Abstract
Background
Osteochondritis dissecans is a condition wherein there is a subchondral bone lesion that causes pain, inflammation, and cartilage damage. Dominant Familial Osteochondritis Dissecans is a rare and severe form of osteochondritis dissecans (OCD). It is caused by heterozygous pathogenic variants in the gene encoding Aggrecan; ACAN. Aggrecan, a proteoglycan, is an essential component of the articular and growth plate cartilage.
Methods
Herein, we report three individuals from one family; the proband who presented with short stature, a lower limb bone exostosis, and bilateral knee and elbow OCD at the age of 13 years old. His twin brother presented with isolated short stature and his father with short stature and lumbar disc herniation.
Results
Next‐generation sequencing of the ACAN gene in the proband identified a frameshift variant which is also present in the brother and father with short stature. The proband was treated surgically with bilateral elbow microfracture, after the failure of conservative therapy.
Conclusion
To the best of our knowledge, this is the first patient with an aggrecanopathy who presents with osteochondritis dissecans due to a frameshift variant. This family presents with variable expressivity which might be attributed to modifier genes.
Keywords: aggrecan, aggrecanopathy, cartilage, chondrodysplasia, familial osteochondritis dissecans, osteochondritis dissecans, skeletal dysplasia
Dominant Familial Osteochondritis Dissecans is a rare and severe form of osteochondritis dissecans (OCD). It is caused by heterozygous pathogenic variants in the gene encoding Aggrecan; ACAN. Herein, we report 3 individuals from one family; the proband who presented with short stature, a lower limb bone exostosis, and bilateral knee and elbow OCD at the age of 13 years old. Next‐generation sequencing of the ACAN gene in the proband identified a frameshift deletion and this variant was also present in the brother and father with short stature. To the best of our knowledge, this is the first patient with an aggrecanopathy who presents with osteochondritis dissecans due to a frameshift mutation.
1. INTRODUCTION
Osteochondritis dissecans (OCD), first described in 1870 by Paget (Ananthaharan & Randsborg, 2018; Edmonds & Polousky, 2013), is a disorder affecting articulations and is characterized by the sterile osteonecrosis of subchondral bone. This condition is not uncommon as the incidence is reported to be 11.5/100 000 in patients 18 years old or younger (Ananthaharan & Randsborg, 2018). The natural history of OCD in developing children has been well studied and the majority of OCD lesions heal spontaneously in patients with open growth plates (Bellelli et al., 2001). When OCD does not resolve, an osteochondral fragment can separate from the articular surface and lead to a “loose body” in the joint space and possibly precipitate osteoarthritis (Gibson & Briggs, 2016). Patients affected by this disease usually present with activity‐related pain, “locking” mechanical symptoms, and stiffness in the articulation (Gkourogianni et al., 2017). Various non‐surgical and surgical treatments have been described to treat OCD; however, no consensus has been established on the best management plan.
A rarer and more severe form of OCD is dominant familial osteochondritis dissecans, also known as Short Stature and Advanced Bone age, with or without Early‐Onset Osteoarthritis and/or Osteochondritis Dissecans (SSOAOD) (MIM 165800). It is caused by a heterozygous pathogenic variant in the gene encoding aggrecan; ACAN (MIM 155760). Aggrecan is a chondroitin sulphated proteoglycan, an essential component of the cartilage growth plate (Gkourogianni et al., 2017; Stattin et al., 2010; Figure 1). Patients with an abnormal aggrecan protein typically present with early onset joint disease and short stature (Tatsi et al., 2017).
FIGURE 1.
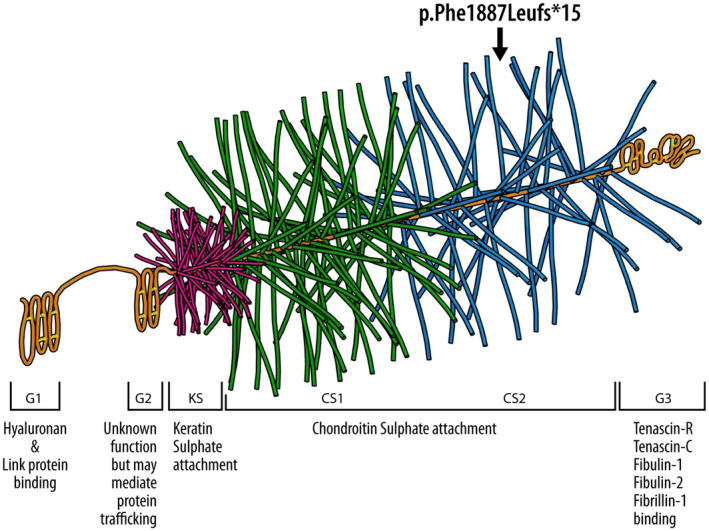
3D illustration of the Agrrecan protein showing the different domains. The pathogenic variant (p. Phe1887Leufs*15) falls on the Chondroitin sulphate attachment domain 2 (CS2)
We report a case of a 13‐year‐old boy with a novel pathogenic variant in the ACAN gene. This novel variant led to the development of symptomatic bilateral knee and elbow osteochondritis dissecans. The same pathogenic variant was also identified in the father and brother of the patient. This is the first report in the literature describing a variant predicted to lead to nonsense‐mediated decay with OCD. In the following case report, we will describe the clinical manifestation and treatment of this condition.
1.1. Case
The patient is a 13‐year‐old Caucasian male, otherwise healthy, who presented with a 1‐month history of left elbow pain and stiffness. He did not have any “locking” mechanical symptoms and there was no history of elbow trauma or prior surgery in the upper extremity. The patient reported a 1‐year history of exercise‐related anterior knee pain bilaterally. He did not complain of any swelling, stiffness or blockage at the knees and did not have prior trauma or surgeries in the lower extremities. Despite the pain he was experiencing, he remained very active in athletic activities, mainly skiing, tennis, and soccer. The family history revealed that he has a fraternal twin brother who has short height, but without the musculoskeletal manifestations, including a normal skeletal survey. The father is also short, measuring 157 cm (−2.7 SD) while the mother who also measures 157 cm is at the 17th percentile for height. The father had lumbar disc herniation, a condition associated with ACAN variants (Dateki et al., 2017; Kawaguchi et al., 1999; Table 1).
TABLE 1.
Main clinical features of the family members
| Family member | Mutation ACAN | Clinical features |
|---|---|---|
| Proband (patient) | ACAN* |
|
| Brother | ACAN* |
|
| Father | ACAN* |
|
| Mother | None |
|
*NM_013227.3:c.5658delG, p. Phe1887Leufs*15.
1.2. Physical examination
Upon initial physical examination at the age of 13 years, his height was 140.4 cm (third percentile). His weight was 47.5 kg, which corresponds to the 40th percentile and his head circumference was 56.5 cm which correspond to the 75th percentile. There was no sign of muscular atrophy and the spinal alignment did not demonstrate any signs of scoliosis; however, there was a slight lumbar hyperlordosis. The shoulder and wrist examinations were normal with a symmetrical range of motion. However, it was found that the left elbow was tender to palpation at the lateral joint line, with a flexion contracture on both active and passive extension of 20°. The active and passive flexion were symmetrical and flexion past 140° elicited pain in the left elbow. A deficit in an active extension of 25° was found on the left elbow and 30° on the right elbow. Palpation of the right elbow revealed a similar but less severe clinical picture with lateral joint line pain, a 5° flexion contracture, and pain elicited beyond 140° of flexion. Both elbows had a stable ligamentous examination and had normal symmetrical pronation and supination at 90° of elbow flexion.
In the lower extremities, the hip and ankle examinations were normal with a symmetrical range of motion and normal ligamentous examination. The bilateral knees had no edema with a symmetrical flexion contracture of 10°. The patient had pain under the medial facet of the right patella and there was otherwise no joint line tenderness, and the ligamentous examination was normal bilaterally. The patient had slight bilateral genu valgum with flexible pes planus. He did not have any dysmorphic features or abnormal skin manifestations. Measurements also showed mild rhizomelia of the upper limbs and mesomelia of the lower limbs.
These physical examination findings were coherent with suspicion of dominant familial OCD lesions which are caused by aggrecans gene mutations. Consequently, magnetic resonance arthrograms (MRA) and genetic testing were ordered.
1.3. Imaging
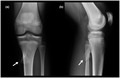
Upon initial presentation, radiographic imaging of both elbows and knees was obtained. The initial elbow radiographs revealed bilateral osteochondral defects which were observed over the capitellum as radiolucent lesions (Figure 2). No signs of instability were present. Hand x‐ray showed a radiological age consistent with his chronological age.
FIGURE 2.
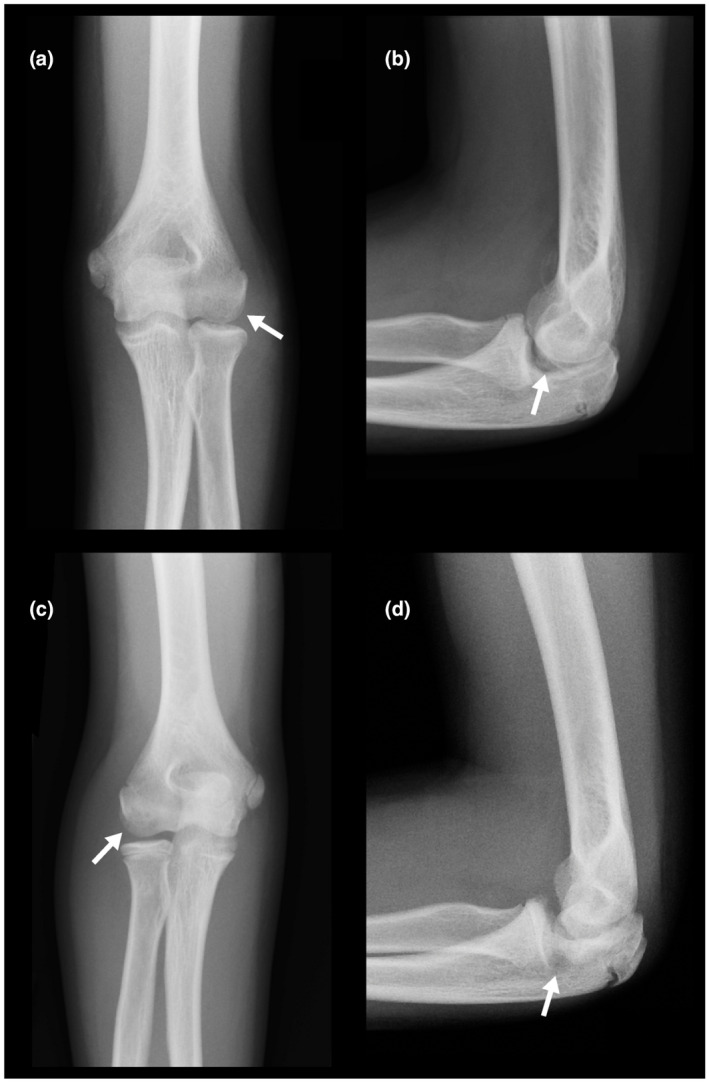
(a‐d) Radiographs of left and right elbow. (arrow) Osteochondral defects are observed bilaterally in capitellum as a radiolucent lesion without signs of fragmentation
One year later, after the failure of conservative treatment, MRI imaging of both elbows was obtained. On the left elbow MRI, a 1.75 × 5.19 mm osteochondral defect was observed in the capitellum. No surrounding edema or signs of instability were detected (Figure 3). Those findings are coherent with a stage II lesion according to the International Cartilage Repair Society (ICRS) scale of osteochondral lesions (Brittberg & Winalski, 2003) (see Annex 1).
FIGURE 3.
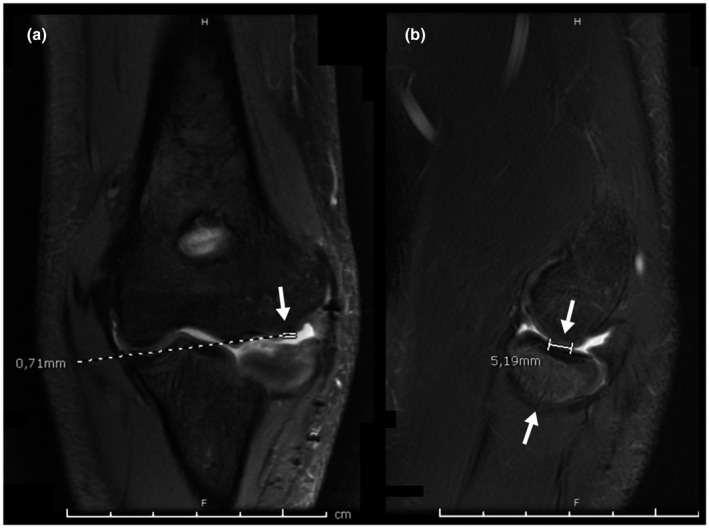
Left elbow Coronal T2 MRI. (Arrow) 1,75 × 5.19 mm cartilage defect in the capitellum without surrounding edema or signs suggestive of instability
On the right elbow MRI, a 4.48 × 5.01 mm subchondral cyst was detected in the capitellum (Figure 4). A 14.67 × 15.01 mm osteochondral defect was also present over the capitellum without signs of surrounding edema. The lesion was classified as stage III due to the presence of a radio‐intense line in the fragment bed suggestive of instability.
FIGURE 4.
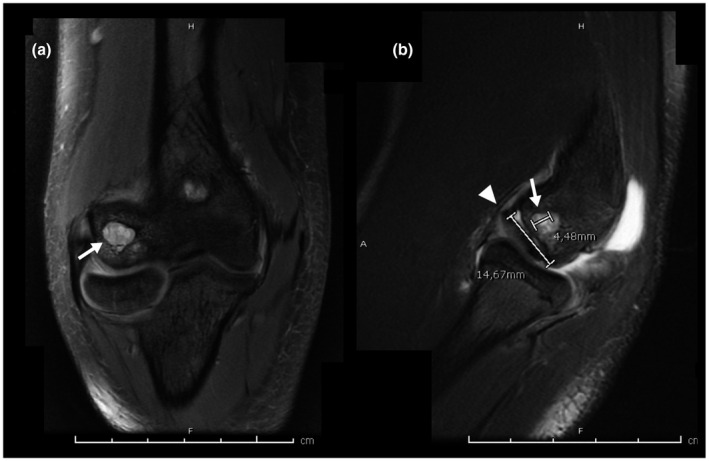
(a,b) Right elbow Coronal T2 MRI. (arrow) A 4.48 × 5.01 mm subchondral cyst in the capitellum. (Arrowhead) An osteochondral defect 14.67 × 15.01 mm is observed in the capitellum without surrounding edema and with a radio‐intense line in the fragment bed suggestive of instability. (ICRS Stage 3)
Radiographs of both knees were also obtained at the initial presentation. On the right knee radiograph, a small osteochondral fragment was observed at the distal edge of the patella, which is coherent with a stage III lesion (Figure 5). The left knee radiograph revealed a tibial osteochondroma but no osteochondral lesions (Figure 6).
FIGURE 5.
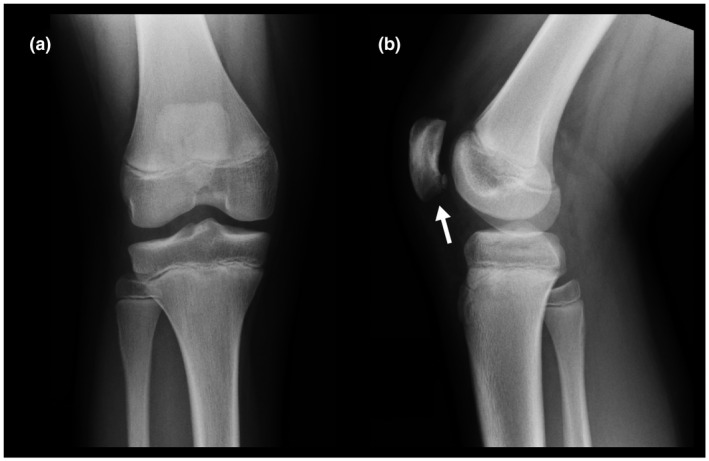
Right knee AP (a) and lateral radiographs (b). Small osteochondral fragment at the distal edge of the medial facet of the patella (Arrow)
FIGURE 6.
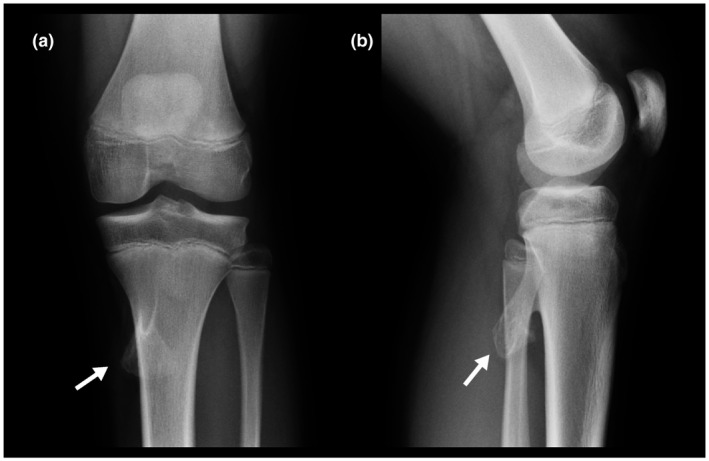
Left knee AP (a) and lateral radiographs (b). Pedunculated osteochondroma on the posterior medial proximal tibia (Arrow) . There are no osteochondral lesions
1.4. Genetic testing
The clinical findings of short stature and multiple OCD were coherent with suspicion of Short Stature and Advanced Bone age, with or without Early‐Onset Osteoarthritis and/or Osteochondritis Dissecans (SSOAOD). Consequently, genetic testing for the ACAN gene only was ordered.
Gene sequencing of ACAN with copy number variant (CNV, i.e., deletions or insertions) detection was performed in a private genetic laboratory. It was performed using whole‐exome sequencing background by NovaSeq 6000 (Illumina), this technique covers the targeted genes plus ~10 bases of non‐coding DNA flanking each exon. Note that only the ACAN gene was analyzed. Familial segregation studies in the father, mother, and twin brother were performed at the same laboratory using Sanger sequencing.
The genetic testing identified a novel heterozygous variant; ACAN (NM_013227.3):c.5658delG, p. Phe1887LeufsTer15 (NC_000015.9) in the proband, his father, and the twin brother (Figure 1). This variant results in a single nucleotide deletion at the 5658 position on the complementary DNA which leads to the frameshift and introduction of the premature stop codon. This variant is classified as a pathogenic variant based on PVS1, PM2, and PP1 as per the ACMG guidelines (Richards et al., 2015).
At the level of mRNA, the premature stop codon is predicted to cause the mRNA to be degraded, a process known as nonsense‐mediated decay. The predicted impact of a premature termination codon on an mRNA and/or a protein product depends on the location of the new termination codon within the most biologically relevant transcript(s) in our case the pathogenic variant falls in exon 12 of 16. Generally, NMD is not predicted to occur if the premature termination codon occurs in the last exon or within the last 50 nucleotides of the penultimate exon (Abou Tayoun et al., 2018). At the protein level, no protein will be produced from this allele, and the cell survives with half the amount of normal protein that is produced from the normal copy of the Aggrecan gene (wild type). The remaining protein will be insufficient to produce a normal phenotype, a genetic phenomenon known as haploinsufficiency.
Genetic testing of the father revealed the same ACAN variant as his son. The mother was negative for any ACAN pathogenic variants. The parents of the patients were informed that data regarding the case would be presented for publication and they consented.
1.5. Surgical intervention and follow‐ups
At the age of 14 years, after 8 months of conservative treatment consisting of physical therapy and activity restriction, the patient's symptoms were not improving. It was then decided to perform left elbow surgery as it was the most symptomatic joint. A left elbow arthroscopy with excision of loose body and microfracture of the OCD lesion was performed. The indications for surgery were the failure of conservative treatment, the presence of a free fragment, loss of end‐range range of motion, swelling, and pain at the joint line. After the surgery, the left elbow was immobilized in a removable night splint for 6 weeks and gradual elbow mobilization was immediately allowed. Physiotherapy was also immediately initiated to preserve and regain range of motion.
At the 6‐week post‐op follow‐up, the patient experienced diminished pain compared to the pre‐operative state and the scar had healed nicely. He followed his physiotherapy and wore his removable splint at night. He also had good recovery of his range of motion. Pronation and supination were 80° for both arms compared to 90° on pre‐operative examination. The range of motion of the left elbow was from 30° to 130°, whereas the right elbow range of motion was from 5° to 140°pre‐operatively. Participation in sports was still being restricted.
During a follow‐up 6 months after the surgery, the left operated elbow was no longer painful during daily activities. The left elbow extension improved from a flexion contracture of 25° pre‐operatively to 20° post‐operatively. Active and passive flexion in both elbows were complete at 150° and the left elbow was no longer painful upon flexion. The right elbow became more painful and was now impeding his daily function. The right elbow active extension had worsened to be limited at −35° compared to −30° prior to left elbow surgery. The patient was still involved in many sports but had to limit participation in tennis and mountain biking due to right elbow pain.
One year after left elbow surgery, extension and flexion were stable compared to the last follow‐up. The pain levels were also unchanged. Recommendations for the progressive return to sports were given to the patient given the stability of the ROM and symptoms. Participation in sports was now limited by the right elbow. The right elbow's active flexion and extension did not vary compared to the pre‐operative state. The right elbow pain was still the main factor limiting his physical activity. A surgical procedure is projected for this right elbow.
2. DISCUSSION
We present herein the clinical, radiological, and molecular genetic characteristics of one family of three affected family members with intrafamilial variable expressivity due to a novel pathogenic variant in ACAN. While the proband presented with short statute and bilateral knee and elbow OCD, his fraternal twin brother presents with milder phenotype of the only short statute, and the father presents with only short stature.
2.1. Aggrecanopathy
Aggrecan is a large proteoglycan bearing numerous sulfate and keratan chains that provide the articular cartilage with its ability to counteract the impact of the compressive forces experienced during the joint use (Roughley & Mort, 2014). The negatively charged nature of aggrecan shows a strong affinity for ions and water, which provides cartilage with a hydrated‐gel structure that helps sustained loads (Kiani et al., 2002). It is essential to have sufficient aggrecan concentration for optimal cartilage function as the aggrecan in aggrecanopathy has abnormal structure, or half the concentration of normal aggrecan in the cases of haploinsufficiency, as in our family, the cartilage will have reduced ability to withstand compressive loads. Over time and/or upon traumatic events, patients affected by aggrecanopathies will be more prone to develop osteochondral lesions compared to healthy subjects (Roughley & Mort, 2014). The short stature phenotype has a similar mechanism since the long bones grow by the process of endochondral ossification, which relies on adequate cartilage matrix secretion. Therefore, a mutated version of aggrecan will alter the growth plate and manifest as short stature in patients affected by familial OCD (Nilsson et al., 2014).
The aggrecan gene provides a good example of allelic heterogeneity, in which different types of variants cause different phenotypes, these are collectively known as Aggrecan‐related bone disorders (Gibson & Briggs, 2016). There is one autosomal recessive disorder, spondylo‐epi‐metaphyseal dysplasia, aggrecan type (MIM 612813). There are two dominant disorders, Kimberley type spondyloepiphyseal dysplasia, (MIM 608361) and SSOAOD discussed in this manuscript (MIM 165800). Indeed, familial osteochondritis dissecans and idiopathic short statute are considered as one entity by the Online Mendelian Inheritance in Man (OMIM, 2017), named: short stature and advanced bone age, with or without early onset osteoarthritis and/or osteochondritis dissecans (SSOAOD), which our proband presented with. This phenotype also includes occasional exostoses and our proband had a single exostosis. There is relative paucity in the genetic literature about familial osteochondritis dissecans.
Gkouroguianni et al. studied the clinical characteristics of 103 individuals from 20 families (including from previously reported individuals) with autosomal dominant short stature and heterozygous pathogenic variant in ACAN. The median adult height is −2.8 standard deviation, 12 families presented with early‐onset osteoarthritis, 11 families presented with back pain due to intervertebral disc disease (confirmed or suspected). Furthermore, only in 3 families out of the 20 families presented with osteochondritis dissecans. Interestingly in these families, the pathogenic missense variants in the ACAN occurred at the C‐type lectin (CLD) of the G3 domain (Gkourogianni et al., 2017) while in our family it is a frameshift variant that occurred at the CS domain. One of the families included in the Gkouroguianni study had previously been reported in detail by Stattin et al who performed genome‐wide linkage analysis in 53 members from 5 generations of a Northern Sweden family, including 15 affected individuals with Dominant familial OCD. They identified a missense pathogenic variant (Val2417Met) in CLD of the G3 domain that disrupts the extracellular matrix interaction (Stattin et al., 2010). Since all 15 affected members in this pedigree had OCD, that missense variant is thus much more penetrant for OCD than the frameshift variant we report here.
2.2. Microfracture
In the literature, there is no consensus on the indications for surgery in patients suffering from osteochondritis dissecans. However, a typical practice is to attempt a 6‐month to 1one‐year course of conservative treatment (immobilization, limited weight‐bearing limitation, and activity restriction) and shift to surgery if the previous treatment fails. Factors such as older age (closed growth plates), a larger lesion size, an unstable lesion, the presence of a loose body, and mechanical problems can indicate that surgery would be a more effective treatment option (Camp et al., 2016; Lewine et al., 2016; Takahara et al., 2008). Regarding the elbow, indications for surgery include unstable lesions, a closed capitellum physis, fragmentation of the OCD or restriction of motion (flexion or extension) over 20° (Takahara et al., 2008). The patient in the presented case report followed an 8‐month course of conservative treatment before shifting to a surgical modality. The limited ROM, the lesion instability, and the presence of a loose body also favored the use of surgical treatment. Elbow OCD lesions greater than 1 cm2 without lateral column involvement should be treated with microfracture drilling, whereas osteochondral grafting should be used if the lateral column is involved (Chappell & ElAttrache, 2008). OCD lesions with lateral column involvement are usually more complex to treat because this structure plays an important role in supporting large compressive forces (Ahmad et al., 2011). In this presented case, once the absence of lateral column involvement was confirmed arthroscopically, the surgeon opted for microfracture drilling over osteochondral grafting as the operative technique.
Microfracture is a widely used surgical technique used in the treatment of OCD lesions. This method typically consists of drilling holes into the lesion in order to promote the vascularization of the necrotic subchondral bone and to elicit multipotent marrow cells which may repair the osteochondral lesion by replacing it with fibrocartilage (Lewine et al., 2016). A major advantage of this procedure is that it can be performed arthroscopically, which reduces postoperative pain and morbidity compared to open procedures while allowing for a non‐invasive diagnostic assessment of the elbow.
The clinical benefits of elbow microfracture have been shown through various studies where 71.4% to 85% of patients had fair to complete clinical or radiographic resolution (Bexkens et al., 2018; Chappell & ElAttrache, 2008). Studies have also shown that a microfracture is a viable option for athletic patients who want a relatively rapid return to sport. It was found in one study that 85.7% of patients returned to any sport and that 66.7% returned to their primary sport after microfracture (Lewine et al., 2016). Another case series resulted in six out of eight athletic patients returning to their primary sport at the same competitive level at an average of 5.1 months post‐surgery (Wulf et al., 2012). Another case series reported a lower rate of return to the sport of 62% (Bexkens et al., 2017).
However, all these articles refer to OCD lesions that were not caused by a diagnosed genetic condition. Consequently, the rates of recovery and return to sports described in the literature might not apply to patients with aggrecanopathies. Functional outcomes and pain relief similar to the above‐mentioned studies were observed with this patient.
2.3. Physical therapy
Physical therapy plays a major role in the treatment management plan of juvenile osteochondral lesions. Studies have demonstrated that stable juvenile OCD lesions lead to better outcomes when managed nonoperatively, therefore, involving physical therapists early on in the course of treatment should be considered for most cases (De Smet et al., 1997; Hughes et al., 2003; Jürgensen et al., 2002). Their role includes addressing typical OCD patients’ impairments, including reduced lower or upper extremity strength, limited range of motion (ROM), and altered neuromuscular control (Paterno et al., 2014).
Following an elbow microfracture surgery, a rehabilitation program should be developed and carried out by a certified physiotherapist. This program should be divided into four phases: the acute, intermediate, advanced, and return‐to‐sport phase (Paterno et al., 2014). Throughout those phases, the physiotherapist helps the patient restore pre‐operative full elbow ROM, reduce swelling and pain, strengthen the affected joint, and initiate weight‐bearing. To achieve the goals, several therapeutic modalities are used including joint mobilizations, electrical stimulation, cryotherapy and plyometric, and strengthening exercises.
3. CONCLUSION
We present a case of bilateral knee and elbow osteochondral lesions arising from an autosomal dominant pathogenic variant in the gene ACAN in a 13‐year‐old male of French Canadian descent. The patient was treated with left elbow arthroscopy with free fragment removal and microfracture of the OCD lesion.
Familial osteochondritis dissecans is a rare disease for which no consensus has been established on the best course of treatment. The patient presented in this case report has shown significant improvements following microfracture surgical intervention. One year after left elbow surgery, the scars had nicely healed. Left elbow extension was deficient by 20°. Flexion was complete and painless. Return to all activities with no restrictions was allowed 16 weeks after the operation. The patient had again been educated about his increased risk of injuries and recommended to avoid sports that put excessive stress on his joints. Constant monitoring will be required as the patient is at increased risk of other joint lesions due to his diagnosed aggrecanopathy.
To the best of our knowledge, this is the first reported case of osteochondritis dissecans due to a novel frameshift pathogenic variant that leads to presumed haploinsufficiency. This family presents with variable expressivity, and since the proband is more severely affected compared to his relatives and to other previously reported patients this might be attributed hypothetically to modifier genes. Such modifier genes could influence aggrecan expression levels, post‐translational processing, or other aspects of matrix biology. Further research is required to improve diagnostic modalities and evidence‐based treatment for patients affected by this condition.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors attest that they meet the current ICMJE criteria for Authorship.
PATIENT CONSENT
The parents of the patients were informed that data regarding the case would be presented for publication and they consented.
ETHICAL COMPLIANCE
Genetic testing was offered after a signed informed consent was completed, in compliance with our Institutional Review Board.
ACKNOWLEDGMENTS
We thank Mark Lepik (Shriners Hospitals for Children) for providing graphic design on all figures.
ANNEX 1. International Cartilage Repair Society (ICRS) OCD lesion classification
| Stage | Definition |
|---|---|
| Stage I | Stable lesion with a continuous but softened area covered by intact articular cartilage |
| Stage II | Lesion with partial articular cartilage discontinuity, stable when probed |
| Stage III | Lesion with complete articular cartilage discontinuity, but no dislocation |
| Stage IV | Empty defect, or defect with a dislocated fragment or loose fragment within the bed |
Brittberg M., Winalski C. S. Evaluation of cartilage injuries and repair. Journal of Bone and Joint Surgery. 2003;85‐A Suppl 2:58–69.
Denis, A. , Chergui, S. , Basalom, S. , Campeau, P. M. , Janelle, C. , & Pauyo, T. (2022). Variable expressivity in a family with an aggrecanopathy. Molecular Genetics & Genomic Medicine, 10, e1773. 10.1002/mgg3.1773
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abou Tayoun, A. N. , Pesaran, T. , DiStefano, M. T. , Oza, A. , Rehm, H. L. , Biesecker, L. G. , & Harrison, S. M. (2018). Recommendations for interpreting the loss of function PVS1 ACMG/AMP variant criterion. Human Mutation, 39(11), 1517–1524. 10.1002/humu.23626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, C. S. , Vitale, M. A. , & ElAttrache, N. S. (2011). Elbow arthroscopy: Capitellar osteochondritis dissecans and radiocapitellar plica. Instructional Course Lectures, 60, 181–190. [PubMed] [Google Scholar]
- Ananthaharan, A. , & Randsborg, P. H. (2018). Epidemiology and patient‐reported outcome after juvenile osteochondritis dissecans in the knee. The Knee, 25(4), 595–601. 10.1016/j.knee.2018.02.005 [DOI] [PubMed] [Google Scholar]
- Bellelli, A. , Avitto, A. , & David, V. (2001). Spontaneous remission of osteochondritis dissecans in 8 pediatric patients undergoing conservative treatment. La Radiologia Medica, 102(3), 148–153. [PubMed] [Google Scholar]
- Bexkens, R. , van Bergen, C. J. , van den Bekerom, M. P. , Kerkhoffs, G. M. , & Eygendaal, D. (2018). Decreased defect size and partial restoration of subchondral bone on computed tomography after arthroscopic debridement and microfracture for osteochondritis dissecans of the capitellum. The American Journal of Sports Medicine, 46(12), 2954–2959. 10.1177/0363546518790455 [DOI] [PubMed] [Google Scholar]
- Bexkens, R. , van den Ende, K. I. , Ogink, P. T. , van Bergen, C. J. , van den Bekerom, M. P. , & Eygendaal, D. (2017). Clinical outcome after arthroscopic debridement and microfracture for osteochondritis dissecans of the capitellum. The American Journal of Sports Medicine, 45(10), 2312–2318. 10.1177/0363546517704842 [DOI] [PubMed] [Google Scholar]
- Brittberg, M. , & Winalski, C. S. (2003). Evaluation of cartilage injuries and repair. Journal of Bone and Joint Surgery, 85(Suppl 2), 58–69. [DOI] [PubMed] [Google Scholar]
- Camp, C. L. , Dines, J. S. , Degen, R. M. , Sinatro, A. L. , & Altchek, D. W. (2016). Arthroscopic microfracture for osteochondritis dissecans lesions of the capitellum. Arthroscopy Techniques, 5, e477–e481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell, J. D. , & ElAttrache, N. S. (2008). Clinical outcome of arthroscopic treatment of OCD lesions of the capitellum. American Orthopaedic Society for Sports Medicine. [Google Scholar]
- Dateki, S. , Nakatomi, A. , Watanabe, S. , Shimizu, H. , Inoue, Y. , Baba, H. , Yoshiura, K. I. , & Moriuchi, H. (2017). Identification of a novel heterozygous mutation of the Aggrecan gene in a family with idiopathic short stature and multiple intervertebral disc herniation. Journal of Human Genetics, 62(7), 717–721. 10.1038/jhg.2017.33 [DOI] [PubMed] [Google Scholar]
- De Smet, A. A. , Ilahi, O. A. , & Graf, B. K. (1997). Untreated osteochondritis dissecans of the femoral condyles: Prediction of patient outcome using radiographic and MR findings. Skeletal Radiology, 26(8), 463–467. 10.1007/s002560050267 [DOI] [PubMed] [Google Scholar]
- Edmonds, E. W. , & Polousky, J. (2013). A review of knowledge in osteochondritis dissecans: 123 years of minimal evolution from König to the ROCK study group. Clinical Orthopaedics and Related Research, 471(4), 1118–1126. 10.1007/s11999-012-2290-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, B. G. , & Briggs, M. D. (2016). The aggrecanopathies; an evolving phenotypic spectrum of human genetic skeletal diseases. Orphanet Journal of Rare Diseases, 11(1), 86. 10.1186/s13023-016-0459-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gkourogianni, A. , Andrew, M. , Tyzinski, L. , Crocker, M. , Douglas, J. , Dunbar, N. , Fairchild, J. , Funari, M. F. , Heath, K. E. , Jorge, A. A. , & Kurtzman, T. (2017). Clinical characterization of patients with autosomal dominant short stature due to aggrecan mutations. The Journal of Clinical Endocrinology & Metabolism, 102(2), 460–469. 10.1210/jc.2016-3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, J. A. , Cook, J. V. , Churchill, M. A. , & Warren, M. E. (2003). Juvenile osteochondritis dissecans: A 5‐year review of the natural history using clinical and MRI evaluation. Pediatric Radiology, 33(6), 410–417. 10.1007/s00247-003-0876-y [DOI] [PubMed] [Google Scholar]
- Jürgensen, I. , Bachmam, G. , Schleicher, I. , & Haas, H. (2002). Osteochondritis dissecans‐an easy classification in MRI. Zeitschrift Fur Orthopadie Und Ihre Grenzgebiete, 140(1), 58–64. [DOI] [PubMed] [Google Scholar]
- Kawaguchi, Y. , Osada, R. , Kanamori, M. , Ishihara, H. , Ohmori, K. , Matsui, H. , & Kimura, T. (1999). Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine, 24, 2456–2460. [DOI] [PubMed] [Google Scholar]
- Kiani, C. , Chen, L. , Wu, Y. J. , Yee, A. J. , & Yang, B. B. (2002). Structure and function of aggrecan. Cell Research, 12, 19–32. [DOI] [PubMed] [Google Scholar]
- Lewine, E. B. , Miller, P. E. , Micheli, L. J. , Waters, P. M. , & Bae, D. S. (2016). Early results of drilling and/or microfracture for grade iv osteochondritis dissecans of the capitellum. Journal of Pediatric Orthopaedics, 36, 803–809. [DOI] [PubMed] [Google Scholar]
- Nilsson, O. , Guo, M. H. , Dunbar, N. , Popovic, J. , Flynn, D. , Jacobsen, C. , Lui, J. C. , Hirschhorn, J. N. , Baron, J. , & Dauber, A. (2014). Short stature, accelerated bone maturation, and early growth cessation due to heterozygous aggrecan mutations. The Journal of Clinical Endocrinology & Metabolism, 99, E1510–E1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Online Mendelian Inheritance in Man, OMIM® . (2017) Johns Hopkins University, Baltimore, MD. MIM Number: 165800: 09/28/2017. https://omim.org/
- Paterno, M. V. , Prokop, T. R. , & Schmitt, L. C. (2014). Physical therapy management of patients with osteochondritis dissecans: A comprehensive review. Clinics in Sports Medicine, 33(2), 353–374. 10.1016/j.csm.2014.01.001 [DOI] [PubMed] [Google Scholar]
- Richards, S. , Aziz, N. , Bale, S. , Bick, D. , Das, S. , Gastier‐Foster, J. , Grody, W. W. , Hegde, M. , Lyon, E. , Spector, E. , & Voelkerding, K. (2015). Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine, 17(5), 405–423. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley, P. J. , & Mort, J. S. (2014). The role of aggrecan in normal and osteoarthritic cartilage. Journal of Experimental Orthopaedics, 1, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stattin, E. L. , Wiklund, F. , Lindblom, K. , Onnerfjord, P. , Jonsson, B. A. , Tegner, Y. , Sasaki, T. , Struglics, A. , Lohmander, S. , Dahl, N. , Heinegard, D. , & Aspberg, A. (2010). A missense mutation in the aggrecan C‐type lectin domain disrupts extracellular matrix interactions and causes dominant familial osteochondritis dissecans. American Journal of Human Genetics, 86, 126–137. 10.1016/j.ajhg.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara, M. , Mura, N. , Sasaki, J. , Harada, M. , & Ogino, T. (2008). Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. The Journal of Bone and Joint Surgery American, 90(Suppl 2 Pt 1), 47–62. [DOI] [PubMed] [Google Scholar]
- Tatsi, C. , Gkourogianni, A. , Mohnike, K. , Dearment, D. , Witchel, S. , Andrade, A. C. , Markello, T. C. , Baron, J. , Nilsson, O. , & Jee, Y. H. (2017). Aggrecan mutations in nonfamilial short stature and short stature without accelerated skeletal maturation. Journal of the Endocrine Society, 1, 1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf, C. A. , Stone, R. M. , Giveans, M. R. , & Lervick, G. N. (2012). Magnetic resonance imaging after arthroscopic microfracture of capitellar osteochondritis dissecans. The American Journal of Sports Medicine, 40(11), 2549–2556. 10.1177/0363546512458765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


