Abstract
Hippo信号通路在进化上高度保守,哺乳动物细胞中该信号通路的核心成员包括MST1/2激酶、LATS1/2激酶和效应蛋白YAP/TAZ。虽然YAP/TAZ及其下游相关研究相对较多,但Hippo信号通路的上游调控因子并不明确,是目前该通路研究的热点方向之一。另外,Hippo信号通路可与Wnt和Notch等其他信号通路发生交叉对话,并在控制器官大小、维持组织稳态、促进组织修复再生等过程中扮演重要角色。Hippo信号通路异常可能会导致多种肿瘤的发生,尤其是肝癌、结直肠癌和胃癌等消化系统肿瘤,其成员在消化系统肿瘤中的异常表达与肿瘤细胞的增殖、凋亡、侵袭和迁移等过程密切相关。Hippo信号通路对肝脏的修复再生至关重要,其失活会导致原发性肝癌的发生,YAP在肝癌中的促肿瘤作用机制主要依赖于TEAD介导的基因转录。Hippo信号通路对于维持肠道稳态也很重要,其失调会导致结直肠癌的发生及复发。在原发性和转移性胃癌中,YAP/TAZ的表达显著上调,但具体分子调控机制并不清楚。本文总结了近年来Hippo信号通路的发现、上游调控因子及其在消化系统肿瘤发生发展过程中的作用和分子调控机制,并对未来的研究方向进行初步探讨。
Abstract
Hippo signaling pathway is highly conservative in evolution. MST1/2, LATS1/2, and the effector protein YAP/TAZ are the core members of this signaling pathway in mammalian cells. There have been many studies on YAP/TAZ and its downstream, however, the upstream regulatory factors of the Hippo signaling pathway remain unclear, and become one of the hot research directions of this pathway at present. In addition, Hippo signaling pathway can cross-talk with other signaling pathways such as Wnt and Notch signaling pathways, and plays an important role in controlling organ size, maintaining tissue homeostasis, and promoting tissue repair and regeneration. Abnormal Hippo signaling pathway may lead to the occurrence of a variety of tumors, especially gastrointestinal cancers such as liver cancer, colorectal cancer and gastric cancer. The abnormal expression of its members in gastrointestinal cancers is related to cancer cell proliferation, apoptosis, invasion and migration. Hippo signaling pathway is vital for liver repair and regeneration. Its inactivation will lead to the occurrence of primary liver cancer. The mechanism of YAP in liver cancer mainly depends on TEAD-mediated gene transcription. Hippo signaling pathway is also important for maintaining intestinal homeostasis, and its imbalance can lead to the occurrence and recurrence of colorectal cancer. In primary and metastatic gastric cancer, the expression of YAP/TAZ is significantly up-regulated, but the specific molecular mechanism is unclear. This article summarizes the recent progress on Hippo signaling pathway and its upstream regulatory factors, its roles in the development of gastrointestinal cancers and related molecular mechanisms; and also discusses the future research directions of Hippo signaling pathway.
Keywords: Digestive system neoplasms; Signal transduction; Hippo signaling pathway; Wnt signaling pathway; Receptors, Notch; Review
Hippo信号通路是一条最初于果蝇中发现的进化上高度保守的信号通路,在调控器官大小、维持细胞和组织稳态、调节组织修复再生等一系列生命活动中发挥重要作用,目前越来越多的文献表明Hippo信号通路的异常与消化系统肿瘤的发生发展密切相关 [ 1] 。消化系统肿瘤是世界范围内高发的一类恶性肿瘤,所致死亡数占所有肿瘤的40 %以上,由于患者早期症状轻、早期诊断方法少,多数患者确诊时常为肿瘤进展期,导致患者治疗手段受限及预后较差,因此深入研究消化系统肿瘤发生发展的分子机制可为该疾病诊断和治疗提供新的理论基础和潜在的防治对策。本文着重讨论Hippo信号通路的作用及其异常在消化系统肿瘤发生发展过程中的调控机制。
1 Hippo信号通路
1.1 Hippo信号通路的核心成员
Hippo信号通路最初在果蝇中发现,进化上高度保守,见 图 1A。自1995年Justice等 [ 2] 发现Hippo信号通路核心成员Warts激酶以来,Salvador和Hippo等核心成员陆续被发现,而且它们在同一条信号通路中形成了激酶级联反应 [ 3- 4] 。目前的研究发现,果蝇中Hippo通路的核心成员包括Hippo、Warts、Salvador、Mats、Yorkie和Scalloped,除Yorkie和Scalloped外,其他核心成员的基因突变或失活均可导致果蝇的眼睛、翅膀和四肢等过度生长;相反,Yorkie的失活则会抑制组织生长 [ 1] 。Hippo通路还在果蝇胃肠道组织的再生和修复中发挥重要作用 [ 5] 。
图1.
果蝇和哺乳动物中Hippo信号通路的关键蛋白
果蝇Hippo信号通路中,上游调控因子Expanded蛋白(Ex)、肾脑表达蛋白(Kibra)和膜联FERM结构域蛋白(Merlin)复合物在外界信号刺激下激活激酶Hippo,进而导致激酶级联反应,激活激酶Warts,使效应蛋白Yorkie进入细胞核中,与转录因子Scalloped结合,促进下游靶基因的转录;哺乳动物Hippo信号通路中,上游调控因子FRMD6、WWCI和神经纤维瘤蛋白2(NF2)复合物在外界信号刺激下激活MST1/2,进而导致激酶级联反应,激活大肿瘤抑制激酶1/2(LATS1/2),使效应蛋白Yes相关蛋白(YAP)/具有PDZ结合基序的转录共激活子(TAZ)进入细胞核中,与转录因子TEA结构域转录因子(TEAD)结合,促进下游靶基因的转录. P:磷酸化基团.
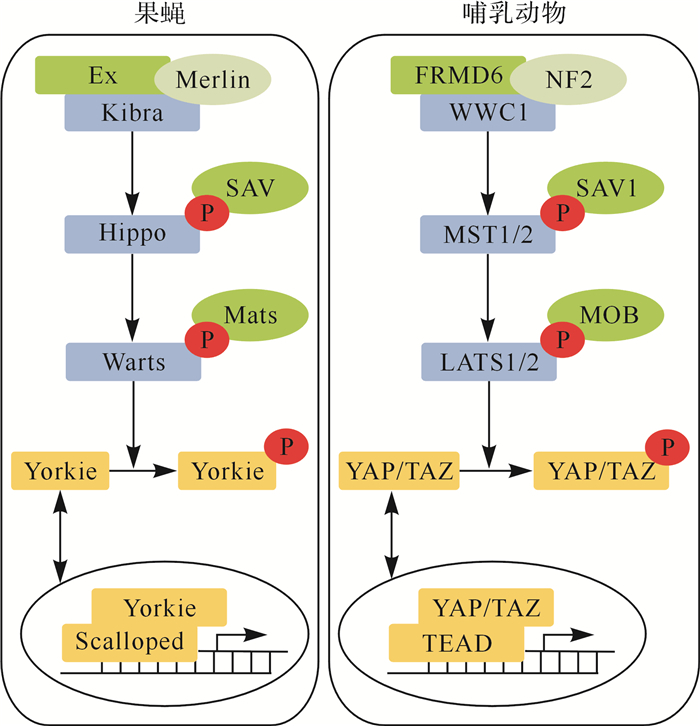
在哺乳动物中,科学家们发现了 Hippo的同源基因 MST1 (mammalian STE20-like protein kinase 1,也称为 STK4 )和 MST2 (也称为 STK3 ),见 图 1B [ 6] 。在上游信号刺激下,MST1/2激酶形成同源二聚体,被磷酸化或发生自磷酸化,随后激活其下游激酶大肿瘤抑制激酶(large tumor suppressor kinase,LATS)1和LATS2,使得Hippo信号通路的效应蛋白yes相关蛋白(yes-associated protein,YAP,位于127或318位点的丝氨酸)和TAZ(transcriptional co-activator with PDZ-binding motif,位于89或311位点的丝氨酸)被磷酸化,磷酸化的YAP和TAZ滞留于细胞质内,被14-3-3蛋白识别,参与细胞间的黏着连接和紧密连接,或被蛋白酶体降解。当Hippo信号通路未被激活时,未磷酸化的YAP/TAZ则会进入细胞核,与转录因子如TEA结构域转录因子(TEA domain transcription factor, TEAD)1~4结合,促进下游相关靶基因如结缔组织生长因子(connective tissue growth factor, CTGF)等转录 [ 7] 。Hippo信号通路在哺乳动物细胞的增殖、凋亡、干性及组织的稳态维持和损伤修复中均具有重要调控作用 [ 6] 。
1.2 Hippo信号通路的上游调控因子
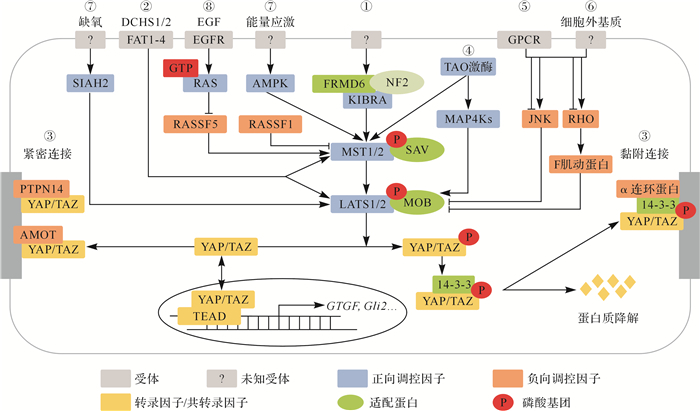
目前,Hippo信号通路的上游调控因子相关研究有限,如 图 2所示,主要有以下几类:①上皮细胞中的顶端-基底极性可以调控Hippo信号通路。在果蝇中,位于细胞顶端的膜联FERM结构域蛋白(Merlin蛋白)、肾脑表达蛋白(kidney and brain expressed protein,KIBRA)和Expanded蛋白形成复合物,协同激活Hippo蛋白,这些蛋白可能在接收同一上游调节因子的信号时形成复合物,也可能分别接收不同上游调节因子的信号 [ 8] 。哺乳动物的上皮细胞也存在类似功能的蛋白:KIBRA蛋白、FRMD6蛋白(FERM domain containing 6,也称为Willin)和神经纤维瘤蛋白2(neurofibromatosis2,NF2)。②在哺乳动物体内,细胞平面极性调节蛋白FAT1-4(FAT atypical cadherin 1-4)和DCHS1/2(dachsous cadherin-related 1/2)可以绕过MST1/2,直接激活LATS1/2激酶的活性 [ 9] 。③紧密连接和黏附连接的相关蛋白可与YAP/TAZ相互作用,调控YAP/TAZ的活性 [ 10] 。当细胞密度增加时,AMOT(angiomotin)蛋白和蛋白酪氨酸磷酸酶14(protein tyrosine phosphatase non-receptor type 14,PTPN14)与YAP/TAZ相互作用,将YAP/TAZ定位于细胞紧密连接处,使其入核减少,抑制YAP/TAZ与TEAD的结合,从而抑制下游靶基因的表达;而在黏附连接中,α连环蛋白(α-catenin)与14-3-3蛋白、磷酸化的YAP/TAZ形成复合物,使YAP/TAZ定位于细胞黏附连接处而不会被蛋白酶体降解。④TAO激酶(TAOK)不仅可直接磷酸化并激活MST1/2,也可通过丝裂原激活蛋白激酶激酶激酶激酶(mitogen-activated protein kinase kinase kinase kinases,MAP4K)间接激活LATS1/2 [ 11] 。⑤G蛋白偶联受体(GPCR)的配体包括质子、代谢物、多肽和分泌蛋白,均可通过受体结合来调节Hippo通路的活性 [ 12] 。⑥YAP对细胞外基质的刚度十分敏感,被认为是一种与机械转导有关的传感器。细胞张力、细胞的几何形状和细胞间接触等均可调控Hippo信号通路。同时,细胞外基质对Hippo信号的调控作用与JNK(c-Jun NH2-terminal kinase)、RHO GTP酶及肌动蛋白骨架(F肌动蛋白)重塑等密切相关 [ 13] 。⑦细胞外氧气浓度和能量代谢的变化也会触发Hippo信号通路。缺氧时,E3泛素酶SIAH2(siah E3 ubiquitin protein ligase 2)可通过泛素化降解LATS1/2,抑制Hippo信号通路 [ 14] ;葡萄糖饥饿时,AMP活化蛋白激酶能激活Hippo信号通路,使YAP/TAZ被磷酸化失活 [ 15] 。⑧RASSF蛋白家族(RAS association domain-containing family protein)具有与MST1/2相同的SARAH结构域,能够与MST1/2结合,抑制MST1/2的自磷酸化。如RASSF5可与MST1/2蛋白单体结合,抑制其形成二聚体,维持其失活状态。同时,RASSF5还具有优先与RAS-GTP结合的特性,当RAS蛋白被上游信号激活时,RASSF5可以介导RAS-GTP激活MST1/2 [ 16] 。
图2.
哺乳动物Hippo信号通路的上游调控因子
Hippo信号通路的上游调控因子主要包括①顶端-基底极性、②平面极性、③紧密连接和黏附连接、④TAO激酶、⑤G蛋白偶联受体、⑥细胞外基质、⑦氧气浓度和能量代谢、⑧RASSF蛋白家族.图中箭头表示激活,T型线表示抑制.PTPN14:蛋白酪氨酸磷酸酶14;MAP4K:丝裂原激活蛋白激酶激酶激酶激酶;GPCR:G蛋白偶联受体;AMPK:AMP活化蛋白激酶;EGF:表皮生长因子;EGFR:表皮生长因子受体; LATS:大肿瘤抑制激酶;YAP:yes相关蛋白;TEAD:TEA结构域转录因子.
1.3 Hippo通路与其他信号通路间的相互作用
Hippo信号通路与其他信号通路存在交叉对话,这些通路间的相互作用在组织生长发育和细胞生命活动中发挥重要的调节作用。
Hippo信号通路与Wnt信号通路之间存在相互调控,见 图 3A。一方面,YAP/TAZ参与Wnt信号通路的激活与失活中,细胞质中的YAP/TAZ可与Wnt信号通路的上游正向调控因子Fz(Frizzled)结合,抑制其活化; YAP/TAZ也可与β连环蛋白(β-catenin)、APC蛋白复合物相互作用,滞留于细胞质中,共同被糖元合成酶激酶3β磷酸化,最终通过泛素化途径降解;细胞核内YAP/TAZ能够促进β连环蛋白与转录因子如T细胞因子/淋巴增强因子(TCF/LEF)结合,增强下游靶基因(如 c-myc)的表达。另一方面,Wnt信号通路也可激活YAP/TAZ活性。Wierzbicki等 [ 17] 认为YAP/TAZ是一种Wnt信号通路的靶基因,起负反馈回路的作用,限制Wnt信号通路的过度激活。
图3.
哺乳动物中Hippo信号通路与Wnt信号通路、Notch信号通路的相互作用
A:Hippo信号通路与Wnt信号通路的交叉对话,Hippo信号通路中的关键蛋白YAP/TAZ是Wnt信号通路的靶基因,同时YAP/TAZ又与Wnt信号通路的受体蛋白Fz及效应蛋白β连环蛋白存在相互作用,可以相互调控;B:Hippo信号通路与Notch信号通路的交叉对话,Notch信号通路的重要受体蛋白Notch2是Hippo信号通路的靶基因,因此Notch信号通路受到Hippo信号通路的调控.图中箭头表示激活,T型线表示抑制, 虚箭头表示作用尚不明确.APC:腺瘤息肉病杆菌;TCF:T细胞因子;LEF:淋巴增强因子;NICD:Notch胞内结构域;Cyclin E:细胞周期素E;CTGF:结缔组织生长因子;TEAD:TEA结构域转录因子;LATS:大肿瘤抑制激酶;YAP:yes相关蛋白;P:磷酸化.
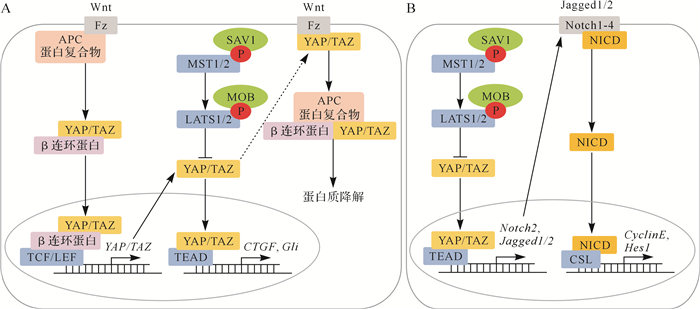
Hippo信号通路还与Notch信号通路存在交叉对话,见 图 3B。哺乳动物有四个Notch受体(Notch1、Notch2、Notch3和Notch4)。当配体如DLL1~3(delta like canonical Notch ligand 1~3)或Jagged1/2(jagged canonical Notch ligand 1/2)激活Notch受体时,Notch受体被蛋白水解,Notch胞内结构域(NICD)从受体中分离出来,并转移进入细胞核,与转录因子如重组信号结合蛋白Jκ(RBPJ)结合,促进下游靶基因如 Hes1 ( hes家族 bHLH转录因子 1 )的表达。 MST1/2 双敲除小鼠肠道细胞中的Notch胞内结构域核定位明显增加,Notch信号通路被激活。同时,在YAP过表达的细胞中,Notch信号通路的核心成员基因 Notch1、 Notch2、 Jagged1/2、 Sox9( SRY盒转录因子 9)和 Hes1 的转录水平明显上调,其中 Notch2、 Jagged1/2可能是Hippo信号通路的直接靶基因 [ 18] 。
2 Hippo信号通路异常在消化系统肿瘤发生发展中的作用
Hippo信号通路是抑制肿瘤的信号通路,Hippo信号通路失活可促进肿瘤细胞增殖能力、抵抗肿瘤细胞凋亡信号、促进肿瘤组织和肿瘤细胞的侵袭和转移,从而促进肿瘤的发生发展。本文主要以肝癌、结直肠癌和胃癌为例,重点探讨Hippo信号通路在消化系统肿瘤中的作用及其分子调控机制。
2.1 Hippo信号通路失活会导致原发性肝癌发生
Hippo信号通路受外源性刺激、体内代谢物和肝炎病毒蛋白等上游因子的调节,能够影响肝癌细胞的增殖、抗凋亡能力及肝脏的修复再生能力,其失调是肝癌发生的重要调控因子之一 [ 19] 。在小鼠肝脏中, MST1/2 双敲除能够导致4~5周龄小鼠的肝脏明显肿大,最终可导致肝癌发生 [ 20] 。在肝上皮细胞和胆管细胞中,YAP的蛋白水平和活性最高,敲除 YAP会使小鼠在出生时胆管发育不全,且随着年龄的增长,胆管逐渐消失 [ 21] 。在成年小鼠中,敲除 YAP并不会引起胆管丢失或肝细胞坏死,但会影响肝损伤修复,因为敲除 YAP的肝细胞对损伤更加敏感,丧失组织再生和自我修复能力,逐渐发展为肝炎和肝纤维化 [ 22] ,提示Hippo信号通路(尤其是YAP)在肝癌发生发展中的重要作用。小鼠肝癌模型和人肝细胞癌组织的全基因组分析发现,染色体11q22上9qA1位点出现反复扩增,基因表达分析证实此位点上的 YAP过度表达,且 YAP过度表达与肝癌的发生密切相关 [ 23] 。Zhang等 [ 24] 利用免疫组织化学检测了115例人肝癌组织样本,发现65 %的肝癌样本中YAP表达上调,95 %正常肝组织中YAP表达量很低,且YAP表达量与肿瘤进展及预后相关。
YAP在肝癌中的促肿瘤作用机制主要依赖于TEAD介导的基因转录。YAP与TEAD相互作用可促进调控肿瘤细胞增殖和肿瘤组织过度生长基因的表达,如 CTGF等。最新研究显示,转录辅助因子退变性蛋白家族成员VGLL4能与YAP竞争性结合TEAD,通过干扰YAP与TEAD的结合,抑制Hippo信号通路下游靶基因的表达 [ 25] 。Shen等 [ 26] 发现miR-130a可结合在 VGLL4 的3′非翻译区,抑制其翻译表达,间接促进YAP与TEAD的结合,同时miR-130a本身又是Hippo信号通路的靶基因, YAP过表达可以促进miR-130a的转录,从而形成了一条正反馈回路。此外,Hippo信号通路还可以通过影响染色体的稳定性促进肝癌发生。通过调控AKT信号通路,诱导E3连接酶S期激酶相关蛋白2(Skp2)的乙酰化。乙酰化的Skp2被滞留于细胞质中,导致细胞周期蛋白依赖性激酶抑制剂p27在细胞核中过度积累,从而抑制了细胞的有丝分裂,诱导了肝细胞多倍体化 [ 27] 。YAP对于肝脏内能量代谢的调控也十分重要,可以提高葡萄糖的利用率,抑制糖异生,优先为肿瘤细胞的生长和增殖提供能量。
2.2 Hippo信号通路失活会导致结直肠癌的发生及复发
Hippo信号通路对肠道稳态的维持至关重要,当Hippo信号通路失活时,肠道中不可控的组织再生可能会导致肠道的恶性转化 [ 28] 。在肠上皮特异性条件敲除的小鼠模型中, MST1/2 基因敲除小鼠在十三周时出现小肠和大肠发育不良和自发腺瘤 [ 29] ; SAV1 基因敲除小鼠在十三个月时出现结肠息肉。 YAP/TAZ敲除虽然不会影响小鼠的肠道结构,但会影响肠道损伤后的再生修复 [ 30] 。Liang等 [ 31] 检测了结直肠癌组织中Hippo信号通路相关基因的表达情况,发现结直肠癌组织中 LATS2 和 MST1 的mRNA水平下调,而 YAP、 TAZ、 TEAD1的mRNA水平上调;结直肠癌中MST1蛋白水平相应下调,YAP和TEAD1蛋白水平上调。Yuen等 [ 32] 分析了522例结直肠癌患者的 TAZ和 YAP及其下游转录靶点 AXL(AXL受体酪氨酸激酶)和 CTGF的表达水平,发现 TAZ、 YAP的mRNA表达水平与 AXL、 CTGF的mRNA表达水平呈正相关,当 YAP、 TAZ、 AXL、 CTGF mRNA表达水平较高时,肿瘤患者的生存期较短。以上数据提示,YAP/TAZ在结直肠癌中高表达,可以作为结直肠癌的预后标志物。此外,YAP可促进结直肠癌细胞的耐药性,且与结直肠癌的复发相关。临床上,5-氟尿嘧啶是晚期结直肠癌患者常用的化疗药。在5-氟尿嘧啶耐药结直肠癌细胞系中,YAP的靶基因表达增加,提示YAP可能会促进结直肠癌细胞对5-氟尿嘧啶产生耐药性 [ 33] 。YAP还可促进结直肠癌细胞对表皮生长因子受体(EGFR)抑制剂的耐药性。下调YAP蛋白水平可以增强结直肠癌细胞对西妥昔单抗(EGFR抑制剂)的敏感性 [ 34] ,提示YAP可以作为结直肠癌治疗的靶点之一,其抑制剂可以提高结直肠癌细胞对西妥昔单抗等药物的敏感性。
结直肠癌中YAP/TAZ的上调可能是由Hippo信号通路上游激酶活性的下调导致 [ 35] ,如LATS1/2基因启动子的甲基化导致LATS1/2表达下降 [ 36] ,促进YAP/TAZ上调;又如,肿瘤微环境中G蛋白偶联受体4被细胞外质子激活后会促进RhoA的活化及F肌动蛋白的重排,抑制了LATS1/2激酶活性,促进YAP/TAZ上调 [ 37] 。另外,结直肠癌中YAP/TAZ上调也可能依赖于其他机制,如上文所述的Hippo信号通路与Wnt信号通路的交叉对话, YAP/TAZ是Wnt信号通路的靶基因,其表达量和稳定性受到β连环蛋白的调控。Wnt信号通路异常在结直肠癌发生中发挥至关重要的作用,大多数结直肠癌患者至少有一个Wnt信号通路基因发生突变,如 β-catenin基因和腺瘤性息肉病杆菌基因突变 [ 38] 。然而,YAP/TAZ在结直肠癌中的促肿瘤作用是否依赖于Wnt信号通路仍未明确。
2.3 Hippo信号通路失活会导致胃癌的发生
已有研究表明,Hippo信号通路与胃癌的发生发展密切相关。在雏鸡的胃间充质中, YAP过表达可以导致胃平滑肌细胞层扩张。在小鼠幽门上皮干细胞中, LATS1/2 双敲除可诱发胃癌发生。 YAP在正常人胃上皮增生区里只有中度表达,而在原发性和转移性胃癌患者中, YAP表达增加 [ 39] 。多项独立研究表明, YAP/TAZ过表达与胃癌患者淋巴转移及预后较差密切相关。胃癌组织中 YAP的过表达受多种因素影响,有报道认为幽门螺杆菌可通过改变信号转导、细胞极性及基因组稳定性等使Hippo信号通路失活,增加YAP/TAZ的活性。胃癌组织中Hippo信号通路上游调控因子相关基因(如 FAT1-4、TAOK等)的失活突变频率较高,也会促进 YAP过表达。此外,在胃癌组织中,许多微小RNA也能促进 YAP的表达,如miR-93-5p在胃癌组织中可直接锚定在 FAT4 和 LATS2 的3′非翻译区,抑制 FAT4 和 LATS1/2 的转录,增加YAP的活性,促进胃癌细胞的增殖、侵袭和耐药能力 [ 40] 。
胃癌中YAP促肿瘤的机制目前研究较少,有文献提示YAP可能是通过抑制线粒体的自噬活性,导致线粒体的凋亡和细胞氧化的应激反应,从而增强胃癌细胞的迁移和生存能力 [ 41] 。也有文章指出,c- Myc是 YAP的下游靶点,可能是 YAP诱发胃癌发生的关键下游调节基因 [ 39] ,但具体机制仍不明确。YAP与部分转录因子的相互作用也会影响胃癌的发生发展。转录因子IRF3与细胞核中的YAP和TEAD4相互作用,增强其相互作用,促进YAP的核易位和活化,在一定程度上促进了胃癌的发生发展 [ 42] 。转录因子FOXP3的缺失可能导致胃腺癌的发生,而FOXP3的表达受到Hippo信号通路的调控 [ 43] 。
3 结语
综上所述,Hippo信号通路的激活对于维持消化系统的稳态是必须的,其失活会促进消化系统肿瘤的发生发展。YAP/TAZ在消化系统肿瘤中的过度活化可以促进肿瘤的发生,为临床干预提供了新的治疗靶点。然而,Hippo信号通路在消化系统肿瘤中的未来研究还需要解决以下几个问题。首先,Hippo信号通路的上游调控机制目前仍不明确,如WWC1-Frmd6-NF2蛋白复合物的上游目前仍不清楚,该蛋白复合物是如何激活MST1/2或LATS1/2的?其次,在不同的消化系统肿瘤中,Hippo信号通路的基因突变存在差异,如Hippo信号通路上游调控因子的突变频率在胃癌和结直肠癌中较高而在肝癌中较低,这些差异对于不同消化系统肿瘤的发生发展是否存在不同的作用机制?再次,目前研究提示YAP/TAZ可以作为消化系统肿瘤的生物靶标,那么靶向于YAP/TAZ的小分子药物是否可以用于消化系统肿瘤的临床治疗?上述问题均有待进一步研究。
Funding Statement
国家自然科学基金(31801132)
References
- 1.YU F X, MENG Z, PLOUFFE S W, et al. Hippo pathway regulation of gastrointestinal tissues. Annu Rev Physiol. 2015;77:201–227. doi: 10.1146/annurev-physiol-021014-071733. [YU F X, MENG Z, PLOUFFE S W, et al. Hippo pathway regulation of gastrointestinal tissues[J]. Annu Rev Physiol, 2015, 77:201-227. DOI:10.1146/annurev-physiol-021014-071733.] [DOI] [PubMed] [Google Scholar]
- 2.JUSTICE R W, ZILIAN O, WOODS D F, et al. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9(5):534–546. doi: 10.1101/gad.9.5.534. [JUSTICE R W, ZILIAN O, WOODS D F, et al. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation[J]. Genes Dev, 1995, 9(5):534-546. DOI:10.1101/gad.9.5.534.] [DOI] [PubMed] [Google Scholar]
- 3.TAPON N, HARVEY K F, BELL D W, et al. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110(4):467–478. doi: 10.1016/s0092-8674(02)00824-3. [TAPON N, HARVEY K F, BELL D W, et al. Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines[J]. Cell, 2002, 110(4):467-478. DOI:10.1016/s0092-8674(02)00824-3.] [DOI] [PubMed] [Google Scholar]
- 4.HAY B A, GUO M. Coupling cell growth, proliferation, and death. Hippo weighs in. Dev Cell. 2003;5(3):361–363. doi: 10.1016/s1534-5807(03)00270-3. [HAY B A, GUO M. Coupling cell growth, proliferation, and death. Hippo weighs in[J]. Dev Cell, 2003, 5(3):361-363. DOI:10.1016/s1534-5807(03)00270-3.] [DOI] [PubMed] [Google Scholar]
- 5.CHAI Y, XIANG K, WU Y, et al. Cucurbitacin B inhibits the hippo-YAP signaling pathway and exerts anticancer activity in colorectal cancer cells. Med Sci Monit. 2018;24:9251–9258. doi: 10.12659/MSM.911594. [CHAI Y, XIANG K, WU Y, et al. Cucurbitacin B inhibits the hippo-YAP signaling pathway and exerts anticancer activity in colorectal cancer cells[J]. Med Sci Monit, 2018, 24:9251-9258. DOI:10.12659/MSM.911594.] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.KANG W, CHENG A S, YU J, et al. Emerging role of Hippo pathway in gastric and other gastrointestinal cancers. World J Gastroenterol. 2016;22(3):1279–1288. doi: 10.3748/wjg.v22.i3.1279. [KANG W, CHENG A S, YU J, et al. Emerging role of Hippo pathway in gastric and other gastrointestinal cancers[J]. World J Gastroenterol, 2016, 22(3):1279-1288. DOI:10.3748/wjg.v22.i3.1279.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SHIMOMURA T, MIYAMURA N, HATA S, et al. The PDZ-binding motif of Yes-associated protein is required for its co-activation of TEAD-mediated CTGF transcription and oncogenic cell transforming activity. Biochem Biophys Res Commun. 2014;443(3):917–923. doi: 10.1016/j.bbrc.2013.12.100. [SHIMOMURA T, MIYAMURA N, HATA S, et al. The PDZ-binding motif of Yes-associated protein is required for its co-activation of TEAD-mediated CTGF transcription and oncogenic cell transforming activity[J]. Biochem Biophys Res Commun, 2014, 443(3):917-923. DOI:10.1016/j.bbrc.2013.12.100.] [DOI] [PubMed] [Google Scholar]
- 8.YU J, ZHENG Y, DONG J, et al. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18(2):288–299. doi: 10.1016/j.devcel.2009.12.012. [YU J, ZHENG Y, DONG J, et al. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded[J]. Dev Cell, 2010, 18(2):288-299. DOI:10.1016/j.devcel.2009.12.012.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MA L, CUI J, XI H, et al. Fat4 suppression induces Yap translocation accounting for the promoted proliferation and migration of gastric cancer cells. Cancer Biol Ther. 2016;17(1):36–47. doi: 10.1080/15384047.2015.1108488. [MA L, CUI J, XI H, et al. Fat4 suppression induces Yap translocation accounting for the promoted proliferation and migration of gastric cancer cells[J]. Cancer Biol Ther, 2016, 17(1):36-47. DOI:10.1080/15384047.2015.1108488.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SHARMA P, MCNEILL H. Fat and Dachsous cadherins. Prog Mol Biol Transl Sci. 2013;116:215–235. doi: 10.1016/B978-0-12-394311-8.00010-8. [SHARMA P, MCNEILL H. Fat and Dachsous cadherins[J]. Prog Mol Biol Transl Sci, 2013, 116:215-235. DOI:10.1016/B978-0-12-394311-8.00010-8.] [DOI] [PubMed] [Google Scholar]
- 11.AVRUCH J, ZHOU D, FITAMANT J, et al. Protein kinases of the Hippo pathway:regulation and substrates. Semin Cell Dev Biol. 2012;23(7):770–784. doi: 10.1016/j.semcdb.2012.07.002. [AVRUCH J, ZHOU D, FITAMANT J, et al. Protein kinases of the Hippo pathway:regulation and substrates[J]. Semin Cell Dev Biol, 2012, 23(7):770-784. DOI:10.1016/j.semcdb.2012.07.002.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LUO J, YU F X. GPCR-Hippo signaling in cancer. Cells. 2019;8(5) doi: 10.3390/cells8050426. [LUO J, YU F X. GPCR-Hippo signaling in cancer[J]. Cells, 2019, 8(5). DOI:10.3390/cells8050426.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.PAN D. The Hippo signaling pathway in development and cancer. Dev Cell. 2010;19(4):491–505. doi: 10.1016/j.devcel.2010.09.011. [PAN D. The Hippo signaling pathway in development and cancer[J]. Dev Cell, 2010, 19(4):491-505. DOI:10.1016/j.devcel.2010.09.011.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MA B, CHEN Y, CHEN L, et al. Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat Cell Biol. 2015;17(1):95–103. doi: 10.1038/ncb3073. [MA B, CHEN Y, CHEN L, et al. Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase[J]. Nat Cell Biol, 2015, 17(1):95-103. DOI:10.1038/ncb3073.] [DOI] [PubMed] [Google Scholar]
- 15.WANG W, XIAO Z D, LI X, et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17(4):490–499. doi: 10.1038/ncb3113. [WANG W, XIAO Z D, LI X, et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis[J]. Nat Cell Biol, 2015, 17(4):490-499. DOI:10.1038/ncb3113.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GALAN J A, AVRUCH J. MST1/MST2 protein kinases:regulation and physiologic roles. Biochemistry. 2016;55(39):5507–5519. doi: 10.1021/acs.biochem.6b00763. [GALAN J A, AVRUCH J. MST1/MST2 protein kinases:regulation and physiologic roles[J]. Biochemistry, 2016, 55(39):5507-5519. DOI:10.1021/acs.biochem.6b00763.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WIERZBICKI P M, RYBARCZY A. The Hippo pathway in colorectal cancer. Folia Histochem Cytobiol. 2015;53(2):105–119. doi: 10.5603/FHC.a2015.0015. [WIERZBICKI P M, RYBARCZY A. The Hippo pathway in colorectal cancer[J]. Folia Histochem Cytobiol, 2015, 53(2):105-119. DOI:10.5603/FHC.a2015.0015.] [DOI] [PubMed] [Google Scholar]
- 18.TSCHAHARGANEH D F, CHEN X, LATZKO P, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530–1542. doi: 10.1053/j.gastro.2013.02.009. [TSCHAHARGANEH D F, CHEN X, LATZKO P, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma[J]. Gastroenterology, 2013, 144:1530-1542. DOI:10.1053/j.gastro.2013.02.009.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.YIMLAMAI D, CHRISTODOULOU C, GALLI G G, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157(6):1324–1338. doi: 10.1016/j.cell.2014.03.060. [YIMLAMAI D, CHRISTODOULOU C, GALLI G G, et al. Hippo pathway activity influences liver cell fate[J]. Cell, 2014, 157(6):1324-1338. DOI:10.1016/j.cell.2014.03.060.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ZHOU D, CONRAD C, XIA F, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16(5):425–438. doi: 10.1016/j.ccr.2009.09.026. [ZHOU D, CONRAD C, XIA F, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene[J]. Cancer Cell, 2009, 16(5):425-438. DOI:10.1016/j.ccr.2009.09.026.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LOFORESE G, MALINKA T, KEOGH A, et al. Impaired liver regeneration in aged mice can be rescued by silencing Hippo core kinases MST1 and MST2. EMBO Mol Med. 2017;9(1):46–60. doi: 10.15252/emmm.201506089. [LOFORESE G, MALINKA T, KEOGH A, et al. Impaired liver regeneration in aged mice can be rescued by silencing Hippo core kinases MST1 and MST2[J]. EMBO Mol Med, 2017, 9(1):46-60. DOI:10.15252/emmm.201506089.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.HONG L, CAI Y, JIANG M, et al. The Hippo signaling pathway in liver regeneration and tumorigenesis. Acta Biochim Biophys Sin (Shanghai) 2015;47:46–52. doi: 10.1093/abbs/gmu106. [HONG L, CAI Y, JIANG M, et al. The Hippo signaling pathway in liver regeneration and tumorigenesis[J]. Acta Biochim Biophys Sin (Shanghai), 2015, 47:46-52. DOI:10.1093/abbs/gmu106.] [DOI] [PubMed] [Google Scholar]
- 23.ZENDER L, SPECTOR M S, XUE W, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125(7):1253–1267. doi: 10.1016/j.cell.2006.05.030. [ZENDER L, SPECTOR M S, XUE W, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach[J]. Cell, 2006, 125(7):1253-1267. DOI:10.1016/j.cell.2006.05.030.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ZHANG L, SONG X, LI X, et al. Yes-associated protein 1 as a novel prognostic biomarker for gastrointestinal cancer:a meta-analysis. Biomed Res Int. 2018;2018:4039173. doi: 10.1155/2018/4039173. [ZHANG L, SONG X, LI X, et al. Yes-associated protein 1 as a novel prognostic biomarker for gastrointestinal cancer:a meta-analysis[J]. Biomed Res Int, 2018, 2018:4039173. DOI:10.1155/2018/4039173.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.JIAO S, LI C, HAO Q, et al. VGLL4 targets a TCF4-TEAD4 complex to coregulate Wnt and Hippo signalling in colorectal cancer. Nat Commun. 2017;8:14058. doi: 10.1038/ncomms14058. [JIAO S, LI C, HAO Q, et al. VGLL4 targets a TCF4-TEAD4 complex to coregulate Wnt and Hippo signalling in colorectal cancer[J]. Nat Commun, 2017, 8:14058. DOI:10.1038/ncomms14058.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.SHEN S, GUO X, YAN H, et al. A miR-130a-YAP positive feedback loop promotes organ size and tumorigenesis. Cell Res. 2015;25(9):997–1012. doi: 10.1038/cr.2015.98. [SHEN S, GUO X, YAN H, et al. A miR-130a-YAP positive feedback loop promotes organ size and tumorigenesis[J]. Cell Res, 2015, 25(9):997-1012. DOI:10.1038/cr.2015.98.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ZHANG S, CHEN Q, LIU Q, et al. Hippo signaling suppresses cell ploidy and tumorigenesis through Skp2. Cancer Cell. 2017;31(5):669–684. doi: 10.1016/j.ccell.2017.04.004. [ZHANG S, CHEN Q, LIU Q, et al. Hippo signaling suppresses cell ploidy and tumorigenesis through Skp2[J]. Cancer Cell, 2017, 31(5):669-684.e7. DOI:10.1016/j.ccell.2017.04.004.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.HONG A W, MENG Z, GUAN K L. The Hippo pathway in intestinal regeneration and disease. Nat Rev Gastroenterol Hepatol. 2016;13(6):324–337. doi: 10.1038/nrgastro.2016.59. [HONG A W, MENG Z, GUAN K L. The Hippo pathway in intestinal regeneration and disease[J]. Nat Rev Gastroenterol Hepatol, 2016, 13(6):324-337. DOI:10.1038/nrgastro.2016.59.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ZHOU D, ZHANG Y, WU H, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A. 2011;108(49):E1312–1320. doi: 10.1073/pnas.1110428108. [ZHOU D, ZHANG Y, WU H, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance[J/OL]. Proc Natl Acad Sci U S A, 2011, 108(49):E1312-1320. DOI:10.1073/pnas.1110428108.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DEHGHANIAN F, HOJATI Z, HOSSEINKHAN N, et al. Reconstruction of the genome-scale co-expression network for the Hippo signaling pathway in colorectal cancer. Comput Biol Med. 2018;99:76–84. doi: 10.1016/j.compbiomed.2018.05.023. [DEHGHANIAN F, HOJATI Z, HOSSEINKHAN N, et al. Reconstruction of the genome-scale co-expression network for the Hippo signaling pathway in colorectal cancer[J]. Comput Biol Med, 2018, 99:76-84. DOI:10.1016/j.compbiomed.2018.05.023.] [DOI] [PubMed] [Google Scholar]
- 31.LIANG K, ZHOU G, ZHANG Q, et al. Expression of hippo pathway in colorectal cancer. Saudi J Gastroenterol. 2014;20(3):188–194. doi: 10.4103/1319-3767.133025. [LIANG K, ZHOU G, ZHANG Q, et al. Expression of hippo pathway in colorectal cancer[J]. Saudi J Gastroenterol, 2014, 20(3):188-194. DOI:10.4103/1319-3767.133025.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.YUEN H F, MCCRUDDEN C M, HUANG Y H, et al. TAZ expression as a prognostic indicator in colorectal cancer. PLoS One. 2013;8(1):e54211. doi: 10.1371/journal.pone.0054211. [YUEN H F, MCCRUDDEN C M, HUANG Y H, et al. TAZ expression as a prognostic indicator in colorectal cancer[J/OL]. PLoS One, 2013, 8(1):e54211. DOI:10.1371/journal.pone.0054211.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SONG R, GU D, ZHANG L, et al. Functional significance of Hippo/YAP signaling for drug resistance in colorectal cancer. Mol Carcinog. 2018;57(11):1608–1615. doi: 10.1002/mc.22883. doi: 10.1002/mc.22883. [SONG R, GU D, ZHANG L, et al. Functional significance of Hippo/YAP signaling for drug resistance in colorectal cancer[J]. Mol Carcinog, 2018, 57(11):1608-1615. DOI:10.1002/mc.22883.] [DOI] [PubMed] [Google Scholar]
- 34.LIU B S, XIA H W, ZHOU S, et al. Inhibition of YAP reverses primary resistance to EGFR inhibitors in colorectal cancer cells. Oncol Rep. 2018;40(4):2171–2182. doi: 10.3892/or.2018.6630. [LIU B S, XIA H W, ZHOU S, et al. Inhibition of YAP reverses primary resistance to EGFR inhibitors in colorectal cancer cells[J]. Oncol Rep, 2018, 40(4):2171-2182. DOI:10.3892/or.2018.6630.] [DOI] [PubMed] [Google Scholar]
- 35.WIERZBICKI P M, ADRYCH K, KARTANOWICZ D, et al. Underexpression of LATS1 TSG in colorectal cancer is associated with promoter hypermethylation. World J Gastroenterol. 2013;19(27):4363–4373. doi: 10.3748/wjg.v19.i27.4363. [WIERZBICKI P M, ADRYCH K, KARTANOWICZ D, et al. Underexpression of LATS1 TSG in colorectal cancer is associated with promoter hypermethylation[J]. World J Gastroenterol, 2013, 19(27):4363-4373. DOI:10.3748/wjg.v19.i27.4363.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MCKEY J, MARTIRE D, DE SANTA BARBARA P, et al. LIX1 regulates YAP1 activity and controls the proliferation and differentiation of stomach mesenchymal progenitors. BMC Biol. 2016;14:34. doi: 10.1186/s12915-016-0257-2. [MCKEY J, MARTIRE D, DE SANTA BARBARA P, et al. LIX1 regulates YAP1 activity and controls the proliferation and differentiation of stomach mesenchymal progenitors[J]. BMC Biol, 2016, 14:34. DOI:10.1186/s12915-016-0257-2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.YU M, CUI R, HUANG Y, et al. Increased proton-sensing receptor GPR4 signalling promotes colorectal cancer progression by activating the hippo pathway. EBioMedicine. 2019;48:264–276. doi: 10.1016/j.ebiom.2019.09.016. [YU M, CUI R, HUANG Y, et al. Increased proton-sensing receptor GPR4 signalling promotes colorectal cancer progression by activating the hippo pathway[J]. EBioMedicine, 2019, 48:264-276. DOI:10.1016/j.ebiom.2019.09.016.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.YAO H, ASHIHARA E, MAEKAWA T. Targeting the Wnt/beta-catenin signaling pathway in human cancers. Expert Opin Ther Targets. 2011;15(7):873–887. doi: 10.1517/14728222.2011.577418. [YAO H, ASHIHARA E, MAEKAWA T. Targeting the Wnt/beta-catenin signaling pathway in human cancers[J]. Expert Opin Ther Targets, 2011, 15(7):873-887. DOI:10.1517/14728222.2011.577418.] [DOI] [PubMed] [Google Scholar]
- 39.CHOI W, KIM J, PARK J, et al. YAP/TAZ initiates gastric tumorigenesis via upregulation of MYC. Cancer Res. 2018;78(12):3306–3320. doi: 10.1158/0008-5472.CAN-17-3487. [CHOI W, KIM J, PARK J, et al. YAP/TAZ initiates gastric tumorigenesis via upregulation of MYC[J]. Cancer Res, 2018, 78(12):3306-3320. DOI:10.1158/0008-5472.CAN-17-3487.] [DOI] [PubMed] [Google Scholar]
- 40.LI L, ZHAO J, HUANG S, et al. MiR-93-5p promotes gastric cancer-cell progression via inactivation of the Hippo signaling pathway. Gene. 2018;641:240–247. doi: 10.1016/j.gene.2017.09.071. [LI L, ZHAO J, HUANG S, et al. MiR-93-5p promotes gastric cancer-cell progression via inactivation of the Hippo signaling pathway[J]. Gene, 2018, 641:240-247. DOI:10.1016/j.gene.2017.09.071.] [DOI] [PubMed] [Google Scholar]
- 41.YAN H, QIU C, SUN W, et al. Yap regulates gastric cancer survival and migration via SIRT1/Mfn2/mitophagy. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=a88bc45e003fd585a22003855bc5bcaf. Oncol Rep. 2018;39(4) doi: 10.3892/or.2018.6252. [YAN H, QIU C, SUN W, et al. Yap regulates gastric cancer survival and migration via SIRT1/Mfn2/mitophagy[J]. Oncol Rep, 2018, 39(4):DOI:1671-1681.10.3892/or.2018.6252.] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.JIAO S, GUAN J, CHEN M, et al. Targeting IRF3 as a YAP agonist therapy against gastric cancer. J Exp Med. 2018;215(2):699–718. doi: 10.1084/jem.20171116. [JIAO S, GUAN J, CHEN M, et al. Targeting IRF3 as a YAP agonist therapy against gastric cancer[J]. J Exp Med, 2018, 215(2):699-718. DOI:10.1084/jem.20171116.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.SUH J H, WON K Y, KIM G Y, et al. Expression of tumoral FOXP3 in gastric adenocarcinoma is associated with favorable clinicopathological variables and related with Hippo pathway. http://cn.bing.com/academic/profile?id=6b75b2653617e1e55c52939b52c8acce&encoded=0&v=paper_preview&mkt=zh-cn. Int J Clin Exp Pathol. 2015;8(11):14608–14618. [SUH J H, WON K Y, KIM G Y, et al. Expression of tumoral FOXP3 in gastric adenocarcinoma is associated with favorable clinicopathological variables and related with Hippo pathway[J]. Int J Clin Exp Pathol, 2015, 8(11):14608-14618.] [PMC free article] [PubMed] [Google Scholar]


