Abstract
Introduction
Cardiovascular dysautonomia comprising postural orthostatic tachycardia syndrome (POTS) and orthostatic hypotension (OH) is one of the presentations in COVID-19 recovered subjects. We aim to determine the prevalence of cardiovascular dysautonomia in post COVID-19 patients and to evaluate an Artificial Intelligence (AI) model to identify time domain heart rate variability (HRV) measures most suitable for short term ECG in these subjects.
Methods
This observational study enrolled 92 recently COVID-19 recovered subjects who underwent measurement of heart rate and blood pressure response to standing up from supine position and a 12-lead ECG recording for 60 s period during supine paced breathing. Using feature extraction, ECG features including those of HRV (RMSSD and SDNN) were obtained. An AI model was constructed with ShAP AI interpretability to determine time domain HRV features representing post COVID-19 recovered state. In addition, 120 healthy volunteers were enrolled as controls.
Results
Cardiovascular dysautonomia was present in 15.21% (OH:13.04%; POTS:2.17%). Patients with OH had significantly lower HRV and higher inflammatory markers. HRV (RMSSD) was significantly lower in post COVID-19 patients compared to healthy controls (13.9 ± 11.8 ms vs 19.9 ± 19.5 ms; P = 0.01) with inverse correlation between HRV and inflammatory markers. Multiple perceptron was best performing AI model with HRV(RMSSD) being the top time domain HRV feature distinguishing between COVID-19 recovered patients and healthy controls.
Conclusion
Present study showed that cardiovascular dysautonomia is common in COVID-19 recovered subjects with a significantly lower HRV compared to healthy controls. The AI model was able to distinguish between COVID-19 recovered patients and healthy controls.
Keywords: Artificial intelligence, Autonomic nervous system, COVID-19, Heart rate variability, Machine learning
1. Introduction
COVID-19 made a huge impact globally leading to unprecedented morbidity and mortality [1]. A significant proportion of patients present with functional limitations and decline in quality of life at various time intervals following COVID-19 recovery. They characteristically occur within 3–12 weeks post recovery and are labelled as Post-acute COVID-19 syndrome or “Long Haul COVID-19” [2]. Although not well characterized, common presenting symptoms pertaining to the cardiovascular system include fatigue, dyspnea, chest pain, orthostatic intolerance, lightheadedness and palpitations. Post COVID-19 cardiovascular dysautonomia (PCCD) is often attributed to SARS-CoV2 virus-related direct damage, cytokine storm mediated or immune-mediated dysregulation of the autonomic nervous system (ANS) [3,4]. It is characterized by failure or increased activity of sympathetic or parasympathetic components of the ANS [5]. PCCD continuum comprises of orthostatic intolerance syndrome including orthostatic hypotension (OH) and postural orthostatic tachycardia syndrome (POTS) as well as inappropriate sinus tachycardia and reflex syncope [5]. Such dysautonomia has been previously reported following multiple viral infections including hepatitis C, human immunodeficiency virus and Epstein-Barr virus [6].
There is scarcity of systematic data on occurrence of cardiovascular dysautonomia in post-acute COVID-19 syndrome [3,[7], [8], [9], [10]]. With recurring waves in this pandemic, the likely burden of this syndrome is going to increase. Hence, there is an urgent need to develop simple non-invasive tools to identify this entity. Heart rate variability (HRV) is a simple non-invasive marker of cardiovascular dysautonomia [11]. Frequency and time-domain measures of HRV have emerged as one of the promising tools to evaluate the balance between sympathetic and parasympathetic components of ANS [11]. It can easily be determined on a 12-lead electrocardiography (ECG) based on the difference in time between two successive heartbeats. HRV time-domain measures can be analyzed from ECG monitoring periods from <1 min to >24 h. These include the standard deviation normal sinus beats (SDNN), root mean square of successive differences between normal heartbeats (RMSSD) and the number of adjacent NN intervals that differ from each other by more than 50 ms (NN50) [12]. Variations in HRV often reflect the presence of pathological conditions with high HRV seen in healthy individuals with normally functioning of ANS while lower HRV is reflective of dysautonomia [13]. There is a paucity of systematically conducted study reporting the utility of HRV as a predictive of autonomic dysfunction in COVID-19 recovered patients [[14], [15], [16], [17]].
Artificial intelligence (AI) has emerged as a powerful tool in the field of biomedical research. AI confirms the traditional statistical results and is able to rank and identify the top features that can distinguish between two classes. In the present study, we determine the prevalence of cardiovascular dysautonomia, as well as its spectrum in post COVID-19 recovered patients based on HRV analysis. We also developed and evaluated an AI model to identify which time domain HRV measure is most suitable for short term ECG to differentiate between post-COVID-19 recovered subjects and healthy controls.
2. Methodology
2.1. Data collection
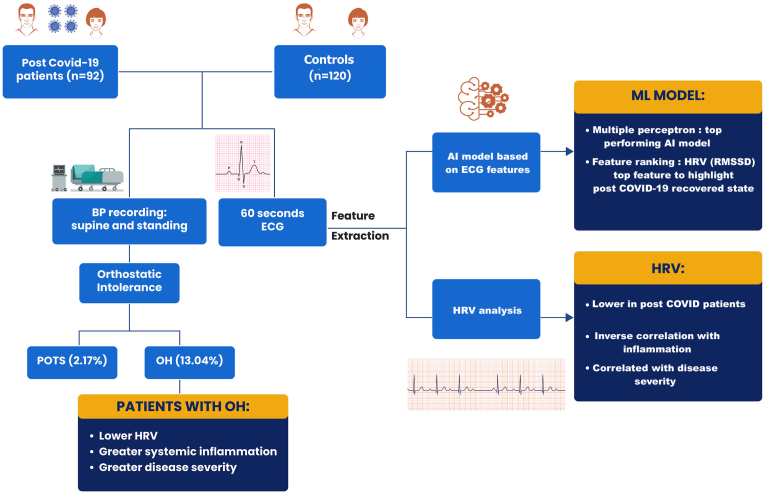
This was a prospective single-centre study in the Department of Cardiology in a tertiary care centre from December 2020 to March 2021. A total of 117 consecutive subjects (≥18 years of age) recently recovered (within 30–45 days) from COVID-19 infection (COVID-19 reverse transcription polymerase chain reaction [RT-PCR negative]) were screened for eligibility. Subjects with presence of one or more pre-existing conditions known to affect HRV analysis (atrial fibrillation, numerous atrial or ventricular extra systoles, bundle branch blocks, malignancy, renal or hepatic failure, diabetes mellitus) and reduced left ventricular function (LVEF<50%) were excluded. A total of 117 patients were screened of whom 25 were excluded due to uninterpretable or noisy ECG data. In the 92 patients included in the study, clinical details (active infection and convalescent phase), comorbidities and severity [18] (mild, moderate, severe) of COVID-19 infection were recorded. Baseline clinical, hematological, biochemical parameters (hemogram, liver and kidney function tests) as well as inflammatory markers [C-reactive protein (CRP), D-dimer, ferritin, interleukin (IL)-6 and lactate dehydrogenase (LDH)] were obtained at the time of admission during COVID-19 infection on a pre-structured proforma. Raw electrocardiogram of the six limb leads (I, II, III, aVL, aVR and aVF) and six chest leads (V1, V2, V3, V4, V5 and V6) was recorded over a 60 s period during supine paced breathing using VESTA 301i (500 Hz). Each subject's ECG data was stored in.dat &.xls format for further feature extraction. In addition, in all these patients heart rate (HR) and blood pressure (BP) response to standing up from supine position was determined. The patients were rested in a supine position for 5 min prior to standing up for a period of 3 min. HR as well as systolic and diastolic BP were measured just before standing in supine position and after every minute for 3 min following active standing. A patient was labelled to be suffering from PCCD if the following abnormal response were recorded (a) orthostatic hypotension (OH) if there was a fall of >20 mmHg systolic and/or >10 mmHg diastolic BP following standing for 3 min [19], (b) postural orthostatic tachycardia syndrome (POTS) if there were orthostatic symptoms (in absence of OH) and increase in heart rate of >30 beats/minute (age: 12–19 years - 40 beats/minute) following standing [20]. One hundred and twenty age and sex-matched controls were also recruited who underwent a 12-lead ECG over 60 s (Fig. 1: central illustration).
Fig. 1.
Central illustration of the heart rate variability (HRV) analysis of COVID-19 recovered subjects and healthy controls. OH: orthostatic hypotension; POTS: postural orthostatic tachycardia syndrome; AI: artificial intelligence; ECG: electrocardiograph; BP: blood pressure; HRV: Heart Rate Variability.
2.2. Pre-processing and features extraction from raw ECG data
Lead II of the 12-lead raw ECG was used to for the study. The samples were visualized after plotting the ECG as a graph, and the samples deemed too noisy were removed. Baseline wandering was present in some samples for which a correction was done (Supplementary Fig. S1). After correcting the R wave noise, feature extraction algorithm was used to extract various ECG features namely (a) average RR interval defined as the average distance between two R peaks, (b) average sig value defined as average value of the ECG signal, (c) HR mean defined as the average value of an individual's HR, (d) HR standard deviation defined as standard deviation of an individual's HR, (e) Heart rate variability [HRV (RMSSD)] defined as the temporal variation between sequences of consecutive heartbeats and (f) SDNN defined as standard deviation of all of the RR intervals.
2.3. Artificial Intelligence (AI) model for feature ranking
The study data was divided into two groups: (a) training dataset to train an AI model and (b) testing data set for testing the trained AI model. The samples of both classes were divided into five folds. Three folds had data of 18 post-COVID-19 subjects, while two folds had 19 post-COVID-19 subjects. Each fold had 24 healthy subjects who belonged to the control population. In the five-fold cross-validation, four folds are used for training, while the 5th fold was used as the test data. This process was repeated every time with a different classifier until each of the folds was used as the test data once. Thus, five classifiers were trained, using one of the folds as the test data. Once all 5 classifiers were trained and tested, overall results of every AI model were quoted after considering the cumulative performance of all the 5 classifiers. To handle class imbalance in this training data, the standard practice of Random Oversampling Technique was employed on the training data. However, to avoid any bias in the test results, no oversampling was carried out on the test fold during the cross-validation. This ensured that the test fold did not contain repeated samples and hence, every sample was treated as the test sample only once for reporting the results of cross-validation. Python sklearn library was used for developing AI models. Nine candidate features were used as input and trained with eight AI algorithms viz. the traditional method of Logistic Regression, four tree-based methods (RandomForests, CatBoost, XGBoost, Extra-tree classifier), Artificial Neural Network (Multiple Perceptron (MLP)) classifier, Support Vector Machine Classifier and AdaBoost Classifier to distinguish between COVID-19 recovered patients and healthy controls. The performance of various AI models was assessed by comparing sensitivity, specificity, receiver operating characteristic (ROC) area under the curve (AUC), precision-recall and Matthews's correlation coefficient (MCC). For the top performing AI model, AI interpretability ShAP algorithm [21] was applied and the feature ranking was done by permutation importance algorithm for all the AI models.
2.4. Statistical analysis
Continuous data was expressed as mean ± SD and categorical data was represented as proportions. Normality of distribution of continuous variables were assessed using the Kolmogorov-Smirnov test. Comparison of means of continuous variables was done using Student's t-test or Mann-Whitney U test as appropriate, while Fisher exact test or χ2 test was used for categorical variables. In addition, ANOVA or Kruskal Wallis was used to compare mean values of continuous variables between the groups based on severity of COVID-19. Correlation between inflammatory markers and HRV was done using Pearson or Spearman correlation coefficient test as appropriate. Multivariate logistic regression analysis was used to determine independent predictors of PCCD. A two-sided p value of ≤ 0.05 was considered to be statistically significant. SPSS version 24.0 (IBM Corp, Armonk, NY) was used for statistical analysis. The study was approved by the Institutional ethics committee and a written informed consent was obtained from each patient prior to enrollment.
3. Results
A total of 92 subjects were included in the study for final analysis. The mean age of the study population was 50.6 ± 12.1 years. The demographic characteristics of all the included patients is shown in Table 1. On the basis of National Institute of Health (NIH) [18] COVID-19 severity classification, 87.1% were symptomatic during the illness. Mild illness was observed in 38 (40.8%), moderate disease was reported in 33 (35.4%) and severe in 10 (10.8%). Following recovery from COVID-19 infection, 39 (41.9%) were likely to be symptomatic with dyspnea (17.4%), palpitations (16.3%), dizziness (14.1%) and fatigue (11.9%) being the commonest presentation. HRV (RMSSD) was significantly lower in post COVID-19 recovered subjects as compared to the control population (13.9 ± 11.8 ms vs 19.9 ± 19.5 ms; P = 0.01) [Supplementary Fig. S2]. Patients with severe COVID-19 infection were more likely to have a lower HRV as compared to asymptomatic or mild cases (HRV [RMSSD]- asymptomatic: 24.2 ± 13.4 ms; mild: 16.3 ± 13.4 ms; moderate: 9.3 ± 6.4 ms; severe: 7.2 ± 3.8 ms; P < 0.0001). Posthoc analysis revealed a significant difference between HRV in mild and moderate cases (P = 0.04). A significant inverse correlation was documented between HRV [RMSSD] and levels of inflammatory markers viz. CRP (r = −0.30; P = 0.02) and IL-6 (r = −0.36; P = 0.005).
Table 1.
Comparative evaluation of the features between COVID-19 recovered and 120 healthy controls subjects.
| Post COVID-19 patients (n = 92) | Controls (n = 120) | p-value | |
|---|---|---|---|
| Age | 50.6 ± 12.1 | 51.8 ± 4.2 | 0.39 |
| Gender (Male) | 54 (58.7%) | 65 (54.1%) | 0.51 |
| Hypertension | 11 (11.9%) | 10 (8.3%) | 0.37 |
| Mean HR | 88.1 ± 15.2 | 77.6 ± 11.3 | <0.0001 |
| HRV (SDNN) | 16.9 ± 12.9 | 22.5 ± 17.6 | 0.01 |
| HRV (RMSSD) | 13.9 ± 11.8 | 19.9 ± 19.5 | 0.01 |
| Avg signal value (microV) | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.907 |
| Avg RR interval (ms) | 704.3 ± 116.1 | 787.8 ± 110.2 | <0.001 |
| COVID-19 recoveredsubjects' symptoms | |||
| Symptoms during COVID-19 | |||
| Fever | 63 (68.5%) | – | |
| Cough | 50 (54.3%) | – | |
| Sore throat | 16 (17.4%) | – | |
| Dyspnoea | 34 (36.9%) | – | |
| Chest pain | 14 (15.2%) | – | |
| Myalgia | 11 (11.9%) | – | |
| Anosmia/Aguesia | 10 (10.9%) | – | |
| Headache | 4 (4.3%) | – | |
| Symptoms post COVID-19 | 39 (42.4%) | – | |
| Dyspnoea | 16 (17.4%) | – | |
| Cough | 8 (8.7%) | – | |
| Fatigue | 11 (11.9%) | – | |
| Palpitations | 15 (16.3%) | – | |
| Orthostatic intolerance | 14 (15.2%) | – | |
| Chest pain | 11 (11.9%) | – | |
| Dizziness | 13 (14.1%) | – | |
| Syncope | 2 (2.1%) | – | |
| Severity | |||
| Asymptomatic | 12 (13.1%) | – | |
| Mild | 38 (41.3%) | – | |
| Moderate | 32 (34.7%) | – | |
| Severe | 10 (10.9%) | – | |
Abbreviations: Avg: average; HR: heart rate; HRV: Heart rate variability; microV: microvolt; ms: millisecond; RMSSD: Root Mean Square Standard Deviation.
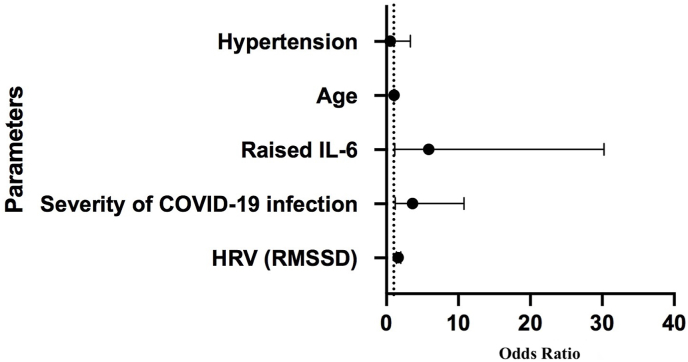
PCCD was observed in 15.21% cases. Orthostatic hypotension was reported in 12 patients (13.04%), while two patients (2.17%) had POTS. The patients with OH were more likely to have suffered from moderate (5/12[41.7%]) or severe COVID-19 (5/12[41.7%]) illness. Patients with OH had a significantly lower HRV (RMSDD) as compared to those without (5.3 ± 3.2 ms vs 15.2 ± 12.1 ms; P = 0.006). They also had significantly higher levels of inflammatory markers (CRP: 56.5 ± 109.8 mg/L vs 15.8 ± 32.5 mg/L; P = 0.03 and IL-6: 36.3 ± 82.2 pg/mL vs 6.7 ± 12.4 pg/mL; P = 0.01) as compared to those without. There was no significant difference in terms of presence of co-morbidities, haemoglobin and creatinine levels as well as left ventricular ejection fraction (LVEF) between patients with OH and those without (Table 2). None of the healthy controls had OH or POTS. The independent predictors of PCCD included (a) HRV (RMSSD) [OR:1.63; 95% CI: 1.39–1.99; P = 0.04], (b) severity of COVID-19 infection [OR:3.60; 95% CI: 1.21–10.78; P = 0.02] and (c) raised IL-6 [OR: 5.88; 95% CI: 1.14–30.25; P = 0.03 ] (Fig. 2).
Table 2.
Comparative evaluation of patients with or without orthostatic hypotension.
| Parameters | Patients with OH (n = 12) | Patients without OH (n = 80) | P-value |
|---|---|---|---|
| Age | 56.42 ± 11.54 | 49.78 ± 12.06 | 0.07 |
| Gender (Male) | 7 (58.3%) | 47 (58.7%) | 0.97 |
| Hypertension | 2 (16.6%) | 19 (23.7%) | 0.58 |
| Symptoms during COVID-19 | |||
| Fever | 11 (91.7%) | 52 (65%) | 0.06 |
| Cough | 9 (75%) | 41 (51.2%) | 0.124 |
| Sore throat | 2 (16.7%) | 14 (17.5%) | 0.943 |
| Dyspnea | 6 (50%) | 28 (35%) | 0.31 |
| Chest pain | 2 (16.6%) | 12 (15%) | 0.881 |
| Myalgia | 3 (25%) | 8 (10%) | 0.135 |
| Symptoms post COVID-19 | |||
| Dyspnea | |||
| Cough | 1 (8.3%) | 7 (8.7%) | 0.96 |
| Fatigue | 4 (33.3%) | 7 (8.7%) | 0.01 |
| Palpitations | 5 (41.7%) | 10 (12.5%) | 0.01 |
| Orthostatic intolerance | 8 (66.7%) | 6 (7.5%) | <0.0001 |
| Chest pain | 2 (16.7%) | 12 (15%) | 0.88 |
| Dizziness | 7 (58.3%) | 6 (7.5%) | <0.0001 |
| Syncope | 2 (16.7%) | 0 | 0.0002 |
| Severity | |||
| Asymptomatic | 0 | 12 (15%) | |
| Mild | 3 (25%) | 35 (43.7%) | 0.002 |
| Moderate | 4 (33.3%) | 28 (35%) | |
| Severe | 5 (41.7%) | 5 (6.25%) | |
| HRV (RMSSD) [ms] | 5.3 ± 3.2 | 15.2 ± 12.1 | 0.006 |
| HRV (SDNN) [ms] | 9.2 ± 6.0 | 18.1 ± 13.3 | 0.02 |
| Mean HR | 99.2 ± 17.8 | 86.4 ± 14.1 | 0.006 |
| IL-6(pg/ml) | 36.3 ± 82.2 | 6.7 ± 12.4 | 0.016 |
| CRP(mg/L) | 56.4 ± 109.8 | 15.8 ± 32.4 | 0.03 |
| D-Dimer(μg/L) | 919.9 ± 1283.8 | 453.2 ± 703.6 | 0.11 |
| LDH(U/L) | 373.4 ± 364.8 | 364.8 ± 182.3 | 0.89 |
| Ferritin(μg/L) | 8111.7 ± 25513.3 | 317.2 ± 298.9 | 0.04 |
| Haemoglobin (gm%) | 12.2 ± 2.5 | 12.4 ± 1.6 | 0.81 |
| TLC (per mm3) | 9283.6 + 2927.7 | 9138.1 + 9258.8 | 0.96 |
| LVEF (%) | 61.3 ± 5.7 | 60.5 ± 5.1 | 0.63 |
| Avg signal value (microV) | 0.002 ± 0.006 | 0.001 ± 0.003 | 0.26 |
| Avg RR interval (ms) | 635.9 ± 127.7 | 714.6 ± 111.5 | 0.028 |
Abbreviations: Avg: average; CRP: C-reactive protein; HR: heart rate; HRV: Heart rate variability; IL-6: interleukin-6; LDH: lactate dehydrogenase; LVEF: left ventricular ejection fraction; ms: millisecond; microV: microvolt; OH: orthostatic hypotension; RMSSD: Root Mean Square Standard Deviation; TLC: total leucocyte count.
Fig. 2.
Forest plot showing the independent predictors of development of post COVID-19 cardiovascular dysfunction.
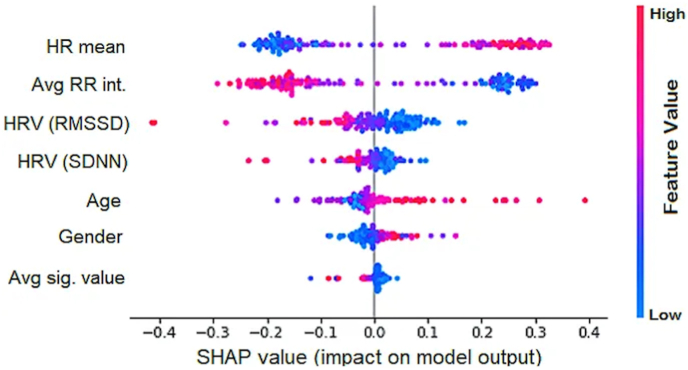
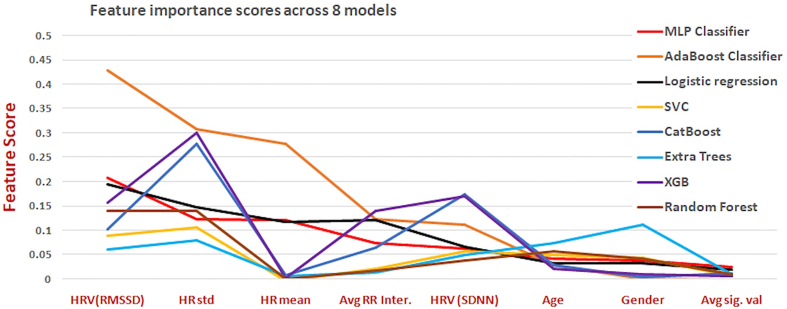
AI models confirmed that HRV (RMSSD) is an important marker for cardiovascular dysautonomia and to distinguish between COVID-19 recovered patients and healthy controls with accuracy ranging from 67 to 90% depending on the model (Table 3). The GridSearch algorithm was used as the parameter tuning methodology. The hyperparameter considered for MLP was the number of nodes in the hidden layer. All combinations of (6, 5, 4, 3) and (5, 4, 3) were used for the two hidden layers. The best combination was (5, 3) for the hidden layers. Further, max iterations were increased to 5000 because the default 2000 iterations did not allow the solution to converge. Multiple perceptron was the top performing AI model with sensitivity of 91.3%, specificity of 87.5%, accuracy of 89%, AUC of 89.8% and MMC of 78% (Table 3 & Table S1). MLP based ShAP interpretability feature importance showed in Fig. 3. According to ShAP summary plot, higher value of HR-mean has a higher impact on the prediction of the post-COVID class. Similarly, HRV (RMSSD) and HRV (SDNN), blue color is on the right side, indicating the lower HRV is a marker of Post-COVID class. Permutation importance feature ranking was applied to all the AI models which revealed HRV (RMSSD) to be the top feature distinguishing between COVID-19 recovered patients and healthy controls (Supplementary Figure- S3).
Table 3.
Comparative evaluation of the performance of various ML models.
| Model | Sensitivity (%) | Specificity (%) | AUC | Accuracy (%) | Accuracy (weighted)[%] | MCC (%) |
|---|---|---|---|---|---|---|
| MLP Classifier | 91.3 | 87.5 | 89.3 | 89.1 | 88.9 | 78.3 |
| Ada Boost Classifier | 85.8 | 66.6 | 76.3 | 75 | 76.2 | 52.5 |
| Logistic Regression | 77.1 | 74.1 | 75.6 | 75.5 | 75.3 | 50.9 |
| SVC | 84.7 | 65.8 | 75.2 | 74.1 | 75.2 | 50.5 |
| Cat Boost Classifier | 79.3 | 68.3 | 73.8 | 73.1 | 73.5 | 47.3 |
| Extra Trees Classifier | 77.1 | 65 | 71.1 | 70.3 | 70.8 | 41.9 |
| XGB Classifier | 79.3 | 58.3 | 68.9 | 67.4 | 69 | 37.8 |
| Random Forest Classifier | 75 | 60 | 67.6 | 66.5 | 67.4 | 34.9 |
Abbreviations: AUC: area under the curve; MCC: Matthews's correlation coefficient; MLP: Multiple Perceptron (MLP); ML: machine learning.
Fig. 3.
SHAP summary plot using MLP AI model. Each point on a feature line is a SHAP value for one subject's feature. The x-axis represents SHAP value, while the y-axis represents features. The right-side (positive (+) SHAP value) of the central line indicates the post-COVID-19 class. A greater positive SHAP value indicates higher impact on the prediction of the Post-COVID class. The color represents the value of the feature from low (blue) to high (red). For example, in the case of HR-mean, there is a clear distinction with red color on the right side. This indicates a higher value of HR-mean has a higher impact on the prediction of the post-COVID class. Similarly, HRV (RMS) and HRV SDNN, blue color is on the right side, indicating the lower HRV is a marker of Post-COVID class.
4. Discussion
In the present study, cardiovascular dysautonomia was common (15.21%) in post COVID-19 recovered patients. This was reflected by lower HRV and presence of OH/POTS in the convalescent phase of COVID-19 infection. In addition, HRV(RMSSD) time domain measures can be considered as a marker for cardiovascular dysautonomia for short time ECG recording (60 s) as confirmed by the AI model ShAP interpretability algorithm, standard statistics, and permutation importance features ranking methods. Cardiovascular dysautonomia in the form of OH or POTS has been previously observed in active COVID-19 infection in some reports. However, there is no systematic study documenting the same in COVID-19 recovered subjects.3,7-10,20Autonomic symptoms in COVID-19 are often thought to be multifactorial with deconditioning, hypovolemia, hyperadrenergic state or viral/immune-mediated damage to the ANS playing an important role [3]. OH is the most common autonomic presentation in COVID-19 followed by POTS. The clinical presentation in POTS varies with most of the patients reporting orthostatic tachycardia, palpitations, dizziness, lightheadedness or chronic fatigue [22]. Data regarding the occurrence of POTS following COVID-19 infection is markedly limited [7,10,23]. Though the exact mechanism for POTS in COVID-19 is still unclear, it has often been attributed to post-viral autoimmune response and damage to ANS [23,24]. In the present study, OH was the most common autonomic dysfunction observed in 12/92 (13.04%) patients. These patients had significantly higher levels of inflammatory markers and greater disease severity at the time of initial presentation. In our study, POTS was less frequent as compared to OH and was reported in 2/92 (2.17%) patients. An initial small study reported OH in 41% patients and POTS in 22% patients [20]. Another series of six patients documented OH in four while one patient had POTS [3].
HRV analysis from ECG has been used as a surrogate marker for cardiovascular dysautonomia. There is paucity of data regarding HRV in both active as well as in those recently recovered from COVID-19 [[14], [15], [16], [17]]. In the present study, we observed significantly lower HRV in COVID-19 recovered subjects as compared to healthy controls. This is the first study to characterise lower HRV according to disease severity and correlate it with inflammatory markers. Previous small studies have reported lower HRV in covid recovered patients [16,17]. However, these studies are limited by the design, lack of severity assessment and absence of correlation with inflammatory markers.
COVID-19 infection is characterized by an intense hyperinflammatory response known as “cytokine storm” with elevation of inflammatory markers such as CRP and IL-6 [25]. They have been reported as independent predictors of disease severity and worse outcomes in COVID-19 [25]. It has been previously established that ANS modulates the inflammatory response. Increased vagal responses has been associated with higher HRV and decreased inflammatory response through the cholinergic anti-inflammatory pathway [26]. Conversely, sympathetic overactivity, a finding observed in pro-inflammatory states is associated with lower HRV and poor outcomes. Low HRV and dysautonomia has been reported in other infectious states such as community acquired pneumonia, dengue and HIV [[27], [28], [29]]. There is inverse correlation between both short and long term HRV recordings and inflammatory markers including CRP [[30], [31], [32]]. In the present study, patients with increased levels of inflammatory markers such as IL-6 (r = −0.36) and CRP (r = −0.30) had a significant negative correlation with HRV. Further, patients recovering from moderate/severe COVID-19 had lower HRV and increased prevalence of OH reflecting higher cardiovascular dysautonomia in these patients.
AI using deep learning neural networks and data from wearable devices have been previously used as an effective method for the early detection of COVID-19 [33]. Both HR and HRV has been used previously in a convolutional neural network model with an AUC of 0.77 ± 0.018 for prediction of onset of COVID-19. They reported lower HRV with increased respiration and heart rate following onset of COVID-19 [34]. In our study, we used ML algorithms to confirm and select the best HRV time domain measure as a non-invasive biomarker for Post- COVID-19 cardiovascular dysautonomia. We found MLP classification as the top performing ML model in terms of its sensitivity (91.3%) and specificity (87.5%) followed by AdaBoost Classifier (sensitivity 85.8% and Specificity 66.6%), Logistic Regression (sensitivity 77% and specificity 74%). MLP based permutation importance feature ranking showed HRV (RMSSD) is the top feature, followed by average RR and HR features to distinguishing between COVID-19 recovered patients and healthy controls. In the present study, HRV(RMSSD) time domain measure is the most relevant and accurate measure of ANS activity on a short-term ECG recording. In low- and middle-income countries, with a huge burden of COVID-19 recovered patients and having “post COVID-19 syndrome”, ML using low-cost ECG is a promising technique to identify those at risk of developing autonomic dysfunction.
5. Limitations
Single centre study and a small sample size are the major limitations of this study. However, this is the first study that examined the use of AI and HRV in post COVID-19 recovered subjects. Additionally, another limitation of our study could possibly be the lack of frequency domain measures of HRV as only time domain measures of HRV were analyzed. In the present study, there is a lack of follow up data, although these patients are currently being followed up every three months to assess HRV and the functional status in order to determine the persistence and clinical impact of dysautonomia in these patients.
6. Conclusion
The findings of our study provide insights into cardiovascular dysautonomia and its spectrum in COVID-19 recovered patients. Reduced HRV analysis using ECG and AI algorithms was found to be a simple, non-invasive biomarker for autonomic dysfunction in post-COVID-19 subjects.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
All authors have no conflict of interest with regards to the present submission.
Acknowledgement
Authors would like to thank the Centre of Excellence in Healthcare, Indraprastha Institute of Information Technology (IIIT)-Delhi, India for the support in this research work. The authors would also like to acknowledge of the contributions of Ms. Shruti Singh, Mrs. Neha Bansal and Mrs. Aarti Gupta for their help in obtaining the ECGs.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ipej.2022.01.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
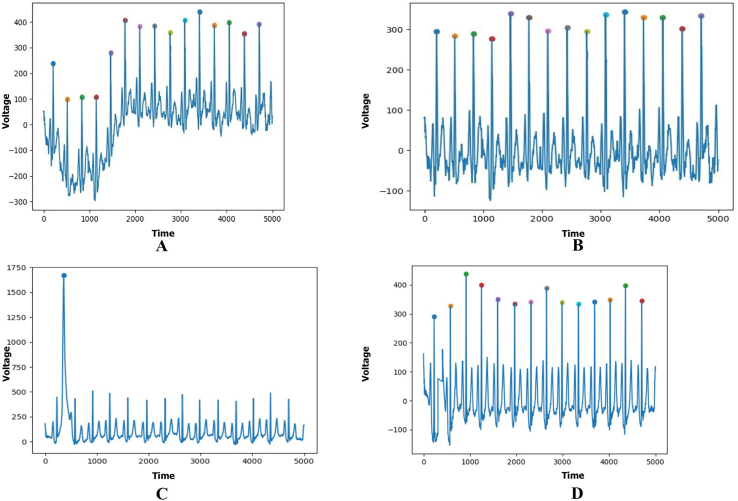
figs1.
Supplementary Figure S1: Raw ECG visualization and preprocessing (Fig S1A: before Baseline wandering correction; Fig S1B: After Baseline wandering correction; Fig S1C: Before R wave Noise correction; Fig S1D: After R wave Noise correction).
figs2.
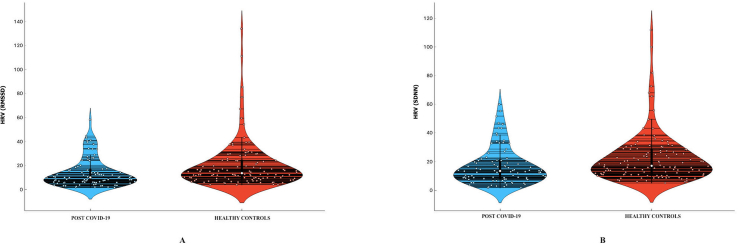
Supplementary Figure S2: Violin plot representation showing the HRV [RMSSD] (Fig S2A) and HRV [SDNN] (Fig S2B) in post COVID-19 recovered patients and heathy controls. The median value of HRV [RMSSD] in post COVID-19 recovered patients was 10.13 ms (IQR:10.93 ms) while that in heathy controls was 13.69 ms (IQR:14.77 ms). Similarly, the median value of HRV [SDNN] in post COVID-19 recovered patients was 13.50 ms (IQR:12.67 ms) while that in heathy controls was 17.10 ms (IQR:16.85 ms).
figs3.
Supplementary Figure S3: Feature importance score of all the eight features across the eight models.
References
- 1.Zhou P., Yang X.L., Wang X.G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunal S., Madan M., Tarke C., et al. Emerging spectrum of post-COVID-19 syndrome. Postgrad Med. 2021 doi: 10.1136/postgradmedj-2020-139585. postgradmedj-2020-139585. [DOI] [PubMed] [Google Scholar]
- 3.Dani M., Dirksen A., Taraborrelli P., et al. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin Med. 2021;21:e63–e67. doi: 10.7861/clinmed.2020-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fudim M., Qadri Y.J., Ghadimi K., et al. Implications for neuromodulation therapy to control inflammation and related organ dysfunction in COVID-19. J Cardiovasc Transl Res. 2020;13:894–899. doi: 10.1007/s12265-020-10031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eshak N., Abdelnabi M., Ball S., Elgwairi E., Creed K., Test V., Nugent K. Dysautonomia: an overlooked neurological manifestation in a critically ill COVID-19 patient. Am J Med Sci. 2020;360:427–429. doi: 10.1016/j.amjms.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carod-Artal F.J. Infectious diseases causing autonomic dysfunction. Clin Auton Res. 2018;28:67–81. doi: 10.1007/s10286-017-0452-4. [DOI] [PubMed] [Google Scholar]
- 7.Miglis M.G., Prieto T., Shaik R., Muppidi S., Sinn D.I., Jaradeh S. A case report of postural tachycardia syndrome after COVID-19. Clin Auton Res. 2020;30:449–451. doi: 10.1007/s10286-020-00727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman B.P., Khoury J.A., Blair J.E., Grill M.F. COVID-19 dysautonomia. Front Neurol. 2021;12:624968. doi: 10.3389/fneur.2021.624968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo Y.L. COVID-19, fatigue, and dysautonomia. J Med Virol. 2021;93:1213. doi: 10.1002/jmv.26552. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein D.S. The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm. 2021;18:508–509. doi: 10.1016/j.hrthm.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 12.Shaffer F., Ginsberg J.P. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst G. Heart-rate variability-more than heart beats? Front Public Health. 2017;5:240. doi: 10.3389/fpubh.2017.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasty F., García G., Dávila C.H., Wittels S.H., Hendricks S., Chong S. Heart rate variability as a possible predictive marker for acute inflammatory response in COVID-19 patients. Mil Med. 2020;186:e34–e38. doi: 10.1093/milmed/usaa405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aragón-Benedí C., Oliver-Forniés P., Galluccio F., et al. Is the heart rate variability monitoring using the analgesia nociception index a predictor of illness severity and mortality in critically ill patients with COVID-19? A pilot study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barizien N., Le Guen M., Russel S., Touche P., Huang F., Vallée A. Clinical characterization of dysautonomia in long COVID-19 patients. Sci Rep. 2021;11:14042. doi: 10.1038/s41598-021-93546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adler T.E., Norcliffe-Kaufmann L., Condos R., et al. Heart rate variability is reduced 3- and 6-months after hospitalization for COVID-19 infection. J Am Coll Cardiol. 2021;77:3062. [Google Scholar]
- 18.COVID-19 Treatment Guidelines Panel Coronavirus disease 2019 (COVID-19) treatment guidelines. National institutes of Health. https://www.covid19treatmentguidelines.nih.gov/ Available at. [PubMed]
- 19.Lahrmann H., Cortelli P., Hilz M., Mathias C.J., Struhal W., Tassinari M. EFNS guidelines on the diagnosis and management of orthostatic hypotension. Eur J Neurol. 2006;13:930–936. doi: 10.1111/j.1468-1331.2006.01512.x. [DOI] [PubMed] [Google Scholar]
- 20.Shouman K., Vanichkachorn G., Cheshire W.P., et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. 2021;31:385–394. doi: 10.1007/s10286-021-00803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundberg S.M., Lee S.I. A unified approach to interpreting model predictions. Proceedings of the 31st international conference on neural information processing systems. 2017:4768–4777. [Google Scholar]
- 22.Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2019;285:352–366. doi: 10.1111/joim.12852. [DOI] [PubMed] [Google Scholar]
- 23.Johansson M., Ståhlberg M., Runold M., et al. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Rep. 2021;3:573–580. doi: 10.1016/j.jaccas.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schondorf R., Low P.A. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 25.Kunal S., Sharma S.M., Sharma S.K., et al. Cardiovascular complications and its impact on outcomes in COVID-19. Indian Heart J. 2020;72:593–598. doi: 10.1016/j.ihj.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huston J.M., Tracey K.J. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med. 2011;269:45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aliberti S., Tobaldini E., Giuliani F., et al. Cardiovascular autonomic alterations in hospitalized patients with community-acquired pneumonia. Respir Res. 2016;17:98. doi: 10.1186/s12931-016-0414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter R., 3rd, Hinojosa-Laborde C., Convertino V.A. Heart rate variability in patients being treated for dengue viral infection: new insights from mathematical correction of heart rate. Front Physiol. 2014;5:46. doi: 10.3389/fphys.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lebech A.M., Kristoffersen U.S., Mehlsen J., et al. Autonomic dysfunction in HIV patients on antiretroviral therapy: studies of heart rate variability. ClinPhysiolFunct Imaging. 2007;27:363–367. doi: 10.1111/j.1475-097X.2007.00760.x. [DOI] [PubMed] [Google Scholar]
- 30.Araújo F., Antelmi I., Pereira A.C., et al. Lower heart rate variability is associated with higher serum high-sensitivity C-reactive protein concentration in healthy individuals aged 46 years or more. Int J Cardiol. 2006;107:333–337. doi: 10.1016/j.ijcard.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 31.Young L.C., Roediger M.P., Grandits G., et al. Relationship between inflammatory and coagulation biomarkers and cardiac autonomic function in HIV-infected individuals. Biomarkers Med. 2014;8:1073–1083. doi: 10.2217/bmm.14.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams D.P., Koenig J., Carnevali L., et al. Heart rate variability and inflammation: a meta-analysis of human studies. Brain Behav Immun. 2019;80:219–226. doi: 10.1016/j.bbi.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Mishra T., Wang M., Metwally A.A., et al. Pre-symptomatic detection of COVID-19 from smartwatch data. Nat Biomed Eng. 2020;4:1208–1220. doi: 10.1038/s41551-020-00640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Natarajan A., Su H.W., Heneghan C. Assessment of physiological signs associated with COVID-19 measured using wearable devices. NPJ Digit Med. 2020;3:156. doi: 10.1038/s41746-020-00363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.