Abstract
Data on factors associated with vaccine acceptance among pregnant women are critical to the rapid scale up of interventions to improve vaccine uptake. When COVID-19 vaccines were still in the testing phases of research, we surveyed pregnant women accessing prenatal care at an academic medical institution in Central Pennsylvania, United States to examine factors associated with vaccine acceptance. Willingness to receive a COVID-19 vaccine once a vaccine became available was asked as part of an ongoing study on the COVID-19 pandemic and pregnancy (n = 196). Overall, 65% of women reported they would be willing or somewhat willing to receive the COVID-19 vaccine. Women who had received an influenza vaccine within the past year were more likely to be willing to receive the COVID-19 vaccine than women who had never received an influenza vaccine or those who received it over one year ago (aOR 4.82; 95% CI 2.17, 10.72). Similarly, women who were employed full-time were more willing to receive the COVID-19 vaccine than women who were not employed full time (aOR 2.22; 95% CI 1.02, 4.81), and women who reported feeling overloaded were more willing to receive the COVID-19 vaccine than women who did not feel overloaded (aOR 2.18; 95% CI 1.02, 4.68). Our findings support the need to increase vaccination education among pregnant women before vaccines are rolled out, especially those who have not received an influenza vaccine within the past year. Improved understanding of willingness to vaccinate among pregnant women will improve future pandemic responses and current vaccination efforts.
Keywords: COVID-19, SARS-CoV-2, Immunization during pregnancy, Vaccine-preventable diseases, Pennsylvania, Pregnant Women, Vaccination
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected over 232 million people and led to more than four million deaths worldwide as of September 30, 2021.(Kaiser Family Foundation, 2021) In the United States (US), vaccines were first available in December 2020 and as of September 30, 2021, over 184 million people have been fully vaccinated.(Centers for Disease Control and Prevention, 2021) There are three approved COVID-19 vaccines in the US; the Pfizer-BioNTech and Moderna COVID-19 vaccines were approved for use under the emergency use authorization (EUA) in December 2020 and followed soon after by an EUA for the Janssen COVID-19 vaccine in February 2021.(Oliver, 2020, Oliver, 2020, MacNeil et al., 2021) Globally; other vaccines are available and approved by the World Health Organization including Sinopharm, Sinovac-CoronaVac, and Oxford/AstraZeneca vaccines. The efficacy for all approved vaccines ranges from 66.9% to 96.3% in preventing moderate to severe COVID-19.(Pilishvili et al., 2021, Sadoff et al., 2021, Ghiasi et al., 2021, Doroftei et al., 2021, Organization, 2021) Although the approved and available vaccines are effective in preventing serious illness; vaccine acceptance varies across the globe from 56.9% in the US to 97.0% in Ecuador to 23.6% in Kuwait.(Sallam et al., 2021) Studies have shown vaccination greatly decreases COVID-19 cases; morbidity, and mortality and most scientists agree vaccination is a public health priority and is critical to curb the pandemic.(Calina et al., 2020, Christie et al., 2021)
The US Centers for Disease Control and Prevention (CDC) reported that pregnant women with SARS-CoV-2 have an increased rate of hospitalization, admission to the intensive care unit, and need for artificial ventilation compared with non-pregnant women with SARS-CoV-2.(Ellington et al., 2020) Pregnant women with COVID-19 have been found to have worse pregnancy outcomes including an increased risk for preeclampsia/eclampsia, severe infections, intensive care unit admission, mortality, preterm birth, and neonatal morbidity and mortality compared with pregnant women without COVID-19.(Villar et al., 2021) The mortality rate for pregnant women with COVID-19 has been shown to be 1.6%.(Villar et al., 2021).
At the time this study was conducted COVID-19 vaccines were in development and little information on their safety and efficacy were available, especially among pregnant women. Since that time, more information on vaccine safety has been made available and vaccination among pregnant women is recommended in order to prevent severe COVID-19 and to provide immunity to the neonate by transferring active IgG to the fetus during pregnancy.(Albrecht and Arck, 2020) Although adverse events after vaccination among pregnant women are very rare, there have been reported cases of gestational hypertension, threatened labor, miscarriage, and premature delivery after receipt of the Pfizer-BioNTech vaccine (Kadali et al., 2021). Other common side effects of the Pfizer-BioNTech vaccination including sore arm, fatigue, headache, chills, myalgia, nausea, fever, and sweating were reported by both pregnant and non-pregnant women at the same rates (Kadali et al., 2021). Additionally, fever in early pregnancy, a possible side effect of vaccination has been found to have adverse pregnancy and child outcomes and may be a concern related to the COVID-19 vaccine.(Gustavson et al., 2019, Waller et al., 2018, Kerr et al., 2017). Although pregnant women experience a high COVID-19 burden and serious health outcomes associated with COVID-19 during pregnancy and vaccination protects against severe COVID-19 and transfers immunity to the neonate, pregnant women are considered a priority group for COVID-19 immunization only in the US and the United Kingdom and pregnant women are offered vaccination in just 11 of the top 20 countries affected by COVID-19.(Ellington et al., 2020, Albrecht and Arck, 2020, Centers for Disease Control and Prevention, 2021, Centers for Disease Control and Prevention, 2021, Sarwal et al., 2021)
In addition to concerns regarding vaccine availability and prioritization, positive vaccination attitudes and trust in the vaccine, health system, and provider are important predictors of vaccination uptake.(Jean-Jacques and Bauchner, 2021) In December 2020; 27% of the general public said they probably or definitely would not get a COVID-19 vaccine according to the Kaiser Family Foundation COVID-19 Vaccine Monitor.(Hamel et al., 2020) The main reasons cited in this study for not planning to get vaccinated were concerns about side effects (59%), safety and efficacy (55%), the vaccine being too new (53%), and the role of politics in the development process (51%).(Hamel et al., 2020) Evidence on COVID-19 vaccine acceptance among pregnant women is beginning to emerge. Between 48 and 61% of pregnant women were willing to receive the COVID-19 vaccine before vaccinations became available; with refusal reasons being concern for side effects, lack of safety data among pregnant women, and concern for their fetus.(Ahlers-Schmidt, 2020, Khubchandani, 2021, Ceulemans, 2021) Another study found pregnant women in the United Kingdom were 2.4 times more likely to be vaccine resistant than non-pregnant individuals.(Murphy, 2021) Recently, the CDC reported pregnant women were less likely to complete the COVID-19 vaccine series than non-pregnant women and vaccination rates among pregnant women remain low at under 40%.(Razzaghi, 2021, Centers for Disease Control and Prevention, 2021).
Although vaccine hesitancy remains higher among pregnant women even for other recommended vaccines (e.g., influenza and TDaP)(Bödeker et al., 2014), little is known about COVID-19 vaccine hesitancy in this group. The aim of this study is to explore factors associated with willingness to receive the COVID-19 vaccine among pregnant women.
2. Methods
This secondary analysis was part of a larger study conducted to study the effects COVID-19 on pregnant women. The study is ongoing, but the analysis conducted includes data collected between May 15, 2020 and December 1, 2020. Pregnant women receiving prenatal care at a mid-size academic medical center in Central Pennsylvania who were over 18 years of age, had internet access for online surveys, and were able to read in English, were eligible to participate in this study. The medical center is the hub of a larger health system that includes four medical centers, three additional hospitals, 126 outpatient practices in 94 locations across 29 counties in central Pennsylvania. The medical center serves a population of predominantly white, middle class, suburban and rural pregnant women. Study participants were recruited through direct email, mail, and phone calls, as well as through social media and fliers. Direct recruitment was conducted by merging the list of pregnant women from the Department of Obstetrics and Gynecology with patient electronic health record contact data. The study aimed to assess multiple factors associated with the COVID-19 pandemic and pregnancy. One aspect of the survey assessed vaccine willingness.
Willingness to be vaccinated was measured by the following survey question: “While there is no COVID-19 vaccine available right now, how willing would you be to accept the vaccine once developed?” with responses as “Willing”; “Somewhat willing”; “Somewhat unwilling”; “Unwilling”; and “Don’t know”. We dichotomized their responses into Yes (willing and somewhat willing) and No (somewhat unwilling, unwilling, and don’t know).(Laurence et al., 2012) We excluded women from the study who did not answer this question.
We collected the following information about enrolled participants using the REDCap secure online survey tool: race (white/ not white), ethnicity (Hispanic/ not Hispanic), age (35 or older/ <35)(Lean et al., 2017), gravida status (first pregnancy/ not first pregnancy), pregnancy trimester (first or second trimester/ third trimester), highest education level (college degree or higher/ less than college degree), employment status (employed full time/ not employed full time), whether the participant or their partner was an essential worker (e.g., healthcare, delivery worker, store worker, security, building maintenance, first responder, warehouse, meatpacking, factory, prison worker, etc.) (yes/ no), annual income level ($100,000 USD or above/ less than $100,000 USD per year), possible to isolate in their home (yes/ no), having more than two adults in the household (yes/ no), having children in the household (yes/ no), exposure to household smoking (yes/ no), time frame of survey completion as denoted by date of survey completion (May-July/ August-December), whether the participant was ever tested and/or diagnosed with COVID-19 (yes/ no), and whether the participant had family members and/or close friends diagnosed with COVID-19 (yes/ no). Further, women were asked about pertinent medical history of obesity, a previous miscarriage, diabetes, and lung disease with all responses being ever or never having a history of the conditions. Additionally, four measurements of stress were included: work related stress, stress about their baby’s health, stress about their family’s health, and feeling overloaded. Each stressor was classified into no stress or any stress (i.e., some stress, moderate stress, or severe stress). Questions from our survey were adopted and adapted from the First Baby Study Prenatal Questionnaire, the Psychosocial Hassles Scale, the Coronavirus Perinatal Experiences Impact Survey, and the COVID-19 Impact Scale.(Stoddard, 2021, Kjerulff, 2013, Da Costa, 1999, Thomason et al., 2020)
3. Ethical approval
The study was approved by the Pennsylvania State University College of Medicine Institutional Review Board.
3.1. Statistical analysis
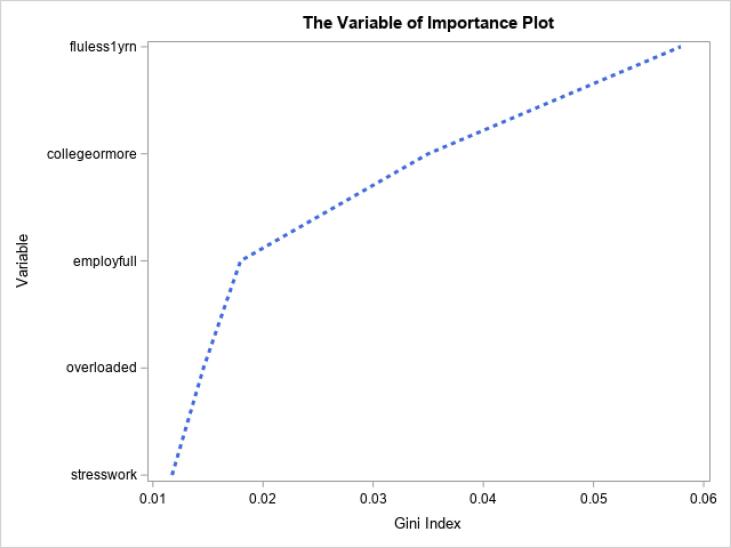
Bivariate analyses were completed to assess the association between factors hypothesized to be associated with willingness to vaccinate including sociodemographic variables, comorbidities, serving as an essential worker, vulnerable living conditions, stressors, and cohort effects (time when survey was completed, whether it was their first pregnancy and what time point in pregnancy the survey was completed) with willingness to vaccinate using univariate logistic regression models.. Next, variables that were significant (p < 0.05) in the unadjusted analyses were included in a full multivariable regression and backwards elimination multivariable regression. Multicollinearity was not found to exist among the independent variables included in the multivariable regression models. For further evaluation and verification, the importance of all variables was assessed based on random forests that quantified how a variable contributes to the homogeneity of the nodes and leaves in the resulting random forest. The forest plot shows each variable on the y-axis and the Gini index on the x-axis. A larger Gini index indicates greater inequality, and thus higher variable importance. All statistical analyses were completed using SAS 9.4.
4. Results
Of the 208 enrolled women, 196 answered their willingness to receive a COVID-19 vaccine. Between May and December 2020, 65% (95% CI 58%, 71%) of study participants reported a willingness to receive the COVID-19 vaccine (willing 36%, somewhat willing 29%, somewhat unwilling 5%, unwilling 14%, and don’t know 17%). The characteristics of the study population (Table 1) show that 20% of women were 35 years or older, 90% were white, and 3% were Hispanic. The majority of women in this study had more than a college degree (70%) and reported having children at home (61%). Nearly 60% of partners had a college degree or higher. Among the study population, 63% were employed full-time and of those employed (full or part-time) 63% were essential workers, and 47% of study participants reported to live with essential workers. Among women in the study, 46% reported a household income of US $100,000 per year or more. The majority of women (78%) reported their ability to isolate in their homes if necessary and 14% reported exposure to indoor smoking. Most women were multigravida (64%), in their first or second trimester of pregnancy (58%), completed the survey between May and July of 2020 (79%) and reported having received a flu vaccine within the last year (77%). Few women in this study had firsthand experience with COVID-19, 11% were ever tested for COVID-19, 4% had a COVID-19 diagnosis, and 13% had a family member or close friend diagnosed with COVID-19. Among study subjects, 25% were obese before pregnancy, 28% had a pervious miscarriage, 15% had a history of lung diseases, and 6% reported a history of diabetes. Finally, women reported high levels of stress with 62% reporting stress at work, 80% reported stress about their baby’s health, 65% reported stress about the health of their family and friends, and 68% reported feeling overloaded.
Table 1.
Characteristics of study population n = 196.
| Demographic Variable | N(%) |
|---|---|
| Willing to receive the COVID-19 vaccine | 127 (65%) |
| Not willing to receive the COVID-19 vaccine | 69 (35%) |
| Age >35 years | 39 (20%) |
| Age <35 years | 157 (80%) |
| White race | 173 (90%) |
| Not white race | 19 (9.9) |
| Hispanic ethnicity | 6 (3%) |
| Not Hispanic ethnicity | 188 (97%) |
| Mother college degree+ | 137 (70%) |
| Mother < college degree | 58 (30%) |
| Father college degree + | 109 (57%) |
| Father < than college degree | 84 (43%) |
| Employed full time | 121 (63%) |
| Not employed full time | 71 (37%) |
| Essential worker | 95 (63%) |
| Not essential worker | 56 (37%) |
| Adults in household essential worker | 92 (47%) |
| No adults in household essential worker | 103 (53%) |
| Possible to isolate in home | 141 (78%) |
| Not possible to isolate at home | 41 (23%) |
| Household >$100,000/yr | 85 (46%) |
| Household <$100,000/yr | 101 (54%) |
| 2+ adults living in household | 25 (13%) |
| <2 adults living in household | 171 (87%) |
| Someone smokes in home | 27 (14%) |
| No one smokes in home | 167 (86%) |
| Has children living in home | 120 (61%) |
| No children living in home | 76 (39%) |
| May-July survey completion | 154 (79%) |
| August-December survey completion | 42 (22%) |
| First or second trimester | 111 (58%) |
| Third trimester | 83 (44%) |
| First pregnancy | 71 (36%) |
| Not first pregnancy | 125 (64%) |
| Had a flu shot <1 year ago | 144 (77%) |
| Had a flu shot 1 year ago or more or never | 44 (23%) |
| Wears a mask every time within 6 feet of someone else | 96 (46%) |
| Does not wear a mask every time within 6 feet of someone else | 112 (54%) |
| Ever been tested for SARS-CoV-2 | 22 (11%) |
| Never tested for SARS-CoV-2 | 174 (89%) |
| Family or friend with COVID-19 | 25 (13%) |
| No family or friend with COVID-19 | 165 (87%) |
| Had a COVID-19 diagnosis | 8 (4%) |
| No COVID-19 diagnosis | 188 (96%) |
| Obese | 47 (25%) |
| Not obese | 140 (75%) |
| Previous miscarriage | 54 (28%) |
| No previous miscarriage | 141 (72%) |
| History of diabetes | 12 (6%) |
| No history of diabetes | 181 (94%) |
| History of lung disease | 28 (15%) |
| No history of lung disease | 164 (85%) |
| Work related stress | 122 (62%) |
| No work related stress | 74 (38%) |
| Stress about baby’s health | 155 (80%) |
| No stress about baby’s health | 40 (20%) |
| Stress about family’s health | 127 (65%) |
| No stress about family’s health | 68 (35%) |
| Stress about own health | 119 (61%) |
| No stress about own health | 76 (39%) |
| Feeling overloaded | 133 (68%) |
| Not feeling overloaded | 62 (32%) |
The bivariate analysis (Table 2) showed having a college degree or higher, being employed full time, having had an influenza vaccine in the last year, reporting work related stress, and reporting feeling overloaded as statistically significant factors associated with willingness to be vaccinated. The multivariable logistic models (Table 2) found that having had an influenza vaccination in the last year (full model: aOR 4.82; 95% CI 2.17, 10.72; backwards elimination model: aOR 4.78; 95% CI 2.25, 10.15), being employed full time (full model: aOR 2.22; 95% CI 1.02, 4.81; backwards elimination model: aOR 2.17; 95% CI 1.10, 4.28), and feeling overloaded (full model: aOR 2.18; 95% CI 1.02, 4.68) were independently associated with a willingness to receive the COVID-19 vaccine. Note the variable of feeling overloaded is detected to be significant in the full model, but not selected in the backwards elimination model. After ranking variables of importance, having had an influenza vaccination in the past year was the most important factor followed by education, being employed full time, feeling overloaded, and reporting work stress (Fig. 1).
Table 2.
Association between willingness to receive the COVID-19 vaccine and sociodemographic and health factors.
| Demographic Variable | No n=69 (35%) | Yes n=127 (65%) | Univariate Models cOR (95% CI) | Full Model aOR (95% CI) | Backwards elimination aOR (95% CI) |
|---|---|---|---|---|---|
| Age >35 years | 13 (33%) | 26 (67%) | 1.11 (0.53, 2.33) | ||
| Age <35 years | 56 (36%) | 101 (64%) | |||
| White race | 62 (36%) | 111 (64%) | 1.05 (0.39, 2.79) | ||
| Not white race | 7 (37%) | 12 (63%) | |||
| Hispanic ethnicity | 1 (17%) | 5 (83%) | 2.77 (0.32, 24.19) | ||
| Not Hispanic ethnicity | 67 (36%) | 121 (64%) | |||
| Mother college degree+ | 38 (28%) | 99 (73%) | 2.79 (1.48, 5.28) | 1.69 (0.78, 3.64) | |
| Mother < college degree | 30 (52%) | 28 (48%) | |||
| Father college degree + | 32 (29%) | 77 (71%) | 1.72 (0.95, 3.13) | ||
| Father < college degree | 35 (42%) | 49 (58%) | |||
| Employed full time | 33 (27%) | 88 (73%) | 2.59 (1.41, 4.79) | 2.22 (1.02, 4.81) | 2.17 (1.10, 4.28) |
| Not employed full time | 35 (49%) | 36 (51%) | |||
| Essential worker | 29 (31%) | 66 (69%) | 1.08 (0.53, 2.19) | ||
| Not essential worker | 18 (32%) | 38 (68%) | |||
| Adults in household essential worker | 35 (38%) | 57 (62%) | 0.80 (0.45, 1.44) | ||
| No adults in household essential worker | 34 (33%) | 69 (67%) | |||
| Possible to isolate in home | 49 (35%) | 92 (65%) | 1.20 (0.59, 2.46) | ||
| Not possible | 16 (39%) | 25 (61%) | |||
| Household > $100,000/yr | 26 (31%) | 59 (69%) | 1.26 (0.68, 2.33) | ||
| Household < $100,000/yr | 36 (36%) | 65 (64%) | |||
| 2+ adults living in household | 11 (44%) | 14 (56%) | 0.65 (0.28, 1.53) | ||
| <2 adults living in household | 58 (34%) | 113 (66%) | |||
| Someone smokes in home | 14 (52%) | 13 (48%) | 0.44 (0.19, 1.01) | ||
| No one smokes in home | 54 (32%) | 113 (68%) | |||
| Has children living in home | 47 (39%) | 73 (61%) | 0.63 (0.34, 1.17) | ||
| No children living in home | 22 (29%) | 54 (71%) | |||
| May-July survey completion | 54 (35%) | 100 (65%) | 1.03 (0.50, 2.09) | ||
| August-December survey completion | 15 (36%) | 27 (64%) | |||
| First or second trimester | 42 (38%) | 69 (62%) | 0.75 (0.41, 1.37) | ||
| Third trimester | 26 (31%) | 57 (69%) | |||
| First pregnancy | 24 (34%) | 47 (66%) | 1.10 (0.59, 2.03) | ||
| Not first pregnancy | 45 (36%) | 80 (64%) | |||
| Had a flu shot<1 year ago | 36 (25%) | 108 (75%) | 5.25 (2.55, 10.79) | 4.82 (2.17, 10.72) | 4.78 (2.25, 10.15) |
| Had a flu shot 1 year ago or more or never | 28 (64%) | 16 (36%) | |||
| Wears a mask every time within 6 feet of someone else | 34 (35%) | 62 (65%) | 0.98 (0.55 1.77) | ||
| Does not wear a mask every time within 6 feet of someone else | 35 (35%) | 65 (65%) | |||
| Ever been tested for SARS-CoV-2 | 10 (45%) | 12 (55%) | 0.62 (0.25, 1.51) | ||
| Never tested for SARS-CoV-2 | 59 (34%) | 115 (66%) | |||
| Family or friend with COVID-19 | 9 (36%) | 16 (64%) | 0.94 (0.39, 2.26) | ||
| No family or friend with COVID-19 | 57 (35%) | 108 (65%) | |||
| Had a COVID-19 Diagnosis | 3 (38%) | 5 (64%) | 0.90 (0.21, 3.89) | ||
| No COVID-19 Diagnosis | 66 (35%) | 122 (65%) | |||
| Obese | 19 (40%) | 28 (60%) | 0.75 (0.38, 1.47) | ||
| Not obese | 47 (34%) | 93 (66%) | |||
| Previous miscarriage | 20 (37%) | 34 (63%) | 0.88 (0.46, 1.69) | ||
| No previous miscarriage | 48 (34%) | 93 (66%) | |||
| History of diabetes | 6 (50%) | 6 (50%) | 0.52 (0.16, 1.68) | ||
| No history of diabetes | 62 (34%) | 119 (66%) | |||
| History of lung disease | 11 (39%) | 17 (61%) | 0.80 (0.35, 1.83) | ||
| No history of lung disease | 56 (34%) | 108 (66%) | |||
| Work related stress | 36 (30%) | 86 (70%) | 1.92 (1.05, 3.51) | 0.71 (0.31, 1.63) | |
| No work related stress | 33 (45%) | 41 (55%) | |||
| Stress about baby’s health | 51 (33%) | 104 (67%) | 1.67 (0.82, 3.39) | ||
| No stress about baby’s health | 18 (45%) | 22 (55%) | |||
| Stress about family’s health | 39 (31%) | 88 (69%) | 1.78 (0.97, 3.28) | ||
| No stress about family’s health | 30 (44%) | 38 (56%) | |||
| Stress about own health | 36 (30%) | 83 (70%) | 1.77 (0.97, 3.22) | ||
| No stress about own health | 33 (43%) | 43 (57%) | |||
| Feeling overloaded | 40 (30%) | 93 (70%) | 2.04 (1.09, 3.80) | 2.18 (1.02, 4.68) | |
| Not feeling overloaded | 29 (47%) | 33 (53%) |
Fig. 1.
The Variable of Importance Plot.
5. Discussion
Our study assessed COVID-19 vaccine acceptance among pregnant women before vaccines were rolled out in the US. Factors associated with a willingness to receive a COVID-19 vaccine included having had an influenza vaccine within the previous year, being employed full time, and a general feeling of being overloaded. Since 2007 there have been six events that were declared as public health emergencies of international concern, including the H1N1 influenza pandemic, Ebola, and Zika. However, these events had a smaller direct impact on most Americans compared with the COVID-19 pandemic.(Wilder-Smith and Osman, 2020) Studies during the H1N1 pandemic on vaccination during pregnancy found many women were concerned with the safety of the vaccine; efficacy of the vaccine, and were not concerned regarding the severity of illness due to H1N1.(Fridman et al., 2011)
The results from this study, which found a 65% COVID-19 vaccine acceptance rate between May-December 2020, are in line with than previous prevalence estimates of COVID-19 vaccine willingness among pregnant women early in the pandemic ranging from to 48%-61%.(Ahlers-Schmidt, 2020, Ceulemans, 2021, Skjefte, 2021) The rates of vaccine acceptance among pregnant women have generally been lower than the rates found in the general population which have been estimated to be 65%-78% during the same time period; which are also similar to the rate found in this study.(Khubchandani, 2021, Murphy, 2021) Furthermore, research has shown higher income and higher education are associated with increased willingness to receive the COVID-19 vaccine.(Khubchandani et al., 2021) The rate of vaccine acceptance among pregnant women found in this study may be due to this study population having a higher education or higher income than women in other studies.(Ahlers-Schmidt, 2020, Ceulemans, 2021)
The strongest association with vaccine willingness in our study population was receiving an influenza vaccine within the last year. In our study population, 77% of women reported receiving an influenza vaccine during the previous year and of those, 75% were willing to receive the COVID-19 vaccine. This is in comparison to the 33% of women who did not report receiving an influenza vaccine in the previous year and of those just 35% were willing to receive the COVID-19 vaccine. Previous and recent influenza vaccination could serve as a proxy for vaccine hesitancy more generally. In our study population, being employed full time was a stronger factor associated with vaccine willingness compared with education and income. Full time employment is likely indicative of higher education and income, but may also point to increased autonomy among women which has also been associated with increased childhood vaccination.(Jung et al., 2018) In our study, 63% of women reported being employed full time and among those women, 73% were willing to receive the COVID-19 vaccine compared to 51% of the 27% of women who reported not being employed full time. Additionally, our study found a significant association between willingness to be vaccinated and generally feeling overloaded. Sixty-eight percent of women in our study reported feeling overloaded and of those women, 70% reported a willingness to receive the COVID-19 vaccination. This is compared with 32% of women who did not report feeling overloaded and of those women only 53% reported being willing to receive the COVID-19 vaccine. The COVID-19 pandemic has altered the pregnancy and birth experience and dramatically impacted daily life, which has led to an increase in psychological distress among pregnant women.(Preis et al., 2020) A recent study showed an increased burden of household responsibilities for women compared to men during the COVID-19 pandemic.(Power et al., 2020) These family dynamics may have played a role in vaccine willingness, as women may be eager to receive the vaccine to increase family support. This finding is underlined by a previous study which found higher levels of stress increased pertussis immunization among pregnant women.(Mohammed et al., 2020)
In this study, factors not associated with willingness to receive the COVID-19 vaccination were notable. First, being employed as an essential worker or living with an essential worker did not impact willingness to receive the COVID-19 vaccine. This was unexpected as being an essential worker could carry higher risks for SARS-CoV-2 infection compared with those who were non-essential and therefore more likely to have a lower level of exposure to SARS-COV-2.(The, 2020) Additionally; having comorbidities that would increase one’s risk for COVID-19 did not impact women’s willingness to receive a vaccine. It was expected that women who were at a higher risk for severe COVID-19 may have a higher level of vaccine acceptance.(Do et al., 2021) Furthermore; neither having had a personal diagnosis, having been tested for COVID-19, nor having a family member or close friend diagnosed with COVID-19 were associated with vaccine willingness. Having a personal connection to COVID-19 may increase one’s willingness to be vaccinated, as personal stories are often reasons people choose to be vaccinated.(Xu, 2019, Gandhi et al., 2020) Moreover, feeling stress related to their own health, the health of their baby, or the health of their family or friends did not impact their willingness to be vaccinated, which was in contrast to previous research showing increased influenza vaccine uptake with a desire to protect family members.(Corace et al., 2013) Finally, although more information became available regarding the COVID-19 vaccine from May 2020 to December 2020, we did not observe any change in women’s willingness to be vaccinated during the study period. This was striking, because we expected an increase in COVID-19 acceptability as vaccine information became clearer. This finding could be due to limited data on the safety and efficacy of the COVID-19 vaccine in pregnant women, because this population was excluded from the clinical trials.(Rasmussen et al., 2021) However, it could also be due to the politics surrounding the COVID-19 vaccine as one study assessing vaccine acceptability between March 2020 and August 2020 found that people who identified with the Democratic party did not change their attitudes towards vaccination and those who identified as Republication had decreasing levels of vaccine acceptability during the study time.(Fridman et al., 2021) We did not ask about political affiliation; and therefore cannot examine this variable in our data.
Factors associated with vaccine acceptance may differ across cultures, contexts, and settings. In more collective cultures, concern for others and the perception of increased social acceptance may be an important variable in the willingness to accept COVID-19 vaccination as was shown in China and Japan.(Machida, 2021, Fu, et al., 2020) Trust and confidence in the healthcare system and vaccine are important factors that could differ across settings and contexts, and have been shown to play a role in vaccine acceptance in studies from the US and other regions of the world.(Al-Mohaithef and Padhi, 2020, Halpin and Reid, 2019, Jamison et al., 2019, Tran et al., 2021, Karafillakis, 2021) Since we did not observe any association between comorbidities and willingness to be vaccinated, it may point to a lack of trust in the healthcare system or little information or understanding of the patient’s level of risk for severe COVID-19. Conversely, the finding that influenza vaccination was strongly associated with a willingness to receive the COVID-19 vaccine likely indicates a general trust in the healthcare system and vaccines.
Some limitations are noted for the current study. This study enrolled a population of mostly highly educated non-Hispanic white women with a high household income living in Pennsylvania and had a small sample size of 196 women. Compared with the demographics of Pennsylvania, women in this study more frequently identified as white (90% white in our study; 69% white in Pennsylvania) and less frequency identified as Hispanic (6% Hispanic in our study; 12% Hispanic in Pennsylvania).(Pennsylvania Department of Health, 2019) Minority populations have been found to have higher vaccine hesitancy compared with white populations.(Razai, et al., 2021, Webb Hooper et al., 2021) This reduces our ability to assess COVID-19 vaccine willingness across racial and ethnic groups as well as our ability to generalize our findings to broader populations. Additionally, although factors such as employment and previous vaccination could carry over to other contexts, the cultural meaning may differ. These factors could be explored further in larger samples in the US and in international contexts. Due to the small sample size of our study, we may not have sufficient power to assess associations in the data. Finally, our study did not include direct and precise questions on measures such as vaccine knowledge, perceptions, and practices that may have evolved over the course of the pandemic. Notwithstanding these limitations, our study provides important information on vaccine willingness before vaccine rollout among pregnant women and can be useful in COVID-19 vaccine intervention development.
Our findings reveal vaccine willingness is not due to a single factor; instead, a complex intersection of many factors likely stemming from a long and complex history of vaccine hesitancy. Findings from this analysis suggest clinicians could offer the COVID-19 vaccine along with the influenza vaccine and remind women who are feeling overloaded that a COVID-19 vaccination could reduce their risk for severe COVID-19. This type of conversation between providers and patients may be an important building block to increase trust in the healthcare system which will be critical when countering wide-spread misinformation and disinformation about the COVID-19 vaccine when caring for pregnant women.(Karafillakis, 2021, Verger and Dubé, 2020) The study results may also help public health officials to address COVID-19 vaccine hesitancy in pregnant women and may help in designing interventions to improve vaccine uptake. Although COVID-19 vaccines are considered safe during pregnancy (Shimabukuro et al., 2021), providers should be cognizant about the conflicting messages pregnant women may receive and their persistent hesitancy, and engage in meaningful discussions with their patients regarding COVID-19 vaccination decision making.
CRediT authorship contribution statement
Kristin K. Sznajder: Conceptualization, Methodology, Visualization, Investigation, Resources, Writing – review & editing. Kristen H. Kjerulff: Software, Software, Formal analysis, Data curation, Writing – original draft, Investigation, Resources, Writing – review & editing. Ming Wang: Investigation, Resources, Writing – review & editing. Wenke Hwang: Investigation, Resources, Writing – review & editing. Sarah I. Ramirez: Investigation, Resources, Writing – review & editing. Chintan K. Gandhi: Investigation, Resources, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Our study was funded by the Pennsylvania State University Huck Institute of Life Sciences #7754. We thank the study participants for their time.
References
- Ahlers-Schmidt C.R., et al. Concerns of women regarding pregnancy and childbirth during the COVID-19 pandemic. Patient Educ. Couns. 2020;103(12):2578–2582. doi: 10.1016/j.pec.2020.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht, M. and P.C. Arck, Vertically Transferred Immunity in Neonates: Mothers, Mechanisms and Mediators. Front. Immunol., 2020. 11: p. 555–555. [DOI] [PMC free article] [PubMed]
- Al-Mohaithef M., Padhi B.K. Determinants of COVID-19 Vaccine Acceptance in Saudi Arabia: A Web-Based National Survey. J. Multidiscip. Healthcare. 2020;13:1657–1663. doi: 10.2147/JMDH.S276771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bödeker B., et al. Cross-sectional study on factors associated with influenza vaccine uptake and pertussis vaccination status among pregnant women in Germany. Vaccine. 2014;32(33):4131–4139. doi: 10.1016/j.vaccine.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Calina D., et al. Towards effective COVID-19 vaccines: Updates, perspectives and challenges (Review) Int. J. Mol. Med. 2020;46(1):3–16. doi: 10.3892/ijmm.2020.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. COVID-19 vaccination among pregnant people aged 18-49 years overall, by race/ethnicity, and date reported to CDC - Vaccine Safety Datalink,* United States. 2021 [cited 2021 September 30]; Available from: https://covid.cdc.gov/covid-data-tracker/#vaccinations-pregnant-women.
- Centers for Disease Control and Prevention. COVID-19 Vaccinations in the United States. COVID Data Tracker 2021 [cited 2021 September 30]; Available from: https://covid.cdc.gov/covid-data-tracker/#vaccinations.
- Centers for Disease Control and Prevention. COVID-19 Vaccination for Pregnant People to Prevent Serious Illness, Deaths, and Adverse Pregnancy Outcomes from COVID-19. 2021 [cited 2021 September 30]; Available from: https://emergency.cdc.gov/han/2021/han00453.asp?utm_source=STAT+Newsletters&utm_campaign=1d9394f060-MR_COPY_01&utm_medium=email&utm_term=0_8cab1d7961-1d9394f060-149826813.
- Centers for Disease Control and Prevention. Vaccination Considerations for People who are Pregnant or Breastfeeding. 2021 [cited 2021 January 8]; Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html.
- Ceulemans M., et al. Vaccine willingness and impact of the COVID-19 pandemic on women’s perinatal experiences and practices—a multinational, cross-sectional study covering the first wave of the pandemic. Int. J. Environ. Res. Public Health. 2021;18(7):3367. doi: 10.3390/ijerph18073367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie A., et al. Decreases in COVID-19 Cases, Emergency Department Visits, Hospital Admissions, and Deaths Among Older Adults Following the Introduction of COVID-19 Vaccine - United States, September 6, 2020-May 1, 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70(23):858–864. doi: 10.15585/mmwr.mm7023e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corace K., et al. Predicting influenza vaccination uptake among health care workers: What are the key motivators? Am. J. Infect. Control. 2013;41(8):679–684. doi: 10.1016/j.ajic.2013.01.014. [DOI] [PubMed] [Google Scholar]
- Da Costa D., et al. Variations in stress levels over the course of pregnancy: Factors associated with elevated hassles, state anxiety and pregnancy-specific stress. J. Psychosom. Res. 1999;47(6):609–621. doi: 10.1016/s0022-3999(99)00064-1. [DOI] [PubMed] [Google Scholar]
- Do T.V.C., et al. COVID-19 vaccine acceptance among rural appalachian healthcare workers (Eastern Kentucky/West Virginia): a cross-sectional study. Cureus. 2021;13(8) doi: 10.7759/cureus.16842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroftei B., et al. Mini-review discussing the reliability and efficiency of COVID-19 vaccines. Diagnostics. 2021;11(4):579. doi: 10.3390/diagnostics11040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington S., et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-June 7, 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(25):769–775. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman D., et al. Predictors of H1N1 vaccination in pregnancy. Am. J. Obstet. Gynecol. 2011;204(6):S124–S127. doi: 10.1016/j.ajog.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Fridman A., Gershon R., Gneezy A. COVID-19 and vaccine hesitancy: A longitudinal study. PloS one. 2021;16(4) doi: 10.1371/journal.pone.0250123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, C., et al., Acceptance and preference for COVID-19 vaccination in health-care workers (HCWs). medRxiv, 2020.
- Gandhi C.K., Patel J., Zhan X. Trend of influenza vaccine Facebook posts in last 4 years: a content analysis. Am. J. Infect. Control. 2020;48(4):361–367. doi: 10.1016/j.ajic.2020.01.010. [DOI] [PubMed] [Google Scholar]
- Ghiasi, N., et al., Efficacy and side effects of Sputnik V, Sinopharm and AstraZeneca vaccines to stop COVID-19; a review and discussion. 2021.
- Gustavson K., et al. Maternal fever during pregnancy and offspring attention deficit hyperactivity disorder. Sci. Rep. 2019;9(1):9519. doi: 10.1038/s41598-019-45920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin C., Reid B. Attitudes and beliefs of healthcare workers about influenza vaccination. Nurs. Older People. 2019;31(2):32–39. doi: 10.7748/nop.2019.e1154. [DOI] [PubMed] [Google Scholar]
- Hamel, L., Kirzinger, A., Munana, C., Brodie, M. KFF COVID-19 Vaccine Monitor: December 2020. 2020 [cited 2021 January 25]; Available from: https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/.
- Jamison A.M., Quinn S.C., Freimuth V.S. “You don't trust a government vaccine”: Narratives of institutional trust and influenza vaccination among African American and white adults. Soc. Sci. Med. 2019;221:87–94. doi: 10.1016/j.socscimed.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean-Jacques M., Bauchner H. Vaccine distribution—equity left behind? JAMA. 2021 doi: 10.1001/jama.2021.1205. [DOI] [PubMed] [Google Scholar]
- Jung M. The effect of maternal decisional authority on children's vaccination in East Asia. PloS one. 2018;13(7) doi: 10.1371/journal.pone.0200333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadali, R.A.K., et al., Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: a cross-sectional study on healthcare workers with detailed self-reported symptoms. American Journal of Obstetrics & Gynecology. [DOI] [PMC free article] [PubMed]
- Kaiser Family Foundation. COVID-19 Coronavirus Tracker – Updated as of September 28, 2022021 [cited 2021 September 30]; Available from: https://www.kff.org/global-health-policy/fact-sheet/coronavirus-tracker/.
- Karafillakis E., et al. Trust, emotions and risks: Pregnant women’s perceptions, confidence and decision-making practices around maternal vaccination in France. Vaccine. 2021;39(30):4117–4125. doi: 10.1016/j.vaccine.2021.05.096. [DOI] [PubMed] [Google Scholar]
- Kerr S.M., et al. Periconceptional maternal fever, folic acid intake, and the risk for neural tube defects. Ann. Epidemiol. 2017;27(12):777–782.e1. doi: 10.1016/j.annepidem.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khubchandani J., et al. COVID-19 vaccination hesitancy in the United States: a rapid national assessment. J. Community Health. 2021;46(2):270–277. doi: 10.1007/s10900-020-00958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjerulff K.H., et al. Mode of first delivery and women's intentions for subsequent childbearing: findings from the First Baby Study. Paediatr. Perinatal Epidemiol. 2013;27(1):62–71. doi: 10.1111/ppe.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence B. Dentists consider medical screening important and are willing to incorporate screening procedures into dental practice. J. Evid. Based Dent. Pract. 2012;12(3 Suppl):32–33. doi: 10.1016/S1532-3382(12)70008-8. [DOI] [PubMed] [Google Scholar]
- Lean S.C., et al. Advanced maternal age and adverse pregnancy outcomes: A systematic review and meta-analysis. PloS One. 2017;12(10) doi: 10.1371/journal.pone.0186287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida M., et al. Acceptance of a COVID-19 Vaccine in Japan during the COVID-19 Pandemic. Vaccines. 2021;9(3):210. doi: 10.3390/vaccines9030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil J.R., et al. Updated recommendations from the advisory committee on immunization practices for use of the Janssen (Johnson & Johnson) COVID-19 vaccine after reports of thrombosis with thrombocytopenia syndrome among vaccine recipients—United States, April 2021. Morb. Mortal. Wkly Rep. 2021;70(17):651. doi: 10.15585/mmwr.mm7017e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed H., et al. Psychosocial determinants of pertussis and influenza vaccine uptake in pregnant women: A prospective study. Vaccine. 2020;38(17):3358–3368. doi: 10.1016/j.vaccine.2020.02.020. [DOI] [PubMed] [Google Scholar]
- Murphy J., et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat. Commun. 2021;12(1):29. doi: 10.1038/s41467-020-20226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver S.E., et al. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. Morb. Mortal. Wkly Rep. 2020;69(50):1922. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, S.E., The advisory committee on immunization practices’ interim recommendation for use of moderna COVID-19 vaccine—United States, December 2020. MMWR. Morbidity and mortality weekly report, 2020. 69. [DOI] [PMC free article] [PubMed]
- Organization, W.H., Background document on the inactivated vaccine Sinovac-CoronaVac against COVID-19: background document to the WHO interim recommendations for use of the inactivated COVID-19 vaccine, CoronaVac, developed by Sinovac, 24 May 2021. 2021, World Health Organization.
- Pennsylvania Department of Health. Birth Statistics. 2019 [cited 2021 September 29]; Available from: https://www.health.pa.gov/topics/HealthStatistics/VitalStatistics/BirthStatistics/Pages/birth-statistics.aspx.
- Pilishvili T., et al. Effectiveness of mRNA Covid-19 Vaccine among US Health Care Personnel. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power K. The COVID-19 pandemic has increased the care burden of women and families. Sustainab.: Sci., Practice Policy. 2020;16(1):67–73. [Google Scholar]
- Preis H., et al. Vulnerability and resilience to pandemic-related stress among US women pregnant at the start of the COVID-19 pandemic. Soc. Sci. Med. 2020 doi: 10.1016/j.socscimed.2020.113348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S.A., et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy: what obstetricians need to know. Obstet. Gynecol. 2021;137(3):408. doi: 10.1097/AOG.0000000000004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razai, M.S., et al., Covid-19 vaccine hesitancy among ethnic minority groups. 2021, British Medical Journal Publishing Group. [DOI] [PubMed]
- Razzaghi H., et al. COVID-19 vaccination coverage among pregnant women during pregnancy — eight integrated health care organizations, United States, December 14, 2020–May 8, 2021. Morb. Mortal. Wkly Rep. 2021;70 doi: 10.15585/mmwr.mm7024e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J., et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N. Engl. J. Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines. 2021;9(2):160. doi: 10.3390/vaccines9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwal Y., Sarwal T., Sarwal R. Prioritizing pregnant women for COVID-19 vaccination. Int. J. Gynecol. Obstet. 2021 doi: 10.1002/ijgo.13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro, T.T., et al., Preliminary findings of mRNA covid-19 vaccine safety in pregnant persons. New England Journal of Medicine, 2021. 384(24): p. 2273–2282. [DOI] [PMC free article] [PubMed]
- Skjefte M., et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur. J. Epidemiol. 2021;36(2):197–211. doi: 10.1007/s10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddard J., et al. The Coronavirus Impact Scale: Construction. Valid., Comp. Diverse Clin. Samples. 2021 [Google Scholar]
- The, L., The plight of essential workers during the COVID-19 pandemic. Lancet (London, England), 2020. 395(10237): p. 1587-1587. [DOI] [PMC free article] [PubMed]
- Thomason, M.E., A. Graham, and M.R. VanTieghem. COPE: Coronavirus Perinatal Experiences - Impact Survey (COPE-IS). 2020 [cited 2021 October 11]; Available from: https://www.nlm.nih.gov/dr2/COPE-Impact_Survey_Perinatal_Pandemic_Survey.pdf.
- Tran V.D., et al. Determinants of COVID-19 vaccine acceptance in a high infection-rate country: a cross-sectional study in Russia. Pharmacy Practice (Granada) 2021;19(1) doi: 10.18549/PharmPract.2021.1.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger, P. and E. Dubé, Restoring confidence in vaccines in the COVID-19 era. 2020, Taylor & Francis. [DOI] [PubMed]
- Villar J., et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatrics. 2021;175(8):817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller D.K., et al. Maternal report of fever from cold or flu during early pregnancy and the risk for noncardiac birth defects, National Birth Defects Prevention Study, 1997–2011. Birth defects Res. 2018;110(4):342–351. doi: 10.1002/bdr2.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. No populations left behind: vaccine hesitancy and equitable diffusion of effective COVID-19 vaccines. J Gen Intern Med. 2021:2130–2133. doi: 10.1007/s11606-021-06698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith A., Osman S. Public health emergencies of international concern: a historic overview. J. Travel Med. 2020;27(8):p. taaa227. doi: 10.1093/jtm/taaa227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. Personal stories matter: topic evolution and popularity among pro- and anti-vaccine online articles. J. Comput. Soc. Sci. 2019;2(2):207–220. [Google Scholar]