Abstract
The fear of SARS-CoV-2 infection is due to its high mortality related to seasonal flu. To date, few medicines have been developed to significantly reduce the mortality of the severe COVID-19 patients, especially those requiring tracheal intubation. The severity and mortality of SARS-CoV-2 infection not only depend on the viral virulence, but are primarily determined by the cytokine storm and the destructive inflammation driven by the host immune reaction. Thus, to target the host immune response might be a better strategy to combat this pandemic. Melatonin is a molecule with multiple activities on a virus infection. These include that it downregulates the overreaction of innate immune response to suppress inflammation, promotes the adaptive immune reaction to enhance antibody formation, inhibits the entrance of the virus into the cell as well as limits its replication. These render it a potentially excellent candidate for treatment of the severe COVID-19 cases. Several clinical trials have confirmed that melatonin when added to the conventional therapy significantly reduces the mortality of the severe COVID-19 patients. The cost of melatonin is a small fraction of those medications approved by FDA for emergency use to treat COVID-19. Because of its self-administered, low cost and high safety margin, melatonin could be made available to every country in the world at an affordable cost. We recommend melatonin be used to treat severe COVID-19 patients with the intent of reducing mortality. If successful, it would make the SARS-CoV-2 pandemic less fearful and help to return life back to normalcy.
Keywords: Melatonin, SARS-CoV-2, COVID-19, Innate immunity, Adaptive immunity, Inflammation, Main protease, Nirmatrelvir, Ritonavir
1. Introduction
The COVID-19 pandemic caused by the SARS-CoV-2 has ravaged the world for two years. It has caused millions of deaths and cost trillions of dollars in economic losses. Based on the experiences of previous two pandemics, i.e., severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) which were caused by the similar corona viruses, scientists have rapidly developed effective vaccines. In addition, several new antiviral medications including monoclonal antibodies, mRNA replication inhibitors, and antiviral and protease inhibitor cocktails which specifically target the SARS-CoV-2 have been rapidly approved by FDA for emergency use to treat COVID-19 patients. These products have flattened the rising curve of the SARS-CoV-2 spread and have saved an untold number of lives. However, the mutation rate of this mRNA virus is high and new variants have continued and may continue to emerge. Unfortunately, some of these variants are more transmissible than the original form of the virus and have acquired some capacity to avoid the antibodies of the vaccines. Thus, the development of effective vaccines usually lags behind the appearance of the new variants. This results in repeated infectious waves such as those fueled by the new variants including Delta [1] and the current Omicron [2] with others already on the horizon. This continuous emergence of potentially vaccine-resistant new variants makes the development of herd immunity to terminate the pandemic difficult even in countries with a high vaccination rate.
Another important issue is that, to date, the intermediate host(s) of SARS-CoV-2 from which the virus was initially transmitted to humans has not been definitively identified. Without this information, it is difficult to predict, and therefore to prevent, any upcoming pandemic caused by similar corona viruses. To improve the chances of termination of the current pandemic (or at least keeping it in check) and/or preventing the inevitably of the appearance of new pandemics caused by different pathogens, all conceivable and safe strategies should be considered. One of these strategies is to increase the tolerance of the immune system to the pathogen invasion. Such a scheme has been successfully developed in bats during evolution since bats can harbor many viruses that are deadly to other mammals but do not harm the bats themselves [3]. This includes the corona virus, the culprit responsible for the current pandemic. A potential mechanism is that bats exhibit a relatively low innate immune response and inflammatory reaction to the pathogens and thus have the higher tolerance to the deadly viruses than do other mammals [4].
Mounting evidence has documented that melatonin is a molecule which can also increase the tolerance of animals to the environmental insults including deadly viral attacks [5]. In principle, melatonin should be useful in protecting against the SARS-CoV2 infection and to reduce the symptoms of COVID-19 patients. Indeed, several clinical studies have confirmed this point [6], [7], [8]. Most importantly, use of melatonin is one of the only treatments which may significantly reduce the mortality of severe COVID patients. Since the primary target of melatonin is the host immune system, its protective effects against a SARS-CoV-2 infection will not be weaker against any of the gene-mutated new variants. This advantage exceeds what any specific vaccine or antiviral drug can achieve. Furthermore, its broad protective effects prepare the host against the future upcoming pandemics with different pathologies. Another important feature of melatonin should also be mentioned, i.e., its safety. Melatonin is a naturally occurring molecule presence in essentially all products that humans consume including vegetables, cereals, meat, fish and wines, etc. [9], [10]. Thus, its consumption for a short interval (for example, several weeks of treatment on SARS-CoV-2 infection) will not produce serious side effects even at large dosage [11]. The exogenous melatonin administration has been proven to not influence the endocrine function and circadian rhythm of the hormone secretions in humans [12]. In addition, it also decreases the circadian misalignment of patients [13]. Compared to most synthetic medicines used in treatment of the COVID-19, melatonin is safe and tolerable for most of the patients. In this review, we briefly discuss the mechanisms of melatonin's protective actions against COVID-19 and summarize the clinical evidence showing that melatonin has been successfully used in treatment of the severe COVID patients.
2. Melatonin targets host: downregulating the overreaction of innate immune response to increase tolerance against pathogen invasion
The symptoms and the outcomes after viral infection not only depend on the load, replication rate and the virulence of the viruses, but are greatly impacted by the host immune response, particularly by the innate immune network. Specific to SARS-CoV-2, the majority of the damage is not caused by virus per se but by the overwhelming inflammatory reaction induced by the so-called cytokine storm [14]. Cytokine storm is a consequence of the byproducts of the excessive innate immune response of the host to the infection. Adequate amounts of cytokines are required to combat the pathogen invasion, but overproduction of these molecules (cytokine storm) is destructive to tissues and organs. The reactive oxygen species (ROS) and reactive nitrogen species (RNS) generated during the cytokine storm and inflammatory reaction attack the afflicted cells, tissues and organs leading to multi-organ oxidative injury [15]. Thus, downregulating the overreaction of the innate immune response, suppressing the inflammatory reaction and its associated free radical generation significantly improve the outcome of a SARS-CoV-2 infection. These are also the basic mechanisms supporting melatonin's use to treat the severe COVID-19 patients [16].
2.1. Antioxidative activity of melatonin
Melatonin is a potent free radical scavenger and antioxidant [17]. It directly detoxifies a variety of ROS and RNS including the highly reactive hydroxyl radical, peroxynitrite anion, hydrogen peroxide, superoxide anion radical and hypochlorous acid which is a major mediator of inflammatory injury. Not only melatonin but also its metabolites readily donate an electron to reduce other oxidizing molecules; one melatonin molecule is estimated to scavenge up to 10 ROS and RNS [18]. This unique feature has not been observed for other classic antioxidants and it renders melatonin a more efficient antioxidant than the classic scavengers, vitamin C, vitamin E, carotenoids and NADH [19]. Compared to other antioxidants, melatonin exhibits favorable cellular distribution with both water and lipid solubilities. It can also bind to proteins and DNA with hydrogen binds to provide on-site protection. In addition to its direct free radical scavenging activity, melatonin also stimulates the activities of several antioxidant enzymes or upregulates their gene expressions. These enzymes including mitochondrial and cytosol superoxide dismutases, catalase, glutathione peroxidase and glutamate synthases [20], [21]. Via this means, melatonin indirectly enhances the antioxidant capacity of cells. Furthermore, melatonin has the ability to reduce the formation of ROS/RNS. This is achieved by melatonin acting on the mitochondrial energy metabolism. Mitochondria are the primary sites of free radical formation by leakage of electron to the oxygen. The leakage mainly occurs during the stage of high mitochondrial membrane potential (MMP). Elevated MMP inhibits the electron flow in the electron transport chain and enhances electron leakage to generate ROS. Reducing the elevated MMP will lower ROS formation. Regulation of the MMP is a function of mitochondrial uncoupling proteins (UCPs) which shunt protons from the mitochondrial intermembrane space into the matrix thereby reducing the MMP. Melatonin stimulates the activities of UCPs including UCP1, 2 and 3 and thus, reduces radical formation [22], [23], [24], [25]. This action is referred as the free radical avoidance action of melatonin [26]. The direct free radical scavenging, stimulation of antioxidant enzymes and free radical avoidance activities of melatonin make this molecule a potent antioxidant under most physio-pathological circumstances. The protective effects of melatonin against cell apoptosis, as well as tissue and organ injury caused by oxidative stress are well documented in animal studies and clinical trials [27], [28].
2.2. Antiinflammation activity of melatonin
Melatonin is also an excellent anti-inflammatory molecule. This was first observed by Cuzzocrea et al. [29], [30]. They reported that melatonin inhibited the unspecific inflammation induced by carrageenan or zymosan with the mechanism involving scavenging the peroxynitrite anion. Thereafter, hundreds of studies have confirmed the anti-inflammatory action of melatonin. The anti-inflammatory mechanisms of melatonin are highly diverse. Here, we only discuss a few of them. 1). Melatonin effectively suppresses the activity or downregulate gene expressions of several important proinflammatory enzymes including cyclooxygenase 2 (COX2), inducible nitric oxide synthase (iNOS), eosinophilic peroxidase and matrix metallopeptidase 2 and 9 (MMP2, 9) [31], [32], [33], [34]. These enzymes catalyze the production of inflammatory mediators including prostaglandins, nitric oxide, hypochlorous acid or directly hydrolyze the proteins to cause tissue damage. Their inhibition reduces tissue inflammation. 2). An important molecular mechanism of melatonin relative to inflammation is that melatonin suppresses NLRP3 inflammasome progression [35], [36]. Its progression leads to caspase one activation and IL-1β, IL-18 maturation which subsequently induce pyroptosis, a final outcome of the typical destructive inflammation. Pyroptosis has been observed in various acute or chronic inflammatory diseases including SARS-CoV-2 infection [37], [38]. By interacting with several signal transduction pathways including SIRT1, microRNA, long non-coding RNA, and Wnt/β-catenin, melatonin effectively inhibits NLRP3 inflammasome formation and thus, diminishes inflammation [39]. 3). NF-κB is also important molecular signaling pathway involved in inflammation. Translocation of NF-κB from cytosol to nucleus initiates transcription of an array of proinflammatory cytokines which constitute the cytokine storm as it occurs in severe SARS-CoV-2 infection [40], [41]. Melatonin inhibits IκBα phosphorylation and thus, diminishes the NF-κB translocation into nucleus [42]. By inhibiting the NF-κB pathway, melatonin suppresses the cytokine storm and its associated destructive inflammation. 4). NF-κB signaling pathway downregulation also promotes autophagic activity. Enhanced autophagy is typically accompanied by reduced inflammasome formation [43]. Activation of autophagy including mitophagy cleans the inflammatory debris and accelerates tissue recovery from inflammation and this has been often observed in melatonin-treated animals [44], [45].
2.3. Immunoregulatory activity of melatonin
Melatonin is an immune regulator. It downregulates the overreaction of innate immune response and promotes adaptive immunity [46]. The innate immune system is the first line of defense against a pathogen invasion. It is driven by pathogen associated molecular pattern (PAMP) receptors and include TLRs, NLRs, ALRs, cGAS, AIM2, etc. The PAMP receptors recognize pathogen RNA, DNA, proteins and lipids, respectively. Their normal activities of PAMP receptors aid the innate immune cells to remove pathogens. However, their overreactions often lead to the tissue damage. For example, TLR4 recognizes bacterial LPS and its overactivation exaggerates septic symptoms [47]. TLR-9 and cGAS recognize the pathogic double strand DNA and their activation induces the NLRP3 inflammasome formation. Melatonin inhibits the activation of TLR4, TLR9 and cGAS and lowers the innate immune response; hence, the tissue damage caused by pathogens, ischemia/reperfusion and other insults is diminished [48], [49], [50], [51].
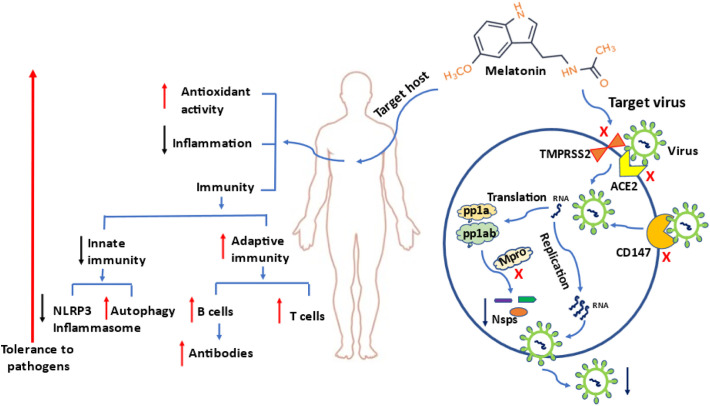
Melatonin also directly impacts innate immune cells, primarily with negative regulatory activities. For example, neutrophil migration to inflammatory sites is the fundamental process of the innate immune system in response to a pathogen; however, neutrophil accumulation often worsens inflammation. By blocking ERK phosphorylation, melatonin inhibits neutrophil migration and its associated tissue damage [52]. Macrophages and mast cells are important antigen processing innate immune cells. Their overreaction leads to the cytokine storm and inflammatory tissue damage. Melatonin receptors and synthetic enzymes have been identified in these cells indicating the likely important physiological roles of melatonin in the immune cells [53], [54]. Melatonin treatment down-regulates mast cell activation and reduces its production of TNF-α and IL-6 as well as inhibits the IKK/NF-κB signal transduction pathway in activated mast cells [55]. For macrophages, SARS-CoV-2 infection in severely-ill patients depletes the M2 anti-inflammatory macrophage and increases the M1 pro-inflammatory phenotype [56]. In contrast, melatonin treatment reverses this conversion by manipulating their glycolytic metabolic status to oxidative metabolism [57]. The increased M2 macrophages help to eliminate SARS-CoV-2 and suppress the dysfunctional hyper-inflammatory response mediated by M1 macrophages [58]. It should be noted that under physiological conditions, melatonin may increase the innate immunity [59] and, thus it will not jeopardize the protective effects of this system on pathogen invasion. What melatonin does is to lower the extreme response of the innate immune system during the viral infection [60]. In contrast to downregulating the overreaction of innate immune response, melatonin enhances the adaptive immune response including balancing the ratio of T lymphocyte populations [61] and increasing the B lymphocyte number and their antibody titer after vaccination [62]. Gurunathan et al. [63] recently suggested that administration of melatonin could increase the potency of the immune response and the duration of the immunity induced by the vaccine. Cardinali and co-workers [64] suggested that melatonin should be given from 2 to 4 weeks after vaccination to enhance the efficacy of vaccine against SARS-CoV-2. The utility of melatonin in the treatment of other viral infections have been extensively reviewed by Boga et al. [65]. Here, we briefly summarize several current developments in this field (Fig. 1 ).
Fig. 1.
The protective mechanisms of melatonin on SARS-CoV2 infection.
Melatonin primarily targets the host to enhance the tolerance against pathogens. In addition, it may also target SARS-CoV-2 by blocking its entrance into cells and its replication. When the viral RNA is released into the cytoplasm and translated in two polyproteins (pp1a and pp1ab), both are cleaved by Mpro and papain-like protease (PLpro) to form 15 new non-structural proteins (nsps) that compose the replication-transcription complex. The newly formed RNA, nucleocapsid proteins and envelope glycoproteins assemble to form viral particles. Melatonin is an Mpro inhibitor and, thus can inhibit SARS CoV-2 replication. Red upward arrows: enhanced action; black downward arrows: reduced action; blue arrows: direction; red cross: blocking action; TMPRSS2: transmembrane protease serine 2; ACE2: angiotensin-converting enzyme 2, CD147: cluster of differentiation 147; Mpro: main protease. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Melatonin targets SARS-CoV-2: inhibiting its cellular entrance and replication
3.1. Blocking the entrance of SARS-CoV-2 into cell by melatonin
In addition to targeting the host, melatonin may also impact a COVD-19 infection by inhibiting virus entrance and replication in cells. It is well documented that angiotensin-converting enzyme 2 (ACE2), transmembrane protease serine 2 (TMPRSS2) and metalloproteinase 17 (ADAM17) are responsible for SARS-CoV-2 entering cells [66]. Briefly, ADAM17 makes ACE2 available on the membrane; ACE2 serves as a membrane receptor of the surface spike (S) glycoprotein of SARS-CoV-2 and TMPRSS2, facilitating virus-cell membrane fusion. Actions on either influences the entrance of SARS-CoV-2 into cells. Several indirect data have indicated that melatonin may also target these molecules to retard the cellular entrance of the coronavirus. For example, ACE2, ADAM17 and TMPRSS2 exhibit daily rhythms of expression in some mammalian tissues and the circadian system is known to modulate COVID-19 progression which may be regulated by the melatonin circadian rhythm [67]. The regulatory activity of melatonin on ADAM17 has been reported [68]. The direct association of melatonin and ACE2 has not been demonstrated. However, ACE2 is a key element in the process of absorption of tryptophan, the necessary precursor of melatonin. When the ACE2 is occupied by SARS-CoV2, it leads to tryptophan depletion as well as a likely melatonin deficiency in COVID-19 patients [69]. Also, melatonin may serve as an indirect regulator of ACE2 via binding to calmodulin [70] or by impacting MMP9 [71]; both calmodulin and MMP9 directly modulate the ACE2 activity. A recent study in animals confirmed the therapeutic potential of melatonin on ACE2. Transgenic mice expressing human ACE2 receptor (K18- hACE2) exhibited an increased susceptibility to SARS-CoV-2 infection and melatonin treatment at a high dose delayed the occurrence of severe clinical symptoms with an improvement of survival [72]. More direct evidence comes from the report of Cecon et al. [73]. They have determined that melatonin allosterically binds to human ACE2 of endothelial cells and modifies its configuration, thus, inhibiting SARS-CoV-2 entry into the cells. In addition to the ACE2/TMPRSS2 major entrance sites, alternative pathways of SARS-CoV2 entering cells have also attracted attention of researchers since targeting ACE2/MPRSS2 pathway has not achieved the expected therapeutic effects. The cluster of differentiation 147 (CD147) transmembrane protein may be another entry receptor and mediator of endocytosis-promoted entry of the SARS-CoV-2 [74], [75]. It has been speculated that melatonin can reduce the increased CD147 activation during a SARS-CoV-2 infection by suppressing HIF-1ɑ expression; the mechanisms for this action have been discussed in an extensive review [76] (Fig. 1).
3.2. Inhibiting replication of SARS-CoV-2 by melatonin
Several studies have shown that melatonin probably inhibits viral replication. For example, melatonin can suppress Dengue virus replication by activating the sirtuin pathway to upregulate the transcription of antiviral genes [77]. It can also inhibit the replication of several swine coronaviruses in a dose-dependent manner under in vitro conditions [78]. There are no animal studies to show that melatonin inhibits SARS-CoV-2 replication; however, some theoretical evidence suggests that melatonin may do so. Tesarik [79] proposed that growth factor signaling is required for SARS-CoV2 replication and its inhibition may prevent SARS-CoV-2 replication in infected cells [80]. Melatonin downregulates growth factor signaling; thus, melatonin also may act against the virus itself via targeting this molecular pathway to inhibit viral replication.
Recently, the main protease (Mpro) [also called 3C like protease (3CLpro)] of SARS-CoV-2 has become a potential target for development of replication inhibitor since Mpro is unique to the virus and is not found in the host cells. Mpro is responsible for processing of the replicase polyproteins during coronavirus replication and transcription (Fig. 1). Its inhibition results in the termination of virus replication. This strategy has been successfully used to treat COVID-19 patients. For instance, the newly developed drug of Paxlovid by Pfizer reduces the hospitalization and death by 89% in the mild to moderately severe COVID-19 patients [72]. The effective ingredients of Paxlovid are nirmatrelvir (PF-07321332) and ritonavir. PF-07321332 is an Mpro inhibitor and it presumably inhibits the catalytic action of Mpro to terminate the SARS-CoV-2 replication. Co-administration of low dose ritonavir is expected to slow down the metabolic degradation of PF-07321332, thus maintaining its high tissue level. Mpro has a cysteine-histidine catalytic dyad at its active site [cysteine 145 (C145) and histidine 41 (H41)]. The sulfur of the cysteine acts as a nucleophile and the imidazole ring of the histidine as a general base to cleave the coronavirus polyprotein at 11 conserved sites [81], [82], [83]. The crystal structure of SARS-CoV-2 Mpro and PF-07321332 complex shows that the PF-07321332 is located in the catalytic center of Mpro. At this site, the Sγ atom of the C145 forms a 1.8-Å C—S covalent bond with the nitrile carbon of PF-07321332 and H41 binds to PF-07321332 via carbon‑hydrogen bond [74], [75]. Interestingly, similar to PF-07321332, melatonin also localizes in the catalytic site of Mpro and binds to the catalytic amino acid residues of C145 and H41 with pi‑sulfur/conventional hydrogen bonds and carbon‑hydrogen bonds, respectively [70]. The similarity of melatonin and PF-07321332 binding to the catalytic center of Mpro suggests that melatonin also functions as Mpro inhibitor as PF-07321332 with high efficiency since, with the exception of the His41 and Cys145, the other residues of Phe140, Ser144, His163, His164, Glu 166, Gln189 and Thr190 are important for the interaction of inhibitors with SARS-CoV-2 Mpro and these amino acid residues are also present on the melatonin binding site [70].
4. Clinical evidence that melatonin reduces the mortality of severe COVID-19 patients
Based on the potentially antiviral capacity and results of the previous studies of melatonin on other viral infection, in the early stages of the pandemic, several groups had already suggested that melatonin could be used to prevent and treat COVID-19 patients [5], [84], [85]. Subsequently, a number clinical trials with different end points were performed using melatonin to treat COVID-19 patients. The endpoints included improvement of the sleep, prophylactic use, shortening of the hospitalization time and others [6], [7], [86], [87]. The size of these trials is small because of limited financial support since melatonin is inexpensive and non-patentable; thus, pharmaceutical companies would not support research on this molecule. However, judging from the data available, we are left with the impression that melatonin represents an effective, convenient and affordable therapy for the SARS-CoV-2 infection. To date, few medicines have been found to significantly reduce the mortality rate of severe COVID-19 patients, except for melatonin. Here we summarize the results of the trials in which melatonin treatment had favorable effects on mortality rate of severe COVID-19 patients. The limitations of these trials are also considered.
Castillo et al. [88] reported on a case series study in which they used high dose melatonin to treat 10 hospitalized COVID-19 patients. Each of these patients had some high-risk factors for COVID-19 associated with mortality including advanced age, hypertension, type 2 diabetes (T2DM), chronic kidney disease (CKD), chronic gout, moderate acute respiratory distress syndrome (ARDS), or pulmonary ground glasslike opacities. With conventional treatment plus high dose (approximately 72 mg/day) of melatonin all the patients survived. In contrast, 12 of the 34 COVID-19 patients admitted during the same period in the same hospital with conventional treatment alone died, and the death rate was 35.3%. It should be noted that those 34 COVID-19 patients originally were not designed as the control group and they were only used as a parallel retrospective comparison.
Another important observation was made by Ramlall et al. [89]. By retrospective analysis of a huge data base of 189,987 patients who sought care at NYP/CUIMC between February 1st, 2020, and August 1st, 2020, they identified 256 severe COVID-19 patients who were over 65 years old with some of the commodities such as chronic kidney or obstructive lung disease, heart disease, diabetes, hypertension, etc. All of them required mechanical ventilation. Among them, 112 received melatonin treatment for different reasons including insomnia, sleep wake cycle, difficulty sleeping, anxiety, delirium, agitation, agitated delirium etc. The multivariate model Cox proportional hazards ratio (HR) analysis indicated that the probable death rate for the patients without melatonin treatment was 75% (108 out of 144 patients died). However, in intubated and melatonin-treated group, the death rate was 13.2% (14 out of 122 patients died). The difference is highly statistically significant (p < 0.001). Unfortunately, the melatonin doses and intake duration were not available from the report.
The above results have been somehow confirmed by Sánchez-González et al. [90]. They also retrospectively analyzed hospitalized 2463 COVID-19 patients of University Hospital (FJDUH) in Madrid, Spain, during the first wave of the pandemic. 224 severe patients (23% required ICE care, 50% with hypertension, 47% with dyslipidemia, 27.2% with cardiovascular and 23.7 with diabetes) received conventional plus melatonin treatment. Additional 224 patients with conventional treatment alone were selected as the control group. To control for basal differences among the two groups a propensity score matching was performed and showed no difference between groups. The melatonin-treated patients showed a much lower mortality rate (10.7% vs 23.7%) compared to the non-melatonin treatment matched group (p < 0.01). The authors claimed these were real world clinical data to support a possible benefit of melatonin in COVID-19 disease. Recently, Hasan et al. [91] completed a prospective clinical trial which was specifically designed to test the protective effects of melatonin in severe COVID-19 patients. All patients received standard therapy with oxygen intubation, remdesivir (as an antiviral), levofloxacin (for protection against secondary bacterial infection), dexamethasone (as an anti-inflammatory) and enoxaparin (as an anticoagulant). Half of them additionally received 10 mg melatonin. The results were highly promising with 13 deaths out of 76 patients in the conventional therapy group (mortality rate of 17.1%) compared to only 1 death out of 82 patients in melatonin group (mortality rate of 1.2%). Thus, the mortality of the severe COVID-19 patient was reduced by 93% as a result of melatonin treatment compared to the conventional treatment alone patients. This was a single-center, open-label, randomized clinical trial. This type of trial cannot completely avoid bias and more well-designed clinical trials should be conducted to confirm this observation. The detailed information on these clinical trials is summarized in the Table 1 .
Table 1.
The effects of melatonin treatment on the mortality in severe COVID-19 patients.
| Trial type | Conventional therapy |
Conventional therapy plus melatonin |
||||||
|---|---|---|---|---|---|---|---|---|
| N | N of deaths | % of deaths | N | N of deaths | % of deaths | Dose and duration | Ref | |
| Prospective | 34 | 12 | 35.3 | 10 | 0 | 0 | 36–72 mg/day, divided into 4 doses for 7 days. | [88] |
| Retrospective | 144 | 108⁎ | 75⁎ | 122 | 14⁎ | 13.2⁎ | Not available | [89] |
| Retrospective | 224 | 53 | 23.7 | 224 | 24 | 10.7 | 2–6 mg/day, at least 7 days. | [90] |
| Prospective | 76 | 13 | 17.1 | 82 | 1 | 1.2 | 10 mg/day, 20–30 min before bed time for 14 days. | [91] |
Majority of the patients included in this table are considered with severe symptoms and some required mechanical ventilation, ICU care and all required hospitalization. Several other clinical trials in which the endpoint did not include mortality or did not justify as severe COVID-19 patients are not included in this table.
The data were extracted from the hazards rate (HR) presented in Table 2 of the report.
5. Discussion
In the past two years, in addition to the rapid introduction of several vaccines, a variety of repurposed medicines and the newly-developed drugs have been used to prevent and/or treat SARS-CoV-2 infected COVID-19 patients. The vast majority of these medications are designed to target the virus per se and a few of them have achieved the expected results, particularly with regard to reducing the mortality of the severely infected patients. This is not surprising since the severity of the disease as well as the mortality do not primarily depend on the viral cytotoxicity, but rather, on the host's immune response. For example, the most of the cellular damage to multiple organs is caused by cytokine storm and the associated destructive inflammation. Thus, to target the host's immune response to reduce the inflammatory tissue damage may be a better strategy to combat this pandemic [46].
Humans are equipped with two different immune responses, the innate and the adaptive. The innate immune system provides a rapid response against any pathogen invasion by recognizing their PAMPs and, thus, it lacks specificity. Lack of specific targets of the innate immunity often results in its overreaction to external insults since the damaged tissues caused by the external insults such as pathogen invasion can also serve as the damage/danger associated molecular pattern (DAMP) to further exacerbate an inflammatory reaction. In contrast, the adaptive immune system is comprised of T lymphocytes which recognize the specific pathogens as well as infected cells and B lymphocytes which produce specific antibodies to attack the pathogen as in the case of SARS-CoV-2 infection. Thus, the adaptive immune system is better suited to neutralize SARS-CoV-2 than is the innate immune system. Thus, the adaptive immune system will not cause wide spread tissue damage as does the innate immune response due to it largely targets the specific pathogens. When an adaptive immune response is built with sufficient T cells and antibodies, the pathogens will be quickly cleaned and the patients will recover from the viral infection. However, the development of the adaptive immune response lags behind the pathogen infection by several weeks. Specific to SARS-CoV-2 infection, the blood antibody levels begin to rise within a week after symptom appearance and reach their peaks approximately after 3 weeks [92], [93]. Correspondingly, the severe symptoms and death of the patients often occur during the interval between the innate immune response and the fully matured adaptive immunity developed. This is evidence that mortality peak often occurs about 16 days after the onset of COVID-19 symptoms [94]. These observations strongly suggest that a downregulation of the overreaction of the innate immune response and reduction of the associated tissue damage is a feasible strategy to limit the severity and mortality caused by SARS-CoV-2 infection. This strategy would allow the patients to increase their tolerance to the infection and gain sufficient time to fully develop their adaptive immune response. Without this, some patients would die during the period between the innate immune response transition to the fully developed adaptive response due to the overreaction of the innate immune response.
Dexamethasone is an example of a drug used for this purpose since it lowers the innate immune response of hosts. It has been successfully used in the UK to reduce the mortality of the severely-ill COVID-19 patients [95], Dexamethasone suffers from serious side effects, since corticosteroids reduce viral clearance, increase secondary infections and perturb metabolism [96], [97]. Similar to dexamethasone, melatonin downregulates the innate immunity but unlike the corticosteroid, melatonin promotes the adaptive immunity as well as inhibits the entrance and replication of the SARS-CcV-2. This triad of functions makes melatonin an excellent candidate to protect against severe SARS-CoV-2 infection.
The clinical trials mentioned above have provided evidence of its beneficial effects on the severe COVID-19 patients. The described mechanisms and the positive clinical observations all support melatonin's use in severe COVID-19 patients, especially considering the general lack of treatments available for those seriously-ill patients. Currently, the pharmaceutical industry, i.e., Merck and Pfizer claimed to have developed antiviral medications which can reduce the hospitalization or death of the COVID-19 patients by approximately 50% (Merck) and 89% (Pfizer), respectively [98], [99]. It should be noted that these medicines have only been effective in mild to moderately severe patients without hospitalization and its effects on the severely ill patient have not been tested or reported. The effective ingredient of Paxlovid of Pfizer is an Mpro inhibitor, PF-07321332, which binds to the catalytic residues of C145 and H41 of Mpro to block its function. Another ingredient of Paxlovid is ritonavir which is co-administered at a low-dose to boost and maintain plasma concentrations of PF-07321332. Ritonavir inhibits or induces an array of drug metabolic enzymes and the caution of its potential for clinically significant drug–drug interactions has been raised [100]. Ritonavir is a known inducer of CYP1A2 which is the primary enzyme to metabolizes melatonin to 6-hydroxylmelatonin in the liver [101]. A melatonin deficiency, induced by drugs or a function of age, has been proposed as the risk factor related to the severity of the disease in COVID-19 patients [102], [103], [104]. The ritonavir-induced melatonin deficiency should be a consideration when Paxlovid is used to treat COVID-19 patients. The means to solve this issue may be co-treated patients with Paxlovid plus melatonin to avoid the ritonavir-induced melatonin deficiency.
Interestingly, melatonin is also an Mpro inhibitor binding to C145 and H41 very similar to the PF-07321332. But melatonin exhibits more favorable pharmacological features than PF-07321332. These includes its high bioavailability, easy penetration into cell and minimal side effects. In addition, melatonin improves the host immune system while also inhibiting SARS-CoV-2 invasion. Collectively, the overall protective effects of melatonin on SARS-CoV-2 infection would be expected to be comparable to or better than those of Paxlovid. Yet, its cost only a fraction of the Paxlovid. Table 2 compares the cost of melatonin and other FDA approved medications for COVID-19 emergency use.
Table 2.
The price comparisons for medicines used to treat SARS-CoV-2 infection.
Because of their high cost, the prescription medicines listed in the table are not affordable in many countries, especially, for the underdeveloped countries where treatments are acutely needed. If the only treatments available depend on these expensive medications, it will dramatically hinder the control of this disease in the economically-depressed countries so the recovery from the pandemic will be slowed. Since the pandemic does not respect national boundaries, lack of its control in these countries will delay the recovery from this devastating disease throughout the world. Melatonin, because of is low cost, could be provided at minimal expense to anyone in the world.
In conclusion, people's fear to the SARS-CoV-2 infection is because of its high mortality compared to the seasonal flu. To protect against a SARS-CoV-2 infection, vaccination is still the best choice. However, due to the continuous emergence of the vaccine resistant new variants, a considerable portion of the population who resists receiving the vaccine and the availability of vaccine being questionable for underdeveloped countries and regions, other alternatives to combat SARS-CoV-2 infection should be addressed. Melatonin is one of such alternatives. Due to the multiple actions by which it negatively impacts the SARS-CoV-2 infection melatonin significantly reduces the mortality of the severely-ill COVID-19 patients. Based on the evidence mentioned above, melatonin should be strongly recommended for use in severe COVID-19 cases to lower the mortality and to reduce the fear people have of this pandemic. This could not only allow the world population to return to more normal activities but also likely improve the mental health status of individuals who suffer with psychological issues resulting from the imposed restrictions.
CRediT authorship contribution statement
Dun-Xian Tan: Conceptualization, Data curation, Writing – original draft. Russel J. Reiter: Data curation, Writing – original draft.
Declaration of competing interest
The authors claim that there are no conflict of interests associated with this paper.
Data availability
No data was used for the research described in the article.
References
- 1.Venkatraja B., Srilakshminarayana G., Kumar B.Krishna. The dominance of severe acute respiratory syndrome coronavirus 2 B.1.617 and its sublineages and associations with mortality during the COVID-19 pandemic in India between 2020 and 2021. Am. J. Trop. Med. Hyg. 2021 doi: 10.4269/AJTMH.21-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohapatra R.K., Sarangi A.K., Kandi V., Azam M., Tiwari R., Dhama K. Omicron (B.1.1.529 variant of SARS-CoV-2); an emerging threat: current global scenario. J. Med. Virol. 2021 doi: 10.1002/JMV.27561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta P., Singh M.P., Goyal K., Tripti P., Ansari M.I., Obli Rajendran V., Dhama K., Malik Y.S. Bats and viruses: a death-defying friendship. Virusdisease. 2021;32:467–479. doi: 10.1007/S13337-021-00716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavlovich S.S., Lovett S.P., Koroleva G., Guito J.C., Arnold C.E., Nagle E.R., Kulcsar K., Lee A., Thibaud-Nissen F., Hume A.J., Mühlberger E., Uebelhoer L.S., Towner J.S., Rabadan R., Sanchez-Lockhart M., Kepler T.B., Palacios G. The egyptian rousette genome reveals unexpected features of bat antiviral immunity. Cell. 2018;173:1098–1110.e18. doi: 10.1016/j.cell.2018.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan D.X., Hardeland R. Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: focus on COVID-19. Melatonin Res. 2020;3:120–143. doi: 10.32794/mr11250052. [DOI] [Google Scholar]
- 6.Bologna C., Madonna P., Pone E. Efficacy of prolonged-release melatonin 2 mg (PRM 2 mg) prescribed for insomnia in hospitalized patients for COVID-19: a retrospective observational study. J. Clin. Med. 2021;10:5857. doi: 10.3390/JCM10245857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mousavi S.A., Heydari K., Mehravaran H., Saeedi M., Alizadeh-Navaei R., Hedayatizadeh-Omran A., Shamshirian A. Melatonin effects on sleep quality and outcomes of COVID-19 patients: an open-label, randomized, controlled trial. J. Med. Virol. 2022;94:263–271. doi: 10.1002/JMV.27312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alizadeh Z., Keyhanian N., Ghaderkhani S., Dashti-Khavidaki S., Shoormasti R.S., Pourpak Z. A pilot study on controlling coronavirus disease 2019 (COVID-19) inflammation using melatonin supplement, Iran. J. Allergy. Asthma. Immunol. 2021;20:494–499. doi: 10.18502/ijaai.v20i4.6959. [DOI] [PubMed] [Google Scholar]
- 9.Tan D.X., Zheng X., Kong J., Manchester L., Hardeland R., Kim S., Xu X., Reiter R. Fundamental issues related to the origin of melatonin and melatonin isomers during evolution: relation to their biological functions. Int. J. Mol. Sci. 2014;15:15858–15890. doi: 10.3390/ijms150915858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan D.X., Zanghi B.M., Manchester L.C., Reiter R.J. Melatonin identified in meats and other food stuffs: potentially nutritional impact. J. Pineal Res. 2014;57:213–218. doi: 10.1111/jpi.12152. [DOI] [PubMed] [Google Scholar]
- 11.Tan D.X., Hardeland R. Estimated doses of melatonin for treating deadly virus infections: focus on COVID-19. Melatonin Res. 2020;3:276–296. doi: 10.32794/mr11250062. [DOI] [Google Scholar]
- 12.Wright J., Aldhous M., Franey C., English J., Arendt J. The effects of exogenous melatonin on endocrine function in man. Clin. Endocrinol. 1986;24:375–382. doi: 10.1111/J.1365-2265.1986.TB01641.X. [DOI] [PubMed] [Google Scholar]
- 13.Marqueze E.C., Nogueira L.F.R., Vetter C., Skene D.J., Cipolla-Neto J., Moreno C.R.C. Exogenous melatonin decreases circadian misalignment and body weight among early types. J. Pineal Res. 2021;71 doi: 10.1111/JPI.12750. [DOI] [PubMed] [Google Scholar]
- 14.Polidoro R.B., Hagan R.S., de Santis Santiago R., Schmidt N.W. Overview: systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19. Front. Immunol. 2020;11 doi: 10.3389/FIMMU.2020.01626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson D.S.A., Oliver P.L. ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants (Basel, Switzerland) 2020;9:1–27. doi: 10.3390/ANTIOX9080743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiter R.J., Abreu-Gonzalez P., Marik P.E., Dominguez-Rodriguez A. Therapeutic algorithm for use of melatonin in patients with COVID-19. Front. Med. 2020;7 doi: 10.3389/FMED.2020.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan D.X., Chen L.C., Poeggeler B., Manchester L.C., Reiter R.J. Melatonin: a potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993;1:57–63. [Google Scholar]
- 18.Tan D.X., Manchester L.C., Terron M.P., Flores L.J., Reiter R.J. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 19.Tan D.X., Hardeland R., Manchester L.C., Poeggeler B., Lopez-Burillo S., Mayo J.C., Sainz R.M., Reiter R.J. Mechanistic and comparative studies of melatonin and classic antioxidants in terms of their interactions with the ABTS cation radical. J. Pineal Res. 2003;34:249–259. doi: 10.1034/J.1600-079X.2003.00037.X. [DOI] [PubMed] [Google Scholar]
- 20.Mayo J.C., Sainz R.M., Antolín I., Herrera F., Martin V., Rodriguez C. Melatonin regulation of antioxidant enzyme gene expression. Cell. Mol. Life Sci. 2002;59:1706–1713. doi: 10.1007/PL00012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szewczyk-Golec K., Rajewski P., Gackowski M., Mila-Kierzenkowska C., Wesołowski R., Sutkowy P., Pawłowska M., Woźniak A. Melatonin supplementation lowers oxidative stress and regulates adipokines in obese patients on a calorie-restricted diet. Oxidative Med. Cell. Longev. 2017:8494107. doi: 10.1155/2017/8494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan P., Zhang H., Su L., Wang X., Liu D. Melatonin balance the autophagy and apoptosis by regulating UCP2 in the LPS-induced cardiomyopathy. Molecules. 2018;23 doi: 10.3390/MOLECULES23030675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu L., Zhang S., Wen H., Liu T., Cai J., Du D., Zhu D., Chen F., Xia C. Melatonin decreases M1 polarization via attenuating mitochondrial oxidative damage depending on UCP2 pathway in prorenin-treated microglia. PLoS One. 2019;14 doi: 10.1371/JOURNAL.PONE.0212138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aslan G., Gül H.F., Tektemur A., Sahna E. Ischemic postconditioning reduced myocardial ischemia-reperfusion injury: the roles of melatonin and uncoupling protein 3, anatol. J. Cardiol. 2020;23:19–27. doi: 10.14744/ANATOLJCARDIOL.2019.72609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan D.X., Manchester L.C., Fuentes-Broto L., Paredes S.D., Reiter R.J. Significance and application of melatonin in the regulation of brown adipose tissue metabolism: relation to human obesity. Obes. Rev. 2011;12:167–188. doi: 10.1111/j.1467-789X.2010.00756.x. [DOI] [PubMed] [Google Scholar]
- 26.Hardeland R. Neuroprotection by radical avoidance: search for suitable agents. Molecules. 2009;14:5054–5102. doi: 10.3390/molecules14125054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Barcelo E.S., Rueda N., Mediavilla M.D., Rodriguez-Cue C., Reiter R.J. Clinical uses of melatonin in neurological diseases and mental and behavioural disorders. Curr. Med. Chem. 2017;24 doi: 10.2174/0929867324666170718105557. [DOI] [PubMed] [Google Scholar]
- 28.Reiter R.J., Tan D.X., Rosales-Corral S., Galano A., Zhou X.J., Xu B. Mitochondria: central organelles for melatonin’s antioxidant and anti-aging actions. Molecules. 2018;23:509. doi: 10.3390/molecules23020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuzzocrea S., Zingarelli B., Gilad E., Hake P., Salzman A.L., Szabó C. Protective effect of melatonin in carrageenan-induced models of local inflammation: relationship to its inhibitory effect on nitric oxide production and its peroxynitrite scavenging activity. J. Pineal Res. 1997;23:106–116. doi: 10.1111/j.1600-079x.1997.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 30.Cuzzocrea S., Zingarelli B., Costantino G., Caputi A.P. Protective effect of melatonin in a non-septic shock model induced by zymosan in the rat. J. Pineal Res. 1998;25:24–33. doi: 10.1111/j.1600-079x.1998.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 31.Costantino G., Cuzzocrea S., Mazzon E., Caputi A.P. Protective effects of melatonin in zymosan-activated plasma-induced paw inflammation. Eur. J. Pharmacol. 1998;363:57–63. doi: 10.1016/s0014-2999(98)00673-6. [DOI] [PubMed] [Google Scholar]
- 32.Mayo J.C., Sainz R.M., Tan D.-X., Hardeland R., Leon J., Rodriguez C., Reiter R.J. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J. Neuroimmunol. 2005;165:139–149. doi: 10.1016/j.jneuroim.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 33.El-Sokkary G.H., Omar H.M., Hassanein A.-F.M.M., Cuzzocrea S., Reiter R.J. Melatonin reduces oxidative damage and increases survival of mice infected with Schistosoma mansoni. Free Radic. Biol. Med. 2002;32:319–332. doi: 10.1016/s0891-5849(01)00753-5. http://www.ncbi.nlm.nih.gov/pubmed/11841922 (accessed January 22, 2018) [DOI] [PubMed] [Google Scholar]
- 34.Kong J., Zhang Y., Liu S., Li H., Liu S., Wang J., Qin X., Jiang X., Yang J., Zhang C., Zhang W. Melatonin attenuates angiotensin II-induced abdominal aortic aneurysm through the down-regulation of matrix metalloproteinases. Oncotarget. 2017;8:14283–14293. doi: 10.18632/oncotarget.15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y., Li X., Grailer J.J., Wang N., Wang M., Yao J., Zhong R., Gao G.F., Ward P.A., Tan D.-X., Li X. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J. Pineal Res. 2016;60:405–414. doi: 10.1111/jpi.12322. [DOI] [PubMed] [Google Scholar]
- 36.Hu X., Li D., Wang J., Guo J., Li Y., Cao Y., Zhang N., Fu Y. Melatonin inhibits endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation in lipopolysaccharide-induced endometritis in mice. Int. Immunopharmacol. 2018;64:101–109. doi: 10.1016/j.intimp.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 37.Kaivola J., Nyman T.A., Matikainen S. Inflammasomes and SARS-CoV-2 infection. Viruses. 2021;13:2513. doi: 10.3390/V13122513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kucia M., Ratajczak J., Bujko K., Adamiak M., Ciechanowicz A., Chumak V., Brzezniakiewicz-Janus K., Ratajczak M.Z. An evidence that SARS-Cov-2/COVID-19 spike protein (SP) damages hematopoietic stem/progenitor cells in the mechanism of pyroptosis in Nlrp3 inflammasome-dependent manner. Leukemia. 2021;35:3026–3029. doi: 10.1038/S41375-021-01332-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashrafizadeh M., Najafi M., Kavyiani N., Mohammadinejad R., Farkhondeh T., Samarghandian S. Anti-inflammatory activity of melatonin: a focus on the role of NLRP3 inflammasome. Inflammation. 2021;44:1207–1222. doi: 10.1007/S10753-021-01428-9. [DOI] [PubMed] [Google Scholar]
- 40.Davies D.A., Adlimoghaddam A., Albensi B.C. The effect of COVID-19 on NF-κB and neurological manifestations of disease. Mol. Neurobiol. 2021;58:4178–4187. doi: 10.1007/S12035-021-02438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manik M., Singh R.K. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 2021 doi: 10.1002/JMV.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H.W., Ying P., Cai Q.Q., Yang Z.H., Wu X.L. Exogenous melatonin alleviates hemorrhagic shock-induced hepatic ischemic injury in rats by inhibiting the NF-κB/IκBα signaling pathway. Mol. Med. Rep. 2021;23 doi: 10.3892/MMR.2021.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding Y., Fu X., Wang Q., Liu H., Wang H., Wu D. The complex interplay between autophagy and NLRP3 inflammasome in renal diseases. Int. J. Mol. Sci. 2021;22 doi: 10.3390/IJMS222312766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin M., Liu Y., Sun M., Li X., Xu J., Zhang L., Jiang H. Protective effects of melatonin on the white matter damage of neonatal rats by regulating NLRP3 inflammasome activity. Neuroreport. 2021;32:739–747. doi: 10.1097/WNR.0000000000001642. [DOI] [PubMed] [Google Scholar]
- 45.Cao S., Shrestha S., Li J., Yu X., Chen J., Yan F., Ying G., Gu C., Wang L., Chen G. Melatonin-mediated mitophagy protects against early brain injury after subarachnoid hemorrhage through inhibition of NLRP3 inflammasome activation. Sci. Rep. 2017;7 doi: 10.1038/S41598-017-02679-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan D.X., Ruediger H. Targeting host defense system and rescuing compromised mitochondria to increase tolerance against pathogens by melatonin may impact outcome of deadly virus infection pertinent to COVID-19. Molecules. 2020;25 doi: 10.3390/MOLECULES25194410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sygitowicz G., Sitkiewicz D. Molecular mechanisms of organ damage in sepsis: an overview. Braz. J. Infect. Dis. 2020;24:552–560. doi: 10.1016/J.BJID.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo J., Song J., Zhang H., Zhang F., Liu H., Li L., Zhang Z., Chen L., Zhang M., Lin D., Lin M., Zhou R. Melatonin mediated Foxp3-downregulation decreases cytokines production via the TLR2 and TLR4 pathways in H. Pylori infected mice. Int. Immunopharmacol. 2018;64:116–122. doi: 10.1016/J.INTIMP.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 49.Jauhari A., Baranov S.V., Suofu Y., Kim J., Singh T., Yablonska S., Li F., Wang X., Oberly P., Minnigh M.B., Poloyac S.M., Carlisle D.L., Friedlander R.M. Melatonin inhibits cytosolic mitochondrial DNA-induced neuroinflammatory signaling in accelerated aging and neurodegeneration. J. Clin. Invest. 2020;130:3124–3136. doi: 10.1172/JCI135026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu X., Wang G., Ai L., Shi J., Zhang J., Chen Y.X. Melatonin suppresses TLR9-triggered proinflammatory cytokine production in macrophages by inhibiting ERK1/2 and AKT activation. Sci. Rep. 2018;8 doi: 10.1038/S41598-018-34011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng R., Adeniran S.O., Huang F., Li Y., Ma M., Zheng P., Zhang G. The ameliorative effect of melatonin on LPS-induced sertoli cells inflammatory and tight junctions damage via suppression of the TLR4/MyD88/NF-κB signaling pathway in newborn calf. Theriogenology. 2022;179:103–116. doi: 10.1016/J.THERIOGENOLOGY.2021.11.020. [DOI] [PubMed] [Google Scholar]
- 52.Ren D.L., Sun A.A., Li Y.J., Chen M., Ge S.C., Hu B. Exogenous melatonin inhibits neutrophil migration through suppression of ERK activation. J. Endocrinol. 2015;227:49–60. doi: 10.1530/JOE-15-0329. [DOI] [PubMed] [Google Scholar]
- 53.Maldonado M.D., Mora-Santos M., Naji L., Carrascosa-Salmoral M.P., Naranjo M.C., Calvo J.R. Evidence of melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol. Res. 2010;62:282–287. doi: 10.1016/j.phrs.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 54.Muxel S.M., Pires-Lapa M.A., Monteiro A.W.A., Cecon E., Tamura E.K., Floeter-Winter L.M., Markus R.P. NF-κB drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine-N-acetyltransferase (AA-NAT) gene. PLoS One. 2012;7 doi: 10.1371/JOURNAL.PONE.0052010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maldonado M.D., García-Moreno H., González-Yanes C., Calvo J.R. Possible involvement of the inhibition of NF-κB factor in anti-inflammatory actions that melatonin exerts on mast cells. J. Cell. Biochem. 2016;117:1926–1933. doi: 10.1002/JCB.25491. [DOI] [PubMed] [Google Scholar]
- 56.Hasan M.Z., Islam S., Matsumoto K., Kawai T. Meta-analysis of single-cell RNA-seq data reveals phenotypic switching of immune cells in severe COVID-19 patients. Comput. Biol. Med. 2021;137 doi: 10.1016/J.COMPBIOMED.2021.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reiter R.J., Sharma R., Ma Q., Liu C., Manucha W., Abreu-Gonzalez P., Dominguez-Rodriguez A. Plasticity of glucose metabolism in activated immune cells: advantages for melatonin inhibition of COVID-19 disease. Melatonin Res. 2020;3:362–379. doi: 10.32794/MR11250068. [DOI] [Google Scholar]
- 58.Duan F., Guo L., Yang L., Han Y., Thakur A., Nilsson-Payant B.E., Wang P., Zhang Z., Ma C.Y., Zhou X., Han T., Zhang T., Wang X., Xu D., Duan X., Xiang J., Tse H.-F., Liao C., Luo W., Huang F.-P., Chen Y.-W., Evans T., Schwartz R.E., tenOever B., Ho D.D., Chen S., Lian Q., Chen H.J. Modeling COVID-19 with human pluripotent stem cell-derived cells reveals synergistic effects of anti-inflammatory macrophages with ACE2 inhibition against SARS-CoV-2. Res. Sq. 2020 doi: 10.21203/RS.3.RS-62758/V1. [DOI] [Google Scholar]
- 59.Cuesta A., Cerezuela R., Esteban M.Á., Meseguer J. In vivo actions of melatonin on the innate immune parameters in the teleost fish gilthead seabream. J. Pineal Res. 2008;45:70–78. doi: 10.1111/J.1600-079X.2008.00557.X. [DOI] [PubMed] [Google Scholar]
- 60.Crespo I., Fernández-Palanca P., San-Miguel B., Álvarez M., González-Gallego J., Tuñón M.J. Melatonin modulates mitophagy, innate immunity and circadian clocks in a model of viral-induced fulminant hepatic failure. J. Cell. Mol. Med. 2020;24:7625–7636. doi: 10.1111/JCMM.15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castrillón P.O., Esquifino A.I., Varas A., Zapata A., Cutrera R.A., Cardinali D.P. Effect of melatonin treatment on 24-h variations in responses to mitogens and lymphocyte subset populations in rat submaxillary lymph nodes. J. Neuroendocrinol. 2000;12:758–765. doi: 10.1046/J.1365-2826.2000.00519.X. [DOI] [PubMed] [Google Scholar]
- 62.Ramos A., Míguez M.P., Morgado S., Sanchez-Correa B., Gordillo J.J., Casado J.G., Tarazona R., Regodón S. Melatonin enhances responsiveness to dichelobacter nodosus vaccine in sheep and increases peripheral blood CD4 T lymphocytes and IgG-expressing B lymphocytes. Vet. Immunol. Immunopathol. 2018;206:1–8. doi: 10.1016/J.VETIMM.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Gurunathan S., Kang M.-H., Choi Y., Reiter R.J., Kim J.-H. Melatonin: a potential therapeutic agent against COVID-19. Melatonin Res. 2021;4:30–69. doi: 10.32794/MR11250081. [DOI] [Google Scholar]
- 64.Cardinali D.P., Brown G.M., Pandi-Perumal S.R. An urgent proposal for the immediate use of melatonin as an adjuvant to anti- SARS-CoV-2 vaccination. Melatonin Res. 2021;4:206–212. doi: 10.32794/MR11250091. [DOI] [Google Scholar]
- 65.Boga J.A., Coto-Montes A., Rosales-Corral S.A., Tan D.X., Reiter R.J. Beneficial actions of melatonin in the management of viral infections: a new use for this “molecular handyman”? Rev. Med. Virol. 2012;22:323–338. doi: 10.1002/RMV.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zipeto D., Argañaraz G.A., Argañaraz E.R., Palmeira J.da F. ACE2/ADAM17/TMPRSS2 interplay may be the main risk factor for COVID-19. Front. Immunol. 2020;11 doi: 10.3389/FIMMU.2020.576745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zlacká J., Stebelová K., Zeman M., Herichová I. Interactions of renin-angiotensin system and COVID-19: the importance of daily rhythms in ACE2, ADAM17 and TMPRSS2 expression. Physiol. Res. 2021:S177–S194. doi: 10.33549/PHYSIOLRES.934754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shukla M., Htoo H.H., Wintachai P., Hernandez J.F., Dubois C., Postina R., Xu H., Checler F., Smith D.R., Govitrapong P., Vincent B. Melatonin stimulates the nonamyloidogenic processing of βaPP through the positive transcriptional regulation of ADAM10 and ADAM17. J. Pineal Res. 2015;58:151–165. doi: 10.1111/jpi.12200. [DOI] [PubMed] [Google Scholar]
- 69.Sen A. Does serotonin deficiency lead to anosmia, ageusia, dysfunctional chemesthesis and increased severity of illness in COVID-19? Med. Hypotheses. 2021;153 doi: 10.1016/j.mehy.2021.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feitosa E.L., Júnior F.T.D.S.S., Neto J.A.D.O.N., Matos L.F.L., Moura M.H.D.S., Rosales T.O., De Freitas G.B.L. COVID-19: rational discovery of the therapeutic potential of melatonin as a SARS-CoV-2 main protease inhibitor. Int. J. Med. Sci. 2020;17:2133–2146. doi: 10.7150/IJMS.48053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hazra S., Chaudhuri A.G., Tiwary B.K., Chakrabarti N. Matrix metallopeptidase 9 as a host protein target of chloroquine and melatonin for immunoregulation in COVID-19: a network-based meta-analysis. Life Sci. 2020;257 doi: 10.1016/J.LFS.2020.118096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cecon E., Izabelle C., Le Poder S., Real F., Zhu A., Tu L., Ghigna M.R., Klonjkowski B., Bomsel M., Jockers R., Dam J. Therapeutic potential of melatonin and melatonergic drugs on K18-hACE2 mice infected with SARS-CoV-2. J. Pineal Res. 2022;72 doi: 10.1111/JPI.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cecon E., Fernandois D., Renault N., Coelho C.F.F., Wenzel J., Bedart C., Izabelle C., Wimez S.G., Le Poder S., Klonjkowski B., Schwaninger M., Prevot V., Dam J., Jockers R. Melatonin drugs inhibit SARS-CoV-2 entry into the brain and virus-induced damage of cerebral small vessels. BioRxiv. 2022 doi: 10.1101/2021.12.30.474561. 2021.12.30.474561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang K., Chen W., Zhang Z., Deng Y., Lian J.Q., Du P., Wei D., Zhang Y., Sun X.X., Gong L., Yang X., He L., Zhang L., Yang Z., Geng J.J., Chen R., Zhang H., Wang B., Zhu Y.M., Nan G., Jiang J.L., Li L., Wu J., Lin P., Huang W., Xie L., Zheng Z.H., Zhang K., Miao J.L., Cui H.Y., Huang M., Zhang J., Fu L., Yang X.M., Zhao Z., Sun S., Gu H., Wang Z., Wang C.F., Lu Y., Liu Y.Y., Wang Q.Y., Bian H., Zhu P., Chen Z.N. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020;5 doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Behl T., Kaur I., Aleya L., Sehgal A., Singh S., Sharma N., Bhatia S., Al-Harrasi A., Bungau S. CD147-spike protein interaction in COVID-19: get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2021;808 doi: 10.1016/J.SCITOTENV.2021.152072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loh D. The potential of melatonin in the prevention and attenuation of oxidative hemolysis and myocardial injury from cd147 SARS-CoV-2 spike protein receptor binding. Melatonin Res. 2020;3:380–416. doi: 10.32794/mr11250069. [DOI] [Google Scholar]
- 77.Morchang A., Malakar S., Poonudom K., Noisakran S., Yenchitsomanus P.T., Limjindaporn T. Melatonin inhibits dengue virus infection via the sirtuin 1-mediated interferon pathway. Viruses. 2021;13 doi: 10.3390/V13040659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhai X., Wang N., Jiao H., Zhang J., Li C., Ren W., Reiter R.J., Su S. Melatonin and other indoles show antiviral activities against swine coronaviruses in vitro at pharmacological concentrations. J. Pineal Res. 2021;71 doi: 10.1111/JPI.12754. [DOI] [PubMed] [Google Scholar]
- 79.Tesarik J. Melatonin attenuates growth factor receptor signaling required for SARS-CoV-2 replication. Melatonin Res. 2020;3:534–537. doi: 10.32794/MR11250077. [DOI] [Google Scholar]
- 80.Klann K., Bojkova D., Tascher G., Ciesek S., Münch C., Cinatl J. Growth factor receptor signaling inhibition prevents SARS-CoV-2 replication. Mol. Cell. 2020;80:164–174.e4. doi: 10.1016/J.MOLCEL.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Coronaviruses. 2015;1282:1. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/SCIENCE.1085658. [DOI] [PubMed] [Google Scholar]
- 83.Ryu Y.B., Park S.J., Kim Y.M., Lee J.Y., Seo W.D., Chang J.S., Park K.H., Rho M.C., Lee W.S. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from tripterygium regelii. Bioorg. Med. Chem. Lett. 2010;20:1873–1876. doi: 10.1016/J.BMCL.2010.01.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., Liu C., Reiter R.J. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou Y., Hou Y., Shen J., Mehra R., Kallianpur A., Culver D.A., Gack M.U., Farha S., Zein J., Comhair S., Fiocchi C., Stappenbeck T., Chan T., Eng C., Jung J.U., Jehi L., Erzurum S., Cheng F. A network medicine approach to investigation and population-based validation of disease manifestations and drug repurposing for COVID-19. PLoS Biol. 2020;18 doi: 10.1371/JOURNAL.PBIO.3000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hosseini A., Ghaleh H.Esmaeili Gouvarchin, Aghamollaei H., Ramandi M.Fasihi, Alishiri G., Shahriary A., Hassanpour K., Tat M., Farnoosh G. Evaluation of Th1 and Th2 mediated cellular and humoral immunity in patients with COVID-19 following the use of melatonin as an adjunctive treatment. Eur. J. Pharmacol. 2021;904 doi: 10.1016/J.EJPHAR.2021.174193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Castillo R.R., Quizon G.R.A., Juco M.J.M., Roman A.D.E., de Leon D.G., Punzalan F.E.R., Guingon R.B.L., Morales D.D., Tan D.-X., Reiter R.J. Melatonin as adjuvant treatment for coronavirus disease 2019 pneumonia patients requiring hospitalization (MAC-19 PRO): a case series. Melatonin Res. 2020;3:297–310. doi: 10.32794/MR11250063. [DOI] [Google Scholar]
- 89.Ramlall V., Zucker J., Tatonetti N. Melatonin is significantly associated with survival of intubated COVID-19 patients. MedRxiv Prepr. Serv. Heal. Sci. 2020 doi: 10.1101/2020.10.15.20213546. [DOI] [Google Scholar]
- 90.Sánchez-González M.Á., Mahíllo-Fernández I., Villar-álvarez F., Llanos L. What if melatonin could help COVID-19 severe patients? J. Clin. Sleep Med. 2021 doi: 10.5664/JCSM.9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hasan Z.T., Atrakji D.M.Q.Y.M.A.A., Mehuaiden D.A.K. The effect of melatonin on thrombosis, sepsis and mortality rate in COVID-19 patients. Int. J. Infect. Dis. 2021 doi: 10.1016/J.IJID.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lei Q., Hou H., Yu C., Zhang Y., Ndzouboukou J.L.B., Lin X., Yao Z., Fu H., Sun Z., Wang F., Fan X. Kinetics of neutralizing antibody response underscores clinical COVID-19 progression. J. Immunol. Res. 2021;2021 doi: 10.1155/2021/9822706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ouoba S., Okimoto M., Nagashima S., Kitahara Y., Miwata K., Ko K., E B., Sugiyama A., Takahashi K., Sakaguchi T., Takafuta T., Tanaka J. Sequential dynamics of virological and serological changes in the serum of SARS-CoV-2 infected patients. J. Med. Virol. 2021 doi: 10.1002/JMV.27518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khalili M., Karamouzian M., Nasiri N., Javadi S., Mirzazadeh A., Sharifi H. Epidemiological characteristics of COVID-19: a systematic review and meta-analysis. Epidemiol. Infect. 2020;148 doi: 10.1017/S0950268820001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.The RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMOA2021436/SUPPL_FILE/NEJMOA2021436_. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang F., Karam J.G. Glycemic profile of intravenous dexamethasone-induced hyperglycemia using continuous glucose monitoring. Am. J. Case Rep. 2021;22 doi: 10.12659/AJCR.930733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.FakhriRavari A., Jin S., Kachouei F.H., Le D., Lopez M. Systemic corticosteroids for management of COVID-19: saving lives or causing harm? Int. J. Immunopathol. Pharmacol. 2021;35 doi: 10.1177/20587384211063976. 205873842110639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mahase E. Covid-19: UK becomes first country to authorise antiviral molnupiravir. BMJ. 2021;375 doi: 10.1136/BMJ.N2697. [DOI] [PubMed] [Google Scholar]
- 99.Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study | Pfizer, (n.d.). https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate (accessed November 24, 2021).
- 100.Heskin J., Pallett S.J.C., Mughal N., Davies G.W., Moore L.S.P., Rayment M., Jones R. Caution required with use of ritonavir-boosted PF-07321332 in COVID-19 management. Lancet (London, England) 2022;399:21–22. doi: 10.1016/S0140-6736(21)02657-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma X., Idle J.R., Krausz K.W., Gonzalez F.J. Metabolism of melatonin by human cytochromes p450. Drug Metab. Dispos. 2005;33:489–494. doi: 10.1124/DMD.104.002410. [DOI] [PubMed] [Google Scholar]
- 102.Cardinali D.P., Brown G.M., Reiter R.J., Pandi-Perumal S.R. Elderly as a high-risk group during COVID-19 pandemic: effect of circadian misalignmentSleep Dysregulation and Melatonin Administration. Sleep Vigil. 2020;4:81–87. doi: 10.1007/S41782-020-00111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Büki A., Okonkwo D.O., Wang K.K., Povlishock J.T. Cytochrome c release and caspase activation in traumatic axonal injury. J. Neurosci. 2000;20:2825–2834. doi: 10.1523/JNEUROSCI.20-08-02825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Simko F., Reiter R.J. Is melatonin deficiency a unifying pathomechanism of high risk patients with COVID-19? Life Sci. 2020;256 doi: 10.1016/J.LFS.2020.117902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Regeneron Announces New U.S. Government Agreement to Purchase Additional Doses of REGEN-COVTM (casirivimab and imdevimab) Antibody Cocktail | Regeneron Pharmaceuticals Inc., (n.d.). https://investor.regeneron.com/news-releases/news-release-details/regeneron-announces-new-us-government-agreement-purchase (accessed November 24, 2021).
- 106.Gilead’s coronavirus treatment remdesivir to cost $3,120 for U.S. insured patients, (n.d.). https://www.cnbc.com/2020/06/29/gileads-coronavirus-treatment-remdesivir-to-cost-3120-for-us-insured-patients.html (accessed November 24, 2021).
- 107.Merck’s COVID Pills Price Could Change From $700 Federal Government Paid, (n.d.). https://www.newsweek.com/merck-covid-pill-cost-molnupiravir-1637550 (accessed January 4, 2022).
- 108.U.S. authorizes Pfizer oral COVID-19 treatment, first for at-home use | Reuters, (n.d.). https://www.reuters.com/business/healthcare-pharmaceuticals/pfizer-oral-covid-19-pill-gets-us-authorization-at-home-use-2021-12-22/ (accessed January 4, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.