Abstract
Background:
While many patients with follicular lymphoma (FL) undergo routine radiographic surveillance during their first remission, no consensus exists on the modality, duration, frequency, or need for routine imaging studies. We retrospectively examined the effect of surveillance imaging on relapse detection and overall survival (OS) in FL patients.
Methods:
Patients with newly diagnosed FL with a response to induction therapy were identified from the Lymphoid Malignancies Enterprise Architecture Database (LEAD) at Emory University and from the Molecular Epidemiology Resource (MER) of the University of Iowa/Mayo Clinic. Patients were evaluated for both relapse and method of relapse detection (i.e. clinical concerns vs radiologic detection via surveillance imaging in an asymptomatic patient).
Results:
Of 148 LEAD cohort patients, 55 (37%) relapsed, with the majority (n=35, 64%) detected clinically. In the MER cohort, 63 (54%) of 117 relapses were detected clinically. There was no significant difference in OS from the date of diagnosis between the two methods of relapse detection in the LEAD (hazard ratio [HR]=0.61 [95% CI (0.13–2.94)], p=0.54) and MER (HR=1.02 [95% CI (0.47–2.21)], p=0.96) cohorts. Similarly, there was no significant difference in OS from the date of relapse between the two methods of relapse detection in the LEAD (HR=0.47 [95% CI (0.10–2.27)], p=0.35) and MER (HR=1.02 [95% CI (0.47–2.21)], p=0.96) cohorts.
Conclusion:
These findings suggest a limited role for routine surveillance imaging in patients with FL who complete front-line therapy. Future studies should evaluate which patients may benefit from a more aggressive surveillance approach and should explore novel methods of relapse detection.
Keywords: Follicular lymphoma, surveillance, relapsed follicular lymphoma, PET/CT, CT
Precis:
Method of relapse detection (asymptomatic surveillance imaging vs clinically-directed assessment) is not associated with improved outcomes for patients with relapsed follicular lymphoma. Radiographic abnormalities identified on asymptomatic surveillance studies are frequently false positive findings.
Introduction:
Follicular lymphoma (FL) is the most common type of indolent non-Hodgkin lymphoma (NHL).1,2 While almost all patients with FL will respond to initial induction therapy, FL is typically considered an incurable disease with conventional therapy, and nearly all patients will relapse after initial induction. Most relapses occur more than 3 years after completing initial therapy, and the median overall survival (OS) with currently available treatments is prolonged and approaches the life expectancy of unaffected patients.2–5 As a result, many patients will remain in remission for years before relapsing and will often experience prolonged survival even after initial relapse.4,6–9 These patients require an evidence-based approach to surveillance that accounts for patient- and disease-related risk stratification such as stage, grade, and FL international prognostic index (FLIPI) risk score.10,11
Due to the high lifetime risk of relapse in FL, current guidelines suggest that patients should undergo routine clinical follow-up for early relapse detection during first remission.11,12 While scheduled surveillance imaging of asymptomatic patients is a common practice in certain regions of the world, no consensus exists on the type, duration, frequency, or need for routine imaging studies as part of follow-up care.13,14 Most guidelines for post-induction computed tomography (CT) surveillance in NHL are founded on data regarding aggressive NHL. A plethora of studies suggest the limited value of surveillance imaging in detecting asymptomatic NHL relapse earlier, relative to symptom-driven investigations, as surveillance imaging has not been associated with improved OS.12,14–20 In addition, scheduled radiographic imaging can be harmful to patients.21 Aside from the increased expense and heightened anxiety, according to Shenoy et al., a five-year course of CT surveillance in NHL could increase the lifetime cancer incidence attributable to radiation exposure to 0.044% for males and 0.057% for females. Significant radiation exposure also results from cumulative positron emission tomography–CT (PET-CT) scans, which are now widely used in surveillance.13,21–23 Because routine asymptomatic surveillance imaging in aggressive NHL is of unclear benefit, the National Comprehensive Cancer Network (NCCN), European Society for Medical Oncology (ESMO), and the American Society of Hematology (ASH) Choosing Wisely Campaign guidelines have recommended limiting its use in most patients with NHL.12,15–17
Not all recommendations based upon data from aggressive lymphoma studies, however, can be accurately applied to indolent lymphomas such as FL, due to differences in disease biology and clinical pattern of relapse. The management of indolent lymphomas differs from that of aggressive lymphomas, as many patients with the former are currently not treated with curative intent and will not relapse until years after completing induction therapy.2,24,25 Despite these differences, there is a paucity of data available to guide clinical management of patients with FL who have achieved first complete or partial remission. Current guidelines from NCCN and ESMO still suggest surveillance imaging every six months for two years following induction, and optionally annually up to five years.15 However, data supporting guideline recommendations is limited as they relate to indolent lymphomas.12 In this study, we evaluated the role of routine surveillance imaging following induction therapy in an institutional cohort of patients with previously untreated FL, described the impact of surveillance on OS, and validated our results with an independent, multi-institutional patient cohort.
Methods:
Patient Population
We performed a retrospective chart review on all patients with previously untreated FL and complete records at Emory University diagnosed between July 1991 and July 2016. Patients were identified using the Institutional Review Board (IRB)-approved institutional Lymphoid Malignancies Enterprise Architecture Database (LEAD), which included patient data for each lymphoma patient evaluated or treated at Emory. Patients eligible for inclusion were those above the age of 18 who had achieved a documented partial response, complete response, or stable disease following first-line induction therapy as reported by clinicians and radiologists and who did not proceed immediately to a second-line regimen (planned maintenance was not considered a second-line regimen).11 All stages and grades of FL were included, except for grade 3b. Patients were excluded if they had incomplete outcome data after initial treatment (i.e. missing or incomplete documentation of clinical outcomes following induction therapy), experienced treatment failure defined as a time to progression of less than 26 weeks, or pursued watchful waiting without therapy during the observation period.
The validation cohort comprised of patients with previously untreated FL within the Molecular Epidemiology Resource (MER) of the University of Iowa and Mayo Clinic Lymphoma Specialized Program of Research Excellence, which prospectively enrolled patients within 9 months of a lymphoma diagnosis, between 2002 and 2015.26 Eligible patients met the same inclusion/exclusion criteria and medical record abstraction protocol as those included in the LEAD cohort. However, only FL patients with documented relapse were included in the MER cohort, with the intent of validating the initial findings on the impact of surveillance imaging on OS in relapsed FL patients.
The medical records were evaluated for patient demographics, disease characteristics at the time of diagnosis, and information regarding treatment.10,11,27,28 Patients were assessed for the type of response to therapy according to standard criteria based on the type of end-of-induction imaging studies. Type and duration of maintenance therapies were also noted, if administered.
Imaging Studies and Relapse Detection
We reviewed clinical documentation and all radiology reports following initial induction therapy, for the entirety of the surveillance period. Central review was not utilized as part of the study protocol. Each individual study was evaluated for imaging modality (CT, PET/CT, magnetic resonance imaging [MRI], other), imaging indication (scheduled asymptomatic surveillance imaging vs concerning signs or symptoms of relapse vs other indication), study impression (concerning for relapse, negative for relapse, equivocal), determined disease status after imaging, and study date. The number and type of each imaging study for every subsequent year after completion of induction therapy until first confirmed relapse were recorded for each patient.
Additionally, the diagnostic modality of relapse detection, the date of declared relapse, and the presence of concerning signs or symptoms at the time of relapse were recorded. The modality of relapse detection was stratified into clinical or radiologic detection of disease relapse. Clinical detection was defined by the documentation of patient-reported symptoms, worrisome physical exam findings potentially warranting biopsy, or abnormal lab values suggestive of relapse that were identified prior to a newly ordered scan or concurrently with a prescheduled surveillance scan at the time of confirmed relapse. Radiologic detection was defined by disease relapse first suggested on imaging, reviewed by a radiologist as part of routine surveillance, and later confirmed by other diagnostic means (i.e. histopathology and/or additional imaging); these patients must have been asymptomatic at the time of detection as determined by a lack of concerning findings on their most recent visit with an oncologist. Clinical notes were individually reviewed at the time of relapse to ensure accuracy in classifying the modality of relapse detection. Date of last follow-up or death were recorded for all patients. Access to the original data forms can be obtained by contacting the corresponding author.
Statistical Considerations
Analysis was conducted both separately for the LEAD and MER cohorts and in combination. Descriptive statistics were used to summarize the patient demographics, disease characteristics, and disease course following first remission. Categorical variables were presented with counts and percentages. The comparison between the clinical detection and radiographic detection groups were tested using the χ2 tests or Fisher’s exact test. Continuous variables were presented using the median and range, and the difference between two groups was determined using ANOVA or the Kruskal-Wallis test.
To mitigate the risk for lead-time bias, OS was analyzed from both the date of diagnosis and the date of relapse until the date of death due to any cause or last known follow-up. PFS was defined as the time from the date of diagnosis to the first date of clinical evaluation or imaging leading to subsequent relapse detection. Survival probability was estimated using the Kaplan-Meier method, and the survivals between different groups, adjusted by clinical detection of relapse or asymptomatic radiographic detection of relapse, were compared using the cox proportional hazards model in the univariate survival analysis. The cox-proportional hazards model was further employed in the multivariable survival analysis to reduce potential confounding factors (Supplemental Tables 1–3). Those variables that were significant at an alpha of 0.2 were used. Variables were selected using backwards elimination method with an alpha of 0.2. For the imaging studies, the cohort of origin was considered a potential confounder for the combined analysis and was therefore included in the multivariable selection model.
The performance of each radiographic imaging modality within the LEAD cohort was also assessed. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were estimated and reported. In order to be considered a true negative scan, a patient needed to have an absence of relapse within 6 months of the negative scan or absence of relapse by the next scheduled scan, whichever was shorter. All statistical analyses were performed using SAS, R and OIsurv packages with two-sided tests. Statistical significance was assessed at the 0.05 level.29
Results:
LEAD Cohort
Patient Characteristics
Out of a total of 395 patients with FL identified in LEAD, 247 were excluded due to missing recorded treatment outcomes after first-line induction therapy (frequently due to patients being seen as a one-time second opinion visit), incomplete documentation regarding baseline disease characteristics at the time of initial diagnosis, lack of response to first-line induction therapy, or pursuit of a watchful-waiting monitoring strategy. Eleven patients were excluded due to early progression. Overall, 148 patients with newly-diagnosed FL and a documented response to first-line therapy met criteria for study inclusion, including 104 patients who achieved CR, 37 patients who achieved PR, 1 patient who achieved SD, and 6 patients who had a response but for which CR vs PR could not be determined.
Of the 148 included patients, 66 (45%) were male. Median age at diagnosis was 57.6 years (range 22–84), and 119 patients (83%) had grade 1–2 disease. Most patients (n=108, 75%) had stage 3–4 disease. Of 127 patients with available data, 56 (44%) had a high-risk FLIPI score (≥3). The median surveillance period for the cohort was 5.9 years (range 0.54–25.70). Additional patient characteristics are included in Table 1.
Table 1.
Baseline characteristics of patients with documented remission after first-line induction therapy.
| LEAD Cohort Relapse (n = 53) | MER Cohort Relapse (n = 113) | Combined Relapse (n=166) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Relapse Detected (LEAD) | Relapse Detected (LEAD + MER) | Total LEAD | Clinical Relapse Detection | Radiographic Relapse Detection | P | Total MER | Clinical Relapse Detection | Radiographic Relapse Detection | P | Total Combined | Clinical Relapse Detection | Radiographic Relapse Detection | P | |
| Total | n = 93 (36.4%) | n = 172 (63.6%) | 148 | 11 = 35 (66%) | n = 18 (34%) | 117 | n = 63 (53.8%) | n = 50 (42.7%) | 265 | n = 98 (59.04%) | n = 68 (40.96%) | |||
| Age#, years | 59.01 [31.50, 83.84] | 58.00 [22.25, 83.84] | 57.54 [22.25, 83.84] | 52.46 [45.42, 64.65] | 60.73 [48.7, 63.46] | 0.453** | 59.00 [35.32, 83.25] | 59 [49, 66] | 60.09 [49, 69.04] | 0.619** | 59.00 [22.25, 83.84] | 57.21 [48, 65] | 60.42 [49, 67.38] | 0.317** |
|
Gender
Male Female |
40 (43%) 53 (57.0%) |
97 (56.4%) 75 (43.6%) |
66 (44.59%) 82 (55.41%) |
14 (40%) 21 (60%) |
10 (55.56%) 8 (44.44%) |
0.281* |
71 (60.70%) 46 (39.30%) |
43 (68.25%) 20 (31.75%) |
26 (52%) 24 (48%) |
0.078* |
137 (51.70%) 128 (48.30%) |
41 (41.84%) 57 (58.16%) |
32 (47.06%) 36 (52.94%) |
0.505* |
|
Ethnicity
White Other |
65 (69.9%) 28 (30.1%) |
150 (88.2%) 20 (11.8%) |
104 (70.27%) 42 (29.73%) |
24 (68.57%) 11 (31.43%) |
14 (77.78%) 4 (22.22%) |
0.539** |
111 (96.50%) 4 (3.50%) |
61 (98.93%) 1 (1.61%) |
47 (94%) 3 (6%) |
0.323** |
215 (81.70%) 48 (18.30%) |
85 (86.73%) 12 (12.24%) |
61 (89.71%) 7 (10.29%) |
0.681* |
|
Stage
1,2 3,4 |
27 (29.3%) 65 (70.7%) |
27 (16.0%) 142 (84.0%) |
36 (25.00%) 108 (75.00%) |
7 (21.88%) 25 (78.13%) |
2 (11.11%) 16 (88.89%) |
0.459** |
18 (15.4%) 99 (84.60%) |
8 (12.7%) 55 (87.3%) |
9 (18%) 41 (82%) |
0.434* |
54 (20.70%) 207 (79.3%) |
15 (15.79%) 80 (84.21%) |
11 (16.18%) 57 (83.82%) |
0.947* |
|
Grade
1,2 3 |
75 (83.3%) 15 (16.7%) |
145 (84.8%) 26 (15.2%) |
119 (82.60%) 25 (17.4%) |
29 (82.86%) 6 (17.14%) |
14 (82.35%) 3 (17.65%) |
1.000** |
101 (86.30%) 16 (13.7%) |
54 (85.71%) 9 (14.29%) |
43 (86%) 7 (14%) |
0.965* |
220 (84.3%) 41 (15.7%) |
83 (84.69%) 15 (15.31%) |
57 (85.07%) 10 (14.93%) |
0.947* |
|
ECOG
0 ≥1 |
43 (47.8%) 47 (52.2%) |
91 (54.4%) 72 (45.2%) |
58 (41.40%) 82 (58.60%) |
8 (25%) 24 (75%) |
7 (43.75%) 9 (56.25%) |
0.186* |
76 (65.50%) 40 (34.5%) |
36 (58.06%) 26 (41.94%) |
36 (72%) 14 (28%) |
0.126* |
134 (52.30%) 122 (47.70%) |
44 (46.81%) 50 (53.19%) |
43 (65.15%) 23 (34.85%) |
0.022 * |
|
FLIPI
Low Intermediate High |
24 (27.9%) 27 (31.4%) 35 (40.7%) |
36 (22.8%) 53 (33.5%) 69 (43.7%) |
31 (24.40%) 40 (31.50%) 56 (44.10%) |
6 (25%) 6 (25%) 12 (50%) |
1 (6.67%) 6 (40%) 8 (53.33%) |
0.018 ** |
29 (24.80%) 40 (34.20%) 48 841.00%) |
15 (23.81%) 24 (38.1%) 24 (38.1%) |
12 (24%) 16 (32%) 22 (44%) |
0.767* |
60 (24.60%) 80 (32.8%) 104 (42.6%) |
21 (24.14%) 30 (34.48%) 36 (41.38%) |
13 (20.00%) 22 (33.85%) 30 (46.15%) |
0.785* |
|
B-Symp.
Yes No |
13 (15.3%) 72 (84.7%) |
31 (18.8%) 134 (81.2%) |
27 (20.00%) 108 (80.00%) |
13 (40.63%) 19 (59.38%) |
1 (5.88%) 16 (94.12%) |
0.453** |
17 (14.80%) 98 (85.20%) |
10 (16.39%) 51 (83.61%) |
7 (14%) 43 (86%) |
0.728* |
44 (17.60%) 206 (82.4%) |
23 (24.73%) 70 (75.27%) |
8 (11.94%) 59 (88.06%) |
0.043 * |
Clinical detection, defined by patient-reported symptoms at the time of confirmed detection of disease, concerning physical exam findings documented during a clinical visit, or abnormal lab values on prior follow-up; surveillance detection, defined by relapse of disease first suggested on imaging reviewed by a radiologist, as part of a routine surveillance schedule, and later confirmed by other diagnostic means; Variables are presented as N (%) unless otherwise noted; p <0.05 are marked in bold.
Abbreviations: Stage, Ann Arbor Staging; ECOG, Eastern Cooperative Oncology Group performance score; FLIPI, follicular lymphoma international prognostic index risk category; B-symp., B-symptoms present on presentation; Bone marrow involved, involvement of bone marrow by follicular lymphoma on presentation; GELF, Groupe d’Etude des Lymphomes Folliculaires score ≥1.
Variables presented as median [range].
Parametric
Non-parametric p-value
The median time from diagnosis to initial treatment was 2 months (range 0–134). Initial treatment included combination chemo-immunotherapy in 73% of patients, single agent rituximab in 16% of patients, and radiation therapy in 12% of patients. Six patients who were treated prior to 2000 received combination chemotherapy without immunotherapy. Among those who received combination chemo-immunotherapy, R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) and BR (bendamustine-rituximab) were the two most common regimens given, administered to 42 (28.4%) and 17 (11.5%) of all treated patients, respectively, followed by VR-CHOP (bortezomib and R-CHOP) administered to 16 (10.8%) patients on a clinical trial30, and R-CVP (rituximab, cyclophosphamide, vincristine sulfate, and prednisone) administered to 12 (8.1%) patients. Eighty-three patients (56.1%) received maintenance therapy.
Method of Relapse Detection in Post-Treatment Patients
Fifty-five (37%) of the 148 included patients experienced a relapse during the surveillance period. The median follow-up for those who did not relapse was 4.3 years (range 0.6–18.3). Of the 55 relapses, 35 (64%) were detected as a result of clinical signs or symptoms, and 18 (33%) cases were detected on routine surveillance imaging in the absence of any other documented clinical suspicion of relapse (Figure 1). Two patients (3%) did not have the method of relapse detection documented. Among the patients with a documented relapse, only FLIPI score (p=0.018) was associated with a difference in method of relapse detection (Table 1).
Figure 1.
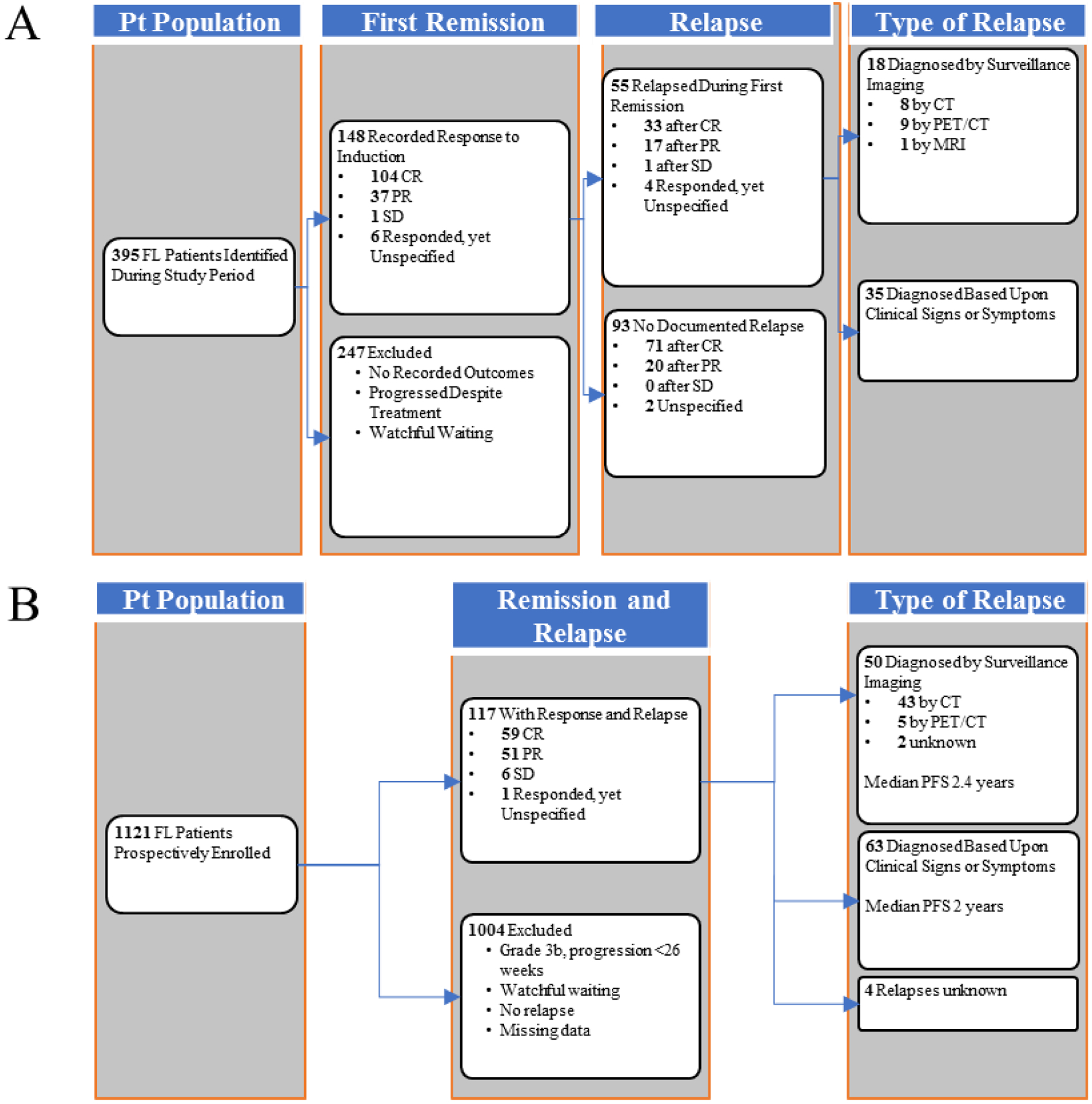
Flowchart of FL patient selection and disease course of selected patients following first remission. (A) LEAD cohort. (B) MER cohort.
Surveillance imaging detection, defined by relapse of disease first suggested on imaging reviewed by a radiologist, as part of a routine surveillance schedule, and later confirmed by other diagnostic means; clinical signs and symptoms. defined by patient-reported symptoms at the time of confirmed detection of disease, concerning physical exam findings documented during a clinical visit, or abnormal lab values on prior follow-up.
Abbreviations: Pt, patient; CR, complete remission; PR, partial response; SD, stable disease; PET/CT, position-emission tomography/computed tomography; MRI, magnetic resonance imaging; PFS, progressionfree survival defined as the time from the date of diagnosis to the first date of clinical evaluation or imaging leading to subsequent relapse detection
Median PFS from the date of diagnosis for those whose first relapse was detected by asymptomatic surveillance imaging was 3.6 years (95% confidence interval[CI] [1.8–4.4]) whereas it was 4.2 years (95% CI [2.5–6]; Table 2) for those whose relapse was detected as a result of clinical signs and symptoms. Three-year PFS was 55.6% and 57.1% for the radiologic surveillance detection group and the clinical detection group, respectively (Table 2). There was no difference in PFS between surveillance imaging and concerning clinical signs/symptoms (HR = 1.37 [95% CI (0.76–2.45)], log rank p = 0.29; Figure 2A]. No clinical variable except age at diagnosis was associated with PFS (HR = 1.02 [95% CI (1.00–1.04), log rank p = 0.07]. After adjusting for age in the multivariable analysis, the association of the type of imaging with PFS was not significant (HR for surveillance = 1.26 [95% CI (0.70–2.26)], p = 0.44; Supplemental Table 1).
Table 2.
PFS KM-probability estimates in FL patients with confirmed relapse, stratified by method of relapse detection
| Cohort | Group | N | Median Survival | 1-Year Survival | 3-Year Survival | 5-Year Survival |
|---|---|---|---|---|---|---|
| LEAD | Clinical Detection | 35 | 4.2 (2.5, 6) | 94.3% (79%, 98.5%) | 57.1% (39.3%, 71.5%) | 40% (24.0%, 55.5%) |
| Surveillance Detection | 18 | 3.6 (1.8, 4.4) | 94.4% (66.6%, 99.2%) | 55.6% (30.5%, 74.8%) | 22.2% (6.9%, 42.9%) | |
| MER | Clinical Detection | 63 | 2.0 (1.6, 2.6) | 88.9% (78.1%, 94.5%) | 33.3% (22.1%, 45.0%) | 9.5% (3.9%, 18.2%) |
| Surveillance Detection | 50 | 2.4 (1.9, 2.8) | 96.0% (84.9%, 99.0%) | 32.0% (19.7%, 45.0%) | 18.0% (8.9%, 29.7%) | |
| Combined | Clinical Detection | 98 | 2.5 (2, 3.2) | 90.8% (83.1%, 95.1%) | 41.8% (32.0%, 51.3%) | 20.4% (13.1%, 28.9%) |
| Surveillance Detection | 68 | 2.5 (1.9, 2.9) | 95.6% (86.9%, 98.6%) | 38.2% (26.8%, 49.6%) | 19.1% (10.8%, 29.2%) |
PFS, progression-free survival, defined as the time from the date of diagnosis to the first date of clinical evaluation or imaging leading to subsequent relapse detection; clinical detection, defined by patient-reported symptoms at the time of confirmed detection of disease, concerning physical exam findings documented during a clinical visit, or abnormal lab values on prior follow-up; surveillance detection, defined by relapse of disease first suggested on imaging reviewed by a radiologist, as part of a routine surveillance schedule, and later confirmed by other diagnostic means.
Variables are presented as N and median survival in years or survival% (95% confidence interval) unless otherwise noted.
Abbreviations: KM, survival probability estimated using the Kaplan-Meir method; N, total number.
Figure 2.
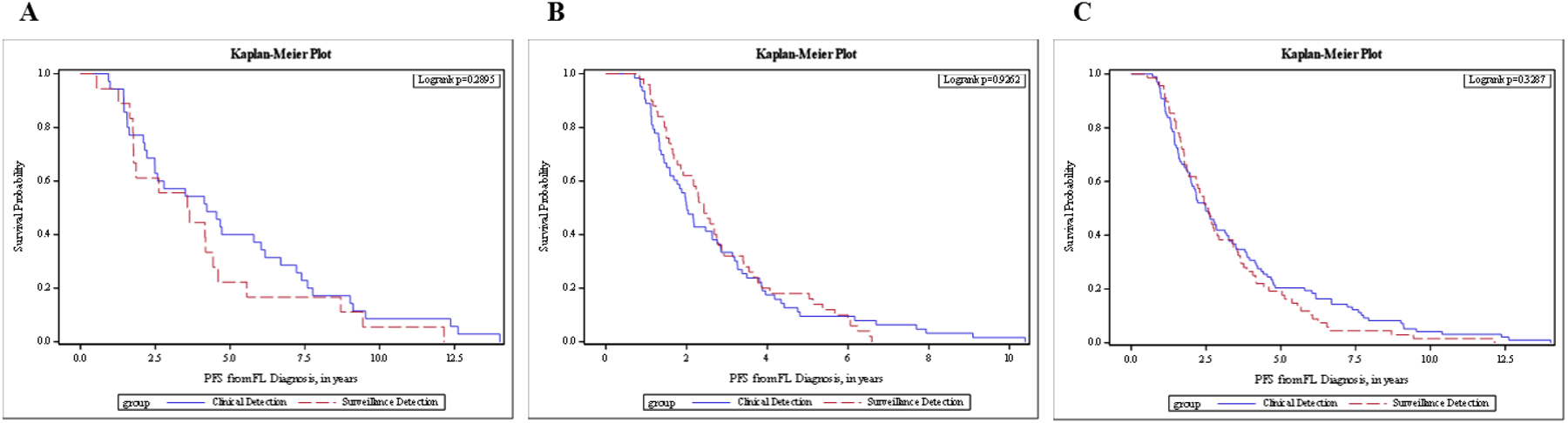
PFS from diagnosis in FL patients with confirmed relapse, stratified by method of relapse detection. (A) LEAD cohort. (B) MER cohort. (C) Combined cohorts.
PFS, progression-free survival, defined as the time from the date of diagnosis to the first date of clinical evaluation or imaging leading to subsequent relapse detection; clinical detection, defined by patient-reported symptoms at the time of confirmed detection of disease, concerning physical exam findings documented during a clinical visit, or abnormal lab values on prior follow-up; surveillance detection, defined by relapse of disease first suggested on imaging reviewed by a radiologist, as part of a routine surveillance schedule, and later confirmed by other diagnostic means.
Similarly, there was no statistically significant difference in OS from the date of diagnosis between those whose first relapse was detected by routine surveillance imaging and those whose first relapse was detected as a result of concerning clinical signs/symptoms in both the univariate analysis (HR=0.61; [95%CI (0.13–2.94), log rank p=0.53; Figure 3A) and after adjusting for age in the multivariable analysis (HR=0.32 [95% CI (0.06 – 1.76)], p=0.19; Supplemental Table 2). Three-year OS was 93.3% for the radiologic surveillance detection group and 97.1% for the clinical detection group (log rank p=0.53; Table 3). No significant difference was found in OS from the date of confirmed relapse to the time of death or last known follow-up (HR for surveillance group 0.46; 95% CI 0.09–2.22; log rank p=0.32; Figure 4A) by univariate analysis and after adjusting for age in the multivariable analysis (HR=0.32 [95% CI (0.06–1.70), p=0.18; Supplemental Table 3).
Figure 3.
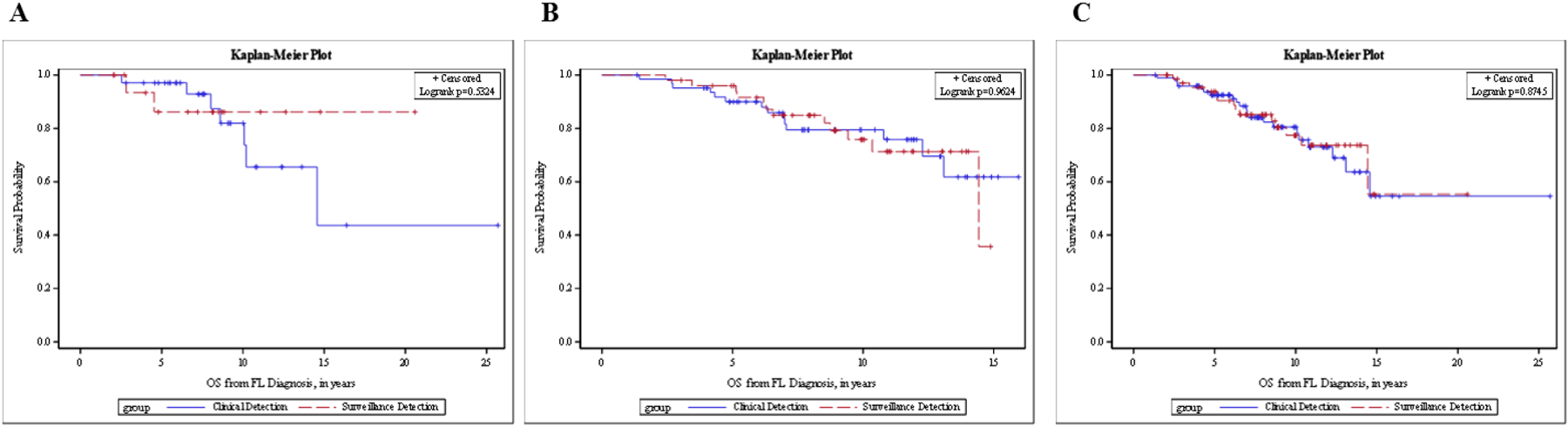
OS from diagnosis in FL patients with confirmed relapse, stratified by method of relapse detection. (A) LEAD cohort. (B) MER cohort. (C) Combined cohorts.
OS, overall survival, defined as the time from the date of diagnosis until the date of death or last known follow-up; clinical detection, defined by patient-reported symptoms at the time of confirmed detection of disease, concerning physical exam findings documented during a clinical visit, or abnormal lab values on prior follow-up; surveillance detection, defined by relapse of disease first suggested on imaging reviewed by a radiologist as part of a routine surveillance schedule, and later confirmed by other diagnostic means.
Table 3.
OS from Diagnosis KM-probability estimates in FL patients with confirmed relapse, stratified by method of relapse detection
| Cohort | Group | N | Events | Censored | 1-Year Survival | 3-Year Survival | 5-Year Survival |
|---|---|---|---|---|---|---|---|
| LEAD | Clinical Detection | 34 | 7 (21%) | 27 (79%) | 100% (NA, NA) | 97.1% (80.9%, 99.6%) | 95.8% (89.1%, 98.4%) |
| Surveillance Detection | 17 | 2 (12%) | 15 (88%) | 100% (NA, NA) | 93.3% (61.3%, 99.0%) | 86.2% (55.0%, 96.4%) | |
| MER | Clinical Detection | 63 | 14 (22%) | 49 (78%) | 100% (NA, NA) | 95.2% (85.7%, 98.4%) | 90.0% (79.0%, 95.4%) |
| Surveillance Detection | 50 | 12 (24%) | 38 (76%) | 100% (NA, NA) | 98.0% (86.6%, 99.7%) | 96.0% (84.8%, 99.0%) | |
| Combined | Clinical Detection | 98 | 21 (21%) | 77 (79%) | 100% (NA, NA) | 88.5% (79.6%, 93.6%) | 83.4% (72.7%, 90.1%) |
| Surveillance Detection | 68 | 14 (21%) | 54 (79%) | 100% (NA, NA) | 91.9% (81.5%, 96.6%) | 85.8% (73.4%, 92.7%) |
OS, overall survival, defined as the time from the date of diagnosis until the date of death or last known follow-up; clinical detection, defined by patient-reported symptoms at the time of confirmed detection of disease, concerning physical exam findings documented during a clinical visit, or abnormal lab values on prior follow-up; surveillance detection, defined by relapse of disease first suggested on imaging reviewed by a radiologist, as part of a routine surveillance schedule, and later confirmed by other diagnostic means.
Variables are presented as N (%) or mean survival% (95% confidence interval) unless otherwise noted.
Abbreviations: KM, survival probability estimated using the Kaplan-Meier method; N, total number.
Figure 4.
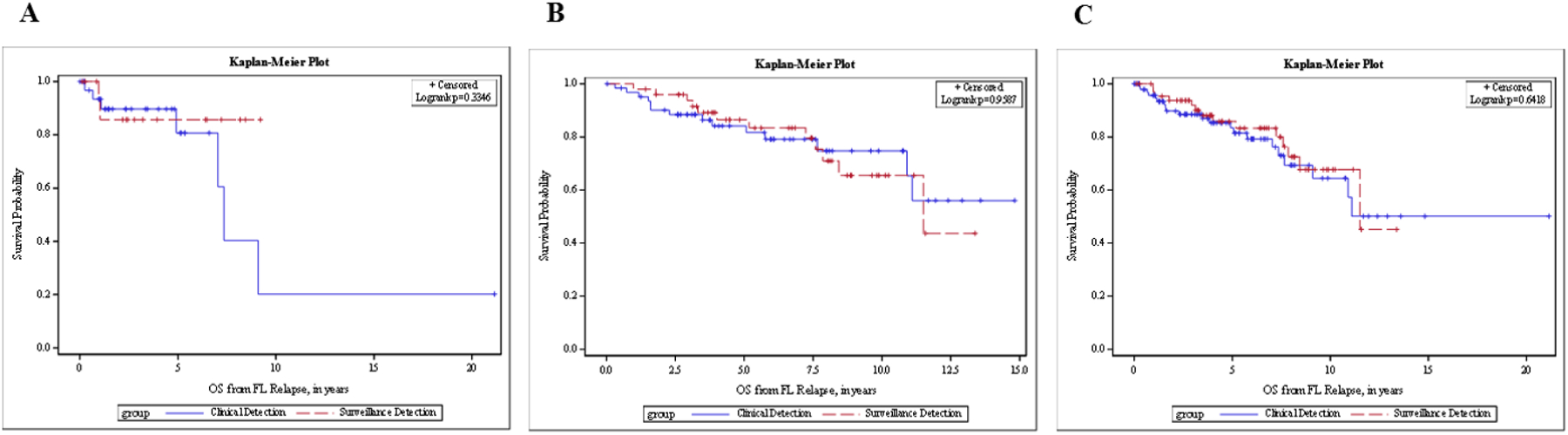
OS from relapse in FL patients with confirmed relapse, stratified by method of relapse detection. (A) LEAD cohort. (B) MER cohort. (C) Combined cohorts.
OS, overall survival, defined as the time from the date of first relapse until the date of death or last known follow-up; clinical detection, defined by patient-reported symptoms at the time of confirmed detection of disease, concerning physical exam findings documented during a clinical visit, or abnormal lab values on prior follow-up; surveillance detection, defined by relapse of disease first suggested on imaging reviewed by a radiologist as part of a routine surveillance schedule, and later confirmed by other diagnostic means.
Individual Imaging Studies
One hundred and thirty-one patients (89%) underwent at least one recorded radiographic imaging study, with a median of 6 recorded studies per patient during the surveillance period (range 1–27). Of 656 total individual imaging studies, 443 (67%) were CT scans, 188 (29%) were PET/CT, 24 (4%) were MRI, and 1 was a mammogram. Seventy-one of 656 scans were performed due to a clinical concern, 584 due to surveillance, and for one scan the indication was not reported. Thirty-two (45%) of the 71 scans completed due to a concern for possible relapse resulted in a confirmation of relapse through biopsy or further imaging. Of the 584 surveillance scans, 68 (11%) were concerning for relapse based on the study impression. Twenty-one of the 68 concerning surveillance scans ultimately resulted in confirmed relapse, showing that only 3.6 % of the 584 surveillance scans led to the identification of a confirmed FL relapse (Figure 5). The total number-needed-to-treat/scan (NNT) to detect one asymptomatic relapse was thus 28 surveillance imaging scans. The surveillance scans had a 91% specificity, 92% sensitivity, 99.6% NPV, and 31% PPV across all imaging modalities. CT scans had a higher specificity than PET/CT scans (95% vs 83%) but a lower sensitivity (88% vs 100%) (Table 5).
Figure 5.
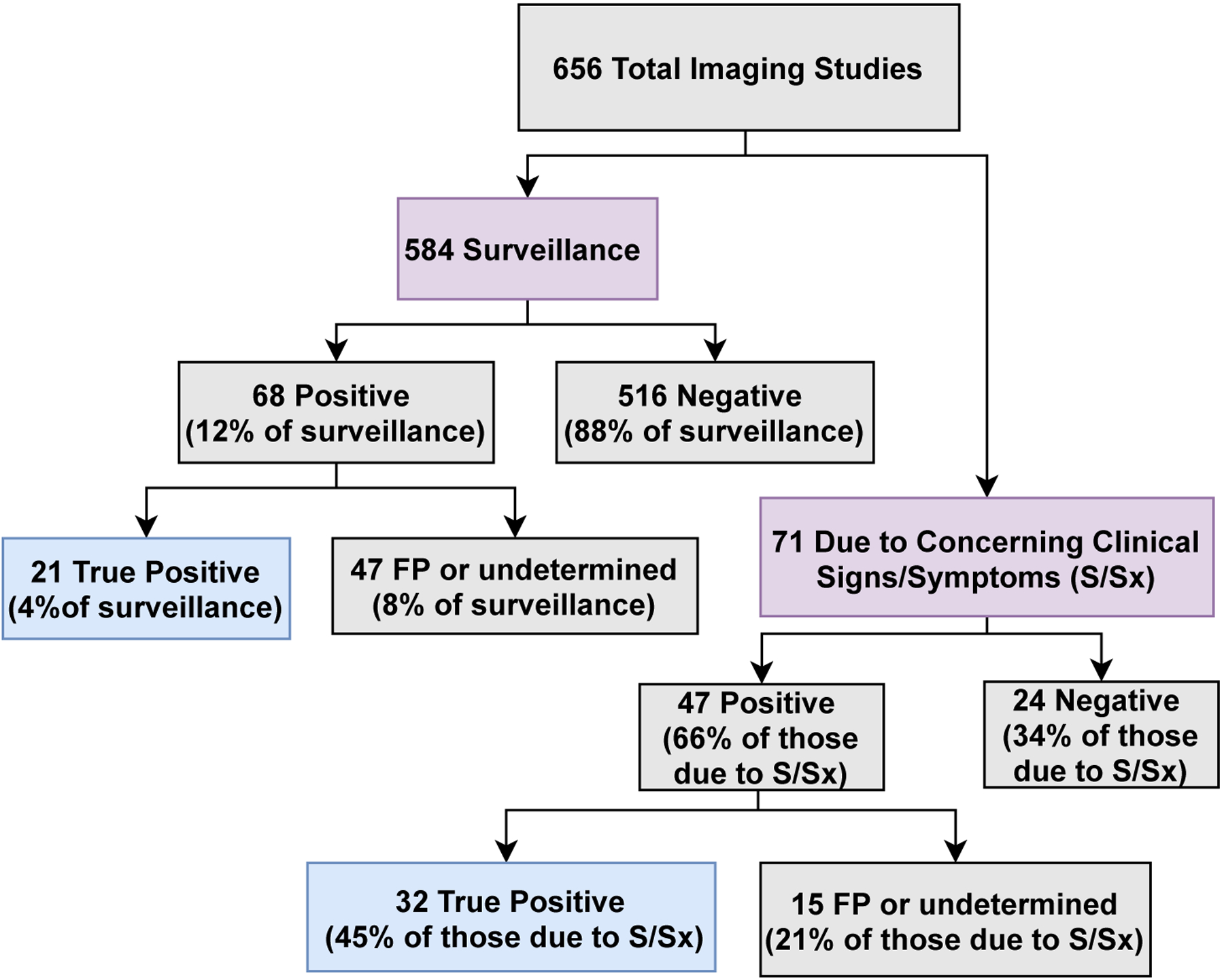
Imaging study indications flowchart for LEAD cohort FL patients in remission after first-line induction therapy
Table 5.
Performance of asymptomatic surveillance scans by radiographic imaging modality within the LEAD cohort.
| Surveillance CT | Surveillance PET | Surveillance Other Imaging | TOTAL | |
|---|---|---|---|---|
| Total Scans | 402 | 160 | 22 | 584 |
| Concerning for Relapse | 29 (7%) | 39 (24%) | 0 (0%) | 68b (12%) |
| Total True Positives | 8 (2%) | 13 (8%) | 0 (0%) | 21 (4%) |
| Sensitivity | 88% | 100% | 0% | 91% |
| Specificity | 95% | 83% | 100% | 92% |
| PPV | 27% | 33% | 0% | 31% |
| NPV | 99.7% | 100% | 100% | 99.6% |
Abbreviations: Concerning for Relapse, radiographic imaging study concerning for relapse as initially read by a radiologist; Total True Positive, imaging study read as positive by radiologist and later confirmed by biopsy or further imaging studies; PET, positron-emission tomography; CT, computed tomography; Other Imaging, includes magnetic resonance imaging and mammography used as part of surveillance for FL; PPV, positive predictive value; NPV, negative predictive value.
MER Cohort
Patient Characteristics
Out of a total of 1121 patients with newly diagnosed FL within MER, 117 met the inclusion criteria for our study and experienced a relapse confirmed by subsequent imaging and/or biopsy during the surveillance period. Of the 117 patients, 71 (61%) were male. Median age at diagnosis was 59.0 years (range 35–83), and 101 (86%) had grade 1–2 disease. Most patients (n=99, 85%) had stage 3–4 disease. Of the 117 patients, 48 (40%) had a high-risk FLIPI score. The median follow-up period for the cohort was 8.9 years (range 1.35–16) from diagnosis. Additional patient characteristics are included in Table 1.
Method of Relapse Detection in Post-Treatment Patients
Within the MER cohort, the median time from diagnosis to first confirmed relapse was 2.17 years (range 0.7–10.4). Of the 117 patients who relapsed, 63 (53.8%) were detected as a result of concerning clinical signs/symptoms, and 50 (42.7%) were detected on routine surveillance imaging in the absence of any other documented clinical suspicion of relapse. Four patients (3.4%) did not have the method of relapse detection documented. With a median follow-up from relapse of 5.8 years (range 0.02–14.82), 26 patients died.
The PFS between those with radiologic surveillance detection and those with clinical detection were not different (HR=0.98 [95% CI (0.67–1.44)], log rank p=0.93, Figure 2B). The median PFS was 2.4 years (95% CI [1.9–2.8]) for the surveillance group vs 2.0 years (95% CI [1.6–2.6]) for those clinically detected (Table 2). There was no statistically significant difference in OS from the date of diagnosis between those whose relapse was detected by routine surveillance imaging and those whose relapse was detected as a result of clinical signs and symptoms (HR 1.02, 95% CI 0.47–2.21, p=0.96; Figure 3B). Three-year OS was 98.0% for the surveillance group and 95.2% for the clinical detection group (Table 3). The HR for OS from diagnosis for surveillance imaging after adjusting for age, ECOG, and FLIPI score was not statistically significant (HR = 1.21 [95% CI (0.54–2.70)], p = 0.65). Additionally, no significant difference was found in OS from the date of confirmed relapse to the time of death or last known follow-up in the univariate analysis (HR 1.02, [95% CI (0.47–2.21)], p=0.96; Figure 4B) and after adjusting for age, ECOG, and FLIPI score (HR = 1.14, 95% CI [0.51–2.54], p=0. 75; Supplemental Table 3).
Combined Cohorts
After validating our initial findings regarding the impact of relapse detection on OS, we subsequently evaluated all relapsed patients in the combined cohorts to further describe PFS and OS outcomes. Among the 117 patients with relapse in the MER cohort and 55 patients with relapse in the LEAD cohort, the median age was 58, and 97 patients (56%) were male. Four patients from MER and 2 patients from LEAD had no information on the type of imaging and were excluded from our analysis, resulting in a total of 68 patients with relapse detected by routine surveillance imaging and 98 by clinical suspicion (n=166). Baseline patient differences between the two methods of relapse detection are described in Table 1.
The median PFS was 2.5 years (95% CI [1.9–2.9]) for surveillance and 2.5 years (95% CI [2.0–3.2]) for clinical detection (Table 2). Based on the univariate analysis, routine surveillance was not statistically significantly associated with PFS (HR = 1.17 [95% CI (0.85–1.60)], log-rank p = 0.33; Figure 2C). Similar results were obtained after adjusting for age, stage, and cohort (HR = 1.05 [95% CI (0.76–1.44)], p = 0.77). Cohort type (LEAD vs MER) was also associated with PFS after adjusting for age, stage, and image type (HR = 0.55 [95% CI (0.38–0.80)], HR p = 0.002). The 3-year OS from diagnosis for the surveillance group was 96.9% [95% CI (88.3–99.2%)] and 95.8% [95% CI (89.3–98.4%)] for those clinically detected (Table 3). Based on OS from relapse, the 3-year survival rate was also comparable (Table 4). Method of relapse detection was not significantly associated with OS from diagnosis or relapse in the multivariable model (Figures 3C and 4C).
Table 4.
OS from Relapse KM-probability estimates in FL patients with confirmed relapse, stratified by method of relapse detection
| Cohort | Group | N | Events | Censored | 1-Year Survival | 3-Year Survival | 5-Year Survival |
|---|---|---|---|---|---|---|---|
| LEAD | Clinical Detection | 34 | 7 (21%) | 27 (79%) | 100% (NA, NA) | 97.1% (80.9%, 99.6%) | 95.8% (89.1%, 98.4%) |
| Surveillance Detection | 17 | 2 (12%) | 15 (88%) | 100% (NA, NA) | 93.3% (61.3%, 99.0%) | 86.2% (55.0%, 96.4%) | |
| MER | Clinical Detection | 63 | 14 (22%) | 49 (78%) | 100% (NA, NA) | 95.2% (85.7%, 98.4%) | 90.0% (79.0%, 95.4%) |
| Surveillance Detection | 50 | 12 (24%) | 38 (76%) | 100% (NA, NA) | 98.0% (86.6%, 99.7%) | 96.0% (84.8%, 99.0%) | |
| Combined | Clinical Detection | 98 | 21 (21%) | 77 (79%) | 95.7% (88.8%, 98.3%) | 88.5% (79.6%, 93.6%) | 83.4% (72.7%, 90.1%) |
| Surveillance Detection | 68 | 14 (21%) | 54 (79%) | 96.9% (88.1%, 99.2%) | 91.9% (81.5%, 96.6%) | 85.8% (73.4%, 92.7%) |
OS, overall survival, defined as the time from the date of first relapse until the date of death or last known follow-up; clinical detection, defined by patient-reported symptoms at the time of confirmed detection of disease, concerning physical exam findings documented during a clinical visit, or abnormal lab values on prior follow-up; surveillance detection, defined by relapse of disease first suggested on imaging reviewed by a radiologist, as part of a routine surveillance schedule, and later confirmed by other diagnostic means.
Variables are presented as N (%) or mean survival% (95% confidence interval) unless otherwise noted.
Abbreviations: KM, survival probability estimated using the Kaplan-Meir method; N, total number.
Discussion:
This study evaluated the survival outcomes of a large cohort of FL patients achieving first remission after induction therapy; we initially investigated the role of asymptomatic surveillance imaging at a major academic medical center and later confirmed our findings in a multi-institutional validation cohort. We conclude that the majority of relapses after first remission are initially suggested by clinical signs and symptoms and that relapse detection via routine surveillance imaging in asymptomatic patients carries no additional survival advantage. The routine, pre-scheduled use of radiographic imaging for surveillance of asymptomatic patients in first remission may therefore need to be reconsidered. Our data suggest that PET and CT scans be used judiciously in FL, and we recommend limiting them to investigations of new clinical findings outside of clinical trials, such as end-of-induction response determination and as a mechanism for relapse confirmation in the presence of concerning clinical signs and symptoms. We recognize that the HR estimates suggest that there may be at least some groups of patients who benefit from a surveillance approach, and future projects should aim to identify these patients whom we feel are a minority of all patients presenting with FL.
While consensus about limiting the use of surveillance scans in NHL exists throughout the literature, most patients with FL continue to receive asymptomatic surveillance imaging.2,12,16,17,24,31 This may be due to the paucity of evidence regarding surveillance during first remission in indolent lymphomas, such as FL. Unlike aggressive lymphomas, FL is not currently treated with curative intent, and most patients will survive for many years, most of which will be without active disease.2,24,25 This study is significant in undertaking the largest retrospective investigation with a validation cohort, to our knowledge, of routine, asymptomatic surveillance imaging in first remission of FL as it relates to survival outcomes.
Of the patients who relapsed during the observation period, roughly 2/3 of patients in both cohorts presented with concerning clinical signs and symptoms that led to relapse detection. While the incidence of relapse detected on asymptomatic surveillance imaging was higher in this study relative to studies of diffuse large B-cell lymphoma, the incidence was consistent with that found in studies of indolent lymphomas.2,13,18,31 In addition, this study found that FL patients with relapse detected on surveillance do not have a significantly improved OS relative to those with relapse detected clinically. There is significant potential for lead-time bias when calculating the OS of FL, as it has an indolent course and can often be safely observed for an extended period of time without initiating chemotherapy immediately upon diagnosis. To mitigate this, we calculated OS from both the time of diagnosis as well as the time of relapse, as the OS calculated from the date of diagnosis to the date of death or last follow-up would be less impacted by lead-time bias. In our study however, while we expected OS post-relapse detection to have been longer in those patients whose relapse was detected by surveillance imaging, we found no difference in OS. This may suggest that our study cohorts were less affected by lead-time bias overall, and thus potentially reinforcing further the lack of benefit with routine surveillance imaging.
Our findings support extending the ASH Choosing Wisely Campaign and ESMO’s call for limiting the number of surveillance scans in asymptomatic NHL during first remission to include indolent lymphomas, although additional studies should be pursued for other NHL cohorts and indolent subtypes.12,15–17 Due to the retrospective nature of this study as well as the small number of patients with relapse detection by surveillance imaging, we were unable to detect a subgroup of patients who might benefit from routine surveillance. However, we found that those with B-symptoms on initial diagnosis were significantly more likely to have clinically significant symptoms on relapse. Given the unlikely eventuality of a randomized controlled trial of investigating the role of surveillance imaging, our understanding of the value of this strategy will rely on a series of retrospective analyses of controlled cohorts such as this one, which may provide greater insight into related individual patient factors that confer a benefit from a particular type of follow-up strategy during remission.
For asymptomatic surveillance imaging in FL, the NNT was 28 scans to detect one confirmed diagnosis of relapse. This finding is a noteworthy consideration when deciding to employ routine surveillance imaging as part of a general follow-up strategy. In addition, across both CT and PET, the PPV of the imaging modalities in FL was low. While PET had superior sensitivity to CT, its specificity was lower. The performance of these imaging studies suggests the non-inferiority of CT relative to PET in relapse detection. While PET is more sensitive, the lower specificity suggests higher rates of false positives. CT and PET scanning do not come without risk, as false positives can lead to heightened anxiety and pursuit of invasive testing, such as biopsy or further radiation exposure.21–23 Furthermore, while cost-benefit analyses were not performed as part of this study, the increasing cost burden to both patients and health systems in pursuing follow-up testing to surveillance imaging, especially in light of such poor PPV and a high NNT, must be considered.13
As part of a retrospective cohort study, our conclusions were limited to observed associations and susceptible to selection bias and confounding variables from a lack of standardized disease management. Differences in acceptable methods of relapse detection and types of therapies available over the expansive observation period likely contributed to these variations. Clinically detected relapses may have theoretically been influenced by a concurrent scan just prior to the scheduled visit. In addition, the types of induction regimens employed, follow-up care, and surveillance strategies were at the discretion of individual physicians. The dissimilarities in the use of scheduled surveillance imaging by different physicians, in the follow-up care of asymptomatic patients further reflects the current lack of a standard approach to managing FL. Given the limitations of our retrospective data collection, we could not capture all imaging studies performed outside the treating institutions. However, it is likely that outside surveillance scans were negative for relapse, as follow-up visits at the treating institutions would have noted progression were it present, and therefore the inclusion of such scans may have further supported our findings. Additionally, while the detection of significant abdominal disease is often an issue in FL, we did not include and analyze serum creatinine values in this study, although patients who would have pursued imaging based on an abnormal lab value and who were found to relapse would have been classified under the clinically detected relapsed group.
Conclusion
In conclusion, most initial relapses of FL are detected as a result of concerning clinical signs or symptoms, and routine surveillance imaging provides no survival benefit. Future multi-centered retrospective cohort studies are needed to reinforce these findings and potentially change current surveillance guidelines after first remission in FL. Investigations of specific prognostic markers to identify higher-risk FL pts who may benefit from surveillance imaging should also be considered. This study provides clinicians with evidence to support the practice of minimizing surveillance imaging in FL.
Supplementary Material
Funding:
We thank the American Society of Hematology and the Williams Family Foundation of Georgia for their generous support of this project through funding via the Abstract Achievement Award and the Travel Award for Innovative Oncology research, respectively.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose related to this manuscript.
References:
- 1.Teras LR, Desantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US Lymphoid Malignancy Statistics by World Health Organization Subtypes. 2016;66(6):443–459. doi: 10.3322/caac.21357. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JB, Flowers CR. Optimal disease surveillance strategies in non-Hodgkin lymphoma. Hematology. 2014;2014(1):481–487. doi: 10.1182/asheducation-2014.1.481 [DOI] [PubMed] [Google Scholar]
- 3.Federico M, Luminari S, Dondi A, et al. R-CVP versus R-CHOP versus R-FM for the initial treatment of patients with advanced-stage follicular lymphoma: Results of the FOLL05 trial conducted by the fondazione italiana linfomi. J Clin Oncol. 2013;31(12):1506–1513. doi: 10.1200/JCO.2012.45.0866 [DOI] [PubMed] [Google Scholar]
- 4.Ghielmini M Follicular lymphoma. Ann Oncol. 2010;21(Supplement 7):151–153. doi: 10.1093/annonc/mdq287 [DOI] [PubMed] [Google Scholar]
- 5.Sarkozy C, Maurer MJ, Link BK, et al. Cause of death in follicular lymphoma in the first decade of the rituximab era: A pooled analysis of French and US cohorts. J Clin Oncol. 2019;37(2):144–152. doi: 10.1200/JCO.18.00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher RI, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23(33):8447–8452. doi: 10.1200/JCO.2005.03.1674 [DOI] [PubMed] [Google Scholar]
- 7.Salles G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet. 2011;377(9759):42–51. doi: 10.1016/S0140-6736(10)62175-7 [DOI] [PubMed] [Google Scholar]
- 8.Nastoupil LJ, Sinha R, Byrtek M, et al. Comparison of the effectiveness of frontline chemoimmunotherapy regimens for follicular lymphoma used in the United States. Leuk Lymphoma. 2015;56(5):1295–1302. doi: 10.3109/10428194.2014.953144 [DOI] [PubMed] [Google Scholar]
- 9.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–1210. doi: 10.1016/S0140-6736(12)61763-2 [DOI] [PubMed] [Google Scholar]
- 10.Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258–1265. doi: 10.1182/blood-2003-12-4434 [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: The lugano classification. J Clin Oncol. 2014;32(27):3059–3067. doi: 10.1200/JCO.2013.54.8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mary Dwyer N, Hema Sundar M, Glenn MJ, et al. NCCN 1.2017 B-cell Lymphomas. 2017.
- 13.Truong Q, Shah N, Knestrick M, et al. Limited utility of surveillance imaging for detecting disease relapse in patients with non-Hodgkin lymphoma in first complete remission. Clin Lymphoma, Myeloma Leuk. 2014;14(1):50–55. doi: 10.1016/j.clml.2013.08.009 [DOI] [PubMed] [Google Scholar]
- 14.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. doi: 10.1200/JCO.2006.09.2403 [DOI] [PubMed] [Google Scholar]
- 15.Dreyling M, Ghielmini M, Rule S, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(August):v83–v90. doi: 10.1093/annonc/mdw400 [DOI] [PubMed] [Google Scholar]
- 16.Ghielmini M, Vitolo U, Kimby E, et al. ESMO guidelines consensus conference on malignant lymphoma 2011 part 1: Diffuse large B-cell lymphoma (DLBCL), Follicular Lymphoma (FL) and Chronic Lymphocytic Leukemia (CLL). Ann Oncol. 2013;24(3):561–576. doi: 10.1093/annonc/mds517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely(R) campaign: five hematologic tests and treatments to question. Blood. 2013;122(24):3879–3883. doi: 10.1182/blood-2013-07-518423 [DOI] [PubMed] [Google Scholar]
- 18.Thompson CA, Ghesquieres H, Maurer MJ, et al. Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. J Clin Oncol. 2014;32(31):3506–3512. doi: 10.1200/JCO.2014.55.7561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Galaly T, Prakash V, Christiansen I, et al. Efficacy of routine surveillance with positron emission tomography/computed tomography in aggressive non-Hodgkin lymphoma in complete remission: Status in a single center. Leuk Lymphoma. 2011;52(4):597–603. doi: 10.3109/10428194.2010.547642 [DOI] [PubMed] [Google Scholar]
- 20.El-Galaly T, Mylam KJ, Bøgsted M, et al. Role of routine imaging in detecting recurrent lymphoma: A review of 258 patients with relapsed aggressive non-Hodgkin and Hodgkin lymphoma. Am J Hematol. 2014;89(6):575–580. doi: 10.1002/ajh.23688 [DOI] [PubMed] [Google Scholar]
- 21.Thompson CA, Charlson ME, Schenkein E, et al. Surveillance CT scans are a source of anxiety and fear of recurrence in long-term lymphoma survivors. Ann Oncol. 2010;21(11):2262–2266. doi: 10.1093/annonc/mdq215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shenoy P, Sinha R, Tumeh JW, Lechowicz MJ, Flowers CR. Surveillance computed tomography scans for patients with lymphoma: is the risk worth the benefits? Clin Lymphoma Myeloma Leuk. 2010;10(4):270–277. doi: 10.3816/CLML.2010.n.056 [DOI] [PubMed] [Google Scholar]
- 23.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–2284. doi: 10.1056/NEJMra072149 [DOI] [PubMed] [Google Scholar]
- 24.Cohen JB, Kurtz DM, Staton AD, Flowers CR. Next-generation surveillance strategies for patients with lymphoma. Futur Oncol. 2015;11(13):1977–1991. doi: 10.2217/fon.15.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armitage JO. Who Benefits From Surveillance Imaging? J Clin Oncol. 2012;30(21):2579–2580. doi: 10.1200/JCO.2012.42.6189 [DOI] [PubMed] [Google Scholar]
- 26.Cerhan JR, Link BK, Habermann TM, et al. Cohort profile: The lymphoma Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource (MER) cohort study. Int J Epidemiol. 2017;46(6):1753–1754. doi: 10.1093/ije/dyx119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brice P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: A randomized study from the Groupe d’Etude des Lymphomes Folliculaires. J Clin Oncol. 1997;15(3):1110–1117. doi: 10.1200/jco.1997.15.3.1110 [DOI] [PubMed] [Google Scholar]
- 28.Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international prognostic index 2: A new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27(27):4555–4562. doi: 10.1200/JCO.2008.21.3991 [DOI] [PubMed] [Google Scholar]
- 29.Liu Y, Nickleach DC, Zhang C, Switchenko JM, Kowalski J. Carrying out streamlined routine data analyses with reports for observational studies: introduction to a series of generic SAS ®macros. F1000Research. 2019;7:1–21. doi: 10.12688/f1000research.16866.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen OC, Counsell N, Rabin N, et al. Bortezomib consolidation post-ASCT as frontline therapy for multiple myeloma deepens disease response and MRD-negative rate whilst maintaining QOL and response to re-treatment at relapse. Br J Haematol. 2019;185(5):948–951. doi: 10.1111/bjh.15649 [DOI] [PubMed] [Google Scholar]
- 31.Guidot DM, Switchenko JM, Nastoupil LJ, et al. Surveillance imaging in mantle cell lymphoma in first remission lacks clinical utility. Leuk Lymphoma. 2017;0(0):1–8. doi: 10.1080/10428194.2017.1361032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


