Abstract
Background
Attrition threatens the success of antiretroviral therapy (ART). In this cohort study, we examined outcomes of people living with human immunodeficiency virus (PLHIV) who were lost to follow-up (LTFU) during 2014–2017 at ART programs in Southern Africa.
Methods
We confirmed LTFU (missed appointment for ≥60 or ≥90 days, according to local guidelines) by checking medical records and used a standardized protocol to trace a weighted random sample of PLHIV who were LTFU in 8 ART programs in Lesotho, Malawi, Mozambique, South Africa, Zambia, and Zimbabwe, 2017–2019. We ascertained vital status and identified predictors of mortality using logistic regression, adjusted for sex, age, time on ART, time since LTFU, travel time, and urban or rural setting.
Results
Among 3256 PLHIV, 385 (12%) were wrongly categorized as LTFU and 577 (17%) had missing contact details. We traced 2294 PLHIV (71%) by phone calls, home visits, or both: 768 (34% of 2294) were alive and in care, including 385 (17%) silent transfers to another clinic; 528 (23%) were alive without care or unknown care; 252 (11%) had died. Overall, the status of 1323 (41% of 3256) PLHIV remained unknown. Mortality was higher in men than women, higher in children than in young people or adults, and higher in PLHIV who had been on ART <1 year or LTFU ≥1 year and those living farther from the clinic or in rural areas. Results were heterogeneous across sites.
Conclusions
Our study highlights the urgent need for better medical record systems at HIV clinics and rapid tracing of PLHIV who are LTFU.
Keywords: tracing, HIV, lost to follow-up, vital status, Southern Africa
A sampling-based approach to tracing clients lost to follow-up can lead to a better understanding of the outcomes in those lost to follow-up and inform interventions tailored to antiretroviral therapy programs in Southern Africa.
Mortality and retention in care are essential indicators of the success of antiretroviral therapy (ART) programs. Obtaining accurate estimates is, however, challenging, given the uncertain vital and care status of people living with human immunodeficiency virus (PLHIV) classified as lost to follow-up (LTFU). Attrition along the care cascade is common [1–5]. In resource-limited settings, the long distances to clinics, costs of travel, and long waiting times, as well as stigma and discrimination, can deter clients from attending appointments [6–11]. Undocumented, silent transfers from one clinic to another can erroneously result in a client being classified as lost to care. Silent transfers are common in sub-Saharan Africa, where national and international migration is frequent and data exchange between clinics is limited [12–14].
Tracing of PLHIV LTFU is an essential part of ART program activities. From a clinical and public health perspective, the aim is to bring clients back into care. From a programmatic and epidemiological perspective, tracing allows ascertaining the outcomes of those LTFU. The implementation of effective tracing in resource-limited settings can be challenging due to limited resources and inadequate documentation systems [15]. A meta-analysis of individual participant data (IPD) from 9 tracing studies in sub-Saharan Africa showed that 29% of PLHIV defined as LTFU remained lost despite tracing [16]. This study also showed that outcomes varied across regions, with mortality ranging from 9% to 50%, depending on the setting.
Most previous tracing studies used disparate protocols and were based on convenience samples, such as PLHIV living near clinics. In this study, we used a standardized protocol to trace a weighted random sample of PLHIV who were classified as LTFU at 8 ART programs in Southern Africa. We report on the success of tracing and vital and care outcomes.
METHODS
The protocol for this cohort study is available on Open Science Framework [17]. PLHIV from 6 Southern African countries were eligible if classified as LTFU between 1 January 2014, and 30 June 2017, based on ART programs’ databases. Participants were traced using a standardized protocol between 1 October 2017 and 30 November 2019.
Study Setting
Eight ART programs in Southern Africa (1 in Lesotho, Mozambique, South Africa, and Zambia, and 2 in Malawi and Zimbabwe) participated in this study. Programs included 73 (range, 1–32) clinics. Some of the rural programs included several smaller clinics, whereas the ART program was typically based at 1 large clinic in urban settings. Sixty-three (86%) of clinics were rural. All ART programs reported having tracing in place but methods varied (Table 1). All programs were part of the International epidemiology Databases to Evaluate AIDS (IeDEA) in Southern Africa [18, 19].
Table 1.
Characteristics of Antiretroviral Therapy Programs
| Characteristic | All | SMART, Lesotho | Dignitas, Malawi | Lighthouse Trust, Malawi | SMART, Mozambique | Themba Lethu, South Africa | MoH-CIDRZ, Zambia | SMART, Zimbabwe | Newlands, Zimbabwe |
|---|---|---|---|---|---|---|---|---|---|
| No. of PLHIV classified as LTFU between Jan 2014 and Jun 2017 | 20 174 | 423 | 3779 | 6713 | 1882 | 1997 | 4777 | 413a | 190 |
| No. of participating health clinics | 73 | 6 | 22 | 2 | 7 | 1 | 2 | 32 | 1 |
| Setting of clinics | |||||||||
| Rural | 63 (86) | 5 (83) | 21 (95) | 0 | 5 (71) | 0 | 0 | 32 (100) | 0 |
| Urban | 10 (14) | 1 (17) | 1 (5) | 2 (100) | 2 (29) | 1 (100) | 2 (100) | 0 | 1 (100) |
| Level of care of clinics | |||||||||
| Health center | 65 (89) | 6 (100) | 21 (95) | 0 | 5 (71) | 0 | 2 (100) | 30 (94) | 1 (100) |
| District hospital | 7 (10) | 0 | 1 (5) | 2 (100) | 2 (29) | 0 | 0 | 2 (6) | 0 |
| Regional hospital | 1 (1) | 0 | 0 | 0 | 0 | 1 (100) | 0 | 0 | 0 |
| Tracing methods in place | |||||||||
| Phone calls | … | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Home visits | … | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: LTFU, lost to follow-up; MoH-CIDRZ, Ministry of Health–Centre for Infectious Disease Research in Zambia; PLHIV, people living with human immunodeficiency virus; SMART, SolidarMed-supported antiretroviral therapy program.
aOnly PLHIV classified as lost to follow-up in 2014 were included.
Sampling
We used a disproportioned stratified random sample design. Strata were defined by sex (women and men), age at last visit (0–15, 16–25, 26–50, and ≥51 years old), and time on ART (≤30, 31–180, 181–364, and ≥365 days since ART initiation). We aimed to sample 500 PLHIV from each ART program with equal allocation within each stratum. For strata containing too few participants, all participants within that strata were sampled, and the remaining strata were oversampled to reach the target of 500. In ART programs with <500 PLHIV classified as lost, all were eligible for tracing.
Tracing Protocol
The standardized tracing protocol consisted of (1) reviewing records to confirm the vital and care status of PLHIV considered LTFU and obtain their contact details, and (2) tracing participants confirmed as lost. Tracing consisted of up to 3 phone calls and up to 3 home visits. All programs used phone calls except in rural Ancuabe, Mozambique, where most people did not have mobile phones. Home visits were conducted at all programs except in Johannesburg, South Africa, because of inaccurate addresses and safety concerns (Table 1).
Data Collection
We used a questionnaire to collect data on demographics, tracing methods used, vital and care outcomes, and whether the participant was found in person or not. Data collection was in English or Portuguese, on paper or Android tablets, using REDCap (Research Electronic Data Capture) [20, 21].
Outcomes
We defined 3 process outcomes: (1) The medical records of participants were found or not found; (2) participants were found or not found through tracing (in person or through informants); and (3) tracing was successful in ascertaining the vital status or not successful. We defined 2 clinical outcomes: vital and care status. We categorized vital status as alive, died, or unknown; and care status as in care (participant never missed an appointment, returned to care, or transferred to another clinic), out of care (stopped taking ART), or unknown.
Definitions
PLHIV were defined as LTFU if they missed an appointment for ≥60 days in Malawi and ≥90 days at all other participating ART programs in keeping with local guidelines. We defined the age of participants as the age at their last clinic visit, and 3 age groups: children (0–9 years), young people (10–24 years), and adults (≥25 years). We defined time on ART as the period between the participants’ ART initiation and last clinic visit and the time since the participant was lost as the period between the last clinic visit and the study start. We defined the study start date at each program as the date when tracing activities were initiated. We determined the travel time to the clinic as the time needed for participants to travel from home to the clinic (1-way), regardless of the means of transport. We classified participants whose medical records showed that they were in fact not LTFU as “false lost” and participants whose vital and care status remained unknown after tracing as “true lost.”
Statistical Analyses
We used descriptive statistics to summarize participants’ characteristics, process outcomes, and vital and care status. We used logistic regression models to calculate odds ratios with 95% confidence intervals of mortality and being in care. We adjusted multivariable models for sex, age, time on ART, time since the participant was lost, travel time, and the clinic setting (urban or rural). We introduced a random intercept for the ART program to account for clustering within programs. The models on mortality included all participants who were traced and for whom the vital status could be determined. The models for being in care included all clients who were traced and found alive. Logistic models used inverse probability weights to adjust for the sampling strategy and dropouts at the different stages of the study to make results representative of all PLHIV lost (Supplementary Text 1). Analyses were performed with Stata 15 (StataCorp, College Station, Texas) or R version 3.6 (R Foundation for Statistical Computing, Vienna, Austria) software.
Ethical Considerations
The Ethics Committee of the Canton of Bern, the Ethics Committee of the University of Cape Town, and the local ethics committees or institutional review boards all approved the contribution of each ART program to research performed within the IeDEA collaboration. All PLHIV provided consent for being traced within routine care.
RESULTS
Participant Recruitment and Characteristics
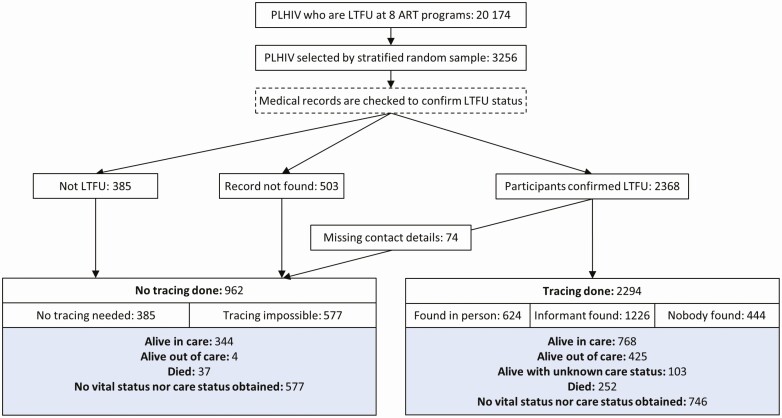
A total of 20 174 PLHIV from 73 clinics and 8 ART programs were eligible. Most clinics were health centers in rural areas. Most programs used both phone calls and home visits to trace lost clients (Table 1). We sampled 3256 PLHIV and thus reached 81% of the planned sample size. The shortfall was explained by <500 eligible PLHIV in some sites and logistical issues in others. Overall, 1837 (56%) participants were female, the median age at the last visit was 32 years (interquartile range, 23–44 years), and 1738 (53%) were on ART for <1 year. About half of the study participants were from smaller health centers and rural areas (Table 2). The selection of participants into the study and their outcomes are shown in Figure 1.
Table 2.
Characteristics of All People Living With Human Immunodeficiency Virus Defined as Lost to Follow-up and Sampled for the Study, Overall and by Antiretroviral Therapy Program
| All | SMART, Lesotho | Dignitas, Malawi | Lighthouse Trust, Malawi | SMART, Mozambique | Themba Lethu Clinic, South Africa | MoH-CIDRZ, Zambia | SMART, Zimbabwe | Newlands, Zimbabwe | |
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | (N = 3256) | (n = 423) | (n = 501) | (n = 506) | (n = 467) | (n = 492) | (n = 264) | (n = 413) | (n = 190) |
| Sex | |||||||||
| Male | 1419 (44) | 160 (38) | 230 (46) | 261 (52) | 216 (46) | 224 (46) | 137 (52) | 115 (28) | 76 (40) |
| Female | 1837 (56) | 263 (62) | 271 (54) | 245 (48) | 251 (54) | 268 (54) | 127 (48) | 298 (72) | 114 (60) |
| Age at last visit, y | |||||||||
| 0–9 | 327 (10) | 8 (2) | 91 (18) | 81 (16) | 69 (15) | 0 | 56 (21) | 19 (5) | 3 (2) |
| 10–24 | 771 (24) | 56 (13) | 134 (27) | 155 (31) | 123 (26) | 74 (15) | 88 (33) | 88 (21) | 53 (28) |
| ≥25 | 2158 (66) | 359 (85) | 276 (55) | 270 (53) | 275 (59) | 418 (85) | 120 (46) | 306 (74) | 134 (70) |
| Median (IQR) | 32 (23–44) | 35 (29–43) | 27 (15–45) | 26 (18–44) | 27 (20–39) | 39 (29–50) | 24 (13–51) | 32 (25–40) | 33 (24–41) |
| Time on ART, at last visit, mo | |||||||||
| 0–11 | 1738 (53) | 145 (34) | 319 (64) | 344 (68) | 221 (47) | 297 (60) | 198 (75) | 177 (43) | 37 (19) |
| ≥12 | 1518 (47) | 278 (66) | 182 (36) | 162 (32) | 246 (53) | 195 (40) | 66 (25) | 236 (57) | 153 (81) |
| Last CD4 count, cells/mm3 | |||||||||
| 0–199 | 522 (16) | 77 (18) | 30 (6) | 63 (12) | 70 (15) | 153 (31) | 10 (4) | 102 (25) | 53 (28) |
| 200–349 | 524 (16) | 75 (17) | 48 (10) | 52 (10) | 102 (22) | 119 (24) | 12 (5) | 78 (19) | 37 (19) |
| 350–499 | 387 (12) | 53 (13) | 44 (9) | 41 (8) | 68 (14) | 77 (16) | 6 (2) | 44 (11) | 41 (22) |
| ≥500 | 551 (17) | 143 (34) | 13 (3) | 27 (5) | 124 (27) | 117 (24) | 18 (7) | 45 (11) | 57 (30) |
| Median (IQR) | 327 (185–520) | 413 (228–704) | 327 (209–407) | 270 (144–433) | 386 (224–578) | 308 (154–500) | 358 (219–594) | 268 (143–422) | 371 (179–534) |
| Missing | 1272 (39) | 75 (18) | 366 (73) | 323 (64) | 103 (22) | 26 (5) | 218 (83) | 144 (35) | 2 (1) |
| Time since the participant was seen for the last time, mo | |||||||||
| 0–11 | 462 (14) | 26 (6) | 84 (17) | 84 (17) | 179 (38) | 42 (9) | 14 (5) | 0 | 33 (17) |
| ≥12 | 2638 (81) | 385 (91) | 391 (78) | 389 (77) | 213 (46) | 446 (91) | 249 (94) | 413 (100) a | 152 (80) |
| Missing | 156 (5) | 12 (3) | 26 (5) | 33 (7) | 75 (16) | 4 (<1) | 1 (<1) | 0 | 5 (3) |
| Travel time from home to the clinic (one-way), h | |||||||||
| <1 | 1785 (55) | 168 (40) | 472 (94) | 478 (94) | 137 (29) | 0 | 245 (93) | 100 (24) | 185 (97) |
| ≥1 | 527 (16) | 169 (40) | 29 (6) | 4 (1) | 292 (63) | 1 (<1) | 15 (6) | 12 (3) | 5 (3) |
| Missing | 944 (29) | 86 (20) | 0 (0) | 24 (5) | 38 (8) | 491 (100) | 4 (2) | 301 (73) | 0 |
| Setting of the clinic | |||||||||
| Rural | 1478 (45) | 391 (92) | 471 (94) | 0 | 203 (43) | 0 | 0 | 413 (100) | 0 |
| Urban | 1778 (55) | 32 (8) | 30 (6) | 506 (100) | 264 (57) | 492 (100) | 264 (100) | 0 | 190 (100) |
| Level of care of the clinic | |||||||||
| Health center | 1753 (54) | 423 (100) | 445 (89) | 0 | 203 (43) | 0 | 264 (100) | 228 (55) | 190 (100) |
| District hospital | 1011 (31) | 0 | 56 (11) | 506 (100) | 264 (57) | 0 | 0 | 185 (45) | 0 |
| Regional hospital | 492 (15) | 0 | 0 | 0 | 0 | 492 (100) | 0 | 0 | 0 |
| LTFU status confirmed, contact details available | |||||||||
| No | 962 (29) | 175 (41) | 64 (13) | 313 (62) | 141 (30) | 28 (6) | 58 (22) | 181 (44) | 2 (1) |
| Yes | 2294 (71) | 248 (59) | 437 (87) | 193 (38) | 326 (70) | 464 (94) | 206 (78) | 232 (56) | 188 (99) |
| No. of clients traced | 2294 | 248 | 437 | 193 | 326 | 464 | 206 | 232 | 188 |
| No. of tracing attempts | |||||||||
| 1 | 1518 (66) | 166 (67) | 437 (100) | 118 (61) | 314 (96) | 218 (47) | 18 (9) | 209 (90) | 38 (20) |
| ≥2 | 776 (34) | 82 (33) | 0 | 75 (39) | 12 (4) | 244 (53) | 188 (91) | 23 (10) | 150 (80) |
| Median (IQR) | 1 (1–2) | 1 (1–2) | 1 (1–1) | 1 (1–2) | 1 (1–1) | 2 (1–2) | 2 (2–4) | 1 (1–1) | 2 (2–4) |
| Tracing method used | |||||||||
| Phone calls only | 761 (33) | 33 (13) | 8 (2) | 101 (52) | 0 | 464 (100) | 0 | 116 (50) | 41 (22) |
| Home visits only | 1096 (48) | 159 (64) | 429 (98) | 52 (27) | 326 (100) | 0 | 18 (9) | 110 (47) | 2 (1) |
| Phone calls and home visits | 437 (19) | 56 (23) | 0 | 40 (21) | 0 | 0 | 188 (91) | 6 (3) | 145 (77) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; LTFU, lost to follow-up; MoH-CIDRZ, Ministry of Health-Centre for Infectious Disease Research in Zambia; PLHIV, people living with HIV; SMART, SolidarMed-supported antiretroviral therapy program.
aOnly people living with human immunodeficiency virus classified as lost to follow-up in 2014 were sampled.
Figure 1.
Study flowchart. Abbreviations: ART, antiretroviral therapy; LTFU, lost to follow-up; PLHIV, people living with HIV.
Vital and Care Status in Medical Records
By checking the medical records, we clarified the vital and care status of 385 (12%) participants who had been erroneously classified as LTFU (“false lost”): 348 (11%) were alive, and 37 (1%) had died. The contact information of 577 (17%) participants was missing, including 503 (15%) for whom we could not find any medical record, and 74 (2%) for whom no contact details were available in the record (Figure 1). We traced the remaining 2294 (71%) participants. The proportion of traced participants among those sampled varied from 38% to 99%, depending on the ART program (Supplementary Table 1).
Tracing Process and Clinical Outcomes
Of 2294 participants, we traced 761 (33%) by phone calls, 1096 (48%) by home visits, and 437 (19%) by a combination of both. We found 624 (27%) of them in person and spoke to 1226 (54%) informants. We did not find the remaining 444 (19%) participants, nor any informant. Overall, 1296 of the 2294 (57%) participants traced were alive, 252 (11%) had died, and 746 (32%) had unknown vital status (“true lost”) (Figure 1). Mortality among the successfully traced was 16% (252 of 1548).
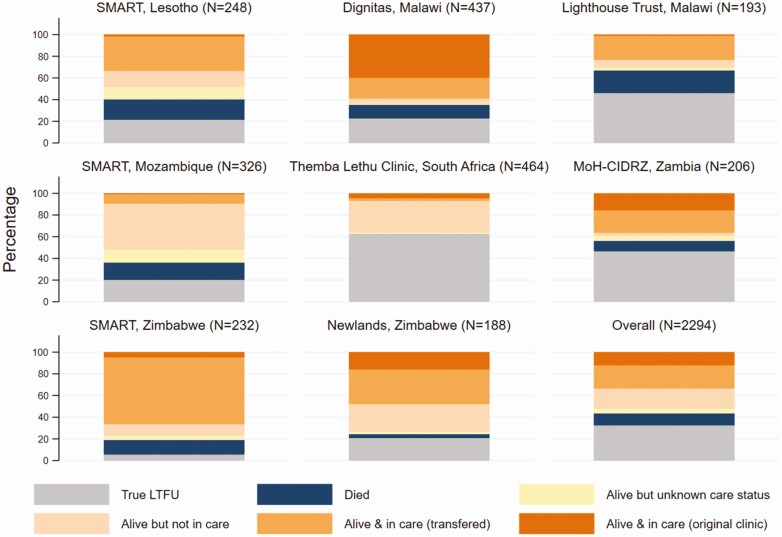
The vital status and care outcomes among the 2294 participants traced are summarized by ART program in Figure 2. Overall, 768 (34%) participants were alive and in care, 425 (19%) were alive and out of care, and 103 (4%) were reported alive by informants with unknown care status. Among the 768 participants who were in care, 491 (64%) had transferred to another clinic. Silent transfers thus accounted for 17% (385 of 2294) of outcomes. There was substantial variation in the distribution of outcomes across ART programs. For example, 59% of participants were found to be alive and in care in an ART program in Malawi, with only 2% not found, compared to 7% alive and in care and 53% not found in the South African program (Figure 2, Supplementary Table 1).
Figure 2.
Vital status and care outcomes among participants who were traced, by antiretroviral therapy program and overall. Abbreviations: LTFU, lost to follow-up; MoH-CIDRZ, Ministry of Health–Centre for Infectious Disease Research in Zambia; SMART, SolidarMed-supported antiretroviral therapy program.
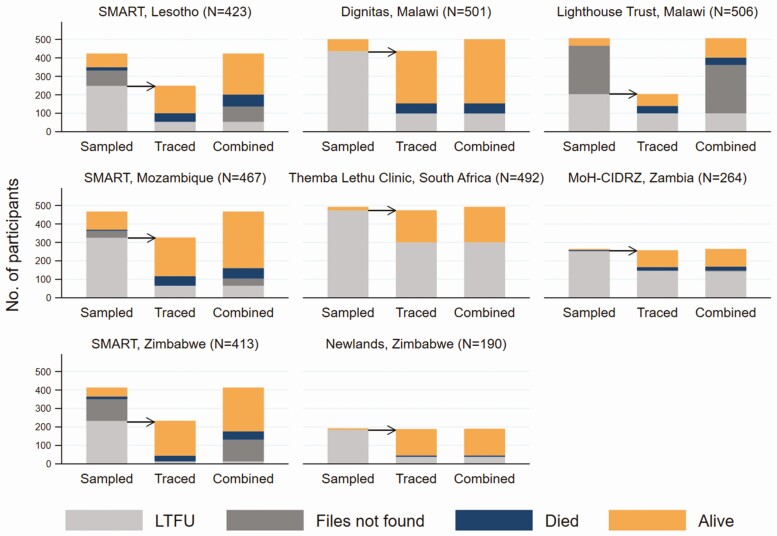
Figure 3 combines outcomes obtained from examining the medical records with those from tracing by ART program. Overall, 1112 of 3256 (34%) participants initially identified as LTFU were alive and in care, 429 (13%) were alive but out of care, 103 (3%) were alive with unknown care status, 289 (9%) had died, and 1323 (41%) remained lost (“true lost”). Among the latter, 577 could not be traced, and 746 were traced but the vital status remained unknown. Again, there was substantial variation across ART programs. For example, the proportion of participants “truly lost” ranged from 20% to 71% depending on the ART program (Supplementary Table 1).
Figure 3.
Differences across antiretroviral therapy programs in the proportions of participants confirmed lost to follow-up, medical records not found, alive or who have died, among those sampled (first bar), the subgroup of those traced (second bar), and the combined outcomes (third bar). Abbreviations: LTFU, lost to follow-up; MoH-CIDRZ, Ministry of Health–Centre for Infectious Disease Research in Zambia; SMART, SolidarMed-supported antiretroviral therapy program.
Predictors of Mortality and Being in Care
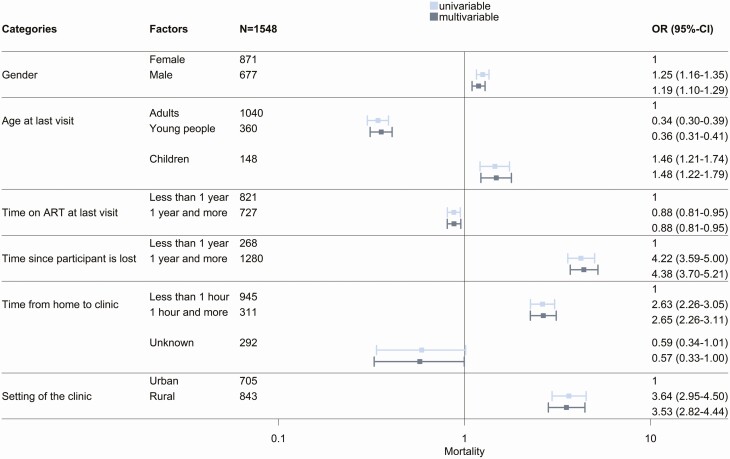
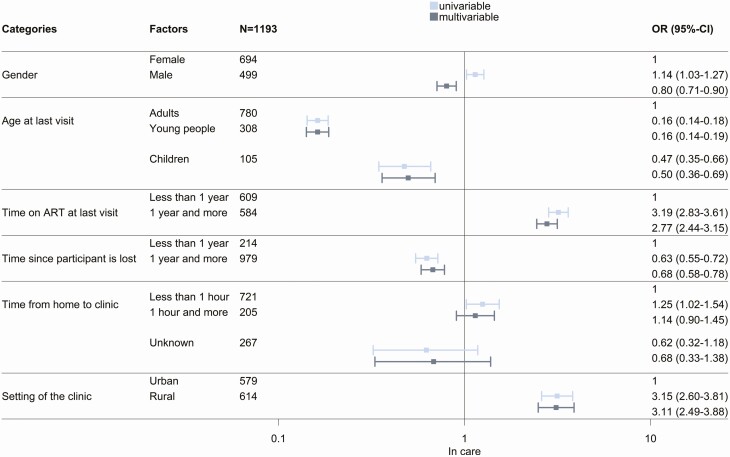
The 1548 participants for whom the vital status was clarified through tracing were included in analyses of mortality. Analyses of being in care included 1296 participants who were found alive. Mortality was higher in men than women, higher in children than in young people or adults, higher in PLHIV who had been on ART <1 year or had been LTFU ≥1, year and higher in PLHIV living in rural areas and living farther from the clinic (Figure 4). For outcome being in care, most of these associations went into the opposite direction (Figure 5), with a few exceptions. There was no association with living further away from the clinic and being in care in the adjusted model. Young people were less likely to be in care than adults and children. Finally, the probability of being in care was higher in rural clinics. There was substantial variation in mortality and retention in care between programs, with standard deviations of the random intercept in adjusted analyses of 1.85 and 2.02, respectively.
Figure 4.
Univariable and multivariable logistic regressions of mortality, among 1548 participants who were traced with determined vital status. Abbreviations: ART, antiretroviral therapy; CI, confidence interval; OR, odds ratio.
Figure 5.
Univariable and multivariable logistic regressions of being in care, among 1193 participants who were traced and found alive. Abbreviations: ART, antiretroviral therapy; CI, confidence interval; OR, odds ratio.
DISCUSSION
The vital status of PLHIV who are LTFU in ART programs is generally unknown but central to estimating program-level outcomes [1, 16, 22, 23]. In lower-income countries, vital registration and national electronic record systems are often weak or absent [24–26]. Physically tracing the clients LTFU is often the only way to obtain reliable information on their vital and care status. We used a standardized protocol to trace a weighted random sample of PLHIV who were classified as lost in 6 Southern African countries, covering steps from identifying PLHIV LTFU in records to tracing and ascertaining outcomes. Overall, the vital status of 41% of PLHIV LTFU remained unknown. Many PLHIV were erroneously classified as lost to care or had missing contact details. Among sampled PLHIV, about a third were alive in care, 13% were alive but out of care, and a tenth had died. Another third of clients could not be found, and hence remained LTFU. Our results underline the difficulty of evaluating program-level mortality of ART with high rates of loss to follow-up [22] and the challenges of tracing PLHIV.
The outcomes differed across programs, underlining the need for locally adapted interventions. The 41% unknown vital and care status hides that this percentage was 21% in a Zimbabwean but 71% in a Malawian program. The medical records showed that 10% to 20% of clients were not LTFU in some clinics whereas this was not an issue in others. In some programs, records could not be located or did not contain the contact information required for tracing. Tracing success also varied, ranging from 37% to >90%. A systematic review of tracing studies [2] found that home visits increased the probability of success compared to phone calls. In this study, tracing success was lowest in a Johannesburg program, which did not visit homes because of inaccurate addresses and safety concerns. Another factor was the delay between loss to follow-up and tracing [16]. The timely tracing of clients lost should, therefore, be a priority. This may be challenging, as the introduction of “treat all” may have overstretched some programs.
Mortality among PLHIV traced was lower than in previous studies, 11% among all traced, and 16% among those traced successfully. A systematic review [2] found that overall, mortality of PLHIV lost and successfully traced was 34%, declining from an estimated 56% in 2003 to 24% in 2011. Another systematic review also found a decline in mortality [12]. Our results indicate that mortality declined further since then, but the studies included in the reviews are not directly comparable between themselves and with the present study. For example, definitions of loss to follow-up varied, from a single missed appointment to no visit for >6 months [2, 12]. In our study, mortality was higher among men than women, in line with an IPD meta-analysis [16] and a recent study from Zambia [27]. Mortality was also higher among those lost for ≥1 year than in those lost for <1 year. In the IPD meta-analysis, mortality plateaued 4 years after the last visit, at 22% [16].
Using a weighted random sampling approach, rather than a convenience sample, and a standardized protocol across different ART programs in Southern Africa are unique strengths of our study. The approach allowed comparisons between different ages, including children and young people. Data on children and young people are scarce. The IPD meta-analysis [16] included one study of adults and children [28] and one study of children only [29]. It showed that mortality was higher in adults older than 30 years, but lacked the power to examine differences between children, adolescents, and adults [16]. Compared to adults, the present study shows that mortality was increased in children and lower in young people. In contrast, the probability of being in care was lower in children and young people than adults. Our study supports calls for distinguishing between children and young people [30, 31].
Silent transfers, whereby clients change facilities without notifying their original clinic, are another barrier to program evaluation. These PLHIV were erroneously classified as lost to care at their original clinic, although they were in care at another clinic. Seventeen percent of the PLHIV who were traced and in care had silently transferred to another clinic, in line with the estimate of 19% from a systematic review and meta-analysis [12]. In the South African electronic monitoring system, undocumented transfers accounted for most misclassified client outcomes [26]. HIV-related laboratory records of South Africa’s National Health Laboratory Service have been used to overcome this problem and estimate retention in care, taking into account transfers between clinics [32]. The Western Cape Provincial Health Data Centre consolidates person-level clinical data across government services using patient registration systems, a unique identification number, and several administrative and clinical digital health systems [33]. The South African experience illustrates the potential and constraints of national information systems.
Our study has several limitations. Only about 80% of the planned sample size was reached, which will have reduced power. The CD4 cell count was missing in many clients. Medical records and contact details were missing in some clients, which prevented their tracing. Among those traced, the vital and care status could be ascertained for only two-thirds. Our study was not designed to determine barriers for remaining in long-term care, which are best addressed using qualitative methods. For example, concerns about stigma and disclosure may prevent some PLHIV from providing accurate contact details or encourage them to change clinic silently [9]. Also, assessing paths in and out of care or how best to implement tracing activities was outside our study’s scope.
In conclusion, our study found that about a third of PLHIV considered to be LTFU at ART programs in Southern Africa were alive and in care. About 40% remained lost due to a combination of unreliable records, missing contact information, and the inability to locate clients despite intensive tracing efforts. Our study underlines the need for nationally linked medical record systems to prioritize PLHIV at high risk of death for tracing and returning to care, including children, and those who are lost after the first year of ART and who live at a greater distance from the clinic. It illustrates the difficulties of evaluating program-level mortality in the presence of high rates of loss to follow-up [1, 16, 22, 23]. A sampling-based approach can lead to a better understanding of the outcomes in those LTFU and inform interventions tailored to the ART program.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. M. B., B. C., N. A., and M. E. designed the study, performed the analyses, and drafted the manuscript. N. A. and F. C. performed the sampling and provided statistical support. J. M., L. J., J. H., J. v. D., M. J. V., M. v. L., C. C., S. J. P., D. O., and M. P. F. reviewed the study design and were in charge of data collection. All authors reviewed, revised, and approved the final manuscript.
Acknowledgments. The authors thank all study participants, tracers, and research collaborators at antiretroviral therapy programs for contributing to this study; Kathrin Zürcher for her input on the study design and data collection; Janet Michel for her contribution to the study initiation; and Martina Reichmuth for her input on the data analysis.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. This work was supported by the NIH’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Fogarty International Center (award number U01AI069924). M. E. was supported by special project funding from the Swiss National Science Foundation (grant number 189498).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Geng EH, Odeny TA, Lyamuya R, et al. Retention in care and patient-reported reasons for undocumented transfer or stopping care among HIV-infected patients on antiretroviral therapy in Eastern Africa: application of a sampling-based approach. Clin Infect Dis 2016; 62:935–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zürcher K, Mooser A, Anderegg N, et al. ; IeDEA and MESH Consortia . Outcomes of HIV-positive patients lost to follow-up in African treatment programmes. Trop Med Int Health 2017; 22:375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health 2010; 15:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep 2010; 7:234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fox MP, Rosen S. Systematic review of retention of pediatric patients on HIV treatment in low and middle-income countries 2008–2013. AIDS (London, England) 2015; 29:493–502. [DOI] [PubMed] [Google Scholar]
- 6. Prust ML, Banda CK, Nyirenda R, et al. Multi-month prescriptions, fast-track refills, and community ART groups: results from a process evaluation in Malawi on using differentiated models of care to achieve national HIV treatment goals. J Int AIDS Soc 2017; 20:21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hubbard J, Phiri K, Moucheraud C, et al. A qualitative assessment of provider and client experiences with 3- and 6-month dispensing intervals of antiretroviral therapy in Malawi. Glob Health Sci Pract 2020;8:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horter S, Bernays S, Thabede Z, et al. “I don’t want them to know”: how stigma creates dilemmas for engagement with treat-all HIV care for people living with HIV in Eswatini. Afr J AIDS Res 2019; 18:27–37. [DOI] [PubMed] [Google Scholar]
- 9. Pantelic M, Casale M, Cluver L, Toska E, Moshabela M. Multiple forms of discrimination and internalized stigma compromise retention in HIV care among adolescents: findings from a South African cohort. J Int AIDS Soc 2020; 23:e25488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc 2013; 16:18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christ B, van Dijk JH, Ballif M, et al. Differentiated antiretroviral therapy delivery in rural Zimbabwe: availability, needs and challenges . OSF Preprints. Posted online 12 August 2020. Available at: https://osf.io/zpq2e/. Accessed 6 January 2021. [Google Scholar]
- 12. Wilkinson LS, Skordis-Worrall J, Ajose O, Ford N. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: systematic review and meta-analysis. Trop Med Int Health 2015; 20:365–79. [DOI] [PubMed] [Google Scholar]
- 13. Hickey MD, Omollo D, Salmen CR, et al. Movement between facilities for HIV care among a mobile population in Kenya: transfer, loss to follow-up, and reengagement. AIDS Care 2016; 28:1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sikazwe I, Eshun-Wilson I, Sikombe K, et al. Patient-reported reasons for stopping care or switching clinics in Zambia: a multi-site, regionally representative estimate using a multi-stage sampling-based approach in Zambia. Clin Infect Dis 2021; 73:e2294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Etoori D, Wringe A, Renju J, Kabudula CW, Gomez-Olive FX, Reniers G. Challenges with tracing patients on antiretroviral therapy who are late for clinic appointments in rural South Africa and recommendations for future practice. Glob Health Action 2020; 13:1755115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chammartin F, Zürcher K, Keiser O, et al. Outcomes of patients lost to follow-up in African antiretroviral therapy programs: individual patient data meta-analysis. Clin Infect Dis 2018; 67:1643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Christ B, Ballif M, Anderegg N, et al. Tracing people living with HIV who are lost to follow-up at ART programs in Southern Africa: study protocol. OSF Preprint. Posted online 24 December 2020. Available at: https://osf.io/52tk8/. Accessed 24 December 2020. [Google Scholar]
- 18. Egger M, Ekouevi DK, Williams C, et al. Cohort profile: the International Epidemiological Databases to Evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012; 41:1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chammartin F, Dao Ostinelli CH, Anastos K, et al. International Epidemiology Databases to Evaluate AIDS (IeDEA) in sub-Saharan Africa, 2012–2019. BMJ Open 2020; 10:e035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Egger M, Spycher BD, Sidle J, et al. ; IeDEA East Africa, West Africa and Southern Africa . Correcting mortality for loss to follow-up: a nomogram applied to antiretroviral treatment programmes in sub-Saharan Africa. PLoS Med 2011; 8:e1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderegg N, Hector J, Jefferys LF, et al. Loss to follow-up correction increased mortality estimates in HIV-positive people on antiretroviral therapy in Mozambique. J Clin Epidemiol 2020; 128:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lopez AD, Setel PW. Better health intelligence: a new era for civil registration and vital statistics? BMC Med 2015; 13:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Forster M, Bailey C, Brinkhof MW, et al. ; ART-LINC collaboration of International Epidemiological Databases to Evaluate AIDS . Electronic medical record systems, data quality and loss to follow-up: survey of antiretroviral therapy programmes in resource-limited settings. Bull World Health Organ 2008; 86:939–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Etoori D, Wringe A, Kabudula CW, et al. Misreporting of patient outcomes in the South African National HIV treatment database: consequences for programme planning, monitoring, and evaluation. Front Public Health 2020; 8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holmes CB, Sikazwe I, Sikombe K, et al. Estimated mortality on HIV treatment among active patients and patients lost to follow-up in 4 provinces of Zambia: findings from a multistage sampling-based survey. PLoS Med 2018; 15:e1002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rachlis B, Ochieng D, Geng E, et al. Implementation and operational research: evaluating outcomes of patients lost to follow-up in a large comprehensive care treatment program in western Kenya. J Acquir Immune Defic Syndr 2015; 68:e46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ardura-Garcia C, Feldacker C, Tweya H, et al. Implementation and operational research. J Acquir Immune Defic Syndr 2015; 70:e160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slogrove AL, Mahy M, Armstrong A, Davies MA. Living and dying to be counted: what we know about the epidemiology of the global adolescent HIV epidemic. J Int AIDS Soc 2017; 20:21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kariminia A, Law M, Davies MA, et al. ; IeDEA . Mortality and losses to follow-up among adolescents living with HIV in the IeDEA global cohort collaboration. J Int AIDS Soc 2018; 21:e25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fox MP, Bor J, Brennan AT, et al. Estimating retention in HIV care accounting for patient transfers: a national laboratory cohort study in South Africa. PLoS Med 2018; 15:e1002589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boulle A, Heekes A, Triffin N, et al. Data centre profile: the Provincial Health Data Centre of the Western Cape Province, South Africa. Int J Pop Data Sci 2019; 4:1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.