Abstract
Patient: Male, 46-year-old
Final Diagnosis: Ectopic metastatic calcification
Symptoms: Left shoulder pain
Medication: —
Clinical Procedure: Decrease serum phosphate levels
Specialty: Nephrology • Orthopedics and Traumatology
Objective:
Unknown etiology
Background:
Non-specific pain of connective tissues and joints is one of the most frequently expressed patient concerns in everyday practice. The most common cause is osteo-degenerative changes in the cartilage and/or joint system. Metastatic calcification is a rare and initially often overlooked cause of persistent, therapy-resistant pain of connective tissues and joint apparatus in end-stage renal disease (ESRD) patients on dialysis therapy. These calcifications are induced by persistent hyperphosphatemia/hyperparathyroidism and can occur in various organs, including joints, tendons, heart valves, soft tissues, and blood vessels.
Case Report:
We report on a 46-year-old male patient with ESRD due to cANCA-associated systemic vasculitis. The patient evolved unfavorably to end-stage renal failure and started continuous ambulatory peritoneal dialysis (CAPD). Four years after initiation of CAPD, the patient reported having painful motion of the left shoulder, and symptomatic physiotherapy and non-steroidal-anti-inflammatory-drugs (NSAIDs) were prescribed. An X-ray examination of the left shoulder showed severe periarticular calcifications. Repeated nutritional counselling was offered, and intensive phosphate-binder therapy was administered, resulting in a reduction in phosphate levels from 2.10 mmol at the time of diagnosis to 1.26 mmol/l 16 months later. Radiological reevaluation showed a near complete resolution of the periarticular calcifications.
Conclusions:
Metastatic calcifications may arise in ESRD patients despite only moderately elevated blood phosphate levels. Intensive measures to reduce the phosphate load to normal levels should be implemented and can lead to almost complete resolution of ectopic calcifications in affected patients.
Keywords: Calcification of Joints and Arteries; Dialysis; Hyperphosphatemia; Peritoneal Dialysis, Continuous Ambulatory; Shoulder Dystocia; Shoulder Pain
Background
As a result of decreased renal phosphate excretion due to reduced nephron mass, severe hyperphosphatemia can arise in patients with end-stage renal disease. Therefore, all such patients routinely receive specialized nutritional counselling to reduce the daily phosphate load, mainly due to intake of dairy products or processed food. Additionally, oral phosphate binders are prescribed to reduce gastrointestinal phosphate up-take. In ESRD patients with sustained hyperphosphatemia, metastatic calcifications can occur in various organs, including joints, tendons, heart valves, and blood vessels, necessitating strict measures to reduce the blood phosphate levels.
Case Report
A 46-year-old male patient had ESRD due to cANCA-associated systemic vasculitis with rapid-progressive glomerulonephritis. Despite intensive immunomodulatory therapy with plasma-pheresis, steroid boluses, and cyclophosphamide, renal failure developed and the patient was started on continuous ambulatory peritoneal dialysis. Over time, residual kidney function declined and phosphate levels rose from 1.07 to about 1.20 mmol/l at the time of radiologic examination. Four years after initiation of CAPD, the patient reported having painful motion of the left shoulder, and symptomatic physiotherapy and NSAIDs were prescribed. Continuing pain prompted an X-ray examination of the left shoulder, showing severe ectopic or metastatic periarticular calcifications (Figure 1). At the time of diagnosis, the following laboratory values were remarkable: calcium 2.38 mmol/L, phosphate 2.10 mmol/L, and parathyroid hormone 283 ng/L. Secondary hyperparathyroidism was controlled with oral calcidiol therapy 10 000 IU per week and 25-OH-D3 levels were 36 nmol/l at the time of diagnoses, but 1-25-OH-D3 levels were not checked. Nevertheless, the patient was not receiving active vitamin D3 therapy. The patient was offered repeated nutritional counselling, and phosphate-binder therapy was intensified using different combinations of phosphate binders, including lanthanum-carbonate, calcium-acetate and magnesium-carbonate, and sevelamer-carbonate. This was done to find the most effective regimen, as the patient reported adverse effects as nausea, constipation, and abdominal pain when maximum dosages of single phosphate binders were prescribed. In addition to supportive pain medication and physiotherapy, he was seen by an orthopedic specialist, but invasive therapies such as local injections of anesthetics were not performed. The estimated glomerular filtration rate approximated less than 5 ml/min/1.73 m2 BSA and the patient was switched to 3-times-weekly hemodialysis due to repeated volume overload with hypertension despite a weekly Kt/V of 1.71 on CAPD. The dialysis regimen prescribed consisted of 3-times-weekly sessions, each 270 min long, resulting in a Kt/V of 1.45. The procedure was performed using post-dilutional online hemodiafiltration with a mean volume of 12–16 liters. This regimen, most presumably the switch to hemodialysis, resulted in a marked decrease in phosphate levels, which were controlled thereafter at normal ranges with a mean value of 1.26 mmol/L. Calcium and parathyroid hormone levels remained stable over time, at 2.29 mmol/L and 255 ng/l, respectively. Over the following 16 months, the symptoms gradually improved and finally resolved completely. Radiological reevaluation showed nearly complete resolution of the periarticular calcifications (Figure 2).
Figure 1.
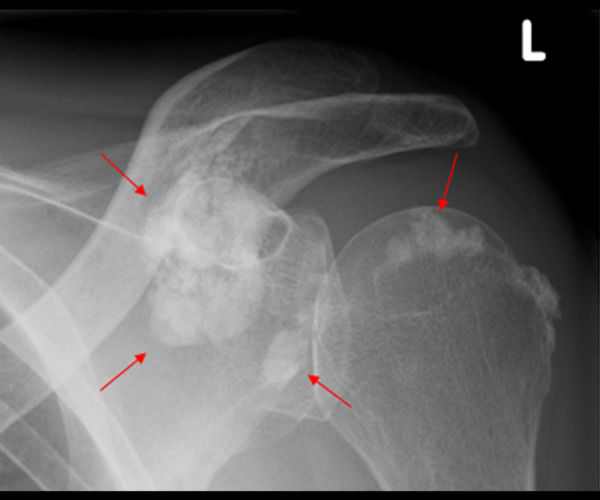
A 46-year-old male patient with ESRD and painful motion of the left shoulder. X-ray examination showed metastatic calcification (red arrows) around the joint structures of the left shoulder.
Figure 2.
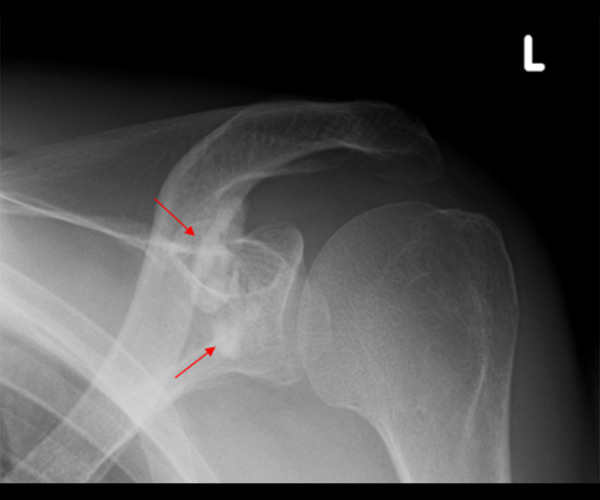
A 46-year-old male patient with ESRD after sustained lowering of phosphate levels to normal levels. The X-ray examination showed markedly reduced metastatic calcification (red arrows) around the joint structures of the left shoulder.
Discussion
Soft-tissue calcifications in uremic patients can occur in several different anatomical locations, including arterial, ocular, subcutaneous, valvula, visceral, and periarticular [1,2]. It is postulated that this disease entity is associated with an increase calcium-phosphate-product and elevated PTH levels in ESRD patients. The reason for the preponderance of certain anatomical regions over others in different patients is still unclear, but the syndrome can lead to disastrous consequences, including paraparesis, if detected late in the course and not treated adequately [3]. Calcifications can arise rapidly within several months, such that negative radiological evaluations in the past medical history cannot reliably rule out de novo ectopic deposition of calcium deposits [4]. In extremely rare cases, ectopic tumoral calcinosis has been described in multiple distinct locations in the same patient [2] and concomitantly with calciphylaxis [5]. Nevertheless, in contrast to calciphylaxis, ectopic calcifications are not associated with warfarin therapy. Few ESRD patients develop ectopic calcification, and this might be due to be a complex interplay of metabolic interactions predisposing certain individuals to this process. Most cases of metastatic calcifications are described in individuals with very high parathyroid hormone levels [6]. In this regard, the case presented here differs in that the patient showed only moderately elevated phosphate levels and normal calcium and PTH levels within recommended target ranges, suggesting other relevant pathophysiological mechanisms. Although high phosphate levels seem to be more associated with ectopic calcifications [7], lower levels of 2.48 to 2.07 mmol/l have also been reported [8,9]. One explanation might be a possible reduced appetite due to chronic pain or adverse effects of pain medication in these patients. We could not detect any predisposing factors to develop ec-topic calcifications, such as prior trauma or surgical procedures, in our patient. Besides these parameters, novel models assume that alterations of vascular smooth muscle cells can have an additive effect in this distinct disorder. Cell cultures of smooth muscle cells can be induced to mineralize by elevating the phosphate levels within the culture milieu [10], thereby adding an active process on the cellular level to the simple model of mineral ion oversaturation and precipitation. Further research has shed some light on a complex interplay of different factors as inducers or inhibitors of the calcification process. Here, 2 different proteins, matrix Gla protein and serum fetuin-A, have been shown to be potent intrinsic inhibitors of calcification [11]. Although, the role of these factors in this case report must remain undetermined, the fact that immediate and sustained lowering of phosphate levels to normal range levels effectively reversed ectopic calcifications is clinically reassuring. The basic approaches used to treat hyperphosphatemia in ESRD patients are changes and/or adaptations in lifestyle and diet followed by the prescription of phosphate binders as tolerated. Furthermore, these measures are not only important in reducing the risk for ectopic calcifications, but also in improving the known high cardiovascular mortality risk in these vulnerable patients. Given the potential catastrophic consequences of this disorder, intensifying the ESRD regimen by switching, as in this case, from CAPD to HD or even emergent transplant might be justified. Nevertheless, this case also demonstrates the overall benefit of simple dietary and medicinal measures, such as increasing the dose of phosphate binders and switching to hemodialysis, in the treatment armamentarium of metastatic calcifications. Therapeutically, such conservative non-operative measures are preferred over operative procedures, as these might lead to non-healing wounds with increased risk of infections.
Conclusions
Metastatic or ectopic calcifications can arise in ESRD patients despite only moderately elevated blood phosphate levels. In cases with unexplained symptoms, metastatic calcifications should be considered as a differential diagnosis, even in patients with moderately elevated blood phosphate levels. Intensive measures to reduce the phosphate load to normal levels should be implemented. These measures may include switching to more intensive ESRD treatment regimens, repeated dietary counselling, high-dose phosphate-binder therapy, and, ultimately, renal transplantation. Importantly, operative measures or surgical re-section of the calcified tissues should be postponed if possible.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Oxlund CS, Hansen H, Hansen S, Rohold A. Progressive valvular calcifications with critical aortic stenosis in a 25-year-old woman with end-stage renal disease on haemodialysis: A case report. Eur Heart J Case Rep. 2021;5:ytab061. doi: 10.1093/ehjcr/ytab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labidi J, Ben Ariba Y, Ben Gabsia A, et al. Severe metastatic calcifications in a hemodialysis patient. Saudi J Kidney Dis Transpl. 2016;27:1037–42. doi: 10.4103/1319-2442.190884. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R, Burns RR, Vergne-Marini P. Paraparesis due to massive ectopic paravertebral calcification in a patient on maintenance hemodialysis. Am J Kidney Dis. 1993;22:717–20. doi: 10.1016/s0272-6386(12)80436-7. [DOI] [PubMed] [Google Scholar]
- 4.Cowlam TE, Bucknall TE. Cutaneous ectopic breast calcification in a haemodialysis patient. Breast. 2003;12:342–44. doi: 10.1016/s0960-9776(03)00104-8. [DOI] [PubMed] [Google Scholar]
- 5.Elghobashy M, Vaquas S, Elshafie M, et al. Unusual presentation of mam-mary calciphylaxis in a patient on long-standing renal dialysis. Pathobiology. 2020;87:317–21. doi: 10.1159/000508537. [DOI] [PubMed] [Google Scholar]
- 6.Kawase K, Takagi K, et al. A case of temporary metastatic pulmonary calcification in a patient with hyperparathyroidism on peritoneal dialysis. Clin Nephrol. 2021;95:161–65. doi: 10.5414/CN110337. [DOI] [PubMed] [Google Scholar]
- 7.Shpilberg KA, Blowe SEb, Som PM. Mass-like and extensive secondary tumoral calcinosis in the neck and body of a patient on peritoneal dialysis. Clin Imaging. 2013;37:972–75. doi: 10.1016/j.clinimag.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Pečovnik-Balon B, Kramberger S. Tumoral calcinosis in patients on hemo-dialysis. Am J Nephrol. 1997;17:93–95. doi: 10.1159/000169078. [DOI] [PubMed] [Google Scholar]
- 9.Pan CW, Chen RF. Tumoral calcinosis in the neck region involving an unusual site in a hemodialysis patient. Laryngoscope. 2016;126:E196–98. doi: 10.1002/lary.25794. [DOI] [PubMed] [Google Scholar]
- 10.Abbasian N. Vascular calcification mechanisms: Updates and renewed insight into signaling pathways involved in high phosphate-mediated vascular smooth muscle cell calcification. Biomedicines. 2021;9:804. doi: 10.3390/biomedicines9070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahalane RM, Barrett HE, Ross AM, et al. On the association between circulating biomarkers and atherosclerotic calcification in a cohort of arterial disease participants. Nutr Metab Cardiovasc Dis. 2021;31:1533–41. doi: 10.1016/j.numecd.2021.02.005. [DOI] [PubMed] [Google Scholar]


