Abstract
Background
Persons who use drugs (PWUD) face substantial risk of Staphylococcus aureus infections. Limited data exist describing clinical and substance use characteristics of PWUD with invasive S. aureus infections or comparing treatment and mortality outcomes in PWUD vs non-PWUD. These are needed to inform optimal care for this marginalized population.
Methods
We identified adults hospitalized from 2013 to 2018 at 2 medical centers in San Francisco with S. aureus bacteremia or International Classification of Diseases–coded diagnoses of endocarditis, epidural abscess, or vertebral osteomyelitis with compatible culture. In addition to demographic and clinical characteristic comparison, we constructed multivariate Cox proportional hazards models for 1-year infection-related readmission and mortality, adjusted for age, race/ethnicity, housing, comorbidities, and methicillin-resistant S. aureus (MRSA).
Results
Of 963 hospitalizations for S. aureus infections in 946 patients, 372 of 963 (39%) occurred in PWUD. Among PWUD, heroin (198/372 [53%]) and methamphetamine use (185/372 [50%]) were common. Among 214 individuals using opioids, 98 of 214 (46%) did not receive methadone or buprenorphine. PWUD had lower antibiotic completion than non-PWUD (70% vs 87%; P < .001). While drug use was not associated with increased mortality, 1-year readmission for ongoing or recurrent infection was double in PWUD vs non-PWUD (28% vs 14%; adjusted hazard ratio [aHR], 2.0 [95% confidence interval {CI}: 1.3–2.9]). MRSA was independently associated with 1-year readmission for infection (aHR, 1.5 [95% CI: 1.1–2.2]).
Conclusions
Compared to non-PWUD, PWUD with invasive S. aureus infections had lower rates of antibiotic completion and twice the risk of infection persistence/recurrence at 1 year. Among PWUD, both opioid and stimulant use were common. Models for combined treatment of substance use disorders and infections, particularly MRSA, are needed.
Keywords: Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, bacteremia, substance-related disorders, patient readmission
Persons who use drugs (PWUD) with invasive Staphylococcus aureus infections had lower rates of antibiotic completion and twice the risk of 1-year infection persistence/recurrence compared to non-PWUD. Methicillin-resistant S. aureus was also associated with 1-year infection readmission.
In step with soaring rates of drug use and overdose in recent years [1, 2], hospitalizations for serious bacterial infections associated with drug use have increased by a factor of 2–10 in the past decade [3–5]. Staphylococcus aureus infections occur commonly in people who use drugs (PWUD) [6, 7] and confer substantial morbidity and mortality [8]. On average, PWUD hospitalized for these infections are younger, have fewer chronic medical conditions, and have higher rates of homelessness than persons not using drugs (non-PWUD) [9–12]. However, detailed comparative outcome data on S. aureus bacteremia and other invasive infections in PWUD vs non-PWUD are limited. So, while there are increasing numbers of invasive S. aureus infections among PWUD, there are comparatively few data defining outcomes among PWUD.
In this study, we sought to (1) characterize clinical and substance use characteristics of PWUD with invasive S. aureus infections, and (2) assess rates of antibiotic treatment completion, hospital readmission for ongoing or recurrent infection, and mortality of PWUD vs non-PWUD.
METHODS
Study Design, Setting, and Participants
We conducted a retrospective cohort study of patients hospitalized from 2013 to 2018 at San Francisco General Hospital, a 226-bed public county hospital/trauma center, and University of California, San Francisco Medical Center, a 600-bed tertiary care/referral hospital.
We identified all hospitalizations of adults (age ≥18 years) during which (1) S. aureus was isolated via blood cultures or culture of another sterile site (tissue, abscess, fluid, or bone) and (2) an International Classification of Diseases, Ninth or Tenth Revision (ICD) diagnosis code compatible with invasive S. aureus infection (bacteremia, endocarditis, epidural abscess, and/or vertebral osteomyelitis; Supplementary Appendix 1) was documented. Given the possibility of incomplete or inaccurate ICD coding, we also included hospitalizations in which S. aureus was isolated in ≥1 blood culture but lacked a compatible ICD code. For all hospitalizations, we conducted structured medical record reviews and excluded hospitalizations where S. aureus was either designated a contaminant and not treated, or did not involve a S. aureus infection of interest. This yielded an analysis set of hospitalizations of adults with S. aureus bacteremia, endocarditis, epidural abscess, and/or vertebral osteomyelitis from 2013 to 2018 (see Supplementary Appendix 2 for modified Consolidated Standards of Reporting Trials [CONSORT] diagram). A priori power calculation indicated that a sample size of 729 would be adequate to detect a 10% difference in readmission rates between groups with 80% power and α = .05.
Chart Review/Definitions
Four authors (A. A., M. A., S. L., J. D.) performed structured medical records review, with a subset of medical records jointly reviewed to ensure standardization of data extraction. We defined PWUD as persons with any nonprescribed substance use noted in hospital notes by the primary admitting team, infectious disease consultants, or social workers. We excluded cannabis, alcohol, and tobacco use from the PWUD definition. Type of drug, administration route, and timing of use were also recorded. We specified a priori our primary outcomes of antibiotic treatment completion, hospital readmission for infection persistence or recurrence, and death. Using local electronic medical record (EMR) data as well as available regional EMR data, we ascertained antibiotic treatment completion, as well as readmission for ongoing or recurrent S. aureus infection within 30 days and at 1 year. Antibiotic treatment was considered incomplete if noted as such in the EMR or if no subsequent records referencing treatment could be located in any health system. We ascertained mortality by medical record review and by cross-comparison to the California state death registry. Deaths occurring out of state were not ascertained.
Statistical Analysis
Demographic and clinical characteristics of PWUD and non-PWUD were summarized using median and interquartile range (IQR) for continuous variables and count and percentage for categorical variables. These characteristics were compared between groups using Wilcoxon rank-sum tests for continuous variables and χ 2 tests for categorical variables. Clinical outcomes and hospital treatments were similarly compared between groups.
To compare survival distributions for both recurrence of/readmission for infection within 1 year of discharge and all-cause mortality (measured from hospital admission to 1 year postdischarge), we examined univariate associations with time to each of the 2 outcomes individually for our primary predictor, drug use, and all variables considered to be potential confounders (including age, race, housing status, medical comorbidities, and methicillin-resistant S. aureus [MRSA] vs methicillin-susceptible S. aureus [MSSA]) using Cox proportional hazards models. We then created multivariable Cox proportional hazards models including variables that attained statistical significance in univariate analyses and had a plausible explanation for acting as confounders. We considered time origin for all-cause mortality to be time of admission to the hospital, in order to account for in-hospital deaths. For time to recurrence, however, those who died in-hospital were excluded, since they were incapable of experiencing a recurrence, and time origin was considered to be date of discharge. For mortality analyses, patients were censored at 1 year postdischarge if known to be alive at that time; if mortality 1-year postdischarge was unknown, they were censored at last time seen and known to be alive. For time to recurrence analyses, patients were censored at time of death (if death occurred before recurrence), at 1 year postdischarge (if known to be alive and recurrence-free at that point), and at last time seen and known to be recurrence-free (if data were otherwise unavailable). To account for the possibility that deaths among the study population represented competing risks for recurrence rather than censoring events, we also performed a sensitivity analysis using treating death as a competing risk, and calculated a sub–hazard ratio (HR) for infection persistence/recurrence. Finally, we performed a subgroup analysis evaluating 1-year mortality in PWUD who did vs did not complete antibiotic treatment.
Ethics Statement
The study protocol was approved by the University of California, San Francisco Committee on Human Subjects Research.
RESULTS
Demographics and Clinical Characteristics of PWUD and Non-PWUD with S. aureus Infections
We identified 1011 hospitalizations from 2013 to 2018 for S. aureus infections, and via structured medical record reviews identified 963 hospitalizations for invasive S. aureus infections that occurred in 946 unique individuals. Of these, 372 of 963 (39%) were among PWUD (Table 1). Compared to non-PWUD, PWUD were younger (median age, 50 vs 60 years; P < .001), more frequently experiencing homelessness (37% vs 5%; P < .001), had fewer chronic health conditions (median Charlson comorbidity index, 2 vs 4; P < .001), and had a higher prevalence of human immunodeficiency virus infection(18% vs 4%; P < .001). Of PWUD, 258 of 372 (69%) reported injection drug use and 264 of 372 (71%) reported drug use within the last month. PWUD reported opioid use (n = 214 [58%]), methamphetamine use (n = 185 [50%]), and concomitant opioid and stimulant use (n = 145 [39%]) (Table 1).
Table 1.
Demographic, Clinical, Substance Use, and Staphylococcus aureus Infection Characteristics Among Persons Who Use Drugs (PWUD) and Non-PWUD Individuals Hospitalized for Invasive Disease
| Characteristic | PWUD (n = 372) | No History of Drug Use (Non-PWUD) (n = 591) | P Valuea |
|---|---|---|---|
| Demographic | |||
| Age, y, median (IQR) | 50 (40–57) | 60 (50–71) | <.001b |
| Male sex | 264 (71) | 407 (69) | .49 |
| Race/ethnicity | <.001 | ||
| White | 218 (59) | 251 (43) | |
| Black/African American | 76 (20) | 79 (13) | |
| Hispanic/Latinx | 47 (13) | 88 (15) | |
| Asian/Pacific Islander | 8 (2) | 128 (22) | |
| Other | 23 (6) | 45 (8) | |
| Experiencing homelessness | 136 (37) | 27 (5) | <.001 |
| Clinical | |||
| Charlson comorbidity score, median (IQR) | 2 (0–4) | 4 (2–7) | <.001b |
| HIV positive | 68 (18) | 22 (4) | <.001 |
| Immunosuppressedc | 77 (21) | 165 (28) | .01 |
| History of hepatitis C infection | 177 (48) | 15 (3) | <.001 |
| Any mental health condition | 129 (35) | 148 (25) | .001 |
| Substance use | |||
| Injection route described | 258 (69) | … | |
| Recent drug use (<1 mo) | 264 (71) | … | |
| Drug type | |||
| Heroin | 198 (53) | … | |
| Any opioid | 214 (58) | … | |
| Cocaine | 139 (37) | … | |
| Methamphetamine | 185 (50) | … | |
| Opioid and stimulant | 145 (39) | … | |
| Other | 17 (5) | … | |
| Risky alcohol use/AUDd | 73 (20) | 69 (12) | .002 |
| S. aureus infection type | |||
| MRSA infection | 180 (48) | 183 (31) | <.001 |
| Type of infection | |||
| Bacteremia | 275 (74) | 514 (87) | <.001 |
| Endocarditis | 76 (20) | 70 (12) | <.001 |
| Vertebral osteomyelitis | 104 (28) | 76 (13) | <.001 |
| Epidural abscess | 92 (25) | 66 (11) | <.001 |
| Two or more of the above | 175 (47) | 142 (24) | <.001 |
| Pitt bacteremia score, median (IQR) | 1 (0–2) | 1 (0–2) | .56b |
| Duration of bacteremia, d, median (IQR)e | 3 (2–5) | 2 (2–4) | <.01b |
| ICU admission during hospital course | 145 (39) | 265 (45) | .001 |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AUD, alcohol use disorder; HIV, human immunodeficiency virus; ICU, intensive care unit; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; PWUD, people who use drugs.
a P values were calculated using χ 2 test unless otherwise indicated.
b P value calculated using Wilcoxon rank-sum test.
cImmunosuppressed defined as those with HIV, chronic exposure to daily prednisone ≥10 mg, biologic agents or disease-modifying anti-rheumatic drugs, hematologic malignancy, or chemotherapy.
dDiagnosed with AUD or noted to consume more than 3 or 4 alcoholic drinks daily in women and men, respectively.
eAmong those with bacteremia.
Among PWUD, 48% of infections were caused by MRSA, compared to 31% in non-PWUD (P < .001). PWUD had higher rates of deep-seated S. aureus infection compared to non-PWUD: 20% vs 12% had endocarditis (P < .001), 28% vs 13% had vertebral osteomyelitis (P < .001), and 25% vs 11% had epidural abscess (P < .001), respectively (Table 1).
Care Delivery
Planned parenteral antibiotic treatment duration for bacteremia, endocarditis, vertebral osteomyelitis, and epidural abscess followed established guidelines and did not differ between PWUD and non-PWUD groups (Table 2). Of patients without preexisting central venous access, fewer PWUD than non-PWUD received a peripherally inserted central catheter (PICC) for parenteral antibiotic treatment (65% vs 84%; P < .001).
Table 2.
Care Delivery During Hospitalizations of Persons Who Use Drugs (PWUD) and Non-PWUD
| Characteristic | PWUD (n = 372) |
Non-PWUD (n = 591) |
P Value |
|---|---|---|---|
| Planned parenteral antibiotic treatment | |||
| Bacteremia alone, median weeks (IQR) | 4 (2–6) | 4 (2–6) | .59a |
| Endocarditis, median weeks (IQR) | 6 (6–6) | 6 (6–6) | .35a |
| Vertebral osteomyelitis, median weeks (IQR) | 6 (6–6) | 6 (6–6) | .63a |
| Epidural abscess, median weeks (IQR) | 6 (6–6) | 6 (6–6) | .43a |
| Inpatient medications for OUD (persons reporting opioid use, n = 214) | |||
| New start methadone ≤40 mg | 36 (17) | … | |
| New start methadone >40 mg | 11 (5) | … | |
| New start buprenorphine | 6 (3) | … | |
| Prior methadone or buprenorphine continued | 63 (29) | … | |
| No inpatient treatment for OUD | 98 (46) | … | |
| Discharge planning | |||
| PICC placedb | 202/309 (65) | 334/400 (84) | <.001c |
| Discharge setting | <.001c | ||
| Remained inpatient for antibiotic course | 65 (18) | 64 (11) | |
| Skilled nursing facility | 157 (42) | 194 (33) | |
| Home | 48 (13) | 208 (35) | |
| Patient-directed discharge from hospital | 46 (12) | 7 (1) | |
| Otherd | 24 (6) | 23 (4) | |
| Died or transitioned to hospice in the hospital | 31 (8) | 95 (16) | |
| Patient-directed discharge (“AMA”) any time during antibiotic coursee | 80 (24) | 12 (2) | <.001c |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AMA, against medical advice; IQR, interquartile range; OUD, opioid use disorder; PICC, peripherally inserted central catheter.
a P value calculated using Wilcoxon rank-sum test.
bNot including those with preexisting central access, those who died, or those who transitioned to hospice in the hospital.
c P value calculated using χ 2 test.
dIncludes transfer back to referring hospital, discharge to medical respite (recuperative services for homeless persons), etc.
eDenominator in those who survived to hospital discharge, not on hospice: n = 340 in PWUD, n = 496 in non-PWUD.
Among PWUD reporting opioid use, 98 of 214 (46%) did not receive opioid use disorder or opioid withdrawal treatment in the hospital. Furthermore, only 53 of 214 (25%) had new medications for opioid use disorder (MOUD) started while hospitalized (Table 2). Most PWUD who did not die or transition to hospice in the hospital were discharged to a skilled nursing facility (157/340 [46%]) or remained in the hospital until their planned or patient-directed discharge (112/340 [33%]). Non-PWUD who did not die or transition to hospice in the hospital more often completed their antibiotic treatment at home (208/496 [42%]) or at a skilled nursing facility (194/496 [39%]). Almost 1 in 4 episodes of S. aureus infection care among PWUD involved a patient-directed discharge (ie, against medical advice, 24%), compared to only 2% of episodes of care in non-PWUD (Table 2; P < .001).
Treatment Completion, Readmission, and Mortality
Table 3 shows unadjusted primary and secondary outcomes. Of persons surviving to hospital discharge, 70% of PWUD vs 87% of non-PWUD completed antibiotic treatment (P < .001). At 1 year following hospital discharge, 28% of PWUD were readmitted to the hospital for ongoing or recurrent S. aureus infection vs 14% in non-PWUD (P < .001). Overall 1-year all-cause mortality was 18% in PWUD vs 30% in non-PWUD (P < .001).
Table 3.
Clinical Outcomes of Staphylococcus aureus Infections
| Outcome | PWUD (n = 340)a | Non-PWUD (n = 496)a |
P Valueb |
|---|---|---|---|
| Completed antibiotic treatment | 239/340 (70%) | 430/496 (87%) | <.001 |
| 30-day hospital readmission due to ongoing or recurrent Staphylococcus aureus infection | 72/340 (21%) | 54/496 (11%) | <.001 |
| 1-year hospital readmission for ongoing or recurrent S. aureus infection | 95/340 (28%) | 67/496 (14%) | <.001 |
| 1-year mortality | 67/372 (18%) | 175/591 (30%) | <.001 |
Abbreviation: PWUD, persons who use drugs.
aDenominator consists of those who survived to hospital discharge, not on hospice.
b P values calculated using χ 2 tests.
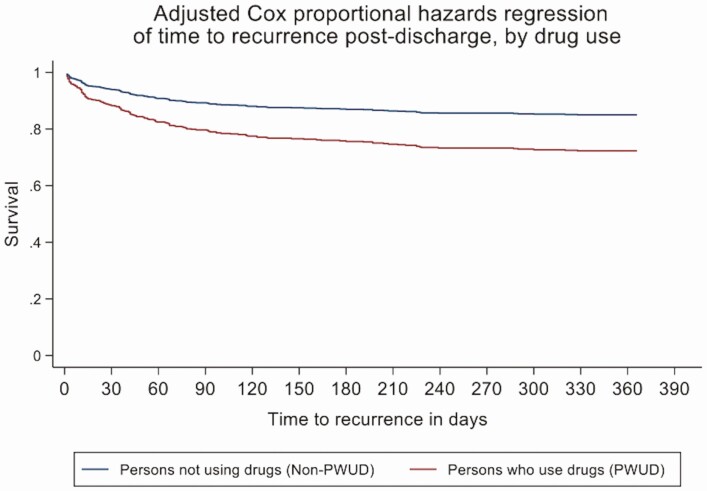
One-year survival without hospital readmission for S. aureus infection persistence or recurrence in PWUD vs non-PWUD is shown in Figure 1. PWUD had a 2-fold increased hazard of readmission for infection (adjusted HR [aHR], 2.00 [95% confidence interval {CI}: 1.36 – 2.94]; P < .001; Table 4). Patients with MRSA also had an independently increased hazard of readmission for infection (HR, 1.54 [95% CI: 1.12–2.12]; P = .01). Neither age, race, housing, nor medical comorbidities conferred excess hazard for readmission (Table 4). Results of analyses treating death without recurrence as a competing risk yielded results consistent with those in which subjects who died without recurrence were censored.
Figure 1.
Infection-related readmission-free survival in persons who use drugs (PWUD) vs non-PWUD: Cox proportional hazards regression. One-year survival without hospital readmission for Staphylococcus aureus infection persistence or recurrence in PWUD (red) vs non-PWUD (blue) demonstrates early and persistent increase in hazard of infection-related hospital readmission among PWUD (adjusted hazard ratio, 2.00 [95% confidence interval: 1.36 – 2.94]; P < .001).
Table 4.
Factors Associated With Hospital Readmission for Staphylococcus aureus Infection Persistence or Recurrence Within 1 Year of Hospital Discharge
| Univariate Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| Characteristic | HR | (95% CI) | P Value | HR | (95% CI) | P Value |
| Drug use | 2.30 | (1.68–3.15) | <.001 | 2.00 | (1.36–2.94) | <.001 |
| Age | 0.88 | (.80–.97) | .01 | 0.94 | (.82–1.08) | .37 |
| Sex | 0.94 | (.67–1.32) | .74 | 1.00 | (.71–1.40) | .99 |
| Race/ethnicity | ||||||
| White | Ref | … | … | … | ||
| Black | 1.03 | (.67–1.57) | .90 | 0.97 | (.63–1.49) | .89 |
| Hispanic/Latinx | 0.57 | (.33–.99) | .05 | 0.66 | (.38–1.15) | .14 |
| Asian/Pacific Islander | 1.00 | (.63–1.60) | 1.00 | 1.67 | (.99–2.80) | .05 |
| Native American | 3.02 | (1.11–8.23) | .03 | 2.48 | (.91–6.81) | .08 |
| Other | 0.62 | (.27–1.43) | .27 | 0.72 | (.31–1.65) | .44 |
| Experiencing homelessness | 1.83 | (1.29–2.61) | .001 | 1.24 | (.83–1.83) | .29 |
| Charlson comorbidity score | 0.94 | (.88–.99) | .03 | 1.01 | (.94–1.08) | .86 |
| HIV | 0.79 | (.64–.97) | .03 | 0.89 | (.71–1.12) | .33 |
| Any immunosuppression | 1.02 | (.71–1.46) | .92 | … | … | |
| Any mental health condition | 1.10 | (.78–1.53) | .59 | … | … | |
| MRSA | 1.72 | (1.26–3.24) | .001 | 1.54 | (1.12–2.12) | .01 |
| Duration of bacteremia | 1.00 | (.99–1.00) | .80 | … | … | |
| Received surgical procedure | 0.96 | (.70–1.31) | .81 | … | … | |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; MRSA, methicillin-resistant Staphylococcus aureus.
Time-to-event analysis focused on all-cause mortality in PWUD vs non-PWUD is shown in Supplementary Figure 2 (Supplementary Appendix 3). In this adjusted Cox proportional hazards model for mortality, drug use was not associated with increased hazard of 1-year mortality (aHR, 0.82 [95% CI: .59–1.15]; Supplementary Appendix 4), and only degree of comorbid illness was independently associated with increased hazard of death (aHR, 1.20 [95% CI: 1.14–1.26]; Supplementary Appendix 4).
In the subgroup of PWUD who survived to hospital discharge, we compared individuals who completed antibiotic therapy vs those who did not complete therapy. Unadjusted 1-year mortality following discharge among PWUD who did not complete antibiotic treatment was 27.6% (95% CI: 18.6%–39.9%) compared to 11.5% (95% CI: 2.9%–38.6%) in PWUD who successfully completed antibiotic treatment.
DISCUSSION
In this large cohort of patients hospitalized for invasive S. aureus infections at academic and public hospitals, we found that PWUD, compared to non-PWUD, had double the risk of readmission for infection persistence or recurrence 1 year after hospital discharge. MRSA infection was also independently associated with decreased readmission-free survival. Additionally, PWUD had markedly decreased antibiotic treatment completion and experienced differences in care delivery. Among PWUD, opioid and stimulant use were common, though substance use disorder treatment rates were low.
In this comparative outcomes study, we found that PWUD hospitalized with invasive S. aureus infections had double the hazard of hospital readmissions for ongoing or persistent infection compared to non-PWUD, though with no meaningful difference in mortality between groups. This finding of more frequent hospital readmissions coupled with comparable or lower rates of mortality among PWUD vs non-PWUD are consistent with prior studies on drug use–associated endocarditis [10, 11, 13]. The higher rate of infection persistence and recurrence we found did not appear to be due to prescription of inadequate antibiotic treatment regimens: in our study, we found no difference in planned antibiotic durations in PWUD vs non-PWUD. This is similar to findings of a smaller recent study evaluating quality metrics in care for PWUD with S. aureus bacteremia [14]. However, higher infection recurrence in our cohort did appear to be associated with higher frequency of antibiotic treatment interruptions and incomplete regimens. In our study, PWUD—who were overall younger and healthier though with more prevalent mental health conditions—were more likely to experience interrupted treatment, leading to ongoing need for treatment or to chronic infection or reinfection. The lower mortality observed among PWUD is consistent with known factors including younger age and fewer advanced medical comorbidities [10, 13].
The toll of S. aureus antibiotic treatment noncompletion is high and associated with increased healthcare costs [15, 16]. More research is needed to understand infection-related outcomes when antibiotics are delivered to PWUD via alternate methods such as oral antibiotic regimens [17], long-acting injectable lipoglycopeptide antibiotic regimens [18], and out-of-facility supported models. Likewise, more research is needed on integrating substance use disorder and infection treatment to create models of care that best fit patients’ needs.
Our study identified 2 care delivery–related factors that may contribute to higher rates of treatment noncompletion among PWUD. First, PWUD were less frequently offered antibiotics via PICC. In tandem, PWUD were less frequently discharged home; their therapy was more often delivered in the hospital. Together, these findings are consistent with existing literature showing that many physicians and home care providers have reservations about offering outpatient parenteral antibiotic therapy (OPAT) for PWUD [19, 20]. While there have been reports of infections related to PICC lines in OPAT patients [21] that underscore the importance of reliable PICC care during therapy, increasing literature supports the overall safety and effectiveness of home-based OPAT for PWUD [22–25]. This emphasizes the importance of providing patient-centered care to PWUD and non-PWUD alike. Our concomitant finding that PWUD had far higher rates of patient-directed discharges (ie, against medical advice) reflects the challenges of prolonged hospitalizations.
Another important difference in care delivery for PWUD was the infrequent provision of opioid use disorder treatment. Similar to previous reports on inpatient care for PWUD, we found that among patients using opioids but not previously on MOUD, these medications were infrequently prescribed. This is a known contributor to patient-directed discharges and other poor health outcomes [10, 26, 27]. Additionally, we found that, of patients initiated on methadone, most received lower doses more appropriate for physiologic withdrawal rather than MOUD treatment doses. This may have contributed to patient-directed discharges and antibiotic treatment noncompletion [27]. Importantly, these pharmacotherapy-based quality metrics do not incorporate the pervasive stigma and undertreated pain that PWUD face in acute care settings [28]. They also leave unaddressed the high prevalence in our sample of patients using stimulants, for whom limited pharmacologic treatments exist.
While substantial national attention has focused on the opioid epidemic given rates of opioid overdose [2] and opioid-related infections [3, 29], we found that stimulants, particularly methamphetamine, were involved in more than half of invasive S. aureus infections among PWUD. Patterns of drug use in the United States are distinctly regional. Data describing injection drug use (IDU)–related bacterial infections in Maine [10] and North Carolina [30] have noted prevalence of stimulant use to be only 9%–20%. However, consistent with our California data, a recent report from Florida described a 43% prevalence of stimulant use among patients hospitalized for IDU-related infections [31]. Many mechanisms likely contribute to stimulant-related infections. Methamphetamine use likely raises infection risk via bacterial inoculation of sterile tissue sites during injection drug use, via diminished innate and cellular immunity leading to increased susceptibility to S. aureus infection [32, 33], and via increased skin picking behavior. More research including PWUD is needed to elucidate these factors and their relative magnitudes.
For patients with stimulant use disorders, there is a limited evidence base for both pharmacotherapy and behavioral treatments (contingency management, cognitive behavioral therapy) in acute care settings, representing an important treatment gap. Given the rising level of use of both opioids and stimulants nationally (ie, the “fourth wave” of the opioid epidemic) [34, 35], infections related to stimulant use may rise in coming years. Therefore, it is crucial to gain a better understanding of infection pathophysiology and develop interventions to co-deliver therapy for stimulant use disorders and infections.
Finally, in addition to drug use, we also found that MRSA infection was independently associated with increased risk of hospital readmission for infection persistence or recurrence. Compared to MSSA infections, MRSA infections have been associated with poorer outcomes [36, 37], though there is debate about whether this is due to varying efficacy of anti-staphylococcal β-lactams and glycopeptides [38, 39], pathogen-related factors [40, 41], host factors, or confounders such as comorbid illnesses such as cancer and renal failure [36, 42, 43]. Our finding that MRSA infection was associated with increased readmission for infection is concordant with a 2014 study of >90 000 US patients with S. aureus bacteremia that found that MRSA infection raised the hazard of both infection-related readmissions, as well as in-hospital mortality. In that study, ICD-coded “drug abuse” was also associated with increased readmission rates [15]; however, only 10.5% of patients had an ICD code for drug abuse, suggesting that PWUD may have been underestimated. The challenge of ascertaining drug use by ICD codes alone is significant [44]. Last, given PWUD have higher rates of MRSA colonization and infection [7, 45, 46], our findings of lower antibiotic completion among PWUD as well as decreased readmission-free survival associated with both drug use and MRSA both suggest that incomplete MRSA treatments in PWUD may be particularly harmful.
Our study has several important limitations. First, our study population was comprised of patients hospitalized at a county and academic medical center in an urban West coast location. As such, our sample may overrepresent patients with homelessness and other socioeconomic hardship, stimulant use, and chronic illness. However, as stimulant use rises nationally, our results may be generalizable to more urban locations [34, 35]. Second, the accuracy of substance use histories documented in EMRs is challenging to assess. While data collected directly from patient interviews would be optimal, the medical record review methods we used have been shown to be more accurate than administrative coding data in recording comorbidities [47, 48]. Finally, it is possible that our retrospective medical record review–based study design may have underestimated or overestimated both treatment completion and mortality among PWUD. However, our medical record review method included use of our EMR’s CareEverywhere feature, allowing us to incorporate clinical follow-up data from regional medical centers. Additionally, we determined mortality not just from participants’ EMRs but also from the California death registry, thereby increasing accuracy.
To our knowledge, our study is among the largest retrospective cohort studies to date evaluating comparative outcomes among PWUD and non-PWUD hospitalized with invasive S. aureus infections. Among PWUD, we found low provision of substance use disorder treatment along with high rates of antibiotic treatment noncompletion and hospital readmission for infection, particularly those with MRSA infection. Given rising rates of serious bacterial infections among PWUD, our findings represent an urgent call to innovate, test, and implement more effective, patient-centered models for co-treatment of S. aureus infections and substance use disorders.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. Authors gratefully acknowledge Michael Jula, Joan “Kim” Stanley, and Lusha Wang for their assistance with data queries and refinement of our study sample.
Disclaimer. None of the funding entities had a role in the study design, implementation, analysis, or reporting of results.
Financial support. This work was supported by the National Institutes of Health (grant numbers 5T32AI007641-17 to A. A., K24DA042720 to P. O. C., and R25DA033211 to A. A.).
Potential conflicts of interest. S. B. D. and H. C. have received funding from the National Institutes of Health (grant number UM1AI104681). S. B. D. discloses receipt of consulting fees from Genentech and Basilea Pharmaceutica, outside the submitted work. P. O. C. reports study drug donations from Gilead Sciences, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users—United States, 2002–2013. MMWR Morb Mortal Wkly Rep 2015; 64:719–25. [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Understanding the epidemic: drug overdose.2020. Available at: https://www.cdc.gov/drugoverdose/epidemic/index.html. Accessed 20 August 2020.
- 3. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections from 2002 to 2012. Health Aff (Millwood) 2016; 35:832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schranz AJ, Fleischauer A, Chu VH, Wu L-T, Rosen DL. Trends in drug use– associated infective endocarditis and heart valve surgery, 2007 to 2017. Ann Intern Med 2018; 170:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCarthy NL, Baggs J, See I, et al. . Bacterial infections associated with substance use disorders, large cohort of United States hospitals, 2012–2017. Clin Infect Dis 2020; 71:e37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon RJ, Lowy FD. Bacterial infections in drug users. N Engl J Med 2005; 353:1945–54. [DOI] [PubMed] [Google Scholar]
- 7. Jackson KA. Invasive methicillin-resistant Staphylococcus aureus infections among persons who inject drugs—six sites, 2005–2016. MMWR Morb Mortal Wkly Rep 2018; 67:625–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wurcel AG, Anderson JE, Chui KKH, et al. . Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis 2016; 3:ofw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thakarar K, Rokas KE, Lucas FL, et al. . Mortality, morbidity, and cardiac surgery in injection drug use (IDU)-associated versus non-IDU infective endocarditis: the need to expand substance use disorder treatment and harm reduction services. PLoS One 2019; 14:e0225460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leahey PA, LaSalvia MT, Rosenthal ES, Karchmer AW, Rowley CF. High morbidity and mortality among patients with sentinel admission for injection drug use-related infective endocarditis. Open Forum Infect Dis 2019; 6:ofz089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chambers HF, Korzeniowski OM, Sande MA. Staphylococcus aureus endocarditis: clinical manifestations in addicts and nonaddicts. Medicine (Baltimore) 1983; 62:170–7. [PubMed] [Google Scholar]
- 13. Ruotsalainen E, Sammalkorpi K, Laine J, et al. . Clinical manifestations and outcome in Staphylococcus aureus endocarditis among injection drug users and nonaddicts: a prospective study of 74 patients. BMC Infect Dis 2006; 6:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Serota DP, Niehaus ED, Schechter MC, et al. . Disparity in quality of infectious disease vs addiction care among patients with injection drug use–associated Staphylococcus aureus bacteremia. Open Forum Infect Dis 2019; 6:ofz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inagaki K, Lucar J, Blackshear C, Hobbs CV. Methicillin-susceptible and methicillin-resistant Staphylococcus aureus bacteremia: nationwide estimates of 30-day readmission, in-hospital mortality, length of stay, and cost in the United States. Clin Infect Dis 2019; 69:2112–8. [DOI] [PubMed] [Google Scholar]
- 16. Kim JH, Fine DR, Li L, et al. . Disparities in United States hospitalizations for serious infections in patients with and without opioid use disorder: a nationwide observational study. PLoS Med 2020; 17:e1003247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marks LR, Liang SY, Muthulingam D, et al. . Evaluation of partial oral antibiotic treatment for persons who inject drugs and are hospitalized with invasive infections. Clin Infect Dis 2020; 71:e650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bryson-Cahn C, Beieler AM, Chan JD, Harrington RD, Dhanireddy S. Dalbavancin as secondary therapy for serious Staphylococcus aureus infections in a vulnerable patient population. Open Forum Infect Dis 2019; 6:ofz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rapoport AB, Fischer LS, Santibanez S, Beekmann SE, Polgreen PM, Rowley CF. Infectious diseases physicians’ perspectives regarding injection drug use and related infections, United States, 2017. Open Forum Infect Dis 2018; 5:ofy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fanucchi L, Leedy N, Fanucchi L, Li J, Thornton AC. Perceptions and practices of physicians regarding outpatient parenteral antibiotic therapy in persons who inject drugs. J Hosp Med 2016; 11:581–2. [DOI] [PubMed] [Google Scholar]
- 21. Rossow JA, Gharpure R, Brennan J, et al. . Injection drug use-associated candidemia: incidence, clinical features, and outcomes, East Tennessee, 2014–2018. J Infect Dis 2020; 222:S442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suzuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis 2018; 5:ofy194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Appa A, Marquez C, Jain V. Home-based outpatient parenteral antibiotic therapy at an urban safety net hospital: comparing outcomes in persons with and without noninjection drug use. Open Forum Infect Dis 2020; 7:ofaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Price CN, Solomon DA, Johnson JA, Montgomery MW, Martin B, Suzuki J. Feasibility and safety of outpatient parenteral antimicrobial therapy in conjunction with addiction treatment for people who inject drugs. J Infect Dis 2020; 222:494–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan C, Shojaei E, Wiener J, Shah M, Koivu S, Silverman M. Risk of new bloodstream infections and mortality among people who inject drugs with infective endocarditis. JAMA Netw Open 2020; 3:e2012974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barocas JA, Morgan JR, Wang J, McLoone D, Wurcel A, Stein MD. Outcomes associated with medications for opioid use disorder among persons hospitalized for infective endocarditis. Clin Infect Dis 2021; 72:472–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Suzuki J, Robinson D, Mosquera M, et al. . Impact of medications for opioid use disorder on discharge against medical advice among people who inject drugs hospitalized for infective endocarditis. Am J Addict 2020; 29:155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus 2020; 41:519–25. [DOI] [PubMed] [Google Scholar]
- 29. Coyle JR, Freeland M, Eckel ST, Hart AL. Trends in morbidity, mortality, and cost of hospitalizations associated with infectious disease sequelae of the opioid epidemic. J Infect Dis 2020; 222:451–7. [DOI] [PubMed] [Google Scholar]
- 30. Sredl M, Fleischauer AT, Moore Z, Rosen DL, Schranz AJ. Not just endocarditis: hospitalizations for selected invasive infections among persons with opioid and stimulant use diagnoses—North Carolina, 2010–2018. J Infect Dis 2020; 222:S458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serota DP, Bartholomew TS, Tookes HE. Evaluating differences in opioid and stimulant use-associated infectious disease hospitalizations in Florida, 2016–2017 [manuscript published online ahead of print 4 September 2020]. Clin Infect Dis 2021; 73:e1649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Potula R, Hawkins BJ, Cenna JM, et al. . Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J Immunol 2010; 185:2867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mihu MR, Roman-Sosa J, Varshney AK, et al. . Methamphetamine alters the antimicrobial efficacy of phagocytic cells during methicillin-resistant Staphylococcus aureus skin infection. mBio 2015; 6:e01622-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gladden RM, O’Donnell J, Mattson CL, Seth P. Changes in opioid-involved overdose deaths by opioid type and presence of benzodiazepines, cocaine, and methamphetamine—25 states, July–December 2017 to January–June 2018. MMWR Morb Mortal Wkly Rep 2019; 68:737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shearer RD, Howell BA, Bart G, Winkelman TNA. Substance use patterns and health profiles among US adults who use opioids, methamphetamine, or both, 2015–2018. Drug Alcohol Dependence 2020; 214:108162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hanberger H, Walther S, Leone M, et al. ; EPIC II Group of Investigators . Increased mortality associated with methicillin-resistant Staphylococcus aureus (MRSA) infection in the intensive care unit: results from the EPIC II study. Int J Antimicrob Agents 2011; 38:331–5. [DOI] [PubMed] [Google Scholar]
- 37. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003; 36:53–9. [DOI] [PubMed] [Google Scholar]
- 38. Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med 2006; 166:2138–44. [DOI] [PubMed] [Google Scholar]
- 39. Soriano A, Marco F, Martínez JA, et al. . Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46:193–200. [DOI] [PubMed] [Google Scholar]
- 40. Holmes NE, Turnidge JD, Munckhof WJ, et al. . Antibiotic choice may not explain poorer outcomes in patients with Staphylococcus aureus bacteremia and high vancomycin minimum inhibitory concentrations. J Infect Dis 2011; 204:340–7. [DOI] [PubMed] [Google Scholar]
- 41. Holland TL, Fowler VG Jr. Vancomycin minimum inhibitory concentration and outcome in patients with Staphylococcus aureus bacteremia: pearl or pellet? J Infect Dis 2011; 204:329–31. [DOI] [PubMed] [Google Scholar]
- 42. Bassetti M, Peghin M, Trecarichi EM, et al. . Characteristics of Staphylococcus aureus bacteraemia and predictors of early and late mortality. PLoS One 2017; 12:e0170236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guillamet MCV, Vazquez R, Deaton B, Shroba J, Vazquez L, Mercier R-C. Host-pathogen-treatment triad: host factors matter most in methicillin-resistant Staphylococcus aureus bacteremia outcomes. Antimicrob Agents Chemother 2018; 62:e01902-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quan H, Li B, Saunders LD, et al. ; IMECCHI Investigators . Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res 2008; 43:1424–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fleisch F, Zbinden R, Vanoli C, Ruef C. Epidemic spread of a single clone of methicillin-resistant Staphylococcus aureus among injection drug users in Zurich, Switzerland. Clin Infect Dis 2001; 32:581–6. [DOI] [PubMed] [Google Scholar]
- 46. Dahlman D, Berge J, Nilsson AC, Kral AH, Bjorkman P, Hakansson AC. Opioid and amphetamine dependence is associated with methicillin-resistant Staphylococcus aureus (MRSA): an epidemiological register study with 73 201 Swedish in- and outpatients 1997–2013. Infect Dis 2017; 49:120–7. [DOI] [PubMed] [Google Scholar]
- 47. Chong WF, Ding YY, Heng BH. A comparison of comorbidities obtained from hospital administrative data and medical charts in older patients with pneumonia. BMC Health Serv Res 2011; 11:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Marks LR, Nolan NS, Jiang L, Muthulingam D, Liang SY, Durkin MJ. Use of ICD-10 codes for identification of injection drug use–associated infective endocarditis is nonspecific and obscures critical findings on impact of medications for opioid use disorder. Open Forum Infect Dis 2020; 7:ofaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.