Pharmacol. 2020;11:1456. doi: 10.3389/fphar.2020.546439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Feng S., Zhou H., Wu D., Zheng D., Qu B., Liu R., et al. Nobiletin and its derivatives overcome multidrug resistance (MDR) in cancer: total synthesis and discovery of potent MDR reversal agents. Acta Pharmaceutica Sinica B. 2020;10(2):327–343. doi: 10.1016/j.apsb.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dobiasová S., Řehořová K., Kučerová D., Biedermann D., Káňová K., Petrásková L., et al. Multidrug resistance modulation activity of silybin derivatives and their anti-inflammatory potential. Antioxidants. 2020;9(5):455. doi: 10.3390/antiox9050455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lopez A.T., Carvajal R.D., Geskin L. Secondary Prevention Strategies for Nonmelanoma Skin Cancer. Oncology (08909091) 2018;32(4) [PubMed] [Google Scholar]
- 126.Lorusso D., Bria E., Costantini A., Di Maio M., Rosti G., Mancuso A. Patients’ perception of chemotherapy side effects: Expectations, doctor–patient communication and impact on quality of life–An Italian survey. European journal of cancer care. 2017;26(2) doi: 10.1111/ecc.12618. [DOI] [PubMed] [Google Scholar]
- 127.Demaria M., O'Leary M.N., Chang J., Shao L., Liu S., Alimirah F., et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer discovery. 2017;7(2):165–176. doi: 10.1158/2159-8290.CD-16-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Griffin L.L., Lear J.T. Photodynamic therapy and non-melanoma skin cancer. Cancers. 2016;8(10):98. doi: 10.3390/cancers8100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kharofa J., Currey A., Wilson J.F. Patient-reported outcomes in patients with nonmelanomatous skin cancers of the face treated with orthovoltage radiation therapy: a cross-sectional survey. International Journal of Radiation Oncology• Biology•. Physics. 2013;87(4):636–637. doi: 10.1016/j.ijrobp.2013.06.2061. [DOI] [PubMed] [Google Scholar]
- 130.Soleymani T., Abrouk M., Kelly K.M. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al] 2017;43(5):615. doi: 10.1097/DSS.0000000000001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hasmat S., Howle J.R., Karikios D.J., Carlino M.S., Veness M.J. Immunotherapy in advanced Merkel cell carcinoma: Sydney west cancer network experience. Journal of medical imaging and radiation oncology. 2021 doi: 10.1111/1754-9485.13243. [DOI] [PubMed] [Google Scholar]
- 132.Cullen J.K., Simmons J.L., Parsons P.G., Boyle G.M. Topical treatments for skin cancer. Adv Drug Deliv Rev. 2020;153:54–64. doi: 10.1016/j.addr.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 133.Thomas D., Zalcberg J. 5-fluorouracil: a pharmacological paradigm in the use of cytotoxics. Clin Exp Pharmacol Physiol. 1998;25(11):887–895. doi: 10.1111/j.1440-1681.1998.tb02339.x. [DOI] [PubMed] [Google Scholar]
- 134.Schön M., Schön M. Imiquimod: mode of action. Br J Dermatol. 2007;157:8–13. doi: 10.1111/j.1365-2133.2007.08265.x. [DOI] [PubMed] [Google Scholar]
- 135.Ramsay J., Suhrbier A., Aylward J., Ogbourne S., Cozzi S.J., Poulsen M., et al. The sap from Euphorbia peplus is effective against human nonmelanoma skin cancers. Br J Dermatol. 2011;164(3):633–636. doi: 10.1111/j.1365-2133.2010.10184.x. [DOI] [PubMed] [Google Scholar]
- 136.Stockfleth E., Meyer T. Sinecatechins (Polyphenon E) ointment for treatment of external genital warts and possible future indications. Expert Opin Biol Ther. 2014;14(7):1033–1043. doi: 10.1517/14712598.2014.913564. [DOI] [PubMed] [Google Scholar]
- 137.Lu Y.-P., Lou Y.-R., Xie J.-G., Peng Q.-Y., Liao J., Yang C.S., et al. Topical applications of caffeine or (−)-epigallocatechin gallate (EGCG) inhibit carcinogenesis and selectively increase apoptosis in UVB-induced skin tumors in mice. Proc Natl Acad Sci. 2002;99(19):12455–12460. doi: 10.1073/pnas.182429899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zuco V., Supino R., Righetti S.C., Cleris L., Marchesi E., Gambacorti-Passerini C., et al. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett. 2002;175(1):17–25. doi: 10.1016/s0304-3835(01)00718-2. [DOI] [PubMed] [Google Scholar]
- 139.Tang J.Q., Hou X.Y., Yang C.S., Li Y.X., Xin Y., Guo W.W., et al. Recent developments in nanomedicine for melanoma treatment. Int J Cancer. 2017;141(4):646–653. doi: 10.1002/ijc.30708. [DOI] [PubMed] [Google Scholar]
- 140.Ye Y, Wang J, Sun W, Bomba HN, Gu Z. Topical and transdermal nanomedicines for cancer therapy. Nanotheranostics for Cancer Applications: Springer; 2019. p. 231-51.
- 141.Bibi N, Ahmed N, Khan GM. Nanostructures in transdermal drug delivery systems. Nanostructures for drug delivery: Elsevier; 2017. p. 639-68.
- 142.Krishnan V., Mitragotri S. Nanoparticles for topical drug delivery: Potential for skin cancer. Adv Drug Deliv Rev. 2020 doi: 10.1016/j.addr.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 143.Mir M, Ahmed N, ur Rehman A. Recent applications of PLGA based nanostructures in drug delivery. Colloids and Surfaces B: Biointerfaces. 2017;159:217-31. [DOI] [PubMed]
- 144.Zhou Q., Zhang L., Yang T., Wu H. Stimuli-responsive polymeric micelles for drug delivery and cancer therapy. Int J Nanomed. 2018;13:2921. doi: 10.2147/IJN.S158696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kesharwani S.S., Kaur S., Tummala H., Sangamwar A.T. Multifunctional approaches utilizing polymeric micelles to circumvent multidrug resistant tumors. Colloids Surf, B. 2019;173:581–590. doi: 10.1016/j.colsurfb.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 146.Song M., Xia W., Tao Z., Zhu B., Zhang W., Liu C., et al. Self-assembled polymeric nanocarrier-mediated co-delivery of metformin and doxorubicin for melanoma therapy. Drug Delivery. 2021;28(1):594–606. doi: 10.1080/10717544.2021.1898703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Daraba O.M., Cadinoiu A.N., Rata D.M., Atanase L.I., Vochita G. Antitumoral drug-loaded biocompatible polymeric nanoparticles obtained by non-aqueous emulsion polymerization. Polymers. 2020;12(5):1018. doi: 10.3390/polym12051018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Batista FA, Fontele SBC, Santos LKB, Filgueiras LA, Nascimento SQ, e Sousa JMdC, et al. Synthesis, characterization of α-terpineol-loaded PMMA nanoparticles as proposed of therapy for melanoma. Materials Today Communications. 2020;22:100762.
- 149.Ferraz L.S., Watashi C.M., Colturato-Kido C., Pelegrino M.T., Paredes-Gamero E.J., Weller R.B., et al. Antitumor potential of S-nitrosothiol-containing polymeric nanoparticles against melanoma. Mol Pharm. 2018;15(3):1160–1168. doi: 10.1021/acs.molpharmaceut.7b01001. [DOI] [PubMed] [Google Scholar]
- 150.Dias M.F., de Figueiredo B.C.P., Teixeira-Neto J., Guerra M.C.A., Fialho S.L., Cunha A.S. In vivo evaluation of antitumoral and antiangiogenic effect of imiquimod-loaded polymeric nanoparticles. Biomed Pharmacother. 2018;103:1107–1114. doi: 10.1016/j.biopha.2018.04.079. [DOI] [PubMed] [Google Scholar]
- 151.Capanema Nd.S., Carvalho I.C., Mansur A.A., Carvalho S.M., Lage A.P., Mansur H.S. Hybrid hydrogel composed of carboxymethylcellulose–silver nanoparticles–doxorubicin for anticancer and antibacterial therapies against melanoma skin cancer cells. ACS Applied Nano Materials. 2019;2(11):7393–7408. [Google Scholar]
- 152.Liu Y., Bailey J.T., Abu-Laban M., Li S., Chen C., Glick A.B., et al. Photocontrolled miR-148b nanoparticles cause apoptosis, inflammation and regression of Ras induced epidermal squamous cell carcinomas in mice. Biomaterials. 2020;256 doi: 10.1016/j.biomaterials.2020.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lan X., She J., Lin D.-a., Xu Y., Li X., Yang W-f, et al. Microneedle-mediated delivery of lipid-coated cisplatin nanoparticles for efficient and safe cancer therapy. ACS Appl Mater Interfaces. 2018;10(39):33060–33069. doi: 10.1021/acsami.8b12926. [DOI] [PubMed] [Google Scholar]
- 154.Dong L., Li Y., Li Z., Xu N., Liu P., Du H., et al. Au nanocage-strengthened dissolving microneedles for chemo-photothermal combined therapy of superficial skin tumors. ACS Appl Mater Interfaces. 2018;10(11):9247–9256. doi: 10.1021/acsami.7b18293. [DOI] [PubMed] [Google Scholar]
- 155.Li X., Xu Q., Zhang P., Zhao X., Wang Y. preparation and characterization nvironment responsive microneedle patch for rapid gene release to treat subdermal tumor. J Control Release. 2019;314:72–80. doi: 10.1016/j.jconrel.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 156.Sabri A.H., Cater Z., Gurnani P., Ogilvie J., Segal J., Scurr D.J., et al. Intradermal delivery of imiquimod using polymeric microneedles for basal cell carcinoma. Int J Pharm. 2020;589 doi: 10.1016/j.ijpharm.2020.119808. [DOI] [PubMed] [Google Scholar]
- 157.Arruda D.C., de Oliveira T.D., Cursino P.H.F., Maia V.S.C., Berzaghi R., Travassos L.R., et al. Inhibition of melanoma metastasis by dual-peptide PLGA NPS. Pept Sci. 2017;108(5) doi: 10.1002/bip.23029. [DOI] [PubMed] [Google Scholar]
- 158.Yao Q., Choi J.H., Dai Z., Wang J., Kim D., Tang X., et al. Improving tumor specificity and anticancer activity of dasatinib by dual-targeted polymeric micelles. ACS Appl Mater Interfaces. 2017;9(42):36642–36654. doi: 10.1021/acsami.7b12233. [DOI] [PubMed] [Google Scholar]
- 159.Xia C., Yin S., Xu S., Ran G., Deng M., Mei L., et al. Low molecular weight heparin-coated and dendrimer-based core-shell nanoplatform with enhanced immune activation and multiple anti-metastatic effects for melanoma treatment. Theranostics. 2019;9(2):337. doi: 10.7150/thno.29026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.El-Sawy H.S., Al-Abd A.M., Ahmed T.A., El-Say K.M., Torchilin V.P. Stimuli-responsive nano-architecture drug-delivery systems to solid tumor micromilieu: past, present, and future perspectives. ACS Nano. 2018;12(11):10636–10664. doi: 10.1021/acsnano.8b06104. [DOI] [PubMed] [Google Scholar]
- 161.Li Z., Zhang F-l. Pan L-l, Zhu X-l, Zhang Z-z. Preparation and characterization of injectable Mitoxantrone poly (lactic acid)/fullerene implants for in vivo chemo-photodynamic therapy. J Photochem Photobiol, B. 2015;149:51–57. doi: 10.1016/j.jphotobiol.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 162.Yamada M., Prow T.W. Physical drug delivery enhancement for aged skin, UV damaged skin and skin cancer: Translation and commercialization. Adv Drug Deliv Rev. 2020;153:2–17. doi: 10.1016/j.addr.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 163.Leone M., Mönkäre J., Bouwstra J., Kersten G. Dissolving microneedle patches for dermal vaccination. Pharm Res. 2017;34(11):2223–2240. doi: 10.1007/s11095-017-2223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Waghule T., Singhvi G., Dubey S.K., Pandey M.M., Gupta G., Singh M., et al. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed Pharmacother. 2019;109:1249–1258. doi: 10.1016/j.biopha.2018.10.078. [DOI] [PubMed] [Google Scholar]
- 165.Hao Y., Dong M., Zhang T., Peng J., Jia Y., Cao Y., et al. Novel approach of using near-Infrared Responsive PEGylated Gold Nanorod Coated Poly (L-lactide) microneedles to enhance the antitumor efficiency of Docetaxel-Loaded MPEG-PDLLA Micelles for Treating an A431 tumor. ACS Appl Mater Interfaces. 2017;9(18):15317–15327. doi: 10.1021/acsami.7b03604. [DOI] [PubMed] [Google Scholar]
- 166.Hao Y., Chen Y., He X., Yang F., Han R., Yang C., et al. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioact Mater. 2020;5(3):542–552. doi: 10.1016/j.bioactmat.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Song G., Jiang G., Liu T., Zhang X., Zeng Z., Wang R., et al. Separable Microneedles for Synergistic Chemo-Photothermal Therapy against Superficial Skin Tumors. ACS Biomater Sci Eng. 2020;6(7):4116–4125. doi: 10.1021/acsbiomaterials.0c00793. [DOI] [PubMed] [Google Scholar]
- 168.Uddin M.J., Scoutaris N., Economidou S.N., Giraud C., Chowdhry B.Z., Donnelly R.F., et al. 3D printed microneedles for anticancer therapy of skin tumours. Mater Sci Eng, C. 2020;107 doi: 10.1016/j.msec.2019.110248. [DOI] [PubMed] [Google Scholar]
- 169.Naguib Y.W., Kumar A., Cui Z. The effect of microneedles on the skin permeability and antitumor activity of topical 5-fluorouracil. Acta Pharmaceutica Sinica B. 2014;4(1):94–99. doi: 10.1016/j.apsb.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lv L., Guo Y., Shen Y., Liu J., Zhang W., Zhou D., et al. Intracellularly Degradable, Self-Assembled Amphiphilic Block Copolycurcumin Nanoparticles for Efficient In Vivo Cancer Chemotherapy. Adv Healthcare Mater. 2015;4(10):1496–1501. doi: 10.1002/adhm.201500075. [DOI] [PubMed] [Google Scholar]
- 171.Xu M., Zhang C.Y., Wu J., Zhou H., Bai R., Shen Z., et al. PEG-detachable polymeric micelles self-assembled from amphiphilic copolymers for tumor-acidity-triggered drug delivery and controlled release. ACS Appl Mater Interfaces. 2019;11(6):5701–5713. doi: 10.1021/acsami.8b13059. [DOI] [PubMed] [Google Scholar]
- 172.Kandekar S.G., del Río-Sancho S., Lapteva M., Kalia Y.N. Selective delivery of adapalene to the human hair follicle under finite dose conditions using polymeric micelle nanocarriers. Nanoscale. 2018;10(3):1099–1110. doi: 10.1039/c7nr07706h. [DOI] [PubMed] [Google Scholar]
- 173.Cheng C.-C., Huang J.-J., Lee A.-W., Huang S.-Y., Huang C.-Y., Lai J.-Y. Highly effective photocontrollable drug delivery systems based on ultrasensitive light-responsive self-assembled polymeric micelles: an in vitro therapeutic evaluation. ACS Applied Bio Materials. 2019;2(5):2162–2170. doi: 10.1021/acsabm.9b00146. [DOI] [PubMed] [Google Scholar]
- 174.Wan D., Li C., Pan J. Polymeric micelles with reduction-responsive function for targeted cancer chemotherapy. ACS Applied Bio Materials. 2020;3(2):1139–1146. doi: 10.1021/acsabm.9b01070. [DOI] [PubMed] [Google Scholar]
- 175.Lapteva M., Mignot M., Mondon K., Möller M., Gurny R., Kalia Y.N. Self-assembled mPEG-hexPLA polymeric nanocarriers for the targeted cutaneous delivery of imiquimod. Eur J Pharm Biopharm. 2019;142:553–562. doi: 10.1016/j.ejpb.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 176.Chinembiri T.N., Gerber M., Du Plessis L., Du Preez J., Du Plessis J. Topical delivery of 5-fluorouracil from Pheroid™ formulations and the in vitro efficacy against human melanoma. AAPS PharmSciTech. 2015;16(6):1390–1399. doi: 10.1208/s12249-015-0328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rancan F., Asadian-Birjand M., Dogan S., Graf C., Cuellar L., Lommatzsch S., et al. Effects of thermoresponsivity and softness on skin penetration and cellular uptake of polyglycerol-based nanogels. J Control Release. 2016;228:159–169. doi: 10.1016/j.jconrel.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 178.Sahu P., Kashaw S.K., Jain S., Sau S., Iyer A.K. Assessment of penetration potential of pH responsive double walled biodegradable nanogels coated with eucalyptus oil for the controlled delivery of 5-fluorouracil: In vitro and ex vivo studies. J Control Release. 2017;253:122–136. doi: 10.1016/j.jconrel.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 179.Gazzi R., Frank L., Onzi G., Pohlmann A., Guterres S.S. New pectin-based hydrogel containing imiquimod-loaded polymeric nanocapsules for melanoma treatment. Drug Delivery and Translational Research. 2020;10(6):1829–1840. doi: 10.1007/s13346-020-00805-5. [DOI] [PubMed] [Google Scholar]
- 180.Siddalingam R., Chidambaram K. Topical nano-delivery of 5-fluorouracil: Preparation and characterization of water-in-oil nanoemulsion. Trop J Pharm Res. 2016;15(11):2311–2319. [Google Scholar]
- 181.Wang X., Shi L., Tu Q., Wang H., Zhang H., Wang P., et al. Treating cutaneous squamous cell carcinoma using 5-aminolevulinic acid polylactic-co-glycolic acid nanoparticle-mediated photodynamic therapy in a mouse model. Int J Nanomed. 2015;10:347. doi: 10.2147/IJN.S71245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nasr S., Rady M., Gomaa I., Syrovets T., Simmet T., Fayad W., et al. Ethosomes and lipid-coated chitosan nanocarriers for skin delivery of a chlorophyll derivative: A potential treatment of squamous cell carcinoma by photodynamic therapy. Int J Pharm. 2019;568 doi: 10.1016/j.ijpharm.2019.118528. [DOI] [PubMed] [Google Scholar]
- 183.Sharma R., Sharma A., Kambhampati S.P., Reddy R.R., Zhang Z., Cleland J.L., et al. Scalable synthesis and validation of PAMAM dendrimer-N-acetyl cysteine conjugate for potential translation. Bioeng Transl Med. 2018;3(2):87–101. doi: 10.1002/btm2.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Akbarzadeh A., Khalilov R., Mostafavi E., Annabi N., Abasi E., Kafshdooz T., et al. Role of dendrimers in advanced drug delivery and biomedical applications: a review. Experimental oncology. 2018;40(3):178–183. [PubMed] [Google Scholar]
- 185.Venuganti V.V.K., Perumal O.P. Effect of poly (amidoamine)(PAMAM) dendrimer on skin permeation of 5-fluorouracil. Int J Pharm. 2008;361(1–2):230–238. doi: 10.1016/j.ijpharm.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 186.Hu W., Qiu L., Cheng L., Hu Q., Liu Y., Hu Z., et al. Redox and pH dual responsive poly (amidoamine) dendrimer-poly (ethylene glycol) conjugates for intracellular delivery of doxorubicin. Acta Biomater. 2016;36:241–253. doi: 10.1016/j.actbio.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 187.O'day SJ, Kim CJ, Reintgen DS. Metastatic melanoma: chemotherapy to biochemotherapy. Cancer control. 2002;9(1):31-8. [DOI] [PubMed]
- 188.Ruan R., Chen M., Sun S., Wei P., Zou L., Liu J., et al. Topical and targeted delivery of siRNAs to melanoma cells using a fusion peptide carrier. Sci Rep. 2016;6(1):1–11. doi: 10.1038/srep29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vile R., Russell S., Lemoine N. Cancer gene therapy: hard lessons and new courses. Gene Ther. 2000;7(1):2–8. doi: 10.1038/sj.gt.3301084. [DOI] [PubMed] [Google Scholar]
- 190.Ain Q.U., Campos E.V., Huynh A., Witzigmann D., Hedtrich S. Gene delivery to the skin–how far have we come? Trends Biotechnol. 2020 doi: 10.1016/j.tibtech.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jing N., Tweardy D.J. Targeting Stat3 in cancer therapy. Anticancer Drugs. 2005;16(6):601–607. doi: 10.1097/00001813-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 192.Pan J., Ruan W., Qin M., Long Y., Wan T., Yu K., et al. Intradermal delivery of STAT3 siRNA to treat melanoma via dissolving microneedles. Sci Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-19463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zheng D.-W., Lei Q., Zhu J.-Y., Fan J.-X., Li C.-X., Li C., et al. Switching apoptosis to ferroptosis: metal–organic network for high-efficiency anticancer therapy. Nano Lett. 2017;17(1):284–291. doi: 10.1021/acs.nanolett.6b04060. [DOI] [PubMed] [Google Scholar]
- 194.Gulzar A., Xu J., Yang D., Xu L., He F., Gai S., et al. Nano-graphene oxide-UCNP-Ce6 covalently constructed nanocomposites for NIR-mediated bioimaging and PTT/PDT combinatorial therapy. Dalton Trans. 2018;47(11):3931–3939. doi: 10.1039/c7dt04141a. [DOI] [PubMed] [Google Scholar]
- 195.Zhang H., Zhang X., Zhu X., Chen J., Chen Q., Zhang H., et al. NIR light-induced tumor phototherapy using photo-stable ICG delivery system based on inorganic hybrid. Nanomed Nanotechnol Biol Med. 2018;14(1):73–84. doi: 10.1016/j.nano.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 196.Xu Q., Li X., Zhang P., Wang Y. Rapidly dissolving microneedle patch for synergistic gene and photothermal therapy of subcutaneous tumor. J Mater Chem B. 2020;8(19):4331–4339. doi: 10.1039/d0tb00105h. [DOI] [PubMed] [Google Scholar]
- 197.Shi S., Wang Y., Wang B., Chen Q., Wan G., Yang X., et al. Homologous-targeting biomimetic nanoparticles for photothermal therapy and Nrf2-siRNA amplified photodynamic therapy against oral tongue squamous cell carcinoma. Chem Eng J. 2020;388 [Google Scholar]
- 198.Yasun E., Gandhi S., Choudhury S., Mohammadinejad R., Benyettou F., Gozubenli N., et al. Hollow Micro and Nanostructures for Therapeutic and Imaging Applications. J Drug Delivery Sci Technol. 2020;102094 doi: 10.1016/j.jddst.2020.102094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hou X., Zhou H., Wang L., Tang J., Chen C., Jiang G., et al. Multifunctional near-infrared dye-magnetic nanoparticles for bioimaging and cancer therapy. Cancer Lett. 2017;390:168–175. doi: 10.1016/j.canlet.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 200.Upponi J.R., Jerajani K., Nagesha D.K., Kulkarni P., Sridhar S., Ferris C., et al. Polymeric micelles: Theranostic co-delivery system for poorly water-soluble drugs and contrast agents. Biomaterials. 2018;170:26–36. doi: 10.1016/j.biomaterials.2018.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shen X., Li T., Chen Z., Geng Y., Xie X., Li S., et al. Luminescent/magnetic PLGA-based hybrid nanocomposites: a smart nanocarrier system for targeted codelivery and dual-modality imaging in cancer theranostics. Int J Nanomed. 2017;12:4299. doi: 10.2147/IJN.S136766. [DOI] [PMC free article] [PubMed] [Google Scholar]