Abstract
铁死亡是近年发现的一种铁依赖的新型细胞死亡方式,其特征是脂质过氧化物和活性氧簇的过量蓄积。大量研究表明,铁死亡不仅在重大慢性疾病的发生发展过程中发挥重要作用,而且在不同的疾病背景下扮演不同角色。目前认为,铁死亡可抑制肿瘤生长并增加多种肿瘤对化疗药物和免疫治疗的敏感性,因此诱导铁死亡的发生拓展了肿瘤治疗思路。然而,在心脑血管疾病和神经退行性疾病中,铁死亡的发生通过引发正常组织器官损伤和功能丧失直接参与疾病的发生、发展及转归,因此针对心脑血管疾病和神经退行性疾病,抑制铁死亡的发生能够有效预防并延缓这些疾病的发生和发展。本文综述了铁死亡在恶性肿瘤、神经退行性疾病和心脑血管疾病三类不同重大慢性疾病中的最新研究进展及其潜在的作用机制,系统讨论了靶向铁死亡在防治重大慢性疾病中的临床应用前景,为重大慢性疾病的防治提供新的依据。
Abstract
Recently, ferroptosis, an iron-dependent novel type of cell death, has been characterized as an excessive accumulation of lipid peroxides and reactive oxygen species. Emerging studies demonstrate that ferroptosis not only plays an important role in the pathogenesis and progression of chronic diseases, but also functions differently in the different disease context. Notably, it is shown that activation of ferroptosis could potently inhibit tumor growth and increase sensitivity to chemotherapy and immunotherapy in various cancer settings. As a result, the development of more efficacious ferroptosis agonists remains the mainstay of ferroptosis-targeting strategy for cancer therapeutics. By contrast, in non-cancerous chronic diseases, including cardiovascular & cerebrovascular diseases and neurodegenerative diseases, ferroptosis functions as a risk factor to promote these diseases progression through triggering or accelerating tissue injury. As a matter of fact, blocking ferroptosis has been demonstrated to effectively prevent ischemia-reperfusion heart disease in preclinical animal models. Therefore, it is a promising field to develope potent ferroptosis inhibitors for preventing and treating cardiovascular & cerebrovascular diseases and neurodegenerative diseases. In this article, we summarize the most recent progress on ferroptosis in chronic diseases, and draw attention to the possible clinical impact of this recently emerged ferroptosis modalities.
Keywords: Iron metabolism disorder, Ferroptosis, Neoplasms, Neurodegeneration, Vascular diseases, Review
慢性疾病是指非传染性、病程长且病情迁延不愈的疾病,主要包括心脑血管疾病、肿瘤、糖尿病及慢性呼吸道疾病等。据WHO统计,2016年全球有大约4100万人死于慢性疾病,占死亡总数的71 % [ 1] 。中国疾病预防控制中心最新统计结果显示,2017年由于慢性疾病导致的死亡人数占总死亡人数的88 % [ 2] 。慢性疾病的发生发展不仅病理机制复杂,而且受遗传、环境及生活方式等诸多因素的影响。长期以来,人们对慢性疾病发病的分子机制缺乏清晰的认识,并且缺少有效的治疗药物。2012年,一种新型细胞死亡方式——铁死亡的发现,为研究慢性疾病的发生发展及其防治提供了新视角 [ 3] 。
铁死亡是一种铁依赖的非凋亡形式的细胞死亡方式,在形态学、遗传学、代谢学和分子生物学等方面均不同于凋亡、焦亡或坏死等细胞死亡方式 [ 3] ,其主要特征是脂质过氧化物和活性氧过量积累 [ 4] 。因此,铁代谢和脂质过氧化在铁死亡通路中发挥重要调控作用 [ 5] ,其可能的分子机制见 图 1。血液循环中的三价铁离子与转铁蛋白结合并运输,通过细胞膜上的转铁蛋白受体1(TFR1)进入细胞内。三价铁离子在细胞内被还原为二价铁离子后,被转运并释放到细胞质铁池中,而过量的铁则被储存到铁蛋白中。有研究提示,铁蛋白选择性自噬通过核受体共活化子4途径促进铁蛋白进入自噬体,从而导致游离铁的释放 [ 6- 7] 。一般认为,过量铁主要通过芬顿反应产生的活性氧而引发铁死亡;相反,运用铁螯合剂能够有效抑制铁死亡 [ 8] 。脂质过氧化物是铁死亡发生过程中的执行者,磷脂酰乙醇胺(PE)是脂质氧化的首选底物,因此过氧化氢-PE(OOH-PE)被认为是铁死亡发生的主要信号 [ 9- 10] 。在脂质过氧化蓄积过程中,NADPH氧化酶、脂氧合酶、酰辅酶A长链家族成员4(ACSL4)和溶血磷脂酰胆碱酰基转移酶3等在铁死亡的发生发展过程中可能发挥重要作用 [ 3, 10- 12] 。常用铁死亡抑制剂ferrostatin-1(Fer-1)、liproxstatin-1(Lip-1)和维生素E等小分子化合物主要通过清除脂质过氧化物而抑制铁死亡 [ 13] 。
图1.
铁死亡分子机制
铁代谢和脂代谢途径通过诱发脂质过氧化最终导致铁死亡,而谷胱甘肽过氧化物酶和铁死亡抑制因子1(FSP1)等通过抑制脂质过氧化而抑制铁死亡.FTH1:铁蛋白重链1;FTL:铁蛋白轻链;GSH:还原型谷胱甘肽;GSSH:氧化型谷胱甘肽;HMOX1:血红素加氧酶1;NADP +:氧化型烟酰胺腺嘌呤二核苷酸磷酸;NADPH:还原型烟酰胺腺嘌呤二核苷酸磷酸;NRF2:核因子E2相关因子2;SLC7A11:溶质载体家族7成员11;system Xc -:谷氨酸/胱氨酸反向转运体;IREB2:铁反应元件结合蛋白2.
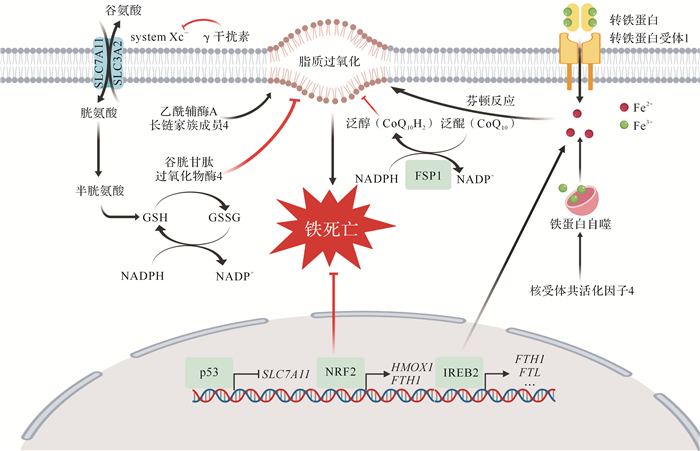
谷胱甘肽过氧化物酶(GPX)4是一种调控铁死亡的关键酶,能够通过催化脂质过氧化物的还原反应,将OOH-PE转化为OH-PE,进而抑制铁死亡的发生 [ 14] 。RSL3和ML162等小分子化合物能够抑制GPX4活性,导致脂肪酸自由基积聚,最终引发铁死亡;还原型谷胱甘肽作为GPX4的辅酶因子,其合成的限速步骤是胱氨酸的吸收 [ 15] 。谷氨酸/胱氨酸反向转运体由跨膜转运蛋白溶质载体家庭7成员11(SLC7A11)和跨膜调节蛋白溶质载体家庭3成员2(SLC3A2)组成,在向细胞内转运胱氨酸的同时外排等量的谷氨酸 [ 16] ,是重要的铁死亡调控因子。Erastin等小分子物质能够抑制谷氨酸/胱氨酸反向转运体,进而引发铁死亡。我们团队首次报道了敲除 Slc7a11 基因能够促进小鼠体内铁死亡的发生 [ 17] 。最近,德国和美国实验室分别独立发现新型铁死亡抑制因子1(FSP1),其在细胞膜上利用NADPH将泛醌(CoQ 10)还原为泛醇(CoQ 10H 2),减少细胞膜脂质过氧化,进而抑制铁死亡 [ 18- 19] 。这一发现对于靶向铁死亡相关药物研发提供了重要依据。
尽管铁死亡的具体机制尚未明确,但随着研究的不断深入,人们逐渐发现铁死亡在重大慢性疾病的发生发展中发挥重要作用 [ 20] 。本文综述了铁死亡在肿瘤、神经退行性疾病及心脑血管疾病中的最新研究进展,以期为重大慢性疾病的防治提供新思路与新策略。
1 铁死亡与肿瘤
肿瘤细胞可以通过规避细胞死亡而增殖;而细胞凋亡、坏死、自噬等死亡方式在肿瘤的发生发展中同样发挥了重要作用。 表 1总结了铁死亡在常见肿瘤中的相关研究进展,提示铁死亡的分子信号机制研究可以为肿瘤防治提供一系列潜在的新靶点。
表1 铁死亡与肿瘤相关研究进展
Table 1 Research progress on the role of ferroptosis in cancer
|
肿瘤类型 |
相关研究 |
参考文献 |
|
肝细胞癌 |
索拉非尼能够诱导肝细胞癌细胞发生铁死亡 |
[ 21] |
|
成视网膜细胞瘤蛋白缺失肝细胞癌细胞对索拉非尼诱导的铁死亡更敏感 |
[ 22] |
|
|
p62-Keap1-NRF2激活导致肝细胞癌细胞对铁死亡耐受 |
||
|
SLC7A11、 Rb和 MT1表达水平与肝细胞癌患者预后相关 |
||
|
胰腺癌 |
青蒿琥酯能够诱导胰腺导管腺癌细胞发生铁死亡 |
|
|
荜苃酰胺能诱导胰腺导管腺癌细胞发生铁死亡 |
[ 31] |
|
|
荜苃酰胺、Cotylenin A和柳氮磺胺吡啶联用通过铁死亡有效抑制胰腺癌 |
[ 31] |
|
|
肾细胞癌 |
相比其他肿瘤细胞,肾透明细胞癌细胞对谷胱甘肽过氧化物酶4抑制诱导的铁死亡更加敏感 |
[ 32] |
|
HIF-2α-HILPDA通路调控肾透明细胞癌细胞对铁死亡的敏感性 |
[ 33] |
|
|
TAZ-EMP1-NOX4通路调控肾透明细胞癌细胞对铁死亡的敏感性 |
||
|
乳腺癌 |
西拉美新和阿帕替尼联用上调铁水平并诱导乳腺癌细胞发生铁死亡 |
[ 36] |
|
黏蛋白1C亚基、SLC7A11和CD44v形成复合物上调还原型谷胱甘肽表达,使三阴性乳腺癌细胞对铁死亡耐受 |
[ 37] |
|
|
柳氮磺胺吡啶能抑制谷氨酰胺营养缺陷型三阴性乳腺癌细胞生长 |
[ 38] |
|
|
SLC7A11与三阴性乳腺癌细胞的耐药和转移有密切联系 |
[ 39] |
|
|
转铁蛋白受体表达水平与乳腺癌预后相关 |
SLC7A11:溶质载体家族7成员11;Rb:成视网膜细胞瘤;MT1:金属硫蛋白1.
1.1 铁死亡与肝细胞癌
铁死亡是治疗肝细胞癌的潜在作用机制之一 [ 21] 。成视网膜细胞瘤(Rb)蛋白缺失的癌细胞对索拉非尼诱导的铁死亡更加敏感 [ 22] ,可能原因是其通过影响线粒体中的活性氧浓度从而导致氧化应激反应增强。此外,Sun等 [ 23] 研究发现,核因子E2相关因子2(NRF2)在索拉非尼诱导的肝细胞癌细胞铁死亡过程中发挥保护作用,提示靶向p62-Keap1-NRF2通路可能克服肝癌细胞对索拉非尼的耐药 [ 23- 26] 。另外,NRF2还通过胱硫醚酶途径诱导促进铁死亡重要负向调节因子金属硫蛋白(MT)1G的表达,导致癌细胞对索拉非尼耐药 [ 28] 。CDGSH铁硫结构域1能够保护肝细胞癌细胞中的线粒体避免铁死亡,同时能被Erastin以铁依赖的方式上调 [ 42] 。此外,肝细胞癌细胞携带的p53 S47突变通过抑制ACSL4导致铁死亡耐受 [ 43] 。
在肝癌中,铁死亡相关基因的表达与患者预后有关。Kinoshita等 [ 27] 比较了130例肝细胞癌组织及癌旁正常组织中 SLC7A11 的mRNA表达水平,发现 SLC7A11 在肝细胞癌组织中的表达量比其在正常组织中增加,且 SLC7A11 高表达的肝癌患者的生存期和无病生存期均明显短于 SLC7A11 低表达的肝癌患者。此外,在索拉非尼治疗肝细胞癌患者的过程中, Rb和 MT1 高表达也与患者的不良预后有关 [ 22, 28] 。
1.2 铁死亡与胰腺癌
胰腺癌主要发生机制为突变的 KRAS基因将胰腺导管腺癌(PDAC)细胞重新编码为对细胞凋亡具有高度抵抗性的状态。青蒿琥酯能通过生成活性氧诱导肿瘤细胞发生凋亡 [ 29] 。Eling等 [ 30] 研究发现,青蒿琥酯能够诱导 KRAS突变的PDAC细胞发生铁死亡,而该过程可被Fer-1有效抑制。Yamaguchi等 [ 31] 发现天然产物荜苃酰胺可通过促进活性氧生成诱发肿瘤细胞铁死亡,其抗肿瘤细胞效果能够被抗氧化剂、铁死亡抑制剂和铁螯合剂等抑制;荜苃酰胺、Cotylenin A(一种植物生长调节素)和柳氮磺胺吡啶联合使用对胰腺癌有很好的协同治疗效果。上述研究结果提示铁死亡诱导剂有望应用于胰腺癌的治疗。
1.3 铁死亡与肾细胞癌
肾细胞癌起源于肾实质泌尿小管上皮系统,是泌尿系统中恶性度较高的肿瘤。Yang等 [ 32] 发现GPX4是肾透明细胞癌细胞铁死亡信号通路中的关键调节因子,肾癌细胞相比其他七种组织来源的肿瘤细胞(肺癌、结肠癌、中枢神经系统、黑色素瘤、卵巢癌、乳腺癌和白血病)对抑制GPX4诱导的铁死亡更敏感。肾癌细胞通过肝细胞因子-1β-1-酰基甘油-3磷酸氧酰基转移酶3(AGPAT3)轴和HIF-2α-HILPDA途径诱导,该途径能够诱导多不饱和脂肪酰基脂质富集的细胞状态,从而增加其对铁死亡的敏感性 [ 33] 。最近,美国杜克大学Yang等 [ 35] 发现肾癌细胞对铁死亡的敏感性受细胞密度及转录调节因子1(TAZ)- TAZ调控表皮膜蛋白1(EMP1)-NOX4通路调控 [ 34- 35] ,提示TAZ是一个潜在的靶向铁死亡的治疗靶点。
1.4 铁死亡与乳腺癌
乳腺癌来源于乳腺上皮组织。Ma等 [ 36] 发现,溶酶体干扰剂西拉美新和酪氨酸激酶抑制剂拉帕替尼能够破坏乳腺癌细胞内铁稳态产生活性氧并诱导细胞发生铁死亡,过表达铁转运蛋白1或铁螯合剂能够减少西拉美新和拉帕替尼诱导产生的活性氧。有研究表明,黏蛋白1C亚基与SLC7A11、CD44v形成复合物后能上调还原型谷胱甘肽表达,抑制三阴性乳腺癌细胞发生铁死亡 [ 37] 。Timmerman等 [ 38] 发现一个谷氨酰胺营养缺陷型的三阴性乳腺癌细胞亚群,高度依赖SLC7A11获取胱氨酸进行谷氨酰胺代谢,SLC7A11抑制剂柳氮磺胺吡啶能够通过促进铁死亡从而抑制肿瘤生长。此外,SLC7A11和谷氨酸/胱氨酸反向转运体的活性能被Keap1/NRF2氧化还原通路调控 [ 44] 。Lanzardo等 [ 39] 认为SLC7A11与三阴性乳腺癌细胞的耐药和转移存在密切联系。乳腺癌细胞中TFR1表达增加与雌激素受体表达呈负相关,且乳腺癌组织TFR1高表达与患者不良预后有关 [ 40- 41] 。
1.5 与肿瘤相关铁死亡调控蛋白
1.5.1 p53调控铁死亡
p53可通过调控细胞周期阻滞、凋亡或早衰等方式抑制肿瘤, p53 基因突变后便失去了抑癌功能 [ 45] 。Jiang等 [ 46] 发现,p53的DNA结合域的三个赖氨酸突变为精氨酸(K117/161/162R,即p53 3KR)后,p53 3KR可以通过抑制SLC7A11基因转录进一步限制胱氨酸摄取,使肿瘤细胞对氧化应激诱导的铁死亡更为敏感。Wang等 [ 47] 发现,p53的DNA结合域中的K98位点对调控 SLC7A11 尤为重要 [ 47] 。此外,p53的S47突变体不能抑制 SLC7A11 的转录,同时引发肝细胞癌细胞对铁死亡耐受,增加小鼠的患癌风险 [ 48] 。此外,p53能够通过抑制二肽基肽酶4活性使得结肠癌细胞对铁死亡不敏感 [ 49] 。
1.5.2 NRF2调控铁死亡
NRF2是氧化反应中重要的转录调控因子 [ 50] ,其过表达可抑制凋亡并导致一些肿瘤产生耐药 [ 51] 。NRF2在保护肝细胞癌细胞免受铁死亡的过程中发挥重要作用 [ 23] 。肝细胞癌细胞经Erastin和索拉非尼处理后,p62抑制NRF2的降解并通过Keap1失活使NRF2在核内聚集,进而调控下游基因转录;生物碱trigonelline抑制NRF2能诱导肝细胞癌细胞发生铁死亡,与化疗药物联合使用具有克服肿瘤耐药的应用前景 [ 23] 。可见,p62-Keap1-NRF2通路的激活能够激活铁死亡逆转肿瘤化疗药物的耐药。
1.5.3 ACSL4调控铁死亡
ACSL4表达于线粒体外膜和内质网上,能将长链脂肪酸转化为脂酰辅酶A,在脂类生物合成和脂肪酸降解过程中扮演了重要角色。ACSL4通过将长链多不饱和ω-6脂肪酸聚集于细胞膜,增加了细胞对铁死亡的敏感性 [ 10] 。研究表明,在基底样乳腺癌细胞系、肝癌细胞、白血病细胞和前列腺癌细胞中,ACSL4的表达水平可用于预测肿瘤细胞对铁死亡的敏感性 [ 10, 43, 52] 。结果提示,ACSL4有望成为肿瘤治疗中靶向铁死亡的潜在靶点及生物学标记物。
1.5.4 FSP1抑制铁死亡
FSP1最初被命名为线粒体凋亡诱导因子2(AIFM2),作为最新发现的GPX4非依赖性铁死亡抑制因子,其表达与肿瘤细胞对铁死亡的敏感性密切相关。近期,Doll和Bersuker两个课题组同时独立筛选发现不同细胞系中FSP1表达水平不同,并且多种肿瘤细胞系对铁死亡的抵抗水平与FSP1的表达水平呈正相关,导致不同肿瘤细胞系对铁死亡的敏感性之间的差异 [ 18- 19] 。这些最新研究成果对肿瘤中靶向铁死亡及其相关药物研发等领域提供了重要参考依据。
2 铁死亡与神经退行性疾病
铁稳态代谢对于大脑和神经发育及其认知功能至关重要,特别是在胎儿期或新生儿早期,铁缺乏会严重影响神经发育,导致记忆和学习能力减退 [ 53] 。随着年龄的增长,铁在大脑中逐渐蓄积,大量研究表明铁蓄积与阿尔茨海默病、帕金森症、肌萎缩侧索硬化等神经退行性疾病有关 [ 54] 。此外,近年研究发现在神经退行性疾病和认知障碍中存在脂质过氧化增加、谷胱甘肽减少和GPx4抑制等铁死亡发生的主要特征。运用铁死亡抑制剂能够有效保护神经元,提高认知能力 [ 55] ,相关研究进展见 表 2。
表2 铁死亡与神经退行性疾病相关研究进展
Table 2 Research progress on the role of ferroptosis in neurodegenerative diseases
|
神经退行性疾病类型 |
相关研究 |
参考文献 |
|
阿尔茨海默病 |
脑脊液中铁蛋白水平能预测阿尔茨海默病发展进程 |
[ 56] |
|
脑 Gpx4诱导性敲除小鼠海马神经元死亡和认知能力下降 |
[ 57] |
|
|
过表达或增加磷酸化τ蛋白能诱导神经元铁死亡,α硫辛酸可抑制τ蛋白诱导的铁死亡 |
[ 58] |
|
|
帕金森病 |
蛋白激酶C激活可引发铁死亡 |
[ 59] |
|
丝氨酸/苏氨酸蛋白激酶参与Erastin诱导的铁死亡 |
[ 60] |
|
|
星形胶质细胞为神经元提供GSTM2,保护神经元免受氧化损伤 |
||
|
肌萎缩侧索硬化 |
神经元 Gpx4诱导性敲除小鼠出现肌萎缩侧索硬化症状 |
[ 57] |
Gpx4 :谷胱甘肽过氧化物酶4;GSTM2:谷胱甘肽S-转移酶Mu2.
2.1 铁死亡与阿尔茨海默病
阿尔茨海默病患者存在金属代谢的失稳态、炎症反应、氧化应激、线粒体功能异常和神经胶质功能受损 [ 54, 64] 。研究表明,大脑铁蓄积与老年斑和神经元纤维缠结形成有关,脑内铁水平升高可增加罹患阿尔茨海默病的风险,且脑脊液中的铁蛋白水平可预测从轻度认知障碍到阿尔茨海默病发展的进程 [ 56, 65- 67] 。此外,阿尔茨海默病伴随的慢性炎症、神经元变性及缺少下游凋亡的指标提示了阿尔茨海默病中存在铁死亡等其他细胞死亡方式 [ 68- 70] 。
在一项研究中,大脑皮层和海马神经元 Gpx4 特异性敲除小鼠在水迷宫实验中表现出认知下降和海马神经元退化,给予富含维生素E的饮食或Lip-1后,小鼠神经元退化得到明显缓解,提示铁死亡在神经元退化过程发挥了重要作用 [ 57] 。另一研究发现,过表达或过磷酸化τ蛋白能够诱导神经元发生铁死亡,而α硫辛酸能够通过下调Tfr1、降低p38磷酸化水平和上调 Slc7a11 、 Gpx4 表达等方式挽救神经元 [ 58] 。此外,阿尔茨海默病小鼠模型喂食氘化多不饱和脂肪酸能够缓解脑组织的脂质过氧化,减少β淀粉样蛋白沉积 [ 71- 72] 。
2.2 铁死亡与帕金森症
帕金森症的一个重要特征是神经元和黑质神经胶质的铁蓄积,且铁蓄积浓度与疾病严重程度呈正相关 [ 73- 74] ;帕金森症患者及小鼠模型中观察到铁调节蛋白1(IRP1)、二价金属转运蛋白1(DMT1)等关键铁稳态蛋白水平发生了显著变化 [ 75- 79] 。 τ基因敲除的小鼠中出现帕金森症状并伴有黑质铁蓄积,这一现象可被铁螯合剂阻止 [ 80- 82] 。帕金森症在病理进程中除了黑质致密部铁升高,还出现还原型谷胱甘肽耗竭和脂质过氧化等铁死亡的特征 [ 83] ,铁螯合剂和N-乙酰半胱氨酸可缓解并改善帕金森症患者和帕金森症小鼠模型的部分症状 [ 80, 82, 84- 89] ,提示铁死亡可能参与了帕金森症的发生和发展。
Do Van等 [ 59] 在LUHMES细胞系、脑组织切片体外培养和1-甲基-4-苯基-1, 2, 3, 6-四氢吡啶(MPTP)诱导的帕金森症模型中发现多巴胺能神经元存在铁死亡,而用Fer-1、Lip-1和铁螯合剂能缓解或逆转帕金森症的症状。Gouel等 [ 60] 发现人血小板裂解液可使LUHMES细胞抵抗Earstin诱导的神经元铁死亡。此外,星形胶质细胞有很强的储铁能力,可防止神经元中的铁过载 [ 63] 。星形胶质细胞为神经元提供谷胱甘肽S-转移酶Mu2(GSTM2)和其他抗氧化因子,以保护神经元免受氧化损伤。综上,星形胶质细胞与神经元相互作用失调可能可导致多巴胺能神经元发生铁死亡 [ 61- 62] 。
2.3 铁死亡与肌萎缩侧索硬化
肌萎缩侧索硬化模型小鼠脑中存在铁蓄积 [ 90- 92] ,铁螯合剂的治疗效果证实了铁在肌萎缩侧索硬化发病中的作用。肌萎缩侧索硬化患者的脑脊液和血浆中脂质过氧化升高,运动皮质中还原型谷胱甘肽水平下降,表明可能存在铁死亡 [ 93- 94] 。小鼠神经元中敲除 Gpx4 可出现肌萎缩侧索硬化症状,主要表现为迅速瘫痪,严重的肌肉萎缩和死亡,这与脊髓运动神经元的铁死亡有关 [ 57] 。而在神经元 Gpx4 诱导性敲除和其他 Gpx4 选择性皮层神经元敲除小鼠模型的皮层中未观察到明显神经变性,提示Gpx4在脊髓运动神经元的铁死亡过程中发挥重要作用 [ 57] 。
3 铁死亡与心脑血管疾病
心脑血管疾病中心肌细胞和神经元死亡与多种细胞死亡方式有关,铁死亡也参与其中,相关研究进展见 表 3。
表3 铁死亡与心脑血管疾病相关研究进展
Table 3 Research progress on the role of ferroptosis in cardiovascular and cerebrovascular diseases
|
心脑血管疾病类型 |
相关研究 |
参考文献 |
|
缺血再灌注 |
离体小鼠心脏缺血再灌注模型中,抑制谷氨酰胺代谢可减轻铁死亡引发的心脏损伤 |
[ 95] |
|
铁死亡抑制剂和铁螯合剂可有效缓解小鼠心脏缺血再灌注引发的心肌损伤 |
[ 96] |
|
|
阿霉素诱导心肌损伤 |
阿霉素通过血红素加氧酶诱导心肌细胞发生铁死亡;铁蓄积和脂质过氧化主要发生于线粒体 |
[ 96] |
|
血红素加氧酶抑制剂、铁死亡抑制剂、线粒体抗氧化抑制剂、铁螯合剂等可有效逆转阿霉素引发的心肌损伤 |
[ 96] |
|
|
心脏移植后心肌损伤 |
铁死亡调控小鼠心脏移植后中性粒细胞的募集 |
[ 97] |
|
缺血性脑卒中 |
缺氧诱导因子脯氨酰羟化酶可能是铁螯合剂抑制神经元铁死亡的潜在靶点 |
[ 98] |
|
抑制铁死亡能够保护大脑中动脉阻塞小鼠神经元,铁与τ蛋白的相互作用存在多效调控 |
[ 99] |
|
|
出血性脑卒中 |
(-)-表儿茶素通过减少大脑铁蓄积和铁死亡相关蛋白表达缓解出血性脑卒中早期脑损伤 |
[ 100] |
|
铁死亡抑制剂可减轻脑切片中神经元死亡及出血性脑卒中小鼠模型中神经元死亡 |
||
|
增加谷胱甘肽过氧化物酶4表达能避免神经元发生铁死亡而改善预后 |
[ 103] |
3.1 铁死亡与心血管疾病
心脏在一些病理情况下能导致铁的过度蓄积、活性氧的产生及膜脂的病理转变,这都是构成铁死亡的重要因素。目前直接将铁死亡与心血管疾病相联系的研究较少,笔者团队2019年的最新研究成果首次揭示了铁死亡在心肌病和缺血再灌注诱发的心脏损伤中的重要作用 [ 96] ,这一里程碑式的发现为心肌病等心脏疾病防治提供了新策略。
3.1.1 铁死亡参与缺血再灌注引发的组织器官损伤
心脏缺血再灌注时会产生过量活性氧、脂质过氧化及血红素中铁释放所致铁蓄积 [ 104- 106] 。Gao等 [ 95] 建立了离体小鼠心脏缺血再灌注模型,发现抑制谷氨酰胺代谢可抑制铁死亡,进而减轻心脏损伤。Fang等 [ 96] 建立了在体心肌缺血再灌注模型,发现Fer-1和铁螯合剂能够明显减轻缺血再灌注的急性和慢性心脏损伤,证实铁死亡在心脏缺血再灌注损伤中的作用。此外,铁死亡还参与肾脏 [ 107] 和肝脏 [ 108] 中的缺血再灌注损伤过程。
3.1.2 铁死亡参与抗肿瘤药物诱导的心肌损伤
阿霉素作为广谱抗肿瘤药物,因其心脏毒性而限制了其在临床的使用。自噬、凋亡及坏死等多种细胞死亡方式参与阿霉素引起的心肌损伤 [ 109- 111] 。Fang等 [ 96] 在凋亡和/或坏死性凋亡缺陷的小鼠中用阿霉素诱导心肌病,发现小鼠心肌细胞出现了铁死亡的特征,并提出血红素加氧酶1可能是其关键调控因子;通过亚细胞定位,发现心肌细胞铁蓄积和脂质过氧化发生于线粒体,靶向线粒体的抗氧化剂MitoTEMPO能够有效抑制铁死亡进而保护心脏。
3.1.3 铁死亡参与心脏移植后心肌损伤
Li等 [ 97] 发现,心脏移植手术后发生的中性粒细胞募集现象是由铁死亡调控的。供体心脏在移植后由于缺血、缺氧等原因可诱导心肌细胞发生铁死亡,细胞内容物释放并通过TLR4/Trif/Ⅰ型干扰素通路募集中性粒细胞,造成坏死性炎症。Fer-1能降低心脏移植后过氧氢花生四烯酰基磷脂酰乙醇胺的水平,减少心肌细胞死亡和中性粒细胞招募。
3.2 铁死亡与脑血管疾病
缺血性脑卒中和出血性脑卒中均可导致神经元发生铁死亡 [ 99, 101] 。
3.2.1 铁死亡与缺血性脑卒中
在铁死亡被发现前,临床及缺血性脑卒中动物模型中已证实铁蓄积能够加重再灌注时的神经元损伤 [ 112- 115] 。铁螯合剂可降低实验动物在缺血性脑卒中后再灌注的损伤 [ 116- 119] 。Speer等 [ 98] 提出,大脑缺血后铁死亡导致了神经元死亡,缺氧诱导因子脯氨酰羟化酶可能是铁螯合剂产生有益作用的靶点。研究发现,在大脑中动脉阻塞小鼠模型中抑制铁死亡能够保护神经元免受缺血再灌注损伤 [ 99] 。
3.2.2 铁死亡与出血性脑卒中
Chang等 [ 100] 发现,(-) -表儿茶素能够通过减少大脑铁蓄积和铁死亡相关蛋白表达来缓解出血性脑卒中的早期脑损伤。之后又发现,Fer-1能缓解血红蛋白诱导的脑切片细胞死亡,以及缓解胶原酶诱导的出血性脑卒中小鼠模型中神经元的死亡 [ 101] 。同时,Zille等 [ 102] 发现Fer-1、去铁胺等铁死亡抑制剂能够抑制小鼠原代神经元死亡。急性出血性脑卒中大鼠 Gpx4 表达水平急剧下降,增加 Gpx4 水平能够避免神经元发生继发的铁死亡损伤并改善出血性脑卒中的预后 [ 103] 。因此,铁死亡通路可能参与脑卒中神经元死亡的过程,推测靶向抑制铁死亡可能是缓解脑卒中的有效治疗方式。
4 结语
除了铁死亡与肿瘤、心脑血管疾病和神经退行性疾病,铁死亡在非酒精性脂肪肝和非酒精性脂肪肝炎等肝病中也有报道 [ 120- 124] 。活性氧诱导的铁死亡可抑制肿瘤生长,增加肿瘤细胞对化疗和放疗的敏感性。与肿瘤治疗策略相反,铁死亡可促进神经退行性疾病和心脑血管疾病的发生、发展,因此相关转化医学研究主要聚焦于发现可有效抑制铁死亡的小分子物质。这些靶向铁死亡的小分子激活剂可直接作为化疗药物,或作为化疗增敏剂与化疗药物联合使用。但铁死亡在不同类型肿瘤及不同基因突变(如 p53 或 RAS突变)环境中表现复杂,在临床前期及临床研究中的可行性亟待深入研究。值得关注的是,GPX4通路非依赖性FSP1的发现和CD8 +T细胞通过释放γ干扰素诱导肿瘤细胞发生铁死亡等新机制和新靶点的发现 [ 18- 19, 125] ,为肿瘤治疗和药物发现提供了全新的视角和策略。
大脑和神经组织中的铁蓄积及铁死亡已被证明与阿尔茨海默病、帕金森症、肌萎缩侧索硬化等多种神经退行性疾病的发病存在直接联系。目前,运用铁螯合剂治疗神经退行性疾病的各种临床试验不断涌现,但针对脑卒中尚缺乏有效治疗手段。鉴于铁死亡在脑卒中后神经元死亡发挥的重要作用,有效抑制铁死亡有望为预防脑卒中引发的神经元死亡提供防治新策略。
与神经退行性疾病发病机制相似,许多心脏疾病出现共同的铁死亡特征,如铁过载、氧化应激、内质网应激和线粒体功能失常等。笔者团队前期研究提示,铁死亡抑制剂能够有效防治因心肌细胞铁过载、阿霉素诱导的心脏毒性及心脏缺血再灌注等诱发的心肌病及心力衰竭 [ 96] 。铁死亡抑制剂、铁螯合剂、线粒体特异性抗氧化剂、血红素加氧酶1抑制剂和低铁膳食等五种不同途径均可有效防治心肌细胞的铁死亡,从而保护心脏,且这些铁死亡抑制剂在小鼠体内相对安全、可行,为靶向铁死亡防治心脏病的临床转化研究提供了乐观的前景 [ 126] 。
着眼临床转化,需要考虑哪种疾病或肿瘤适合靶向铁死亡的治疗方法?在临床或临床前的实验中,靶向铁死亡的药物需要有很高的组织器官特异性和较少的不良反应,纳米靶向给药系统体现了一定优势 [ 127- 128] 。尽管人们对铁死亡的认识日益深入,但铁死亡相关的关键科学问题仍亟待解决,如铁死亡中关键执行分子是什么?脂质过氧化在多大程度上与铁死亡有关?生理过程中是否存在铁死亡?铁死亡在进化过程中是否保守?路漫漫,其修远兮,随着铁死亡相关研究的不断深入与拓展,一定会为靶向铁死亡防治重大慢性疾病的临床转化提供基础。
Funding Statement
国家重点研发计划(2018YFC2000400)
References
- 1.World Health Organization. World health statistics 2018: monitoring health for the SDGs[R]. Geneva: World Health Organization, 2018.
- 2.中华人民共和国国家卫生健康委员会 . 2018中国卫生统计年鉴. 北京: 中国协和医科大学出版社; 2018. [中华人民共和国国家卫生健康委员会.2018中国卫生统计年鉴[M].北京:中国协和医科大学出版社, 2018.] [Google Scholar]
- 3.DIXON S J, LEMBERG K M, LAMPRECHT M R, et al. Ferroptosis:an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. [DIXON S J, LEMBERG K M, LAMPRECHT M R, et al. Ferroptosis:an iron-dependent form of nonapoptotic cell death[J]. Cell, 2012, 149(5):1060-1072. DOI:10.1016/j.cell.2012.03.042.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CAO J Y, DIXON S J. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73(11-12):2195–2209. doi: 10.1007/s00018-016-2194-1. [CAO J Y, DIXON S J. Mechanisms of ferroptosis[J]. Cell Mol Life Sci, 2016, 73(11-12):2195-2209. DOI:10.1007/s00018-016-2194-1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DIXON S J, STOCKWELL B R. The role of iron and reactive oxygen species in cell death. Nat Chem Biol. 2014;10(1):9–17. doi: 10.1038/nchembio.1416. [DIXON S J, STOCKWELL B R. The role of iron and reactive oxygen species in cell death[J]. Nat Chem Biol, 2014, 10(1):9-17. DOI:10.1038/nchembio.1416.] [DOI] [PubMed] [Google Scholar]
- 6.MANCIAS J D, WANG X, GYGI S P, et al. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509(7498):105–109. doi: 10.1038/nature13148. [MANCIAS J D, WANG X, GYGI S P, et al. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy[J]. Nature, 2014, 509(7498):105-109. DOI:10.1038/nature13148.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.HOU W, XIE Y, SONG X, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425–1428. doi: 10.1080/15548627.2016.1187366. [HOU W, XIE Y, SONG X, et al. Autophagy promotes ferroptosis by degradation of ferritin[J]. Autophagy, 2016, 12(8):1425-1428. DOI:10.1080/15548627.2016.1187366.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.YANG W S, STOCKWELL B R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234–245. doi: 10.1016/j.chembiol.2008.02.010. [YANG W S, STOCKWELL B R. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells[J]. Chem Biol, 2008, 15(3):234-245. DOI:10.1016/j.chembiol.2008.02.010.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WENZEL S E, TYURINA Y Y, ZHAO J, et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell. 2017;171(3):628–641. doi: 10.1016/j.cell.2017.09.044. [WENZEL S E, TYURINA Y Y, ZHAO J, et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals[J]. Cell, 2017, 171(3):628-641.e26. DOI:10.1016/j.cell.2017.09.044.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DOLL S, PRONETH B, TYURINA Y Y, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–98. doi: 10.1038/nchembio.2239. [DOLL S, PRONETH B, TYURINA Y Y, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition[J]. Nat Chem Biol, 2017, 13(1):91-98. DOI:10.1038/nchembio.2239.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.HANGAUER M J, VISWANATHAN V S, RYAN M J, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551(7679):247–250. doi: 10.1038/nature24297. [HANGAUER M J, VISWANATHAN V S, RYAN M J, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition[J]. Nature, 2017, 551(7679):247-250. DOI:10.1038/nature24297.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DIXON S J, WINTER G E, MUSAVI L S, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem Biol. 2015;10(7):1604–1609. doi: 10.1021/acschembio.5b00245. [DIXON S J, WINTER G E, MUSAVI L S, et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death[J]. ACS Chem Biol, 2015, 10(7):1604-1609. DOI:10.1021/acschembio.5b00245.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.XIE Y, HOU W, SONG X, et al. Ferroptosis:process and function. Cell Death Differ. 2016;23(3):369–379. doi: 10.1038/cdd.2015.158. [XIE Y, HOU W, SONG X, et al. Ferroptosis:process and function[J]. Cell Death Differ, 2016, 23(3):369-379. DOI:10.1038/cdd.2015.158.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FRIEDMANN ANGELI J P, CONRAD M. Selenium and GPX4, a vital symbiosis. Free Radic Biol Med. 2018;127:153–159. doi: 10.1016/j.freeradbiomed.2018.03.001. [FRIEDMANN ANGELI J P, CONRAD M. Selenium and GPX4, a vital symbiosis[J]. Free Radic Biol Med, 2018, 127:153-159. DOI:10.1016/j.freeradbiomed.2018.03.001.] [DOI] [PubMed] [Google Scholar]
- 15.MAIORINO M, CONRAD M, URSINI F. GPx4, lipid peroxidation, and cell death:discoveries, rediscoveries, and open issues. Antioxid Redox Signal. 2018;29(1):61–74. doi: 10.1089/ars.2017.7115. [MAIORINO M, CONRAD M, URSINI F. GPx4, lipid peroxidation, and cell death:discoveries, rediscoveries, and open issues[J]. Antioxid Redox Signal, 2018, 29(1):61-74. DOI:10.1089/ars.2017.7115.] [DOI] [PubMed] [Google Scholar]
- 16.LU L, HOPE B T, SHAHAM Y. The cystine-glutamate transporter in the accumbens:a novel role in cocaine relapse. Trends Neurosci. 2004;27(2):74–76. doi: 10.1016/j.tins.2003.11.007. [LU L, HOPE B T, SHAHAM Y. The cystine-glutamate transporter in the accumbens:a novel role in cocaine relapse[J]. Trends Neurosci, 2004, 27(2):74-76. DOI:10.1016/j.tins.2003.11.007.] [DOI] [PubMed] [Google Scholar]
- 17.WANG H, AN P, XIE E, et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017;66(2):449–465. doi: 10.1002/hep.29117. [WANG H, AN P, XIE E, et al. Characterization of ferroptosis in murine models of hemochromatosis[J]. Hepatology, 2017, 66(2):449-465. DOI:10.1002/hep.29117.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.BERSUKER K, HENDRICKS J M, LI Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692. doi: 10.1038/s41586-019-1705-2. [BERSUKER K, HENDRICKS J M, LI Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis[J]. Nature, 2019, 575(7784):688-692. DOI:10.1038/s41586-019-1705-2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DOLL S, FREITAS F P, SHAH R, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698. doi: 10.1038/s41586-019-1707-0. [DOLL S, FREITAS F P, SHAH R, et al. FSP1 is a glutathione-independent ferroptosis suppressor[J]. Nature, 2019, 575(7784):693-698. DOI:10.1038/s41586-019-1707-0.] [DOI] [PubMed] [Google Scholar]
- 20.STOCKWELL B R, FRIEDMANN ANGELI J P, BAYIR H, et al. Ferroptosis:a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [STOCKWELL B R, FRIEDMANN ANGELI J P, BAYIR H, et al. Ferroptosis:a regulated cell death nexus linking metabolism, redox biology, and disease[J]. Cell, 2017, 171(2):273-285. DOI:10.1016/j.cell.2017.09.021.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LOUANDRE C, EZZOUKHRY Z, GODIN C, et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int J Cancer. 2013;133(7):1732–1742. doi: 10.1002/ijc.28159. [LOUANDRE C, EZZOUKHRY Z, GODIN C, et al. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib[J]. Int J Cancer, 2013, 133(7):1732-1742. DOI:10.1002/ijc.28159.] [DOI] [PubMed] [Google Scholar]
- 22.LOUANDRE C, MARCQ I, BOUHLAL H, et al. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett. 2015;356(2 Pt B):971–977. doi: 10.1016/j.canlet.2014.11.014. [LOUANDRE C, MARCQ I, BOUHLAL H, et al. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells[J]. Cancer Lett, 2015, 356(2 Pt B):971-977. DOI:10.1016/j.canlet.2014.11.014.] [DOI] [PubMed] [Google Scholar]
- 23.SUN X, OU Z, CHEN R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173–184. doi: 10.1002/hep.28251. [SUN X, OU Z, CHEN R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells[J]. Hepatology, 2016, 63(1):173-184. DOI:10.1002/hep.28251.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SUZUKI T, MOTOHASHI H, YAMAMOTO M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol Sci. 2013;34(6):340–346. doi: 10.1016/j.tips.2013.04.005. [SUZUKI T, MOTOHASHI H, YAMAMOTO M. Toward clinical application of the Keap1-Nrf2 pathway[J]. Trends Pharmacol Sci, 2013, 34(6):340-346. DOI:10.1016/j.tips.2013.04.005.] [DOI] [PubMed] [Google Scholar]
- 25.HARRISON P M, AROSIO P. The ferritins:molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;1275(3):161–203. doi: 10.1016/0005-2728(96)00022-9. [HARRISON P M, AROSIO P. The ferritins:molecular properties, iron storage function and cellular regulation[J]. Biochim Biophys Acta, 1996, 1275(3):161-203. DOI:10.1016/0005-2728(96)00022-9.] [DOI] [PubMed] [Google Scholar]
- 26.ARLT A, SEBENS S, KREBS S, et al. Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene. 2013;32(40):4825–4835. doi: 10.1038/onc.2012.493. [ARLT A, SEBENS S, KREBS S, et al. Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity[J]. Oncogene, 2013, 32(40):4825-4835. DOI:10.1038/onc.2012.493.] [DOI] [PubMed] [Google Scholar]
- 27.KINOSHITA H, OKABE H, BEPPU T, et al. Cystine/glutamic acid transporter is a novel marker for predicting poor survival in patients with hepatocellular carcinoma. Oncol Rep. 2013;29(2):685–689. doi: 10.3892/or.2012.2162. [KINOSHITA H, OKABE H, BEPPU T, et al. Cystine/glutamic acid transporter is a novel marker for predicting poor survival in patients with hepatocellular carcinoma[J]. Oncol Rep, 2013, 29(2):685-689. DOI:10.3892/or.2012.2162.] [DOI] [PubMed] [Google Scholar]
- 28.SUN X, NIU X, CHEN R, et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64(2):488–500. doi: 10.1002/hep.28574. [SUN X, NIU X, CHEN R, et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis[J]. Hepatology, 2016, 64(2):488-500. DOI:10.1002/hep.28574.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.EFFERTH T, DUNSTAN H, SAUERBREY A, et al. The anti-malarial artesunate is also active against cancer. Int J Oncol. 2001;18(4):767–773. doi: 10.3892/ijo.18.4.767. [EFFERTH T, DUNSTAN H, SAUERBREY A, et al. The anti-malarial artesunate is also active against cancer[J]. Int J Oncol, 2001, 18(4):767-773. DOI:10.3892/ijo.18.4.767.] [DOI] [PubMed] [Google Scholar]
- 30.ELING N, REUTER L, HAZIN J, et al. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience. 2015;2(5):517–532. doi: 10.18632/oncoscience.160. [ELING N, REUTER L, HAZIN J, et al. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells[J]. Oncoscience, 2015, 2(5):517-532. DOI:10.18632/oncoscience.160.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.YAMAGUCHI Y, KASUKABE T, KUMAKURA S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int J Oncol. 2018;52(3):1011–1022. doi: 10.3892/ijo.2018.4259. [YAMAGUCHI Y, KASUKABE T, KUMAKURA S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis[J]. Int J Oncol, 2018, 52(3):1011-1022. DOI:10.3892/ijo.2018.4259.] [DOI] [PubMed] [Google Scholar]
- 32.YANG W S, SRIRAMARATNAM R, WELSCH M E, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1-2):317–331. doi: 10.1016/j.cell.2013.12.010. [YANG W S, SRIRAMARATNAM R, WELSCH M E, et al. Regulation of ferroptotic cancer cell death by GPX4[J]. Cell, 2014, 156(1-2):317-331. DOI:10.1016/j.cell.2013.12.010.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ZOU Y, PALTE M J, DEIK A A, et al. HIF-2α drives an intrinsic vulnerability to ferroptosis in clear cell renal cell carcinoma. BioRxiv. 2018 doi: 10.1101/388041. [ZOU Y, PALTE M J, DEIK A A, et al. HIF-2α drives an intrinsic vulnerability to ferroptosis in clear cell renal cell carcinoma[J]. BioRxiv, 2018. DOI:10.1101/388041.] [DOI] [Google Scholar]
- 34.WU J, MINIKES A M, GAO M, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572(7769):402–406. doi: 10.1038/s41586-019-1426-6. [WU J, MINIKES A M, GAO M, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling[J]. Nature, 2019, 572(7769):402-406. DOI:10.1038/s41586-019-1426-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.YANG W H, DING C C, SUN T, et al. The hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma. Cell Rep. 2019;28(10):2501–2508. doi: 10.1016/j.celrep.2019.07.107. [YANG W H, DING C C, SUN T, et al. The hippo pathway effector TAZ regulates ferroptosis in renal cell carcinoma[J]. Cell Rep, 2019, 28(10):2501-2508.e4. DOI:10.1016/j.celrep.2019.07.107.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MA S, HENSON E S, CHEN Y, et al. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7:e2307. doi: 10.1038/cddis.2016.208. [MA S, HENSON E S, CHEN Y, et al. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells[J/OL]. Cell Death Dis, 2016, 7:e2307. DOI:10.1038/cddis.2016.208.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.HASEGAWA M, TAKAHASHI H, RAJABI H, et al. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells. Oncotarget. 2016;7(11):11756–11769. doi: 10.18632/oncotarget.7598. [HASEGAWA M, TAKAHASHI H, RAJABI H, et al. Functional interactions of the cystine/glutamate antiporter, CD44v and MUC1-C oncoprotein in triple-negative breast cancer cells[J]. Oncotarget, 2016, 7(11):11756-11769. DOI:10.18632/oncotarget.7598.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.TIMMERMAN L A, HOLTON T, YUNEVA M, et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target. Cancer Cell. 2013;24(4):450–465. doi: 10.1016/j.ccr.2013.08.020. [TIMMERMAN L A, HOLTON T, YUNEVA M, et al. Glutamine sensitivity analysis identifies the xCT antiporter as a common triple-negative breast tumor therapeutic target[J]. Cancer Cell, 2013, 24(4):450-465. DOI:10.1016/j.ccr.2013.08.020.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LANZARDO S, CONTI L, ROOKE R, et al. Immunotargeting of antigen xCT attenuates stem-like cell behavior and metastatic progression in breast cancer. Cancer Res. 2016;76(1):62–72. doi: 10.1158/0008-5472.CAN-15-1208. [LANZARDO S, CONTI L, ROOKE R, et al. Immunotargeting of antigen xCT attenuates stem-like cell behavior and metastatic progression in breast cancer[J]. Cancer Res, 2016, 76(1):62-72. DOI:10.1158/0008-5472.CAN-15-1208.] [DOI] [PubMed] [Google Scholar]
- 40.HABASHY H O, POWE D G, STAKA C M, et al. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res Treat. 2010;119(2):283–293. doi: 10.1007/s10549-009-0345-x. [HABASHY H O, POWE D G, STAKA C M, et al. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen[J]. Breast Cancer Res Treat, 2010, 119(2):283-293. DOI:10.1007/s10549-009-0345-x.] [DOI] [PubMed] [Google Scholar]
- 41.TONIK S E, SHINDELMAN J E, SUSSMAN H H. Transferrin receptor is inversely correlated with estrogen receptor in breast cancer. Breast Cancer Res Treat. 1986;7(2):71–76. doi: 10.1007/bf01806791. [TONIK S E, SHINDELMAN J E, SUSSMAN H H. Transferrin receptor is inversely correlated with estrogen receptor in breast cancer[J]. Breast Cancer Res Treat, 1986, 7(2):71-76. DOI:10.1007/bf01806791.] [DOI] [PubMed] [Google Scholar]
- 42.YUAN H, LI X, ZHANG X, et al. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478(2):838–844. doi: 10.1016/j.bbrc.2016.08.034. [YUAN H, LI X, ZHANG X, et al. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation[J]. Biochem Biophys Res Commun, 2016, 478(2):838-844. DOI:10.1016/j.bbrc.2016.08.034.] [DOI] [PubMed] [Google Scholar]
- 43.CHEN W C, WANG C Y, HUNG Y H, et al. Systematic analysis of gene expression alterations and clinical outcomes for long-chain acyl-coenzyme a synthetase family in cancer. PLoS One. 2016;11(5):e0155660. doi: 10.1371/journal.pone.0155660. [CHEN W C, WANG C Y, HUNG Y H, et al. Systematic analysis of gene expression alterations and clinical outcomes for long-chain acyl-coenzyme a synthetase family in cancer[J/OL]. PLoS One, 2016, 11(5):e0155660. DOI:10.1371/journal.pone.0155660.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.HABIB E, LINHER-MELVILLE K, LIN H X, et al. Expression of xCT and activity of system xc(-) are regulated by NRF2 in human breast cancer cells in response to oxidative stress. Redox Biol. 2015;5:33–42. doi: 10.1016/j.redox.2015.03.003. [HABIB E, LINHER-MELVILLE K, LIN H X, et al. Expression of xCT and activity of system xc(-) are regulated by NRF2 in human breast cancer cells in response to oxidative stress[J]. Redox Biol, 2015, 5:33-42. DOI:10.1016/j.redox.2015.03.003.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LI T, KON N, JIANG L, et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149(6):1269–1283. doi: 10.1016/j.cell.2012.04.026. [LI T, KON N, JIANG L, et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence[J]. Cell, 2012, 149(6):1269-1283. DOI:10.1016/j.cell.2012.04.026.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.JIANG L, KON N, LI T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi: 10.1038/nature14344. [JIANG L, KON N, LI T, et al. Ferroptosis as a p53-mediated activity during tumour suppression[J]. Nature, 2015, 520(7545):57-62. DOI:10.1038/nature14344.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.WANG S J, LI D, OU Y, et al. Acetylation is crucial for p53-mediated ferroptosis and tumor suppression. Cell Rep. 2016;17(2):366–373. doi: 10.1016/j.celrep.2016.09.022. [WANG S J, LI D, OU Y, et al. Acetylation is crucial for p53-mediated ferroptosis and tumor suppression[J]. Cell Rep, 2016, 17(2):366-373. DOI:10.1016/j.celrep.2016.09.022.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.JENNIS M, KUNG C P, BASU S, et al. An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev. 2016;30(8):918–930. doi: 10.1101/gad.275891.115. [JENNIS M, KUNG C P, BASU S, et al. An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model[J]. Genes Dev, 2016, 30(8):918-930. DOI:10.1101/gad.275891.115.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.XIE Y, ZHU S, SONG X, et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep. 2017;20(7):1692–1704. doi: 10.1016/j.celrep.2017.07.055. [XIE Y, ZHU S, SONG X, et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity[J]. Cell Rep, 2017, 20(7):1692-1704. DOI:10.1016/j.celrep.2017.07.055.] [DOI] [PubMed] [Google Scholar]
- 50.MA Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [MA Q. Role of nrf2 in oxidative stress and toxicity[J]. Annu Rev Pharmacol Toxicol, 2013, 53:401-426. DOI:10.1146/annurev-pharmtox-011112-140320.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WANG X J, SUN Z, VILLENEUVE N F, et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29(6):1235–1243. doi: 10.1093/carcin/bgn095. [WANG X J, SUN Z, VILLENEUVE N F, et al. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2[J]. Carcinogenesis, 2008, 29(6):1235-1243. DOI:10.1093/carcin/bgn095.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.YUAN H, LI X, ZHANG X, et al. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478(3):1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [YUAN H, LI X, ZHANG X, et al. Identification of ACSL4 as a biomarker and contributor of ferroptosis[J]. Biochem Biophys Res Commun, 2016, 478(3):1338-1343. DOI:10.1016/j.bbrc.2016.08.124.] [DOI] [PubMed] [Google Scholar]
- 53.RADLOWSKI E C, JOHNSON R W. Perinatal iron deficiency and neurocognitive development. Front Hum Neurosci. 2013;7:585. doi: 10.3389/fnhum.2013.00585. [RADLOWSKI E C, JOHNSON R W. Perinatal iron deficiency and neurocognitive development[J]. Front Hum Neurosci, 2013, 7:585. DOI:10.3389/fnhum.2013.00585.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.BELAIDI A A, BUSH A I. Iron neurochemistry in Alzheimer's disease and Parkinson's disease:targets for therapeutics. J Neurochem. 2016;139(Suppl 1):179–197. doi: 10.1111/jnc.13425. [BELAIDI A A, BUSH A I. Iron neurochemistry in Alzheimer's disease and Parkinson's disease:targets for therapeutics[J]. J Neurochem, 2016, 139 Suppl 1:179-197. DOI:10.1111/jnc.13425.] [DOI] [PubMed] [Google Scholar]
- 55.WEILAND A, WANG Y, WU W, et al. Ferroptosis and its role in diverse brain diseases. Mol Neurobiol. 2019;56(7):4880–4893. doi: 10.1007/s12035-018-1403-3. [WEILAND A, WANG Y, WU W, et al. Ferroptosis and its role in diverse brain diseases[J]. Mol Neurobiol, 2019, 56(7):4880-4893. DOI:10.1007/s12035-018-1403-3.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.AYTON S, FAUX N G, BUSH A I, et al. Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE. Nat Commun. 2015;6:6760. doi: 10.1038/ncomms7760. [AYTON S, FAUX N G, BUSH A I, et al. Ferritin levels in the cerebrospinal fluid predict Alzheimer's disease outcomes and are regulated by APOE[J]. Nat Commun, 2015, 6:6760. DOI:10.1038/ncomms7760.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.HAMBRIGHT W S, FONSECA R S, CHEN L, et al. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017;12:8–17. doi: 10.1016/j.redox.2017.01.021. [HAMBRIGHT W S, FONSECA R S, CHEN L, et al. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration[J]. Redox Biol, 2017, 12:8-17. DOI:10.1016/j.redox.2017.01.021.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.ZHANG Y H, WANG D W, XU S F, et al. α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol. 2018;14:535–548. doi: 10.1016/j.redox.2017.11.001. [ZHANG Y H, WANG D W, XU S F, et al.α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice[J]. Redox Biol, 2018, 14:535-548. DOI:10.1016/j.redox.2017.11.001.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DO VAN B, GOUEL F, JONNEAUX A, et al. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC. Neurobiol Dis. 2016;94:169–178. doi: 10.1016/j.nbd.2016.05.011. [DO VAN B, GOUEL F, JONNEAUX A, et al. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC[J]. Neurobiol Dis, 2016, 94:169-178. DOI:10.1016/j.nbd.2016.05.011.] [DOI] [PubMed] [Google Scholar]
- 60.GOUEL F, DO VAN B, CHOU M L, et al. The protective effect of human platelet lysate in models of neurodegenerative disease:involvement of the Akt and MEK pathways. J Tissue Eng Regen Med. 2017;11(11):3236–3240. doi: 10.1002/term.2222. [GOUEL F, DO VAN B, CHOU M L, et al. The protective effect of human platelet lysate in models of neurodegenerative disease:involvement of the Akt and MEK pathways[J]. J Tissue Eng Regen Med, 2017, 11(11):3236-3240. DOI:10.1002/term.2222.] [DOI] [PubMed] [Google Scholar]
- 61.CUI Z, ZHONG Z, YANG Y, et al. Ferrous iron induces Nrf2 expression in mouse brain astrocytes to prevent neurotoxicity. J Biochem Mol Toxicol. 2016;30(8):396–403. doi: 10.1002/jbt.21803. [CUI Z, ZHONG Z, YANG Y, et al. Ferrous iron induces Nrf2 expression in mouse brain astrocytes to prevent neurotoxicity[J]. J Biochem Mol Toxicol, 2016, 30(8):396-403. DOI:10.1002/jbt.21803.] [DOI] [PubMed] [Google Scholar]
- 62.ISHII T, WARABI E, MANN G E. Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis. Free Radic Biol Med. 2019;133:169–178. doi: 10.1016/j.freeradbiomed.2018.09.002. [ISHII T, WARABI E, MANN G E. Circadian control of BDNF-mediated Nrf2 activation in astrocytes protects dopaminergic neurons from ferroptosis[J]. Free Radic Biol Med, 2019, 133:169-178. DOI:10.1016/j.freeradbiomed.2018.09.002.] [DOI] [PubMed] [Google Scholar]
- 63.CODAZZI F, PELIZZONI I, ZACCHETTI D, et al. Iron entry in neurons and astrocytes:a link with synaptic activity. Front Mol Neurosci. 2015;8:18. doi: 10.3389/fnmol.2015.00018. [CODAZZI F, PELIZZONI I, ZACCHETTI D, et al. Iron entry in neurons and astrocytes:a link with synaptic activity[J]. Front Mol Neurosci, 2015, 8:18. DOI:10.3389/fnmol.2015.00018.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.BUSH A I, CURTAIN C C. Twenty years of metallo-neurobiology:where to now? Eur Biophys J. 2008;37(3):241–245. doi: 10.1007/s00249-007-0228-1. [BUSH A I, CURTAIN C C. Twenty years of metallo-neurobiology:where to now?[J]. Eur Biophys J, 2008, 37(3):241-245. DOI:10.1007/s00249-007-0228-1.] [DOI] [PubMed] [Google Scholar]
- 65.CONNOR J R, SNYDER B S, BEARD J L, et al. Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimer's disease. J Neurosci Res. 1992;31(2):327–335. doi: 10.1002/jnr.490310214. [CONNOR J R, SNYDER B S, BEARD J L, et al. Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimer's disease[J]. J Neurosci Res, 1992, 31(2):327-335. DOI:10.1002/jnr.490310214.] [DOI] [PubMed] [Google Scholar]
- 66.LOVELL M A, ROBERTSON J D, TEESDALE W J, et al. Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci. 1998;158(1):47–52. doi: 10.1016/s0022-510x(98)00092-6. [LOVELL M A, ROBERTSON J D, TEESDALE W J, et al. Copper, iron and zinc in Alzheimer's disease senile plaques[J]. J Neurol Sci, 1998, 158(1):47-52. DOI:10.1016/s0022-510x(98)00092-6.] [DOI] [PubMed] [Google Scholar]
- 67.BILGIC B, PFEFFERBAUM A, ROHLFING T, et al. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping. Neuroimage. 2012;59(3):2625–2635. doi: 10.1016/j.neuroimage.2011.08.077. [BILGIC B, PFEFFERBAUM A, ROHLFING T, et al. MRI estimates of brain iron concentration in normal aging using quantitative susceptibility mapping[J]. Neuroimage, 2012, 59(3):2625-2635. DOI:10.1016/j.neuroimage.2011.08.077.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.HAMBRIGHT W S, FONSECA R S, CHEN L, et al. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017;12:8–17. doi: 10.1016/j.redox.2017.01.021. [HAMBRIGHT W S, FONSECA R S, CHEN L, et al. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration[J]. Redox Biol, 2017, 12:8-17. DOI:10.1016/j.redox.2017.01.021.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.KHANDELWAL P J, HERMAN A M, MOUSSA C E. Inflammation in the early stages of neurodegenerative pathology. J Neuroimmunol. 2011;238(1-2):1–11. doi: 10.1016/j.jneuroim.2011.07.002. [KHANDELWAL P J, HERMAN A M, MOUSSA C E. Inflammation in the early stages of neurodegenerative pathology[J]. J Neuroimmunol, 2011, 238(1-2):1-11. DOI:10.1016/j.jneuroim.2011.07.002.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.RAINA A K, HOCHMAN A, ZHU X, et al. Abortive apoptosis in Alzheimer's disease. Acta Neuropathol. 2001;101(4):305–310. doi: 10.1007/s004010100378. [RAINA A K, HOCHMAN A, ZHU X, et al. Abortive apoptosis in Alzheimer's disease[J]. Acta Neuropathol, 2001, 101(4):305-310. DOI:10.1007/s004010100378.] [DOI] [PubMed] [Google Scholar]
- 71.RAEFSKY S M, FURMAN R, MILNE G, et al. Deuterated polyunsaturated fatty acids reduce brain lipid peroxidation and hippocampal amyloid beta-peptide levels, without discernable behavioral effects in an APP/PS1 mutant transgenic mouse model of Alzheimer's disease. Neurobiol Aging. 2018;66:165–176. doi: 10.1016/j.neurobiolaging.2018.02.024. [RAEFSKY S M, FURMAN R, MILNE G, et al. Deuterated polyunsaturated fatty acids reduce brain lipid peroxidation and hippocampal amyloid beta-peptide levels, without discernable behavioral effects in an APP/PS1 mutant transgenic mouse model of Alzheimer's disease[J]. Neurobiol Aging, 2018, 66:165-176. DOI:10.1016/j.neurobiolaging.2018.02.024.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.YANG W S, KIM K J, GASCHLER M M, et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113(34):E4966–E4975. doi: 10.1073/pnas.1603244113. [YANG W S, KIM K J, GASCHLER M M, et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis[J/OL]. Proc Natl Acad Sci U S A, 2016, 113(34):E4966-E4975. DOI:10.1073/pnas.1603244113.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DEXTER D T, WELLS F R, AGID F, et al. Increased nigral iron content in postmortem parkinsonian brain. Lancet. 1987;2(8569):1219–1220. doi: 10.1016/s0140-6736(87)91361-4. [DEXTER D T, WELLS F R, AGID F, et al. Increased nigral iron content in postmortem parkinsonian brain[J]. Lancet, 1987, 2(8569):1219-1220. DOI:10.1016/s0140-6736(87)91361-4.] [DOI] [PubMed] [Google Scholar]
- 74.DEXTER D T, WELLS F R, LEES A J, et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. J Neurochem. 1989;52(6):1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DEXTER D T, WELLS F R, LEES A J, et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease[J]. J Neurochem, 1989, 52(6):1830-1836. DOI:10.1111/j.1471-4159.1989.tb07264.x.] [DOI] [PubMed] [Google Scholar]
- 75.AYTON S, LEI P, DUCE J A, et al. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann Neurol. 2013;73(4):554–559. doi: 10.1002/ana.23817. [AYTON S, LEI P, DUCE J A, et al. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease[J]. Ann Neurol, 2013, 73(4):554-559. DOI:10.1002/ana.23817.] [DOI] [PubMed] [Google Scholar]
- 76.BOLL M C, SOTELO J, OTERO E, et al. Reduced ferroxidase activity in the cerebrospinal fluid from patients with Parkinson's disease. Neurosci Lett. 1999;265(3):155–158. doi: 10.1016/s0304-3940(99)00221-9. [BOLL M C, SOTELO J, OTERO E, et al. Reduced ferroxidase activity in the cerebrospinal fluid from patients with Parkinson's disease[J]. Neurosci Lett, 1999, 265(3):155-158. DOI:10.1016/s0304-3940(99)00221-9.] [DOI] [PubMed] [Google Scholar]
- 77.OLIVIERI S, CONTI A, IANNACCONE S, et al. Ceruloplasmin oxidation, a feature of Parkinson's disease CSF, inhibits ferroxidase activity and promotes cellular iron retention. J Neurosci. 2011;31(50):18568–18577. doi: 10.1523/JNEUROSCI.3768-11.2011. [OLIVIERI S, CONTI A, IANNACCONE S, et al. Ceruloplasmin oxidation, a feature of Parkinson's disease CSF, inhibits ferroxidase activity and promotes cellular iron retention[J]. J Neurosci, 2011, 31(50):18568-18577. DOI:10.1523/JNEUROSCI.3768-11.2011.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.SALAZAR J, MENA N, HUNOT S, et al. Divalent metal transporter 1(DMT1) contributes to neurodegeneration in animal models of Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105(47):18578–18583. doi: 10.1073/pnas.0804373105. [SALAZAR J, MENA N, HUNOT S, et al. Divalent metal transporter 1(DMT1) contributes to neurodegeneration in animal models of Parkinson's disease[J]. Proc Natl Acad Sci U S A, 2008, 105(47):18578-18583. DOI:10.1073/pnas.0804373105.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.FAUCHEUX B A, MARTIN M E, BEAUMONT C, et al. Lack of up-regulation of ferritin is associated with sustained iron regulatory protein-1 binding activity in the substantia nigra of patients with Parkinson's disease. J Neurochem. 2002;83(2):320–330. doi: 10.1046/j.1471-4159.2002.01118.x. [FAUCHEUX B A, MARTIN M E, BEAUMONT C, et al. Lack of up-regulation of ferritin is associated with sustained iron regulatory protein-1 binding activity in the substantia nigra of patients with Parkinson's disease[J]. J Neurochem, 2002, 83(2):320-330. DOI:10.1046/j.1471-4159.2002.01118.x.] [DOI] [PubMed] [Google Scholar]
- 80.LEI P, AYTON S, APPUKUTTAN A T, et al. Clioquinol rescues Parkinsonism and dementia phenotypes of the tau knockout mouse. Neurobiol Dis. 2015;81:168–175. doi: 10.1016/j.nbd.2015.03.015. [LEI P, AYTON S, APPUKUTTAN A T, et al. Clioquinol rescues Parkinsonism and dementia phenotypes of the tau knockout mouse[J]. Neurobiol Dis, 2015, 81:168-175. DOI:10.1016/j.nbd.2015.03.015.] [DOI] [PubMed] [Google Scholar]
- 81.LEI P, AYTON S, FINKELSTEIN D I, et al. Tau protein:relevance to Parkinson's disease. Int J Biochem Cell Biol. 2010;42(11):1775–1778. doi: 10.1016/j.biocel.2010.07.016. [LEI P, AYTON S, FINKELSTEIN D I, et al. Tau protein:relevance to Parkinson's disease[J]. Int J Biochem Cell Biol, 2010, 42(11):1775-1778. DOI:10.1016/j.biocel.2010.07.016.] [DOI] [PubMed] [Google Scholar]
- 82.LEI P, AYTON S, MOON S, et al. Motor and cognitive deficits in aged tau knockout mice in two background strains. Mol Neurodegener. 2014;9:29. doi: 10.1186/1750-1326-9-29. [LEI P, AYTON S, MOON S, et al. Motor and cognitive deficits in aged tau knockout mice in two background strains[J]. Mol Neurodegener, 2014, 9:29. DOI:10.1186/1750-1326-9-29.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.SIAN J, DEXTER D T, LEES A J, et al. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36(3):348–355. doi: 10.1002/ana.410360305. doi: 10.1002/ana.410360305. [SIAN J, DEXTER D T, LEES A J, et al. Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting basal ganglia[J]. Ann Neurol, 1994, 36(3):348-355. DOI:10.1002/ana.410360305.] [DOI] [PubMed] [Google Scholar]
- 84.LEI P, AYTON S, FINKELSTEIN D I, et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med. 2012;18(2):291–295. doi: 10.1038/nm.2613. [LEI P, AYTON S, FINKELSTEIN D I, et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export[J]. Nat Med, 2012, 18(2):291-295. DOI:10.1038/nm.2613.] [DOI] [PubMed] [Google Scholar]
- 85.DEVOS D, MOREAU C, DEVEDJIAN J C, et al. Targeting chelatable iron as a therapeutic modality in parkinson's disease. Antioxid Redox Signal. 2014;21(2):195–210. doi: 10.1089/ars.2013.5593. [DEVOS D, MOREAU C, DEVEDJIAN J C, et al. Targeting chelatable iron as a therapeutic modality in parkinson's disease[J]. Antioxid Redox Signal, 2014, 21(2):195-210. DOI:10.1089/ars.2013.5593.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.COLES L D, TUITE P J, ÖZ G, et al. Repeated-dose oral n-acetylcysteine in Parkinson's disease:pharmacokinetics and effect on brain glutathione and oxidative stress. J Clin Pharmacol. 2018;58(2):158–167. doi: 10.1002/jcph.1008. [COLES L D, TUITE P J, ÖZ G, et al. Repeated-dose oral n-acetylcysteine in Parkinson's disease:pharmacokinetics and effect on brain glutathione and oxidative stress[J]. J Clin Pharmacol, 2018, 58(2):158-167. DOI:10.1002/jcph.1008.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.PARK S W, KIM S H, PARK K H, et al. Preventive effect of antioxidants in MPTP-induced mouse model of Parkinson's disease. Neurosci Lett. 2004;363(3):243–246. doi: 10.1016/j.neulet.2004.03.072. [PARK S W, KIM S H, PARK K H, et al. Preventive effect of antioxidants in MPTP-induced mouse model of Parkinson's disease[J]. Neurosci Lett, 2004, 363(3):243-246. DOI:10.1016/j.neulet.2004.03.072.] [DOI] [PubMed] [Google Scholar]
- 88.PERRY T L, YONG V W, CLAVIER R M, et al. Partial protection from the dopaminergic neurotoxin N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine by four different antioxidants in the mouse. Neurosci Lett. 1985;60(2):109–114. doi: 10.1016/0304-3940(85)90229-0. [PERRY T L, YONG V W, CLAVIER R M, et al. Partial protection from the dopaminergic neurotoxin N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine by four different antioxidants in the mouse[J]. Neurosci Lett, 1985, 60(2):109-114. DOI:10.1016/0304-3940(85)90229-0.] [DOI] [PubMed] [Google Scholar]
- 89.MONTI D A, ZABRECKY G, KREMENS D, et al. N-acetyl cysteine may support dopamine neurons in Parkinson's disease:preliminary clinical and cell line data. PLoS One. 2016;11(6):e0157602. doi: 10.1371/journal.pone.0157602. [MONTI D A, ZABRECKY G, KREMENS D, et al. N-acetyl cysteine may support dopamine neurons in Parkinson's disease:preliminary clinical and cell line data[J/OL]. PLoS One, 2016, 11(6):e0157602. DOI:10.1371/journal.pone.0157602.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.GAJOWIAK A, STYS' A, STARZYN'SKI R R, et al. Misregulation of iron homeostasis in amyotrophic lateral sclerosis. Postepy Hig Med Dosw (Online) 2016;70(0):709–721. doi: 10.5604/17322693.1208036. [GAJOWIAK A, STYS' A, STARZYN'SKI R R, et al. Misregulation of iron homeostasis in amyotrophic lateral sclerosis[J/OL]. Postepy Hig Med Dosw (Online), 2016, 70(0):709-721. DOI:10.5604/17322693.1208036.] [DOI] [PubMed] [Google Scholar]
- 91.MOREAU C, DANEL V, DEVEDJIAN J C, et al. Could conservative iron chelation lead to neuroprotection in amyotrophic lateral sclerosis? Antioxid Redox Signal. 2018;29(8):742–748. doi: 10.1089/ars.2017.7493. [MOREAU C, DANEL V, DEVEDJIAN J C, et al. Could conservative iron chelation lead to neuroprotection in amyotrophic lateral sclerosis?[J]. Antioxid Redox Signal, 2018, 29(8):742-748. DOI:10.1089/ars.2017.7493.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.VEYRAT-DUREBEX C, CORCIA P, MUCHA A, et al. Iron metabolism disturbance in a French cohort of ALS patients. Biomed Res Int. 2014;2014:485723. doi: 10.1155/2014/485723. [VEYRAT-DUREBEX C, CORCIA P, MUCHA A, et al. Iron metabolism disturbance in a French cohort of ALS patients[J]. Biomed Res Int, 2014, 2014:485723. DOI:10.1155/2014/485723.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.CHOI I Y, LEE P, STATLAND J, et al. Reduction in cerebral antioxidant, glutathione (GSH), in patients with ALS:A preliminary study (P6.105) http://cn.bing.com/academic/profile?id=6a7e58b2e8c584d25233f716b3007a33&encoded=0&v=paper_preview&mkt=zh-cn. Neurology. 2015;84(14 Supplement) [CHOI I Y, LEE P, STATLAND J, et al. Reduction in cerebral antioxidant, glutathione (GSH), in patients with ALS:A preliminary study (P6.105)[J]. Neurology, 2015, 84(14 Supplement).] [Google Scholar]
- 94.SIMPSON E P, HENRY Y K, HENKEL J S, et al. Increased lipid peroxidation in sera of ALS patients:a potential biomarker of disease burden. Neurology. 2004;62(10):1758–1765. doi: 10.1212/wnl.62.10.1758. [SIMPSON E P, HENRY Y K, HENKEL J S, et al. Increased lipid peroxidation in sera of ALS patients:a potential biomarker of disease burden[J]. Neurology, 2004, 62(10):1758-1765. DOI:10.1212/wnl.62.10.1758.] [DOI] [PubMed] [Google Scholar]
- 95.GAO M, MONIAN P, QUADRI N, et al. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59(2):298–308. doi: 10.1016/j.molcel.2015.06.011. [GAO M, MONIAN P, QUADRI N, et al. Glutaminolysis and transferrin regulate ferroptosis[J]. Mol Cell, 2015, 59(2):298-308. DOI:10.1016/j.molcel.2015.06.011.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.FANG X, WANG H, HAN D, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A. 2019;116(7):2672–2680. doi: 10.1073/pnas.1821022116. [FANG X, WANG H, HAN D, et al. Ferroptosis as a target for protection against cardiomyopathy[J]. Proc Natl Acad Sci U S A, 2019, 116(7):2672-2680. DOI:10.1073/pnas.1821022116.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.LI W, FENG G, GAUTHIER J M, et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J Clin Invest. 2019;129(6):2293–2304. doi: 10.1172/JCI126428. [LI W, FENG G, GAUTHIER J M, et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation[J]. J Clin Invest, 2019, 129(6):2293-2304. DOI:10.1172/JCI126428.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.SPEER R E, KARUPPAGOUNDER S S, BASSO M, et al. Hypoxia-inducible factor prolyl hydroxylases as targets for neuroprotection by "antioxidant" metal chelators:From ferroptosis to stroke. Free Radic Biol Med. 2013;62:26–36. doi: 10.1016/j.freeradbiomed.2013.01.026. [SPEER R E, KARUPPAGOUNDER S S, BASSO M, et al. Hypoxia-inducible factor prolyl hydroxylases as targets for neuroprotection by "antioxidant" metal chelators:From ferroptosis to stroke[J]. Free Radic Biol Med, 2013, 62:26-36. DOI:10.1016/j.freeradbiomed.2013.01.026.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.TUO Q Z, LEI P, JACKMAN K A, et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry. 2017;22(11):1520–1530. doi: 10.1038/mp.2017.171. [TUO Q Z, LEI P, JACKMAN K A, et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke[J]. Mol Psychiatry, 2017, 22(11):1520-1530. DOI:10.1038/mp.2017.171.] [DOI] [PubMed] [Google Scholar]
- 100.CHANG C F, CHO S, WANG J. (-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann Clin Transl Neurol. 2014;1(4):258–271. doi: 10.1002/acn3.54. [CHANG C F, CHO S, WANG J. (-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways[J]. Ann Clin Transl Neurol, 2014, 1(4):258-271. DOI:10.1002/acn3.54.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.LI Q, HAN X, LAN X, et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight. 2017;2(7):e90777. doi: 10.1172/jci.insight.90777. [LI Q, HAN X, LAN X, et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain[J/OL]. JCI Insight, 2017, 2(7):e90777. DOI:10.1172/jci.insight.90777.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.ZILLE M, KARUPPAGOUNDER S S, CHEN Y, et al. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis . Stroke. 2017;48(4):1033–1043. doi: 10.1161/STROKEAHA.116.015609. [ZILLE M, KARUPPAGOUNDER S S, CHEN Y, et al. Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of ferroptosis and necroptosis[J]. Stroke, 2017, 48(4):1033-1043. DOI:10.1161/STROKEAHA.116.015609. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.ZHANG Z, WU Y, YUAN S, et al. Glutathione peroxidase 4 participates in secondary brain injury through mediating ferroptosis in a rat model of intracerebral hemorrhage. Brain Res. 2018;1701:112–125. doi: 10.1016/j.brainres.2018.09.012. [ZHANG Z, WU Y, YUAN S, et al. Glutathione peroxidase 4 participates in secondary brain injury through mediating ferroptosis in a rat model of intracerebral hemorrhage[J]. Brain Res, 2018, 1701:112-125. DOI:10.1016/j.brainres.2018.09.012.] [DOI] [PubMed] [Google Scholar]
- 104.CADENAS S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med. 2018;117:76–89. doi: 10.1016/j.freeradbiomed.2018.01.024. [CADENAS S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection[J]. Free Radic Biol Med, 2018, 117:76-89. DOI:10.1016/j.freeradbiomed.2018.01.024.] [DOI] [PubMed] [Google Scholar]
- 105.DAS D K, ENGELMAN R M, LIU X, et al. Oxygen-derived free radicals and hemolysis during open heart surgery. Mol Cell Biochem. 1992;111(1-2):77–86. doi: 10.1007/bf00229577. [DAS D K, ENGELMAN R M, LIU X, et al. Oxygen-derived free radicals and hemolysis during open heart surgery[J]. Mol Cell Biochem, 1992, 111(1-2):77-86. DOI:10.1007/bf00229577.] [DOI] [PubMed] [Google Scholar]
- 106.MEERSON F Z, KAGAN V E, YUP K, et al. The role of lipid peroxidation in pathogenesis of ischemic damage and the antioxidant protection of the heart. Basic Res Cardiol. 1982;77(5):465–485. doi: 10.1007/bf01907940. [MEERSON F Z, KAGAN V E, YUP K, et al. The role of lipid peroxidation in pathogenesis of ischemic damage and the antioxidant protection of the heart[J]. Basic Res Cardiol, 1982, 77(5):465-485. DOI:10.1007/bf01907940.] [DOI] [PubMed] [Google Scholar]
- 107.LINKERMANN A, SKOUTA R, HIMMERKUS N, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A. 2014;111(47):16836–16841. doi: 10.1073/pnas.1415518111. [LINKERMANN A, SKOUTA R, HIMMERKUS N, et al. Synchronized renal tubular cell death involves ferroptosis[J]. Proc Natl Acad Sci U S A, 2014, 111(47):16836-16841. DOI:10.1073/pnas.1415518111.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.FRIEDMANN ANGELI J P, SCHNEIDER M, PRONETH B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180–1191. doi: 10.1038/ncb3064. [FRIEDMANN ANGELI J P, SCHNEIDER M, PRONETH B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice[J]. Nat Cell Biol, 2014, 16(12):1180-1191. DOI:10.1038/ncb3064.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.LU L, WU W, YAN J, et al. Adriamycin-induced autophagic cardiomyocyte death plays a pathogenic role in a rat model of heart failure. Int J Cardiol. 2009;134(1):82–90. doi: 10.1016/j.ijcard.2008.01.043. [LU L, WU W, YAN J, et al. Adriamycin-induced autophagic cardiomyocyte death plays a pathogenic role in a rat model of heart failure[J]. Int J Cardiol, 2009, 134(1):82-90. DOI:10.1016/j.ijcard.2008.01.043.] [DOI] [PubMed] [Google Scholar]
- 110.TAKEMURA G, KANOH M, MINATOGUCHI S, et al. Cardiomyocyte apoptosis in the failing heart——a critical review from definition and classification of cell death. Int J Cardiol. 2013;167(6):2373–2386. doi: 10.1016/j.ijcard.2013.01.163. [TAKEMURA G, KANOH M, MINATOGUCHI S, et al. Cardiomyocyte apoptosis in the failing heart——a critical review from definition and classification of cell death[J]. Int J Cardiol, 2013, 167(6):2373-2386. DOI:10.1016/j.ijcard.2013.01.163.] [DOI] [PubMed] [Google Scholar]
- 111.ZHANG T, ZHANG Y, CUI M, et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22(2):175–182. doi: 10.1038/nm.4017. [ZHANG T, ZHANG Y, CUI M, et al. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis[J]. Nat Med, 2016, 22(2):175-182. DOI:10.1038/nm.4017.] [DOI] [PubMed] [Google Scholar]
- 112.DIETRICH R B, BRADLEY WG J R. Iron accumulation in the basal ganglia following severe ischemic-anoxic insults in children. Radiology. 1988;168(1):203–206. doi: 10.1148/radiology.168.1.3380958. [DIETRICH R B, BRADLEY WG J R. Iron accumulation in the basal ganglia following severe ischemic-anoxic insults in children[J]. Radiology, 1988, 168(1):203-206. DOI:10.1148/radiology.168.1.3380958.] [DOI] [PubMed] [Google Scholar]
- 113.LIPSCOMB D C, GORMAN L G, TRAYSTMAN R J, et al. Low molecular weight iron in cerebral ischemic acidosis in vivo . Stroke. 1998;29(2):487–492. doi: 10.1161/01.str.29.2.487. [LIPSCOMB D C, GORMAN L G, TRAYSTMAN R J, et al. Low molecular weight iron in cerebral ischemic acidosis in vivo[J]. Stroke, 1998, 29(2):487-492; discussion 493. DOI:10.1161/01.str.29.2.487. ] [DOI] [PubMed] [Google Scholar]
- 114.DING H, YAN C Z, SHI H, et al. Hepcidin is involved in iron regulation in the ischemic brain. PLoS One. 2011;6(9):e25324. doi: 10.1371/journal.pone.0025324. [DING H, YAN C Z, SHI H, et al. Hepcidin is involved in iron regulation in the ischemic brain[J/OL]. PLoS One, 2011, 6(9):e25324. DOI:10.1371/journal.pone.0025324.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.PARK U J, LEE Y A, WON S M, et al. Blood-derived iron mediates free radical production and neuronal death in the hippocampal CA1 area following transient forebrain ischemia in rat. Acta Neuropathol. 2011;121(4):459–473. doi: 10.1007/s00401-010-0785-8. [PARK U J, LEE Y A, WON S M, et al. Blood-derived iron mediates free radical production and neuronal death in the hippocampal CA1 area following transient forebrain ischemia in rat[J]. Acta Neuropathol, 2011, 121(4):459-473. DOI:10.1007/s00401-010-0785-8.] [DOI] [PubMed] [Google Scholar]
- 116.PATT A, HORESH I R, BERGER E M, et al. Iron depletion or chelation reduces ischemia/reperfusion-induced edema in gerbil brains. J Pediatr Surg. 1990;25(2):224–227. doi: 10.1016/0022-3468(90)90407-z. [PATT A, HORESH I R, BERGER E M, et al. Iron depletion or chelation reduces ischemia/reperfusion-induced edema in gerbil brains[J]. J Pediatr Surg, 1990, 25(2):224-227; discussion 227-228. DOI:10.1016/0022-3468(90)90407-z.] [DOI] [PubMed] [Google Scholar]
- 117.DAVIS S, HELFAER M A, TRAYSTMAN R J, et al. Parallel antioxidant and antiexcitotoxic therapy improves outcome after incomplete global cerebral ischemia in dogs. Stroke. 1997;28(1):198–204. doi: 10.1161/01.str.28.1.198. [DAVIS S, HELFAER M A, TRAYSTMAN R J, et al. Parallel antioxidant and antiexcitotoxic therapy improves outcome after incomplete global cerebral ischemia in dogs[J]. Stroke, 1997, 28(1):198-204; discussion 204-205. DOI:10.1161/01.str.28.1.198.] [DOI] [PubMed] [Google Scholar]
- 118.PRASS K, RUSCHER K, KARSCH M, et al. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro . J Cereb Blood Flow Metab. 2002;22(5):520–525. doi: 10.1097/00004647-200205000-00003. [PRASS K, RUSCHER K, KARSCH M, et al. Desferrioxamine induces delayed tolerance against cerebral ischemia in vivo and in vitro[J]. J Cereb Blood Flow Metab, 2002, 22(5):520-525. DOI:10.1097/00004647-200205000-00003. ] [DOI] [PubMed] [Google Scholar]
- 119.HANSON L R, ROEYTENBERG A, MARTINEZ P M, et al. Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. J Pharmacol Exp Ther. 2009;330(3):679–686. doi: 10.1124/jpet.108.149807. [HANSON L R, ROEYTENBERG A, MARTINEZ P M, et al. Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke[J]. J Pharmacol Exp Ther, 2009, 330(3):679-686. DOI:10.1124/jpet.108.149807.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.HANDA P, THOMAS S, MORGAN-STEVENSON V, et al. Iron alters macrophage polarization status and leads to steatohepatitis and fibrogenesis. J Leukoc Biol. 2019;105(5):1015–1026. doi: 10.1002/JLB.3A0318-108R. [HANDA P, THOMAS S, MORGAN-STEVENSON V, et al. Iron alters macrophage polarization status and leads to steatohepatitis and fibrogenesis[J]. J Leukoc Biol, 2019, 105(5):1015-1026. DOI:10.1002/JLB.3A0318-108R.] [DOI] [PubMed] [Google Scholar]
- 121.WOODHOO A, IRUARRIZAGA-LEJARRETA M, BERAZA N, et al. Human antigen R contributes to hepatic stellate cell activation and liver fibrosis. Hepatology. 2012;56(5):1870–1882. doi: 10.1002/hep.25828. [WOODHOO A, IRUARRIZAGA-LEJARRETA M, BERAZA N, et al. Human antigen R contributes to hepatic stellate cell activation and liver fibrosis[J]. Hepatology, 2012, 56(5):1870-1882. DOI:10.1002/hep.25828.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.ZHANG Z, YAO Z, WANG L, et al. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14(12):2083–2103. doi: 10.1080/15548627.2018.1503146. [ZHANG Z, YAO Z, WANG L, et al. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells[J]. Autophagy, 2018, 14(12):2083-2103. DOI:10.1080/15548627.2018.1503146.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.ATARASHI M, IZAWA T, KUWAMURA M, et al. The role of iron overload in the progression of nonalcoholic steatohepatitis (NASH) Nihon Yakurigaku Zasshi. 2019;154(2):61–65. doi: 10.1254/fpj.154.61. [ATARASHI M, IZAWA T, KUWAMURA M, et al. The role of iron overload in the progression of nonalcoholic steatohepatitis (NASH)[J]. Nihon Yakurigaku Zasshi, 2019, 154(2):61-65. DOI:10.1254/fpj.154.61.] [DOI] [PubMed] [Google Scholar]
- 124.TSURUSAKI S, TSUCHIYA Y, KOUMURA T, et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 2019;10(6):449. doi: 10.1038/s41419-019-1678-y. [TSURUSAKI S, TSUCHIYA Y, KOUMURA T, et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis[J]. Cell Death Dis, 2019, 10(6):449. DOI:10.1038/s41419-019-1678-y.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.WANG W, GREEN M, CHOI J E, et al. CD8 + T cells regulate tumour ferroptosis during cancer immunotherapy . Nature. 2019;569(7755):270–274. doi: 10.1038/s41586-019-1170-y. [WANG W, GREEN M, CHOI J E, et al. CD8 + T cells regulate tumour ferroptosis during cancer immunotherapy[J]. Nature, 2019, 569(7755):270-274. DOI:10.1038/s41586-019-1170-y. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.方 学贤, 蔡 昭贤, 王 浩, et al. 铁过载及铁死亡在心脏疾病中的研究进展. 科学通报. 2019;64(28-29):2974–2987. doi: 10.1360/TB-2019-0242. [方学贤, 蔡昭贤, 王浩, 等.铁过载及铁死亡在心脏疾病中的研究进展[J].科学通报, 2019, 64(28-29):2974-2987. DOI:10.1360/TB-2019-0242.] [DOI] [Google Scholar]
- 127.ZHENG D W, LEI Q, ZHU J Y, et al. Switching apoptosis to ferroptosis:metal-organic network for high-efficiency anticancer therapy. Nano Lett. 2017;17(1):284–291. doi: 10.1021/acs.nanolett.6b04060. [ZHENG D W, LEI Q, ZHU J Y, et al. Switching apoptosis to ferroptosis:metal-organic network for high-efficiency anticancer therapy[J]. Nano Lett, 2017, 17(1):284-291. DOI:10.1021/acs.nanolett.6b04060.] [DOI] [PubMed] [Google Scholar]
- 128.YOU L, WANG J, LIU T, et al. Targeted brain delivery of rabies virus glycoprotein 29-modified deferoxamine-loaded nanoparticles reverses functional deficits in parkinsonian mice. ACS Nano. 2018;12(5):4123–4139. doi: 10.1021/acsnano.7b08172. [YOU L, WANG J, LIU T, et al. Targeted brain delivery of rabies virus glycoprotein 29-modified deferoxamine-loaded nanoparticles reverses functional deficits in parkinsonian mice[J]. ACS Nano, 2018, 12(5):4123-4139. DOI:10.1021/acsnano.7b08172.] [DOI] [PubMed] [Google Scholar]


