Abstract
Background
Coronavirus disease 2019 (COVID-19) is a new health problem discovered in 2019 thus requires biomarkers that can detect early tissue damage. Soluble receptor for advanced glycation end-products (sRAGE) is a biomarker that can be used to identify early lung damage.
Objective
Analyzing the association of serum sRAGE on COVID-19 severity.
Methods
This study employed a cross-sectional design with a consecutive sampling method. It was conducted from May 2020–October 2021. The number of participants in this study was 145 participants which were divided into 2 groups (non-severe = 47 and severe = 98). Association of sRAGE serum on COVID-19 severity was analyzed using the chi-square test, Fisher's exact test, independence t-test, Mann Withney test, and Spearman's rank test with p-value <0.05.
Results
The results of blood analysis showed several blood components such as leukocytes (9896.51 ± 4949.64/μL; z = 2.431; p = 0.015), lymphocytes (13.55 ± 8.48%; z = 2.256; p = 0.024), neutrophils (78.91 ± 10.50%; z = 2.464; p = 0.014), procalcitonin (0.92 ± 3.22 ng/mL; z = 3.323; p = 0.001), CRP (8.59 ± 7.62 mg/L; z = 2.114; p = 0.034), D-dimer (4360.29 ± 7797.81 ng/mL; z = 2.186; p = 0.029), and fibrinogen (474.58 ± 168.90 mg/dL; t = 0.383; p = 0.703). There was a significant comparison in serum sRAGE values in the non-severe group (0.78 [0.63–1.00] ng/mL) and severe group (1.47 [0.97–2.25] ng/mL; r = 7.154; p <0.001). There was a significant association between serum sRAGE and COVID-19 severity (r = 0.598; p <0.001). The cut-off value for serum sRAGE between the severe and non-severe groups was 0.985 ng/mL. This study obtained sensitivity of 73.5%, specificity of 74.5% OR 8.077 and AUC 0.868 95% CI.
Conclusion
There is a significant association between serum sRAGE and COVID-19 severity and there is also a significant difference in serum sRAGE in the two groups.
Keywords: Serum sRAGE, COVID-19 severity, Infectious disease
Highlights
-
•
Serum sRAGE can be used to identify COVID-19 severity.
-
•
The level of serum sRAGE in each COVID-19 patient is different.
-
•
The blood components of each COVID-19 severity are different.
1. Introduction
Coronavirus disease 2019 or better known as COVID-19 caused by SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) became a worldwide pandemic at the end of 2019 with various systemic complaints but was more dominant in respiratory disorders. The worldwide mortality rate was 2.1% by February 12, 2020 [1]. The February 2020 data by Johns Hopkins University's Center for Systems Science and Engineering (CSSE) showed a total case of more than 60,331 patients, with a total death of more than 1369 patients and an improvement of more than 6061 patients [2]. On December 27, 2020, the total number of worldwide cases was more than 79 million, including 1,751,311 deaths. Incidents in Indonesia were 706,837 confirmed cases of COVID-19 and 20,994 cases of death [3].
The severity of COVID-19 according to WHO is divided into mild, moderate, severe, and critical [4,5]. The most frequently encountered clinical symptoms are pneumonia symptoms. Biomarkers are frequently used to determine the severity of pneumonia such as procalcitonin, C-reactive protein (CRP), copeptin, pro-ANP (atrial natriuretic peptide), adrenomedullin, cortisol, and D-dimers [6]. These biomarkers are good in determining infection in pneumonia but have not been able to detect early tissue damage, as patients often go to the hospital with a more severe condition. Recent studies in immunology have examined soluble receptors for advanced glycation end-products (sRAGE) as a biomarker of the severity of community pneumonia and can detect tissue damage in ARDS early [7].
Pathophysiology occurred in COVID-19 includes the inflammatory process. One of the inflammatory processes during pneumonia is characterized by an increase in receptors for advanced glycation end-products (RAGE). RAGE is one of the non-enzymatic receptors of Advanced Glycation End-Products (AGEs) which has a multi-ligand receptor, namely a V-type domain, two C-type domains, a transmembrane domain, and a cytoplasmic tail. RAGE has several ligands including AGEs, S100/calgranulins, and HMGB I which are present in different vascular cells such as endothelial cells, neuronal cells, smooth muscle cells, or inflammatory cells (monocytes). HMGB I is one of the RAGE ligands that play a role in the occurrence of sepsis which can stimulate the formation of cytokines along with TLRs in the immune system cells (B cells) [8]. The interaction between RAGE and its ligands will cause the formation of Reactive Oxygen Species (ROS) which will activate NADPH oxidation. The process will mediate the formation of inflammatory cells. Trianta et al. stated two processes of RAGE interaction with its ligands that are related to the inflammatory process, namely its interaction with leukocytes and on endothelial cells, RAGE is an adhesive receptor and directly forms inflammatory cells. The accumulation of RAGE ligands is predicted to cause chronic cell stimulation and tissue damage [9,10].
RAGE is expressed in the membrane-bound form (fl-RAGE or mRAGE) and the soluble form in the transmembrane domain. Soluble RAGE is produced by proteolytic cleavage of fl-RAGE and alternative splicing mRNA [7]. The administration of sRAGE in experimental animals can also interact with the RAGE ligand [10]. Based on these studies, the role of sRAGE becomes very important in determining COVID-19 diagnosis based on the severity quickly, so that effective and adequate treatment planning can be carried out early to reduce the morbidity and mortality of COVID-19 patients. In addition, the level of sRAGE in serum can detect early tissue damage which in turn can affect the severity of COVID-19 patients as common biomarkers have not been able to detect the process of tissue damage early. Research on sRAGE in the serum of COVID-19 patients is still limited and has never been carried out in Indonesia despite a few studies having been conducted in other countries. This biomarker is also easy to use and at a more affordable cost, so we are interested in analyzing the association of serum sRAGE on COVID-19 severity.
2. Methods
2.1. Participants
Participants in this study were COVID-19 patients diagnosed with real-time polymerase chain reaction (PCR) [5]. Participants' inclusion criteria included patients diagnosed with COVID-19 and aged >21 years. Participants' exclusion criteria included patients with a history of respiratory tract infection, myocardia infarct, cancer, and cerebral vascular attack. Participants who were willing to take part in the research first received an explanation of the rights and obligations of the participants, in which they voluntarily filled out the informed consent form.
2.2. Study design
This study used a cross-sectional design with a consecutive sampling method. It was carried out from May 2020–October 2020. This study collected participant characteristics, serum sRAGE, and COVID-19 severity. This study reported the data based on the strengthening the reporting of cohort studies in surgery (STROCSS) 2021 guideline [11]. The number of participants in this study was 145 participants that were divided into 2 groups (non-severe = 47 and severe = 98). The non-severe group consisted of participants identified as having COVID-19 in the mild and moderate category, while the severe group consisted of participants identified as having COVID-19 in the severe and critical categories [5].
2.3. Assessment of COVID-19 severity
The severity of COVID-19 in this study was assessed using WHO criteria at the time of the initial examination of the patient, which distinguished the severity of COVID-19 from being non-severe (mild-moderate category) and severe (severe-critical categories). Mild is a symptomatic patient who meets the COVID-19 case definition without evidence of viral pneumonia or hypoxia. Moderate include clinical symptoms of pneumonia (fever, cough, dyspnoea, rapid breathing) but no signs of severe pneumonia, including SpO2 90% in room air or PaO2 60 mmHg (PaO2 measurements were obtained from patient medical records). Severe shows clinical symptoms of pneumonia (fever, cough, shortness of breath, rapid breathing) plus one of respiratory rate >30 times/minute; severe respiratory distress or SpO2 <90% or PaO2 59 mmHg (PaO2 measurements were obtained from patient medical records). Critical when patients have ARDS, sepsis, and septic shock. Mild ARDS: 200 mmHg < PaO2/FiO2a 300 mmHg (with PEEP or CPAP 5 cmH2O). Moderate ARDS: 100 mmHg < PaO2/FiO2 200 mmHg (with PEEP 5 cmH2O). ARDS weight: PaO2/FiO2 100 mmHg (with PEEP 5 cmH2O) [5].
2.4. sRAGE serum examination
The sRAGE is soluble forms in the transmembrane domain of RAGE which the serum levels of sRAGE are determined using a specific sandwich human ELISA kit BioAssay (MyBioSource Inc, San Diego, USA). The sRAGE measurement is in the range of 0.31–2.00 ng/mL. These results were obtained from taking 5 cc venous blood samples [12].
2.5. Statistical analysis
The analysis in this study used descriptive analysis and bivariate analysis. The descriptive analysis includes a descriptive presentation of the results using a distribution table, mean, median, standard deviation, maximum value, and minimum value. The analysis was conducted using IBM SPSS Statistics software version 21.0 (IBM Corp., Armonk, NY, USA). Participants' characteristic data were analyzed using the chi-square test or Fisher's exact test. Meanwhile, the data from this study were first tested for normality using the Kolmogorov-Smirnov test. Analysis of the association of sRAGE serum with COVID-19 severity using the independence t-test or Mann Whitney test. The comparison between the two variables is significant if p < 0.05. In addition, Spearman's rank test was used to analyze the association between two variables.
3. Results
3.1. Characteristics of participants
The demographic characteristics of participants included age and gender. The average age of participants was 50.54 ± 12.70 years (non-severe group = 49.11 ± 12.44 years and severe group = 51.23 ± 12.83 years). The median age of participants was 52.00 (43.00–59.00) years of which the youngest participant was 22.00 years old and the oldest participant was 80.00 years old. Most participants were in the age range of 35.00–55.00 years, consisting of 25 participants (53.2%) in the non-severe group and 51 participants (52.0%; p = 0.705) in the severe group. Most participants were male (90 participants; 62.1%), consisting of 25 participants (53.2%) in non-severe group and 65 participants in severe group (66.3%; OR = 0.577; p = 0.179; Table 1).
Table 1.
Characteristics of participants.
| Characteristics | COVID-19 Severity |
p | |
|---|---|---|---|
| Non-severe | Severe | ||
| Age (years) | 0.705 | ||
| 21-35 | 8 (17.0) | 12 (12.2) | |
| 35-55 | 25 (53.2) | 51 (52.0) | |
| 55-65 | 8 (17.0) | 24 (24.5) | |
| >65 | 6 (12.8) | 11 (11.2) | |
| Gender | 0.179 | ||
| Male | 25 (53.2) | 65 (66.3) | |
| Female | 22 (46.8) | 33 (33.7) | |
| Clinical symptoms | |||
| Shortness of breath | 30 (63.8) | 92 (93.9) | <0.001** |
| Fever | 22 (46.8) | 39 (39.8) | 0.535 |
| Cough | 28 (59.6) | 42 (42.9) | 0.088 |
| Painful swallowing | 1 (2.1) | 3 (3.1) | 1.000 |
| Diarrhea | 3 (6.4) | 4 (4.1) | 0.682 |
| Outcome | <0.001** | ||
| Recovered | 41 (87.2) | 47 (48.0) | |
| Died | 6 (12.8) | 51 (52.0) | |
| Comorbid | |||
| Hypertension | 13 (27.7) | 28 (28.6) | 1.000 |
| Diabetes | 20 (42.6) | 46 (46.9) | 0.750 |
| Obesity | 9 (19.1) | 26 (26.5) | 0.444 |
Note: *significant <0.05; **significant <0.01.
There were several clinical symptoms appeared, including shortness of breath in 122 participants (84.1%; 63.8% vs 93.9%; OR = 8689; p <0.001), fever in 61 participants (42.1%; 46.8% vs 39.8%; OR = 0.751; p = 0.535), cough in 70 participants (70%; 59.6% vs. 42.9%; OR = 0.509; p = 0.088), painful swallowing in 4 participants (2.8%; 2.1% vs 3.1%; OR = 1.453; p = 1.000), and diarrhea in 7 participants (4.8%; 6.4% vs. 4.1%; OR = 0.624; p = 0.682). Based on the outcome of the COVID-19 treatment, most of non-severe participants recovered as many as 41 participants (87.2%) and most of severe participants were declared dead as many as 51 participants (52%; p <0.001). Overall, 88 participants (60.7%) were recovered. Several participants were declared to have comorbidities, including hypertension as many as 41 participants (28.3%; 27.7% vs 28.6%; OR = 1.046; p = 1.000), diabetes as many as 66 participants (45.5%; 42.6% vs 46.9%; OR = 1.194; p = 0.750), and obesity as many as 35 participants (24.1%; 19.1% vs. 26.5%; OR = 1.525; p = 0.444; Table 1).
3.2. Association of soluble receptor for Advanced Glycation End-Products (sRAGE) serum with COVID-19 severity
The results of blood analysis showed several blood components such as leukocytes (9896.51 ± 4949.64/μL), lymphocytes (13.55 ± 8.48%), neutrophils (78.91 ± 10.50%), procalcitonin (0.92 ± 3.22 ng/mL), CRP (8.59 ± 7.62 mg/L), D-dimer (4360.29 ± 7797.81 ng/mL), and fibrinogen (474.58 ± 168.90 mg/dL). The average value of serum sRAGE was 1.48 ± 0.98 ng/mL, with a median value of 1.07 (0.85–1.84) ng/mL. The lowest and highest value of participants' serum sRAGE was 0.44 ng/mL and 5.14 ng/mL, respectively. The results of the COVID-19 severity measurement were divided into 4: mild as many as 2 participants (1.4%), moderate as many as 45 participants (31.0%), severe as many as 96 participants (66.2%), and critical as many as 2 participants (1.4%). Meanwhile, in this study, COVID-19 severity was divided into 2 groups, namely the non-severe group with 47 participants (32.88%) and the severe group with 98 participants (68.53%).
There was a significant difference in blood component in the non-severe group and the severe group as follows: leukocyte value was 8005.00 (6157.50–9687.50) vs 9840.00 (7420.00–12,830.00/μL; z = 2.431; p = 0.015), lymphocyte was 14.40 (8.83–21.65) vs 10.20 (6.60–16.80%; z = 2.256; p = 0.024), neutrophils was 77.40 (68.90–83.28) vs. 82.60 (76.00–87.10%; z = 2464; p = 0.014), procalcitonin was 0.11 (0.07–0.22) vs 0.27 (0.13–0.46 ng/mL; z = 3.323; p = 0.001), CRP was 4.65 (0.80–11.35) vs. 8.70 (2.30–13.60 mg/L; z = 2.114; p = 0.034), and D-dimer was 810.00 (535.00–2430.00) vs. 1460.00 (740.00–4025 ng/mL; z = 2.186; p = 0.029). Meanwhile, there was no significant difference in the levels of fibrinogen between participants in the two groups (465.50 ± 176.04 vs. 480.06 ± 165.92 mg/dL; t = 0.383; p = 0.703; Table 2).
Table 2.
Comparison of blood component based on COVID-19 severity.
| Blood Analysis | COVID-19 Severity |
p | |
|---|---|---|---|
| Non-severe | Severe | ||
| Leukocytes (n = 139) | 8622.10 ± 4204.47 | 10,526.86 ± 5185.37 | 0.015* |
| Lymphocyte (n = 139) | 15.50 ± 8.22 | 12.58 ± 8.49 | 0.024* |
| Neutrophile (n = 139) | 76.06 ± 10.36 | 80.32 ± 10.34 | 0.014* |
| Procalcitonin (n = 143) | 1.01 ± 4.67 | 0.88 ± 2.22 | 0.001* |
| CRP (n = 90) | 6.52 ± 6.71 | 9.53 ± 7.87 | 0.034* |
| Fibrinogen (n = 85) | 465.50 ± 176.04 | 480.06 ± 165.92 | 0.703 |
| D-Dimer (n = 139) | 2790.64 ± 5558.74 | 5162.17 ± 8641.11 | 0.029* |
| s-RAGE (n = 143) | 0.82 ± 0.23 | 1.80 ± 1.04 | <0.001** |
Note: CRP = C-reactive protein; s-RAGE = soluble receptor for advanced glycation end products; *significant <0.05; **significant <0.001.
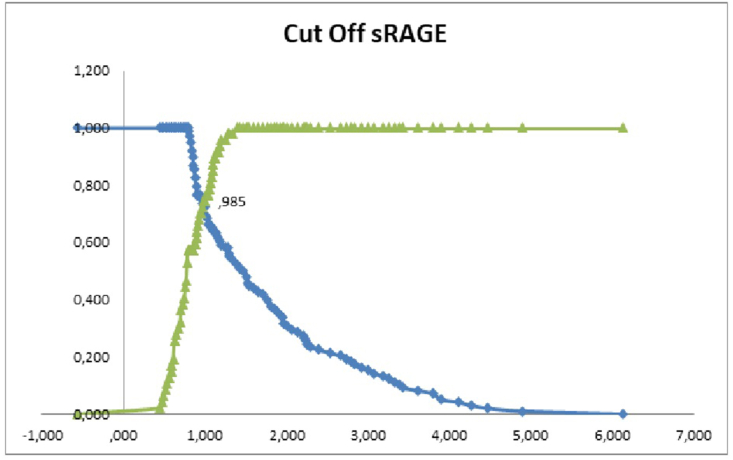
There was a significant difference between serum sRAGE in the non-severe group and the severe group of 0.78 (0.63–1.00) vs 1.47 (0.97–2.25 ng/mL; r = 7.154; p < 0.001; Table 2). There was a significant association between serum sRAGE and COVID-19 severity (r = 0.598; p <0.001). The cut-off value for serum sRAGE between the severe and non-severe group was 0.985 ng/mL. This study obtained sensitivity of 73.5%, specificity of 74.5%, OR of 8.077 and AUC 0.868 CI 95% (Fig. 1).
Fig. 1.
Cut-off Serum sRAGE level based on severe and non-severe groups of COVID-19 patients.
4. Discussion
This study assessed serum sRAGE based on the severity of COVID-19. The results of this study are consistent with previous studies that examined sRAGE as a biomarker for COVID-19. A study examined the association of sRAGE with severity and as an indicator of mechanical ventilation requirements, ARDS, and mortality in COVID-19 patients. The results showed an increase in serum sRAGE concentrations in COVID-19 patients based on severity [13]. These results are consistent with another study which stated a significant increase in serum sRAGE of ARDS patients admitted to non-isolated ICUs [14].
There is a significant association between serum sRAGE and COVID-19 severity. The serum sRAGE values in the severe group show a significant difference from serum sRAGE values in the non-severe group. The results are consistent with previous studies that showed an increase in serum sRAGE values in COVID-19 patients with a degree of severity. Increased sRAGE values can also help predict respiratory disorders that require mechanical ventilation and the mortality rate of COVID-19 patients [13]. Increased serum sRAGE is commonly found in ARDS patients admitted to the ICU [15]. As many as 20% of COVID-19 patients progress to the third phase called the involvement of the respiratory tract and progression to ARDS [16].
Increased serum sRAGE values can occur due to a viral infection process that will trigger an immune response, namely the innate immune system. Pattern-recognition receptors (PRR) recognize pathogen-associated molecular patterns (PAMPs) involving toll-like receptors (TLR) that detect components of infection and signaling tissue damage, one of which is HMGB1. Then it continues to the process of indirect lung tissue damage, namely damage-associated molecular patterns (DAMPs) that involve RAGE, NLR, TLR, and CLR which can exacerbate the occurrence of tissue damage that has occurred previously. The process of interaction of sRAGE with its ligand becomes more frequent due to an increase in HMGB1 that result in the increased inflammatory response in the form of IL-1 and TNF-Alpha activation [17,18].
Other tissue damage processes can also occur when SARS-CoV2 invades AT2 cells located in the periphery and subpleural so that the patient begins to feel hypoxia. SARS-CoV2 replicates in AT2 lead to cell damage and death. Dead AT2 cells release toxins and damage surrounding cells. Infected cells send signals that are detected by the immune system which then releases cytokines such as IL-1, IL-6, and TNF-α. These cytokine release aims to kill the virus, but it also causes damage to lung cells, namely diffuse alveolar damage, formation of hyaline membranes, and multinuclear giant cells. Abnormal wound healing leads to fibrosis [16,19].
This study, however, has limitations, including the need for a future study that compares healthy individuals and pneumonia patients without COVID-19.
5. Conclusion
sRAGE is a biomarker that can be used to determine COVID-19 severity. The patients' COVID-19 severity in this study is categorized into 2, namely non-severe and severe. Based on blood component analysis, there are significant differences between the non-severe and severe groups. The differences consist of leukocytes, lymphocytes, neutrophils, procalcitonin, CRP, and D-dimer. The sRAGE values in the two groups also show a significant difference. In addition, there is a significant association between serum sRAGE and COVID-19 severity.
Ethical approval
We have conducted an ethical approval base on the Declaration of Helsinki with registration research at the Health Research Ethics Committee in Dr. Soetomo General Academic Hospital, Surabaya, Indonesia.
Funding
Dr. Soetomo General Academic Hospital, Surabaya, Indonesia.
Guarantor
Resti Yudhawati.
Author contributor
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Registration of research studies
-
1.
Name of the registry: Health Research Ethics Committee in the Dr. Soetomo General Academic Hospital, Surabaya, Indonesia.
-
2.
Unique Identifying number or registration ID: 1954/KEPK/IV/2020.
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): -.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgment
We would like to thank the COVID-19 patients and Guardian. We would also thank Dr. Soetomo General Academic Hospital as the place of our research, and our editor “Fis Citra Ariyanto”.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103303.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harapan H., Itoh N., Yufika A., Winardi W., Keam S., Te H., et al. Coronavirus disease 2019 (COVID-19): a literature review. J.of infection and public health. 2020;13(5):667–673. doi: 10.1016/j.jiph.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Djalante R., Lassa J., Setiamarga D., Sudjatma A., Indrawan M., Haryanto B., et al. Review and analysis of current responses to COVID-19 in Indonesia: period of january to march 2020. Progress in disaster science. 2020;6 doi: 10.1016/j.pdisas.2020.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Son K.B., Lee T.J., Hwang S.S. Disease severity classification and COVID-19 outcomes, Republic of Korea. Bull. World Health Organ. 2021;99(1):62–66. doi: 10.2471/blt.20.257758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suryananda T.D., Yudhawati R. Association of serum KL-6 levels on COVID-19 severity: a cross-sectional study design with purposive sampling. Ann. Med. Surg. 2021;69 doi: 10.1016/j.amsu.2021.102673. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seligman R., Ramos-Lima L.F., Oliveira Vdo A., Sanvicente C., Pacheco E.F., Dalla Rosa K. Biomarkers in community-acquired pneumonia: a state-of-the-art review. Clinics. 2012;67(11):1321–1325. doi: 10.6061/clinics/2012(11)17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narvaez-Rivera R.M., Rendon A., Salinas-Carmona M.C., Rosas-Taraco A.G. Soluble RAGE as a severity marker in community acquired pneumonia associated sepsis. BMC Infect. Dis. 2012;12:15. doi: 10.1186/1471-2334-12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparvero L.J., Asafu-Adjei D., Kang R., Tang D., Amin N., Im J., et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavakis T., Bierhaus A., Nawroth P.P. RAGE (receptor for advanced glycation end products): a central player in the inflammatory response. Microb. Infect. 2004;6(13):1219–1225. doi: 10.1016/j.micinf.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Clynes R., Moser B., Yan S.F., Ramasamy R., Herold K., Schmidt A.M. Receptor for AGE (RAGE): weaving tangled webs within the inflammatory response. Curr. Mol. Med. 2007;7(8):743–751. doi: 10.2174/156652407783220714. [DOI] [PubMed] [Google Scholar]
- 11.Mathew G., Agha R. Strocss 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int. J. Surg. 2021;96 doi: 10.1016/j.ijsu.2021.106165. [DOI] [PubMed] [Google Scholar]
- 12.García-Salido A., Melen G., Gómez-Piña V., Oñoro-Otero G., Serrano-González A., Casado-Flores J., et al. Circulating soluble RAGE and cell surface RAGE on peripheral blood mononuclear cells in healthy children. J. Pediatr. Endocrinol. Metab. : JPEM (J. Pediatr. Endocrinol. Metab.) 2018;31(6):649–654. doi: 10.1515/jpem-2017-0512. [DOI] [PubMed] [Google Scholar]
- 13.Lim A., Radujkovic A., Weigand M.A., Merle U. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COVID-19 disease severity and indicator of the need for mechanical ventilation, ARDS and mortality. Ann. Intensive Care. 2021;11(1):50. doi: 10.1186/s13613-021-00836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jabaudon M., Berthelin P., Pranal T., Roszyk L., Godet T., Faure J.S., et al. Receptor for advanced glycation end-products and ARDS prediction: a multicentre observational study. Sci. Rep. 2018;8(1):2603. doi: 10.1038/s41598-018-20994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Zoelen M.A., Schouten M., de Vos A.F., Florquin S., Meijers J.C., Nawroth P.P., et al. The receptor for advanced glycation end products impairs host defense in pneumococcal pneumonia. J. Immunol. (Baltimore, Md : 1950. 2009;182(7):4349–4356. doi: 10.4049/jimmunol.0801199. [DOI] [PubMed] [Google Scholar]
- 16.Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020;55(4) doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mondrinos M.J., Kennedy P.A., Lyons M., Deutschman C.S., Kilpatrick L.E. Protein kinase C and acute respiratory distress syndrome. Shock. 2013;39(6):467–479. doi: 10.1097/SHK.0b013e318294f85a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horiguchi H., Loftus T.J., Hawkins R.B., Raymond S.L., Stortz J.A., Hollen M.K., et al. Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front. Immunol. 2018;9:595. doi: 10.3389/fimmu.2018.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carcaterra M., Caruso C. Alveolar epithelial cell type II as main target of SARS-CoV-2 virus and COVID-19 development via NF-Kb pathway deregulation: a physio-pathological theory. Med. Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.