Abstract
Background.
Neuroinflammation is a well-known feature of early Alzheimer disease (AD) yet astrocyte activation has not been extensively evaluated with in vivo imaging in Mild Cognitive Impairment (MCI) due to amyloid plaque pathology. Unlike neurons, astrocytes metabolize acetate, which has potential as a glial biomarker in neurodegeneration in response to AD pathologic features. Since the medial temporal lobe (MTL) is a hotspot for AD neurodegeneration and inflammation, we assessed astrocyte activity in the MTL and compared to amyloid and cognition.
Methods.
We evaluate spatial patterns of in vivo astrocyte activation and their relationships to amyloid deposition and cognition in a cross-sectional pilot study of 6 participants with MCI and 5 cognitively normal (CN) participants. We measure 11C-acetate and 18F-florbetaben amyloid standardized uptake values ratios (SUVRs) and kinetic flux compared to cerebellum on positron emission tomography (PET), with magnetic resonance imaging and neurocognitive testing.
Results.
Medial temporal lobe (MTL) 11C-acetate SUVR was significantly elevated in MCI compared to CN participants (P = 0.03; Cohen d = 1.76). Moreover, MTL 11C-acetate SUVR displayed significant associations with global and regional amyloid burden in MCI. Greater MTL 11C-acetate retention was significantly related with worse neurocognitive measures including the Montreal Cognitive Assessment (P = 0.001), Word List Recall memory (P = 0.03), Boston Naming Test (P = 0.04) and Trails B test (P = 0.04).
Conclusions.
While further validation is required, this exploratory pilot study suggests a potential role for 11C-acetate PET as a neuroinflammatory biomarker in MCI and early AD to provide clinical and translational insights into astrocyte activation as a pathological response to amyloid.
Keywords: Alzheimer Disease, Cognition, Amyloid, Neuroinflammation, Astrocyte, Acetate, PET
INTRODUCTION
Neuroinflammation is a major pathological feature of Alzheimer disease (AD). Alzheimer and Fischer’s 1907 discoveries identified the association between activated astrocytes and amyloid plaques [1,2], which was subsequently confirmed [3-5]. In AD, astrocyte activation is observed particularly in the hippocampus within the medial temporal lobe (MTL) [6]. Neuroinflammation in mild cognitive impairment (MCI) and AD has been studied most often through microglial imaging using positron emission tomography (PET) [7]. However, methods to evaluate astrocyte activation with in vivo metabolite-based imaging have not been extensively studied in MCI and AD.
Here, we explore astrocyte activation in MCI due to AD pathology using 11C-acetate PET. Acetate is a metabolic substrate for astrocytes but not neurons, particularly when used for fatty acid synthesis and glial proliferation [8,9]. Astrocyte activation is a dynamic process comprising different activity states [6]. Ex vivo and in vitro findings demonstrate that activated astrocytes linked to AD will display intracellular accumulation of fatty acids and lipids [1,10,11]. These observations in AD support in vivo imaging results from other brain diseases showing that astrocyte proliferation and anabolism are associated with higher 11C-acetate retention [12,13]. Since 11C-acetate PET has already been applied to evaluate activated astrocytes in patients with multiple sclerosis [14], alcoholism [15] and glioma [16], we sought to test 11C-acetate PET as a marker of astrocyte neuroinflammation in patients with MCI and amyloid pathology.
Guided by literature and analysis of ex vivo astrocyte markers (Supplement), we predicted that in vivo 11C-acetate retention is elevated in the MTL. Furthermore, we posited that 11C-acetate retention correlated with amyloid PET burden and with worse performance on tests of MTL-associated memory and global cognition. We emphasize that this pilot study is exploratory; while this inquiry cannot definitively answer these hypotheses, it can provide insight into the potential applications of 11C-acetate PET in MCI and AD.
MATERIALS AND METHODS
Study Design and Participants
This cross-sectional imaging study (NIH NCT02811744) was approved by the Institutional Review Board of our hospital and evaluated participants over the age of 60 years old from 2016 to 2018. Informed consent was obtained by a physician. Participants with normal cognition and MCI were assessed for competence and accompanied by a study partner. Participants with MCI had amnestic phenotype and amyloid-positive PET, thus characterized as having MCI due to AD pathology. Both amyloid-positive and amyloid-negative participants with normal cognition were recruited. Though this diverse “control group” reduces the power to identify group differences in MCI, this approach more realistically captures the heterogeneity in amyloid levels found in cognitively normal older adults. Moreover, such a cognitively normal (CN) group with both amyloid-negative and amyloid-positive participants provides a dynamic range of subclinical cerebral amyloid levels to identify possible relationships between regional 11C-acetate and amyloid PET measures.
Magnetic Resonance Imaging
Isotropic T1 magnetization-prepared rapid gradient echo (MPRAGE; 0.8mm3) magnetic resonance imaging (MRI) on a 3 Tesla scanner (Magnetom Prisma or Trio model, Siemens, Erlangen, Germany) with a 64-channel head coil were obtained within 111±182 days of 11C-acetate PET scan. There was no significant difference of time between 11C-acetate and MRI for cognitively normal and MCI groups. MRIs were used for defining regions of interest (ROIs) and adjusting for atrophy-related cerebrospinal fluid dilution.
11C-Acetate PET
PET was performed on an Ingenuity TF PET/computed tomography (CT) scanner (Phillips Healthcare, Amsterdam, Netherlands) with low dose CT for attenuation correction. Reconstruction used BLOB-OS-TF with trans-axial field of view of 256mm and 2mm isotropic voxels. 11C-Acetate was produced at our GMP cyclotron facility per established protocol [17]. Each subject was injected with about 20mCi (range 19-24mCi) as a rapid bolus with simultaneous initiation of a 60 minute, 50 frame dynamic acquisition (24x5 seconds, 6x10 seconds, 3x20 seconds, 2x30 seconds, 5x60 seconds, 10x300 seconds). Venous blood samples were collected at 2, 5, 10, 20, 40, and 60 minutes post-injection of 11C-acetate to quantify the amount of the metabolite 11C-CO2 in blood over time. Whole blood samples were mixed into a basic solution of 1-part 0.9M NaHCO3, 3-parts isopropanol, and 1-part 0.1N NaOH and an acidic solution containing 1-part 0.9M NaHCO3, 3-parts isopropanol, and 1-part 6N HCl. The basic solution was sealed, while the acidic solution was placed in a positive pressure manifold and exposed to an air current to release metabolized 11C-CO2. Samples were counted in Wizard2 2480 gamma counter (PerkinElmer, Waltham, MA, United States) to calculate percentage un-metabolized parent for kinetic analyses of tracer uptake.
Regional time-activity curves (TACs) were quantified using 30 ROIs determined from fused T1-weighted MRI in the PMOD package v3.7 (PMOD Technologies, Zurich, Switzerland) and the Automated Anatomical Labeling merged atlas [18]. Examples of TACs from the venous blood sampling and individual brain ROIs are shown in Figures S1 and S2, respectively. Regional TACs were quantified without and with partial volume correction (PVC) using geometric transfer ratio method implementation in PMOD [19]. Results are presented with PVC as findings without PVC are similar. Since AD and amnestic MCI demonstrate relatively symmetric, bilateral pathology of amyloid deposition [20-22], atrophy [23] and glial-mediated neuroinflammation [7], left and right ROIs were combined via volume-weighted average in regional analyses. Our cohort displayed right and left symmetry for both amyloid and 11C-acetate imaging. The Heschl gyrus ROI was excluded from study analyses due to small size (<1cm3) relative to our PET scanner’s spatial resolution of 5mm full width at half maximum [24].
For static analysis, uptake from 40-60 minutes post-injection dynamic frames were averaged to generate standardized uptake value (SUV) parametric images. This timeframe was selected to preferentially capture 11C-acetate trapped in biosynthetic pathways in activated astrocytes, rather than what was catabolized to 11C-CO2 [9,14,16,25]. SUV was normalized to cerebellar gray matter (GM) as a physiological comparator region to calculate SUV ratio (SUVR) [14,16]. Until late stages of AD (well beyond early stages in our sample), cerebellar GM is mostly free of amyloid deposition [7,26-28] and glial activation [7,29-31]. Unlike cerebellar white matter, the GM contains more abundant astrocytes, the cell type of interest. Thus, cerebellar GM astrocytes in MCI are not exposed to local amyloid-related neuropathologic changes so this region can serve as a comparator for astrocyte metabolism in the absence of Alzheimer pathology [29-31].
For kinetic modeling, image-derived arterial input functions were calculated per previously published methods [32] and were scaled using individual venous blood data from 10-60 minutes post-injection. Kinetic parameters of regional brain 11C-acetate uptake were modeled in PMOD. TACs were fitted using Patlak graphical methods [33,34]. The model inputs included individual blood input functions and the population average 11C-acetate parent fractions (Figure S1B). Plasma 11C-acetate concentrations were assumed to be equal to whole blood concentrations. Brain blood volume fractions were fixed at 5%. Patlak linear analyses were performed to derive kinetic flux (Ki). Due to anticipated errors in kinetic modeling for 11C-acetate from lack of invasive cannulated arterial input function, inherent limitations of image-derived input functions [35] and potential variability in biodistribution of the 11C-CO2 metabolite, we evaluated a flux ratio (KiR) based on nuclear medicine literature [36-40] with cerebellar GM as comparator region, similar to astrocyte imaging studies with 11C-deuterium-L-deprenyl [29-31]. Kinetic flux was compared to cerebellar GM to normalize for variability in 11C-acetate delivery, washout and background due to metabolism to 11C-CO2 in a region of baseline astrocyte activity without the presence of amyloid. Time stability curves of regional distribution volumes (VT) were created with the Logan method [41] with PMOD. Dynamic PET scans were visualized on MIM (MIM Software Inc, Cleveland, OH, United States).
18F-Florbetaben PET
Amyloid PET was performed as 20-minute scans after 8.1mCi 18F-florbetaben intravenous injection as per standard clinical protocol within 176±108 days prior to 11C-acetate PET scan. SUV analyses were performed similar to methods described above. The SUVR reference region was the cerebellum, as described in literature [26,27]. A trained neuroradiologist interpreted amyloid scans as amyloid-positive or amyloid-negative per established qualitative guidelines [27]. There was no significant difference in the time between 11C-acetate and 18F-florbetaben PET scans for cognitively normal and MCI groups. One cognitively normal participant had 18F-florbetapir PET and was excluded from comparisons of regional amyloid 18F-florbetaben vs. 11C-acetate but was included for other analyses.
Cognitive Evaluation
Cognitive testing was performed the day of 11C-acetate PET and included the Word List Recall memory (10 word test) [42], Montreal Cognitive Assessment (MoCA) [43], Boston Naming Test (BNT) [44], Trail Making B Test [45] and Rey Auditory-Verbal Learning Test [46] per NIH AD Research Center (ADRC) guidelines.
Statistical Analysis
Descriptive statistics show mean and standard deviation unless otherwise specified. Analysis was performed with 2-sample independent means t-tests with 2-tailed P values. Effect size was computed with the Cohen d statistic. Due to the exploratory nature of these pilot study investigations, P values were not corrected for multiple comparisons and a threshold of P < 0.05 was considered statistically significant. With the goal of 0.80 power and 0.05 type I error, we predicted the sample size would be sufficient to detect mean SUVR difference of 0.1. Regressions for cognition and 11C-acetate measures were evaluated with Spearman correlations. To evaluate amyloid and 11C-acetate relationships across the 30 brain ROIs sampled within subjects, linear mixed-effects regression model analyses were performed using the nlme package in R v4.0.2 [47]. Fixed effects included regional 11C-acetate measures and amyloid SUVR for each ROI in each participant. The participants served as random effects to account for the regional variation between individuals. Differences in correlations were determined by 2-tailed Fisher R-to-Z transformation. Differences in regressions were compared by 2-sample t-tests between slopes. Given the sample size, leave-one-participant-out analysis was performed to assess if individual participants were disproportionately influencing correlations; this did not change the results.
RESULTS
There were 6 participants with MCI (mean age 72±6 years) compared to 5 CN participants (74±10 years, 3 amyloid-positive, 2 amyloid-negative). As expected, patients with MCI had significantly worse scores on the Montreal Cognitive Assessment (MoCA) (P = 0.02) and Word List Recall memory test (P = 0.04), as well as lower bilateral MTL volume (P < 0.05) compared to CN participants (Table 1).
TABLE 1.
Study participant characteristics. Abbreviations: standard deviation (SD), Montreal Cognitive Assessment (MoCA), Boston Naming Test (BNT), medial temporal lobe (MTL). P values are from 2-sample independent t-tests.
| Study Characteristic | Cognitively Normal |
MCI |
P value |
|---|---|---|---|
| Total participants | 5 | 6 | |
| Female (%) | 2 (40%) | 4 (67%) | |
| Age, mean (SD) [range], years | 74 (10) [61-85] | 72 (6) [65-84] | 0.75 |
| Amyloid PET positive (%) | 3 (60%) | 6 (100%) | |
| MoCA, mean (SD) [range] | 29 (2) [25-30] | 24 (2) [21-27] | 0.02 |
| Word List Recall, mean (SD) [range] | 8 (2) [6-10] | 5 (2) [2-7] | 0.04 |
| BNT, mean (SD) [range] | 28 (1) [27-30] | 26 (4) [21-30] | 0.20 |
| Trails Time, mean (SD) [range], seconds | 63 (19) [34-88] | 135 (76) [68-260] | 0.07 |
| MTL Volume, mean (SD), cm3 | 16.1 (1.9) | 13.5 (1.9) | <0.05 |
Elevated MTL 11C-Acetate in MCI
Despite these distinctions, there was no significant global difference in 11C-acetate SUVR between MCI and CN groups (P = 0.50). However, lack of global 11C-acetate elevation does not preclude a localized astrocyte response to amyloid. Ex vivo glial activation has been noted in the hippocampus, a critical memory node within the medial temporal lobe (MTL) [6]. Indeed, post-mortem biomarker expression analysis revealed elevations of astrocyte and acetate metabolism markers in the hippocampi of patients with AD relative to CN participants (Figure S3). See Supplementary Methods for additional information.
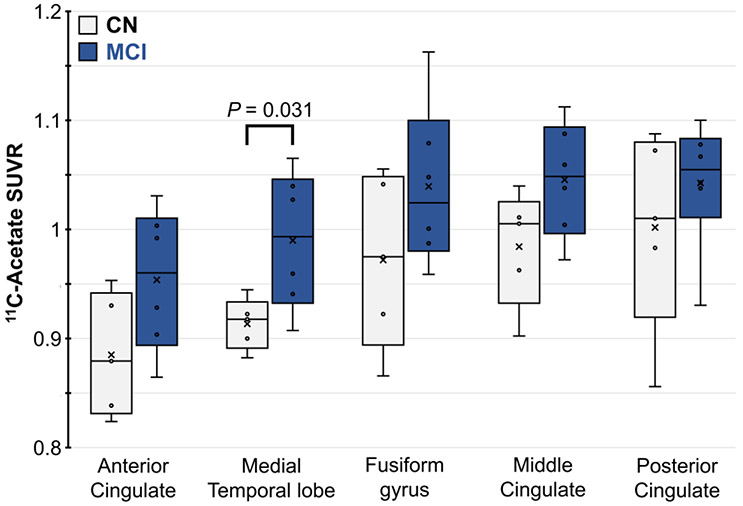
Thus, we hypothesized that MTL 11C-acetate retention is elevated in MCI participants with amyloid pathology. We found that participants with MCI had significantly higher mean 11C-acetate SUVR in the MTL (0.99±0.06) compared to CN participants (0.91±0.02; Cohen d = 1.76; P = 0.03) (Table 1, Figure 1). The magnitude of this observation is consistent with effect sizes of other glial activation radiotracers; indeed, Cohen d effect sizes of regional 11C-PBR28 activity range from 0.8-1.7 [48]. Additionally, limbic structures, such as cingulate and fusiform gyri, trended towards modest 11C-acetate elevation in MCI (Figure 2, Table S1). These findings were replicated with KiR data (Table S2). The time stability curve (Figure S4) further depicts relatively stable VT from 40-60 minutes consistent with non-accumulation of labelled metabolites, such that activity uptake and washout is likely due to the parent radiotracer.
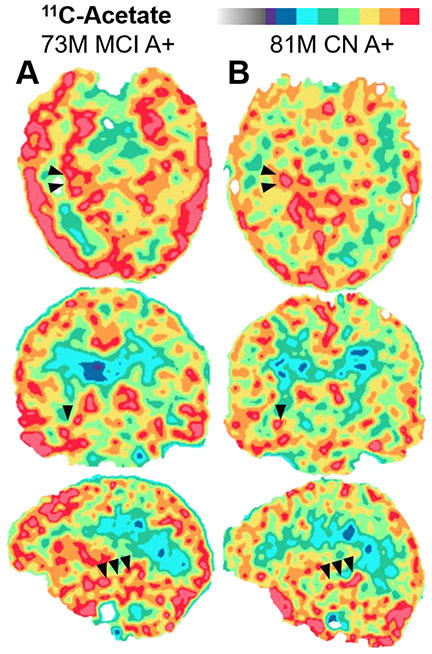
FIGURE 1.
Example 11C-acetate SUVR maps in representative amyloid-positive (A+) participants with (A) mild cognitive impairment (MCI) or (B) normal cognition (CN). Arrowheads depict the hippocampus in the right medial temporal lobe.
FIGURE 2.
11C-Acetate SUVR in limbic brain ROIs of participants with MCI (blue) and normal cognition (CN, gray). P values < 0.05 are shown.
Association of 11C-Acetate and Amyloid PET
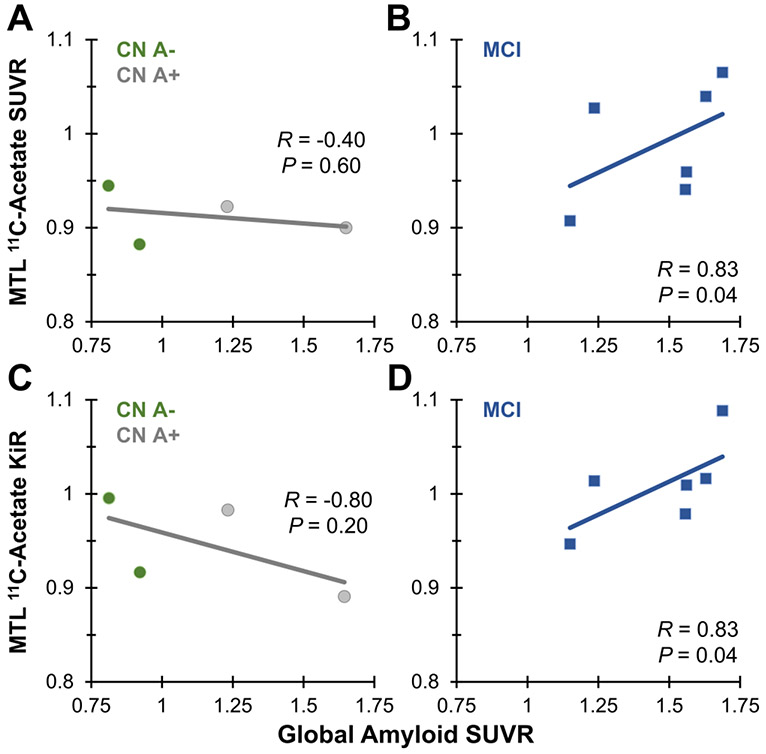
Our cohort was also tested for amyloid plaque levels by in vivo PET (Figure S5). Since overall neocortical amyloid deposition is associated with localized MTL neurodegeneration and neuroinflammation [49], we further examined the relationships between 11C-acetate in the MTL and global amyloid burden in the supratentorial gray matter. MTL 11C-acetate was positively correlated with global amyloid burden in the MCI group (R = 0.83; P = 0.04) but not in the normal cognition group (Figure 3A,B). These results were consistent with KiR data (Figure 3C,D). The differences in correlations of the CN and MCI groups by Fisher R-to-Z transformation were statistically significant (P values < 0.05), thereby supporting a potential association between local astrocyte activity and amyloid burden in MCI.
FIGURE 3.
Comparison of MTL 11C-acetate SUVR vs. global amyloid SUVR in (A) 4 CN and (B) 6 MCI participants. Comparison of MTL 11C-acetate Patlak flux ratio (KiR) vs. global amyloid SUVR in (C) 4 CN and (D) 6 MCI participants. Linear regressions, Spearman correlations and P values are shown.
Activated astrocytes co-localize with amyloid plaques in several neuropathological studies [3,4,49], so we hypothesized that we would find greater 11C-acetate retention in regions with greater amyloid burden. Since amyloid deposition varies between and within subjects, we studied regional association of astrocyte activation and amyloid across participants and regions with 11C-acetate and 18F-florbetaben amyloid PET, respectively, as a mixed linear effects model (Figure S6A,B). Intriguingly, for cognitively normal (CN) participants, regional 11C-acetate retention revealed a significant negative association with regional amyloid deposition (R = −0.36; P < 0.001). For MCI, regional 11C-acetate positively associated with regional amyloid (R = 0.33; P < 0.001). Between CN and MCI cohorts, significant group differences were noted for comparisons of both correlations and slopes (P values < 0.01). Furthermore, these amyloid correlation findings were replicated with 11C-acetate kinetic data (Figure S6C-F). With the 11C-acetate flux measures, differences between both correlations and slopes for CN and MCI were also significant (P values < 0.01).
Association of MTL 11C-Acetate and Cognition
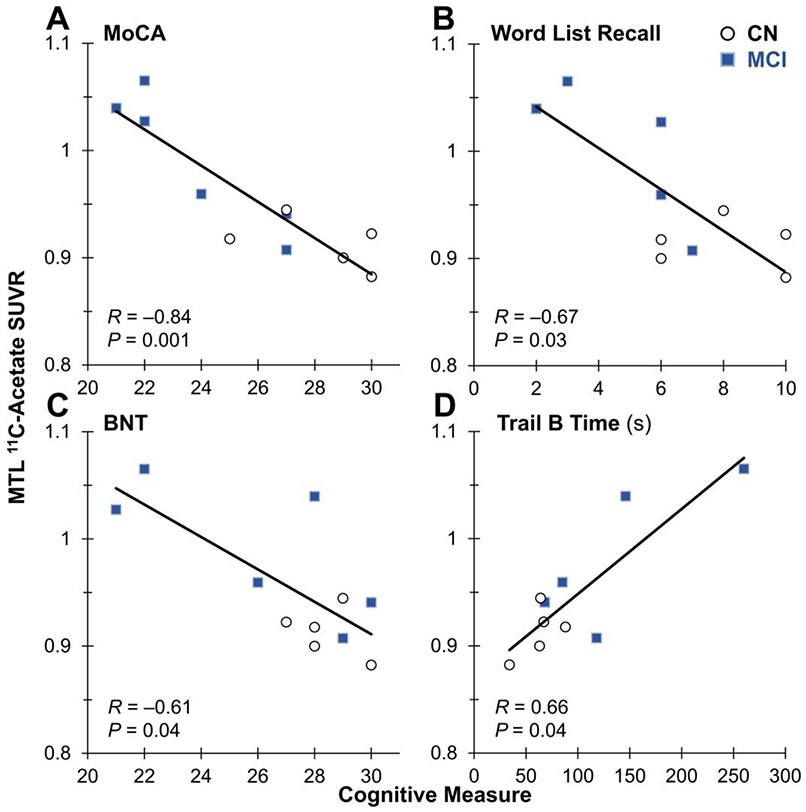
Next, we determined how MTL 11C-acetate varied with cognition, including tests sensitive to episodic memory (Figure 4). Across all participants, elevated MTL 11C-acetate SUVR was significantly associated with worse cognitive performance on tests such as the MoCA (R = −0.84; P = 0.001), Word List Recall memory (R = −0.67; P = 0.03), BNT (R = −0.61; P = 0.04) and Trails B Test (R = 0.66; P = 0.04).
FIGURE 4.
Comparison across all participants of MTL 11C-acetate SUVR vs. cognitive tests of (A) Montreal Cognitive Assessment (MoCA), (B) Word List Recall (a test of hippocampal memory), (C) Boston Naming Test (BNT) and (D) Trails B time (in seconds). Linear regressions, Spearman correlations and P values are shown.
After excluding CN participants from the regression analysis, associations between 11C-acetate and cognition in participants with MCI remained significant for the MoCA (P = 0.02) and trended towards significance with Word List Recall (P = 0.05; Figure S7). The KiR flux replicated significant associations between 11C-acetate and cognition for the MoCA, BNT and Trails B Test (P values < 0.05), with a trend for Word List Recall (P = 0.08) (Figure S8).
DISCUSSION
This exploratory pilot study assessed 11C-acetate PET as a biomarker in MCI with amyloid pathology. Astrocyte activation is seen on post-mortem assessments in early AD [1-4], however in vivo imaging of astrocyte metabolism biomarkers has not been surveyed extensively. Acetate uptake characterizes proliferating astrocytes [8,9,12-16] and may measure astrocyte activation in MCI. Overall, our findings depict regional correlation between amyloid and 11C-acetate retention. Activation in the MTL also correlates with a global pattern of amyloid.
The MTL has been shown to be a hotspot of neuroinflammation in cognitive impairment and neurodegeneration [3-6,23,49,50]; this is concordant with our post-mortem expression analysis and in vivo 11C-acetate imaging study. In addition to the significant group difference in the MTL, there is a general though modest elevation of 11C-acetate retention across additional limbic brain regions, though this observation is limited by the exploratory nature of the pilot study. Notably, similar effect sizes are seen with other radiotracers of glial activity such as 11C-PBR28 [7,29,30,48]. Limbic regions were shown in one study to have higher amyloid load and microglial inflammation in AD [7]; here, they displayed modest trends towards 11C-acetate elevation in MCI. Consistent with other inflammation radiotracers [7,29,30], this trend may represent a relatively wider increase of astrocyte activity associated with diffuse cerebral amyloid pathology. However, the MTL might have the most discernible effect size for participants with MCI and early AD. Our pilot study was only sufficiently powered to detect an average 11C-acetate SUVR difference of about 0.1. Based off prior 11C-acetate neuroimaging studies, such a difference is consistent with disease-associated astrocyte activation [14,16].
The associations of 11C-acetate, amyloid and clinical status are intricate. Significant elevation of MTL 11C-acetate was found in participants with MCI; this effect is opposite of the well-known AD-related neuronal metabolic reductions measured with 18F-fluorodeoxyglucose PET but compatible with prior literature on acetate as a biomarker of astrocyte activation [12-16]. Previous gene-expression studies have isolated distinct subtypes of reactive astrocytes, including pro-inflammatory, neurotoxic astrocytes enriched in AD and homeostatic astrocytes found in cognitively normal subjects [5,6]. While 11C-acetate imaging may not be able to distinguish between distinct types of astrocyte activation, our observed associations of 11C-acetate and amyloid retention are consistent with amyloid-induced, astrocyte-mediated inflammation [3-5]. Indeed, greater regional and global amyloid uptake were associated with higher regional 11C-acetate in MCI, a correlation not mirrored in amyloid-positive, cognitively normal adults. Though additional studies are required, this directional difference raises the possibility that 11C-acetate elevation is an astrocyte marker for the evolution of a proliferative inflammatory response and perhaps an amyloid-related neurodegenerative process in early AD that is not found in amyloid-positive, cognitively normal subjects. To this point, elevated MTL 11C-acetate correlated with worse cognition, suggesting that 11C-acetate is a marker of disease severity. Together, these in vivo imaging and post-mortem expression findings indicate 11C-acetate is a potential neuroinflammation marker of early astrocyte changes in early AD.
Our study has limitations. First, this exploratory pilot study had small sample size, limiting the power to detect changes in 11C-acetate and perhaps increasing potential for sampling-related bias. Power in MCI vs. CN group comparisons was likely further reduced by the inclusion of amyloid-positive and amyloid-negative participants in the CN group. Nevertheless, this heterogeneous CN group more faithfully represents the variability of amyloid levels in cognitively unimpaired older adults and allows the evaluation of astrocyte activity in response to amyloid plaques at a dynamic range of subclinical amyloid burden. Next, the lack of detection of regional differences between MCI and CN groups outside the MTL may be due to sample size. Since this between-group effect size was modest, it is not clear that 11C-acetate PET would be useful in all individual cases, at least in the absence of a separate amyloid PET study. Tau status was not known, and variability in pathological tau aggregates could potentially affect localization and extent of astrocyte activation [3,49,50]. Though it is possible that additional non-neuronal cells may contribute to 11C-acetate uptake, much of the literature has focused on acetate uptake as an astrocyte marker [8,9,13]. Moreover, acetate metabolism is associated with reduced microglial activation [51], compared to greater astrocyte activity [8,9].
Additionally, PET signal was assessed using SUVR, kinetic flux and flux compared to cerebellum, which may correct for variability in tracer delivery, washout and background due to 11C-acetate metabolism to 11C-CO2 [14,16]. Time-activity curves stabilized after 20 minutes (Figure S2 and S4) and we measured SUVR from 40-60 minutes, consistent with previous studies [9,14]. At this later time point after 11C-acetate injection, most of the signal was likely attributed to 11C radionuclide from the parent tracer incorporated into macromolecules and thus represents a measure of astrocyte anabolic function and proliferative activity rather than a marker of just astrocyte energy catabolism or accumulation of radiometabolite alone. This is consistent with prior in vivo PET studies of homeostatic and proliferative astrocytes in healthy brains and patients with glioma [52,53]. Utilizing radiotracers that produce 11C-CO2 metabolites (such as 11C-thymidine), these studies have demonstrated a relatively ubiquitous 11C-CO2 uptake with minimal tissue trapping similar to the results seen here. We had predicted that the lack of arterial input may impact the quality of the data used in the kinetic flux analysis, which was confirmed by our observation of inter-subject variability in Ki measurements (Figure S6). Thus, we utilized a flux measure [36-40] compared to cerebellar GM [29-31] to address such biases. In addition to quality of the data used, another limitation may pertain to the analytical approach. These findings suggest that the assumption of the Patlak method, that the radiotracer irreversibly binds to the specific target (k4 = 0) from injection until the end of the imaging session, may not be entirely appropriate here, and may be related to the reversible exchange and lack of tissue trapping of the metabolite 11C-CO2. Hence, the flux ratio was utilized to reduce potential biases related to data and model limitations when comparing between participants and further work will compare the flux ratio to methods that do not require irreversible binding using 11C-acetate PET data acquired with arterial blood sampling and metabolite analysis.
Future directions include the validation of these pilot study results with a larger cohort of study participants and longitudinal study design. Given the differences in dynamic ranges and spatial localization of 11C-acetate and 18F-florbetaben uptake, additional studies may acquire multiple scans to evaluate test-retest variability and changes over time to further illuminate the dynamic pathophysiological processes in early AD. Correlation of astrocyte activation through 11C-acetate PET with additional molecular imaging markers (such as tau PET) and post-mortem neuropathological assessment can also strengthen these findings.
CONCLUSIONS
This exploratory pilot imaging study in patients with amnestic MCI due to Alzheimer pathology suggests that astrocyte activation is associated with amyloid burden and can be detected with 11C-acetate PET. We observed relationships between regional amyloid deposition with 11C-acetate retention across the brain as well as associations between global amyloid burden with 11C-acetate in the medial temporal lobe. Further, medial temporal lobe 11C-acetate was significantly higher in participants with MCI and correlated with cognition. This study was limited by small sample size and larger trials with follow-up analyses and additional testing modalities are warranted. Together, 11C-acetate imaging may be a biomarker of early astrocyte activation in neurodegeneration.
Supplementary Material
Acknowledgments:
The authors gratefully acknowledge contributions from Dr. David A. Mankoff in analysis and review, Dr. Robert H. Mach in study design and Ms. Erin K. Schubert in protocol development.
Funding:
Research reported in this publication was supported by Alzheimer’s Disease Research Center funding from the National Institute on Aging under grant number P30-AG010124, the National Center for Advancing Translational Sciences under award number UL1TR001878 and the Transdisciplinary Program in Translational Medicine and Therapeutics from the Institute for Translational Medicine and Therapeutics at the University of Pennsylvania.
REFERENCES
- 1.Alzheimer A Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiat. 1907;64:146–148. [Google Scholar]
- 2.Fischer O Miliare Nekrosen mit drusigen Wucherungen der Neurofibrillen, eien regelmässige Veränderung der Hirnrinde bei seniler Demenz. Monatsschr Psychiat Neurol. 1907;22:361–372. [Google Scholar]
- 3.De Strooper B, Karran E. The Cellular Phase of Alzheimer's Disease. Cell. 2006;164(4):603–615. [DOI] [PubMed] [Google Scholar]
- 4.Serrano-Pozo A, Muzikansky A, Gomez-Isla T, et al. Differential relationships of reactive astrocytes and microglia to fibrillar amyloid deposits in Alzheimer disease. J Neuropathol Exp Neurol. 2013. 72(6):462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y-T, Seo J, Gao F, et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer's Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron. 2018;98:1141–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parbo P, Ismail R, Hansen KV, et al. Brain inflammation accompanies amyloid in the majority of mild cognitive impairment cases due to Alzheimer’s disease. Brain. 2017;140(7):2002–2011. [DOI] [PubMed] [Google Scholar]
- 8.Muir D, Berl S, Clarke DD. Acetate and fluoroacetate as possible markers for glial metabolism in vivo. Brain Res. 1986;380(2):336–340. [DOI] [PubMed] [Google Scholar]
- 9.Wyss MT, Magistretti PJ, Buck A, et al. Labeled acetate as a marker of astrocytic metabolism. J Cereb Blood Flow Metab. 2011;31(8):1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.TCW J, Liang SA, Qian L, et al. Cholesterol and matrisome pathways dysregulated in human APOE ε4 glia. bioRχiv, 2019. [Google Scholar]
- 11.Farmer BC, Kluemper J, Johnson LA, Apolipoprotein E4 Alters Astrocyte Fatty Acid Metabolism and Lipid Droplet Formation. Cells. 2019;8(2):182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang L, Gulanski BI, De Feyter HM, et al. Increased brain uptake and oxidation of acetate in heavy drinkers. J Clin Invest. 2013;123(4):1605–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashimo T, Pichumani K, Vemireddy V, et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159(7):1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takata K, Kato H, Shimosegawa E, et al. 11C-acetate PET imaging in patients with multiple sclerosis. PLOS ONE. 2014;9(11):e111598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkow ND, Kim SW, Wang GJ, et al. Acute alcohol intoxication decreases glucose metabolism but increases acetate uptake in the human brain. Neuroimage. 2013;64:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto Y, Nishiyama Y, Kimura N. 11C-acetate PET in the evaluation of brain glioma: comparison with 11C-methionine and 18F-FDG-PET. Mol Imaging Biol. 2008;10(5):281–287. [DOI] [PubMed] [Google Scholar]
- 17.Pike VW, Eakins MN, Allan RM, Selwyn AP, Preparation of [1-11C]acetate-an agent for the study of myocardial metabolism by positron emission tomography. Int J Appl Radiat Isot. 1982;33(7):505–512. [DOI] [PubMed] [Google Scholar]
- 18.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. [DOI] [PubMed] [Google Scholar]
- 19.Aston JA, Cunningham VJ, Asselin MC, et al. Positron emission tomography partial volume correction: estimation and algorithms. J Cereb Blood Flow Metab. 2002;22(8):1019–1034. [DOI] [PubMed] [Google Scholar]
- 20.Ikonomovic MD, Buckley CJ, Heurling K, et al. Post-mortem histopathology underlying β-amyloid PET imaging following flutemetamol F18 injection. Acta Neuropathol Commun. 2–16;4:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raji CA, Becker JT, Tsopelas ND, et al. Characterizing regional correlation, laterality and symmetry of amyloid deposition in mild cognitive impairment and Alzheimer’s disease with Pittsburgh Compound B. J Neurosci Methods. 2008;172:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moossy J, Zubenko GS, Martinez AJ, et al. Bilateral symmetry of morphologic lesions in Alzheimer's disease. Arch Neurol. 1988;45(3):251–254. [DOI] [PubMed] [Google Scholar]
- 23.Johnson KA, Fox NC, Sperling RA and Klunk WE. Brain Imaging in Alzheimer Disease. Cold Spring Harb Perspect Med, 2012:a006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolthammer JA, Su KH, Grover A, et al. Performance evaluation of the ingenuity TF PET/CT scanner with a focus on high count-rate conditions. Phys Med Biol. 2014;59(14):3843–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grassi I, Nanni C, Allegri V, et al. The clinical use of PET with 11C-acetate. Am J Nucl Med Mol Imaging. 2012;2(1):33–47. [PMC free article] [PubMed] [Google Scholar]
- 26.Catafau AM, Bullich S, Seibyl JP, et al. Cerebellar Amyloid-β Plaques: How Frequent Are They, and Do They Influence 18F-Florbetaben SUV Ratios? J Nucl Med. 2016;57:1740–1745. [DOI] [PubMed] [Google Scholar]
- 27.Sabri O, Seibyl J, Rowe C, Barthel H. Beta-amyloid imaging with florbetaben. Clin Transl Imaging. 2015;3:13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weigel J, Wisniewski HM, Dziewiatkotski J, et al. Cerebellar Atrophy in Alzheimer's Disease-Clinicopathological Correlations. Brain Res. 1999;6(818):41–50. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Vieitez E, Saint-Aubert L, Carter SF, et al. Diverging longitudinal changes in astrocytosis and amyloid PET in autosomal dominant Alzheimer's disease. Brain. 2016;139:922–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scholl M, Carter SF, Westman E, et al. Early astrocytosis in autosomal dominant Alzheimer's disease measured in vivo by multi-tracer positron emission tomography. Sci Rep. 2015;5:16404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carter SF, Scholl M, Almkvist O, et al. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11C-Pittsburgh compound B and 18F-FDG. J Nucl Med. 2012;53:37–46. [DOI] [PubMed] [Google Scholar]
- 32.O’Sullivan F, Muzi M, Mankoff DA, Eary JF, Spence AM and Krohn KA. Voxel-Level Mapping of Tracer Kinetics in PET Studies: a Statistical Approach Emphasizing Tissue Life Tables. Ann Appl Stat. 2014;8(2):1065–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patlak CS, Blasberg RG and Fenstermacher JD. Graphical Evaluation of Blood-to-Brain Transfer Constants from Multiple-Time Uptake Data. J Cereb Blood Flow Metab. 1983;3:1–7. [DOI] [PubMed] [Google Scholar]
- 34.Patlak CS, Blasberg RG. Graphical Evaluation of Blood-to-Brain Transfer Constants from Multiple-Time Uptake Data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–590. [DOI] [PubMed] [Google Scholar]
- 35.Zanotti-Fregonara P, Chen K, Liow J-S, Fujita M and Innis RB. Image-derived input function for brain PET studies: many challenges and few opportunities. J Cereb Blood Flow Metab. 2011;31(10):1986–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dhawan V, Ma Y, Pillai V, et al. Comparative Analysis of Striatal FDOPA Uptake in Parkinson’s Disease: Ratio Method Versus Graphical Approach. J Nucl Med. 2002;43(10):1324–1330. [PubMed] [Google Scholar]
- 37.Kawatsu S, Kato T, Nagano-Saito A, Hatano K, Ito K and Ishigaki T. New Insight Into the Analysis of 6-[18F]fluoro-L-DOPA PET Dynamic Data in Brain Tissue Without an Irreversible Compartment: Comparative Study of the Patlak and Logan Analyses. Radiat Med. 2003;21(1):47–54. [PubMed] [Google Scholar]
- 38.Alves IL, Meles SK, Willemsen ATM, et al. Dual time point method for the quantification of irreversible tracer kinetics: A reference tissue approach applied to [18F]-FDOPA brain PET. J Cereb Blood Flow Metab. 2017;37(9):3124–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Ye W, Brašić JR, Wong DF. Multi-graphical analysis of dynamic PET. Neuroimage. 2010;49(4):2947–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu YG, Zhou Y, Bao BL, et al. Using the rPatlak plot and dynamic FDG-PET to generate parametric images of relative local cerebral metabolic rate of glucose. Sci Bull. 2020;57:3811–3818. [Google Scholar]
- 41.Logan J, Fowler JS, Volkow ND, et al. Distribution Volume Ratios Without Blood Sampling from Graphical Analysis of PET Data. J Cereb Blood Flow Metab 1996;16(5):834–840. [DOI] [PubMed] [Google Scholar]
- 42.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. [DOI] [PubMed] [Google Scholar]
- 43.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan E, Goodglass H and Weintraub S. The Boston Naming Test. Lea & Febiger, Philadelphia, PA, 1983. [Google Scholar]
- 45.Reitan RM. Trail Making Test: Manual for administration and scoring. Reitan Neuropsychology Laboratory, Tucson, AZ,1992. [Google Scholar]
- 46.Schmidt M Rey Auditory Verbal Learning Test: RAVLT: a handbook. Western Psychological Services, Los Angeles, CA, 1996. [Google Scholar]
- 47.Pinhheiro J, Bates D, Debroy S, Sarkar D and R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. 3rd. ed., 2016. [Google Scholar]
- 48.Bloomfield PS, Selvaraj S, Veronese M, et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia; an [11C]PBR28 PET brain imaging study. Am J Psychiatry. 2016;173(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinney JW, Bemiller SM, Murtishaw AS, et al. Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement. 2018;4:575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duong MT, Nasrallah IM, Wolk DA, Chang CCY and Chang T-Y. Cholesterol, Atherosclerosis, and APOE in Vascular Contributions to Cognitive Impairment and Dementia (VCID): Potential Mechanisms and Therapy. Front Aging Neurosci. 2021;13:647990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soliman ML, Puig KL, Combs CK and Rosenberger TA. Acetate reduces microglia inflammatory signaling in vitro. J Neurochem. 2013;123(4):555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wells JM, Mankoff DA, Muzi M, O'Sullivan F, Eary JF, Spence AM, Krohn KA. Kinetic analysis of 2-[11C]thymidine PET imaging studies of malignant brain tumors: compartmental model investigation and mathematical analysis. Mol Imaging. 2002. Jul;1(3):151–9. [DOI] [PubMed] [Google Scholar]
- 53.Wells JM, Mankoff DA, Eary JF, Spence AM, Muzi M, O'Sullivan F, Vernon CB, Link JM, Krohn KA. Kinetic analysis of 2-[11C]thymidine PET imaging studies of malignant brain tumors: preliminary patient results. Mol Imaging. 2002. Jul;1(3):145–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.