Abstract
Sporadic clusters of health care-associated COVID-19 infection occurred in a highly vaccinated health care-workers and patient population, over a 3-month period during ongoing community transmission of the B.1.617.2 variant. Enhanced infection-prevention measures and robust surveillance systems, including routine-rostered-testing of all inpatients and staff and usage of N95-respirators in all clinical areas, were insufficient in achieving zero health care-associated transmission. The unvaccinated and immunocompromised remain at-risk and should be prioritized for enhanced surveillance.
Key words: SARS-CoV-2, Hospital, Antigen test, Outbreak, Infection control, Nosocomial
Infection-prevention measures in health care settings may mitigate transmission of severe-acute-respiratory-syndrome-coronavirus-2 (SARS-CoV-2), resulting in lower secondary-attack-rates compared to community settings.1 However, novel variants with higher transmissibility, including the SARS-CoV-2 delta variant (B.1.617.2), challenge containment efforts. Despite usage of appropriate personal-protective-equipment (PPE), healthcare-associated outbreaks of B.1.617.2 have occurred.2 , 3
In Singapore, hospitals instituted extensive infection-prevention measures early-on.3 , 4 However, community outbreaks of B.1.617.2 increased potential spillover into health care-facilities. A large nosocomial cluster arising from B.1.617.2 was reported in end-April 2021,3 providing impetus for routine-rostered-testing (RRT) via polymerase-chain-reaction (PCR) testing for inpatients and health care-workers (HCWs).5 We evaluated health care-associated transmission of SARS-CoV-2 in a large health care-campus during an ongoing community surge of B.1.617.2.
Methodology
Institutional setting and study period
Our health care-campus handles COVID-19 and non-COVID-19-admissions, hosting a 1,785-bed acute-hospital, a 545-bed community-hospital, and 4 specialist's centers. Patients were admitted in cohorted general-wards (5-12 beds/bay, ∼1.5 meters apart). Almost 13,000 HCWs work on-campus. The study-period lasted from 27th June 2021 to 29th September 2021.
Admission triage strategies
Patients with epidemiological risk (close-contact) were admitted directly to the isolation-ward (negative-pressure isolation-rooms with antechamber), whereas patients without epidemiological risk presenting with clinical-syndromes compatible with COVID-19 were isolated in modified cohort cubicles with reduced bed-density in the “respiratory-surveillance-ward (RSW)” while awaiting PCR.4 From 27th June 2021, all inpatient admissions were additionally screened using the BD-Veritor-SARS-CoV-2-antigen rapid-test-kit.6 Patients with positive antigen-tests were isolated till PCR confirmation.
Enhanced surveillance via RRT of staff and inpatients
From April 2021, RRT via PCR-testing of respiratory samples for SARS-CoV-2 was conducted fortnightly for asymptomatic vaccinated HCWs and weekly for non-vaccinated HCWs.5 Symptomatic HCWs received free testing at our staff clinic. From 19th June 2021, universal inpatient screening was instituted. Asymptomatic patients were tested on admission and weekly subsequently; testing could be performed more frequently at clinician-discretion if patients turned symptomatic.5
Enhanced campus-wide infection-control measures
All HCWs in general-ward donned N95-respirators as a mandatory-minimum. HCWs in isolation-ward/RSW donned N95-respirators and disposable gloves, gowns and faceshields. COVID-19 vaccination uptake amongst HCWs was high, with 89.6% fully-vaccinated by end-April 2021. Similarly, 75.0% of inpatients were fully-vaccinated. Pre-pandemic inpatient-areas were cleaned with 1:1,000 hypochlorite-based disinfectant 3x-a-day. Regular cleaning and hand-hygiene-compliance were reinforced.4 UV-C disinfection was also utilized for terminal-cleaning in isolation-areas. All visitors donned masks and if visiting for ≥30 minutes, required antigen-testing.7 Two asymptomatic, fully-vaccinated visitors/inpatient/day were allowed at maximum.7
Definition of health care-associated COVID-19 infection
All newly-diagnosed inpatient cases were classified into community-onset or health care-associated infection:8
-
•
Community-onset: PCR-positive ≤2 days postadmission
-
•
Indeterminate-health care-associated: PCR-positive 3-7 days postadmission
-
•
Probable-health care-associated: PCR-positive 8-14 days postadmission
-
•
Definite-health care-associated: PCR-positive ≥15 days postadmission
Epidemiological clusters were defined as ≥2 cases in patients or HCWs associated with the same setting, ending when no cases were diagnosed for 14 days.8 Significant close-contact was defined as contact within 2-metres of the index-case for ≥15 minutes, during the index-case's infectious-period.8 Infectious periods were defined from 4 days before to 7 days after a positive PCR.9 Patients and HCWs with significant close-contact underwent PCR on D1/D4/D7/D10 postexposure, regardless of symptoms. All exposed-patients were isolated; only HCWs who had not donned N95 respirators were furloughed. Whole-genome-sequencing was performed for inpatient and HCW-cases in the epidemiological clusters (Supplementary Material 1).
Results
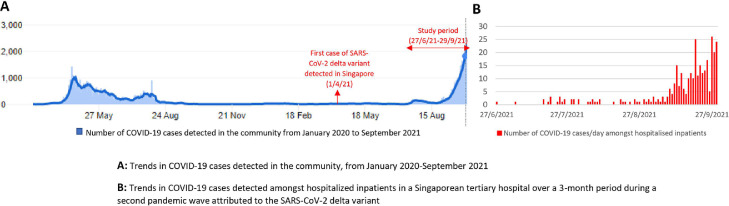
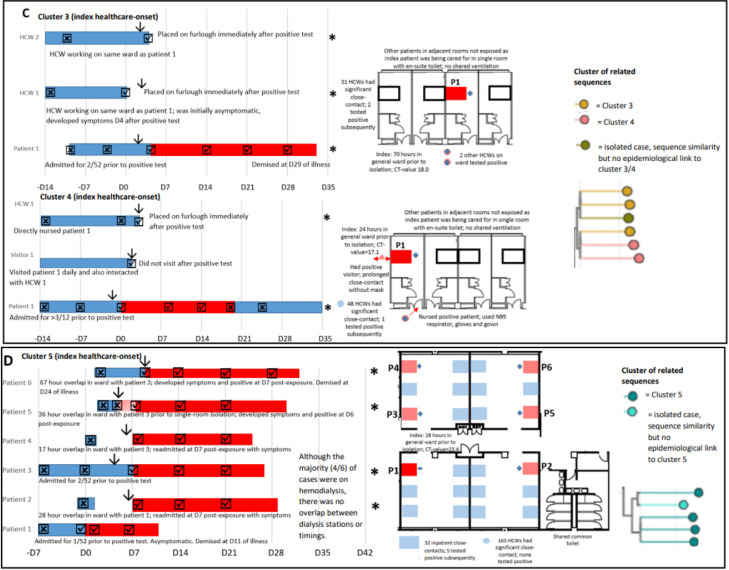
Over a 3-month community surge (Fig 1 A), 6.7% (1219/17676) of admissions had concurrent COVID-19 infection. One-quarter (26.3%, 321/1219) were newly diagnosed during hospitalization (Fig 1B); the rest tested positive elsewhere prior. A minority of newly-diagnosed cases (6.9%, 22/321) were health care-associated, with the vast majority classified as community-onset (N = 299). Antigen-testing combined with epidemiological/clinical risk-stratification was highly successful in triaging suspected community-onset cases to isolation, with a sensitivity of 98.3% (95% CI = 96.1-99.5) (Supplementary Table 1). Almost all community-onset cases (95.0%, 284/299) were triaged to isolation from onset. Community-onset cases initially triaged outside of the isolation ward (N = 15) spent 8.7 hours (SD = 14.6) prior to isolation, seeding 2 clusters. The first cluster involved an asymptomatic index-case admitted to a cohorted ward with a negative antigen-test/PCR; PCR on D2 of admission returned positive (Fig 2 A). A secondary cluster was seeded on another ward, with sequencing links between patients on both wards (Fig 2A). The second cluster involved a symptomatic index-case in a cohorted RSW (Fig 2B).
Fig. 1.
Trends in COVID-19 cases detected in the community and amongst hospitalized inpatients in a Singaporean tertiary hospital over a 3-month period during a second pandemic wave attributed to the SARS-CoV-2 delta variant.
Fig. 2.
Epidemiological clusters of potential health care-associated COVID-19 cases amongst hospitalized inpatients in a Singaporean tertiary hospital with high vaccination uptake, enhanced infection-prevention, and surveillance measures.
Amongst health care-associated cases (N = 22), 7 cases were definite, 12 probable, and the remaining 3 cases indeterminate. The majority (12/22) were unvaccinated; one-third (36.3%, 8/22) received immunosuppression or had malignancy; and one-third (36.3%, 8/22) received hemodialysis. Two-fifths (36.3%, 9/22) were first identified outside of isolation; the remainder were already on enhanced surveillance due to significant inpatient-exposure. Health care-associated COVID-19 cases initially detected outside of isolation (N = 9) spent 33.3 hours (SD = 22.2) prior to isolation, seeding 3 clusters. Two clusters comprised definite health care-associated inpatient-cases and fully-vaccinated HCWs, with sequencing links (Fig 2C). A final cluster occurred on a cohorted renal ward (Fig 2D).
Amongst the 5 clusters (Fig 2A-D), 498 HCWs and 107 inpatients had significant close-contact; 1.7% (6/498) of HCWs and 13.1% (14/107) of exposed-inpatients subsequently tested positive. The odds-ratio of acquisition amongst exposed-inpatients, compared with HCWs, was 12.3 (95% CI = 4.6-32.9, P < .001). One-quarter (23.1%, 6/26) of unvaccinated/partially-vaccinated exposed-inpatients subsequently tested positive, compared with 9.8% (8/81) amongst fully-vaccinated exposed-inpatients (odds-ratio = 2.7, 95%CI = 0.9-8.8, P = .08).
Discussion
Although enhanced infection-prevention measures mitigated potential health care-associated transmission of COVID-19, it was insufficient in achieving zero health care-associated transmission during widespread community spread. Admission-triage strategies ensured that 95% of community-onset cases were initially isolated, but a single asymptomatic case with negative antigen-testing still resulted in secondary transmission. Enhanced surveillance and rigorous contact-tracing remains crucial in outbreak containment; genomic analysis supplemented epidemiology investigations and facilitated rapid confirmation of clusters, allowing prioritization of infection-prevention resources. The small number of breakthrough infections amongst vaccinated HCWs caring for patients with unsuspected COVID-19 highlights the potential for transmission despite high PPE compliance (≥90%)10 and widespread usage of N95 respirators. Asymptomatic visitors may escape detection at symptom-based triage and have been implicated in nosocomial clusters; however, screening asymptomatic visitors remains logistically challenging.7 At our campus, ≥1200 visitors entered daily. No-visitor policies have been considered, but this poses potential psychological distress to patients. The unvaccinated and immunocompromised remain at-risk and should be prioritized for enhanced-surveillance.
Footnotes
Conflicts of interest: The authors report no conflicts of interest.
Ethics approval and consent to participate: This study was conducted as part of outbreak-investigation; ethics approval was not required under our institutional-review-board guidelines.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2022.01.009.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.Thompson HA, Mousa A, Dighe A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) setting-specific transmission rates: a systematic review and meta-analysis. Clin Infect Dis. 2021;73:e754–e764. doi: 10.1093/cid/ciab100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shitrit P, Zuckerman NS, Mor O, Gottesman BS, Chowers M, et al. Nosocomial outbreak caused by the SARS-CoV-2 Delta variant in a highly vaccinated population, Israel, July 2021. Euro Surveill. 2021;6:2100822. doi: 10.2807/1560-7917.ES.2021.26.39.2100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow A, Guo H, Kyaw WM, Li AL, Lim RHF, Ang B. Rostered routine testing for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection among healthcare personnel-Is there a role in a tertiary-care hospital with enhanced infection prevention and control measures and robust sickness-surveillance systems? Infect Control Hosp Epidemiol, in press [DOI] [PMC free article] [PubMed]
- 4.Wee LE, Sim XYJ, Conceicao EP, et al. Containing COVID-19 outside the isolation ward: the impact of an infection control bundle on environmental contamination and transmission in a cohorted general ward. Am J Infect Control. 2020;48:1056–1061. doi: 10.1016/j.ajic.2020.06.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wee LE, Conceicao EP, Aung MK, et al. Rostered routine testing for healthcare workers and universal inpatient screening: the role of expanded hospital surveillance during an outbreak of COVID-19 in the surrounding community. Infect Control Hosp Epidemiol, in press [DOI] [PMC free article] [PubMed]
- 6.Wee LE, Conceicao EP, Sim XYJ, et al. Utilisation of rapid antigen assays for detection of SARS-CoV-2 in a low-incidence setting at emergency department triage: does risk-stratification still matter? Infect Control Hosp Epidemiol, in press [DOI] [PubMed]
- 7.Wee LE, Conceicao EP, Sim JX, Venkatachalam I, Wijaya L. Utilisation of SARS-CoV-2 rapid antigen assays in screening asymptomatic hospital visitors: mitigating the risk in low-incidence settings. Int J Infect Dis. 2022;114:132–134. doi: 10.1016/j.ijid.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control. Surveillance definitions for COVID-19. 2021. Accessed 27, September 2021 https://www.ecdc.europa.eu/en/covid-19/surveillance/surveillance-definitions.
- 9.Lumley SF, Constantinides B, Sanderson N, et al. Epidemiological data and genome sequencing reveals that nosocomial transmission of SARS-CoV-2 is underestimated and mostly mediated by a small number of highly infectious individuals. J Infect. 2021;83:473–482. doi: 10.1016/j.jinf.2021.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wee LE, Sim JXY, Conceicao EP, Aung MK, Ng IM, Ling ML. Re: 'Personal protective equipment protecting healthcare workers in the Chinese epicenter of COVID-19′ by Zhao et al. Clin Microbiol Infect. 2020;26:1719–1721. doi: 10.1016/j.cmi.2020.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.