Abstract
Despite advances in assisted reproductive techniques in the 4 decades since the first human birth after in vitro fertilisation, 1–2% of couples experience recurrent implantation failure, and some will never achieve a successful pregnancy even in the absence of a confirmed dysfunction. Furthermore, 1–2% of couples who do conceive, either naturally or with assistance, will experience recurrent early loss of karyotypically normal pregnancies. In both cases, embryo-endometrial interaction is a clear candidate for exploration. The impossibility of studying implantation processes within the human body has necessitated the use of animal models and cell culture approaches. Recent advances in 3-dimensional modelling techniques, namely the advent of organoids, present an exciting opportunity to elucidate the unanswerable within human reproduction. In this review, we will explore the ontogeny of implantation modelling and propose a roadmap to application and discovery.
Lay summary
A significant number of couples experience either recurrent implantation failure or recurrent pregnancy loss. Often, no underlying disorder can be identified. In both cases, the interaction of the embryo and maternal tissues is key. The lining of the womb, the endometrium, becomes receptive to embryo implantation during each menstrual cycle and provides a nourishing and supportive environment to support ongoing pregnancy. It is not possible to study early pregnancy directly, therefore, modelling embryo-endometrium interactions in the laboratory is essential if we wish to understand where this goes wrong. Advances in the lab have resulted in the development of organoids in culture: 3D cellular structures that represent the characteristics of a particular tissue or organ. We describe past and present models of the endometrium and propose a roadmap for future work with organoid models, from fundamental understanding of the endometrial function and implantation processes to the development of therapeutics to improve pregnancy outcomes and gynaecological health.
Key Words: embryo implantation, endometrium, organoid, assembloid
Introduction
Although reported success rates in assisted reproduction approach or even surpass natural conceptions, 1–2% of couples will experience recurrent implantation failure, defined as the absence of a positive pregnancy test after three transfers of high-quality embryos (Mascarenhas et al. 2021). Some will never achieve a successful pregnancy even in the absence of a confirmed dysfunction. Furthermore, 1–2% of couples who do conceive, either naturally or with assistance, will experience recurrent early loss of karyotypically normal pregnancies with no identifiable cause even after extensive clinical investigation (Bender Atik et al. 2018). In both cases, the loss of overtly ‘normal’ embryos suggests that defects of embryo-endometrial interaction might be a plausible explanation.
The purpose of any model system is to generate a physical, theoretical, or mathematical representation of a real phenomenon that is difficult or impractical to observe directly (Rogers 2012), a situation entirely descriptive of the study of human implantation. The ethical and practical impossibility of studying implantation processes within the human body has necessitated the use of animal models and cell culture approaches. These approaches have revealed considerable detail about the requirements for successful implantation but remain far from ideal. Recent advances in 3-dimensional (3D) modelling techniques, namely the advent of organoids, present an exciting opportunity to elucidate the unanswerable within human reproduction. In this review, we will explore the ontogeny of implantation modelling and propose a roadmap to application and discovery.
The endometrium and implantation
The mucosal lining of the uterus, the endometrium, is a complex tissue comprising luminal and glandular epithelium, as well as stromal, endothelial, and immune cells and provides a nourishing, immune-privileged environment to support successful embryo implantation (Gellersen & Brosens 2014). Formed of two distinct layers, the endometrium undergoes hormone-dependent cyclic renewal with proliferation, differentiation, shedding (menstruation), and regeneration of the upper functional layer in each cycle. Spontaneous, embryo-independent transformation of the tissue, termed decidualisation, occurs in the midluteal phase of each cycle facilitating the development of the decidua of pregnancy to support placentation and foetal development until parturition. Humans are one of only a handful of species in which spontaneous decidualisation has evolved. This is proposed to reflect the need for maternal control over the challenge posed by genetically diverse and mosaic embryos in species where deep haemochorial placentation requires complete maternal investment in the pregnancy (Gellersen & Brosens 2014).
Functional defects in the endometrium, and particularly in the decidualisation process, have been associated with a spectrum of reproductive disorders, ranging from implantation failure and miscarriages to major obstetrical syndromes such as preeclampsia, foetal growth restriction, and preterm birth (reviewed by Lucas et al. 2013). However, other than histological descriptions of embryo implantation in hysterectomy samples from the early and mid-20th century (Hertig et al. 1956), we know very little about the earliest stages of implantation of the human blastocyst and the pivotal interactions taking place between the midluteal endometrium and the embryo. In vivo studies of dynamic implantation processes have been dependent on the use of animal models, while the generation of in vitro systems has facilitated the study of human embryo development and endometrial physiology and pathology, albeit largely in isolation. The development of embryo-endometrium co-cultures that faithfully mimic in vivo processes will truly unlock the black box of human reproduction.
Modelling implantation
Animal models
In the absence of human in vivo studies, murine, ovine, and bovine models, among others, have contributed much to the study of endometrial biology and pregnancy. However, the extent to which animal models represent human physiology is limited due to marked interspecies differences in reproductive strategy, implantation, and placentation. These have been reviewed extensively elsewhere (Lee & DeMayo 2004, Carter 2007, 2020, Morrison et al. 2018).
Livestock models have been utilised to pioneer many techniques used in modern assisted reproductive technologies (ART) (reviewed by Morrison et al. 2018). Sheep and cattle have been used to model human pregnancy while also advancing their commercial productivity with similar goals to human ART: to improve conception and live birth rates and reduce the incidence of pregnancy loss. Considerable differences in maternal recognition of pregnancy, implantation timing, and placental morphology make direct inference to human implantation difficult, nonetheless, conserved mechanisms are present (Tinning et al. 2020). Notwithstanding the value of sheep to model human foetal development (Morrison et al. 2018), the utility of large domestic animals to model early human pregnancy is limited (Carter 2020). Mouse models have been used to explore all aspects of reproduction, including implantation and the establishment of pregnancy. The mouse has been a preferred model for researchers for a number of reasons including litter-bearing status, rapid conception and gestation, haemochorial placentation, and genetic tractability; all of which provide an abundance of material for study and the ability to target specific pathways of interest to determine the impact on fecundity and fertility (Carter 2020). However, progesterone receptor expression dynamics vary greatly between humans and mice (Teilmann et al. 2006), and decidualisation in mouse is dependent on an embryonic stimulus (Lopes et al. 2004) rather than a spontaneous cyclic process. Overall, despite the many advances in our understanding of early pregnancy from animal models, interspecies differences have hindered the translation of understanding to human embryo implantation research.
In vitro models
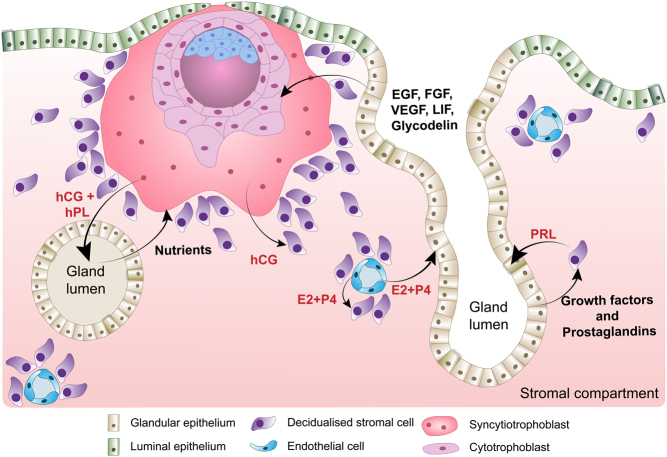
To circumvent interspecies variability at implantation, in vitro models have been developed using primary endometrial epithelial or stromal cell cultures as well as endometrium-derived cell lines. In vitro endometrial models aim to represent at least certain aspects of the human implantation environment while retaining levels of experimental control and manipulability (Fig. 1 and Table 1). In combination with a range of molecular techniques and other assays, these models present effective tools to close the knowledge gap regarding human embryo implantation and answer a diverse panel of questions.
Figure 1.
In vitro endometrial models to study implantation. Schematic representations of existing models for modelling endometrium-embryo interactions. The main benefits (+) and shortfalls (-) of each system are indicated.
Table 1.
In vitro models to study human endometrium-embryo interaction.
| Model format/cell types | Embryo/spheroid | Measures of interaction | References | |||
|---|---|---|---|---|---|---|
| Embryo/spheroid | Stromal cells | Epithelial cells | Trophoblast | |||
| Monolayer | ||||||
| Primary human endometrial stromal cells | Hatched human blastocysts, high- and low-quality human embryos, human embryo-conditioned media, mouse blastocysts, human trophoblast spheroids, embryonic stem cell-derived trophoblast spheroids | Attachment (apposition and anchoring), trophoblast outgrowth and invasion, hCG secretion | Cell migration, secretion of implantation-associated cytokines, gene expression profiles, calcium signalling, ‘biosensing’ embryo quality | Carver et al. (2003), Grewal et al. (2008, 2010), Teklenburg et al. (2010), Weimar et al. (2012), Brosens et al. (2014), Lee et al. (2015), Berkhout et al. (2018) | ||
| Human endometrial stromal cell lines (T-HESC; hTERT-immortalised endometrial stromal cells) | Human trophoblast spheroids (AC-1M88, Sw.71) | *Attachment, expansion, outgrowth and invasion, CEACAM1 expression | Mmigration | Holmberg et al. (2012), Gellersen et al. (2013) | ||
| Primary human endometrial epithelial cells | Cleavage stage human embryos and blastocysts, human embryo-conditioned media, mouse blastocysts, human trophoblast spheroids (trophoblast stem cell-, embryonic stem cell-and/or choriocarcinoma -derived) | Blastocyst rate and quality, blastocyst adhesion, spheroid attachment and outgrowth | Gene expression and secretion of implantation-associated chemokines, chemokine receptor expression and localisation, gene expression profiles, morphological assessment | Lindenberg et al. (1985), Simón et al. (1997, 1999), Meseguer et al. (2001), Caballero-Campo et al. (2002), Dominguez et al. (2003), Lee et al. (2015), Le Saint et al. (2019), Evans et al. (2020). | ||
| Human endometrial epithelial cell lines (Ishikawa, RL95-2) | Hatched and unhatched human blastocysts, hatched mouse blastocysts, human trophoblast spheroids (trophoblast stem cell-, embryonic stem cell-and/or choriocarcinoma -derived) | Attachment and outgrowth/invasion | Gene expression profiles | Lee et al. (2015), Huang et al. (2017), Berneau et al. (2019), Evans et al. (2020), Ruane et al. (2020). | ||
| Layered culture | ||||||
| Primary human endometrial epithelial and stromal cells | Expanded/hatching human blastocysts, trophoblast spheroids (choriocarcinoma, JAr) | Embryo attachment, trophoblast invasion and syncytium formation, spheroid attachment and outgrowth/invasion | Bentin-Ley et al. (2000), Wang et al. (2012) | |||
| Human endometrial epithelial cell lines (RL95-2, HEC-1A, Ishikawa), immortalised and primary human endometrial stromal cells | Trophoblast spheroids (Jar choriocarcinoma) | *Attachment and outgrowth/invasion | Evron et al. (2011), Wang et al. (2012) | |||
| Transwell culture | ||||||
| Primary human endometrial stromal cells and immortalised cell lines (St-T1b; T-HESC) | Trophoblast cells (AC-1M88) | Mmotility and invasion | Invasiveness | Gellersen et al. (2010, 2013) | ||
| Organoids | ||||||
| Primary human endometrial epithelial cells | Embryo conditioned media | Pinopode development, glycodelin secretion profile. | Luddi et al. (2021) | |||
| Assembloid | ||||||
| Primary human endometrial epithelial and stromal cells | Hatched human blastocysts | †Expansion and attachment; EM cells: morphology and motility | Rawlings et al. (2021) | |||
*Spheroid; †Embryos.
EM cells, endometrial cells.
Monolayer cultures
Two-dimensional (2D) cultures of endometrial stromal and epithelial cells have both been utilised to investigate implantation processes (Fig. 1). Since the first report using monolayer epithelial cells to describe interactions between the luminal epithelium and an implanting embryo (Lindenberg et al. 1985), many studies have used this approach to study early feto-maternal interactions (Weimar et al. 2013, Aplin & Ruane 2017). In response to embryonic signals, co-cultured epithelial cells upregulate expression of the receptivity genes CXC chemokine receptor type 1 (CXCR1), CXCR4, and CXCR5, and the chemokine interleukin (IL)-8 (Caballero-Campo et al. 2002, Dominguez et al. 2003), encoded by CXCL8, as well as epithelial cell surface molecules implicated in embryo implantation, such as mucin 1 (MUC1) (Simón et al. 1997, Meseguer et al. 2001) and osteopontin (OPN) (von Wolff et al. 2001, Erikson et al. 2009, Berneau et al. 2019) (Table 1). Co-culture of in vitro fertilised (IVF) embryos with an autologous epithelial monolayer prior to embryo transfer increased the frequency of high-quality blastocyst formation and implantation rates (Simón et al. 1999, Le Saint et al. 2019). However, a significant benefit of the co-culture to live birth rates remains to be confirmed (Le Saint et al. 2019). Trophoblast spheroids, as a surrogate for human blastocysts, have also been used successfully in co-culture with epithelial monolayers to investigate embryo attachment to the luminal epithelium (Weimar et al. 2013, Lee et al. 2015, Huang et al. 2017, Evans et al. 2020, Ruane et al. 2020) (Table 1). These studies demonstrate the reciprocal relationship between luminal epithelium and preimplantation embryos in preparation for human implantation. However, endometrial epithelial cells are technically challenging to propagate as monolayers and cannot be cultured long-term (Varma et al. 1982, Fitzgerald et al. 2021). They also do not represent the normal physiology and architecture of glands in vivo (Gray et al. 2001).
Primary human endometrial stromal cells have been used extensively to study human decidualisation and the peri-implantation endometrial environment. Studies of primary stromal monolayers have demonstrated embryo-stroma interactions and the retention of patient phenotypes by these cells. Carver and colleagues (2003) co-cultured stromal cell monolayers with hatched day five human blastocysts for 3 days and demonstrated that blastocysts could attach to and invade a decidualised stromal monolayer but did not interact with undifferentiated cells. In midluteal endometrium, decidualising stromal cells transiently attain a myo-fibroblastic phenotype, in which they become migratory and can produce extracellular matrix (ECM)-degrading matrix metallopeptidases (MMP) (Anacker et al. 2011), a process which is essential to allow trophoblast outgrowth (Grewal et al. 2008, 2010). Decidualised stromal cells are sensitive to embryo quality, selectively migrating towards high-quality human embryos but downregulating implantation-associated genes and the migratory phenotype in the presence of poor-quality or arresting embryos or conditioned media (Teklenburg et al. 2010, Weimar et al. 2012, Brosens et al. 2014, Berkhout et al. 2018) (Table 1).
Monolayer stromal co-cultures with blastocysts have also been used to investigate endometrial function and dysfunction in reproductive failure. Decidualising stromal cells of recurrent pregnancy loss (RPL) patients fail to inhibit implantation genes when co-cultured with poor-quality embryos (Weimar et al. 2012). This suggests a defect in embryo quality ‘biosensing’ in the endometrium of these women, manifesting as a loss of embryo selectivity. Also referred to as the ‘selection failure hypothesis, this failure to prevent implantation of poor-quality embryos is predicted to lead to subsequent miscarriage (Aplin et al. 1996, Macklon & Brosens 2014).
Overall, monolayer endometrial cell models provide a simple and robust model for studying early embryo implantation and embryo-endometrial interactions in humans. A major disadvantage of monolayer models is the restriction to a single endometrial cell type, disregarding stromal-epithelial interactions within the in vivo environment, as well as the roles of resident immune and endothelial cell populations. Additionally, although these models have been utilised to study the initial attachment and invasion of embryos, they lack the 3D architecture essential to study trophoblast invasion and the foundations of placentation.
Layered co-cultures
Glandular development and differentiation are dependent on stromal secretions (reviewed by Fitzgerald et al. 2021), exemplifying the dependence of the peri-implantation endometrial environment on synchronous paracrine signals, cell–cell, and cell-matrix interactions between multiple cell types of the decidualising endometrium. To study these interactions, more complex co-culture models have been developed (Fig. 1 and Table 1) and these will be explored briefly here.
To establish 3D cultures, cells are seeded into or on top of a scaffold to mimic their spatial environment within the structure of the ECM. The most common scaffold is the hydrogel, a hydrophilic polymer chain network that can be easily fine-tuned to mimic the structural properties of a native tissue ECM. Layered co-cultures of endometrial epithelial cells grown over a stromal cell-containing collagen hydrogel resemble a simplified human endometrium and aim to replicate the complexity of the in vivo endometrium more closely (Bentin-Ley et al. 1994, Evron et al. 2011, Wang et al. 2012). Luminal epithelium polarisation and embryo attachment within 48 h were revealed in one such model by scanning electron microscopy, with apparent penetration of syncytiotrophoblast through the epithelium into the underlying stromal layer (Bentin-Ley et al. 2000). In place of embryos, trophoblast cell line spheroids, such as the JAr cell line (John et al. 1993), have also been utilised to study implantation in layered co-culture models (Evron et al. 2011, Wang et al. 2012). The attachment of trophoblast spheroids to epithelial cell lines was improved by co-culturing with stromal cells (Wang et al. 2012) and dependent on the receptivity status of the stromal cells (Evron et al. 2011). Occasional spontaneous gland-like structures were observed in these cultures (Wang et al. 2012) although layered models generally fail to form glandular structures reproducibly and thus while useful for studying very early implantation processes, are not truly representative of the tissue at implantation.
Transwell co-culture models enhanced the ability to study trophoblast invasion: using dual-chambered systems containing an extracellular matrix (Matrigel™)-coated porous filter insert, adherent trophoblast cells can invade the matrix and migrate through the filter (Aplin 2006, Lash et al. 2007) (Fig. 1). By seeding endometrial epithelial cells on top of the Matrigel™ in the transwell insert and culturing stromal cells in the well beneath the filter (Arnold et al. 2001, Pierro et al. 2001, Bläuer et al. 2005), the transwell approach provides an easily manipulatable assay to assess trophoblast invasion in the presence of endometrial cells. The presence of trophoblasts also improves the invasiveness of decidualised stromal cells in transwell systems (Gellersen et al. 2010, 2013). However, cell–cell contact between epithelial and stromal cells is not established in these models and so critical interactions to orchestrate trophoblast invasion and endometrial tissue remodelling are likely to be missing.
Clearly, layered and transwell co-culture approaches improve upon monolayer cultures by incorporating both stromal and epithelial cells into a 3D space, providing the architecture for a more physiological model to study implantation. However, these models conspicuously lack glandular structures, a functional unit of the endometrium essential for embryo implantation.
Modelling endometrial glands: the missing link?
Secretory transformation of endometrial glands results in the production of histotroph, or ‘uterine milk’. Histotroph composition has been studied extensively during the menstrual cycle, consisting largely of glycogen and glycoproteins, as well as other components such as amino acids and lipid droplets (Burton et al. 2002). This rich secreted product provides nutrition for the embryo until the onset of placental perfusion (Burton et al. 2007, 2010). Studies using animal models, such as the uterine gland knockout sheep, produced by a progestin-induced gland knock out, demonstrate that without endometrial glands, embryo implantation is inhibited (Gray et al. 2001). Progestin uterine gland knockout mice also exhibit implantation failure, with reduced expression of key implantation genes (Kelleher et al. 2016).
Besides providing nutritional support to the implanting embryo, endometrial glands upregulate genes required for embryo receptivity and implantation, including numerous secreted factors essential for implantation (Fig. 2). For example, osteopontin (OPN), encoded by phosphoprotein 1 (SPP1), contains the integrin-binding motif RGD, a tripeptide of amino acids arginine, glycine, and aspartate, which facilitates embryo adhesion to the luminal epithelium (Singh & Aplin 2009, Berneau et al. 2019). Leukaemia inhibitory factor, encoded by LIF, is a protein related to blastocyst adhesion, as well as having roles in embryonic development and trophoblast differentiation (Salleh & Giribabu 2014). Glycodelin, a dimeric glycoprotein, encoded by progestogen-associated endometrial protein (PAEP), also participates in interactions between the implanting blastocyst and luminal epithelium. In vitro, induction of glycodelin secretion improves trophoblast spheroid attachment while silencing PAEP inhibits attachment (Uchida et al. 2007, So et al. 2012).
Figure 2.
Embryo-endometrium crosstalk at implantation. Schematic representation of the crosstalk between endometrial glands, decidualised stromal cells, corpus luteum, and trophoblasts cells of the invading embryo. During pregnancy, the syncytiotrophoblast cells produce hCG to maintain the corpus luteum so progesterone can be continually produced. Progesterone is essential for the maintenance of the decidualised cell and differentiated glands. A key secretion of the decidual cells at implantation is PRL, which acts on the glands through PRLR to maintain the glandular secretory phenotype. Additionally, trophoblast cells act directly on the glands through hCG and hPL secretion, resulting in the production of growth factors, nutrients, and receptivity markers such as LIF and glycodelin. In turn, these factors improve trophoblast invasion, thus forming a positive feedback loop.
Human endometrial glands also secrete the growth factors EGF, vascular endothelial growth factor (VEGF), and transforming growth factor-beta (TGFB), the cognate receptors for which are expressed by the trophectoderm of the implanting blastocyst. EGF stimulates proliferation of cytotrophoblast and secretion of human chorionic gonadotropin (hCG) and human placental lactogen (hPL) by the syncytiotrophoblast (Ladines-Llave et al. 1991, Maruo et al. 1997) (Fig. 2). VEGF enhances the adhesion of the trophoblast to the luminal epithelium (Binder et al. 2014), while TGFB increases ECM remodelling, via the secretion of fibronectin, for example, thereby facilitating endometrial adhesion for invading trophoblasts (Feinberg et al. 1994).
Overall, the glands and their secretions are essential for implantation, and therefore, being able to model the glands in vitro is an essential step towards studying and understanding human embryo implantation and early pregnancy events. Recent development of glandular organoid models may have brought us closer than ever to establish a physiological model of the human endometrium.
Organoids of the reproductive system
‘Organoid’ describes 3D structures that resemble organs or tissues ex vivo in a supportive hydrogel droplet, such as Matrigel™, replacing the ECM of the originating tissue. Organoids arise from single cells or small clusters of cells with stem or progenitor properties in cultures with complex growth media designed to mimic organ- or tissue-specific signalling pathways and recapitulate the niche environment (Kim et al. 2020b). Organoids differ from previous in vitro model systems in that they spontaneously organise into architectures that resemble their corresponding in vivo tissue or organ in a culture plate. Additionally, organoid models functionally mimic their tissue of origin and can recapitulate developmental processes that take place in vivo, thus allowing the study of developing and differentiating cells in real time. Growth factor and inhibitor combinations in these complex media support proliferation and renewal as well as differentiation and substitute for the absence of the tissue complexity present in vivo. Most reported models employ a consistent core set of factors that are reviewed elsewhere (Kretzschmar & Clevers 2016, Alzamil et al. 2021). Organoids have been generated from the vast majority of endoderm-derived tissues including the colon (Sato et al. 2011), intestine (Fujii et al. 2018), stomach (Bartfeld et al. 2015, Schlaermann et al. 2016), liver (Huch et al. 2015, Hu et al. 2018), pancreas (Loomans et al. 2018), lung/airway (Sachs et al. 2019) and bladder (Lee et al. 2018).
Several recent reports have demonstrated progress in modelling human reproductive epithelia using organoid systems. In the female reproductive system, endometrium (Boretto et al. 2017, Turco et al. 2017), fallopian tube (Kessler et al. 2015), cervix (Chumduri et al. 2021, Maru et al. 2020, Lohmussaar et al. 2021), and ovarian cancer (Kopper et al. 2019, Maenhoudt et al. 2020) models have been described (Fig. 3), as well as mammary gland (Linnemann et al. 2015). These models facilitate the study of the general biology and histology of the reproductive tissues since they recapitulate epithelial cell morphology, function, and cellular heterogeneity while being genetically stable. These organoids are hormone-responsive and can be differentiated under specific conditions (reviewed by Alzamil et al. 2021). In the male reproductive tract, organoid systems are described for both testis (Alves-Lopes et al. 2017, Baert et al. 2017, Pendergraft et al. 2017) and prostate (Chua et al. 2014, Karthaus et al. 2014). Trophoblast organoids to model placental development (Haider et al. 2018, Turco et al. 2018), as well as embryo-like organoids, known as blastoids have also been described (Zheng et al. 2019, Liu et al. 2021, Yu et al. 2021). Human blastoids recapitulate the key morphology of preimplantation blastocysts, including cell-lineage composition and allocation, with transcriptomic similarities but lack important structures like the zona pellucida (Liu et al. 2021, Yu et al. 2021). Blastoids and trophoblast organoids both offer the opportunity to study implantation and early developmental processes at a greater scale than possible with research embryos, and in cases where embryos are unavailable for research use or such applications are prohibited.
Figure 3.
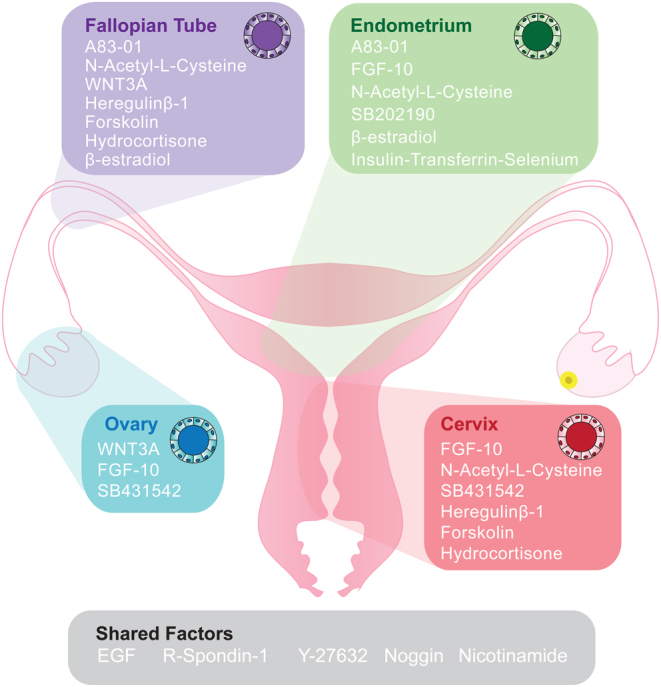
Organoid models of the female reproductive tract. In the human female reproductive tract, organoid models have been derived from the endometrium, fallopian tube, ovary, and cervix tissues and recapitulate the epithelial structure of the tissue of origin. All systems have been cultured in basal medium including Advanced DMEM/F12, B-27, and N-2 supplements, antibiotics, and L-Glutamine but also include a cocktail of growth factors and inhibitors to promote proliferation and maintain undifferentiated or progenitor cell-like conditions. Common to all models is the growth factor EGF and the WNT signalling activator R-Spondin-1, as well as the ROCK inhibitor Y-27632 and BMP pathway inhibitor Noggin, and Nicotinamide, a survival factor, and ROCK inhibitor. Other factors which are often added include the TGFB receptor inhibitor A83-01, the activin/ BMP/TGFB inhibitor, SB431542, and the p38 MAPK inhibitor SB202190; Growth factors FGF-10 (fibroblast growth factor) and WNT3A as well as other factors such as N-acetyl-L-cysteine (antioxidant), β-estradiol (mitogen), heregulin-β (growth factor), forskolin (adenylyl cyclase activator), and hydrocortisone (glucocorticoid).
The emergence of these organoid models for human reproduction and fertility represents a huge leap forward for the field, presenting a considerable opportunity to advance our knowledge of many ‘hidden’ processes within these systems. Limitations remain, for example, ease of accessibility means comparisons with healthy tissue are limited in some cases, while optimisation of other models is still required.
Endometrial gland organoids
Organoid-like structures established from endometrial epithelial cells were first reported in 1988, derived from primary gland fragments seeded into Matrigel™ (Rinehart et al. 1988). Glandular structures were retained after tissue digest collapsed and monolayer colony outgrowth was observed within 7 to 10 days. Following several weeks of culture, these colonies formed large cystic organoid structures of polarised columnar epithelial cells with luminal microvilli and could be maintained in culture for at least 6 months. Endometrial gland organoids were also produced in Matrigel™ and grown in a transwell system, in co-culture with stromal cells grown in the lower chamber (Bläuer et al. 2005). In this system, the organoids were responsive to oestradiol treatment in the presence of the stromal cell layer. These reports failed to recapitulate the gland phenotype fully, and genetic stability was not confirmed, nor were the culture conditions chemically defined. Renewed interest in endometrial gland organoids was stimulated recently by parallel reports delivering a step-change in our ability to study the function of these structures (Boretto et al. 2017, Turco et al. 2017) (Fig. 3). The resulting organoids are genetically stable, have the clonogenic capacity, cellular heterogeneity, and can recapitulate molecular and histological similarities of the glands in vivo (Boretto et al. 2017, Turco et al. 2017, Fitzgerald et al. 2019). They can be derived from physiologically diverse endometrial tissues throughout the menstrual cycle, after menopause, and from decidual pregnancy samples (Turco et al. 2017). Like other organoid models, endometrial gland organoids are cultured in Matrigel™ and are dependent on a chemically defined complex ‘expansion medium’ containing a variety of growth factors required to promote proliferation and inhibit differentiation, including fibroblast growth factor 10 (FGF10), EGF, Wingless and Int1 (WNT) signalling activator R-spondin 1 (RSPO1), bone morphogenetic protein (BMP) inhibitor Noggin, and the TGFβ antagonist, A83-01. Unlike the initial report (Rinehart et al. 1988), these recent models see rapid organoid formation from gland fragments within 7–10 days. Oestrogen treatment induces proliferation of the cells with an increase in Ki67+ cells (Boretto et al. 2017) as well as stimulating ciliogenesis (Haider et al. 2019) and, when combined with progesterone, secretion of glycodelin-A corresponds to tissue profiles in mid-secretory endometrium (Luddi et al. 2020). Glycodelin profiles were further modulated after incubation of organoids with embryo-conditioned medium (Luddi et al. 2021), confirming embryo-endometrial crosstalk is possible within the model (Table 1). Metabolomic analysis of the secretome of endometrial organoids also demonstrates unique characteristics of the apical (inter-organoid) and basolateral (extra-organoid) secretory profiles, characteristic of that predicted in vivo (Simintiras et al. 2021). Through exposure to pregnancy signals of the stroma (cyclic AMP and prolactin (PRL)) and trophoblast (hCG and hPL), the organoids acquired a decidual-like phenotype akin to early pregnancy (Turco et al. 2017).
Thus far, endometrial gland organoid models are still in their infancy, albeit one that surpasses prior systems physiologically. The opportunity to address questions of normal endometrial physiology and implantation processes, therefore, lies along our path.
Endometrial gland organoids and the future: a roadmap to utility
Endometrial gland organoids, assembloids, and modelling embryo implantation
Despite their structural and functional recapitulation of endometrial gland phenotypes, endometrial organoid models do not faithfully represent the midluteal endometrium, due to being grown in isolation without the influence of other endometrial cell types present in the tissue. Endometrial stromal cells are essential for glandular regeneration, expansion, and differentiation throughout the menstrual cycle. For example, through the secretion of PRL, decidualising stroma regulates gland differentiation via the PRL receptor (PRLR), whose expression peaks in the endometrial glands during the mid-secretory phase (Jones et al. 1998). PRL induces glandular differentiation by stimulating the Janus kinase (JAK)/STATs and mitogen-activated protein kinase (MAPK) pathways. In parallel, apoptosis in the glandular epithelium is inhibited by activation of the phosphatidylinositol 3 kinase (PI3K) pathway (Jabbour et al. 2002).
Recently, several protocols have described stromal-epithelial co-cultures retaining physiological structure. Slices of full thickness endometrial tissue cultured in collagen gel maintain true histoarchitecture of the endometrium, and a decidual response similar to the in vivo profile can be induced following differentiation with oestradiol (E2) and progesterone (P4) (Muruganandan et al. 2020). Others have reported the use of porous 3D scaffolds to establish co-cultures. Using a collagen-based scaffold, Abbas and colleagues demonstrated that stromal cells were able to proliferate within the scaffold pores and deposit their own ECM, while fragments of endometrial gland organoids seeded on top of the scaffolds formed a luminal epithelium reminiscent of the tissue structure in vivo (Abbas et al. 2020). As well as solid scaffolds, synthetic hydrogels, such as polyethylene glycol (PEG), functionalised with ECM- and integrin-binding peptides have been utilised as a replacement for animal-derived hydrogels. When cultured in PEG hydrogels, endometrial stromal cells proliferate and decidualise in response to hormonal stimulation (Cook et al. 2017). Modulating the PEG hydrogel altered cell behaviour, highlighting the importance of matrix conditions when developing a representative model of the endometrium (Cook et al. 2017). Finally, another recent protocol reported self-aggregation of epithelial and stromal cells in a scaffold-free environment to form structures containing a stromal cell centre and an outer layer of epithelial cells within an agarose mould (Wiwatpanit et al. 2020).
An alternative approach to produce a structurally and functionally physiological model of the endometrium is to incorporate endometrial stromal cells into endometrial organoid cultures. Co-culturing of organoids with stromal cells has been reported in other organoid systems, such as the bladder and brain, and these models are designated as ‘assembloids’ (Kim et al. 2020a, Andersen et al. 2020, Miura et al. 2020). In the case of bladder, the applicability of organoids to in vivo pathology and disease is uncertain because, in isolation, they do not recapitulate the native tissue architecture and microenvironment. To overcome these limitations, bladder organoids were mixed with components of the bladder stroma, such as stromal, endothelial, and immune cells, and a muscle layer to form bladder assembloids (Kim et al. 2020). The assembloids represent a more physiological model of the native organ or tissue, and therefore, should provide a more faithful model for testing drug responses and mimicking disease phenotypes.
Recently, we have reported the establishment of endometrial assembloids whereby the gland organoid model was modified to incorporate stromal cells (Rawlings et al. 2021). Gland organoids were expanded from primary epithelial cells while in parallel the stromal fraction was propagated by standard monolayer culture (Fig. 4). Single-cell stromal suspensions were combined with manually digested organoids, seeded into a collagen hydrogel, and cultured in an expansion medium supplemented with E2. In this model, gland organoid formation was unperturbed by stromal co-culture, and assembloids resemble the architecture of native endometrium more closely than the gland organoids alone. The addition of stromal cells should abrogate the need for many components of the complex medium of growth factors and inhibitors to maintain secretory gland organoids, and indeed a minimal differentiation medium supported robust glandular differentiation in the assembloid model (Rawlings et al. 2021). Based on single-cell transcriptomic analysis, decidualised assembloids closely resemble the mid-secretory endometrium, containing several subpopulations of both stromal and epithelial cells, including senescent stromal and epithelial cells, which secrete many canonical implantation factors. Co-culturing human blastocysts with decidualised assembloids demonstrated that in the presence of senescent decidual cells, the assembloids engender a dynamic implantation environment, enabling embryo expansion and attachment, although persistent endometrial senescence led to the gradual disintegration of the assembloid matrix, likely through the actions of MMPs (Freitas-Rodriguez et al. 2017). Pharmacological inhibition of stress responses in pre-decidual cells by a tyrosine kinase inhibitor inhibited the propagation of decidual senescence, impeding assembloid breakdown. However, the lack of senescent cells resulted in the entrapment of the blastocysts in the largely static assembloid matrix. This study not only demonstrated that the endometrial assembloid model could be used as a novel embryo implantation model but also confirms previous reports that decidual senescence controls endometrial fate decisions during implantation and early pregnancy (Brighton et al. 2017, Lucas et al. 2020). Challenges for the endometrial assembloid model are to introduce a luminal epithelium, in order to study sequential blastocyst attachment and invasion, as well as the incorporation of immune cells such as uterine natural killer cells to mimic tissue remodelling processes predicted to take place during implantation (Brighton et al. 2017, Kong et al. 2021). Moreover, long-term maintenance and propagation of endometrial assembloids to mimic the cycling tissue have yet to be developed.
Figure 4.
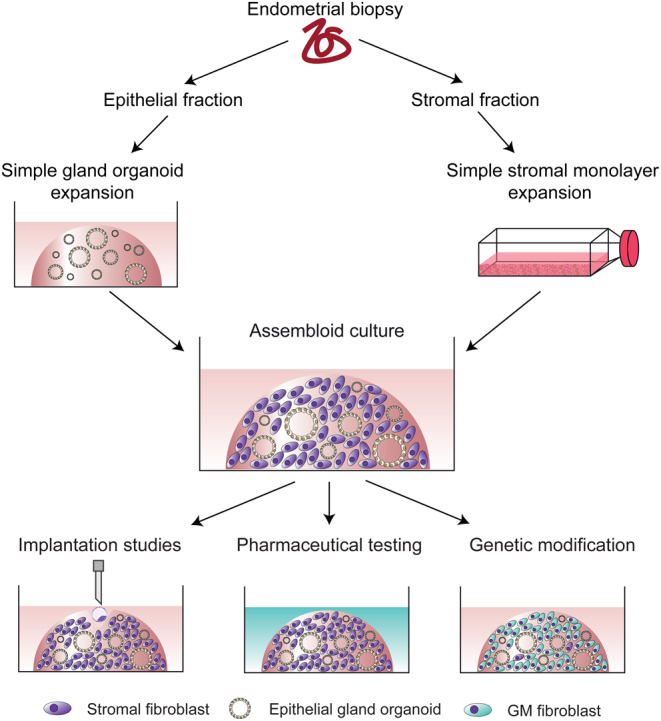
Establishment of an endometrial assembloid model. Schematic representation of the establishment of the endometrial assembloid model. Endometrial pipelle biopsies are digested and separated into epithelial and stromal fractions. Epithelial cells are expanded in Matrigel using the simple gland organoid culture approach for two passages, while stromal cells are expanded in monolayer culture. At passage 2, digested organoids and stromal cells are combined and encapsulated in collagen hydrogel to form an assembloid culture which then follows growth and differentiation protocols as required. Current and future applications for the assembloids include, but are not limited to embryo implantation studies, pharmaceutical testing (e.g. drugs or small molecules) and genetic modification of the different cell populations.
The major challenge in increasing complexity for a physiological model of the endometrium suitable for use in implantation studies is being able to balance the needs of different cell types within the model. For example, the basement membrane preparation Matrigel™, used frequently for organoid culture, is unsuitable as an extracellular matrix for stromal cells (Arnold et al. 2001). Beyond the co-culture of stromal cells with epithelial organoids, endothelial cells may be required to directly invade trophoblasts for placentation (Weiss et al. 2016). Additionally, immune cells, such as resident uterine natural killer cells, have been demonstrated to be essential for providing a robust decidual matrix suitable for successful embryo implantation (Kong et al. 2021). A microfluidic approach may be the solution to this potential dilemma.
Endometrial pathologies, genetic manipulation, and biomarker discovery
Beyond understanding the fundamentals of embryo implantation, endometrial pathologies that impede fertility must also be studied to advance the provision of therapeutics (Fig. 5). The development of medical treatments for many human diseases is limited by patient variation, difficulties in predicting outcomes, and lengthy preclinical drug testing. This is restricted further for applications in human reproduction by our limited ability to study in vivo processes in normal tissues. Therefore, organoid and assembloid cultures based on specific pathologies and even on individual patients are expected to develop into powerful tools for precision therapy, permitting a personalised approach to drug discovery, development, and screening. Primary cancers, infectious diseases, and developmental diseases can be replicated with ex vivo biopsy samples from patients. Testing prospective pharmacological treatments on human organoids could facilitate the identification of patient group-specific responses and lead to targeted therapeutic approaches. Indeed, initial explorations into the utility of gland organoids in studying endometrial pathology have been reported, demonstrating the value of patient-specific cultures to study pathologies such as endometriosis and cancer (Boretto et al. 2017, 2019).
Figure 5.
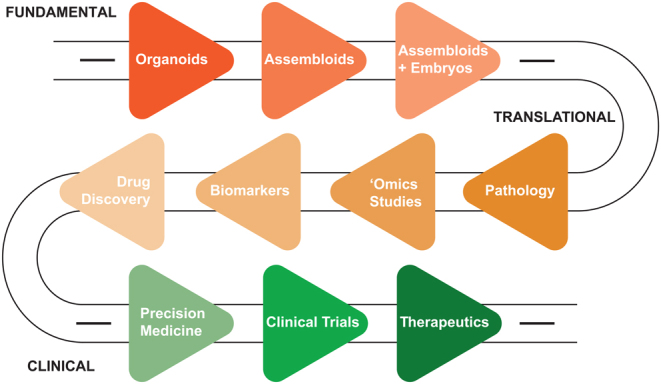
A roadmap to application for endometrial organoids. Schematic representation of the proposed roadmap to application for the endometrial organoids. The roadmap is divided into fundamental (red), translational (orange) and clinical (green) approaches to implantation research. This roadmap presents the trajectory from defining the embryo-endometrial interactions during and after implantation to disease modelling and biomarker- and drug- discovery, through developing successful therapeutic interventions for pathologies that impede successful pregnancy.
Endometriosis is an endometrial disorder characterised by the ectopic growth of endometrial tissue. Endometriosis is associated with infertility and affects 10% of women of reproductive age. The aetiology and pathogenesis of this disease remain unclear, and therefore, effective treatments are limited. Mouse models of endometriosis, while useful, do not model the complexity of the disease fully (Greaves et al. 2017). Gland organoids established from endometriotic lesions have been shown to differ from organoids derived in parallel from patient-matched eutopic endometrium, maintaining a heterogeneous disease profile, including aberrant signalling in integrin, PI3K-AKT, and WNT pathways, and thus identifying potential drug targets (Boretto et al. 2019). Furthermore, differences in glycodelin secretion mirror those in patients and controls (Luddi et al. 2020). However, this model is limited to only glandular epithelial cells. Sampson’s theory of retrograde menstruation proposes that endometriotic lesions derive from endometrial cells seeding the peritoneum at menstruation (Filby et al. 2020). Incorporating decidualised stromal cells from endometriotic lesions to generate endometriosis assembloids could provide a more encompassing model for lesion establishment and behaviour, while crossover experiments with healthy and pathological cell subpopulations could also aid in pinpointing the exact mechanisms for endometrial diseases.
Endometrial cancer is another potential candidate for organoid modelling since the pathology of endometrial cancer is largely unknown, and treatment is limited, mainly due to a lack of reliable preclinical models. Tumour-derived cell lines do not recapitulate clinical cancer heterogeneity, and genetic mouse models show aberrations inconsistent with clinical endometrial cancer (Boretto et al. 2019). Tumour-derived organoids from endometrial cancers have been shown to maintain clinical heterogeneity under long-term expansion but required medium optimisation to recapitulate the tumour niche, owing to non-cancerous organoids outcompeting cancerous organoids in the standard protocol (Boretto et al. 2019). This key observation highlights the necessity to consider the nuances of the local environment when developing future endometrial organoid-based disease models. Pre-cancerous organoid models have also been developed, such as organoids derived from hyperplastic endometrium, which faithfully reproduced the disease genotype. These organoids may provide a model that can be used to identify patient-specific biomarkers for early detection and intervention (Boretto et al. 2019).
The development of pathological endometrial organoid and assembloid models, in concert with healthy controls, provides promising tools to interrogate endometrial biology and pathology. In models that are physiologically or pathologically relevant, single-cell omics and functional assays may provide initial targets for gene editing. Owing to their clonal capacity, organoids provide an excellent candidate model for gene editing through CRISPR/Cas9 (Jinek et al. 2012, Cong et al. 2013, Mali et al. 2013) and this has been achieved in numerous organoid systems already, including the brain (Wang et al. 2015, Ogawa et al. 2018), liver (Artegiani et al. 2019), kidney (Freedman et al. 2015), and intestine (Matano et al. 2015, Roper et al. 2018). To our knowledge, only one study has demonstrated CRISPR/Cas9 in endometrial organoids (Chen et al. 2021), derived from mouse tissue, and therefore, efforts to advance the manipulation of human endometrial organoids will be welcomed. Genetic and gene regulatory manipulations will be essential for underpinning the key mechanisms and aberrations that lead to disease states, and as well as to open new avenues for biomarker investigation and drug discovery.
Endometrial precision medicine, clinical trials, and therapeutic potential
The clearest benefit of patient-derived organoids and assembloids is to provide a novel opportunity to discover personalised treatments. Unlike previous models, patient-derived organoids have been demonstrated to maintain the heterogeneity and complexity of their clinical derivatives, allowing for the study of patient-specific phenotypes. Therefore, the next step in the development of pathological endometrial organoid models is to utilise them in preclinical drug screening tools. Organoids from other systems have already been used for drug screening. For example, cystic fibrosis (CF) is a genetic disorder caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene and causes particularly severe damage to the pulmonary and digestive systems (Dekkers et al. 2013). Clinical trials with CFTR-targeting drugs have shown variable efficacy among individuals. Organoids can be harnessed to target different mutations in the CFTR protein with the intention to discover personalised and effective treatments for CF (Dekkers et al. 2013, 2016, Berkers et al. 2019, Ramalho et al. 2021).
Endometrial organoids have already been used in pre-clinical drug screening studies for endometrial cancer (Chen et al. 2021). Unbiased drug screening of tumour organoids from mouse endometrial cancer identified MI-136 as a potential inhibitor of endometrial cancer through regulation of the HIF pathway, a novel mechanism distinct from those in acute myeloid leukaemia and prostate cancer (Chen et al. 2021). Additionally, the study reported that MI-136 also inhibited growth significantly in primary cancer organoids derived from patients (Chen et al. 2021). This provides evidence that endometrial organoids could be used as in pre-clinical drug screening studies.
To maximise the effective use of therapeutics, stratification of subjects in clinical trials is essential, especially in the case of a heterogeneous disease. Stratification and personalised medicine could be extrapolated to endometrial organoids and assembloids and the treatment of infertility. Our recent research has demonstrated that excessive decidual cellular senescence is associated with recurrent pregnancy loss (Lucas et al. 2020). Biomarkers for excessive decidual senescence could be utilised for screening for organoid- and assembloid-based pre-clinical drug interventions to reduce senescence and possibly reduce the burden of miscarriage. Only patients experiencing cycles with excessive senescence would likely see a benefit in reducing senescence, and therefore, it is essential to target this subset of patients. Primary endometrial stromal cells in vitro retain phenotypic characteristics of the donating patient. For example, endometrial stromal cells from women with recurrent pregnancy loss exhibit an imbalance of mature decidual and senescent decidual cells and an inability to recognise poor-quality embryos (Weimar et al. 2012, Lucas et al. 2020). Therefore, endometrial assembloid models should be able to mimic the behaviour of the tissue in vivo and provide a first step approach to patient-specific testing and stratification for treatment. Indeed, since organoid cultures can be bio-banked for future drug testing (Boretto et al. 2019), reducing the requirement for repeated biopsies, directed testing should be possible on specified cohorts of bio-banked samples.
Overall, organoids appear to be a promising model for drug discovery, pre-clinical evaluation, and stratification of subject groups for clinical trials and our challenge lie in driving the models from the fundamental study into the translational application to realise their full potential (Fig. 5).
Conclusions
Organoid and assembloid models of the endometrium offer the opportunity to study embryo-endometrium interactions at the earliest timepoints in implantation, with increased physiological relevance, and thus present a promising tool for future research. Advancing our knowledge of the mechanisms and requirements for successful implantation also offers the prospect of identifying the routes through which the process fails, thus indicating possible therapeutic avenues. Through the ongoing development and characterisation of patient-specific organoid and assembloid models, targeted pre-conception therapies can be developed and tested extensively in pre-clinical studies. These exciting possibilities mean much work ahead: the full potential of organoid and assembloid models has not yet been realised, and the challenge now is to ensure that we drive the models forward to translational outcomes.
Declaration of interest
E S L is an ordinary member of SRF council. The authors have no financial interests to declare.
Funding
T M R is supported by a fellowship from the Warwick-Wellcome Trust Translational Partnership initiative. K M is funded by a Warwick Medical School scholarship. M T is funded by a EUTOPIA Co-tutelle PhD Scholarship between the University of Warwick and Vrije Universiteit Brussel (VUB). E S L is funded through a Wellcome Trust Investigator Award to Professor Jan Brosens (212233/Z/18/Z).
Author contribution statement
T M R and E S L conceptualised the article. T M R, K M, M T, and E S L drafted the article. K M, M T, T M R, and E S L prepared the figures. E S L edited the article. All authors approved the final version.
References
- Abbas Y, Brunel LG, Hollinshead MS, Fernando RC, Gardner L, Duncan I, Moffett A, Best S, Turco MY, Burton GJ.et al. 2020. Generation of a three-dimensional collagen scaffold-based model of the human endometrium. Interface Focus 10 20190079. ( 10.1098/rsfs.2019.0079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves-Lopes JP, Söder O, Stukenborg JB. 2017. Testicular organoid generation by a novel in vitro three-layer gradient system. Biomaterials 130 76–89. ( 10.1016/j.biomaterials.2017.03.025) [DOI] [PubMed] [Google Scholar]
- Alzamil L, Nikolakopoulou K, Turco MY. 2021. Organoid systems to study the human female reproductive tract and pregnancy. Cell Death and Differentiation 28 35–51. ( 10.1038/s41418-020-0565-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker J, Segerer SE, Hagemann C, Feix S, Kapp M, Bausch R, Kämmerer U. 2011. Human decidua and invasive trophoblasts are rich sources of nearly all human matrix metalloproteinases. Molecular Human Reproduction 17 637–652. ( 10.1093/molehr/gar033) [DOI] [PubMed] [Google Scholar]
- Andersen J, Revah O, Miura Y, Thom N, Amin ND, Kelley KW, Singh M, Chen X, Thete MV, Walczak EM.et al. 2020. Generation of functional human 3D cortico-motor assembloids. Cell 183 1913, .e26–1929.e26. ( 10.1016/j.cell.2020.11.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin JD.2006. In vitro analysis of trophoblast invasion. In Placenta and Trophoblast: Methods and Protocols, Vol. 2. Eds Soares MJ, Hunt JS. Totowa, NJ: Humana Press. ( 10.1385/1-59259-989-3:45) [DOI] [Google Scholar]
- Aplin JD, Ruane PT. 2017. Embryo-epithelium interactions during implantation at a glance. Journal of Cell Science 130 15–22. ( 10.1242/jcs.175943) [DOI] [PubMed] [Google Scholar]
- Aplin JD, Hey NA, Li TC. 1996. MUC1 as a cell surface and secretory component of endometrial epithelium: reduced levels in recurrent miscarriage. American Journal of Reproductive Immunology 35 261–266. ( 10.1111/j.1600-0897.1996.tb00042.x) [DOI] [PubMed] [Google Scholar]
- Arnold JT, Kaufman DG, Seppälä M, Lessey BA. 2001. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Human Reproduction 16 836–845. ( 10.1093/humrep/16.5.836) [DOI] [PubMed] [Google Scholar]
- Artegiani B, Van Voorthuijsen L, Lindeboom RGH, Seinstra D, Heo I, Tapia P, López-Iglesias C, Postrach D, Dayton T, Oka R.et al. 2019. Probing the tumor suppressor function of BAP1 in CRISPR-engineered human liver organoids. Cell Stem Cell 24 927, .e6–943.e6. ( 10.1016/j.stem.2019.04.017) [DOI] [PubMed] [Google Scholar]
- Baert Y, De Kock J, Alves-Lopes JP, Söder O, Stukenborg JB, Goossens E. 2017. Primary human testicular cells self-organize into organoids with testicular properties. Stem Cell Reports 8 30–38. ( 10.1016/j.stemcr.2016.11.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfeld S, Bayram T, Van De Wetering M, Huch M, Begthel H, Kujala P, Vries R, Peters PJ, Clevers H. 2015. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148 126, .e6–136.e6. ( 10.1053/j.gastro.2014.09.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin-Ley U, Pedersen B, Lindenberg S, Larsen JF, Hamberger L, Horn T. 1994. Isolation and culture of human endometrial cells in a three-dimensional culture system. Journal of Reproduction and Fertility 101 327–332. ( 10.1530/jrf.0.1010327) [DOI] [PubMed] [Google Scholar]
- Bentin-Ley U, Horn T, Sjogren A, Sorensen S, Falck Larsen J, Hamberger L. 2000. Ultrastructure of human blastocyst-endometrial interactions in vitro. Journal of Reproduction and Fertility 120 337–350. ( 10.1530/jrf.0.1200337) [DOI] [PubMed] [Google Scholar]
- Berkers G, Van Mourik P, Vonk AM, Kruisselbrink E, Dekkers JF, De Winter-De Groot KM, Arets HGM, Marck-Van Der Wilt REP, Dijkema JS, Vanderschuren MM.et al. 2019. Rectal organoids enable personalized treatment of cystic fibrosis. Cell Reports 26 1701, .e3–1708.e3. ( 10.1016/j.celrep.2019.01.068) [DOI] [PubMed] [Google Scholar]
- Berkhout RP, Lambalk CB, Huirne J, Mijatovic V, Repping S, Hamer G, Mastenbroek S. 2018. High-quality human preimplantation embryos actively influence endometrial stromal cell migration. Journal of Assisted Reproduction and Genetics 35 659–667. ( 10.1007/s10815-017-1107-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berneau SC, Ruane PT, Brison DR, Kimber SJ, Westwood M, Aplin JD. 2019. Characterisation of osteopontin in an in vitro model of embryo implantation. Cells 8 432. ( 10.3390/cells8050432) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder NK, Evans J, Gardner DK, Salamonsen LA, Hannan NJ. 2014. Endometrial signals improve embryo outcome: functional role of vascular endothelial growth factor isoforms on embryo development and implantation in mice. Human Reproduction 29 2278–2286. ( 10.1093/humrep/deu211) [DOI] [PubMed] [Google Scholar]
- Bläuer M, Heinonen PK, Martikainen PM, Tomás E, Ylikomi T. 2005. A novel organotypic culture model for normal human endometrium: regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Human Reproduction 20 864–871. ( 10.1093/humrep/deh722) [DOI] [PubMed] [Google Scholar]
- Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, Amant F, Timmerman D, Tomassetti C, Vanhie A.et al. 2017. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 144 1775–1786. ( 10.1242/dev.148478) [DOI] [PubMed] [Google Scholar]
- Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, Heremans R, Perneel L, Kobayashi H, Van Zundert I.et al. 2019. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nature Cell Biology 21 1, 041–1. ( 10.1038/s41556-019-0360-z) [DOI] [PubMed] [Google Scholar]
- Brighton PJ, Maruyama Y, Fishwick K, Vrljicak P, Tewary S, Fujihara R, Muter J, Lucas ES, Yamada T, Woods L.et al. 2017. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. eLife 6 e31274. ( 10.7554/eLife.31274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosens JJ, Salker MS, Teklenburg G, Nautiyal J, Salter S, Lucas ES, Steel JH, Christian M, Chan YW, Boomsma CM.et al. 2014. Uterine selection of human embryos at implantation. Scientific Reports 4 3894. ( 10.1038/srep03894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Watson AL, Hempstock J, Skepper JN, Jauniaux E. 2002. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. Journal of Clinical Endocrinology and Metabolism 87 2954–2959. ( 10.1210/jcem.87.6.8563) [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, Charnock-Jones DS. 2007. Human early placental development: potential roles of the endometrial glands. Placenta 28 (Supplement A) S64–S69. ( 10.1016/j.placenta.2007.01.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E, Charnock-Jones DS. 2010. The influence of the intrauterine environment on human placental development. International Journal of Developmental Biology 54 303–312. ( 10.1387/ijdb.082764gb) [DOI] [PubMed] [Google Scholar]
- Caballero-Campo P, Domínguez F, Coloma J, Meseguer M, Remohí J, Pellicer A, Simón C. 2002. Hormonal and embryonic regulation of chemokines IL-8, MCP-1 and RANTES in the human endometrium during the window of implantation. Molecular Human Reproduction 8 375–384. ( 10.1093/molehr/8.4.375) [DOI] [PubMed] [Google Scholar]
- Carter AM.2007. Animal models of human placentation – a review. Placenta 28 (Supplement A) S41–S47. ( 10.1016/j.placenta.2006.11.002) [DOI] [PubMed] [Google Scholar]
- Carter AM.2020. Animal models of human pregnancy and placentation: alternatives to the mouse. Reproduction 160 R129–R143. ( 10.1530/REP-20-0354) [DOI] [PubMed] [Google Scholar]
- Carver J, Martin K, Spyropoulou I, Barlow D, Sargent I, Mardon H. 2003. An in-vitro model for stromal invasion during implantation of the human blastocyst. Human Reproduction 18 283–290. ( 10.1093/humrep/deg072) [DOI] [PubMed] [Google Scholar]
- Chen J, Zhao L, Peng H, Dai S, Quan Y, Wang M, Wang J, Bi Z, Zheng Y, Zhou S.et al. 2021. An organoid-based drug screening identified a menin-MLL inhibitor for endometrial cancer through regulating the HIF pathway. Cancer Gene Therapy 28 112–125. ( 10.1038/s41417-020-0190-y) [DOI] [PubMed] [Google Scholar]
- Chua CW, Shibata M, Lei M, Toivanen R, Barlow LJ, Bergren SK, Badani KK, Mckiernan JM, Benson MC, Hibshoosh H.et al. 2014. Single luminal epithelial progenitors can generate prostate organoids in culture. Nature Cell Biology 16 951–96. ( 10.1038/ncb3047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumduri C, Gurumurthy RK, Berger H, Dietrich O, Kumar N, Koster S, Brinkmann V, Hoffmann K, Drabkina M, Arampatzi P. et al. 2021. Opposing Wnt signals regulate cervical squamocolumnar homeostasis and emergence of metaplasia Nature Cell Biology 23 184-–197.. ( 10.1038/s41556-020-00619-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA.et al. 2013. Multiplex genome engineering using CRISPR/Cas systems. Science 339 819–823. ( 10.1126/science.1231143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CD, Hill AS, Guo M, Stockdale L, Papps JP, Isaacson KB, Lauffenburger DA, Griffith LG. 2017. Local remodeling of synthetic extracellular matrix microenvironments by co-cultured endometrial epithelial and stromal cells enables long-term dynamic physiological function. Integrative Biology 9 271–289. ( 10.1039/c6ib00245e) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers JF, Van Der Ent CK, Beekman JM. 2013. Novel opportunities for CFTR-targeting drug development using organoids. Rare Diseases 1 e27112. ( 10.4161/rdis.27112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers JF, Berkers G, Kruisselbrink E, Vonk A, de Jonge HR, Janssens HM, Bronsveld I, van de Graaf EA, Nieuwenhuis EE, Houwen RH.et al. 2016. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Science Translational Medicine 8 344ra84. ( 10.1126/scitranslmed.aad8278) [DOI] [PubMed] [Google Scholar]
- Dominguez F, Galan A, Martin JJL, Remohi J, Pellicer A, Simón C. 2003. Hormonal and embryonic regulation of chemokine receptors CXCR1, CXCR4, CCR5 and CCR2B in the human endometrium and the human blastocyst. Molecular Human Reproduction 9 189–198. ( 10.1093/molehr/gag024) [DOI] [PubMed] [Google Scholar]
- Erikson DW, Burghardt RC, Bayless KJ, Johnson GA. 2009. Secreted phosphoprotein 1 (SPP1, osteopontin) binds to integrin Alphavbeta6 on porcine trophectoderm cells and integrin Alphavbeta3 on uterine luminal epithelial cells, and promotes trophectoderm. Biology of Reproduction 81 814–825. ( 10.1095/biolreprod.109.078600) [DOI] [PubMed] [Google Scholar]
- ESHRE Guideline Group on RPL, Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, Nelen W, Peramo B, Quenby S.et al. 2018. ESHRE guideline: recurrent pregnancy loss. Human Reproduction Open 2018 hoy004. ( 10.1093/hropen/hoy004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Walker KJ, Bilandzic M, Kinnear S, Salamonsen LA. 2020. A novel ‘embryo-endometrial’ adhesion model can potentially predict ‘receptive’ or ‘non-receptive’ endometrium. Journal of Assisted Reproduction and Genetics 37 5–16. ( 10.1007/s10815-019-01629-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evron A, Goldman S, Shalev E. 2011. Effect of primary human endometrial stromal cells on epithelial cell receptivity and protein expression is dependent on menstrual cycle stage. Human Reproduction 26 176–190. ( 10.1093/humrep/deq296) [DOI] [PubMed] [Google Scholar]
- Feinberg RF, Kliman HJ, Wang CL. 1994. Transforming growth factor-beta stimulates trophoblast oncofetal fibronectin synthesis in vitro: implications for trophoblast implantation in vivo. Journal of Clinical Endocrinology and Metabolism 78 1241–1248. ( 10.1210/jcem.78.5.8175984) [DOI] [PubMed] [Google Scholar]
- Filby CE, Rombauts L, Montgomery GW, Giudice LC, Gargett CE. 2020. Cellular origins of endometriosis: towards novel diagnostics and therapeutics. Seminars in Reproductive Medicine 38 201–215. ( 10.1055/s-0040-1713429) [DOI] [PubMed] [Google Scholar]
- Fitzgerald HC, Dhakal P, Behura SK, Schust DJ, Spencer TE. 2019. Self-renewing endometrial epithelial organoids of the human uterus. PNAS 116 23132–23142. ( 10.1073/pnas.1915389116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald HC, Schust DJ, Spencer TE. 2021. In vitro models of the human endometrium: evolution and application for women’s health. Biology of Reproduction 104 282–293. ( 10.1093/biolre/ioaa183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV.et al. 2015. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nature Communications 6 8715. ( 10.1038/ncomms9715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas-Rodriguez S, Folgueras AR, Lopez-Otin C. 2017. The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochimica et Biophysica Acta: Molecular Cell Research 1864 2015–2025. ( 10.1016/j.bbamcr.2017.05.007) [DOI] [PubMed] [Google Scholar]
- Fujii M, Matano M, Toshimitsu K, Takano A, Mikami Y, Nishikori S, Sugimoto S, Sato T. 2018. Human intestinal organoids maintain self-renewal capacity and cellular diversity in niche-inspired culture condition. Cell Stem Cell 23 787, .e6–793.e6. ( 10.1016/j.stem.2018.11.016) [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens JJ. 2014. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocrine Reviews 35 851–905. ( 10.1210/er.2014-1045) [DOI] [PubMed] [Google Scholar]
- Gellersen B, Reimann K, Samalecos A, Aupers S, Bamberger AM. 2010. Invasiveness of human endometrial stromal cells is promoted by decidualization and by trophoblast-derived signals. Human Reproduction 25 862–873. ( 10.1093/humrep/dep468) [DOI] [PubMed] [Google Scholar]
- Gellersen B, Wolf A, Kruse M, Schwenke M, Bamberger A-M. 2013. Human endometrial stromal cell-trophoblast interactions: mutual stimulation of chemotactic migration and promigratory roles of cell surface molecules CD82 and CEACAM11. Biology of Reproduction 88 80. ( 10.1095/biolreprod.112.106724) [DOI] [PubMed] [Google Scholar]
- Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. 2001. Developmental biology of uterine glands. Biology of Reproduction 65 1311–1323. ( 10.1095/biolreprod65.5.1311) [DOI] [PubMed] [Google Scholar]
- Greaves E, Critchley HOD, Horne AW, Saunders PTK. 2017. Relevant human tissue resources and laboratory models for use in endometriosis research. Acta Obstetricia et Gynecologica Scandinavica 96 644–658. ( 10.1111/aogs.13119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal S, Carver JG, Ridley AJ, Mardon HJ. 2008. Implantation of the human embryo requires Rac1-dependent endometrial stromal cell migration. PNAS 105 16189–16194. ( 10.1073/pnas.0806219105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal S, Carver J, Ridley AJ, Mardon HJ. 2010. Human endometrial stromal cell rho GTPases have opposing roles in regulating focal adhesion turnover and embryo invasion in vitro. Biology of Reproduction 83 75–82. ( 10.1095/biolreprod.109.080630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider S, Meinhardt G, Saleh L, Kunihs V, Gamperl M, Kaindl U, Ellinger A, Burkard TR, Fiala C, Pollheimer J.et al. 2018. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Reports 11 537–551. ( 10.1016/j.stemcr.2018.07.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider S, Gamperl M, Burkard TR, Kunihs V, Kaindl U, Junttila S, Fiala C, Schmidt K, Mendjan S, Knöfler M.et al. 2019. Estrogen signaling drives ciliogenesis in human endometrial organoids. Endocrinology 160 2282–2297. ( 10.1210/en.2019-00314) [DOI] [PubMed] [Google Scholar]
- Hertig AT, Rock J, Adams EC. 1956. A description of 34 human ova within the first 17 days of development. American Journal of Anatomy 98 435–493. ( 10.1002/aja.1980306) [DOI] [PubMed] [Google Scholar]
- Holmberg JC, Haddad S, Wünsche V, Yang Y, Aldo PB, Gnainsky Y, Granot I, Dekel N, Mor G. 2012. An in vitro model for the study of human implantation. American Journal of Reproductive Immunology 67 169–178. ( 10.1111/j.1600-0897.2011.01095.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Gehart H, Artegiani B, Löpez-Iglesias C, Dekkers F, Basak O, van Es J, Chuva de Sousa Lopes SM, Begthel H, Korving J.et al. 2018. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell 175 1591, .e19–1606.e19. ( 10.1016/j.cell.2018.11.013) [DOI] [PubMed] [Google Scholar]
- Huang X, Liu H, Li R. 2017. Prostaglandin E2 promotes BeWo spheroids implantation in RL95-2 cell monolayers. Gynecological Endocrinology 33 548–552. ( 10.1080/09513590.2017.1296125) [DOI] [PubMed] [Google Scholar]
- Huch M, Gehart H, Van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, Ellis E, Van Wenum M, Fuchs SA, De Ligt J.et al. 2015. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 160 299–312. ( 10.1016/j.cell.2014.11.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour HN, Gubbay O, Critchley HOD. 2002. Prolactin action and signalling in the human endometrium. Reproductive Medicine Review 10 117–132. ( 10.1017/S0962279902236) [DOI] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337 816–821. ( 10.1126/science.1225829) [DOI] [PMC free article] [PubMed] [Google Scholar]
- John NJ, Linke M, Denker HW. 1993. Quantitation of human choriocarcinoma spheroid attachment to uterine epithelial cell monolayers. In Vitro Cellular and Developmental Biology: Animal 29 461–468. ( 10.1007/BF02639380) [DOI] [PubMed] [Google Scholar]
- Jones RL, Critchley HOD, Brooks J, Jabbour HN, Mcneilly AS. 1998. Localization and temporal expression of prolactin receptor in human endometrium. Journal of Clinical Endocrinology and Metabolism 83 258–262. ( 10.1210/jcem.83.1.4506) [DOI] [PubMed] [Google Scholar]
- Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, Van Boxtel R, Wongvipat J, Dowling CM, Gao D, Begthel H, Sachs N.et al. 2014. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159 163–175. ( 10.1016/j.cell.2014.08.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher AM, Burns GW, Behura S, Wu G, Spencer TE. 2016. Uterine glands impact uterine receptivity, luminal fluid homeostasis and blastocyst implantation. Scientific Reports 6 38078–38078. ( 10.1038/srep38078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M, Hoffmann K, Brinkmann V, Thieck O, Jackisch S, Toelle B, Berger H, Mollenkopf HJ, Mangler M, Sehouli J.et al. 2015. The Notch and Wnt pathways regulate stemness and differentiation in human Fallopian tube organoids. Nature Communications 6 8989. ( 10.1038/ncomms9989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Choi S, Kang B, Kong J, Kim Y, Yoon WH, Lee HR, Kim S, Kim HM, Lee H.et al. 2020a. Creation of bladder assembloids mimicking tissue regeneration and cancer. Nature 588 664–669. ( 10.1038/s41586-020-3034-x) [DOI] [PubMed] [Google Scholar]
- Kim J, Koo BK, Knoblich JA. 2020b. Human organoids: model systems for human biology and medicine. Nature Reviews: Molecular Cell Biology 21 571–584. ( 10.1038/s41580-020-0259-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong CS, Ordonez AA, Turner S, Tremaine T, Muter J, Lucas ES, Salisbury E, Vassena R, Tiscornia G, Fouladi-Nashta AA.et al. 2021. Embryo biosensing by uterine natural killer cells determines endometrial fate decisions at implantation. FASEB Journal 35 e21336. ( 10.1096/fj.202002217R) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopper O, De Witte CJ, Lõhmussaar K, Valle-Inclan JE, Hami N, Kester L, Balgobind AV, Korving J, Proost N, Begthel H.et al. 2019. An organoid platform for ovarian cancer captures intra-and interpatient heterogeneity. Nature Medicine 25 838–849. ( 10.1038/s41591-019-0422-6) [DOI] [PubMed] [Google Scholar]
- Kretzschmar K, Clevers H. 2016. Organoids: modeling development and the stem cell niche in a dish. Developmental Cell 38 590–600. ( 10.1016/j.devcel.2016.08.014) [DOI] [PubMed] [Google Scholar]
- Ladines-Llave CA, Maruo T, Manalo AS, Mochizuki M. 1991. Cytologic localization of epidermal growth factor and its receptor in developing human placenta varies over the course of pregnancy. American Journal of Obstetrics and Gynecology 165 1377–1382. ( 10.1016/2-9378(9190372-x) [DOI] [PubMed] [Google Scholar]
- Lash GE, Hornbuckle J, Brunt A, Kirkley M, Searle RF, Robson SC, Bulmer JN. 2007. Effect of low oxygen concentrations on trophoblast-like cell line invasion. Placenta 28 390–398. ( 10.1016/j.placenta.2006.06.001) [DOI] [PubMed] [Google Scholar]
- Le Saint C, Crespo K, Bourdiec A, Bissonnette F, Buzaglo K, Couturier B, Bisotto S, Phillips SJ, Stutz M, Gouze JN.et al. 2019. Autologous endometrial cell co-culture improves human embryo development to high-quality blastocysts: a randomized controlled trial. Reproductive Biomedicine Online 38 321–329. ( 10.1016/j.rbmo.2018.12.039) [DOI] [PubMed] [Google Scholar]
- Lee KY, DeMayo FJ. 2004. Animal models of implantation. Reproduction 128 679–695. ( 10.1530/rep.1.00340) [DOI] [PubMed] [Google Scholar]
- Lee YL, Fong SW, Chen ACH, Li T, Yue C, Lee CL, Ng EHY, Yeung WSB, Lee KF. 2015. Establishment of a novel human embryonic stem cell-derived trophoblastic spheroid implantation model. Human Reproduction 30 2614–2626. ( 10.1093/humrep/dev223) [DOI] [PubMed] [Google Scholar]
- Lee SH, Hu W, Matulay JT, Silva MV, Owczarek TB, Kim K, Chua CW, Barlow LJ, Kandoth C, Williams AB.et al. 2018. Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell 173 515, .e17–528.e17. ( 10.1016/j.cell.2018.03.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberg S, Nielsen MH, Lenz S. 1985. In vitro studies of human blastocyst Implantationa. Annals of the New York Academy of Sciences 442 368–374. ( 10.1111/j.1749-6632.1985.tb37541.x) [DOI] [PubMed] [Google Scholar]
- Linnemann JR, Miura H, Meixner LK, Irmler M, Kloos UJ, Hirschi B, Bartsch HS, Sass S, Beckers J, Theis FJ.et al. 2015. Quantification of regenerative potential in primary human mammary epithelial cells. Development 142 3239–3251. ( 10.1242/dev.123554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Tan JP, Schröder J, Aberkane A, Ouyang JF, Mohenska M, Lim SM, Sun YBY, Chen J, Sun G.et al. 2021. Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 591 627–632. ( 10.1038/s41586-021-03372-y) [DOI] [PubMed] [Google Scholar]
- Lohmussaar K, Oka R, Espejo Valle-Inclan J, Smits MHH, Wardak H, Korving J, Begthel H, Proost N, Van De Ven M, Kranenburg OW.et al. 2021. Patient-derived organoids model cervical tissue dynamics and viral oncogenesis in cervical cancer. Cell Stem Cell 28 1380, .e6–1396.e6. ( 10.1016/j.stem.2021.03.012) [DOI] [PubMed] [Google Scholar]
- Loomans CJM, Giuliani NW, Balak J, Ringnalda F, Van Gurp L, Huch M, Boj SF, Sato T, Kester L, De Sousa Lopes SMC.et al. 2018. Expansion of adult human pancreatic tissue yields organoids harboring progenitor cells with endocrine differentiation potential. Stem Cell Reports 10 712–724. ( 10.1016/j.stemcr.2018.02.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes FL, Desmarais JA, Murphy BD. 2004. Embryonic diapause and its regulation. Reproduction 128 669–678. ( 10.1530/rep.1.00444) [DOI] [PubMed] [Google Scholar]
- Lucas ES, Salker MS, Brosens JJ. 2013. Uterine plasticity and reproductive fitness. Reproductive Biomedicine Online 27 506–514. ( 10.1016/j.rbmo.2013.06.012) [DOI] [PubMed] [Google Scholar]
- Lucas ES, Vrljicak P, Muter J, Diniz-Da-Costa MM, Brighton PJ, Kong CS, Lipecki J, Fishwick KJ, Odendaal J, Ewington LJ.et al. 2020. Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Communications Biology 3 37. ( 10.1038/s42003-020-0763-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luddi A, Pavone V, Semplici B, Governini L, Criscuoli M, Paccagnini E, Gentile M, Morgante G, Leo V, Belmonte G.et al. 2020. Organoids of human endometrium: a powerful in vitro model for the endometrium-embryo cross-talk at the implantation site. Cells 9 1121. ( 10.3390/cells9051121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luddi A, Pavone V, Governini L, Capaldo A, Landi C, Ietta F, Paccagnini E, Morgante G, De Leo V, Piomboni P. 2021. Emerging role of embryo secretome in the paracrine communication at the implantation site: a proof of concept. Fertility and Sterility 115 1054–1062. ( 10.1016/j.fertnstert.2020.10.058) [DOI] [PubMed] [Google Scholar]
- Macklon NS, Brosens JJ. 2014. The human endometrium as a sensor of embryo quality. Biology of Reproduction 91 98. ( 10.1095/biolreprod.114.122846) [DOI] [PubMed] [Google Scholar]
- Maenhoudt N, Defraye C, Boretto M, Jan Z, Heremans R, Boeckx B, Hermans F, Arijs I, Cox B, Van Nieuwenhuysen E.et al. 2020. Developing organoids from ovarian cancer as experimental and preclinical models. Stem Cell Reports 14 717–729. ( 10.1016/j.stemcr.2020.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P, Yang L, Esvelt KM, Aach J, Guell M, Dicarlo JE, Norville JE, Church GM. 2013. RNA-guided human genome engineering via Cas9. Science 339 823–826. ( 10.1126/science.1232033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru Y, Kawata A, Taguchi A, Ishii Y, Baba S, Mori M, Nagamatsu T, Oda K, Kukimoto I, Osuga Y.et al. 2020. Establishment and molecular phenotyping of organoids from the squamocolumnar junction region of the uterine cervix. Cancers 12 694. ( 10.3390/cancers12030694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruo T, Matsuo H, Otani T, Mochizuki M. 1997. Epidermal growth factor (EGF) regulates trophoblast proliferation and endocrine function in synergy with thyroid hormone. Placenta 18 27–39. ( 10.1016/S0143-4004(0580158-4) [DOI] [Google Scholar]
- Mascarenhas M, Jeve Y, Polanski L, Sharpe A, Yasmin E, Bhandari HM. 2021. Management of recurrent implantation failure: British Fertility Society Policy and Practice Guideline. Human Fertility 1–25. ( 10.1080/14647273.2021.1905886) [DOI] [PubMed] [Google Scholar]
- Meseguer M, Aplin JD, Caballero-Campo P, O’Connor JE, Martín JC, Remohí J, Pellicer A, Simón C. 2001. Human endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocyst. Biology of Reproduction 64 590–601. ( 10.1095/biolreprod64.2.590) [DOI] [PubMed] [Google Scholar]
- Miura Y, Li MY, Birey F, Ikeda K, Revah O, Thete MV, Park JY, Puno A, Lee SH, Porteus MH.et al. 2020. Generation of human striatal organoids and cortico-striatal assembloids from human pluripotent stem cells. Nature Biotechnology 38 1421–1430. ( 10.1038/s41587-020-00763-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Berry MJ, Botting KJ, Darby JRT, Frasch MG, Gatford KL, Giussani DA, Gray CL, Harding R, Herrera EA.et al. 2018. Improving pregnancy outcomes in humans through studies in sheep. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 315 R1123–R1153. ( 10.1152/ajpregu.00391.2017) [DOI] [PubMed] [Google Scholar]
- Muruganandan S, Fan X, Dhal S, Nayak NR. 2020. Development of A 3D tissue slice culture model for the study of human endometrial repair and regeneration. Biomolecules 10 136. ( 10.3390/biom10010136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa J, Pao GM, Shokhirev MN, Verma IM. 2018. Glioblastoma model using human cerebral organoids. Cell Reports 23 1220–1229. ( 10.1016/j.celrep.2018.03.105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergraft SS, Sadri-Ardekani H, Atala A, Bishop CE. 2017. Three-dimensional testicular organoid: a novel tool for the study of human spermatogenesis and gonadotoxicity in vitro. Biology of Reproduction 96 720–732. ( 10.1095/biolreprod.116.143446) [DOI] [PubMed] [Google Scholar]
- Pierro E, Minici F, Alesiani O, Miceli F, Proto C, Screpanti I, Mancuso S, Lanzone A. 2001. Stromal-epithelial interactions modulate estrogen responsiveness in normal human endometrium. Biology of Reproduction 64 831–838. ( 10.1095/biolreprod64.3.831) [DOI] [PubMed] [Google Scholar]
- Ramalho AS, Fürstová E, Vonk AM, Ferrante M, Verfaillie C, Dupont L, Boon M, Proesmans M, Beekman JM, Sarouk I.et al. 2021. Correction of CFTR function in intestinal organoids to guide treatment of cystic fibrosis. European Respiratory Journal 57 1902426. ( 10.1183/13993003.02426-2019) [DOI] [PubMed] [Google Scholar]
- Rawlings TM, Makwana K, Taylor DM, Molè MA, Fishwick KJ, Tryfonos M, Odendaal J, Hawkes A, Zernicka-Goetz M, Hartshorne GM.et al. 2021. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. bioRxiv 2021.03.02.433560. ( 10.1101/2021.03.02.433560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart CA, Lyn-Cook BD, Kaufman DG. 1988. Gland formation from human endometrial epithelial cells in vitro. In Vitro Cellular and Developmental Biology 24 1037–1041. ( 10.1007/BF02620878) [DOI] [PubMed] [Google Scholar]
- Rogers K.2012. Scientific Modeling [Online]. Encyclopedia Britannica. (available at: https://www.britannica.com/science/scientific-modeling). Accessed on 21 February 2021. [Google Scholar]
- Ruane PT, Buck CJ, Babbington PA, Aboussahoud W, Berneau SC, Westwood M, Kimber SJ, Aplin JD, Brison DR. 2020. The effects of hyaluronate-containing medium on human embryo attachment to endometrial epithelial cells in vitro. Human Reproduction Open 2020 hoz033. ( 10.1093/hropen/hoz033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N, Papaspyropoulos A, Zomer-Van Ommen DD, Heo I, Böttinger L, Klay D, Weeber F, Huelsz-Prince G, Iakobachvili N, Amatngalim GD.et al. 2019. Long-term expanding human airway organoids for disease modeling. EMBO Journal 38 e100300. ( 10.15252/embj.2018100300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleh N, Giribabu N. 2014. Leukemia inhibitory factor: roles in embryo implantation and in nonhormonal contraception. ScientificWorldJournal 2014 201514. ( 10.1155/2014/201514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaermann P, Toelle B, Berger H, Schmidt SC, Glanemann M, Ordemann J, Bartfeld S, Mollenkopf HJ, Meyer TF. 2016. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut 65 202–213. ( 10.1136/gutjnl-2014-307949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simintiras CA, Dhakal P, Ranjit C, Fitzgerald HC, Balboula AZ, Spencer TE. 2021. Capture and metabolomic analysis of the human endometrial epithelial organoid secretome. PNAS 118 e2026804118. ( 10.1073/pnas.2026804118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simón C, Gimeno MJ, Mercader A, O’Connor JE, Remohí J, Polan ML, Pellicer A. 1997. Embryonic regulation of integrins β3,α 4, and α1 in human endometrial epithelial cells in vitro. Journal of Clinical Endocrinology and Metabolism 82 2607–2616. ( 10.1210/jcem.82.8.4153) [DOI] [PubMed] [Google Scholar]
- Simón C, Mercader A, Garcia-Velasco J, Nikas G, Moreno C, Remohí J, Pellicer A. 1999. Coculture of human embryos with autologous human endometrial epithelial cells in patients with implantation failure. Journal of Clinical Endocrinology and Metabolism 84 2638–2646. ( 10.1210/jcem.84.8.5873) [DOI] [PubMed] [Google Scholar]
- Singh H, Aplin JD. 2009. Adhesion molecules in endometrial epithelium: tissue integrity and embryo implantation. Journal of Anatomy 215 3–13. ( 10.1111/j.1469-7580.2008.01034.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- So KH, Lee CL, Yeung WS, Lee KF. 2012. Glycodelin suppresses endometrial cell migration and invasion but stimulates spheroid attachment. Reproductive Biomedicine Online 24 639–645. ( 10.1016/j.rbmo.2012.03.004) [DOI] [PubMed] [Google Scholar]
- Teilmann SC, Clement CA, Thorup J, Byskov AG, Christensen ST. 2006. Expression and localization of the progesterone receptor in mouse and human reproductive organs. Journal of Endocrinology 191 525–535. ( 10.1677/joe.1.06565) [DOI] [PubMed] [Google Scholar]
- Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C.et al. 2010. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS ONE 5 e10258. ( 10.1371/journal.pone.0010258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinning H, Taylor A, Wang D, Constantinides B, Sutton R, Oikonomou G, Velazquez MA, Thompson P, Treumann A, O’Connell MJ.et al. 2020. The role of CAPG in molecular communication between the embryo and the uterine endometrium: is its function conserved in species with different implantation strategies? FASEB Journal 34 11015–11029. ( 10.1096/fj.202882RR) [DOI] [PubMed] [Google Scholar]
- Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, Hollinshead M, Marsh SGE, Brosens JJ, Critchley HO.et al. 2017. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nature Cell Biology 19 568–577. ( 10.1038/ncb3516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco MY, Gardner L, Kay RG, Hamilton RS, Prater M, Hollinshead MS, Mcwhinnie A, Esposito L, Fernando R, Skelton H.et al. 2018. Trophoblast organoids as a model for maternal–fetal interactions during human placentation. Nature 564 263–267. ( 10.1038/s41586-018-0753-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida H, Maruyama T, Ono M, Ohta K, Kajitani T, Masuda H, Nagashima T, Arase T, Asada H, Yoshimura Y. 2007. Histone deacetylase inhibitors stimulate cell migration in human endometrial adenocarcinoma cells through up-regulation of glycodelin. Endocrinology 148 896–902. ( 10.1210/en.2006-0896) [DOI] [PubMed] [Google Scholar]
- Varma VA, Melin SA, Adamec TA, Dorman BH, Siegfried JM, Walton LA, Carney CN, Norton CR, Kaufman DG. 1982. Monolayer culture of human endometrium: methods of culture and identification of cell types. In Vitro 18 911–918. ( 10.1007/BF02796347) [DOI] [PubMed] [Google Scholar]
- von Wolff M, Strowitzki T, Becker V, Zepf C, Tabibzadeh S, Thaler CJ. 2001. Endometrial osteopontin, a ligand of beta3-integrin, is maximally expressed around the time of the ‘implantation window’. Fertility and Sterility 76 775–781. ( 10.1016/s0015-0282(0102015-5) [DOI] [PubMed] [Google Scholar]
- Wang H, Pilla F, Anderson S, Martínez-Escribano S, Herrer I, Moreno-Moya JM, Musti S, Bocca S, Oehninger S, Horcajadas JA. 2012. A novel model of human implantation: 3D endometrium-like culture system to study attachment of human trophoblast (Jar) cell spheroids. Molecular Human Reproduction 18 33–43. ( 10.1093/molehr/gar064) [DOI] [PubMed] [Google Scholar]
- Wang P, Lin M, Pedrosa E, Hrabovsky A, Zhang Z, Guo W, Lachman HM, Zheng D. 2015. CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in neurodevelopment. Molecular Autism 6 55. ( 10.1186/s13229-015-0048-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimar CH, Kavelaars A, Brosens JJ, Gellersen B, De Vreeden-Elbertse JM, Heijnen CJ, Macklon NS. 2012. Endometrial stromal cells of women with recurrent miscarriage fail to discriminate between high- and low-quality human embryos. PLoS ONE 7 e41424. ( 10.1371/journal.pone.0041424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimar CH, Post Uiterweer ED, Teklenburg G, Heijnen CJ, Macklon NS. 2013. In-vitro model systems for the study of human embryo-endometrium interactions. Reproductive Biomedicine Online 27 461–476. ( 10.1016/j.rbmo.2013.08.002) [DOI] [PubMed] [Google Scholar]
- Weiss G, Huppertz B, Siwetz M, Lang I, Moser G. 2016. Arterial endothelial cytokines guide extravillous trophoblast invasion towards spiral arteries; an in-vitro study with the trophoblast cell line ACH-3P and female non-uterine endothelial cells. Placenta 38 49–56. ( 10.1016/j.placenta.2015.12.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiwatpanit T, Murphy AR, Lu Z, Urbanek M, Burdette JE, Woodruff TK, Kim JJ. 2020. Scaffold-free endometrial organoids respond to excess androgens associated with polycystic ovarian syndrome. Journal of Clinical Endocrinology and Metabolism 105 769–780. ( 10.1210/clinem/dgz100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Wei Y, Duan J, Schmitz DA, Sakurai M, Wang L, Wang K, Zhao S, Hon GC, Wu J. 2021. Blastocyst-like structures generated from human pluripotent stem cells. Nature 591 620–626. ( 10.1038/s41586-021-03356-y) [DOI] [PubMed] [Google Scholar]
- Zheng Y, Xue X, Shao Y, Wang S, Esfahani SN, Li Z, Muncie JM, Lakins JN, Weaver VM, Gumucio DL.et al. 2019. Controlled modelling of human epiblast and amnion development using stem cells. Nature 573 421–425. ( 10.1038/s41586-019-1535-2) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a