Abstract
Polymersomes are self-assembled nano-vesicles composed of amphiphilic block copolymers. These building blocks can be selected from a large number of hydrophilic and hydrophobic polymers in order to achieve required properties of the final system, such as biodegradability, sustainable and multiple stimuli-response drug release, long blood circulation, and low toxicity. Moreover, the surface of polymersomes can be functionalized to induce targeting character. Polymersomes are able to encapsulate a broad range of hydrophilic or/and hydrophobic molecules either in the aqueous core or membrane bilayer, respectively. In addition, colloidal stability and low membrane fluidity make polymersomes attractive nano-sized drug carriers. The review describes polymersomes compositions, their applications in pharmaceutical delivery, and preparation methods.
Keywords: Polymersomes, amphiphilic block copolymers, pharmaceutical delivery, hydrophobic drugs, hydrophilic drugs, co-loading, preparation methods
1. INTRODUCTION
Conventional pharmaceutical drugs have many shortcomings, such as poor solubility, lack of target specificity, poor biodistribution and unfavorable pharmacokinetic behavior, which may cause damage to healthy tissues. In order to improve pharmacological and therapeutic properties of drugs, different drug delivery systems have been developed. When pharmaceuticals are encapsulated within, or attached to, a carrier composed of polymers, lipids or other materials, pharmacokinetics and biodistribution can be significantly altered. Further development of new materials, especially smart delivery systems and approaches, will greatly improve therapeutic efficacy.
In last few decades many research works on nanocarriers based on amphiphilic block copolymers for pharmaceutical drug delivery have been published [1–7]. Amphiphilic block copolymers have a number of advantages which are very useful in drug delivery systems. For example, they can form various types of nanoparticles, including micelles, nanocapsules, nanospheres and polymersomes [8]. During the formulation of nanoparticles from amphiphilic block copolymers, different properties of the system can be easily tuned, such as size, surface charge, stimuli-responsiveness, drug loading method (covalent or non-covalent), surface decoration with ligands, drug-release profile, etc. [9]. Therefore, nanocarriers consisting of amphiphilic block copolymers are very promising drug delivery applications.
Recently, polymersomes have attracted much attention as versatile carriers for pharmaceutical compounds. Polymersomes are composed of amphiphilic block copolymers, which under certain conditions form vesicles consisting of an aqueous core within lipid-like bilayer [10]. Thereby, polymersomes have an ability to encapsulate both hydrophilic and hydrophobic molecules (Fig. 1). Polymersomes exhibit suitable features for drug carriers, including: high stability, tunable membrane permeability, long circulation time, surface functionality and ability to encapsulate broad range of drugs [10, 11]. Moreover, it is feasible to develop stimuli-responsive polymersomes to achieve controlled manner release of contents at the site of action [12]. All these properties expand the applications of polymersomes as nanocarriers in biomedical and pharmaceutical fields. In this review, we will discuss the formation conditions of polymersomes and describe their building blocks. In addition, the loading strategies of hydrophilic and/or hydrophobic anti-cancer drugs are summarized. General methods of polymersomes fabrication are also reviewed.
Fig. (1).
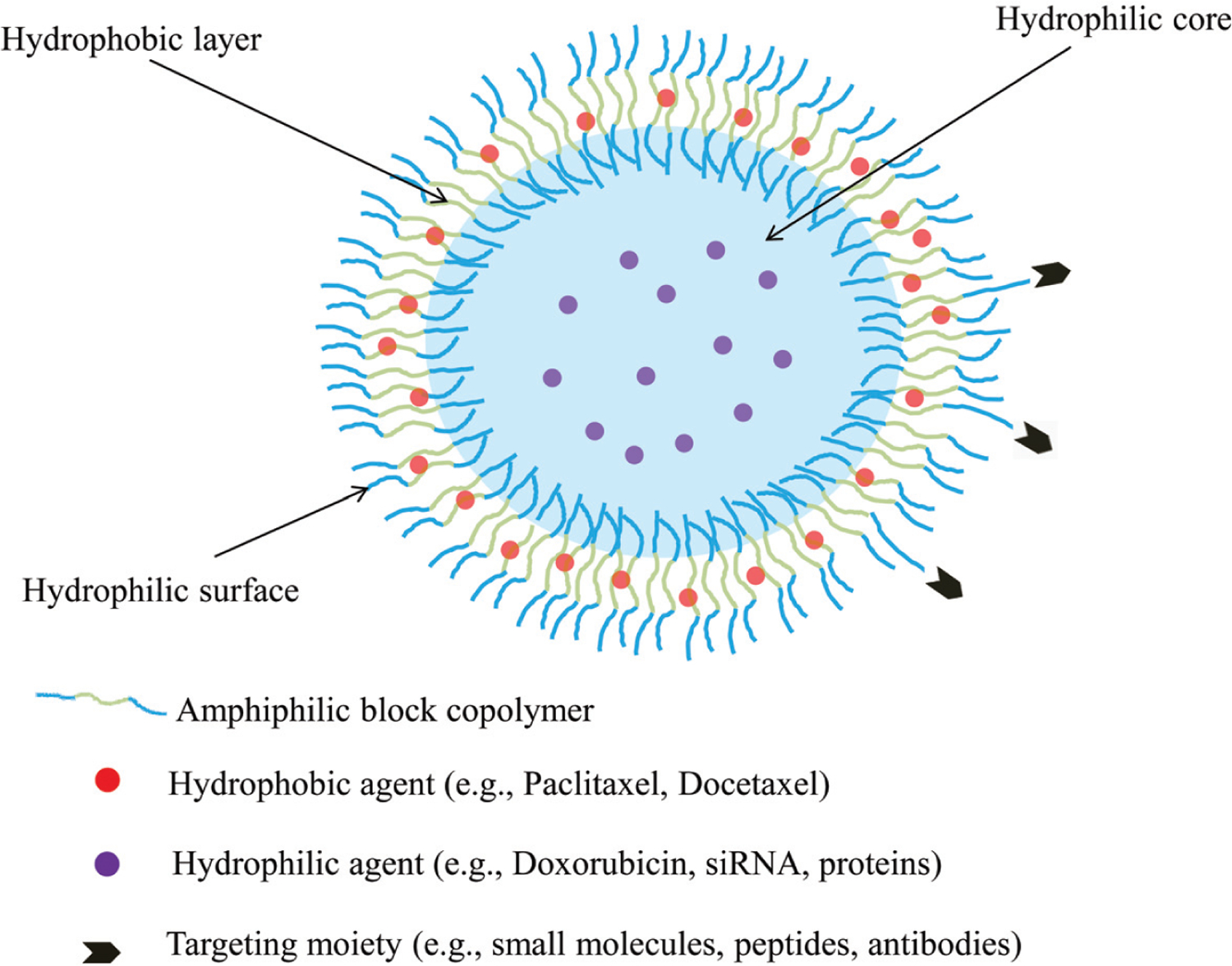
Schematic illustration of polymersomes structure. The structure allows loading of hydrophilic and/or hydrophobic agents. Polymersomes surface can also be modified with various targeting moieties.
2. COMPOSITIONS OF POLYMERSOMES
2.1. Self-assembly of Polymersomes
Structural units of polymersomes are similar to lipids in that they are self-assemble amphiphilic block copolymers. Usually block copolymers consist of two or more homopolymer blocks, which are polymerized with a monomer or a combination of monomers [11]. At low concentrations in aqueous solutions these amphiphilic copolymers exist as free, randomly distributed single molecules, however, as their concentration increase until sufficient quantity in the solution, amphiphiles reduce their free energy by assembling into aggregated structures [5, 10, 13]. Thereby, the concentration of amphiphilic molecules in aqueous medium at which they self-assemble into nanosized structures (i.e., vesicles, micelles) is called the critical micelle concentration (CMC). Amphiphilic block copolymers can form a wide range of morphologies, including spherical micelles, cylindrical micelles or vesicles [11]. The morphology is determined by many factors, such as the character of the polar head group, the length and number of the hydrophobic tails, and the temperature [14, 15]. The optimum hydrophilic head group and hydrophobic tail length can be expressed as a dimensionless packing factor (p):
v is the volume of the hydrophobic part of the copolymer, a is the optimal area per molecule, and l is the length of the hydrophobic part of the copolymer. The packing factor determines which morphology will form, for instance, spherical micelles are formed when ≤ p 1/3, cylindrical micelles are observed when 1/3 ≤ p < 1/2, and the vesicles when 1/2 ≤ p < 1 [11, 14, 16, 17]. The morphology of nanocarriers also depends on relative mass or volume fraction of each block of the amphiphiles. It was demonstrated that hydrophilic fraction (f) dictates [10, 16] aggregate morphology of the system. Generally speaking, f reflects the ratio of hydrophilic part to total mass of block copolymer. Self-assembly of amphiphiles in water solutions into vesicular structures are predominantly formed when f is 25–40%, cylindrically shaped micelles tend to form at f = 40–50%, and at f > 50% the formation of spherical micelles prevails [10, 16]. Moreover, the polymersomes formation depends on such parameters as the copolymer composition and concentration, the type of organic solvent, the amount of water contained in the solution, and the presence of additives (i.e., surfactants, ions, homopolymers) [15]. The characteristics of polymersomes are largely determined by membrane thickness which is associated with molecular weight of the copolymers that form the vesicle systems. High molecular weight leads to the formation of thick and tough layer with high density [14], which endows polymersomes with a number of properties required for nanocarriers, including colloidal and mechanical stability, high drug loading capacity, reduced membrane fluidity, and high storage capability [18].
2.2. Hydrophilic Blocks
A variety of polymers have been used as a hydrophilic part of amphiphilic block copolymers. Among them the well-known poly (ethylene glycol) (PEG) remains the most widely used hydrophilic block due to its suitable properties. PEG is a highly hydrophilic nonionic molecule which is completely miscible with water. Hydrophobic surfaces which coated with PEG become highly saturated with water molecules, as a result, the solubility of such complexes in water improves. High hydrophilicity of PEG prevents opsonization and the adsorption of proteins in plasma, resulting in a prolonged circulation time of PEG based systems [2, 5, 12]. Therefore, the attachment of PEG is often used to improve the biocompatibility of foreign objects. In addition, PEG has low toxicity and immunogenicity that makes it an optimal material for biomedical and pharmaceutical delivery systems [19].
The use of polypeptides as hydrophilic blocks also has a number of advantages. For instance, the lack of biofunctionality of polymersomes can be improved by modification of outer shell with multiple targeting agents and cell-penetrating moieties due to presence of various terminal functional groups in polypeptide segments. Polypeptides with ionizable side groups can also have electrostatic interactions with oppositely charged molecules, such as DNAs, RNAs, and proteins [20]. In addition, it is well established that polypeptides are biodegradable and exhibit high biocompatibility, low toxicity and immunogenicity [20, 21]. A number of polypeptides, such as poly(L-glutamic acid) (PGA) [22, 23], poly(L-lysine) (PLLys) [24], poly(sarcosine) (PSar) [25] were used as hydrophilic blocks for vesicles formation. Among them, PSar is the most promising material, due to its high hydrophilicity and the possibility of being degraded by endogenous sarcosine dehydrogenase. Moreover, the high density of hydrophilic chains of PSar hinders the clearance of vesicles by reticuloendothelial system (RES) which leads to prolongation of circulation time [20].
Examples of other less frequently used hydrophilic polymers include poly(acrylic acid) (PAA). PAA is an anionic polymer and this feature can be taken into account during polymersomes designing. For instance, PAA can be used in order to stabilize loaded cationic drugs via electrostatic interactions [26]. Due to its good water solubility as well as targeting capability for CD44 receptors, the natural glycosaminoglycan hyaluronan (HYA) was also employed as a hydrophilic block of copolymers that were used for polymersomes formation [27, 28]. The hydrophilic part of polymersomes can also be presented by phosphorylcholine-based polymers. Phosphorylcholine is composed of a negatively charged phosphate group and positively charged choline group. It can work as a hydrophilic head group in some phospholipids that form lipid bilayer of cell membranes. Phosphorylcholine-containing outer shell of vesicles mimics natural cells surfaces and therefore, can provide vesicles with long-term blood circulation due to less protein adsorption and cell attachment [29].
2.3. Hydrophobic Blocks
Unlike polymers which constitute hydrophilic building blocks in amphiphilic block copolymers, polymers used as hydrophobic blocks are more abundant. At the initial stage, non-biodegradable hydrophobic polymers such as poly(butadiene) (PBD), poly(dimethylsiloxane) (PDMS), poly(ethyl ethylene) (PEE) and poly(styrene) (PS) were utilized as hydrophobic blocks for polymersomes fabrication [11]. However, most of these formulations have limited applications in loading and delivery of anticancer drugs and further in vitro and in vivo studies, since non-biodegradable constituents cannot be degraded into non-toxic materials with further elimination from the body via natural metabolic pathways.
In recent decades, biodegradable hydrophobic polymers are extensively explored as hydrophobic parts of amphiphilic block copolymers, which are widely used for the fabrication of various nanosized drug delivery systems, including polymersomes. Generally, according to the type of monomer, biodegradable hydrophobic polymers can mainly be classified as follows: polyesters, polycarbonates, polypeptides and other blended polymers.
Polyesters are one of the most widely utilized biodegradable polymers for drug delivery. The polymer backbone of polyesters can be hydrolyzed in the body, eventually leading to H2O and CO2 as degradation products. Such polyesters, as polylactic acid (PLA) [30, 31], polyglycolic acid (PGA) [32], poly(D,L-lactic-co-glycolic acid) (PLGA) [33, 34] and poly(ε-caprolactone) (PCL) [35, 36] are approved by the FDA for biomedical applications in humans.
Polycarbonates are another category of biodegradable polymers employed for drug delivery purposes. Polycarbonates are degraded in vivo by surface corrosion, which is in contrast with the bulk degradation mode of polyesters. An additional point of interest derives from their stimuli responsiveness and low toxicity. Some polycarbonates, such as poly(trimethylene carbonate) (PTMC) and poly(2,4,6-trimethoxybenzylidenepentaerythritolcarbonate) (PTMBPEC), are exploited as hydrophobic building blocks with hydrophilic PEG to fabricate polymer vesicles [37–39].
Polypeptides are also introduced into construction of biodegradable polymersomes due to their good biocompatibility and easy degradation of amido bond by hydrolysis. Moreover, polypeptides can reversibly change conformation in response to environmental stimuli, such as pH or temperature, which is of great importance in the programmed drug release [20, 21]. Some hydrophobic polypeptides or modified polypeptides are used as hydrophobic parts in polymersomes, including poly(L-leucine), polyglycine, poly(ε-benzyloxycarbonyl-L-Lysine), poly(L-phenylalanine) and poly(γ-benzyl L-glutamate) [28].
Through combining hydrophilic polymer candidates with hydrophobic ones at proper ratios as stated in part 2.1, various amphiphilic polymers can be custom-tailored to prepare polymersomes. Some examples of non-biodegradable and biodegradable block copolymers are summarized in Table 1 and Table 2.
Table 1.
Examples of non-biodegradable polymersomes.
| Amphiphilic block copolymer | Abbreviation | Refs. |
|---|---|---|
| poly(ethylene glycol)- b-poly(butadiene) | PEG-PBD | [64] |
| poly(2-methyl-2-oxazoline)-b-poly(dimethylsiloxane)-b-poly(2-methyl-2-oxazoline) | PMOXA-PDMS-PMOXA | [65] |
| poly(ethylene glycol)-b-poly(ethyl ethylene) | PEG-PEE | [64] |
| poly(ethylene glycol)-b-poly(styrene) | PEG-PS | [66] |
Table 2.
Summary of polymersomes fabricated from biodegradable copolymers for anti-cancer drug delivery.
| Copolymer | Refs. | |
|---|---|---|
| Polyesters as hydrophobic blocks | PEG-PLA, PEG-PCL | [36, 67] |
| PEO-PCL | [68] | |
| PEG-b-PLGA | [34, 69] | |
| Polycarbonates as hydrophobic blocks | PEG-PTMBPEC | [38] |
| PEG-PTMC | [70] | |
| Polypeptide based biodegradable polymersomes | PGA-PTMC | [71] |
| PBLG-HYA | [72] | |
| Other blended biodegradable polymersomes | PEG-PLA + PEG-PBD | [73, 74] |
| PEG-b-PCL-g-PAE | [75] | |
PEG-PLA: poly(ethylene glycol)-polylactic acid.
PEG-PCL: poly(ethylene glycol)-poly(ε-caprolactone).
PEO-PCL: poly(ethylene oxide)-poly(ε-caprolactone).
PEG-b-PLGA: poly(ethylene glycol)-b-poly(lactic-co-glycolic acid). PEG-PTMBPEC: poly(ethylene glycol)-poly(2,4,6-trimethoxybenzylidenepentaerythritol carbonate).
PEG-PTMC: poly(ethyleneglycol)-b-poly(trimethylene carbonate).
PGA-PTMC: poly(L-glutamic acid)-b-poly(trimethylene carbonate).
PBLG-HYA: poly(γ-benzyl L-glutamate)-b-hyaluronan.
PEG-PBD: poly(ethylene glycol)-polybutadiene.
PEG-b-PCL-g-PAE: methoxy-poly(ethylene glycol)-b-poly(ε-caprolactone)-g-poly(β-amino ester.
3. APPLICATIONS OF POLYMERSOMES FOR DIFFERENT DRUG LOADING STRATEGIES
The structure of polymersomes can be designed in order to achieve such required properties for drug carriers as biodegradability, in vivo stability, prolonged time circulation, controlled drug release, and surface upgradability. However, one of the major advantages of polymersomes is the ability to load hydrophilic and/or hydrophobic molecules due to their optimal structures. The aqueous core of vesicles acts as a reservoir for hydrophilic compounds, while the bilayer membrane can load hydrophobic agents. In this regard, polymer vesicles can be utilized for hydrophilic, hydrophobic or dual drug loading and delivery (Table 3). In the following part we will discuss a number of polymersomes which were demonstrated to have a potential to improve the utility of various anticancer agents.
Table 3.
Examples of multi-functional polymersomes used for anticancer drug loading and delivery.
| Copolymers | Targeting Moiety | Target | Loaded Cargo | Refs. |
|---|---|---|---|---|
| Polymersomes used for hydrophilic drugs loading | ||||
| PEG-PLGA | EpCAM aptamer | EpCAM | Doxorubicin | [34] |
| PEGGM-PDSGM | Des-octanoyl ghrelin/Folic acid | Brain/ Folate receptor | Doxorubicin | [40] |
| PEG-PTTMA-PAA | Non-targeted | ---------- | Doxorubicin | [26] |
| R-PEG-b-P(DEAEMA-stat –BMA) | Folic acid | Folate receptor | Doxorubicin | [76] |
| PBLG-b-HYA | Hyaluronic acid | CD44 receptor of MCF-7 cells | Doxorubicin | [72] |
| PB-PEO | GRGDSP peptide | α5β1 integrin | siRNA | [43] |
| PEG-PTTMA-PAA | Anisamide | Sigma receptor | Granzyme B | [42] |
| Polymersomes used for hydrophobic drugs loading | ||||
| PBLG-b-HYA | Hyaluronic acid | CD44 receptor of MCF-7 cells | Docetaxel | [27] |
| PEO-b-PBLG-b-PLL | Non-targeted | ---------- | Doxorubicin or Paclitaxel | [44] |
| Polymersomes used for simultaneous hydrophilic and hydrophobic drugs loading | ||||
| PEG-PLA/PEG-PCL | Non-targeted | ---------- | Doxorubicin+Paclitaxel | [74] |
| PTMBPEC-PEG | Non-targeted | ---------- | Doxorubicin+Paclitaxel | [38] |
| PMPC- PDPA | Non-targeted | ---------- | Doxorubicin+Paclitaxel | [46] |
| PTMC-b-PGA | Can be guided in an external magnetic field gradient | ---------- | Doxorubicin+γ-Fe2O3 | [47] |
PEGGM-PDSGM: poly(ethylene glycol-g-glutamate)-co-poly(distearin-g-glutamate).
PEG-PTTMA-PAA: poly(ethylene glycol)-b-poly(2,4,6-trimethoxybenzylidene-1,1,1-tris(hydroxymethyl)ethane methacrylate)-b-poly(acrylic acid).
PEG-b-P(DEAEMA-stat –BMA): poly(ethylene glycol)-block-poly[2-(diethylamino) ethyl methacrylate-stat −2-hydroxy-4-(methacryloyloxy) benzophenone].
PB-PEO: poly(1,2-butadiene)-b-poly(ethylene oxide).
PEO-b-PBLG-b-PLL: poly(ethylene oxide)-b- poly(g-benzyl-(d7) L-glutamate)-b- poly(L-lysine hydrochloride).
PMPC- PDPA: poly 2-(methacryloyloxy)ethyl phosphorylcholine- poly 2-(diisopropylamino)ethyl methacrylate.
3.1. Polymersomes as Carriers for Hydrophilic Molecules
Due to properties of polymersomes mentioned above and the presence of aqueous interior in the structure, polymersomes have received great attention as versatile vehicles for multiple hydrophilic agents. One of the most common hydrophilic chemotherapeutic agents doxorubicin hydrochloride (DOX) was used in many studies as a model hydrophilic drug for loading in the polymersomes core. Encapsulation of DOX inside the vesicles can significantly improve therapeutic properties of the drug and reduce its side effects, including cardiotoxicity. For example, polymersomes based on PEG-b-PLGA copolymer and loaded with DOX demonstrated high encapsulation efficiency of drug (higher than 90%) and high stability during 6 months storage period of the lyophilized powder at 4 °C [33]. Further animal studies showed that the DOX-loaded polymersomes were more effective in comparison to free DOX against a murine breast cancer tumor model. Moreover, the DOX-polymersomes showed higher survival rate of mice than free DOX, due to decreased cardiotoxicity of DOX incorporated in the polymersomes. In another work Chen et al. formulated polymersomes composed of poly(ethylene glycol-g-glutamate)-co-poly (distearing-glutamate) (PEGGM-PDSGM) block copolymer and loaded them with DOX [40]. In order to facilitate the delivery through BBB (blood-brain barrier) and achieve improved tumor targeting against glioma cells, the surface of vesicles was decorated with des-octanoyl ghrelin and folate respectively. Functionalized polymer vesicles exhibited enhanced penetrating and targeting properties in BBB model and C6 glioma cells. Moreover, compared with non-decorated vesicles, modified polymersomes demonstrated more efficient inhibition of cancer cells. In addition, PEGGM-PDSGM polymersomes with conjugated ligands improved the survival of tumor-bearing mice in comparison to free DOX, liposomal DOX, unmodified vesicles or single ligand conjugated polymersomes.
Along with diblocks, triblock copolymers can also be used as polymersome units. For example, Du with co-workers formulated pH-sensitive polymersomes based on asymmetric poly(ethyleneglycol)-b-poly(2,4,6-trimethoxybenzylidene-1,1,1-tris (hydroxymethyl)ethane methacrylate)-b-poly(acrylic acid) (PEG-PTTMA-PAA) triblock copolymer. This system consists of two hydrophilic blocks with different chain length: PEG is longer than PAA. Thus, negatively charged PAA formed the inner hydrophilic layer and provided the efficient and stable loading of DOX via electrostatic interaction. Polymersomes demonstrated high stability at physiological pH 7.4, while they disassembled at acidic pH 4.0 and 5.0, suggesting that these vesicles have pH-dependent drug release profile. In addition, polymersomes exhibited successful delivery of DOX into the nuclei of HeLa cells and high antitumor activity of drug loaded polymersomes while empty vesicles were practically non-toxic [26].
Natural polymers can also be introduced as a part of amphiphilic polymers in order to fabricate polymersomes [41]. For instance, Upadhyay et al. loaded DOX inside the polymersomes which were synthesized from amphiphilic polypeptide-block-polysaccharide copolymers poly(g-benzyl L-glutamate)-block-hyaluronan (PBLG-b-HYA) [21]. Owing to its water solubility, as well as targeting capability HYA played a dual role in this system: as a hydrophilic and stabilizing fragment of vesicles and as a targeting moiety for CD44 receptors. It was found that the drug loading content and encapsulation efficiency were determined to be 12±1 wt% and 50% respectively. In vitro experiments demonstrated high cytotoxic effect of drug-loaded polymersomes against MCF-7 cells. Enhanced cellular uptake and accumulation were achieved in MCF-7 cells in comparison to U87 cells due to higher expression of CD44 glycoproteins in MCF-7 cancer cells. Further in vivo experiments showed significant tumor growth suppression when animals were treated by drug-loaded polymersomes.
Besides DOX, other anticancer hydrophilic compounds including siRNA and proteins have been encapsulated inside the polymersomes and used in cancer therapy. For example, aforementioned PEG-PTTMA-PAA triblock copolymer was decorated with anisamide and used as a carrier for apoptotic protein Granzyme B [42]. The protein encapsulation efficiency was 40.5–100% and loading content was up to 16.8 wt%. Anisamide-decorated vesicles demonstrated high targeting ability to cancer cells (H460 and PC-3) with over-expressed sigma receptors, due to high affinity of anisamide ligand to these receptors. Granzyme B-loaded targeting vesicles revealed high anti-tumor efficacy, with low half maximal inhibitory concentration (IC50) toward H460 cells. In addition, polymersomes exhibited fast internalization and intracellular protein release in cancer cells.
In another study, Pangburn et al. loaded siRNA in poly(1,2-butadiene)-b-poly(ethylene oxide) (PB-PEO) based polymersomes and functionalized their surface with PR_b (α5β1 integrin binding peptide) in order to achieve precise drug delivery. PR_b-decorated polymersomes demonstrated higher delivery efficiency to the cancerous T47D cells in comparison to normal MCF10A cells. Further experiments revealed successful early endosome release of siRNA and efficient suppression of targeted Orai3 gene, suggesting the effective intracellular delivery of encapsulated hydrophilic agent by PB-PEO vesicles [43].
3.2. Polymersomes as Carriers for Hydrophobic Molecules
Due to its bilayer morphology membrane of polymersomes can be considered as a reservoir for hydrophobic molecules. Since a large number of chemotherapeutics is hydrophobic (including such common drugs as paclitaxel, docetaxel, and hydrophobic doxorubicin), applying of polymersomes as carriers for hydrophobic molecules makes them a promising tool for anticancer therapy. For instance, previously mentioned nanocarrier based on PBLG-b-HYA was used for encapsulation of hydrophobic anticancer drug [27]. In this study, authors substituted hydrophilic DOX by hydrophobic docetaxel (DOC) and loaded it within the membrane of the polymersome. Drug loading content of DOC was lower than that of DOX (9.8±0.7%), but encapsulation efficiency was the same (50%). In this work the cytotoxicity of DOC-loaded vesicles also was higher for MCF-7 cells in comparison to U87 cells, because of higher expression of CD44 glycoproteins in MCF-7 cancer cells. The data of pharmacokinetic study suggested that unlike free DOC, drug-loaded polymersomes had prolonged blood circulation time. Biodistribution results revealed that polymersomes were successfully accumulated at the tumor site. In addition, hemolysis test displayed that DOC loaded polymersomes do not cause hemolysis in the range of 10 to 100 mg • mL−1 DOC equivalent concentration.
Similarly, Iatrou et al. explored PEO-b-PBLG-b-PLL based polymersomes as carriers for hydrophilic or hydrophobic drugs [44]. Temperature- and pH-sensitive polymersomes were fabricated of asymmetric amphiphilic triblock terpolymer poly(ethylene oxide)-b-poly(g-benzyl-(d7) L-glutamate)-b-poly(L-lysine hydrochloride). The polymersomes were separately loaded with hydrophilic DOX or hydropobic paclitaxel (PTX) with average size distribution of 115 nm and 125 nm respectively. The highest drug encapsulation efficiency of DOX loaded vesicles was 33% and loading content of 19.5%. For PTX loaded polymersomes the encapsulation efficiency and loading content were 25% and 13% respectively. In vitro release assays showed both drugs exhibit a sustainable release in a temperature dependent manner. However, higher pH caused faster release of PTX, whereas DOX-loaded vesicles demonstrated the highest release rate at low pH. For in vitro activity experiments AsPC-1 and BxPC-3 cancer cells were treated with drugs loaded polymersomes. PTX loaded polymers were found to be more active comparing with DOX loaded vesicles, which exhibited slight antiproliferative activity against BxPC-3 cells. In addition, blank PEO-b-PBLG-b-PLL polymersomes did not demonstrate any toxic effect up to 200 mg • kg−1.
3.3. Polymersomes as Carriers for Multiple Molecules
One of the general goals of combination chemotherapy is to synergistically enhance the treatment efficacy of drugs in cancer therapy. Other principle of combination treatment is using of chemotherapeutic drugs with different mechanisms of action in order to reduce the emergence of multidrug resistance. Moreover, the combination of drugs leads to reduction of dose with consequent decreasing of toxicity to healthy tissues [45]. Thereby, the development of nanosized vehicles, which are able to carry more than one drug, is getting considerable attention. Due to their structures, polymersomes can encapsulate both hydrophilic and hydrophobic molecules simultaneously, and this feature makes them a suitable carrier for combinational therapy. For instance, Chen with co-workers applied pH-sensitive degradable polymersomes composed of poly(2,4,6-trimethoxybenzylidenepentaerythritolcarbonate)-b-poly(ethylene glycol) (PTMBPEC-PEG) for simultaneous encapsulation and delivery of hydrophobic PTX and hydrophilic DOX [38]. Polymersomes demonstrated drug encapsulation efficiencies of 30.0 – 37.7% for PTX and 19.5 – 26.2% for DOX, respectively. The release studies showed the stability of polymersomes at normal physiological pH = 7.4 and pH-dependent release of drugs at acidic pH of 4.0 and 5.0. Colley et al. used pH-sensitive polymersomes based on poly 2-(methacryloyloxy)ethyl phosphorylcholinepoly 2-(diisopropylamino)ethyl methacrylate (PMPC-PDPA) for dual drug delivery of DOX and PTX [46]. Results revealed that formulated dual drug loaded polymersomes had encapsulation efficiency of 37.1% ± 13.5% and 49.1% ± 4.4% for single and dual loaded drugs respectively. Polymersomes showed fast drug release under endosomal low pH conditions. Cell lines experiments revealed that cellular uptake is more rapid for oral head and neck squamous cell carcinoma (HNSCC) cells when compared with normal oral cells, due to high affinity of polymersomes to scavenger receptors which are higher expressed in cancer cells. Cytotoxic effect of dual loaded polymersomes after 24 hours of treating of cancer cells is higher, in comparison to the cells treated with free drugs or polymersomes encapsulated with PTX or DOX alone. However, after 48 hours no difference in cell viability was observed after treating cells with polymersome-loaded drugs and free drugs, suggesting that the advantage observed in this polymersome-mediated therapy is in virtue of the initial rapid drug uptake. Further analysis confirmed short exposure time is needed to cause a strong cytotoxic effect in cancer cells. Moreover, three-dimensional tumor models displayed that PMPC-PDPA polymersomes were able to penetrate deep into the 3D cell culture, causing extensive cell damage.
Polymersomes are versatile vesicles which can also be applied as carriers for inorganic materials. For example, Sanson et al. demonstrated the possibility of polymer vesicles to encapsulate iron oxide magnetic nanoparticles (MNPs) [47]. Biodegradable polymersomes fabricated from poly(trimethylene carbonate)-b-poly(L-glutamic acid) (PTMC-b-PGA) block copolymer have been exploited as carriers for hydrophobic γ-Fe2O3 that was encapsulated in the membrane of vesicles with loading content up to 70 wt.%. DOX was used as a model hydrophilic agent loaded within the core of the polymersomes. These superparamagnetic theranostic nano-vesicles provided enhanced contrast properties which are potentially useful for magnetic resonance imaging (MRI). They can also be guided by an external magnetic field gradient to improve their targeted properties. Moreover, controlled DOX release by radio frequency magnetic hyperthermia was achieved, showing the multi-functional features of the system.
4. POLYMERSOMES FORMULATION TECHNIQUES
Polymersomes preparation techniques are basically similar to methods commonly applied in the formulation of liposomes and other vesicle-type systems. These methods are generally grouped into two: solvent displacement and rehydration techniques [48–50]. However, other methods such as direct hydration and electroformation methods have also been used in the formulation of polymersomes [51–53].
4.1. Solvent Displacement Techniques
The solvent displacement techniques involve the use of a suitable organic solvent to dissolve the components of the amphiphilic block copolymer followed by the hydration of this organic polymer solution by either injecting an aqueous solvent into the organic polymer solution or vice-versa [48, 54]. Increased interfacial tension between the hydrophobic block and water triggers the copolymer self-assembly into polymersomes [55]. Organic solvent could then be removed either by dialysis method or evaporation [49]. The type of solvent used, polymer concentration and order of polymer hydration determines the size and size distribution of the resulting polymersomes [56]. Nanoprecipitation and emulsion-solvent evaporation are the two widely used solvent displacement techniques [18].
4.1.1. Nanoprecipitation Technique
In the nanoprecipitation method, the drug and polymer are dissolved in the organic solvent and then injected under magnetic stirring into an aqueous solution (with or without a surfactant). The organic solvent may or may not be miscible with water, but the hydrophobic moiety of the copolymer initiates the precipitation of the polymer out of solution and forms the vesicular polymersomes which are left in the aqueous solvent after the evaporation of the organic solvent (Fig. 2) [18, 57]. Choice of the organic solvent or co-solvent at different ratios can influence the polymersomes particles size [58].
Fig. (2).
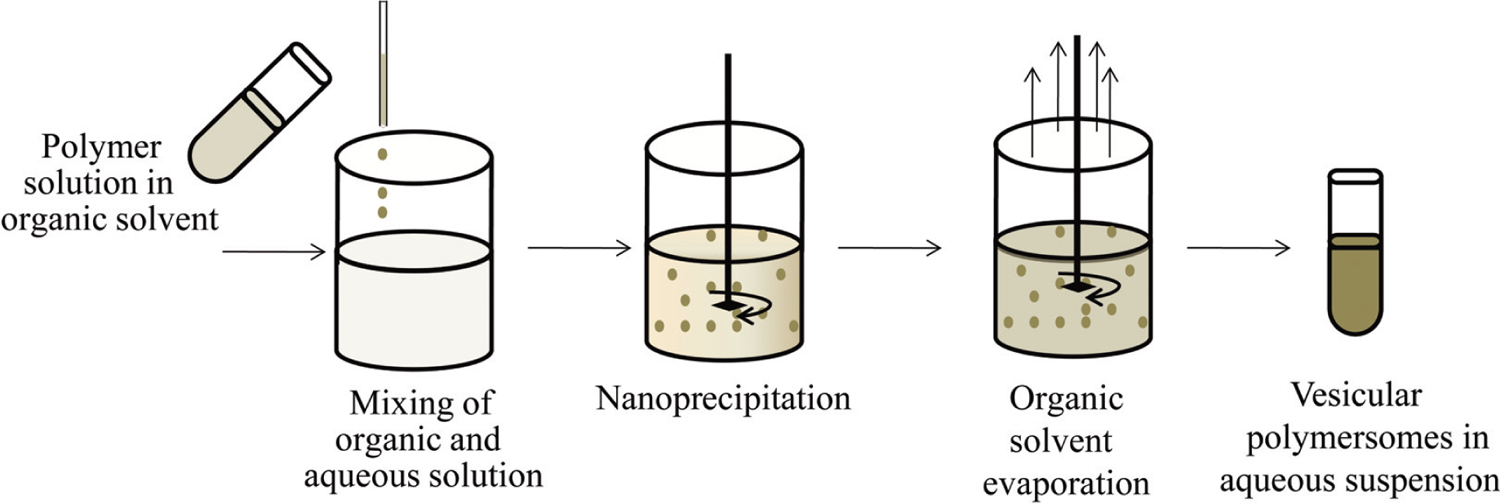
Formulation steps for polymersomes by nanoprecipitation method.
4.1.2. Emulsion-Solvent Evaporation Technique
Emulsification of a copolymer solution in a water immiscible solvent with a surfactant containing aqueous solution will yield nanosized polymersomes after evaporation of the organic solvent at reduced pressure [18]. The surfactant modulates the force of the phase separation and provides a shielding layer such that well-ordered polymersomes remain in aqueous solution after evaporation of the organic solvent [59]. The primary emulsion could be oil-in-water (o/w) single emulsion or a water-in-oil-in-water (w/o/w) double emulsion (Fig. 3). The double emulsion is more suitable for loading hydrophilic drugs such as peptides into the polymersomes [60].
Fig. (3).
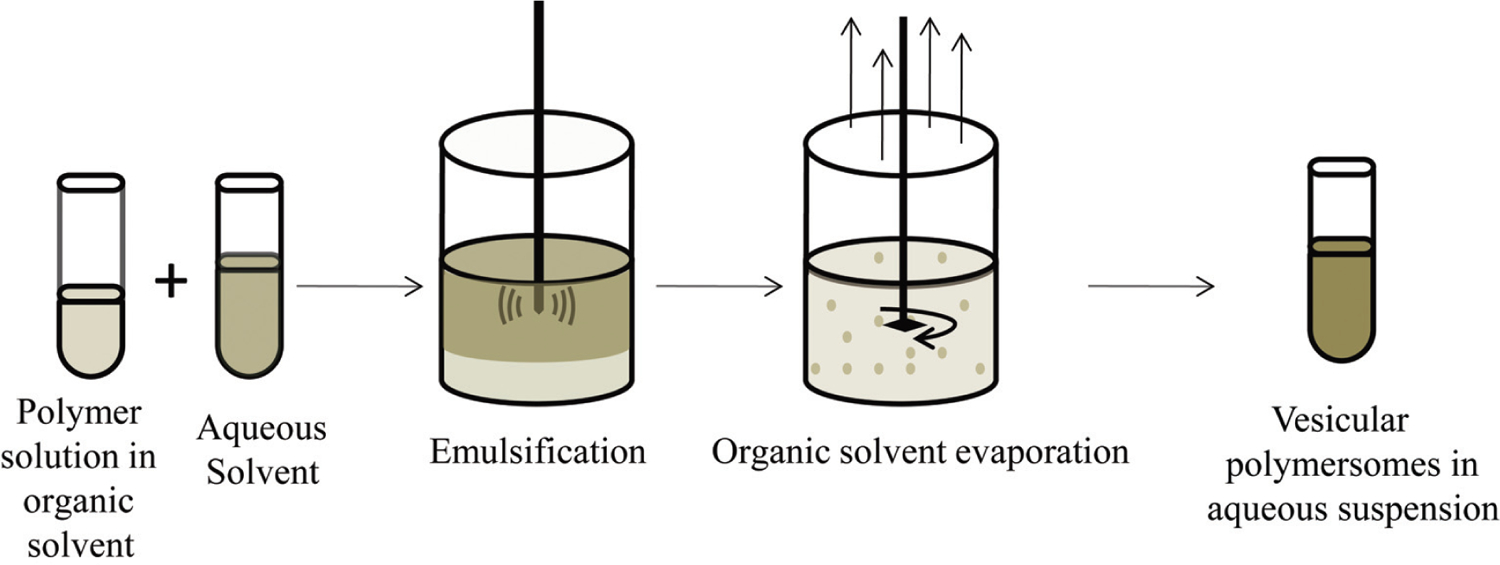
Formulation steps for polymersomes by nanoemulsion-solvent evaporation method.
4.2. Rehydration Techniques
The rehydration technique is an organic solvent free technique for polymersomes formulation. Though an organic solvent is used to dissolve the polymer at the beginning, it is then evaporated under reduced pressure as the polymer is deposited as a thin film on the wall of the flask [49]. The thin film is then hydrated with water or a buffer solution, which penetrates through defects on the thin film and bulge into polymersomes (Fig. 4). This formulation method yields polymersomes with a wide particles size distribution and would require filtration through pre-calibrated filter membranes or sonication to achieve a desired narrow size distribution [61]. Furthermore, in electroformation technique (another organic solvent free technique), an electric field can be applied during the hydration process to produce polymersomes with a narrow particles size range [62]. In this method, the thin film is deposited on a pair of electrodes made of either platinum wire, gold wire or indium-tin-oxide coated glass plates [63].
Fig. (4).
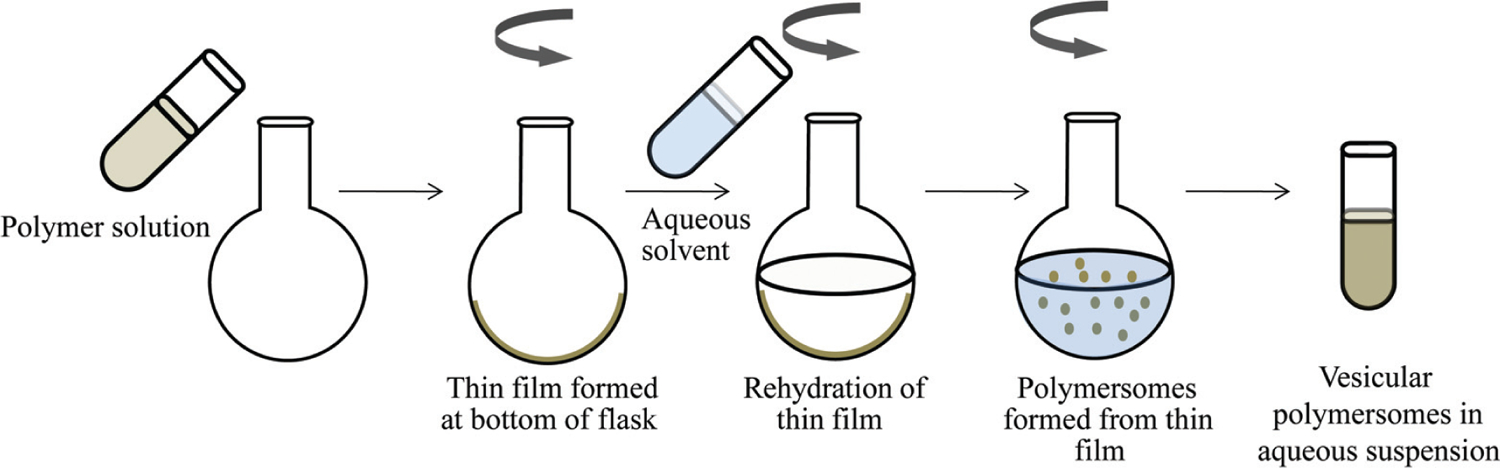
Formulation steps for polymersomes by thin film rehydration method.
CONCLUSION AND PERSPECTIVES
Owing to their special features polymersomes have been utilized as drug carriers for pharmaceutical delivery. Various drugs, proteins, nucleic acids, enzymes, as well as imaging agents can be encapsulated within either hydrophilic core or hydrophobic bilayer using two general formulation methods. Polymersomes can be exploited as nanocarriers for combination therapy because of their ability to simultaneously encapsulate multiple molecules. Due to the possibility to customize and regulate distinct properties of polymersomes at the molecular level, it has become feasible to develop polymersomes with desirable characteristics. For example, by using certain materials for polymersomes formulation, it is possible to achieve stimuli-responsive release profiles. In the future, it will be necessary to develop new stimuli-responsive polymersomes that can respond sensitively to internal stimuli such as pH value, temperature, enzymes or glucose level. This feature will facilitate the programmable delivery of therapeutic agents and their precise release in pathological area, which can significantly enhance therapeutic efficacy and reduce undesirable side effects. Moreover, hydrophilic surface of the vesicles can provide “stealth” properties, as well as possibility to decorate it with different ligands. Modifying the polymersomes with targeting ligands will provide selective delivery of therapeutics to their site of action with minimum damage to normal tissues. Further development of high-specific targeting ligands and their conjugation with polymersomes may lead to improvement of current existing approaches to diagnosis and treatment of various diseases including cancer. In addition, ability of polymersomes to co-load imaging and therapeutic agents can make them excellent theranostic nanoparticles.
Although, polymersomes have a number of advantages in comparison to other nanosized carriers, some serious challenges still remain. For example, biocompatibility of polymersomes is still an issue. For successful further medical use of polymersomes, it is important to study the impact of polymersomes on biological components and their in vivo behavior. In this regard, analysis of the polymersomes pharmacokinetics, particularly toxicokinetics, becomes essential for evolving them for clinical use.
ACKNOWLEDGEMENTS
This work was supported by the Chinese Natural Science Foundation key project (31430031) and National Distinguished Young Scholars grant (31225009), State High-Tech Development Plan (2012AA020804 and SS2014AA020708). This work was supported in part by NIH/NIMHD 8 G12 MD007597 and USAMRMC W81XWH-10-1-0767 grants. The authors also appreciate the support by the “Strategic Priority Research Program” of the Chinese Academy of Sciences, Grant No. XDA09030301 and support by the external cooperation program of BIC, Chinese Academy of Science, Grant No. 121D11KYSB20130006.
Biographies

Ruslan G. Tuguntaev

Chukwunweike Ikechukwu Okeke
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Rösler A, Vandermeulen GWM, Klok H-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv Drug Deliv Rev 2012; 64(Supplement): 270–9. [DOI] [PubMed] [Google Scholar]
- [2].Adams ML, Lavasanifar A, Kwon GS. Amphiphilic block copolymers for drug delivery. J Pharm Sci 2003; 92: 1343–55. [DOI] [PubMed] [Google Scholar]
- [3].Förster S, Antonietti M. Amphiphilic block copolymers in structure-controlled nanomaterial hybrids. Adv Mater 1998; 10: 195–217. [Google Scholar]
- [4].Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release 2001; 70: 1–20. [DOI] [PubMed] [Google Scholar]
- [5].Tyrrell ZL, Shen Y, Radosz M. Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers. Prog Polym Sci 2010; 35: 1128–43. [Google Scholar]
- [6].Pillai O, Panchagnula R. Polymers in drug delivery. Curr Opin Chem Biol 2001; 5: 447–51. [DOI] [PubMed] [Google Scholar]
- [7].Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv Drug Deliv Rev 2001; 47: 113–31. [DOI] [PubMed] [Google Scholar]
- [8].Letchford K, Burt H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur J Pharm Biopharm 2007; 65: 259–69. [DOI] [PubMed] [Google Scholar]
- [9].Kabanov AV, Okano T. Challenges in polymer therapeutics. In: Maeda H, Kabanov AV, Kataoka K, Okano T, Eds. Polymer Drugs in the Clinical Stage. New York: Kluwer Academic/Plenum Publishers; 2003; pp. 1–27. [Google Scholar]
- [10].Discher DE, Ahmed F. Polymersomes. Annu Rev Biomed Eng 2006; 8: 323–41. [DOI] [PubMed] [Google Scholar]
- [11].Lee JS, Feijen J. Polymersomes for drug delivery: Design, formation and characterization. J Control Release 2012; 161: 473–83. [DOI] [PubMed] [Google Scholar]
- [12].Meng F, Zhong Z, Feijen J. Stimuli-responsive polymersomes for programmed drug delivery. Biomacromolecules 2009; 10: 197–209. [DOI] [PubMed] [Google Scholar]
- [13].Torchilin VP. Micellar nanocarriers: Pharmaceutical perspectives. Pharm Res 2007; 24: 1–16. [DOI] [PubMed] [Google Scholar]
- [14].Mai Y, Eisenberg A. Self-assembly of block copolymers. Chem Soc Rev 2012; 41: 5969–85. [DOI] [PubMed] [Google Scholar]
- [15].Lim Soo P, Eisenberg A. Preparation of block copolymer vesicles in solution. J Polym Sci Part B: Polym Phys 2004; 42: 923–38. [Google Scholar]
- [16].Guan L, Rizzello L, Battaglia G. Polymersomes and their applications in cancer delivery and therapy. Nanomedicine 2015; 10: 2757–80. [DOI] [PubMed] [Google Scholar]
- [17].Anajafi T, Mallik S. Polymersome-based drug-delivery strategies for cancer therapeutics. Ther Deliv 2015; 6: 521–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Krishnamoorthy B, Karanam V, Chellan VR, Siram K, Natarajan TS, Gregory M. Polymersomes as an effective drug delivery system for glioma-a review. J Drug Target 2014; 22: 469–77. [DOI] [PubMed] [Google Scholar]
- [19].Sherman MR, Williams LD, Saifer MGP, French JA, Kwak LW, Oppenheim JJ. Conjugation of high-molecular weight poly(ethylene glycol) to cytokines: Granulocyte-macrophage colony-stimulating factors as model substrates. In: Harris JM, Zalipsky S, Eds. Poly(ethylene Glycol). Washington DC: American Chemical Society; 1997; pp. 155–69. [Google Scholar]
- [20].Zhao L, Li N, Wang K, Shi C, Zhang L, Luan Y. A review of polypeptide-based polymersomes. Biomaterials 2014; 35: 1284–301. [DOI] [PubMed] [Google Scholar]
- [21].Morell M, Puiggalí J. Hybrid block copolymers constituted by peptides and synthetic polymers: An overview of synthetic approaches, supramolecular behavior and potential applications. Polymers 2013; 5: 188–224. [Google Scholar]
- [22].Chen P, Qiu M, Deng C, et al. pH-responsive chimaeric pepsomes based on asymmetric poly (ethylene glycol)-b-poly (L-leucine)-b-poly (L-glutamic acid) triblock copolymer for efficient loading and active intracellular delivery of doxorubicin hydrochloride. Biomacromolecules 2015; 16: 1322–30. [DOI] [PubMed] [Google Scholar]
- [23].Goñi-de-Cerio F, Thevenot J, Oliveira H, et al. Cellular uptake and cytotoxic effect of epidermal growth factor receptor targeted and plitidepsin loaded co-polymeric polymersomes on colorectal cancer cell lines. J Biomed Nanotechnol 2015; 11: 2034–49. [DOI] [PubMed] [Google Scholar]
- [24].Iatrou H, Frielinghaus H, Hanski S, et al. Architecturally induced multiresponsive vesicles from well-defined polypeptides. Formation of gene vehicles. Biomacromolecules 2007; 8: 2173–81. [DOI] [PubMed] [Google Scholar]
- [25].Makino A, Kizaka-Kondoh S, Yamahara R, et al. Near-infrared fluorescence tumor imaging using nanocarrier composed of poly (L-lactic acid)-block-poly (sarcosine) amphiphilic polydepsipeptide. Biomaterials 2009; 30: 5156–60. [DOI] [PubMed] [Google Scholar]
- [26].Du Y, Chen W, Zheng M, Meng F, Zhong Z. pH-sensitive degradable chimaeric polymersomes for the intracellular release of doxorubicin hydrochloride. Biomaterials 2012; 33: 7291–9. [DOI] [PubMed] [Google Scholar]
- [27].Upadhyay KK, Bhatt AN, Castro E, et al. In vitro and in vivo evaluation of docetaxel loaded biodegradable polymersomes. Macromol Biosci 2010; 10: 503–12. [DOI] [PubMed] [Google Scholar]
- [28].Upadhyay KK, Bhatt AN, Mishra AK, et al. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly(β-benzyl l-glutamate)-b-hyaluronan polymersomes. Biomaterials 2010; 31: 2882–92. [DOI] [PubMed] [Google Scholar]
- [29].Lewis AL. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Colloids Surf B 2000; 18: 261–75. [DOI] [PubMed] [Google Scholar]
- [30].Zhu J, Xu X, Hu M, Qiu L. Co-encapsulation of combretastatin-A4 phosphate and doxorubicin in polymersomes for synergistic therapy of nasopharyngeal epidermal carcinoma. J Biomed Nanotechnol 2015; 11: 997–1006. [DOI] [PubMed] [Google Scholar]
- [31].Nahire R, Haldar MK, Paul S, et al. Multifunctional polymersomes for cytosolic delivery of gemcitabine and doxorubicin to cancer cells. Biomaterials 2014; 35: 6482–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lai P, Daear W, Löbenberg R, Prenner EJ. Overview of the preparation of organic polymeric nanoparticles for drug delivery based on gelatine, chitosan, poly (d, l-lactide-co-glycolic acid) and polyalkylcyanoacrylate. Colloids Surf B 2014; 118: 154–63. [DOI] [PubMed] [Google Scholar]
- [33].Alibolandi M, Sadeghi F, Abnous K, Atyabi F, Ramezani M, Hadizadeh F. The chemotherapeutic potential of doxorubicin-loaded PEG-b-PLGA nanopolymersomes in mouse breast cancer model. Eur J Pharm Biopharm 2015; 94: 521–31. [DOI] [PubMed] [Google Scholar]
- [34].Alibolandi M, Ramezani M, Sadeghi F, Abnous K, Hadizadeh F. Epithelial cell adhesion molecule aptamer conjugated PEG–PLGA nanopolymersomes for targeted delivery of doxorubicin to human breast adenocarcinoma cell line in vitro. Int J Pharm 2015; 479: 241–51. [DOI] [PubMed] [Google Scholar]
- [35].Lee JS, Feijen J. Biodegradable polymersomes as carriers and release systems for paclitaxel using Oregon Green® 488 labeled paclitaxel as a model compound. J Control Release 2012; 158: 312–8. [DOI] [PubMed] [Google Scholar]
- [36].Zou T, Dembele F, Beugnet A, et al. Nanobody-functionalized PEG-b-PCL polymersomes and their targeting study. J Biotechnol 2015; 214: 147–55. [DOI] [PubMed] [Google Scholar]
- [37].Ohya Y, Takahashi A, Nagahama K. Biodegradable polymeric assemblies for biomedical materials. In: Kunugi S, Yamaoka T, Eds. Polymers in nanomedicine. Berlin: Springer Berlin Heidelberg; 2012; pp. 65–114. [Google Scholar]
- [38].Chen W, Meng F, Cheng R, Zhong Z. pH-sensitive degradable polymersomes for triggered release of anticancer drugs: A comparative study with micelles. J Control Release 2010; 142: 40–6. [DOI] [PubMed] [Google Scholar]
- [39].Chen W, Meng F, Cheng R, Deng C, Feijen J, Zhong Z. Advanced drug and gene delivery systems based on functional biodegradable polycarbonates and copolymers. J Control Release 2014; 190: 398–414. [DOI] [PubMed] [Google Scholar]
- [40].Chen YC, Chiang CF, Chen LF, Liang PC, Hsieh WY, Lin WL. Polymersomes conjugated with des-octanoyl ghrelin and folate as a BBB-penetrating cancer cell-targeting delivery system. Biomaterials 2014; 35: 4066–81. [DOI] [PubMed] [Google Scholar]
- [41].Bakalova R, Lazarova D, Nikolova B, et al. Delivery of size-controlled long-circulating polymersomes in solid tumours, visualized by quantum dots and optical imaging in vivo. Biotechnol Biotec Equip 2015; 29: 175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lu L, Zou Y, Yang W, et al. Anisamide-decorated pH-sensitive degradable chimaeric polymersomes mediate potent and targeted protein delivery to lung cancer cells. Biomacromolecules 2015; 16: 1726–35. [DOI] [PubMed] [Google Scholar]
- [43].Pangburn TO, Georgiou K, Bates FS, Kokkoli E. Targeted polymersome delivery of siRNA induces cell death of breast cancer cells dependent upon Orai3 protein expression. Langmuir 2012; 28: 12816–30. [DOI] [PubMed] [Google Scholar]
- [44].Iatrou H, Dimas K, Gkikas M, Tsimblouli C, Sofianopoulou S. Polymersomes from polypeptide containing triblock co- and terpolymers for drug delivery against pancreatic cancer: Asymmetry of the external hydrophilic blocks. Macromol Biosci 2014; 14: 1222–38. [DOI] [PubMed] [Google Scholar]
- [45].Pinto AC, Moreira JN, Simões S. Combination chemotherapy in cancer: Principles, evaluation and drug delivery strategies. In: Ozdemir O, Ed. Current cancer treatment - novel beyond conventional approaches. Rijeka: InTech; 2011; pp. 693–715. [Google Scholar]
- [46].Colley HE, Hearnden V, Avila-Olias M, et al. Polymersome-mediated delivery of combination anticancer therapy to head and neck cancer cells: 2D and 3D in vitro evaluation. Mol Pharm 2014; 11: 1176–88. [DOI] [PubMed] [Google Scholar]
- [47].Sanson C, Diou O, Thévenot J, et al. Doxorubicin loaded magnetic polymersomes: Theranostic nanocarriers for MR imaging and magneto-chemotherapy. ACS Nano 2011; 5: 1122–40. [DOI] [PubMed] [Google Scholar]
- [48].LoPresti C, Lomas H, Massignani M, Smart T, Battaglia G. Polymersomes: Nature inspired nanometer sized compartments. J Mater Chem 2009; 19: 3576–90. [Google Scholar]
- [49].Du J, O’Reilly RK. Advances and challenges in smart and functional polymer vesicles. Soft Matter 2009; 5: 3544–61. [Google Scholar]
- [50].Lee JS, Feijen J. Polymersomes for drug delivery: Design, formation and characterization. J Control Release 2012; 161: 473–83. [DOI] [PubMed] [Google Scholar]
- [51].O’Neil CP, Suzuki T, Demurtas D, Finka T, Hubbell JA. A novel method for the encapsulation of biomolecules into polymersomes via direct hydration. Langmuir 2009; 25: 9025–9. [DOI] [PubMed] [Google Scholar]
- [52].Sanson C, Schatz C, Le Meins JF, Brûlet A, Soum A, Lecommandoux S. Biocompatible and biodegradable poly(trimethylene carbonate)-b-poly(L-glutamic acid) polymersomes: Size control and stability. Langmuir 2010; 26: 2751–60. [DOI] [PubMed] [Google Scholar]
- [53].Discher BM, Won YY, Ege DS, et al. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999; 284: 1143–6. [DOI] [PubMed] [Google Scholar]
- [54].Luo L, Eisenberg A. Thermodynamic stabilization mechanism of block copolymer vesicles. J Am Chem Soc 2001; 123: 1012–3. [DOI] [PubMed] [Google Scholar]
- [55].Discher DE, Eisenberg A. Polymer vesicles. Science 2002; 297: 967–73. [DOI] [PubMed] [Google Scholar]
- [56].Battaglia G, Ryan AJ. Pathways of polymeric vesicle formation. J Phys Chem B 2006; 110: 10272–9. [DOI] [PubMed] [Google Scholar]
- [57].Yildiz ME, Prud’homme RK, Robb I, Adamson DH. Formation and characterization of polymersomes made by a solvent injection method. Polym Adv Technol 2007; 18: 427–32. [Google Scholar]
- [58].Chiu HC, Lin YW, Huang YF, Chuang CK, Chern CS. Polymer vesicles containing small vesicles within interior aqueous compartments and pH-responsive transmembrane channels. Angew Chem Int Ed 2008; 47: 1875–8. [DOI] [PubMed] [Google Scholar]
- [59].Marsden HR, Quer CB, Sanchez EY, Gabrielli L, Jiskoot W, Kros A. Detergent-aided polymersome preparation. Biomacromolecules 2010; 11: 833–8. [DOI] [PubMed] [Google Scholar]
- [60].Kim JH, Bae YH. Albumin loaded microsphere of amphiphilic poly(ethylene glycol)/ poly(alpha-ester) multiblock copolymer. Eur J Pharm Sci 2004; 23: 245–51. [DOI] [PubMed] [Google Scholar]
- [61].Malinova V, Belegrinou S, de Bruyn Ouboter D, Meier WP. Biomimetic block copolymer membranes. In: Grasser T, Meller G, Li L, Eds. Organic electronics. Berlin: Springer Berlin Heidelberg; 2010; pp. 213–58. [Google Scholar]
- [62].Lomas H, Massignani M, Abdullah KA, et al. Non-cytotoxic polymer vesicles for rapid and efficient intracellular delivery. Faraday Discuss 2008; 139: 143–59. [DOI] [PubMed] [Google Scholar]
- [63].Liao J, Wang C, Wang Y, Luo F, Qian Z. Recent advances in formation, properties, and applications of polymersomes. Curr Pharm Des 2012; 18: 3432–41. [DOI] [PubMed] [Google Scholar]
- [64].Bermudez H, Brannan AK, Hammer DA, Bates FS, Discher DE. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules 2002; 35: 8203–8. [Google Scholar]
- [65].Nardin C, Hirt T, Leukel J, Meier W. Polymerized ABA triblock copolymer vesicles. Langmuir 2000; 16: 1035–41. [Google Scholar]
- [66].Kabanov AV, Bronich T, Kabanov V, Yu K, Eisenberg A. Spontaneous formation of vesicles from complexes of block ionomers and surfactants. J Am Chem Soc 1998; 120: 9941–2. [Google Scholar]
- [67].Ahmed F, Discher DE. Self-porating polymersomes of PEG-PLA and PEG–PCL: hydrolysis-triggered controlled release vesicles. J Control Release 2004; 96: 37–53. [DOI] [PubMed] [Google Scholar]
- [68].Ghoroghchian PP, Li G, Levine DH, et al. Bioresorbable vesicles formed through spontaneous self-assembly of amphiphilic poly (ethylene oxide)-block-polycaprolactone. Macromolecules 2006; 39: 1673–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Alibolandi M, Sadeghi F, Abnous K, Atyabi F, Ramezani M, Hadizadeh F. The chemotherapeutic potential of doxorubicin-loaded PEG-b-PLGA nanopolymersomes in mouse breast cancer model. Eur J Pharm Biopharm 2015; 94: 521–31. [DOI] [PubMed] [Google Scholar]
- [70].Li S, Meng F, Wang Z, et al. Biodegradable polymersomes with an ionizable membrane: Facile preparation, superior protein loading, and endosomal pH-responsive protein release. Eur J Pharm Biopharm 2012; 82: 103–11. [DOI] [PubMed] [Google Scholar]
- [71].Sanson C, Schatz C, Le Meins J-F, et al. A simple method to achieve high doxorubicin loading in biodegradable polymersomes. J Control Release 2010; 147: 428–35. [DOI] [PubMed] [Google Scholar]
- [72].Upadhyay KK, Bhatt AN, Mishra AK, et al. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly (γ-benzyl l-glutamate)-b-hyaluronan polymersomes. Biomaterials 2010; 31: 2882–92. [DOI] [PubMed] [Google Scholar]
- [73].Rodríguez-Hernández J, Lecommandoux S. Reversible inside-out micellization of pH-responsive and water-soluble vesicles based on polypeptide diblock copolymers. J Am Chem Soc 2005; 127: 2026–7. [DOI] [PubMed] [Google Scholar]
- [74].Ahmed F, Pakunlu RI, Srinivas G, et al. Shrinkage of a rapidly growing tumor by drug-loaded polymersomes: pH-triggered release through copolymer degradation. Mol Pharm 2006; 3: 340–50. [DOI] [PubMed] [Google Scholar]
- [75].Kang SW, Li Y, Park JH, Lee DS. pH-triggered unimer/vesicle-transformable and biodegradable polymersomes based on PEG-b-PCL–grafted poly (β-amino ester) for anti-cancer drug delivery. Polymer 2013; 54: 102–10. [Google Scholar]
- [76].Yassin MA, Appelhans D, Wiedemuth R, et al. Overcoming concealment effects of targeting moieties in the PEG corona: Controlled permeable polymersomes decorated with folate-antennae for selective targeting of tumor cells. Small 2015; 11: 1580–91. [DOI] [PubMed] [Google Scholar]


