Abstract
目的
探究经典瞬时受体电位通道C(TRPC)相关蛋白在阻塞性睡眠呼吸暂停低通气综合征(OSAHS)大鼠心脏和肾脏损害中的作用。
方法
18只SD雄性大鼠随机分为实验组和对照组,每组9只。实验组大鼠在间歇性低氧舱中,每天暴露于间歇性低氧环境8 h(10:00—18:00)。此后通过实时荧光定量PCR和蛋白质印迹法分别检测大鼠心脏和肾脏组织中TRPC mRNA和相关蛋白的表达。
结果
实验组心脏组织中TRPC3、TRPC4、TRPC5的mRNA表达较对照组升高(均 P < 0.05),而肾脏组织TRPC1、TRPC3、TRPC4、TRPC5、TRPC6、TRPC7的mRNA表达在两组之间差异无统计学意义(均 P>0.05);实验组肾脏组织中TRPC4、TRPC5、TRPC6的mRNA表达低于心脏组织(均 P < 0.05),对照组肾脏组织TRPC7的mRNA表达高于心脏组织( P < 0.05)。实验组心脏组织中的TRPC5蛋白表达较对照组升高( P < 0.05),而肾脏组织TRPC5、TRPC6、TRPC7相关蛋白的表达在两组之间差异无统计学意义(均 P>0.05)。
结论
TRPC5可能参与OSAHS心脏损害的病理生理过程,有望成为治疗OSAHS所致心脏损害的药物新靶点。
Abstract
Objective
To investigate the expression of transient receptor potential canonical channels (TRPCs) in the heart and kidney of rat model of obstructive sleep apnea hypopnea syndrome (OSAHS).
Methods
Eighteen male SD rats were randomly assigned to intermittent hypoxia (IH) group ( n=9) and control group ( n=9). In IH group, rats were placed in a chamber and exposed to intermittent hypoxia for 8h (10AM-6PM) daily. The expression of TRPC-related mRNA and protein in the heart and kidney tissue were detected by qRT-PCR and Western blotting, respectively.
Results
The mRNA expressions of TRPC3/TRPC4/TRPC5 in heart tissues of IH group were increased significantly compared with the control group (all P>0.05); while there were no significant differences in the mRNA expressions of TRPC1/TRPC3/TRPC4/TRPC5/TRPC6/TRPC7 in kidney tissue between two groups (all P < 0.05). The mRNA expressions of TRPC4, TRPC5 and TRPC6 in kidney tissues of IH group were lower than that in heart tissues (all P < 0.05). The mRNA expression of TRPC7 in kidney tissues of control group was significantly higher than that in heart tissues ( P < 0.05). The expression of TRPC5 protein in heart tissues of IH group was significantly higher than that in the control group ( P < 0.05); while there was no significant differences in the expression of TRPC5/TRPC6/TRPC7 protein in kidney tissue between two groups (all P>0.05).
Conclusion
The IH rat model shows that TRPC5 channel is likely to be involved in the OSAHS induced pathophysiological changes in the myocardium and may become a target to prevent OSAHS related cardiac damage.
Keywords: Transient receptor potential channels/metabolism; Proteins; Sleep apnea syndromes/physiopathology; Hypoxia/pathology; Heart/metabolism; Kidney/metabolism; Disease models, animal; Case-control studies
阻塞性睡眠呼吸暂停低通气综合征(obstructive sleep apnea hypopnea syndrome, OSAHS)是一种被严重低估的全身性疾病。OSAHS患者常合并心血管、肾脏等器官功能损害,如左心室重构、血管内皮损伤、冠心病、心力衰竭、心律失常、微量白蛋白尿、肾功能损害等,严重影响OSAHS患者的生活质量及预后 [ 1- 3] 。间歇性低氧/复氧(intermittent hypoxia,IH)是OSAHS最根本的机制,其中钙超载扮演了重要角色。经典瞬时受体电位通道C(transient receptor potential canonical channel, TRPC)是一种非电压依赖性的离子通道蛋白,主要参与钙离子的调控 [ 4- 5] 。目前研究发现TRPC与心血管、肾脏等疾病的发生、发展密切相关 [ 6- 8] ,但TRPC是否参与OSAHS病理生理过程,目前研究甚少。本研究通过建立IH大鼠模型,利用实时荧光定量PCR(quantitative real time polymerase chain reaction, qRT-PCR)及蛋白质印迹法检测大鼠心脏及肾脏组织中相关TRPC mRNA及蛋白的表达,以期探讨TRPC与OSAHS相关心脏和肾脏损害的关系。
1 材料与方法
1.1 试剂与仪器
Anti-ACTB rabbit polyclonal antibody(D110001)为生工生物工程(上海)股份有限公司产品;Trans2K DNA标记(BM101)、TransZol Up(ET111)、TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix(AT311)、蛋白预染标记(DM131)、Easy Ⅱ Protein Quantitative Kit(DQ111-01)为北京全式金生物技术有限公司产品;QuantiNava SYBR Green Kit(208054)为凯捷公司产品;RIPA裂解液(AR0105)、蛋白酶抑制剂(AR1178)为武汉博士德生物工程有限公司产品;山羊抗小鼠IgG H&L(ab205719)、山羊抗兔IgG H&L(ab205718)、抗TRP 7抗体[N64A/36](ab93618)为英国Abcam公司产品;PVDF Transfer Membrane 0.45 μm(IPVH00010)为美国Millipore公司产品;SuperSignal West Prico Chemiluminescent Substrate(34080)为美国Thermo Scientific产品;TRPC6(D3G1Q)Rabbit mAb(16716S)为美国CST公司产品;TRPC5抗体1C8(sc-293259)为美国Santa cruz公司产品。
核酸蛋白定量仪(K5500)为北京凯奥科技发展有限公司产品;凝胶成像系统(2500)为上海天能科技有限公司产品;PCR仪(MyCycler Thermal Cycler)、蛋白转膜仪(Mini-PROTEAN Tetra system)为美国Bio-Rad公司产品;ABI QuantStudio TM 6 Flex Real-Time PCR System为美国ABI公司产品;酶标仪(xMark TM)为Bio-Rad(中国)公司产品;化学发光成像仪系统(ChemiScope 3000)为上海勤翔科学仪器有限公司产品。
1.2 动物分组及处理
Sprague Dawley(SD)大鼠由新疆医科大学动物实验中心提供。本实验所有动物研究均通过新疆医科大学动物伦理委员会审批。
选取18只15~17周龄的雄性SD大鼠,常规适应性饲养1周后用抽签法将大鼠随机分成实验组和对照组,各9只。将实验组大鼠置于自制间歇性低氧舱,实验期间每天10:00—18:00向舱内循环充入氮气和排出混合空气,每次循环8 min,即4 min充入氮气,维持舱内氧气最低浓度达8.5%,维持时间1 min,随后2 min排出舱内混合空气,使舱内氧浓度恢复至21%维持1 min,至下一循环。每天持续8 h,4周后检测IH模型是否建立成功。建模成功标准:缺氧最低点时大鼠动脉血氧分压为20.9~29.7 mmHg(1 mmHg=0.133 kPa),血氧饱和度为31.2%~58.3%,当恢复至正常氧浓度(21%)时血氧分压为71.6~106.4 mmHg,血氧饱和度为92.2%~97.4%,符合人类重度OSAHS的血氧饱和度诊断标准动脉血氧饱和度(SaO 2)低于80%。建模期间,实验组和对照组分别有1只大鼠于饲养第8天、第12天死亡。其余大鼠建模成功后被快速处死取出心脏、肾脏,提取组织迅速放入液氮中,以用于后期实验。
1.3 实时荧光定量PCR测定大鼠心脏和肾脏组织中TRPC mRNA表达
将大鼠心脏及肾脏组织(对照组3例,实验组5例)在液氮冻存下研磨成粉,取25~50 mg放置于加入有1 mL TRLZOL的离心管中,混匀。室温放置15 min以上,提取总RNA。按照RT-PCR试剂盒说明书将2 μL RNA逆转录为cDNA,取1 μg进行PCR。大鼠TRPC mRNA及内参照的大鼠管家基因 GAPDH的PCR引物序列见 表 1。
表1 荧光定量PCR引物
Table 1 Primers used for the amplification of TRPC genes
|
引物名称 |
序列(5′→3′) |
引物大小(bp) |
|
TRPC1 |
正向:CTGCTTATCTTCATGTGCGGTC |
138 |
|
反向:GAAGCTGTGGTAGGCTCTGT |
||
|
TRPC3 |
正向:ACGCAGTACGGCAACATC |
209 |
|
反向:CGCACATAGCCTTTGCTGAT |
||
|
TRPC4 |
正向:AAGGATTAGCTTCACGGGGTG |
198 |
|
反向:CCTCCTCCTGGGCGTGTTTC |
||
|
TRPC5 |
正向:CCATACAGAGACCGCATCCC |
283 |
|
反向:CCTTGCGGATGGCATAGAGT |
||
|
TRPC6 |
正向:AAACAGACTGACTCACCGGC |
238 |
|
反向:CGCCAACTGTAGGGCATTCT |
||
|
TRPC7 |
正向:TTGGGGAGCAACACCTTCAA |
97 |
|
反向:TGAACATGTAGGCAGGACCC |
||
|
GAPDH |
正向:CAGGGCTGCCTTCTCTTGTG |
172 |
|
反向:GATGGTGATGGGTTTCCCGT |
TRPC:瞬时受体电位通道C.
反应参数:95 ℃预变性2 min;95 ℃变性5 s、60 ℃退火30 s、60 ℃延伸30 s,进行40个循环;循环结束后取PCR产物10 μL 1.8%琼脂糖凝胶电泳,150 V,10 min,电泳完毕后使用凝胶成像仪观察结果。采用2 -ΔΔCt计算公式计算Ct值 [ 9- 11] 。
1.4 蛋白质印迹法测定大鼠心脏和肾脏组织中TRPC蛋白表达
将大鼠心脏及肾脏组织(对照组3例,实验组5例)在液氮下研磨,均质化后加入400 μL RIPA裂解液,充分混匀,4 ℃放置60 min后,24 149× g,4 ℃,离心15 min收集上清液。获得蛋白质提取物,并用Pierce BCA蛋白质测定法定量蛋白质浓度。将等量的蛋白质与适量5×SDS-PAGE上样缓冲液(含β-巯基乙醇)于100 ℃沸水加热处理5 min,使蛋白充分变性,24 149× g离心5 min,取上清液加载到8%、12%分离胶与5%浓缩胶中,电泳结束后冰浴下恒流(300 mA)湿法转膜2 h,在质量分数为5%的脱脂奶粉室温封闭1 h后,与相应一抗4 ℃共孵育过夜,抗体稀释比例分别为:TRPC6(D3G1Q) Rabbit mAb(1:500);抗TRP 7抗体[N64A/36](1:500);TRPC5抗体(1C8)(1:500)次日TBS-T洗液(酸碱度为7.6)洗涤后,与HRP标记的二抗共孵育,用化学发光仪检测、拍照,并利用AlphaEaseFC 4.0灰度分析软件定量分析,以β-actin的灰度值标化蛋白表达。
1.5 统计学方法
运用SPSS 19.0软件进行统计学分析。所有数据以均数±标准差( x ± s)描述,采用独立样本 t检验。 P<0.05为差异有统计学意义。
2 结果
2.1 模型大鼠心脏和肾脏组织中TRPC mRNA表达变化
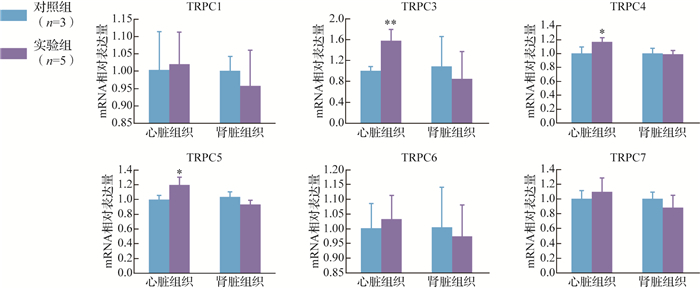
在心脏组织中,实验组TRPC3、TRPC4、TRPC5的mRNA表达均较对照组增加( P<0.05或 P<0.01);在肾脏组织中,实验组TRPC1、TRPC3、TRPC4、TRPC5、TRPC6、TRPC7 mRNA的表达与对照组差异均无统计学意义(均 P>0.05),见 图 1。
图1.
实验组与对照组大鼠瞬时受体电位通道C(TRPC) mRNA水平比较
与对照组比较, * P<0.05, ** P<0.01.
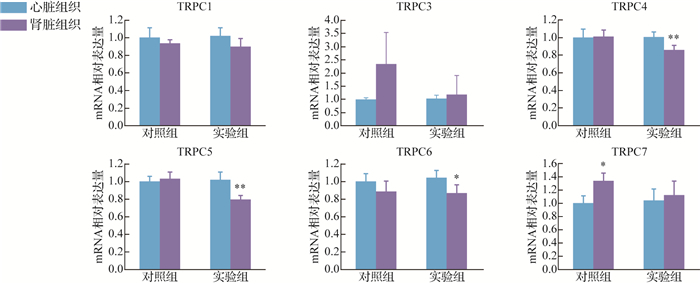
对照组肾脏组织中TRPC7 mRNA表达较心脏组织增加( P<0.05),而TRPC1、TRPC3、TRPC4、TRPC5、TRPC6 mRNA在心脏和肾脏组织中的表达差异均无统计学意义(均 P>0.05)。实验组肾脏组织中TRPC4、TRPC5、TRPC6 mRNA表达较其在心脏组织中减少( P<0.05或 P<0.01),而TRPC1、TRPC3、TRPC4、TRPC7 mRNA在心脏和肾脏组织中的表达差异均无统计学意义(均 P>0.05),见 图 2。
图2.
大鼠心脏组织与肾脏组织中瞬时受体电位通道C(TRPC) mRNA水平比较
与心脏组织比较, * P<0.05, ** P<0.01.对照组: n=3;实验组: n=5.
结果提示,TRPC3、TRPC4、TRPC5可能参与IH环境下的心脏组织病理生理改变。
2.2 模型大鼠心脏和肾脏组织中TRPC蛋白表达变化
预实验结果提示,TRPC1、TRPC3、TRPC4的转印膜上没有目的蛋白条带,提示组织中不含此蛋白或者含量过低,而TRPC5、TRPC6、TRPC7转印膜上可见目的蛋白条带( 图 3)。实验组心脏组织中TRPC5蛋白表达较对照组增加( P<0.05),而TRPC6、TRPC7蛋白表达两组间差异无统计学意义(均 P>0.05);在肾脏组织中,TRPC5、TRPC6、TRPC7蛋白表达在两组间差异均无统计学意义(均 P>0.05),见 表 2。结果提示,在IH环境中,TRPC5蛋白可能参与心脏损害的病理生理过程。
图3.
瞬时受体电位通道C(TRPC)蛋白在大鼠心脏和肾脏组织中表达电泳图
1:实验组;2:对照组.
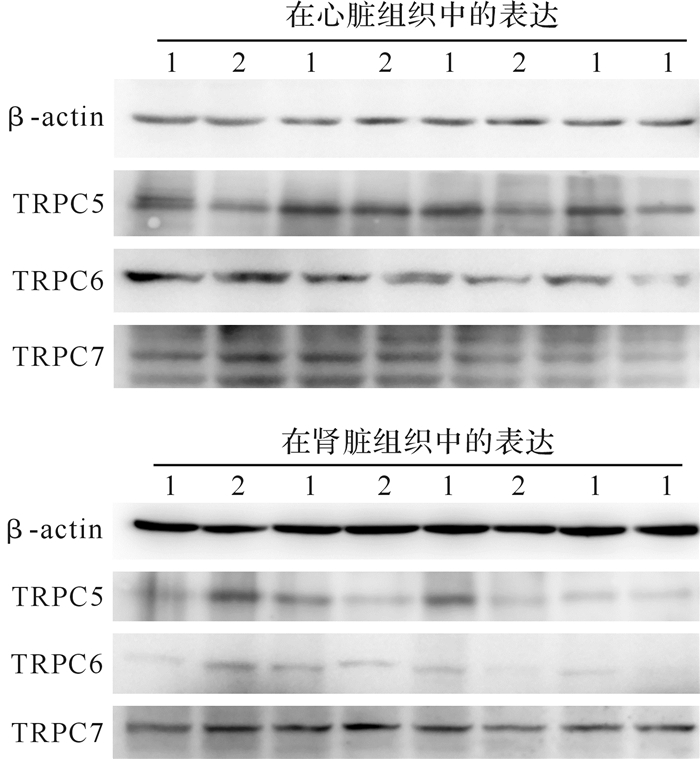
表2 实验组和对照组大鼠心脏和肾脏组织中瞬时受体电位通道C(TRPC)各蛋白表达水平比较
Table 2 Transient receptor potential canonical channel (TRPC) protein levels in heart and kidney tissues of intermittent hypoxia rats and control rats ( x ± s)
|
组别 |
n |
TRPC5 |
TRPC6 |
TRPC7 |
|||||
|
心脏组织 |
肾脏组织 |
心脏组织 |
肾脏组织 |
心脏组织 |
肾脏组织 |
||||
|
对照组 |
3 |
6.822±1.909 |
0.164±0.192 |
1.460±0.537 |
0.018±0.011 |
6.030±0.675 |
0.136±0.023 |
||
|
实验组 |
5 |
11.448±2.601 * |
0.135±0.112 |
1.530±0.767 |
0.014±0.005 |
5.028±1.836 |
0.117±0.018 |
与对照组比较, * P<0.05.
3 讨论
本研究建立的IH动物模型可有效地模拟OSAHS,操作简便,重复性好,目前已运用于OSAHS相关的研究中 [ 12- 13] 。本研究采用的间歇性低氧舱可避免由舱内气压骤变产生的安全性隐患,并能克服采用面罩低氧浓度呼吸时呼气阻力较高、容易出现低氧生理反应失真的问题,可自然地模拟间歇性低氧环境。大鼠在该环境中饲养4周,缺氧最低点时大鼠动脉血氧分压为20.9~29.7 mmHg,血氧饱和度为31.2%~58.3%,当恢复至正常氧浓度(21%)时血氧分压为71.6~106.4 mmHg,血氧饱和度为92.2%~97.4%,符合人类重度OSAHS的血氧饱和度诊断标准SaO 2 < 80%,说明该模型能模拟OSAHS的血氧特点。
OSAHS的主要病理生理改变是夜间睡眠过程中反复发生间歇性低氧,二氧化碳潴留,睡眠片段,正常睡眠结构遭到破坏,从而引发或加剧心、肾等器官功能损害。OSAHS对心、肾损害的机制涉及间歇性低氧、交感神经兴奋、氧化应激、炎症反应等 [ 14- 16] 。但具体机制尚未有统一定论。OSAHS的基本机制IH类似缺血/再灌注损伤,由于细胞缺氧导致细胞膜结构的破坏,细胞外及细胞内钙离子涌入细胞质;再灌注后,细胞外的钙离子增多以及氧自由基破坏膜结构等原因,造成细胞内钙急剧增多,导致钙超载的发生 [ 17] 。细胞内钙离子的异常反过来又导致线粒体损害,致使能量代谢障碍,加重钙超载。
钙离子是人体生理活动中不可或缺的离子,也是各个信号转导通路的参与者,钙离子失衡则会引起细胞一系列病理生理变化,如氧化应激、炎症反应等 [ 18] ,而细胞内钙超载则会导致细胞凋亡。TRPC是广泛存在于哺乳动物细胞中的一种非电压依赖性钙离子通道,TRPC分为7种亚型(TRPC1~7), 其中TRPC2在人类组织不表达 [ 19] 。近年来研究发现,TRPC的激活与高血压 [ 20] 、心室重构 [ 21] 、扩张性心肌病及动脉粥样硬化 [ 7, 22] 等心血管疾病发生、发展关系密切 [ 6] , TRPC5、TRPC6与慢性肾脏疾病有关 [ 8, 23- 24] 。
TRPC在正常成年心肌细胞中表达较低,但在心肌病理状态下表达增加 [ 25] 。目前TRPC通道被认为是引起病理性心肌肥大、心肌重构的钙离子依赖信号通路的发起者 [ 26] 。通过对心脏组织比较发现, 实验组TRPC3、TRPC4、TRPC5相关mRNA的表达明显高于对照组,提示TRPC3、TRPC4、TRPC5可能参与IH环境下的心脏组织病理生理改变。但心脏组织中高表达的mRNA是否会翻译成相关蛋白质而发挥生物学效应?为此进一步检测心脏组织中TRPC相关蛋白的表达水平,结果发现实验组中的TRPC5蛋白表达较对照组增加,提示在心脏组织中TRPC5可能在IH病理过程中发挥作用。近年来,国内外对TRPC5通道的研究围绕肾脏疾病、肿瘤、神经疾病等方面,对于心血管疾病的研究多与动脉粥样硬化相关 [ 23, 27- 29] 。本文资料提示, TRPC5与OSAHS心脏损害相关,这将为下一步研究打下基础。
大鼠正常肾脏组织中,TRPC1在肾小球入球及出球小动脉均有表达,并介导了血管紧张素的收缩作用,肾小球前阻力血管TRPC3、TRPC6的mRNA及蛋白均为高表达水平,其中TRPC3的表达水平更高,而TRPC7的mRNA在大鼠肾脏血管中不表达,但在肾脏其他组织中低表达 [ 19, 30- 31] 。本研究对照组肾脏组织的TRPC7 mRNA表达较心脏组织增多,这提示TRPC7参与了肾脏正常生理过程,为后期关于TRPC7参与肾脏正常生理的研究提供了科学依据。
OSAHS患者存在肾功能损害,且肾功能损害程度随着OSAHS程度的加重而加重 [ 32] 。本研究在肾脏组织中均未发现TRPC mRNA及相关蛋白表达,这表明在IH环境下,肾脏组织中无相关TRPC mRNA及蛋白发挥生物学效应,TRPC可能不参与OSAHS所致的病理生理损害,或TRPC不是最主要的参与因素。目前TRPC通道参与OSAHS相关肾脏损害的机制尚未见报道。
综上所述,在肾脏组织中,TRPC可能不参与OSAHS所致的病理生理损害,或TRPC不是最主要的参与因素。在心脏组织中,TRPC5通道可能参与OSAHS所致IH引起心脏损害的病理生理过程,且TRPC5有望成为治疗OSAHS所致心脏损害的药物新靶点。TRPC5通过何种机制影响并参与OSAHS心脏损害的病理生理过程,是下一步研究的方向。
Funding Statement
国家自然科学基金(82060058)
References
- 1.MORAND J, ARNAUD C, PEPIN J L, et al. Chronic intermittent hypoxia promotes myocardial ischemia-related ventricular arrhythmias and sudden cardiac death. Sci Rep. 2018;8(1):2997. doi: 10.1038/s41598-018-21064-y. [MORAND J, ARNAUD C, PEPIN J L, et al. Chronic intermittent hypoxia promotes myocardial ischemia-related ventricular arrhythmias and sudden cardiac death[J]. Sci Rep, 2018, 8(1):2997. DOI:10.1038/s41598-018-21064-y.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MA L, ZHANG J, LIU Y. Roles and mechanisms of obstructive sleep apnea-hypopnea syndrome and chronic intermittent hypoxia in atherosclerosis:evidence and prospective. Oxid Med Cell Longev. 2016;2016:8215082. doi: 10.1155/2016/8215082. [MA L, ZHANG J, LIU Y. Roles and mechanisms of obstructive sleep apnea-hypopnea syndrome and chronic intermittent hypoxia in atherosclerosis:evidence and prospective[J]. Oxid Med Cell Longev, 2016, 2016:8215082. DOI:10.1155/2016/8215082.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LEUNG R S. Sleep-disordered breathing:autonomic mechanisms and arrhythmias. Prog Cardiovasc Dis. 2009;51(4):324–338. doi: 10.1016/j.pcad.2008.06.002. [LEUNG R S. Sleep-disordered breathing:autonomic mechanisms and arrhythmias[J]. Prog Cardiovasc Dis, 2009, 51(4):324-338. DOI:10.1016/j.pcad.2008.06.002.] [DOI] [PubMed] [Google Scholar]
- 4.NILIUS B, FLOCKERZI V. Mammalian transient receptor potential (TRP) cation channels. Preface. http://europepmc.org/abstract/MED/25296415. Handb Exp Pharmacol. 2014;223:5–6. [NILIUS B, FLOCKERZI V. Mammalian transient receptor potential (TRP) cation channels. Preface[J]. Handb Exp Pharmacol, 2014, 223:5-6.] [PubMed] [Google Scholar]
- 5.CIOFFI D L. Redox regulation of endothelial canonical transient receptor potential channels. Antioxid Redox Signal. 2011;15(6):1567–1582. doi: 10.1089/ars.2010.3740. [CIOFFI D L. Redox regulation of endothelial canonical transient receptor potential channels[J]. Antioxid Redox Signal, 2011, 15(6):1567-1582. DOI:10.1089/ars.2010.3740.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.HOF T, CHAIGNE S, RÉCALDE A, et al. Transient receptor potential channels in cardiac health and disease. Nat Rev Cardiol. 2019;16(6):344–360. doi: 10.1038/s41569-018-0145-2. [HOF T, CHAIGNE S, RÉCALDE A, et al. Transient receptor potential channels in cardiac health and disease[J]. Nat Rev Cardiol, 2019, 16(6):344-360. DOI:10.1038/s41569-018-0145-2.] [DOI] [PubMed] [Google Scholar]
- 7.KONISHI T, KASHIWAGI Y, FUNAYAMA N, et al. Obstructive sleep apnea is associated with increased coronary plaque instability:an optical frequency domain imaging study. Heart Vessels. 2019;34(8):1266–1279. doi: 10.1007/s00380-019-01363-8. [KONISHI T, KASHIWAGI Y, FUNAYAMA N, et al. Obstructive sleep apnea is associated with increased coronary plaque instability:an optical frequency domain imaging study[J]. Heart Vessels, 2019, 34(8):1266-1279. DOI:10.1007/s00380-019-01363-8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DRYER S E, ROSHANRAVAN H, KIM E Y. TRPC channels:Regulation, dysregulation and contributions to chronic kidney disease. Biochim Biophys Acta Mol Basis Dis. 2019;1865(6):1041–1066. doi: 10.1016/j.bbadis.2019.04.001. [DRYER S E, ROSHANRAVAN H, KIM E Y. TRPC channels:Regulation, dysregulation and contributions to chronic kidney disease[J]. Biochim Biophys Acta Mol Basis Dis, 2019, 1865(6):1041-1066. DOI:10.1016/j.bbadis.2019.04.001.] [DOI] [PubMed] [Google Scholar]
- 9.FROSTH S, KÖNIG U, NYMAN A K, et al. Sample pooling for real-time PCR detection and virulence determination of the footrot pathogen Dichelobacter nodosus. Vet Res Commun. 2017;41(3):189–193. doi: 10.1007/s11259-017-9686-9. [FROSTH S, KÖNIG U, NYMAN A K, et al. Sample pooling for real-time PCR detection and virulence determination of the footrot pathogen Dichelobacter nodosus[J]. Vet Res Commun, 2017, 41(3):189-193. DOI:10.1007/s11259-017-9686-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RODRIGUES A, MAGALHÃES R D, ROMCY K, et al. A new whole mitochondrial genome qPCR (WMG-qPCR) with SYBR Green ® to identify phlebotomine sand fly blood meals . Vet Parasitol. 2017;238:17–23. doi: 10.1016/j.vetpar.2017.03.007. [RODRIGUES A, MAGALHÃES R D, ROMCY K, et al. A new whole mitochondrial genome qPCR (WMG-qPCR) with SYBR Green ® to identify phlebotomine sand fly blood meals[J]. Vet Parasitol, 2017, 238:17-23. DOI:10.1016/j.vetpar.2017.03.007. ] [DOI] [PubMed] [Google Scholar]
- 11.CAMPBELL S J, NERY S V, WARDELL R, et al. Water, sanitation and hygiene (WASH) and environmental risk factors for soil-transmitted helminth intensity of infection in Timor-Leste, using real time PCR[J/OL]. PLoS Negl Trop Dis, 2017, 11(3): e0005393. DOI: .10.1371/journal.pntd.0005393. [DOI] [PMC free article] [PubMed]
- 12.李 秀翠, 蔡 晓红, 温 正旺, et al. 间歇性低氧动物模型的建立及验证. 医学研究杂志. 2012:41. doi: 10.3969/j.issn.1673-548X.2012.07.019. [李秀翠, 蔡晓红, 温正旺, 等.间歇性低氧动物模型的建立及验证[J].医学研究杂志, 2012:41(7):57-61. DOI:10.3969/j.issn.1673-548X.2012.07.019.] [DOI] [Google Scholar]
- 13.FLETCHER E C, LESSKE J, QIAN W, et al. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19(6 Pt 1):555–561. doi: 10.1161/01.hyp.19.6.555. [FLETCHER E C, LESSKE J, QIAN W, et al. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats[J]. Hypertension, 1992, 19(6 Pt 1):555-561. DOI:10.1161/01.hyp.19.6.555.] [DOI] [PubMed] [Google Scholar]
- 14.MBATA G, CHUKWUKA J. Obstructive sleep apnea hypopnea syndrome. Ann Med Health Sci Res. 2012;2(1):74–77. doi: 10.4103/2141-9248.96943. [MBATA G, CHUKWUKA J. Obstructive sleep apnea hypopnea syndrome[J]. Ann Med Health Sci Res, 2012, 2(1):74-77. DOI:10.4103/2141-9248.96943.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LAVIE L. Obstructive sleep apnoea syndrome——an oxidative stress disorder. Sleep Med Rev. 2003;7(1):35–51. doi: 10.1053/smrv.2002.0261. [LAVIE L. Obstructive sleep apnoea syndrome——an oxidative stress disorder[J]. Sleep Med Rev, 2003, 7(1):35-51. DOI:10.1053/smrv.2002.0261.] [DOI] [PubMed] [Google Scholar]
- 16.LAVIE L, LAVIE P. Molecular mechanisms of cardiovascular disease in OSAHS:the oxidative stress link. Eur Respir J. 2009;33(6):1467–1484. doi: 10.1183/09031936.00086608. [LAVIE L, LAVIE P. Molecular mechanisms of cardiovascular disease in OSAHS:the oxidative stress link[J]. Eur Respir J, 2009, 33(6):1467-1484. DOI:10.1183/09031936.00086608.] [DOI] [PubMed] [Google Scholar]
- 17.BOMPOTIS G C, DEFTEREOS S, ANGELIDIS C, et al. Altered calcium handling in reperfusion injury. Med Chem. 2016;12(2):114–130. doi: 10.2174/1573406411666150928112420. [BOMPOTIS G C, DEFTEREOS S, ANGELIDIS C, et al. Altered calcium handling in reperfusion injury[J]. Med Chem, 2016, 12(2):114-130. DOI:10.2174/1573406411666150928112420.] [DOI] [PubMed] [Google Scholar]
- 18.GRANGER D N, RUTILI G, MCCORD J M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981;81(1):22–29. doi: 10.1016/0016-5085(81)90648-X. [GRANGER D N, RUTILI G, MCCORD J M. Superoxide radicals in feline intestinal ischemia[J]. Gastroenterology, 1981, 81(1):22-29.] [DOI] [PubMed] [Google Scholar]
- 19.NILIUS B, OWSIANIK G. The transient receptor potential family of ion channels. Genome Biol. 2011;12(3):218. doi: 10.1186/gb-2011-12-3-218. [NILIUS B, OWSIANIK G. The transient receptor potential family of ion channels[J]. Genome Biol, 2011, 12(3):218. DOI:10.1186/gb-2011-12-3-218.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LIU D, YANG D, HE H, et al. Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension. 2009;53(1):70–76. doi: 10.1161/HYPERTENSIONAHA.108.116947. [LIU D, YANG D, HE H, et al. Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats[J]. Hypertension, 2009, 53(1):70-76. DOI:10.1161/HYPERTENSIONAHA.108.116947.] [DOI] [PubMed] [Google Scholar]
- 21.ONOHARA N, NISHIDA M, INOUE R, et al. TRPC3 and TRPC6 are essential for angiotensin Ⅱ-induced cardiac hypertrophy. EMBO J. 2006;25(22):5305–5316. doi: 10.1038/sj.emboj.7601417. [ONOHARA N, NISHIDA M, INOUE R, et al. TRPC3 and TRPC6 are essential for angiotensin Ⅱ-induced cardiac hypertrophy[J]. EMBO J, 2006, 25(22):5305-5316. DOI:10.1038/sj.emboj.7601417.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.HARADA M, LUO X, QI X Y, et al. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation. 2012;126(17):2051–2064. doi: 10.1161/CIRCULATIONAHA.112.121830. [HARADA M, LUO X, QI X Y, et al. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation[J]. Circulation, 2012, 126(17):2051-2064. DOI:10.1161/CIRCULATIONAHA.112.121830.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SHARMA S H, PABLO J L, MONTESINOS M S, et al. Design, synthesis and characterization of novel N-heterocyclic-1-benzyl-1H-benzo[D]imidazole-2-amines as selective TRPC5 inhibitors leading to the identification of the selective compound, AC1903[J]. Bioorg Med Chem Lett, 2019, 29(2): 155-159. DOI: .10.1016/j.bmcl.2018.12.007. [DOI] [PMC free article] [PubMed]
- 24.STARUSCHENKO A. TRPC6 in diabetic kidney disease:good guy or bad guy? Kidney Int. 2019;95(2):256–258. doi: 10.1016/j.kint.2018.10.027. [STARUSCHENKO A. TRPC6 in diabetic kidney disease:good guy or bad guy?[J]. Kidney Int, 2019, 95(2):256-258. DOI:10.1016/j.kint.2018.10.027.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MAKAREWICH C A, ZHANG H, DAVIS J, et al. Transient receptor potential channels contribute to pathological structural and functional remodeling after myocardial infarction. Circ Res. 2014;115(6):567–580. doi: 10.1161/CIRCRESAHA.115.303831. [MAKAREWICH C A, ZHANG H, DAVIS J, et al. Transient receptor potential channels contribute to pathological structural and functional remodeling after myocardial infarction[J]. Circ Res, 2014, 115(6):567-580. DOI:10.1161/CIRCRESAHA.115.303831.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.SABOURIN J, BARTOLI F, ANTIGNY F, et al. Transient receptor potential canonical (TRPC)/orai1-dependent store-operated Ca 2+ channels:NEW TARGETS OF ALDOSTERONE IN CARDIOMYOCYTES . J Biol Chem. 2016;291(25):13394–13409. doi: 10.1074/jbc.M115.693911. [SABOURIN J, BARTOLI F, ANTIGNY F, et al. Transient receptor potential canonical (TRPC)/orai1-dependent store-operated Ca 2+ channels:NEW TARGETS OF ALDOSTERONE IN CARDIOMYOCYTES[J]. J Biol Chem, 2016, 291(25):13394-13409. DOI:10.1074/jbc.M115.693911. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ZHANG P, LIU X, LI H, et al. TRPC5-induced autophagy promotes drug resistance in breast carcinoma via CaMKKβ/AMPKα/mTOR pathway. Scientific Reports. 2017;7(1):3158. doi: 10.1038/s41598-017-03230-w. [ZHANG P, LIU X, LI H et al. TRPC5-induced autophagy promotes drug resistance in breast carcinoma via CaMKKβ/AMPKα/mTOR pathway[J]. Scientific Reports, 2017, 7(1):3158.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.HONG C, SEO H, KWAK M, et al. Increased TRPC5 glutathionylation contributes to striatal neuron loss in Huntington's disease. Brain. 2015;138(Pt 10):3030–3047. doi: 10.1093/brain/awv188. [HONG C, SEO H, KWAK M, et al. Increased TRPC5 glutathionylation contributes to striatal neuron loss in Huntington's disease[J]. Brain, 2015, 138(Pt 10):3030-3047. DOI:10.1093/brain/awv188.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.季 一楠, 郭 瑞威. 经典瞬时受体电位C亚族5与动脉粥样硬化发生发展的关系. 心脑血管病防治. 2019;19(1):82–83,95. doi: 10.3969/j.issn.1009-816X.2019.01.012. [季一楠, 郭瑞威.经典瞬时受体电位C亚族5与动脉粥样硬化发生发展的关系[J].心脑血管病防治, 2019, 19(1):82-83, 95. DOI:10.3969/j.issn.1009-816X.2019.01.012.] [DOI] [Google Scholar]
- 30.TAKENAKA T, SUZUKI H, OKADA H, et al. Transient receptor potential channels in rat renal microcirculation:actions of angiotensin Ⅱ. Kidney Int. 2002;62(2):558–565. doi: 10.1046/j.1523-1755.2002.00484.x. [TAKENAKA T, SUZUKI H, OKADA H, et al. Transient receptor potential channels in rat renal microcirculation:actions of angiotensin Ⅱ[J]. Kidney Int, 2002, 62(2):558-565. DOI:10.1046/j.1523-1755.2002.00484.x.] [DOI] [PubMed] [Google Scholar]
- 31.FACEMIRE C S, MOHLER P J, ARENDSHORST W J. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation. Am J Physiol Renal Physiol. 2004;286(3):F546–F551. doi: 10.1152/ajprenal.00338.2003. [FACEMIRE C S, MOHLER P J, ARENDSHORST W J. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation[J]. Am J Physiol Renal Physiol, 2004, 286(3):F546-F551. DOI:10.1152/ajprenal.00338.2003.] [DOI] [PubMed] [Google Scholar]
- 32.郭世放.阻塞性睡眠呼吸暂停低通气综合征肾功能损害的危险因素研究[D].郑州: 郑州大学, 2017.


