Abstract
Background
Invasive fungal infections in the United States are chronically underdiagnosed and a lack of coordinated surveillance makes the true burden of disease difficult to determine. The purpose of this analysis was to capture mortality-associated burden of risk conditions and fungal infections.
Methods
We analyzed data from the National Vital Statistics System from 1999 through 2018 to estimate the mortality attributed to risk conditions and related fungal disease.
Results
The number of risk conditions associated with fungal disease is steadily rising in the United States, with 1 047 422 diagnoses at time of death in 2018. While fungal disease decreased substantially from 1999 to 2010, primarily due to the control of human immunodeficiency virus (HIV) infection, the number of deaths with fungal diagnosis has increased in the non-HIV cohort, with significant increases in patients with diabetes, cancer, immunosuppressive disorders, or sepsis.
Conclusions
The landscape of individuals at risk for serious fungal diseases is changing, with a continued decline in HIV-associated incidence but increased diagnoses in patients with cancer, sepsis, immunosuppressive disorders, and influenza. Additionally, there is an overall increase in the number of fungal infections in recent years, indicating a failure to control fungal disease mortality in these new immunocompromised cohorts. Improvement in the prevention and management of fungal diseases is needed to control morbidity and mortality in the rising number of immunocompromised and at-risk patients in the United States.
Keywords: mycoses, fungal disease, mortality analysis, United States
Mortality for individuals at risk of invasive fungal infections, as well as those with fungal diagnoses, has risen over the past 20 years. This trend underscores an urgent need for improvement in fungal disease prevention, diagnostics and therapeutics.
Serious and invasive fungal infections generally occur in conjunction with other health issues that have resulted in mild to severe immunocompromised states. In the early 1980s, increased attention regarding the clinical importance of invasive fungal infections (IFIs) was a sequela of the eruption of the human immunodeficiency virus (HIV)/AIDS epidemic. The opportunistic fungal pathogen, Pneumocystis jirovecii, the causative agent of Pneumocystis pneumonia (PCP), was identified as an early AIDS-defining illness and the leading cause of morbidity and mortality in these patients for many years [1]. The advent of antiretroviral therapy substantially increased control of HIV, and thus PCP, as well as morbidity and mortality associated with other related opportunistic fungal pathogens.
Recently, there has been a shift in the populations most frequently associated with IFIs away from those who are HIV positive to patients with drug-induced immunosuppression. These include solid-organ and stem cell transplant recipients [2], patients with cancer [3], patients receiving immunomodulators for autoimmune disease and inflammatory diseases [4–6], as well a small cohort of patients with newly diagnosed HIV and those with poor viral control. Fungal infections are also found in patients with less severe comorbidities, including diabetes [7, 8], asthma [9], cystic fibrosis [10, 11], tuberculosis [12], influenza [13, 14], and most recently, coronavirus disease 2019 (COVID-19) [15, 16]. While these immunocompromised cohorts are primarily affected by opportunistic pathogens like Candida, Aspergillus, and Pneumocystis, endemic mycoses, such as Coccidioides, Histoplasma, and Blastomycoses, can cause disease in both immunocompetent and immunocompromised hosts, with a state of impaired immunity resulting in more severe disease [17, 18].
Despite increasing appreciation of the role of fungal disease in immunocompromised patients, active improvement in diagnostic and therapeutic options has only recently gained momentum [19]. The development of new antifungal drugs has primarily focused on the azole and echinocandin classes, with reformulations as a priority, while some vaccine candidates work their way towards clinical trials [20]. However, in the clinical setting, poor patient outcomes continue to be exacerbated by delayed diagnosis and treatment due to the nonspecific symptoms of invasive fungal disease, such as fever, fatigue, cough, and difficulty breathing. Furthermore, a lack of standard diagnosis and treatment guidelines means there is still a high degree of variability in the prognosis of infected individuals [21]. Even with prophylactic strategies with standard antifungals, breakthrough infections occur, and these regimens fail to provide long-term protection once discontinued. There are additional concerns regarding drug–drug interactions and the potential for the development of antifungal drug resistance with extended use for prophylaxis and treatment [22–24].
In addition to mortality concerns, IFIs in the United States resulted in at least $7.2 billion in healthcare costs in 2017, demonstrating a considerable economic burden [25]. The true healthcare cost is likely much higher due to extensive underdiagnosis and a lack of public health surveillance [26], resulting in a continued inability to fully assess the burden of fungal disease in the United States. A more comprehensive understanding of the extent of IFIs in the immunocompromised population, as well as IFI-associated mortality, is needed to highlight the importance of fungal disease management. We used data from the National Vital Statistics System to identify the trends in risk conditions and fungal disease in people who died in the United States from 1999 through 2018.
METHODS
The National Vital Statistics System’s multiple-cause-of-death mortality files for 1999–2018 (including all ages) were used for this analysis. International Classification of Diseases, 10th Revision (ICD–10), codes (Table 1) were used to identify high-risk patients, coinfections, and fungal disease in any of the 20 “entity axis” data fields for multiple causes of death. In this analysis, deaths “from” a particular fungal disease include any mention of fungal disease on the death certificate, regardless of whether it was identified as the primary cause of death, as all listed diagnoses must have contributed to death. The total number of infections were used for all fungal analyses in order to more equally assess all fungal pathogens. Total counts were assessed longitudinally in Figures 1 and 2 for risk conditions and fungal infections, respectively. For the remaining analyses, only mortality records that contained at least 1 fungal infection and at least 1 risk condition were utilized.
Table 1.
Number of Risk Conditions and Fungal Cases Diagnosed at Time of Death in 2018, United States
| ICD-10 Code | Cases Diagnosed by Time of Death | |
|---|---|---|
| Risk condition | ||
| Asthma | J45-J46 | 12 349 |
| Autoimmune conditions | G35, G70, K90, L93, M05, M35 | 13 012 |
| Celiac disease | K90 | 792 |
| Lupus | L93 | 1210 |
| Rheumatoid arthritis | M05 | 198 |
| Polymyalgia rheumatica | M35 | 2030 |
| Multiple sclerosis | G35 | 6380 |
| Myasthenia gravis | G70 | 2479 |
| Cancer | C00-C97 | 327 046 |
| Breast | C50 | 52 674 |
| Colon, rectum, anus | C18–21 | 61 384 |
| Leukemia | C91–C95 | 28 895 |
| Lung, trachea, bronchus | C33–C34 | 153 331 |
| Non-Hodgkin’s lymphoma | C82–C85 | 25 746 |
| Ovary, uterus, cervix | C53–56 | 32 249 |
| Pancreas | C25 | 47 320 |
| Stomach | C16 | 12 055 |
| Urinary | C64–C68 | 39 000 |
| Other | C00–C15, C17, C22–C24, C26–C32, C37–C49, C51–C52, C57–C60, C62–C63, C69–C81, C88, C90, C96–C97 | 269 618 |
| Cystic fibrosis | E84 | 501 |
| Diabetes mellitus | E10–E14 | 276 528 |
| HIV | B20–B24 | 7456 |
| Immunosuppressive disorders | D80–D89 | 4325 |
| Influenza | J09–J11 | 14 481 |
| Pneumonia | J12–J18 | 182 950 |
| Sepsis | A40–A41 | 207 739 |
| Transplant complications | T86 | 1845 |
| Tuberculosis | A16–A19 | 1035 |
| Fungal disease | ||
| Aspergillus | B44 | 807 |
| Invasive | B44.0, B44.1, B44.7 | 456 |
| Noninvasive | B44.2, B44.8 | 9 |
| Candida | B37 | 1031 |
| Invasive | B37.1, B37.5, B37.6, B37.7 | 595 |
| Noninvasive | B37.0, B37.2, B37.3, B37.4, B37.8 | 237 |
| Coccidioides | B38 | 268 |
| Cryptococcus | B45 | 392 |
| Histoplasma | B39 | 167 |
| Mucor | B46 | 151 |
| Pneumocystis | B59 | 554 |
| Other | B35, B36, B40, B41, B42, B43, B47, B48 | 460 |
| Unspecified mycoses | B49 | 1771 |
Abbreviations: HIV, human immunodeficiency virus; ICD-10, International Classification of Diseases, 10th Revision.
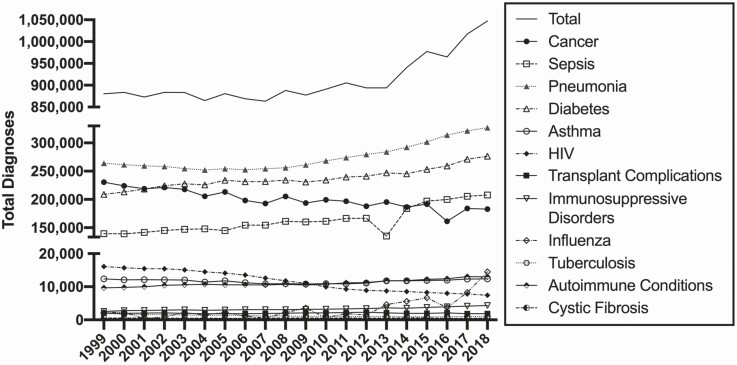
Figure 1.
Burden of immunosuppressive diseases and conditions. Total number of diagnoses of associated risk conditions and diseases at time of death over time. Abbreviation: HIV, human immunodeficiency virus.
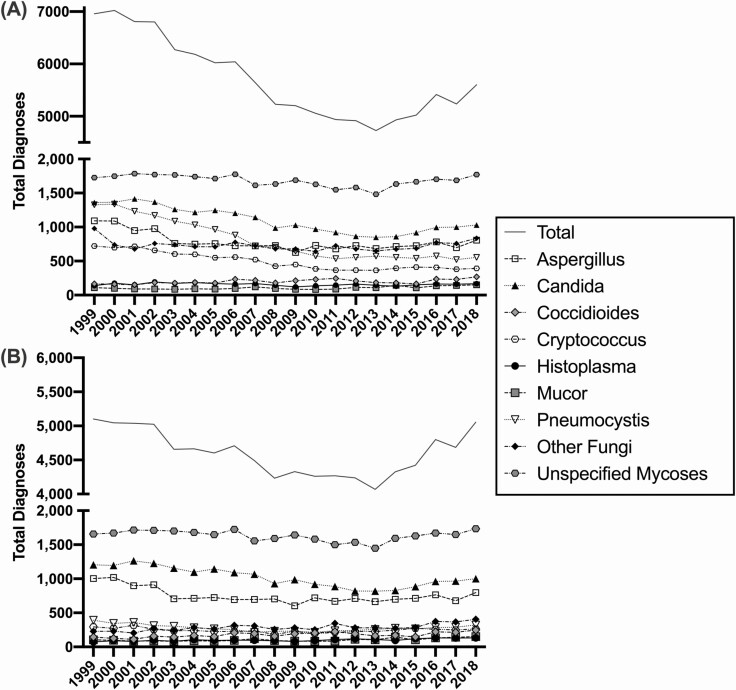
Figure 2.
Burden of fungal disease. Total number of diagnosed fungal infections by type in (A) all recorded deaths and (B) in persons without an HIV diagnosis, over time. Abbreviation: HIV, human immunodeficiency virus.
Age-adjusted rates were calculated using the direct method and the 2000 US standard population. All statistical analysis was performed with SAS version 9.4 (SAS Institute, Inc, Cary, NC). Figures were created using GraphPad Prism (GraphPad Software, La Jolla, CA). To test recent trends in the number of fungal diagnoses in each high-risk cohort, linear regression evaluated the relationship between year, ranging from 2009 to 2018, and number of fungal infections. Risk ratio analysis, with corresponsing 95% confidence internals (CI), was conducted to determine if a single fungal pathogen was more likely associated with a given risk condition over all other fungal pathogens, using the most recent data from 2018.
RESULTS
Of the 2.8 million people who died in the United States in 2018, 429 447 (15.1%) had 1 049 267 diagnoses associated with elevated risk of IFIs. There has been a steady rise in the absolute number of immunosuppressed conditions diagnosed at time of death, most notably over the past 10 years (Figure 1). The greatest decrease in immunosuppressed populations during this time was a steady decline in the incidence of HIV infections. Deaths related to cancer also declined, while the total number of diagnoses for pneumonia, diabetes, and sepsis all rose appreciably.
While the total number of diagnoses of fungal infections in all deaths declined steadily from 1999 through 2010 (Figure 2A), the total number of infections plateaued in 2010 and has since started to rise. Figure 2B depicts the total number of fungal infections in all mortality unrelated to an HIV diagnosis, demonstrating that the decrease in cases was primarily attributable to HIV infections, and the number of infections in other at-risk groups has risen from 2013 through 2018. In 2018, 5601 fungal infections were diagnosed in 3186 (0.112%) of the 2.8 million people who died in the United States. These results demonstrate that patients were often diagnosed with more than 1 fungal infection at time of death (average: 1.76; range: 1–4). Of the 3186 deaths, 40.9% (1304) had fungal infections listed as the primary cause of death. Additionally, 5016 (89.6%) of the diagnosed fungal infections depicted in Figure 2A were associated with a risk condition listed in Figure 1. The fungal pathogens least accounted for by these risk conditions were primarily endemic, with only 72.8% of Coccidioides, 82.5% of Histoplasma, and 76.1% other (including Blastomycoses), while opportunistic pathogens Candida and Pneumocystis each had 99.4% of their diagnoses accounted for by association with these risk conditions.
Rates of Fungal Infections by Associated Condition
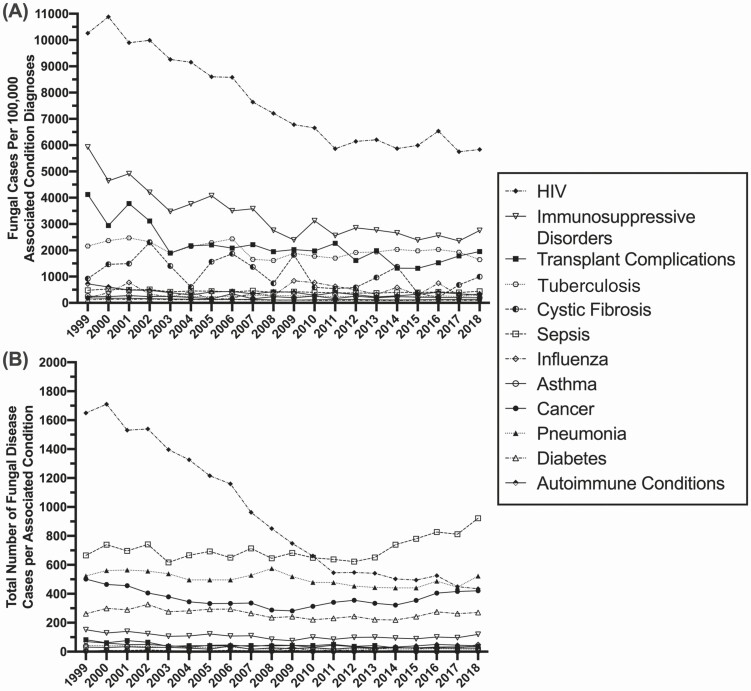
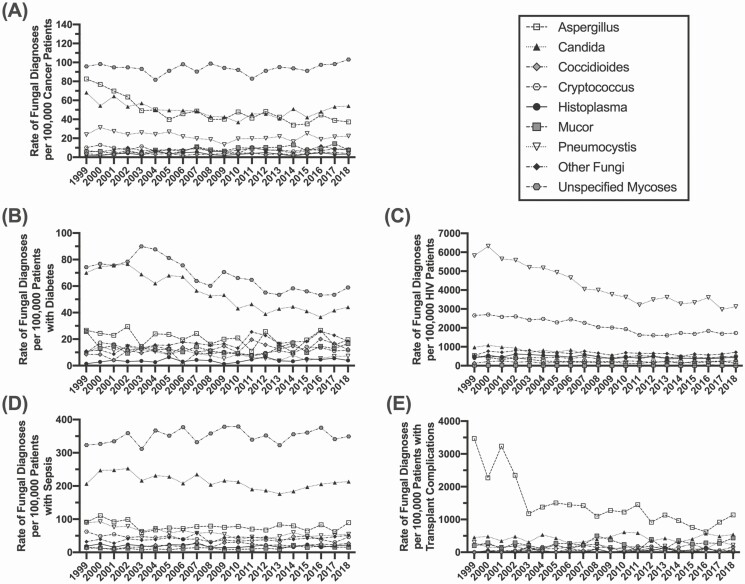
Figure 3A highlights the rate of fungal infections within each at-risk cohort, with the rate calculated as the number of infections within that at-risk cohort over the total number of at-risk individuals within that cohort per year. Patients with HIV are still diagnosed at the greatest rate at time of death, but a subset of transplant recipients (noted for specific transplant complications) and patients with tuberculosis or underlying immunosuppressive disorders have additionally elevated rates of fungal disease diagnosis. The rates of fungal diagnosis in these cohorts have been relatively stable for the past decade, indicating that there has not been significant improvement in diagnostics or therapeutic options/strategies in patients who died. Figure 3B depicts the absolute number of diagnosed fungal infections in each at-risk cohort. Initially, patients with HIV were the group with the highest number of diagnosed fungal infections, but rates in this group have steadily declined since 2004; however, the number of cases diagnosed in this cohort has been stable since 2011. Since 2010, the highest frequency of fungal diagnoses has been in individuals with sepsis, with a steady rise in that cohort, as well as patients with cancer. Supplementary Figure 1 highlights cohorts with lower rates (<1000 per associated condition diagnosis) and total numbers (<100 per associated condition diagnosis) of fungal infections, including patients with asthma, influenza, or autoimmune conditions. It is more difficult to highlight trends in these cohorts, given the smaller sample size; however, the total number of fungal infections in patients with influenza has increased within the past decade.
Figure 3.
Fungal disease diagnosis by underlying condition. A) Age adjusted rate and B) total number of diagnosed fungal infections by underlying at-risk condition at time of death. Abbreviation: HIV, human immunodeficiency virus.
Trends in Fungal Diagnoses by Associated Condition
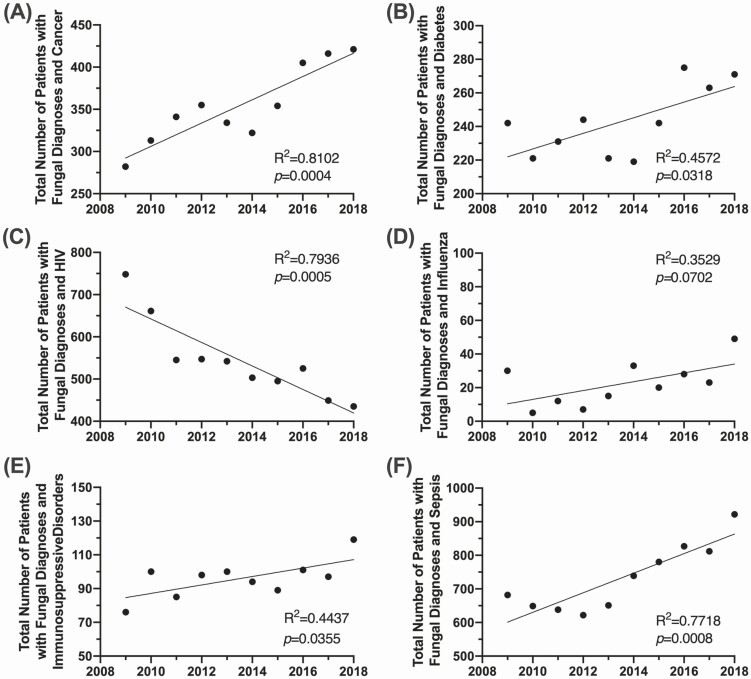
Trends in fungal disease by underlying condition are displayed in Figure 4, including linear regression results. Focusing within the past decade (2009–2018), the number of fungal infections is significantly increasing in the non-HIV cohorts. In patients with cancer, total fungal diagnoses increased by 149% (Figure 4A) (P = .0004), with additional significant increases in patients with diabetes (Figure 4B) (P = .0318), immunosuppressive disorders (Figure 4E) (P = .0355), or sepsis (Figure 4F) (P = .0008). The steady decrease in the HIV-positive cohort has continued over the decade and this is the only risk group with a significant regression that has a negative coefficient (Figure 4C) (P = .0005). There is a steady upward trend in patients with influenza, although the significance of this trend was tempered by the spike in cases during the 2009 influenza pandemic (Figure 4D) (P = .0702). Risk conditions with a net fungal case count of less than 10 were categorized as not demonstrating a clinically significant change in trend (Supplementary Figure 2). These included patients with asthma (P = .7880), autoimmune diseases (P = .3839), cystic fibrosis (P = .3845), pneumonia (P = .7611), transplant complications (P = .0515), and tuberculosis (P = .2686).
Figure 4.
Trends in fungal disease diagnosis by underlying condition. Linear regression of the total number of fungal disease diagnoses reported from 2009 to 2018 in patients with (A) cancer, (B) diabetes, (C) HIV, (D) influenza, (E) immunosuppressive disorders, and (F) sepsis. Abbreviation: HIV, human immunodeficiency virus.
Pathogen Diversity by Associated Condition
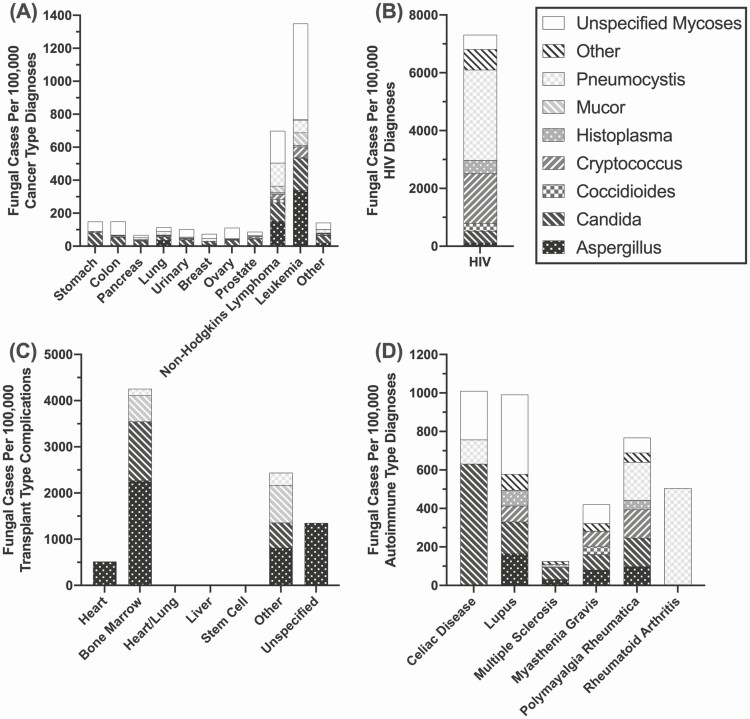
Within the cancer, transplant, and autoimmune disease cohorts, clinical diversity in these underlying conditions translates to a wide variety of fungal diagnoses and related complications, including death. Patients with leukemia and non-Hodgkin’s lymphoma were predominantly affected by unspecified fungal infections, followed by Aspergillus, at much higher rates compared with the larger cancer cohort (Figure 5A). Within the HIV-positive cohort, Pneumocystis and Cryptococcus were the primary diagnoses in this group (Figure 5B). Transplant analysis was limited to patients with reported complications at time of death, as opposed to all transplant recipients. However, Aspergillus was a major contributor in patients with complications related to bone marrow transplant (Figure 5C). There were no transplant complications in the mortality reports for heart/lung, liver, or stem cell transplant recipients. Patients with autoimmune conditions, which may include those receiving immunomodulating therapies, had a diverse prevalence of fungal diagnoses with Candida, primarily diagnosed in patients with celiac disease, while those with rheumatoid arthritis were more likely to be diagnosed with Pneumocystis and those with lupus were most likely to be diagnosed with an unspecified fungal infection (Figure 5D).
Figure 5.
Burden of fungal infections in diverse risk condition types, 2018. Rate of fungal infections in patients with (A) cancer, (B) HIV, (C) transplant complications, or (D) autoimmune disease, by type. Abbreviation: HIV, human immunodeficiency virus.
As noted in Figure 5, some risk conditions are predominantly associated with a single fungal pathogen and these associations should be taken into consideration when diagnosing infections in these cohorts. In Figure 6, longitudinal analysis of the rate of fungal diagnoses by risk condition further highlights these relationships, with risk ratio (RR) analysis conducted for the 2018 data. Notably, unspecified mycoses were diagnosed at the highest rate in patients with cancer, diabetes, and sepsis. However, secondary to unspecified mycoses, the prevalence of Aspergillus and Candida has remained elevated in patients with diabetes compared with other fungal diagnoses, with a combined risk of diagnosis of 1.33 (confidence interval [CI]: 1.2–1.4; P < .0001). Cancer and sepsis were both defined by Candida infections as well (RR [CI]: 1.57 [1.4–1.8], P < .0001; 2.2 [2.0–2.4], P < .0001). Individuals with transplant complications were significantly more likely to be diagnosed with Aspergillus than any other fungal pathogen (RR: 2.34; CI: 1.7–2.9; P < .0001). Influenza and Aspergillus diagnoses have been more closely associated in recent years with an RR of 1.96 (Supplementary Figure 3) (CI: 1.4–2.5; P = .0004). Finally, HIV diagnosis continues to be most closely associated with Pneumocystis (RR: 4.93; CI: 4.3–5.6; P < .0001). Pathogen-specific screening in these cohorts may increase the odds of a more accurate and rapid diagnosis. No significant association with a single pathogen was found in patients with asthma, autoimmune diseases, cystic fibrosis, immunosuppressive disorders, pneumonia, or tuberculosis (Supplementary Figure 3)
Figure 6.
Fungal disease type by associated condition. Rate of fungal diagnoses by type in patients with (A) cancer, (B) diabetes mellitus, (C) HIV, (D) sepsis, or (E) transplant complications. Abbreviation: HIV, human immunodeficiency virus.
DISCUSSION
This analysis provides the first update on the burden of risk conditions and fungal disease at time of death in the United States since 2001 [27]. Our findings demonstrate that the landscape of the immunosuppressed population and those at risk for fungal infections in the United States is changing. With the advent of improved HIV prevention and treatment, as well as the increase in other high-risk populations, the incidence of serious fungal infections and mortality has shifted. Following the trends observed by McNeil et al [27], fungal infections substantially decreased in the early 2000s with HIV control and public health intervention. However, after stabilizing around 2009, the number of deaths with fungal diagnosis has increased, primarily in the cohort without HIV, with significant upward trends in patients with cancer, diabetes, influenza, immunosuppressive disorders, or sepsis. These results reflect the transformation of those affected by fungal disease, both in an expanding recognition of at-risk groups as well as the increase in lifespans and the size of these at-risk patient populations. With improvements in transplant surgeries and management regimens (Organ Procurement and Transplantation Network data as of 8 January 2019), immunomodulators, and cancer treatments [28], this translates to larger patient populations undergoing immunosuppressive treatments moving forward.
In 2018, the patients impacted by fungal disease at the highest rate at time of death were those with HIV, transplant complications, immunosuppressive disorders, or tuberculosis, while patients with cancer, sepsis, HIV, or pneumonia were diagnosed with the highest total numbers. We observed that the relationships between immunosuppression and frequency of specific fungal diagnosis is consistent with previous findings regarding Aspergillus infections and asthma complications [9], Candida and Aspergillus infections associated with diabetes mellitus [7, 8], and Pneumocystis in persons with HIV. Interestingly, transplant complications reported at time of death appear to be most closely associated with Aspergillus infections, as studies in the US-based Transplant-Associated Infection Surveillance Network (TRANSNET) found Candida to be the predominant pathogen associated with solid-organ transplantation [2] and Aspergillus in bone marrow transplantation [29]. The majority of transplant complications reported here were in patients who had received a bone marrow transplant, so these results broadly reflect the TRANSNET findings. While pathogen-specific screening may increase speed and accuracy of diagnosis, and thus improve morbidity and mortality in IFIs, there is emerging evidence of other fungal infections associated with chronic diseases, such as Pneumocystis in asthma [30, 31] and chronic obstructive pulmonary disease [32] and Mucor in transplant recipients [33]. Additionally, the high rate of unspecified mycoses reporting highlights clinical gaps in specific fungal diagnostics, which is especially notable in patients with cancer or sepsis.
The findings observed in this dataset echo a field that is constantly expanding the known clinical implications of fungal disease. Here, we found that an emerging relationship of Aspergillus with influenza, likely due to clinical observations of Aspergillus superinfections in the 2009/2010 influenza pandemic [34, 35]. However, the connection between Aspergillus and viral coinfections has recently extended to include severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and COVID-19–associated pulmonary aspergillosis. While COVID-19–related mortality could not be assessed in this dataset, given that it predates the pandemic, patients with COVID-19 who are mechanically ventilated have high rates of Aspergillus coinfection (10–33%) [16, 36]. Furthermore, additional underlying immunosuppression has been found to correlate with increased risk of invasive mold infections (including aspergillosis) in patients with COVID-19 in intensive care, with solid-organ transplant recipients having 4.66 times the odds and patients on long-term corticosteroids having 8.55 times the odds of invasive aspergillosis compared with those without underlying immunosuppression [37].
The analysis conducted in the National Vital Statistics System dataset had a number of limitations. There was a high rate of unspecified fungal infections, especially within Aspergillus (31.2%) and Candida (19.3%), limiting a clear assessment of invasive disease. Furthermore, unspecified mycoses made up one-third (33.7%) of the fungal infections reported in 2018, somewhat limiting pathogen-specific analysis. Patient conditions and history unrelated to their death are not listed as part of this dataset. For example, the ICD-10 code related to transplants was specific to transplant complications while the broader code for history of organ transplantation was not included. This primarily limited the analysis of transplant cohorts as some common transplant types, including stem cell, were not included in any patient mortality report, indicating that a transplant complication was not identified by the physician as a comorbidity contributing to patient death. Further analysis of the morbidity of fungal disease in high-risk groups in the hospitalization setting will also be informative in evaluating the impact of these pathogens.
While this analysis focused on a rise in fungal infections as a result of the increasing size of at-risk populations, these are a number of reasons for fungal disease to be of major clinical concern. From agriculturally linked azole resistance in Aspergillus species [38] to multidrug-resistant Candida auris in healthcare settings [39], fungal pathogens are matching and outcompeting our current standard of care. With nonspecific clinical symptoms and a lack of pathogen-specific diagnostic options or standardized diagnostic procedures, fungal infections are chronically underdiagnosed. In this dataset, patients were often diagnosed with more than 1 fungal condition related to mortality, if they were diagnosed with any at all. Additionally, endemic mycoses, including Coccidioides and Histoplasma, make up a smaller proportion of fungal infections compared with opportunistic pathogens. As noted in the Results section, infections caused by endemic pathogens were less likely to be reported than opportunistic pathogens in association with high-risk conditions, fitting with the literature that documents endemic mucosal infections in immunocompetent hosts [18]. While these infections are less frequent, these mycoses are increasingly reported in areas where they were not previously considered endemic. It has been proposed that these changes are associated with human migration, agricultural practices, deforestation, and climate change [40].
Invasive fungal infections present a growing risk in the United States. The number of immunocompromised and at-risk patients is increasing, observed through reported deaths annually. There has been a demonstrated increasing trend in fungal infections, especially within non-HIV cohorts. A lack of advancement in diagnostics, standardized prophylactic regimens, and therapeutics has likely contributed to the increased number of fungal disease deaths in the past decade. These results continue to highlight a critical need for improvement in fungal disease surveillance, prevention, and management.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Dr Mary Kay Rayens and Dr William Rayens at the University of Kentucky for helpful discussion and SAS technical support.
Financial support. This work was supported by National Institutes of Health grant number 1R01 AI148365-01A1 (K. A. N.), the Georgia Research Alliance (K. A. N), and the University of Georgia Research Foundation.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Masur H, Michelis MA, Greene JB, et al. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med 1981; 305:1431–8. [DOI] [PubMed] [Google Scholar]
- 2. Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010; 50:1101–11. [DOI] [PubMed] [Google Scholar]
- 3. Sipsas NV, Kontoyiannis DP. Invasive fungal infections in patients with cancer in the intensive care unit. Int J Antimicrob Agents 2012; 39:464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bishu S, Su EW, Wilkerson ER, et al. Rheumatoid arthritis patients exhibit impaired Candida albicans-specific Th17 responses. Arthritis Res Ther 2014; 16:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martínez-Martínez MU, Herrera-Van Oostdam D, Román-Acosta S, Magaña-Aquino M, Baranda-Cándido L, Abud-Mendoza C. Invasive fungal infections in patients with systemic lupus erythematosus. J Rheumatol 2012; 39:1814–8. [DOI] [PubMed] [Google Scholar]
- 6. Silva MF, Ferriani MP, Terreri MT, et al. A multicenter study of invasive fungal infections in patients with childhood-onset systemic lupus erythematosus. J Rheumatol 2015; 42:2296–303. [DOI] [PubMed] [Google Scholar]
- 7. Rodrigues CF, Rodrigues ME, Henriques M. Candida sp. infections in patients with diabetes mellitus. J Clin Med 2019; 8:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghanaat F, Tayek JA. Weight loss and diabetes are new risk factors for the development of invasive aspergillosis infection in non-immunocompromized humans. Clin Pract (Lond) 2017; 14:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denning DW, O’Driscoll BR, Hogaboam CM, Bowyer P, Niven RM. The link between fungi and severe asthma: a summary of the evidence. Eur Respir J 2006; 27:615–26. [DOI] [PubMed] [Google Scholar]
- 10. Green HD, Bright-Thomas RJ, Mutton KJ, Guiver M, Jones AM. Increased prevalence of Pneumocystis jirovecii colonisation in acute pulmonary exacerbations of cystic fibrosis. J Infect 2016; 73:1–7. [DOI] [PubMed] [Google Scholar]
- 11. Amin R, Dupuis A, Aaron SD, Ratjen F. The effect of chronic infection with Aspergillus fumigatus on lung function and hospitalization in patients with cystic fibrosis. Chest 2010; 137:171–6. [DOI] [PubMed] [Google Scholar]
- 12. Bongomin F. Post-tuberculosis chronic pulmonary aspergillosis: an emerging public health concern. PLoS Pathog 2020; 16:e1008742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vanderbeke L, Spriet I, Breynaert C, Rijnders BJA, Verweij PE, Wauters J. Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis 2018; 31:471–80. [DOI] [PubMed] [Google Scholar]
- 14. Coste A, Frerou A, Raute A, et al. The extend of aspergillosis in critically ill patients with severe influenza pneumonia: a multicenter cohort study. Crit Care Med 2021; Epub ahead of Print. [DOI] [PubMed] [Google Scholar]
- 15. Marr KA, Platt A, Tornheim JA, et al. Aspergillosis complicating severe coronavirus disease. Emerg Infect Dis 2021; 27:18–25. Available at: 10.3201/eid2701.202896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meijer EFJ, Dofferhoff ASM, Hoiting O, Meis JF. COVID-19-associated pulmonary aspergillosis: a prospective single-center dual case series. Mycoses 2021; 64:457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 2010; 36:1–53. [DOI] [PubMed] [Google Scholar]
- 18. Kauffman CA. Endemic mycoses: blastomycosis, histoplasmosis, and sporotrichosis. Infect Dis Clin North Am 2006; 20:645–62, vii. [DOI] [PubMed] [Google Scholar]
- 19. Sanguinetti M, Posteraro B, Beigelman-Aubry C, et al. Diagnosis and treatment of invasive fungal infections: looking ahead. J Antimicrob Chemother 2019;74:ii27–37. [DOI] [PubMed] [Google Scholar]
- 20. Scorzoni L, de Paula E Silva AC, Marcos CM, et al. Antifungal therapy: new advances in the understanding and treatment of mycosis. Front Microbiol 2017; 8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Formanek PE, Dilling DF. Advances in the diagnosis and management of invasive fungal disease. Chest 2019; 156:834–42. [DOI] [PubMed] [Google Scholar]
- 22. Khodavaisy S, Mortaz E, Mohammadi F, Aliyali M, Fakhim H, Badali H. Pneumocystis jirovecii colonization in chronic obstructive pulmonary disease (COPD). Curr Med Mycol 2015; 1:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chowdhary A, Sharma C, Meis JF. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 2017; 13:e1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riat A, Plojoux J, Gindro K, Schrenzel J, Sanglard D. Azole resistance of environmental and clinical aspergillus fumigatus isolates from Switzerland. Antimicrob Agents Chemother 2018; 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Benedict K, Jackson BR, Chiller T, Beer KD. Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis 2019; 68:1791–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 2017; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McNeil MM, Nash SL, Hajjeh RA, et al. Trends in mortality due to invasive mycotic diseases in the United States, 1980-1997. Clin Infect Dis 2001; 33:641–7. [DOI] [PubMed] [Google Scholar]
- 28. Cancer Trends Progress Report National Cancer Institute, NIH, DHHS, Bethesda, MD, March 2020. Available at: https://progressreport.cancer.gov. [Google Scholar]
- 29. Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis 2010; 50:1091–100. [DOI] [PubMed] [Google Scholar]
- 30. Goldman DL, Chen Z, Shankar V, Tyberg M, Vicencio A, Burk R. Lower airway microbiota and mycobiota in children with severe asthma. J Allergy Clin Immunol 2018; 141:808–11, e7. [DOI] [PubMed] [Google Scholar]
- 31. Rayens E, Noble B, Vicencio A, Goldman DL, Bunyavanich S, Norris KA. Relationship of Pneumocystis antibody responses to paediatric asthma severity. BMJ Open Respir Res 2021; 8:e000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Norris KA, Morris A. Pneumocystis infection and the pathogenesis of chronic obstructive pulmonary disease. Immunol Res 2011; 50:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Song Y, Qiao J, Giovanni G, et al. Mucormycosis in renal transplant recipients: review of 174 reported cases. BMC Infect Dis 2017; 17:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. ; Dutch-Belgian Mycosis Study Group . Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med 2018; 6:782–92. [DOI] [PubMed] [Google Scholar]
- 35. Waldeck F, Boroli F, Suh N, et al. Influenza-associated aspergillosis in critically-ill patients—a retrospective bicentric cohort study. Eur J Clin Microbiol Infect Dis 2020; 39:1915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Permpalung N, Chiang TP, Massie AB, et al. COVID-19 associated pulmonary aspergillosis in mechanically ventilated patients. Clin Infect Dis 2021; Epub ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fekkar A, Lampros A, Mayaux J, et al. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am J Respir Crit Care Med 2021; 203:307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guegan H, Prat E, Robert-Gangneux F, Gangneux JP. Azole resistance in aspergillus fumigatus: a five-year follow up experience in a tertiary hospital with a special focus on cystic fibrosis. Front Cell Infect Microbiol 2020; 10:613774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spivak ES, Hanson KE. Candida auris: an emerging fungal pathogen. J Clin Microbiol 2018; 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ashraf N, Kubat RC, Poplin V, et al. Re-drawing the maps for endemic mycoses. Mycopathologia 2020; 185:843–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.