Abstract
ProAKAP4 is synthetized as a precursor polypeptide that must be converted into mature AKAP4 in living spermatozoa and is considered as a functional marker of spermatozoa. The gene is well-conserved in mammals although uncharacterized in Camelidae. In the present study, we investigate the expression metabolism of proAKAP4 and AKAP4 proteins and evaluate their seasonal dynamics relative to semen quality in dromedary camels. Semen parameters including volume and viscosity and characteristics of sperm including concentration, total production, total and progressive motility, vitality, acrosome integrity and morphological abnormalities were assessed in semen samples collected weekly from six camels during the rutting season, from November to April. Only total sperm production varied, peaking in January. Both the precursor proAKAP4 and AKAP4 proteins were investigated and shown to express biochemical properties similar to those described in other mammals. ProAKAP4 concentrations expressed in ng/10 million spermatozoa as assayed using a specific ELISA showed a strong positive correlation with ejaculate volume (P = 0.045), viscosity (P < 0.001) and sperm total motility (P = 0.049). Furthermore, their concentrations exhibited clear seasonal variations in camel semen. In conclusion, the assessment of proAKAP4 concentrations in camel sperm provides a novel parameter to assess sperm quality. Further studies should be performed to investigate proAKAP4 concentrations relative to fertility in Camelidae that may help to define the right time for mating and semen collection and increase the success of breeding programs.
Lay summary
Breeding related to the seasons/time of year in the camel has been reported in several studies. A better knowledge of semen quality during the breeding season would assist in determining the best period for mating in camels. However, conventional sperm parameters are held to be unsatisfactory because they cannot predict breeding potential. ProAKAP4 a sperm-specific protein has been described as a functional marker of sperm and a key fertility marker in several species but has not been described in camels. Motility or membrane integrity parameters of semen collected throughout the breeding season and also the presence of proAKAP4 protein were investigated. ProAKAP4 was identified for the first time in camels and their concentrations exhibited clear seasonal variations in camel semen showing strong correlations with ejaculate volume and total motility and viscosity. Further studies should be performed to investigate proAKAP4 concentrations relative to fertility in camels to define the right time for mating and increase the success of breeding programs.
Keywords: dromedary camel, PROAKAP4, spermatozoa, seasonality
Introduction
Seasonality in the male dromedary and its influence on reproductive variables has been reported in several studies (Yagil & Etzion 1980, Deen 2008, El-Harairy & Attia 2010, Swelum et al. 2018a). The breeding season will vary with the geographical location, management and nutritional status. A better knowledge and definition of semen quality during the rutting season would assist in determining the best month for semen collection, cryopreservation and also for mating in Camelidae.
Spermatogenesis in dromedary camels takes place throughout the year though with greater intensity from November to March (rutting season: Tingari et al. 1984, El-Kon et al. 2011) when a significant increase in testicle weight and size has been reported (Zeidan et al. 2001, Pasha et al. 2011). In a recent study, Al-Bulushi et al. (2019) confirmed the seasonal changes of testis function and accessory gland size through the year in the Gulf region of the Middle East countries, showing greater values from December to February and the least in June and July. These anatomical changes are associated with sperm parameter variations including greater volume, motility, velocities and viability during the rutting months (Deen 2008). Meanwhile, no seasonal differences were found in sperm morphology, findings similar to those of Swelum et al. (2018b).
However, conventional sperm parameters are held to be unsatisfactory because they do not not correlate well with fertility and cannot predict breeding potential (Khatun et al. 2018). Thus, new test methods have been established to better investigate relationships between sperm physiology and functionality. To this end, several studies have investigated concentrations of the protein ProAKAP4 as they relate to predictions of male fertility (Sergeant et al. 2019).
ProAKAP4 (A-kinase anchor protein 4) has been described as a functional marker of spermatozoa and a key fertility marker (Delehedde et al. 2018, Nixon et al. 2019, Sergeant et al. 2019, Greither et al. 2020). This sperm-specific protein is highly conserved from reptiles to mammals (Hu et al. 2009, Blommaert et al. 2019, Nixon et al. 2019, Sergeant et al. 2019). Structurally, proAKAP4 is expressed as a precursor protein that must be converted by proteolytic cleavage in living spermatozoa into the mature AKAP4 protein. This conversion process occurs during spermatogenesis starting at spermatid stages and only in living motile spermatozoa. Lack of proAKAP4 conversion is encountered in transgenic models invalidated for intraflagellar transport proteins 81 and 174 both of which cause defective spermiogenesis and male infertility (Qu et al. 2020, Zhang et al. 2020). Both proAKAP4 and AKAP4 proteins are among the most abundant proteins of the principal piece of the flagellum and they are essential for flagellum formation and for the structure of the fibrous sheath that surrounds the axoneme (Fang et al. 2019, Xue et al. 2020). Moreover, AKAP4 actively coordinates the core transduction signals that together regulate sperm function including motility, capacitation and fertility (Sergeant et al. 2019, 2020). Spermatozoa are immotile and animals are infertile in transgenic mice invalidated for proAKAP4/AKAP4 expression (Miki et al. 2002, Fang et al. 2019, Fu et al. 2019). Additional evidences show that a striking under-representation of AKAP4 is reported in spermatozoa of infertile human patients (Moretti et al. 2007, Redgrove et al. 2012, Frapsauce et al. 2014, Delehedde et al. 2019a) and recently in hybrids of cattle and yaks that are sterile (Wu et al. 2020). Positive correlations between the levels of proAKAP4 expression, with key sperm quality and fertility indicators, have been reported in a number of livestock species including bulls, pigs, rams and stallions (Peddinti et al. 2008, Blommaert et al. 2019, Sergeant et al. 2019, Riesco et al. 2020).
In this study, we investigated classic parameters of semen collected throughout the rutting season and also expression of proAKAP4 and AKAP4 sperm proteins as they relate to season and semen quality.
Materials and methods
Animals and semen collection
The study was conducted between November (2018) and April (2019) at the Camel Reproduction Centre, Dubai, UAE. The average humidity and temperature per month have been included in Table 1 (latitude 25.272577°N; longitude 55.364445°E).
Table 1.
Semen parameters analyzed from November to April.
| Variables | Nov | Dec | Jan | Feb | Mar | Apr |
|---|---|---|---|---|---|---|
| Temperature (°C average) | 27 | 23 | 22 | 22 | 23 | 29 |
| Humidity (%; average) | 55 | 57 | 57 | 55 | 55 | 40 |
| Volume (mL) | 2.19 ± 0.44 | 3.40 ± 1.18 | 4.00 ± 0.58 | 4.30 ± 0.73 | 4.10 ± 0.51 | 2.90 ± 0.95 |
| Viscosity (1–5) | 2.50 ± 0.34 | 2.60 ± 0.51 | 2.67 ± 0.33 | 3.00 ± 0.00 | 2.80 ± 0.20 | 2.60 ± 0.24 |
| Concentration (M/mL) | 460.00 ± 94.36 | 310.00 ± 128.34 | 610.00 ± 376.34 | 434.00 ± 64.70 | 386.3 ± 51.59 | 439.00 ± 125.86 |
| Total production | 1188 ± 297a,b | 905 ± 375c,d,e,f | 2796 ± 67g,h,i | 1766 ± 286b,d,g | 1569 ± 647e,h | 1576 ± 1997f,i |
| Total motility (%) | 49.36 ± 7.12 | 45.58 ± 13.19 | 75.77 ± 4.32 | 50.92 ± 4.12 | 60.26 ± 7.41 | 57.12 ± 12.90 |
| Progressive motility (%) | 21.49 ± 4.63 | 16.46 ± 4.68 | 34.37 ± 2.10 | 10.58 ± 1.19 | 7.46 ± 2.08 | 4.52 ± 1.58 |
| Vitality (%) | 55.25 ± 5.33 | 52.60 ± 12.21 | 77.33 ± 7.80 | 55.00 ± 4.25 | 59.20 ± 7.44 | 59.80 ± 12.75 |
| Acrosome integrity (%) | 80.75 ± 6.63 | 80.40 ± 6.37 | 87.67 ± 4.70 | 71.40 ± 4.55 | 69.10 ± 16.43 | 72.80 ± 7.00 |
| Morphological abnormalities (%) | 11.38 ± 2.15 | 12.40 ± 3.44 | 9.67 ± 1.67 | 12.80 ± 1.56 | 13.20 ± 1.93 | 11.60 ± 2.68 |
| Head (%) | 3 ± 0.7 | 6 ± 2.59 | 6.33 ± 2.85 | 5.83 ± 1.53 | 2.0 ± 1.22 | 2.0 ± 0.71 |
| Tail (%) | 5.12 ± 1.78 | 3.20 ± 1.39 | 1 ± 0 | 2.4 ± 0.75 | 5.6 ± 3.78 | 7.2 ± 5.45 |
| Principal (%) | 0.13 ± 0.17 | 1.0 ± 1.0 | 0.0 ± 0.0 | 0.4 ± 0.4 | 0.6 ± 0.55 | 0.4 ± 0.89 |
| Droplets (%) | 3.13 ± 0.98 | 2.20 ± 1.71 | 2.33 ± 1.20 | 4.20 ± 1.16 | 5.00 ± 2.55 | 2.00 ± 1.22 |
| proAKAP4 ng/10 M spermatozoa | 4.72 ± 0.26 | 4.46 ± 1.24 | 5.78 ± 1.51 | 12.43 ± 1.31 | 18.45 ± 11.82 | 11.42 ± 9.4 |
aNov vs Jan: P < 0.0001; bNov vs Feb: P = 0.016; cDec vs Jan: P < 0.0001; dDec vs Feb: P = 0.0001; eDec vs Mar: P = 0.0086; fDec vs Apr: P = 0.0079; gJan vs Feb: P = 0.0004; hJan vs Mar: P < 0.0001; iJan vs Apr: P < 0.0001.
Six adult dromedary camel males, 8 to 10 years old, of proven fertility were used in this study. Ejaculates were obtained weekly from each male using an artificial vagina (Skidmore et al. 2013), between November and April. Full ejaculates were immediately taken to the laboratory and placed in a 37°C water bath. Non sequential ejaculate fractions can differ in camel ejaculates. All animal procedures were approved by the Animal Welfare and Ethics Committee of the Camel Reproduction Centre, UAE.
Semen assessment
The volume and viscosity of the ejaculate were recorded immediately after the collection. Semen viscosity was scored on a scale of 1–5. Briefly, semen was drawn into a 2 mL plastic pipette and the difficulty of pipetting it up and down was assessed: 1: no viscosity; 2: some viscosity but it can be pipetted easily; 3: some difficulties to be pipetted, more strength must be applied; 4: it can barely be pipetted up; 5: it cannot be pipetted at all.
Fresh ejaculates were extended 1:4 in pre-warmed Tris–citrate–fructose buffer (TCF, pH 6.9; 340 mOsm) composed of 300 mM Tris, 94.7 mM citric acid and 27.8 mM fructose (Evans & Maxwell 1987). BSA (0.05%) and EDTA (10 mM) were added with 4% (v:v) egg-yolk and the solution was filter-sterilized (0.22 µm).
Semen samples were incubated at room temperature with gentle pipetting (30–45 min), to achieve liquefaction, then assessed for sperm motility, concentration, total sperm production, morphology, vitality and acrosome. One mL of each sample was stored at −80°C for the proAKAP4 analysis. They were subsequently thawed for 1 min at 37°C.
Motility
To determine sperm total and progressive motility and initial concentration, aliquots of semen samples (2 µL) were placed in a disposable chamber slide (Cytonix, Beltsville, MD; USA) that to be analyzed using CASA (CEROS II®; Hamilton Thorne; MA; USA) under negative phase contrast microscopy and ×10 objective. For each evaluation, a 3 μL aliquot of the sperm sample was placed in a disposable capillary counting chamber (MicroToolTM, Cytonix, USA) which provides a chamber depth of 20 µm; four fields were analyzed (approximately 400 sperm in total) at a frame rate of 30/s. Particles of size between 13 and 101 μm were considered to be spermatozoa. Setting for progressive motility was: STR > 70% and VAP > 40 μm/s. Semen was diluted to obtain between 100 and 120 spermatozoa per field. Total sperm production was calculated by multiplying the initial volume by the initial concentration.
Morphological abnormalities
Eosin-nigrosin staining was used to assess morphology (Agarwal et al. 2016). One drop of the sample was mixed with one drop of stain and spread on a glass slide. The smear was dried and immediately observed under the microscope to assess the percentages of abnormal heads, middle pieces and tails as well as the presence of droplets. A total of 200 sperm were evaluated.
Membrane integrity
Plasma membrane integrity was evaluated using the fluorescent probes SYBR-14 (SY) and propidium iodide (PI) according to the manufacturer’s instructions (L-7011, Live/Dead Sperm Viability Kit; Molecular Probes Europe). Briefly, 30 µL of liquefied semen was diluted with 6 µL SYBR-14 solution (final concentration: 20 µM) and incubated 10 min at 38°C in 5% CO2 and then 1 µL of PI solution (final concentration: 12 µM) was added (Malo et al. 2019). Paraformaldehyde (1 µL of 4%) was added to the sample to immobilize the sperm and then an additional 30 µL of extender was added. Spermatozoa were allocated to 'intact membrane' or 'dead' classifications if they exhibited SY+/PI− (green) and SY−/PI+ (red) staining, respectively. A total of 200 sperm were counted per sample and the percentage of viable sperm was calculated.
Acrosome status
Acrosome status was assessed by FITC conjugated with peanut agglutinin (FITC-PNA) as described by Malo et al. (2019). Briefly, 30 µL of liquefied semen was diluted with 170 µL of green buffer (IMV, France), 6 µL FITC-PNA solution (2 mg/mL) (final concentration 0.06 ng/mL) and 0.5 µL of 4% paraformaldehyde. Spermatozoa were allocated to 'damaged acrosome', or 'intact acrosome' classifications if they exhibited PNA+ (green acrosome) and PNA− (absence of color) staining, respectively. In total, 200 spermatozoa were assessed per sample, and the percentages of intact acrosome sperm were calculated.
ProAKAP4
Quantification of ProAKAP4 concentration by ELISA
ProAKAP4 concentrations were quantified using the ELISA assay Camel 4MID® Kit (4BioDx, France). Thawed samples (50 µL) were mixed with 450 µL of the camel lysis buffer and subjected to ELISA quantification according to the manufacturer’s instructions (Ref. 4VDX-19K11, 4BioDx, France). Then, 100 µL of the lysed semen sample preparation was loaded into each well of the 96-well plate of the Camel 4MID® Kit and incubated at ambient temperature on an orbital shaker at 300 rpm (4BioDx, France). The resulting color intensity was proportional to the amount of ProAKAP4 present and measured as the optical density by spectrophotometry at a wavelength of 450 nm. Results of ProAKAP4 concentrations were expressed in ng/10 million spermatozoa (ng/10 M spermatozoa) following the camel 4MID® Kit User instructions.
Analysis of camel ProAKAP4 and AKAP4 by Western blotting
For Western blotting analysis, one volume of thawed semen was first added to one volume of 10 mM Tris–HCl pH 6.8 containing 2% SDS. Total protein concentrations of the semen preparation were quantified by using Bradford’s method (BioRad). Then 50 µL of the Tris-SDS sample was added to one volume of 2× concentrated NuPAGE LDS sample buffer (ThermoFisher) to reach equal protein concentration in each sample. NuPAGE sample reducing agent (ThermoFisher) was added (10 µL) to each sample that was then rapidly mixed using a vortex and heated in a dry-bath for 10 min at 80°C. Then equal amounts (25 µg) of sperm protein preparation were loaded on polyacrylamide gels (4–12% NuPage Precast Gels) and run up to 45 min. After gel electrophoresis, proteins were transferred onto 0.45 µm nitrocellulose membranes (G&E Healthcare) using a Liquid Transfer System (Life Technologies). The membranes were then blocked in a 25 mM Tris–HCl (pH 8.0), 150 mM NaCl and 0.1% (v/v) Tween 20 (TNT Buffer) solution with 5% skimmed milk added. Membranes were rinsed three times (10 min each) with TNT before being placed at 4°C overnight in the presence of the first antibody at a dilution of 1:4000 either with the anti-AKAP4 clone 10F8 (4BioDx, 4BDX-1805) or with the clone 6F12, a MAB anti-ProAKAP4 (4BioDx, 4BDX-1701, France). After three washing steps in TNT over a period of 10 min, the secondary anti-mouse antibody coupled to horseradish peroxidase (dilution at 1:50,000 from Vector Laboratories, Burlingame, CA USA) was added to the nitrocellulose membranes then revealed using the ECL™ chemiluminescence kit (G&E Healthcare). Images were acquired using the Image Quant™ LAS 4000 system (G&E Healthcare).
Electron microscopy
Thawed camel spermatozoa were first fixed for 1 h at 4°C in a solution of 0.1 M phosphate buffer (pH 7.4) containing 2% (v/v) paraformaldehyde and 0.05% glutaraldehyde (v/v), then dehydrated by immersion in increasing concentrations of ethanol and embedded in LR White Resin (EMS, USA). After sectioning, the samples on grids were incubated for 30 min at room temperature in TBS buffer (Tris–HCl (pH 8.0, 150 mM NaCl, 0.1% (v/v), pH 8.0) containing 1% (v/v) normal goat antiserum and 1% (w/v BSA). They were subsequently incubated with the monoclonal anti-proAKAP4 antibody clone 6F12 diluted at 1:50 in the same buffer. After three washes in TBS, the grids were incubated with a 12 nm colloidal gold-conjugated goat anti-mouse IgG (H+L) antibody (Jackson Immunoresearch Laboratories) diluted 1:50 in the same buffer. Control grids were prepared with colloidal-conjugated antibody alone. Following three washes in TBS, all grids were fixed and counterstained with 2% uranyl acetate. Grids were observed and photographed using a Zeiss transmission 900 electron microscope.
Statistical analysis
Statistical analyses were performed using Prism 8.2 GraphPad software (GraphPad Software). Linear correlation analyses were performed with the Spearman’s rank correlation test, and differences between groups were determined by a non-parametric paired samples t-test, Mann–Whitney U-test or a two-way ANOVA test for comparison of sperm parameters. Statistical tests were considered as significant for P values below 0.05 (*P < 0.05; **P < 0.01; ***P < 0.001). Values are given as the means ± s.e.m.
Results
ProAKAP4 homology sequence analysis
AKAP4 is a well-known marker of sperm quality although proAKAP4 or AKAP4 polypeptides are not yet fully characterized in Camelidae semen. First, the homology of proAKAP4 protein sequences referenced in repository protein banks was compared between Camelus dromedarius (XP_010979579.1), Camelus bactrianus (XP_010962425.1) and Camelus bactrianus ferus (S9W656). Protein sequence homology between these three Camelidae species shares more than 95.85% (Fig. 1). Furthermore, AKAP4 protein sequence of C. dromedarius is more than 85% homologous to that of goat, ram and bull proAKAP4 and 79.32% homologous with human proAKAP4 protein sequence (Fig. 2A) which is also consistent with the ontology analysis showing that AKAP4 orthologues are in this same genetic cluster with goat, ram, bull, yak and alpaca. ProAKAP4 is converted in AKAP4 functional protein following the cleavage at an asparagine-rich stretch followed by glutamine residue 180 (according to the numbering the sequence number from 177 to 180 of C. dromedarius XP_010979579.1, Fig. 1) corresponding to a highly conserved sequence of the prodomain cleavage site necessary for the conversion of proAKAP4 into AKAP4. This sequence and cleavage site of the Camelus proAKAP4 amino acid sequence is 100% homologous between mammalian species, suggesting a highly conserved mechanism of proAKAP4 conversion to AKAP4 among these species for regulating the amount of released AKAP4 functional protein.
Figure 1.
Reference sequences of proAKAP4/AKAP4 protein from Camelus dromedarius, Camelus bactrianus and Camelus ferus used for the homology analysis. The protein repository references of the predicted proteins sequences are indicated in FASTA format.
Figure 2.
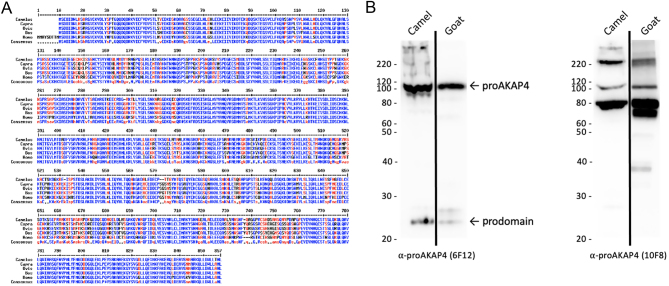
Both proAKAP4 and AKAP4 are expressed in camel spermatozoa. (A) Sequence alignment of proAKAP4 from Camelus, Capra, Ovis, Bos taurus or Homo sapiens using Mulatlin alignment application (http://multalin.toulouse.inra.fr/multalin/). Homologous amino acid residue is indicated in blue. In red are the amino acids from the same biochemical family whereas differences are in black and indicated by dots. (B) Western blot analysis of proAKAP4 and AKAP4 expression in total protein from semen of camel or goat. Proteins were separated using (4–12%) SDS-PAGE gels. ProAKAP4 on the left panel was stained with the proAKAP4 monoclonal antibodyMAB clone 6F12 while AKAP4 MAB clone 10F8 s8 staining is shown on the right panel. Apparent molecular weights from 220 to 20 kDa are indicated on the left of the Western-blot lanes.
Both proAKAP4 and AKAP4 polypeptides are expressed in camel semen
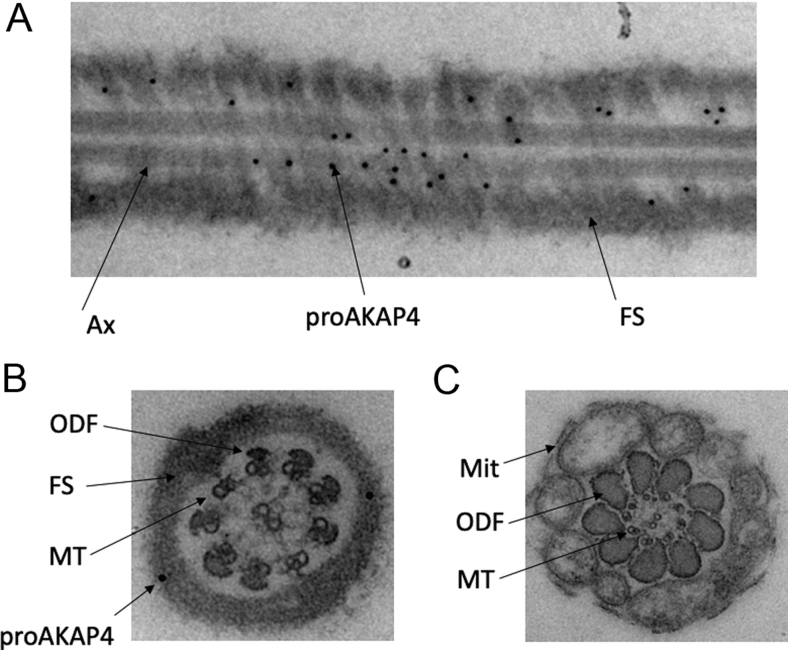
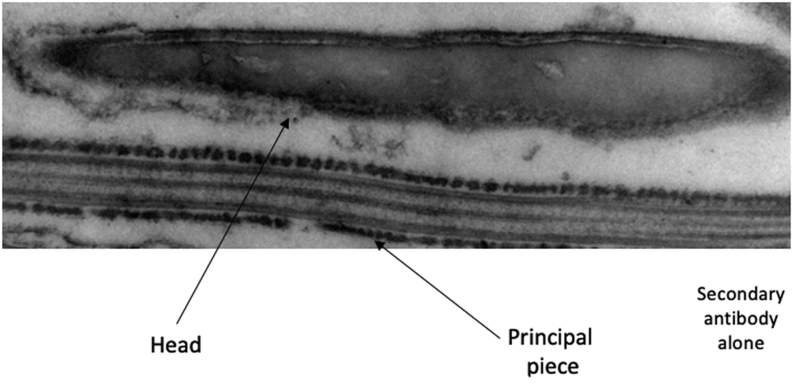
ProAKAP4 and AKAP4 expression in camel semen were analyzed by immunoblotting using specific AKAP4 antibodies against the prodomain of the AKAP4 precursor (anti-proAKAP4 clone 6F12) and an anti-AKAP4 antibody (clone 10F8) against an epitope localized at the C-terminus of the protein. Goat semen was also included as a positive control as the homology with the proAKAP4 protein sequence of C. dromedarius is theoretically the highest. Both in camel and goat semen, the anti-ProAKAP4 clone 6F12 immunoblotting showed two bands at identical molecular weights of 100 kDa (proAKAP4) and 82 kDa (AKAP4). Together with the 100and 82 kDa bands, an additional band at 25 kDa was observed (Fig. 2B) that corresponds to the cleavage product generated by the conversion process of proAKAP4 into AKAP4. This 25 kDa polypeptide is specifically and only detected with proAKAP4 (clone 6F12) antibody, not with the AKAP4 antibody, and was then referred to as the prodomain of proAKAP4 (Fig. 2B). Both proAKAP4 and AKAP4 proteins were detected in camel semen with 10F8 MAB, at 100 and 82 kDa, respectively (Fig. 2B). The 100 kDa band corresponds to the full-length proAKAP4, and the second band at 82 kDa corresponds to the mature AKAP4 protein resulting from the conversion of proAKAP4 at the glutamine residue 188 of C. dromedarius AKAP4 protein sequence (Fig. 1). The prodomain band at 25 kDa was not observed with the 10F8 antibody that only detected C-terminus proAKAP4 and AKAP4 m4 molecules. Then the proAKAP4/AKAP4 localization inside spermatozoa was assessed by immunogold labeling and electron microscopy. Ultrastructure immuno labeling with monoclonal anti-proAKAP4 antibodies showed a restricted localization in the fibrous sheath of the principal piece of the flagellum, as confirmed with both longitudinal and coronal sections of the camel spermatozoa flagellum (Fig. 3A and B). Immunogold particles were never observed in other structures (outer dense fibers or microtubules) of the flagellum including the intermediate piece (Fig. 3C) or in other parts of the spermatozoa such as the head, the acrosome or the neck (data not shown). The head or principal piece of the flagellum was not stained by the secondary gold- labeled antibody alone (Fig. 4). Taken together, these results show that proAKAP4 protein is expressed in camel spermatozoa and converted into AKAP4 and is specifically localized in the flagellum and, more precisely, in the fibrous sheath of the principal piece as previously described in other mammal species. Quantifying the variations of proAKAP4 concentrations in camel semen appears then a relevant approach to further investigate semen quality in camels during the breeding season.
Figure 3.
Localization of proAKAP4 marker in camel spermatozoa by electron microscopy. (A) Electron microscopy images of proAKAP4 immunogold staining (black dots of 20 nm gold particles), in a longitudinal section of the proximal part and medial part of the principal piece of the flagellum. (B) proAKAP4 immunogold labeling in the coronal section of a camel spermatozoa. (C) Coronal section of the intermediate piece of the flagellum of camel spermatozoa. The microtubules (MT) of the axoneme (Ax) as well as the outer dense fibers (ODF) and mitochondria (MIT) are indicated by arrows.
Figure 4.
Electron microscopy of the head and principal piece of the flagellum incubated with the secondary antibody alone. The head (Head) and principal piece are indicated. Note the absence of immunogold labeling.
In vitro sperm quality parameters and seasonality
The data for the mean volume, viscosity, concentration, total and progressive sperm motility, vitality, acrosome integrity and morphological abnormalities are reported for each month from December to April in columns (Table 1). A two-way ANOVA statistical analysis was performed and shown that variance between months accounts for 0.8013% of the total variance (F = 2.19; DFn = 5, DFd = 332 and the P value = 0.0553). Sperm parameters account for 73.05% of the total variance (F = 76.71; DFn = 13, DFd = 322 and P value is below 0.0001, ***). While considering month and sperm parameters, the interaction accounts for 7.627% of the total variance (F = 160; DFn = 65, DFd = 322 and P value = 0.0045, **). Differences were observed for total sperm production along the rutting season (Table 1). November presented significantly higher values than December, and January sperm production increased when compared to December and February. Greater sperm production was seen in March and April than in December and November (Table 1), all of which were significantly correlated. As shown in Table 1, no differences were observed in acrosome integrity, abnormalities, droplets and viscosity parameters during the rutting season. In contrast, other sperm parameters were significantly modified during the rutting season. January exhibited the highest values of sperm production and total motility and progressive motility (Table 1) whereas proAKAP4 concentrations were at their highest in March.
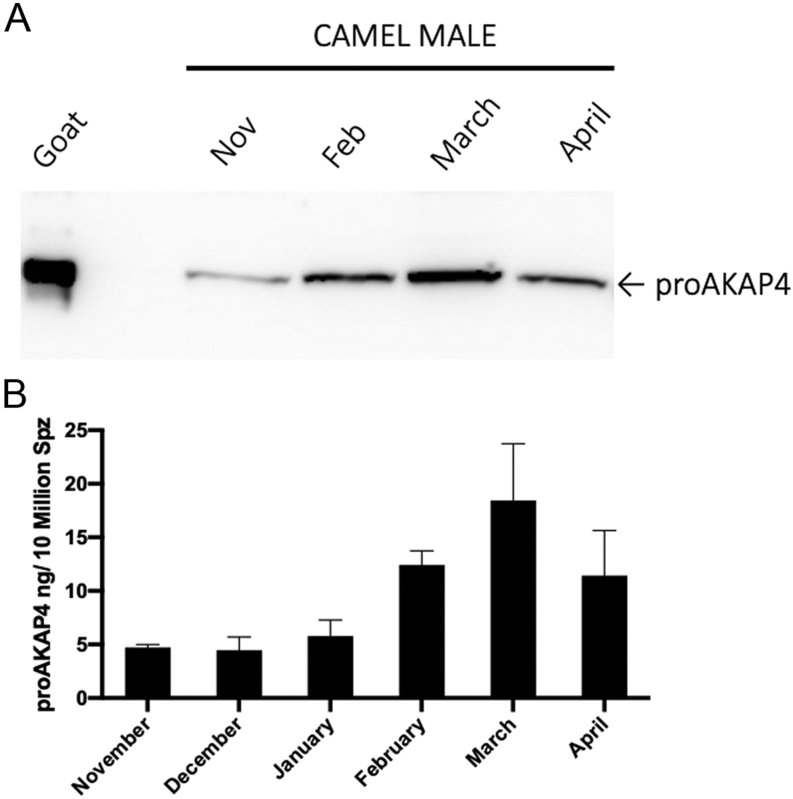
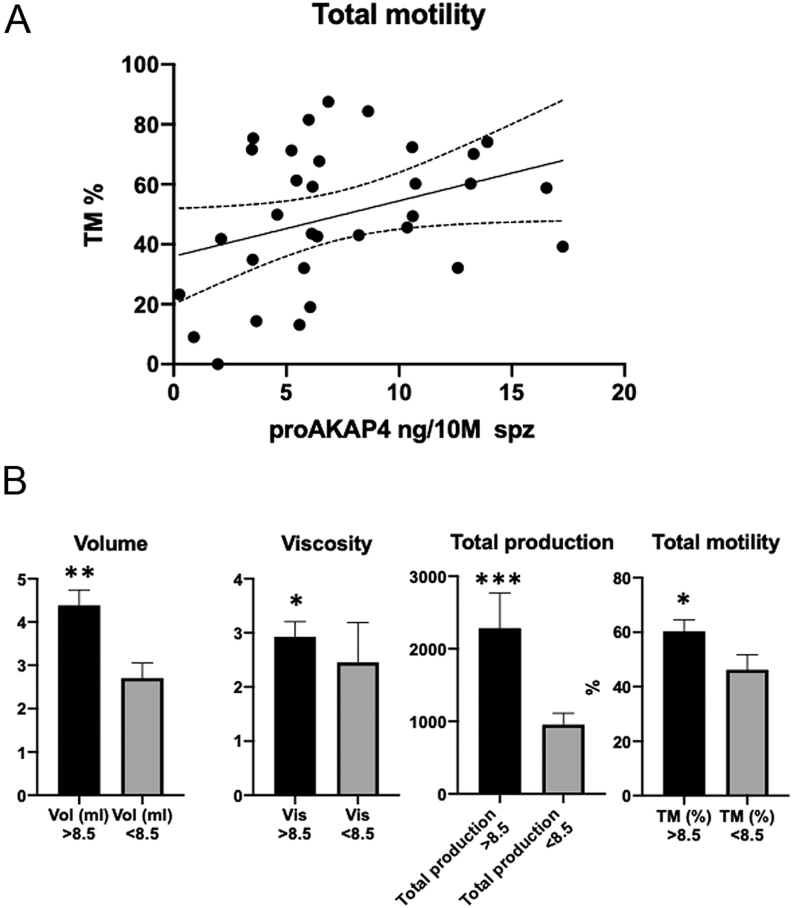
ProAKAP4 concentrations were then analyzed and compared to classic sperm parameters. As observed by Western blotting, the total amount of proAKAP4 in camel semen was modulated from November to April (Fig. 5A). The proAKAP4 100 kDa band intensity progressively increased from November to March and then decreased in April, showing modifications of proAKAP4 expression along autumn, winter and spring. The concentration of proAKAP4 using the Camel 4MID® ELISA Kit to quantify these variations in semen collected month by month was assessed then (Table 1). The concentrations of proAKAP4 were expressed in ng per 10 million spermatozoa (ng/10 M spermatozoa). Average proAKAP4 concentrations seasonally remained similar in November and December, started to increase from January with a maximum observed in March when ProAKAP4 concentration was up to five-fold higher than the concentrations observed in November/December (Fig. 5B). Therefore, the average ProAKAP4 sperm parameter values clearly show monthly differences with the ProAKAP4 expression significantly higher in March. While considering ProAKAP4 in individual ejaculates, the ProAKAP4 concentrations ranged from a minimum of 0.25 ng/10 M spermatozoa to a maximum of 39.24 ng/10 M spermatozoa, exhibiting large variations among males that therefore account for about a 120-fold difference between individuals in semen quality based on the proAKAP4 sperm parameter. More precisely, among samples, the mean proAKAP4 concentration ± s.e.m. was 8.701 ± 1.28 ng/10 M, the median, 25% and 75% percentiles were at 6.16, 3.67 and 10.73 ng/10 M spermatozoa, respectively. Interestingly a positive correlation (P = 0.016) was found between proAKAP4 concentrations and total sperm production. proAKAP4 sperm parameters were also positively correlated with ejaculate volume (P = 0.045), viscosity (P < 0.001) and with the total motility (P = 0.049). ProAKAP4 concentration was significantly correlated with total motility (n = 33, r = 0.35, P = 0.049) (Fig. 6A). The proAKAP4 concentrations in ng/10 M were also positively correlated with the volume of the ejaculates (Spearman rank test; n = 33; r = 0.35; P = 0.042) and the viscosity (n = 33; r = 0.54; P = 0.0009) (Fig. 6A). Based on the average concentration of proAKAP4 of all camel semen samples assessed, the mean value of each sperm parameter obtained below and above the threshold of 8.5 ng of proAKAP4/10 M spermatozoa was compared. Interestingly, we observed that the viscosity was significantly increased as well as the total production and the total motility sperm parameters above the threshold of 8.5 ng of proAKAP4/10 M spermatozoa (Fig. 6B).
Figure 5.
Western-blot analysis of the expression of proAKAP4 in camel ejaculates collected from November to April. (A) proAKAP4 precursor was detected with the anti-proAKAP4 mouse MAB clone 6F12 from semen samples collected in November, February, March and April. ProAKAP4 staining from goat semen is also showed as a positive control. (B) Quantification of proAKAP4 using the Camel 4MID® Kit in semen collected in November, December, January, February, March and April. The concentration is expressed in ng/10 millions spermatozoa (ng / 10 million Spz).
Figure 6.
ProAKAP4 concentrations and classical camel sperm parameters. (A) Correlation between proAKAP4 concentrations in ng/10 M of spz as measured by ELISA in sperm samples and total motility expressed in percentage. (B) Comparative changes in volume, viscosity, total production, total motility at a threshold concentration of proAKAP4 8.5 ng / 10 millions of spz. Comparative analyses were performed between averaged concentrations over and below the proAKAP4 concentration of 8.5 ng /10 millions spz. Test were considered as statistically significant with P values over 0.05 (*), 0.01 (**) and 0.001 (***).
Discussion
In this study, the precursor protein of AKAP4 called proAKAP4 was analyzed for the first time in camel semen and compared to the sperm parameters during the rutting season, from November up to April.
Using orthogonal methods, we showed that proAKAP4 is expressed in C. dromedarius sperm and converted into AKAP4. Camel proAKAP4 and AKAP4 are strictly localized in the fibrous sheath of the principal piece of the flagellum which overall is structurally similar to that of other mammalian flagellum principal pieces (Jumeau et al. 2018, Blommaert et al. 2019, Delehedde et al. 2019a), being responsible for the propagation of sperm motility. One of the specific functions of AKAP4 is indeed at the level of the fibrous sheath of the principal piece of the flagellum and therefore the formation of sperm flagellum (Fanget al. 2019, Xu et al. 2020). This fibrous sheath surrounds the axoneme of the flagellum and contains the molecular machinery necessary to regulate and maintain long-lasting motility. As a major structural and abundant protein of the principal piece of the flagellum, AKAP4 has a central regulatory function of sperm motility, hypermotility, capacitation and sperm fertility (Delehedde et al. 2019b, Sergeant et al. 2019). Lack of AKAP4 expression in transgenic models leads to a normal total amount of spermatozoa but with defective fibrous structure, that results in immotile sperm and infertile animals (Mikiet al. 2002, Fang et al. 2019, Xu et al. 2020).
In this study was demonstrated that the proAKAP4 polypeptide is synthesized and metabolized in modulating concentrations in camel ejaculates. During spermatogenesis, the AKAP4 gene has first to be translated into the proAKAP4 polypeptide, that can then be converted in active AKAP4 through the proteolytic cleavage of the prodomain to ensure motility. The cleavage site is a highly conserved motif consisting of a stretch of three asparagine followed by glutamine, that appears to not vary between mammals. This conversion cleavage occurs during spermatogenesis coincident with flagellum elongation in the spermatid stage and will occur in mature spermatozoa during motility acquisition, in the ejaculate and along all living spermatozoa pathways up to the fecundation site (Nipper et al. 2006, Blommaert et al. 2019, Dewulf et al. 2019, Nixon et al. 2019, Sergeant et al. 2019). The conversion process ensures a constant delivery of AKAP4 to maintain the spermatozoa flagellum structure and maintenance of functional motility. This process was well-established in several mammal species including, humans, bulls, pigs and horses suggesting that this conversion process is preserved, and the motility-regulating functions are then similar in camels as in other mammals. Furthermore, a strong positive correlation of proAKAP4 concentration with total sperm motility was observed with camel spermatozoa. AKAP4 expression was previously shown to be significantly correlated with total and progressive motility parameters in humans, pigs, dogs, bulls and in stallions (Blommaert et al. 2018, 2019, Delehedde et al. 2018, 2019b, Jumeau et al. 2018, Le Couazer et al. 2019, Ruelle et al. 2019, Sergeant et al. 2019).
Concentrations of proAKAP4 changed during the camel rutting period with their highest values occurring toward the end of the season (March) when they correlated with high total semen production. The proAKAP4 dosage sperm quality and functionality parameter in the dromedary camel may reflect the best fertility period. Low levels of AKAP4, and/or that of the proAKAP4 precursor molecule, were previously described as a pertinent sperm parameter for sperm quality assessments and to predict fertility in pig and bulls (Peddinti et al. 2008, Sergeant et al. 2016, 2019, Delehedde et al. 2018, Ruelle et al. 2019). ProAKAP4 concentrations have been correlated with fertility in pigs (Sergeant et al. 2019), bulls (Peddinti et al. 2008, Singh et al. 2019) and mice (Miki et al. 2002, Fang et al. 2019, Xu et al. 2020). It will be interesting to study the correlation between ProAKAP4 and fertility in camels.
This study showed for the first time that the proAKAP4 sperm protein parameter is seasonally expressed in camel semen. Sperm parameters were significantly increased with proAKAP4 concentrations above the threshold of 8.5 ng/10 M of spermatozoa from January until April. ProAKAP4 concentrations are associated with better sperm quality including semen volume, viscosity, sperm motility and total production. ProAKAP4 in ng/10 M spermatozoa has provided a good indication of sperm quality, showing that it is still high in March that may translate to semen reaching its high quality at this time. Previously Sarkar et al. (2016) described the AKAP4 gene to be strictly associated with active spermatogenesis in both mouse and lizard testis and that it is downregulated in the regressed phase of the testis during the non-breeding season of lizards (Sarkar et al. 2016). Only total sperm production showed differences among the months. Similarly, Al-Bulushi et al. (2019) reported an increase in the quality of the ejaculate from December to February. In that study, more differences were found in the semen parameters (volume, concentration, motility) as they evaluated the seasonal changes throughout the year, with values increased in January and decreased from May to September. Our findings of no differences in sperm morphology within the rutting season agree with other reports (Swelum et al. 2018a, Al-Bulushi et al. 2019). Interestingly, Swelum et al. (2018b) demonstrated that short artificial lightening and low temperature induced rutting out of season in camels, improving seme quality. Similarly, Swelum et al. (2019) demonstrated that melatonin implants improved the reproductive performance of bulls during the non-breeding and subsequently, breeding season. Unfortunately, we do not have information about the non-breeding season in this study to compare with.
Spermatozoa in the ejaculate will result from spermatogenesis that begins at least a month before or more. Interestingly high proAKAP4 concentrations were observed in camel semen from January until March suggesting that they result from spermatogenesis that began in November/December. Elevated concentrations from February to April with a peak occurring in March suggest that sperm quality for banking, artificial insemination or natural mating reach their optimal functionality toward the end of the rutting season. That ProAKAP4 concentrations are indicative of better semen quality, higher fertility and high-quality embryos in other mammals have been shown in several studies (Delehedde et al. 2019a, Ruelle et al. 2019). Using proAKAP4 concentrations as a sperm parameter and as an indicator for semen preservation should be evaluated in further trials.
Furthermore, since proAKAP4 expression can be impaired by several environmental factors including oxidative stress (Delehedde et al. 2019b, Nixon et al. 2019), proAKAP4 may offer a useful marker to assess sperm damage during camel semen preservation.
In conclusion, this study described for the first time the proAKAP4 protein in camel semen, its localization in the principal piece of the flagellum and the changes in proAKAP4 concentrations during the rutting season with a peak in March. Correlations with classic sperm parameters offer valuable information for the possible use of this protein assay to predict sperm quality as it relates to natural mating practices and assisted reproduction technologies in this camel species.
Declaration of interest
M D and N S are co-founders of the company SPQI S.A.S (Lille, France). There is no conflict of interest in the other authors.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author contribution statement
C M, M D and N S conceived the study, analyzed the data and wrote the paper. C M performed all semen analysis. S C, M D and N S performed the proAKAP4 evaluations. J S helped with the writing and English grammar.
Acknowledgements
The authors are thankful to Quentin Dewulf, Fanette Chambonnet and Anne Loyens for their great technical help, Pr Claude Alain Maurage (the University of Lille, Histology lab) for his advice and the Region Hauts-de-France and the BPI Innovation for their financial supports. The expertise of semen collector Asif Rehman is gratefully acknowledged. The authors would like to thank Dr EG Crichon for his help with editing the manuscript. This study was sponsored by H.H. General Sheikh Mohammed bin Rashid Al Maktoum, Ruler of Dubai.
References
- Agarwal A, Gupta S, Sharma R.2016Eosin-nigrosin staining procedure. Andrological Evaluation of Male Infertility 73–78. ( 10.1007/978-3-319-26797-5_8) [DOI] [Google Scholar]
- Al-Bulushi S, Manjunatha BM, de Graaf SP, Rickard JP.2019Reproductive seasonality of male dromedary camels. Animal Reproduction Science 20210–20. ( 10.1016/j.anireprosci.2018.12.013) [DOI] [PubMed] [Google Scholar]
- Blommaert D, Sergeant N, Delehedde M, Franck T, Lejeune JP, Serteyn D.2018Significant correlation between the proAKAP4 concentration and the total and progressive motility in stallion sperm after thawing. Journal of Equine Veterinary Science 66 43. ( 10.1016/j.jevs.2018.05.019) [DOI] [Google Scholar]
- Blommaert D, Sergeant N, Delehedde M, Jouy N, Mitchell V, Franck T, Donnay I, Lejeune JP, Serteyn D.2019Expression, localization, and concentration of A-kinase anchor protein 4 (AKAP4) and its precursor (ProAKAP4) in equine semen: promising marker correlated to the total and progressive motility in thawed spermatozoa. Theriogenology 13152–60. ( 10.1016/j.theriogenology.2019.03.011) [DOI] [PubMed] [Google Scholar]
- Deen A.2008Testosterone profiles and their correlation with sexual libido in male camels. Research in Veterinary Science 85220–226. ( 10.1016/j.rvsc.2007.10.012) [DOI] [PubMed] [Google Scholar]
- Delehedde M, Blommaert D, Jouy N, Scabello J, Miersman H, Franck T, Serteyn D, Mitchell V, Sergeant N.2018Concentration of ProAKAP4 as a pertinent read-out of sperm quality in mammals. Animal Reproduction Science 194 e24. ( 10.1016/j.anireprosci.2018.04.053) [DOI] [Google Scholar]
- Delehedde M, Carracedo S, Selleslagh M, Eddarkaoui S, Amirat-Briand L, Sergeant N.2019aProAKAP4 polypeptide as a biomarker of sperm functionality and male fertility disorders. International Journal of Gynecology and Reproductive Sciences 21, 3–19. [Google Scholar]
- Delehedde M, Demouveaux B, Remy G, Selleslagh M, Dewulf Q, Desseyn JL, Moreau M, Gosset P, Muriel Pichavant M, Sergeant N.2019bProAKAP4 concentrations as an indicator of good spermatogenesis and sperm quality under oxidative stress conditions. Andrology 7 86. ( 10.1111/andr.12624) [DOI] [Google Scholar]
- Dewulf Q, Briand-Amirat L, Eddarkaoui S, Chambonnet F, Delehedde M, Sergeant N.2019The effects of freeze-thaw cycles and of storage time on the stability of ProAKAP4 polypeptide in raw sperm samples: implications for semen analysis assessment in breeding activities. Journal of Dairy and Veterinary Sciences 131–7. ( 10.19080/JDVS.13.555861) [DOI] [Google Scholar]
- El-Harairy MA, Attia KA.2010Effect of age, pubertal stage and season on testosterone concentration in male dromedary camel. Saudi Journal of Biological Sciences 17227–230. ( 10.1016/j.sjbs.2010.04.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kon I, Heleil B, Mahmoud S.2011Effect of age and season on the testicular sperm reserve and testosterone profile in camel (Camelus dromedarius). Animal Reproduction 868–72. [Google Scholar]
- Evans G, Maxwell WMC. Salamon's artificial insemination of sheep and goats. Butterworths, Sydney, 1987. [Google Scholar]
- Fang X, Huang LL, Xu J, Ma CQ, Chen ZH, Zhang Z, Liao CH, Zheng SX, Huang P, Xu WMet al. 2019Proteomics and single-cell RNA analysis of Akap4-knockout mice model confirm indispensable role of Akap4 in spermatogenesis. Developmental Biology 454118–127. ( 10.1016/j.ydbio.2019.06.017) [DOI] [PubMed] [Google Scholar]
- Frapsauce C, Pionneau C, Bouley J, Delarouziere V, Berthaut I, Ravel C, Antoine JM, Soubrier F, Mandelbaum J.2014Proteomic identification of target proteins in normal but non fertilizing sperm. Fertility and Sterility 102372–380. ( 10.1016/j.fertnstert.2014.04.039) [DOI] [PubMed] [Google Scholar]
- Fu Q, Pan L, Huang D, Wang Z, Hou Z, Zhang M.2019Proteomic profiles of buffalo spermatozoa and seminal plasma. Theriogenology 13474–82. ( 10.1016/j.theriogenology.2019.05.013) [DOI] [PubMed] [Google Scholar]
- Greither T, Schumacher J, Dejung M, Behre HM, Zischler H, Butter F, Herlyn H. 2020Fertility Relevance Probability Analysis Shortlists Genetic Markers for Male Fertility Impairment. Cytogenetic and Genome Research 25 1–17. ( 10.1159/000511117) [DOI] [PubMed] [Google Scholar]
- Hu Y, Yu H, Pask AJ, O’Brien DA, Shaw G, Renfree MB.2009A-kinase anchoring protein 4 has a conserved role in mammalian spermatogenesis. Reproduction 137645–653. ( 10.1530/REP-08-0337) [DOI] [PubMed] [Google Scholar]
- Jumeau F, Sigala J, Dossou-Gbete F, Frimat K, Barbotin AL, Buée L, Béhal H, Sergeant N, Mitchell V.2018A-kinase anchor protein 4 precursors (pro-AKAP4) in human sperm. Andrology 6854–859. ( 10.1111/andr.12524) [DOI] [PubMed] [Google Scholar]
- Khatun A, Rahman MS, Pang MG.2018Clinical assessment of the male fertility. Obstetrics and Gynecology Science 61179–191. ( 10.5468/ogs.2018.61.2.179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couazer D, Sergeant N, Jouy N, Michaud S, Loyens A, Delehedde M, Amirat-Briand L, Bencharif D.2019Expression of proAKAP4 in dog semen as promising marker of sperm quality. Reproduction in Domestic Animals 54 73. ( 10.1111/rda.13527) [DOI] [Google Scholar]
- Malo C, Grundin J, Morrell JM, Skidmore JA.2019Individual male dependent improvement in post-thaw dromedary camel sperm quality after addition of catalase. Animal Reproduction Science 209106168. ( 10.1016/j.anireprosci.2019.106168) [DOI] [PubMed] [Google Scholar]
- Miki K, Willis WD, Brown PR, Goulding EH, Fulcher KD, Eddy EM.2002Targeted disruption of the Akap4 gene causes defects in sperm flagellum and motility. Developmental Biology 248331–342. ( 10.1006/dbio.2002.0728) [DOI] [PubMed] [Google Scholar]
- Moretti E, Scapigliati G, Pascarelli NA, Baccetti B, Collodel G.2007Localization of AKAP4 and tubulin proteins in sperm with reduced motility. Asian Journal of Andrology 9641–649. ( 10.1111/j.1745-7262.2007.00267.x) [DOI] [PubMed] [Google Scholar]
- Nipper RW, Jones BH, Gerton GL, Moss SB.2006Protein domains govern the intracellular distribution of mouse sperm AKAP4. Biology of Reproduction 75189–196. ( 10.1095/biolreprod.106.050963) [DOI] [PubMed] [Google Scholar]
- Nixon B, Bernstein IR, Cafe SL, Delehedde M, Sergeant N, Anderson AL, Trigg NA, Eamens AL, Lord T, Dun MDet al. 2019A kinase anchor protein 4 is vulnerable to oxidative adduction in male germ cells. Frontiers in Cell and Developmental Biology 7 319. ( 10.3389/fcell.2019.00319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasha RH, Qureshi AS, Lodhi LA, Jamil H.2011Biometric and ultrasonographic evaluation of the testis of one-humped camel (Camelus dromedarius). Pakistan Veterinary Journal 31129–133. [Google Scholar]
- Peddinti D, Nanduri B, Kaya A, Feugang JM, Burgess SC, Memili E.2008Comprehensive proteomic analysis of bovine spermatozoa of varying fertility rates and identification of biomarkers associated with fertility. BMC Systems Biology 219. ( 10.1186/1752-0509-2-19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu W, Yuan S, Quan C, Huang Q, Zhou Q, Yap Y, Shi L, Zhang D, Guest T, Li Wet al. 2020The essential role of intraflagellar transport protein IFT81 in male mice spermiogenesis and fertility. American Journal of Physiology: Cell Physiology 318C1092–C1106. ( 10.1152/ajpcell.00450.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrove KA, Nixon B, Baker MA, Hetherington L, Baker G, Liu DY, Aitken RJ.2012The molecular chaperone HSPA2 plays a key role in regulating the expression of sperm surface receptors that mediate sperm-egg recognition. PLoS ONE 7e50851. ( 10.1371/journal.pone.0050851) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesco M, Anel-Lopez L, Neila-Montero M, Palacin-Martinez C, Montes-Garrido R, Alvarez M, de Paz P, Anel L.2020ProAKAP4 as novel molecular marker of sperm quality in ram: an integrative study in fresh, cooled and cryopreserved sperm. Biomolecules 10 1046. ( 10.3390/biom10071046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruelle I, Charreaux F, Bencharif D, Thorin C, Michaud S, Schmitt E, Sergeant N, Briand-Amirad L, Delehedde M.2019Assessment of the sperm specific protein proAKAP4 as a marker to evaluate sperm quality and fertility in Holstein bulls. Revista Brasileira Reproduccion Animal 43 472. [Google Scholar]
- Sarkar H, Arya S, Rai U, Majumdar SS.2016A study of differential expression of testicular genes in various reproductive phases of Hemidactylus flaviviridis (Wall Lizard) to derive their association with onset of spermatogenesis and its relevance to mammals. PLoS ONE 11e0151150. ( 10.1371/journal.pone.0151150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant N, Jumeau F, Eddarkaoui S, Sigala J, Dossou GF, Delehedde M, Buee L, Yvoz JF, Mitchell V.2016Investigating proteomic methods and tools to assess sperm quality. Animal Reproduction Science 16999–135. ( 10.1530/REP-20-0284) [DOI] [Google Scholar]
- Sergeant N, Briand-Amirat L, Bencharif D, Delehedde M.2019The sperm specific protein ProAKAP4 as an innovative marker to evaluate sperm quality and fertility. Journal of Dairy Veterinary Science 111–19. ( 10.19080/jvds.11.555803) [DOI] [Google Scholar]
- Sergeant N, Carracedo S, Blommaert D, Aubry S, Jouy N, Lejeune JP, Franck T, Maurage CA, Serteyn D, Buée Let al. 2020Proteolysis of proAKAP4 in semen as a regulatory sensor of sperm quality and functionality. Andrology 8 (Supplement 1) 44 – 45.33615720 [Google Scholar]
- Singh R, Junghare V, Hazra S, Singh U, Sengar GS, Raja TV, Kumar S, Tyagi S, Das AK, Kumar Aet al. 2019Database on spermatozoa transcriptogram of catagorised Frieswal crossbred (Holstein Friesian X Sahiwal) bulls. Theriogenology 129130–145. ( 10.1016/j.theriogenology.2019.01.025) [DOI] [PubMed] [Google Scholar]
- Skidmore JA, Morton KM, Billah M.2013Artificial insemination in dromedary camels. Animal Reproduction Science 136178–186. ( 10.1016/j.anireprosci.2012.10.008) [DOI] [PubMed] [Google Scholar]
- Swelum AA, Saadeldin IM, Ba-Awadh H, Alowaimer AN.2018aShortened daily photoperiod during the non-breeding season can improve the reproductive performance of camel bulls (Camelus dromedarius). Animal Reproduction Science 195334–344. ( 10.1016/j.anireprosci.2018.06.014) [DOI] [PubMed] [Google Scholar]
- Swelum AA, Saadeldin IM, Ba-Awadh H, Alowaimer AN.2018bEffects of melatonin implants on the reproductive performance and endocrine function of camel (Camelus dromedarius) bulls during the non-breeding and subsequent breeding seasons. Theriogenology 11918–27. ( 10.1016/j.theriogenology.2018.06.017) [DOI] [PubMed] [Google Scholar]
- Swelum AA, Saadeldin IM, Ba-Awadh H, Al-Mutary MG, Alowaimer AN.2019Effect of short artificial lighting and low temperature in housing rooms during non-rutting season on reproductive parameters of male dromedary camels. Theriogenology 131133–139. ( 10.1016/j.theriogenology.2019.03.038) [DOI] [PubMed] [Google Scholar]
- Tingari MD, Ramos AS, Gaili ES, Rahma BA, Saad AH.1984Morphology of the testis of the one-humped camel in relation to reproductive activity. Journal of Anatomy 139133–143. [PMC free article] [PubMed] [Google Scholar]
- Wu S, Mipam T, Xu C, Zhao W, Shah MA, Yi C, Luo H, Cai X, Zhong J.2020Testis transcriptome profiling identified genes involved in spermatogenic arrest of cattle yak. PLoS ONE 15 e0229503. ( 10.1371/journal.pone.0229503) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Yang L, Zhang L, Qi H.2020Lack of AKAP3 disrupts integrity of the subcellular structure and proteome of mouse sperm and causes male sterility. Development 147 2. ( 10.1242/dev.181057) [DOI] [PubMed] [Google Scholar]
- Yagil R, Etzion Z.1980Hormonal and behavioural patterns in the male camel (Camelus dromedarius). Journal of Reproduction and Fertility 5861–65. ( 10.1530/jrf.0.0580061) [DOI] [PubMed] [Google Scholar]
- Zeidan A, Habeeb A, Ahmadi E, Amer H, Abd El-Razik M.2001Testicular and physiological changes of the male dromedary camels in relation to different ages and seasons of the year. In Second International Conference on Animal Production and Health, Semi-Arid Areas, pp. 147–160. [Google Scholar]
- Zhang S, Liu Y, Huang Q, Yuan S, Liu H, Shi L, Yap YT, Li W, Zhen J, Zhang Let al. 2020Murine germ cell-specific disruption of Ift172 causes defects in spermiogenesis and male fertility. Reproduction 159409–421. ( 10.1530/REP-17-0789) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a