Abstract
Background
Direct oral anticoagulants (DOACs) are preferred over warfarin for stroke prevention in atrial fibrillation (AF). Meta-analyses using individual patient data offer significant advantages over study-level data.
Methods
We used individual patient data from the COMBINE AF database, which includes all patients randomized in the 4 pivotal trials of DOACs vs warfarin in AF (RE-LY, ROCKET AF, ARISTOTLE, ENGAGE AF-TIMI 48), to perform network meta-analyses using a stratified Cox model with random effects comparing standard-dose DOAC, lower-dose DOAC, and warfarin. Hazard ratios (95% CIs) were calculated for efficacy and safety outcomes. Covariate-by-treatment interaction was estimated for categorical covariates and for age as a continuous covariate, stratified by sex.
Results
A total of 71,683 patients were included (29,362 on standard-dose DOAC, 13,049 on lower-dose DOAC, 29,272 on warfarin). Compared with warfarin, standard-dose DOACs were associated with a significantly lower hazard of stroke/systemic embolism (883/29312 [3.01%] vs 1080/29229 [3.69%]; HR 0.81, 95% CI 0.74–0.89), death (2276/29312 [7.76%] vs 2460/29229 [8.42%]; HR 0.92, 95% CI 0.87–0.97) and intracranial bleeding (184/29270 [0.63%] vs 409/29187 [1.40%]; HR 0.45, 95% CI 0.37–0.56), but no statistically different hazard of major bleeding (1479/29270 [5.05%] vs 1733/29187 [5.94%]; HR 0.86, 95% CI 0.74–1.01), whereas lower-dose DOACs were associated with no statistically different hazard of stroke/systemic embolism (531/13049 [3.96%] vs 1080/29229 [3.69%]; HR 1.06, 95% CI 0.95–1.19) but a lower hazard of intracranial bleeding (55/12985 [0.42%] vs 409/29187 [1.40%]; HR 0.28, 95% CI 0.21–0.37), death (1082/13049 [8.29%] vs 2460/29229 [8.42%]; HR 0.90, 95% CI 0.83–0.97), and major bleeding (564/12985 [4.34%] vs 1733/29187 [5.94%]; HR 0.63, 95% CI 0.45–0.88). Treatment effects for standard- and lower-dose DOACs versus warfarin were consistent across age and sex for stroke/systemic embolism and death, whereas standard-dose DOACs were favored in patients with no history of vitamin K antagonist use (p=0.01) and lower creatinine clearance (p=0.09). For major bleeding, standard-dose DOACs were favored in patients with lower body weight (p=0.02). In the continuous covariate analysis, younger patients derived greater benefits from standard-dose (interaction p=0.02) and lower-dose DOACs (interaction p=0.01) versus warfarin.
Conclusions
Compared with warfarin, DOACs have more favorable efficacy and safety profiles among patients with AF.
Keywords: Atrial fibrillation, Anticoagulation, Direct Oral Anticoagulant, Non-vitamin K Antagonist, Warfarin, Stroke, Meta-Analysis
BACKGROUND
Direct oral anticoagulants (DOACs) are recommended by both European and North American guidelines as first-line therapy for prevention of ischemic stroke in patients with atrial fibrillation (AF).1, 2 Four DOACs (dabigatran, rivaroxaban, apixaban, and edoxaban) have obtained regulatory approval and guideline recommendations for stroke prevention in patients with AF based on data from 4 pivotal randomized trials comparing DOAC versus warfarin.3–6 These trials excluded patients with moderate or severe mitral stenosis and with mechanical prosthetic valves (i.e. “valvular AF”), leading to a product-labeled indication for all DOACs for non-valvular AF that will henceforth be referred to as AF in the present report.
Previously-published trial-level meta-analyses used aggregate data from the 4 pivotal trials and demonstrated that DOAC use is associated with significant reductions in stroke, intracranial hemorrhage and death compared with warfarin, with no statistically different risk of major bleeding.7 Study-level meta-analyses however are subject to significant limitations.8 Meta-analyses using individual patient data offer important advantages over study-level data. Individual patient data meta-analyses allow for the analyses of individual patient-level time-to-event censored survival data and application of consistent follow-up time across trials, rather than simply pooling study-level hazard ratios that were estimated under different settings across individual trials. Individual patient-level meta-analyses further allow for the analyses of continuous variables and a more thorough assessment of treatment effect heterogeneity.9–11
The COMBINE AF (A COllaboration between Multiple institutions to Better Investigate Non-vitamin K antagonist oral anticoagulant usE in Atrial Fibrillation) database contains individual patient data from the 4 pivotal trials of DOACs versus warfarin in patients with AF.12 We used data from the COMBINE AF database to perform network meta-analyses, aimed at assessing the overall safety and efficacy of DOACs versus warfarin, including two different DOAC treatment strategies (standard-dose and lower-dose). In these network meta-analyses, we aimed to leverage the strengths of individual patient data and estimate treatment effects by standardizing follow-up duration for time-to-event outcomes and study population across trials and to assess effect modification with a Cox regression model as well as across the spectrum of age as a continuous covariate.
METHODS
Anonymized data from the COMBINE AF database are shared by members of the COMBINE AF executive committee and their corresponding institutions, however these data are unable to be shared outside of these institutions due to preexisting data privacy restrictions. Individual investigators may reach out directly to a member of the COMBINE AF executive committee to discuss opportunities for collaboration.
Study Selection, data sources, and treatment strategies
The design and rationale for COMBINE AF has been previously published12 and a list of COMBINE AF investigators can be found in the online supplement (Supplement Table 1). COMBINE AF contains individual patient data from RE-LY (Randomized Evaluation of Long Term Anticoagulation Therapy),3 ROCKET AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation),4 ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation),5 and ENGAGE AF-TIMI 48 (Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation – Thrombolysis in Myocardial Infarction 48),6 which studied dabigatran, rivaroxaban, apixaban, and edoxaban (respectively) versus warfarin in patients with AF. All individual trials were performed in accordance with local data protection regulations that were in place at the time of study conduct, were approved by the local Institutional Review Board, and all study subjects provided written informed consent. Creation of the COMBINE AF database and the statistical analyses for this manuscript were approved by the Duke University Institutional Review Board. COMBINE AF is registered with PROSPERO (CRD42020178771).
For these analyses, a standard-dose DOAC treatment strategy was defined as dabigatran 150mg twice daily (RE-LY), rivaroxaban 20mg (or 15mg if dose reduction criteria were met) once daily (ROCKET AF), apixaban 5mg (or 2.5mg if dose reduction criteria were met) twice daily (ARISTOTLE), or edoxaban 60mg (or 30mg if dose reduction criteria were met) once daily (ENGAGE AF-TIMI 48). A lower-dose DOAC treatment strategy was defined as dabigatran 110mg twice daily (RE-LY) or edoxaban 30mg (or 15mg if dose reduction criteria were met) once daily (ENGAGE AF-TIMI 48). Patients in our meta-analyses were analyzed according to their randomization group regardless of whether they were treated with dose reduction by individual trial criteria.
Outcomes
All outcomes were adjudicated as described in the individual trials and were assessed as time-to-first-event. Charter definitions of the adjudicated outcomes were similar across each individual trial. The primary efficacy outcome was a composite outcome of any stroke (ischemic, hemorrhagic, or other) or systemic embolism (i.e. stroke/systemic embolism). Secondary efficacy outcomes included all-cause death, cardiovascular death, ischemic stroke, systemic embolism, hemorrhagic stroke, any stroke, and a composite efficacy outcome consisting of ischemic stroke, systemic embolism, or cardiovascular death.
The primary safety outcome was major bleeding as defined by the International Society on Thrombosis and Haemostasis (ISTH).13 Secondary safety outcomes included fatal bleeding, major or clinically relevant non-major bleeding, any bleeding (including fatal, major, clinically relevant non-major, or minor bleeding), intracranial bleeding, and gastrointestinal bleeding (adjudicated major bleeding events determined to be from gastrointestinal bleeding events only).
Two net clinical benefits were assessed. These were the composite of any stroke, systemic embolism, all-cause death, or major bleeding and the composite of any stroke, systemic embolism, all-cause death, or intracranial bleeding.
Study population
Efficacy outcomes were assessed using an intention-to-treat population, while safety outcomes and net clinical benefits were assessed in the safety population as defined by the individual trials. A common definition of safety populations across trials was patients who received ≥1 dose of study drug and were followed for events occurring between the dates the patient began study drug and ≤2 days (or ≤3 in ENGAGE-AF TIMI 48) following the last dose of study drug. To account for differences in follow-up duration between trials, patients were censored at 32-months, which was the point at which < 10% of patients remained at risk across all studies (Supplement Table 2).
Statistical analyses
We performed network meta-analyses to compare time-to-event measures of efficacy and safety outcomes and net clinical benefits for 3 treatment strategies (standard-dose DOAC, lower-dose DOAC, and warfarin).14 For the primary analyses, Kaplan-Meier curves were generated for key outcomes and univariable stratified Cox proportional hazard models were fitted including treatment strategy as an independent variable. Cox models were stratified by trial allowing random effects to account for cross-trial heterogeneity. To evaluate treatment strategies, we compared hazard ratios (HRs) with 95% confidence intervals (CIs) for standard-dose or lower-dose DOACs versus warfarin. Between-trial heterogeneity was assessed by the estimated standard deviation of random effects. A larger standard error of the HR estimate is expected with large between-study heterogeneity compared with that estimated under fixed effect Cox models. We evaluated the proportional hazards assumption using graphical approach of Kaplan-Meier curves and the global Schoenfeld test.15 There was no strong evidence of violation of the proportional hazard assumption for any of the examined outcomes. To report trial-specific HRs, we fitted a Cox model to individual trials. The secondary analyses assessed effect modification by fitting stratified Cox proportional hazards models with random effects including baseline covariate-by-treatment interaction. For categorical covariates, HRs for standard-dose or lower-dose DOACs compared with warfarin were calculated and the associated 95% CIs were reported with p-values for interaction.
Event rates (% per year) were calculated by categorical baseline body weight and creatinine clearance for each randomized treatment arm. To assess treatment effect variation across age, we fitted a separate model and calculated change in HR per unit change in age with associated 95% CIs and interaction p-values. In addition, we fitted an extended model including a three-way interaction of age, sex, and treatment to further assess if the age-by-treatment interaction differs by sex. We did not adjust interaction p-values for multiplicity as these analyses were not confirmatory but rather exploratory.16 When assessing an interaction, we considered a p-value of <0.1 to indicate potentially meaningful evidence of effect modification, as testing for interactions has limited statistical power.16, 17
We conducted sensitivity analyses limiting the study population only to trials of factor Xa inhibitors (i.e. excluding all patients from RE-LY) (Supplement Figure 1). For these analyses, the lower-dose edoxaban arm from ENGAGE AF-TIMI 48 was not analyzed, as these analyses would have served only to replicate findings from the individual trial.
All analyses were conducted using the coxme (version 2.2)18 and survival (version 3.2)19 packages on R, version 3.5.3 (The R Foundation).
RESULTS
Patient characteristics
A total of 71,683 patients were included in these analyses (Supplement Figure 1; n=29,362 randomized to standard-dose DOAC, n=13,049 randomized to lower-dose DOAC, and n=29,272 randomized to warfarin). After censoring at 32-months, the median (25th, 75th percentile) follow-up duration was 26.6 (18.9, 32.0) months. Baseline characteristics by treatment strategy can be found in Table 1. No clinically meaningful differences were observed across randomized treatment groups. Baseline characteristics by trial and extent of missing baseline variables from individual trials have previously been published.12
Table 1.
Baseline characteristics by randomized treatment assignment (i.e. standard-dose DOAC, lower-dose DOAC, or warfarin).
| Pooled by Treatment Strategy |
||||
|---|---|---|---|---|
| Demographics | All Patients n=71,683 |
Standard-dose DOAC n=29,362 |
Lower-dose DOAC n=13,049 |
Warfarin n=29,272 |
| Age (years) | 72 (65–77) | 72 (65–77) | 72 (66–77) | 72 (65–77) |
| < 65 | 24.0 | 24.7 | 21.4 | 24.6 |
| 65 – 75 | 37.4 | 36.9 | 39.1 | 37.2 |
| ≥ 75 | 38.6 | 38.5 | 39.5 | 38.2 |
| Female | 37.3 | 37.4 | 37.4 | 37.1 |
| Race | ||||
| White | 80.2 | 80.7 | 77.8 | 80.7 |
| Black | 1.2 | 1.3 | 1.1 | 1.2 |
| Asian | 14.2 | 14.1 | 14.8 | 14.2 |
| Other | 4.4 | 3.9 | 6.2 | 4.0 |
| Hispanic | 9.7 | 11.3 | 2.7 | 11.3 |
| Vital Signs | ||||
| Weight (kg) | 81 (70–95) | 81 (70–95) | 81 (70–95) | 81 (70–94) |
| < 60 | 9.3 | 9.4 | 9.0 | 9.5 |
| 60 – 120 | 85.7 | 85.6 | 86.1 | 85.6 |
| ≥ 120 | 4.8 | 4.9 | 4.9 | 4.8 |
| Systolic BP (mmHg) | 130 (120–140) | 130 (120–140) | 130 (120–140) | 130 (120–140) |
| BMI (kg/m2) | 28.3 (25.2–32.2) | 28.4 (25.2–32.2) | 28.4 (25.2–32.2) | 28.3 (25.1–32.2) |
| < 25 | 23.7 | 23.6 | 23.5 | 23.9 |
| 25 – 30 | 38.1 | 37.9 | 38.7 | 38.2 |
| ≥ 30 | 37.9 | 38.3 | 37.6 | 37.6 |
| Alcohol* | ||||
| None/Rare | 60.2 | 62.6 | 48.4 | 63.0 |
| Light/Moderate | 13.2 | 15.3 | 4.6 | 15.0 |
| Heavy | 1.3 | 1.4 | 0.9 | 1.4 |
| Medical History | ||||
| Diabetes | 30.8 | 31.1 | 30.3 | 30.8 |
| Hypertension | 87.7 | 87.8 | 86.7 | 88.0 |
| CAD | 29.9 | 29.7 | 30.9 | 29.8 |
| MI | 14.6 | 14.7 | 14.0 | 14.9 |
| CABG* | 5.2 | 5.5 | 3.7 | 5.4 |
| PCI* | 6.0 | 6.5 | 4.0 | 6.5 |
| Heart failure | 46.4 | 46.8 | 45.3 | 46.6 |
| Stroke or TIA | 28.1 | 28.8 | 24.5 | 29.0 |
| Paroxysmal AF | 23.2 | 21.6 | 28.8 | 22.2 |
| Smoking (ever) | 43.6 | 43.4 | 46.3 | 42.6 |
| CHADS2 score | ||||
| 0–1 | 16.7 | 17.2 | 15.1 | 16.9 |
| 2 | 34.5 | 32.6 | 41.3 | 33.3 |
| ≥3 | 48.8 | 50.2 | 43.6 | 49.8 |
| Prior GI bleeding* | 2.8 | 3.0 | 1.9 | 3.1 |
| Prior non-GI bleeding* | 5.6 | 6.0 | 4.0 | 5.8 |
| Baseline Medications | ||||
| Prior VKA use (ever) | 68.2 | 67.6 | 71.5 | 67.4 |
| Aspirin | 33.8 | 33.6 | 33.9 | 33.9 |
| Thienopyridine | 3.0 | 2.8 | 4.0 | 2.9 |
| Beta blocker | 64.3 | 64.3 | 64.8 | 64.1 |
| Calcium channel blocker | 30.5 | 30.1 | 31.9 | 30.3 |
| NSAID | 4.1 | 4.3 | 2.8 | 4.5 |
| Digoxin | 31.9 | 32.1 | 29.4 | 32.7 |
| PPI | 11.9 | 12.2 | 10.5 | 12.3 |
| Amiodarone | 10.6 | 10.6 | 10.9 | 10.6 |
| Laboratory Studies | ||||
| Creatinine clearance (mL/min) | 70.0 (54.0–90.3) | 70.0 (54.0–91.0) | 69.5 (53.6–90.0) | 70.0 (54.0–90.0) |
| ≤50 | 19.6 | 19.6 | 19.8 | 19.4 |
| 51–80 | 44.4 | 44.0 | 45.1 | 44.5 |
| >80 | 35.8 | 36.1 | 35.1 | 35.9 |
| LVEF* | ||||
| Normal | 46.5 | 47.2 | 42.5 | 47.5 |
| Mild | 11.6 | 11.9 | 10.1 | 12.0 |
| Moderate | 8.2 | 8.6 | 6.8 | 8.5 |
| Severe | 3.8 | 3.8 | 3.2 | 3.9 |
Continuous variables listed as median (25th-75th percentile). Categorical variables listed as %. Creatinine clearance calculated using Cockcroft-Gault equation.
“Standard-dose DOAC” includes the dabigatran 150mg twice daily arm from RE-LY, the rivaroxaban arm from ROCKET AF, the apixaban arm from ARISTOTLE, and the standard-dose edoxaban arm from ENGAGE AF-TIMI 48. “Lower-dose DOAC” includes the dabigatran 110mg twice daily from RE-LY and the lower-dose edoxaban arm from ENGAGE AF-TIMI 48.
= data missing from 1 or more of the individual trials. See reference #12 for detailed information regarding missing data.
BMI = body mass index, CABG = coronary artery bypass graft, CAD = coronary artery disease, MI = myocardial infarction, NSAID = non-steroidal anti-inflammatory, PCI = percutaneous coronary intervention, PPI = proton pump inhibitor, TIA = transient ischemic attack, VKA = vitamin K antagonist
In the study population, the median (25th, 75th percentile) age was 72 (65, 77) years, 37.3% were female, 34.5% had a CHADS2 score of 2, and 48.8% had a CHADS2 score ≥3 (Table 1). Over 2/3 (68.2%) of patients had used a vitamin K antagonist (VKA) prior to randomization and 33.8% were on aspirin at baseline. A total of 19.6% of patients had creatinine clearance of ≤50 at baseline.
Efficacy outcomes
All efficacy outcomes showed little or no between-trial heterogeneity with estimated standard deviation of random effects across trials close to zero (Supplement Table 4). HR’s for the primary efficacy outcome from each individual trial can be found in Supplement Table 5.
Compared with warfarin, patients randomized to standard-dose DOAC had a lower hazard of stroke/systemic embolism (883/29312 (3.01%) vs 1080/29229 (3.69%); HR 0.81, 95% CI 0.74–0.89) and a lower hazard of all-cause death, cardiovascular death, systemic embolism, hemorrhagic stroke, any stroke, and the composite efficacy outcome (ischemic stroke, systemic embolism, or cardiovascular death) over the duration of follow-up (Figure 1). Kaplan-Meier curves for key efficacy outcomes can be found in Figure 2.
Figure 1.
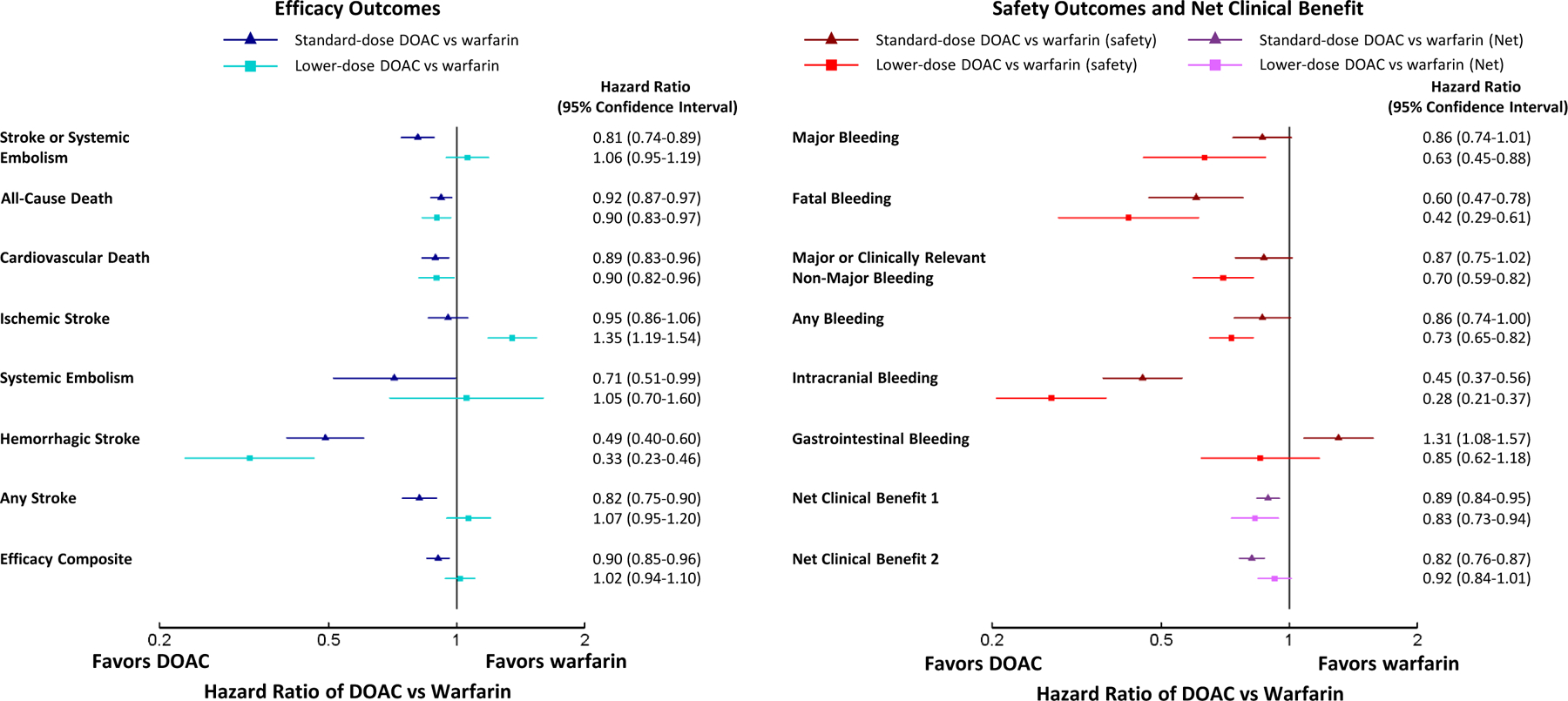
Forest plots showing hazard ratios comparing standard-dose and lower-dose DOAC versus warfarin for efficacy outcomes (left) and safety/net clinical benefit outcomes (right).
Figure 2.
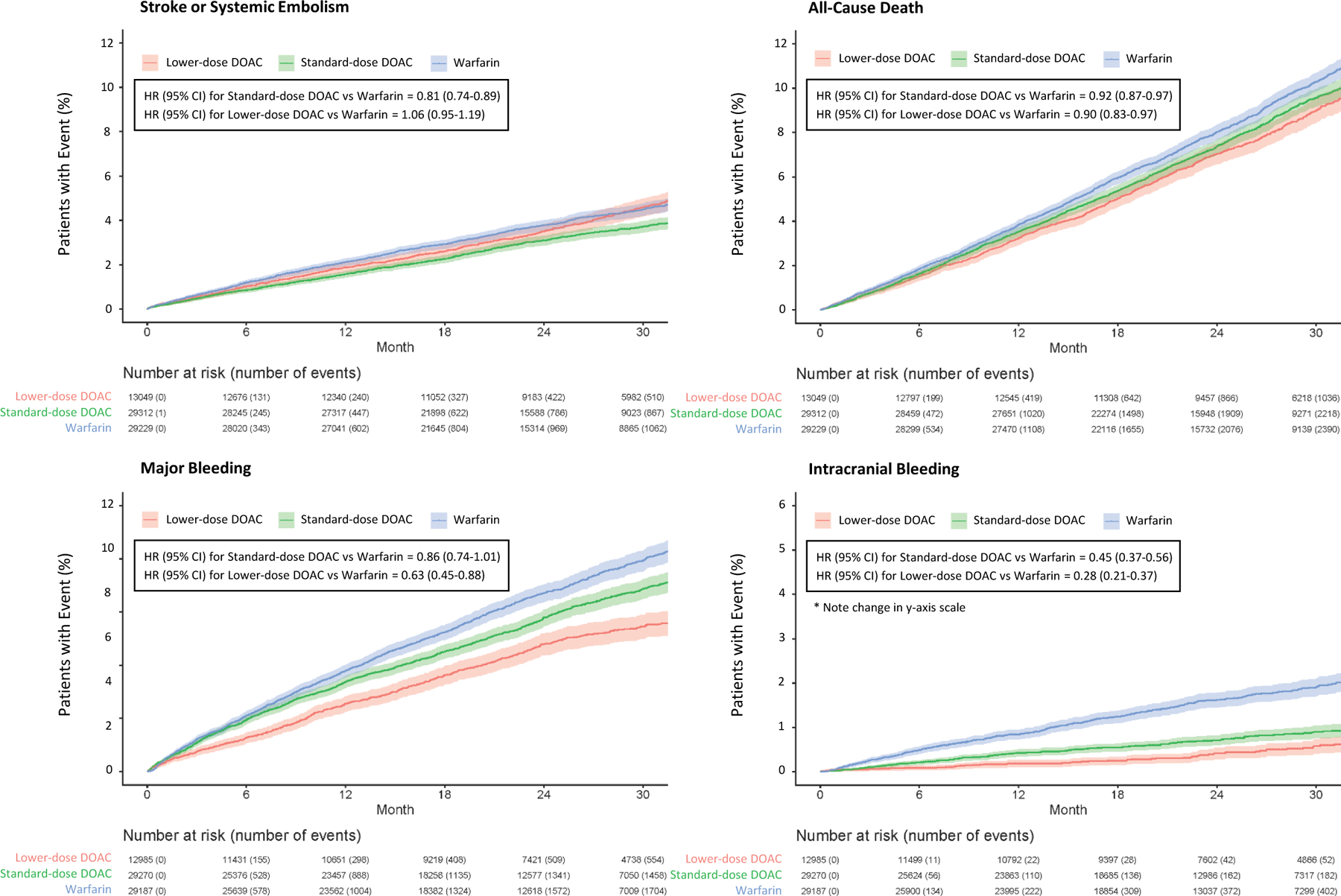
Kaplan-Meier curves for stroke or systemic embolism (top left), all-cause death (top right), major bleeding (bottom left), and intracranial bleeding (bottom right).
Compared with warfarin, patients randomized to lower-dose DOAC (i.e. dabigatran 110mg or edoxaban 30/15mg) had no statistically different hazard of stroke/systemic embolism (531/13049 (4.07%) vs 1080/29229 (3.69%); HR 1.06, 95% CI 0.95–1.19), but had a lower hazard of all-cause death, cardiovascular death, and hemorrhagic stroke (Figure 1). Patients randomized to lower-dose DOAC had a significantly higher hazard of ischemic stroke compared with warfarin (454/13049 (3.48%) vs 685/29229 (2.34%); HR 1.35, 95% CI 1.19–1.54).
HRs with 95% CIs for pairwise comparison of standard-dose DOAC vs lower-dose DOAC can be found in the online supplement (Supplement Table 3). Compared with lower-dose DOAC, patients randomized to standard-dose DOAC had a lower hazard of stroke/systemic embolism (883/29312 (3.01%) vs 531/13049 (4.07%); HR 0.76, 95% CI 0.68–0.86) and a lower hazard of any stroke, ischemic stroke, and the composite efficacy outcome.
Safety outcomes and net clinical benefits
All safety outcomes except fatal bleeding and hemorrhagic stroke showed moderate between-trial heterogeneity with estimated standard deviation of random effects across trials larger than 0.1 (Supplement Table 4). HR’s for the primary safety outcome from each individual trial can be found in Supplement Table 5.
Compared with warfarin, patients randomized to standard-dose DOAC had no statistically different hazard of major bleeding (1479/29270 (5.05%) vs 1733/29187 (5.94%); HR 0.86, 95% CI 0.74–1.01), but a lower hazard of fatal bleeding and intracranial bleeding (Figure 1). Patients randomized to standard-dose DOAC had a significantly higher hazard of major gastrointestinal bleeding compared with warfarin (744/29270 (2.54%) vs 569/29187 (1.95%); HR 1.31, 95% CI 1.08–1.57). Kaplan-Meier curves for key safety outcomes can be found in Figure 2.
Compared with warfarin, patients randomized to lower-dose DOAC had a lower hazard of major bleeding (564/12985 (4.34%) vs 1733/29187 (5.94%); HR 0.63, 95% CI 0.45–0.88) and a lower hazard of fatal bleeding, major or clinically relevant non-major bleeding, any bleeding, and intracranial bleeding (Figure 1). Patients randomized to lower-dose DOAC had no statistically different risk of major gastrointestinal bleeding compared with warfarin (271/12985 (2.09%) vs 569/29187 (1.95%); HR 0.85, 95% CI 0.62–1.18).
Compared with lower-dose DOAC, patients randomized to standard-dose DOAC had no statistically different hazard of major bleeding (1479/29270 (5.05%) vs 564/12985 (4.34%); HR 1.37, 95% CI 0.95–1.96) and fatal bleeding, but a higher hazard of major or clinically relevant non-major bleeding, intracranial bleeding, hemorrhagic stroke, and gastrointestinal bleeding (Supplement Table 3).
Effect modification by categorical baseline covariates
For stroke/systemic embolism, potentially meaningful interactions suggesting a greater benefit of standard-dose DOAC over warfarin were observed in patients with no history of prior VKA use and in patients with lower creatinine clearance (Figure 3). Potentially meaningful interactions suggesting a greater benefit of lower-dose DOAC over warfarin were observed in patients with no history of prior VKA use, no history of prior gastrointestinal bleeding, and in patients with older age (Supplement Table 6). Stroke/systemic embolism event rates for standard-dose and lower-dose DOAC vs warfarin by categorical baseline body weight and creatinine clearance can be found in Supplement Table 7 and Supplement Table 8, respectively.
Figure 3.
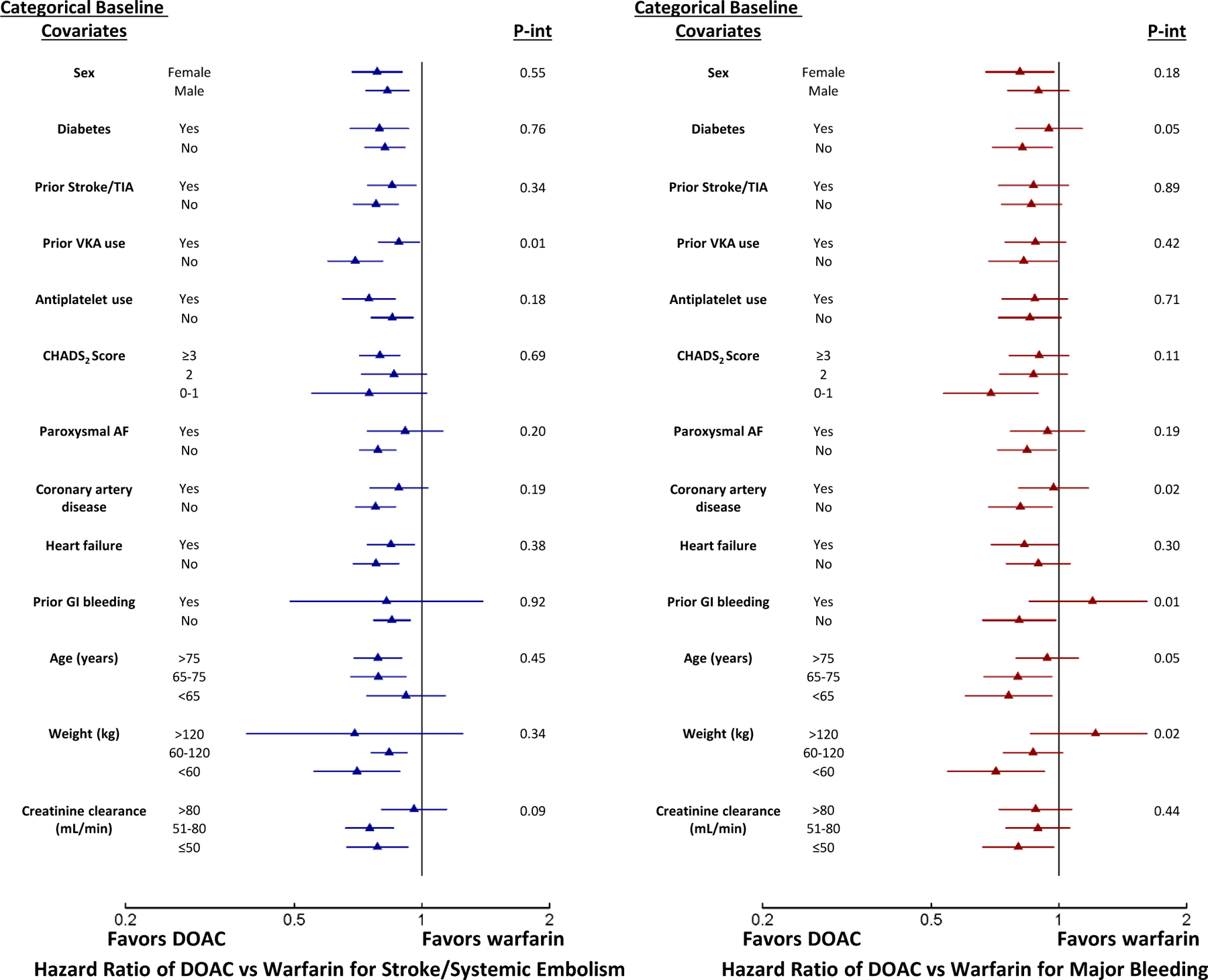
Forest plots showing hazard ratios for standard-dose DOAC versus warfarin with stratification by baseline categorical covariates and interaction p-values for stroke or systemic embolism (left) and major bleeding (right).
For major bleeding, potentially meaningful interactions suggesting a benefit of standard-dose DOAC over warfarin were observed in patients without diabetes, those with no history of coronary artery disease, no history of gastrointestinal bleeding, with younger age, and lower weight (Figure 3). Potentially meaningful interactions suggesting a benefit of lower-dose DOAC over warfarin were observed in patients with no history of coronary artery disease, no history of heart failure, and younger age (Supplement Table 9). Major bleeding event rates for standard-dose and lower-dose DOAC vs warfarin by categorical baseline body weight and creatinine clearance can be found in Supplement Table 7 and Supplement Table 8, respectively.
Effect modification by continuous age and sex
Baseline age in the study population ranged from 19 to 101 years. The 5th and 95th percentiles for age were 54 and 84 years. For stroke/systemic embolism, no meaningful interaction was observed for standard-dose or lower-dose DOAC versus warfarin across the spectrum of ages (Table 2, Figure 4). Male and female sex did not show any meaningful treatment-by-age interaction (Table 2, Supplement Figure 2).
Table 2.
Percent change in hazard ratios comparing DOAC versus warfarin over the spectrum of baseline age as a continuous covariate for the primary efficacy outcome, the primary safety outcome, and all-cause death (top) with stratification by sex (bottom).
| % change in HR per 10-year increase in age | P-value | ||||
|---|---|---|---|---|---|
| Primary Efficacy Outcome (Stroke/Systemic Embolism) | |||||
| Standard-dose DOAC vs warfarin | 5.1% decrease (−5.1%, 14.3%) | 0.31 | |||
| Lower-dose DOAC vs warfarin | 9.1% decrease (−3.2%, 20.0%) | 0.14 | |||
| Primary Safety Outcome (Major Bleeding) | |||||
| Standard-dose DOAC vs warfarin | 10.2% increase (1.3%, 19.9%) | 0.02 | |||
| Lower-dose DOAC vs warfarin | 17.6% increase (3.4%, 33.7%) | 0.01 | |||
| All-Cause Death | |||||
| Standard-dose DOAC vs warfarin | 2.1% decrease (−4.7%, 8.4%) | 0.54 | |||
| Lower-dose DOAC vs warfarin | 1.5% increase (−7.0%, 10.7%) | 0.75 | |||
| % change in HR per 10-year increase in age | |||||
| Male | P-value | Female | P-value | Interaction P-value | |
| Primary Efficacy Outcome (Stroke/Systemic Embolism) | |||||
| Standard-dose DOAC vs warfarin | 8.0% decrease (−4.7%, 19.1%) | 0.21 | 1.4% increase (−14.6%, 20.4%) | 0.88 | 0.38 |
| Lower-dose DOAC vs warfarin | 8.0% decrease (−8.0%, 21.7%) | 0.31 | 11.0% decrease (−9.0%, 27.3%) | 0.26 | 0.80 |
| Primary Safety Outcome (Major Bleeding) | |||||
| Standard-dose DOAC vs warfarin | 13.1% increase (2.1%, 25.3%) | 0.02 | 6.5% increase (−8.5%, 23.9%) | 0.42 | 0.52 |
| Lower-dose DOAC vs warfarin | 12.2% increase (−3.6%, 30.6%) | 0.14 | 32.8% increase (5.9%, 66.7%) | 0.01 | 0.22 |
| All-Cause Death | |||||
| Standard-dose DOAC vs warfarin | 0.1% decrease (−8.2%, 7.7%) | 0.98 | 6.6% decrease (−6.2%, 17.8%) | 0.30 | 0.39 |
| Lower-dose DOAC vs warfarin | 3.7% decrease (−6.7%, 13.1%) | 0.47 | 15.2% increase (−2.7, 36.4%) | 0.10 | 0.08 |
For HR point estimates, a decrease in HR signifies increasing favorability of DOAC over warfarin, whereas an increase in HR signifies decreasing favorability of DOAC over warfarin. For 95% confidence intervals, a negative value signifies an opposite direction (decreasing/increasing) of the reported increasing/decreasing HR point estimate.
HR = hazard ratio, DOAC = direct oral anticoagulant
Figure 4.
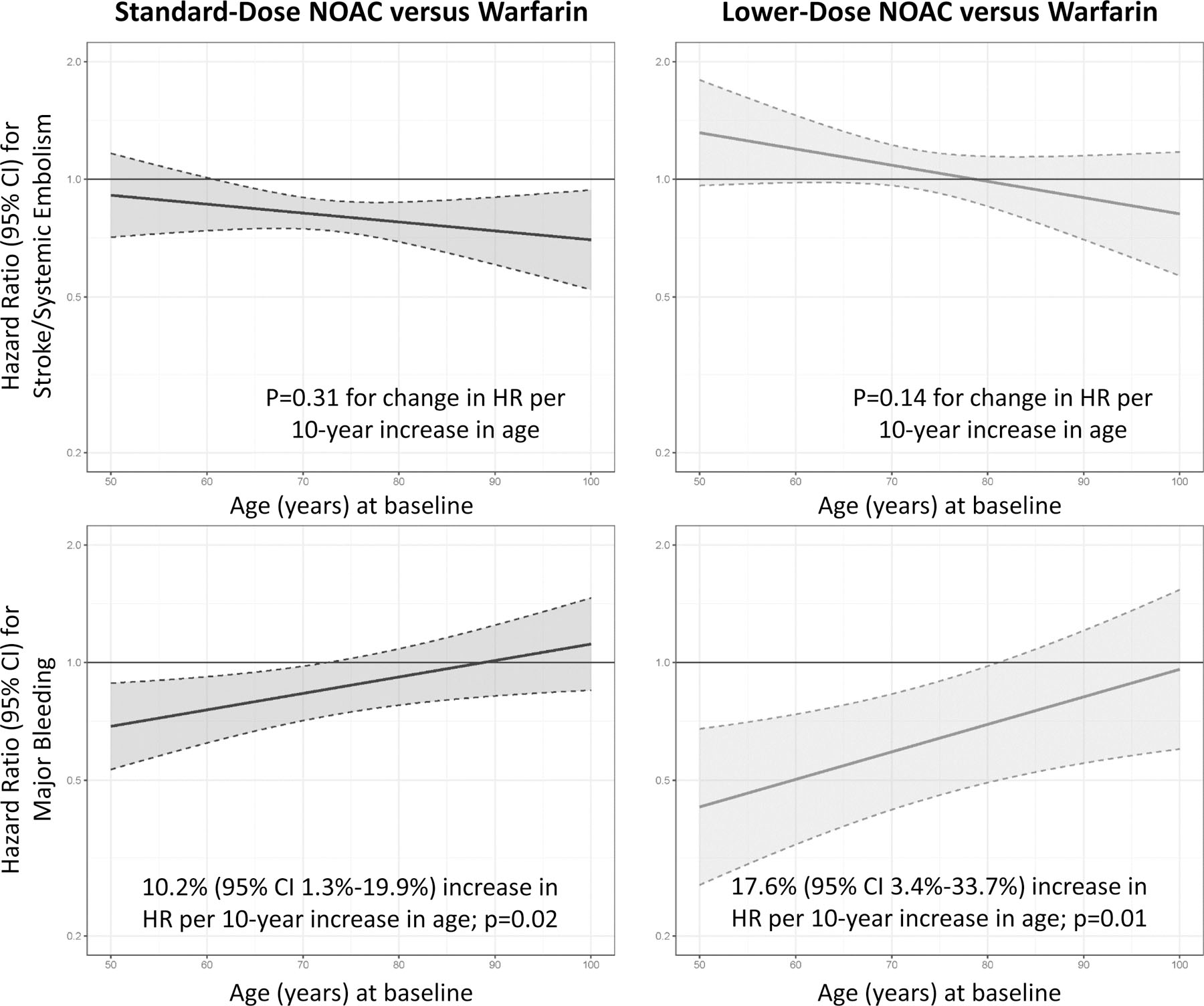
Hazard ratios for the primary efficacy (top) and safety (bottom) outcomes plotted over the range of baseline age. Hazard ratio > 1 favors warfarin over DOAC, whereas a hazard ratio < 1 favors DOAC over warfarin.
Footnote: X-axis truncated to include 5th and 95th percentiles.
For major bleeding, a potentially meaningful interaction suggesting a greater benefit for standard-dose DOAC versus warfarin was observed in younger patients (HR for standard-dose DOAC versus warfarin increases by 10.2% [95% CI 1.3%−19.9%] for every 10-year increase in age; p=0.02). A similar potentially meaningful interaction for major bleeding was observed for lower-dose DOAC versus warfarin in younger patients (HR for lower-dose DOAC versus warfarin increases by 17.6% [95% CI 3.4%−33.7%] for every 10-year increase in age; p=0.01) (Table 2). No meaningful interaction for major bleeding was observed after stratification by sex for either treatment strategy (Table 1, Supplement Figure 2).
For all-cause death, no meaningful interaction was observed for standard-dose or lower-dose DOAC versus warfarin across the spectrum of age or after stratification by sex (Table 2).
Sensitivity analyses
In the sensitivity analyses, after exclusion of patients from RE-LY and exclusion of patients from the lower-dose DOAC arm from ENGAGE AF-TIMI 48 so as to include only factor Xa inhibitors in the standard-dose DOAC treatment strategy groups, HRs for stroke/systemic embolism and major bleeding for standard-dose DOAC vs warfarin did not differ from those from the primary analyses (Supplement Figure 3). The estimated HR for major gastrointestinal bleeding with standard-dose DOAC vs warfarin was found to be lower in magnitude compared with the primary analyses. For standard-dose DOAC vs warfarin, the increase in major gastrointestinal bleeding observed in the primary analyses was no longer statistically significant (551/23211 (2.37%) vs 436/23189 (1.88%); HR 1.24 [95% CI 0.98–1.58]). All other efficacy and safety outcomes were consistent with those from the primary analyses.
DISCUSSION
In these results from network meta-analyses using individual patient-level data from the pivotal randomized trials of DOACs vs warfarin in patients with AF, we found that a standard-dose DOAC treatment strategy results in significant reductions in the risk of stroke/systemic embolism, intracranial bleeding, and all-cause death compared with warfarin. No statistically significant difference in major bleeding was observed, although moderate heterogeneity across trials was observed. The benefit of standard-dose DOACs over warfarin for stroke/systemic embolism was more pronounced in patients without prior VKA use and in patients with lower creatinine clearance, but was consistent across the entire range of patient age and was consistent after stratification by sex. For major bleeding, statistically significant interaction was observed suggesting a greater benefit for standard-dose DOAC over warfarin in patients with lower body weight and younger age, regardless of sex.
Results from these analyses provide the most robust evidence to date demonstrating the collective benefits of DOACs over warfarin in patients with AF. While prior meta-analyses have examined the relative efficacy and safety of DOACs versus warfarin using aggregate published data,7 such results are subject to the previously noted limitations of study-level data.8 Our analyses leverage the strengths of individual patient data through consistent follow-up for time-to-event outcomes, assessment of effect modification in the Cox regression model in a consistent manner, and by examining interactions across the spectrum of age as a continuous covariate.
Results from the primary analyses more clearly define the benefit of standard-dose DOACs over warfarin for reducing intracranial bleeding, one of the most feared and devastating complications of oral anticoagulants, with approximately as much benefit as the lower-dose DOAC treatment strategy. Standard-dose DOACs were associated with an increased hazard of major gastrointestinal bleeding compared with warfarin, a phenomenon that has been hypothesized to be due to incomplete DOAC gastrointestinal absorption and dose-dependent variation in the relative anticoagulant intensity produced at the local gastrointestinal mucosal surface by different DOACs.20, 21 Gastrointestinal bleeding events in these analyses included only events that met criteria for major bleeding, thus these are clinically significant events. In the primary analyses, history of gastrointestinal bleeding was identified as an important driving factor for the increased hazard of major bleeding with standard-dose DOACs. Moderate between-trial heterogeneity for the major bleeding outcome was detected, which is consistent with prior reports based on study-level data.7 While results from the primary and sensitivity analyses demonstrate a consistent pattern of increased risk for gastrointestinal bleeding with standard-dose DOACs, it is likely that drug class (direct thrombin inhibitor vs factor Xa inhibitor) and drug dose/exposure also influence an individual patient’s risk. The increased hazard of gastrointestinal bleeding with standard-dose DOACs is counterbalanced by significant reductions in thromboembolism, intracranial bleeding, and fatal bleeding, which are of far greater consequence.
Several key findings in these analyses add to previously published data. Prior VKA use was identified as a significant effect modifier for stroke/systemic embolism. While patients with and without prior VKA use were found to derive a benefit from standard-dose DOACs over warfarin for reduction in thromboembolic events, those with no prior VKA use derived a potentially greater benefit from standard-dose DOACs. Prior secondary analyses from individual randomized trials of DOACs vs warfarin with stratification by prior VKA use have yielded inconsistent results, with some trials demonstrating greater benefits of DOACs in patients without prior VKA use22 and others showing no significant interaction.23–25 The differential effect of standard-dose DOACs over warfarin based on prior VKA use is important given guideline recommendations for DOACs as first-line therapy in patients without contraindications.1, 2 Data from these analyses confirm that clinicians should have no hesitancy to start DOACs in eligible patients regardless of prior VKA use.
We identified evidence of interaction favoring standard-dose DOACs over warfarin with respect to the major bleeding outcome for the sub-group of patients with low baseline body weight. Interaction testing from 3 of the 4 individual trials have shown no statistically significant interaction for major bleeding by baseline body weight4, 5, 26, while 1 of the 4 individual trials however showed findings similar to those from our meta-analyses with respect to a treatment interaction favoring DOACs in lower body weight27. The interaction may relate to the finding that the incidence of major bleeding was higher among patients with lower body weight, which in turn is related to other factors like older age and worse kidney function, both of which are associated with higher risk for major bleeding and tendency for greater safety with DOACs. Dedicated analyses from COMBINE AF analyzing body weight as a continuous variable are forthcoming.
We further identified evidence of interaction favoring standard-dose DOACs over warfarin with respect to the stroke/systemic embolism outcome for the sub-group of patients with low baseline creatinine clearance. While prior study-level meta-analyses have similarly suggested a greater benefit of standard-dose DOACs over warfarin in patients with lower baseline creatinine clearance28, these analyses have been limited by the use of categorical creatinine clearance cutoffs that restrict the generalizability of the results. Dedicated analyses from COMBINE AF analyzing creatinine clearance as a continuous variable are forthcoming.
An important strength of these analyses is the ability to assess effect modification using continuous baseline variables. We demonstrate consistent benefits of standard-dose DOACs vs warfarin for stroke/systemic embolism across the continuous spectrum of age. For the major bleeding outcome, younger patients experienced a greater reduction in bleeding with standard-dose DOACs versus warfarin, perhaps due to a lower prevalence of competing comorbidities such as prior gastrointestinal bleeding or kidney dysfunction. Prior reports assessing treatment interaction by age are limited by the use of categorical data, with a typical age cut point of < or ≥75 years.7 There is generally more information in a continuous variable when assessed as such. Moreover, data derived from categorical cut points are challenging to interpret as within each category exists a wide spectrum of competing comorbidities, some of which are factors influencing DOAC dose reduction for 3 of the 4 individual trials. Although there was little or no between-trial heterogeneity detected in these analyses for the examined efficacy outcomes, moderate between-trial heterogeneity with respect to bleeding outcomes was detected, thus aggregate findings for bleeding outcomes must be interpreted with caution. One trial (RE-LY) showed an increase in major bleeding with higher-dose dabigatran (150mg) versus warfarin in patients ≥80 years old29 while the other 3 trials showed no significant interaction across age groups (with 2 of the 3 trials showing statistically significant reduction in major bleeding with DOACs).30 Heterogeneity may partly explain the findings from these analyses with respect to the bleeding interaction across continuous age. These data add depth to prior studies of DOACs in elderly patients, which are limited to sub-group analyses from individual trials,30 aggregate meta-analyses,7 and observational data.31 These findings reinforce guideline recommendations supporting preferential use of DOACs over warfarin in all age categories given consistent stroke reduction among elderly patients with at worst no difference in the incidence of major bleeding.
These analyses have several limitations. We performed univariable analyses for individual outcomes separately and tested each effect modifier individually. These analyses may therefore introduce type I error inflation due to multiplicity. Furthermore, as we did not employ a multivariable approach in the effect modification analyses, potential multivariable collinearity between covariates (such as age and creatinine clearance and treatment effect) may not be accounted for. Patients in the COMBINE AF database are from randomized trials with specific inclusion and exclusion criteria, thus do not represent an unselected group of patients with AF in general practice. Randomization however offers reliable assessment of relative efficacy and safety, which cannot be reliably determined from non-randomized observational comparisons. Although the individual trials within COMBINE AF employed robust efficacy and safety outcomes, few patient-reported outcomes were collected, which may limit the application of our findings. Furthermore data describing temporary discontinuation of study drug and study drug adherence were not included in the COMBINE AF database. Adherence in the randomized trials, for example as measured by INR time in therapeutic range, was at least as good as what has been described in most studies of patients in general practice. Lastly, aggregating trials with different study drugs and doses for meta-analyses may obscure subtle differences between outcomes that are specific to individual study drugs.
In summary, these analyses of individual patient data from the 4 pivotal trials of DOACs versus warfarin in patients with AF show that standard-dose DOACs reduce the risk of stroke/systemic embolism, death, and intracranial bleeding compared with warfarin with no significant difference in risk of major bleeding. For stroke/systemic embolism, standard-dose DOACs performed consistently better than warfarin across every examined categorical subgroup as well as across the continuous spectrum of age. For major bleeding, standard-dose DOACs performed better than warfarin in younger patients and were no different than warfarin in elderly patients, regardless of sex. These data reinforce and provide more granular detail describing the widely accepted beneficial effects of DOACs over warfarin in a broad population of patients with AF.
Supplementary Material
Clinical Perspective.
What is new?
When individual patient data from randomized trials of DOACs versus warfarin are analyzed collectively, standard-dose DOAC use results in lower incidence of stroke, death, and intracranial hemorrhage with no difference in major bleeding.
The relative benefits of standard-dose DOACs over warfarin for stroke prevention were consistent across nearly all sub-groups, including across the entire continuous spectrum of age, with no evidence of interaction by sex. These benefits may be even greater in patients with lower creatinine clearance.
For major bleeding, younger patients and patients with lower body weight may derive an even greater benefit from standard-dose DOAC over warfarin.
What are the clinical implications?
The totality of efficacy and safety data from randomized clinical trials supports the use of standard-dose DOACs over warfarin for stroke prevention in non-valvular atrial fibrillation, regardless of age or sex.
Sources of Funding
RE-LY was funded by Boehringer Ingelheim. ROCKET AF was funded by Johnson & Johnson and Bayer. ARISTOTLE was funded by Bristol Myers Squibb and Pfizer. ENGAGE AF-TIMI 48 was funded by Daiichi Sankyo. No outside funding was obtained to support the creation of the COMBINE AF database or for these analyses.
Non-standard Abbreviations and Acronyms:
- AF
Atrial Fibrillation
- ARISTOTLE
Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation
- COMBINE AF
A Collaboration between Multiple Institutions to Better Investigate Non-vitamin K Antagonist Oral Anticoagulant Use in Atrial Fibrillation
- CI
Confidence Interval
- DOAC
Direct Oral Anticoagulant
- ENGAGE AF-TIMI 48
Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation – Thrombolysis in Myocardial Infarction 48
- HR
Hazard Ratio
- ISTH
International Society on Thrombosis and Haemostasis
- RE-LY
Randomized Evaluation of Long Term Anticoagulation Therapy
- ROCKET AF
Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation
- VKA
Vitamin K Antagonist
Footnotes
Conflict of Interest Disclosures
APC reports grants from National Institutes of Health, during the conduct of the study. HH has nothing to disclose. SJC reports personal fees from BMS, personal fees from Bayer, personal fees from Boehringer Ingelheim, personal fees from daiichi Sankyo, personal fees from Portola, during the conduct of the study. JE reports honoraria and grant support from Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol-Myers-Squibb/Pfizer, Daiichi Sankyo, Glaxo Smith Kline, Janssen, sanofi aventis and Eli Lilly as well as a personnel award from the Heart and Stroke Foundation. RPG reports personal fees from Amarin, personal fees from American College of Cardiology, grants and personal fees from Amgen, grants from Anthos Therapeutics, personal fees from AstraZeneca, personal fees from Boehringer-Ingelheim, personal fees from Bristol-Myers-Squibb, personal fees from CryoLife, personal fees from CVS Caremark, grants and personal fees from Daiichi-Sankyo, personal fees from Dr. Reddy’s Laboratories, personal fees from Eli Lilly and Company, personal fees from Esperion, personal fees from Gilead, personal fees from GlaxoSmithKline, personal fees from Janssen, personal fees from Lexicon, grants and personal fees from Merck, personal fees from Pfizer, personal fees from St. Lukes, personal fees from SAJA Pharmaceuticals, personal fees from Samsung, personal fees from Servier, outside the submitted work. DAM reports being member of the TIMI Study Group which has received institutional research grant support through Brigham and Women’s Hospital from: Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., Daiichi-Sankyo, Eisai, Intarcia, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Inc., Roche, Siemens Healthcare Diagnostics, Inc., The Medicines Company, Zora Biosciences. MRP reports grants from ASTRA ZENECA, grants from BAYER, personal fees from BAYER, grants from JANSSEN, personal fees from JANSSEN, personal fees from MYTONOMY, grants from PROCYRION, personal fees from PROCYRION, grants from HEARTFLOW, outside the submitted work. LW reports grants from AstraZeneca, grants from Boehringer Ingelheim, grants from Bristol-Myers Squibb/Pfizer, grants from GlaxoSmithKline, grants from Merck & Co., grants from Roche Diagnostics, personal fees from Abbot, outside the submitted work. JHA reports grants from Bayer, grants and personal fees from Bristol Myers Squibb, grants and personal fees from CryoLife, personal fees from Janssen, personal fees from Pfizer, personal fees from Portola, and grants from XaTek outside the submitted work. MCB reports other from Pfizer, other from VIFOR, other from CSL behring, outside the submitted work. APB has nothing to disclose. EAB reports being member of the TIMI Study Group which has received institutional research grant support through Brigham and Women’s Hospital from: Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., Daiichi-Sankyo, Eisai, Intarcia, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Inc., Roche, Siemens Healthcare Diagnostics, Inc., The Medicines Company, Zora Biosciences. TFC has nothing to disclose. LD reports funding from Boehringer Ingelheim, during the conduct of the study. ME reports grants and other from Boehringer Ingelheim,, other from Sanofi – Aventis, other from Boston Scientific, other from Alta Thera, other from Anthos, other from Biogen Idec, other from Boston Scientific, grants from Pfizer, grants from J & J, grants from Daiichi Sankyo Pharma Development, during the conduct of the study. KAAF reports grants and personal fees from Bayer/Janssen, grants from AstraZeneca, personal fees from Verseon, outside the submitted work. BG has nothing to disclose. JLH reports personal fees from Boehringer Ingelheim, Bayer Healthcare, Ortho-McNeil-Janssen, Pfizer, Bristol Myers Squibb, and Daiichi Sankyo during the conduct of the study and personal fees from Boehringer Ingelheim, Ortho-McNeil Janssen, the ATLAS Group, Duke Clinical Research Institute and TIMI Group outside the submitted work. ZH reports consulting and lecture fees from Boehringer Ingelheim and Pfizer/BMS, grants from The Swedish Society for Medical Research (grant no. S17–0133) and The Swedish Heart-Lung Foundation (grant no. 20200722), during the conduct of the study; fees paid to his institution for advisory boards and lectures from Roche Diagnostics, outside the submitted work. SHH reports personal fees from BI, personal fees from BMS, personal fees from Pfizer, personal fees from Daiichi Sankyo, personal fees from Bayer Healthcare, personal fees from Medtronic, personal fees from sanofi, personal fees from zoll, outside the submitted work. KH has nothing to disclose. EH reports consulting fees from Anthos Therapeutics, Bristol Myers Squibb/Pfizer, Janssen, Medtronic, Honoraria from Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, and Advisory Board fees from Anthos Therapeutics, CryoLife, outside of the submitted work. ETK reports personal fees from Daiichi-Sankyo, personal fees from AstraZeneca, personal fees from Bristol-Myers Squibb, grants and personal fees from Ono Pharmaceutical, personal fees from MSD KK, personal fees from Pfizer, from Tanabe-Mitsubishi, personal fees from Bayer, from Boehringer Ingelheim, grants from Abbott Japan, personal fees from Amgen, personal fees from Takeda, during the conduct of the study. JK reports grants from Daiichi-Sankyo, during the conduct of the study; and I am a member of the TIMI Study Group which has received institutional research grant support through Brigham and Women’s Hospital from: Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., Daiichi-Sankyo, Eisai, Intarcia, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Inc., Roche, Siemens Healthcare Diagnostics, Inc., The Medicines Company, Zora Biosciences. RDL reports personal fees from Bayer, personal fees from Boehringer Ingleheim, grants from Bristol-Myers Squibb, personal fees from Bristol-Myers Squibb, personal fees from Daiichi Sankyo, personal fees from Glaxo Smith Kline, grants from Glaxo Smith Kline, personal fees from Medtronic, grants from Medtronic, personal fees from Merck, grants from Pfizer, personal fees from Pfizer, personal fees from Portola, personal fees from Sanofi, grants from Sanofi, outside the submitted work. KWM reports grants from Afferent, grants from AHA, grants and personal fees from Amgen, personal fees from Anthos, personal fees from Applied Therapeutics, grants from Apple, Inc, grants and personal fees from AstraZeneca, grants and personal fees from Bayer, grants from Cardiva Medical, Inc, personal fees from CSL Behring, grants from Eidos, personal fees from Elsevier, grants from Ferring, grants from Gilead, grants from Google (Verily), personal fees from Inova, personal fees from Intermountain Health, grants and personal fees from Johnson & Johnson, grants from Luitpold, personal fees from Medscape, grants from Medtronic, grants from Merck, personal fees from Mount Sinai, personal fees from Mundi Pharma, personal fees from Myokardia, grants and personal fees from Novartis, personal fees from Novo Nordisk, personal fees from Otsuka, personal fees from Portola, grants from Sanifit, grants and personal fees from Sanofi, personal fees from SmartMedics, grants from St. Jude, personal fees from Theravance, outside the submitted work. JO reports fees to his institution, for consultant/advisory boards (including study steering committees and data safety monitoring boards) and lectures, from Alexion, AstraZeneca, Bayer, BMS, Boehringer-Ingelheim, Daichii-Sankyo, Janssen, Novartis, Pfizer, Roche Diagnostics and Sanofi, outside the submitted work. JPP reports grants from Johnson & Johnson, during the conduct of the study; grants from Bayer, grants and personal fees from Boston Scientific, grants and personal fees from Abbott, personal fees from BMS, outside the submitted work. CTR reports grants from Daiichi Sankyo, during the conduct of the study; grants and personal fees from Anthos, grants and personal fees from Boehringer Ingelheim, grants from Daiichi Sankyo, grants from AstraZeneca, grants from National Institutes of Health, personal fees from Bayer, personal fees from Bristol Myers Squibb, personal fees from Janssen, personal fees from Pfizer, personal fees from Portola, outside the submitted work; and Ýr. Ruff is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from: Abbott, Amgen, Anthos Therapeutics, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., Daiichi-Sankyo, Eisai, Intarcia, MedImmune, Merck, Novartis, Pfizer, Quark Pharmaceuticals, Regeneron Pharmaceuticals, Inc., Roche, Siemens Healthcare Diagnostics, Inc., The Medicines Company, Zora Biosciences. JS reports personal fees from Amgen, personal fees from Astra Zeneca, grants and personal fees from Bayer Healthcare, personal fees from Boehringer-Ingelheim, grants and personal fees from Biosense Webster, grants and personal fees from Boston Scientifc, personal fees from Bristol-Myers Squibb, grants and personal fees from Daiichi-Sankyo, grants and personal fees from Medtronic, personal fees from Novartis, personal fees from Pfizer, personal fees from Portola / Alexion, grants and personal fees from Abbott, other from CorXL, grants and personal fees from Biotronik, personal fees from Medscape, personal fees from WebMD, personal fees from Merck / MSD, personal fees from Berlin Chemie / Menarini, personal fees from Roche Diagnostics, personal fees from Saja Pharmaceuticals, personal fees from Servier, outside the submitted work. DW has nothing to disclose. CBG reports personal fees from Bayer, grants and personal fees from Boehringer Ingelheim, personal fees from Boston Scientific, grants and personal fees from Bristol Myers Squibb, grants from Daiichi Sankyo, grants and personal fees from Janssen, grants and personal fees from Pfizer, during the conduct of the study; personal fees from Abbvie, grants from Akros, grants from AstraZeneca, personal fees from Espero, grants from FDA, grants from Galxo Smith Kline, personal fees from Medscape, personal fees from Medtronic Inc., grants from Medtronic Foundation, personal fees from Merck, personal fees from NIH, personal fees from Novo Nordisk, grants and personal fees from Novartis, personal fees from Roche, grants and personal fees from The Medicine’s Co., grants from Apple, personal fees from Rho Pharmaceuticals, personal fees from CeleCor, personal fees from Correvio, personal fees from Philips, personal fees from Abiomed, personal fees from Anthos Therapeutics, outside the submitted work.
References
- 1.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr., Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et a 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125–e151. [DOI] [PubMed] [Google Scholar]
- 2.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 3.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 4.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 5.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 6.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Spinar J, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 7.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–962. [DOI] [PubMed] [Google Scholar]
- 8.Lyman GH and Kuderer NM. The strengths and limitations of meta-analyses based on aggregate data. BMC Med Res Methodol 2005;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong H, Fu H, Price KL and Carlin BP. Incorporation of individual-patient data in network meta-analysis for multiple continuous endpoints, with application to diabetes treatment. Stat Med 2015;34:2794–2819. [DOI] [PubMed] [Google Scholar]
- 10.Ioannidis J Next-generation systematic reviews: prospective meta-analysis, individual-level data, networks and umbrella reviews. Br J Sports Med 2017;51:1456–1458. [DOI] [PubMed] [Google Scholar]
- 11.Chaimani A. Conduct and reporting of individual participant data network meta-analyses need improvement. BMC Med 2020;18:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carnicelli AP, Hong H, Giugliano RP, Connolly SJ, Eikelboom J, Patel MR, Wallentin L, Morrow DA, Wojdyla D, Hua K, et al. Individual Patient Data from the Pivotal Randomized Controlled Trials of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation (COMBINE AF): Design and Rationale: From the COMBINE AF (A COllaboration between Multiple institutions to Better Investigate Non-vitamin K antagonist oral anticoagulant usE in Atrial Fibrillation) Investigators. Am Heart J 2021;233:48–58. [DOI] [PubMed] [Google Scholar]
- 13.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–694. [DOI] [PubMed] [Google Scholar]
- 14.Smith CT, Williamson PR and Marson AG. Investigating heterogeneity in an individual patient data meta-analysis of time to event outcomes. Stat Med 2005;24:1307–1319. [DOI] [PubMed] [Google Scholar]
- 15.Schoenfeld D Chi-squared goodness-of-fit tests for the proportional hazards regression model. Biometrika 1980;67:145–153. [Google Scholar]
- 16.Li G, Taljaard M, Van den Heuvel ER, Levine MA, Cook DJ, Wells GA, Devereaux PJ and Thabane L. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol 2017;46:746–755. [DOI] [PubMed] [Google Scholar]
- 17.Brookes ST, Whitely E, Egger M, Smith GD, Mulheran PA and Peters TJ. Subgroup analyses in randomized trials: risks of subgroup-specific analyses; power and sample size for the interaction test. J Clin Epidemiol 2004;57:229–236. [DOI] [PubMed] [Google Scholar]
- 18.Therneau T coxme: Mixed Effects Cox Models. R package version 2.2–16 2020.
- 19.Therneau T and Grambsch P. Modeling Survival Data: Extending the Cox Model New York: Springer; 2000. [Google Scholar]
- 20.Desai J, Kolb JM, Weitz JI and Aisenberg J. Gastrointestinal bleeding with the new oral anticoagulants--defining the issues and the management strategies. Thromb Haemost 2013;110:205–212. [DOI] [PubMed] [Google Scholar]
- 21.Vanassche T, Hirsh J, Eikelboom JW and Ginsberg JS. Organ-specific bleeding patterns of anticoagulant therapy: lessons from clinical trials. Thromb Haemost 2014;112:918–923. [DOI] [PubMed] [Google Scholar]
- 22.O’Donoghue ML, Ruff CT, Giugliano RP, Murphy SA, Grip LT, Mercuri MF, Rutman H, Shi M, Kania G, Cermak O, Braunwald E, et al. Edoxaban vs. warfarin in vitamin K antagonist experienced and naive patients with atrial fibrillation. Eur Heart J 2015;36:1470–1477. [DOI] [PubMed] [Google Scholar]
- 23.Ezekowitz MD, Wallentin L, Connolly SJ, Parekh A, Chernick MR, Pogue J, Aikens TH, Yang S, Reilly PA, Lip GY, et al. Dabigatran and warfarin in vitamin K antagonist-naive and -experienced cohorts with atrial fibrillation. Circulation 2010;122:2246–2253. [DOI] [PubMed] [Google Scholar]
- 24.Mahaffey KW, Wojdyla D, Hankey GJ, White HD, Nessel CC, Piccini JP, Patel MR, Berkowitz SD, Becker RC, Halperin JL, et al. Clinical outcomes with rivaroxaban in patients transitioned from vitamin K antagonist therapy: a subgroup analysis of a randomized trial. Ann Intern Med 2013;158:861–868. [DOI] [PubMed] [Google Scholar]
- 25.Garcia DA, Wallentin L, Lopes RD, Thomas L, Alexander JH, Hylek EM, Ansell J, Hanna M, Lanas F, Flaker G, et al. Apixaban versus warfarin in patients with atrial fibrillation according to prior warfarin use: results from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2013;166:549–558. [DOI] [PubMed] [Google Scholar]
- 26.Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, Yang S, Alings M, Kaatz S, Hohnloser SH, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation 2011;123:2363–2372. [DOI] [PubMed] [Google Scholar]
- 27.Boriani G, Ruff CT, Kuder JF, Shi M, Lanz HJ, Antman EM, Braunwald E and Giugliano RP. Edoxaban versus Warfarin in Patients with Atrial Fibrillation at the Extremes of Body Weight: An Analysis from the ENGAGE AF-TIMI 48 Trial. Thromb Haemost 2021;121:140–149. [DOI] [PubMed] [Google Scholar]
- 28.Del-Carpio Munoz F, Gharacholou SM, Munger TM, Friedman PA, Asirvatham SJ, Packer DL and Noseworthy PA. Meta-Analysis of Renal Function on the Safety and Efficacy of Novel Oral Anticoagulants for Atrial Fibrillation. Am J Cardiol 2016;117:69–75. [DOI] [PubMed] [Google Scholar]
- 29.Lauw MN, Eikelboom JW, Coppens M, Wallentin L, Yusuf S, Ezekowitz M, Oldgren J, Nakamya J, Wang J and Connolly SJ. Effects of dabigatran according to age in atrial fibrillation. Heart 2017;103:1015–1023. [DOI] [PubMed] [Google Scholar]
- 30.Schafer A, Flierl U, Berliner D and Bauersachs J. Anticoagulants for Stroke Prevention in Atrial Fibrillation in Elderly Patients. Cardiovasc Drugs Ther 2020;34:555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell A, Watson MC, Welsh T and McGrogan A. Effectiveness and Safety of Direct Oral Anticoagulants versus Vitamin K Antagonists for People Aged 75 Years and over with Atrial Fibrillation: A Systematic Review and Meta-Analyses of Observational Studies. J Clin Med 2019;8:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


