Highlights
-
•
Ultrasound assisted crystallization of Azithromycin for control on particle size.
-
•
Understanding into effect of operating parameters and type of crystallization.
-
•
Characterization studies to analyse effect of ultrasound on structure and shape of azithromycin.
-
•
Ultrasound improves the particle characteristics especially in terms of mean size and shape.
Keywords: Azithromycin, Ultrasound, Size reduction, Cooling crystallization, Antisolvent crystallization
Abstract
The primary motive of the current work is to achieve smaller mean particle size with narrow size distribution that can enhance the bioavailability of azithromycin (ARZ), an essential requirement due to its poor water solubility. Recrystallization of ARZ was evaluated using cooling as well as antisolvent crystallization approaches in the presence of ultrasonic irradiation with detailed study into effect of different parameters such as ultrasonic power, time and temperature. Ultrasound assisted antisolvent crystallization at low temperatures (<10℃) yielded best size reduction up to 80% with narrower distribution and also gave better yield of the product, that too within 5 min of sonication. With scale up considerations, recirculation mode of operation was also evaluated which offered promising results for the size reduction. Images captured using optical microscope and SEM revealed a nearly uniform rod/plate-shaped geometry. Increase in amorphous nature of ARZ was confirmed based on XRD analysis. FTIR analysis showed no significant changes in the functional groups when compared to the original sample. Overall, the work demonstrated an improved reprocessing approach based on the use of ultrasound with insights into effect of operating parameters and effect of ultrasound on various characteristics.
1. Introduction
Crystallization of a solute is the evolution of a crystalline state from its solution or melt [1]. The final form of crystals is obtained in two stages namely nucleation and crystal growth. Based on the supersaturation levels, nucleation is attained and subsequently the nuclei grows into well – defined crystal forms [2]. The mode of supersaturation, say solvents used in crystallization or the rate of cooling, plays a major role in affecting the habit of crystalline material [3]. Different crystallization methods such as melt, cooling, anti-solvent, and evaporative, mostly varying in terms of mode of supersaturation, can be employed for the manufacture of solid compounds. Among these, cooling and anti-solvent crystallization approaches are the most common, and the rate of inducing the supersaturation say in terms of rate of cooling or the rate of addition of antisolvent affects the crystal shape and size distribution [4].
Crystallization is also a key isolation step or purification step during the synthesis of Active Pharmaceutical Ingredients (APIs). High chemical purity and correct polymorphic form of the final API is of utmost importance and needs to adhere to strict norms. For example, excessive fines and wide particle size distribution cause hindrances in down streaming operations like filtration and drying or the entrapment of mother liquid which compromises the purity of the dried product. Hence, crystallization has to be controlled properly to avoid bottlenecks in the API manufacturing process [5]. To improve stability and to control the particle size and particle size distribution (PSD), the nucleation rate has to be controlled along with control on the inhibition of the particle growth, or agglomeration by steric or electrostatic stabilization [6]. Faster nucleation can be achieved by high level of supersaturation in a very short time interval. This can help to overcome the metastable zone quickly, and the nucleation process will dominate the crystal growth process [7]. Modifying crystallization process of pharmaceutical compounds to change their physical properties is also called particle engineering [8]. For APIs with limited water solubility, a high surface area is desirable to maximize and enhance bioavailability which can be realized by reducing the particle size.
Agglomeration is the resulting consequence of several factors such as amount of solid in suspension, agitation rate, and degree of supersaturation achieved at the time of nucleation or seeding. An appropriate choice of solvent, careful seeding and temperature control can largely decrease the degree of agglomeration [2]. In addition to these factors, ultrasound also offers a promising approach to facilitate deagglomeration. Ultrasound has a proven history of being utilized in crystallization to enhance nucleation rates and also to achieve size reduction through breakage action on the crystal agglomerates [9], [10], [11], [12]. In addition, use of ultrasound also helps in obtaining the desired polymorphs. For example, Nii and Takayanagi established that ultrasound aided the formation of a desired polymorph, i.e. α-glycine based on the application in antisolvent crystallization [12]. In another study, Jordens et al. [14] analysed the particle breakage mechanisms due to implosion of stable and transient cavitation bubbles by means of Kapur function analysis and reported that the lowest frequency employed gave the best breakage [13]. Cavitation induced by ultrasound helps in size reduction based on the energy released due to the cavity bubbles after implosion and also generation of high temperature and pressure [10] that helps in enhancing the nucleation. The ultrasonic waves propagate through the medium generating streaming and shock waves. All these phenomena together cause particle breakage in the near proximity areas as well as easy generation of higher number of nuclei ultimately helping to control the particle size. Overall, ultrasound has a great potential in the pharmaceutical industry since the API synthesised must possess specific particle size distribution and polymorphs. The different advantages of ultrasound seen in crystallization include reduction in the induction time, lowering of supersaturation limit and in some cases increased yield. Sonocrystallization can also eliminate the need to add seed crystals, which proves advantageous in sterile operations [14].
Azithromycin (ARZ) is an antibiotic that is used to treat bacterial infections of the respiratory tract, ear, nose, throat, lungs, skin, and eyes, among other. Typhoid fever and several sexually transmitted disorders, such as gonorrhoea, are also treated using ARZ. It operates by inhibiting the formation of critical proteins that bacteria need to perform their functions. But there are some lacunae of this drug especially the meagre solubility in water that reduces its bioavailability. To improve the bioavailability, it is necessary to reduce the particle size and increase the surface area, since the surface area exposed to the dissolution media determines the rate of dissolution [8]. Furthermore, particle size reduction aids in reducing the diffusion layer thickness surrounding the drug particles and this helps to improve the concentration gradient thus enhancing the bioavailability [15]. Considering this aspect of particle size requirements, it is important to study the crystallization of ARZ or its reprocessing with an aim of tailoring the particle size and its distribution. We now present an overview of the earlier studies on crystallization of ARZ with an objective of highlighting the novelty of the current work.
Pouretedal [8] used different stabilizers such as hydroxypropyl methyl cellulose (HPMC), hydroxypropyl methyl cellulose phthalate (HPMC-P), sodium lauryl sulfate (SLS), polyvinyl acetate (PVA), methyl cellulose, and polyethylene glycol 400 (PEG 400) for antisolvent crystallization of azithromycin and studied their effect on the distribution of nanoparticles. Hou and co-workers employed a combined approach of reactive precipitation and freeze-drying along with a biocompatible stabilizer to achieve 200 nm size of azithromycin in the nanosuspensions [16]. From the literature, it was inferred that limited techniques have been explored to reduce the size of azithromycin and also, none focus on the ultrasound assisted approach of size reduction. Ultrasound also offers an additional advantage of avoidance of use of other chemicals that can affect the drug purity and polymorphic form. The main objective of this study is to recrystallize ARZ using cooling and anti-solvent crystallization methods in the presence of acoustic cavitation with an objective to establish best conditions to reduce its particle size and improve its bioavailability based on a faster dissolution rate. The properties of recrystallized ARZ such as mean particle size, crystalline state, bulk density and water dissolution have also been investigated so as to capture the effect of ultrasound on drug characteristics.
2. Materials and methods
2.1. Materials
ARZ, the API used in this study, was procured from a local supplier in Mumbai, India. Ethanol (L.R. grade) was used as the solvent and was purchased from M/s Thomas Baker, Mumbai, India. Freshly prepared deionized water was used as antisolvent.
Ultrasound probe (Make: Dakshin, Mumbai) attached to a generator capable of generating a power up to 150 W with a fixed frequency of 20 kHz was used for sonication. The probe has titanium alloy tip with diameter of 13 mm and was used for treatment of ARZ in batch as well as recirculation modes of operation. A schematic representation of the experimental setup used for batch cooling / antisolvent crystallization and recirculation cooling / antisolvent crystallization in a jacketed vessel has been depicted in Fig. 1a, Fig. 1b respectively. The vessel used for batch processing had a height of 7 cm and diameter of 5 cm and that used for recirculation processing had height and diameter of 9 cm and 5 cm respectively. Ultrasonic Flow Cell (Sonics 1500 HV; Sonics Inc., USA) with a maximum power dissipation of 1.5 kW was also used for scale up studies. The ultrasonic flow cell dimensions were 16 cm (height) and 10 cm (diameter). The schematic representation of the experimental setup involving flow cell is depicted in Fig. 1c. Ultrasonic probe was placed at the centre for batch and recirculation processing in glass reactors, and the flow cell probe is also at the centre by default when the setup is assembled.
Fig. 1a.
Schematic representation of batch cooling / antisolvent crystallization of ARZ in a jacketed vessel using ultrasonic probe.
Fig. 1b.
Schematic representation of recirculation cooling / antisolvent crystallization of ARZ in a jacketed vessel using ultrasonic probe.
Fig. 1c.
Schematic representation of recirculation cooling / antisolvent crystallization of ARZ using ultrasonic flowcell.
2.2. Solubility studies
Water solubility of ARZ is very low and was found to be 0.0685 g in 100 ml water at 25 °C as determined experimentally. On the contrary, it is soluble in ethanol. At a room temperature of 25 °C, 5 g of ARZ dissolves in 100 ml ethanol. At higher temperature of 68 °C, 6.58 g ARZ dissolves in 100 ml ethanol and the solution becomes saturated. This concentration was used for crystallization studies. It was also found that, 80% (v/v) ethanol – water solution could also dissolve the same amount of azithromycin.
2.3. Experimental procedure
Initially, conventional size reduction by grinding the ARZ powder was carried out using mortar pestle. Subsequently, wet milling by irradiating ARZ dispersed in water by ultrasound was studied. For comparing the efficacy of grinding and wet milling with ultrasound based method, recrystallization studies in the presence of ultrasound were also undertaken.
For batch studies, 2 g of ARZ was dissolved in 32 ml ethanol at approximately 68 °C to reach the saturation conditions. The various procedures followed for crystallization included cooling crystallization, antisolvent crystallization and combination of cooling crystallization with antisolvent addition. Recirculation cooling crystallization with antisolvent addition was also studied. All these methods were carried out in the presence of ultrasound and using the conventional approach to compare the efficacy of ultrasound.
2.3.1. Cooling crystallization using ultrasound
A saturated ARZ – ethanol solution at 70℃ was transferred to a jacketed vessel maintained at lower temperatures and irradiated with ultrasound at different power dissipation levels (60 W to 120 W). The experiments were carried out at different driving temperatures for cooling between 5 °C and 30 °C.
2.3.2. Antisolvent crystallization using ultrasound
Antisolvent (water) was added to the saturated ARZ – ethanol solution so that ARZ crystallizes out. A specific quantity of antisolvent was added to the solution using a peristaltic pump so that flowrate could be controlled. The antisolvent addition flowrate was kept at 10 ml/min for experimental setups based on the use of ultrasonic horn as depicted in Fig. 1a, Fig. 1b, and at 60 ml/min for the flowcell (shown in Fig. 1c). The applied addition rate was fixed on the basis of hold-up volume of the vessel. After antisolvent addition, the mixture was irradiated with ultrasound for time intervals between 2 and 20 min and powers ranging from 40 W to 130 W to investigate the effect of operating parameters on the particle size.
2.3.3. Batch cooling crystallization with antisolvent addition in presence of ultrasound
A jacketed batch reactor, maintained at 0-3˚C was used to achieve cooling crystallization coupled with antisolvent addition along with sonication at various power dissipation levels from 60 W to 130 W and for variable time duration from 2 min to 20 min.
2.3.4. Recirculation cooling crystallization with antisolvent addition
A recirculation jacketed reactor was used in which the ARZ – ethanol – water mixture was pumped at 50 ml/min. The jacket temperature was maintained at 0-3˚C. Once, the entire quantity of required antisolvent was added, the mixture was recirculated in the reactor / flowcell in the presence of ultrasound. The sonication was carried out for 10 min. In the case of jacketed vessel, the ultrasound power was kept fixed at 150 W, while that in the case of flowcell was set at 900 W.
2.3.5. Analytical methods
Particle size was measured using Bettersizer 2600E (Bettersize Instruments, China) working on the principle of laser diffractometry. Optical microscope (Olympus 51X-TF) was also used to study the variations in crystal shape. Mettler Toledo Mark 3 moisture analyser was used to analyse the moisture content of all the ARZ samples. XRD (Shimadzu 6100 diffractometer, Japan) was used to compare crystallinity of the original sample and treated samples and also measure the percent crystallinity. Bruker Alpha FTIR was employed to verify the presence of characteristic functional groups in the drug and establish any changes based on the treatment using ultrasound. Scanning electron microscope (Carl Zeiss, Model: Supra 55, Germany) was used to analyse the crystal surface morphologies.
3. Results and discussion
3.1. Size reduction without crystallization
The original ARZ showed irregular geometry with a mean size of 34.84 μm and span of 2.137, where span is a dimensionless number corresponding to (D90 – D10) / D50. Values closer to unity indicate narrow size distribution, while larger values indicate wide or multimodal distributions. Grinding ARZ with a mortar-pestle yielded a mean particle size of 29.16 μm. Ultrasonic wet milling of ARZ slurry in water was carried out at 90 W power dissipation for 10 min and the mean particle size obtained was 14.64 μm. Span values for the particles obtained using these two methods were 2.565 and 2.259 respectively, indicating a wide size distribution. The resulting particle size distribution in both the cases was either bimodal or multi-modal. Moreover, the shapes of particles were found to be non-uniform and uneven as per the data represented in Table 1. Considering that there was no significant size reduction and the irregular shape, further studies were directed on investigating the size reduction based on recrystallization.
Table 1.
Final mean size, shape and size distribution of original ARZ and ARZ obtained using different size reduction approaches.
| Sr. No. | Method | Time (min) | Ultrasonic Power (W) | Mean Particle Size (µm) | Span | Microscopic Image (40X) | Particle Size distribution |
|---|---|---|---|---|---|---|---|
| 1 | ARZ – Original | – | – | 34.84 | 2.137 |  |
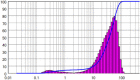 |
| 2 | Grinding | 5 | – | 29.16 | 2.565 |  |
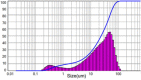 |
| 3 | Wet milling using ultrasound | 10 | 90 | 14.64 | 2.259 |  |
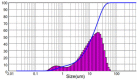 |
3.2. Size reduction using recrystallization
3.2.1. Cooling crystallization in the presence of ultrasound and understanding the effect of ultrasonic power
In this method, the saturated solution of ARZ in ethanol which was at 70℃ was cooled to different temperatures to crystallize out ARZ using conventional method. The dissolved ARZ sample was cooled using an ice bath kept at a constant temperature till a final temperature of 5˚C and 30˚C and the obtained mean particle size was found to be 36.89 µm and 49.98 µm respectively. This recrystallization method did not yield the required size reduction and therefore ultrasonic treatment was used. For cooling crystallization applied in the presence of sonication, effect of driving temperature on the mean crystal size was investigated under constant set of operating conditions as time of 10 min and power dissipation of 90 W. Initially no temperature controlling mechanism was used and the temperature increased to 48℃ after sonication, and the mean particle size was found to be 14.4 µm. Final temperature of the sample was high due to the energy dissipated by ultrasound. After this, the same procedure of cooling crystallization while sonication using ultrasonic horn was repeated by providing a continued cooling during the entire operation so that the final temperatures were 30℃, 20℃ and 10℃. The maintaining of the temperatures at 30℃, 20℃ and 10℃ resulted in the mean particle size of 37.88 µm, 29.91 µm and 31.25 µm respectively. The trend of reaching same final mean size for cooling crystallization at much lower temperatures can be a result of supersaturation reaching its maximum level beyond a cooling temperature of 20 °C, indicating that using lower temperatures below 20℃ is not useful and hence considered to be optimum.
After the study of temperature, the input power was varied with different values as 60 W, 90 W, and 120 W at cooling temperature of 20℃. The final mean particle size obtained at these power dissipations were 41.83 µm, 37.88 µm, and 19.98 µm, respectively and is depicted in Fig. 2. The span for these sizes were 1.311, 1.507 and 2.639 respectively. When ultrasound was applied simultaneously with cooling, the mean particle size was found to decrease with an increase in the power of ultrasound, however, high span at 120 W signifies uneven particle breakage leading to wide size distribution. The smaller size obtained at higher power can be explained by the fact that at higher power dissipation, the intensity of cavitation being high, results in the formation of more nuclei, as well as a greater extent of turbulence, arrests crystal growth and drives particle breakage, thus smaller size of particles is obtained. A similar trend of reduced agglomeration with an increase in power has been reported in previous studies. Castro and Capote [17] reviewed the positive effects of ultrasound in crystallization of different compounds with a general conclusion that greater ultrasonic power gives faster nucleation, decreases the meta stable zone width and reduces particle size to a much larger extent. Gielen et al. [18] used pulsed ultrasound in batch and recirculation mode for the size reduction of paracetamol. The authors observed a D50 value of 281 μm without ultrasound and D50 values of 47 μm and 65 μm for batch and recirculation based approach with ultrasound.
Fig. 2.
Variations in mean particle size for ultrasound assisted crystallization of ARZ at constant irradiation time of 10 min.
Cooling crystallization in recirculation mode was also performed using a jacketed flow reactor with the same ultrasonic horn at an ultrasonic power of 150 W to evaluate the outcomes of the process in recirculation flow. Prior to this, a recirculation cooling crystallization of ARZ was carried out without the use of ultrasound where the mean size obtained was 30.23 µm. The resulting mean particle size in the case of ultrasonic horn operated in recirculation mode was lower at 24.40 µm (span: 1.817) and the particles had a substantially better morphology than batch processed samples, which can be due to the fact that ARZ particles are exposed to a limited time to ultrasound which aids sufficient growth to attain a rod-like shape, but with a bimodal size distribution. Further, an ultrasonic flow cell at 900 W was used for similar cooling crystallization in recirculation mode and the mean size obtained in this case was 28.86 µm with a wide size distribution. As similar to the previous case, here too, the time of exposure to ultrasound was less, but the ultrasonic power intensity being quite large, uneven particle breakage occurs and yields a bimodal size distribution. All these results are shown in Table 2, Table 3.
Table 2.
Results for ultrasound assisted batch crystallization.
| Sr. No. | Method | Time (min) | Ultrasonic Power (W) | Mean Particle Size (µm) | Span | Microscopic Image (40X) | Particle Size distribution |
|---|---|---|---|---|---|---|---|
| 1 | Batch Cooling Crystallization | 10 | 90 | 13.13 | 1.814 |  |
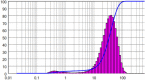 |
| 2 | Batch Antisolvent Crystallization | 10 | 90 | 18.61 | 1.627 |  |
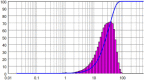 |
| 3 | Batch Cooling + Antisolvent Crystallization | 5 | 120 | 7.95 | 1.548 |  |
 |
Table 3.
Results for ultrasound assisted recirculation crystallization.
| Sr. No. | Method | Time (min) | Ultrasonic Power (W) | Mean Particle Size (µm) | Span | Microscopic Image (40X) | Particle Size distribution |
|---|---|---|---|---|---|---|---|
| 1 | Recirculation Cooling Crystallization in Jacketed vessel | 10 | 150 | 24.40 | 1.816 |  |
 |
| 2 | Recirculation Cooling + Antisolvent Crystallization in Jacketed vessel | 10 | 150 | 18.97 | 1.446 |  |
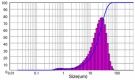 |
| 3 | Recirculation Cooling Crystallization in Flowcell | 10 | 900 | 28.86 | 2.639 |  |
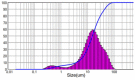 |
| 4 | Recirculation Antisolvent Crystallization in Flowcell | 10 | 900 | 11.63 | 1.719 |  |
 |
3.2.2. Antisolvent crystallization in presence of ultrasound and understanding the effect of ultrasonic power
Water was added as antisolvent to the saturated solution of ARZ in ethanol while stirring to crystallize ARZ. During this process, antisolvent was added at room temperature and no temperature control was provided to the system. The operation was then followed by sonication at different input ultrasonic powers from 40 W to 130 W. The results showed that for an increase in power from 40 W to 130 W, the particle size reduced from 28.86 µm (span: 1.552) to 14.50 µm (span: 2.046). Higher ultrasonic power ensured the breakage of the formed crystals which dominated the crystal growth process as also seen in many of the previous studies [14], [15], [19]. A wider size distribution as shown by greater span value is a consequence of inconsistent breakage of particles. It is therefore necessary to select a proper input ultrasonic power so as to get the desired particle size distribution. Sabnis et al. in their work on size reduction of DADPS by antisolvent crystallization of DADPS achieved a final size of 9.74 μm by sonication at 300 W as compared to 28.42 µm at 240 W using the same ultrasonic probe [10]. In another study, Narducci et al. [19] reported that adipic acid particle size could be lowered by increasing the amplitude of ultrasound. A general explanation to this phenomenon is that the higher energy released due to higher ultrasonic power increases the magnitude of particle breakage. The samples derived from antisolvent crystallization represented a well-defined rod-shaped geometry. The crystal edges were fairly visible, and the sizes of the treated ARZ appeared to be nearly homogeneous due to the deagglomeration caused by the increasing power of ultrasound. Untreated ARZ had a mean particle size of 38.84 µm, whereas treated ARZ samples showed up to 62% overall reduction in mean particle size. Due to faster supersaturation attainment by antisolvent addition, the yield of these experimental sets was at least 8% higher than cooling method.
3.2.3. Combined cooling antisolvent crystallization and understanding the effect of ultrasonic power
Since both the previous methods of cooling and antisolvent crystallization (as discussed in sections 3.2.1 and 3.2.2) have their individual lacunae, a combination of two approaches with ultrasound was experimented. For this, a jacketed batch reactor / vessel was used and was maintained at 5 °C for the entire duration. Antisolvent (water) was added to the saturated ARZ – ethanol solution and subjected to ultrasound at different powers of 60 W, 90 W, 120 W, and 130 W. The process resulted in lower mean particle sizes with actual values of 9.591 µm, 9.047 µm, 7.957 µm, and 10.23 µm, and the corresponding span values were 1.636, 1.843, 1.847 and 2.298 respectively. The obtained mean particle sizes and corresponding power levels are represented in Fig. 2. The lowest mean size was obtained at 120 W, which increased by a small value for power of 130 W. Such an optimum value is dependent only on the solute properties. Although a higher power indicates more intense cavitation events, these do not alter the solute particle size because the conditions may not be favourable due to cushioning effects and bubble cloud formation [20] at ultrasonic powers higher than specific limits. We can infer that using lower temperatures conditions for antisolvent addition in batch processes resulted in smaller particles. The microscopic images depicted in Table 1, Table 2, Table 3 also show uniform rod/plate type geometry of the crystals.
The same process was replicated using a jacketed flow reactor and also separately using the flow cell as a scale-up measure. Recirculation cooling + antisolvent crystallization without ultrasound in jacketed vessel resulted into a mean of 25.05 µm, span of 1.78, while sonication using ultrasonic horn at 150 W for 10 min gave a mean size of 18.97 µm and a span of 1.446 with a distinct rod shape geometry of crystallized ARZ (represented in Table 1), while for the flow cell, 11.63 µm was the mean particle size obtained with 10 min of sonication with a span of 1.719. Another reason for the flow cell giving better results is that the ultrasonic horn dissipates the power in terms of cavitation bubbles in the downward direction only. Conversely, the probe in the flow cell being of step or cascading type, dissipates energy in both, horizontal and downward directions and nearly covers the entire volume of the reactor. It is also important to note that the power dissipated through the flow cell being many folds larger, gave better size reduction with comparable rod-like particle shape. The particle size distributions are comparatively narrower for cooling antisolvent crystallization than cooling crystallization alone carried out in both the ultrasonic devices (Table 3).
3.3. Effect of sonication time
Ultrasound irradiation periods were varied from 2 min to 60 min for cooling crystallization keeping ultrasonic power constant at 90 W. The solution was cooled till 10℃. The lowest mean size was obtained for 10 min operation with value as 13.13 μm. Irradiating ultrasound for 2 min yielded a mean size of 20.55 μm while that for 60 min gave a size of 34.42 μm. For very short sonication times, there is a possibility that the solute may not get required driving force for a sufficient duration and hence larger size is obtained. The generated crystals from the solution are irregularly shaped and of various sizes. Thus, prolonged sonication time improved mixing, exposed majority of the crystals to cavitation bubbles and thus, prevented undesired crystal growth leading to lower sizes till treatment of 10 min. Kougoulos et al. [19] investigated the effects of sonication duration on crystal size of adipic acid in a continuous crystallizer and found a significant decrease in crystal size by increasing sonication duration. The observed results were attributed to sono-fragmentation induced by imploding cavities and shock waves that also dislodge the formed particle clusters [21]. It was reported that as sonication time increases, crystal size decreases and crystals also become more uniform. It is important to note that there is an optimum value of sonication time so that desired quality of the output crystals is obtained depending on the solute – solvent system under consideration. In some cases, longer durations of ultrasound exposure may cause abrasion or uneven breakage of the crystals or in some, redissolution of the formed crystals in the solvent [10]. Therefore, care must be taken to select proper sonication time. In the present work as well, the minimum size for cooling crystallization was determined to be 13.13 μm for 10 min of irradiation, but no distinctive trend was detected subsequently with increasing irradiation duration.
In the case of antisolvent crystallization, mean particle size decreased as irradiation duration increased, but again the difference was not significant. However, when antisolvent crystallization was performed in a batch jacketed reactor at 5 °C, i.e., batch cooling + antisolvent crystallization, the resulting mean particle size decreased below 10 μm for 5 min treatment, but extending the sonication period from 5 to 10 min resulted in a marginal increase in the final mean size from 9.047 to 10.43 μm at the same ultrasonic power of 90 W. The overall trend showed that there is no noticeable change in the mean particle size with an increase in sonication time to 10 min. This means that for this particular solute – solvent – antisolvent combination, a 5 min sonication would prove sufficient in a batch process.
3.4. Effect of temperature
The effect of cooling temperature was also studied for the process of cooling crystallization, both with and without antisolvent addition. For cooling crystallization, lower driving temperature yielded smaller particle size. Reducing cooling temperature from 30℃ to 5℃ reduced the final size from 49.98 µm to 36.89 µm for sonication time of 10 min at 90 W. On the other hand, antisolvent approach produced mean particle sizes of 20.55 µm, 18.61 µm, and 18.06 µm for sonication time of 5 min, 10 min and 20 min respectively at ultrasound power of 90 W, as per the data shown in Fig. 3. But when cooling mechanism was provided along with antisolvent addition, a consistent mean size of < 10 µm could be obtained. This leads to the inference that temperature is the governing factor as the solubility of ARZ increases with an increase in temperature. A sudden supersaturation is induced when temperature is rapidly dropped based on use of lower driving cooling temperature. The lowest mean size of 7.95 µm was obtained as a result of cooling the solution below 5 °C along with the use of antisolvent. Gielen et al. also demonstrated that lower temperature of cooling helped to achieve a faster nucleation of paracetamol crystals [18]. Antisolvent addition into the mixture at this stage of lower cooling temperature also gave a stimulus to supersaturation. These together make the nucleation phase more dominant than crystal growth phase and generate a greater number of nuclei [8], which eventually will grow to a lower size. The nuclei formation is also enhanced by ultrasonic irradiation that also induces particle breakage or avoids the agglomeration. All these factors contribute towards a better size reduction based on the generation of more nuclei. Lindenberg et al. studied cooling antisolvent crystallization of acetylsalicylic acid and also concluded that combination of both these approaches together helped realizing early nucleation and faster processing with narrower size distribution for the obtained crystals [22].
Fig. 3.
Changes in mean size with respect to time of irradiation at constant ultrasonic power at 90 W.
3.5. Characterization of ARZ
3.5.1. XRD analysis
The X-ray diffractograms of the original ARZ and those recrystallized using the cooling, antisolvent and cooling + antisolvent crystallization approaches are shown in Fig. 4. Azithromycin diffraction peaks were seen at different diffraction angles (2θ) of 10.68°, 17.31°, 17.52°, 19.59°, 20.67°, 22.80°, 24.97° and 26.55° which match with those reported in the previous studies [8], [23]. In the case of recrystallized ARZ using cooling – antisolvent method, the peak intensity is seen to reduce, though with similar peaks as in the original ARZ. Some prominent peaks in the ARZ obtained by cooling crystallization show a shift of about 5° towards higher diffraction angle when compared with the other two diffractograms. In the case of antisolvent crystallization in presence of ultrasound, a minor shift of about 2 to 2.5° for major peaks can be seen. A prominent peak at 36° is also observed which is absent in the other diffractograms. These shifts of diffraction peaks roughly indicate a compression in the crystal lattice due to the cooling provided to crystallize the solute.
Fig. 4.
XRD diffractograms of original and treated ARZ samples.
Percentage crystallinity and mean crystallite size of all the four samples has been calculated using a trial version of Match3! Software. The obtained values are shown in Table 4. It was seen that use of only cooling crystallization in presence of ultrasound increased both the crystallinity (31.87%) and the mean crystallite size. For antisolvent crystallization with ultrasound, the percent crystallinity is around 29.88% with lower mean crystallite size than the original ARZ sample. It can be thus said that both these methods when combined gave better results as stated in previous sections which is again confirmed in the XRD analysis. The combined method yields lower crystallite size as well as better crystallinity. The mean crystallite size is the lowest at 1561.8 Å for ultrasound assisted cooling + antisolvent approach with reduction in the degree of crystallinity indicating a rise in amorphous nature of the compound due to the ultrasonic processing. It has been stated by Aucamp et al. that amorphous azithromycin has a better water solubility than its crystalline counterpart [24]. The work has allowed to establish that combined antisolvent with cooling approach is better compared to only cooling in terms of obtaining the desired nature of ARZ.
Table 4.
Percentage crystallinity and average crystallite size as calculated from XRD.
| Sample | Percentage Crystallinity (%) | Average Crystallite Size (Å) |
|---|---|---|
| ARZ – Original | 29.07 | 2029.1 |
| ARZ – Cooling + Ultrasound | 31.87 | 2852.2 |
| ARZ – Antisolvent + Ultrasound | 29.88 | 1887.2 |
| ARZ – Cooling + Antisolvent + Ultrasound | 25.71 | 1561.8 |
3.5.2. IR studies
IR studies conducted using Fourier Transform Infrared spectroscopy under dry conditions established that there are minimal changes in the functional groups based on the interaction with the solvent i.e., ethanol and water (antisolvent) as well as use of ultrasound. The FTIR spectrum of original ARZ and the one processed using combined cooling antisolvent method are depicted in Fig. 5 (a) and (b), respectively. The peaks at 1724 cm−1 (C O stretch), 1190 cm−1 (C—O—C asymmetrical stretching) and 1049 cm−1 (C—O—C symmetrical stretching) [23] which are the characteristic peaks for azithromycin are clearly visible for the original ARZ sample. In the case of treated ARZ, these characteristic peaks are seen at 1720 cm−1 (C O stretch), 1183 cm−1 (C—O—C asymmetrical stretching) and 1045 cm−1 (C—O—C symmetrical stretching). The very minor shift can be attributed to the Van der Waals forces developed between azithromycin and solvent – antisolvent mixture [23]. From the figures, it can be very well concluded that there was no undesirable chemical interaction between the drug, solvent and antisolvent and also there was no strong negative effect of ultrasound processing over the applied operating conditions, and formation of any hydrates in the processed sample was ruled out. The main peaks of ethanol (1021 cm−1 and 3061 cm−1) are also clearly distinguished in the spectrum.
Fig. 5.
(a): FTIR spectra of the original ARZ sample; (b): FTIR spectra of the ARZ sample obtained by batch cooling + antisolvent crystallization.
3.5.3. SEM analysis
Scanning electron microscopy was also employed to analyze the surface morphology and exact shape of the samples and obtained images are illustrated in Fig. 6 (a-h). It was seen that Original ARZ (Fig. 6 a) constitutes irregular shaped particles, with corrugations but it can be said that they have generally a cuboid shape with sharp edges and also matches with the images reported in the literature [23]. ARZ recrystallized by cooling alone (Fig. 6 b), cooling + ultrasound (Fig. 6 d) and antisolvent + ultrasound (Fig. 6 e), showed inconsistent geometry and particle agglomerations are clearly seen. Fig. 6 c gives the first impression of a defined plate shape geometry by antisolvent crystallization, although there is a wide size distribution. The morphology is further refined in the case of cooling + antisolvent approach in presence of ultrasound (Fig. 6 f, g). The edges are smooth and particles have a consistent rod / plate shape which is desirable for tableting purposes. Use of ultrasonic flowcell at high powers (900 W) resulted into particle breakage of plate type crystals which can be seen in Fig. 6 h. Wu et al. [25] in their studies of azithromycin using different solvents also reported formation of columnar (or rod-like) crystals after recrystallization process.
Fig. 6.
SEM images of original and processed ARZ (a): Original ARZ (500X); (b): Batch cooling (5000X); (c): Batch antisolvent (500X); (d): Batch cooling + Ultrasound (5000X); (e): Batch antisolvent + Ultrasound (5000X) (f): Batch cooling + antisolvent (2000X); (g): Recirculation cooling + antisolvent (2000X) (h): Recirculation cooling + antisolvent – flowcell (2000X).
3.6. Physical properties of ARZ
3.6.1. Moisture content
Moisture is generally present as water of crystallization and/or as adsorbed water in APIs. Moisture content beyond a certain specific limit can lead to microbial contamination and affect the product stability [26]. If this moisture content is not within an acceptable limit, it may affect manufacturing of the solid formulation in terms of the poor powder flow and irregular tablet parameter performance which is undesirable [26], [27]. The moisture levels of original and treated ARZ were analysed and the obtained data are tabulated in Table 5. It can be seen that there is a minor increment in the moisture content, which can be said to be well within the permissible tableting limit.
Table 5.
Moisture content of original and recrystallized ARZ samples.
| Sample | Moisture content (%) |
|---|---|
| ARZ – Original | 2.19 |
| ARZ – Cooling + Ultrasound | 2.23 |
| ARZ – Antisolvent + Ultrasound | 2.29 |
| ARZ – Cooling + Antisolvent + Ultrasound | 2.31 |
3.6.2. Bulk density
The flow ability of APIs impacts formulation as well as drug product performance and therefore considered an important property. Poor flow behaviour of the API makes it troublesome operation during formulation. Thus, it is beneficial to develop protocols or processes that impart API with good flow properties. Bulk density is considered as an indicator of flow properties with low values indicating poor flow due to the presence of large agglomerates [25], [28]. Original ARZ is a free flowing solid having a bulk density of 0.334 g/cc, while the sample obtained using ultrasound assisted cooling crystallization demonstrated bulk density of 0.345 g/cc. Table 6 represents the bulk densities of ARZ processed using different means. The samples processed with conventional cooling and antisolvent approaches gave bulk densities ranging from 0.338 g/cc to 0.48 g/cc. Use of ultrasound in these approaches resulted in an improvement in the bulk densities and the highest bulk density was obtained for ARZ recrystallized using antisolvent in presence of ultrasound under cooling conditions with actual value as 0.374 g/cc under batch mode. Ultrasonic flow cell yielded a bulk density of 0.362 g/cc. From Fig. 6 f and g, it is clearly observed that antisolvent approach for recrystallization in presence of ultrasound under cooling conditions gives plate / rod type crystals unlike corrugated, porous or spherical structures observed in the case of only cooling approaches. Presence of uniform plate / rod type geometry prevents formation of air pockets, reduces pore volume and thus increases the bulk density of the powder. Thus, it can be said that ultrasound assisted recrystallized azithromycin using combined method of cooling and antisolvent addition proves better in terms of flow properties and can give better performance in tableting due to enhanced bulk density.
Table 6.
Bulk densities of original and recrystallized ARZ.
| Sample | Bulk density (g/cc) |
|---|---|
| ARZ – Original | 0.334 |
| ARZ – Batch Cooling | 0.348 |
| ARZ – Batch Cooling + Antisolvent | 0.341 |
| ARZ – Recirculation Cooling | 0.338 |
| ARZ – Recirculation Cooling + Antisolvent | 0.345 |
| ARZ – Cooling + Ultrasound | 0.345 |
| ARZ – Antisolvent + Ultrasound | 0.357 |
| ARZ – Cooling + Antisolvent + Ultrasound | 0.374 |
| ARZ – Recirculation Cooling + Antisolvent + Ultrasound (in flowcell) | 0.362 |
3.6.3. Post processing dissolution studies
Azithromycin is sparingly soluble in water and the dissolution rate is also lower, which typically depends on the particle size. Therefore, for better assimilation into the body, its dissolution rate has to be improved. So, water dissolution studies were performed for the original and ultrasound treated ARZ by adding a fixed quantity of ARZ to 100 ml distilled water and maintaining a temperature of 37 °C for 24 h under constant stirring conditions. It was seen from the results that the untreated ARZ showed a dissolution extent of 13.7% while the treated sample yielded a dissolution extent of 24.8%. It is thus demonstrated that the size reduction yielded about 58% improvement in the dissolution extent of ARZ in water.
3.7. Recovery of solvent
From recirculation cooling + antisolvent recrystallization process, ethanol – water mixture was recovered as the filtrate. The mixture was distilled in a rotary evaporator to separate ethanol. The distillate (ethanol) recovery was found to be 84.2%. Previously, it was found that a 80% v/v ethanol – water solution is capable of dissolving the same amount of ARZ as in pure ethanol. So, the recovered ethanol was tested as solvent for ARZ and it showed satisfactory solubility (∼6.5 g/100 ml) indicating that solvent can be recycled and reused for processing the ARZ, which can give a cost effective operation at commercial scale.
4. Conclusions
Azithromycin being a water insoluble drug, was recrystallized in the presence of ultrasound to reduce its particle size which can increase its surface area. Three different approaches namely cooling, antisolvent and cooling + antisolvent crystallization were investigated. Among these, cooling + antisolvent crystallization proved to be best approach since, a narrow particle size distribution was obtained with lower mean size. The mean size obtained by this method was 7.95 μm yielding upto 80% size reduction as compared to the untreated ARZ. XRD analysis revealed that cooling crystallization showed a rightward shift in the XRD pattern suggesting changes in the crystal lattice of ARZ, while there was a slight drop in the crystallinity for the ARZ sample obtained using cooling + antisolvent crystallization. FTIR spectrum also confirmed the presence of azithromycin characteristic bonds in the treated sample, meaning no unfavourable changes due to the use of ultrasound. SEM analysis showed that well defined plate / rod shaped crystals with smooth surfaces were obtained as a result of cooling + antisolvent crystallization in the ultrasound, while only cooling mechanisms yielded rugged crystals with inconsistent shapes. A scale up approach was also successfully demonstrated using ultrasonic flow cell at substantially higher volumes and optimum input ultrasonic power. Also, studies of solvent recovery made it possible to reuse the solvent again for processing the same drug. It was demonstrated that recrystallization of ARZ in presence of ultrasound is an effective way in obtaining a preferred size for the crystals, at the same time maintaining or slightly improving the characteristics of the processed ARZ sample.
CRediT authorship contribution statement
Sarvesh S. Sabnis: Methodology, Investigation, Writing – original draft. Shikhar D. Singh: Investigation, Writing – original draft. Parag R. Gogate: Writing – review & editing, Supervision, Project administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors are grateful to the DAE-ICT Centre for Chemical Engineering Education and Research at ICT, Mumbai for financial assistance to SSS.
References
- 1.J.W. Mullin, Crystallization., 4th ed., Reed Educational and Professional Publishing Ltd, 2001. 10.1016/B978-0-7506-4833-2.X5000-1.
- 2.Sriamornsak P., Burapapadh K. Characterization of recrystallized itraconazole prepared by cooling and anti-solvent crystallization. Asian J. Pharm. Sci. 2015;10(3):230–238. doi: 10.1016/j.ajps.2015.01.003. [DOI] [Google Scholar]
- 3.Bennema P., van Eupen J., van der Wolf B.M.A., Los J.H., Meekes H. Solubility of molecular crystals: polymorphism in the light of solubility theory. Int. J. Pharm. 2008;351:74–91. doi: 10.1016/j.ijpharm.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 4.Paulino A.S., Rauber G.S., Campos C.E.M., Maurício M.H.P., de Avillez R.R., Cuffini S.L., Cardoso S.G. Hollow crystal anti-solvent preparation process as a promising technique to improve dissolution of poorly soluble drugs. J. Cryst. Growth. 2013;366:76–81. doi: 10.1016/j.jcrysgro.2012.12.013. [DOI] [Google Scholar]
- 5.Lipinski C. Poor aqueous solubility – an industry wide problem in drug discovery. Am. Pharm. Rev. 2002;5:82–85. [Google Scholar]
- 6.Mersmann A., Heyer C., Eble A. Taylor & Francis Group; LLC, New York: 2001. Crystallization Technology Handbook. [Google Scholar]
- 7.Dalvi S.V., Dave R.N. Controlling particle size of a poorly water-soluble drug using ultrasound and stabilizers in antisolvent precipitation. Ind. Eng. Chem. Res. 2009;48(16):7581–7593. [Google Scholar]
- 8.Pouretedal H.R. Preparation and characterization of azithromycin nanodrug using solvent / antisolvent method. Int. Nano Lett. 2014;4 doi: 10.1007/s40089-014-0103-x. [DOI] [Google Scholar]
- 9.Ramisetty K.A., Pandit A.B., Gogate P.R. Ultrasound-assisted antisolvent crystallization of benzoic acid: effect of process variables supported by theoretical simulations. Ind. Eng. Chem. Res. 2013;52:17573–17582. doi: 10.1021/ie402203k. [DOI] [Google Scholar]
- 10.Sabnis S.S., Raikar R., Gogate P.R. Evaluation of different cavitational reactors for size reduction of DADPS. Ultrason. Sonochem. 2020;69 doi: 10.1016/j.ultsonch.2020.105276. [DOI] [PubMed] [Google Scholar]
- 11.Amara N., Ratsimba B., Wilhelm A., Delmas H. Crystallization of potash alum: effect of power ultrasound. Ultrason. Sonochem. 2001;8:265–270. doi: 10.1016/s1350-4177(01)00087-6. [DOI] [PubMed] [Google Scholar]
- 12.Nii S., Takayanagi S. Growth and size control in anti-solvent crystallization of glycine with high frequency ultrasound. Ultrason. Sonochem. 2014;21(3):1182–1186. doi: 10.1016/j.ultsonch.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Jordens J., Appermont T., Gielen B., Van Gerven T., Braeken L. Sonofragmentation: effect of ultrasound frequency and power on particle breakage. Cryst. Growth Des. 2016;16:6167–6177. doi: 10.1021/acs.cgd.6b00088. [DOI] [Google Scholar]
- 14.Hatkar U.N., Gogate P.R. Process intensification of anti-solvent crystallization of salicylic acid using ultrasonic irradiations. Chem. Eng. Process. Process Intensif. 2012;57–58:16–24. doi: 10.1016/j.cep.2012.04.005. [DOI] [Google Scholar]
- 15.Dizaj S.M., Vazifehasl Z., Salatin S., Adibkia K., Javadzadeh Y. Nanosizing of drugs: effect on dissolution rate. Res. Pharm. Sci. 2015;10:95–108. [PMC free article] [PubMed] [Google Scholar]
- 16.Hou C.-D., Wang J.-X., Le Y., Zou H.-K., Zhao H. Preparation of azithromycin nanosuspensions by reactive precipitation method. Drug Dev. Ind. Pharm. 2012;38(7):848–854. doi: 10.3109/03639045.2011.630394. [DOI] [PubMed] [Google Scholar]
- 17.Luque de Castro M.D., Priego-Capote F. Ultrasound-assisted crystallization (sonocrystallization) Ultrason. Sonochem. 2007;14(6):717–724. doi: 10.1016/j.ultsonch.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Gielen B., Kusters P., Jordens J., Thomassen L.C.J.J., Van Gerven T., Braeken L. Energy efficient crystallization of paracetamol using pulsed ultrasound. Chem. Eng. Process. - Process Intensif. 2017;114:55–66. doi: 10.1016/j.cep.2017.01.001. [DOI] [Google Scholar]
- 19.Narducci O., Jones A.G., Kougoulos E. Continuous crystallization of adipic acid with ultrasound. Chem. Eng. Sci. 2011;66(6):1069–1076. doi: 10.1016/j.ces.2010.12.008. [DOI] [Google Scholar]
- 20.Bagal M.V., Gogate P.R. Wastewater treatment using hybrid treatment schemes based on cavitation and Fenton chemistry: A review. Ultrason. Sonochem. 2014;21(1):1–14. doi: 10.1016/j.ultsonch.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Sander J.R.G., Zeiger B.W., Suslick K.S. Sonocrystallization and sonofragmentation. Ultrason. Sonochem. 2014;21(6):1908–1915. doi: 10.1016/j.ultsonch.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Lindenberg C., Krättli M., Cornel J., Mazzotti M., Brozio J. Design and optimization of a combined cooling/antisolvent crystallization process. Cryst. Growth Des. 2009;9(2):1124–1136. doi: 10.1021/cg800934h. [DOI] [Google Scholar]
- 23.Adeli E. Preparation and evaluation of azithromycin binary solid dispersions using various polyethylene glycols for the improvement of the drug solubility and dissolution rate, Brazilian. J. Pharm. Sci. 2016;52(1):1–13. doi: 10.1590/S1984-82502016000100002. [DOI] [Google Scholar]
- 24.Aucamp M., Odendaal R., Liebenberg W., Hamman J. Amorphous azithromycin with improved aqueous solubility and intestinal membrane permeability. Drug Dev. Ind. Pharm. 2015;41(7):1100–1108. doi: 10.3109/03639045.2014.931967. [DOI] [PubMed] [Google Scholar]
- 25.Wu S., Shen H., Li K., Yu B., Xu S., Chen M., Gong J., Hong Hou B. Agglomeration mechanism of azithromycin dihydrate in acetone-water mixtures and optimization of the powder properties. Ind. Eng. Chem. Res. 2016;55:4905–4910. doi: 10.1021/acs.iecr.5b04437. [DOI] [Google Scholar]
- 26.M. Tomar, S.A. Kumar, S.A. Raj, Effect of moisture content of exicipient (microcrystalline cellulose) on direct compressible solid dosage forms, Int. J. Pharm. Sci. Res. 8 (2017) 282. 10.13040/IJPSR.0975-8232.8(1).282–88.
- 27.Christensen L.H., Johansen H.E., Schaefer T. Moisture-activated dry granulation in a high shear mixer. Drug Dev. Ind. Pharm. 1994;20(14):2195–2213. doi: 10.3109/03639049409050233. [DOI] [Google Scholar]
- 28.P. Grigorov, A. Lekhal, D. Zarkadas, Optimization of API Attributes and Flow Properties Via Crystallization and Size Reduction Techniques, in: AIChE Annu. Meet., Minneapolis, USA, 2011.










