Highlights
-
•
Oral smokeless tobacco (SLT) products are non-combusted forms of tobacco that can be dependence producing.
-
•
SLT use may pose health risks to users such as cardiovascular disease (CVD) through various pathways including influencing hemodynamics, endothelial dysfunction, inflammation, insulin resistance, hyperlipidemia, and arrhythmogenesis.
-
•
Past studies have suggested a small, elevated risk of CVD among SLT users compared to never tobacco users.
-
•
This study advances the literature by exploring how the duration of regular SLT use relates to CVD prevalence.
-
•
In this study of ≥ 40-year-old men only, we did not find a consistent dose–response trend for years of SLT use and prevalence of CVD.
Keywords: Smokeless tobacco, Cigarette smoking, Cardiovascular disease, Dose–response
Abstract
The purpose of this period prevalence study is to compare the prevalence of cardiovascular disease (CVD) in current/former established smokeless tobacco (SLT) users (ever SLT users who have used the product fairly regularly) to those who were: 1) never established cigarette smokers and SLT users, and 2) current/former established exclusive cigarette smokers (have smoked at least a 100 or more cigarettes in lifetime) only, adjusting for known risk factors for CVD. Analyses included 4,703 men ≥ 40 years of age who participated in the Population Assessment of Tobacco and Health (PATH) Study, Waves: 1–4, conducted between 2013 and 2017. Current users were those using SLT products daily or on some days, whereas former users had not used SLT and/or cigarettes in the past 12 months. CVD prevalence was defined as a self-reported diagnosis of congestive heart failure, stroke, or myocardial infarction. Among current/former established SLT users, years of use defined exposure history, while pack-years defined exposure history for smokers. Adjusted odds ratios (AOR) and 95% confidence intervals (CI) were reported with trend tests to examine dose–response associations. Current/former established exclusive SLT users were not significantly more likely to have had any CVD compared to never established cigarette and SLT users (OR = 1.7 [0.8–3.7]), or current/former established exclusive cigarette smokers (OR = 0.9 [0.5–1.8]). Current/former established exclusive cigarette smokers were more likely to have had any CVD compared to those who were never established cigarette and SLT users (OR = 1.6 [1.1–2.3]).
1. Introduction
Oral smokeless tobacco (SLT) products are non-combusted forms of tobacco that are available in three main types in the United States (U.S.): chewing tobacco, moist snuff, and snus (Food and Administration, 2018). Between 1992 and 2003, SLT use prevalence in the total adult population decreased at an annual percent change of 4.5% per year but has been approximately constant or slightly increasing since then (Chang et al., 2016). By contrast, cigarette smoking prevalence has been falling since the mid-1960s although the prevalence of current cigarette smoking is still 4-fold greater compared to SLT (Cornelius et al., 2020). Some public health researchers have attributed the differential trends in SLT and cigarette prevalence to increasing restrictions on indoor smoking, increased availability of snus products in the U.S., and the marketing of appealing flavored SLT products (Levy et al., 2018, Chaffee et al., 2017).
The Population Assessment of Tobacco and Health (PATH) Study is an ongoing, nationally representative, longitudinal cohort study of adults (18-years or older) and youth (12-17 years of age) (Tomar et al., 2010, Campbell et al., 2015, Cheng et al., 2020, Rostron et al., 2015, US Department of Health and Human Services, 1986, US Department of Health and Human Services, 2010) that can be used to estimate and track the prevalence of tobacco use behaviors in the U.S. (Hyland et al., 2017). In 2013–2014, an estimated 16.5% of adults had ever used any SLT type, and 2.9% were current established users (i.e., used every day or some days) (Kasza et al., 2017, Sharma et al., 2020). The PATH Study and other studies have found that SLT is almost exclusively regularly used by men, and is more common in younger adults, non-Hispanic White people, and nonurban residents (Sharma et al., 2020, Lipari and Van Horn, 2002, Cheng et al., 2017, Jones et al., 2017). SLT is also often used in conjunction with other tobacco products, particularly cigarettes (Kasza et al., 2017, Sharma et al., 2020, Cheng et al., 2017, Jones et al., 2017, Tomar et al., 2010).
SLT products are known to expose users to some of the same harmful chemicals found in cigarette smoke, including nicotine and some carcinogens (Campbell et al., 2015, Cheng et al., 2020, Rostron et al., 2015). However, the overall health risks associated with cigarette smoking are considerably greater than those associated with the use of SLT because burning tobacco exposes the user to more toxicants (Cornelius et al., 2020). Epidemiologic studies have suggested that SLT use may be associated with several adverse health outcomes, including cancer (e.g., oral cavity, pancreatic, and esophageal cancers), gum disease, and various types of cardiovascular disease (CVD) (i.e., myocardial infarction, stroke, congestive heart failure) (Cornelius et al., 2020, US Department of Health and Human Services, 1986, US Department of Health and Human Services, 2010, Timberlake et al., 2017, Accortt et al., 2002, Fisher et al., 2019, Henley et al., 2005, Inoue-Choi et al., 2019). Most of the evidence on the association of SLT and CVD outcomes is based upon studies conducted in Sweden where snus pouches have replaced cigarettes as the most popular form of tobacco consumed (Lee, 2007, Rostron et al., 2018, Vidyasagaran et al., 2016, Boffetta and Straif, 2009, Gupta et al., 2004, Piano et al., 2010). Swedish snus is a specially processed type of SLT that has been found to have fewer carcinogens than are typically found in moist snuff products sold in the U.S. (Stepanov et al., 2008). In Sweden, the results are mixed with one large occupational study suggesting that the use of snus pouches was associated with an increased risk of myocardial infarction, with several other studies of snus pouch users failing to confirm this association (Asplund et al., 2003, Haglund et al., 2007, Bolinder et al., 1994, Hergens et al., 2005, Wennberg et al., 2007, Johansson et al., 2005, Huhtasaari et al., 1992). However, given the difference in the content of snus pouches between products sold in Sweden and the U.S., it is unclear if the findings from Sweden can generalize to SLT users from the U.S. (Boffetta and Straif, 2009).
Two prospective studies conducted in the U.S., the Cancer Prevention Study I (CPS-I; initiated in 1959) and the Cancer Prevention Study II (CPS-II; initiated in 1982), observed that men who reported current use of SLT at the time of enrollment had significantly higher death rates during follow-up from all causes combined and from different cardiovascular endpoints (i.e., coronary heart disease, cerebrovascular disease, other CVDs) when compared to men who reported never using any tobacco product (Henley et al., 2005). However, the associations observed between CVD mortality and SLT were generally weaker in CPS-II compared to CPS-I. The authors of this study cautioned that the association observed between SLT use and increased risk of CVD may not be causal, but instead may reflect confounding by unmeasured factors associated with the characteristics of those using SLT including differences in CVD risk factors and socioeconomic status (Henley et al., 2005). However, similar associations between SLT use and CVD have been reported in other U.S. studies (Inoue-Choi et al., 2019, Timberlake et al., 2017, Accortt et al., 2002, Fisher et al., 2019, Yatsuya and Folsom, 2010, Mushtaq et al., 2010, Henley et al., 2007).
Mechanistically, exposure to nicotine in SLT and other tobacco products may contribute to an increased risk of CVD through various pathways including influencing hemodynamics, endothelial dysfunction, inflammation, insulin resistance, hyperlipidemia, and arrhythmogenesis (Cheng et al., 2020, Rostron et al., 2015, US Department of Health and Human Services, 1986, US Department of Health and Human Services, 2010). It is well established that nicotine is associated with acute increases in blood pressure and heart rate (Gupta et al., 2004, Piano et al., 2010, Cheng et al., 2020, Rostron et al., 2015, US Department of Health and Human Services, 1986, US Department of Health and Human Services, 2010). Nicotine may also contribute to atherosclerotic disease by actions on lipid metabolism and coagulation (Cheng et al., 2020, Rostron et al., 2015, US Department of Health and Human Services, 1986, US Department of Health and Human Services, 2010). Several studies have also examined the association between SLT and risk factors for CVD such as high blood pressure and type 2-diabetes with mixed findings (Siegel et al., 1992, Keith et al., 2016, Östenson et al., 2012, Persson et al., 2000, Bolinder et al., 1992). Also, biomarkers of inflammation appear to be slightly altered in SLT users, although lower compared to biomarker levels found in cigarette smokers and concurrent users of both cigarettes and SLT (i.e., dual users) (Cheng et al., 2020, Rostron et al., 2015, Prasad et al., 2016, Sgambato et al., 2018).
Understanding SLT use and potential health consequences has become even more pertinent after the passage of the 2009 Family Smoking Prevention and Tobacco Control Act, which gave the U.S. Food and Drug Administration the authority to regulate tobacco products, including SLT (US Food and Drug Administration, 2009). The purpose of this period prevalence study is to compare the self-reported prevalence of CVD across the first four waves of the PATH Study conducted between 2013 and 2017 in U.S. adult males, ≥40 years, who were current/former established SLT users including exclusive SLT users or those who used SLT in combination with cigarettes to those who were: 1) never established cigarette and SLT users, and 2) current/former established exclusive cigarette smokers, while controlling for known risk factors for CVD. This study advances the existing published literature on the health risks of SLT use by also exploring how the duration of regular SLT use (measured in years of use) relates to CVD prevalence.
2. Methods
2.1. Study design and population
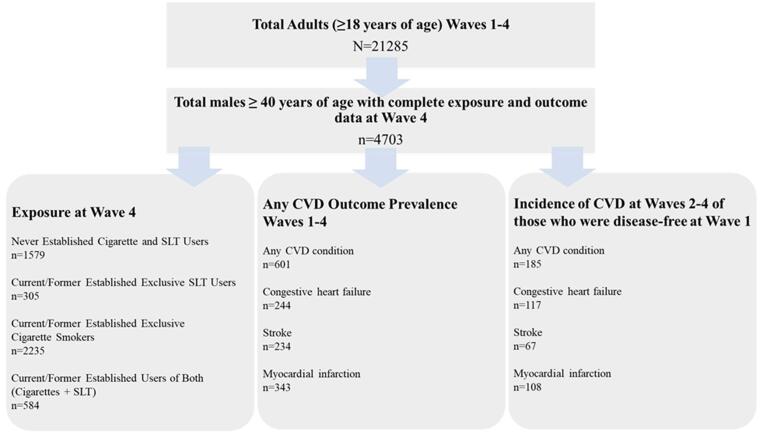
The study population was restricted to the 21,285 participants who participated in the first four waves of the PATH Study conducted between 2013 and 2017. Although the PATH Study is a longitudinal study, we were limited to a period prevalence design because the number of incident CVD cases that occurred among SLT users in the PATH Study Wave 1 Cohort followed up between 2013 and 2017 was too small to allow for meaningful analyses as shown in Fig. 1. In the analyses reported herein, we focused on CVD prevalence maximizing CVD reporting by inclusion of data across all four waves. Tobacco exposure measures were based on self-reports obtained in Wave 4. The study population was restricted to males because of the low rate of established SLT use among females and to those ≥ 40 years of age because CVD prevalence is low in those under age 40 (Sharma et al., 2020). Complete responses for the tobacco exposure and CVD outcome measures across Wave 1–4 were required yielding 4,703 males ≥ 40 years of age. Missing data on sex was imputed as described in the PATH Study Restricted Use Files (RUF) User Guide at https://doi.org/10.3886/Series606.
Fig. 1.
Selection of unweighted analytic sample used in the study.
2.2. PATH Study procedures
The PATH Study uses audio computer-assisted self-interviews (ACASI) available in English and Spanish to collect self-reported information on tobacco-use patterns and associated health behaviors in representative samples of adults and youth (Tomar et al., 2010, Campbell et al., 2015, Cheng et al., 2020, Rostron et al., 2015, US Department of Health and Human Services, 1986, US Department of Health and Human Services, 2010) in the U.S. The study recruitment employed a stratified address-based, area-probability sampling design at Wave 1 (Wave 1: September 12, 2013 to December 14, 2014) that oversampled adult tobacco users, young adults (Timberlake et al., 2017, Accortt et al., 2002, Fisher et al., 2019, Henley et al., 2005, Inoue-Choi et al., 2019, Lee, 2007, Rostron et al., 2018), and African American adults. An in-person screener was used at Wave 1 to randomly select youths and adults from households for participation (Wave 1 Cohort). A probability replenishment sample of adults and youth was selected from the U.S. civilian noninstitutionalized population (CNP) at the time of Wave 4 (Wave 4: December 1, 2016 through January 3, 2018), including persons who were not in the CNP at the time of Wave 1 (recent immigrants or those returning from the military). Members of the Wave 1 Cohort who remained in the CNP at the time of Wave 4 were combined with the Wave 4 replenishment sample to form the new Wave 4 Cohort. This analysis only included adults from the Wave 1 Cohort. For the group originating at Wave 1, the weighted response rate for the Wave 1 household screener was 54.0%. Among adults selected during screening, the weighted response rate at Wave 1 was 74.0% for the adult (18yearsandolder) interview. The weighted Wave 4 response rate for the Wave 1 Cohort, conditional upon Wave 1 participation, was 73.5% for adults. Full-sample and replicate weights that adjust for the complex sample design (e.g., oversampling of demographic groups) and nonresponse were used. This analysis used Wave 4 all-waves weights to obtain statistically valid estimates from longitudinal analysis that examine the Wave 1 Cohort across Waves 1 through 4. Weighted estimates are representative of the Wave 1 civilian, noninstitutionalized population of the U.S. at Wave 4.
Further details regarding the PATH Study design and methods for the Wave 1 Cohort are published elsewhere (Hyland et al., 2017, Kasza et al., 2017, Tourangeau et al., 2019). Details on interview procedures, questionnaires, sampling, weighting, response rates, and accessing the data are described in the PATH Study Restricted Use Files User Guide at https://doi.org/10.3886/Series606. The study was conducted by Westat and approved by the Westat Institutional Review Board. All respondents ages 18 and older provided informed consent.
2.3. Measures
Supplementary Table 1 provides a description of the measures used to assess CVD outcomes, lifetime SLT use, lifetime cigarette use, and various covariates that were considered in analyses.
2.4. CVD outcome measures
At Wave 1, all adult participants were asked, ‘Has a doctor or other health professional ever told you that you had any of the following conditions?’ Responses included congestive heart failure, stroke, heart attack (also called myocardial infarction), or needed bypass surgery with yes/no options. At subsequent waves, participants were asked, ‘In the past 12 months, has a doctor or other health professional told you that you had any of the following conditions?’ Participants who ever reported that they had been told they had congestive heart failure, stroke, or heart attack were classified as having a CVD condition. The outcome was defined as the report of any of the above-mentioned conditions in any of the 4 waves of data.
2.5. Smokeless and other tobacco use measures
The PATH Study interviews ask about tobacco use behaviors for cigarettes, electronic nicotine delivery systems (ENDS), traditional cigars, cigarillos, filtered cigars, pipe tobacco, hookah, snus pouches, and other SLT. The interview describes SLT as products which are put in the mouth and frequently chewed, sucked or spitted, and snus pouches, a type of SLT that comes in a small pouch that is put inside the lip. Generic pictures and descriptions of SLT products are displayed on the screen for respondents prior to questioning, and common brands such as Redman, Levi Garrett, Beechnut, Skoal, Grizzly, Nordic Ice, and Copenhagen, are provided as examples.
2.6. Measures of SLT use
Established users of SLT include those in Wave 4 who reported ever use of tobacco products which are put in the mouth and frequently chewed, sucked or spitted, and snus pouches fairly regularly, while never established users did not. Current users were those reporting daily or someday use, whereas former users had not used SLT or snus pouches in the past 12 months. Exclusive SLT users included those who only reported use of SLT or snus pouches and never established cigarette use during their lifetime.
Current established SLT users were also asked about frequency of use of SLT and classified as either every day or some-day users. Former SLT users were not asked about frequency of past use of SLT. Current and former established SLT users were asked to indicate the approximate number of years they used SLT and/or snus pouches fairly regularly. Duration of SLT use was grouped into 1–19 years, 20–39 years, and ≥ 40 years. These exposure cut-points divided the sample into three groups of approximately equal size.
2.7. Measures of cigarette use
Established cigarette smokers were defined as those in the Wave 4 PATH Study interview who reported having smoked ≥ 100 cigarettes in their lifetime, while never established users did not exceed this threshold. Current established smokers were those reporting daily or some-day use, whereas former established smokers had not smoked the past 12 months. Both current and former established cigarette smokers were asked to report the approximate number of years they smoked cigarettes and the number of cigarettes smoked per day. Pack-year history of smoking was computed as the number of cigarette packs smoked per day multiplied by the number of years they have smoked fairly regularly. Never established cigarette smokers were defined as including those in Wave 4 of the PATH Study who had not smoked ≥ 100 cigarettes in their lifetime and those who were classified as established cigarette smokers (i.e., ≥100 cigarettes in their lifetime) who reported having accumulated less than one pack-year of cigarette use during their lifetime.
Cigarette exposure was grouped into 1–19 pack years, 20–39 pack years, and ≥ 40 pack years. These exposure cut-points divided the sample into three groups of approximately equal size.
2.8. Measures of combined cigarette and SLT use
A combined measure of cigarette smoking and SLT use was used to jointly characterize SLT and cigarette smoking as follows: 1) never established cigarette smoker and SLT users; 2) current/former established exclusive SLT users; 3) current/former established exclusive cigarette smokers; 4) current/former established users of both cigarettes and SLT.
2.9. Measures of regular non-cigarette combustible tobacco product use
Wave 4 adult respondents who reported any level of current or former established use of any other combustible tobacco product (traditional cigars, cigarillos, filtered cigars, pipe or hookah) without consideration for cigarettes were coded as having a history of regular non-cigarette combustible tobacco product use. Those who reported not having current or former established use of all these products were coded as not having history of regular non-cigarette combustible tobacco product use. Those who had missing data on current or former established use of any of these products were coded as having missing history of regular non-cigarette combustible tobacco product use.
2.10. Other covariates
Covariates assessed in this study included reported current or past regular use of cigarettes (i.e., pack-years of exposure), and any regular use of non-cigarette combustible tobacco products (i.e., any mention of the following combustible tobacco products traditional cigars, cigarillos, filtered cigars, pipe or hookah). Other covariates considered in the analyses include age categorized into four groups (i.e., 40–49 years of age, 50–59 years of age, 60–69 years of age, 70 years of age and older), urbanicity (see Supplementary Table 1 for definition of urban and not-urban), educational attainment (less than high school or GED, high school graduate, some college (no degree) or Associates degree, Bachelor’s or advanced degree), race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Non-Hispanic Other, Hispanic).
Five CVD risk factors were assessed by asking respondents in the Wave 4 PATH Study interview to self-report having a history of: (Food and Administration, 2018) high blood pressure, (Chang et al., 2016) high cholesterol, (Cornelius et al., 2020) diabetes, (Levy et al., 2018) family history of premature cardiac disease, and (Chaffee et al., 2017) obesity (body mass index [BMI] ≥ 35). Adult participants were asked, ‘Has a doctor or other health professional ever told you that you had any of the following conditions?’ Responses included high blood pressure, high cholesterol, and diabetes (including sugar diabetes, high blood sugar, or borderline diabetes). Family history of premature cardiac disease was assessed by asking participants, ‘Were any of your close biological or blood relatives ever told by a health professional that they had a heart attack or needed bypass surgery?’ If yes, ‘Were they told they had a heart attack or needed bypass surgery before the age of 50?’ Finally, obesity was determined by BMI ≥ 35 calculated for each participant based on self-reported height and weight. Responses to these items were used to create a risk factor index (range 0 [none reported] to 5 [all 5 reported]).
2.11. Data analysis
Unweighted frequencies and weighted percentages were reported for categorical variables. Weighted means were provided for continuous variables and standard errors were reported for all variables. Weighted logistic regression models were implemented to determine the association between tobacco use (independent variable) and CVD (dependent variable). Weighted odds ratios (OR) and 95% confidence intervals (95% CI) were reported for adjusted associations. Full sample weights and replicate weights were used to estimate the variance according to the balanced repeated replication method (BRR) (McCarthy, 1969, Judkins, 1990). All statistical analyses were performed in SAS 9.4 (SAS Institute, Cary, NC).
In analyses comparing the prevalence of CVD in current/former established SLT users against never established cigarette and SLT users, lifetime use of cigarettes assessed by measuring pack-years of exposure was included as a covariate. In analyses comparing the prevalence of CVD among current/former established exclusive cigarette smokers and current/former established exclusive SLT users with similar duration of exposure, years of use of SLT were compared to years of use of cigarettes rather than pack years for consistency. Duration of exclusive SLT use (e.g., 1–19 years) was compared to the same duration of exclusive cigarettes use (e.g., 1–19 years) by a logistic regression model setting 1–19 years of exclusive cigarettes use as the referent group. The same logistic regression model was run with alternative referent groups to compare 20–39 years of exclusive SLT use to 20–39 years of exclusive cigarettes use, and ≥ 40 years of exclusive SLT use to ≥ 40 years of exclusive cigarettes use.
We also performed a trend tests to evaluate how years of use of SLT related to CVD prevalence. Trend tests were run with years of use of SLT as a continuous variable and also and also as an ordinal variable where never users of SLT were given a value of 0, those who used SLT for a period of 1–19 years were given a value of 10, those who used SLT for a period of 20–39 years were given a value of 30, and those who used SLT for 40 years or more were given a value of 50. The results were the same regardless of whether years of use of SLT was treated as a continuous variable or ordinal variable. In this paper we have chosen to present the ordinal results where a logistic regression model was run with CVD prevalence as the dependent variable and the ordinal years of SLT exposure variable with 4 categories (0, 10, 30, 50) was the independent variable. The logistic regression model generated a type-3p-value for the ordinal variable which is the p-value for the linear trend test.
3. Results
Table 1 provides an overview of the study population of males ≥ 40 years of age. The average age was 58.9 years. The prevalence of established exclusive cigarette smoking (current and former users combined) was 36.4% and that of established exclusive SLT use (current and former combined) was 4.7%. The prevalence of established users of both cigarettes and SLT (current and former users combined) was 8.1%; while 50.7% were never established cigarette and SLT users. The average cigarette pack year history for males ≥ 40 years of age was 31.9 pack years (current established smokers = 33.1 pack years; former established smokers = 31.2 pack-years). Among current established exclusive cigarette smokers, 86.3% were daily smokers. Among current/former established SLT users, the average duration of use was 23.1 years and was similar among current/former users of both (cigarettes and SLT) (22.2 years) and current/former established exclusive SLT users (24.5 years). As expected, it was considerably longer among current established SLT users (34.0 years) compared to former established SLT users (17.2 years) [data not shown]. About three-fourths of current established SLT users (78.4%) were daily users. The prevalence of any CVD outcome among males ≥ 40 years of age was 12.0%; 4.7% congestive heart failure, 4.1% stroke, and 7.3% myocardial infarction. Among males ≥ 40 years of age, 72.7% had at least one CVD risk factor; 50.7% hypertension, 45.5% hypercholesterolemia, 27.6% type-2 diabetes, 12.0% family history of premature heart disease, and 12.1% had BMI ≥ 35.
Table 1.
Weighted characteristics of adult males ≥ 40 years of age by tobacco use status in PATH Study Waves 1–4.
| Characteristic |
Total |
Never Established Cigarette and SLT Users |
Current/Former Established Exclusive SLT Users |
Current/Former Established Exclusive Cigarette Smokers |
Current/Former Established Users of Both (Cigarettes + SLT) |
|---|---|---|---|---|---|
| N1 = 4703 | n = 1579 (50.7%, 1.0) | n = 305 (4.7%, 0.4) | n = 2235 (36.4%, 0.9) | n = 584 (8.1%, 0.5) | |
| Age in years, Mean (SEM) | 58.9 (0.2) | 57.9 (0.3) | 54.7 (0.9) | 61.2 (0.3) | 56.8 (0.7) |
| Age in years, n (%, SE%) | |||||
| 40–49 years | 1397 (25.9, 0.7) | 496 (28.4, 1.3) | 122 (35.2, 3.8) | 545 (19.0, 1.0) | 234 (36.4, 2.5) |
| 50–59 years | 1512 (29.7, 0.8) | 510 (31.6, 1.4) | 112 (41.4, 3.7) | 716 (26.2, 1.1) | 174 (26.1, 2.2) |
| 60–69 years | 1137 (24.5, 0.9) | 339 (21.8, 1.2) | 43 (13.7, 2.4) | 650 (30.6, 1.4) | 105 (20.3, 2.8) |
| ≥70 years | 657 (19.9, 0.5) | 234 (18.2, 1.0) | 28 (9.7, 2.6) | 324 (24.2, 1.2) | 71 (17.1, 2.5) |
| Urbanicity, n (%, SE%) | |||||
| Urban | 3428 (74.5, 2.0) | 1282 (79.7, 2.0) | 159 (57.9, 4.9) | 1648 (73.5, 2.5) | 339 (56.2, 3.2) |
| Not urban | 1275 (25.5, 2.0) | 297 (20.3, 2.0) | 146 (42.1, 4.9) | 587 (26.5, 2.5) | 245 (43.8, 3.2) |
| Educational attainment, n (%, SE%) | |||||
| Less than high school or GED | 1080 (18.4, 0.5) | 231 (14.0, 0.8) | 50 (12.1, 1.9) | 636 (23.9, 1.1) | 163 (24.8, 2.6) |
| High school graduate | 954 (22.7, 0.6) | 255 (19.8, 1.2) | 78 (29.4, 3.8) | 492 (25.1, 1.3) | 129 (25.9, 2.8) |
| Some college (no degree) or associate degree | 1388 (28.1, 0.6) | 411 (25.6, 1.1) | 100 (30.7, 3.3) | 683 (29.9, 1.2) | 194 (34.9, 3.1) |
| Bachelor’s or advanced | 1281 (30.8, 0.5) | 682 (40.6, 1.1) | 77 (27.8, 3.3) | 424 (21.1, 1.3) | 98 (14.3, 1.9) |
| Race/Ethnicity, n (%, SE%) | |||||
| Non-Hispanic white | 3174 (71.6, 0.8) | 939 (65.4, 1.4) | 257 (87.0, 3.2) | 1498 (74.7, 1.2) | 480 (87.5, 1.6) |
| Non-Hispanic black | 707 (11.7, 0.5) | 271 (13.1, 0.9) | 14 (3.2, 1.0) † | 394 (12.6, 0.8) | 28 (3.8, 0.9) |
| Non-Hispanic other | 286 (6.0, 0.6) | 94 (7.1, 1.0) | 21 (7.6, 3.2) † | 120 (4.8, 0.8) | 51 (4.3, 0.7) |
| Hispanic | 536 (10.7, 0.5) | 275 (14.4, 0.8) | 13 (2.2, 0.7) | 223 (7.9, 0.7) | 25 (4.5, 1.3) |
| Any ever fairly regular use of non-cigarette combustible tobacco products, n (%, SE%) | |||||
| No | 2201 (56.3, 0.9) | 1000 (71.7, 1.2) | 142 (48.3, 4.2) | 889 (41.2, 1.4) | 170 (32.1, 3.1) |
| Yes | 1368 (21.5, 0.9) | 236 (8.2, 0.7) | 71 (21.3, 3.1) | 762 (33.7, 1.6) | 299 (49.4, 3.3) |
| Missing | 1134 (22.3, 0.8) | 343 (20.1, 1.2) | 92 (30.4, 4.1) | 584 (25.1, 1.3) | 115 (18.5, 2.2) |
| Average pack-year cigarette history (Mean SEM) 2, 3 | 31.9 (0.9) | NA | NA | 31.6 (1.0) | 33.2 (1.9) |
| Categorical pack-year cigarette history, n (%, SE%) | |||||
| 1–19 pack-years | 1099 (39.7, 1.3) | NA | NA | 886 (40.2, 1.4) | 213 (37.7, 2.5) |
| 20–39 pack-years | 965 (32.9, 1.3) | NA | NA | 750 (32.5, 1.4) | 215 (35.0, 2.7) |
| ≥40 pack-years | 755 (27.3, 1.4) | NA | NA | 599 (27.3, 1.5) | 156 (27.3, 2.3) |
| Average pack-year cigarette history by category, Mean (SEM) | |||||
| 1–19 pack years | 9.7 (0.2) | NA | NA | 9.6 (0.2) | 10.1 (0.5) |
| 20–39 pack years | 28.4 (0.3) | NA | NA | 28.3 (0.3) | 28.8 (0.6) |
| ≥40 pack-years | 68.5 (2.2) | NA | NA | 68.0 (2.3) | 70.7 (4.2) |
| Average duration of cigarette use in years, Mean (SEM) 3 | 31.2 (0.4) | NA | NA | 31.6 (0.5) | 29.7 (0. 9) |
| Categorical duration (years) of cigarette use, n (%, SE%) | |||||
| 1–19 years | 404 (23.6, 1.4) | NA | NA | 304 (23.3, 1.4) | 100 (25.0, 2.7) |
| 20–39 years | 1285 (44.9, 1.4) | NA | NA | 968 (43.5, 1.6) | 317 (51.0, 2.7) |
| ≥40 years | 1125 (31.2, 1.3) | NA | NA | 959 (32.9, 1.6) | 166 (23.8, 2.4) |
| Average duration (years) of cigarette use by category, Mean (SEM) | |||||
| 1–19 years | 12.0 (0.3) | NA | NA | 12.0 (0.3) | 12.0 (0.6) |
| 20–39 years | 29.5 (0.2) | NA | NA | 29.5 (0.3) | 29.7 (0.4) |
| ≥40 years | 48.2 (0.2) | NA | NA | 48.2 (0.3) | 48.3 (0.8) |
| Daily cigarette smokers among current established smokers, n (%, SE%)4 | 1400 (85.6, 1.0) | NA | NA | 1163 (86.3, 1.0) | 237 (82.2, 3.1) |
| Average duration (years) of SLT use, Mean (SEM) 3 | 23.1 (0.7) | NA | 24.5 (1.4) | NA | 22.2 (0.8) |
| Categorical duration (years) of SLT use, n (%, SE%) | |||||
| 1–19 years | 310 (39.6, 2.6) | NA | 71 (35.2, 4.2) | NA | 239 (42.2, 3.3) |
| 20–39 years | 375 (40.2, 2.0) | NA | 156 (45.5, 3.8) | NA | 219 (37.0, 2.7) |
| ≥40 years | 149 (12.9, 1.4) | NA | 69 (15.5, 2.5) | NA | 80 (11.3, 1.6) |
| Average duration (years) of SLT by category, Mean (SEM) | |||||
| 1–19 years | 8.4 (0.4) | NA | 6.7 (0.9) | NA | 9.3 (0.5) |
| 20–39 years | 29.3 (0.5) | NA | 30.3 (0.8) | NA | 28.6 (0.6) |
| ≥40 years | 48.8 (0.9) | NA | 47.8 (1.0) | NA | 49.7 (1.5) |
| Daily SLT use among current established SLT users, n (%, SE%)5 | 293 (78.4, 2.2) | NA | 156 (82.0, 3.1) | NA | 137 (74.9, 3.7) |
| Ever CVD outcomes, n (%, SE%) | |||||
| Congestive Heart Failure | 244 (4.7, 0.4) | 50 (2.8, 0.5) | 12 (2.5, 1.0) † | 145 (7.5, 0.9) | 37 (5.4, 1.2) |
| Stroke | 234 (4.1, 0.4) | 56 (2.9, 0.5) | 13 (4.0, 1.4) † | 131 (5.6, 0.7) | 34 (4.9, 1.1) |
| Myocardial Infarction | 343 (7.3, 0.5) | 79 (4.8, 0.7) | 18 (6.9, 2.1) † | 194 (10.6, 1.1) | 52 (9.0, 1.8) |
| Any of the above | 601 (12.0, 0.7) | 137 (8.2, 0.9) | 31 (10.8, 2.7) | 347 (17.1, 1.2) | 86 (13.7, 2.0) |
| Number of ever CVD events, n (%, SE%) | |||||
| None | 4102 (88.0, 0.7) | 1442 (91.8, 0.9) | 274 (89.2, 2.7) | 1888 (82.9, 1.2) | 498 (86.3, 2.0) |
| One | 416 (8.5, 0.6) | 96 (6.2, 0.8) | 19 (8.2, 2.6) † | 244 (11.6, 1.0) | 57 (9.1, 1.7) |
| Two or more | 185 (3.5, 0.4) | 41 (2.0, 0.4) | 12 (2.6, 0.9) † | 103 (5.5, 0.8) | 29 (4.6, 1.1) |
| CVD risk factors, n (%, SE%) | |||||
| Hypertension | 2411 (50.7, 0.9) | 717 (45.5, 1.6) | 154 (47.5, 3.5) | 1210 (56.4, 1.6) | 330 (59.2, 3.1) |
| Hypercholesterolemia | 2071 (45.5, 1.0) | 654 (41.3, 1.7) | 132 (45.0, 3.7) | 1015 (50.5, 1.5) | 270 (49.2, 2.8) |
| Type 2 Diabetes | 1287 (27.6, 0.9) | 437 (27.0, 1.4) | 79 (26.7, 3.4) | 619 (28.4, 1.7) | 152 (28.0, 2.5) |
| Family history of premature heart disease | 661 (12.0, 0.6) | 176 (10.2, 0.9) | 37 (10.9, 2.1) | 321 (12.8, 0.9) | 127 (20.9, 2.7) |
| BMI ≥ 35 | 605 (12.1, 0.6) | 207 (12.1, 0.9) | 54 (16.1, 2.4) | 258 (11.3, 1.0) | 86 (13.4, 2.4) |
| Any of the above | 3426 (72.7, 0.8) | 1110 (69.4, 1.4) | 217 (70.0, 4.0) | 1651 (76.8, 1.3) | 448 (76.9, 2.6) |
| Number of CVD risk factors overall, n (%, SE%) | |||||
| None | 1230 (26.3, 0.8) | 455 (29.7, 1.3) | 87 (29.8, 4.0) | 558 (22.4, 1.3) | 130 (21.1, 2.4) |
| One | 1231 (26.2, 0.9) | 443 (27.3, 1.3) | 74 (24.2, 3.6) | 562 (25.9, 1.5) | 152 (22.2, 1.9) |
| Two | 1119 (24.4, 0.8) | 343 (22.8, 1.3) | 68 (21.3, 3.9) | 573 (26.9, 1.4) | 135 (25.3, 2.6) |
| Three or more | 1076 (22.1, 0.9) | 324 (19.3, 1.2) | 75 (24.4, 3.3) | 516 (24.0, 1.5) | 161 (29.4, 2.7) |
SEM: standard error of mean
SE%: standard error of percent
NA: Not applicable because the corresponding user group was not included in analyses for the measure.
CVD: any cardiovascular disease defined as congestive heart failure, stroke, or heart attack
SLT: tobacco products which are put in the mouth and frequently chewed, sucked or spitted, and snus pouches, a type of SLT that comes in a small pouch that is put inside the lip.
N and n’s are unweighted; (%), SE%, mean, and SEM are weighted.
1Frequencies may not add up to total N or user group n because of one or more of the following reasons: a) missing data, b) analyses were limited to selected subgroups, and c) using the categorical version of a measure as presented in this table.
2The outliers of the pack-year cigarette history measure were trimmed down to the 99th percentile.
3 Measures were fixed to zero for the Never Established Cigarette or SLT Users and Current/Former Established Exclusive users of a product as presented; User groups marked with “NA” were not included in analyses of the total estimates but still contribute to the regression analyses of Table 2.
4There were a total 1616 current established smokers, 1336 exclusive smokers and 280 users of both (cigarettes + SLT).
5There were a total 377 current established SLT users, 189 exclusive SLT users and 188 users of both (cigarettes + SLT).
†: Estimate should be interpreted with caution because it has low statistical precision. It is based on a denominator sample size of less than 50, or the coefficient of variation of the estimate or its complement is larger than 30%.
Supplementary Table 2 provides the unadjusted prevalence rates of CVD outcomes for multiple tobacco product user groups, including examining current and former tobacco users separately and compared exclusive SLT users to those who also smoked cigarettes at some time during their lifetime. The prevalence of any CVD outcome among the never established cigarette and SLT users was 8.2%, 12.6% among current/former established users of SLT, 10.8% among current/former established exclusive users of SLT, 13.7% among current/former established users of both (cigarettes and SLT), and 17.1% among current/former established exclusive cigarette smokers.
Table 2 shows the odds of having any type of CVD among current/former established SLT users compared to never established cigarette and SLT users controlling for pack-years of smoking, any regular use of other combustible tobacco, age, urbanicity, educational attainment, race/ethnicity, and other CVD risk factors (OR = 1.2 [0.7–2.1]), which was not statistically significant. A non-significant association was also found when comparing current/former established exclusive SLT users to never established cigarettes and SLT users (OR = 1.7 [0.8–3.7]) after adjustment for covariates.
Table 2.
Weighted associations of SLT use and cigarette smoking with prevalence of any CVD.
| N4 | OR (95% CI) | |
|---|---|---|
| Never Established Cigarette and SLT users1 | 1565 | 1.0 (ref.) |
| Current/Former Established SLT users1, 2 | 827 | 1.2 (0.7–2.1) |
| Years of SLT use | ||
| 1–19 | 308 | 1.0 (0.5–1.9) |
| 20–39 | 373 | 1.2 (0.6–2.5) |
| ≥40 | 146 | 1.5 (0.8–3.1) |
| p-value for trend3 | 0.2371 | |
| Current/Former Established Exclusive SLT users1 | 295 | 1.7 (0.8–3.7) |
| Years of SLT use | ||
| 1–19 | 71 | 0.8 (0.1–4.9) |
| 20–39 | 156 | 2.7 (1.1–6.8) |
| ≥40 | 68 | 1.5 (0.5–4.2) |
| p-value for trend3 | 0.1012 | |
| Current/Former Established Exclusive Cigarette Smokers1 | 2209 | 1.6 (1.1–2.3) |
| Pack years of cigarettes smoking | ||
| 1–19 | 872 | 1.3 (0.8–2.1) |
| 20–39 | 742 | 1.5 (1.0–2.4) |
| ≥40 | 595 | 2.1 (1.4–3.1) |
| p-value for trend3 | 0.0004 | |
| Current/Former Established Exclusive Cigarette Smokers | 2205 | 1.0 (ref.) |
| Current/Former Established Exclusive SLT users1 | 295 | 0.9 (0.5–1.8) |
| Years of use | ||
| 1–19 years of exclusive SLT use (ref. 1–19 years of exclusive cigarettes smoking) | 71 (ref. 300) | 0.5 (0.1–3.7) |
| 20–39 years of exclusive SLT use (ref. 20–39 years of exclusive cigarettes smoking) | 156 (ref. 952) | 1.6 (0.7–4.0) |
| ≥40 years of exclusive SLT use (ref. ≥ 40 years of exclusive cigarettes smoking) | 68 (ref. 953) | 0.5 (0.2–1.4) |
N and n’s are unweighted; OR’s 95%CI’s are weighted
OR: odds ratio
CI: confidence interval
CVD: any cardiovascular disease defined as congestive heart failure, stroke, or heart attack
SLT: tobacco products which are put in the mouth and frequently chewed, sucked or spitted, and snus pouches, a type of SLT that comes in a small pouch that is put inside the lip
1Covariates adjusted for include: age (40 s, 50 s, 60 s, 70 s + ), urbanicity (urban, not urban household residence created after cumulating total urban population and total population from the 2010 decennial census block-level data to the segment level. A segment was classified as “urban” if the majority of its total population resides in areas classified as urban according to the 2010 census, and “not urban” otherwise), educational attainment (less than high school or GED, high school graduate, some college (no degree) or associates degree, Bachelor’s or advanced degree), Race/Ethnicity (Non-Hispanic white, Non-Hispanic black, Non-Hispanic other, Hispanic), use of other combustible (Yes/No/Missing), and CVD risk factor score (0, 1, 2, 3 + ).
2In addition to the covariates adjusted for above, we also adjusted for pack-years of cigarette smoking (only for current/former cigarette smokers; pack-years was fixed to 0 for Never Established Cigarette or SLT users and Current/Former Established Exclusive SLT users).
3An ordinal form of the years-of-use variable (with categorical values coded as 0 years, 10 years, 30 years, and 50 years, respectively) was used in the logistic regression model as a continuous predictor, other covariates remained the same in the model.
4Some observations were excluded due to listwise deletion analysis.
By contrast, current/former established exclusive cigarette smokers had significantly elevated CVD prevalence compared to never established cigarette and SLT users (OR = 1.6 [1.1–2.3]).
Table 2 also shows the odds of having any type of CVD in current/former established exclusive SLT users compared to current/former established exclusive cigarette smokers controlling for covariates. The association with CVD in current/former established exclusive SLT users was not significantly different compared to current/former established exclusive cigarette smokers (OR = 0.9 [0.5–1.8]).
Table 2 also shows the dose–response associations between any CVD in those with different durations of established SLT use and/or established cigarette smoking compared to those without an established history of SLT use and cigarette smoking. A consistent dose–response association between duration of established SLT use (measured by years of use) and risk of any CVD was not observed, with OR = 1.0 [0.5–1.9] for 1–19 years of established SLT use, OR = 1.2 [0.6–2.5] for 20–39 years of established SLT use, and OR = 1.5 [0.8–3.1] for ≥ 40 years of established SLT use, when compared to never established cigarette and SLT users (p-trend = 0.24). The results were similar for established exclusive SLT users compared to never established cigarette and SLT users (p-trend = 0.10).
Table 2 also shows the association between CVD in established exclusive cigarette smokers stratified by pack-years of exposure compared to those without an established history of SLT and cigarette smoking. A significant dose–response association (p-trend = 0.0004) was found between pack-years of cigarette exposure and prevalence of any CVD compared to never established cigarette and SLT users (OR = 1.3, [0.8–2.1] for 1–19 pack years; OR = 1.5, [1.0–2.4] for 20–39 pack years; OR = 2.1, [1.4–3.1] for ≥ 40 pack years).
4. Discussion
The published literature (Campbell et al., 2015, Cheng et al., 2020, Rostron et al., 2015, US Department of Health and Human Services, 1986, US Department of Health and Human Services, 2010, Timberlake et al., 2017, Accortt et al., 2002, Fisher et al., 2019, Henley et al., 2005, Inoue-Choi et al., 2019, Lee, 2007, Rostron et al., 2018, Vidyasagaran et al., 2016, Boffetta and Straif, 2009, Gupta et al., 2004, Piano et al., 2010, Asplund et al., 2003, Haglund et al., 2007, Bolinder et al., 1994, Hergens et al., 2005, Wennberg et al., 2007, Johansson et al., 2005, Huhtasaari et al., 1992, Yatsuya and Folsom, 2010, Mushtaq et al., 2010, Henley et al., 2007) on association between SLT use and CVD is mixed, with some studies showing a slight elevated risk of CVD compared to non-users and others reporting no association. The present study found that the unadjusted prevalence of having had any CVD was slightly elevated in current and former established SLT users compared to never established users of cigarettes and SLT. However, the association was not statistically significant after adjustment for covariates. The evidence favoring an association would have been strengthened if a dose–response association was present. However, trend tests failed to show a clear-cut monotonic trend between years of exposure and the prevalence of any CVD. Consistent with other published studies, we did observe a significant dose–response relationship between the prevalence of any CVD and exclusive established cigarette smoking, based on pack-years of exposure (US Department of Health and Human Services, 2010).
This period prevalence study of SLT use in relation to the prevalence of CVD outcome has some unique strengths. First, the study has a rare degree of external validity because it was based on a representative sample of U.S. males ≥ 40 years of age. Second, because the PATH Study focuses on tobacco use, the measurement of the tobacco use variables has a degree of precision that is rarely observed in population-based epidemiologic studies. Participants provided detailed information on life-time history of SLT and other tobacco use enabling exclusive SLT use to be distinguished from dual use of SLT and combustible tobacco cigarettes.
There are limitations to the present study that need to be acknowledged. First, this study relied upon self-reported histories of three types of CVD – myocardial infarction, stroke, and congestive heart disease-- that were not validated by medical records. However, the reported prevalence rates for the three CVD diagnoses assessed were consistent with those that have been reported and validated in other national samples (Mahoney et al., 2020). Second, our study only includes prevalence of CVD, not incidence rates. Even though the PATH Study is a large study with multiple years of follow-up, there were only 202 incident cases of CVD in the sample of ≥40 year old males over the approximately four-year follow-up period, making it difficult to reliably assess the association between SLT use and CVD incidence. This may explain, for example, why CVD prevalence tended to be higher in established former versus current SLT users, as the diagnosis of CVD could have resulted in increased likelihood of quitting SLT use. A challenge to all studies that attempt to characterize the health effects of SLT use is the fact that SLT users often also smoke combustible tobacco cigarettes, making it difficult to isolate the association for SLT use. For example, 63.1% of SLT users had a history of established current and/or past cigarette smoking. A larger sample of exclusive SLT users would improve the reliability of the associations between SLT use and CVD, but this will be difficult to achieve in practice because the reality is that the majority of SLT users currently or previously smoked cigarettes and/or used other forms of combustible tobacco. Thus, the reported odds ratios for all SLT users regardless of past or current cigarette smoking are reasonable approximations of the risk of CVD as it might be observed in the general population. Fourth, while this study improves upon earlier published studies evaluating the health risks of SLT by incorporating a measure of duration of regular use, the PATH Study interview does not capture information on former SLT users’ frequency of SLT use nor type of SLT product used. Frequency and type of SLT product used were captured on current users only, but even in this group of current users, information available on product type and frequency may not reflect historical patterns of use (Stepanov et al., 2008). Nevertheless, despite these weaknesses, a measure of reassurance is provided by the associations between combustible cigarette smoking and CVD outcomes, which were of similar direction and magnitude as those typically observed in prospective cohort studies (Cornelius et al., 2020, Inoue-Choi et al., 2019). Another challenge facing the field of research into the health effects of SLT is that given the evolution of the SLT market during the past several decades it is unclear whether a finding of an association between history of SLT use and disease risk would be relevant to newer products currently on the market (Stepanov et al., 2008).
In summary, this study found that CVD prevalence was not significantly higher in established users of SLT compared to never established users of cigarettes and SLT after adjustment for co-variates. Moreover, we did not observe a consistent dose–response trend for years of SLT use and prevalence of CVD, although this conclusion is weakened by the limited statistical power to test the dose–response trend. As the Wave 1 of the PATH Study Cohort ages, the ability to test for associations between SLT use and CVD will be enhanced.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
K. Michael Cummings provides expert testimony on the health effects of smoking and industry tactics in lawsuits filed against cigarette manufacturers. He has also received payment as a consultant to Pfizer, Inc., for services on an external advisory panel to assess ways to improve smoking cessation delivery in health care settings.
All other co-authors report no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2021.101650.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- US Food and Drug Administration. Smokeless tobacco products, including dip, snuff, Snus, and chewing tobacco, 2018. Available: https://www.fda.gov/tobacco-products/products-guidance-regulations/products-ingredients-components [Accessed 14 Oct 2020].
- Chang J.T., Levy D.T., Meza R. Trends and factors related to smokeless tobacco use in the United States. Nicotine Tob. Res. 2016;18(8):1740–1748. doi: 10.1093/ntr/ntw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius, M.E., Wang, T.W., Jamal, A., Loretan, C., Neff, L. 2020. Tobacco Product Use Among Adults – United States, 2019. Morbidity and Mortality Weekly Report, 69(issue 46), 1736–1742. [DOI] [PMC free article] [PubMed]
- Levy, D.T., Mays, D., Boyle, R.G., Tam, J., Chaloupka, F.J. 2017. The effect of tobacco control policies on US smokeless tobacco use: a structured review. Nicotine Tob. Res. 13, 20(1), 3-11. [DOI] [PMC free article] [PubMed]
- Chaffee B., Urata J., Couch E., Gansky S. Perceived flavored smokeless tobacco ease-of-use and youth susceptibility. Tob. Regul. Sci. 2017;3(3):367–373. doi: 10.18001/TRS.3.3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A., Ambrose B.K., Conway K.P., Borek N., Lambert E., Carusi C., Taylor K., Crosse S., Fong G.T., Cummings K.M., Abrams D., Pierce J.P., Sargent J., Messer K., Bansal-Travers M., Niaura R., Vallone D., Hammond D., Hilmi N., Kwan J., Piesse A., Kalton G., Lohr S., Pharris-Ciurej N., Castleman V., Green V.R., Tessman G., Kaufman A., Lawrence C., van Bemmel D.M., Kimmel H.L., Blount B., Yang L., O'Brien B., Tworek C., Alberding D., Hull L.C., Cheng Y.-C., Maklan D., Backinger C.L., Compton W.M. Design and methods of the population assessment of tobacco and health (PATH) study. Tobacco Control. 2017;26(4):371–378. doi: 10.1136/tobaccocontrol-2016-052934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza K.A., Ambrose B.K., Conway K.P., Borek N., Taylor K., Goniewicz M.L., Cummings K.M., Sharma E., Pearson J.L., Green V.R., Kaufman A.R., Bansal-Travers M., Travers M.J., Kwan J., Tworek C., Cheng Y.-C., Yang L., Pharris-Ciurej N., van Bemmel D.M., Backinger C.L., Compton W.M., Hyland A.J. Tobacco product use by adults and youths in the United States in 2013 and 2014. N. Engl. J. Med. 2017;376(4):342–353. doi: 10.1056/NEJMsa1607538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma E., Edwards K.C., Halenar M.J., Taylor K.A., Kasza K.A., Day H., Gardner L.D., Anic G., Bansal-Travers M., Limpert J., Hammad H.T., Borek N., Kimmel H.L., Compton W.M., Hyland A., Stanton C.A. Longitudinal pathways of exclusive and polytobacco smokeless use among youth, young adults and adults in the USA: findings from the PATH Study Waves 1–3 (2013–2016) Tob. Control. 2020;29(Suppl 3):s170–s177. doi: 10.1136/tobaccocontrol-2020-055628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipari, R.N. and Van Horn, S.L. Trends in smokeless tobacco use and initiation: 2002 to 2014. The CBHSQ Report: May 31, 2017. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. https://www.samhsa.gov/data/sites/default/files/report_2740/ShortReport-2740.html [Accessed 14 Oct 2020].
- Cheng Y.-C., Rostron B.L., Day H.R., Stanton C.A., Hull L.C., Persoskie A., Travers M.J., Taylor K., Conway K.P., Ambrose B.K., Borek N. Patterns of Use of Smokeless Tobacco in US Adults, 2013–2014. Am. J. Public Health. 2017;107(9):1508–1514. doi: 10.2105/AJPH.2017.303921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.M., Majeed B.A., Weaver S.R., Sterling K., Pechacek T.F., Eriksen M.P. Prevalence and factors associated with smokeless tobacco use, 2014–2016. Am J Health Behav. 2017;41(5):608–617. doi: 10.5993/AJHB.41.5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar S.L., Alpert H.R., Connolly G.N. Patterns of dual use of cigarettes and smokeless tobacco among us males: findings from national surveys. Tob. Control. 2010;19(2):104–109. doi: 10.1136/tc.2009.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell L.R., Brown B.G., Jones B.A., Marano K.M., Borgerding M.F. Study of cardiovascular disease biomarkers among tobacco consumers, part 1: biomarkers of exposure. Inhal. Toxicol. 2015;27(3):149–156. doi: 10.3109/08958378.2015.1013228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.-C., Reyes-Guzman C.M., Christensen C.H., Rostron B.L., Edwards K.C., Wang L., Feng J., Jarrett J.M., Ward C.D., Xia B., Kimmel H.L., Conway K., Leggett C., Taylor K., Lawrence C., Niaura R., Travers M.J., Hyland A., Hecht S.S., Hatsukami D.K., Goniewicz M.L., Borek N., Blount B.C., van Bemmel D.M. Biomarkers of exposure among adult smokeless tobacco users in the population assessment of tobacco and health study (Wave 1, 2013–2014) Cancer Epidemiol. Biomarkers Prev. 2020;29(3):659–667. doi: 10.1158/1055-9965.EPI-19-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostron B.L., Chang C.M., van Bemmel D.M., Xia Y., Blount B.C. Nicotine and toxicant exposure among U.S. smokeless tobacco users: results from 1999 to 2012 national health and nutrition examination survey data. Cancer Epidemiol. Biomarkers Prev. 2015;24(12):1829–1837. doi: 10.1158/1055-9965.EPI-15-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services . DHHS publication (NIH); Washington, DC: 1986. The Health Consequences of Using Smokeless Tobacco: A Report of the Advisory Committee to the Surgeon General; pp. 86–2874. [Google Scholar]
- U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010. [PubMed]
- Timberlake D.S., Nikitin D., Johnson N.J., Altekruse S.F. A longitudinal study of smokeless tobacco use and mortality in the United States. Int. J. Cancer. 2017;141:264–270. doi: 10.1002/ijc.30736. [DOI] [PubMed] [Google Scholar]
- Accortt N.A., Waterbor J.W., Beal C., Howard G. Chronic disease mortality in a cohort of smokeless tobacco users. Am. J. Epidemiol. 2002;156:730–737. doi: 10.1093/aje/kwf106. [DOI] [PubMed] [Google Scholar]
- Fisher M.T., Tan-Torres S., Gaworski C.L., Black R.S.A., rkar M.A. Smokeless tobacco mortality risks: an analysis of two contemporary nationally representative longitudinal mortality studies. Harm. Reduction J. 2019;16(27) doi: 10.1186/s12954-019-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley S.J., Thun M.J., Connell C., Calle E.E. Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States) Cancer Causes Control. 2005;16(4):347–358. doi: 10.1007/s10552-004-5519-6. [DOI] [PubMed] [Google Scholar]
- Inoue-Choi M. Shiels MS, McNeel TS, Graubard BI, Hatsukami D, Freedman ND. Contemporary associations of exclusive cigarette, cigar, pipe , and smokeless tobacco use with overall and cause-specific mortality in the United States. JNCI Cancer Spectrum, 2019; Epub 2019/05/17. doi.org/10.1093/jncics/pkz036. [DOI] [PMC free article] [PubMed]
- Lee P.N. Circulatory disease and smokeless tobacco in Western populations: a review of the evidence. Int. J. Epidemiol. 2007;36(4):789–804. doi: 10.1093/ije/dym039. [DOI] [PubMed] [Google Scholar]
- Rostron B.L., Chang J.T., Anic G.M., Tanwar M., Chang C.M., Corey C.G. Smokeless tobacco use and circulatory disease risk: a systematic review and meta-analysis. Open Heart. 2018;5(2):e000846. doi: 10.1136/openhrt-2018-000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidyasagaran A.L., Siddiqi K., Kanaan M. Use of smokeless tobacco and risk of cardiovascular disease: a systematic review and meta-analysis. Eur. J. Prevent. Cardiol. 2016;23(18):1970–1981. doi: 10.1177/2047487316654026. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Straif K. Use of smokeless tobacco and risk of myocardial infarction and stroke: systematic review with meta-analysis. BMJ. 2009; 339:b3060 doi:10.1136/bmj.b3060. [DOI] [PMC free article] [PubMed]
- Gupta R., Gurm H., Bartholomew J.R. Smokeless tobacco and cardiovascular risk. Arch. Intern. Med. 2004;164(17):1845. doi: 10.1001/archinte.164.17.1845. [DOI] [PubMed] [Google Scholar]
- Piano M.R., Benowitz N.L., FitzGerald G.A., Corbridge S., Heath J., Hahn E., Pechacek T.F., Howard G. Impact of smokeless tobacco products on cardiovascular disease: implications for policy, prevention, and treatment: a policy statement from the American heart association. Circulation. 2010;122(15):1520–1544. doi: 10.1161/CIR.0b013e3181f432c3. [DOI] [PubMed] [Google Scholar]
- Asplund K., Nasic S., Janlert U., Stegmayr B. Smokeless tobacco as a possible risk factor for stroke in men A nested case-control study. Stroke. 2003;34(7):1754–1759. doi: 10.1161/01.STR.0000076011.02935.A1. [DOI] [PubMed] [Google Scholar]
- Haglund B., Eliasson M., Stenbeck M., Rosen M. Is moist snuff use associated with excess risk of IHD or stroke? A longitudinal follow-up of snuff users in Sweden. Scand. J. Public Health. 2007;35(6):618–622. doi: 10.1080/14034940701436949. [DOI] [PubMed] [Google Scholar]
- Bolinder G., Alfredsson L., Englund A., de Faire U. Smokeless tobacco use and increased cardiovascular mortality among Swedish construction workers. Am. J. Public Health. 1994;84(3):399–404. doi: 10.2105/ajph.84.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergens M.-P., Ahlbom A., Andersson T., Pershagen G. Swedish moist snuff and myocardial infarction among men. Epidemiology. 2005;16(1):12–16. doi: 10.1097/01.ede.0000147108.92895.ba. [DOI] [PubMed] [Google Scholar]
- Wennberg P., Eliasson M., Hallmans G., Johansson L., Boman K., Jansson J.-H. The risk of myocardial infarction and sudden cardiac death amongst snuff users with or without a previous history of smoking. J. Intern. Med. 2007;262(3):360–367. doi: 10.1111/j.1365-2796.2007.01813.x. [DOI] [PubMed] [Google Scholar]
- Johansson S.-E., Sundquist K., Qvist J., Sundquist J. Smokeless tobacco and coronary heart disease: a 12-year follow-up study. Eur. J. Cardiovasc. Prevent. Rehab. 2005;12(4):387–392. doi: 10.1097/01.hjr.0000169189.22302.99. [DOI] [PubMed] [Google Scholar]
- Huhtasaari F., Asplund K., Lundberg V., Stegmayr B., Wester P.O. Tobacco and myocardial infarction: is snuff less dangerous than cigarettes? BMJ. 1992;305(6864):1252–1256. doi: 10.1136/bmj.305.6864.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsuya H., Folsom A.R. Risk of incident cardiovascular disease among users of smokeless tobacco in the atherosclerosis risk in communities (ARIC) study. Am. J. Epidemiol. 2010;172(5):600–605. doi: 10.1093/aje/kwq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq N., Beebe L.A., Thompson D.M., Skaggs V.J. Smokeless tobacco and prevalence of cardiovascular disease. J. Okla. State Med. Assoc. 2010;103(11–12):539–544. [PubMed] [Google Scholar]
- Henley, S.J., Connell, C.J., Richter, P., Husten, C., Pechacek, T., Calle, E.E., Thun, M.J. 2007. Tobacco-related disease mortality among men who switched from cigarettes to spit tobacco. Tobacco Control. 16, 22–28. [DOI] [PMC free article] [PubMed]
- Siegel D., Benowitz N., Ernster V.L., Grady D.G., Hauck W.W. Smokeless tobacco, cardiovascular risk factors, and nicotine and cotinine levels in professional baseball players. Am. J. Public Health. 1992;82(3):417–421. doi: 10.2105/ajph.82.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith R.J., Al Rifai M., Carruba C., De Jarnett N., McEvoy J.W., Bhatnagar A., et al. Tobacco use, insulin resistance, and risk of type 2 diabetes: results from the multi-ethnic study of atherosclerosis. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157592Tobacco. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Östenson C.-G., Hilding A., Grill V., Efendic S. High consumption of smokeless tobacco (“snus”) predicts increased risk of type 2 diabetes in a 10-year prospective study of middle-aged Swedish men. Scand. J. Public Health. 2012;40(8):730–737. doi: 10.1177/1403494812459814. [DOI] [PubMed] [Google Scholar]
- Persson P.-G., Carlsson S., Svanstrom L., Ostenson C.-G., Efendic S., Grill V. Cigarette smoking, oral moist snuff use and glucose Intolerance. J. Intern. Med. 2000;248(2):103–110. doi: 10.1046/j.1365-2796.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- Bolinder, G.M., Ahlbor, B.O., Lindell, J.H. 1992. Use of smokeless tobacco: blood pressure elevation and other health hazards found in a large-scale population survey. J. Int. Med. 232, 327-334. [DOI] [PubMed]
- Prasad G.L., Jones B.A., Chen P., Gregg E.O. A cross-sectional study of biomarkers of exposure and effect in smokers and moist snuff consumers. Clin. Chem. Lab. Med. 2016;54:633–642. doi: 10.1515/cclm-2015-0594. [DOI] [PubMed] [Google Scholar]
- Sgambato J.A., Jones B.A., Caraway J.W., Prasad G.L. Inflammatory profile analysis reveals differences in cytokine expression between smokers, moist snuff users, and dual users compared to nontobacco consumers. Cytokine. 2018;107:43–51. doi: 10.1016/j.cyto.2017.11.013. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration. Family Smoking Prevention And Tobacco Control And Federal Retirement Reform. Public Law 111–31—June 22, 2009. https://www.govinfo.gov/content/pkg/PLAW-111publ31/pdf/PLAW-111publ31.pdf [Accessed 14 Oct 2020].
- Tourangeau, R., Yan, T., Sun, H., Hyland, A, Stanton, C. A. (2019). Population Assessment of Tobacco and Health (PATH) reliability and validity study: Selected reliability and validity estimates. Tobacco Control. 28(6), 663–668. [DOI] [PubMed]
- McCarthy P.J. Pseudoreplication: further evaluation and applications of the balanced half-sample technique. Vital Healt. Stat. 2. 1969;31:1–24. [PubMed] [Google Scholar]
- Judkins D.R. Fay’s method for variance estimation. J. Off. Stat. 1990;6(3):223–239. [Google Scholar]
- Mahoney, M.C., Rivard, C., Hammad, H., Blanco, C., et al. 2020. Cardiovascular risk factor and disease measures from the Population Assessment of Tobacco and Health (PATH) Study. In progress. [DOI] [PMC free article] [PubMed]
- Stepanov I., Jensen J., Hatsukami D., Hecht S.S. New and traditional smokeless tobacco: comparison of toxicant and carcinogen levels. Nicotine Tob. Res. 2008;10(12):1773–1782. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.