Abstract
Background
Wearable sensors, particularly accelerometers alone or combined with gyroscopes and magnetometers in an inertial measurement unit (IMU), are a logical alternative for gait analysis. While issues with intrusive and complex sensor placement limit practicality of multi-point IMU systems, single-point IMUs could potentially maximize patient compliance and allow inconspicuous monitoring in daily-living. Therefore, this review aimed to examine the validity of single-point IMUs for gait metrics analysis and identify studies employing them for clinical applications.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Guidelines (PRISMA) were followed utilizing the following databases: PubMed; MEDLINE; EMBASE and Cochrane. Four databases were systematically searched to obtain relevant journal articles focusing on the measurement of gait metrics using single-point IMU sensors.
Results
A total of 90 articles were selected for inclusion. Critical analysis of studies was conducted, and data collected included: sensor type(s); sensor placement; study aim(s); study conclusion(s); gait metrics and methods; and clinical application. Validation research primarily focuses on lower trunk sensors in healthy cohorts. Clinical applications focus on diagnosis and severity assessment, rehabilitation and intervention efficacy and delineating pathological subjects from healthy controls.
Discussion
This review has demonstrated the validity of single-point IMUs for gait metrics analysis and their ability to assist in clinical scenarios. Further validation for continuous monitoring in daily living scenarios and performance in pathological cohorts is required before commercial and clinical uptake can be expected.
Keywords: Accelerometry, gait analysis, wearable electronic devices
Introduction
Human gait is affected by ageing as well as numerous musculoskeletal and neurological ailments. Consequently, gait analysis has wide-ranging clinical applications from diagnosis and severity assessment as well as evaluation of intervention and rehabilitation efficacy in neurological and orthopedic conditions (1-8) to identifying falls risk and frailty status (9,10). Qualitative and subjective measures generally constitute routine clinical gait analysis, with patient self-reporting and clinician observation sometimes integrated with clinical tests such as the Timed-Up-And-Go and 6-minute walking test (6MWT) (11). These approaches impose significant interobserver inaccuracies and deny appreciation of kinematic and kinetic intricacies that can be obtained from quantitative gait assessment (12).
The gold-standard for quantitative gait analysis, optoelectronic stereophotogrammetry, features infrared cameras that capture three-dimensional trajectories of reflective markers placed on the subject that are processed to accurately assess spatio-temporal and kinematic variables of gait (13). Stereophotogrammetry is often combined with force plates that measure ground-reaction forces (GRF) to determine kinetic forces and electromyography (EMG) systems to measure muscle activity during gait. However, these systems are expensive, time-consuming, and require expert operation and equipment. Furthermore, restriction of their performance to dedicated laboratory settings limits portability, access and external validity of measures obtained to free-living gait (11).
In response, wearable sensors (goniometers, EMG systems, sensing fabric etc.), particularly accelerometers alone or combined with gyroscopes and magnetometers in an inertial measurement unit (IMU), are proving to be the logical alternative for gait analysis. Cheap, small and portable, wearables could potentially enable continuous gait metrics analysis in daily living (14-17). Furthermore, fast preparation and processing negate the need for expert operation, enhancing practicality. Multi-point IMU systems have been validated against standards (18-23) and employed clinically for gait metrics analysis (24-28); however, issues with intrusiveness and consistency of complex sensor placement limit real-life adoption (29). Although not as accurate and reliable as multi-point IMU systems (30), single-point IMUs have nonetheless demonstrated enormous potential. They have clinical uses, such as with the assessment of Parkinson’s Disease (PD) severity (31,32) and the evaluation of falls risk in preventative health care (33,34), and personal uses, such as with the tallying of daily steps in consumer-grade watches (4,35). Commonly used single-point IMUs and their specifications are detailed for comparison in Table 1.
Table 1. Common single-point IMUs and their specifications.
| References | Sensor | Placement | Gait metrics demonstrated to be captured | Component MEMS sensors | Other known specifications |
|---|---|---|---|---|---|
| (31,36-40) | Locometrix | Lower back, L3–4 | GV and stride frequency, length, symmetry and regularity | Tri-axial accelerometer | 100 Hz frequency of data capture, resolution 0.001 g |
| (41-45) | MT product line IMUs (Xsens, Enschede, The Netherlands) | Lower back, L4 | MTx was able to capture GV, cadence, step regularity, stride regularity, gait symmetry, step time, step time variability, RMS and HR | Tri-axial accelerometer, gyroscope, and magnetometer | According to MTi (newer than MTx) specifications: Output frequency up to 2 kHz. Accelerometer range 16 g. Gyroscope range 2,000 deg/s. 12.1×12.1×2.55 mm3 (without encasing). 0.6 grams (without encasing) |
| (46,47) | Pi-node (Philips, The Netherlands) | Lower back, L4 | GV, step time, step time variability, cadence, stride length, stride length variability and non-linear measures (gait variability and symmetry) | Tri-axial accelerometer | 100 Hz frequency of data capture |
| (48-59) | DynaPort IMUs (McRoberts, The Hague, The Netherlands) | Lower back | Step count, GV, stride time variability, stride regularity and cadence | Tri-axial accelerometer, and gyroscope | 100 Hz frequency of data capture. 106.6×58×11.5 mm3. 55 grams. 14-day maximum measurement duration |
| (60-64) | G-walk (BTS Bioengineering Milan, Italy) | Lower back, L5 | GV, SL, stride length and cadence. Gait cycle duration, stance duration, swing duration and double support duration | Tri-axial accelerometer, gyroscope, and magnetometer | Accelerometer range 2, 4, 8, or 16 g (configurable). Gyroscope range 250, 500, 1,000, or 2,000 deg/s (configurable). Magnetometer range ±1,200 uT. Accelerometer sampling rate 4–1,000 Hz (configurable). Gyroscope sampling rate 4 to 8,000 Hz (configurable). 37 grams |
| (65,66) | Physilog product line (Gaitup, Lausanne, Switzerland) | Chest, 5 cm below sternal notch | GV, non-linear measures (gait stability), RMS and walk-ratio | Tri-axial accelerometer and gyroscope | According to Physilog5 specifications: Accelerometer range 16 g. Gyroscope up to 2,000 deg/s. 26.5×10×47.5 mm3 |
| (67,68) | GENEActiv (Activinsights, Kimbolton, England) | Lower back or wrist | Step count, stride time, stride time variability, step time, step time variability and non-linear measures (gait variability) | Tri-axial accelerometer | 100 Hz sampling frequency |
| (34,69-71) | Axivity AX3 (Axivity, York, England) | Lower back, L5 | Step count, mean bout length, step time, swing time, stance time, step time asymmetry, swing time asymmetry, stance time asymmetry, SL asymmetry, step velocity, SL, swing time variability, step time variability, stance time variability, GV and SL variability | Tri-axial accelerometer | Up to 8 g. 100 Hz. Waterproof. 23×32.5×7.6 mm3, 11 g weight. Accelerometer range 2, 4, 8, or 16 g (configurable). Accelerometer sampling rate 12.5–3,200 Hz (configurable) |
| (72) | MIMAMORI-Gait system (LSI Medience Corp, Tokyo, Japan) | Back of waist | GV, SL, cadence, step time, step time variability and double support time | Tri-axial accelerometer | 100 Hz frequency of data capture 75×50×20 mm3, 120 grams |
| (73) | activPAL (PAL Technologies, Glasgow, Scotland) | Upper thigh | Step count, cadence | Uni-axial accelerometer | 20 Hz frequency of data capture. 53.0×35.0×7.0 mm3. 20 grams |
| (74,75) | Actigraph wGT3X-BT activity monitor (Actigraph, Pensacola, FL, USA) | Lateral waist | Cadence, stride regularity and non-linear measures (intensity, dynamic stability) | Tri-axial accelerometer | 50 Hz frequency of data capture. Acceleration range 8 g |
| (76,77) | e-AR sensor | Behind ear | Stride duration and step time asymmetry. Gait asymmetry | Tri-axial accelerometer | |
| (4) | Apple Watch (Apple, San Francisco, CA, USA) | Wrist | Daily step count, gait velocity, estimated caloric expenditure, distance travelled | Tri-axial accelerometer and gyroscope |
IMU, inertial measurement unit; g, magnitude of acceleration due to gravity, 9.8 m/s2; MEMS, microelectromechanical systems; GV, gait velocity; SL, step length; RMS, root-mean square; HR, harmonic ratio.
Moreover, presuming validated accuracy is demonstrated, single-point IMUs could potentially maximize patient compliance and comfort and allow inconspicuous monitoring in daily-living. Consequently, any future mass uptake of IMUs in clinical and commercial settings is likely to be dependent upon the validation and applications of single-point IMUs and not of multi-point IMU systems. Therefore, this review will provide a synopsis of inertial sensor principles, practical considerations and gait metrics analysis capabilities before examining the validity and clinical applications of single-point devices for gait analysis. We present the article in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Guidelines (PRISMA) checklist (available at https://dx.doi.org/10.21037/mhealth-21-17).
Inertial sensors: principles and practical considerations
Inertial sensors, accelerometers and gyroscopes, are often fabricated into microelectromechanical systems (MEMS) alone or together as an IMU and employed for gait metrics analysis (78).
Accelerometers measure acceleration along their sensitive axis, ranging from uni-axial to the commonly employed tri-axial sensitivity which allows appreciation of movement along the antero-posterior, horizontal and vertical planes. However, current devices are susceptible to drift errors due to change in mechanical or electrical properties and noise from amplified mechanical motions. Furthermore, the measured acceleration comprises both the inertial acceleration associated with changes in velocity, and gravitational acceleration superimposed along the accelerometer’s sensitive axes. Removing this confounding effect of gravity can be difficult (78). To appreciate velocity and distance, numerical integration of acceleration data is required, causing noise and drift errors to accumulate, imposing significant limitations on long-term accelerometer employment. Without compensation for this drift, readings become useless. Compensation requires frequent accelerometer recalibration, achieved through zero-velocity updates (ZUPT), using an external event indicating an instantaneous null in movement such as a footstep (79).
Gyroscopes measure angular velocity, demonstrating greater accuracy than accelerometers as measurement is absolute with no external information considered (80). However, gyroscopes only return rate of change of angular position; to detect relative orientation, integration of the signal is required. This leads to accumulation of drift errors and noise, similar to accelerometers (80). Furthermore, lack of an initial reference compared to accelerometers means gyroscopes cannot be recalibrated, resulting in accumulation of errors and limited long-term precision (80). This limitation is often minimized by incorporating a magnetometer in the IMU, able to calibrate sensor orientation with reference to the Earth’s magnetic field. However, these devices are prone to interference by magnetic fields created by other devices (80).
Inertial sensor-based gait analysis
While single-point inertial sensors are unable to appreciate kinetic and many kinematic variables of gait, they can determine spatio-temporal parameters. Spatio-temporal parameters are of importance clinically, as they objectively characterize key gait events (GE) and common gait abnormalities (81). A plethora of spatio-temporal parameters are employed in the literature, with some [such as gait velocity (GV) and gait regularity in predicting the staging of PD severity] being more relevant than others in different clinical scenarios (31,32). This review focusses on spatio-temporal parameters based on a validated model (82,83) and clinical guidelines from The Biomathics and Canadian Gait Consortiums Initiative (84). These parameters encompass the mean, variability and asymmetry of temporal (cadence, step time, stride time, stance duration, swing duration, single-support duration, double-support duration) and spatial [step length (SL), stride length, GV step width] components of gait.
As acceleration data retains a time-series nature when extracted, by determining GEs such as heel-strike (HS) and toe-off (TO) within the gait cycle, mean temporal parameters can be quantified. Methods for GE detection are based on signal feature extraction of peaks, valleys or zero-crossings from raw accelerometric or gyroscopic data that may indicate a HS or TO (78). This can be complemented by applying hidden Markov models or Gaussian continuous wavelet-transformation (CWT) to increase GE detection accuracy (29). These methods have been implemented for single sensors placed on the trunk (85-87), waist (88,89), shank (90), ear (76) and foot (91,92). A thigh-based single-point IMU, the Activpal (PAL Technologies, Glasgow, Scotland), has also been used to measure mobility (73,93). However, this sensor has, to our knowledge, not analysed gait metrics beyond step identification in a single-point system.
Spatial parameter estimation proves more difficult due to the aforementioned technical limitations of inertial sensors (29). Current methods are based on abstraction models (e.g., machine-learning, linear regressive models), locomotion models [e.g., inverted pendulum (IP), double-IP] and numerical integration. Those employed for single-point sensors include: direction integration (88), linear regression models (89), IP model with double-integration of antero-posterior (86,87) or vertical acceleration from trunk sensors (85); IP model with double integration of AP-acceleration from a foot sensor (91); double-IP model with integration of angular velocity of the shank (90); and autocorrelation procedures also able to determine temporal parameters, and a measure of regularity and symmetry (94). However, the requirement of numerical integration in these models causes accumulation of drift errors (78). Drift compensation is performed using kinematical reset through ZUPT by assuming foot velocity as zero (91) and shank inclination as vertical during midstance (90); however, these methods only prevent growth of drift error without minimizing the already accumulated error (29). As zero-velocity reset is not possible with trunk sensors due to continuous pelvic motion, drift correction is achieved by applying a high-pass (86) or Kalman filter (88) to retrospectively correct errors, occasionally in combination with direct and inverse integration at every step (88). However, correction efficacy may be limited in pathological gait where vertical trunk acceleration amplitude is lower and variability higher (78).
Linear measures of spatio-temporal variability and asymmetry are subsequently determined from mean spatio-temporal values, commonly expressed as the standard deviation or coefficient of variation and the absolute difference between left and right mean values respectively (95).
In addition to these traditional spatio-temporal parameters, accelerometer-based systems and non-linear calculations introduce new measures (11). Although these complex non-linear, autocorrelation and acceleration-based measures are dimensionless and unable to be validated against a standard, they are employed extensively clinically. Non-linear measures derived from the theory of stochastic dynamics (e.g., phase plot analysis, fractal-scaling index, sample entropy, Lyapunov exponents) allow appreciation of dynamic fluctuations and patterns between gait cycles throughout a walking bout, contrary to traditional linear measures that treat each as independent to the last (11,49,83). These measures represent the smoothness, regularity, stability, variability, complexity and symmetry of gait, showing sensitivity delineating between pathological and healthy subjects (96-98) equal, or superior to the sensitivity of linear measures (99). Similarly, autocorrelation measures of regularity and symmetry represent a clinically relevant (31,44,67), dynamic substitute for spatio-temporal variability and symmetry respectively (36,94). Other measures, including harmonic ratio (HR) based on Fourier analysis and the root-mean square (RMS) of acceleration magnitudes, are clinically relevant indicators of the smoothness, rhythmicity and symmetry of gait (33,100-102).
Methods
Literature Search
The PRISMA guidelines were followed for this systematic review (103) utilizing the following databases: PubMed; MEDLINE; EMBASE; Cochrane. Firstly, key search terms “gait” AND “accelerometer or inertial” were used to locate studies using inertial sensors to monitor gait. Next, “spatiotemporal” or “temporal” or “phase” or “stride” or “length” or “velocity” were used to locate publications that measure clinically relevant gait metrics beyond step-count and activity. Finally, the terms “clinical or valid or validity or test or reference or standard” were included to reflect studies that had tested the validity of these wearable technologies or applied them clinically. Relevant MeSH (Medical Subject Heading) terms, variations and synonyms were adjusted for each database.
Study selection
Studies from the above databases were collated and duplicate studies removed. Primary screening by an independent reviewer (JP) was performed based on the title and abstract of the remaining studies following the developed inclusion and exclusion criteria detailed below. Subsequent eligibility assessment was performed based on the full texts of remaining articles by an independent reviewer (JP) following the inclusion and exclusion criteria.
Inclusion criteria
Articles involving wearable technology/ies.
The wearable technology features an inertial sensor (accelerometer, gyroscope) or is an IMU.
The wearable technology is a single-point sensor.
Articles written in English.
Journal papers.
Exclusion criteria
Wearable technology/ies only capable of identifying activity or step count.
Wearable technology/ies features multiple sensor points.
Wearable technology/ies classed as robotic or exoskeletons.
Systematic reviews, books, or conference papers.
Data collection
Following final article selection, results were classified as validation, clinical application or both. Data for validation studies was collected including sensor type(s); sensor placement; study aim(s); conclusions of study; primary gait metrics and methods. Critical analysis of validation studies was also included. Data collected for clinical applications studies included: sensor type(s); sensor placement; primary gait metrics and methods; and clinical application.
Bias analysis
Three different tools were used for risk of bias assessment based on the nature of the studies. Validation and reliability studies were assessed using the Scottish Intercollegiate Guidelines Network (SIGN) methodology checklist for diagnostic tests (104,105). Of the clinical application studies, observational studies were assessed using the Newcastle-Ottawa Scale (106) while randomized-controlled trials (RCTs) were assessed using the SIGN checklist for RCTs (105,107). Case series and case reports were excluded from the use of these bias assessment scales as questions pertaining to comparability no longer apply. Studies were assessed by 3 independent reviewers (WJC), (SMR) and (MM) with at least 2 different reviewers for each study. Discrepancies in assessment were resolved by discussion and reaching a consensus.
Results
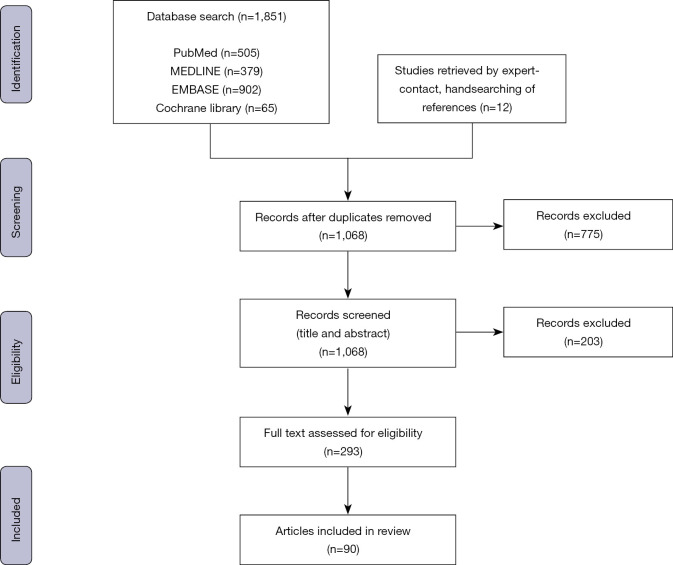
From the 1,068 articles retrieved after duplicate removal, 90 articles were selected for inclusion (Figure 1). Thirty-two articles assessed the validity of single-point sensors and 48 used single-point sensor gait metrics analysis for clinical applications. Ten articles concurrently assessed validity and employed the device in a clinical application.
Figure 1.
PRISMA methodology.
Validity of single-point sensors
Among the 42 articles (Figure 2), 30 studies utilized trunk-based sensor methods; while 12 studies used alternate locations for the sensor placement which include four studies at the subjects’ ear; two at the subjects’ shank, two at the subjects’ foot, one at the waist; while three other studies utilized smart devices with inertial sensors. The parameters used for validating the sensors include: HS, stride length/duration/regularity, SL/count/duration/length variability/variability/time asymmetry/cycle time/regularity/frequency, GV, cadence, traversed distance, walking time, stance duration, swing duration, single/double support duration, HR, TO and time averaged acceleration.
Figure 2.
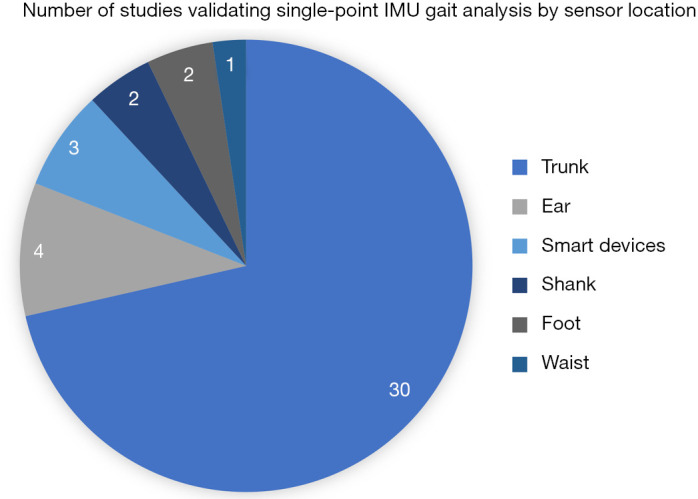
Number of studies validating single-point IMU gait metrics analysis by sensor location. Smart devices have been attached on various locations, some separate to where they were designed to be worn – the iPod touch G4 (iOS 6, Apple Inc.) over L3 (108), the Apple Watch (Apple, San Francisco, CA, USA) on the wrist (4), and the Apple iPhone 5 (iPhone 5, Apple Inc., Cupertino, CA, USA) on the lateral waist (109). IMU, inertial measurement unit.
Validation studies by subject cohort
Among the 42 studies, 59 different cohort of patient population were studied (Figure 3). Twenty-five studies involved healthy adults, 11 studies involved elderly subjects, seven studies involved patients with PD, four studies in post-stroke patients with ataxia and three studies in Huntington’s Disease (HD). There were two studies each for patients with lower limb amputees and diabetic patients. The remainders were single studies in subjects comprising healthy children, multiple sclerosis (MS) patients, orthopedic patients, muscular dystrophy patients, and patients suffering from motor neuron disease (Table S1).
Figure 3.
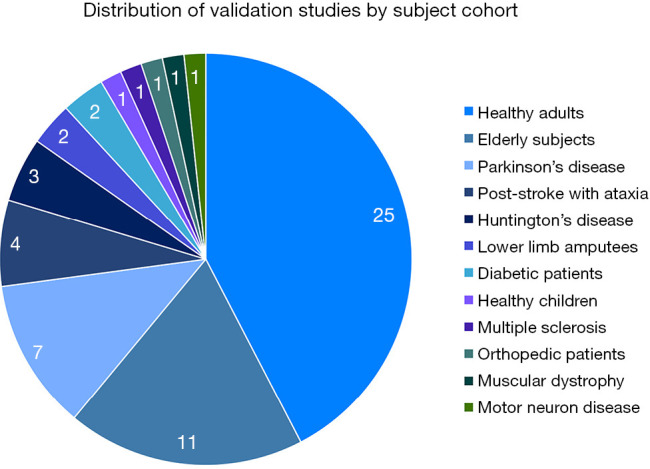
Distribution of validation studies by subject cohort.
Clinical application of single-point IMUs
Fifty-eight articles discussed the use of single-point IMUs in clinical setting. Of these, 12 articles discussed the application of sensors in diagnosis and assessing severity of diseases (PD, MS, PN, Alzheimer’s disease, age-related changes, frailty and foot & ankle health); 12 studies applied the sensors in monitoring rehabilitation and intervention efficacy (orthopedic, neurosurgical and oncological patients, foot orthoses, medical and physical intervention in neurodegenerative diseases); and 31 studies used sensors to characterize patients with different conditions from healthy subjects. Three articles described both diagnosis and severity of assessment as well as delineating healthy and participants with pathologies (Table 2, with more detailed findings of these articles in (Tables S1,S2).
Table 2. Clinical applications of single-point IMUs.
| Reference | Application |
|---|---|
| Diagnosis and severity assessment | |
| Demonceau et al., 2015 (31) | Determine PD severity |
| Herman et al., 2014 (57) | Classify PD subtypes |
| Dalton et al., 2013 Collett et al., 2014 Pau et al., 2016(47,62,110) |
Determine MS severity |
| Esser et al., 2018 (111) | Detect PN |
| De Bruin et al., 2012 (53) | Determine PN severity |
| Gillain et al., 2016 (39) | Predict risk of Alzheimer’s development |
| Kosse et al., 2016 Terrier et al., 2015(65,108) |
Predict age-related gait change |
| Soangra et al., 2018 Martinez-Ramirez et al., 2015 (43,109) |
Predict frailty status/determine severity |
| Van Schooten et al., 2016 Del Din et al., 2017 (33,34) |
Predict falls/determine falls risk |
| Angthong et al., 2018 (112) | Assessment of foot/ankle conditions |
| Rehabilitation and intervention efficacy | |
| Atallah et al., 2014 Rapp et al., 2015 (76,113) |
Total hip and knee replacement efficacy and recovery |
| Jarchi et al., 2016 (77) | Anterior cruciate ligament repair recovery |
| Mobbs et al., 2018 (4) | Lumbar microdiscectomy recovery |
| Hojan et al., 2014 (54) | Effect of breast prostheses after mastectomy |
| Mutoh et al., 2016 Manikowska et al., 2013(5,56) |
Hippotherapy efficacy in cerebral palsy |
| Henderson et al., 2016 (114) | Rivastigmine efficacy in PD |
| Terrier et al., 2009 (115) | Prescription footwear efficacy in foot/ankle fractures |
| Doi et al., 2013 Pau et al., 2014 Perrochon et al., 2015(38,60,101) |
Improvement in gait after physical activity in elderly and cognitive impairment |
| Delineating pathological subjects from healthy controls | |
| Barden et al., 2016 Clermont et al., 2016 Bolink et al., 2012 (67,68,116) |
Knee osteoarthritis subjects |
| Lamoth et al., 2010 Houdijk et al., 2008 (48,49) |
Amputee subjects |
| Terrier et al., 2017 (74) | Chronic lower limb pain subjects |
| Arvin et al., 2015 (58) | Hip abductor fatigue Subjects |
| Esser et al., 2011 Mizuike et al., 2009 Meijer et al., 2011 (41,52,102) |
Muscular dystrophy/Motor neuron disease/Stroke subjects |
| Hickey et al., 2016 Matsushima et al., 2015 (70, 117) |
Ataxia disorder subjects |
| Demonceau et al., 2015 Del Din et al., 2016 Esser et al., 2013 Hatanaka et al., 2016 Yang et al., 2011 (31,46,69,72,118) |
PD/Progressive supranuclear palsy subjects |
| Pau et al., 2017 Pau et al., 2018 Storm et al., 2018 (63,119,120) |
MS subjects |
| Dalton et al., 2013 Collett et al., 2014 (47,110) |
HD subjects |
| Manikowska et al., 2013 (55) | Menopausal women |
| Tanigawa et al., 2018 (121) | Pregnant patients with lumbopelvic pain |
| Iosa et al., 2013 Saether et al., 2014(42,78) |
Cerebral palsy subjects |
| Moe-Nilssen et al., 2003 (122) | Dyslexia subjects |
| Awotidebe et al., 2016 (61) | Type 2 diabetes subjects |
| Chung et al., 2012 Maquet et al., 2010 Martinez-Ramirez et al., 2016 Lamoth et al., 2011(37,44,51,123) |
Cognitively impaired subjects |
| Auvinet et al., 2003 Bautmans et al., 2011 (36,50) |
Falls risk/fallers |
IMU, inertial measurement unit; PD, Parkinson’s Disease; MS, multiple sclerosis; PN, peripheral neuropathy; HD, Huntington’s Disease. Detailed findings of these articles can be found in (Tables S1,S2)
Bias assessment
Risk of bias assessment of validity studies did not reveal studies with an unacceptable level of bias. 31 studies were of high quality while nine were of acceptable quality in minimizing bias. The breakdown of the assessment and interpretation is included in Table S3. Areas in which many studies had ‘unclear’ levels of bias were in the patient selection domain criteria of selecting a consecutive sequence or random selection of participants. Understandably, many of these studies had small sample sizes and recruited volunteers or practiced convenience sampling of patients to achieve this. Many studies were able to reduce bias by conducting simultaneous testing of the IMU and reference standard. Criteria related to the use of a reference standard were not applied to reliability studies that did not use a reference test. The criteria related to pre-specified thresholds of the index test were also largely not applicable.
Of the clinical applicability studies, 30 were scored as “good” quality, eight as “fair” and 12 as “poor”. Two RCTs were deemed having acceptable quality and one RCT as high quality in minimizing bias. The breakdown of the bias assessment results and interpretation is included in Appendix 1. Most studies generally missed out a score on ascertainment of outcome using a blind investigation. This may be attributed to a lack of investigators needed to separate carrying out the test and interpreting data. However, this was regarded as not having a large influence on overall bias assessment as measurements using IMUs are automatically recorded to software and not requiring direct human measurement. It would also be difficult to blind assessors to diseased patients with an obvious gait pathology to healthy controls. The RCTs also had unclear blinding of subjects and investigators to treatment groups. The strengths of these studies were the randomization process and standardization of testing and analysis between treatment and control groups.
Discussion
In our review, single-point IMUs have been reasonably validated in the measurement of spatial and temporal gait parameters (85,86,124). However, IMUs have shown difficulty in estimating variability and asymmetry metrics (48,70,118,125-127). Alongside this, whilst IMUs have shown promise in their clinical applications, such as in the diagnosis of disease (31,32,47,53) and the assessment of treatment efficacy (56,113,128), these studies have predominantly relied on straight-line gait metrics. This critically limits external validity to free-living analysis where day-to-day movements typically represent more complex patterns of acceleration and deceleration. Moreover, studies focusing on clinical application have predominantly described obvious gait changes and have not necessarily demonstrated IMUs to be useful in evaluating subtle differences in gait patterns. Therefore, additional studies focusing on validation and clinical application are required before any mass clinical and commercial uptake of single-point wearable sensors can occur.
Validating IMU gait metrics analysis
Proposed methods and sensor locations for determination of gait metrics from single-point IMU acceleration data are generally validated against a standard or by test-retest reliability (129). The large portion of validation research employing lower trunk sensors compared with alternative sensor locations is reflected in Figure 2.
Trunk-based sensor methods
Association of lower trunk accelerations with HS and TO, and the ability to predict these accelerations with an IP model of the body’s center of mass (COM) trajectory, has prompted the proposal of several methods for GE and spatio-temporal parameter estimation from these signals.
Methods by Zijlstra & Hof (86)
Peak detection and IP methods proposed by Zijlstra & Hof (86) utilizing a tri-axial accelerometer over the lower trunk demonstrated accurate detection of GEs and limited mean spatio-temporal parameters when compared to GRF from a treadmill. However, the straight-walking protocol employed limits external validity to daily-living analysis. Similarly, 100% of GEs were detected in only nine subjects of a small sample (n=15) of healthy adults, with 12% of GEs identified falsely in the remaining subjects. Furthermore, SL calculation which requires input of individual leg-length was consistently underestimated before application of a 1.25 correction factor, revealing limitations in the model itself. Further validation in healthy adults has demonstrated accurate estimation of mean spatio-temporal parameters when compared to stereophotogrammetry and dynamometry, however significant differences were found for gait phase durations that rely on determining TO which isn’t explicitly detected by the model (124). SL estimation was again detected with less accuracy due to errors implied by double-integration in this method (124). Further model limitations were identified in neurological populations (41) as neither a generic (1.25) or pathology-specific correction factor could be applied for accurate SL estimation, alluding to the need for individual corrections in pathological cohorts. This finding was reflected when validated in child (130) and older cohorts (131). The model has been further validated in small samples of PD subjects against motion capture (132), MS subjects against an instrumented walkway (110) and through test-retest reliability (47) for mean and variability spatio-temporal measures. These studies also assessed reliability of non-linear variability measures (47) and demonstrated feasibility of anterior trunk sensor placement, representing an attenuated version of COM accelerations (110). Methods from Zijlstra and Hof (86) have been incorporated into commercially available IMUs, G-Walk (BTS, Milan, Italy) and DynaPort (McRoberts, The Hague, Netherlands). DynaPort accuracy has been assessed in children (130), lower-limb prosthesis (48), diabetes (53), healthy elderly (125,126) and falls risk subjects (50), while G-walk accuracy has been assessed in healthy adult (133-135), PD (136) and MS (137) subjects. Despite determining mean spatio-temporal parameters accurately in most studies, caution is recommended for interpretation of linear variability and asymmetry parameters (48,125,126). The interpretation of gait phase durations reliant on TO (stance, swing, single, double support time) is also uncertain since this event is not explicitly detected by the algorithm (133,136,137). Furthermore, testing limited to small samples in controlled straight-walking conditions limits power of inference to gait metrics analysis in pathological cohorts or scenarios of daily-living.
Autocorrelation methods utilizing lower trunk accelerations from a tri-axial accelerometer (94) have demonstrated test-retest reliability in a small sample (n=20) of healthy subjects for RMS and mean spatio-temporal parameter assessment (138) in straight-walking protocols. Despite lacking further validation for traditional spatio-temporal parameters, the test-retest reliability of autocorrelation measures of regularity and symmetry have been demonstrated in small samples of elderly, falls-risk and HD subjects in straight-walking protocols (47,50,139).
Methods by McCamley et al. (85)
CWT for GE detection and IP methods for spatial parameter estimation proposed by McCamley et al. (85) using a lower trunk IMU have been validated against stereophotogrammetry and force plates. It has also been compared against previous methods from Zijlstra & Hof (86) and González et al. (87). Despite limitations of a small sample (n=18) of healthy subjects and a straight-walking protocol of only 3.6 m, the method by McCamley et al. (85) provided an improved estimate of SL and GE detection compared to previous methods. Validity for temporal parameter means and variability has been subsequently demonstrated in both controlled and free-living conditions against pressure insoles, despite observed accuracy decreases in free-living conditions (140). Similar protocols in MS patients validated measurement of mean temporal parameters (120). However, variability measures were highly overestimated, with inaccuracies increasing with length of walking bout, detracting from applicability to continuous monitoring. The lower accuracy for which this method detects TO compared to HS, may account for difficulty estimating variability measures, shown to be highly sensitive to incorrect GE identification compared to mean parameters (120). Method accuracy was also shown to be speed-dependent and decrease with increasing disability, hindering reliability in pathological cohorts (120).
Method by Godfrey et al. (95,141) combining those by Zijlstra & Hof (86) and McCamley et al. (85)
Combining methods by McCamley et al. (85) for temporal parameter and Zijlstra & Hof (86) for SL estimation, Godfrey et al. (95,141) demonstrated validity against an instrumented walkway and through test-retest reliability in large populations of young and older adults (cumulative n=92) in protocols reflecting daily-living. Despite acceptable agreement for mean spatio-temporal parameters, SL underestimation was again attributed to limitations with generic correction factors, straight-walking dependent IP model and mathematical integration errors. Both methods’ measures of spatio-temporal variability and asymmetry were poor, concurring with findings of other studies using an instrumented walkway as a control (70,125-127). In defense of IMUs, Godfrey et al. (95) demonstrated that discrepancies in variability and asymmetry were due to inherent differences between IMUs and instrumented walkways used in these studies, rather than IMU inaccuracy. This highlights the importance of caution when choosing a standard for validation purposes. Demonstrated discriminatory power between pathological and healthy cohorts based on IMU asymmetry and variability measures, despite poor agreement with an instrumented walkway, reinforces these conclusions (70).
Other methods of GE and spatio-temporal parameter estimation
Waist-placed sensor and algorithm development has also been validated. Direct integration methods for SL estimation based on a waist placed IMU (88) were validated against stereophotogrammetry. However, limited gait parameters, a small sample (n=9) of healthy patients and accuracy discrepancies between left and right steps due to anatomically asymmetrical sensor placement limit clinical applicability (88).
To combat the plethora of COM methods, a comparison of five methods (85,86,88,89,142) against stereophotogrammetry and force plates for determination of temporal parameters was conducted by Trojaniello et al. (143). The different GE identification methods incorporated largely either the zero-crossing or wavelet-based method. Zjilstra & Hof (86) used a zero-crossing method where foot contact was taken as peak forward acceleration preceding the change of sign of acceleration from positive to negative. González et al. also used a zero-crossing method to approximate a search window prior to applying certain heuristic rules to determine the peak associated with the contact event (142). In conjunction with the zero-crossing method, Shin et al. used a sliding window summing technique to reduce noise (89). The method by McCamley et al. involved integrating and differentiating the acceleration signal using Gaussian continuous wavelet transforms prior to identifying initial and final contact events from the minima and maxima of the smoothed signal (85). Köse et al. used a wavelet-based method to identify windows of interest prior to decomposition and reconstruction of the original signal based on certain threshold application. Heel strike was then detected as the timepoint between signals of the different local frame axes (88). No statistically significant difference was found between methods for stride and step duration and the standard. However, methods that detect TO in addition to HS to allow determination of gait phase durations (85,87,88) showed a statistically significant difference, due to difficulties detecting the smoother acceleration signals indicating TO (143). Omitting assessment of spatial parameter methods limits completeness of the study. While despite examining a large sample, comparison of methods in healthy controls limits external validity to pathological cohorts. In response, assessment of the three best-performing methods (85,86,89) in 10 hemiparetic, 10 PD and 10 HD subjects against an instrumented walkway was undertaken (144). This revealed a universal decrease in GE detection and temporal parameter accuracy compared to healthy subjects. However, no statistically significant differences were revealed regarding accuracy between IMU methods in any cohort, apart from PD subjects for which methods from Zijlstra & Hof (86) outperformed.
New methods are continuously being formulated for lower trunk IMU analysis of spatial and temporal parameters. Oyake et al. (145) recently proposed a new algorithm for SL symmetry determination, validated in stroke subjects, while Sejdić et al. (146) validated novel methods against motion capture data in PD and peripheral neuropathy (PN) subjects.
Alternative sensor placements
Despite trunk IMUs maintaining the lion’s-share of research, methods based on alternative sensor positions have also garnered validation attention.
An ear-worn tri-axial accelerometer has been validated in healthy and lower-limb orthopedic subjects against an instrumented treadmill for estimation of mean step time and symmetry (76). A similar sensor has also been validated for the detection of GE’s and limited gait parameters in small samples of healthy (147), PD (148) and orthopedic (77) subjects in laboratory conditions.
With placement closer to the ground allowing better GE detection, sensors on the lower limb have also been proposed and validated. GV estimation from a single shank IMU was validated on a treadmill across numerous speeds and slope gradients (90). However, decrease in effectiveness of vertical shank inclination as a ZUPT re-calibration and subsequent GV accuracy with changing incline limits daily-living application. Maqbool et al. (149) further validated an algorithm for detection of GEs across walking speeds and slopes in eight healthy and two amputee subjects using a shank gyroscope. Straight-walking protocols and need for the instrumented shank to take the first step limit these studies’ clinical applicability. IP methods using one foot IMU have been validated against a treadmill for GV estimation in five healthy subjects (91). However, difficult attachment of the IMU to the shoe, straight-walking protocols and an accuracy decrease with increasing incline limit applicability. Temporal parameter detection has also been validated using a foot IMU in eight healthy adults (92), showing strong correlation with motion capture. Limited validation of comprehensive gait metrics and inability to assess asymmetry and complex trunk-accelerometer measures with lower-limb sensor placement limits clinical applicability (88).
Smart device gait analysis
Smart devices embedded with inertial sensors have become ubiquitous in everyday life, making them an obvious solution for maximizing patient compliance and allowing inconspicuous, portable gait analysis.
Initial proof of concept using an iPhone attached to the lateral malleolus demonstrated test-retest reliability for quantification of time averaged acceleration and step duration (150). However, unrealistic device placement, limited gait parameters and a sample of only one healthy patient limit validity and clinical applicability. Following this, utilizing trunk-based methods for GE detection (85-87) and SL estimation (86), high correlations were found for an iPhone against stereophotogrammetry for the identification of GE and mean spatio-temporal parameters in eleven healthy subjects (151). However, similar unrealistic device placement over the lumbar spine and waist has limited applicability to daily living. Addressing this, the reliability of smartphone locations: body, belt, bag, pocket and hand and validity against an instrumented walkway has been tested (152). Hand positions demonstrated poor reliability and agreement with the standard at slow speeds which only marginally improved at higher velocity, while high validity and excellent reliability were demonstrated in body, bag or belt positions at fast/comfortable speeds, lending traction to their incorporation into everyday gait monitoring. However, universal inaccuracies assessing gait at slow speeds limits application to pathological groups. Furthermore, limited emulation of free-living scenarios was employed with only five to nine steps investigated per trial (152).
Validation status of single-point IMUs
Despite lacking validation for a comprehensive set of gait metrics in alternative positions, current COM systems are a proven alternative for calculation of a range of traditional spatio-temporal measures. However, these algorithms still need development, with caution recommended with interpretation of spatio-temporal variability and symmetry parameters, measures of gait phases and estimation of spatial parameters. Further validation is required in larger samples of pathological groups; Furthermore, their accuracy for continuous monitoring in scenarios of daily living needs to be assessed. Incorporation of these algorithms into commercial devices and smart devices is promising for clinical practicality and uptake.
Clinical applications
Although the vision of single-point IMUs for gait metrics analysis in daily-living is in its infancy (4,34,69,120), these devices have been employed extensively in clinical environments to aid diagnosis and severity assessment, determine rehabilitation and intervention efficacy, and delineate pathological groups from healthy controls (Table 2). Of these applications, trunk-based IMUs are uniformly employed with the exception of a limited number of studies utilizing ear (76,77), foot (112,123) and smart-device (4,108,109) analysis.
Diagnosis and severity assessment
Single-point IMU gait metrics analysis has been employed as a method of assessment of ageing, orthopedic and neurological conditions.
Gait metrics analysis with single-point devices has aided diagnosis and severity assessment in numerous neurological diseases. Lower trunk sensor gait metrics analysis has been used to determine PD severity, demonstrating significantly reduced gait regularity and GV with increasing Hoeh and Yahr stage severity (31,32). Utilizing the commercially available DynaPort, Herman et al. (57) have also classified PD subtypes based on increased gait impairment and demonstrated classification superiority based on objective gait measures compared to conventional schemes. In MS, mean spatio-temporal parameter changes have been correlated with increasing disease severity using a G-Walk sensor, while also demonstrating high correlations between gait characteristics and patient-reported outcomes, reinforcing the applicability of gait metrics analysis as a clinical measure (62). Dalton et al. (110) also demonstrated significant differences between MS severity groups based on spatio-temporal mean and variability parameters as well as autocorrelation regularity and symmetry. Determining HD severity from trunk-based gait metrics analysis has also shown to correlate with clinical scales using both linear and non-linear measures (47). In diabetes subjects, gait parameters from a trunk IMU have shown good discriminatory power in detecting those with PN in a pilot study with a small sample (111), while De Bruin et al. (53) demonstrated the discriminatory power of SL to discern PN severity in type 2 diabetes patients in free-living gait conditions.
Similarly, gait metrics analysis has aided assessment in orthopedic conditions. Using a foot IMU, Angthong & Veljkovic (112) demonstrated significant correlations between obtained spatio-temporal parameters and subjective validated patient-reported outcomes and quality of life scores in patients with foot and ankle conditions such as arthritis, injury and tendinopathy. This is suggestive of the validity of gait assessment in clinical practice as an objective outcome measure.
In cognitive impairment, analysis of mean spatio-temporal and autocorrelation measures using a trunk IMU has been shown to predict risk of decline from mild cognitive impairment (MCI) to Alzheimer’s Disease in small sample sizes (n=23) (39). Furthermore, single-point gait quantification has been used to delineate between dementia subtypes (153).
Decline in physical and cognitive capacity with age is associated with frailty and disability, with consequences including falls, hospitalization and death. Numerous studies using single-point sensors and smart-devices have attempted to predict age-related gait changes (65,108), predict frailty status and determine severity (43,109) and predict falls and determine risk (33,34) to allow early-intervention to reduce adverse outcomes.
Rehabilitation and intervention efficacy
Gait metrics analysis using single-point IMUs has been used as an objective measure of rehabilitation and intervention efficacy in a range of conditions.
Single-point IMUs have been employed in the assessment of surgical outcomes and rehabilitation. A range of objective gait measures from trunk IMUs have been obtained to determine operation efficacy and rehabilitation progress after total hip replacement (113) and decompressive laminectomy for lumbar spine stenosis (128). An ear-worn sensor has been used to evaluate recovery from anterior cruciate ligament repair based on gait symmetry (147) as well as total hip and knee replacements based on stride duration and gait symmetry in small samples (76). Similarly, employment of the consumer available Apple Watch (Apple, San Francisco, CA, USA) for detection of GV was claimed invaluable in monitoring recovery through continuous gait monitoring in daily life following lumbar microdiscectomy in a single patient (4).
Assessment of non-operable intervention efficacy has also been determined using trunk IMUs. In cerebral palsy subjects, improvement in spatio-temporal mean and symmetry parameters after one hippotherapy session (56) and increase in mean parameters over a two-year intervention course (5) has been demonstrated. While linear measures of gait variability from trunk accelerations have been used to determine the effectiveness of Rivastigmine in in PD subjects over 32 weeks (114). In orthopedics, the effectiveness of prescription footwear has been assessed through trunk-based autocorrelation gait metrics analysis in severe foot and ankle fractures (115). While in women following single-breast mastectomy, significant influence of external breast prosthesis on spatio-temporal parameters of gait has been demonstrated (54).
Prescribed physical activity programs have been shown to improve physical functioning and reduce risk of falls and adverse outcomes (154). Pau et al. (60) utilized a trunk accelerometer to determine the increased effectiveness of vigorous compared to light physical activity on mean spatio-temporal parameters in elderly over 36 weeks. Similarly, the positive effect of PA on a range of spatio-temporal and accelerometer-based measures was demonstrated in MCI (101) and dementia subjects (38) using trunk-based accelerometry.
The efficacy of neurorehabilitation has also been assessed using trunk-based gait analysis. Santoyo et al. (155) demonstrated an increase in mean spatio-temporal parameters following a five-month neurorehabilitation program in 45 MS patients. Furthermore, Zanetta et al. (156) demonstrated similar improvements in cadence and GV after a four-week program, and significant correlation of gait parameters with validated clinical assessments (Berg Balance Scale, 6MWT), validating the usefulness of gait metrics analysis as an objective measure of outcomes.
Delineating pathological and healthy subjects
Most single-point IMU clinical applications focus on delineating between pathological groups with gait impairments and healthy controls. This application enables clinical validation of gait metrics and appreciation of metrics relevant to different pathologies.
Quantification of gait through single-point sensors in musculoskeletal disorders has been assessed. Knee osteoarthritis patients have been delineated from healthy controls based on numerous mean, autocorrelation and accelerometer-based spatio-temporal measures (67,68,116). In amputee gait, measures of stability, regularity and variability (49) as well as mean spatio-temporal parameters (48) have shown significant difference from healthy controls. Hip adductor fatigue has been demonstrated to significantly impact variability and symmetry of gait (58), while patients with chronic lower limb pain have demonstrated significant differences in cadence, variability and symmetry measures compared to controls (74). Using a trunk IMU in pregnant patients, significant differences have also been noted in trunk movement asymmetries between those with and without lumbopelvic pain. (121)
Numerous neurological conditions show altered gait quality using single-point IMUs. In stroke, RMS, autocorrelation measures of regularity and symmetry (102) and GV (52) have shown significant differences compared to controls. The gait impairments of various ataxia disorders have been assessed, demonstrating significant differences in mean, variability and asymmetry of spatio-temporal characteristics as well as autocorrelation and accelerometer-based measures (70,117). In PD, Esser et al. (46) demonstrated superior sensitivity of GV and non-linear variability measures compared to mean parameters for delineation from healthy controls in a small sample (n=24). Further works have demonstrated the ability of mean and symmetry spatio-temporal parameters as well as autocorrelation measures in both laboratory and free-living gait to delineate between PD subjects and healthy controls (31,69,72,118). Accelerometer-based measures of gait smoothness (HR) have also been shown to delineate between MS and healthy subjects prior to any measurable changes in mean spatio-temporal parameters (119), while dual-task gait in these patients has shown to result in a significant difference in mean spatio-temporal parameters compared to controls (63). In HD, significant differences have been demonstrated for autocorrelation measures, mean spatio-temporal parameters and both linear and non-linear variability measures against healthy subjects (47,110). Furthermore, in type 2 diabetes (61) and normal pressure hydrocephalus (157), altered spatio-temporal parameters have been demonstrated compared to healthy controls. In child pathological cohorts, differences in gait parameters have also been demonstrated in cerebral palsy (42,78) and dyslexia subjects (122) compared to healthy controls.
The effect of cognitive impairment on gait has also been quantified compared to healthy controls using single-point sensors (37,52,123,153). Gait differences have been quantified in those at risk or with a history of falls, with autocorrelation measures of regularity and symmetry, GV, stride length and step-time asymmetry showing significant discriminative capacity against healthy controls (36,50).
Limitation of single-point IMUs in delineating diseases
Clinical use of IMUs discussed above were mainly describing an obvious gait change (e.g., MS or Parkinson disease) rather than minute structural damages such as a torn hip labrum. Therefore, current use of a single-point IMU has to be considered within the appropriate clinical context. Additionally, the clinical application studies included are largely limited to straight line gait metrics assessment which does not fully reflect real-life movements. Along with the maturation of single-point IMUs in terms of validity, future studies should attempt to assess validity of these sensors in picking up complex movements that reflect real life movements such as falls.
Future prospects
The ability for wearable devices in detecting gait and posture is maturing and undergoing continuous development. Multiple studies have demonstrated the use of wearable technologies in aiding postural analysis as well as serving as a tool for the general population in everyday postural/activity tracking (158,159). A novel scoring algorithm incorporating gait and postural scores has also been proposed to report patients’ outcome in a manner which is simple and conducive to a continuous stream of data that can be remotely monitored by clinicians (160). This ability to remotely measure and record continuous data gives wearable devices an upper hand compared to lab-based instruments which are geographically sparse and perform gait analysis at discrete time points, though validity and standardization remains a drawback currently (4,23,158-160).
Future studies are required before the implementation of IMUs can be recommended to clinicians. In particular, there is an urgent need to validate IMU accuracy in free-living home environments, with most current validation studies instead measuring gait metrics on straight-line pathways. Other parameters such as U-turns, complex acceleration and deceleration that mimics day-to-day movements such as slowing down when approaching a door or chair could be studied. In addition, single-point IMUs have not been consistently shown to have high accuracy when measuring variability and asymmetry metrics. Future studies may assess other models for GE detection, which are continuously being developed, that may more accurately capture these metrics. Moreover, many of the studies focusing on clinical application have described obvious gait changes. While IMUs are still useful in objectively quantifying these changes, more evidence is required to demonstrate the clinical applications of IMUs in the measurement of subtle gait differences. Future studies to compare user acceptability and compliance between single-point IMUs and multi-point IMUs should also be conducted. Studies to determine ideal placement location of single-point IMUs at various body parts could also be conducted. Further developments and validation may one day bridge the gap for incorporating wearable technologies into actual clinical setting in aiding diagnosis and monitoring patient progression.
Conclusions
This review has demonstrated the validity of single-point IMUs as an alternative to current quantitative methods and their ability to assist in clinical scenarios. The accuracy of these systems for detection of traditional metrics as well as the demonstrated clinical relevance of novel, accelerometer-based measures is promising for practicality and efficacy in the clinical context. Further validation for long-term, continuous monitoring in daily living scenarios is required as is performance in larger samples of pathological cohorts before mass commercial and clinical uptake can be expected.
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Footnotes
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://dx.doi.org/10.21037/mhealth-21-17
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/mhealth-21-17). The authors have no conflicts of interest to declare.
References
- 1.Cook RE, Schneider I, Hazlewood ME, et al. Gait analysis alters decision-making in cerebral palsy. J Pediatr Orthop 2003;23:292-5. 10.1097/01241398-200305000-00004 [DOI] [PubMed] [Google Scholar]
- 2.Hodgins A, Manning O, Ritsma B, et al. Validity of Wearable Sensors in Measuring Gait Quality Following Stroke. World Stroke Congress 2018. [Google Scholar]
- 3.Lecat M, Decavel P, Magnin E, et al. Multiple Sclerosis and Clinical Gait Analysis before and after Fampridine: A Systematic Review. Eur Neurol 2017;78:272-86. 10.1159/000480729 [DOI] [PubMed] [Google Scholar]
- 4.Mobbs RJ, Katsinas CJ, Choy WJ, et al. Objective monitoring of activity and Gait Velocity using wearable accelerometer following lumbar microdiscectomy to detect recurrent disc herniation. J Spine Surg 2018;4:792-7. 10.21037/jss.2018.12.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mutoh T, Mutoh T, Takada M, et al. Application of a tri-axial accelerometry-based portable motion recorder for the quantitative assessment of hippotherapy in children and adolescents with cerebral palsy. J Phys Ther Sci 2016;28:2970-4. 10.1589/jpts.28.2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steultjens MP, Dekker J, van Baar ME, et al. Range of joint motion and disability in patients with osteoarthritis of the knee or hip. Rheumatology (Oxford) 2000;39:955-61. 10.1093/rheumatology/39.9.955 [DOI] [PubMed] [Google Scholar]
- 7.Tzallas AT, Tsipouras MG, Rigas G, et al. PERFORM: a system for monitoring, assessment and management of patients with Parkinson's disease. Sensors (Basel) 2014;14:21329-57. 10.3390/s141121329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang HH, Yan SH, Fang C, et al. Clinical Evaluation and Gait Characteristics before and after Total Knee Arthroplasty Based on a Portable Gait Analyzer. Orthop Surg 2016;8:360-6. 10.1111/os.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verghese J, Holtzer R, Lipton RB, et al. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci 2009;64:896-901. 10.1093/gerona/glp033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwenk M, Mohler J, Wendel C, et al. Wearable sensor-based in-home assessment of gait, balance, and physical activity for discrimination of frailty status: baseline results of the Arizona frailty cohort study. Gerontology 2015;61:258-67. 10.1159/000369095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S, Lach J, Lo B, et al. Toward Pervasive Gait Analysis With Wearable Sensors: A Systematic Review. IEEE J Biomed Health Inform 2016;20:1521-37. 10.1109/JBHI.2016.2608720 [DOI] [PubMed] [Google Scholar]
- 12.Saleh M, Murdoch G. In defence of gait analysis. Observation and measurement in gait assessment. J Bone Joint Surg Br 1985;67:237-41. 10.1302/0301-620X.67B2.3980533 [DOI] [PubMed] [Google Scholar]
- 13.Cappozzo A, Della Croce U, Leardini A, et al. Human movement analysis using stereophotogrammetry. Part 1: theoretical background. Gait Posture 2005;21:186-96. [DOI] [PubMed] [Google Scholar]
- 14.Spine News International. Wearables in spine surgery: Beginnings, research and real-world applications 2017. Available online: https://spinalnewsinternational.com/wearables/
- 15.Rehan K. Wearing Your Spine Health on Your Sleeve Spine Universe. 2017. Available online: https://www.spineuniverse.com/treatments/emerging/wearing-your-spine-health-your-sleeve
- 16.Mobbs RJ, Phan K, Maharaj M, et al. Physical Activity Measured with Accelerometer and Self-Rated Disability in Lumbar Spine Surgery: A Prospective Study. Global Spine J 2016;6:459-64. 10.1055/s-0035-1565259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan K, Mobbs RJ. Long-Term Objective Physical Activity Measurements using a Wireless Accelerometer Following Minimally Invasive Transforaminal Interbody Fusion Surgery. Asian Spine J 2016;10:366-9. 10.4184/asj.2016.10.2.366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agostini V, Gastaldi L, Rosso V, et al. A Wearable Magneto-Inertial System for Gait Analysis (H-Gait): Validation on Normal Weight and Overweight/Obese Young Healthy Adults. Sensors (Basel) 2017;17:2406. 10.3390/s17102406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donath L, Faude O, Lichtenstein E, et al. Mobile inertial sensor based gait analysis: Validity and reliability of spatiotemporal gait characteristics in healthy seniors. Gait Posture 2016;49:371-4. 10.1016/j.gaitpost.2016.07.269 [DOI] [PubMed] [Google Scholar]
- 20.Kluge F, Gaßner H, Hannink J, et al. Towards Mobile Gait Analysis: Concurrent Validity and Test-Retest Reliability of an Inertial Measurement System for the Assessment of Spatio-Temporal Gait Parameters. Sensors (Basel) 2017;17:1522. 10.3390/s17071522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maffiuletti NA, Gorelick M, Kramers-de Quervain I, et al. Concurrent validity and intrasession reliability of the IDEEA accelerometry system for the quantification of spatiotemporal gait parameters. Gait Posture 2008;27:160-3. 10.1016/j.gaitpost.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 22.Orlowski K, Eckardt F, Herold F, et al. Examination of the reliability of an inertial sensor-based gait analysis system. Biomed Tech (Berl) 2017;62:615-22. 10.1515/bmt-2016-0067 [DOI] [PubMed] [Google Scholar]
- 23.Rao PJ, Phan K, Maharaj MM, et al. Accelerometers for objective evaluation of physical activity following spine surgery. J Clin Neurosci 2016;26:14-8. 10.1016/j.jocn.2015.05.064 [DOI] [PubMed] [Google Scholar]
- 24.Barth J, Klucken J, Kugler P, et al. Biometric and mobile gait analysis for early diagnosis and therapy monitoring in Parkinson's disease. Annu Int Conf IEEE Eng Med Biol Soc 2011;2011:868-71. 10.1109/IEMBS.2011.6090226 [DOI] [PubMed] [Google Scholar]
- 25.Guo Y, Wu D, Liu G, et al. A low-cost body inertial-sensing network for practical gait discrimination of hemiplegia patients. Telemed J E Health 2012;18:748-54. 10.1089/tmj.2012.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu YL, Chung PC, Wang WH, et al. Gait and balance analysis for patients with Alzheimer's disease using an inertial-sensor-based wearable instrument. IEEE J Biomed Health Inform 2014;18:1822-30. 10.1109/JBHI.2014.2325413 [DOI] [PubMed] [Google Scholar]
- 27.Mackey AH, Stott NS, Walt SE. Reliability and validity of an activity monitor (IDEEA) in the determination of temporal-spatial gait parameters in individuals with cerebral palsy. Gait Posture 2008;28:634-9. 10.1016/j.gaitpost.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 28.Tadano S, Takeda R, Sasaki K, et al. Gait characterization for osteoarthritis patients using wearable gait sensors (H-Gait systems). J Biomech 2016;49:684-90. 10.1016/j.jbiomech.2016.01.017 [DOI] [PubMed] [Google Scholar]
- 29.Tunca C, Pehlivan N, Ak N, et al. Inertial Sensor-Based Robust Gait Analysis in Non-Hospital Settings for Neurological Disorders. Sensors (Basel) 2017;17:825. 10.3390/s17040825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sprager S, Juric MB. Inertial Sensor-Based Gait Recognition: A Review. Sensors (Basel) 2015;15:22089-127. 10.3390/s150922089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demonceau M, Donneau AF, Croisier JL, et al. Contribution of a Trunk Accelerometer System to the Characterization of Gait in Patients With Mild-to-Moderate Parkinson's Disease. IEEE J Biomed Health Inform 2015;19:1803-8. 10.1109/JBHI.2015.2469540 [DOI] [PubMed] [Google Scholar]
- 32.Cheng JZ, Von Coelln R, Schrader K, et al. Accelerometry-based quantitative analysis of mobility in Parkinson disease. Neurology 2018;90:P3.044.
- 33.van Schooten KS, Pijnappels M, Rispens SM, et al. Daily-Life Gait Quality as Predictor of Falls in Older People: A 1-Year Prospective Cohort Study. PLoS One 2016;11:e0158623. 10.1371/journal.pone.0158623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Din S, Galna B, Godfrey A, et al. Analysis of Free-Living Gait in Older Adults With and Without Parkinson's Disease and With and Without a History of Falls: Identifying Generic and Disease-Specific Characteristics. J Gerontol A Biol Sci Med Sci 2019;74:500-6. 10.1093/gerona/glx254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Espinosa HG, Thiel DV, Sorell M, et al. Can We Trust Inertial and Heart Rate Sensor Data from an APPLE Watch Device? Proceedings 2020;49:128. 10.3390/proceedings2020049128 [DOI] [Google Scholar]
- 36.Auvinet B, Berrut G, Touzard C, et al. Gait abnormalities in elderly fallers. J Aging Phys Act 2003;11:40-52. 10.1123/japa.11.1.40 [DOI] [Google Scholar]
- 37.Maquet D, Lekeu F, Warzee E, et al. Gait analysis in elderly adult patients with mild cognitive impairment and patients with mild Alzheimer's disease: simple versus dual task: a preliminary report. Clin Physiol Funct Imaging 2010;30:51-6. 10.1111/j.1475-097X.2009.00903.x [DOI] [PubMed] [Google Scholar]
- 38.Perrochon A, Tchalla AE, Bonis J, et al. Effects of a Multicomponent Exercise Program on Spatiotemporal Gait Parameters, Risk of Falling and Physical Activity in Dementia Patients. Dement Geriatr Cogn Dis Extra 2015;5:350-60. 10.1159/000435772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillain S, Dramé M, Lekeu F, et al. Gait speed or gait variability, which one to use as a marker of risk to develop Alzheimer disease? A pilot study. Aging Clin Exp Res 2016;28:249-55. 10.1007/s40520-015-0392-6 [DOI] [PubMed] [Google Scholar]
- 40.Walking and running analysis gait analysis devices Paris: Centaure Metrix. [cited 2021 Jun 28]. Available online: http://www.centaure-metrix.com/Index_en.html
- 41.Esser P, Dawes H, Collett J, et al. Assessment of spatio-temporal gait parameters using inertial measurement units in neurological populations. Gait Posture 2011;34:558-60. 10.1016/j.gaitpost.2011.06.018 [DOI] [PubMed] [Google Scholar]
- 42.Saether R, Helbostad JL, Adde L, et al. Gait characteristics in children and adolescents with cerebral palsy assessed with a trunk-worn accelerometer. Res Dev Disabil 2014;35:1773-81. 10.1016/j.ridd.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 43.Martínez-Ramírez A, Martinikorena I, Gómez M, et al. Frailty assessment based on trunk kinematic parameters during walking. J Neuroeng Rehabil 2015;12:48. 10.1186/s12984-015-0040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martínez-Ramírez A, Martinikorena I, Lecumberri P, et al. Dual Task Gait Performance in Frail Individuals with and without Mild Cognitive Impairment. Dement Geriatr Cogn Disord 2016;42:7-16. 10.1159/000447451 [DOI] [PubMed] [Google Scholar]
- 45.MTi-1 IMU Enschede: Xsens. [cited 2021 Jun 28]. Available online: https://www.xsens.com/mti-1-imu
- 46.Esser P, Dawes H, Collett J, et al. Insights into gait disorders: walking variability using phase plot analysis, Parkinson's disease. Gait Posture 2013;38:648-52. 10.1016/j.gaitpost.2013.02.016 [DOI] [PubMed] [Google Scholar]
- 47.Collett J, Esser P, Khalil H, et al. Insights into gait disorders: walking variability using phase plot analysis, Huntington's disease. Gait Posture 2014;40:694-700. 10.1016/j.gaitpost.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 48.Houdijk H, Appelman FM, Van Velzen JM, et al. Validity of DynaPort GaitMonitor for assessment of spatiotemporal parameters in amputee gait. J Rehabil Res Dev 2008;45:1335-42. 10.1682/JRRD.2007.12.0209 [DOI] [PubMed] [Google Scholar]
- 49.Lamoth CJ, Ainsworth E, Polomski W, et al. Variability and stability analysis of walking of transfemoral amputees. Med Eng Phys 2010;32:1009-14. 10.1016/j.medengphy.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 50.Bautmans I, Jansen B, Van Keymolen B, et al. Reliability and clinical correlates of 3D-accelerometry based gait analysis outcomes according to age and fall-risk. Gait Posture 2011;33:366-72. 10.1016/j.gaitpost.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 51.Lamoth CJ, van Deudekom FJ, van Campen JP, et al. Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. J Neuroeng Rehabil 2011;8:2. 10.1186/1743-0003-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meijer R, Plotnik M, Zwaaftink EG, et al. Markedly impaired bilateral coordination of gait in post-stroke patients: Is this deficit distinct from asymmetry? A cohort study. J Neuroeng Rehabil 2011;8:23. 10.1186/1743-0003-8-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Bruin ED, Hubli M, Hofer P, et al. Validity and reliability of accelerometer-based gait assessment in patients with diabetes on challenging surfaces. J Aging Res 2012;2012:954378. 10.1155/2012/954378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hojan K, Manikowska F, Molinska-Glura M, et al. The impact of an external breast prosthesis on the gait parameters of women after mastectomy. Cancer Nurs 2014;37:E30-6. 10.1097/NCC.0b013e3182919576 [DOI] [PubMed] [Google Scholar]
- 55.Manikowska F, Hojan K, Chen PJ, et al. The gait pattern in post-menopausal women. Pilot study. Ortop Traumatol Rehabil 2013;15:575-83. 10.5604/15093492.1091513 [DOI] [PubMed] [Google Scholar]
- 56.Manikowska F, Jóźwiak M, Idzior M, et al. The effect of a hippotherapy session on spatiotemporal parameters of gait in children with cerebral palsy - pilot study. Ortop Traumatol Rehabil 2013;15:253-7. 10.5604/15093492.1058420 [DOI] [PubMed] [Google Scholar]
- 57.Herman T, Weiss A, Brozgol M, et al. Gait and balance in Parkinson's disease subtypes: objective measures and classification considerations. J Neurol 2014;261:2401-10. 10.1007/s00415-014-7513-6 [DOI] [PubMed] [Google Scholar]
- 58.Arvin M, Hoozemans MJ, Burger BJ, et al. Effects of hip abductor muscle fatigue on gait control and hip position sense in healthy older adults. Gait Posture 2015;42:545-9. 10.1016/j.gaitpost.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 59.MoveMonitor The Hague: McRoberts. [cited 2021 Jun 28]. Available online: https://www.mcroberts.nl/products/movemonitor/
- 60.Pau M, Leban B, Collu G, et al. Effect of light and vigorous physical activity on balance and gait of older adults. Arch Gerontol Geriatr 2014;59:568-73. 10.1016/j.archger.2014.07.008 [DOI] [PubMed] [Google Scholar]
- 61.Awotidebe TO, Ativie RN, Oke KI, et al. Relationships among exercise capacity, dynamic balance and gait characteristics of Nigerian patients with type-2 diabetes: an indication for fall prevention. J Exerc Rehabil 2016;12:581-8. 10.12965/jer.1632706.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pau M, Caggiari S, Mura A, et al. Clinical assessment of gait in individuals with multiple sclerosis using wearable inertial sensors: Comparison with patient-based measure. Mult Scler Relat Disord 2016;10:187-91. 10.1016/j.msard.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 63.Pau M, Corona F, Pilloni G, et al. Texting while walking differently alters gait patterns in people with multiple sclerosis and healthy individuals. Mult Scler Relat Disord 2018;19:129-33. 10.1016/j.msard.2017.11.021 [DOI] [PubMed] [Google Scholar]
- 64.G-Walk: Wearable system for the functional analysis of movement Milan: BTS Bioengineering. [cited 2021 Jun 28]. Available online: https://www.btsbioengineering.com/products/g-walk-inertial-motion-system/
- 65.Terrier P, Reynard F. Effect of age on the variability and stability of gait: a cross-sectional treadmill study in healthy individuals between 20 and 69 years of age. Gait Posture 2015;41:170-4. 10.1016/j.gaitpost.2014.09.024 [DOI] [PubMed] [Google Scholar]
- 66.Compact and versatile wireless inertial measurement unit Lausanne: Gaitup. [cited 2021 Jun 28]. Available online: https://research.gaitup.com/physilog/
- 67.Barden JM, Clermont CA, Kobsar D, et al. Accelerometer-Based Step Regularity Is Lower in Older Adults with Bilateral Knee Osteoarthritis. Front Hum Neurosci 2016;10:625. 10.3389/fnhum.2016.00625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clermont CA, Barden JM. Accelerometer-based determination of gait variability in older adults with knee osteoarthritis. Gait Posture 2016;50:126-30. 10.1016/j.gaitpost.2016.08.024 [DOI] [PubMed] [Google Scholar]
- 69.Del Din S, Godfrey A, Galna B, et al. Free-living gait characteristics in ageing and Parkinson's disease: impact of environment and ambulatory bout length. J Neuroeng Rehabil 2016;13:46. 10.1186/s12984-016-0154-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hickey A, Gunn E, Alcock L, et al. Validity of a wearable accelerometer to quantify gait in spinocerebellar ataxia type 6. Physiol Meas 2016;37:N105-17. [DOI] [PubMed]
- 71.AX3 York: Axivity. [cited 2021 Jun 28]. Available online: https://axivity.com/product/ax3
- 72.Hatanaka N, Sato K, Hishikawa N, et al. Comparative Gait Analysis in Progressive Supranuclear Palsy and Parkinson's Disease. Eur Neurol 2016;75:282-9. 10.1159/000445111 [DOI] [PubMed] [Google Scholar]
- 73.Edwardson CL, Winkler EAH, Bodicoat DH, et al. Considerations when using the activPAL monitor in field-based research with adult populations. J Sport Health Sci 2017;6:162-78. 10.1016/j.jshs.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Terrier P, Le Carre J, Connaissa ML, et al. Monitoring of Gait Quality in Patients With Chronic Pain of Lower Limbs. IEEE Trans Neural Syst Rehabil Eng 2017;25:1843-52. 10.1109/TNSRE.2017.2688485 [DOI] [PubMed] [Google Scholar]
- 75.ActiGraph wGT3X-BT. Pensacola: Actigraph. [cited 2021 Jun 28]. Available online: https://actigraphcorp.com/actigraph-wgt3x-bt/
- 76.Atallah L, Wiik A, Lo B, et al. Gait asymmetry detection in older adults using a light ear-worn sensor. Physiol Meas 2014;35:N29-40. [DOI] [PubMed]
- 77.Jarchi D, Lo B, Wong C, et al. Gait Analysis From a Single Ear-Worn Sensor: Reliability and Clinical Evaluation for Orthopaedic Patients. IEEE Trans Neural Syst Rehabil Eng 2016;24:882-92. 10.1109/TNSRE.2015.2477720 [DOI] [PubMed] [Google Scholar]
- 78.Iosa M, Morelli D, Marro T, et al. Ability and stability of running and walking in children with cerebral palsy. Neuropediatrics 2013;44:147-54. 10.1055/s-0033-1336016 [DOI] [PubMed] [Google Scholar]
- 79.Potter MV, Ojeda LV, Perkins NC, et al. Effect of IMU Design on IMU-Derived Stride Metrics for Running. Sensors (Basel) 2019;19:2601. 10.3390/s19112601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Felisberto F, Fdez-Riverola F, Pereira A. A ubiquitous and low-cost solution for movement monitoring and accident detection based on sensor fusion. Sensors (Basel) 2014;14:8961-83. 10.3390/s140508961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perry J, Schoneberger B. Gait Analysis: Normal and Pathological Function. SLACK, 1992. [Google Scholar]
- 82.Lord S, Galna B, Verghese J, et al. Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci 2013;68:820-7. 10.1093/gerona/gls255 [DOI] [PubMed] [Google Scholar]
- 83.Lord S, Galna B, Coleman S, et al. Mild depressive symptoms are associated with gait impairment in early Parkinson's disease. Mov Disord 2013;28:634-9. 10.1002/mds.25338 [DOI] [PubMed] [Google Scholar]
- 84.Beauchet O, Allali G, Sekhon H, et al. Guidelines for Assessment of Gait and Reference Values for Spatiotemporal Gait Parameters in Older Adults: The Biomathics and Canadian Gait Consortiums Initiative. Front Hum Neurosci 2017;11:353. 10.3389/fnhum.2017.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCamley J, Donati M, Grimpampi E, et al. An enhanced estimate of initial contact and final contact instants of time using lower trunk inertial sensor data. Gait Posture 2012;36:316-8. 10.1016/j.gaitpost.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 86.Zijlstra W, Hof AL. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture 2003;18:1-10. 10.1016/S0966-6362(02)00190-X [DOI] [PubMed] [Google Scholar]
- 87.González RC, Alvarez D, López AM, et al. Ambulatory estimation of mean step length during unconstrained walking by means of COG accelerometry. Comput Methods Biomech Biomed Engin 2009;12:721-6. 10.1080/10255840902896000 [DOI] [PubMed] [Google Scholar]
- 88.Köse A, Cereatti A, Della Croce U. Bilateral step length estimation using a single inertial measurement unit attached to the pelvis. J Neuroeng Rehabil 2012;9:9. 10.1186/1743-0003-9-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shin SH, Park CG. Adaptive step length estimation algorithm using optimal parameters and movement status awareness. Med Eng Phys 2011;33:1064-71. 10.1016/j.medengphy.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 90.Li Q, Young M, Naing V, et al. Walking speed estimation using a shank-mounted inertial measurement unit. J Biomech 2010;43:1640-3. 10.1016/j.jbiomech.2010.01.031 [DOI] [PubMed] [Google Scholar]
- 91.Sabatini AM, Martelloni C, Scapellato S, et al. Assessment of walking features from foot inertial sensing. IEEE Trans Biomed Eng 2005;52:486-94. 10.1109/TBME.2004.840727 [DOI] [PubMed] [Google Scholar]
- 92.Song M, Kim J. An Ambulatory Gait Monitoring System with Activity Classification and Gait Parameter Calculation Based on a Single Foot Inertial Sensor. IEEE Trans Biomed Eng 2018;65:885-93. 10.1109/TBME.2017.2724543 [DOI] [PubMed] [Google Scholar]
- 93.Baroudi L, Newman MW, Jackson EA, et al. Estimating Walking Speed in the Wild. Front Sports Act Living 2020;2:583848. 10.3389/fspor.2020.583848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moe-Nilssen R, Helbostad JL. Estimation of gait cycle characteristics by trunk accelerometry. J Biomech 2004;37:121-6. 10.1016/S0021-9290(03)00233-1 [DOI] [PubMed] [Google Scholar]
- 95.Godfrey A, Del Din S, Barry G, et al. Instrumenting gait with an accelerometer: a system and algorithm examination. Med Eng Phys 2015;37:400-7. 10.1016/j.medengphy.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sekine M, Akay M, Tamura T, et al. Fractal dynamics of body motion in patients with Parkinson's disease. J Neural Eng 2004;1:8-15. 10.1088/1741-2560/1/1/002 [DOI] [PubMed] [Google Scholar]
- 97.Akay M, Sekine M, Tamura T, et al. Fractal dynamics of body motion in post-stroke hemiplegic patients during walking. J Neural Eng 2004;1:111-6. 10.1088/1741-2560/1/2/006 [DOI] [PubMed] [Google Scholar]
- 98.Hausdorff JM. Gait dynamics in Parkinson's disease: common and distinct behavior among stride length, gait variability, and fractal-like scaling. Chaos 2009;19:026113. 10.1063/1.3147408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rochester L, Chastin SF, Lord S, et al. Understanding the impact of deep brain stimulation on ambulatory activity in advanced Parkinson's disease. J Neurol 2012;259:1081-6. 10.1007/s00415-011-6301-9 [DOI] [PubMed] [Google Scholar]
- 100.Brach JS, McGurl D, Wert D, et al. Validation of a measure of smoothness of walking. J Gerontol A Biol Sci Med Sci 2011;66:136-41. 10.1093/gerona/glq170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doi T, Makizako H, Shimada H, et al. Effects of multicomponent exercise on spatial-temporal gait parameters among the elderly with amnestic mild cognitive impairment (aMCI): preliminary results from a randomized controlled trial (RCT). Arch Gerontol Geriatr 2013;56:104-8. 10.1016/j.archger.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 102.Mizuike C, Ohgi S, Morita S. Analysis of stroke patient walking dynamics using a tri-axial accelerometer. Gait Posture 2009;30:60-4. 10.1016/j.gaitpost.2009.02.017 [DOI] [PubMed] [Google Scholar]
- 103.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Scottish Intercollegiate Guidelines Network (SIGN). Methodology Checklist 5: Studies of Diagnostic Accuracy. 2014. Available online: https://www.sign.ac.uk/what-we-do/methodology/checklists/
- 105.NHMRC. Guidelines for Guidelines: Assessing risk of bias. 2019. Available online: https://nhmrc.gov.au/guidelinesforguidelines/develop/assessing-risk-bias
- 106.GA Wells, B Shea, D O'Connell, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 1999. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 107.Scottish Intercollegiate Guidelines Network (SIGN). Methodology checklist 2: randomised controlled trials 2014. Available online: https://www.sign.ac.uk/what-we-do/methodology/checklists/
- 108.Kosse NM, Vuillerme N, Hortobágyi T, et al. Multiple gait parameters derived from iPod accelerometry predict age-related gait changes. Gait Posture 2016;46:112-7. 10.1016/j.gaitpost.2016.02.022 [DOI] [PubMed] [Google Scholar]
- 109.Soangra R, Lockhart TE. Inertial Sensor-Based Variables Are Indicators of Frailty and Adverse Post-Operative Outcomes in Cardiovascular Disease Patients. Sensors (Basel) 2018;18:1792. 10.3390/s18061792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dalton A, Khalil H, Busse M, et al. Analysis of gait and balance through a single triaxial accelerometer in presymptomatic and symptomatic Huntington's disease. Gait Posture 2013;37:49-54. 10.1016/j.gaitpost.2012.05.028 [DOI] [PubMed] [Google Scholar]
- 111.Esser P, Collett J, Maynard K, et al. Single Sensor Gait Analysis to Detect Diabetic Peripheral Neuropathy: A Proof of Principle Study. Diabetes Metab J 2018;42:82-6. 10.4093/dmj.2018.42.1.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Angthong C, Veljkovic A. Relationships among subjective patient-reported outcome, quality of life, and objective gait characteristics using wearable foot inertial-sensor assessment in foot-ankle patients. Eur J Orthop Surg Traumatol 2019;29:683-7. 10.1007/s00590-018-2346-0 [DOI] [PubMed] [Google Scholar]
- 113.Rapp W, Brauner T, Weber L, et al. Improvement of walking speed and gait symmetry in older patients after hip arthroplasty: a prospective cohort study. BMC Musculoskelet Disord 2015;16:291. 10.1186/s12891-015-0755-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Henderson EJ, Lord SR, Brodie MA, et al. Rivastigmine for gait stability in patients with Parkinson's disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016;15:249-58. 10.1016/S1474-4422(15)00389-0 [DOI] [PubMed] [Google Scholar]
- 115.Terrier P, Dériaz O, Meichtry A, et al. Prescription footwear for severe injuries of foot and ankle: effect on regularity and symmetry of the gait assessed by trunk accelerometry. Gait Posture 2009;30:492-6. 10.1016/j.gaitpost.2009.07.122 [DOI] [PubMed] [Google Scholar]
- 116.Bolink SA, van Laarhoven SN, Lipperts M, et al. Inertial sensor motion analysis of gait, sit-stand transfers and step-up transfers: differentiating knee patients from healthy controls. Physiol Meas 2012;33:1947-58. 10.1088/0967-3334/33/11/1947 [DOI] [PubMed] [Google Scholar]
- 117.Matsushima A, Yoshida K, Genno H, et al. Clinical assessment of standing and gait in ataxic patients using a triaxial accelerometer. Cerebellum Ataxias 2015;2:9. 10.1186/s40673-015-0028-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang CC, Hsu YL, Shih KS, et al. Real-time gait cycle parameter recognition using a wearable accelerometry system. Sensors (Basel) 2011;11:7314-26. 10.3390/s110807314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pau M, Mandaresu S, Pilloni G, et al. Smoothness of gait detects early alterations of walking in persons with multiple sclerosis without disability. Gait Posture 2017;58:307-9. 10.1016/j.gaitpost.2017.08.023 [DOI] [PubMed] [Google Scholar]
- 120.Storm FA, Nair KPS, Clarke AJ, et al. Free-living and laboratory gait characteristics in patients with multiple sclerosis. PLoS One 2018;13:e0196463. 10.1371/journal.pone.0196463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tanigawa A, Morino S, Aoyama T, et al. Gait analysis of pregnant patients with lumbopelvic pain using inertial sensor. Gait Posture 2018;65:176-81. 10.1016/j.gaitpost.2018.07.165 [DOI] [PubMed] [Google Scholar]
- 122.Moe-Nilssen R, Helbostad JL, Talcott JB, et al. Balance and gait in children with dyslexia. Exp Brain Res 2003;150:237-44. 10.1007/s00221-003-1450-4 [DOI] [PubMed] [Google Scholar]
- 123.Chung P, Hsu Y, Wang C, et al. Gait analysis for patients with Alzheimer'S disease using a triaxial accelerometer. 2012 IEEE International Symposium on Circuits and Systems (ISCAS), 2012:1323-6. [Google Scholar]
- 124.Bugané F, Benedetti MG, Casadio G, et al. Estimation of spatial-temporal gait parameters in level walking based on a single accelerometer: validation on normal subjects by standard gait analysis. Comput Methods Programs Biomed 2012;108:129-37. 10.1016/j.cmpb.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 125.Hartmann A, Luzi S, Murer K, et al. Concurrent validity of a trunk tri-axial accelerometer system for gait analysis in older adults. Gait Posture 2009;29:444-8. 10.1016/j.gaitpost.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 126.Hartmann A, Murer K, de Bie RA, et al. Reproducibility of spatio-temporal gait parameters under different conditions in older adults using a trunk tri-axial accelerometer system. Gait Posture 2009;30:351-5. 10.1016/j.gaitpost.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 127.Byun S, Han JW, Kim TH, et al. Test-Retest Reliability and Concurrent Validity of a Single Tri-Axial Accelerometer-Based Gait Analysis in Older Adults with Normal Cognition. PLoS One 2016;11:e0158956. 10.1371/journal.pone.0158956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ohtaki Y, Mamizuka N, Hirano A, et al. Pre- and Post-operative Evaluations of Patients with Lumbar Spinal Stenosis by Clinical Walk Test Using an Inertial Sensor. Transactions of Japanese Society for Medical and Biological Engineering 2014;52:167-74. [Google Scholar]
- 129.Jarchi D, Pope J, Lee TKM, et al. A Review on Accelerometry-Based Gait Analysis and Emerging Clinical Applications. IEEE Rev Biomed Eng 2018;11:177-94. 10.1109/RBME.2018.2807182 [DOI] [PubMed] [Google Scholar]
- 130.Brandes M, Zijlstra W, Heikens S, et al. Accelerometry based assessment of gait parameters in children. Gait Posture 2006;24:482-6. 10.1016/j.gaitpost.2005.12.006 [DOI] [PubMed] [Google Scholar]
- 131.Zijlstra A, Zijlstra W. Trunk-acceleration based assessment of gait parameters in older persons: a comparison of reliability and validity of four inverted pendulum based estimations. Gait Posture 2013;38:940-4. 10.1016/j.gaitpost.2013.04.021 [DOI] [PubMed] [Google Scholar]
- 132.Esser P, Dawes H, Collett J, et al. Validity and inter-rater reliability of inertial gait measurements in Parkinson's disease: a pilot study. J Neurosci Methods 2012;205:177-81. 10.1016/j.jneumeth.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 133.De Ridder R, Lebleu J, Willems T, et al. Concurrent Validity of a Commercial Wireless Trunk Triaxial Accelerometer System for Gait Analysis. J Sport Rehabil 2019;28:jsr. 10.1123/jsr.2018-0295 [DOI] [PubMed] [Google Scholar]
- 134.Lim SY, Lee WH. Comparison of accelerometer-based and treadmill-based analysis systems for measuring gait parameters in healthy adults. J Phys Ther Sci 2017;29:651-3. 10.1589/jpts.29.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park G; BHSc; Woo Y. Comparison between a center of mass and a foot pressure sensor system for measuring gait parameters in healthy adults. J Phys Ther Sci 2015;27:3199-202. 10.1589/jpts.27.3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zago M, Sforza C, Pacifici I, et al. Gait evaluation using inertial measurement units in subjects with Parkinson's disease. J Electromyogr Kinesiol 2018;42:44-8. 10.1016/j.jelekin.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 137.Gudesblatt M, Trebing S, Burke C, et al. Multiple sclerosis, gait and digital devices: A comparison of two FDA approved validated devices that provide multidimensional quantifiable gait parameters in people with multiple sclerosis (PWMS). Neurology Conference: 70th Annual Meeting of the American Academy of Neurology, AAN. 2018;90: P4.400. [Google Scholar]
- 138.Henriksen M, Lund H, Moe-Nilssen R, et al. Test-retest reliability of trunk accelerometric gait analysis. Gait Posture 2004;19:288-97. 10.1016/S0966-6362(03)00069-9 [DOI] [PubMed] [Google Scholar]
- 139.Grimpampi E, Oesen S, Halper B, et al. Reliability of gait variability assessment in older individuals during a six-minute walk test. J Biomech 2015;48:4185-9. 10.1016/j.jbiomech.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 140.Storm FA, Buckley CJ, Mazzà C. Gait event detection in laboratory and real life settings: Accuracy of ankle and waist sensor based methods. Gait Posture 2016;50:42-6. 10.1016/j.gaitpost.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 141.Godfrey A, Del Din S, Barry G, et al. Within trial validation and reliability of a single tri-axial accelerometer for gait assessment. Annu Int Conf IEEE Eng Med Biol Soc 2014;2014:5892-5. 10.1109/EMBC.2014.6944969 [DOI] [PubMed] [Google Scholar]
- 142.González RC, López AM, Rodriguez-Uría J, et al. Real-time gait event detection for normal subjects from lower trunk accelerations. Gait Posture 2010;31:322-5. 10.1016/j.gaitpost.2009.11.014 [DOI] [PubMed] [Google Scholar]
- 143.Trojaniello D, Cereatti A, Della Croce U. Accuracy, sensitivity and robustness of five different methods for the estimation of gait temporal parameters using a single inertial sensor mounted on the lower trunk. Gait Posture 2014;40:487-92. 10.1016/j.gaitpost.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 144.Trojaniello D, Ravaschio A, Hausdorff JM, et al. Comparative assessment of different methods for the estimation of gait temporal parameters using a single inertial sensor: application to elderly, post-stroke, Parkinson's disease and Huntington's disease subjects. Gait Posture 2015;42:310-6. 10.1016/j.gaitpost.2015.06.008 [DOI] [PubMed] [Google Scholar]
- 145.Oyake K, Yamaguchi T, Sugasawa M, et al. Validity of gait asymmetry estimation by using an accelerometer in individuals with hemiparetic stroke. J Phys Ther Sci 2017;29:307-11. 10.1589/jpts.29.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sejdić E, Lowry KA, Bellanca J, et al. Extraction of stride events from gait accelerometry during treadmill walking. IEEE J Transl Eng Health Med 2016. doi: . 10.1109/JTEHM.2015.2504961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jarchi D, Lo B, Ieong E, et al. Validation of the e-AR Sensor for Gait Event Detection Using the Parotec Foot Insole with Application to Post-Operative Recovery Monitoring. 2014 11th International Conference on Wearable and Implantable Body Sensor Networks; 2014:127-31. [Google Scholar]
- 148.Jarchi D, Peters A, Lo B, et al. Assessment of the e-AR sensor for gait analysis of Parkinson;s Disease patients. 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN). 2015:1-6. [Google Scholar]
- 149.Maqbool HF, Husman MAB, Awad MI, et al. A Real-Time Gait Event Detection for Lower Limb Prosthesis Control and Evaluation. IEEE Trans Neural Syst Rehabil Eng 2017;25:1500-9. 10.1109/TNSRE.2016.2636367 [DOI] [PubMed] [Google Scholar]
- 150.Lemoyne R, Mastroianni T, Cozza M, et al. Implementation of an iPhone as a wireless accelerometer for quantifying gait characteristics. Annu Int Conf IEEE Eng Med Biol Soc 2010;2010:3847-51. 10.1109/IEMBS.2010.5627699 [DOI] [PubMed] [Google Scholar]
- 151.Pepa L, Verdini F, Spalazzi L. Gait parameter and event estimation using smartphones. Gait Posture 2017;57:217-23. 10.1016/j.gaitpost.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 152.Silsupadol P, Teja K, Lugade V. Reliability and validity of a smartphone-based assessment of gait parameters across walking speed and smartphone locations: Body, bag, belt, hand, and pocket. Gait Posture 2017;58:516-22. 10.1016/j.gaitpost.2017.09.030 [DOI] [PubMed] [Google Scholar]
- 153.Ardle RM, Galna B, Thomas A, et al. Continuous Monitoring of Gait: What Can It Tell Us About Dementia? Alzheimer's and Dementia 2018;14:P192-3. 10.1016/j.jalz.2018.06.2031 [DOI] [Google Scholar]
- 154.Suttanon P, Hill KD, Said CM, et al. Feasibility, safety and preliminary evidence of the effectiveness of a home-based exercise programme for older people with Alzheimer's disease: a pilot randomized controlled trial. Clin Rehabil 2013;27:427-38. 10.1177/0269215512460877 [DOI] [PubMed] [Google Scholar]
- 155.Santoyo C, Fabregas D, Janer M, et al. Changes in spatio-temporal gait parameters after physical rehabilitation in Multiple Sclerosis: A descriptive analysis. Mult Scler J 2018;24:866. [Google Scholar]
- 156.Zanetta C, Pisa M, Guerrieri S, et al. Intensive neurorehabilitation is associated with improved gait kinematic analysis in progressive multiple sclerosis. Mult Scler J 2017;23:144. [Google Scholar]
- 157.Ono Y, Ora H, Kiko Y, et al. Gait evaluation of normal pressure hydrocephalus using inertial sensor. J Neurol Sci 2017;381:722. 10.1016/j.jns.2017.08.2035 [DOI] [Google Scholar]
- 158.Chakravorty A, Mobbs RJ, Anderson DB, et al. The role of wearable devices and objective gait analysis for the assessment and monitoring of patients with lumbar spinal stenosis: systematic review. BMC Musculoskelet Disord 2019;20:288. 10.1186/s12891-019-2663-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yoong NKM, Perring J, Mobbs RJ. Commercial Postural Devices: A Review. Sensors (Basel) 2019;19:5128. 10.3390/s19235128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Simpson L, Maharaj MM, Mobbs RJ. The role of wearables in spinal posture analysis: a systematic review. BMC Musculoskelet Disord 2019;20:55. 10.1186/s12891-019-2430-6 [DOI] [PMC free article] [PubMed] [Google Scholar]