Abstract
Intrahepatic cholangiocarcinoma (iCCA) is a relatively rare, but highly lethal and biologically complex primary biliary epithelial cancer arising within liver. After hepatocellular carcinoma, iCCA is the second most common primary liver cancer, accounting for approximately 10–20% of all primary hepatic malignancies. Over the last 10–20 years, iCCA has become the focus of increasing concern largely due to its rising incidence and high mortality rates in various part of the world, including the USA. The challenges posed by iCCA are daunting and despite recent progress in the standard of care and management options for iCCA, the prognosis for this cancer continues to be dismal. In an effort to provide a framework for advancing our understanding of iCCA malignant aggressiveness and therapy resistance, this review will highlight key etiological, biological, molecular, and microenvironmental factors hindering more effective management of this hepatobiliary cancer. Particular focus will be on critically reviewing the cell origins and morpho-molecular heterogeneity of iCCAs, providing mechanistic insights into high risk fibroinflammatory cholangiopathies associated with iCCA development, and notably discussing the deleterious role played by the tumor reactive desmoplastic stroma in regulating iCCA malignant progression, lymphangiogenesis, and tumor immunobiology.
Keywords: morpho-molecular classification, fibroinflammatory risk conditions, tumor reactive microenvironment, cancer-associated fibroblasts, extracellular matrix, transforming growth factor-β, periostin, immune milieu, M2 macrophages, therapeutic targeting
1. Introduction
Intrahepatic cholangiocarcinoma (iCCA) denotes a rare heterogeneous class of epithelial cancers arising within liver, which exhibits characteristics of cholangiocyte differentiation (Sirica et al., 2009). After hepatocellular carcinoma (HCC), iCCA is the second most common primary liver cancer, accounting for approximately 3.0% all gastrointestinal malignancies (Khan et al., 2019; Banales et al., 2020) and comprising around 10–20% hepatobiliary cancers (Blechacz, 2017; Gupta & Dixson, et al., 2017; Massarweh et al., 2017; Banales et al., 2020) and an estimated 8–20% of total biliary tract cholangiocarcinomas (Gupta & Dixson, et al., 2017; Vijgen et al., 2017; Rizvi et al., 2018; Khan et al., 2019).
Over the past few decades, iCCA has become the focus of heightened interest and concern, largely due to its increasing incidence in various countries around the world (Saha et al., 2016; Banales et al., 2016; Cardinale et al., 2018), together with its globally high mortality rates also rising in several areas of the world (Bertuccio et al., 2019), including the USA (Yao et al., 2016; Beal et al., 2017). Specific high risk factors for CCA include liver fluke infection with Opisthorchis viverrini (Thailand) or Clonorchis sinensis (Korea), primary sclerosing cholangitis (PSC), hepatolithiasis, and fibropolycystic liver diseases, such as Caroli disease (Massarweh & El-Serag, 2017; Khan et al., 2019). The highest rates of iCCA are reported in Southeast and East Asia, particularly where parasite infection with liver flukes is endemic (Sripa et al., 2018), whereas PSC with or without ulcerative colitis has been established as having the strongest association with CCA development in Western populations (Kirstein & Vogel, 2016; Khan et al., 2019). Hepatitis B (HBV), hepatitis C, and cirrhosis have also been identified as strong risk factors for iCCA (Kirstein & Vogel, 2016; Gupta & Dixon, 2017; Clements et al., 2020) and may account in part for the rising incidence of iCCA observed in different countries of the world.
In Western countries, most iCCAs (i.e., 50–70%) arise sporadically without any identifiable risk factors except for advancing age (Kirsten & Vogel, 2016; Khan et al., 2019). Furthermore, in contrast to HCC, iCCAs usually develop in non-cirrhotic liver (Lee & Lee, 2017), but it is likely that the presence of liver cirrhosis in iCCA may have been underestimated (Jesper et al, 2018). It is also becoming increasingly evident that existing global trends in iCCA incidence rates associated with geographically variable risk factors need to be interpreted with caution based on recently reported concerns about historical under-reporting, country-based variations in data recording, and notably, iCCA misclassification in cancer registries (Khan et al., 2019; Cardinale, 2019; Labib et al. 2019).
The vast majority of iCCAs are diagnosed at an advanced incurable stage [multicentric disease within the liver, lymph node and/or peritoneal metastasis] (Sirica et al., 2019), accounting for the dismally high mortality rates for this hepatobiliary cancer. Moreover, the indolence of the early disease, the malignant aggressiveness and therapeutic refractoriness of the advanced disease, and the high reported rates (> 60%) of iCCA recurrence following curative-intent surgical resection (Chan et al., 2018; Zhang et al., 2018; Wang et al., 2019; Hu et al., 2019) all underscore the importance of advancing our current understanding of interactive cellular and molecular mechanisms that drive the development and progression of iCCA, with the ultimate aim of devising more effective biomarker-driven targeted approaches for managing and/or preventing this challenging and most often fatal hepatobiliary cancer.
In sharp contrast to conventional hepatocellular carcinomas (HCC), iCCAs are typically characterized by a prominent desmoplastic and usually hypovascularized tumor stroma (Sirica & Gores, 2014; Bösmüller et al., 2018), which in many cases represents the dominant histological feature of the tumor. This complex tumor reactive stroma is largely composed of a dense collagen-fiber enriched extracellular matrix (ECM) together with matricellular proteins and proteinases, and containing an abundance of activated cancer-associated fibroblasts (CAFs), and to a lesser extent tumor associated macrophages (TAMs) and varying numbers of vascular and innate immune cells (Sirica & Gores, 2014; Brivio et al., 2017; Affo et al., 2017). The cellular and ECM components of the desmoplastic stroma of iCCA are not structurally nor functionally static, but should be viewed as providing an evolving, interactive and mutable microenvironment that plays a preeminent role in promoting iCCA progression and invasive growth, cancer cell survival, and resistance to chemotherapeutic drugs, targeted agents, and immunotherapies (Cadamuro et al., 2017; Gentilini et al., 2018; Sirica et al., 2019; Loeuillard et al., 2019; Fabris et al., 2019a)
This review will highlight timely research findings aimed at providing mechanistic insights into the biological and translational significance of the desmoplastic microenvironment in iCCA. Our focus will be on (1) iCCA pathological and molecular classification and cell origins, (2) fibroinflammatory cholangiopathies and mechanisms, (3) the desmoplastic reaction and iCCA progression, (4) the immune milieu of iCCA and immune resistance, and (5) clinical implications and challenges.
2. iCCA Classification, Cell Origins, and Molecular Subtypes
2.1. Macroscopic and Microscopic Classification
iCCAs are macroscopically and microscopically diverse. Classically, iCCA can arise from both small intrahepatic bile ducts (septal & interlobular BD) and large intrahepatic bile ducts (segmental and area BD) within liver (Nakanuma et al., 2010; Vijgen et al., 2017; Kendall et al., 2019). Peribiliary glands associated with large intrahepatic BD may also give rise to iCCA (Nakanuma et al., 2010; Nakagawa et al. (2018).
Based on macroscopic growth patterns, iCCAs have been grossly classified as mass-forming (MF), periductular infiltrating (PI), intraductal growing (IG) and MF + PI types (Shimada et al., 2007; Guglielmi et al. 2009; Nakanuma et al., 2010; Vijgen et al., 2017; Kendall et al., 2019). The MF growth pattern is the most common form of the macroscopic types, accounting for about 65% of all iCCAs (Guglielmi et al., 2009; Vijgen et al. 2017; Kendall et al., 2019). The PI and IG types are much less common, comprising 6.0%−14%% and 4.0%, respectively, of all iCCAs (Guglielmi et al., 2009; Tsukahara et al., 2016; Vijgen et al., 2017; Kendall et al., 2019). Mixed MF + PI iCCAs, on the other hand, account for about 25% of CCA in liver (Guglielmi et al., 2009; Vijgen et al., 2017; Kendall et al., 2019). The MF + PI type has the worst prognosis with higher rates of early recurrence following curative-intent surgical resection when compared with other types of iCCA (Shimada et al; 2007; Guglielmi et al. 2009; Blechaz et al, 2011; Vijgen et al., 2017; Bagante et al., 2018).
The MF type of iCCA is generally thought to preferentially arise from small intrahepatic bile ducts (Cardinale et al., 2018; Kendall et al., 2019). This type commonly presents as a singular solid nodular mass that is gray to gray-white in color, firm and solid, non-encapsulated, and polylobulated with no macroscopically discernable connection with a bile duct (Nakanuma et al., 2010; Vijgen et al. 2017). MF-iCCAs can be quite large, often ranging between 5 and10 cm in diameter at the time of diagnosis (Buettner et al., 2017). Advanced MF type of iCCA may also exhibit satellite or multifocal tumor growth within liver, consisting of various sized nodules that may coalesce. In comparison, iCCAs arising within the large intrahepatic bile ducts are usually of the PI, IG, or the more frequent MF+PI type (Vijgen et al., 2017). The PI type of iCCA does not form a nodular mass, but rather grows longitudinally along the wall of the large intrahepatic bile ducts and spreads along the portal tracts, resulting in strictures of the affected bile ducts and dilatation of the smaller proximal bile ducts (Nakanuma et al., 2010; Vijgen et al., 2017; Kendall et al., 2019). The IG type of iCCA typically presents as a slow growing polypoid or papillary tumor growing within a dilated bile duct lumen (Nakanuma et al., 2010; Vijgen et al. 2017; Kendall et al., 2019). IG-iCCA can be divided into two groups, those with tumors showing a predominantly papillary growth pattern and those whose tumors exhibited a predominantly tubular growth pattern (Chung et al., 2009; Tsukahara et al., 2016). Both the papillary and tubular subtypes of IG-iCCA have been found to have similar favorable survival outcomes, although tumors with the tubular growth pattern showed increased incidences of lymphatic and venous invasion and a higher rate of liver metastasis than those with the papillary growth pattern. Bagante et al. (2018) have also reported that while patients who had undergone curative-intent surgery for either MF or MF + PI types of iCCA were more likely to experience early recurrence, IG-iCCA patients were more at risk for late recurrence after 5-years from surgery.
Obstructive jaundice may be manifested in patients with the PI and IG types (Vijgen et al., 2017), but is significantly less common in MF type of iCCA originating at the periphery of the biliary tree (Liau et al., 2014; Akita et al., 2018). Jaundice is significantly associated with the more advanced MF + PI type (Shimada et al., 2007). The MF type of iCCA tends to invade the hepatic parenchyma via the portal vein system and at advanced stages via the lymphatics, whereas the PI type has a tendency to spread along the Glisson sheath via lymphatics (Yang & Yan, 2008; Blechacz et al., 2012; Meng et al., 2017).
Histologically, 90–95% of iCCAs are classified as adenocarcinomas (typically well- to-moderately differentiated) displaying variable architectural patterns [i.e., tubular, acinar, papillary, tubular-papillary, anastomosing glandular] and characterized by varying degrees (usually prominent) of desmoplasia, which is a hallmark feature of iCCA (Sirica et al., 2009; Nakanuma et al., 2010; Sirica, 2013; Akita et al., 2017; Blechacz, 2017; Kendall et al., 2019). In a classification scheme proposed by Nakanuma et al. (2010) classic or conventional iCCA is subclassified according to histological features and anatomic location into two main types: small bile duct type and large bile duct type. Grossly, the small bile duct type of iCCA is almost exclusively of the MF type and is often associated with chronic non-bile duct liver diseases, such as viral hepatitis (B & C) and cirrhosis (Cardinale et al; 2018; Alita et al., 2019; Kendall et al., 2019), whereas the large bile duct type typically characterizes the PI and PI + MF types of iCCAs, such as those associated with predisposing high risk fibroinflammatory cholangiopathies like primary sclerosing cholangitis (PSC), hepatolithiasis, liver-fluke infestation, and Caroli’s disease. Small bile duct type iCCAs, also described as “mixed type” (Cardinale et al., 2018) or “cholangiolar type” (Liau et al., 2014), display no or low mucin production, while large duct type iCCAs and those arising from associated peribiliary glands are mucin-producing adenocarcinomas (Cardinale et al., 2012a; Nakagawa, 2018; Vijgen et al., 2017; Siegel et al., 2018; Kendall et al., 2019).
Cholangiolocellular carcinoma (CLC) is a rare malignancy first described by Steiner & Higginson (Steiner & Higginson, 1959), which accounts for 0.6–1.0% of primary liver cancers. (Yamamoto et al., 2018). CLC is considered to originate from cells within the ductules (cholangioles)/canals of Hering of liver (Komuta et al., 2008; Brunt et al., 2018). This tumor consists of thin, malignant ductular-like structures characterized by low grade cellular atypia that may appear to radiate from or surround a portal tract in a tubular, cord-like, anastomosing pattern within a dense hyalinized (fibrous) stroma, and which may show trabecular and replacing growth at its interface with the surrounding nontumorous liver (Brunt et al., 2018). CLC often exhibits a similar MF type at the periphery of the liver as small duct type of iCCA, and like small duct type MF-iCCA, is mucin negative (Brunt et al., 2018) and associated with cirrhosis or chronic viral hepatitis (Arizumi & Yamamoto, 2015; Yamamoto et al., 2018). Patients with CLC who have undergone curative-intent hepatectomy have been reported to have a significantly better survival rates than those with classic iCCA (Arizumi et al., 2014; Kusano et al., 2020). Integrative genomic analysis revealed CLC to be a distinct biliary-derived entity with chromosomal stability and active transforming growth factor-β (TGF-β) signaling (Moeini et al., 2017). More recent immunochemical features and mutational profiling of CLC (see Molecular Classification subsection below) have further supported classifying CLC as a histological subtype of well differentiated iCCA (Balitzer et al., 2019).
Classic combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is a rare primary MF-type liver cancer, which presents with a more aggressive malignant behavior and a grim prognosis with worse survival outcomes than for HCC and similar to or worse than for iCCA (Gera et al., 2017; Stavraka et al., 2018). Previous reported incidence has ranged between 0.4% and 14.2 % (Stavraka et al., 2018; Wang et al., 2019) and 1.0%−4.7% (Gera et al., 2017) of primary liver tumors, but the true incidence of cHCC-CCA is unclear due to misdiagnosis and a previous lack of consensus on the nomenclature (Stavraka et al., 2018; Wang et al., 2019). Similar to iCCA, the incidence and mortality trends overall for cHCC-CCA have also been reported to have increased in the USA from 2000 to 2014 (Wang et al., 2019).
As the term implies, classic cHCC-CCA contain areas of both unequivocally differentiated HCC and typical iCCA (Gera et al., 2017; Brunt et al., 2018). The HCC component usually is in the form of thickened trabeculae composed of polygonal cells with abundant granular eosinophilic cytoplasm and scant stroma, whereas the iCCA component distinctly presents as an adenocarcinoma with malignant glands comprised of small bile duct-like cells supported by a conspicuous desmoplastic stroma (Gera et al., 2017; Brunt et al., 2018). The HCC and iCCA components may be intermixed or occupy separate regions of a tumor, but focal areas of intermingling are often observed (Brunt et al., 2018). Mucin production is most often negative. Both MF-iCCA and the cholangiocarcinoma component of cHCC-iCCA in cirrhotic liver were further shown to exhibit higher density of arteries and microvessels than classical iCCA in non-cirrhotic liver, which usually presents with a hypovascular stroma (Xu et al., 2012).
cHCC-iCCA with “stem/progenitor” cell features/phenotypes and intermediate cell carcinoma comprised of tumor cells intermediate between hepatocytes and cholangiocytes are considered non-classical subtypes of HCC or iCCA (Gera et al., 2017; Brunt et al., 2018). CLC, which may be entirely distinct as a primary liver cancer can also be observed variably as a component in either HCC, iCCA, or cHCC-iCCA (Brunt et al., 2018). Other unconventional subtypes of iCCA include rare variants such as adenosquamous type, squamous type, intestinal type, signet ring type, clear cell type, lymphoepithelial type, and others (Vijgen et al., 2017; Kendall et al., 2019). The intestinal-type of iCCA is a particularly interesting variant exemplifying the cellular plasticity of biliary cells in cholangiocarcinogenesis. Reported cases of this rare subtype of large duct iCCA showed absorptive columnar cells, Paneth cell and goblet cell metaplasia, and occasional neuroendocrine cells within carcinomatous epithelium of the tumor (Kozuka et al.,1984; Bae et al., 2002;). A similar pattern of intestinal-type differentiation has also been demonstrated in mucin producing iCCA induced by furan in rat livers (Elmore & Sirica, 1993; Elmore & Sirica, Ren et al., 2000).
Lymphoepithelioma-like iCCA is also a distinctive rare sub-type. This primary hepatic tumor is composed of undifferentiated epithelial cells with a dense lymphoplasmacytic infiltration and is characterized by lower rates of recurrence and better overall survival after surgical resection than classic iCCA (Aosasa et al., 2015; Kendall et al., 2019). A recently identified “inflamed” iCCA subtype that presented with a massive T-lymphocyte infiltration and activation of inflammatory and immune checkpoint pathways was also shown to be associated with enhanced patient survival (Job et al., 2019).
2.2. Precursor Lesions of iCCA Subtypes and Cell Origins
Precursor lesions
Presently, no precursor lesions of the MF type of iCCA are known (Kendall et al., 2019), although bile duct adenoma-like and atypical bile duct lesions involving the small bile ducts have been suggested as possibly related to the development of the MF type of iCCA (Nakanuma et al., 2014). However, three preinvasive lesions: (1) biliary epithelial neoplasia (BillN), (2) intraductal papillary neoplasms of the bile duct (IPNBs), and (3) intraductal tubulopapillary neoplasms of the bile duct (ITPNs), which are most frequently observed in high risk conditions affecting the large intrahepatic bile ducts, are now considered as morphologically established precursor lesions for iCCA (Vijgen et al., 2017; Kendall, et al., 2019; Nakanuma et al., 2019; Zaccari et al., 2019). Pancreatic counterparts to these premalignant intraepithelial neoplasms of the biliary tract are also well recognized (Nakanuma et al., 2019; Zaccari et al., 2019).
BillN is a microscopically identifiable dysplastic lesion exhibiting a flat, micropapillary, or pseudopapillary pattern of growth (Nakanuma et al. 2019; Zaccari et al., 2019), which is graded as BillN1 (low grade dysplasia), Billn2 (intermediate dysplasia), and BillN3 (carcinoma in situ). BillNs are frequently observed in areas of mucosa proximal to classic nodular/sclerosing iCCA and in the context of preneoplastic cholangiopathies, such as PSC, hepatolithiasis, liver fluke infection, and Caroli’s disease (Kendall et al., 2019; Zaccari et al., 2019). BillN has also been documented in patients with non-biliary chronic diseases, such as HCV (Vijgen et al., 2017; Kendall et al., 2019), and may also be seen in peribiliary glands (Vijgen et al., 2017; Zaccari et al., 2019).
IPNB can be considered as the biliary counterpart to pancreatic intraductal papillary mucinous neoplasms, while ITNB appears to be the biliary counterpart to intraductal tubular neoplasm of the pancreas (Vijgen et al,, 2017; Nakanuma et al., 2019; Kendall et al., 2019). IPNBs precede the growth of IG type and papillary type iCCAs (Nakanuma et al., 2016) and are more commonly encountered in the Far East in patients with hepatolithiasis and liver fluke infection, and less frequently in the West (Wan et al., 2013; Kendall et al., 2019). Macroscopically, IPNBs grow as single or multiple yellow, friable papillary mass within large size intrahepatic and extrahepatic bile ducts, which may show unilocular or multilocular cystic dilation (Nakanuma et al., 2019; Kendall et al., 2019; Hucl, 2019). Histologically, IPNBs are classified into four subtypes based on the lining epithelial cells and architecture, including fine fibrovascular stalks: pancreaticobiliary, intestinal, gastric, and oncocytic. The pancreaticobiliary and intestinal subtypes are the most common, with IPNBs often graded as high grade (Kendall et al., 2019; Nakanuma et al., 2019). Invasive iCCA can be found in association with each of the IPNB subtypes, although it is more often associated with the pancreaticobiliary type (Kendall et al., 2019). The invasive parts of IPNBs usually show tubular adenocarcinoma with desmoplastic reaction and only occasionally show oncocytic or colloid (mucinous) carcinoma (Nakanuma et al., 2019). However, invasive iCCAs associated with the intestinal subtype of IPNB are often mucinous adenocarcinomas (Kendall et al., 2019). With respect to mucin types, MUC1, which is associated with iCCA aggressiveness and poor prognosis, is commonly expressed in the pancreaticobiliary subtype, whereas MUC2 is a goblet cell mucin characteristic of the intestinal phenotype of IPNB and of mucinous adenocarcinoma. The gastric and oncocytic subtypes are positive for MUC6, while MUC5AC can be detected in either of the four subtypes (Ishikawa et al., 2004; Rocha et al., 2012; Kasprzak & Adamek, 2019).
ITPN is also a polypoid lesion with a predominant tubular growth pattern. Key histological features of ITPNs are tubular glands densely packed back to back with abortive papillary elements, uniform high grade dysplasia throughout the tumor, no cytoplasmic mucin, and focal necrosis (Vijgen et al., 2017; Kendall et al., 2019; Nakanuma et al., 2019) Although ITPNs have a high risk of malignancy, they generally have a more favorable prognosis when compared with IPNBs (Kendall et al., 2019). 2019).
Cell origins
Histopathological observations of precancerous and borderline lesions that precede the development of iCCA livers of patients with fibroinflammatory cholangiopathies (Cannito et al., 2018; see below) have led to the recognition that iCCA develops through a multistage carcinogenic process, which like other epithelial cancers, may proceed through a hyperplasia-dysplasia-carcinoma in situ sequence (Shimonishi et al., 2000; Kendall et al., 2019). Hyperplasia and dysplasia of the lining biliary epithelial cells and peribiliary glands are characteristically observed in patients with hepatolithiasis and those with liver fluke infection, and also in biliary lining epithelium of patients with PSC and Caroli’s disease (Ludwig et al., 1992; Shimonishi et al., 2000; Jang et al., 2014; Zimmermann, 2016; Nakagawa et al., 2018; Carpino et al., 2019). It has also become apparent that the epidemiological, morphological and molecular heterogeneity of iCCA subtypes is linked to potentially diverse cellular origins (Cardinale et al., 2012; Sia et al., 2017; Overi et al., 2018; Bragazzi et al., 2018).
Much of our current understanding of the cell origins of iCCA comes from studies performed using genetic mouse models (Vicent et al., 2019; Erice et al., 2019; Zhu & Kwong, 2020); cell lineage tracing (Fan et al., 2012; Guest et al., 2013; Wang et al., 2018), and immunophenotyping of “stem/progenitor” cells (Sia et al., 2017; Nakagawa et al., 2018; Overi et al., 2018; Vicent et al., 2019). As exemplified in Table 1, and as proposed by Cardinale et al. (2012b), iCCA can potentially develop from multiple cells of origin, including immature or more mature cholangiocytes, hepatic stem/progenitor cells localized to the canals of Hering and bile ductules, biliary tree stem/progenitor cells (BTSCs) found in the peribiliary glands, and from transdifferentiation of mature hepatocytes.
Table 1.
Representative Genetic Mouse Models Supporting Different Cells of Origin of Intrahepatic Cholangiocarcinoma
| Model | Method | Tumor | Suggested Cell Origin2 | Reference |
|---|---|---|---|---|
| AhCreERT;KrasV12/+;Ptenf/f (PTEN loss; KRAS activation) | GEMM1 | Non-invasive papillary neoplasms of intrahepatic biliary tract, including major interlobular bile ducts and small bile duct radicles | BEC/HPC | Marsh et al., (2013) |
| Alb-Cre+;LSL-KrasG12D/+;Ptenf/f | GEMM | Well differentiated, desmoplastic intrahepatic cholangiocarcinoma | BEC/HPC |
Ikenoue et al., (2016); Lin et al., (2018); |
| Sox9-CreERT2;LSL-KrasG12D/+;Ptenf/f | GEMM | Intrahepatic and extrahepatic cholangiocarcinomas3 | BEC | Lin et al., (2018) |
| Alb-Cre,Smad4f/f; Ptenf/f (Smad4 and PTEN ablation) | GEMM | Bile duct dysplasia, carcinoma in situ, well differentiated, desmoplastic intrahepatic cholangiocarcinoma | BEC/HPC | Xu et al., (2006) |
| Ck19-CreERTeYFPR26p53f/f + thioacetamide (p53 deletion + chronic liver injury) | GEMM | Intrahepatic cholangiocarcinoma | BEC4 | Guest et al., (2013) |
| Sox9-CreERT2;KrasLSL-G12D;Tp53f/f (KRAS activation; Tp53 deletion) | GEMM | BilIN lesions5; desmoplastic intrahepatic cholangiocarcinoma | BEC | Hill et al., (2018) |
| AAV8-TBG-Cre;KrasLSL-G12D;Tp53f/f plus 2 week dietary treatment with DCC6 (KRAS activation; Tp53 deletion; dietary-induced liver injury) | GEMM | Intrahepatic cholangiocarcinoma; hepatocellular carcinoma; mixed hepatocellular carcinoma-cholangiocarcinoma | HEP4 | Hill et al., (2018) |
| Alb-Cre;LSL-IDH2R172K; KrasG12D (mutant IDH expression; KRAS activation) | GEMM | Intrahepatic cholangiocarcinoma | HPC | Saha et al., (2014) |
| Alb-Cre;NotchIC (Notch overexpression) | GEMM | Desmoplastic intrahepatic cholangiocarcinoma | HPC | Zender et al., (2013) |
| AAV8-TBG-Cre;Ptenf/f;TBR2f/f (PTEN and TGFβR2 ablation) | GEMM | Desmoplastic intrahepatic cholangiocarcinoma | HEP4 | Mu et al., (2016) |
| Prom1-CreERT2;Ptenf/f;TBR2f/f or K19-CreERT; Ptenf/f;TBR2f/f | GEMM | Desmoplastic intrahepatic cholangiocarcinoma | BEC4 | Mu et al., (2016) |
| Alb-Cre;Nƒ2lox/lox (Neurofibromatosis type 2 gene deletion) | GEMM | Oval cell proliferation; hepatocellular carcinoma; intrahepatic cholangiocarcinoma | HPC | Benhamouche et al., (2010) |
| K19-CreERT;LSL-KrasGD12D; Tgfbr2f/f + IL-33 (KRAS activation, TGFβR2 loss, IL-33 mediated biliary epithelial injury response | GEMM | Extrahepatic cholangiocarcinoma | PBG4 | Nakagawa et al., (2017) |
| AAV8-Tte-Cre; Hydrodynamic tail vein injection NICD/AKT plasmids (activated Notch and AKT) | SB7 transposon-transfection | Intrahepatic cholangiocarcinoma lacking desmoplastic stroma | HEP4 | Fan et al., (2012) |
| Hydrodynamic tail vein injection of HA-tagged pT3-EF5α-PIK3CAH1047R and Flag-tagged pT3-EF5αYapS127A (Co-activation of PI3CA and Yap) | SB transposon transfection | Hepatocellular carcinoma; intrahepatic cholangiocarcinoma; mixed hepatocellular carcinoma-intrahepatic cholangiocarcinoma | HPC/HEP | Li et al., (2015) |
| Intrabiliary injection of murine myr-AKT and human YAP127A combined with systemic IL-33 (or IL-6) injection and lobar bile duct ligation (Transduction of constituitively activated AKT and Yap facilitated by a IL-6-mediated process and lobar bile duct obstruction) | SB transposon transfection | Desmoplastic intrahepatic cholangiocarcinoma | BEC | Yamada et al., (2015) |
GEMM, genetically engineered mouse model
BEC, biliary epithelial cell; HPC, hepatic progenitor cell; HEP, hepatocyte; PBG, peribiliary gland cell
Pancreatic ductal adenocarcinomas also developed in this model
Lineage tracing employed
BilIN, biliary intraepithelial neoplasia, which only preceded development of intrahepatic cholangiocarcinoma from BEC and not hepatocytes in this model
DDC, 3,5-diethoxycarbonyl-1,4 dihydrocollidine
SB, Sleeping Beauty
While the experimental findings favor a diverse cellular origin of iCCA, it remains unclear as to the extent to which human iCCAs are developed from cholangiocytes versus those being derived from hepatic stem/progenitor cells or transdifferentiated hepatocytes. As previously noted, the majority of human iCCAs develop sporadically in livers in the absence of discernable chronic inflammatory liver injury. Also, as has been mentioned, a multistep carcinogenic process indicative of a hyperplasia-dysplasia-cancer in situ sequence is well recognized in the pathogenesis of iCCA arising from cholangiocytes within different levels of the hepatic biliary tree. However, the concept of a hepatic stem/progenitor cell origin is increasingly recognized as being relevant to the development of human MF-iCCA arising in a background of chronic viral hepatitis and cirrhosis, and is particularly apparent in the development of cHCC-CCA with stem cell features (Terada, 2013; Brunt et al., 2018; Stavraka et al., 2019; Cai et al. 2020). In a limited number of molecular studies involving tissue microarray, gene set enrichment analysis, and integrative genomics, expression of hepatic stem/progenitor traits have been demonstrated in cohorts of surgically resected cHCC-CCA (Woo et al., 2010; Coulouarn et al., 2012). Coulouarn et al. (2012) have further shown that cHCC-CCA exhibit down-regulation of the hepatocyte differentiation program and a commitment to the biliary lineage, as well as an activation of the Wnt/β-catenin and TGF-β signaling pathways. Both of these pathways are involved in biliary differentiation. More recently, Xue et al. (2019) performing a large-scale integrative analysis of 133 cases of human cHCC-CCA, which included comprehensive genomic and transcriptomic profiling, showed that tumors with clearly defined areas of HCC and iCCA (denoted as combined-type of cHCC-CCA) and those with intimately mixed components of HCC and iCCA in the same tumor without clear boundaries (defined as mixed-type HCC-CCA) both showed stem-like features, were monoclonal in origin, and exhibited a high level of expression of Nestin, a liver stem cell/progenitor cell protein (Gleiberman et al., 2005). Interestingly, the HCC components of the combined-type cHCC-CCA were not clustered into classic HCC subgroups nor were the iCCA components clustered into classic iCCA subgroups, but rather clustered together, suggesting that the combined-type of cHCC-CCA is not identical to traditional HCC and iCCA, but derived from a common progenitor cell. In this context, is of interest that loss of p53 has been shown facilitate dedifferentiation of mature hepatocytes into nestin-positive liver progenitor-like cells, which then may be poised to potentially develop into HCC, iCCA, or cHCC-CCA (Tschaharganeh et al., 2014).
Experimental studies have also supported the possibility of adult hepatocytes transdifferentiating into cholangiocytes under conditions of severe chronic hepatic injury and cholestasis (Michalopoulos et al., 2005; Michalopoulos & Khan, 2015; Sadri et al., 2016; Ko et al., 2020). Morphological and phenotypic evidence for putative transdifferentiation of rare ductular hepatocytes from bile duct epithelium within interlobular bile ducts has also been described in human liver in various severe hepatic disease states, including chronic viral hepatitis and end stage cirrhosis (Nomoto et al., 1992), as well as in hyperplastic bile ductular structures formed in rat liver in associated with extreme hepatotoxic injury induced by furan (Sirica et al.,1994). Of particular interest are the findings of Seehawer et al. (2018) who showed that the hepatic microenvironment epigenetically shapes lineage commitment in mosaic mouse models of liver tumorigenesis. Specifically, these investigators observed that a necroptosis-associated hepatic cytokine microenvironment promotes oncogenetically-transformed hepatocytes to give rise to iCCAs. In contrast, when hepatocytes containing the same oncogenic drivers were surrounded by an apoptotic microenvironment, they formed HCCs. Notably, the transcription factors Prdm5 and Tbx3 were singled out as being major microenvironment-dependent and epigenetically regulated lineage commitment factors for ICCA and HCC, respectively (i.e., Prdm5 overexpression and Thx3 knockdown resulted in HNF4α-negative and cytokeratin 19 (CK-19)-positive iCCA, whereas Tbx3 overexpression and Prdm5 knockdown yielded HNF4α-positive, CK19-negative HCC). Hepatocyte associated transcription factors (HNF4α and HNF6) have been demonstrated in cholangiocytes in chronic HCV infected liver with end stage cirrhosis (Limaye et al., 2008). However, while the apparent transdifferentiation of hepatocytes to cholangiocytes or of rare cholangiocytes to hepatocytes have been observed to occur in various relevant human liver diseases, it remains to be determined if this facultative progenitor cell mechanism plays a plausible role in human iCCA or cHCC-CCA development, since it is more likely to be predominantly manifested in end stage liver diseases where the normal regenerative capacity of liver is severely impaired.
Peribiliary glands are tubuloaveolar glands with mucinous and serous acini that are located in the wall of the large intrahepatic bile ducts and extrahepatic bile ducts, being largely distributed at the branching points of the biliary tree. They are connected to the bile ducts through small canals/tubes and are considered to modulate bile composition by secreting serous and mucinous components, although their pathophysiological role has not been definitively established (Cardinale et al., 2012b; Nakagawa et al., 2018). Peribiliary glands have been implicated in the origin of human large duct mucin-producing CCA and IPNB (Nakanuma & Sato, 2012; Cardinale et al., 2012a; Sato et al., 2014; Carpino et al., 2019).
BTSCs have been localized to the bottom of human peribiliary glands near the fibromuscular layer and have been demonstrated to express specific stem/progenitor cell markers, including Sox9, Sox17, Pdx1, Foxa2, CD133, CD44, CXCR4, EpCAM, NCAM, Sall4, and Lgr5 (Cardinale et al., 2011; Overi et al., 2018; Nakagawa et al., 2018), but lacked markers of mature cells, such as secretin receptor-SR, albumin, and insulin (Overi et al., 2018). A subpopulation of BTSCs were also found to express markers used to characterize pluripotent stem cells (i.e., Oct4, Nanog, Sox2) (Overi et al., 2018). That the peribiliary gland is a niche for multipotent BTSMs is further highlighted by the demonstration that BTSCs present in peribiliary glands of human extrahepatic biliary tree can give rise to hepatocytes, cholangiocytes, and beta-islet cells in culture and in vivo (Cardinale et al., 2011). Sox17+ and/or Pdx1+ cells of the peribiliary glands and peribiliary network of mice were also shown to proliferate in response to viral-induced biliary injury and to biliary obstructive cholestasis caused by bile duct ligation (DiPaola et al., 2013). A recently established novel mouse model of biliary injury-related extrahepatic CCA from peribiliary glands developed by ductal cell-specific activation of KRAS, and deletion of TGF-β receptor 2 and of E-cadherin, together with IL-33 treatment further adds to the likelihood of large duct CCAs arising in humans with underlying fibroinflammatory risk conditions as originating from peribiliary glands harboring BTSCs (Nakagawa et al., 2017; Nakagawa et al., 2018; Nakagawa et al., 2019). Additional support for this possibility also comes from the recent findings of Carpino et al. (2019) who demonstrated marked proliferation of BTSCs, expansion of peribiliary glands and dysplasia, and high expression of BTSC markers in mucin-producing CCAs, which emerged in the PSC patients. However, while these findings are compelling, definitive evidence establishing a direct cell lineage relationship between BTSCs and cholangiocarcinoma cells arising within large duct CCAs has yet not been conclusively demonstrated.
2.3. Molecular Classification of iCCA Subtypes
Molecular profiling of human iCCA subtypes has revealed a complex mutational landscape with broad inter- and intra-tumor heterogeneity that are attributed in part to diverse multifactorial etiologies, histological tumor differences and cell origins, and evolving malignant progression (Sirica et al., 2019). However, small duct type and large duct type of iCCAs can be grouped into two distinctive morpho-molecular groups based on unique differences in their gene mutation profiles (Kendall et al., 2019). Isocitrate dehydrogenase 1/2 (IDH1/2) mutations and Fibroblast Growth Factor Receptor 2 (FGFR2) fusions are almost exclusively detected in small duct type of iCCAs when compared with large type iCCAs (Liau et al., 2014; Akita et al., 2019, Goeppert et al., 2019; Kendall et al., 2019; Wang et al., 2019; Ma et al., 2020). In contrast to small duct type iCCAs, large duct type iCCAs, as well as extrahepatic CCAs (perihilar and distal) typically show higher mutation frequencies for oncogenes (i.e., KRAS) and tumor suppressor genes (i.e., TP53) (Liau et al., 2014; Jusakul et al., 2017; Kendall et al., 2019). Loss of BAP1 was also reported to be restricted to the small bile duct type of iCCA, whereas in the same study, loss of SMAD4, as well as MDM2 amplification were more frequently detected in the large bile duct type (Akita et al., 2019). Genomic profiling of iCCA in cirrhosis (n=10) demonstrated alterations similar to those of iCCA in non-cirrhotic liver, including IDH1/2 mutations (30%), FGFR2 fusions (20%), as well as BAP1 (10%) and ARID1A (10%) mutations (Joseph et al., 2019). The genomic profile (IDH1/2 mutations, FGFR2 fusions, chromatin-remodeling gene mutations, such as ARID1A and PBRM1 and copy number alterations) were also found to be similar in a small cohort of analyzed cases of human CLC (n=5), iCCA (n=7), and mixed CLC-iCCA (n=5), with mutations typical of small duct type of iCCA, including IDH1/2 mutations and FGFR2 fusions shown to be present in 90% of the cases with a CLC component (Balitzer et al., 2019). Unlike small duct type iCCAs, a limited number of studies have revealed human cHCC-CCA to harbour highly recurrent TP53 and TERT promoter mutations (Sasaki et al, 2017; Liu et al., 2018; Joseph et al., 2019), with TP53 mutations frequently present in both the HCC and CCA components of the tumor (Joseph et al., 2019). None of the cases of cHCC-CCA (n=20) analyzed by Joseph et al. (2019) demonstrated IDH1/2, FGFR2, or BAP1 mutations. On the other hand, the mutational landscape of cHCC-CCA appears to be more similar to that of HCC and distinctly different from that of iCCA, even in cirrhosis (Liu et al., 2018; Joseph et al., 2019). Mutations in TERT promoter, ARID1A, KRAS and TP53 were further shown to correlate with different clinical phenotypes of cHCC-iCCA. TERT promoter mutations correlated with HBV, an intermediate subtype-predominant histology, higher clinical stage, and higher N-factor (lymph node involvement). ARID1A mutations correlated with alcoholic liver disease, smaller tumor size, a lower grade of coexistent HCC, and α-fetoprotein positivity, and were associated with a CLC subtype predominance. KRAS mutations correlated with greater histological diversity and a higher M-factor (distant metastasis), while TP53 mutations correlated with α-fetoprotein positivity (Sasaki et al., 2017).
Integrative molecular analysis was also used to identify two distinct molecular subclasses of human iCCA, respectively designated as an inflammation subclass and a proliferation subclass (Sia et al., 2013; Sia et al., 2017). The inflammation subclass iCCAs was characterized by activation of inflammatory signaling pathways, overexpression of cytokines, including interleukin (IL)-6, IL-10, and IL-17, and STAT3 constitutive activation, whereas the proliferation subclass was based on activation of oncogenic signaling pathways (i.e., KRAS/RAF/ERK, IGF1R, EGFR, MET, NOTCH, ErbB2,, PI3K/AKT/mTOR), oncogenic mutations (i.e., KRAS, BRAF, EGFR, TP53), DNA hypermethylation, and focal aberrations, including DNA amplifications at Chr 11q13.2 and deletions at Chr 14q22. The proliferation class also included a subtype of iCCA with stem cell-like features, chromosome instability and IDH1/2 mutations. This study also revealed a resemblance between the proliferation class of iCCA and HCC subtypes with poor prognosis and stem cell features (Sia et al., 2017).
The molecular classification profiles described above have significant relevance for the prognosis and treatment of patients with iCCA subtypes. For example, the proliferation subclass of iCCA was demonstrated to exhibit a more aggressive clinical behavior, where as the inflammation subclass defined a class of iCCA with a more favorable prognosis (Sia et al., 2013, Sia et al., 2017). In a separate study, iCCA with CLC differentiation whose transcriptomic profile resembled that of an inflammation-related subtype exhibited less aggressive histopathological features and more favorable survival outcomes and time to recurrence than classic iCCA resembling the proliferation subtype (Rhee et al., 2018). Integrative clustering of genetic and epigenetic data from an analysis of 40 small duct type iCCAs and 12 large duct type iCCAs identified four subgroups with prognostic relevance. These were designated as IDH, high (H), medium (M), and low (L) genetic and epigenetic alteration groups. The IDH group consisted of all samples with IDH1 or IDH2 mutations and showed together with the H group a highly disruptive genome. DNA methylation was highest in both the IDH and H subgroup, intermediate in the M subgroup, and low in the L subgroup, which also had few mutations and a lack of copy number alterations. Patient three-year survival rates were 91% for the L subgroup, 65% for the IDH group, 50% for the H group, and 36 % for the M group (Goeppert et al., 2019). An impressive integrated whole-genome analysis and epigenetic analysis of 489 CCAs from 10 countries carried out by the International Cancer Genome Consortium further demonstrated that an anatomic subset of tumors composed of almost entirely intrahepatic iCCAs, which were mostly liver-fluke negative and characterized by IDH1/2 and BAP1 mutations and FGFR alterations, had significantly better overall survival relative to other subsets comprised mostly of liver fluke positive tumors (large duct type) and which were enriched in TP53 mutations and ERBB2 amplifications (Jusakul et al., 2017). The clinical significance of morpho-molecular subclassification of iCCAs is further highlighted by encouraging phase III clinical trial data supporting the use of IDH1 inhibitors after progression to chemotherapy for the treatment of iCCA with IDH1 mutations and phase II trial data demonstrating FGFR inhibitors to show therapeutic activity against iCCAs harboring FGFR2 fusions (Lamarca et al., 2020).
3. Fibroinflammatory Cholangiopathies, Mechanisms, and Cholangiocarcinogenesis
Cholangiopathies are a class of liver diseases of various etiologies that specifically affect the biliary tree (Cannito et al., 2018; Fabris et al., 2019b). Chronic biliary inflammation, bile duct hyperplasia, fibrosis, and cholestasis are common characteristics of cholangiopathies that have been identified as high-risk conditions for cholangiocarcinoma (Cannito et al., 2018; Roy et al., 2019; Guicciardi et al., 2020). As already noted, PSC and liver fluke infestations are two of the most important examples of definitive high risk cholangiopathies for which progressive biliary inflammation and fibrosis have been linked to cholangiocarcinoma development. The main features of PSC and liver fluke infestations are described below in the context of their relationship to cholangiocarcinogenesis.
3.1. Primary Sclerosing Cholangitis
PSC is a rare chronic biliary disease typically diagnosed in the third and fourth decade of life (Lindor et al., 2015; Lazaridis & LaRusso, 2016). PSC is characterized by strong peribiliary fibrosis and inflammation, which can affect both the small and large bile ducts, leading to large bile duct biliary strictures, impaired bile flow, and ultimately to cirrhosis complicated by portal hypertension. Histopathological features of PSC include fibroinflammatory destruction of the interlobular bile ducts with thick concentric fibrosis (“onion skinning”) associated with progressive bile ductopenia, bile duct hyperplasia, and varying degrees of cholestasis (Cannito et al., 2018; Guicciardi et al., 2020). Based on experimental data and anatomic location, it has recently been proposed that in PSC, senescent cholangiocytes and portal fibroblasts participate mainly in the development of persistent peribiliary fibrosis, whereas ductular reactive cholangiocytes and hepatic stellate cells would be involved in the formation of liver parenchymal fibrosis (Guicciardi et al., 2020).
Patients with PSC have been reported to have a 400-to-1500-fold increased lifetime risk of developing cholangiocarcinoma than those without PSC (Roy et al., 2019; Fung et al., 2019), with 20–25% of PSC cases resulting in cholangiocarcinoma (Aron et al., 2009; Karlsen et al., 2010; Karlsen et al., 2013; Williamson & Chapman, 2015, Fung et al., 2019; Buckholz & Brown, 2020). The etiology and pathogenesis of PSC remains unclear (Karlsen et al., 2017, Vesterhus & Karlsen, 2020), but several studies have implicated chronic biliary inflammation as a critical component sustaining the pathogenesis of this disease (Yan et al., 2020). The known association of PSC with inflammatory bowel diseases, the changes in the bile and gut microbiotas, together with genome wide association study data demonstrating the presence of genetic variants related to immune pathways (i.e., IL2, IL2RA, CARD9, and REL) all suggest the involvement of innate immune mechanisms in PSC’s pathogenesis. (Melum et al., 2011; Janse et al., 2011, Liu et al., 2013; Mells et al., 2013).
The role of changes in the microbiota is still debated (Hov & Karlsen, 2017). Bacteria and bacterial components, also known as pathogen associated molecular patterns (PAMPs), could be transported to the liver through the enterohepatic circulation, stimulating sustained innate immune responses targeting cholangiocytes (Karlsen et al., 2010; Trauner et al., 2014; Strazzabosco et al., 2018). Katt et al. (2013) have further shown that IL-17a is upregulated in livers from patients with PSC and that Th17 cells are increased in peripheral blood from patients with PSC following challenges with candida extracts or toll-like receptor 5 agonists (i.e., flagellin). More recently Tedesco et al. (2018) showed in the Mdr2−/− mouse model of PSC that gut dysbiosis leads to the translocation of Lactobacillus gasseri to the liver and to the stimulation of IL-17 secretion by γδ TCR+ cells. Notably, this response was blocked by inhibition of the γδ TCR+ cell receptor, resulting in attenuation of peribiliary fibrosis. In addition, Nakamoto et al. (2019) reported that the liver of PSC patients is colonized by Klebsiella pneumonia, Proteus mirabilis, and Enterococcus gallinarum, stimulating Th17 immune responses and suggesting a possible role of IL-17 and the biliary epithelial cell response to this cytokine in the pathogenesis of PSC.
The persistence of the inflammatory insult in PSC lead to the secretion of numerous fibroinflammatory mediators, in particular of IL-6, interferon (IFN)γ, and tumor necrosis factor (TNF)-α, which, in turn, are able to stimulate inducible nitric oxide synthase (iNOS) expression and activity (Jaiswal et al., 2000; Jaiswal et al., 2001; Spirlì et al., 2003). Increased nitric oxide (NO) synthesized by iNOS stimulates inflammatory cells present in the portal areas to secrete a wide range of cyto- and chemokines, including chemokine (C-C motif) ligand (CCL) 1, 2, and 3, chemokine (C-X-C motif) ligand 2 (CXCL2), IFNβ, IL-6, IL-8, TGFβ, and TNF-α (Cadamuro et al., 2020). Proliferating cholangiocytes also display increased secretions of proinflammatory cytokines, such as TNF-α, and IL-6, as well as chemokines (monocyte chemoattractant protein 1) and growth factors [i.e., platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF] (Guicciardi et al., 2020).The local accumulation of NO2- generate reactive oxidative species (ROS), such as peroxynitrites (ONOO−), by interacting with superoxidedismutase-derived O2- (Jaiswal et al., 2000; Fang et al., 2004). ROS and NO are involved in the neoplastic transformation inducing lipid peroxidation of the cell membrane, nitrosylation of several proteins, single or double-strand breaks in DNA, and inactivating enzymes involved in DNA proofreading and repair, such as 8-oxo-deoxiguanine DNA glycosylase 1 (Jaiswal et al., 2000). These mechanisms may favour the accumulation of genetic mutations in biliary epithelial cells, prodromal to neoplastic transformation. Moreover, NO and ROS could generate two mutagenic compounds, 8-oxo-7,8-dihydro-20-deoxyguanosine (8-oxodG) and 8-nitroguanine (Jaiswal et al., 2000; Jaiswal et al., 2001; Correia da Costa et al., 2014). The nitrosylation of cysteine residues of the active site of caspase 3, 8, and 9 could inhibit their activity, making tumor cells resistant to apoptosis. NO accumulation also activates the p38 MAPK/JNK pathway, stimulating cyclooxygenase (COX) 2 to generate prostaglandin E2, which can directly stimulate the proliferation of cholangiocarcinoma cells through interaction with its receptor EP1, or by transactivating the epidermal growth factor receptor (EGFR) through Src protein (Zhang et al., 2007). The resultant activation of the PI3K/AKT encourages increased cell proliferation and chemoresistance. Carpino et al. (2019) have further shown that neoplastic ducts of PSC patients are surrounded by an expanded peribiliary plexus and secrete higher amounts of IL-6, IL-8, TGFβ, and VEGF compared to non-neoplastic ducts.
Cholangiocarcinoma developed on a background of PSC is associated with KRAS and TP53 mutations (Ahrendt et al., 2000; Boberg et al., 2000), mutations of the p16INK4a promoter (Taniai et al., 2002), and FGFR2 fusion IDH 1 and 2 mutations (Wang et al., 2013). Another important mechanism in cholangiocarcinogenesis is the deregulation of specific miRNAs involved in cell proliferation, stemness, and migration. IL-6 is, in fact, able to depress the expression of the tumor suppressor NF2, by upregulating miR-let-7a, and of p16INK4a by increasing miR-148a and miR-152 (Braconi et al., 2010), whilst stimulating p38 MAPK phosphorylation by reducing miR-370 (Meng et al., 2008).
3.2. Fluke Infestations
Liver fluke infestations of Opisthorchis viverrini and Clonochis sinensis are endemic in several Asian countries (Thailand, Cambodia, Vietnam, Korea, and China), whereas Opisthorchis felineus is prevalent in Eastern Europe [Russia, Siberia, Ukraine, Belarus, and Kazakhstan] (Qian et al., 2016; Fedorova et al., 2017; Sripa et al., 2018). The infestation of the biliary network of human patients with O. viverrini is due to the ingestion of fluke metacercariae in undercooked or raw fish. Parasites proliferate in bile ducts, releasing eggs that are expelled with stools that contaminate water ways. Here, the eggs are eaten by water snails, the main food source for the fish (Sripa et al., 2007; Qian et al., 2016).
The association between fluke infestation and cholangiocarcinoma is well documented and the International Agency for Research on Cancer (IARC) has included O. viverrini and C. sinensis as Group I agents/biological carcinogens (van Tong et al., 2017). Within the biliary tree, liver flukes generate a strong immune response and induce an exacerbated chronic inflammatory response that is determined by duration of the infection, by number of infesting flukes, and probably by their modulation of the gut and liver microbiome (Plieskatt et al., 2013; Saltykova et al., 2018). This chronic fibroinflammatory cholangiopathy, as exemplified by O. viverrini infestation, is characterized by, cholangitis, eosinophilia, and progressive peribiliary fibrosis (Sripa et al., 2018). Reactive biliary epithelial changes in response to O. viverrini include cholangiocyte hyperplasia, goblet cell metaplasia, adenomatous hyperplasia, and dysplasia. Extracellular vesicles from O. viverrini have been also shown to be actively internalized by human cholangiocytes in vitro, where they promote cell proliferation and stimulate secretion of the proinflammatory cytokine IL-6 (Chaiyadet et al., 2015a & b).
The O. viverrini excretory/secretory products (OvESP), similarly to PAMPs, stimulate the signal cascade initiated by TLR4 activation that induce the NF-kB-mediated hypersecretion of several cytokines, including IL-6 and IL-8. This mechanism was developed as a host defence to microbial or parasitic infection, because it stimulates the action of COX2 and iNOS, leading to the local generation free oxygen and nitric radicals with antimicrobial activity, but which could also promote the malignant transformation of the cholangiocytes (Prueksapanich et al., 2018). C. sinensis infestation could also stimulate liver fibrogenesis by activating both TLR2 and 4, leading to the secretion of a wide range of ILs (1β, 4, 6, and 10), TNF- α, and IFNγ. These fibroinflammatory mediators contribute to the recruitment of inflammatory cells and to the proliferation and activation of resident fibroblasts (Sripa et al., 2007; Prueksapanich et al., 2018) leading to chronic inflammation and CCA development. However, it should also be noted that O. viverrini infection alone rarely shows cholangiocarcinoma development (Sripa et al., 2018). In this context, fluke-induced carcinogenesis has some peculiar characteristics. Specifically, opisthorchiasis is characterised by the accumulation of hormone-like molecules, such as oxysterol, a compound derived from the oxidation of conjugated bile acids by cytochrome P450. Its metabolites mediate pro-tumorigenic responses in bile ducts generating DNA adducts, increasing nitrosylative and oxidative damages due the hyperexpression and activation of iNOS and COX2, and deregulating the proliferation/death ratio of cholangiocytes (Vale et al., 2020). Similar effects were also induced by catechol estrogen quinone-like hormones of fluke origin, which were able to inhibit the DNA editing action of 8-oxodG and 8-nitroguanine (Correia da Costa et al., 2014). In addition, opisthorchiasis may enhance colonization of the biliary tree by species of Helicobacter, including H. pylori, a known GI cancer risk factor (Sripa et al., 2018).
Lastly, using a whole genome sequencing approach, Chan-on et al. (2013) depicted the mutational patterns of iCCA on a background of O.viverrini infection relative to iCCA without fluke infestation. In a cohort of 108 patients, these authors reported that non-O. viverrini related iCCAs showed a higher incidence of mutation in BAP1, IDH1 and IDH2 genes, while O. viverrini-related iCCAs were characterized by a low frequency of IDH1/2 mutations and a significantly higher frequency of TP53 and SMAD4 mutations. Consistent with the findings of Chan-on et al., and as previously noted, Jusakul et al., (2017) had also identified a cluster of liver fluke-associated iCCAs uniquely enriched in TP53 mutations (and ERBB2 amplifications) together with a low frequency of expression of BAP1 and IDH1/2 mutations. Conversely, these investigators also found fluke-negative cholangiocarcinomas to be characterized by increased BAP1 and IDH1/2 mutations, together with FGFR2 gene rearrangements. Collectively, these molecular genomic findings indicate that non-O. viverrini cholangiocarcinomas and O. viverrini-positive tumors have distinct mutational profiles, strongly implying that different etiologies may induce distinct mutational landscapes even within the same tumor type.
4. The Desmoplastic Reaction and iCCA Progression
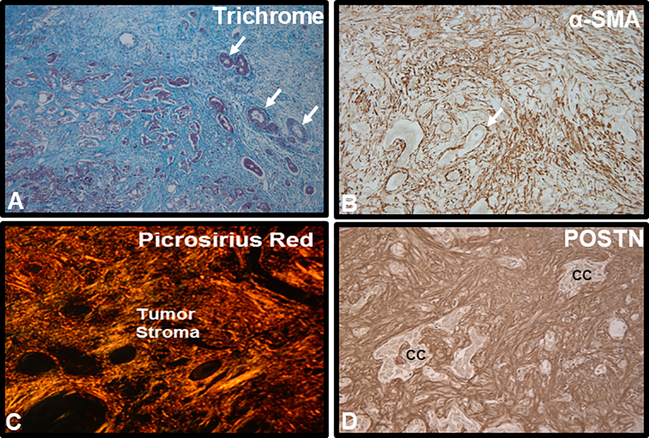
Irrespective of etiology (sporadic versus known risk factors), iCCAs are most often characterized by a prominent desmoplastic reaction typified by the formation of a hard, stiff, and hypovascularized scaffold comprised of a dense fibro-collagenous enriched matrix along with other ECM proteins, and containing an abundance of activated CAFs positive for α-SMA (a biomarker of myofibroblast differentiation), together with variable numbers inflammatory and vascular cell types (Figure 1, Sirica & Gores, 2014; Brivio et al., 2017; Cadamuro et al., 2018; Banales et al., 2020). In recent years, the desmoplastic stroma of iCCA, also known as tumor reactive stroma or TRS (Brivio et al., 2017), has become the focus of a growing research interest due to its prognostic potential and possibilities for therapeutic targeting. Moreover, there is now a growing body of evidence to convincingly implicate the tumor reactive microenvironment in iCCA as playing an active and crucial role in promoting and or modulating the major hallmarks of cancer (Hanahan & Weinberg, 2011). The stromal cellular and ECM components of iCCA, which frequently comprises the bulk of the tumor, provide critical molecular and biomechanical signals for perpetuating cholangiocarcinoma cell proliferation, promoting iCCA progression, facilitating iCCA invasion and metastasis, resisting apoptotic cell death, regulating iCCA angiogenesis and lymphangiogenesis, mediating epigenome and metabolic reprogramming, enabling resistance to chemo- and targeted agent therapies, and avoiding immune destruction (Sirica, 2012; Sirica & Gores, 2014; Affo et al., 2017; Brivio et al., 2017; Cadamuro et al., 2018; Mancinelli, et al., 2019; Cadamuro et al., 2019; Fabris et al., 2019; O’Rourke et al., 2019; Pant et al., 2020; Banales et al., 2020).
Figure 1.
Representative histological images illustrating characteristic features of the desmoplastic microenvironment in human iCCA. A. Masson trichrome staining of a moderately to poorly-differentiated mass-forming iCCA demonstrating the tumor to be largely comprised of a prominent desmoplastic stroma strongly stained for collagen (blue staining). Arrows point to representative small clusters of cholangiocarcinoma. B. CAFs comprising the vast majority of cell types populating the desmoplastic stroma of iCCA are seen to be strongly immunoreactive for α- smooth muscle actin (α- SMA), a biomarker of myofibroblast differentiation, whereas cholangiocarcinoma cells (arrow) are negatively stained for α- SMA. C. Picrosirius red staining for collagen (orange-staining under polarized light) typically reveals the extracellular matrix of desmoplastic CCA to be comprised of thick collagen fiber bundles that are largely comprised of collagen type I. D. Immunostaining for matricellular periostin (Postn), produced by α-SMA+CAFs and which has a binding site for collagen, is exclusively localized to the desmoplastic stroma of iCCA. cc, cholangiocarcinoma. Increased numbers of α-SMA+CAFs within the iCCA microenvironment together with corresponding strong stromal immunoreactivity for Postn have been shown to be predictors of poor survival outcomes for iCCA patients following curative-intent surgical resection.
A critical aspect of the relationship between the desmoplastic reaction in iCCA and increased malignant behavior is the deleterious interplay between cholangiocarcinoma cells and α-SMA+CAFs, which are considered the most important host-derived stromal cells within the tumor microenvironment. Activated CAFs in iCCA are a major source of secreted growth factors [e.g., hepatocyte growth factor (HGF), PDGFs (-BB & -D), heparin-binding epidermal growth factor (HB-EGF)], chemokines /cytokines [e.g., IL-6, TGF-β, stromal derived factor-1 (SDF-1), also known as CXCL12,] ECM proteins [e.g., fibrillar collagens, fibronectin], matricellular proteins [e.g., periostin (Postn), tenascin-C, and osteopontin], proteinases [e.g., metalloproteinases (MMPs)-2 and −9], and angiogenic modifiers [e.g., VEGFs A & C, thrombospondin-1], which collectively function to promote or modulate cholangiocarcinoma cell behavior and progression. (Sirica et al., 2009; Fingas et al., 2011; Sirica, 2012; Clapéron et al., 2013; Cadamuro et al., 2013; Sirica & Gores, 2014; Affo et al., 2017; Brivio et al., 2017; Cadamuro et al., 2018; Gentilini et al., 2018; Cadamuro et al., 2019; Nissen et al., 2019; Labib et al., 2019; Banales et al., 2020; Vaquero et al., 2020). Exosome crosstalk (Goulet et al., 2018; Yang et al., 2019), exchange of metabolites, such as pyruvate and lactate (Fiori et al., 2019; Yoshida et al., 2019), activation of mechanosensitive signaling cascades associated with activation of Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ), desmoplastic ECM stiffness, and hypoxia (Sahai et al., 2020, Banales et al., 2020), and aberrant integrin, sonic hedgehog, and Wnt/β-catenin signaling (Utispan et al., 2012; Gascard & Tisty, 2016; Labib et al., 2019; Banales et al., 2020) are also important to the interactive relationships that exist between cancer cells and CAFs, as well as other cell types of the iCCA tumor reactive microenvironment.
4.1. CAF Origins and Heterogeneity in iCCA
CAFs in iCCA are phenotypically heterogenous and are likely derived from various cell lineages (Sirica et. al., 2011; Sirica & Gores, 2014; Affo et al., 2017; Vaquero et al., 2020), although the actual cellular origins of these tumor stromal cells remains unclear due largely to a lack of in vivo cell fate tracing data. Moreover, CAFs are not normal fibroblasts, and their differentiation and phenotypic biomarker profiles can be influenced by the tumor microenvironment and molecular cross talk with cancer cells. However, based on what is known of fibrogenic cell responses in cirrhosis and fibroinflammatory/cholestatic liver diseases like PSC, it is generally believed that the most likely major source of CAFs in iCCA are likely originated from resident hepatic nonparenchymal cells, notably hepatic stellate cells and activated portal fibroblasts (Okabe et al., 2016; Manzanares et al., 2017; Affo et al., 2017; Vaquero et al., 2020).
Taking into account the limitations of using potential phenotypic biomarkers to identify the cell origins of CAFs in iCCA, such analyses can still be of value in suggesting possible cell lineage relationships, as well as potential prognostic relevance. Okabe et al. (2009) first reported that hepatic stellate cell markers (i.e., desmin, glial fibrillary acidic protein) were expressed in α-SMA+CAFs in human iCCA, suggesting a hepatic stellate cell origin. In contrast, Itou et al. (2019) more recently showed that α-SMA+CAFs in the stroma of primary human iCCAs expressed portal fibroblast markers [i.e., fibulin-2, thymus cell antigen-1 (Thy-1], and except for a small subset, were largely negative for fascin, a hepatic stellate cell biomarker. It was also reported by these investigators that α-SMA+CAFs in metastatic lymph nodes from iCCA patients, while positive for Thy-1 and negative for fascin, were also negative for fibulin-2. On the other hand, hepatic stellate cells in human cirrhotic liver were found to be negative for both Thy-1 and fibulin-2. In addition, Itou et al. also detected a small number of α-SMA+CAFs in the primary human iCCAs that were immunoreactive for bone-marrow derived fibrocyte biomarkers (CD34, CD45), suggesting that bone marrow derived cells may also contribute to some population of iCCA α-SMA+CAFs. Overall the findings by Itoh et al. imply that CAFs at the primary site of iCCA are similar to activated portal fibroblasts, but different from hepatic stellate cells and from CAFs in metastatic lymph nodes. Further, small subsets of CAFs may have also been derived from bone marrow derived fibrocytes, and in the case of the metastases, from resident cells of lymph nodes.
Manzanares et al. (2017) further showed by transcriptomic analysis and by immunophenotyping that α-SMA+CAFs purified from a rat orthotopic desmoplastic iCCA prominently overexpressed portal fibroblast biomarkers (e.g., gremlin 1, fibulin-2, Thy-1, cofilin-1, NTPDase2, elastin), but were negative for desmin, and lacked biomarkers for bone marrow derived fibrocytes or hematopoietic stem cells. Moreover, in this study, only a small number of desmin-positive fibroblastic cells were detected in the desmoplastic stroma of iCCA from which the α-SMA+CAFs were isolated from, as well as in human iCCA stroma.. Moreover, biomarker and karyotypic analyses supported a resident liver fibroblastic origin of the orthotopic iCCA-derived α-SMA+CAFs and argued against epithelial-mesenchymal transitional (EMT) as a source of these myofibroblastic cells. Lineage cell tracing has also presented evidence against the possibility of epithelial mesenchymal transition of neoplastic cholangiocytes as being a likely contributing source for α-SMA+CAFs accumulating within desmoplastic stroma of iCCA (Cadamuro et al., 2013).
It is also not known to what extent other cells, such as endothelial cells, may give rise to iCCAs. Recently, Zhang et al. (2020) using a droplet-based single-cell RNA sequencing platform to profile single cells from human iCCA identified six distinct fibroblast clusters, five of which were mainly enriched in the iCCA tissues and one primarily present in the adjacent tissues. All six subclusters expressed high levels of ACTA2 that encodes for α-SMA, in addition to other canonical CAF markers. Interestingly, one of these subclusters (vCAFs), which accounted for 57.6% of the iCCA fibroblast population, was characterized by microvascular signature genes, including CD146 (an endothelial cell marker), as well as high levels of IL-6 expression. CD146 expression has also been demonstrated in human liver endothelial cells and pericytes surrounding the portal vasculature (Strauss et al., 2017), as well as in mouse liver Thy-1-positive periportal fibroblasts and hepatic stellate cells, although at more variable levels in the hepatic stellate cells (Katsumata et al., 2017). Without cell fate tracing, however, it is not possible to conclude that vCAFs were derived from resident portal fibroblasts, hepatic stellate cells or subsets of hepatic endothelial cells and vascular associated pericytes.
In addition to α-SMA, other biomarkers used to delineate CAFs in iCCA include fibroblast specific protein 1 [FSP-1, also known as S100A4], fibroblast activating factor [FAP] and platelet-derived growth factor receptor-β [PDGFR-β] (Zhang et al., 2017; Sha et al., 2018; Itou et al., 2019; Vaquero et al., 2020). In a number of independent studies, iCCA patients whose tumor stroma was enriched in α-SMA+CAFs were demonstrated to have significantly shorter overall survival and worse recurrence-free survival rates following surgical resection than those whose iCCAs expressed low or negative levels of stromal α-SMA (Okabe et al., 2009; Chuaysri et al., 2009; Sha et al., 2018). High expression of FAP in iCCA has also been reported to be predictive of poor prognosis in iCCA patients (Yang et al., 2016). Zhang et al. (2017) have further shown histological categorization of CAFs in human iCCA to be a potentially useful predictor for prognosis, with surgically resected iCCA patients whose tumors showed a more normal fibroblastic-like morphology (termed mature CAFs) having a significantly better overall survival than those that were characterized by a myofibroblast morphology (termed immature CAFs). In this study, high stromal α-SMA expression was found to be associated with poor iCCA differentiation, but surprisingly, not with improved overall survival. This discrepancy may be due to a relatively small sample size and use of a different immunohistochemistry scoring system than those used by others.
Survival rates of iCCA resected patients with stromal CAFs immunoreactive for the transmembrane glycoprotein podoplanin (D2–40-positive myofibroblasts) were found to be significantly lower than those of patients without podoplanin expressing CAFs (Aishima et al., 2008). Podoplanin has also been shown to be a potentially useful stromal prognostic marker for perihilar cholangiocarcinoma (Obulkasim et al., 2018). Furthermore, CD10 (neprilysine), a membrane bound metalloprotease expressed in CAFs, may be more involved with the progression of perihilar and extrahepatic CCAs than of iCCAs (Nishihara et al., 2009). High tissue and serum Postn, which in both human and rat is produced solely by α-SMA+CAFs in iCCA tissue (Utispan et al., 2010; Dumur et al., 2010) have also been demonstrated to be a particularly promising predictor of poor prognosis in human iCCA patients (Utispan et al., 2010); Thuwajit et al., 2017) and increased malignant tumor grade and progression in a well established syngeneic rat model of desmoplastic iCCA (Manzanares et al., 2018). In this same rat model, high accumulation of α-SMA+CAFs in the tumor stroma was also found to be associated with increased iCCA tumor grade and enhanced malignant aggressiveness (Sirica et al., 2011).
4.2. PDGF-D and TGF-β and the Desmoplastic Reaction in iCCA
PDGF-D and TGF-β play key roles in driving the recruitment and expansion of CAFs in iCCA. Cadamuro et al., (2013) have presented compelling data supporting a model of CAF recruitment into iCCA based on a PDGF-D-mediated paracrine fibroblastic cell recruitment by cholangiocarcinoma cells. In this model, PDGF-D, which is up-regulated in cholangiocarcinoma cells by a hypoxia-mediated mechanism, is secreted into the tumor cell microenvironment, where it in its active ligand form binds to and activates its cognate receptor PDGFRβ expressed by resident liver fibroblastic cells/myofibroblasts and CAFs. This interaction, in turn, promotes proliferation and elicits a strong migratory response in these cells via activation of Rho GTPases (i.e., Rac1, Cdc42) and JNK, thereby providing a mechanism for CAF recruitment in iCCA as illustrated in Sirica et al. (2019).
It has also been shown that PDGF-D, and also PDGFBB, each of which are abundantly produced by cholangiocarcinoma cells, can sensitize activated CAFs to apoptotic cell death triggered by BH3 mimetics, such as navitoclax and ABT-199 (Rizvi et al., 2014). Similar apoptotic sensitization was demonstrated in co-cultures of myofibroblasts and cholangiocarcinoma cells. Furthermore, this study also showed that PDGF-linked apoptotic priming of CAFs occurs via Puma-mediated Bak activation, which can then be converted to full-blown apoptosis induced by navitoclax or ABT-199. Notably, navitoclax or ABT199 have been demonstrated to significantly reduce iCCA tumor burden in mouse (Rizvi et al., 2014) and rat cholangiocarcinoma models (Mertens et al., 2014; Cadamuro et al., 2019) by depleting CAFs from the tumor stroma. Navitoclax treatment was also demonstrated to be associated with decreased lymphatic vascularization and lymph node metastasis in the rat cholangiocarcinoma model (Cadamuro et al., 2019).
TGF-β plays a key role in the acquisition of the CAF phenotype and is well known inducer of myofibroblast differentiation (i.e. α-SMA induction) and fibrosis (Giscard, P. & Tlsty, 2016; Caja et al., 2018). Exosomal TGF-β has also been identified as a molecular mechanism involved in CAF activation (Goulet et al., 2018). In relation to iCCA, TGF-β, expressed in both cholangiocarcinoma cells and in α-SMA+CAFs, was demonstrated to be essential for provoking a dramatic desmoplastic-like reaction in vitro (prominent overproduction of dense fibrocollagenous matrix concomitant with significantly enhanced accumulation and proliferation of α-SMA+CAFs) within the matrix of a three-dimensional organotypic cholangiocarcinoma culture model that closely resembled the desmoplastic features of the in situ tumor. This model was established by co-culturing of cholangiocarcinoma cells derived from a syngeneic rat iCCA with α-SMA+CAFs exhibiting portal fibroblast biomarkers, obtained from the same tumor type, within a dilute collagen type I hydrogel (Manzanares et al., 2017). Three-dimensional co-culturing of the activated CAFs with the cholangiocarcinoma cells was further found to markedly increase mature TGF-β production within the gel cultures over that elaborated by CAFs alone, to cause marked gel contraction (shrinkage), an indicator of increased substratum stiffness, and to promote significantly increased cholangiocarcinoma cell growth and elevated expression of phenotypic markers of malignant progression (i.e., Muc1). Cholangiocarcinoma spheroid ductal-like structures which formed in the gel cultures were further observed to become increasingly more anaplastic, as well as invasive when co-cultured in the presence of α-SMA+CAFs (Campbell et al., 2012; Manzanares et al; 2017; Manzanares et al., 2018; Sirica et al., 2019). TGF-α, an epidermal growth factor receptor (EGFR) ligand expressed in iCCA, was further determined to be a key factor for further enhancing cholangiocarcinoma anaplasia, hyperproliferation, and higher malignant grading in this 3-D culture model (Manzanares et al; 2017).
The interplay between activated CAFs and cholangiocarcinoma cells through TGF-β expression and EGFR activation in relation to cholangiocarcinoma progression is further emphasized by the interesting findings of Clapéron et al. (2013), who showed that cholangiocarcinoma cells produced TGF-β1, which, in turn, induced HB-EGF expression in CAFs leading to paracrine activation of EGFR on the cancer cells, promoting cholangiocarcinoma cell invasion. Of further interest, TGF-β1 expression in cholangiocarcinoma cells was also found to be enhanced by HB-EGF stimulation. These results support a model as first proposed by Clapéron et al. depicting a reciprocal paracrine loop between cholangiocarcinoma cells and CAFs through the HD-EGF/EGFR axis that contributes to cholangiocarcinoma progression and also triggers TGF-β1 production in cholangiocarcinoma cells, which stimulates CAF activation and functions. TGF-β1 has also been demonstrated to promote iCCA tumor growth and metastasis in a rat cholangiocarcinoma model (Huang et al., 2016).
Mesothelin (Msln) is a glycosylphosphatidylinositol-anchored cell membrane glycoprotein that is expressed in liver portal myofibroblasts (Fausther et al., 2017; Koyama et al., 2017), as well as shown to be highly overexpressed in human and rat cholangiocarcinoma cells, but less prominently expressed or not detected in CAFs in desmoplastic human or rat iCCA (Yu et al., 2010; Tang et al., 2013; Manzanares et al., 2017; Manzanares et al., 2018). Msln, however, has been detected by Western blotting in α-SMA+CAFs expressing portal myofibroblast biomarkers, including Thy-1, which were derived from a syngeneic rat iCCA, but expressed at a lower level than Msln protein amounts detected in cell lysates from intrahepatic and extrahepatic metastatic cholangiocarcinoma cells obtained from the same iCCA model (Manzanares et al., 2018).
Msln overexpression has been suggested to be a prognostic factor for patients with intrahepatic cholangiocarcinoma (Nomura et al., 2013) and more recently demonstrated to predict malignant progression in the rat iCCA model described above (Manzanares et al., 2018). In relation to cholestatic liver fibrosis associated with early activation of portal fibroblasts, Koyama et al. (2017) have shown Msln to regulate TGF-β1-inducible activation of portal fibroblasts by disrupting the formation of an inhibitory Thy-1-TGFβR1 complex, as well as to facilitate fibroblast growth factor-mediated proliferation of these cells. However, while it may be assumed, it remains to be determined if Msln effects Thy-1 and TGFβR1 in portal fibroblast-derived CAFs in cholangiocarcinoma in a similar manner. Of added interest is the identification of two distinct molecular weight forms of Msln being expressed in rat cholangiocarcinoma cells in vivo and in 3-dimensional culture, notably a 40 kDa form (mature form), which was associated with a cytoplasmic and diffuse cell membrane immunostaining pattern, and whose increased expression predicted a more aggressive iCCA phenotype compared with a more heavily glycosylated 50 kDa form that was expressed predominantly at the apical luminal surface of well differentiated cholangiocarcinoma ducts and which predicted a less aggressive iCCA phenotype. Complementary to these findings, it could be further shown in 3-dimensional culture that co-culturing α-SMA +CAFs with cholangiocarcinoma cells contributed to a significant increase in the production of the 40 kDa form of Msln, which correlated with an increase in cholangiocarcinoma cell anaplasia and disruption of cholangiocarcinoma “ductal-like” polarity (Manzanares et al., 2018). In comparison, highly tumorigenic rat cholangiocytes harboring mutationally activated rat neu oncogene (homolog of erbB2) were found to primarily express the 40 kDa form of Msln, which was associated with enhanced malignant cell features (e.g., higher malignant grade, disruption of polarized morphogenesis and underexpression of cell polarity regulating proteins, oncogenic neu activation with corresponding overexpression of phosphorylated Erk1/2 and AKT, and invasiveness) even when cultured in the absence of CAFs (Mazanares et al., 2018; Wei et al., 2018).The relationship between Msln isoforms, CAFs, and cholangiocarcinoma progression is illustrated in Figure 2. Further studies are needed now to extend these preclinical findings to human iCCA.
Figure 2.
Relationship between mesothelin (Msln) immunostaining pattern, disruption of polarized morphogenesis, and invasiveness in a syngeneic orthotopic rat tumor model and corresponding 3-dimensional (3-D) organotypic culture model of cholangiocarcinoma progression. A. Immunohistochemical demonstration of luminal Msln immunoreactivity expressed in differentiated cholangiocarcinoma ductal structures (white arrows) contrasted with diffuse cell membrane/cytoplasmic Msln immunoreactivity (black arrows) expressed in invasive cholangiocarcinoma emanating from the ductal structures in an orthotopic rat iCCA (TDECC iCCA). B. Phase contrast image of viable spheroids and well-differentiated ductal-like structures with distinct lumens (L) formed in 3-D organotypic culture of a cholangiocarcinoma cell strain (TDECC cells) derived from rat TDECC iCCA. C. Hematoxylin & eosin stained section demonstrating polarized ductal-like structure formed from rat TDECC cholangiocarcinoma cells in 3-D culture. D. In the absence of CAFs, polarized ductal-like structures formed from TDECC cholangiocarcinoma cells in 3-D culture exhibit strong luminal surface immunoreactivity for Msln (white arrow heads). E. In sharp contrast, polarized morphogenesis is dramatically disrupted when TDECC cholangiocarcinoma cells are maintained in 3-D co-cultured with α- SMA+CAFs, also derived from a rat TDECC iCCA. Under these co-culture conditions, the cholangiocarcinoma cells can organized into hyperproliferative, invasive structures exhibiting diffuse cell membrane/cytoplasmic immunoreactivity for Msln (black arrows). In both the orthotopic tumor and organotypic culture models, a more heavily glycosylated 50-kDa form of Msln was demonstrated to be associated with the apical surface pattern of expression of Msln, whereas a 40-kDA Msln form was shown to be associated with the diffuse cell membrane/cytoplasmic immunostaining pattern. Data based on results presented in Manzanares et al., 2018. Images in 2A and 2E are slightly modified versions of those published as Figure 2B and Figure 7B, respectively, in Manzanares et al, 2018.
TGF-β is also a known inducer of α-SMA+CAF-derived Postn, which is highly germane to supporting and sustaining iCCA’s desmoplastic microenvironment and promoting ICCA invasiveness (Utispan et al., 2012; Sirica et al., 2014) Moreover, Postn along with other matricellular proteins, such as tenascin-C and Msln, may be playing a pivotal role in conditioning metastatic niches and the formation of metastases (Ma et al., 2012; González & Alonso, 2018; Lowy & Oskarsson, 2015; Coelho et al., 2020). Postn, like TGF-β, has also been shown to stimulate liver fibrogenesis by activating lysyl oxidase in hepatic stellate cells, inducing the expression of collagen type I and fibronectin, and stimulating the phosphorylation of SMAD2/3, as well as of focal adhesion kinase and AKT in hepatic stellate cells (Kumar et al., 2018). A novel TGF-β induced long noncoding RNA has further been demonstrated to promote an inflammatory microenvironment in human intrahepatic cholangiocarcinoma (Merdrignac et al., 2018). In addition, preclinical targeting of TGF-β in rat cholangiocarcinoma models has been demonstrated attenuate the desmoplastic reaction generated by co-culturing α-SMA+CAFs with cholangiocarcinoma cells in 3-dimensional culture (Manzanares et al., 2017) and to reverse preexisting fibrosis induced by thioacetamide in vivo (Ling et al., 2013), leading in both cases to a significant reduction in cholangiocarcinoma.
4.3. The Matrisome, Tumor Stiffness, and Hypovascularity in iCCA
In a recent (albeit limited) analysis by Carpino et al., (2019b) to characterize the matrisome or ECM proteome of human iCCA, the proteomic profile revealed a unique signature of ECM proteins in iCCA when compared with that of paired samples of non-cancerous liver tissue (NCT). As expected, the iCCA ECM exhibited high levels of fibrillar collagen type I components (COL1A1 and COL1A2) and of the matricellular protein Postn, which is known to interact with and regulate collagen fibrillogenesis (Norris et al., 2012; Sirica, A.E., et al., 2014), as well as bind to other ECM proteins (i.e, tenascin-C) promoting iCCA malignant behavior (Sirica et al., 2014). Col3A1, a component of fibrillar collagen type III was also observed to be overexpressed in 6 of 9 analyzed samples of iCCA ECM when compared to NCT and further shown to be a component of tumor-associated aligned collagen fibers. Also, in contrast to the NCT, the iCCA ECM was characterized by an almost total absence of elastic fibers and a low expression of proteoglycans and downregulation of laminins, type IV collagen, and basement membrane-specific heparan sulphate proteoglycan. The angiogenesis factors angiopoietin-related protein 6 and sushi repeat protein X-linked 2 were also seen to be dramatically downregulated in the iCCA samples. Taken together, these findings are compatible with an increased tumor stiffness, a breakdown of basement membrane leading to disruption of cholangiocarcinoma cell polarity, and a hypovascularized iCCA stroma.
Increased tumor stiffness, as exemplified by desmoplastic epithelial cancers like iCCA not only affects malignant cell progression by triggering cell survival, proliferation and motility signaling cascades within the cancer cells (Noguchi et al., 2018; Kalli & Stylianopoulos, 2018; Sahai et al., 2020), but also impacts on surrounding stromal cells, whereby tumor stiffness mechanoactivates YAP/TAZ in fibroblastic cells, leading them to acquire a CAF phenotype (Noguchi et al., 2018; Yoshida et al., 2019). Moreover, YAP activation is enhanced by ECM matrix stiffness, thus establishing a feed-forward self-reinforcing loop to maintain the CAF phenotype. Increased mechanical stress associated with tumor stiffness produced by desmoplasia can also lead to collapse of micro-blood vessels within the tumor, leading to hypovascularization, hypoxia, and reduced bioavailability for therapeutic drugs (Sahai et al., 2020). Enhanced expression of thrombospondin-1, immunohistochemically localized to both CAFs cholangiocarcinoma cells in iCCA, has also been reported to be related to diminished microvessels in human iCCA (Kawahara et al., 1998; Aishima et al., 2002; Tang et al., 2006).It is also relevant here that thrombospondin-1 is an activator of latent TGF-β (Murphy-Ullrich & Suto, 2018).
5. Immune Milieu in iCCA.
5.1. The Innate Immune System in iCCA
The iCCA tumor microenvironment is populated to various degrees by cells of the innate immune response, including macrophages (in particular M2 or alternatively activated macrophages), neutrophils, and natural killer (NK) cells. In the TRS of iCCA, two populations of macrophages are present, those derived from Kupffer cells localized to the perisinusoidal space of Disse, and TAMs.
TAMs are CD86 +/CD206 + M2 macrophages, mainly derived from CD14+/CD16+ circulating monocytes, which play a major role in suppressing T lymphocyte activity and proliferation, thereby having a pronounced anti-inflammatory action (Rőszer et al., 2015). M2 TAMs promote angiogenesis, induce tissue remodeling, and stimulate the apoptosis of M1 macrophages (Duluc et al., 2007; Rőszer et al., 2015). Monocytes accumulated within TRS are promoted by tumor cells through the local secretion of a variety of cytokines, including IL-1β, IL-4, IL-8, IL-10, IL-13, colony-stimulating factor 1, and growth factors (i.e., VEGF-A) and through the secretion of CCL2 by Kupffer cells (Li et al., 2018).
In iCCA, TAMs mainly localize to the tumor invasive front, with high counts of M2-TAMs in iCCA patients shown to correlate with poor disease-free survival (Hasita et al., 2010) and poor overall survival in surgically resected iCCA patients (Sun et al., 2020). M2 TAMs could, in fact, sustain tumor invasive ICCA growth and promote metastasis by various mechanisms, including (1) via the release of proangiogenic growth factors (e.g., angiopoietin 1, IL-8, VEGF-A) (Leyva-Illades et al., 2012; Corliss et al., 2016), (2) secretion of soluble factors that stimulate the migration of neoplastic cells (e.g., VEGF, fibroblast growth factor (FGF)-1/−2, PDGF, and TGF-β) (Roy et al., 2019), and (3) through the action of MMP-9, a key metalloproteinase involved in tumor spread (Raggi et al., 2017). Furthermore, TAMs promote cholangiocarcinoma cell proliferation by secreting IL-6 and VEGF into the tumor microenvironment, which, in turn, can stimulate the activation of the p42/p44 MAPK and AKT/mTOR signaling pathways, (Banales et al., 2016; Roy et al., 2019).TAMs may drive iCCA progression by their increased expression of WNT ligands, such as WNT3a and WNT7b (Loilome et al., 2014; Boulter et al., 2015; Saito et al., 2018) and enzymes, such as COX2 and iNOS (Chariyalertsak et al., 2001; Wόjcik et al., 2012). ECM modification in iCCA may also modulate the accumulation of M2 TAMs within the tumor microenvironment. Notably, Postn has been shown to stimulate the migration of TAMs in vitro and to spatially correlate with M2 TAM density in primary human iCCA tissue. (Zeng et al., 2018).
Tumor associated neutrophils (TANs) represent another cell population belonging to the tumor reactive microenvironment that is of increasing importance in cancer (Jaillon et al., 2020). CD66b+ neutrophils are recruited through the local secretion of CXCL5 (Zhou et al., 2014), but very little is known about the pathogenic mechanisms of these cells in iCCA. Current data show that accumulation of TANs within the TRS of iCCA is predictor of poor prognosis (Mao et al., 2015), and that a high neutrophil-to-lymphocyte ratio is an independent predictor of poor survival outcome and insensitivity to chemotherapy and radioembolization (Tan et al., 2016; Lin et al., 2016; Wang et al., 2018; Filippi et al., 2020). One may speculate that accumulation of TANs contributes to the generation of a microenvironment prone to immunosuppression that favors neoplastic cell growth through the local release of CCL2 and CCL17 and recruitment of M2 macrophages.
NK cells, immunophenotypically recognized as CD3-CD56+ cells, represent between 30 and 40% of the total normal human liver lymphocyte population (Björkstrom et al., 2016, Martín-Sierra et al., 2019). Their biological function is to protect against bacterial invasion via the release of cytotoxic granules, which also produce lytic death of tumor cells. In iCCA, NK are recruited in the tumor area in response to the secretion by cholangiocarcinoma cells of CXCL9, a INFγ-dependent chemokine. Notably, the ablation of CXCL9 in a mouse model of iCCA was observed to lead to a reduced accumulation of NK cells in peritumoral area, resulting in an increased volume of the tumor (Fukuda et al., 2020). In a xenotransplant model of human cholangiocarcinoma, the infusion of mice with NK cells was also shown to significantly reduce tumor growth as a result of the lytic and cytotoxic actions of the infused cells (Jung et al., 2018). The mechanisms regulating the antitumor effects of NK cells in iCCA are not well understood, but may involve the secretion of several immunomodulatory mediators, such as adenosine, PGE2, and TGF-β (Melaiu et al., 2020).
Dendritic cells (DCs) represent a bridge between innate and adaptive immune response due to their ability to function as antigen presenting cells to prime CD4+ and CD8+ T-lymphocytes. CD83+ DCs accumulated at the invasive front of iCCA have been shown to significantly correlate with the number of CD4+ and CD8+ T effector cells in the cancerous region, leading to a significantly lower incidence of lymph node metastasis and a better survival outcome for the CD83+ patients than that of CD83-negative patients. (Takagi et al., 2004). Suppression of the expression of receptors for TGF-β and IL10 (immunosuppressive cytokines) on DCs was further demonstrated to result in an increased activation of the adaptive immune response, consequently leading to an enhanced secretion of IFNγ and increased cytolytic action of T-lymphocytes against cholangiocarcinoma cells (Thepmalee et al., 2018; Thepmalee et al., 2020). Furthermore, a limited number of studies on small cohorts of cholangiocarcinoma patients have yielded promising results when coupling chemotherapy (i.e., with gemcitabine) with immunotherapy using protein or RNA-pulsed DCs (Higuchi et al., 2006; Kobayashi et al., 2013; Junking et al., 2017; Sawasdee et al., 2020).
5.2. Adaptive Immune System and Tumor-infiltrating Lymphocytes in iCCA
The adaptive immune response, which involves tumor infiltrating lymphocytes (TILs) and their modulating effects on iCCA growth and spread, is becoming more apparent. This is particularly highlighted by the fact that reduced recruitment of TILs or their inactivation has been shown to correlate with worse patient survival outcomes and increased tumor growth and malignant aggressiveness (Zheng et al., 2020). In an analysis of human iCCA biopsies, TILs accounted for about 8.0% of the inflammatory cells present in the tumor sample (Martìn-Sierra et al., 2019). CD4+ T cells have been shown to be mainly localized at the tumor invasive front of iCCA, whilst CD8+ T cells were usually, but not exclusively, recruited into the tumor mass (Kasper et al., 2009). Patients with iCCA whose tumors exhibited an increased presence of CD3+ and CD8+ infiltrating T cells were also found to exhibit higher overall and disease free survival rates, as well as a lower tumor recurrence risk after curative-intent tumor resection (Vigano et al., 2019; Tian et al., 2019; Tian et al., 2020). In contrast, patients whose iCCAs were characterized by increased infiltration of Foxp3+ regulatory T cells (Tregs) exhibited worse overall survival rates (Vigano et al., 2019), likely due to the ability of Tregs to secrete IL-10 and TGF-β, cytokines able repress the cytotoxic activity of CD8+ T and of NK cells in the tumor microenvironment (Tu et al., 2014). CD20+ B lymphocytes are unfrequently detected in iCCA (Kasper et al., 2009), but their presence also seems to be predictive of a better overall survival when compared with iCCA patients in which B cells were absent (Goeppert et al., 2013).
One of the salient mechanisms used by the cancer cells to survive is to escape immune surveillance of immune cells by modulating the expression of several immune checkpoints, such as programmed death-1 (PD-1) and its ligand PD-L1, or cytotoxic T-lymphocyte antigen-4 (CTLA-4). PD-L1 is variably expressed by cholangiocarcinoma cells, (Sabbatino et al., 2016; Kitano et al., 2020). The rational for the use of a PD-1 based immunotherapy in iCCA is that cholangiocarcinoma cells could promote their survival by inducing depletion of PD-1-expressiong T cells through the PD-L1/PD-1 route. Moreover, CTLA-4 is expressed by Tregs that are known to depress the activity of DCs by binding to its specific surface receptor CD80, thus blocking the cytotoxic action of DCs (Goeppert et al., 2013). In addition, it was shown that targeting of PD-1 and CTLA-4 expressed by iCCA-derived TILs could induce, ex vivo, an increased activation of tumor-infiltrating T cells (Zhou et al., 2019). Notwithstanding this, data regarding the importance of PD-L1 expression in cholangiocarcinoma cells are conflicting. In conflict with studies implicating the expression of this ligand as an independent prognostic factor for predicting poor overall survival of iCCA patients (Sabbatino et al., 2016; Kitano et al., 2020), a recent metanalysis of 1066 cholangiocarcinoma patients subdivided in 11 studies showed that the association among PD-L1 expression and overall or disease free survival was not significant (Xu et al., 2019).
To date, various clinical trials for iCCA based on PD-1/PD-L1 modulation are now in the recruiting phase (e.g., NCT04157985, NCT04440943, NCT02834013, NCT04295317, NCT03111732), but no data are yet available. As alluded to in a previous section of this review, Job et al. (2019) reclassified ICCA on the basis of the different inflammatory infiltrates into 4 subtypes that included immune desert (non-inflammatory), lymphoid, myeloid, and mesenchymal types. The lymphoid (immunogenic) subtype, which represented 11% of the total iCCAs analyzed, was characterized by a massive T-lymphocyte infiltration, an activation of inflammatory and immune checkpoint pathways, and was associated with the best patient survival outcome when compared with the other subtypes. Job et al. (2019) further suggested that this distinct inflamed iCCA subtype could be amenable for treatment with checkpoint blocking immunotherapy due to the unique composition of the inflammatory infiltrate.
6. Challenges and Clinical Implications
Despite improvements in the standard of care and multidisciplinary management of iCCA, the overall global 5-year survival of patients with iCCA has not changed appreciably over the past 20–30 years, currently reported as ≤ 10% (Dhanasekaran et al., 2013; Mody et al., 2018; Simile et al., 2019). As already noted in the Introduction, the majority of patients with iCCA (70–80%) present with advanced disease where therapeutic options are limited to palliative treatments. Gemcitabine plus cisplatin remains the standard of care as a first line systemic treatment for non-resectable cholangiocarcinoma. This palliative treatment, however, provides only a modest survival benefit (Plentz & Malek, 2016; Buettner et al., 2017; Vienot & Neuzillet, 2019). Furthermore, iCCAs, which most often develop sporadically in the absence of known risk factors, are asymptomatic in their early stage. Even in patients with known risk factors, early diagnosis is problematic, and further, most patients that are diagnosed with late disease (locally advanced or metastatic iCCA) usually present with ambiguous or nonspecific symptoms.
Currently there are no curative medical treatments for iCCA and the only hope for a potentially curative therapy is complete surgical resection. However, only a minority of iCCA patients (-i.e., 10%-to ~25%) are eligible for curative-intent surgical resection. (Buettner et al., 2017; Ma et al., 2020; Banales et al., 2020). Moreover, even with curative-intent resection, an estimated median disease free survival in various patient series has been reported to range from 12 to 36 months (Rizvi et al., 2018), with overall 5-year survival rates after resection ranging between 20% and 35% in some series (Mavros et al., 2014; Chun & Javle, 2017; Waisberg et al., 2018; Ma et al., 2020).
The BILCAP phase 3 randomized trial (NCT00363584) aimed at evaluating capecitabine as an adjuvant treatment for biliary tract cancers of all types (including iCCA) following curative-intent surgical resection did not meet its end point of improving overall survival, but demonstrated a median overall survival of 51.1 months in the capecitabine group versus 36.4 months in the observational group not receiving capecitabine (Primrose et al., 2019). The PRODIGE12-ACCORD 18-UNICANCER GI phase 3 trial (NCT01313377), which evaluated adjuvant gemcitabine and oxiplatin in resected biliary tract cancers of all types, failed to show any demonstrable benefit (Edeline et al., 2019).
Molecular profiling of iCCA has revealed a complex and evolving genomic and epigenomic landscape characterized by extensive inter-tumor and intra-tumor heterogeneity that has been attributed in part to diverse multifactorial etiologies, histological tumor differences, adverse stromal microenvironments, and evolving tumor progression (Jusakul et al., 2017; Pellino et al., 2018; Rhee et al., 2018; Sirica et al., 2019; Ma et al., 2019; Goeppert et al., 2019; Banales et al., 2020). This inter-tumor and intra-tumor heterogeneity serves as a major obstacle to the development of molecular targeted and immune-based therapies for iCCA. It has also hindered the development of non-invasive serological tests for iCCA early diagnosis, as well as for monitoring iCCA treatment responses and prognosis. Research aimed at developing noninvasive serological tests based on multiplexing of discreet cholangiocarcinoma cell and tumor stromal markers that may include tumor exosomes, mRNAs, and distinct protein biomarkers linked to malignant progression, such as Muc1, Postn and Msln, could be highly useful towards advancing this clinical need.
The results from earlier clinical trials aimed molecular therapeutic targeting of altered signaling pathways in iCCA, including EGFR, ERBB2, MET, PDGFR, VEGF, and VEGFR have, to date, been disappointing (Mertens et al., 2018; Pellino et al, 2018; Labib et al., 2018; Simile et al., 2019). Direct therapeutic inhibition of activated KRAS has also proved elusive (Mertens et al., 2018). However, with the advent of next-generation sequencing technology to identify oncogenic driver mutations, there is now a powerful tool to identify novel molecular targets and to allow personalized targeted approaches to iCCA therapy. (Mertens et al., 2018; Pellino et al. 2018, Dabney et al., 2019).
The value of the personalized approach to iCCA targeted therapy is exemplified by the rapid translation of FGFR2 fusion/translocation and IDH1/2 mutations, respectively, into promising therapeutic targets for particular subsets of iCCA patients (Pellino et al., 2018; Dabney et al., 2019; Krook et al., 2020). Mutated FGFR2 has been described in approximately 10%-to-23% of patients with iCCAs (Nakamura et al., 2015; Pellino et al., 2018; Dabney et al., 2019; Simile et al., 2019; Krook et al., 2020). Sia et al. (2015), using RNA- and exome-sequencing analysis, reported a higher rate of FGFR2 fusions (45%) in a cohort of 107 resected iCCA patients, which represented the most recurrent targetable alteration. IDH1/2 mutations also commonly occur in patients with iCCA at reported rates of 13% up to 30% (Pellino et al., 2018; Mertens et al., 2018; Goeppert et al., 2019; Lowery et al. 2019; Abou-Alfa et al., 2020a).
The efficacy of pemigatinib, a selective oral FGFR 1,2, and 3 inhibitor, was recently investigated in FIGHT-202 (NCT02924376), a multicenter open-label single arm phase II study that included a cohort of 107 cholangiocarcinoma patients with locally advanced or metastatic cholangiocarcinoma harboring FGFR2 gene rearrangements/fusions and who had received at least one systemic cancer therapy that did not include selective FGFR inhibitors. Among these 107 patients, permigatinib elicited an overall response rate of ~36% (38 of 107 patients), with 3 patients having a complete response. Kaplan-Meier estimates of overall survival further demonstrated a 12-month survival rate of 68% in the permigatinib-treated cholangiocarcinoma patients with FGFR2 fusions or rearrangements compared to 23% for treated cholangiocarcinoma patients with other FGF/FGFR alterations and 13% for those with no FGF/FGFR alterations (Abou-Alfa et al. 2020b). Based on these results, the FDA fast tracked the approval of pemigatinib for the treatment of adult patients with previously treated, unresectable, locally advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements, making it the first targeted agent FDA approved for cholangiocarcinoma therapy. Of further note, in a limited study involving six patients with advanced FGFR2 fusion-positive iCCA, treatment with TAS-120 (an irreversible FGFR inhibitor) was shown to overcome acquired resistance that had developed in these patients to previous treatments with the ATP-competitive FGFR inhibitors BGJ39 or Debio (Goyal et al., 2019).
ClarlDHy, an international multicenter, randomized, double-blind, placebo-controlled, phase III study, evaluated the therapeutic efficacy and safety of ivosidenib (AG-120), a small molecule targeted inhibitor of mutated IDH1, when administered to patients with chemotherapy-refractory advanced IDH1 mutant iCCA. (Abou-Alfa et al. 2020a). This clinical trial demonstrated encouraging therapeutic benefits for the IDH-1 mutant iCCA patients over those treated with matched placebo. Specifically, in the ivosidenib-treated group, progression free survival was reported to be 32% at 6 months and 22% at 12 months, versus no patients in the placebo group being free from progression at 6 months or more.
The complex microenvironment of desmoplastic iCCA and the deleterious interplay between activated CAFs, inflammatory and immune cells, ECM, and vascular components clearly provides a rationale for devising novel therapeutic strategies aimed at targeting stromal components of iCCA. Activated CAFs, by virtue of being the most prominent cell component and driver of the desmoplastic reaction, are emerging as an appealing target for iCCA therapy. As previously cited, preclinical results with TGF-β inhibitors and with BH3 mimetics offer proof of concept that depleting CAFs and ablating the desmoplastic stroma can potentially have significant therapeutic efficacy for iCCA. In further support of this concept, photothermal depletion of CAFs preferentially loaded with hybrid iron oxide-gold nanoparticles was recently shown to normalize tumor stiffness and produce tumor regression in desmoplastic human EGI-1 cholangiocarcinoma formed in a mouse xenograft model (Nicolás- Boluda et al., 2020). The FDA-approved antifibrotic drug nintedanib has also been recently demonstrated to reduce xenografted iCCA growth and activated CAFs expressing α-SMA, and further, combinational treatment with nintedanib and gemcitabine against CAFs and iCCA cells was shown to exhibit the strongest inhibition of tumor growth compared with control and single-treatment groups (Yamanaka et al., 2020).
Another potential strategy suggested for targeting CAFs for clinical benefit relates to developing treatments that would cause activated CAFs to revert to a phenotype comparable to that of normal fibroblasts, as exemplified by the targeting of the vitamin D receptor in pancreatic cancer (Sahai et al., 2020), or by the normalizing effect of dasatinib, a FDA approved inhibitor of PDGFR, on CAFs from primary lung cancer (Haubeiss et al., 2010). Targeting specific tumor promoting factors produced by CAFs, such as Postn, SDF-1, or FAP may also provide a potentially useful strategy for iCCA therapy (Sirica et al., 2014; Liepelt & Tacke, 2016; Lin et al., 2019).
Activated CAFs have also emerged as central players in immune regulation that shapes the immune microenvironment, including that of iCCA (Monteran & Erez, 2019; Fabris et al., 2019). FAP+CAFs have been shown to be a major source of CCL2, which promotes recruitment of myeloid-derived suppressor cells (MDSC) and immunosuppression via a STAT3-CCL2 signaling. FAP, phospho-STAT3 and CCL2 are expressed in the desmoplastic stroma of iCCA (Yang et al., 2016). Furthermore, TGF-β and VEGF, each of which are hypersecreted into the iCCA microenvironment, are both potent suppressors of the tumor immune response (Thepmalee et al., 2018; Batlle & Massagué, 2019; Fukumura et al., 2018; Ma et al., 2019).
As alluded to earlier in this Review, a majority of desmoplastic iCCAs have been found to exhibit a sub-optimal response rate of less than 10% to immune check point blockade monotherapy (Loeuillard et al., 2020). This is consistent with data already cited that most iCCAs are characterized by an immune desert phenotype (Job et al, 2019), with a non-T cell infiltrated tumor immune microenvironment. Loeuillard et al. (2020) have recently shown that TAMs are the major source of PD-L1 in murine and human cholangiocarcinomas and dual inhibition of TAMs and granulocytic-MDSC potentiated PD-1 blockade in a murine cholangiocarcinoma model.
With respect to other gastrointestinal cancer types, but relevant to iCCA, combined targeting of TGF-β and PD-L1 receptors has been shown to promote T-cell mediated tumor regression and improved survival in a genetic mouse model (KPC) of advanced pancreatic ductal adenocarcinoma (Principe et al., 2019). When tested against the highly immunogenic mouse MC38 colon adenocarcinoma model, combinational inhibition of TGF-β signaling and PD-L1 blockade was also found to be significantly more effective than corresponding single agent treatments in improving survival, which was associated with an influx of CD8+ T-cells in the tumor microenvironment. (Sow et al., 2019). In addition, anti-PD-L1 therapy was demonstrated to sensitize RT2-PNET murine pancreatic neuroendocrine tumors to antiangiogenic therapy, and conversely to improve anti-PD-L1 therapy specifically though the generation of high endothelial venules in the tumor, which in turn, promoted cytotoxic T-cell infiltration, activity, and tumor destruction (Allen et al., 2017). Of added interest, inhibition of the ROS-producing enzyme NOX4, which is upregulated in activated CAFs, has most recently been shown in murine colorectal (as well as lung and breast tumor models), to normalize CAF myofibroblastic cells to a quiescent state. This effect, in turn, was found to re-sensitize the CAF-rich tumors to PD-1 check point inhibition by overcoming activated CAF-mediated CD8+ T-cell exclusion (Ford et al., 2020).
As implied by the findings described above, therapeutic strategies that incorporate targeting of the tumor stroma and novel immunotherapies in conjunction with standardized chemotherapy or targeted agents against cholangiocarcinoma cells offer the possibility of increased survival outcomes and possibly even cures, particularly when considered as a second line treatment for distinct subsets of iCCA patients with recurrent disease. Mou et al. (2018) ostensibly was the first to describe in a case report the complete remission of a postoperatively recurred metastatic PD-L1-positive iCCA with a high tumor mutational burden following combination treatment with the anti-PD-1 antibody pembrolizumab together with chemotherapy with oxiplatin, and tegafur. A significant response to anti-PD-1 based immunotherapy with nivolumab administered in combination with the multi-kinase tyrosine kinase inhibitor lenvatinib was also demonstrated against recurrent iCCA with bone metastasis (Chen et al., 2019). The patient’s initial primary iCCA, which had a high tumor mutation burden and actionable mutations in KIT, NRAS, TP53, MET, and PDGFR, recurred at 5 months after surgery and adjuvant chemotherapy. Following combinational treatment with nivolumab plus lenvatinib, the recurred liver tumor completely resolved and the bone metastases were stable after 9 months of therapy.
Bintrafusp alpha is a bifunctional fusion protein composed of a monoclonal antibody against PD-L1 fused to a TGF-β “trap” (Lind et al., 2020). In various preclinical mouse carcinoma models, bintrafusp alpha, previously designated M7824), demonstrated superior anti-tumor activity over that produced by treatments with anti-PD-L1 antibody alone or TGF-β trap alone by a mechanism involving simultaneous blockade of immunosuppressive PD-L1, as well as of TGF-β in the tumor microenvironment (Lan et al., 2018). In this study, treatment with bintrafusp alpha was also shown to reduce α-SMA expression and stromal collagen fiber density in syngeneic EMT-6 mouse breast tumors. In addition, combinations of bintrafusp alpha with chemotherapy (oxaliplatin/5-fluorouracil) or radiotherapy was demonstrated to be effective at improving antitumor efficacy and enhancing CD8+ T-cell activation in various preclinical mouse carcinoma models (Lan et al., 2018; Lind et al. 2020).
An open-label Phase I clinical trial of bintrafusp (MSB0011359C) has been completed, showing early signs of efficacy in patients with heavily pretreated advanced solid cancers without prior immune checkpoint inhibitor treatment (Strauss et al, 2018). More recently, Yoo et al. (2020) described results from an expansion cohort of a Phase I open-label trial of bintrafusp in 30 Asian patients with metastatic or locally advanced biliary tract cancers. Included in this trial were 10 patients with iCCA. The objective response rate for the iCCA patients treated with bintrafusp was reported to be 30%, with one patient showing a complete response. While these findings are encouraging, they are limited by the fact that this study lacked of a comparator group, had a small patient enrollment, and did not include non-Asian patients as part of the cohort. Multiple clinical trials are currently ongoing involving the use of bistrafusp alpha as a monotherapy or in combination with other immunotherapeutics, chemotherapy, or radiation therapy in patients with a variety of solid cancer types, including breast, prostate, biliary, and pancreas (Lind et al., 2020). The results of these trials should hopefully provide new insights concerning the effectiveness of targeting the tumor microenvironment as a strategy for enhancing immunotherapies and other treatment modalities against desmoplastic cancers, such as iCCA.
A variety of combination regimens with PD-1/PD-L1 immune checkpoint inhibitors (ICI) are also now being tested in ongoing key clinical trials against various advanced gastrointestinal malignancies, including biliary cancers (Wang et al., 2019). In this context, there is increasing preclinical and clinical trial evidence to support combined antiangiogenic therapy and immunotherapy with ICIs as a promising strategy for advanced solid cancer treatment (Fukumura et al., 2018; Song et al. 2020). VEGF, which is abundantly expressed in iCCA, impairs dendritic cell maturation, resulting in reduced T-cell priming, as well as stimulates expansion of Tregs, M2-TAMs and MDSCs. These actions, in turn, lead to an immunosuppressive tumor microenvironment and weakened anti-tumor immune response, together with dysregulating tumor neovascularization associated with impaired T cell infiltration and cell death (Fukamura et al., 2018; Kudo, 2020). Anti-VEGF agents have been shown to reprogram the tumor milieu from an immunosuppressive to an immune permissive microenvironment by normalizing the tumor vasculature, decreasing the activity of MDSCs, Tregs, and TAMs, and by suppressing dendritic cell maturation (Fukamuro et al., 2018; Kudo et al., 2020; Song et al., 2020). Immune checkpoint blockade has also been shown to facilitate anti-angiogenesis by downregulating VEGF and alleviating hypoxia (Song et al., 2020). These findings, in turn, have served as the basis for multiple clinical trials, many of which have demonstrated improved anti-cancer efficacy and prolonged survival when anti-angiogenic agents were combined with ICIs in the treatment of variety of advanced carcinoma types (Fukamura et al., 2018; Song et al., 2020).
The therapeutic benefit of combining anti-angiogenic therapy with ICIs is exemplified by the results of the phase III IMbrave 150 study-NCT03434379 (Cheng et al., 2019; Kudo, 2020; Nakano et al., 2020). This study evaluated combinational therapy with the anti-PD-L1 antibody atezolizumab + the anti-VEGF antibody bevacizumab for unresectable HCC without prior systemic therapy versus sorafenib monotherapy (the standard of care for advanced HCC). The results revealed that atezolizumab + bevacizumab-treated group had a significantly improved overall response rate and progression free survival compared to the sorafenib-treated group. Moreover, six percent of the HCC patients treated with the atezolizumab + bevacizumab combination were found to be in complete remission. The patients in the combination group also reported a considerably better quality of life than those in the sorafenib group.
In July 2019, the FDA granted a Breakthrough Therapy designation for lenvatinib, a multikinase inhibitor targeting VEGFR 1–3, FGFR 1–4, PDGFR-α, RET, and KIT) in combination with the PD-1inhibitor pembrolizumab for the first-line treatment of patients with unresectable HCC, based on the results from the phase 1b KEYNOTE-524/Study 116 trial-NCT03006926 (Nakano et al., 2020; Finn et al., 2020). An ongoing double-blind randomized controlled phase III study of lenvatinib plus pembrolizumab as a first-line treatment for HCC versus lenvatinib plus placebo (NCT03713593) to further assess the efficacy and safety of this combination in patients with HCC is now ongoing (Finn et al., 2020), the results of which are also likely to have potential implications for cHCC-CCA and iCCA, notably those formed in association with viral hepatitis and cirrhosis.
Other suggested considerations for targeting of tumor stroma and immune checkpoints in iCCA include the use of BH3 mimetics such as navitoclax to deplete activated CAFs, together with PD-L1 inhibition to reconstruct T-cell response and anti-tumor immunity (Mertens et al., 2018). A number of phase I/II clinical trials (e.g., NCT02341625, NCT03644550, NCT03371381) assessing targeted therapies for Msln expressing cancers based on antibody-based drugs (8BMS-98614; LMB-100) or vaccines (live attenuated Lysteria monocytogenes) in combination with immune checkpoint inhibitors (nivolumab; pembrolizumab) are currently recruiting or ongoing. In addition, a limited number of clinical trials (e.g., NCT03615313; NCT03030001) for targeted therapy of Msln positive advanced solid tumors with PD-1 antibody expressing mesothelin specific chimeric antigen receptor T (CAR-T) cells have also been reported to be in development (Lv & Li, 2019). In support of this strategy, Lal et al (2019) have recently demonstrated that CAR-T cells targeting Msln and secreting PD-L1 antibodies enhanced antitumor efficacy when compared with Msln-targeted CAR-T cells in xenograft mouse models of mesothelioma, pancreatic, and ovarian cancers. The results of these trials are awaited with anticipation, since they may have important implications for the treatment Msln-expressing iCCA.
Last, a better understanding of cellular heterogeneity and functions of the desmoplastic reaction in iCCA is needed inorder to successfully exploit its potential as a therapeutic target while avoiding any possibility of accelerating malignant aggressiveness. Multiple CAF subtypes demonstrating inter- and intra-tumoral heterogeneity have been demonstrated in human desmoplastic pancreatic desmoplastic adenocarcinoma, which may account for inconsistencies in preclinical results and the failure of some stromal targeted agents (Neuzillett et al., 2019). Two separate classes of CAFs, one expressing myofibroblastic features (α-SMA) and the other expressing inflammatory fibroblast features (i.e., IL-6 secretion) have also been shown to coexist in the desmoplastic stroma of pancreatic cancer and to show distinct transcriptional profiles (Öhlund et al., 2019). Furthermore, as previously alluded to, the reactive microenvironment in primary iCCA is distinct from that of metastatic iCCA. In this context, careful attention needs to be given to advancing the development of in vivo models closely reproducing the morpho-molecular pathology and TRS of early, advanced, and metastatic human iCCA in an effort to precisely identify and test primary and adjuvant combinational therapeutic strategies against advanced disease that can predictively translate into more novel systemic targeted agent and immunotherapeutic treatments for iCCA patients, as well as for those with other more common desmoplastic cancers.
Acknowledgments
Grant Support: NIH grant R01DK101528 to M.S., and support from the Clinical and Translational Core of the Yale Liver Center- grant DK034989, Silvio Conte Digestive Disease Centers.
Footnotes
Conflict of Interest Statement: The authors (A.E.S., M.S, and M.C.) have no financial or personal disclosures relevant to this manuscript.
7. REFERENCES
- Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, et al. (2020a). Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarlDHy): a multicentre, randomized, double-blind, placebo-controlled, phase 3 study. Lancet Oncology, 21: 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. (2020b). Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncology, 21: 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affo S, Yu L-Y, & Schwabe RF (2017). The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annual Review of Pathology: Mechanisms of Disease, 12: 153–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrendt SA, Rashid A, Chow JT, Eisenberger CF, Pitt HA, & Sidransky D (2000). p53 overexpression and K-ras gene mutations in primary sclerosing cholangitis-associated biliary tract cancer. Journal of Hepato-Biliary-Pancreatic Surgery, 7: 426–431. [DOI] [PubMed] [Google Scholar]
- Aishima S, Taguchi K, Sugimachi K, Asayama Y, Nishi H, Shimada M, et al. (2002). The role of thymidine phosphorylase and thrombospondin-1 in angiogenesis and progression of intrahepatic cholangiocarcinoma. International Journal of Surgical Pathology, 10: 47–56. [DOI] [PubMed] [Google Scholar]
- Aishimi S, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, Maehara Y, et al. (2008). Lymphatic spread is related to VEGF-C expression and D2–40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Modern Pathology, 21: 256–264. [DOI] [PubMed] [Google Scholar]
- Akita M, Fujikura K, Ajiki T, Fukumoto T, Otani K, Azuma T, et al. (2017). Dichotomy in intrahepatic cholangiocarcinomas based on histological similarities to hilar cholangiocarcinomas. Modern Pathology, 30: 986–997. [DOI] [PubMed] [Google Scholar]
- Akita M, Sofue K, Fujikura K, Otani K, Itoh T, Ajiki T, et al. (2018). Histological and molecular characterization of intrahepatic bile duct cancers suggest an expanded definition of perihilar cholangiocarcinoma. HPB, 21: 226–234 [DOI] [PubMed] [Google Scholar]
- Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, et al. (2017). Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Science Translational Medicine, 9(385): eaak9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aosasa S, Maejima T, Kimura A, Nishiyama K, Edo H, Shinmoto H, et al. (2015). Intrahepatic cholangiocarcinoma with lymphoepithelioma-like carcinoma components not associated with Epstein-Barr virus: report of a case. International Surgery, 100: 689–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi S, Kotera Y, Katagiri S, Nakano M, Nakanuma Y, Saito A, et al. (2014). Long-term survival of patients with cholangiolocellular carcinoma after curative surgery. Annals of Surgical Oncology, 21: 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariizumi S & Yamamoto M (2015). Intrahepatic cholangiocarcinoma and cholangiolocellular carcinoma in cirrhosis and chronic viral hepatitis. Surgery Today, 45: 682–687. [DOI] [PubMed] [Google Scholar]
- Aron JH & Bowlus CL (2009). The immunobiology of primary sclerosing cholangitis. Seminars in Immunopathology, 31, 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae JY, Park YN, Nakanuma Y, Lee WJ, Kim Y, Park C, et al. (2002). Intestinal type cholangiocarcinoma of intrahepatic large bile duct associated with hepatolithiasis--a new histologic subtype for further investigation. Hepatogastroenterology, 49: 628–630. [PubMed] [Google Scholar]
- Bagante F, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, et al. (2018). Long-term outcomes of patients with intraductal growth sub-type of intrahepatic cholangiocarcinoma. HPB, 20: 1189–1197. [DOI] [PubMed] [Google Scholar]
- Balitzer D, Joseph NM, Ferrell L, Shafizadeh N, Jain D, Zhang X, et al. , (2019). Immunohistochemical and molecular features of cholangiolocellular carcinoma are similar to well-differentiated intrahepatic cholangiocarcinoma. Modern Pathology, 32: 1486–1494. [DOI] [PubMed] [Google Scholar]
- Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. (2016). Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nature Reviews. Gastroenterology & Hepatology, 13: 261–280. [DOI] [PubMed] [Google Scholar]
- Banales JM, Marin JJ, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. (2020). Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nature Reviews Gastroenterology & Hepatology, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E & Massagué (2019). Transforming growth factor-β signaling in immunity and cancer. Immunity, 50: 924–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal EW, Tumin D, Moris D, Zhang X, Chakedis J, Dilhoff M, et al. (2018). Cohort contributions to trends in the incidence and mortality of intrahepatic cholangiocarcinoma. HepatoBiliary Surgery and Nutrition, 7: 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamouche S, Curto M, Saotome I, Gladden AB, Liu C-H, Giovannini M, et al. (2010). Nƒ2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes & Development, 24: 1718–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, et al. (2019). Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. Journal of Hepatology, 71: 104–114. [DOI] [PubMed] [Google Scholar]
- Björkström NK, Ljunggren HG, & Michaëlsson J (2016). Emerging insights into natural killer cells in human peripheral tissues. Nature Reviews Immunology, 16: 310–320. [DOI] [PubMed] [Google Scholar]
- Blechacz B, Komuta M, Roskams T, & Gores GJ (2012). Clinical diagnosis and staging of cholangiocarcinoma. Nature Reviews Gastroenterology & Hepatology, 8: 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechacz B (2017). Cholangiocarcinoma: current knowledge and new developments. Gut and Liver, 11: 13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg KM, Schrumpf E, Bergquist A, Broomé U, Pares A, Remotti H, et al. (2000). Cholangiocarcinoma in primary sclerosing cholangitis: K-ras mutations and Tp53 dysfunction are implicated in the neoplastic development. Journal of Hepatology, 32: 374–380. [DOI] [PubMed] [Google Scholar]
- Bösmüller H, Pfefferle V, Bittar Z, Scheble V, Horger M, Sipos B, et al. (2018). Microvessel density and angiogenesisin primary hepatic malignancies: differential expression of CD31 and VEGFR-2 in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Pathology Research and Practice, 214: 1136–1141. [DOI] [PubMed] [Google Scholar]
- Boulter L, Guest RV, Kendall TJ, Wilson DH, Wojtacha D, Robson AJ, et al. (2015). WNT signaling drives cholangiocarcinoma growth and can be pharmacologically inhibited. The Journal of Clinical Investigation, 125: 1269–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braconi C, Huang N, & Patel T (2010). MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor suppressor gene expression by interleukin-6 in human malignant cholangiocytes. Hepatology, 51: 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragazzi MC, Ridola L, Safarikia S, Di Matteo S, Costantini D, Nevi L, et al. (2018) New insights into cholangiocarcinoma: multiple stems and related cell lineages of origin. Annals of Gastroenterology, 31: 42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brivio S, Cadamuro M, Strazzabosco M, & Fabris L (2017). Tumor reactive stroma in cholangiocarcinoma: the fuel behind cancer aggressiveness. World Journal of Hepatology, 9: 455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt E, Aishima S, Clavien P-A., Fowler K, Goodman Z, Gores G, et al. (2018). cHCC-CCA: consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentiation. Hepatology, 68: 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholz AP & Brown RS Jr (2020). Cholangiocarcinoma: diagnosis and management. Clinics in Liver Disease, 24: 421–436. [DOI] [PubMed] [Google Scholar]
- Buettner S, van Vugt JLA, IJzermans JNM, & Koerkamp B,G (2017). Intrahepatic cholangiocarcinoma: current perspectives. OncoTargets and Therapy, 10: 1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadamuro M, Nardo G, Indraccolo S, Dall’Olmo L, Sambado L, Moserle L, et al. (2013). Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology, 58: 1042–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadamuro M, Stecca T, Brivio S, Mariotti V, Fiorotto R, Spirli C, et al. (2018). The deleterious interplay between tumor epithelia and stroma in cholangiocarcinoma. Biochimica et Biophysica Acta-Molecular Basis of Disease, 1864 (4PtB): 1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadamuro M, Brivio S, Mertens J, Vismara M, Moncsek A, Milani C, et al. (2019). Platelet-derived growth factor-D enables liver myofibroblasts to promote tumor lymphangiogenesis in cholangiocarcinoma. Journal of Hepatology, 70: 700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadamuro M, Girardi N, Gores GJ, Strazzabosco M, & Fabris L (2020). The emerging role of macrophages in chronic cholangiopathies featuring biliary fibrosis: an attractive therapeutic target for orphan diseases. Frontiers in Medicine, 7: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, et al. (2018). TGF-β and the tissue microenvironment: relevance in fibrosis and cancer. International Journal of Cancer, 19: 1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DJW, Dumur CI, Lamour NF, DeWitt JL, & Sirica AE (2012). Novel organotypic culture model of cholangiocarcinoma progression. Hepatology Research, 42: 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannito S, Milani C, Cappon A, Parola M, Strazzabosco, & Cadamuro M (2018). Fibroinflammatory liver injuries as preneoplastic conditions in cholangiopathies. International Journal of Molecular Sciences, 19: 3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale V, Wang Y, Carpino G, Cui C-B, Gatto M, Rossi M, et al. (2011). Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology, 54: 2159–2172. [DOI] [PubMed] [Google Scholar]
- Cardinale V, Wang Y, Carpino G, Reid LM, Gaudio E, & Alvaro D (2012a). Mucin-producing cholangiocarcinoma might derive from biliary tree stem/progenitor cells located in peribiliary glands. Hepatology, 55: 2041–2042. [DOI] [PubMed] [Google Scholar]
- Cardinale V, Carpino G, Reid L, Gaudio E, & Alvaro D (2012b). Multiple cells of origin in cholangiocarcinoma underlie biological, epidemiological, and clinical heterogeneity. World Journal of Gastrointestinal Oncology, 4: 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale V, Bragazzi MC, Carpino G, Di Matteo S, Overi D, Nevi L, et al. (2018) Intrahepatic cholangiocarcinoma: review and update. Hepatoma Research, 4: 20. [Google Scholar]
- Cardinale V (2019). Classification and misclassification in cholangiocarcinoma. Liver International, 39: 260–262. [DOI] [PubMed] [Google Scholar]
- Carpino G, Cardinale V, Reid L, Alvaro D, & Gaudio E (2012). Cells of origin and cancer stem cells in cholangiocarcinoma. Translational Gastrointestinal Cancer, 1: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpino G, Cardinale V, Folseraas T, Overi D, Grzyb K, Costantini D, et al. (2019a). Neoplastic transformation of the peribiliary stem cell niche in cholangiocarcinoma arisen in primary sclerosing cholangitis. Hepatology, 69: 622–638. [DOI] [PubMed] [Google Scholar]
- Carpino G, Overi D, Melandro F, Grimaldi A Cardinale V, Matteo S et al. (2019b). Matrisome analysis of intrahepatic cholangiocarcinoma unveils a peculiar cancer-associated extracellular matrix structure. Clinical Proteomics, 16: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiyadet S, Smout M, Johnson M, Whitchurch C, Turnbull L, Kaewkes S, et al. (2015a). Excretory/secretory products of the carcinogenic liver fluke are endocytosed by human cholangiocytes and drive cell proliferation and IL6 production. International Journal for Parasitology, 45: 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiyadet S, Sotillo J, Smout M, Cantacessi C, Jones MK, Johnson MS, et al. (2015b). Carcinogenic liver fluke secretes extracellular vesicles that promote cholangiocytes to adopt a tumorigenic phenotype. The Journal of Infectious Diseases, 212: 1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K-M, Tsai C-Y, Yeh C-N, Yeh T-S, Lee W-C, Jan Y-Y, et al. (2018). Characterization of intrahepatic cholangiocarcinoma after curative resection: outcome, prognostic factor, and recurrence. BMC Gastroenterology, 18:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-on W, Nairismägi M, Ong CK, Lim WK, Dima S, Pairojkul C, et al. (2013). Exome sequencing identifies distinct mutational patterns in liver fluke-related and non-infection-related bile duct cancers. Nature Genetics, 45: 1474–1478. [DOI] [PubMed] [Google Scholar]
- Chariyalertsak S, Sirikulchayanonta V, Mayer D, Kopp-Schneider A, Fürstenberger G, Marks F, & Müller-Decker K (2001). Aberrant cyclooxygenase isozyme expression in human intrahepatic cholangiocarcinoma. Gut, 48: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Li G, Hu Z, Zhu P, Zhang B, & Ding Z (2019). Significant response to anti-PD-1 based immunotherapy plus lenvatinib for recurrent intrahepatic cholangiocarcinoma with bone metastasis-a case report and literature review, Medicine, 98: 45(e17832). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A-L, Qin S, Ikeda M, Galle P, Ducreux M, Zhu A, et al. , (2019). LBA3-IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma. Annals of Oncology, 30 (Suppl 9): ix186–ix 187. [Google Scholar]
- Chuaysri C, Thuwajit P, Paupairoj A, Chau-In S, Suthiphongchai T & Thuwajit C (2009). Alpha-smooth muscle actin positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncology Reports, 21: 957–969. [DOI] [PubMed] [Google Scholar]
- Chun YS & Javle M (2017). Systemic and adjuvant therapies for intrahepatic cholangiocarcinoma. Cancer Control, 24: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YE, Kim M, Park YN, Choi J-Y, Pyo JY, Kim YC, et al. (2009). Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. RadioGraphics, 29: 683–700. [DOI] [PubMed] [Google Scholar]
- Clapéron A, Mergey M, Aoudjehane L, Ho-Bouldoires THN, Wendum D, Prignon A, et al. (2013). Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology, 58: 2001–2011. [DOI] [PubMed] [Google Scholar]
- Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, & Khan SA (2020). Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. Journal of Hepatology, 72: 95–103. [DOI] [PubMed] [Google Scholar]
- Coelho R, Ricardo S, Amaral AL, Huang Y-L, Nunes M, Neves JP, et al. (2020). Regulation of invasion and peritoneal dissemination of ovarian cancer by mesothelin manipulation. Oncogenesis, 9: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corliss BA, Azimi MS, Munson JM, Peirce SM, & Murfee WL (2016). Macrophages: an inflammatory link between angiogenesis and lymphangiogenesis. Microcirculation, 23: 95–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia da Costa JM, Vale N, Gouveia MJ, Botelho MC, Sripa B, Santos LL, et al. (2014). Schistosome and liver fluke derived catechol-estrogens and helminth associated cancers. Frontiers in Genetics, 5: 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulouarn C, Cavard C, Rubbia-Brandt L, Audebourg A, Dumont F, Jacques S, et al. (2012). Combined hepatocellular-cholangiocarcinomas exhibit progenitor features and activation of Wnt and TGFβ signaling pathways. Carcinogenesis, 33: 1791–1796. [DOI] [PubMed] [Google Scholar]
- Dabney RS, Khalife M, Shahid K, & Phan AT (2019). Molecular pathways and targeted therapy in cholangiocarcinoma. Clinical Advances in Hematology & Oncology, 17: 630–637. [PubMed] [Google Scholar]
- Dhanasekaran R, Hemming AW, Zendejas I, George T, Nelson DR, Soldevila-Pico C, et al. (2013). Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncology Reports, 29: 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaola F, Shivakumar P, Pfister J, Walters S, Sabla G, & Bezerra JA (2013). Identification of intramural epithelial networks linked to peribiliary glands that express progenitor cell markers and proliferate after injury. Hepatology, 58: 1486–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duluc D, Delneste Y, Tan F, Moles M-P., Grimaud, L., Lenoir, J., et al. (2007). Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood, 110: 4319–4330. [DOI] [PubMed] [Google Scholar]
- Dumur CI, Campbell DJW, DeWitt JL, Oyesanya RA, & Sirica AE (2010). Differential gene expression profiling of cultured neu-transformed versus spontaneously-transformed rat cholangiocytes and of corresponding cholangiocarcinomas. Experimental and Molecular Pathology, 89: 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline J, Benabdelghani M, Bertaut A, Watelet J, Hammel P, Joly J-P, et al. (2019). Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. Journal of Clinical Oncology, 37: 658–667. [DOI] [PubMed] [Google Scholar]
- Elmore LW, & Sirica AE, (1991). Phenotypic characterization of metaplastic intestinal glands and ductular hepatocytes in cholangiofibrotic lesions rapidly induced in the caudate liver lobe of rats treated with furan. Cancer Research, 51: 5752–5759. [PubMed] [Google Scholar]
- Elmore LW & Sirica AE (1993). “Intestinal-type” of adenocarcinoma preferentially induced in right/caudate liver lobes of rats treated with furan. Cancer Research, 53: 254–259. [PubMed] [Google Scholar]
- Fabris L, Perugorria MJ, Mertens J, Björkström NK, Cramer T, Lleo A, et al. (2019a) The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver International, 39: 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabris L, Fiorotto R, Spirli C, Cadamuro M, Mariotti V, Perugorria MJ, et al. (2019b). Pathobiology of inherited biliary diseases: a roadmap to understand acquired liver diseases. Nature Reviews Gastroenterology & Hepatology, 16: 497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan B, Malato Y, Calvisi DF, Nagqi S, Razumilava N, Ribback S, et al. (2012). Cholangiocarcinoma can originate from hepatocytes in mice. Journal of Clinical Investigation, 122: 2911–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC (2004). Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nature Reviews Microbiology, 2: 820–832. [DOI] [PubMed] [Google Scholar]
- Fausther M, Lavoie EG, & Dranoff JA (2017). Liver myofibroblasts of murine origins express mesothelin: identification of novel rat mesothelin splice variants. Plos One, 12: e0184499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova OS, Kovshirina YV, Kovshirina AE, Fedotova MM, Deev IA, Petrovskiy FI, et al. (2017). Opisthorchis felineus infection and cholangiocarcinoma in the Russian Federation: a review of medical statistics. Parasitology International, 66: 365–371. [DOI] [PubMed] [Google Scholar]
- Filippi L, Di Costanzo GG, Tortora R, Pelle G, Saltarelli A, Marino Marsilia G, et al. (2020). Prognostic value of neutrophil-to-lymphocyte ratio and its correlation with fluorine-18-fluorodeoxyglucose metabolic parameters in intrahepatic cholangiocarcinoma submitted to 90Y-radioembolization. Nuclear Medicine Communications, 41: 78–86. [DOI] [PubMed] [Google Scholar]
- Fingas CD, Bronk SF, Werneburg NW, Mott JL, Guicciardi SC, Cazanave SC, et al. J.C. (2011). Myofibroblast-derived PDGF-BB promotes hedgehog survival signaling in cholangiocarcinoma cells. Hepatology, 54: 2076–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. (2020). Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. Journal of Clinical Oncology, 38: 2960–2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiori ME, Di Franco S, Villanova L, Bianci P, Stassi G, & De Maria R (2019). Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Molecular Cancer, 18: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford K, Hanley CJ, Mellone M, Szyndralewiez C, Heitz F, Wiesel P, et al. (2020). NOX4 inhibition potentiates immunotherapy by overcoming cancer-associated fibroblast-mediated CD8 T-cell exclusion from tumors. Cancer Research, 80: 1846–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukamura D, Kloepper J, Amoozgar Z, Duda DG, & Jain RK (2018). Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nature Reviews Clinical Oncology, 15: 325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Asaoka T, Eguchi H, Yokota Y, Kubo M, Kinoshita M, et al. (2020). Endogenous CXCL9 affects prognosis by regulating tumor-infiltrating natural killer cells in intrahepatic cholangiocarcinoma. Cancer Science, 111: 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung BM, Lindor KD, & Tabibian JH (2019). Cancer risk in primary sclerosing cholangitis: epidemiology, prevention, and surveillance strategies. World Journal of Gastroenterology, 25: 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascard P, & Tlsty TD (2016). Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes & Development, 30: 1002–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentilini A, Pastore M, Marra F, & Raggi C (2018). The role of stroma in cholangiocarcinoma: the intriguing interplay between fibroblastic component, immune cell subsets, and tumor epithelium. International Journal of Molecular Sciences, 19: 2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gera S, Ettel M, Acosta-Gonzales G, & Xu R (2017). Clinical features, histology, and histogenesis of combined hepatocellular-cholangiocarcinoma. World Journal of Hepatology, 9: 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleiberman AS, Encinas JM, Mignone JL, Michurina T, Rosenfeld MG, & Enikolopov G (2005). Expression of nestin-GFP transgene marks oval cells in the adult liver. Developmental Dynamics, 234: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeppert B, Frauenschuh L, Zucknick M, Stenzinger A, Andrulis M, Klauschen F, et al. (2013). Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. British Journal of Cancer, 109: 2665–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeppert B, Toth R, Singer S, Albrecht T, Lipka DB, Lutsik P, et al. (2019). Integrative analysis defines distinct prognostic subgroups of intrahepatic cholangiocarcinoma. Hepatology, 69: 2091–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Gonzáles L & Alonzo J (2018). Periostin: a matricellular protein with multiple functions in cancer development and progression. Frontiers in Oncology, 8: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulet CR, Bernard G, Tremblay S, Chabaud S, Bolduc S, & Pouliot F (2018). Exosomes induce fibroblast differentiation into cancer-associated fibroblasts through TGFβ signaling. Molecular Cancer Research, 16: 1196–1204. [DOI] [PubMed] [Google Scholar]
- Goyal L, Shi L, Liu LY, Fece de la Cruz F, Lennerz JK, Raghavan S, et al. (2019). TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 fusion-positive intrahepatic cholangiocarcinoma. Cancer Discovery, 9: 1064–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest RV, Boulter L, Kendall TJ, Minnis-Lyons SE, Walker R, Wigmore SJ, et al. (2013). Cell lineage tracing reveals a biliary origin of intrahepatic cholangiocarcinoma. Cancer Research, 74: 1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Nicoli P, et al. (2009). Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World Journal of Surgery, 33: 1247–1254. [DOI] [PubMed] [Google Scholar]
- Guicciardi ME, Trussoni CE, LaRusso NF, & Gores GJ (2020). The spectrum of reactive cholangiocytes in primary sclerosing cholangitis. Hepatology, 71: 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A & Dixon E (2017). Epidemiology and risk factors: intrahepatic cholangiocarcinoma. HepatoBiliary Surgery and Nutrition, 6: 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D & Weinberg RA (2011). Hallmarks of cancer: the next generation. Cell, 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Hasita H, Komohara Y, Okabe H, Masuda T, Ohnishi K, Lei XF, et al. (2010). Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Science, 101: 1913–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R, Yamamoto M, Hatori T, Shimizu K, Imai K, & Takasaki K (2006). Intrahepatic cholangiocarcinoma with lymph node metastasis successfully treated by immunotherapy with CD3-activated T cells and dendritic cells after surgery: report of a case. Surgery Today, 36: 559–562. [DOI] [PubMed] [Google Scholar]
- Hill MA, Alexander WB, Guo B, Kato Y, Patra K, O’Dell MR, et al. (2018) Kras and Tp53 mutations cause cholangiocyte- and hepatocyte-derived cholangiocarcinoma. Cancer Research, 78: 4445–4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hov JR, & Karlsen TH (2017). The Microbiome in Primary Sclerosing Cholangitis: Current Evidence and Potential Concepts. Seminars in Liver Disease, 37: 314–331. [DOI] [PubMed] [Google Scholar]
- Hu L-S, Zhang X-F, Weiss M, Popescu I, Marques HP, Aldrighetti L, et al. (2019). Recurrence patterns and timing courses following curative-intent resection for intrahepatic cholangiocarcinoma. Annuals of Surgical Oncology, 26: 2549–2557. [DOI] [PubMed] [Google Scholar]
- Huang C-K, Aihara A, Iwagami Y, Yu T, Carlson R, Koga H, et al. (2016). Expression of transforming growth factor β1 promotes cholangiocarcinoma development and progression. Cancer Letters, 380: 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucl T (2019). Precursors to cholangiocarcinoma. Gastroenterology Research and Practice, Article ID 1389289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenoue T, Terakado Y, Nakagawa H, Hikiba Y, Fujii T, Matsubara D, et al. (2016). A novel mouse model of intrahepatic cholangiocarcinoma induced by liver-specific Kras activation and Pten deletion. Scientific Reports, 6: 23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Sasaki M, Ohira S, Ohta T, Oda K, Nimura Y, et al. (2004). Aberrant expression of CDX2 is closely related to the intestinal metaplasia and MUC2 expression in intraductal papillary neoplasms of the liver in hepatolithiasis. Laboratory Investigation, 84: 629–638. [DOI] [PubMed] [Google Scholar]
- Itou RA, Uyama N, Hirota S, Kawada N, Wu S Miyashita, S. et al. (2019). Immunohistochemical characterization of cancer-associated fibroblasts at the primary sites and in the metastatic lymph nodes of human intrahepatic cholangiocarcinoma. Human Pathology, 83: 77–89. [DOI] [PubMed] [Google Scholar]
- Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, & Mantovani A (2020). Neutrophil diversity and plasticity in tumour progression and therapy. Nature Reviews. Cancer, in press. [DOI] [PubMed] [Google Scholar]
- Jaiswal M, LaRusso NF, Burgart LJ, & Gores GJ (2000). Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Research, 60: 184–190. [PubMed] [Google Scholar]
- Jaiswal M, LaRusso NF, Shapiro RA, Billiar TR, & Gores GJ (2001). Nitric oxide-mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology, 120: 190–199. [DOI] [PubMed] [Google Scholar]
- Jang MH, Lee YJ, & Kim H (2014). Intrahepatic cholangiocarcinoma arising in Caroli’s disease. Clinical and Molecular Hepatology, 20: 402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse M, Lamberts LE, Franke L, Raychaudhuri S, Ellinghaus E, Muri Boberg K, et al. (2011). Three ulcerative colitis susceptibility loci are associated with primary sclerosing cholangitis and indicate a role for IL2, REL, and CARD9. Hepatology, 53: 1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesper D, Heyn SG, Schellhass B, Pfeifer L, Goertz R, Ruediger S, et al. (2018). Effects of liver cirrhosis and patient condition on clinical outcomes in intrahepatic cholangiocarcinoma: a retrospective analysis of 156 cases in a single center. European Journal of Gastroenterology & Hepatology, 30: 552–556. [DOI] [PubMed] [Google Scholar]
- Joseph NM, Tsokos CG, Umetsu SE, Shain AH, Kelley RK, Onodera C, et al. (2019). Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. Journal of Pathology, 248: 164–178. [DOI] [PubMed] [Google Scholar]
- Jung IH, Kim DH, Yoo DK, Baek SY, Jeong SH, Jung DE, et al. (2018). In Vivo Study of Natural Killer (NK) Cell Cytotoxicity Against Cholangiocarcinoma in a Nude Mouse Model. In Vivo, 32: 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junking M, Grainok J, Thepmalee C, Wongkham S, & Yenchitsomanus PT (2017). Enhanced cytotoxic activity of effector T-cells against cholangiocarcinoma by dendritic cells pulsed with pooled mRNA. Tumour Biology, 39: 1010428317733367. [DOI] [PubMed] [Google Scholar]
- Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, et al. (2017). Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discovery, 7: 1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalli M & Stylianopoulos T (2018). Defining the role of solid stress and matrix stiffness in cancer cell proliferation and metastasis. Frontiers in Oncology, 8: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsen TH, & Boberg KM (2013). Update on primary sclerosing cholangitis. Journal of Hepatology, 59: 571–582. [DOI] [PubMed] [Google Scholar]
- Karlsen TH, Folseraas T, Thorburn D, & Vesterhus M (2017). Primary sclerosing cholangitis - a comprehensive review. Journal of Hepatology, 67: 1298–1323. [DOI] [PubMed] [Google Scholar]
- Karlsen TH, Schrumpf E, & Boberg KM (2010). Update on primary sclerosing cholangitis. Digestive and Liver Disease, 42: 390–400. [DOI] [PubMed] [Google Scholar]
- Kasper HU, Drebber U, Stippel DL, Dienes H-P, & Gillessen A (2009). Liver tumor infiltrating lymphocytes: comparison of hepatocellular and cholangiolar carcinoma. World Journal of Gastroenterology, 15: 5053–5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzak A & Adamek A (2019). Mucins: the old, the new and the promising factors in hepatobiliary carcinogenesis. International Journal of Molecular Sciences, 20: 1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsumata LW, Miyajima A, & Itoh T (2017) Portal fibroblasts marked by the surface antigen Thy1 contribute to fibrosis in mouse models of cholestatic liver injury. Hepatology Communications, 1: 198–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katt J, Schwinge D, Schoknecht T, Quaas A, Sobottka I, Burandt E, et al. (2013). Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology, 58: 1084–1093. [DOI] [PubMed] [Google Scholar]
- Kendall T, Verheij J, Gaudio E, Evert M, Guido M, Goeppert B, et al. (2019). Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver International, 39: 7–18. [DOI] [PubMed] [Google Scholar]
- Khan SA, Tavolari S & Brandi G (2019). Cholangiocarcinoma: epidemiology and risk factors. Liver International, 39: 19–31. [DOI] [PubMed] [Google Scholar]
- Kirstein MM & Vogel A (2016). Epidemiology and risk factors of cholangiocarcinoma. Visceral Medicine, 32: 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano Y, Yamashita Y-I, Nakao Y, Itoyama R, Yusa T, Umezaki N, et al. (2020). Clinical significance of PD-L1 expression in both cancer and stroma cells of cholangiocarcinoma patients. Annals of Surgical Oncology, 27: 599–607. [DOI] [PubMed] [Google Scholar]
- Ko S, Russell JO, Molina LM, & Monga SP (2020). Liver progenitors and adult cell plasticity in hepatic injury and repair: knowns and unknowns. Annual Review of Pathology: Mechanisms of Disease, 15: 23–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Sakabe T, Abe H, Tanii M, Takahashi H, Chiba A, et al. (2013). Dendritic cell-based immunotherapy targeting synthesized peptides for advanced biliary tract cancer. Journal of Gastrointestinal Surgery, 17: 1609–1617. [DOI] [PubMed] [Google Scholar]
- Komuta M, Spee B, Borght SV, De Vos R, Verslype C, Aerts R, et al. (2008). Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology, 47: 1544–1556. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Wang P, Liang S, Iwaisako K, Liu X, Xu J, et al. (2017). Mesothelin/mucin16 signaling in activated portal fibroblasts regulates cholestatic liver fibrosis. The Journal of Clinical Investigation, 127: 1254–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka S, Kurashina M, Tsubone M, Hachisuka K, & Yasui A (1984). Significance of intestinal metaplasia for the evolution of cancer in the biliary tract. Cancer, 54: 2277–2285. [DOI] [PubMed] [Google Scholar]
- Krook MA, Lenyo A, Wilberding M, Barker H, Dantuono M, Bailey KM, et al. (2020). Efficacy of FGFR inhibitors and combination therapies for acquired resistance in FGFR2-fusion cholangiocarcinoma. Molecular Cancer Therapeutics, 19: 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo M (2020). A new era in systemic therapy for hepatocellular carcinoma: atezolizumab plus bevacizumab combination therapy. Liver Cancer, 9: 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Smith T, Raeman R, Chopyk DM, Brink H, Liu, et al. (2018). Periostin promotes liver fibrogenesis by activating lysyl oxidase in hepatic stellate cells. Journal of Biological Chemistry, 293: 12781–12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano H, Naito Y, Mihara Y, Kondo R, Ogasawara S, Akiba J, et al. (2020). Distinctive clinicopathological features and KRAS and IDH1/2 mutation status of cholangiolocellular carcinoma. Hepatology Research, 50: 84–91. [DOI] [PubMed] [Google Scholar]
- Labib PL, Goodchild G, & Pereira SP (2019). Molecular pathogenesis of cholangiocarcinoma. BMC Cancer, 19: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal S, Wang G, Chen Z, Xiang X, Liang C, Huang XF, et al. (2019). Co-expression of PD-L1 antibodies enhances the anti-tumor efficacy of chimeric antigen receptor T cells. Journal of Cancer Research and Immuno-oncology, 5: 119. [Google Scholar]
- Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J, et al. (2018). Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Science Translational Medicine, 10: eaan5488. [DOI] [PubMed] [Google Scholar]
- Lamarca A, Barriuso J, McNamara MG, & Valle JW (2020). Molecular targeted therapies: ready for “prime time” in biliary tract cancer. Journal of Hepatology, 73: 170–185. [DOI] [PubMed] [Google Scholar]
- Lazaridis KN, & LaRusso NF (2016). Primary Sclerosing Cholangitis. The New England Journal of Medicine, 375: 2501–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH & Lee JM, (2017) Primary malignant tumors in non-cirrhotic liver. European Journal of Radiology. 95: 349–361. [DOI] [PubMed] [Google Scholar]
- Leyva-Illades D, McMillin M, Quinn M, & Demorrow S (2012). Cholangiocarcinoma pathogenesis: Role of the tumor microenvironment. Translational Gastrointestinal Cancer, 1: 71–80. [PMC free article] [PubMed] [Google Scholar]
- Li L, Wei W, Li Z, Chen H, Li Y, Jiang W, et al. (2018). The spleen promotes the secretion of CCL2 and supports an M1 dominant phenotype in hepatic macrophages during liver fibrosis. Cellular Physiology and Biochemistry, 51: 557–574. [DOI] [PubMed] [Google Scholar]
- Li X, Tao J, Cigliano A, Sini M, Calderaro J, Azoulay D, et al. (2015). Co-activation of PIK3CA and Yap promotes development of hepatocellular and cholangiocellular tumors in mouse and human liver. Oncotarget, 6: 10102–10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau J, Tsai J-H, Yuan R-H, Chang C-N, Lee H-J, & Jeng Y-M (2014). Morphological subclassification of intrahepatic cholangiocarcinoma: etiological, clinicopathological, and molecular features. Modern Pathology, 27: 1163–1173. [DOI] [PubMed] [Google Scholar]
- Liepelt A & Tacke F (2016). Stromal cell-derived factor-1 (SDF-1) as a target in liver diseases. American Journal of Physiology-Gastrointestinal Liver Physiology, 311: G203–G209. [DOI] [PubMed] [Google Scholar]
- Limaye PB, Alarcόn G, Walls AL, Nalesnik MA, Michalopoulos GK, Demetris AJ, et al. (2008). Expression of specific hepatocyte and cholangiocyte transcription factors in human liver disease and embryonic development. Laboratory Investigation, 88: 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Liu Y, Li S, Mao Y, Wang J, Shuang Z, et al. (2016). Elevated neutrophil-to-lymphocyte ratio is an independent poor prognostic factor in patients with intrahepatic cholangiocarcinoma. Oncotarget, 7: 50963–50971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-K, Fang Z, Jiang T-Y, Wan Z-H, Pan Y-F, Ma Y-H, et al. (2018). Combination of Kras activation and PTEN deletion contributes to murine hepatopancreatic ductal malignancy. Cancer Letters, 421: 161–169. [DOI] [PubMed] [Google Scholar]
- Lind H, Gameiro SR, Jochems C, Donahue RN, Strauss J Gulley JL, et al. (2020). Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-BRII agent: status of preclinical and clinical advances. Journal for ImmunoTherapy of Cancer, 8: e000433–e1426519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor KD, Kowdley KV, Harrison ME, & American College of Gastroenterology (2015). ACG Clinical Guideline: Primary Sclerosing Cholangitis. The American Journal of Gastroenterology, 110: 646–660. [DOI] [PubMed] [Google Scholar]
- Ling H, Roux E, Hempel D, Tao J, Smith M, Lonning S, et al. (2013). Transforming growth factor β neutralization ameliorates pre-existing hepatic fibrosis and reduces cholangiocarcinoma in thioacetamide-treated rats. Plos One, 8: e54499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Hov JR, Folseraas T, Ellinghaus E, Rushbrook SM, Doncheva NT, et al. (2013). Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nature Genetics, 45: 670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z-H, Lian B-F, Dong Q-Z, Sun H, Wei J-W, Sheng Y-Y, et al. (2018) Whole -exome mutational and transcriptional landscapes of combined hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma reveal molecular diversity. Biochimica et Biophysica Acta-Molecular Basis of Disease, 1864: 2360–2368. [DOI] [PubMed] [Google Scholar]
- Lv J & Li P (2019). Mesothelin as a biomarker for targeted therapy. Biomarker Research, 7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeuillard E, Conboy CB, Gores GJ, & Rizvi S (2019). Immunobiology of cholangiocarcinoma. JHEP Reports, 1: 297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeuillard E, Yang J, Buckarma E, Wang J, Liu Y, Conboy C, et al. (2020). Targeting tumor-associated macrophages and granulocytic-myeloid-derived suppressor cells augments PD-1 blockade in cholangiocarcinoma. The Journal of Clinical Investigation, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loilome W, Bungkanjana P, Techasen A, Namwat N, Yongvanit P, Puapairoj A, et al. , (2014). Activated macrophages promote Wnt/β-catenin signaling in cholangiocarcinoma cells. Tumour Biology, 35: 5357–5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery MA, Burris III HA, Janku F, Shroff RT, Cleary JM, Azad NS, et al. , Safety and activity of ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: a phase 1 study. The Lancet Gastroenterology & Hepatology, 4: 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy CM & Oskarsson T (2015). Tenascin C in metastasis: a view from the invasive front. Cell Adhesion & Migration, 9: 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig J, Wahlstrom HE, Batts KP, & Wiesner RH (1992). Papillary bile duct dysplasia in primary sclerosing cholangitis. Gastroenterology, 102: 2134–2138. [DOI] [PubMed] [Google Scholar]
- Ma B, Meng H, Tian Y, Wang Y, Song T, Zhang T, et al. (2020). Distinct clinical and prognostic implications of IDH1/2 mutation and other most frequent mutations in large duct and small duct subtypes of intrahepatic cholangiocarcinoma. BMC Cancer, 20: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CH, Hwang DW, Song KB, Kim SC, Shin SH, Lee JH, et al. (2020). Prognostic factors predicting survival rate over 10 years of patients with intrahepatic cholangiocarcinoma after hepatic resection. Annals of Surgical Treatment and Research, 98: 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Tang WK, Esser L, Pastan I, & Xia D (2012). Recognition of mesothelin by the therapeutic antibody MORAb-009: structural and mechanistic insights. Journal of Biological Chemistry, 287: 33123–33131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Hernandes MO, Zhao Y, Mehta M, Tran B, Kelly M, et al. , (2019). Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell, 36: 418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli R, Mammola CL, Sferra R, Pompili S, Vetuschi A, & Pannarale L (2019). Role of the angiogenic factors in cholangiocarcinoma. Applied Sciences, 9: 1393. [Google Scholar]
- Manzanares MÁ, Usui A, Campbell DJ, Dumur CI, Maldonado GT, Fausther M, et al. (2017) Transforming growth factors α and β are essential for modeling cholangiocarcinoma desmoplasia and progression in a three-dimensional organotypic culture model. American Journal of Pathology, 187: 1068–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares MÁ, Campbell DJW, Maldonado GT, & Sirica AE (2018). Overexpression of periostin and distinct mesothelin forms predict malignant progression in a rat cholangiocarcinoma model. Hepatology Communications, 2: 155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao ZY, Zhu GQ, Xiong M, Ren L, & Bai L (2015). Prognostic value of neutrophil distribution in cholangiocarcinoma. World Journal of Gastroenterology, 21: 4961–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh V, Davies EJ, Williams GT & Clarke AR (2013). PTEN loss and KRAS activation cooperate in murine biliary tract malignancies. Journal of Pathology, 230: 165–173. [DOI] [PubMed] [Google Scholar]
- Martín-Sierra C, Martins R, Laranjeira P, Coucelo M, Abrantes AM, Oliveira RC, et al. (2019). Functional and phenotypic characterization of tumor-infiltrating leukocyte subsets and their contribution to the pathogenesis of hepatocellular carcinoma and cholangiocarcinoma. Translational Oncology, 12: 1468–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarweh NN & El-Serag HB (2017). Epidemiology of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control, 24: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavros MN, Economopoulos KP, Alexiou VG, & Pawlik TM (2014). Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surgery, 149: 565–574. [DOI] [PubMed] [Google Scholar]
- Melaiu O, Lucarini V, Cifaldi L, & Fruci D (2020). Influence of the tumor microenvironment on NK cell function in solid tumors. Frontiers in Immunology, 10: 3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mells GF, Kaser A, & Karlsen TH (2013). Novel insights into autoimmune liver diseases provided by genome-wide association studies. Journal of Autoimmunity, 46: 41–54. [DOI] [PubMed] [Google Scholar]
- Melum E, Franke A, Schramm C, Weismüller TJ, Gotthardt DN, Offner FA, et al. (2011). Genome-wide association analysis in primary sclerosing cholangitis identifies two non-HLA susceptibility loci. Nature Genetics, 43: 17–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, Wehbe-Janek H, Henson R, Smith H, & Patel T (2008). Epigenetic regulation of microRNA-370 by interleukin-6 in malignant human cholangiocytes. Oncogene, 27: 378–386. [DOI] [PubMed] [Google Scholar]
- Meng Z-W, Pan W, Hong H-J, Chen J-Z, & Chen Y-L (2017). Macroscopic types of intrahepatic cholangiocarcinoma and the eighth edition of AJCC/UICC TNM staging system. Oncotarget, 8: 101165–101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdrignac A, Angenard G, Allain C, Petitjean K, Bergeat D, Bellaud P et al. , (2018). A novel transforming growth factor beta-induced long noncoding RNA promotes an inflammatory microenvironment in human intrahepatic cholangiocarcinoma. Hepatology Communications, 2: 254–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, Werneburg NW, et al. (2013). Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Research, 73: 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens JC, Rizvi S, & Gores GJ (2018). Targeting cholangiocarcinoma. Biochimica et Biophysica Acta, 1864: 1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK & Khan Z (2015). Liver stem cells: experimental findings and implications for human liver disease. Gastroenterology, 149: 876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeini A, Sia D, Zhang Z, Camprecios G, Stueck A, Dong H, et al. (2017). Mixed hepatocellular cholangiocarcinoma tumors: cholangiolocellular carcinoma is a distinct molecular entity. Journal of Hepatology, 66: 952–961. [DOI] [PubMed] [Google Scholar]
- Monteran L & Erez N (2019). The dark side of fibroblasts: cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Frontiers in Immunology, 10: 1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H, Yu L, Liao Q, Hou X, Wu Y, Cui Q, et al. (2018). Successful response to the combination of immunotherapy and chemotherapy in cholangiocarcinoma with high tumour mutational burden and PD-L1 expression: a case report. BMC Cancer, 18: 1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, Pradere J-P, Affò S, Dapito DH, Friedman R, Lefkovitch JH, et al. , (2016). Epithelial transforming growth factor-β signaling does not contribute to liver fibrosis but protects mice from cholangiocarcinoma. Gastroenterology, 150: 720–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich JE & Suto MJ (2018). Thrombospondin-1 regulation of latent TGF-β activation: a therapeutic target for fibrotic disease. Matrix Biology, 68–69: 28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Suzuki N, Hirata Y, Hikiba Y, Hayakawa Y, Kinoshita H, et al. (2017). Biliary epithelial injury-induced regenerative response by IL-33 promotes cholangiocarcinogenesis from peribiliary glands. Proceedings of the National Academy of Sciences, 114: E3806–E3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Hayata Y, Yamada T, Kawamura S, Suzuki N & Koike K (2018). Peribiliary glands as the cellular origin of biliary tract cancer. International Journal of Molecular Sciences, 19: 1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Suzuki N, & Koike K (2019). Mouse model for cholangiocarcinoma from peribiliary glands. Methods in Molecular Biology, 1905: 237–245. [DOI] [PubMed] [Google Scholar]
- Nakamoto N, Sasaki N, Aoki R, Miyamoto K, Suda W, Teratani T, et al. (2019). Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nature Microbiology, 4: 492–503. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, et al. , (2015). Genomic spectra of biliary tract cancer. Nature Genetics, 47: 1003–1010. [DOI] [PubMed] [Google Scholar]
- Nakano S, Eso Y, Okada H, Takai A Takahashi K & Seno H (2020). Recent advances in immunotherapy for hepatocellular carcinoma. Cancers, 12: 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanuma H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, et al. (2015). Genomic spectra of biliary tract cancer. Nature Genetics, 47: 1003–1010. [DOI] [PubMed] [Google Scholar]
- Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, & Ikeda H (2010). Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World Journal of Hepatology, 2: 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanuma Y & Sato Y (2012). Cystic and papillary neoplasm involving peribiliary glands: a biliary counterpart of branch-type intraductal papillary mucinous cystic neoplasm. Hepatology, 55: 2040–2041. [DOI] [PubMed] [Google Scholar]
- Nakanuma Y, Tsutsui A, Ren XS, Harada K, Sato Y, & Sasaki M (2014). What are the precursor and early lesions of peripheral intrahepatic cholangiocarcinoma? International Journal of Hepatology, 2014: 805973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanuma Y, Miyata T, & Uchida T (2016). Latest advances in the pathological understanding of cholangiocarcinomas. Expert Review of Gastroenterology & Hepatology, 10: 113–127. [DOI] [PubMed] [Google Scholar]
- Nakanuma Y, Kakuda Y, & Uesaka K (2019). Characterization of intraductal papillary neoplasm of the bile duct with respect to the histopathologic similarities to pancreatic intraductal ikosi, mucinous neoplasm. Gut and Liver, 13: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzillet C, Tijeras-Raballand A, Ragulan C, Cros J, Patil Y, Martinet M, et al. (2019). Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. Journal of Pathology, 248: 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholás-Boluda A, Vaquero J, Laurent G, Renault G, Bazzi R, Donnadieu E, et al. (2020). Photothermal depletion of cancer-associated fibroblasts normalizes tumor stiffness in desmoplastic cholangiocarcinoma. ACS Nano, 14: 5738–5753. [DOI] [PubMed] [Google Scholar]
- Nishihara Y, Aishima S, Hayashi A, Iguchi T, Fujita N, Taketomi A, et al. (2009). CD10+ fibroblasts are more involved in the progression of hilar/extrahepatic cholangiocarcinoma than peripheral intrahepatic cholangiocarcinoma. Histopathology, 55: 423–431. [DOI] [PubMed] [Google Scholar]
- Nissen NI, Karsdal M, & Willumsen N (2019). Collagens and cancer associated fibroblasts in the reactive stroma and its relation to cancer biology. Journal of Experimental & Clinical Cancer Research, 38: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S, Saito A, & Nagase T (2018). YAP/TAZ signaling as a molecular link between fibrosis and cancer. International Journal of Molecular Sciences, 19: 3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto M, Uchikosi Y, Kajikazawa N, Tanaka Y, & Asakura H (1992). Appearance of hepatocytelike cells in the interlobular bile ducts of human liver in various liver disease states. Hepatology, 16: 1199–1205. [PubMed] [Google Scholar]
- Nomura R, Fujii H, Abe M, Sugo H, Ishizaki Y, Kawasaki S, et al. (2013). Mesothelin expression is a prognostic factor in cholangiocellular carcinoma. International Surgery, 98: 164–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris RA., Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, et al. (2007). Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissue. Journal of Cellular Biochemistry, 101: 695–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obulkasim H, Shi X, Wang J, Li J, Dai B, Wu P, et al. (2018). Podoplanin is an important stromal prognostic marker in perihilar cholangiocarcinoma. Oncology Letters, 15: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, et al. (2017). Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. Journal of Experimental Medicine, 214: 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe H, Beppu T, Hayashi H, Horino K, Masuda T, Komori H, et al. (2009). Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Annals of Surgical Oncology, 16: 2555–2564. [DOI] [PubMed] [Google Scholar]
- Okabe H, Hayashi H, Nakagawa S, Imai K, Nitta H, Arima K, et al. (2016). Inducible factors for cancer-associated fibroblasts in liver cancer versus myofibroblasts in inflammatory liver disease. Histology and Histopathology, 31: 141–148. [DOI] [PubMed] [Google Scholar]
- O’Rourke CJ, Lafuente-Barquero J, & Andersen JB (2019). Epigenome remodeling in cholangiocarcinoma. Trends in Cancer, 5: 335–349. [DOI] [PubMed] [Google Scholar]
- Overi D, Carpino G, Cardinale V, Franchitto A, Safarikia S, Onori P, et al. (2018). Contribution of resident stem cells to liver and biliary tree regeneration in human diseases. International Journal of Molecular Sciences, 19: 2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant K, Richard S, Peixoto E, & Gradilone SA (2020). Role of glucose metabolism reprogramming in the pathogenesis of cholangiocarcinoma. Frontiers in Medicine, 7: Article 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellino A, Loupakis F, Cadamuro M, Dadduzio V, Fassan M, Guido M, et al. , (2018) Precision medicine in cholangiocarcinoma. Translational Gastroenterology and Hepatology, 3: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plentz RR & Malek NP (2016). Systemic therapy of cholangiocarcinoma. Visceral Medicine, 32: 427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plieskatt JL, Deenonpoe R, Mulvenna JP, Krause L, Sripa B, Bethony JM, & Brindley PJ (2013). Infection with the carcinogenic liver fluke Opisthorchis viverrini modifies intestinal and biliary microbiome. FASEB Journal, 27: 4572–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. (2019). Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomized, controlled, multicentre, phase 3 study. Lancet Oncology, 20: 663–673. [DOI] [PubMed] [Google Scholar]
- Principe DR, Park A, Dorman MJ, Kumar S, Viswakarma N, Rubin J, et al. (2019). TGFβ blockade augments PD-1 inhibition to promote T-cell mediated regression of pancreatic cancer. Molecular Cancer Therapeutics, 18: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prueksapanich P, Piyachaturawat P, Aumpansub P, Ridtitid W, Chaiteerakij R, & Rerknimitr R (2018). Liver fluke-associated biliary tract cancer. Gut and Liver, 12: 236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian MB, Utzinger J, Keiser J, & Zhou XN (2016). Clonorchiasis. Lancet, 387: 800–810. [DOI] [PubMed] [Google Scholar]
- Raggi C, Correnti M, Sica A, Andersen JB, Cardinale V, Alvaro D, et al. (2017). Cholangiocarcinoma stem-like subset shapes tumor-initiating niche by educating associated macrophages. Journal of Hepatology, 66: 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P, Silberg DG, & Sirica AE (2000). Expression of an intestine-specific transcription factor (CDX1) in intestinal metaplasia and in subsequently developed intestinal type of cholangiocarcinoma in rat liver. Americam Journal of Pathology, 156: 621–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee H, Ko JE, Chung T, Jee BA, Kwon SM, Nahm JH, et al. (2018). Transcriptomic and histopathological analysis of cholangiolocellular differentiation trait in intrahepatic cholangiocarcinoma. Liver International, 38: 113–124. [DOI] [PubMed] [Google Scholar]
- Rizvi S, Mertens JC, Bronk SF, Hirsova P, Dai H, Roberts LR, (2014). Platelet -derived growth factor primes cancer-associated fibroblasts for apoptosis. Journal of Biological Chemistry, 289: 22835–22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi S, Khan SA, Hallemeier CL, Kelley RK, & Gores GJ (2018). Cholangiocarcinoma-evolving concepts and therapeutic strategies. Nature Reviews Clinical Oncology, 15: 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D’Angelica MI, et al. (2012). Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology, 56: 1352–1360. [DOI] [PubMed] [Google Scholar]
- Rőszer T (2015). Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators of Inflammation, 2015: 816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Glaser S, & Chakraborty S (2019). Inflammation and progression od cholangiocarcinoma: role of angiogenic and lymphangiogenic mechanisms. Frontiers in Medicine, 6: Article 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatino F, Villani V, Yearley JH, Deshpande V, Cai L, Konstantinidis IT, et al. (2016). PD-L1 and HLA Class I Antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clinical Cancer Research, 22: 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri A-R, Jeschke MG, & Amini-Nik S (2016). Advances in liver regeneration: revisiting hepatic stem/progenitor cells and their origin. Stem Cells international, 2016; 7920897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha SK, Parachoniak CA, Ghanta KS, Fitamant J, Ross KN, Najem MS, et al. (2014). Mutant IDH inhibits HNF4α to block hepatocyte differentiation and promote biliary cancer. Nature, 513: 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha SK, Zhu AX, Fuchs CS, & Brooks GA (2016). Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. The Oncologist, 21: 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Astsaturov I, Cukierman E, De Nardo DG, Egeblad M, Evans RM, et al. (2020). A framework for advancing our understanding of cancer-associated fibroblasts. Nature Reviews Cancer, 20: 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Nakaoka T, Muramatsu T, Ojima H, Sukeda A, Sugiyama Y, et al. (2018). Induction of differentiation of intrahepatic cholangiocarcinoma cells to functional hepatocytes using an organoid culture system. Scientific Reports, 8: 2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltykova IV, Petrov VA, & Brindley PJ (2018). Opisthorchiasis and the microbiome. Advances in Parasitology, 102: 1–23. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Sato Y, & Nakanuma Y (2017). Mutational landscape of combined hepatocellular carcinoma and cholangiocarcinoma, and its clinicopathological significance. Histopathology, 70: 423–434. [DOI] [PubMed] [Google Scholar]
- Sawasdee N, Thepmalee C, Sujjitjoon J, Yongpitakwattana P, Junking M, Poungvarin N, et al. (2020). Gemcitabine enhances cytotoxic activity of effector T-lymphocytes against chemo-resistant cholangiocarcinoma cells. International Immunopharmacology, 78: 106006. [DOI] [PubMed] [Google Scholar]
- Sato Y, Harada K, Sasaki M, & Nakanuma Y (2014). Cystic and micropapillary epithelial changes of peribiliary glands might represent a precursor lesion of biliary epithelial neoplasms. Virchows Archives, 464: 157–163. [DOI] [PubMed] [Google Scholar]
- Seehawer M, Heinzmann F, D’Artista L, Harbig J, Roux P-F, Hoenicke L et al. , (2018). Necroptosis microenvironment directs lineage commitment in liver cancer. Nature, 562: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha M, Jeong S, Qiu B-j, Tong Y, Xia L, Xu N, et al. (2018). Isolation of cancer-associated fibroblasts and its promotion to the progression of intrahepatic cholangiocarcinoma. Cancer Medicine, 7L 4665–4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Sano T, Sakamoto Y, Esaki M, Kosuge T, & Ojima H (2007). Surgical outcomes of the mass-forming plus periductal infiltrating types of intrahepatic cholangiocarcinoma: a comparative study with the typical mass-forming type of intrahepatic cholangiocarcinoma. World Journal of Surgery, 31: 2016–2022. [DOI] [PubMed] [Google Scholar]
- Shimonishi T, Sasaki M, & Nakanuma Y (2000). Precancerous lesions of intrahepatic cholangiocarcinoma. Journal of Hepatobiliary and Pancreatic Surgery, 7: 542–550. [DOI] [PubMed] [Google Scholar]
- Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J Tabak, B., et al. , (2013). Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology, 144: 829–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia D, Losic B, Moeini A, Cabellos L, Hao K, Revill K, et al. (2015). Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nature Communications, 6: 6087. [DOI] [PubMed] [Google Scholar]
- Sia D, Villanueva A, Friedman SL, & Llovet JM (2017). Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology, 152: 745–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel CS, Drill E, Zhou Y, Basturk O, Askan G, Pak LM, Vakiani E, et al. (2018). Intrahepatic cholangiocarcinomas have histologically and immunophenotypically distinct small and large duct patterns. American Journal of Surgical Pathology, 42: 1334–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simile MM, Bagella P, Vidili G, Spanu A, Manetti R, Seddaiu MA, et al. (2019). Targeted therapies in cholangiocarcinoma: emerging evidence from clinical trials. Medicina, 55:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirica AE (2012). The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nature Reviews Gastroenterology & Hepatology, 9: 44–54. [DOI] [PubMed] [Google Scholar]
- Sirica AE, Almenara JA, & Li C (2014). Periostin in intrahepatic cholangiocarcinoma: pathobiological insights and clinical implications. Experimental and Molecular Pathology, 97: 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirica AE, Campbell DJ, & Dumur CI (2011). Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma. Current Opinion in Gastroenterology, 27: 276–284. [DOI] [PubMed] [Google Scholar]
- Sirica AE, Dumur CI, Campbell DJW, Almenara JA, Olorunseun OO, & Dewitt JL (2009). Intrahepatic cholangiocarcinoma progression: prognostic factors and basic mechanisms. Clinical Gastroenterology and Hepatology, 7: S68–S78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirica AE, Gainey TW, & Mumaw VR (1994). Ductular hepatocytes-evidence for a bile ductular cell origin in furan-treated rats. American Journal of Pathology, 145: 375–383. [PMC free article] [PubMed] [Google Scholar]
- Sirica AE & Gores GJ (2014). Desmoplastic stroma and cholangiocarcinoma: clinical implications and therapeutic targeting. Hepatology, 59: 2397–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirica AE., Gores GJ, Groopman JD, Selaru FM, Strazzabosco M, Wang XW, et al. (2019). Intrahepatic cholangiocarcinoma: continuing challenges and translational advances. Hepatology, 69: 1803–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Fu Y, Xie Q, Zhu B, Wang J, & Zhang B (2020). Anti-angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Frontiers in Immunology, 11: 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sow HS, Ren J, Camps M, Ossendorp F & ten Dijke P (2019). Combined inhibition of TGF-β signaling and the PD-L1 immune checkpoint is differentially effective in tumor models. Cells, 8: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirlì C, Fabris L, Duner E, Fiorotto R, Ballardini G, Roskams T, et al. (2003). Cytokine-stimulated nitric oxide production inhibits adenylyl cyclase and cAMP-dependent secretion in cholangiocytes. Gastroenterology, 124: 737–753. [DOI] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, et al. (2007). Liver fluke induces cholangiocarcinoma. PLoS Medicine, 4: e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripa B, Tangkawattana S, & Brindley PJ (2018). Update on pathogenesis of opisthorchiasis and cholangiocarcinoma. Advances in Parasitology, 102: 97–113. [DOI] [PubMed] [Google Scholar]
- Stavraka C, Rush H, & Ross P (2019). Combined hepatocellular cholangiocarcinoma (cHCC-CC): an update of genetics, molecular biology, and therapeutic interventions. Journal of Hepatocellular Carcinoma, 6: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss O, Phillips A, Ruggiero K, Bartlett A & Dunbar PR (2017). Immunofluorescence identifies distinct subsets of endothelial cells in the human liver. Scientific Reports, 7: 44356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazzabosco M, Fiorotto R, Cadamuro M, Spirli C, Mariotti V, Kaffe E, et al. (2018). Pathophysiologic implications of innate immunity and autoinflammation in the biliary epithelium. Biochimica et Biophysica Acta Molecular Basis of Disease, 1864: 1374–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Luo T, Dong P, Zhang N, Chen J, Zhang S, et al. (2020). CD86+/CD206+ tumor-associated macrophages predict prognosis of patients with intrahepatic cholangiocarcinoma. PeerJ, 8: e8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Miyagawa S, Ichikawa E, Soeda J, Miwa S, Miyagawa Y, et al. (2004). Dendritic cells, T-cell infiltration, and Grp94 expression in cholangiocellular carcinoma. Human Pathology, 35: 881–886. [DOI] [PubMed] [Google Scholar]
- Tan D, Fu, Su, Guan, Kong, Wang S-Q, & Wang HL (2016). Prognostic significance of neutrophil to lymphocyte ratio in oncologic outcomes of cholangiocarcinoma: a meta-analysis. Scientific Reports, 6: 33789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Qian M, & Ho M (2013). The role of mesothelin in tumor progression and targeted therapy. Anticancer Agents in Medicinal Chemistry, 13: 276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniai M, Higuchi H, Burgart LJ, & Gores GJ (2002). p16INK4a promoter mutations are frequent in primary sclerosing cholangitis (PSC) and PSC-associated cholangiocarcinoma. Gastroenterology, 123: 1090–1098. [DOI] [PubMed] [Google Scholar]
- Tedesco D, Thapa M, Chin CY, Ge Y, Gong M, Li J, et al. (2018). Alterations in intestinal microbiota lead to production of interleukin 17 by intrahepatic γδ T-Cell receptor-positive cells and pathogenesis of cholestatic liver disease. Gastroenterology, 154: 2178–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada T (2013). Combined hepatocellular-cholangiocarcinoma with stem cell features, ductal plate malformation subtype: a case report and proposal of a new subtype. International Journal of Clinical and Experimental Pathology, 6: 737–748. [PMC free article] [PubMed] [Google Scholar]
- Thepmalee C, Panya A, Junking M, Chieochansin T, & Yenchitsomanus PT (2018). Inhibition of IL-10 and TGF-β receptors on dendritic cells enhances activation of effector T-cells to kill cholangiocarcinoma cells. Human Vaccines & Immunotherapeutics, 14: 1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thepmalee C, Panya A, Sujjitjoon J, Sawasdee N, Poungvarin N, Junking M, et al. (2020). Suppression of TGF-β and IL-10 receptors on self-differentiated dendritic cells by short-hairpin RNAs enhanced activation of effector T-cells against cholangiocarcinoma cells. Human Vaccines & Immunotherapeutics, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuwajit C, Thuwajit P, Jamjantra P, Pairojkul C, Wongkham S, Bhudhisawasdi V, et al. (2017). Clustering of patients with intrahepatic cholangiocarcinoma based on serum periostin may be predictive of prognosis. Oncology Letters, 14: 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M-X, Zhou Y-F, Qu W-F, Liu W-R, Jin L, Jiang X-F, et al. (2019). Histopathology-based immunoscore predicts recurrence for intrahepatic cholangiocarcinoma after hepatectomy. Cancer Immunology, Immunotherapy, 68: 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M, Liu W, Tao C, Tang Z, Zhou Y, Song S, et al. (2020). Prediction of overall survival in resectable intrahepatic cholangiocarcinoma: ISICC -applied prediction model. Cancer Science, 111: 1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauner M, Halilbasic E, Kazemi-Shirazi L, Kienbacher C, Staufer K, Traussnigg S, et al. (2014). Therapeutic role of bile acids and nuclear receptor agonists in fibrosing cholangiopathies. Digestive Diseases, 32: 631–636. [DOI] [PubMed] [Google Scholar]
- Tschaharganeh DF, Xue W, Calvisi DF, Evert M, Michurina TV, Dow LE, et al. (2014). p53 dependent Nestin regulation links tumor suppression to cellular plasticity in liver cancer. Cell, 158: 579–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T, Shimoyama Y, Ebata T, Yokoyama Y, Igami T, Sugawara G, et al. (2016). Cholangiocarcinoma with intraductal tubular growth pattern versus intraductal papillary growth pattern. Modern Pathology, 29: 293–301. [DOI] [PubMed] [Google Scholar]
- Tu E, Chia PZ, & Chen W (2014). TGFβ in T cell biology and tumor immunity: angel or devil? Cytokine & Growth Factor Reviews, 25: 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utispan K, Thuwajit P, Abiko Y, Charngkaew K, Paupairoj A, Chau-in S, et al. (2010). Gene expression profiling of cholangiocarcinoma derived fibroblasts reveals alterations related to tumor progression and indicates periostin as a poor prognostic marker. Molecular Cancer, 9: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utispan K, Sonongbua J, Thuwajit P, Chau-In S, Pairojkul C, Wongkham S, et al. (2012). Periostin activates integrin α5β1 through a PI3K/AKT-dependent pathway in invasion of cholangiocarcinoma. International Journal of Oncology, 41: 1110–1118. [DOI] [PubMed] [Google Scholar]
- Vale N, Gouveia MJ, Gärtner F, & Brindley PJ (2020). Oxysterols of helminth parasites and pathogenesis of foodborne hepatic trematodiasis caused by Opisthorchis and Fasciola species. Parasitology Research, 119: 1443–1453. [DOI] [PubMed] [Google Scholar]
- Vaquero J, Aoudjehane L, & Fouassier L (2020). Cancer-associated fibroblasts in cholangiocarcinoma. Current Opinion in Gastroenterology, 36: 63–69. [DOI] [PubMed] [Google Scholar]
- van Tong H, Brindley PJ, Meyer CG, & Velavan TP (2017). Parasite infection, carcinogenesis and human malignancy. EBioMedicine, 15: 12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterhus M & Karlsen TH (2020). Emerging therapies in primary sclerosing cholangitis: pathophysiological basis and clinical opportunities. Journal of Gastroenterology, 55: 588–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicent S, Lieshout R, Saborowski A, Verstegen MMA,, Raggi, C., Recalcati, S., et al. (2019). Experimental models to unravel the molecular pathogenesis, cell of origin and stem cell properties of cholangiocarcinoma. Liver International, 39: 79–97. [DOI] [PubMed] [Google Scholar]
- Vienot A & Neuzillet C (2019). Cholangiocarcinoma: the quest for a second-line systemic treatment. Translational Cancer Research. 8 (Suppl 3): S275–S288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano L, Soldani C, Franceschini B, Cimino M, Lleo A, Donadon M, et al. (2019). Tumor-infiltrating lymphocytes and macrophages in intrahepatic cholangiocellular carcinoma. Impact on prognosis after complete surgery. Journal of Gastrointestinal Surgery, 23: 2216–2224. [DOI] [PubMed] [Google Scholar]
- Vijgen S, Terris B, & Rubbia-Brandt. (2017). Pathology of intrahepatic cholangiocarcinoma. HepatoBiliary Surgery and Nutrition, 6: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisberg DR, Pinheiro RS, Nacif LS, Rocha-Santos V, Martino RB, Arantes RM (2018). Resection for intrahepatic cholangiocarcinoma: new advances. Translational Gastroenterology and Hepatology, 3: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X-S, Xu, Qian J-Y, Yang X-B, Wang A-Q, He L, et al. (2013). Intraductal papillary neoplasm of the bile duct. World Journal of Gastroenterology, 19: 8595–8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Pang S, Si-Ma H, Yang N, Zhang H, Fu Y, et al. (2019). Specific risk factors contributing to early and late recurrences of intrahepatic cholangiocarcinoma after curative resection. World Journal of Surgical Oncology, 17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Lin J, Yang X, Long J, Bai Y, Yang X, et al. (2019). Combination regimens with PD-1/PD-L1 immune checkpoint inhibitors for gastrointestinal malignancies. Journal of Hematology & Oncology, 12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Bo X, Suo T, Liu H, Ni X, Shen S, et al. (2018). Tumor-infiltrating neutrophils predict prognosis and adjuvant chemotherapeutic benefit in patients with biliary cancer. Cancer Science, 109: 2266–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li E, Yang H, Wu J, cheng Lu H, Yi C, et al. (2019). Combined hepatocellular -cholangiocarcinoma: a population level analysis of incidence and mortality trends. World Journal of Surgical Oncology, 17: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Dong Q, Zhang C, Kuan P-F, Liu Y, Jeck WR, et al. (2013). Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene, 32: 3091–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Drill E, Vakiani E, Pak LM, Boerner T, Askan G, et al. (2019). Distinct histomorphological features are associated with IDH1 mutation in intrahepatic cholangiocarcinoma. Human Pathology, 91: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson KD, & Chapman RW (2015). Primary sclerosing cholangitis: a clinical update. British Medical Bulletin, 114: 53–64. [DOI] [PubMed] [Google Scholar]
- Wójcik M, Ramadori P, Blaschke M, Sultan S, Khan S, Malik IA, et al. (2012). Immunodetection of cyclooxygenase-2 (COX-2) is restricted to tissue macrophages in normal rat liver and to recruited mononuclear phagocytes in liver injury and cholangiocarcinoma. Histochemistry and Cell Biology, 137: 217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Sun L, Li Y, Xie F, Zhou X, Yang H, et al. (2019). The clinicopathological and prognostic value of PD-L1 expression in cholangiocarcinoma: a meta-analysis. Frontiers in Oncology, 9: 897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Igarashi S, Sasaki M, Matsubara T, Yoneda N, Kozaka K, et al. (2012). Intrahepatic cholangiocarcinomas in cirrhosis are hypervascular in comparison to those in normal liver. Liver International, 32: 1156–1164. [DOI] [PubMed] [Google Scholar]
- Xu X, Kobayashi S, Qiao W, Li C, Xiao C, Radaeva S, et al. (2006) Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and Pten in mice. The Journal of Clinical Investigation, 116: 1843–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R, Chen L, Zhang C, Fujita M, Li R, Yan S-M., et al. (2019). Genomic and transcriptomic profiling of combined hepatocellular and intrahepatic cholangiocarcinoma reveals distinct molecular subtypes. Cancer Cell, 35: 932–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada D, Rizvi S, Razumilava N, Bronk SF, Davila JI, Champion MD, et al. (2015). IL-33 facilitates oncogene induced cholangiocarcinoma in mice by an IL-6 sensitive mechanism. Hepatology, 61: 1627–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Harimoto N, Yokobori T, Muranushi R, Hoshino K, Hagiwara K, et al. (2020). Nintedanib inhibits intrahepatic cholangiocarcinoma aggressiveness via suppression of cytokines extracted from cancer-associated fibroblasts. British Journal of Cancer, 122: 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Oshita A, Nishisaka T, Nakahara H, & Itamoto T (2018). Synchronous double primary hepatic cancer consisting of hepatocellular carcinoma and cholangiolocellular carcinoma: a case report. Journal of Medical Case Reports, 12: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Koda S, Wu J, Zhang BB, Yu Q, Netea MG, et al. (2020). Roles of trained immunity in the pathogenesis of cholangiopathies: a novel therapeutic target. Hepatology, In press. [DOI] [PubMed] [Google Scholar]
- Yang J & Yan L-N. (2008). Current status of intrahepatic cholangiocarcinoma. World Journal of Gastroenterology, 14: 6289–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Li Y, Zou L, & Zhu Z (2019). Role of exosomes in crosstalk between cancer-associated fibroblasts and cancer cells. Frontiers in Oncology, 9: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W, et al. (2016). FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Research, 76: 4124–4135. [DOI] [PubMed] [Google Scholar]
- Yao KJ, Jabbour S, Parekh N, Lin Y, & Moss RA (2016). Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterology, 16: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W, Qian Y, Campbell D, Wang J, & Sirica AE (2018). Underexpression of phosphor-LKB1, atypical PKCι, and Hes1 associated with disruption of polarized morphogenesis in a 3-dimensional culture model of rat cholangiocarcinoma progression mediated by activated NEU receptor. Gastroenterology, 154: S–1152. [Google Scholar]
- Yoo C, Oh D-Y, Choi HJ, Kudo M, Ueno M, Kondo S, et al. , (2020). Phase I study of bintrafusp alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with pretreated biliary tract cancer. Journal for Immunotherapy of Cancer, 8: e000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida GJ, Azuma A, Miura Y, & Orimo A (2019). Activated fibroblast program orchestrates tumor initiation and progression: molecular mechanisms and the associated therapeutic strategies. International Journal of Molecular Sciences, 20: 2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Feng M, Kim H, Phung Y, Kleiner DE, Gores GJ, et al. (2010). Mesothelin as a potential therapeutic target in human cholangiocarcinoma. Journal of Cancer, 1: 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccari P, Cardinale V, Severi C, Pedica F, Carpino G, Gaudio E, et al. (2019) Common features between neoplastic and preneoplastic lesions of the biliary tract and the pancreas. World Journal of Gastroenterology, 25: 4343–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender S, Nickeleit I, Wuestefeld T, Sörensen I, Dauch D, Bozko P, et al. (2013). A critical role for notch signaling in the formation of cholangiocellular carcinoma. Cancer Cell, 23: 784–795. [DOI] [PubMed] [Google Scholar]
- Zeng J, Liu Z, Sun S, Xie J, Cao L, Lv P, et al. (2018). Tumor-associated macrophages recruited by periostin in intrahepatic cholangiocarcinoma stem cells. Oncology Letters, 15: 8681–8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Jiang L, Sun Q, Peng T, Lou K, Liu N, & Leng J (2007). Prostaglandin E2 enhances mitogen-activated protein kinase/Erk pathway in human cholangiocarcinoma cells: involvement of EP1 receptor, calcium and EGF receptors signaling. Molecular and Cellular Biochemistry, 305: 19–26. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yang H, Wan L, Wang Z, Wang H, Ge C, et al. (2020). Single-cell transcriptomic architecture and intercellular crosstalk of human intrahepatic cholangiocarcinoma. Journal of Hepatology, In press. [DOI] [PubMed] [Google Scholar]
- Zhang X-F, Beal EW, Bagante F, Chakedis J, Weiss M, Popescu I, et al. (2018). Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. British Journal of Surgery, 105: 848–856. [DOI] [PubMed] [Google Scholar]
- Zheng BH, Ma JQ, Tian LY, Dong LQ, Song GH, Pan JM, et al. (2020). The distribution of immune cells within combined hepatocellular carcinoma and cholangiocarcinoma predicts clinical outcome. Clinical and Translational Medicine, 10: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Sprengers D, Mancham S, Erkens R, Boor P, van Beek AA, et al. (2019). Reduction of immunosuppressive tumor microenvironment in cholangiocarcinoma by ex vivo targeting immune checkpoint molecules. Journal of Hepatology, 71: 753–762. [DOI] [PubMed] [Google Scholar]
- Zhou SL, Dai Z, Zhou Z-J, Chen Q, Wang Z, Xiao Y-S, et al. (2014). CXCL5 contributes to tumor metastasis and recurrence of intrahepatic cholangiocarcinoma by recruiting infiltrative intratumoral neutrophils. Carcinogenesis, 35: 597–605. [DOI] [PubMed] [Google Scholar]
- Zimmermann A (2017). Tumors and tumor-like lesions of peribiliary glands. In: Tumors and Tumor-Like Lesions of the Hepatobiliary Tract. pp 779–788. Springer, Cham, Switzerland. [Google Scholar]