Abstract
Purpose
This study aims to describe the late retinal and optic nerve vascular complications due Coronavirus disease 2019 (COVID-19) in a Spanish young population.
Methods
We describe 15 eyes of 15 young patients without any other systemic risk factors, except controlled arterial hypertension in 5 of them, with the diagnosis of Central retinal vein occlusion (CRVO), Branch retinal vein occlusion (BRVO), Central retinal artery occlusion (CRAO), Branch retinal artery occlusion (BRAO), Mixed occlusions (Artery and Vein) and Non-arteritic ischemic optic neuropathy (NAION) with a previous COVID-19 infection demonstrated with a positive COVID-19 IgG Test (COVID-19 IgG/IgM Rapid Test Cassette, Lambra Laboratories, Madrid, Spain.
Results
9 males and 6 females, with a mean age of presentation of 49.7 ± 9 years old were included. The mean time between infection and diagnosis of the disease was 3.5 ± 1.2 months. The most common retinal or optic nerve vascular complication was CRVO (6 cases), following by CRAO (4 cases), Mixed arterial and venous occlusions (2 cases), NAION (2 cases) and BRAO (1 case).
Conclusions
The presence of a retinal or optic nerve vascular event in a young patient without any other hypercoagulable or genetic thrombophilic disorder, should make us rule out a previous COVID-19 infection. Ophthalmologists must be awared that retinal circulation could be another potential site for thromboembolic and optic nerve circulatory insufficiency complications of COVID-19. To our knowledge, this is the longest case series of retinal or optic nerve vascular events described after COVID-19 infection.
Keywords: COVID-19, Hypercoagulable state, Retinal vascular occlusion, Optic neuropathy
1. Introduction
Coronavirus disease 2019 (COVID-19) is demonstrated to cause coagulation disorders and marked predisposition to both venous and arterial thromboembolic disease1 due to the excessive inflammation produced by the virus, involving high levels of IL-6, C-reactive protein and fibrinogen,2 hypoxia and diffuse intravascular coagulation (DIC).1 As a result of these events a hypercoagulable state occurs and manifests systemically as ischemic strokes, myocardial infarcts or pulmonary embolism.2
COVID-19 infects the host using the angiotensin-converting enzyme 2 (ACE-2) receptor which is highly expressed in lung alveolar cells, cardiac myocytes and the vascular endothelium including retinal endothelial cells, causing endotheliitis and vasculitis in both arterial and venous circulations. Inflammation of endothelial cells induces edema, congestion and thrombosis of small vessels, which eventually results in organ ischemia.3
Retinal vascular diseases could be complications of systemic vascular disorders, including inflammatory diseases and conditions leading to a hypercoagulable state as occurs in COVID-19 infection.3 In the eye, specifically in the retina, has been reported to manifest as retinal vein and artery occlusions,4, 5, 6, 7, 8 Non-Arteritic Anterior Ischemic Optic Neuropathy (NAION)9 and retinal microangiopathy, in some cases manifested as cotton wool spots (CWS).10,11
The aim of this study is to report a series of cases with late retinal and optic nerve vascular complications due COVID-19, in a younger spanish population than could be expected for this type of problems, and alert ophthalmologists that retinal circulation could be another potential site for thromboembolic complications of COVID-19.
2. METHODS
A retrospective study of the patients with the diagnosis of Central retinal vein occlusion (CRVO), Branch retinal vein occlusion (BRVO), Central retinal artery occlusion (CRAO), Branch retinal artery occlusion (BRAO), Mixed occlusions (Artery and Vein) and NAION with a previous COVID-19 infection demonstrated with a positive COVID-19 IgG Test (COVID-19 IgG/IgM Rapid Test Cassette, Lambra Laboratories, Madrid, Spain), took at the moment of the visit after the diagnosis was made. All patients were diagnosed between April 1st, 2020 and March 30th, 2021.
A complete medical history was performed and a thromboembolic risk analysis (including the known risk factors in young people, as homocystein, antiphospholipid síndrome, S and C protein, activated protein C resistance, Leiden factor V, antithrombin 3, prothrombin and collagenopathys and systemic diseases) were ruled out. The ocular examination included Visual acuity (VA) with Snellen Scale and Early Treatment Diabetic Retinopathy Study (ETDRS), biomicroscopy, ocular fundus, ocular coherence tomography (OCT) and OCT- Angiography (OCT-A). Other complementary exams were individualized according to medical criteria like Fluorescein Angiography (FA) in CRVO or BRVO and Visual Fields in patients with NAION.
3. RESULTS
Fifteen eyes of 15 patients (9 men and 6 women) with a mean age of 49.06 ± 9 years (range; 27–61 years, 9 patients under 50 years) were included. (Table 1). The only risk factors found were controlled systemic hypertension in 5 patients (33.33%). None of them had history of antiacoagulants treatment.
Table 1.
C-HTN, Controlled Hypertension; NAION, Non Arteritic Isquemic Optic Neuropathy; BRAO, Branch Retinal Artery Occlusion; CRAO, Central Retinal Artery Occlusion; BRVO, Branch Retinal Vein Occlusion, CRVO, Central Retinal Vein Occlusion; NA Not available* Didn't know the exact date when they passed COVID-19 as they were asymptomatic; IRNL, Internal retinal nasal layers; RNFL, Retinal nerve fiber layers; MA, Macular atrophy; ME, Macular edema; N, Normal; VA, Visual Acuity; VEGF, Vascular Endothelial Growth Factor; PRP, Panretinalphotocoagulation.
| Age/Gender | Risk Factors | Type of retinal/optic nerve vascular disease | COVID symptoms | Mean time (months) between COVID diagnosis and ocular symptoms | Hospitalization required by COVID? | OCT findings | Initial VA LogMAR | Treatment | Final VA LogMAR | |
|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 39, F | NO | NAION | No | NA* | No | Reduction in the RNFL | 1,3 | No | 1,3 |
| Case 2 | 58, M | NO | NAION | Yes | 4 | No | Reduction in the RNFL | 0,39 | No | 0,47 |
| Case 3 | 45, M | NO | BRAO | No | NA* | No | Thickenning of the IRNL | 0 | No | 0 |
| Case 4 | 61, F | C-HTN | CRAO | Yes | 5 | Yes | MA | 1 | No | 1 |
| Case 5 | 60, F | NO | CRAO | No | NA* | No | MA | 1,3 | No | 1,3 |
| Case 6 | 56, F | C-HTN | CRAO | No | NA* | No | MA | 2 | No | 2 |
| Case 7 | 46, M | NO | CRAO | Yes | 4 | No | MA | 1,3 | No | 1,3 |
| Case 8 | 27, M | NO | BRVO, BRAO | Yes | 4 | No | ME | 0,17 | Anti-VEGF | 0,09 |
| Case 9 | 60, F | C-HTN | CRVO, BRAO | No | 4 | No | ME | 1 | Anti-VEGF/PRP | 0,69 |
| Case 10 | 43, F | NO | CRVO | Yes | 4 | No | ME | 0,3 | Anti-VEGF | 0,17 |
| Case 11 | 46, M | C-HTN | CRVO | Yes | 4 | No | ME | 0,17 | Anti-VEGF | 0,09 |
| Case 12 | 45, M | NO | CRVO | No | 3 | No | ME | 0,17 | Anti-VEGF | 0,09 |
| Case 13 | 47, M | NO | CRVO | Yes | 4 | No | ME | 0,09 | Anti-VEGF | 0 |
| Case 14 | 57, M | C-HTN | CRVO | No | NA* | No | ME | 0,3 | Anti-VEGF | 0 |
| Case 15 | 45, M | NO | CRVO | Yes | 5 | No | N | 0,3 | Anti-VEGF | 0,09 |
Five patients were asymptomatic and did not know they had COVID-19 infection, 9 patients knew they had the infection and had minor symptoms (fever, general discomfort, dry cough etc.) and 1 had major symptoms (difficulty breathing, oxygen desaturation) that required hospitalization but did not require endotracheal intubation. None of them were previously vaccinated for COVID-19.
The mean time between infection and the diagnosis of retinal or optic nerve vascular disease was 3.70 ± 0,82 months. The most common retinal disease diagnosed with 6 cases was CRVO (Fig. 1), following with 4 CRAO (Fig. 2), 1 BRAO (Fig. 3), 2 Mixed Arterial/Vein occlusions (1 CRVO/BRAO and 1 BRVO/BRAO) (Fig. 4) and 2 NAION (Fig. 5).
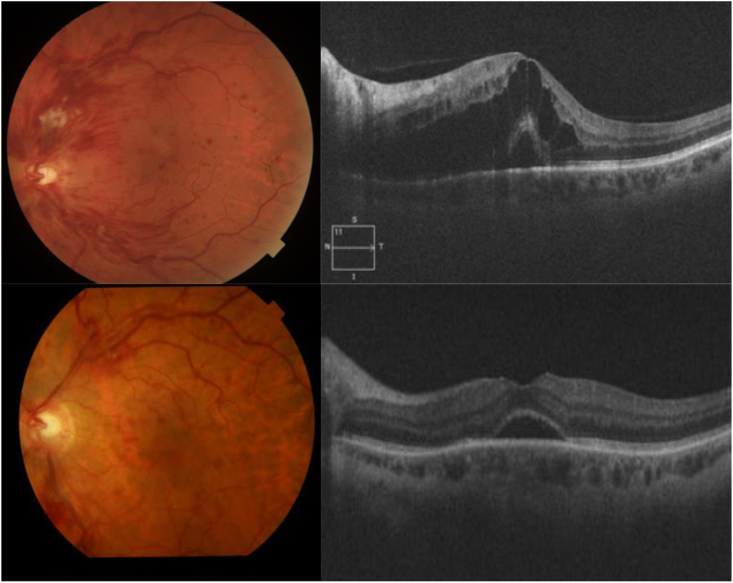
Fig. 1.
Case 12, 45-year-old male patient with no cardiovascular nor haemathologic risk factors, diagnosed with a CRVO in the left eye, developed 4 months after COVID infection, going through minor symptoms and no hospitalization required (A) and (B) Retinography and OCT B-scan through the macula shows cystic macular edema associated with foveal subretinal fluid. (C) and (D) Fundus photograph and OCT after 3 Aflibercept intravitreal injections showing a decrease in the intraretinal hemorrhages and the complete resolution of the intraretinal cysts with a remanent of subretinal fluid.
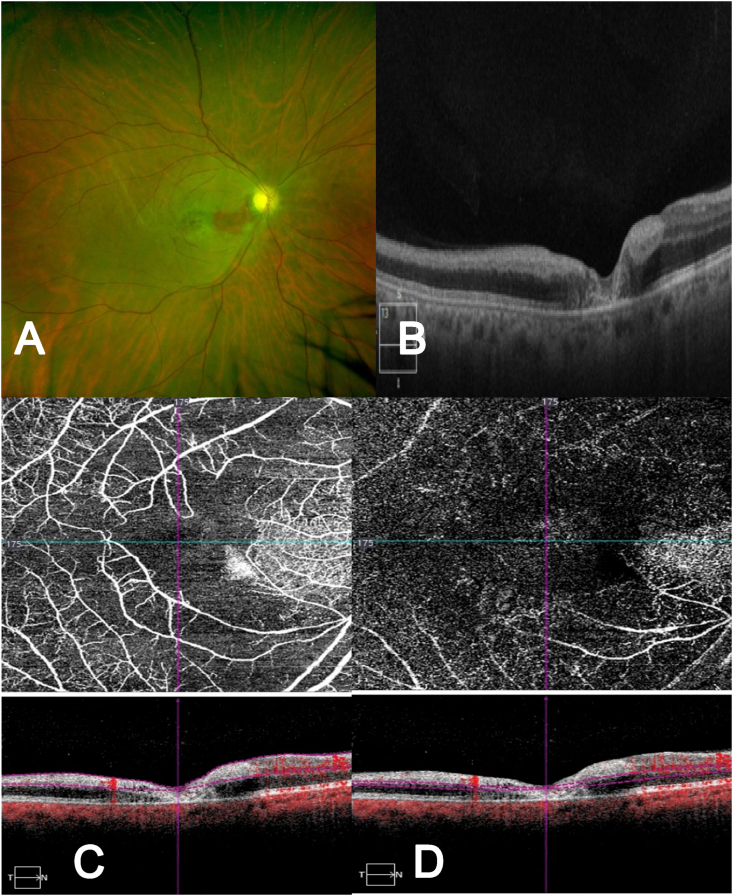
Fig. 2.
Case 7, 46-year-old male, with no cardiovascular nor haemathologic risk factors, diagnosed with a CRAO with cilioretinal artery sparing in the left eye, developed 4 months after COVID infection, going through minor symptoms and no hospitalization required (A) Fundus photograph showing the CRAO with cilioretinal artery sparing (B) OCT B-scan through the foveal region with a thickening of the internal retinal layers and a foveal atrophy of all the intermediate and external layers. (C) OCT-A (8 × 8) superficial layer with a decrease in the blood flow. (D) OCT-A (8 × 8) of the deep layer, corresponding with the intermediate and deep capillary plexus, showing a large zone of decrease of the capillary network.
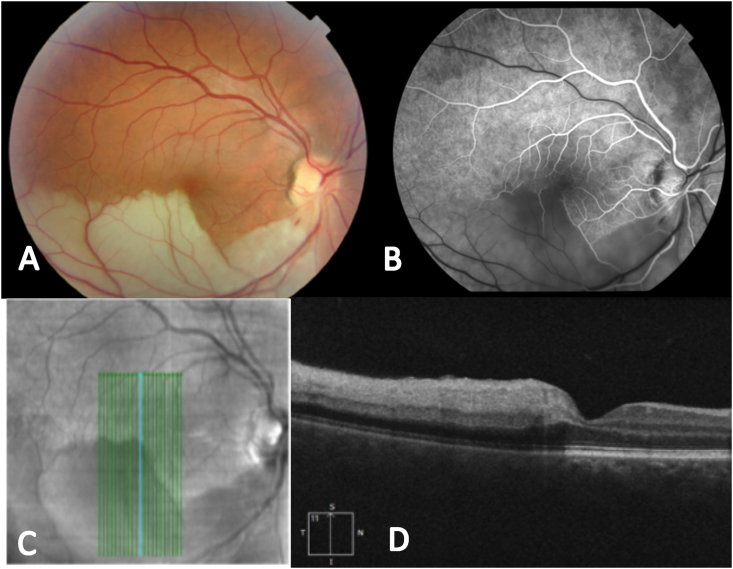
Fig. 3.
Case 3, 45 years old male, with no cardiovascular nor haemathologic risk factors, didn't know that passed COVID-19 infection and tested IgG (+) at the moment of the visit (A) Fundus photograph showing the inferior temporal artery occlusion (B) Early fluorescein angiography phase (C.D) OCT B-Scan showing an inferior to superior cut in order to show the differences between the affected area with hiperreflectivity in the internal layers and hiporreflectivity in the outer nuclear layer and below, and the normal retina (superior in C, right in D).
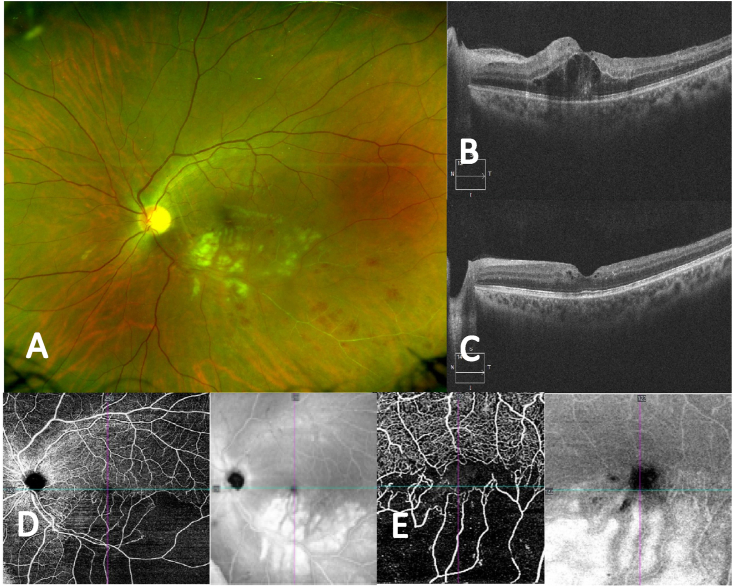
Fig. 4.
Case 8, 27-year-old male with no cardiovascular nor haemathologic risk factors, diagnosed with a mixed BRVO and BRAO in the left eye, developed 4 months after COVID infection, going through minor symptoms and no hospitalization required, (A) Fundus photograph showing the inferotemporal branch artery and vein occlusion with multiple CWS and intraretinal hemorrhages (B) OCT B-scan through the macula shows some parafoveal intraretinal cysts. (C) OCT B-scan 3 months after 3 Ranibizumab intravitreal injections showing an important reduction in the intraretinal cysts. (D) OCT-A (12 × 12): Superficial layer shows a reduction of the capillary network in the inferotemporal zone. (E) OCT-A (3 × 3) Foveal area detailed.
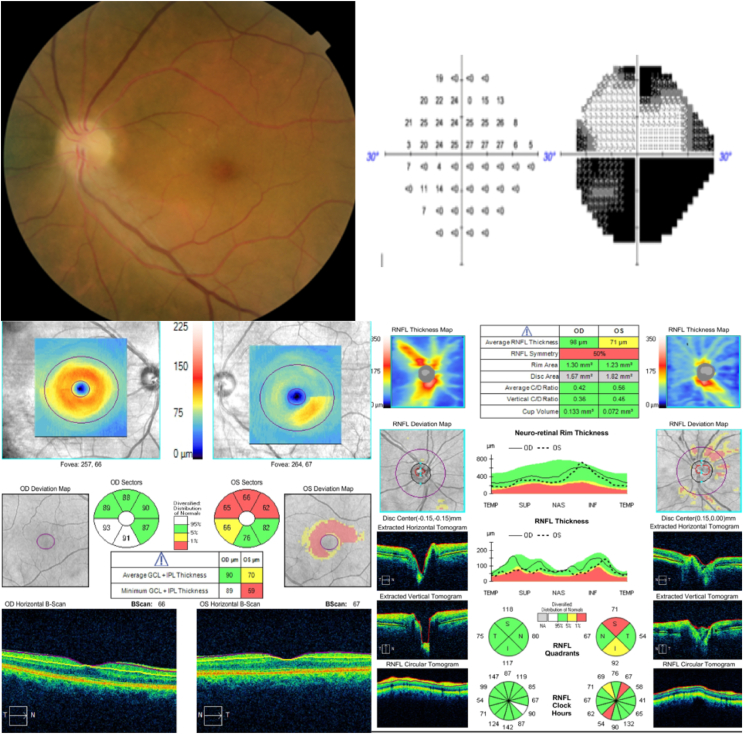
Fig. 5.
Case 2, 58-year-old female patient without any risk factors, diagnosed with a NAION in the left eye, developed 4 months after COVID infection, going through minor symptoms and no hospitalization required, (A)Fundus photography showing an optic disk atrophy (B) Visual Field 24-2 with altitudinal defect. (C) Ganglion c ell layer comparison between right and left eye shows a marked reduction of the ganglion cells in the left eye (D) Retinal Nerve fiber layer (RNFL) comparison with a marked superior and inferior reduction in left eye.
Mean initial VA was 0.39 ± 0.39 logMAR, 7 of the 15 patients (46%) presented cotton wool spots (CWS) at the moment of the diagnosis, 10 (66,6%) patients had retinal ischemia diagnosed by OCT-A and/or AGF secondary to BRAO (1 case), BRVO/BRAO (1 case), CRVO/BRAO (1 case), CRVO (3 cases) and more frequently CRAO (4 cases), 53.33% had macular edema seen by OCT due to either branch or central vein occlusion only, 26.66% had macular atrophy due to CRAO, 13.33% had thickening of the internal retinal nasal layers (IRNL) secondary to BRAO and 40% had optic disk atrophy (4 OACR and 2 NOIAN).
Patients with CRVO, CRVO/BRAO and BRAO/BRVO received treatment (8 eyes). 7 eyes received Anti Vascular Endothelial Growth Factor (Anti-VEGF) intravitreal injection (Ranibizumab or Aflibercept), with a mean number of intravitreal injections of 3 and 1 patient with CRVO/BRAO received 3 injections plus Panretinalphotocoagulation (PRP). The arterial occlusions did not receive any kind of the treatment at the moment of diagnosis, because they were out of therapeutic window.
Mean final VA in treated patients improved from 0.39 logMAR. to 0.17 ± 0.69 logMAR.
4. Discussion
The complete spectrum of clinical manifestations associated with COVID-19 is not fully elucidated, as far as new clinical symptoms are often described.
In the eye, acute COVID-19 infection can cause conjunctivitis in up to 31.6% of patients,12 conjunctival hyperemia, chemosis, epiphora, and increased secretions have been reported mostly during the middle phase of the disease.13
Microangiopathy has been described in multiple organs in COVID-19 autopsies.14 In the retina microvascular ischemia, cotton wool spots, micro-hemorrhages and hyperreflectivity lesions at the level of ganglion cell and inner plexiform layer have been described in symptomatic and asymptomatic COVID-19 patients.10,11,15 Nerve fiber layer infarcts have been described in multiple viral diseases like Dengue, HIV and West Nile virus infections. Inmune-complex deposition and increased plasma viscosity have been proposed as possible pathogenic mechanism16 in viral infections like HIV. The current pandemic caused by COVID-19 is associated with coagulation activation and disproportionate systemic inflammatory response.17,18
As previously mentioned COVID-19 precipitates the onset of a systemic inflammatory response syndrome, resulting in the activation of the coagulation cascade that induces to a hypercoagulable state. However, whether the coagulation cascade is directly activated by the virus or whether is the result of local or systemic inflammation is not completely understood. These findings are consistent with the close connection between thrombosis, that in the eye could manifest as retinal vein or artery occlusion and inflammation.18
In the other hand, the mechanism with which COVID-19 develops a NAION is different. The pathophysiology and its association to COVID is thought to be developed from a circulatory insufficiency of the posterior ciliary arteries supplying the optic nerve. Patients with COVID-19 infection can manifest with hypercoagulability and hypoxemia, both of which may contribute to the development of NAION.9
Patients with moderate to severe infection are more likely to have thrombotic complications that usually occur in late stages of the disease.1 Klok reported that the most common in critically ill ICU patients with covid-19 were pulmonary embolism and deep-vein thrombosis of the leg1. In our study we observed that patients with mild or asymptomatic COVID-19 infection can also develop late thrombotic complications in the retinal vascular circulation as the mean time between being diagnosed with COVID-19 and developing retinal vascular occlusion (RVO) was 4,10 (±0,5) months.
Our mean age of presentation was 49 (±9) years old in patients with no risk factors and published literature states that retinal vascular disease and NAION are rare in people under 60 years old.4 Sang et al. reported an incidence of 26.2% of CRVO in patients under 50 years old with a high prevalence of risk factors such as hematologic disease, active smoker and recent physical or psychological stress.19
Accute coagulopathy events are associated with elevations in fibrinogen an D-dimer levels18; but in ocular thrombosis we did not find an association with it, as there were not alterations in the blood analysis, comprising complete blood count, glycemia, lipidic profile, homocysteinemia, anticardiolipin IgM and Ig G antibodies, and screening for genetic thrombophilias (Factor V Leiden and prothrombin mutations, antithrombin II and proteins C and S deficiencies). Also prothrombin time (PT) and activated partial thromboplastin time (APTT) were normal. The only cardiovascular risk factor that was present in our patients was systemic hypertension, but in all patients was under treatment and controlled, without any other complications.
Most remarkable ocular findings were very low vision or important scotoma at presentation with or without afferent pupillary defect, many CWS and the presence of ischemia related with arterial narrowing, outer retina atrophy and/or decreased capillary flow in the superficial retinal plexus over the lesions area seen by OCT-A.
In a case series of 27 asymptomatic subjects, a 22% of retinal microangiopathy manifested as elliptical CWS was described.10 Although initial COVID-19 retinal microangiopathy could be a direct viral effect, the presence of late vascular complications, several months after acute infection, suggest the presence of other mechanisms. It seems that shares the diabetic mechanism of ACE2R downregulation that might play a major role in inducing the development of retinal ischemia and even acts as a marker of endothelial disease.20
A weakness of the study could be that there is no direct evidence linking these processes to COVID-19, but that this is very probably the case, since they were all young patients without underlying pathologies. To our knowledge, this is the longest series of ocular vascular events described after COVID-19 infection.
5. Conclusion
The presence of an ocular occlusive event in a young patient without any other hypercoagulable disorder or genetic thrombophilia study should make us think about previous infection by COVID-19. Ophthalmologists must be prepared and aware that beyond the involvement of the ocular surface there may be an increase in the incidence of young patients with retinal vascular or optic nerve diseases due to the hyperinflammatory a hypercoagulable stage triggered by COVID-19 infection.
The patient(s) consented to publication of the case in writing/orally.
Disclosures
None.
Acknowledgments
None.
References
- 1.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voicu S., Ketfi C., Stépanian A., Bn Chousterman, Mohamedi N., et al. Vol. 11. January 2021. (Pathophysiological Processes Underlying the High Prevalence of Deep Vein Thrombosis in Critically Ill COVID-19 Patients). Article 608788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karampelas M., Dalamaga M., Karampela I. Does COVID-19 involve the retina? Ophthalmol Ther. 2020;9:693–695. doi: 10.1007/s40123-020-00299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yahalomi T., Pikkel J., Arnon R., Pessach Y. Central retinal vein occlusion in a young healthy COVID-19 patient: a case report. Am. J. Ophthalmol. Case Rep. 2020;20:100992. doi: 10.1016/j.ajoc.2020.100992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acharya S., Diamond M., Anwar Shamsuddin, Glaser A., Tyagi P. Unique case of central retinal artery occlusion secondary to COVID-19 disease. IDCases. 2020;21 doi: 10.1016/j.idcr.2020.e00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walinjkar J.A., Makhija S.C., Sharma H.R., Morekar S.R., Natarajan S. Central retinal vein occlusion with COVID-19 infection as the presumptive etiology. Indian J Ophthalmol. 2020;68:2572–2574. doi: 10.4103/ijo.IJO_2575_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montesel A., Bucolo C., Mouvet V., Moret E., Eandi C. Case report: central retinal artery occlusion in a COVID-19 patient. December. 2020;ume 11 doi: 10.3389/fphar.2020.588384. Article 588384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Invernizzi A., Pellegrini M., Messenio D., et al. 2020. Impending Central Retinal Vein Occlusion in a Patient with Coronavirus Disease 2019 (COVID-19), Ocular Immunology and Inflammation. [DOI] [PubMed] [Google Scholar]
- 9.Rho J., Dryden S.C., Mcguffey C.D., et al. A case of non-arteritic anterior ischemic optic neuropathy with COVID-19. Cureus. 2020;12(12) doi: 10.7759/cureus.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landecho M.F., Yuste J.R., Gándara E., et al. COVID-19 retinal microangiopathy as an in vio biomarker of systemic vascular disease? J Intern Med. 2021;289:116–120. doi: 10.1111/joim.13156. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Lopez J.J., Felix Espinar B., Ye-Zhu C. Symptomatic retinal microangiophaty in a patient with coronavirus disease 2019 (COVID-19): single case report. Ocul Immunol Inflamm. 2021;29:642. doi: 10.1080/09273948.2020.1852260. [DOI] [PubMed] [Google Scholar]
- 12.Shi H., Han X., Jiang N., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu P., Duan F., Luo C., et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei province, China. JAMA Ophthalmol. 2020;138(5):575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao X.H., Li T.Y., He Z.C., et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 15.Marinho P.M., Marcos A.A.A., Romano A.C., NascimentoH BelfortRJr. Retinal findings in patients with COVID-19. Lancet. 2020;395:1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feroze K.B., GulickPG . StatPearls; Treasure Island (FL): 2020. HIV Retinopathy. [Google Scholar]
- 17.Marietta M., Ageno W., Artoni A., et al. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET) Blood Transfus. 2020;18(3):167–169. doi: 10.2450/2020.0083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker R. COVID-19 update: covid-19 associated coagulopathy. J Thromb Thrombolysis. May 2020 doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eah K.S., Kim Y.N., Park Y.J. Central retinal vein occlusion in young patients, retina. J Retin Vit Dis. 2021;41 doi: 10.1097/IAE.0000000000002872. number 3. [DOI] [PubMed] [Google Scholar]
- 20.Zhnger J.M., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Invasive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]