Abstract
One of the most prevalent malignancies, which have severe effects on women's health, is breast cancer. Quercetin, a flavonoid found in vegetables, tea, and fruits, is known to have bioactive properties, such as anti-inflammatory, anti-oxidant, as well as anti-cancer. Long non-coding RNAs (lncRNAs) have been recognized to function as primary regulators of diverse cellular processes, including differentiation, development, and cell fate. INXS and UCA1 are lncRNAs that are up regulated and down regulated respectively in cancer cells. This research aimed to assess the impact of quercetin on the expression of INXS and UCA1 genes in MCF-7 cells. Various quercetin concentrations at different times were used to treat MCF-7 cells. The cell viability and IC50 values were determined using MTT assay. Then, MCF-7 cells were incubated with various quercetin concentrations for 24, 48, and 72 h. Cell cycle analyses were evaluated by flow cytometry. The levels of INXS and UCA1 gene expression compared with the GAPDH gene at different concentrations of quercetin were quantified using real-time PCR method. Based on the results, quercetin exerted a dose- and time-dependent inhibitory impact on the viability of MCF-7 cells. Furthermore, quercetin induced cell cycle arrest at the G2 phase in MCF-7 cells. Also, quercetin induced INXS upregulation and UCA1 downregulation in the MCF-7 cell line. These data suggest that quercetin might increase cell death by up regulating INXS and down regulating UCA1 lncRNAs in MCF-7 cells.
Key Words: Breast cancer, quercetin, MCF-7 cells, INXS, UCA1
In women, breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death, followed by colorectal and lung cancer for incidence, and vice versa for mortality (1). Several methods exist for treating breast cancer, including chemotherapy and radiotherapy. However, such therapies have certain side effects on healthy cells (2).
It has been assessed that the human genome contains 23,000 long non-coding RNA (lncRNA) genes, which are more abundant than 20,000 protein-coding genes (3). LncRNAs are recognized as a new class of non-coding RNA that are longer than 200 nucleotides (4). They have an essential role in various malignancies. Recent researchers have shown that an array of long non-coding RNAs express abnormally in breast cancer. Although the molecular and the biological functions of lncRNAs remain enigmatic (5). Some of the lncRNAs are involved in the regulation of the apoptosis process. The upregulation of intronic BCL-XS-inducing (INXS) lncRNA results in a momentous activation in caspases 3, 7, and 9 in the intrinsic apoptosis pathway. It may decrease tumor size in vivo, hence have tumor suppressor features (6). Urothelial carcinoma associated 1 (UCA1) lncRNA triggers cell proliferation, migration, invasion, and cell apoptosis inhabitation (7).
Among the anti-cancer and cancer-preventing drugs, flavonoids may interfere with particular phases of the carcinogenic procedure, inhibit cell growth, and induce apoptosis in many kinds of cancer cells (8). One such natural flavonoid is quercetin (C15H10O7) that has multiple biological actions, such as anti-viral, anti-oxidant, anti-inflammatory, anti-cancer, apoptosis-inducing, and cell cycle modulatory and angiogenesis inhibitory impacts. It is abundantly found in diverse plant materials, as well as common foods and drinks, such as onions, apples, broccoli, berries, tomato, kale, cherry, and tea (9). When quercetin is consumed, it passes into the large intestine without any change (10). As in many cases, cancer treatment leads to poor outcomes (11), in the ongoing battle against cancer, the development of new therapeutic strategies remains an essential goal.
In this research, the effect of quercetin on MCF-7 cells viability was investigated. Further, the expression of INXS and UCA1 genes and the cell cycle analysis was determined. Moreover, the association between the INXS and UCA1 gene expressions with the cell cycle status was determined. Accordingly, it was attempted to assess the impacts of quercetin on the expression of INXS and UCA1 lncRNAs genes for the first time.
Materials and methods
Cell culture
The MCF-7 breast cancer cell line (12) was purchased from the National Cell Bank of Iran, Pasteur Institute of Iran, Tehran, Iran (NCBI No: C135). Recently, the MCF-7 cell line has been tested for mycoplasma contamination (13). MCF-7 cells were cultured in RPMI-1640 supplemented with 2 mM glutamine, 10% fetal bovine serum (FBS), and antibiotic (all from Gibco, Scotland), at 37 oC in an incubator with 5% CO2 and 95% O2.
Cell treatment and IC 50 determination
Using MTT assay, the cytotoxicity of quercetin was defined in the MCF-7 cell line. Quercetin (Sigma, USA) was dissolved in DMSO (Sigma, USA), In culture media, the DMSO under no circumstances exceeded 0.1% (v/v) (14). 1 × 104 cells were seeded into 96-well plates in the full growth culture medium. Once every 12 h, the medium was replaced with 100 μL growth medium holding a series of different quercetin concentr- ations (0, 25, 50, 75, 150, and 300 μM) (15, 16) for 24, 48, and 72 h. From each well, the culture medium was detached after a specified period. Then, approximately 100 μL of the full medium with 20 μL MTT solutions were added to every well. The media were removed after 5 h incubation, and the crystals of formazan were solubilized for 10 min with 150 μL DMSO. Then, the formazan quantity was defined from the OD at 570 nm. The values of half-maximal inhibitory concentration (IC50) were defined through probit analysis applying Pharm PCS statistical package (Springer Verlag, New York, NY). To evaluate cell cycle and gene expression, the IC50 concentrations of quercetin were used to treat MCF-7 cells at different times.
Flow cytometric analysis
The viability of cells incubated with quercetin and stained with propidium iodide (PI) was measured using the flow cytometry method. Briefly, floating and trypsinized adherent cells were collected, and the harvested cells were aliquoted up to 1 × 106 cells/100 μL into FACS tubes. The cells were washed twice by adding PBS up to 2 ml, centrifuging at 300 × g for 5 min, and decanting the buffer from the pelleted cells. Then, the cells were re-suspended in 100 µL staining buffer (PBS with 2% bovine serum albumin, 2 mM EDTA, and 2 mM sodium azide). Five microliters of PI staining solution (10 mg/mL) were added to the cells, mixed gently, and incubated on ice for 1 min in the dark. Then, the PI fluorescent cells were measured by flow cytometer instrument (FACSCalibure, BD, USA). The stained cells were gated based on the forward scatter (FSC) vs side scatter (SSC), and then the percentage of PI positive cells was measured on the FL3 channel. The frequency of PI+ cells, and the cell cycle phases was determined using FlowJo software version 10 https://www. flowjo.com/solutions/flowjo).
RNA extraction, cDNA synthesis, and primer design
RNA was isolated using Trizol solution. To remove DNA contamination, the isolated RNA was treated with DNase I enzyme. To evaluate the purity and concentration of the isolated RNA, a photo nanometer at 230, 260, and 280 nm was applied. RNA samples with the A260/A280 and A260/A230 ratios higher than 1.7 were chosen for cDNA synthesis. Up to 1 μg RNA was reversely transcribed into cDNA. cDNA synthesis was performed using the cDNA synthesis kit (Favorgen, Thailand) based on the manufacturer's instruction.
In the present study, the GAPDH gene was used as the reference, and UCA1 and INXS genes were selected as target genes. Primer Express and Gene Runner software v.3.0 (Applied Biosystems, Foster City, CA) were used to design oligonuc-leotide primers and analyze them. Primers were confirmed through BLAST analysis to avoid non-specific PCR product formation (17).
The order of forward and reverse primers for the PCR amplification of UCA1 and INXS transcripts was 5′-TTAGGCTGGCAACCATCAG ATC-3′ and 5′-TGTTGTCCTGGATGCTGGTCT-3′ (amplicon size, 127 bp) and 5′- TGATGTTGA AGGCCCGAGAC-3′ and 5′- AATCCCCAACTG CCACGTTC -3′ (amplicon size, 85 bp), respecti- vely.
The order of forward and reverse primers for GAPDH was 5′- CATGAGAAGTATGACAACAG CCT-3′ and 5′- AGTCCTTCCACGATACCAA AGT -3′, respectively (amplicon size, 113 bp).
Quantitative real-time PCR
The SYBR Green PCR Master Mix (Takara, Japan) was applied to carry out quantitative RT-PCR on the Rotor-Gene 6000 (Corbett Research, Australia) with the thermal-cycling settings of 10 min at 95 oC followed by 40 cycles of 15 s at 95 oC and 1 min at 60 oC. Every complete amplification phase was accompanied by a melting phase. To determine non-specific amplification, melting curves were used in this study. Non-template controls (NTC) were included in each run. By plotting Ct values facing log cDNA concentrations of five serial two-fold dilution, a dynamic range of the target and reference genes were assessed to compute the PCR efficiency. The response efficacy was computed via the equation below: E = [10(-1/slope)–1] (18). The GAPDH gene was selected to normalize target genes’ expression. 2-ΔΔCT was used to calculate the mRNA comparative expression level of each target gene (19).
Statistical analysis
The statistical data, including mean ± SD and correlation coefficients (R2), graph preparation, were performed using Microsoft office excel 2010 software. In this work, the statistical significance of differences was calculated via a Student’s t-test, together with one-way variance analysis. The P value <0.05 was regarded as statistically significant.
Results
Quercetin cytotoxicity and IC 50 determination
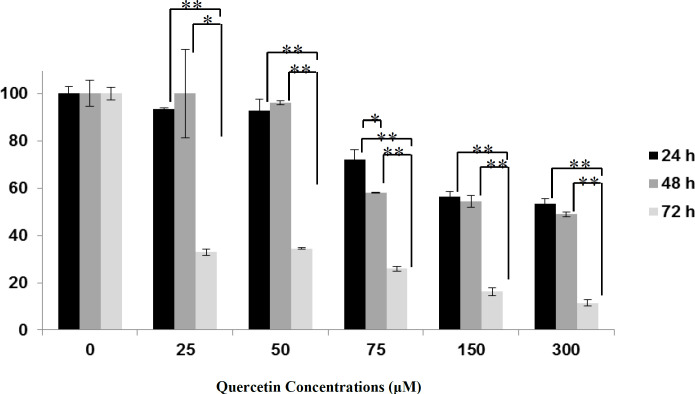
In MCF-7 cells, the cytotoxicity of quercetin was evaluated by MTT assay. Cells were incubated with various quercetin doses for 24, 48, and 72 h. Cytotoxic effects of the quercetin concentrations are illustrated in figure 1. Data show that quercetin decreases the viability of MCF-7 cells dose-dependently. Compared to the controls, the lower dose of quercetin (25 μM) decreased the total cell number about 6.59% ± 0.60 (P> 0.05), 0.11% ± 18.70 (P> 0.05) and 67.87% ± 1.39 (P <0.001) after 24, 48 and 72 h, respectively, while its higher dose (300 μM) decreased the total cell number about 46.76% ± 2.44 (P <0.01), 51.00% ± 1.00 (P <0.01) and 88.64% ± 1.35 (P <0.001) after 24, 48 and 72 h, respectively. For 25, 50, 75, 150, and 300 μM quercetin, significant differences in cell viability were observed at 24 h vs 72 h and 48 h vs 72 h. For 75 μM quercetin significant differences were observed at 24 h vs 48 h after treatment (P <0.05). The IC50 of quercetin after 24, 48, and 72 h was 265 µΜ, 150 µΜ, and 10 µΜ, respectively.
Fig.1.
Concentration- and time-dependent effects of quercetin on MCF-7 cells viability. Results are expressed as the percentage of viability compared with the control, and are presented as mean±SD. Data were analyzed by one-way ANOVA. Significance was set at *P < 0.05, **P < 0.001, *** P < 0.0001
Induction of cell cycle arrest by quercetin
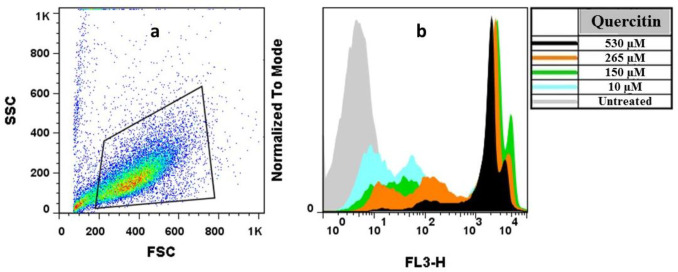
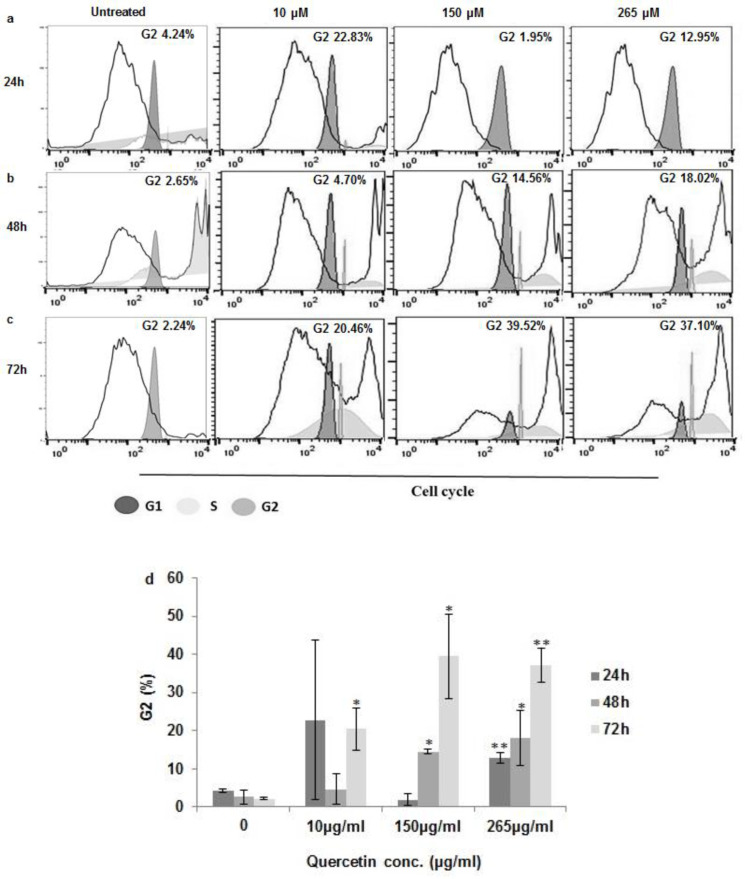
Quercetin has proven multiple biological activities on cell viability and cell cycle. For this purpose quercetin-treated MCF7 was stained with PI and was analyzed via flow cytometry (Figure 2) to examine whether the quercetin-induced toxicity is due to its impact on cell viability and proliferation. The findings showed that quercetin for 24 h did not induce significant changes in MCF-7 cells at 10 and 150 μM, while at 265 μM, a significant increase in the G2/M phase cells was reached (Figure 3a, 3d). Furthermore, quercetin induced significant changes in MCF-7 cells at 150 and 265 μM for 48 h and also significant changes in MCF-7 cells at 10, 150, and 265 μM for 72 h (Figure 3bd).
Fig.2.
Flow cytometry analysis for MCF-7 cells treated by different concentrations of quercetin. a) MCF7 cells were gated based on the forward scatter (FSC) vs. side scatter (SSC); b) the effects of different concentrations of quercetin on cell viability was determined among the gated populations stained with propidium iodide
Fig.3.
Cell cycle arrest by quercetin. MCF-7 cells incubated with various concentrations of quercetin (a) at 24h, (b) 48h and, (c) 72h cells were stained with propidium iodide to determine cell cycle distribution by FACS flow cytometry. No treated cells were used as control. (d) Statistical significances of difference throughout this research were computed using one-way variance analysis. Values with the asterisks are significantly different from the controls *P<0.05, **P<0.001
Real-time PCR validation
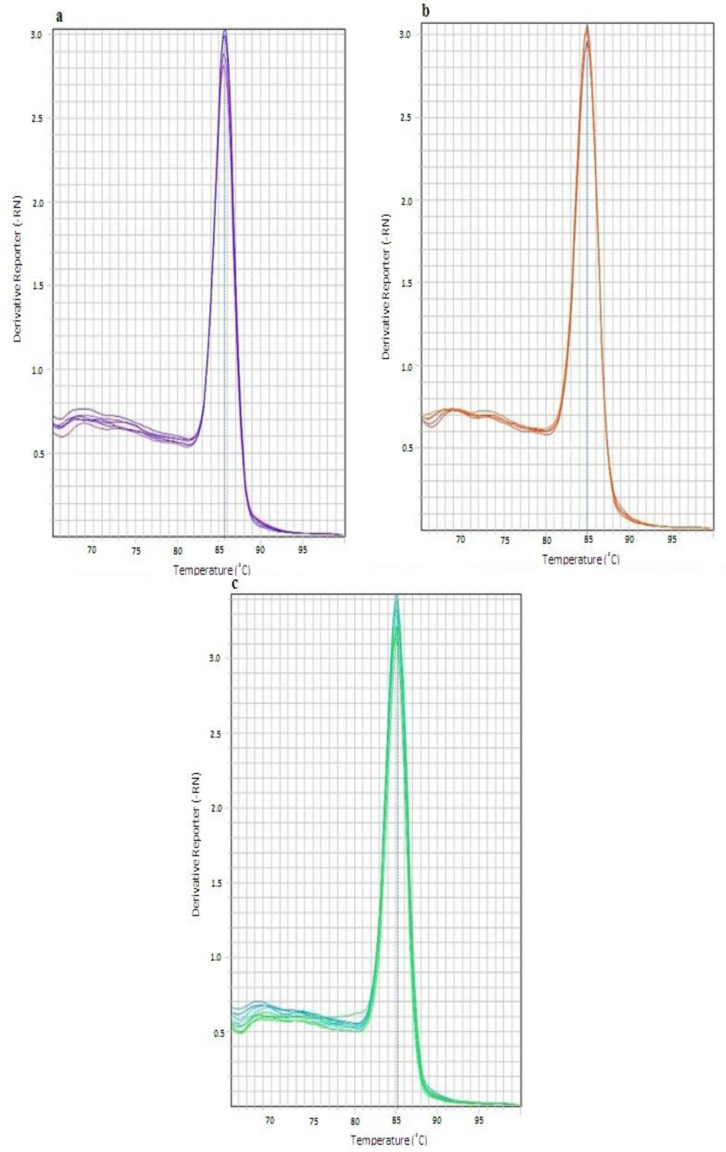
Melting curve analysis was used to confirm the formation of a single amplicon for every gene. The melting peaks were achieved at 85.88°C for the UCA1 gene, 84.93 °C for the INXS gene, and 85.23 for the GAPDH gene (Figure 4)
Fig.4.
The melting analysis of the Real time PCR assays. Melting peaks at (a) 85.88°C for UCA1 gene, (b) 84.93°C for INXS gene and (c) 85.23 for GAPDH gene indicate the formation of the tree specific products with different Tm temperatures
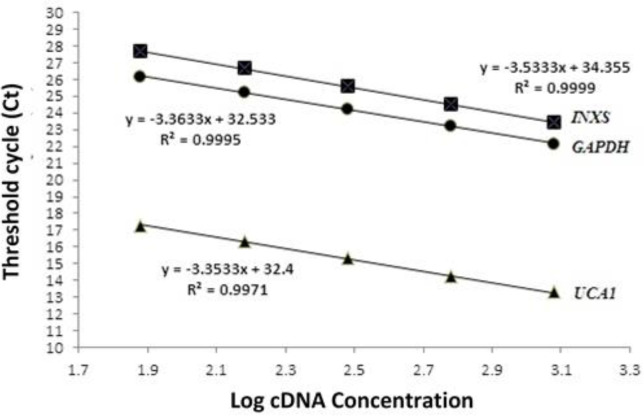
According to the initial data, the expression differences between control and target genes in the treated samples were not more than tenfold. Thus, in the standard curves, two-fold serial dilution was used to confirm the test accuracy. This allowed us to increase the dynamic range resolution. The slope of the standard curves was -3.35, -3.53, and -3.36 for UCA1, INXS, and GAPDH, respectively. The efficiency of PCR was computed as 98.60% for UCA1, 91.86% for INXS, and 98.15% for GAPDH (Figure 5).
Fig.5.
Standard curve for INXS, UCA1 and GAPDH. Standard curve of INXS, slope=−3.53, y-intercept=34.35, R2=0.999. Standard curve of UCA1, slope=−3.35, y-intercept= 32.4, R2=0.997. Standard curve of GAPDH, slope=−3.36, y-intercept= 32.53, R2=0.999
Relative quantification of INXS and UCA1 expression
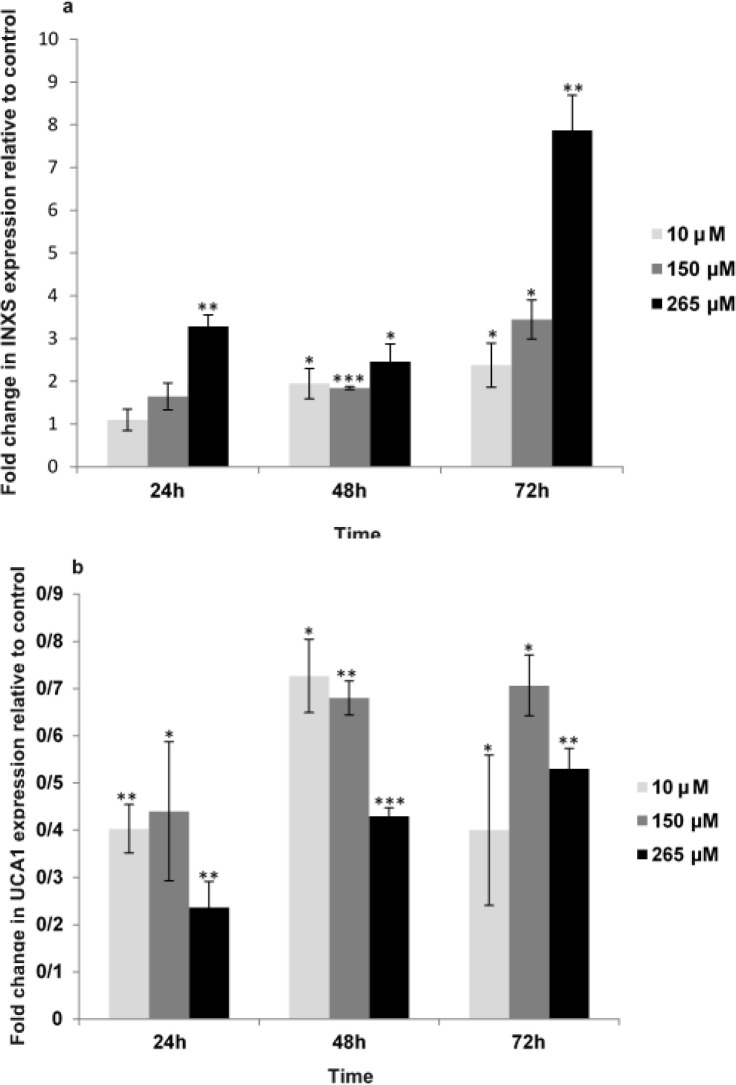
The relative gene expression among two samples (treated and untreated) was defined using the difference in their Ct values. The mCt value for GAPDH was 24.35 ± 0.61 in various quercetin concentrations. The INXS/GAPDH and UCA1/ GAPDH gene expression ratios in MCF-7 cells treated with different quercetin concentrations (10, 150, 265 µΜ) was obtained through the 2-ΔΔCT formula. The most INXS/GAPDH ratio was computed as 7.87 for 72 h (Figure 6a), and the less UCA1/GAPDH ratio was calculated as 0.23 for 24 h (Figure 6b).
Fig.6.
(a) Quercetin effect on LncRNA INXS expression. (b) Quercetin effect on LncRNA UCA1 expression. Bars represent fold differences of the mean of normalized expression values ± SD. Values with asterisk are significantly different from the controls *P<0.05, **P<0.001, *** P<0.001
Discussion
Very little is known about the association between lncRNAs and dietary factors. In this research, INXS and UCA1 lncRNAs expression were investigated in MCF-7 cells treated with quercetin. Our data revealed that lncRNAs levels may change by treatment with quercetin. In a time and dose-dependent manner, quercetin treatment decreased the viability of MCF-7 cells. The induction of G2 phase arrest in the MCF-7 cells was confirmed by flow cytometric analysis. These changes were in association with the upregulation of INXS and the downregulation of UCA1 expressions. To the author's knowledge, the present study is the first to explore the quercetin effects on the expression of INXS and UCA1 lncRNAs. Diet has a crucial role in maintaining a healthy life. Our daily diet may contain some products like flavonoids, which can prevent cancer progression. In recent years, chemists and dietitians found that quercetin is a dietary component and a distinctive bioactive flavonoid, that has various health‐promoting impacts (20). Epidemiologic research has proposed that high flavonoid consumption might correlate with a reduced risk of different types of tumors (21). Quercetin also shows direct pro-apoptotic impacts on cancer cells, which may prevent the progression of many human cancers (20). The anti-cancer effects of quercetin have been documented in many previous investigations involving cell lines and animal models. On the other hand, quercetin has minimal damages or side effects on healthy cells despite its high-toxic impact against tumor cells (21).
Some studies have shown that the incubation of breast cancer cell lines with quercetin leads to G1 phase arrest together with apoptosis (20, 22). Furthermore, quercetin has demonstrated an inhibitory effect on MCF‐7 and MDA‐MB‐231 cells through different mechanisms, including the upregulation of miR‐146a expression and activation of caspase‐3 (23). Quercetin also can cause cell cycle arrest and induce apoptosis by modulating pro-apototic, PI3K/Akt, cyclins and mitogen-activated protein kinase (MAPK) molecular pathways (24). Seo et al. found that, quercetin inhibits the clonogenic survival and proliferation of breast tumor cells. These growth inhibitions were accompanied with an increase in sub-G0/G1 apoptotic populations. Also, quercetin could induce the upregulation of cleaved caspase‐3 and ‐8, resulting in the poly ADP ribose polymerase (PARP) cleavage. However, it did not influence the levels of BAX and BCL‐2, and did not induce apoptosis via the intrinsic apoptosis pathway (25).
In the present study, quercetin decreased cell viability through G2 phase arrest. The investigation by Zhao et al. found that in colon cancer cells, quercetin triggered G2 phase cell cycle arrest. It also induced autophagic cell death via extracellular-signal-regulated kinase stimulation (26, 27). In MDA-MB-231 cells, quercetin induces apoptosis and cell cycle arrest (28). Incubation of 143B cell line with quercetin noticeably led to proliferation inhibition, apoptosis promotion, and G2/M phase cell cycle arrest (29). Quercetin might raise the proportion of cells in the S and G2 phases, and decline the ratio of the G0/G1 phase cells. Down regulation of nuclear factor‐κB and BCL‐2 genes, upregulation of the BAX gene, activation of caspase‐3, and promotion of leukemic cell apoptosis, are other outcomes of quercetin function. On the other hand, it was shown that quercetin might reduce leukemia cell proliferation and promote apoptosis (30). Quercetin was also shown to inhibit cell viability, induce DNA damage , promote G2-M cell cycle arrest, and activate both apoptotic pathways in cervical cancer cells (31).
In the present study, quercetin treatment upregulated the INXS lncRNA, and downregulated the UCA1 lncRNA in the MCF-7 cells. This is the first research to our knowledge, which investigates the effect of quercetin treatment on INXS and UCA1 lncRNAs gene expressions in this cell line. Many reports specify that lncRNAs may regulate apoptosis through different patterns and levels (32). There are a few instances of dietary effects on lncRNAs expression. In a recent study, we have evaluated the expression of MALAT1 and MIAT lncRNA genes in HUVEC cells treated with quercetin. Quercetin treatment reduced the viability of HUVEC cells in a dose- and time-dependent manner. We also observed the downregulation of MALAT1 and MIAT lncRNAs in HUVEC cells treated with quercetin. Thus, this study suggested that quercetin can inhibit angiogenesis via MALAT1 and MIAT downregulation (33). INXS is an lncRNA that can shift the BCL-X alternative splicing from the anti-apoptotic BCL-XL to the pro-apoptotic BCL-XS. INXS up regulation leading to the accumulation of BCL-XS, activation of caspases, and induction of apoptosis (34). The UCA1 lncRNA overexpresses wingless-type MMTV integration site family member 6, enhancing the BAX inhibition via protein kinase B and conferring resistance to cisplatin-induced apoptosis (35, 36). UCA1 silencing in LLC9 and LLC2 cell lines increases tamoxifen drug sensitivity by inducing apoptosis and arresting the G2/M phase cell cycle. Notably, the induced upregulation of UCA1 in T47D and MCF7 breast cancer cells decreases the tamoxifen drug sensitivity (37). Some reports explored how various dietary patterns change the expression of H19 lncRNA that regulates cell proliferation (38, 39). In an additional study, in colorectal cancer, resveratrol was shown to regulate the Wnt/β-catenin signaling pathway by downregulating MALAT1 (40).
Here, we showed that quercetin upregulates the INXS expression and downregulates the UCA1 expression in the MCF-7 cell line. Phytochemicals, such as quercetin with their regulatory effects on lncRNAs, may be helpful as a natural component for different cancer therapies. Significantly, they are essential natural compounds with no signs of side effects or toxicity.
Our study has some limitations as only one human breast cancer cell line was studied. Therefore, the obtained results cannot be generalized to other cell lines, and the clinical outcomes should be interpreted with caution. Also, in the current investigation, we evaluated the expression of INXS and UCA1 lncRNAs without performing a full transcriptome analysis.
Conflict of Interest
The authors state no conflict of interest.
Acknowledgment
The present study was extracted from the thesis of Fatemeh Rezaie (MSc student of Cell and Molecular Biology- Genetic). The authors wish to thank all of the participants in the current research (project no. 25630520972002, Date of approval. 15 JAN 2019). The authors did not receive any specific grant from any funding source.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Tinoco G, Warsch S, Glück S, Avancha K, Montero AJ. Treating breast cancer in the 21st century: emerging biological therapies. Journal of Cancer. 2013;4(2):117. doi: 10.7150/jca.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaaib J F Nassief, Al-Assie A. Simple salting – out method for genomic DNA extraction from whole blood . 2011. [Google Scholar]
- 4.Zhou X-R, Song N, Luo J-Y, Zhai H, Li X-M, Zhao Q, et al. Expression profiles and potential functions of long non-coding RNA in stable angina pectoris patients from Uyghur population of China. Bioscience reports. 2019;39(9) doi: 10.1042/BSR20190364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang T, Hu H, Yan G, Wu T, Liu S, Chen W, et al. Long non-coding RNA and breast cancer. Technology in cancer research & treatment. 2019;18:1533033819843889. doi: 10.1177/1533033819843889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeOcesano-Pereira C, Amaral MS, Parreira KS, et al. Long non-coding RNA INXS is a critical mediator of BCL-XS induced apoptosis. Nucleic Acids Res. 2014;42:8343–55. doi: 10.1093/nar/gku561. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Zhou Y, Chen Y, Ding W, Hua Z, Wang L, Zhu Y, et al. LncRNA UCA1 impacts cell proliferation, invasion, and migration of pancreatic cancer through regulating miR‐96/ FOXO3. Iubmb Life. 2018;70(4):276–90. doi: 10.1002/iub.1699. [DOI] [PubMed] [Google Scholar]
- 8.Hashemzaei M, Delarami Far A, Yari A, Heravi RE, Tabrizian K, Taghdisi SM, et al. Anticancer and apoptosis‑inducing effects of quercetin in vitro and in vivo. Oncology reports. 2017;38(2):819–28. doi: 10.3892/or.2017.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David AVA, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacognosy reviews. 2016;10(20) doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown J. A review of the genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutation Research/Reviews in Genetic Toxicology. 1980;75(3):243–77. doi: 10.1016/0165-1110(80)90029-9. [DOI] [PubMed] [Google Scholar]
- 11.Maeda H, Khatami M. Analyses of repeated failures in cancer therapy for solid tumors: poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clinical and translational medicine. 2018;7(1):1–20. doi: 10.1186/s40169-018-0185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soule H, Vazquez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. Journal of the national cancer institute. 1973;51(5):1409–16. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 13.Kazemiha VM, Azari S, Habibi-Anbouhi M, Amanzadeh A, Bonakdar S, Shokrgozar MA, et al. Effectiveness of Plasmocure™ in Elimination of Mycoplasma Species from Contaminated Cell Cultures: A Comparative Study versus Other Antibiotics. Cell Journal (Yakhteh). 2019;21(2) doi: 10.22074/cellj.2019.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mokhtari MJ, Motamed N, Shokrgozar MA. Evaluation of silibinin on the viability, migration and adhesion of thehuman prostate adenocarcinoma (PC‐3) cell line. Cell biology international. 2008;32(8):888–92. doi: 10.1016/j.cellbi.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Kittl M, Beyreis M, Tumurkhuu M, Fuerst J, Helm K, Pitschmann A, et al. Quercetin stimulates insulin secretion and reduces the viability of rat INS-1 beta-cells. Cellular Physiology and Biochemistry. 2016;39(1):278–93. doi: 10.1159/000445623. [DOI] [PubMed] [Google Scholar]
- 16.Pereira DF, Cazarolli LH, Lavado C, Mengatto V, Figueiredo MSRB, Guedes A, et al. Effects of flavonoids on α-glucosidase activity: potential targets for glucose homeostasis. Nutrition. 2011;27(11-12):1161–7. doi: 10.1016/j.nut.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Bustin SA, Beaulieu J-F, Huggett J, Jaggi R, Kibenge FS, Olsvik PA, et al. MIQE precis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BioMed Central. 2010 doi: 10.1186/1471-2199-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mokhtari MJ, Koohpeima F, Mohammadi H. A comparison inhibitory effects of cisplatin and MNPs‐PEG‐cisplatin on the adhesion capacity of bone metastatic breast cancer. Chemical Biology & Drug Design. 2017;90(4):618–28. doi: 10.1111/cbdd.12985. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Rauf A, Imran M, Khan IA, ur‐Rehman M, Gilani SA, Mehmood Z, et al. Anticancer potential of quercetin: A comprehensive review. Phytotherapy Research. 2018;32(11):2109–30. doi: 10.1002/ptr.6155. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez- García C, Sánchez-Quesada C, Gaforio JJ. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants. 2019;8(5) doi: 10.3390/antiox8050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao H, Bao X, Zhu J, Qu J, Sun Y, Ma X, et al. O-Alkylated derivatives of quercetin induce apoptosis of MCF-7 cells via a caspase-independent mitochondrial pathway. Chemico-biological interactions. 2015;242:91–8. doi: 10.1016/j.cbi.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Tao S-f, He H-f, Chen Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Molecular and cellular biochemistry. 2015;402(1-2):93–100. doi: 10.1007/s11010-014-2317-7. [DOI] [PubMed] [Google Scholar]
- 24.Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. International journal of molecular sciences. 2019;20(13):3177. doi: 10.3390/ijms20133177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seo H-S, Ku JM, Choi H-S, Choi YK, Woo J-K, Kim M, et al. Quercetin induces caspase-dependent extrinsic apoptosis through inhibition of signal transducer and activator of transcription 3 signaling in HER2-overexpressing BT-474 breast cancer cells. Oncology reports. 2016;36(1):31–42. doi: 10.3892/or.2016.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J, Liu J, Wei T, Ma X, Cheng Q, Huo S, et al. Quercetin-loaded nanomicelles to circumvent human castration-resistant prostate cancer in vitro and in vivo. Nanoscale. 2016;8(9):5126–38. doi: 10.1039/c5nr08966b. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Fan D, Zheng ZP, Li ET, Chen F, Cheng KW, et al. 8‐C‐(E‐phenylethenyl) quercetin from onion/beef soup induces autophagic cell death in colon cancer cells through ERK activation. Molecular nutrition & food research. 2017;61(2):1600437. doi: 10.1002/mnfr.201600437. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen LT, Lee Y-H, Sharma AR, Park J-B, Jagga S, Sharma G, et al. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. The Korean journal of physiology & pharmacology. 2017;21(2):205–13. doi: 10.4196/kjpp.2017.21.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berndt K, Campanile C, Muff R, Strehler E, Born W, Fuchs B. Evaluation of quercetin as a potential drug in osteosarcoma treatment. Anticancer research. 2013;33(4):1297–306. [PubMed] [Google Scholar]
- 30.Han Y-Q, Hong Y, Su X-L, Wang J-R. Quercetin enhances the anti-leukemic effect of adriamycin. Zhongguo shi yan xue ye xue za zhi. 2014;22(6):1555–60. doi: 10.7534/j.issn.1009-2137.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Kedhari Sundaram M, Raina R, Afroze N, Bajbouj K, Hamad M, Haque S, et al. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Bioscience reports. 2019;39(8):BSR20190720. doi: 10.1042/BSR20190720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su Y, Wu H, Pavlosky A, Zou L-L, Deng X, Zhang Z-X, et al. Regulatory non-coding RNA: new instruments in the orchestration of cell death. Cell death & disease. 2016;7(8):e2333. doi: 10.1038/cddis.2016.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esteghlal S, Mokhtari MJ, Beyzaei Z. Quercetin can inhibit angiogenesis via the down regulation of MALAT1 and MIAT LncRNAs in human umbilical vein endothelial cells. International Journal of Preventive Medicine. 2021;12(1):59. doi: 10.4103/ijpvm.IJPVM_103_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeOcesano-Pereira C, Amaral MS, Parreira KS, Ayupe AC, Jacysyn JF, Amarante-Mendes GP, et al. Long non-coding RNA INXS is a critical mediator of BCL-XS induced apoptosis. Nucleic acids research. 2014;42(13):8343–55. doi: 10.1093/nar/gku561. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Zhang X, Xie K, Zhou H, Wu Y, Li C, Liu Y, et al. Role of non-coding RNAs and RNA modifiers in cancer therapy resistance. Molecular cancer. 2020;19(1):1–26. doi: 10.1186/s12943-020-01171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, et al. Long non‐coding RNA UCA 1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. The FEBS journal. 2014;281(7):1750–8. doi: 10.1111/febs.12737. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Yu D, Li H, Lv Y, Li S. Long non‑coding RNA UCA1 confers tamoxifen resistance in breast cancer endocrinotherapy through regulation of the EZH2/p21 axis and the PI3K/AKT signaling pathway. International journal of oncology. 2019;54(3):1033–42. doi: 10.3892/ijo.2019.4679. [DOI] [PubMed] [Google Scholar]
- 38.Lin Y, Zhuo Y, Fang Z-f, Che L-q, Wu D. Effect of maternal dietary energy types on placenta nutrient transporter gene expressions and intrauterine fetal growth in rats. Nutrition. 2012;28(10):1037–43. doi: 10.1016/j.nut.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Zhong X, Huang S, Liu D, Jiang Z, Jin Q, Li C, et al. Galangin promotes cell apoptosis through suppression of H19 expression in hepatocellular carcinoma cells. Cancer medicine. 2020;9(15):5546–57. doi: 10.1002/cam4.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L, et al. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PLoS One. 2013;8(11):e78700. doi: 10.1371/journal.pone.0078700. [DOI] [PMC free article] [PubMed] [Google Scholar]