Abstract
This study assessed the futility of proceeding with a Phase 3 clinical trial of minocycline as a disease-modifying treatment for Huntington’s disease (HD). One hundred fourteen research participants with HD were randomized, 87 to minocycline (200 mg/d) and 27 to placebo. The change in Total Functional Capacity (TFC) score from baseline to Mo 18 was prespecified as the primary measure of HD progression. By using a futility design, we tested the null hypothesis that minocycline would reduce the mean decline in TFC score by at least 25% compared to a fixed value obtained from a historical database, with a one-tailed significance level of 10%. The placebo group was included to facilitate blinding. Rejection of the null hypothesis would discourage a major definitive trial of minocycline in HD. For the primary analysis, missing data were handled by carrying forward the last available observation; a secondary analysis used multiple imputations. The mean TFC decline in the minocycline group was 1.55 (SD 1.85), and futility was not declared (P = 0.12) for the primary analysis. When multiple imputation was used to handle missing data, the mean TFC decline in the minocycline group of 1.71 (SD 1.96, P = 0.07) suggested futility, as was the case for prespecified secondary outcome measures. There were no safety abnormalities attributable to minocycline. Based on the threshold of 25% improvement in TFC, further study of minocycline 200 mg/d in HD was not warranted. Futility designs aid in screening potential therapies for HD.
Keywords: Huntington’s disease, minocycline, clinical trial, futility designs
Huntington’s disease (HD) is an autosomal dominant, neurodegenerative disorder caused by an expanded repetition of the trinucleotide “CAG” in the coding region of the HD gene on the short arm of chromosome 4. There is no effective treatment to slow disease progression.1
Minocycline is a tetracycline antibiotic that inhibits release of apoptogenic factors from mitochondria and also inhibits reactive microgliosis,2,3 factors that may play a role in the mechanisms of neuropathology in HD. Two laboratories demonstrated that minocycline delays disease progression and extends survival by approximately 11% and 14% in the HD mouse model.4,5 A third laboratory, using different methods of drug administration and dosing, did not replicate these findings.6 Minocycline also has demonstrated neuroprotective properties in models of stroke, spinal cord injury, amyotrophic lateral sclerosis, multiple sclerosis, and Parkinson’s disease.7
To determine whether to test minocycline in a large Phase 3 trial, we conducted a clinical trial of minocycline in HD using a futility design. This approach minimizes the number of subjects required for the study and provides an indication of whether or not to pursue larger and more definitive studies. We designed a study to declare futile an agent that did not slow functional decline, a relevant clinical measure of disease progression, by at least 25%. Although a high therapeutic index is preferable for a chronic therapy, because HD is a devastating disease and there are no neuroprotective therapies currently, even very low therapeutic and protective indexes would be acceptable for the first such agent.
METHODS
Study Design
A randomized, double-blind, controlled study to assess the safety and futility of minocycline at 200 mg/d in HD was conducted at 12 Huntington Study Group (HSG) clinical research sites. The study was conducted under an Investigational New Drug (IND 60943) and Health Canada Clinical Trial Application approval (control 097576) and was approved by the institutional review boards at all participating centers.
A futility study design was chosen as an efficient approach to determine whether minocycline would be worthy of future investigation in a larger, more definitive trial. Our study was designed to compare the mean value for the primary outcome measure, change in total functional capacity (TFC) score from baseline to Mo 18, between the minocycline arm and a prespecified fixed value representing a 25% reduction in the expected mean decline without treatment; this fixed value was obtained using data from a previously completed large clinical trial in HD.15 The null hypothesis is that minocycline will reduce the mean decline in TFC score over 18 mo by at least 25%, compared with the expected (fixed) value obtained from the historical database. If the null hypothesis is rejected, then the drug would be considered futile for further investigation in HD. If the null hypothesis is not rejected, minocycline would be considered for a Phase 3 trial. Our reason for including a placebo arm was to facilitate blinding to treatment assignment and also to permit a descriptive assessment of the validity of the assumed change in TFC over time in the historical control group; it was not used as a basis of comparison for the active treatment arm.
Eligibility Criteria
Eligible participants had a confirmed hereditary diagnosis of HD, age ≥17 yr, mild to moderate functional impairment as defined by a TFC score ≥7, and ability to take oral medications, provide informed consent, and comply with study procedures. Women of childbearing potential used adequate birth control methods. Excluded were individuals with a history of minocycline intolerability, white blood cell count <3,800/mm3, screening creatinine >2.0 mg/dl, alanine aminotransferase > twice the upper normal limit, unstable medical or psychiatric illness, pregnancy, or history of vestibular disease.
Study Intervention
Participants began study drug at a dosage of 100 mg twice daily and remained on that dosage for the 18-mo treatment period. Minocycline was supplied by Wyeth (Philadelphia, PA) and Triax (Cranford, NJ). The Investigational Drug Service Pharmacy at the University of Rochester made the matching placebo. Minocycline 100 mg and placebo (lactose USP powder) doses were identical in appearance.
Randomization and Enrollment
One hundred fourteen participants were randomized (3:1) to receive either minocycline 100 mg twice daily or matching placebo. The computer-generated randomization plan was independently developed by the Biostatistics Center at the University of Rochester. It included stratification by site and blocking.
Study Procedures
Study procedures included assessment of eligibility criteria, medical history, physical and neurological examinations, concomitant medication review, assessment of adverse events, administration of the TFC and Unified Huntington’s Disease Rating Scale (UHDRS’99),8 and safety laboratory tests. Participants returned for visits after 1 mo, 3 mo, and then every 3 mo through Mo 18. Vital signs and safety skin and vestibular testing occurred at each follow-up visit. Safety laboratory tests and the TFC score were obtained every 3 mo. The full UHDRS’99 was performed every 6 mo. Compliance was assessed by capsule counts and measurement of serum minocycline level at Mo 6. These were assayed at study completion without knowledge of treatment assignment.
Outcome Measures
The primary outcome measure was the change from baseline to Mo 18 in the TFC scale. The TFC is a widely used instrument that assesses a subject’s overall functioning. The TFC consists of five ordinally scaled items assessing a person’s capacity: (1) occupation; (2) financial affairs; (3) domestic responsibilities; (4) activities of daily living; and (5) independent living. The sum of this scale is reported as the TFC score, which ranges from 0 (extreme incapacity) to 13 (full capacity).9
Secondary outcome variables included changes from baseline to Mo 18 in the other components of the UHDRS’99 and safety and tolerability measures. Tolerability was defined as the ability to complete 18 mo of treatment on study medication.
Sample Size
The primary statistical analysis involved a comparison of the mean change in the active minocycline group with a prespecified fixed value, constituting a 25% reduction in the expected mean decline over 18 mo without treatment. The expected mean changes in TFC and UHDRS’99 outcomes were obtained from a database that included 173 participants with HD who were not treated with coenzyme Q10 and were followed for up to 30 mo in a clinical trial of remacemide and coenzyme Q10 (CARE-HD).10 The mean decline in TFC from baseline to Mo 18 in these CARE-HD participants was 1.75 units; thus, a mean decline of 1.31 units represents a 25% reduction in the expected mean decline without treatment.
Data from the CARE-HD trial suggest that the standard deviation of the change in TFC from baseline to Mo 18 is approximately 1.83 units. A sample size of 78 participants was chosen to provide approximately 80% power to detect a difference of 0.44 units between the mean decline in TFC score in the active minocycline group compared with the fixed value of a 25% reduction in the expected mean decline (1.75 units vs. 1.31 units), using a t test and a significance level of 10% (one tailed). To account for an anticipated 10% dropout rate, a sample size of 87 participants assigned to the minocycline group was planned.
Statistical Analysis
The primary outcome variable was analyzed by comparing the mean decline in TFC in the active minocycline group with the prespecified fixed value of 1.31 using a one-sample t test. Originally a one-tailed significance level of 10% was planned, but the results of a multicenter, double-blind, placebo-controlled trial of minocycline 400 mg/d in ALS were reported in May 2007 that indicated a faster rate of functional decline in the minocycline group than in the placebo group.19 As a result, an interim analysis was introduced to detect potential early evidence of futility after 50% of the participants completed 18 mo of follow-up. An O’Brien-Fleming stopping boundary was used whereby a significance level of 2% was used for the interim analysis, and the significance level for the final analysis was slightly adjusted to 9.4% (both one tailed). Likewise, an 81.2% confidence interval for the mean decline in TFC was computed; if the lower bound of this interval fell above the value of 1.31, the null hypothesis was to be rejected (suggesting futility). The principal secondary outcome variables arising from the UHDRS’99 were analyzed in a similar manner, with fixed values representing a 25% reduction from the expected mean worsening derived from the CARE-HD database. The decision after the interim analysis was to allow the trial to continue to its planned conclusion.
The primary analyses were performed according to the intention-to-treat principle and included all random ized subjects. Two strategies were used to address the problem of missing data. The prespecified primary method was to carry forward the last observed value for subsequent visits with missing values. A secondary method involved the use of regression-based multiple imputation.11,12 The imputation model in this case included the outcomes at previous visits, treatment group, and center as independent variables. One hundred imputations were used for the final analyses. When deriving the values representing 25% reductions from the expected mean worsening from the CARE-HD database, identical imputation methods (last observation carried forward or multiple imputations) were used.
RESULTS
Participant Enrollment
One hundred thirty-four potential participants were identified and evaluated. Of these, 114 were eligible and were enrolled and randomly assigned to receive either minocycline or matching placebo (Fig. 1). All treatment groups, including the historical control group from CARE-HD, exhibited similar baseline demographic and clinical characteristics (Table 1).
Figure 1:
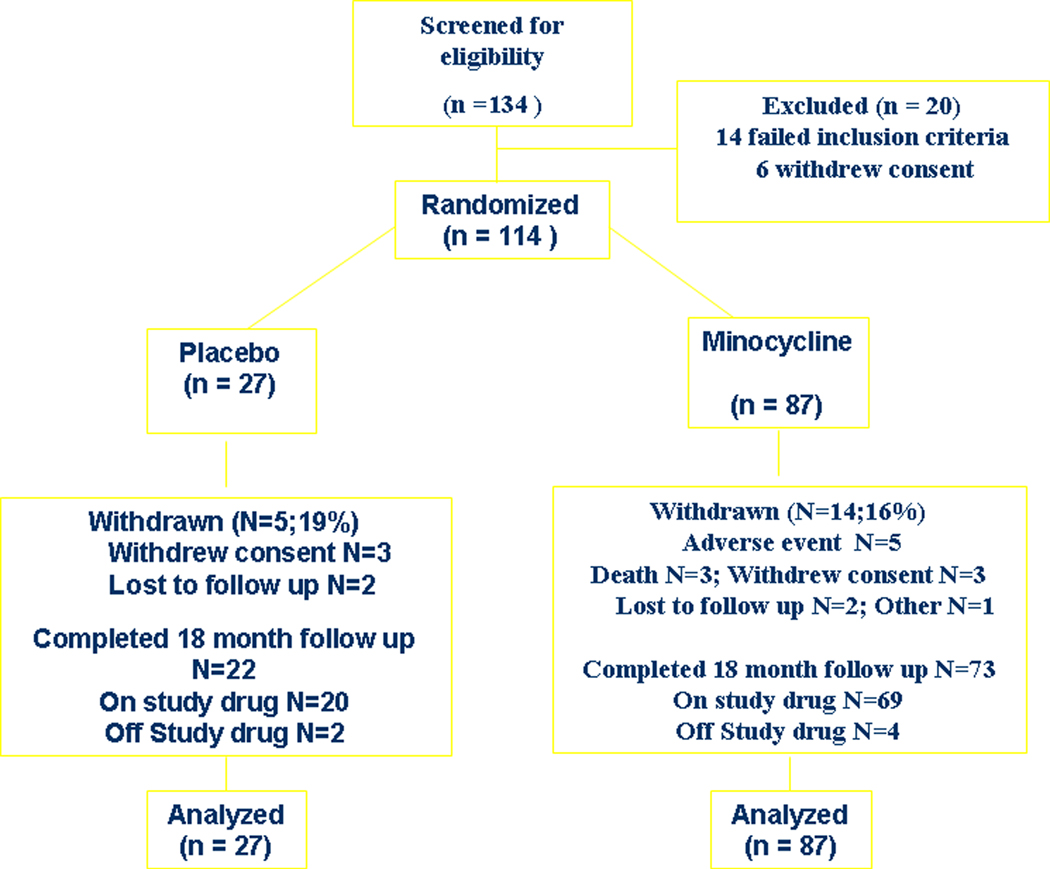
Participant Flow
TABLE 1.
Demographic and clinical characteristics of trial participants and historical controls
| Minocycline (n = 87) | Placebo (n = 27) | CARE-HD (n = 173) | |
|---|---|---|---|
| Age (yr) | 47.1 (10.3) | 47.8 (10.6) | 48.0 (10.7) |
| Female (%) | 47.1 | 55.6 | 47.4 |
| Caucasian (%) | 98.9 | 100.0 | 94.2 |
| Years since HD onset | 6.4 (5.4) | 5.9 (4.8) | 6.2 (5.1) |
| Total Motor Score | 36.5 (15.1) | 33.4 (15.4) | 31.5 (14.1) |
| TFC | 9.9 (1.8) | 10.0 (2.0) | 10.1 (1.8) |
| Functional assessment | 21.5 (3.2) | 21.9 (2.8) | 22.3 (2.4) |
| Independence scale | 85.9 (9.7) | 86.1 (9.9) | 86.4 (9.3) |
Values are mean (standard deviation) unless otherwise indicated.
TFC, total functional capacity; CARE-HD, coenzyme Q10 and remacemide in HD clinical trial (historical control group).
Futility Analysis
The mean 18-mo TFC decline in the minocycline group [1.55, 81.2% confidence interval (CI) 1.29–1.82] was greater than the prespecified criterion of a 25% slowing of the expected decline without treatment (1.31), but the difference did not reach statistical significance (P = 0.12, Table 2). The mean 18-mo TFC decline in the concurrent placebo cohort was 1.15 ± 1.70 (95% CI 0.48–1.82), which was somewhat slower than that observed in the CARE-HD control group (1.75), although the pattern of change over time in all three groups was similar over most of the trial (Fig. 2). However, futility was suggested for some secondary outcome measures, including the UHDRS ‘99 Independence Scale (P = 0.06) and Total Motor Score (P = 0.02, Table 2). The conclusions of the analysis of the TFC were affected by how missing data were handled. When using multiple imputation, the criterion for futility was reached for the TFC (P = 0.07), as well as the Independence Scale (P = 0.02) and the Total Motor Score (P = 0.01, Table 2).
TABLE 2.
Results of futility analyses
| Outcome | Minocycline (n = 87) | Desired level* | 81.2% CI | P |
|---|---|---|---|---|
| Last observation carried forward | ||||
| TFC | 1.55 (1.85) | 1.31 | (1.29, 1.82) | 0.12 |
| Functional assessment | 2.00 (3.78) | 1.90 | (1.46, 2.54) | 0.40 |
| Independence scale | 7.53 (10.34) | 5.83 | (6.06, 9.00) | 0.06 |
| Total Motor Score | 7.01 (7.70) | 5.21 | (5.92, 8.11) | 0.02 |
| Multiple imputation | ||||
| TFC | 1.71 (1.96) | 1.37 | (1.40, 2.01) | 0.07 |
| Functional assessment | 2.40 (4.04) | 2.00 | (1.79, 3.00) | 0.19 |
| Independence scale | 8.61 (10.77) | 6.05 | (6.95, 10.26) | 0.02 |
| Total Motor Score | 7.66 (8.11) | 5.46 | (6.40, 8.93) | 0.01 |
Values in the Minocycline column are mean (standard deviation). All othervalues are expressed as worsening.
Desired level is the value of the mean change that represents a 25% reduction in the expected mean worsening over 18 months without treatment.
TFC, total functional capacity.
Figure 2:
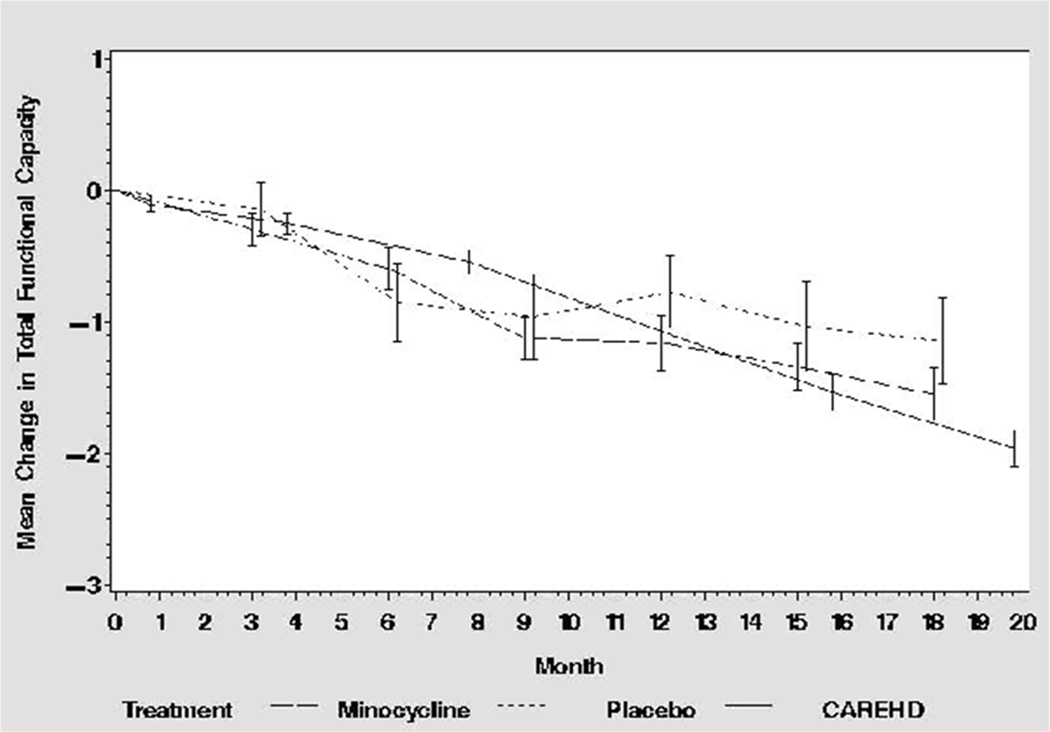
Change in TFC
Tolerability
Minocycline was well tolerated (Fig. 1). Fourteen participants assigned to minocycline (16%) did not complete the 18-mo treatment, five of whom withdrew due to adverse events, three had unrelated deaths, three withdrew consent, one was lost to follow-up, one subject moved, and one subject was withdrawn by the site investigator because of poor drug and visit compliance. Five participants assigned to placebo (19%) withdrew early; three withdrew consent, and two were lost to follow-up.
The adverse events that led to early study withdrawal in the minocycline-treated group included dizziness, abnormal coordination/insomnia, myocardial infarction, rash, and vomiting. Study drug was permanently discontinued in six participants who went on the complete the trial off drug: four in the minocycline group (oral thrush/depression, diarrhea, dysphagia, and cancer) and two in the placebo group (both noncompliance). In addition, there were 12 suspensions of study drug in 9 participants (6 in the minocycline group and 3 in the placebo group) in whom study drug was successfully reinitiated.
Safety
Minocycline at 200 mg/d was safe over 18 mo. There were no clinically relevant changes in vital signs, weight, or safety laboratory values with minocycline treatment. There were no adverse events that occurred more frequently with minocycline than with placebo. There were two reports of tooth discoloration and one report of nail yellowing in the minocycline treatment group. Twelve serious adverse events were reported, including 11 (12.6%) in the minocycline-treated group and 1 (3.7%) in the placebo group (P = 0.29, Fisher’s exact test). Three deaths occurred, all in the minocycline group, but these participants had been off of study medication for 3, 8, and 9 mo when they died. All serious adverse events, including the deaths, were judged by the site investigators to be either unlikely or not related to study medication.
Compliance
Mean compliance was similar between the randomized groups (93.7 ± 8.9% minocycline vs. 91.4 ± 11.2% placebo). Serum for minocycline was drawn at Mo 6 on 73 participants on study drug (56 assigned to minocycline and 17 assigned to placebo). Twenty-two participants assigned to minocycline (30%) did not have measurable levels of minocycline. One participant in the placebo group (6%) had a detectable serum level of minocycline (0.70 μg/ml). The mean serum minocycline level in the 34 participants with measurable levels in the minocycline group was 1.15 ± 0.55 μg/ml.
DISCUSSION
This study is the first trial in HD using a futility design. Using a prespecified futility threshold of 25% slowing of decline in function as measured by the TFC, the results suggest that minocycline treatment is not futile. However, given the dependence of the conclusion on the methods for handling missing data and the results for the secondary outcome measures, interpretation of the overall evidence requires further consideration.
Given that there is invariable functional decline in HD and given the lack of any intervention to improve this situation, the minimally “clinically relevant” benefit is thought to be small. For an intervention known to be associated with mild to moderate side effects, like minocycline, a consensus emerged that a benefit of a 20 to 25% relative slowing of functional decline would be minimally clinically relevant.
By using the prespecified primary analysis and method of handling missing data, the primary null hypothesis that minocycline slows functional decline in HD by at least 25% could not be rejected (P = 0.12), using a historical control group from a previous clinical trial as a basis for comparison. This conclusion differed, however, when using a different approach to handling missing data and futility was also supported by some secondary outcome measures. Taking into account the accumulated evidence, we concluded that it would be futile to proceed with another trial of minocycline involving more participants for longer period of observation. Limitations of the futility design, however, include the inabilities to determine whether or not minocycline is effective in slowing disease progression and whether the conclusion of futility could reasonably be made using a less demanding threshold for futility (e.g., 15% slowing of TFC decline).
The conclusions of this study rely on the validity of the external control group to represent what would have occurred in a concurrent, randomized placebo control group. The concurrent placebo group used in this trial was included mainly to facilitate blinding and was too small (n = 27) to permit any conclusions about the validity of the CARE-HD control group. However, it did not appear that the CARE-HD control group overestimated the rate of progression (Fig. 2). The use of a randomized concurrent placebo control as a basis for comparison would be preferred in terms of trial design, but it would come at the cost of a nearly 4-fold increase in the required sample size.
Minocycline at 200 mg/d was safe and well tolerated. We chose a dosage of 200 mg/d based on its safety profile in an initial 8-week study in HD.13 It is not known whether a higher dosage may have yielded more promising results; however, in a study of minocycline at 400 mg/d in amyotrophic lateral sclerosis, significant toxicity was found.14 A study of minocycline at 200 mg/d was nonfutile in a study in Parkinson’s disease15 and showed efficacy in stroke.16 The minocycline levels measured at 6 mo were undetectable in 30% of minocycline-treated participants with this measurement. This may reflect the timing of the blood draws relative to last dose, a problem with the laboratory assay, or noncompliance. An exploratory analysis revealed that participants with measurable minocycline levels had a greater mean decline in TFC than minocycline-treated participants with undetectable serum levels (data not shown).
The results of this study, although not strictly supporting the futility of minocycline in the prespecified primary analysis, provide insufficient evidence to justify a larger and longer trial of minocycline in HD. The study illustrates the feasibility of screening potential treatments in HD using a futility design.
Supplementary Material
Acknowledgments:
The study was supported by the FDA Office of Orphan Products Development (5R01 FD002588-03). We thank the study participants and their families, and the Safety Monitoring Committee members, Drs. Carl Leventhal, Richard Bedlack, and Jason Roy.
Footnotes
Financial Disclosures: Dr. Cudkowicz has received consultancy payments from GlaxoSmithKline, Trophos and Cytrx.
See Appendix for a complete list of participating Huntington Study Group authors of this report and their affiliations.
Potential conflict of interest: The authors have no personal conflicts of interest related to the study or study drug. There was no ghost writing.
REFERENCES
- 1.Marshall F, Shoulson I. Huntington’s disease: clinical features and therapy. In: Watts R, Kollers W, editors. Movement disorders: neurologic principles and practice. New York: McGraw HIll; 1997. p.491–502. [Google Scholar]
- 2.Wang X, Zhu S, Drozda M, et al. Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington’s disease. PNAS 2003;100:10483–10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu S, Stavrovskiaya I, Drozda M, et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 2002;417:74–78. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Ona C, Li M, et al. Minocycline inhibits caspase-1 andcaspase-2 expression and delays mortality in a transgenic mouse model of Huntington disease. Nature Med 2000;6:797–801. [DOI] [PubMed] [Google Scholar]
- 5.Stack E, Smith K, Ryu H, et al. Combination therapy using minocycline and coenzyme Q10 in R6/2 transgenic Huntington’s disease mice. Biochim Biophys Acta 2006;1762:373–380. [DOI] [PubMed] [Google Scholar]
- 6.Smith D, Woodman B, Mahal A, et al. Minocycline and doxycycline are not beneficial in a model of Huntington’s disease. Ann Neurol 2003;54:186–196. [DOI] [PubMed] [Google Scholar]
- 7.Apoptosis Friedlander R. and caspases in neurodegenerative diseases. New Engl J Med 2003;348:1365–1375. [DOI] [PubMed] [Google Scholar]
- 8.Huntington Study Group. Unified Huntington’s disease rating scale: reliability and consistency. Mov Disord 1996;11:136–142. [DOI] [PubMed] [Google Scholar]
- 9.Shoulson I, Odoroff C, Oakes D, et al. A controlled clinical trial of baclofen as protective therapy in early Huntington’s disease. Ann Neurol 1989;25:252–259. [DOI] [PubMed] [Google Scholar]
- 10.Huntington Study Group (Kieburtz K, principal author). A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington’s disease. Neurology 2001;57:397–404. [DOI] [PubMed] [Google Scholar]
- 11.Little R, Yau L. Intent-to-treat analysis for longitudinal studies with drop-outs. Biometrics 1996;52:1324–1333. [PubMed] [Google Scholar]
- 12.Little R, Rubin D. Statistical analysis with missing data. Hoboken: John Wiley and Sons, Inc.; 2002. [Google Scholar]
- 13.Huntington Study Group. Minocycline safety and tolerability in Huntington’s disease. Neurology 2004;63:547–549. [DOI] [PubMed] [Google Scholar]
- 14.Gordon PH, Moore DH, Florence JM, et al. Results of the phase III randomized controlled trial of minocycline in ALS. Neurology 2007;68(suppl 1):A90–A91. [Google Scholar]
- 15.The NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 2006;66:664–671. [DOI] [PubMed] [Google Scholar]
- 16.Lampl Y, Boaz M, Gilad R, et al. Minocycline treatment in acute stroke. An open-label, evaluator-blinded study. Neurology 2007;69:1404–1410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


