To the Editor: Psoriatic skin was recently described as a potential host site for SARS-CoV-2, the cause of COVID-19, due to the high expression of angiotensin-converting enzyme 2 (ACE2), which is the main viral host receptor in the epidermis of lesional skin.1,2 There are positive correlations between ACE2 expression, Psoriasis Area Severity Index scores, and interleukin 17C (IL-17C) expression at baseline,3 whereas treatment with an anti-IL-17 antibody reduces the risk of COVID-19 in psoriatic patients by downregulating ACE2 expression in affected skin.3 , 4 However, the effect of other molecule-targeted therapies on ACE2 expression in psoriatic skin remains unknown.
To determine this effect, we collected microarray data (GSE13355, GSE14905, GSE30999, GSE34248, GSE41662, GSE47751, GSE50790, GSE53552, GSE57376, GSE78097, GSE106992, GSE117239, GSE136757, GSE31652, and GSE137218) from the Gene Expression Omnibus database. The samples included normal skin (n = 89), psoriatic nonlesional skin (n = 456), and lesional skin (n = 502) at baseline. Samples were also obtained from lesional skin after treatment with placebo (n = 23), with 140 mg, 350 mg, or 700 mg brodalumab (n = 4, 4 or 8), with LY2439821 (n = 6), with secukinumab (n = 14), with etanercept (n = 60), with 45 mg or 90 mg ustekinumab (n = 18 or 55), with tofacitinib (n = 8), or with 30 mg or 100 mg PF-06700841 (n = 7 or 5) at the end time point. The data were analyzed with the Transcriptome Analysis Console software 4.0 (Applied Biosystems, Thermo Fisher Scientific). The least square means by group and fold change were calculated. Hypotheses were tested using 1-way analysis of variance with Tukey’s test. P < .05 was considered statistically significant.
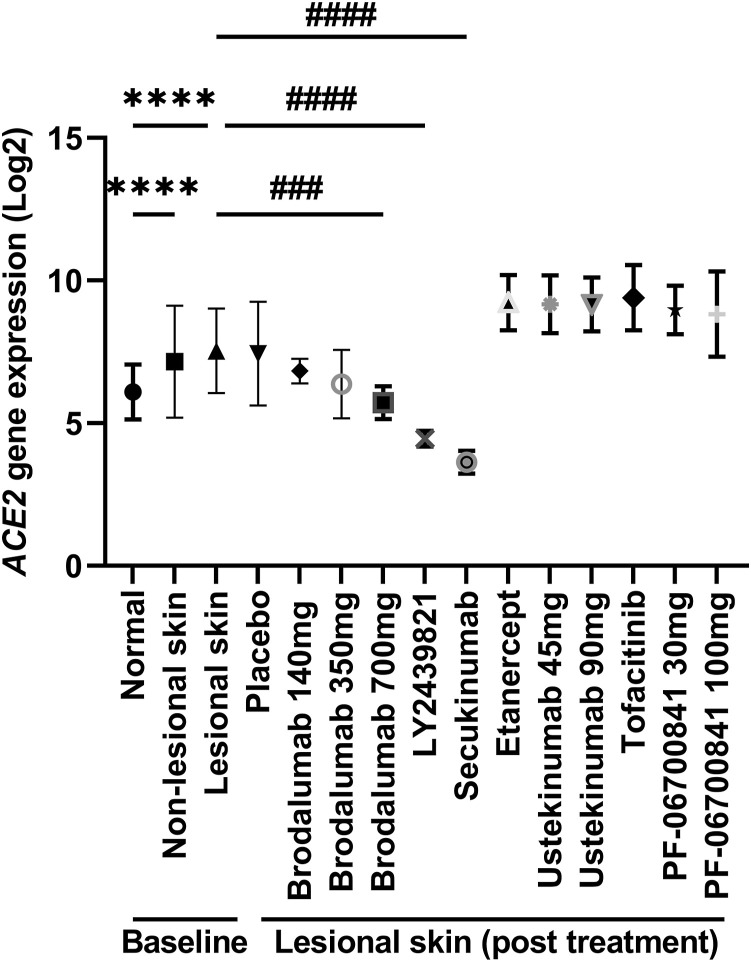
At baseline, ACE2 expression was significantly upregulated in psoriatic lesional skin compared to nonlesional or normal skin (Fig 1 ), which is consistent with previous studies.1 , 2 Interestingly, after treatment with placebo or the different molecule-targeted treatments, only the IL-17 receptor A subunit inhibitor (brodalumab) and anti-IL-17A monoclonal antibodies (LY2439821 and secukinumab), but not the other treatments, such as the tumor nuclear factor-α inhibitor (etanercept), anti-IL-12/-23 antibody (ustekinumab), Janus kinase inhibitor (tofacitinib), or tyrosine kinase 2 inhibitor (PF-06700841), remarkably reduced ACE2 expression in psoriatic skin. Notably, although no significant difference in ACE2 expression was observed between lesional skin at baseline and lesional skin after being treated with lower concentrations of brodalumab, dose-dependent ACE2 expression was observed in the brodalumab-treated groups (Fig 1).
Fig 1.
ACE2 expression in normal, nonlesional, and lesional skin of patients with psoriasis before and after the indicated treatments. ∗∗∗∗ P < .0001, the fold change between the lesional and the nonlesional or the normal skin at baseline. ### P < .001, #### P < .0001, the fold change between the lesional skin at baseline and the lesional skin at the end time point.
Our report, together with previous studies,3 , 4 suggests that the ACE2 levels in psoriatic lesional skin are reduced by IL-17 blockade and that perhaps psoriatic patients who become COVID-19 infected may benefit from IL-17−targeted treatment. Although there is no evidence that COVID-19 spreads through skin contact, if future studies suggest that ACE2 expression in psoriatic lesional skin correlates with COVID-19 susceptibility or severity, then perhaps IL-17−targeted therapies may be preferred in psoriasis patients at risk for COVID-19. This is worth confirming by further investigation.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
Key words: ACE2; COVID-19; IL-17; molecule-targeted therapy; psoriasis; SARS-CoV-2.
References
- 1.Sun Y., Zhou R., Zhang H., et al. Skin is a potential host of SARS-CoV-2: A clinical, single-cell transcriptome-profiling and histologic study. J Am Acad Dermatol. 2020;83:1755–1757. doi: 10.1016/j.jaad.2020.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tembhre M.K., Parihar A.S., Sharma V.K., et al. Enhanced expression of angiotensin-converting enzyme 2 in psoriatic skin and its upregulation in keratinocytes by interferon-γ: implication of inflammatory milieu in skin tropism of SARS-CoV-2. Br J Dermatol. 2021;184(3):577–579. doi: 10.1111/bjd.19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krueger J.G., Murrell D.F., Garcet S., et al. Secukinumab lowers expression of ACE2 in affected skin of patients with psoriasis. J Allergy Clin Immunol. 2021;147(3):1107–1109.e2. doi: 10.1016/j.jaci.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Q., Chen L., Li X., Zheng J. If skin is a potential host of SARS-CoV-2, IL-17 antibody could reduce the risk of COVID-19. J Am Acad Dermatol. 2021;84(3):e173. doi: 10.1016/j.jaad.2020.10.084. [DOI] [PMC free article] [PubMed] [Google Scholar]