Abstract
This study explored factors associated with durable viral suppression (DVS) among two groups of people living with HIV (PLWH) and problem substance use in the context of universal antiretroviral treatment initiation. Participants (N=99) were recruited between 2014–2017 from public sexual health clinics [SHC] and a hospital detoxification unit [detox]). DVS (NYC HIV surveillance registry) was defined as two consecutive viral load tests ≤ 200 copies/mL, ≤ 90 days apart, with all other viral loads suppressed over 12 or 18 months. Detox participants were significantly older, with more unstable housing/employment, substance use severity, and longer-term HIV vs. SHC participants. Older age, opioid and stimulant use disorder were significantly associated with lower odds of DVS, while fulltime employment and stable housing were significantly associated with higher odds of DVS at 12-month follow-up. Patterns held at 18-month follow-up. Co-located substance use and HIV services, funding for supportive housing, and collaborative patient-provider relationships could improve DVS among populations with syndemic of problem substance use, poverty, and long-term HIV.
Keywords: Adherence, Antiretroviral therapy, HIV/AIDS, Substance Use, Viral Load
INTRODUCTION
Despite the known benefits of achieving and sustaining HIV viral suppression, only 62.7% of people living with HIV (PLWH) in the United States were virally suppressed as of 2017 [1]. The proportion of people with durable viral suppression (DVS; defined as at least one pair of viral load tests of ≤ 200 copies/mL, ≤ 90 days apart, with all other viral loads suppressed over a two-year period) is even smaller. Data from the current study show that, of all PLWH newly diagnosed in New York City (NYC) from 2014 to 2017, only 54% remained virally suppressed over an 18-month period. This reflects a minimal improvement from 2011, when 52% of all PLWH in NYC had reached DVS [2].
Although rates of DVS have improved in NYC more recently (e.g., 69% of people in HIV medical care in 2019 had reached DVS [3]), ongoing challenges persist, including barriers to antiretroviral treatment (ART) initiation and adherence. One study found that close to a quarter of NYC PLWH who received a new HIV diagnosis in 2015 had not initiated ART [4]. DVS may be particularly challenging to achieve among people with problem substance use who face barriers across the HIV care continuum, from to being offered ART, accessing ART, retention in care, and adhering to ART at the level needed to reach DVS [5, 6]. Problem substance use is also frequently associated with multiple other adverse determinants of health, such as unemployment, unstable housing, and discrimination and stigma that may impact HIV-related health [6].
On World AIDS Day, December 1, 2011, the New York City Department of Health and Mental Hygiene (NYC DOHMH) first recommended “universal ART”–that ART be offered to all newly diagnosed PLWH regardless of CD4 cell count or other markers of HIV progression [7], with the goal of 80% achieving DVS within 12 months of diagnosis [2]. Universal ART was implemented as a means of increasing treatment for PLWH, reducing possible HIV transmission, and lowering community viral load (i.e., the concept of “treatment as prevention”) [8–15].
In addition to universal ART, other subsequent HIV prevention strategies such as Seek, Test, Treat, and Retain [12, 16, 17], free condom distribution, treatment for opioid use disorders [18, 19], needle and syringe exchange services [20], citywide promotion of pre- and post-exposure prophylaxis (PrEP and PEP), and provision of PrEP and PEP in the city’s public sexual health clinics (SHCs) [21], have sought to positively impact indicators along the HIV care continuum [22] and to contribute to ending the HIV epidemic in NYC [23]. Despite the implementation of these strategies in NYC, as described above, a substantial proportion of PLWH have not maintained DVS.
A closer exploration of the factors associated with successful and ongoing viral suppression is necessary to better understand the barriers that persist. A syndemics approach is especially indicated to understand how intersectional individual and structural factors converge to perpetuate the problem of unsuppressed viral load [24, 25]. A syndemic framework is particularly useful in considering the multiple and severe stressors of our study population, many of whom are individuals involved with substances and with varying histories of living with HIV [25].
Purpose
Using data from two groups recruited from NYC DOHMH SHCs and a hospital-based alcohol/drug detoxification unit (detox) from 2014 to 2017, we conducted an exploratory analysis to examine factors associated with DVS at 12 and 18 months after enrollment into the study; and to compare DVS proportions in our study to the 52% benchmark for all NYC PLWH in 2011 [2], the year the current study was conceptualized. We sought to better understand the range of viral suppression outcomes in NYC, particularly in the context of several recent NYC HIV-related initiatives. The two groups also had markedly different demographic characteristics, with the detox-recruited group comprised mainly of people living with HIV for at least 15 years and with lower rates of full-time employment, more housing instability, and greater substance use severity compared to the SHC-recruited group.
METHODS
Participants
This study enrolled participants from 2014 to 2017. In total, 259 individuals screened for the study, 208 were eligible, and 99 enrolled (two participants who were in continuous care but elected not to take ART were excluded for the purposes of this analysis). To be eligible, participants: (a) reported problem substance use, operationalized broadly to capture variability in severity: any illicit drug use in the past year and/or heavy drinking in the past month (in a single day, more than four drinks for those assigned male sex at birth; more than three drinks for those assigned female sex at birth) [26]; b) were able to speak and understand English; c) resided in one of the five NYC boroughs (Bronx, Brooklyn, Manhattan, Queens, Staten Island); and d) were HIV-positive (self-report and cross-checked in the NYC DOHMH HIV surveillance registry).
Procedures
Potential participants were approached after testing HIV-positive in the NYC DOHMH SHCs (n=36), or in a hospital-based detox unit (n=54) and given information about the study. Nine additional participants were recruited via study flyers and word-of-mouth from detox participants already enrolled in the study and were thus included in the detox-recruited group (total detox n=63). Those interested in participating completed a brief eligibility screening interview either over the phone (SHC) or in person (detox) after providing verbal consent.
After screening, eligible participants completed a confidential in-person baseline interview where they provided written informed consent and completed an interviewer-administered assessment. As part of the consent process, participants also gave permission to match research data with their records in the NYC DOHMH HIV surveillance registry. All enrolled participants were invited to participate in confidential follow-up assessments every six months for a period of two years. Participants were provided a round-trip NYC subway MetroCard for screening (completed by phone) and $30 plus a round-trip MetroCard at each of the assessment visits. Participants also received a round-trip MetroCard (or equivalent payment in cash) for updating their contact information at follow-up assessments. Those who expressed a need for additional mental health support were offered referrals to local services and resources. Institutional Review Board (IRB) approval was obtained from the Icahn School of Medicine at Mount Sinai and the NYC DOHMH.
Measures
All demographic and clinical characteristics (except viral suppression) were collected at the baseline interview.
Demographic characteristics included age in years, race/ethnicity; gender identity; education in years; employment, housing, and health insurance status; year of HIV diagnosis; and whether participants reported being a cisgender man who had had sex with men and had engaged in injection drug use in the past six months.
Problem substance use was measured in three ways, using: (1) the Diagnostic and Statistical Manual – 5th Edition (DSM-5) to determine if participants met criteria for each of the following disorders: alcohol; cannabis; opioid; and/or stimulant use disorder (defined as endorsement of at least two of 11 DSM-5 symptoms in the past 12 months) [27]; (2) the Timeline Follow-Back method (TLFB) [28] to determine number of heavy drinking days (in a single day, more than 4 drinks [for participants who were assigned male sex at birth]; more than 3 drinks [for participants assigned female sex at birth]) [29] in the past six months and number of days on which drugs and/or alcohol were used (systematic TLFB methods were used to assist participant recall, which has demonstrated reliability up to 12 months [30]); and (3) an 8-panel urine drug toxicology screen (cannabis, cocaine, opioids, methamphetamine, amphetamines, benzodiazepines). Participants were also asked whether they currently smoked cigarettes.
Mental health and social support were assessed using the following measures. The Kessler-10, a global measure of distress, assessed participants’ anxiety and depression over the previous 30 days [31]. Participants reported the total number of their close friends and relatives as well as the number of family members and the number of friends, respectively, who knew their HIV status. The Medical Outcomes Study (MOS) Social Support Scale measured availability of social support [32]. The Attitudes Toward HIV Health Care Providers Scale measured participants’ perceptions of professionalism and emotional support from their medical providers [33]. The Medical Mistrust Index assessed participants’ attitudes toward medical systems [34].
HIV surveillance registry variables:
Participants’ study records were matched with NYC HIV surveillance registry data, including HIV viral load test results. Due to changes in viral load testing technology and recent discussions in the field related to measurement of viral suppression [35, 36], the current definition of DVS (at least two viral load tests of ≤ 200 copies/mL at least 90 days apart within a 12 or 18-month period, with all other viral loads suppressed during the same timeframe) was adjusted from that of the definition used to calculate the original 52% DVS benchmark in 2011 (at least two viral load test of ≤ 400 copies/mL at least two weeks apart within a two-year period with all other viral loads suppressed within the same timeframe) [2]. The date of DVS was based on the second suppressed viral load test that would qualify a participant as having DVS, with DVS among study participants examined at 12 and 18 months after enrollment into the study. DVS was also examined among all people newly diagnosed with HIV and living in NYC at time of diagnosis (excluding participants in the study) between January 1, 2014 and December 31, 2017 to explore how study participants compared to the general NYC PLWH population.
Data Analysis
Baseline demographic and psychosocial characteristics were assessed using descriptive statistics and χ2 (or Fisher’s exact test if the expected cell counts were less than five) and t-tests (or Wilcoxon rank-sum tests, in the case of small sample size or observed non-normal data distribution) to compare the SHC and detox participant characteristics at baseline. Year of HIV diagnosis was not included in bivariate analyses as it mapped closely onto recruitment groups (SHC and detox). A one-sample proportion test was used to determine if DVS was significantly different than 52% (a priori benchmark) for the total sample and for each recruitment group (those recruited from the SHCs and detox) at 12- and 18-months post-study enrollment. We also used χ2 (or Fisher’s exact test) and t-tests (or Wilcoxon rank-sum test) to assess differences in DVS among the overall sample by various demographic and psychosocial characteristics of the participants. All analyses were performed in SAS® 9.4 (SAS Institute, Cary NC) on level of significance 5%.
RESULTS
Demographic characteristics
As described in Table 1, of the total sample of 99 participants, 36% were recruited from the SHCs and 64% from detox. On average, participants were 44 years old (SD: 13.20) and had 13 years of education (SD: 2.47). Participants were primarily non-Hispanic Black/African American (52%) or Hispanic/Latinx (35%), cisgender men (86%), and living in their own apartment or house (59%). Most had had health insurance in the past year (92%). Only 27% were employed full time. Years living with diagnosed HIV varied, with 28% diagnosed before 1995, 19% between 1995–1999, 17% in 2000–2003, and 35% in 2014–2017.
Table 1.
Baseline Demographic Characteristics, Substance Use, Mental Health, Social Support, and Attitudes Toward Medical Care among People Living with HIV and Problem Substance Use, New York City Department of Health and Mental Hygiene Sexual Health Clinic and Hospital Detoxification Patients, N = 99
| TOTAL SAMPLE (N = 99) a n (%)/Mean ± SD/Median (IQR) |
Sexual Health Clinic (n = 36) n (%)/Mean ± SD/Median (IQR) |
Detox (n = 63) n (%)/Mean ± SD/Median (IQR) |
Test Statistic | p | |
|---|---|---|---|---|---|
|
| |||||
| Demographic Characteristics | |||||
|
| |||||
| Age in Years | 43.63 ± 13.20 | 28.14 ± 5.96 | 52.49 ± 6.02 | t(97)=−19.43 | <.001 |
| Years of Education | 13.11 ± 2.47 | 14.89 ± 2.01 | 12.10 ± 2.11 | t(97)=6.45 | <.001 |
| Race/Ethnicity | * | <.001 | |||
| Non-Hispanic Black/African American | 51 (51.52) | 11 (30.56) | 40 (63.49) | ||
| Hispanic/Latinx, Any Race | 35 (35.35) | 15 (41.67) | 20 (31.75) | ||
| Non-Hispanic White | 12 (12.12) | 9 (25.00) | 3 (4.76) | ||
| Other Race/Ethnicity | 1 (1.01) | 1 (2.78) | 0 (0.00) | ||
| Gender Identity | * | .004 | |||
| Cisgender Man | 85 (85.86) | 35 (97.22) | 50 (79.37) | ||
| Cisgender Woman | 12 (12.12) | 0 (0.00) | 12 (19.05) | ||
| Transgender Woman | 2 (2.02) | 1 (2.78) | 1 (1.59) | ||
| Employed Full-Time a | 26 (26.53) | 23 (63.89) | 3 (4.84) | χ2(1)=40.74 | <.001 |
| Housing Status | χ2(2)=19.36 | <.001 | |||
| Your Own House/Apartment b | 58 (58.59) | 23 (63.89) | 35 (55.56) | ||
| Someone Else’s House/Apartment | 20 (20.20) | 13 (36.11) | 7 (11.11) | ||
| Unstably Housed c | 21 (21.21) | 0 (0.00) | 21 (33.33) | ||
| Had Health Insurance in Past Year | 91 (91.92) | 28 (77.78) | 63 (100.00) | * | <.001 |
| Year of HIV Diagnosis | * | <.001 | |||
| Before 1995 | 28 (28.28) | 0 (0.00) | 28 (44.44) | ||
| 1995–1999 | 19 (19.19) | 0 (0.00) | 19 (30.16) | ||
| 2000–2013 | 17 (17.17) | 3 (8.33) | 14 (22.22) | ||
| 2014–2017 | 35 (35.35) | 33 (91.67) | 2 (3.17) | ||
| Cisgender Man who Had Sex with Men – Past 6 Months | 43 (43.43) | 35 (97.22) | 8 (12.69) | χ2(1)=63.22 | <.001 |
| Injection Drug Use – Past 6 Months | 15 (15.15) | 3 (8.33) | 12 (19.05) | χ2(1)=1.30 | .244 |
|
| |||||
| Substance Use | |||||
|
| |||||
| Alcohol Use Disorder d | 56 (56.57) | 19 (52.78) | 37 (58.73) | χ2(1)=0.33 | .565 |
| Cannabis Use Disorder d | 34 (34.34) | 15 (41.67) | 19 (30.16) | χ2(1)=1.35 | .246 |
| Opioid Use Disorder d | 26 (26.26) | 1 (2.78) | 25 (39.68) | χ2(1)=16.11 | <.001 |
| Stimulant Use Disorder d | 60 (60.61) | 13 (36.11) | 47 (74.60) | χ2(1)=14.22 | <.001 |
| Days of Heavy Drinking, e Past 6 months (180 Days) | 8 (1.00–31.00) | 4 (1.00–14.00) | 10 (0.00–90.00) | Z=1.46 | .143 |
| Days of Drug and/or Alcohol Use, Past 6 Months (180 Days) | 92 (49.00–165.00) | 71.5 (37.5–135.5) | 140 (68.00–166.00) | Z=1.83 | .068 |
| Positive Urine Toxicology Screen, Any Substance | 62 (62.63) | 15 (41.67) | 47 (74.60) | χ2(1)=10.62 | .001 |
| Marijuana | 31 (31.31) | 11 (35.48) | 20 (35.09) | χ2(1)=0.00 | .970 |
| Cocaine | 39 (39.39) | 3 (9.68) | 36 (63.16) | χ2(1)=20.86 | <.001 |
| Opioids | 16 (16.16) | 0 (0.00) | 16 (28.07) | * | .001 |
| Methamphetamine | 4 (4.04) | 3 (9.68) | 1 (1.75) | * | .123 |
| Amphetamines | 2 (2.02) | 1 (3.23) | 1 (1.75) | * | 1.00 |
| Benzodiazepines | 19 (19.19) | 1 (3.23) | 18 (31.58) | χ2(1)=7.65 | .002 |
| Currently Smokes Cigarettes | 69 (69.70) | 18 (50.00) | 51 (80.95) | χ2(1)=10.39 | .001 |
|
| |||||
| Mental Health & Social Support | |||||
|
| |||||
| Distress (Anxiety, Depression), Past 30 Days f | 22.92 ± 7.88 | 24.14 ± 8.22 | 22.22 ± 7.65 | t(97)=1.17 | .246 |
| Number of Close Friends and Relatives | Z=0.06 | .950 | |||
| 0–2 | 27 (27.55) | 11 (30.56) | 16 (25.81) | ||
| 3–6 | 39 (39.80) | 12 (33.33) | 27 (43.55) | ||
| 7 or More | 32 (32.65) | 13 (36.11) | 19 (30.65) | ||
| Number Family Members Who Know Your HIV Status | 3 (0.00–8.00) | 0 (0.00–1.00) | 5 (2.00–12.00) | Z=−4.47 | <.001 |
| Number Friends Who Know Your HIV Status | 3 (0.00–9.50 | 2 (0.00–6.00) | 3 (0.00–10.00) | Z=−1.14 | .257 |
| Availability of Social Support g | 55.74 ±16.78 | 58.39 ± 15.95 | 54.22 ± 17.18 | t(97)=1.19 | .240 |
|
| |||||
| Attitudes Toward Medical Care | |||||
|
| |||||
| Attitudes Toward HIV Health Care Providers h | 83.27 ± 14.19 | 76.68 ± 22.09 | 86.35 ± 6.57 | t(29)=−2.27 | .031 |
| Attitudes Toward Medical Systems i | 18.62 ± 3.30) | 17.67 ± 3.36 | 19.16 ± 3.17 | t(97)=−2.21 | .030 |
NOTES:
IQR = interquartile range
Fisher’s Exact test
Responses may not add up to 100% due to missing data.
Includes partner’s house/apartment
Includes no fixed address; room rented on a daily basis; shelter welfare residence, or single room occupancy; incarcerated; residential treatment/hospital; or something else
Substance Use Disorder = Endorsement of ≥ 2 DSM-5 symptoms in the past 12 months
In a single day: ≥ 4 drinks (assigned male at birth); ≥ 3 drinks (assigned female at birth)
Kessler-10 Score, Possible Range = 10–50, Higher score = more distress
MOS Social Support Score, Possible Range = 0–100, Higher Score = More frequent availability of different types of support when needed
Possible Range = 19–114, Higher Score = More positive attitude toward HIV health care providers
Medical Mistrust Index, Possible Range = 7–28, Higher Score = Greater Mistrust
SHC and detox participants significantly differed on several demographic characteristics (all p<.001). Compared to detox participants, SHC participants were significantly younger (M=28.14 years, SD = 5.96 vs. M=52.49 years, SD=6.02, t(97)=−19.43) and had more years of education (M=14.89 years, SD=2.01 vs. M=12.10 years, SD=2.11, t(97)=6.45). A significantly larger proportion of SHC participants were white (25% vs. detox 5%, Fisher’s exact test), employed full-time (SHC 64% vs. detox 5%, χ2(1)=40.74), lived in their own house or apartment (64% vs. detox 56%, χ2(2)=19.36), and were diagnosed with HIV between 2014–2017 (92% vs. detox 3%, Fisher’s exact test). A significantly larger proportion of detox participants had been living with long-term HIV infection (74% diagnosed in 1999 or earlier vs. all SHC in the year 2000 or later, Fisher’s exact test; see Table 1). Although we did not collect data on when detox participants first initiated ART, nearly all detox participants self-reported a prescription for ART within the last 30 days (94%) vs. only 47% of SHC participants (χ2(1)=27.69, p<.001). The low proportion of current ART prescription among SHC participants may have been because many had received an HIV diagnosis within a few days of study enrollment and had yet to initiate ART (data not shown in tables).
Problem Substance use
Among the entire sample, 85 participants (86%) met DSM-5 criteria for any substance use disorder (SUD; data not shown in tables). Over half the sample met DSM-5 criteria for an alcohol (57%) or a stimulant (61%) use disorder; about a quarter for opioid use disorder (26%) and approximately a third for cannabis use disorder (34%; see Table 1). A significantly larger proportion of detox participants (as expected given recruitment from a detox facility) met DSM-5 criteria for opioid use disorder (40% vs. SHC 3%, χ2(1)=16.11, p<.001) and stimulant use disorder (75% vs. SHC 36%, χ2(1)=14.22; p<.001) compared to SHC participants. See Table 1 for additional outcomes on substance use, mental health, social support, and attitudes toward medical care.
Durable viral suppression - HIV surveillance registry data
Of the total sample of 99 participants with viral load data in the surveillance system within 12 months from date of study enrollment, 47 (47%) achieved DVS within 12 months of enrollment. Of the 73 participants with viral load data within 18 months of enrollment (74% of the total sample), 39 (53%) had achieved DVS within 18 months of enrollment. Neither 12-month (Z=−0.90, p=.368) nor 18-month (Z=0.24, p=.808) DVS for the overall sample was significantly different than 52% (see Table 2).
Table 2.
Durable Viral Load Suppression, a New York City Department of Health and Mental Hygiene Sexual Health Clinic and Hospital Detoxification Patients, N = 99
| TOTAL | 12-Month Follow-Up | Test Statistic | p b | 18-Month Follow-Up | Test Statistic | p b | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Total with data at 12-month follow-up, n (%) | Durably suppressed at 12-month follow-up, n (%) | Total with data at18-month follow-up, n (%) | Durably suppressed at 18-Month follow-up, n (%) | ||||||
|
| |||||||||
| Total Sample | 99 | 99 (100.00) | 47 (47.47) | Z=−0.90 | .368 | 73 (73.74) | 39 (53.42) | Z=0.24 | .808 |
| Recruitment Location | |||||||||
| Sexual Health Clinic | 36 | 36 (100.00) | 26 (72.22) | Z=2.43 | .015 | 31 (86.11) | 23 (74.19) | Z=2.47 | .013 |
| Detoxification Center | 63 | 63 (100.00) | 21 (33.33) | Z=−2.97 | .003 | 42 (66.67) | 16 (38.10) | Z=−1.80 | .071 |
| New Yorkers Newly Diagnosed with HIV, 2014–2017 c | 8438 | 8438 (100.00) | 4339 (51.42) | Z=−1.07 | .283 | 6803 (80.62) | 3699 (54.37) | Z=3.92 | <.001 |
Durable viral suppression = two consecutive viral load tests at least 90 days apart, of ≤ 200 copies/mL, with no intervening unsuppressed viral load tests
P-value from One-Sample Proportion Test of Suppression Rate vs. 52%
Includes all new HIV diagnoses at a New York City provider from January 1, 2014 through December 31, 2017 that were reported to the HIV Surveillance Registry and living in NYC at the time of diagnosis, excluding participants in the current study
The proportion of SHC participants who had achieved DVS at the 12-month follow-up was significantly higher than 52% (n=26, 72%, Z=2.43, p=.015), as was the proportion who achieved DVS at the 18-month follow-up (n=23 of n=31 with viral load data at 18-month follow-up, 74%; Z=2.47, p=.013) (see Table 2 and Fig. 1). In contrast to the SHC participants, the proportion of detox participants with DVS at 12-month follow-up (n=21, 33%) was significantly lower than 52% (Z=−2.97, p=.003), but was only marginally lower than 52% at the 18-month follow-up (n=16 of n=42 with viral load data at 18-month follow-up, 38%; Z=−1.80, p=.071) (see Table 2 and Fig 1). Although all participants had viral load data available in the NYC DOHMH HIV surveillance registry at their 12-month study follow-up, some were missing data by the 18-month follow-up, possibly because they were disengaged in care in NYC, or had moved out of the jurisdiction [37].
Fig. 1.
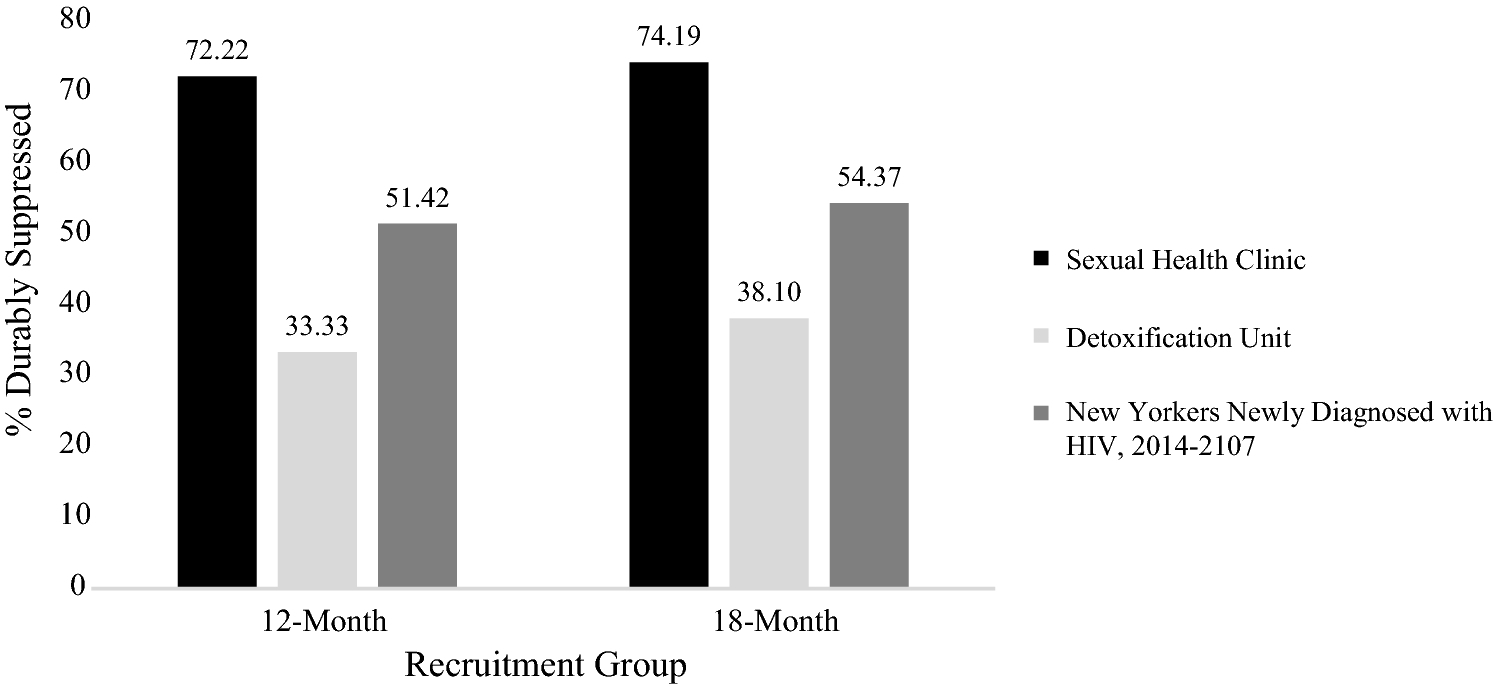
Durable Viral Suppression by Recruitment Group at 12- and 18-Month Follow-Up (N=99), and Among New Yorkers Newly Diagnosed with HIV, 2014–2017 (N=8,438)
Percentages for all people newly diagnosed with HIV (excluding participants in the current study) by a NYC provider between January 1, 2014 and December 31, 2017 and living in NYC at the time of diagnosis provide a useful frame of reference. Of those individuals (n=8438), 51% (n=4339) had achieved DVS within 12 months and 54% (n=3699 of n=6803 with 18-month data) had done so within 18 months of diagnosis; the latter was significantly greater than 52% (Z=3.92, p<.001; see Table 2).
Factors associated with DVS – Full Sample
Older age (odds ratio [OR]: 0.94, 95% confidence interval [CI]: 0.92–0.98, χ2(1)=12.81, p<.001), Black/African American race (vs. white, OR: 0.10, 95% CI: 0.01–0.56, χ2(1)=7.70, p=.006) having an opioid (OR: 0.30, 95% CI: 0.11–0.81, χ2(1)=6.17, p=.013) or stimulant use disorder (OR: 0.39, 95% CI: 0.27–0.89, χ2(1)=5.14, p=.023), a positive urine toxicology screen (OR: 0.38, 95% CI: 0.17–0.89, χ2(1)=5.15, p=.023), currently smoking cigarettes (OR: 0.40, 95% CI: 0.16–0.96, χ2(1)=4.37, p=.037), and having fewer family members who knew one’s HIV status (OR: 0.95, 95% CI: 0.91–1.00, χ2(1)=4.20, p=.040) were all associated with lower odds of DVS 12 months after enrollment (see Table 3). Having more years of education (OR: 1.33, 95% CI: 1.10–1.60, χ2(1)=10.13, p=.002), being employed full-time (vs. not, OR: 4.52, 95% CI: 1.68–12.17, χ2(1)=9.94, p=.002), and living in one’s own house or apartment (vs. being unstably housed; OR: 3.67, 95% CI: 1.18–11.36, χ2(1)=5.10, p=.024) were all associated with higher odds of 12-month DVS (see Table 3). Patterns largely held within 18 months of enrollment. At 18-month follow-up, having an alcohol use disorder (OR: 2.25, 95% CI: 1.00–5.08, χ2(1)=3.90, p=.048) also predicted higher odds of achieving DVS (see Table 3).
Table 3.
Predictors of Durable Viral Suppression at 12- and 18-Months after Enrollment by Baseline Demographic Characteristics, Substance Use, Mental Health, Social Support, and Attitudes Toward Medical Care among People Living with HIV and Problem Substance Use, N = 99
| 12-Month Follow-Up OR (95% CI) | Test Statistic | 18-Month Follow-Up OR (95% CI) | Test Statistic | |||
|---|---|---|---|---|---|---|
| p | p | |||||
|
| ||||||
| Demographic Characteristics | ||||||
|
| ||||||
| Age in Years | 0.94 (0.92–0.98) | χ2(1)=12.81 | <.001 | 0.95 (0.91–0.98) | χ2(1)=12.08 | <.001 |
| Years of Education | 1.33 (1.10–1.60) | χ2(1)=10.13 | .002 | 1.34 (1.11–1.61) | χ2(1)=9.09 | .003 |
| Race/Ethnicity | ||||||
| Non-Hispanic Black/African American | 0.10 (0.01–0.56) | χ2(1)=7.70 | .006 | 0.17 (0.04–0.70) | χ2(1)=6.03 | .014 |
| Hispanic/Latinx, Any Race | 0.24 (0.02–1.40) | χ2(1)=2.89 | .089 | 0.50 (0.11–2.18) | χ2(1)=0.85 | .356 |
| Non-Hispanic White | 1.00 | 1.00 | ||||
| Gender Identity a | ||||||
| Cisgender Man | 1.00 | |||||
| Cisgender & Transgender Woman | 1.13 (0.36, 3.49) | χ2(1)=0.04 | .838 | 0.77 (0.25–2.40) | χ2(1)=0.21 | .649 |
| Employed Full-Time (Yes v. No) b | 4.52 (1.68–12.17) | χ2(1)=9.94 | .002 | 5.56 (1.98–15.55) | χ2(1)=12.34 | <.001 |
| Housing Status | ||||||
| Your Own House/Apartment c | 3.67 (1.18–11.36) | χ2(1)=5.10 | .024 | 4.22 (1.36–13.08) | χ2(1)=6.24 | .013 |
| Someone Else’s House/Apartment | 3.91 (1.03–14.87) | χ2(1)=4.00 | .045 | 3.20 (0.84–12.13) | χ2(1)=2.93 | .087 |
| Unstably Housed d | 1.00 | 1.00 | ||||
| Had Health Insurance in Past Year (Yes vs. No) | 0.28 (0.03–1.65) | χ2(1)=2.73 | .098 | 0.29 (0.05–1.49) | χ2(1)=2.54 | .111 |
|
| ||||||
| Substance Use | ||||||
|
| ||||||
| Alcohol Use Disorder (Yes vs. No) e | 1.49 (0.67–3.32) | χ2(1)=0.96 | .326 | 2.25 (1.00–5.08) | χ2(1)=3.90 | .048 |
| Cannabis Use Disorder (Yes vs. No) e | 1.17 (0.51–2.68) | χ2(1)=0.13 | .716 | 0.92 (0.40–2.10) | χ2(1)=0.04 | .837 |
| Opioid Use Disorder (Yes vs. No) e | 0.30 (0.11–0.81) | χ2(1)=6.17 | .013 | 0.58 (0.23–1.44) | χ2(1)=1.43 | .232 |
| Stimulant Use Disorder (Yes vs. No) e | 0.39 (0.27–0.89) | χ2(1)=5.14 | .023 | 0.59 (0.26–1.33) | χ2(1)=1.62 | .203 |
| Days of Heavy Drinking, f Past 6 months (180 Days) | 1.00 (0.98–1.01) | χ2(1)=0.29 | .588 | 0.98 (0.97–1.00) | χ2(1)=3.85 | .050 |
| Days of Drug and/or Alcohol Use, Past 6 Months (180 Days) | 0.99 (0.98–1.00) | χ2(1)=2.53 | .112 | 0.99 (0.98–1.00) | χ2(1)=2.16 | .141 |
| Positive Urine Toxicology Screen, Any Substance (Yes vs. No) | 0.38 (0.17–0.89) | χ2(1)=5.15 | .023 | 0.49 (0.22–1.13) | χ2(1)=2.86 | .091 |
| Currently Smokes Cigarettes (Yes vs. No) | 0.40 (0.16–0.96) | χ2(1)=4.37 | .037 | 0.34 (0.14–0.84) | χ2(1)=5.77 | .016 |
|
| ||||||
| Mental Health & Social Support | ||||||
|
| ||||||
| Distress (Anxiety, Depression), Past 30 Days g | 1.03 (0.98–1.09) | χ2(1)=1.39 | .238 | 0.98 (0.93–1.03) | χ2(1)=0.49 | .486 |
| Number of Close Friends and Relatives | 0.60 (0.35–1.01) | χ2(1)=3.77 | .052 | 1.11 (0.67–1.86) | χ2(1)=0.16 | .686 |
| Number Family Members Who Know Your HIV Status | 0.95 (0.91–1.00) | χ2(1)=4.20 | .040 | 0.97 (0.93–1.02) | χ2(1)=1.62 | .203 |
| Number Friends Who Know Your HIV Status | 1.00 (0.97–1.04) | χ2(1)=0.05 | .829 | 1.01 (0.98–1.05) | χ2(1)=0.42 | .516 |
| Availability of Social Support h | 0.99 (0.97–1.02) | χ2(1)=0.36 | .546 | 0.99 (0.96–1.01) | χ2(1)=1.30 | .255 |
|
| ||||||
| Attitudes Toward Medical Care | ||||||
|
| ||||||
| Attitudes Toward HIV Health Care Providers i | 0.84 (0.31–2.27) | χ2(1)=0.12 | .728 | 1.22 (0.45–3.33) | χ2(1)=0.15 | .694 |
| Attitudes Toward Medical Systems j | 0.95 (0.84–1.08) | χ2(1)=0.63 | .426 | 0.90 (0.79–1.02) | χ2(1)=3.11 | .078 |
NOTES:
OR = Odds Ratio, CI = Confidence Interval
Due to the small sample size, transgender women (n = 2) were grouped with cisgender women in the bivariate analyses.
Responses may not add up to 100% due to missing data.
Includes partner’s house/apartment
Includes no fixed address; room rented on a daily basis; shelter welfare residence, or single room occupancy; incarcerated; residential treatment/hospital; or something else
Substance Use Disorder = Endorsement of ≥ 2 DSM-5 symptoms in the past 12 months
In a single day: ≥ 4 drinks (assigned male at birth); ≥ 3 drinks (assigned female at birth)
Kessler-10 Score, Possible Range = 10–50, Higher score = more distress
MOS Social Support Score, Possible Range = 0–100, Higher Score = More frequent availability of different types of support when needed
Possible Range = 19–114, Higher Score = More positive attitude toward HIV health care providers
Medical Mistrust Index, Possible Range = 7–28, Higher Score = Greater Mistrust
Post Hoc Analyses
We also conducted bivariate analyses (χ2/Fisher’s exact test and t-tests/Wilcoxon rank-sum test) to further explore syndemic factors associated with DVS among the two groups. The small sample size, however, limited our power to detect significant differences; the only significant finding was that number of close friends was inversely associated with DVS among the SHC subsample. Given the small sample size and the relative homogeneity among each of the two subsamples, there is reasonable likelihood that this finding could be due to random chance; thus, data are not presented within the tables.
As an additional post hoc analysis to better understand participants’ movement into and out of viral suppression, we examined whether participants experienced fluctuations in viral suppression over the course of their participation in the study. SHC participants who were virally suppressed remained so throughout the study. The median for SHC participants’ proportion of viral load records with suppressed viral load was 100% (IQR: 67%, 100%); however, detox participants moved from being suppressed to unsuppressed, as well as from unsuppressed to suppressed, a median of 1 time (IQR: 0, 1) respectively over the course of the study. The median for detox participants’ proportion of viral load records with suppressed viral load was 63% (IQR: 30%, 100%).
DISCUSSION
The objective of this study was to explore factors associated with DVS among two markedly different sub-samples of PLWH in NYC who also reported problem substance use in the era of universal ART. DVS proportions differed based on characteristics of participants aligned with recruitment site. We found that compared to SHC participants, participants recruited from the detox unit were more likely to have characteristics that were also associated with lower odds of DVS. The proportion of SHC participants with DVS at both 12-month and 18-month follow-up was significantly higher than 52% (a priori NYC benchmark contemporary to study recruitment). The proportion of detox participants with DVS, however, was significantly lower than 52% at the 12-month (but not the 18-month) follow-up.
Syndemic of long-term HIV infection, problem substance use, and poverty
Low proportions of DVS among the current detox sample may best be understood in the context of syndemic stressors. Our results showed that older age, as well as opioid and stimulant use disorders, were associated with lower odds of achieving DVS, whereas indicators of higher socioeconomic status such as full-time employment, higher education, and stable housing were associated with increased odds of DVS. Detox participants were significantly older and had also been living with HIV (and possibly other comorbid conditions) longer than participants recruited from SHCs. Substance use severity was greater among detox participants, who were significantly more likely to meet DSM-5 criteria for SUD than SHC participants. Although substantial proportions of SHC participants also met criteria for SUD, this did not seem to fully explain differences in DVS across the recruitment groups. Differences may be attributed instead to the syndemic of problem substance use and poverty-related stressors in the context of long-term HIV infection.
Compared to SHC participants, those recruited from detox has less education, and were more likely to lack full-time employment and to have unstable housing. Such structural factors are well-documented predictors of adverse HIV outcomes [8, 38, 39]. For example, in a recent systematic review of 152 studies (n=139,757 PLWH), unstable housing consistently and independently predicted adverse outcomes, including lack of viral suppression [8]. Employment and resultant financial stress and housing instability present obstacles to engagement in routine HIV care, thus leading to suboptimal viral suppression [38, 40]. The cost of medications, even with payment assistance and Medicaid support, may also be prohibitive to individuals with few economic and social resources.
A significantly larger proportion of detox participants were people of color compared to SHC participants. Systemic racism (i.e., the intersection of cultural, political, and institutional oppression of people of color) contributes to poor HIV and other health outcomes, and thus likely contributed to detox participants’ lower proportions of DVS. Systemic racism also contributes to fewer opportunities for educational attainment and employment [25, 41], and perpetuates income inequality and higher levels of poverty, among people of color in the U.S. [42].
SHC participants were diagnosed not only in the era of universal ART, but also in the context of substantial advances in HIV prevention, care, and messaging, especially in New York. In 2014, New York State launched an End the Epidemic (EtE) initiative with the goal of identifying people with undiagnosed HIV and linking and retaining them in care, and providing PrEP to individuals at higher risk for HIV [43]. In February 2017, the NYC DOHMH announced the JumpstART program, which includes the offer of immediate ART initiation within the SHCs upon testing HIV-positive with subsequent support for linkage to ongoing care [21]. Thus, some SHC participants were given a further advantage of comprehensive, onsite services to facilitate near-instantaneous medication initiation and linkage to care. The fact that the proportions of SHC participants achieving DVS at both the 12- (72%) and 18-month (74%) follow-up were significantly higher than the SHC benchmark of 52% demonstrates that DOHMH initiatives, including the universal ART recommendation, have been successful for many people newly diagnosed with HIV in NYC.
Implications for public health practice
The results of this study suggest that targeted and tailored strategies are needed to improve HIV outcomes among people facing the syndemic of SUD and poverty and added challenges of systemic racism and long-term HIV infection. Such strategies may include improved HIV care and messaging that speaks to the unique needs of long-term HIV survivors and delivering HIV care in the context of evidence-based SUD treatment and harm reduction services (including syringe exchange services), supportive housing, and comprehensive case management.
Improved HIV care and messaging:
To our knowledge, few if any existing behavioral interventions are available to assist those with long-term HIV infection to address treatment fatigue and associated challenges with maintaining viral suppression over time [44]. Newer biomedical strategies like single-dose, combination ART, have been shown to improve medication adherence [45]. Long acting injectable ART administered on a monthly basis is a promising new method of HIV treatment delivery [46]; this method may be particularly effective for those with treatment fatigue surrounding daily medication administration for people living with long-term HIV.
Having a supportive and collaborative relationship with one’s medical provider can also reduce medication fatigue [44, 47]. In the current sample, detox participants reported higher medical mistrust than the SHC participants, yet more positive attitudes toward their HIV providers. This suggests that individual providers who are supportive of and collaborative with their patients can successfully build trusting patient-provider relationships even in the context of larger medical mistrust. Providers could serve as a source of support related to ART adherence and associated improved viral suppression. Providers may also need to tailor universal ART messaging toward individuals who have been living with HIV for longer periods of time, including messaging that ensures wide dissemination of scientific advances like “undetectable=untransmittable” (U=U; the fact that PLWH who are taking ART and have maintained viral suppression for at least six months cannot sexually transmit HIV) [48, 49] and the potential for monthly injectable ART.
Access to evidence-based SUD treatment:
In addition to contending with more severe SUD than SHC participants, a significantly greater proportion of detox participants also had an opioid use disorder (OUD). However, only 15 of 25 detox participants with OUD reported receiving a medication for OUD (buprenorphine/naloxone n=2; methadone n=13). Patients being discharged from detox centers could thus benefit from initiation onto a medication for OUD prior to release, which can support greater retention in SUD treatment and lower likelihood of overdose, especially during times of greater risk such as immediately following detox [50]. Increasing SUD treatment retention (for any SUD) could also improve viral suppression outcomes by increasing HIV treatment retention and creating a context in which adherence to ART is more likely.
Supportive housing:
Providing housing for PLWH, particularly those with problem substance use, facilitates achieving DVS by decreasing the instability that can interrupt retention in HIV care and adherence to ART [38, 40]. Housing PLWH is also cost-effective; stable housing reduces risk of comorbid health conditions and associated medical costs and prevents the spread of HIV by facilitating viral suppression [51, 52]. “Housing first” is an evidence-based strategy that involves providing stable and permanent shelter with concurrent ancillary services to all individuals who need it, irrespective of previous housing status or comorbid mental health conditions [53].
Comprehensive case management and navigation services could address multiple barriers to achieving DVS among people living with HIV and problem substance use, particularly those who are long-term survivors of HIV. The NYC DOHMH Ryan White Care Coordination program is a model for these services. This program offers patients support for ART adherence and retention in care; assistance with accessing social services; access to care coordinators who can support patients in locating and obtaining necessary resources, including HIV services, and interacting with providers; and counseling to support patients in becoming fully independent [54]. Case management services should be made available not only through Ryan White programs but more broadly at all locations where PLWH access HIV care and other services.
Limitations
The primary limitation of this study is the relatively small sample, largely due to the substantial decline in the number of people newly diagnosed with HIV during study recruitment. This decline was particularly strong for persons with histories of injecting drug use [55, 56]. The small sample size did not allow sufficient power to conduct multivariable logistic regression models to test associations between predictors of DVS, or to explore interactions or recruitment group analyses. As mentioned above, changes in viral load testing technology and recent conversations in the field pertaining to measurement of viral suppression led to a shift in the definition of DVS from that used in 2011 to calculate the original 52% DVS benchmark. The relatively dramatic DVS presentations of the two recruitment groups, however, are unlikely to be substantially different, despite changes in the DVS definition. Finally, the two recruitment groups had distinct differences in time of HIV diagnosis and various psychosocial characteristics. Nevertheless, diversity across the sample may have helped to illuminate clear gaps in the provision of consistent HIV treatment.
CONCLUSION
This study demonstrates that a group of people who have basic resources (i.e., employment, housing) and who are diagnosed in a setting emphasizing immediate linkage to HIV primary care, like the SHC group, are able to achieve DVS despite problem substance use. People who have been living with HIV for long periods of time who have SUD and more limited resources seem to face greater challenges with DVS, even in the context of universal ART. There are likely different facilitators and barriers to effective treatment among newly diagnosed people compared to maintenance among those living with HIV longer-term. Ongoing and enhanced resources, intervention, and support are required to successfully improve DVS among people living with HIV long-term, particularly those who experience the syndemic of unemployment, housing instability, and greater substance use severity, many of whom also may experience overarching systemic racism.
Acknowledgements:
The authors would like to acknowledge our research assistants (Laurel Weaver, Jeannie Ortiz, and Martha Nelson) and our study participants.
Funding: This work was supported by grants from the National Institutes of Health, National Institute on Drug Abuse R01 DA035707 (Des Jarlais and Campbell) and R01 DA003574 (Des Jarlais), and National Institute of Mental Health T32 MH019139 (Sandfort).
Footnotes
Conflict of Interest/Competing Interests: The authors declare that they have no conflict of interest.
Declarations
Ethics Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for Publication: No individually identifying information is included in this article.
Availability of Data and Material: Due to the nature of this research (i.e., data on HIV status), the New York City Department of Health and Mental Hygiene does not allow data to be shared publicly; therefore, supporting data are not available.
Code Availability: Due to the nature of this research (i.e., data on HIV status), the New York City Department of Health and Mental Hygiene does not allow code to be shared publicly; therefore, supporting code is not available.
References
- 1.Harris NS, Johnson AS, Huang Y-LA, Kern D, Fulton P, Smith DK, et al. Vital signs: Status of human immunodeficiency virus testing, viral suppression, and HIV preexposure prophylaxis—United States, 2013–2018. Morbidity and Mortality Weekly Report. 2019;68(48):1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stadelmann L, Terzian A, Irvine M, Braunstein S, Shepard C, editors. Changes in HIV viral load suppression among HIV-infected New Yorkers, 2006–2007 to 2010–2011. CROI 2013 Proceedings of the 20th Conference on Retroviruses and Opportunistic Infections; 2013; Atlanta, GA. [Google Scholar]
- 3.New York City Department of Health and Mental Hygiene. HIV surveillance annual report, 2019. 2020. [Available from: https://www1.nyc.gov/assets/doh/downloads/pdf/dires/hiv-surveillance-annualreport-2019.pdf].
- 4.Robertson MM, Braunstein SL, Hoover DR, Li S, Nash D. Timeliness of Human Immunodeficiency Virus diagnosis and antiretroviral treatment initiation in the era of universal test and treat. The Journal of Infectious Diseases. 2019;220(4):648–56. 10.1093/infdis/jiz148. [DOI] [PubMed] [Google Scholar]
- 5.Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang C-J, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: Comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33(8):1417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas GM. Substance abuse, adherence with antiretroviral therapy, and clinical outcomes among HIV-infected individuals. Life Sci. 2011;88(21–22):948–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.New York City Department of Health and Mental Hygiene. NYSDOH and NYC DOHMH now recommend offering antiretroviral treatment to any person living with HIV, regardless of the person’s CD4 cell count. Dear Colleague Letter. December 1, 2011. [Google Scholar]
- 8.Aidala AA, Wilson MG, Shubert V, Gogolishvili D, Globerman J, Rueda S, et al. Housing status, medical care, and health outcomes among people living with HIV/AIDS: A systematic review. Am J Public Health. 2016;106(1):e1–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen MS, Holmes C, Padian N, Wolf M, Hirnschall G, Lo Y-R, et al. HIV treatment as prevention: How scientific discovery occurred and translated rapidly into policy for the global response. Health Aff. 2012;31(7):1439–49. [DOI] [PubMed] [Google Scholar]
- 11.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: Results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montaner JS, Hogg R, Wood E, Kerr T, Tyndall M, Levy AR, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368(9534):531–6. [DOI] [PubMed] [Google Scholar]
- 13.National Institute of Allergy and Infectious Diseases. Starting antiretroviral treatment early improves outcomes for HIV-infected individuals. 2015. [Available from: https://www.nih.gov/news-events/news-releases/starting-antiretroviral-treatment-early-improves-outcomes-hiv-infected-individuals].
- 14.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. Department of Health and Human Services. 2018. [Available from: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf]. [Google Scholar]
- 15.Wood E, Milloy MJ, Montaner JS. HIV treatment as prevention among injection drug users. Curr Opin HIV AIDS. 2012;7(2):151–6. [DOI] [PubMed] [Google Scholar]
- 16.Dieffenbach CW, Fauci AS. Universal voluntary testing and treatment for prevention of HIV transmission. JAMA. 2009;301(22):2380–2. [DOI] [PubMed] [Google Scholar]
- 17.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: A mathematical model. Lancet. 2009;373(9657):48–57. [DOI] [PubMed] [Google Scholar]
- 18.Des Jarlais DC, Semaan S. HIV prevention for injecting drug users: the first 25 years and counting. Psychosom Med. 2008;70(5):606–11. [DOI] [PubMed] [Google Scholar]
- 19.Gowing L, Farrell M, Bornemann R, Sullivan L, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2011;10(8):CD004145. 10.1002/14651858.CD004145.pub4. [DOI] [PubMed] [Google Scholar]
- 20.Abdul-Quader AS, Feelemyer J, Modi S, Stein ES, Briceno A, Semaan S, et al. Effectiveness of structural-level needle/syringe programs to reduce HCV and HIV infection among people who inject drugs: a systematic review. AIDS Behav. 2013;17(9):2878–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.New York City Department of Health and Mental Hygiene. Health department announces historic expansion of HIV and STI services at sexual health clinics. 2017. [Available from: https://www1.nyc.gov/site/doh/about/press/pr2017/pr003-17.page].
- 22.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell AN, Des Jarlais D, Hannah C, Braunstein S, Tross S, Kersanske L, et al. Antiretroviral medication treatment for all HIV-infected individuals: A protocol using innovative multilevel methodologies to evaluate New York City’s universal ART policy among problem substance users. BMC Health Serv Res. 2016;16:341. DOI: 10.1186/s12913-016-1554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer M, Clair S. Syndemics and public health: Reconceptualizing disease in bio - social context. Med Anthropol Q. 2003;17(4):423–41. [DOI] [PubMed] [Google Scholar]
- 25.Wilson PA, Nanin J, Amesty S, Wallace S, Cherenack EM, Fullilove R. Using syndemic theory to understand vulnerability to HIV infection among Black and Latino men in New York City. J Urban Health. 2014;91(5):983–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Institute on Alcohol Abuse and Alcoholism. Rethinking drinking: Alcohol and your health. 2016. [Available from: https://pubs.niaaa.nih.gov/publications/RethinkingDrinking/Rethinking_Drinking.pdf].
- 27.American Psychological Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 28.Sobell LC, Sobell MB. Timeline followback user’s guide: A calendar method for assessing alcohol and drug use. Toronto, Ontario, Canada: Addiction Research Foundation; 1996. [Google Scholar]
- 29.U.S. Department of Health and Human Services, U.S. Department of Agriculture. Appendix 9. Alcohol. 2015–2020 dietary guidelines for Americans. 8th edition 2015. [Available from: https://health.gov/dietaryguidelines/2015/guidelines/appendix-9/]
- 30.Janssen T, Braciszewski JM, Vose-O’Neal A, Stout RL. A comparison of long-vs. short-term recall of substance use and HIV risk behaviors. J Stud Alcohol Drugs. 2017;78(3):463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand S-L, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32(6):959–76. [DOI] [PubMed] [Google Scholar]
- 32.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–14. [DOI] [PubMed] [Google Scholar]
- 33.Bodenlos JS, Grothe KB, Kendra K, Whitehead D, Copeland AL, Brantley PJ. Attitudes toward HIV health care providers scale: Development and validation. AIDS Patient Care STDS. 2004;18(12):714–20. [DOI] [PubMed] [Google Scholar]
- 34.LaVeist TA, Isaac LA, Williams KP. Mistrust of health care organizations is associated with underutilization of health services. Health Serv Res. 2009;44(6):2093–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson B New viral load technologies: Potential for real-time virologic mess. 2011. [Available from: https://www.thebodypro.com/article/new-viral-load-technologies-potential-for-a-real-t].
- 36.Leierer G, Grabmeier-Pfistershammer K, Steuer A, Sarcletti M, Geit M, Haas B, et al. Editor’s choice: A single quantifiable viral load is predictive of virological failure in Human Immunodeficiency Virus (HIV)-infected patients on combination antiretroviral therapy: The Austrian HIV cohort study. Open Forum Infect. 2016;3(2):ofw089. DOI: 10.1093/ofid/ofw089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Udeagu C-CN, Shah S, Misra K, Sepkowitz KA, Braunstein SL. Where are they now? Assessing if persons returned to HIV care following loss to follow-up by public health case workers were engaged in care in follow-up years. AIDS Patient Care STDS. 2018;32(5):181–90. [DOI] [PubMed] [Google Scholar]
- 38.Geter A, Sutton MY, Hubbard McCree D. Social and structural determinants of HIV treatment and care among black women living with HIV infection: A systematic review: 2005–2016. AIDS Care. 2018;30(4):409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiewel EW, Borrell LN, Jones HE, Maroko AR, Torian LV. Neighborhood characteristics associated with achievement and maintenance of HIV viral suppression among persons newly diagnosed with HIV in New York City. AIDS Behav. 2017;21(12):3557–66. [DOI] [PubMed] [Google Scholar]
- 40.Walcott M, Kempf M-C, Merlin JS, Turan JM. Structural community factors and sub-optimal engagement in HIV care among low-income women in the Deep South of the USA. Cult Health Sex. 2016;18(6):682–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palacio H, Kahn JG, Richards TA, Morin SF. Effect of race and/or ethnicity in use of antiretrovirals and prophylaxis for opportunistic infection: A review of the literature. Public Health Rep. 2002;117:233–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau. Income and poverty in the United States: 2017. 2018. [Available from: https://www.census.gov/content/dam/Census/library/publications/2018/demo/p60-263.pdf].
- 43.New York State Department of Health. Ending the AIDS epidemic in New York State. 2020. [Available from: https://www.health.ny.gov/diseases/aids/ending_the_epidemic/].
- 44.Claborn KR, Meier E, Miller MB, Leffingwell TR. A systematic review of treatment fatigue among HIV-infected patients prescribed antiretroviral therapy. Psychol Health Med. 2015;20(3):255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clay PG, Nag S, Graham C, Narayanan S. Meta-analysis of studies comparing single and multi-tablet fixed dose combination HIV treatment regimens. Medicine. 2015;94(42):e1677. DOI: 10.1097/MD.0000000000001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orkin C, Arastéh K, Hernândez-Mora MG, Pokrovsky V, Overton ET, Girard, et al. Long-acting cabotegravir + rilpivirine for hiv maintenance: Flair week 48 results. CROI 2019 Proceedings of the 26th Conference on Retroviruses and Opportunistic Infections; Seattle, Washington. 2019. [Google Scholar]
- 47.Roy M, Czaicki N, Holmes C, Chavan S, Tsitsi A, Odeny T, et al. Understanding sustained retention in HIV/AIDS care and treatment: A synthetic review. Curr HIV/AIDS Rep. 2016;13(3):177–85. [DOI] [PubMed] [Google Scholar]
- 48.Rendina HJ, Parsons JT. Factors associated with perceived accuracy of the Undetectable= Untransmittable slogan among men who have sex with men: Implications for messaging scale - up and implementation. J Int AIDS. 2018;21(1):e25055. DOI: 10.1002/jia2.25055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paschen-Wolff MM, Campbell AN, Tross S, Castro M, Berg H, Braunstein S, et al. HIV treatment knowledge in the context of “Treatment as Prevention” (TasP). AIDS Behav. 2020;24:2984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basu A, Kee R, Buchanan D, Sadowski LS. Comparative cost analysis of housing and case management program for chronically ill homeless adults compared to usual care. Health Serv Res. 2012;47(1pt2):523–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holtgrave DR, Wolitski RJ, Pals SL, Aidala A, Kidder DP, Vos D, et al. Cost-utility analysis of the housing and health intervention for homeless and unstably housed persons living with HIV. AIDS Behav. 2013;17(5):1626–31. [DOI] [PubMed] [Google Scholar]
- 53.U.S. Interagency Council on Homelessness . Opening doors: Federal strategic plan to prevent and end homelessness. 2015. [Available from: https://www.usich.gov/resources/uploads/asset_library/USICH_OpeningDoors_Amendment2015_FINAL.pdf].
- 54.New York City Department of Health and Mental Hygiene. HIV care coordination. 2020. [Available from: https://www1.nyc.gov/site/doh/health/health-topics/aids-hiv-care-coord.page].
- 55.New York City Department of Health and Mental Hygiene. HIV epidemiology & field services semiannual report. 2012. [Available from: https://www1.nyc.gov/assets/doh/downloads/pdf/dires/2012-2nd-semi-rpt.pdf].
- 56.New York City Department of Health and Mental Hygiene. HIV surveillance annual report, 2017. 2017. [Available from: https://www1.nyc.gov/assets/doh/downloads/pdf/dires/hiv-surveillance-annualreport-2017.pdf].


