Abstract
Microplastic contamination is ubiquitous in aquatic and terrestrial environments, found in water, sediments, within organisms and in the atmosphere and the biological effects on animal and plant life have been extensively investigated in recent years. There is growing evidence that humans are exposed to microplastics via ingestion of food and drink and through inhalation. Despite the prevalence of contamination, there has been limited research on the effects of microplastics on human health and most studies, to date, analyse the effects on model organisms with the likely impacts on human health being inferred by extrapolation. This review summarises the latest findings in the field with respect to the prevalence of microplastics in the human–environment, to what extent they might enter and persist in the body, and what effect, if any, they are likely to have on human health. Whilst definitive evidence linking microplastic consumption to human health is currently lacking, results from correlative studies in people exposed to high concentrations of microplastics, model animal and cell culture experiments, suggest that effects of microplastics could include provoking immune and stress responses and inducing reproductive and developmental toxicity. Further research is required to explore the potential implications of this recent contaminant in our environment in more rigorous clinical studies.
Keywords: Human health risks, Inhalation, Ingestion, Microplastics, POPs
Introduction
Large scale production of plastics dates back to around the 1950s (Boucher and Billard 2019). In 2010, 275 million metric tons (MT) of plastic waste was generated in 192 coastal countries (Jambeck et al. 2015). By 2017 this rose to 335 million MT of plastic waste (Boucher and Billard 2019). It is also estimated that between 4.8 and 12.7 million MT of this waste enters the world’s oceans (Jambeck et al. 2015). The impact of macroplastics (> 5 mm in at least one dimension) through entanglement, choking and strangulation on animals has been well documented and is the more conspicuous plastic waste that is often seen in photographs depicting the scale of the plastic waste problem. Although not as obvious to the naked eye, smaller pieces of plastic debris deemed “microplastics” (MPs) (particles < 5 mm in diameter but larger than 1 µm (Hartmann et al. 2019) are the most abundant form of solid waste on Earth. MPs can be further categorized into primary and secondary. Primary MPs are originally made to be microsized, usually for use in cosmetic products such as microbeads (Hartmann et al. 2019). Secondary MPs refers to those that have been broken down by photo degradation or mechanical weathering over time and now fall into the < 5 mm definition.
MPs have been found in the ocean (Law and Thompson 2014; Auta et al. 2017; Boucher and Friot 2017), in freshwater, (Horton et al. 2017; Vaughan et al. 2017; Li et al. 2018a, b), in sediments (Abidli et al. 2018; Reed et al. 2018), in soils (Watteau et al. 2018; Zhang et al. 2018) and in the air (Prata 2018; Wright et al. 2020). Some of these locations are quite remote, far from human settlements. MPs have been found in remote polar regions, specifically with high concentrations seen in sea ice cores (Peeken et al. 2018). Most of the MPs detected in these ice cores were smaller than 50 μm and an average of 67% of the particles were within the smallest detectable class size of 11 μm (Peeken et al. 2018). They are also found in the deepest parts of the world’s oceans; the Mariana Trench sediment was found to have between 200 and 2200 pieces per litre, with the majority being plastic microfibres measuring 1–3 mm in length in seawater and 0.1–0.5 mm in length in sediment (Peng et al. 2018). MPs appearing in remote locations can be explained by the plastic cycle (Horton and Dixon 2018), whereby MPs accumulated in the world’s oceans are so small that they can be present in the evaporation that forms our rain clouds, this rainfall containing MPs in then deposited in mountainous regions and other remote locations. The subsequent lakes and rivers transport the MPs back to the ocean, forming the plastic cycle (Geyer et al. 2017; Bank and Hansson 2019). China’s largest inland lake—Qinghai Lake—was found to have MPs present, with small MPs (0.1–0.5 mm) mostly on the surface water and larger MPs (1–5 mm) were more abundant in the connected river samples (Xiong et al. 2018). Surface water and sediment samples were collected from 6 sites along 5 different rivers in the Tibet Plateau (Jiang et al. 2019). The surface water had 483–967 items m−3 and the sediment 50–195 items kg−1. These examples emphasise how widespread MP contamination has become.
We know that MPs are prevalent in oceans, lakes and rivers but are humans exposed to them? A review of MPs in commercial salt for human consumption found that in 128 brands of salt from 38 countries contained MPs (Peixoto et al. 2019). Similarly, MPs in bottled drinking water was found to be from the caps and could also have long-term exposure implications (Choudhary et al. 2020). MPs have also been found in beer, energy drinks and other soft drinks (Kosuth et al. 2018; Shruti et al. 2020) and more recently MPs (< 10 µm in diameter) have been found within the flesh of fruit and vegetables (Oliveri Conti et al. 2020).
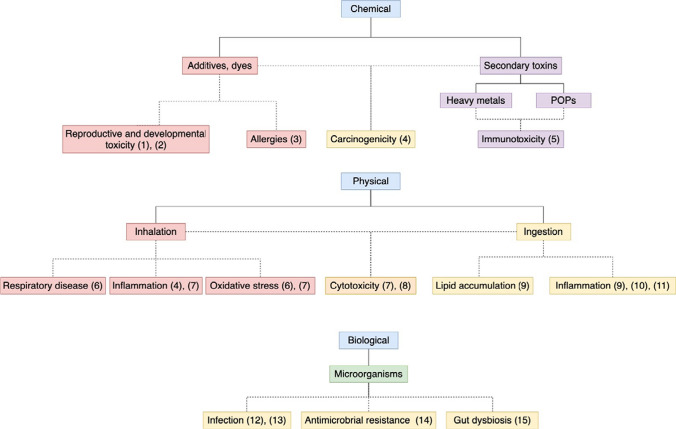
There are many studies on how these MPs are ingested or inhaled and the effects this might have on wildlife (Lehner et al. 2019; Prata et al. 2020). The question is, to what extent are humans exposed and how could it affect humans? Despite the lack of knowledge about direct impacts of human health, it is acknowledged that plastic and micro plastic debris needs to be addressed (Katyal et al. 2020). Literature on the effects of MPs on other wildlife can be used as an indication of how they may impact human health and are summarised in this review. The effects of MPs on human health can be separated into three main categories; chemical, physical and biological effects and then further divided by exposure route and the potential clinical effects, as illustrated by the diagram in Fig. 1.
Fig. 1.
Flow diagram to illustrate the potential human health effects of microplastics. Dotted lines represent current speculative research. (1) Latini et al. (2003), (2) Peretz et al. (2014), (3) Ait Bamai et al. (2014b), (4) Gasperi et al. (2018) (5) Tang et al. (2020), (6) Valavanidis et al. (2013), (7) Prata (2018), (8) Gallagher et al. (2015), (9) Lu et al. (2016), (10) Qiao et al. (2019), (11) Li et al. (2020a, b), (12) Morris and Acheson (2003), (13) Kirstein et al. (2016), (14) Yang et al. (2019a), (15) Lu et al. (2019)
Chemical effects
Toxic additives
There is evidence to suggest that additives such as dyes or plasticisers could cause toxicity, carcinogenicity and mutagenicity (Gasperi et al. 2018). Additives, dyes and pigments could leach from MPs and accumulate on surfaces and in water sources, with the health consequences of this unknown (Gasperi et al. 2018).
Phthalates are commonly used as plasticizers to provide flexibility to plastics. They are an additive, therefore not chemically bound (covalently bonded) to the polymer and so are more likely to be released and transfer to the environment. Over 80% of plasticizers used worldwide are phthalates. They have been shown to appear in household dust (Abb et al. 2009; Ait Bamai et al. 2014a), human urine (Jornet-Martínez et al. 2015) and breastmilk (Main et al. 2006; Högberg et al. 2008). There is some evidence to suggest an association between the level of phthalates and occurrence of asthma and allergies, especially in children (Ait Bamai et al. 2014b). Exposure to phthalates has also been shown to have a biological effect in utero and could be associated with a shorter pregnancy duration (Latini et al. 2003). Bisphenol-A (BPA) has also been studied similarly to phthalates and shown to be a reproductive toxicant, being associated with adverse birth outcomes (Peretz et al. 2014). Monitoring of human tissues and body fluids allows us to see what concentrations of environmental contaminants are present. Biomonitoring has shown that chemicals used in the manufacture of plastics, such as BPA, phthalates and styrene, are present in the human population (Galloway 2015). Some of these chemicals have a widespread presence in the general population at concentrations capable of causing harm in animal models which raises a public health concern (Talsness et al. 2009).
Data from a study on short-tailed shearwater birds suggested that there was a transfer of plastic derived chemicals from ingested plastics to the tissues of the birds (Tanaka et al. 2013). They found brominated chemicals that were not present in the natural prey of the bird but likely from the plastic that was also found in the stomachs of some of the birds.
In a study assessing hazard levels, 31 out of 55 polymers were composed of monomers that were assigned to the most severe hazard levels (Lithner et al. 2011). Polyvinyl chloride (PVC) has a carcinogenic monomer and several hazardous additives making it arguably the most dangerous plastic in terms of toxicity. An investigation into whether various plastic products emitted hazardous chemical substances into water containing Daphnia magna found that 9 of the 32 products caused acute toxic effects (immobility) (Lithner et al. 2009). It was also found that PVC and polyurethane leachates were the only plastic types tested that displayed toxicity.
There have been a few studies on how MPs and their additives can cause toxic effect at a cellular level, looking at cytotoxicity, oxidative stress and cell viability. Human cerebral and epithelial cells were exposed to different levels of contaminants and showed oxidative stress when MPs were introduced but there was no significant reduction in cell viability (Schirinzi et al. 2017). Another study found that the positive control induced a high degree of toxicity in all in vitro tests using direct contact (Van Tienhoven et al. 2006). An additional study found that direct contact of polypropylene MPs with human cells could induce productions of cytokines and histamines (Hwang et al. 2019).
Polybrominated diphenyl ethers (PBDEs) are additives used as flame retardants in many commercial products. Plastic can integrate up to 15% PBDEs and they are not chemically bound so are likely to leach during production, disposal and recycling processes (Domingo 2012). Concentrations of PBDEs have increased over the years in the bodies of wildlife and humans, with the long lasting effects unknown (Linares et al. 2015). There has been a handful of studies that have indicated that bioaccumulation could cause impaired neurological development (Bellés et al. 2010; Reverte et al. 2014) and endocrine disruption (Alonso et al. 2010), however, all studies were conducted on mice or rats, therefore no conclusions can be drawn on the danger to humans.
Secondary toxins
The interaction of MPs and chemical pollutants is an area widely studied (Crawford and Quinn 2017). Persistent organic pollutants (POPs) are extensively recognised to be throughout the environment, including Oceans. These pollutants are hydrophobic and have been found to readily adsorb to MPs (Velzeboer et al. 2014). There are many examples of this interaction in the literature, (Rios et al. 2010; Zarfl and Matthies 2010; Frias et al. 2010; Bakir et al. 2012; Driedger et al. 2015) as well as the MP interaction with heavy metals, (Ashton et al. 2010; Holmes et al. 2012; Holmes and Thompson 2014; Rochman et al. 2014). For example, polyethylene mulching sheets, used in agriculture, easily fragment into MPs. The longer the plastic mulching sheets are used in agriculture, the more microplastics that can be found in the soil indicating that they are a major source of MPs into arable soil (Huang et al. 2020a). They can also adsorb pesticides that are either in the soil or already sprayed onto the plastic (Wang et al. 2020). Carbendazim, dipterex and malathion are examples of the pesticides that can adsorb to MPs. It is suggested that MPs could become the source or carrier of pesticides into other environments, such as water, and have the potential for environmental and human safety risks. A study showed that by increasing the MP dosage there was increased removal of pesticides from solution reaching equilibrium in 120 min (Wang et al. 2020). They concluded that the adsorption was a spontaneous and exothermic process. In this way, additive or sorbed chemicals including polycyclic aromatic hydrocarbons (PAHs), antimicrobials, and halogenated flame retardants (HFRs) have been found in laboratory studies to be transferred from MPs to marine organisms (Browne et al. 2013; Avio et al. 2015) however the accumulation of chemical burdens from ingested MPs is not always unidirectional and depends on the concentration gradient between the ingested plastics and the gut of the organism (Koelmans et al. 2016; Bakir et al. 2016). For example, if an animal which already has a high concentration of chemical contamination from the environment ingests microplastics with low concentrations of chemicals, the transfer is expected to be from the gut to the microplastics, essentially “cleaning out” the animal.
Additionally, antibiotics can adsorb to MPs in contaminated waters and result in them being transported long distances. A study found that polyamide (PA) had the highest adsorption of antibiotics (Li et al. 2018a, b). Five antibiotics (sulfadiazine, amoxicillin, tetracycline, ciprofloxacin and trimethoprim) and five MPs (polyethylene, polystyrene, polypropylene, PA and PVC) were investigated in both freshwater and seawater, with a higher adsorption rate of antibiotics found in freshwater compared to seawater. The same adsorption kinetics have been studied with steroid hormones, although in less depth. It has been found that 17β-estradiol and 17α-ethynylestradiol, types of synthetic hormone, will readily adsorb to MPs (Lu et al. 2020).
The potential ingestion of MPs and subsequent pollutants poses a toxic problem for the food web. Some studies have looked at how these pollutants might affect organisms (Besseling et al. 2013; Browne et al. 2013) and also look at the bioaccumulation of MPs and the pollutants (Koelmans et al. 2013). One study collected edible oysters from a coastal city in China and found MPs in all of the oyster tissue samples (Zhu et al. 2020). In addition, there was bioaccumulation of trace metals in higher concentrations than normal in the oyster tissue. It was concluded that this could pose a potential danger to humans if marine life were exposed to MPs and contaminants are then consumed.
Polychlorinated biphenyls (PCBs), an organic pollutant, with concentrations ranging from 0.1 to > 18 000 ng/g plastic have been found on pre-production plastic pellets (a.k.a. nurdles) globally (Verla et al. 2019). Despite effective bans in the 1970s and 1980s, PCBs are very persistent and recent studies have found that they continue to accumulate in the tissues of marine mammals, such as dolphins and orcas (Simmonds 2017). Worryingly, concentrations often exceeding mammalian toxicity thresholds and, therefore, possibly leading to reproductive failure and health issues (Jepson et al. 2016).
MPs and POPs have also been found to lead to immunotoxicity in blood clams, however, the larger MP size of 30 μm compared to the smaller 500 nm diameter appeared to mitigate the toxicity (Tang et al. 2020). On the other hand, one study concluded that the importance of MPs being a vector of toxic substances to marine organisms was of limited importance, in relation to other exposure pathways (Gouin et al. 2011). Additionally, a study on Talitrus saltatory, a type of amphipod, demonstrated that ingestion of contaminated MPs (polybrominated diphenyl ether) transferred organic pollutants to its tissues. However, uncontaminated MPs ingested by a contaminated amphipod removed the organic pollutants instead (Scopetani et al. 2018). This two-way transfer could support the view that toxic substance adsorption to MPs has an equilibrium effect on marine life and is therefore a less important pathway. More research should be carried out to establish the potential of exposure to toxic pollutants carried by MPs and how they might affect human health (Rodrigues et al. 2019).
Physical effects
Inhalation
There have been many studies that show there are MPs in the atmosphere that can be readily inhaled (Liu et al. 2019b; Zhang et al. 2020b; Huang et al. 2020b). Production of plastic textile fibres has increased more than 6% per year and makes up around 16% of the worlds plastic production (Gasperi et al. 2018). Small fibres can shed from clothing due to general wear and washing, with just one garment predicted to release 1900 fibres per wash into waste water (Browne et al. 2011). The scale of plastic fibre production worldwide and the subsequent potential to be inhaled or ingested suggests investigations on their effects to human health should be considered.
There is research that has been conducted and is continuing to be produced, that is estimating the volume of airborne MPs across the globe. A study conducted in Central London tested atmospheric MP deposition and found it was 20 times greater than in a more remote location (Wright et al. 2020). They also found that fibrous MPs made up the vast majority of the plastics found (92%). Suspended atmospheric MPs were tested for in Shanghai, finding 0–4.18 m−3 (items per cubic metre of air) (Liu et al. 2019a). Of these 67% were microfibres, 30% fragments and 3% granules, leading to the assumption that the likely source of the majority of MPs were synthetic textiles. They also estimated that people in Shanghai inhaled approximately 21 MP particles per day whilst outdoors. An earlier study of atmospheric fallout in Dongguan City also found the dominant MP type to be fibres (Cai et al. 2017). Similar quantities of MPs were sampled over the West Pacific Ocean, suspended in marine air, with 60% microfibres, 31% fragments and 8% granules (Liu et al. 2019c). This supports the conclusion that the vast majority of atmospheric MPs comes from synthetic textiles. It was found that daytime collection had twice the amount as the night time collection. MPs have also been found in dust in Tehran with 88–605 MP particles per 30 g of dry dust (Dehghani et al. 2017). They also estimate that outdoor activity can lead to an estimated exposure of 3223 particles per year for children and 1063 particles per year for adults. A similar study in Iran found on average 900 MP particles in 15 g of street dust (Abbasi et al. 2019).
Atmospheric MPs can also be sourced from deposition, or rain. A study in Paris detected MPs in atmospheric fallout, with the results finding 29–280 particles m−2 day−1 (Dris et al. 2015). Atmospheric deposition has also been tested in remote environments (Zhang et al. 2019). One study found 249 fragments, 73 films and 44 fibres per square metre in the catchment area of the French Pyrenees (Allen et al. 2019). They concluded that the MPs could travel up to 95 km to reach more remote areas via atmospheric transport. A similar study instead looked at a glacier in the Italian Alps and found 74.4 MP items kg−1 of sediment (Ambrosini et al. 2019). This not only contained most commonly polyesters, but also polyamide, polyethylene and polypropylene. Furthermore, they estimate that the whole glacier could have 131–162 million plastic items.
The effects on human health of inhaling these fibrous MPs is little understood. It is thought that the majority of fibres can be cleared from the respiratory system, however, some will go on to cause inflammatory responses and even respiratory lesions (Prata 2018), especially in those with compromised clearance mechanisms (Gasperi et al. 2018). Of 114 lung specimens from patients undergoing lung resection for removal of a tumour, 87% were observed to contain cellulosic or plastic fibres, demonstrating that these small fibres are respirable and accumulate in lung tissue (Pauly et al. 1998). Synthetic textiles are thought to be the main source of airborne MPs, especially indoors where the concentration is greater (Dris et al. 2017). It has been studied previously that inhalation of fibres during factory work can cause some cancers (Gallagher et al. 2015), however, some studies on nylon flock workers suggested that there was no evidence of an increased cancer risk, but there was a higher prevalence of respiratory irritation (Wright and Kelly 2017).
We know that the lungs are exposed daily to pollutants which act as oxidants and this leads to oxidative stress, inflammation and carcinogenesis (Valavanidis et al. 2013). There is also an association between the increased incidence of respiratory disease and lung cancers from the exposure to low levels of respirable fibres (Valavanidis et al. 2013). However, little research has been conducted on the potential adverse health effects on human lungs when inhaling synthetic fibres and is therefore difficult to attribute this increase in respiratory disease to inhaled MPs (Gasperi et al. 2018). A study investigating proinflammatory responses in rats to various sizes of polystyrene particles found that the smaller particles (64 nm) gave a significantly greater neutrophil influx in the lungs compared to the larger particles (202–535 nm) (Brown et al. 2001). It is thought that this is due to the larger surface area of smaller particles, leading to increased inflammation. There is evidence to suggest that MPs could also translocate to other tissues once inhaled or ingested, with one study finding that fluorescent polystyrene microspheres delivered intranasally to mice could be found in the spleen 10 days later (Eyles et al. 2001). It was also found that once there, they could incite immunological functions. There is little to no information available on any human studies looking at health effects of MP fibre or particle inhalation, something that should be investigated in the future.
Ingestion
A considerable amount of MP research is conducted on ingestion by aquatic life, (Possatto et al. 2011; Lusher et al. 2013; Phillips and Bonner 2015; Romeo et al. 2015; Barboza et al. 2018) on seabirds, (van Franeker et al. 2011; Lavers et al. 2014; Amélineau et al. 2016) and other wildlife (Huerta Lwanga et al. 2016), with limited studies conducted on human ingestion (Ribeiro et al. 2019). One study compared human ingestion of MPs from mussels with the inhalation of microfibres whilst eating that same meal and found that you inhale more synthetic microfibres sitting down for a meal than you would from eating the mussels (Catarino et al. 2018). There is however some proof that humans ingest MPs, when one study tested 8 human stool samples and found MPs in all of them (Schwabl et al. 2019). They found that polypropylene and polyethylene terephthalate were the most abundant types of plastic. It is also known that MPs are present in seafood (Li et al. 2016), water, salt and beer (Kosuth et al. 2018) and in potentially more food or drink items that are regularly consumed by humans (Zhang et al. 2020a).
An evaluation of the number of MPs consumed from the average intake of food found that the average annual consumption was in the range of 39 000–52 000 particles (Cox et al. 2019). This could increase to between 74 000–121 000 when the inhalation of MPs was considered. They also found that if you included water intake from only a bottle source then an individual could be ingesting a further 90 000 particles compared to 40 000 particles consumed from tap water. Infant feeding bottles were found to release 1 310 000 ± 130 000 to 16 200 000 ± 1 300 000 microplastic particles per litre, equating to 14 600–4 550 000 particles per day (Li et al. 2020a, b). This is ~ 2600 times the total adult consumption of MPs from water, food and air (up to 600 particles per day for adults (Cox et al. 2019) and is partly due to the intense heat used to sterilise the bottles (Li et al. 2020a, b).
Edible fruits and vegetables provide further example of the potential ingestion of MPs by humans. A study found that apples were the fruit most contaminated with a median of 223 000 p/g of MPs. It was further calculated that the estimated daily intake of MPs from apples was 4.62 E+05 for adults and 1.41E+05 for children (Oliveri Conti et al. 2020).
One study in China comparing MPs in fish and bivalves in cities with other countries in the world, found that MPs are prevalent in commercial fish and bivalves sold in city markets and that the risk to human health is greater from these markets than other countries in the world (Fang et al. 2019). A more recent study looked at 150 fish from 3 species and found 49% had MPs, of this 32% was found in the dorsal muscle of the fish with a mean number of 0.054 items per gram (Barboza et al. 2020). Based on the fish muscle data and the recommended human consumption of fish per capita in selected European and American countries, researchers were able to estimate that adults potentially consume 518–3078 MP items/year/capita. These numbers are considerably smaller than the 39 000 mentioned earlier, however, the figure was based on data from one type of fish; consumption of other products containing MPs were not considered.
Although most studies look at non-human MP ingestion, they can be used to look at the effects this might have on tissues and organs. A study on tissue accumulation of polystyrene in zebrafish found 5 μm diameter MPs in the gills, liver and gut and 20 μm diameter accumulation in just the gills and gut. This caused inflammation and lipid accumulation. They also found that exposure to MPs induced alterations of metabolic profiles in the liver and disturbed lipid and energy metabolism (Lu et al. 2016).
In a separate study, Zebrafish were exposed to three shapes of MPs (bead, fragment and fibre). There was accumulation of the MPs in the gut, with the fibre shape resulting in the more severe intestinal toxicity than fragments and beads. The accumulation caused mucosal damage, increased permeability, inflammation, metabolism disruption and microbiota dysbiosis (Qiao et al. 2019). A different study found that fish with MPs had significantly higher lipid peroxidation levels in the brain, gills and dorsal muscle and increased brain acetylcholinesterase activity than fish with no MPs, suggesting lipid oxidative damage (Barboza et al. 2020). Medaka fish larvae and juveniles were fed food spiked with environmental MPs in a different study. They found that in those fed the spiked food it could cause death, decreased head/body ratios, increased Ethoxyresorufin-O-deethylase (EROD) activity, DNA breaks and alterations to swimming behaviour (Pannetier et al. 2020).
There was also a study that looked at polystyrene MPs in mice based on toxicity-based toxicokinetic/toxicodynamic modelling to quantify organ bioaccumulation and biomarker responses. The gut had the highest bioaccumulation factor and overall the smaller MP size (5 μm) exhibited higher values compared to the larger MP size (20 μm) (Yang et al. 2019b). Another study on mice and polystyrene MPs looked at tissue distribution, accumulation and tissue-specific health risks. They were found to accumulate in the liver, kidneys and gut, depending on particle size. Biochemical biomarkers suggested exposure induced disturbance of energy and lipid metabolism as well as oxidative stress (Deng et al. 2017). There is however, an editorial that is critical of this Deng et al. study, suggesting that the conclusion of health effects by MPs is not sufficiently supported by the data presented (Braeuning 2019). They also question the values given for the accumulation of plastic in organs as the figures seem to far exceed the doses administered.
A different study looked at the exposure to differing amounts of polyethylene MPs in mice. The high concentration of MPs increased the numbers of gut microbial species, bacterial abundance and flora diversity. Serum levels of interleukin 1a in all feeding groups were significantly greater than in the blank group. The intestines of mice fed high concentrations of MPs showed inflammation and higher TLR4, AP-1 and IRF5 expression (Li et al. 2020a, b). A contrasting study looked at the intestinal particle uptake and health-related effects of oral polystyrene in vitro and in vivo. It suggested that oral exposure to polystyrene in the conditions tested did not pose any relevant acute health risks to mammals; no inflammatory response or lesions and no interference of macrophages (Stock et al. 2019).
There should also be consideration for the effects of MPs on offspring during gestation. In a recent study, pregnant mice were exposed to polystyrene MPs in their drinking water and the offspring observed and tested. They found that there was no significant effect on the offspring’s growth, however, there was indication of the offspring having fatty acid metabolic disorders, which was related to the MP particle size (Luo et al. 2019). Another study used an ex vivo human placental perfusion model and fluorescently labelled polystyrene beads from 50 to 500 nm in diameter to see if the particles could cross the placental barrier and affect the foetus (Wick et al. 2010). They found that a diameter of up to 240 nm was able to cross the placental barrier but did not affect the viability of the placental explant.
As mentioned previously there is a wide occurrence and reporting of MP ingestion by aquatic fauna but there are questions as to how much of this actually transfers to humans in terms of ingestion. Most studies that look at the health effects are produced in laboratory conditions which are less relevant when applied to the environment. In particular there is a need to be realistic with the concentrations of MPs used and aligning them with what would most likely be found in the environment (de Sá et al. 2018; Wang et al. 2019).
Prosthetics
There has been limited studies on MPs generated by wear and corrosion of joint replacement prostheses, however one published in 2000, details identification of metallic and polyethylene particles from post-mortems in 29 patients (Urban et al. 2000). They found polyethylene particles in the para-aortic lymph nodes in 68% of 28 patients and in the liver or spleen of 14% of 29 patients. The majority of the particles were less than 1 μm in size and mostly in low concentrations, with little pathological importance. However, in one case granulomas formed in the liver, spleen and abdominal lymph nodes in response to heavy accumulation of wear debris from a hip prosthesis. Additional studies related to plastic prosthesis particle contamination have been carried out in vitro or using in vivo models and seek to explore the health effects of the wear debris. An in vitro study found that when adding polymethylmethacrylate (PMMA) particles to each developmental stage of osteoclasts there was an increase in bone resorption in mature osteoclasts (Zhang et al. 2008). It is thought that inflammation is caused by PMMA particles increasing osteoclast formation resulting in prosthetic failure. Similarly, ultra-high molecular weight polyethylene (UHMWPE) particles were introduced to bone implants in mice and then treated with erythromycin 2 weeks after implantation (Markel et al. 2009). Results showed that exposure to UHMWPE particles induced inflammation and increased bone resorption, but with erythromycin treatment, this was reduced. A study in 2011 looked at toll-like receptors (TLRs) and their role in recognising orthopaedic implant wear-debris particles, ultimately causing inflammation (Pearl et al. 2011). They found that TLRs signal through myeloid differentiation factor 88 (MyD88) and by inhibiting MyD88 there was a decrease in PMMA particle induced production of macrophages and therefore, inflammation.
Biological effects
Microorganisms
It has been shown that bacteria can rapidly colonize MP surfaces in the marine environment (Harrison et al. 2014; Wagner et al. 2014), as well as form microbial biofilms (Lobelle and Cunliffe 2011). Zettler et al. studied plastic marine debris using a scanning electron microscope (SEM) and found a diverse microbial community coined a ‘Plastisphere’ (Zettler, Mincer, and Amaral-Zettler 2013). They found that the hydrophobic surface of these plastics is ideal for microbial colonization and biofilm formation, discovering that the most abundant genus was Vibrio, amongst many others. Although there is sufficient research showing that microorganisms can colonise MPs, there is little evidence of whether or not they are capable of degrading MPs in the field. Laboratory studies, however, indicate that fungi, bacteria and biofilms are capable of degrading MPs of a variety of polymer types including polyethylene, polystyrene and polylactic acid (Yuan et al. 2020). Some plastics provide organic carbon sources that, in theory, are able to be metabolized by specific microorganisms, however for the majority of non-biodegradable plastics there is only weak evidence of microbial degradation because studies lack confirmation of microbial growth on the polymer or differentiation between the degradation of the polymer and its additives or losses due to leaching (Lear et al. 2021).
Potentially pathogenic Vibrio spp. were found to be present on floating MPs in water samples from the North and Baltic Sea, which suggests MPs could function as vectors for the dispersal of pathogens (Kirstein et al. 2016). The Vibrio spp. Pathogen could cause serious infections in humans if ingested (Morris and Acheson 2003), illustrating that the presence of MPs in seafood is an area to be investigated further.
There is some evidence to suggest that interactions between MPs, microorganisms and gut microbiota could lead to health implications (Lu et al. 2019). It is known that gut microbiota plays an important role in the hosts health and it is also known that MPs can carry potential pesticides, fungicides and pathogens. Once ingested these hitchhikers may affect health by changing the composition of gut microbiota (Lu et al. 2019).
A further area of interest is the antibiotic resistant bacteria (ARB) that has been found on some MPs (Yang et al. 2019a). A recent study found ARB counts on MPs were 100–500 times higher than those in water and the ratios of ARB to total bacteria from MPs were higher than those in water (Zhang et al. 2020c). They also looked at multi-antibiotic resistant bacteria (MARB) and found penicillin, sulfafurazole, erythromycin and tetracycline resistant bacteria, accounted for 25.4% on MPs compared with 23.9% in water. Further studies looking at antibiotic resistant genes (ARG) showed a detection rate up to 80% on MPs and 65% in water (Zhang et al. 2020c). It was concluded that MPs can provide a beneficial surface for ARB to form a biofilm and facilitate horizontal gene transfer, which they would otherwise be unable to do in water alone and this could lead to the enrichment of superbugs.
Conclusion
There is a growing body of literature demonstrating that the atmosphere and human food and water sources are contaminated by MPs and may, therefore, be inhaled or ingested by humans. Studies using model organisms indicate that ingestion of microplastics might cause harm to organisms via their physical presence (abrasive effects leading to inflammation, oxidative stress and cytotoxicity), their chemical burden (leaching of additives or adsorbed chemicals from the environment causing reproductive and developmental toxicity or invoking an immune response) or their microbial communities (pathogens causing infection, gut dysbiosis or antimicrobial resistant microbes entering the body). In addition, inhalation of plastic microfibres has become a key research focus with recent estimates suggesting the general population inhales hundreds of plastic fibres each day. Correlative research links inhalation of plastic fibres to respiratory disease, inflammation and oxidative stress making inhalation of microfibres a key area of concern given the growing dominance of synthetic fibres in the clothing industry. The actual concentrations of inhaled and ingested microplastics that are accumulated within the human body are, however, not yet known. There is still a dearth of data on the direct human health implications and future work should target the direct effects of MPs on human health by focusing on inflammation and cellular damage at concentrations realistically reflecting environmental exposure.
Acknowledgements
We would like to thank The Fort Foundation and Anglia Ruskin University QR for funding this research. Thank you to Dr. Clett Erridge for your support and guidance.
Biographies
Kirsty Blackburn
is an Advanced Research Assistant at the Wellcome Sanger Institute. Previously Research Fellow at Anglia Ruskin University. Their research interests include, microplastics, forensics and toxicology.
Dannielle Green
is a Senior Lecturer at the Anglia Ruskin University. Their research interests include ecology, ecotoxicology and pollution.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kirsty Blackburn, Email: kirstyjblackburn@gmail.com.
Dannielle Green, Email: dannielle.green@aru.ac.uk.
References
- Abb M, Heinrich T, Sorkau E, Lorenz W. Phthalates in house dust. Environment International. 2009;35:965–970. doi: 10.1016/J.ENVINT.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Abbasi S, Keshavarzi B, Moore F, Turner A, Kelly FJ, Dominguez A, Jaafarzadeh N. Distribution and potential health impacts of microplastics and microrubbers in air and street dusts from Asaluyeh County, Iran. Environmental Pollution. 2019;244:153–164. doi: 10.1016/J.ENVPOL.2018.10.039. [DOI] [PubMed] [Google Scholar]
- Abidli S, Antunes JC, Ferreira JL, Lahbib Y, Sobral P, Trigui El Menif N. Microplastics in sediments from the littoral zone of the North Tunisian Coast (Mediterranean Sea) Estuarine, Coastal and Shelf Science. 2018;205:1–9. doi: 10.1016/J.ECSS.2018.03.006. [DOI] [Google Scholar]
- Ait Bamai Y, Araki A, Kawai T, Tsuboi T, Saito I, Yoshioka E, Kanazawa A, Tajima S, et al. Associations of phthalate concentrations in floor dust and multi-surface dust with the interior materials in Japanese Dwellings. Science of the Total Environment. 2014;468–469:147–157. doi: 10.1016/j.scitotenv.2013.07.107. [DOI] [PubMed] [Google Scholar]
- Ait Bamai Y, Shibata E, Saito I, Araki A, Kanazawa A, Morimoto K, Nakayama K, Tanaka M, et al. Exposure to house dust phthalates in relation to asthma and allergies in both children and adults. Science of the Total Environment. 2014;485–486:153–163. doi: 10.1016/j.scitotenv.2014.03.059. [DOI] [PubMed] [Google Scholar]
- Allen S, Allen D, Phoenix VR, Le Roux G, Durántez Jiménez P, Simonneau A, Binet S, Galop D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nature Geoscience. 2019;12:339–344. doi: 10.1038/s41561-019-0335-5. [DOI] [Google Scholar]
- Alonso V, Linares V, Bellés M, Albina ML, Pujol A, Domingo JL, Sánchez DJ. Effects of BDE-99 on hormone homeostasis and biochemical parameters in adult male rats. Food and Chemical Toxicology. 2010;48:2206–2211. doi: 10.1016/j.fct.2010.05.048. [DOI] [PubMed] [Google Scholar]
- Ambrosini R, Azzoni RS, Pittino F, Diolaiuti G, Franzetti A, Parolini M. First evidence of microplastic contamination in the supraglacial debris of an alpine glacier. Environmental Pollution. 2019;253:297–301. doi: 10.1016/J.ENVPOL.2019.07.005. [DOI] [PubMed] [Google Scholar]
- Amélineau F, Bonnet D, Heitz O, Mortreux V, Harding AMA, Karnovsky N, Walkusz W, Fort J, et al. Microplastic pollution in the greenland sea: Background levels and selective contamination of planktivorous diving seabirds. Environmental Pollution. 2016;219:1131–1139. doi: 10.1016/J.ENVPOL.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Ashton K, Holmes L, Turner A. Association of metals with plastic production pellets in the marine environment. Marine Pollution Bulletin. 2010;60:2050–2055. doi: 10.1016/J.MARPOLBUL.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Auta HS, Emenike CU, Fauziah SH. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environment International. 2017;102:165–176. doi: 10.1016/J.ENVINT.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Avio C, Gorbi S, Milan M, Benedetti M, Fattorini D, D’Errico G, Pauletto M, Bargelloni L, et al. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environmental Pollution. 2015;198:211–222. doi: 10.1016/j.envpol.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Bakir A, O’Connor I, Rowland S, Hendriks A, Thompson R. Relative importance of microplastics as a pathway for the transfer of hydrophobic organic chemicals to marine life. Environmental Pollution. 2016;219:56–65. doi: 10.1016/j.envpol.2016.09.046. [DOI] [PubMed] [Google Scholar]
- Bakir A, Rowland SJ, Thompson RC. Competitive sorption of persistent organic pollutants onto microplastics in the marine environment. Marine Pollution Bulletin. 2012;64:2782–2789. doi: 10.1016/J.MARPOLBUL.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Bank MS, Hansson SV. The plastic cycle: A novel and holistic paradigm for the anthropocene. Environmental Science and Technology. 2019;53:7177–7179. doi: 10.1021/acs.est.9b02942. [DOI] [PubMed] [Google Scholar]
- Barboza LGA, Dick Vethaak A, Lavorante B, Lundebye AK, Guilhermino L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Marine Pollution Bulletin. 2018;133:336–348. doi: 10.1016/J.MARPOLBUL.2018.05.047. [DOI] [PubMed] [Google Scholar]
- Barboza LGA, Lopes C, Oliveira P, Bessa F, Otero V, Henriques B, Raimundo J, Caetano M, et al. Microplastics in wild fish from North East Atlantic ocean and its potential for causing neurotoxic effects, lipid oxidative damage, and human health risks associated with ingestion exposure. Science of the Total Environment. 2020;717:134625. doi: 10.1016/j.scitotenv.2019.134625. [DOI] [PubMed] [Google Scholar]
- Bellés M, Alonso V, Linares V, Albina ML, Sirvent JJ, Domingo JL, Sánchez DJ. Behavioral effects and oxidative status in brain regions of adult rats exposed to BDE-99. Toxicology Letters. 2010;194:1–7. doi: 10.1016/j.toxlet.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Besseling E, Wegner A, Foekema E, van den Heuvel-Greve M, Koelmans A. Effects of microplastic on fitness and PCB bioaccumulation by the Lugworm Arenicola Marina (L.) Environmental Science and Technology. 2013;47:593–600. doi: 10.1021/es302763x. [DOI] [PubMed] [Google Scholar]
- Boucher J, Billard G. The challenges of measuring plastic pollution. Field Actions Science Reports. 2019;19:68–75. [Google Scholar]
- Boucher J, Friot D. Primary microplastics in the oceans: A global evaluation of sources. Switzerland: IUCN Gland; 2017. [Google Scholar]
- Braeuning A. Uptake of microplastics and related health effects: A critical discussion of Deng et al., Scientific Reports 7:46687, 2017. Archives of Toxicology. 2019;93:219–220. doi: 10.1007/s00204-018-2367-9. [DOI] [PubMed] [Google Scholar]
- Brown DM, Wilson MR, MacNee W, Stone V, Donaldson K. Size-dependent proinflammatory effects of ultrafine polystyrene particles: A role for surface area and oxidative stress in the enhanced activity of ultrafines. Toxicology and Applied Pharmacology. 2001;175:191–199. doi: 10.1006/TAAP.2001.9240. [DOI] [PubMed] [Google Scholar]
- Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environmental Science and Technology. 2011;45:9175–9179. doi: 10.1021/es201811s. [DOI] [PubMed] [Google Scholar]
- Browne MA, Niven SJ, Galloway TS, Rowland SJ, Thompson RC. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Current Biology. 2013;23:2388–2392. doi: 10.1016/j.cub.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Cai L, Wang J, Peng J, Tan Z, Zhan Z, Tan X, Chen Q. Characteristic of microplastics in the atmospheric fallout from Dongguan City, China: Preliminary research and first evidence. Environmental Science and Pollution Research. 2017;24:24928–24935. doi: 10.1007/s11356-017-0116-x. [DOI] [PubMed] [Google Scholar]
- Catarino AI, Macchia V, Sanderson WG, Thompson RC, Henry TB. Low levels of microplastics (MP) in wild mussels indicate that MP ingestion by humans is minimal compared to exposure via household fibres fallout during a meal. Environmental Pollution. 2018;237:675–684. doi: 10.1016/J.ENVPOL.2018.02.069. [DOI] [PubMed] [Google Scholar]
- Choudhary D, Kurien C, Srivastava AK. Microplastic contamination and life cycle assessment of bottled drinking water. In: Siddiqui NA, Tauseef SM, Dobhal R, editors. Advances in water pollution monitoring and control. Singapore: Springer; 2020. pp. 41–48. [Google Scholar]
- Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE. Human consumption of microplastics. Environmental Science and Technology. 2019;53:7068–7074. doi: 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- Crawford CB, Quinn B. The interactions of microplastics and chemical pollutants. Microplastic Pollutants. 2017 doi: 10.1016/B978-0-12-809406-8.00006-2. [DOI] [Google Scholar]
- de Sá LC, Oliveira M, Ribeiro F, Rocha T, Futter MN. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Science of the Total Environment. 2018;645:1029–1039. doi: 10.1016/J.SCITOTENV.2018.07.207. [DOI] [PubMed] [Google Scholar]
- Dehghani S, Moore F, Akhbarizadeh R. Microplastic pollution in deposited urban dust, Tehran Metropolis, Iran. Environmental Science and Pollution Research. 2017;24:20360–20371. doi: 10.1007/s11356-017-9674-1. [DOI] [PubMed] [Google Scholar]
- Deng Y, Zhang Y, Lemos B, Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Scientific Reports. 2017;7:46687. doi: 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo JL. Polybrominated diphenyl ethers in food and human dietary exposure: A review of the recent scientific literature. Food and Chemical Toxicology. 2012;50:238–249. doi: 10.1016/j.fct.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Driedger AGJ, Dürr HH, Mitchell K, Van Cappellen P. Plastic debris in the Laurentian great lakes: A review. Journal of Great Lakes Research. 2015;41:9–19. doi: 10.1016/J.JGLR.2014.12.020. [DOI] [Google Scholar]
- Dris R, Gasperi J, Mirande C, Mandin C, Guerrouache M, Langlois V, Tassin B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environmental Pollution. 2017;221:453–458. doi: 10.1016/J.ENVPOL.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Dris R, Gasperi J, Rocher V, Saad M, Renault N, Tassin B. Microplastic contamination in an urban area: A case study in greater Paris. Environmental Chemistry. 2015;12:592–599. doi: 10.1071/EN14167. [DOI] [Google Scholar]
- Eyles JE, Bramwell VW, Williamson ED, Alpar HO. Microsphere translocation and immunopotentiation in systemic tissues following intranasal administration. Vaccine. 2001;19:4732–4742. doi: 10.1016/S0264-410X(01)00220-1. [DOI] [PubMed] [Google Scholar]
- Fang C, Zheng R, Chen H, Hong F, Lin L, Lin H, Guo H, Bailey C, et al. Comparison of microplastic contamination in fish and bivalves from two major cities in Fujian Province, China and the implications for Human Health. Aquaculture. 2019;512:734322. doi: 10.1016/J.AQUACULTURE.2019.734322. [DOI] [Google Scholar]
- Frias JPGL, Sobral P, Ferreira AM. Organic pollutants in microplastics from two beaches of the Portuguese Coast. Marine Pollution Bulletin. 2010;60:1988–1992. doi: 10.1016/J.MARPOLBUL.2010.07.030. [DOI] [PubMed] [Google Scholar]
- Gallagher LG, Li W, Ray RM, Romano ME, Wernli KJ, Gao D, Thomas DB, Checkoway H. Occupational exposures and risk of stomach and esophageal cancers: Update of a cohort of female textile workers in Shanghai, China. American Journal of Industrial Medicine. 2015;58:267–275. doi: 10.1002/ajim.22412. [DOI] [PubMed] [Google Scholar]
- Galloway, T.S. 2015. Micro- and nano-plastics and human health BT—marine anthropogenic litter. In ed. M. Bergmann, L. Gutow, and M. Klages, 343–66 pp. Cham: Springer International Publishing. 10.1007/978-3-319-16510-3_13.
- Gasperi J, Wright SL, Dris R, Collard F, Mandin C, Guerrouache M, Langlois V, Kelly FJ, Tassin B. Microplastics in air: Are we breathing it in? Current Opinion in Environmental Science & Health. 2018;1:1–5. doi: 10.1016/J.COESH.2017.10.002. [DOI] [Google Scholar]
- Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Science Advances. 2017;3:e1700782. doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin T, Roche N, Lohmann R, Hodges G. A thermodynamic approach for assessing the environmental exposure of chemicals absorbed to microplastic. Environmental Science and Technology. 2011;45:1466–1472. doi: 10.1021/es1032025. [DOI] [PubMed] [Google Scholar]
- Harrison JP, Schratzberger M, Sapp M, Osborn AM. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiology. 2014;14:232. doi: 10.1186/s12866-014-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann NB, Hüffer T, Thompson RC, Hassellöv M, Verschoor A, Daugaard AE, Rist S, Karlsson T, et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environmental Science and Technology. 2019;53:1039–1047. doi: 10.1021/acs.est.8b05297. [DOI] [PubMed] [Google Scholar]
- Högberg J, Hanberg A, Berglund M, Skerfving S, Remberger M, Calafat AM, Filipsson AF, Jansson B, et al. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environmental Health Perspectives. 2008;116:334–339. doi: 10.1289/ehp.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes LA, Thompson RC. Interactions between trace metals and plastic production pellets under estuarine conditions. Marine Chemistry. 2014;167:25–32. doi: 10.1016/J.MARCHEM.2014.06.001. [DOI] [Google Scholar]
- Holmes LA, Turner A, Thompson RC. Adsorption of trace metals to plastic resin pellets in the marine environment. Environmental Pollution. 2012;160:42–48. doi: 10.1016/J.ENVPOL.2011.08.052. [DOI] [PubMed] [Google Scholar]
- Horton AA, Dixon SJ. Microplastics: An introduction to environmental transport processes. WIREs Water. 2018;5:e1268. doi: 10.1002/wat2.1268. [DOI] [Google Scholar]
- Horton AA, Svendsen C, Williams R, Spurgeon DJ, Lahive E. Large microplastic particles in sediments of tributaries of the River Thames, UK—abundance, sources and methods for effective quantification. Marine Pollution Bulletin. 2017;114:218–226. doi: 10.1016/J.MARPOLBUL.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu Q, Jia W, Yan C, Wang J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environmental Pollution. 2020;260:114096. doi: 10.1016/j.envpol.2020.114096. [DOI] [PubMed] [Google Scholar]
- Huang Y, Qing X, Wang W, Han G, Wang J. Mini-review on current studies of airborne microplastics: Analytical methods, occurrence, sources, fate and potential risk to human beings. TrAC, Trends in Analytical Chemistry. 2020;125:115821. doi: 10.1016/J.TRAC.2020.115821. [DOI] [Google Scholar]
- Huerta Lwanga E, Gertsen H, Gooren H, Peters P, Salánki T, van der Ploeg M, Besseling E, Koelmans AA, Geissen Violette. Microplastics in the terrestrial ecosystem: Implications for lumbricus terrestris (Oligochaeta, Lumbricidae) Environmental Science and Technology. 2016;50:2685–2691. doi: 10.1021/acs.est.5b05478. [DOI] [PubMed] [Google Scholar]
- Hwang J, Choi D, Han S, Choi J, Hong J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Science of the Total Environment. 2019;684:657–669. doi: 10.1016/J.SCITOTENV.2019.05.071. [DOI] [PubMed] [Google Scholar]
- Jambeck JR, Geyer R, Wilcox C, Siegler T, Perryman M, Andrady A, Narayan R, Law KL. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- Jepson PD, Deaville R, Barber JL, Aguilar À, Borrell A, Murphy S, Barry J, Brownlow A, et al. PCB pollution continues to impact populations of orcas and other dolphins in European waters. Scientific Reports. 2016;6:18573. doi: 10.1038/srep18573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Yin L, Li Z, Wen X, Luo X, Hu S, Yang H, Long Y, et al. Microplastic pollution in the rivers of the Tibet Plateau. Environmental Pollution. 2019;249:91–98. doi: 10.1016/J.ENVPOL.2019.03.022. [DOI] [PubMed] [Google Scholar]
- Jornet-Martínez N, Antón-Soriano C, Campíns-Falcó P. Estimation of the presence of unmetabolized dialkyl phthalates in untreated human urine by an on-Line miniaturized reliable method. Science of the Total Environment. 2015;532:239–244. doi: 10.1016/J.SCITOTENV.2015.05.124. [DOI] [PubMed] [Google Scholar]
- Katyal D, Kong E, Villanueva J. Microplastics in the environment: Impact on human health and future mitigation strategies. Environmental Health Review. 2020;63:27–31. doi: 10.5864/d2020-005. [DOI] [Google Scholar]
- Kirstein I, Kirmizi S, Wichels A, Garin-Fernandez A, Erler R, Löder M, Gerdts G. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio Spp. on microplastic particles. Marine Environmental Research. 2016;120:1–8. doi: 10.1016/j.marenvres.2016.07.004. [DOI] [PubMed] [Google Scholar]
- Koelmans AA, Bakir A, Burton GA, Janssen CR. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environmental Science and Technology. 2016;50:3315–3326. doi: 10.1021/acs.est.5b06069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelmans AA, Besseling E, Wegner A, Foekema EM. Plastic as a carrier of POPs to aquatic organisms: A model analysis. Environmental Science and Technology. 2013;47:7812–7820. doi: 10.1021/es401169n. [DOI] [PubMed] [Google Scholar]
- Kosuth M, Mason SA, Wattenberg EV. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE. 2018;13:e0194970. doi: 10.1371/journal.pone.0194970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P. In utero exposure to Di-(2-Ethylhexyl)phthalate and duration of human pregnancy. Environmental Health Perspectives. 2003;111:1783–1785. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavers JL, Bond AL, Hutton I. Plastic ingestion by flesh-footed shearwaters (Puffinus Carneipes): Implications for fledgling body condition and the accumulation of plastic-derived chemicals. Environmental Pollution. 2014;187:124–129. doi: 10.1016/J.ENVPOL.2013.12.020. [DOI] [PubMed] [Google Scholar]
- Law KL, Thompson RC. Microplastics in the seas. Science. 2014;345:144–145. doi: 10.1126/science.1254065. [DOI] [PubMed] [Google Scholar]
- Lear G, Kingsbury JM, Franchini S, Gambarini V, Maday SDM, Wallbank JA, Weaver L, Pantos O. Plastics and the microbiome: Impacts and solutions. Environmental Microbiome. 2021;16:2. doi: 10.1186/s40793-020-00371-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner R, Weder C, Petri-Fink A, Rothen-Rutishauser B. Emergence of nanoplastic in the environment and possible impact on human health. Environmental Science and Technology. 2019;53:1748–1765. doi: 10.1021/acs.est.8b05512. [DOI] [PubMed] [Google Scholar]
- Li B, Ding Y, Cheng X, Sheng D, Xu Z, Rong Q, Wu Y, Zhao H, Ji Xiaofei, Zhang Ying. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere. 2020;244:125492. doi: 10.1016/J.CHEMOSPHERE.2019.125492. [DOI] [PubMed] [Google Scholar]
- Li D, Shi Y, Yang L, Xiao L, Kehoe D, Gun’ko YK, Boland J, Wang JJ. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nature Food. 2020;1:746–754. doi: 10.1038/s43016-020-00171-y. [DOI] [PubMed] [Google Scholar]
- Li J, Liu H, Chen JP. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Research. 2018;137:362–374. doi: 10.1016/J.WATRES.2017.12.056. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang K, Zhang H. Adsorption of antibiotics on microplastics. Environmental Pollution. 2018;237:460–467. doi: 10.1016/j.envpol.2018.02.050. [DOI] [PubMed] [Google Scholar]
- Li J, Qu X, Su L, Zhang W, Yang D, Kolandhasamy P, Li D, Shi H. Microplastics in mussels along the coastal waters of China. Environmental Pollution. 2016;214:177–184. doi: 10.1016/J.ENVPOL.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Linares V, Bellés M, Domingo JL. Human exposure to PBDE and critical evaluation of health hazards. Archives of Toxicology. 2015;89:335–356. doi: 10.1007/s00204-015-1457-1. [DOI] [PubMed] [Google Scholar]
- Lithner D, Damberg J, Dave G, Larsson Å. Leachates from plastic consumer products—screening for toxicity with Daphnia Magna. Chemosphere. 2009 doi: 10.1016/j.chemosphere.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Lithner D, Larsson Å, Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Science of the Total Environment. 2011;409:3309–3324. doi: 10.1016/J.SCITOTENV.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Liu K, Wang X, Fang T, Xu P, Zhu L, Li D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Science of the Total Environment. 2019;675:462–471. doi: 10.1016/J.SCITOTENV.2019.04.110. [DOI] [PubMed] [Google Scholar]
- Liu K, Wang X, Wei N, Song Z, Li D. Accurate quantification and transport estimation of suspended atmospheric microplastics in megacities: Implications for human health. Environment International. 2019;132:105127. doi: 10.1016/j.envint.2019.105127. [DOI] [PubMed] [Google Scholar]
- Liu K, Wu T, Wang X, Song Z, Zong C, Wei N, Li D. Consistent transport of terrestrial microplastics to the ocean through atmosphere. Environmental Science and Technology. 2019;53:10612–10619. doi: 10.1021/acs.est.9b03427. [DOI] [PubMed] [Google Scholar]
- Lobelle D, Cunliffe M. Early microbial biofilm formation on marine plastic debris. Marine Pollution Bulletin. 2011;62:197–200. doi: 10.1016/J.MARPOLBUL.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Lu L, Luo T, Zhao Y, Cai C, Fu Z, Jin Y. Interaction between microplastics and microorganism as well as gut microbiota: A consideration on environmental animal and human health. Science of the Total Environment. 2019;667:94–100. doi: 10.1016/J.SCITOTENV.2019.02.380. [DOI] [PubMed] [Google Scholar]
- Lu J, Wu J, Wu J, Zhang C, Luo Y. Adsorption and desorption of steroid hormones by microplastics in seawater. Bulletin of Environmental Contamination and Toxicology. 2020 doi: 10.1007/s00128-020-02784-2. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zhang Y, Deng Y, Jiang W, Zhao Y, Geng J, Ding L, Ren H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio Rerio) and toxic effects in liver. Environmental Science and Technology. 2016;50:4054–4060. doi: 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
- Luo T, Zhang Y, Wang C, Wang X, Zhou J, Shen M, Zhao Y, Fu Z, Jin Yuanxiang. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environmental Pollution. 2019;255:113122. doi: 10.1016/J.ENVPOL.2019.113122. [DOI] [PubMed] [Google Scholar]
- Lusher AL, McHugh M, Thompson RC. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the english channel. Marine Pollution Bulletin. 2013;67:94–99. doi: 10.1016/J.MARPOLBUL.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Main K, Mortensen G, Kaleva M, Boisen K, Damgaard I, Chellakooty M, Schmidt I, Suomi AM, et al. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environmental Health Perspectives. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markel D, Zhang R, Shi T, Hawkins M, Ren W. Inhibitory effects of erythromycin on wear debris-induced VEGF/Flt-1 gene production and osteolysis. Inflammation Research. 2009;58:413. doi: 10.1007/s00011-009-0007-9. [DOI] [PubMed] [Google Scholar]
- Morris JG, Jr, Acheson D. Cholera and other types of vibriosis: A story of human pandemics and oysters on the half shell. Clinical Infectious Diseases. 2003;37:272–280. doi: 10.1086/375600. [DOI] [PubMed] [Google Scholar]
- Oliveri Conti G, Ferrante M, Banni M, Favara C, Nicolosi I, Cristaldi A, Fiore M, Zuccarello P. Micro- and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environmental Research. 2020;187:109677. doi: 10.1016/j.envres.2020.109677. [DOI] [PubMed] [Google Scholar]
- Pannetier P, Morin B, Le Bihanic F, Dubreil L, Clérandeau C, Chouvellon F, Van Arkel K, Danion M, Cachot J. Environmental samples of microplastics induce significant toxic effects in fish larvae. Environment International. 2020;134:105047. doi: 10.1016/J.ENVINT.2019.105047. [DOI] [PubMed] [Google Scholar]
- Pauly JL, Stegmeier SJ, Allaart HA, Cheney RT, Zhang PJ, Mayer AG, Streck RJ. Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiology, Biomarkers and Prevention. 1998;7(5):419–428. [PubMed] [Google Scholar]
- Pearl J, Ma T, Irani AR, Huang Z, Robinson WH, Smith RL, Goodman SB. Role of the toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials. 2011;32:5535–5542. doi: 10.1016/j.biomaterials.2011.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeken I, Primpke S, Beyer B, Gütermann J, Katlein C, Krumpen T, Bergmann M, Hehemann L, Gerdts Gunnar. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nature Communications. 2018;9:1505. doi: 10.1038/s41467-018-03825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto D, Pinheiro C, Amorim J, Oliva-Teles L, Guilhermino L, Vieira M. Microplastic pollution in commercial salt for human consumption: A review. Estuarine, Coastal and Shelf Science. 2019;219:161–168. doi: 10.1016/J.ECSS.2019.02.018. [DOI] [Google Scholar]
- Peng X, Chen M, Chen S, Dasgupta S, Xu H, Ta K, Du M, Li J, Guo Z, Bai S. Microplastics contaminate the deepest part of the World’s Ocean. Geochemical Perspectives Letters. 2018;9:1–5. doi: 10.7185/geochemlet.1829. [DOI] [Google Scholar]
- Peretz J, Vrooman L, Ricke WA, Hunt PA, Ehrlich S, Hauser R, Padmanabhan V, Taylor HS, et al. Bisphenol a and reproductive health: Update of experimental and human evidence, 2007–2013. Environmental Health Perspectives. 2014;122:775–786. doi: 10.1289/ehp.1307728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips MB, Bonner TH. Occurrence and amount of microplastic ingested by fishes in watersheds of the Gulf of Mexico. Marine Pollution Bulletin. 2015;100:264–269. doi: 10.1016/J.MARPOLBUL.2015.08.041. [DOI] [PubMed] [Google Scholar]
- Possatto, F.E., M. Barletta, M.F. Costa, J.A. Ivar do Sul, and D.V. Dantas. 2011. Plastic debris ingestion by marine catfish: An unexpected fisheries impact. Marine Pollution Bulletin 62: 1098–1102. 10.1016/j.marpolbul.2011.01.036. [DOI] [PubMed]
- Prata JC. Airborne microplastics: Consequences to human health? Environmental Pollution. 2018;234(March):115–126. doi: 10.1016/J.ENVPOL.2017.11.043. [DOI] [PubMed] [Google Scholar]
- Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T. Environmental exposure to microplastics: An overview on possible human health effects. Science of the Total Environment. 2020;702:134455. doi: 10.1016/J.SCITOTENV.2019.134455. [DOI] [PubMed] [Google Scholar]
- Qiao R, Deng Y, Zhang S, Wolosker M, Zhu Q, Ren H, Zhang Y. Accumulation of different shapes of microplastics initiates intestinal injury and gut microbiota dysbiosis in the gut of zebrafish. Chemosphere. 2019;236:124334. doi: 10.1016/J.CHEMOSPHERE.2019.07.065. [DOI] [PubMed] [Google Scholar]
- Reed S, Clark M, Thompson R, Hughes KA. Microplastics in marine sediments near Rothera Research Station, Antarctica. Marine Pollution Bulletin. 2018;133:460–463. doi: 10.1016/J.MARPOLBUL.2018.05.068. [DOI] [PubMed] [Google Scholar]
- Reverte I, Domingo JL, Colomina MT. Neurodevelopmental effects of decabromodiphenyl ether (BDE-209) in APOE transgenic mice. Neurotoxicology and Teratology. 2014;46:10–17. doi: 10.1016/j.ntt.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Ribeiro F, O’Brien J, Galloway T, Thomas K. Accumulation and fate of nano- and micro-plastics and associated contaminants in organisms. TrAC, Trends in Analytical Chemistry. 2019;111:139–147. doi: 10.1016/J.TRAC.2018.12.010. [DOI] [Google Scholar]
- Rios LM, Jones PR, Moore C, Narayan UV. Quantitation of persistent organic pollutants adsorbed on plastic debris from the Northern Pacific Gyre’s ‘Eastern Garbage Patch’. Journal of Environmental Monitoring. 2010;12:2226–2236. doi: 10.1039/C0EM00239A. [DOI] [PubMed] [Google Scholar]
- Rochman CM, Hentschel BT, Teh SJ. Long-term sorption of metals is similar among plastic types: Implications for plastic debris in aquatic environments. PLoS ONE. 2014;9:e85433. doi: 10.1371/journal.pone.0085433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues J, Duarte AC, Santos-Echeandía J, Rocha-Santos T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC, Trends in Analytical Chemistry. 2019;111:252–260. doi: 10.1016/j.trac.2018.11.038. [DOI] [Google Scholar]
- Romeo T, Pietro B, Pedà C, Consoli P, Andaloro F, Fossi MC. First evidence of presence of plastic debris in stomach of large pelagic fish in the Mediterranean Sea. Marine Pollution Bulletin. 2015;95:358–361. doi: 10.1016/J.MARPOLBUL.2015.04.048. [DOI] [PubMed] [Google Scholar]
- Schirinzi GF, Pérez-Pomeda I, Sanchís J, Rossini C, Farré M, Barceló D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environmental Research. 2017;159:579–587. doi: 10.1016/J.ENVRES.2017.08.043. [DOI] [PubMed] [Google Scholar]
- Schwabl P, Köppel S, Königshofer P, Bucsics T, Trauner M, Reiberger T, Liebmann B. Detection of various microplastics in human stool: A prospective case series. Annals of Internal Medicine. 2019;171:453–457. doi: 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- Scopetani C, Cincinelli A, Martellini T, Lombardini E, Ciofini A, Fortunati A, Pasquali V, Ciattini S, Ugolini Alberto. Ingested microplastic as a two-way transporter for PBDEs in talitrus saltator. Environmental Research. 2018;167:411–417. doi: 10.1016/j.envres.2018.07.030. [DOI] [PubMed] [Google Scholar]
- Shruti VC, Pérez-Guevara F, Elizalde-Martínez I, Kutralam-Muniasamy G. First study of its kind on the microplastic contamination of soft drinks, cold tea and energy drinks—future research and environmental considerations. Science of the Total Environment. 2020;726:138580. doi: 10.1016/j.scitotenv.2020.138580. [DOI] [PubMed] [Google Scholar]
- Simmonds, M.P. 2017. Of poisons and plastics: an overview of the latest pollution issues affecting marine mammals BT—marine mammal welfare: human induced change in the marine environment and its impacts on marine mammal welfare. In ed. A. Butterworth, 27–37. Cham: Springer. 10.1007/978-3-319-46994-2_3.
- Stock V, Böhmert L, Lisicki E, Block R, Cara-Carmona J, Pack LK, Selb R, Lichtenstein D, et al. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Archives of Toxicology. 2019;93:1817–1833. doi: 10.1007/s00204-019-02478-7. [DOI] [PubMed] [Google Scholar]
- Talsness CE, Andrade AJM, Kuriyama SN, Taylor JA, vom Saal FS. Components of plastic: Experimental studies in animals and relevance for human health. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:2079–2096. doi: 10.1098/rstb.2008.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Takada H, Yamashita R, Mizukawa K, Fukuwaka M, Watanuki Y. Accumulation of plastic-derived chemicals in tissues of seabirds ingesting marine plastics. Marine Pollution Bulletin. 2013;69:219–222. doi: 10.1016/J.MARPOLBUL.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Tang Y, Rong J, Guan X, Zha S, Shi W, Han Y, Du X, Wu F, Huang Wei, Liu Guangxu. Immunotoxicity of microplastics and two persistent organic pollutants alone or in combination to a bivalve species. Environmental Pollution. 2020;258:113845. doi: 10.1016/j.envpol.2019.113845. [DOI] [PubMed] [Google Scholar]
- Tienhoven EAE, Van D, Korbee L, Schipper HW Verharen, De Jong WH. In vitro and in vivo (Cyto)toxicity assays using PVC and LDPE as model materials. Journal of Biomedical Materials Research, Part A. 2006;78A(1):175–182. doi: 10.1002/jbm.a.30679. [DOI] [PubMed] [Google Scholar]
- Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement*. The Journal of Bone and Joint Surgery. 2000;82:457. doi: 10.2106/00004623-200004000-00002. [DOI] [PubMed] [Google Scholar]
- van Franeker JA, Blaize C, Danielsen J, Fairclough K, Gollan J, Guse N, Hansen PL, Heubeck M, et al. Monitoring plastic ingestion by the Northern Fulmar Fulmarus Glacialis in the North Sea. Environmental Pollution. 2011;159:2609–2615. doi: 10.1016/J.ENVPOL.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. International Journal of Environmental Research and Public Health. 2013 doi: 10.3390/ijerph10093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan R, Turner SD, Rose NL. Microplastics in the sediments of a UK Urban Lake. Environmental Pollution. 2017;229:10–18. doi: 10.1016/J.ENVPOL.2017.05.057. [DOI] [PubMed] [Google Scholar]
- Velzeboer I, Kwadijk CJAF, Koelmans AA. Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes. Environmental Science and Technology. 2014;48:4869–4876. doi: 10.1021/es405721v. [DOI] [PubMed] [Google Scholar]
- Verla AW, Enyoh CE, Verla EN, Nwarnorh KO. Microplastic–toxic chemical interaction: A review study on quantified levels, mechanism and implication. SN Applied Sciences. 2019;1:1400. doi: 10.1007/s42452-019-1352-0. [DOI] [Google Scholar]
- Wagner M, Scherer C, Alvarez-Muñoz D, Brennholt N, Bourrain X, Buchinger S, Fries E, Grosbois C, et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environmental Sciences Europe. 2014;26:12. doi: 10.1186/s12302-014-0012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Gao H, Jin S, Li R, Na G. The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: A review. Ecotoxicology and Environmental Safety. 2019;173:110–117. doi: 10.1016/J.ECOENV.2019.01.113. [DOI] [PubMed] [Google Scholar]
- Wang T, Yu C, Chu Q, Wang F, Lan T, Wang J. Adsorption behavior and mechanism of five pesticides on microplastics from agricultural polyethylene films. Chemosphere. 2020;244:125491. doi: 10.1016/J.CHEMOSPHERE.2019.125491. [DOI] [PubMed] [Google Scholar]
- Watteau F, Dignac M, Bouchard A, Revallier A, Houot S. Microplastic detection in soil amended with municipal solid waste composts as revealed by transmission electronic microscopy and pyrolysis/GC/MS. Frontiers in Sustainable Food Systems. 2018 doi: 10.3389/fsufs.2018.00081. [DOI] [Google Scholar]
- Wick P, Malek A, Manser P, Meili D, Maeder-Althaus X, Diener L, Diener PA, Zisch A, Krug Harald F, von Mandach U. Barrier capacity of human placenta for nanosized materials. Environmental Health Perspectives. 2010;118:432–436. doi: 10.1289/ehp.0901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SL, Kelly FJ. Plastic and human health: A micro issue? Environmental Science and Technology. 2017;51:6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- Wright SL, Ulke J, Font A, Chan KLA, Kelly FJ. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environment International. 2020;136:105411. doi: 10.1016/J.ENVINT.2019.105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Zhang K, Chen X, Shi H, Luo Z, Wu C. Sources and distribution of microplastics in china’s largest inland lake—Qinghai lake. Environmental Pollution. 2018;235:899–906. doi: 10.1016/J.ENVPOL.2017.12.081. [DOI] [PubMed] [Google Scholar]
- Yang Y, Liu G, Song W, Ye C, Lin H, Li Z, Liu W. Plastics in the marine environment are reservoirs for antibiotic and metal resistance genes. Environment International. 2019;123:79–86. doi: 10.1016/j.envint.2018.11.061. [DOI] [PubMed] [Google Scholar]
- Yang YF, Chen CY, Lu TH, Liao CM. Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. Journal of Hazardous Materials. 2019;366:703–713. doi: 10.1016/J.JHAZMAT.2018.12.048. [DOI] [PubMed] [Google Scholar]
- Yuan J, Ma J, Sun Y, Zhou T, Zhao Y, Yu F. Microbial degradation and other environmental aspects of microplastics/plastics. Science of the Total Environment. 2020;715:136968. doi: 10.1016/j.scitotenv.2020.136968. [DOI] [PubMed] [Google Scholar]
- Zarfl C, Matthies M. Are marine plastic particles transport vectors for organic pollutants to the arctic? Marine Pollution Bulletin. 2010;60:1810–1814. doi: 10.1016/J.MARPOLBUL.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Zettler ER, Mincer TJ, Amaral-Zettler LA. Life in the ‘plastisphere’: Microbial communities on plastic marine debris. Environmental Science and Technology. 2013;47:7137–7146. doi: 10.1021/es401288x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao T, Kang S, Sillanpää M. Importance of atmospheric transport for microplastics deposited in remote areas. Environmental Pollution. 2019;254:112953. doi: 10.1016/J.ENVPOL.2019.07.121. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ricciardi BF, Yang X, Shi Y, Camacho NP, Bostrom MPG. Polymethylmethacrylate particles stimulate bone resorption of mature osteoclasts in vitro. Acta Orthopaedica. 2008;79:281–288. doi: 10.1080/17453670710015166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Yang X, Gertsen H, Peters P, Salánki T, Geissen V. A simple method for the extraction and identification of light density microplastics from soil. Science of the Total Environment. 2018;616–617:1056–1065. doi: 10.1016/J.SCITOTENV.2017.10.213. [DOI] [PubMed] [Google Scholar]
- Zhang, Q., Y. Zhao, J. Li, and H. Shi. 2020a. Microplastics in food: Health risks. In. Springer. 10.1007/698_2020_453.
- Zhang Y, Kang S, Allen S, Allen D, Gao T, Sillanpää M. Atmospheric microplastics: A review on current status and perspectives. Earth-Science Reviews. 2020;203:103118. doi: 10.1016/J.EARSCIREV.2020.103118. [DOI] [Google Scholar]
- Zhang Y, Lu J, Wu J, Wang J, Luo Y. Potential risks of microplastics combined with superbugs: Enrichment of antibiotic resistant bacteria on the surface of microplastics in mariculture system. Ecotoxicology and Environmental Safety. 2020;187:109852. doi: 10.1016/j.ecoenv.2019.109852. [DOI] [PubMed] [Google Scholar]
- Zhu X, Qiang L, Shi H, Cheng J. Bioaccumulation of microplastics and its in vivo interactions with trace metals in edible oysters. Marine Pollution Bulletin. 2020;154:111079. doi: 10.1016/j.marpolbul.2020.111079. [DOI] [PubMed] [Google Scholar]